Abstract
An entirely unknown species of coronavirus (COVID-19) outbreak occurred in December 2019. COVID-19 has already affected more than 180 million people causing ~3.91 million deaths globally till the end of June 2021. During this emergency, the food nutraceuticals can be a potential therapeutic candidate. Curcumin is the natural and safe bioactive compound of the turmeric (Curcuma longa L.) plant and is known to possess potent anti-microbial and immuno-modulatory properties. This review paper covers the various extraction and quantification techniques of curcumin and its usage to produce functional food. The potential of curcumin in boosting the immune system has also been explored. The review will help develop insight and new knowledge about curcumin's role as an immune-booster and therapeutic agent against COVID-19. The manuscript will also encourage and assist the scientists and researchers who have an association with drug development, pharmacology, functional foods, and nutraceuticals to develop curcumin-based formulations.
Keywords: curcumin, separation methods, human immune system, immunological activity, human health, functional food, COVID-19
Introduction
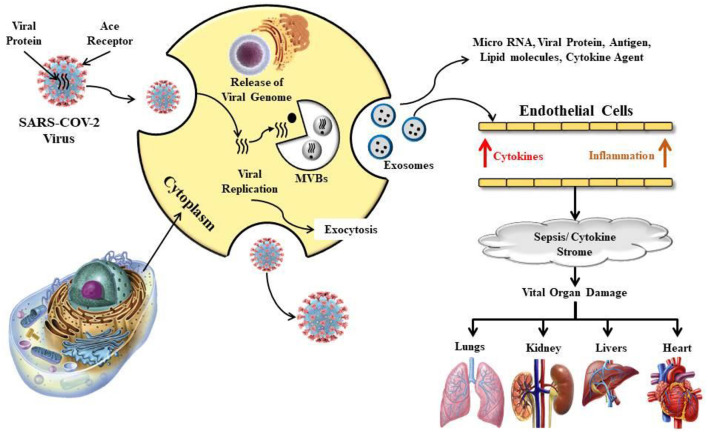
The human immune system is the key to our body's defense against invading pathological microorganisms, extrinsic agents and conditions like cancer. An individual with a weakened immune system is more likely to suffer from infections caused by pathogens like bacteria, viruses, parasites and fungi (1). An outbreak of new viral infection was first recorded in China at the end of December 2019, which has wreaked havoc across the globe since then. This latest virus, known as Severe Acute Respiratory Syndrome Coronavirus 2 or SARS-CoV-2, is a highly infectious pathogen with high levels of morbidity and mortality, leading to a global pandemic (2–5). Coronaviruses are huge, pleomorphic but mostly spherically enveloped, non-segmented, positive-stranded RNA viruses i.e., +ssRNA having 5′ -cap structure and 3′-poly -A tail, and possess the largest genome (27–32 kb) in all RNA viruses (6). The infectious SARS-CoV-2 varies in size from 50 to 200 nm (dia) and is comprised of the following major structural proteins: (i) envelope (E), (ii) membrane (M), (iii) nucleocapsid (N), and (iv) spike (S) (trimeric) (6, 7). They contain protrusions (80–120 nm dia) of glycoproteins above the surface (8). M protein and E protein are involved in the virus assembly whereas the S protein creates the big projections above the surface (9, 10). Through these protrusions, S protein attaches to the cell membrane of a host by targeting the angiotensin-converting enzyme 2 (ACE2) receptors of the host cell, mainly found in the respiratory epithelium and alveoli of the lungs (11, 12). ACE2 protein is also expressed in several organs of humans including kidney and intestine, which are therefore, the main targets of CoV (13). The molecular mechanisms of SARS-COV-2 virus infection in the human respiratory system, as well as the harmful consequences on other vital organs of the human body have been presented in Figure 1.
Figure 1.
Schematic presentation on the molecular mechanisms involved in the SARS-COV-2 virus infection in the human respiratory system, as well as the detrimental effects on other important organs of the human body.
The most likely cause of the SARS-CoV-2 infection-related symptoms (COVID-19) is attributed to its impact on the human immune response. Like SARS and MERS, this coronavirus also affects the host's innate immune system and causes elevated levels of pro-inflammatory cytokines like IL1B, IFN-γ, IP10, MCP1, MIP1A, and TNF-α and also reduces the body's lymphocyte count, thus suppress in the adaptive immune system (14, 15). Although supportive management guidelines and protocols are being regularly updated, no anti-viral drug having specific activity against SARS-CoV-2 has been identified yet. Presently, several drugs across the globe are in clinical trials for the management of COVID-19 and convalescent plasma therapy could be a future alternative for serious patients (15–18). International and national organizations dedicated to public health, advocate certain practices to prevent the spread of this novel virus-like, such as maintaining social distance, regular handwashing with soap or alcohol sanitizer, the use of an approved face mask to cover the mouth and nose, and the strengthening the body's immune system (3, 4, 15, 16, 19). While there are several medicines available in the market which claim to be immunoboosters; however, the most healthy, safe and cost-effective way to strengthen our immune system is by consuming healthy functional foods. Functional food can therefore play an important role in improving human immunity against deadly viruses like SARS-CoV-2.
The term “functional food” can be described as “food with one or more target beneficial effects on the human body other than nutritional effects, and food should reduce the risk of any disease or improve human health,” as Functional Food Science in Europe (FUFOSE) indicated (20). Functional foods are either natural food or one or more additional compounds that can boost consumer efficiency at any age or in a particular age group (21–24). Furthermore, there is scientific evidence of a beneficial impact on human health in the functional food produced by incorporating plant parts that have known or unknown bioactive substances (23, 25). The biological properties of such bioactive compounds can influence human health as antioxidant activity, anti-inflammatory activity, antimicrobial activity, anti-diabetic activity, and anticancer activity, etc. (5, 25, 26). Several bioactive components like flavonoids, phenolic acid, alkaloids, etc. are present in plants such as Allium sativum, Curcuma longa, Ocimum tenuiflorum, Phyllanthus emblica, Piper nigrum, Tinospora cordifolia, etc. which contribute to their therapeutic characteristics, as presented in Table 1 (25, 51, 60–63). As found in previous studies, these plants are immunologically active, such as enhanced antibody production and macrophage mobility, cell-mediated immunity (64). Therefore, these plants can be included in food formulations to develop functional foods, due to their bioactive substances and medicinal activities.
Table 1.
Summary of bioactive compounds present in different food samples along with their mode of action.
| Food sample | Particular bioactive compound | Immunity against | Bioassay | Doses | Mode of action | References |
|---|---|---|---|---|---|---|
Amla (Phyllanthus emblica) 
|
Chlorogenic acid, quercitrin, and myricetin | SARS-CoV-2 | Docking simulation using Schrödinger maestro 2018-1 MM share version | – | Bind to NSP15 endoribonuclease, main protease, and receptor binding domain of prefusion spike protein | (27) |
| 7-ketositosterol, quercetin, epigallocatechin, and phyllaemblic acid-C | COVID-19 Mpro | GLIDE docking protocol | – | Inhibited the COVID-19 Mpro activity by binding to it. | (28) | |
| – | Bacterial and viral infection | Flow cytometric analysis, and ELISA test | 500 mg/kg | Enhanced immunomodulatory efficacy by increasing CD4, CD8, CD16, CD19, IgM, and IgG levels in the blood, and albumin and globulins in serum. | (29) | |
| Astragalin, catechin, chebulagic acid, corilagin, ellagic acid, gallic acid, geraniin, hypophyllanthin, niranthin, phyllanthin, phyltetralin, and quercetin | Immune-related disorder | – | – | Inhibited the NF-κB signaling pathway; thus, influencing innate and adaptive immunity. | (30) | |
| Gallic acid, and ellagic acid | Macrophage cell lines, RAW 264.7 | MTT assay, reverse transcription-polymerase chain reaction (qRT-PCR), and western blotting assay | 2 mg/mL | Down-regulated NF-κB, COX-2, and iNOS. | (31) | |
| – | Tuberculosis | Nitric Oxide (NO) release assay, and Assay of macrophage phagocytic activity | - | Enhanced proliferation of PBMCs, increased NO release, and improved macrophages phagocytic activity. | (32) | |
Black Pepper (Piper nigrum) 
|
Coumaperine | Bacterial infection | NF-κB-Luciferase Reporter Gene Assay | – | Reduction in transcription Nuclear Factor kappa B (NF-κB) activity. | (33) |
| Piperine | Tumor | Cytokine array, and flow cytometry | 200 mg/kg | Suppressed some cytokine and chemokine levels including CXCL7, sICAM-1, and L-selectin. Promoted type 1 T helper cell, and suppressed neutrophil, basophil, type 2 T helper cell, and regulatory T cell. | (34) | |
| Piperine | Food allergy | ELISA test, and RT-qPCR | 100 mg/kg | Decreased levels of IgE and mMCP-1. The expressions of Th2 and Th17 cell-associated cytokines were down-regulated and the levels of Treg cell-associated cytokines were up-regulated. | (35) | |
| Piperine | Upper respiratory tract injury | ELISA test, RT-PCR, and Western blotting analysis | 50 mg/kg | Improves the epithelial barrier dysfunction via enhancing the activation of Nrf2/HO-1 signaling | (36) | |
| Piperine | SARS-CoV-2 | Molecular docking | – | Piperine docked to nucleocapsid protein as a potential inhibitor of the RNA-binding site. | (37) | |
| Piperine | Inflammatory metabolic diseases | Nitrite assay, western blot analysis, and immunofluorescence | 50 mg/kg | Inhibited inducible nitric oxide synthase (iNOS)-mediated NO, and IL-1β, IL-6, TNF-α. Suppressed IκB degradation and further inhibited the cytosol-nucleus translocation of the p65 subunit by targeting IKK-β | (38) | |
| Piperine | – | – | 1,000 mg/kg | Enhance the immune function via augmentation of the immunoglobulin (IgM) levels. Increased IgM and IgG levels. | (39) | |
Cumin (Cuminum cyminum) 
|
Cuminaldehyde, eugenol | Human campylobacteriosis | Histopathological analyses, in situ immunohistochemistry, and pro-inflammatory mediators | 200 mg/kg | Alleviated enteropathogenic-induced apoptotic cell responses in colonic epithelia. Secretion of pro-inflammatory mediators, including nitric oxide and IFN-γ to mesenteric lymph nodes. | (40) |
| – | – | Immunohistochemical investigations | 500 mg/kg | Trk-A immunoreactivity. | (41) | |
| (3,4,5-trihydroxy-6-((4-isopropylbenzyl) oxy)-tetrahydro-2H-pyran-2-yl) methyl (E)-3-(4-propoxyphenyl) acrylate | Lipopolysaccharide (LPS)-stimulated RAW264.7 cells | Immunoblotting, and ELISA test | – | Suppressed the expression levels of inducible nitric oxide synthase and cyclooxygenase-2. Suppressed the phosphorylation of NF-κB/p65, p-IKK-α/β, and p-IκB. | (42) | |
| Cuminol, cuminique alcohol, cuminaldehyde, and cymine | Avian influenza (H9N1) and Newcastle disease | Assay of serum antibody titers | 250–500 mg/kg | Improved the antibody titers and humoral immune response. | (43) | |
| Coumarin, and anthraquinone | Bacterial and viral diseases | Enzyme ZellBio Test and alkaline phosphatase (AKP) activity test | 10 g/kg | Improved the glutathione peroxidase (GPX) enzyme GPX and AKP activity. | (44) | |
Garlic (Allium sativum) 
|
- | ZEN toxicity | Immunity and oxidative stress biomarkers | 30 g/kg | Down-regulation of interleukin-4 (IL-4) and interleukin 1 beta (IL-1β) genes alongside significant up-regulation of tumor necrosis factor-alpha (TNF-α) and heat shock protein 70 (HSP70) genes. | (45) |
| Sulfur-containing compound | – | Hematological profile | 10 g/kg | Improved total leucocyte count (TLC), lymphocytes, and monocyte along with neutrophil count which ultimately strengthened the innate and adaptive immunity. | (46) | |
| Allicin | Copper sulfate-induced toxicity | Hematological and immunological parameters test | 10 g/kg | RBCs, Hb, PCV%, MCV, WBCs were increased and by modulating the deranged differential leukocyte count and phagocytic activity as well as a serum level of nitric oxide, lysozyme activity, and IgM. | (47) | |
| Allicin, alliin | – | Commercial test kit | 0.25–0.075 g/kg | Improved immunoglobulin M (IgM), and IgG. | (48) | |
| Allicin | Infectious diseases | Immunological parameters test | – | Improved superoxide dismutase (SOD) and catalase (CAT) activities. | (49) | |
| – | COVID-19 | – | – | Decreased the expression of proinflammatory cytokines and reverse the immunological abnormalities. | (50) | |
Giloy (Tinospora cordifolia) 
|
20a hydroxy ecdysone, amritoside, apigen-6-C-glucosyl7-O-glucoside, epicatechin, and tinosporine B, | COVID-19 Mpro | GLIDE docking protocol | – | Inhibit the COVID-19 Mpro activity by binding to it | (28) |
| Cordifolioside-A, Magnoflorine, β-Ecdysone, and Palmatine | SARS-CoV-2 spike-protein | Cytological study | 142 μg/kg | Reduced lipopolysaccharide (LPS) induced expression of TNF-α, IL-6, and IL-1β. | (51) | |
| Berberine | COVID-19 3CLpro | Molecular docking analysis | - | Inhibit viral replication by binding to the active site. | (52) | |
Ginger (Zingiber officinale) 
|
Gingerols | – | Hematological profile | 10 g/kg | Improved total leucocyte count (TLC), lymphocytes, and monocyte along with neutrophil count which ultimately strengthened the innate and adaptive immunity. | (46) |
| Zingiberene | Aeromonas hydrophila infection | Immunological assay | 10 g/kg | By increasing the production of H2O2 and NO and SOD and lysozyme activities. | (53) | |
| – | Aeromonas hydrophila infection | Plasma humoral immune analysis | 10 g/kg | Lowered the expression of tumor necrosis alpha (tnfa), interleukin 1 beta (il1b), and interleukin 8 (il8), and higher interleukin 10 (il10) genes. | (54) | |
| Zingiberene, gingerol, and zingerone | – | Mucosal parameters test | 1–4 g/kg | Increased mucosal immune parameters such as lysozyme, alkaline phosphatase, and total Immunoglobulins. | (55) | |
| Gingerol, gingerdiol, and gingerdione | Bacterial diseases | RT-PCR | 30 g/kg | Lysozyme mRNA expression levels were up-regulated. | (56) | |
| Gingerol, shogaols, gingerdiol, and gingerdione | Bacterial diseases (Escherichia coli) | qRT-PCR | 15 g/kg | Regulating the IL-1β, IL-6, and IFN-γ cytokines expression levels. | (57) | |
Tulsi (Ocimum tenuiflorum) 
|
– | Asthma | Immunologic and inflammatory markers measurement | 0.75, 1.50, and 3.00 mg/mL | By increasing the IL-4, IgE, PLA2, and TP levels, and decreasing the IFN-γ/IL-4 ratio. | (58) |
| – | Staphylococcus aureus and Escherichia coli | RT-PCR | 5–10 g/kg | Improved the relative mRNA expression levels of TLR 2, TLR 4, and TLR 7. | (59) |
- Not reported.
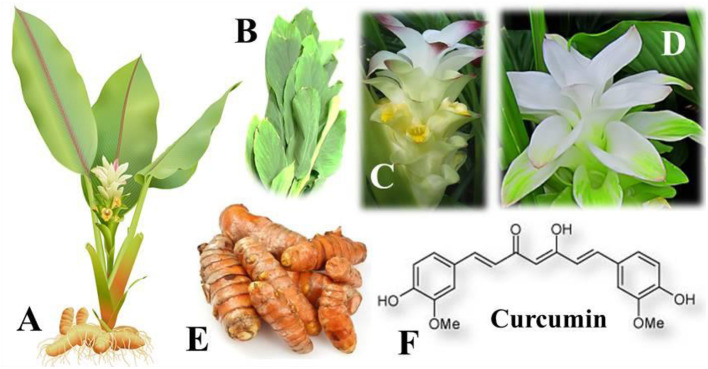
Among many bioactive compounds, curcumin, a natural and major bioactive compound found in rhizomes of turmeric (Curcuma longa L.) plant (Figure 2) which belongs to the family, Zingiberaceae. It is a hydrophobic phenolic compound with the chemical name 1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. There have been 3 major compounds, including curcumin (diferuloylmethane), demethoxycurcumin, and bisdemethoxycurcumin, which are chemically part of the “curcuminoid” family. Curcumin is the most biologically active form of them. The native Indian plant is currently farmed in Asian countries, such as Indonesia and China that are characterized by warm and wet tropical climates. Apart from its uses as a spice or flavoring or coloring agent in food preparations from centuries, it is commonly used to cure various viral infections such as several cases of flu and cold symptoms (65). Curcumin from plant materials is obtained using several methodologies, from traditional extraction processes, like Soxhlet extraction, maceration, and solvent extraction to recent extraction technologies, such as extraction by means of ultrasound, microwaves, enzymes, and supercritical liquids. The isolation and purification of curcumin from crude extracts is accomplished by techniques such as column chromatography, high-performance liquid chromatography (HPLC), high-speed counter-current chromatography, supercritical fluid chromatography, either alone or in combination (66–68). The biological effect of curcumin on some of the global ailments, including cardiovascular diseases, diabetes, metabolic syndrome, and arthritis, is defined by molecular targets and physiological effects on animals (69). Multiple mechanisms have been derived from these molecules to protect the health, owing to its biological properties including immuno-modulatory or immunity-boosting characteristics (Figure 3) (3, 65).
Figure 2.
Turmeric (Curcuma longa L.) plants are with the following features: (A) A highly branched standing C. longa plant with cylindrical rhizomes of yellowish to orange color. (B) Broad long and simple leaves with long petioles (leaf stems) grow from branching rhizomes that lie just below the surface of the soil. (C) Inflorescence is terminal, spike-shaped, and cylindrical, having laterally green united bracts with reddish spots. (D) It produces very pretty, tall white flower spikes. (E) C. longa rhizomes with yellowish to orange color. (F) The natural and major bioactive compound of the C. longa plant.
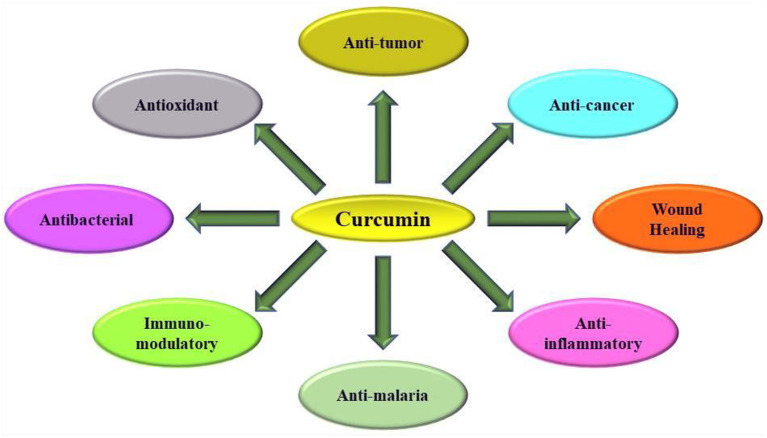
Figure 3.
Important biological activities performed by curcumin in human health (3, 65).
Pure curcumin is a yellow-orange-colored crystalline substance, which normally comes in a powder form. However, the direct application of curcumin is much less due to a slightly bitter taste, poor water solubility, poor chemical stability, particularly under alkaline conditions, and low bioavailability, attributed to low bioaccessibility and chemical transformation due to metabolic enzymes in the gastrointestinal (GI) tract. Further, during storage curcumin is more susceptible to chemical degradation, especially when exposed to high temperatures, alkaline conditions and light (70, 71). It is essential to overcome these barriers by developing efficient approaches so that curcumin can be successfully incorporated into functional foods, supplements, and pharmaceuticals. Therefore, many researchers are investigating various methods to conquer such hurdles and among that encapsulation technology is found to be one of the most significant means of improving the bioavailability, water solubility, and protecting curcumin against chemical degradation (72, 73). Both microencapsulation and nanoencapsulation have been found to be efficient processes to overcome the above problems. These techniques can even mask the bitter flavor of curcumin when consumed directly or in a food product.
Further, curcumin is safe for consumption, even at relatively higher levels as per toxicity studies. Because of its broad-spectrum biological activity and low toxicity, it has been extensively researched as a nutraceutical component for use in functional foodstuffs (73). Such curcumin-incorporated food products will improve the immunity of the human body and may combat the viral infections including coronavirus. However, an essential step toward functional development is the investigation of the biological effects and immune-enhancement properties of curcumin in the healthy population and people diagnosed with non-compatible diseases by giving greater information about its therapeutic advantages (with respect to in-vitro and animal studies). Therefore, the selection of optimal extraction conditions to obtain extracts and the biological activity of the extracts play a crucial role in recognizing and using curcumin in the manufacture of functional foods (74).
Taking into account the above aspects, the effective extraction and purification techniques which should be safe, eco-friendly, economical and efficient, must be followed to get pure curcumin. Therefore, the review addressed the different methods of curcumin extraction, isolation, and quantification in order to give the researchers a better understanding of the processes. The objective of this review is to assess the immunomodulatory impact of curcumin on the novel coronavirus and its potential application in the prevention of COVID-19. It also focuses on the use of curcumin to produce functional foods that can improve human body immunity to novel coronavirus. The main purpose of the review is therefore to address curcumin's immunological activity that will help food scientists and researchers formulate functional food to minimize the outbreak of the coronavirus.
Curcumin Associated Functional Foods, Human Immune System, and COVID-19
The use of food supplements like curcumin may be important if the immune system is to be strengthened and disease conditions like respiratory infections are to be prevented (3, 4, 75–78). It can provide a promising option in terms of functional food products and is an important part for elderly patients or people at risk, whether in hospitals or nursing homes. At every stage of human growth and development, the proper intake of essential nutrients is needed, particularly in certain physiological conditions (old age, severe shortcomings in nutrition, and pathophysiologically stressful situations). The natural bioactive compounds like curcumin extracted from plants have become important in human health nowadays, leading to food scientist's application into food and functional food formulation (3, 4, 22–24, 77, 79). Although the development of COVID-19 vaccines is considered an optimal choice toward the prevention of the disease, their development requires an expensive and time-consuming process besides evaluation of toxicity and effectiveness in the population (4, 80–84). Therefore, it may be vital to determine the impact of certain food ingredients as possible candidates for management with a targeted approach. The use of curcumin has been demonstrated in traditional herbal medicine (2, 4). Curcumin possesses a wide variety of biological activities, improving human health, as illustrated in Figure 3, which has piqued the interest of many researchers and scientists (75, 81, 85–89). In addition to these functions, immunological activity is the most essential property of curcumin and it has therefore been shown to be used against anti-immune diseases (77, 90–92).
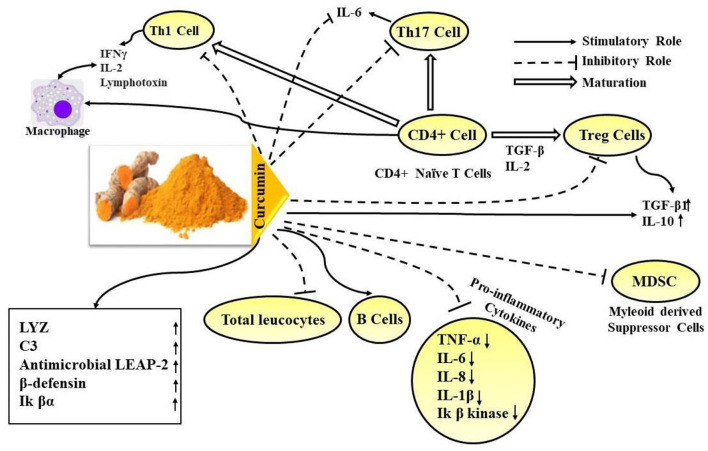
Recently, some studies have identified potential molecular targets in the viral replication cycle to determine the potential role of curcumin in the fight against SARS-CoV-2 (4, 84, 93–98). White blood cells (WBCs) are the key players in human immune systems and specifically lymphocytes have been found to produce increased levels of immunoglobulins (IgG and IgM) when Nawab et al. (77) investigated the effect of curcumin on the immune profiles of the blood. Many researchers have worked on curcumin and reported the effects of curcumin on immunity as shown in Table 2. The immunomodulatory effects of curcumin with a specific focus on the possible effects on the different types of WBCs have been described in Figure 4. The image shows the overall effects of curcumin on different types of WBCs (especially the different types of T cells).
Table 2.
Some recent findings on the effect of Curcumin on the immune system.
| Bioassay | Dose | Remark | References |
|---|---|---|---|
| ELISA assay | 100–200 mg/kg diet | Significant increase in estradiol, follicle-stimulating hormone levels, IgA, IgG, luteinizing hormone, and complement C3 activity in the serum (P < 0.05). | (99) |
| Flow cytometry | - | Curcumin suppressed inflammatory monocytes across the blood-brain barrier (BBB) in Experimental Autoimmune Encephalomyelitis (EAE) mice, suppressed the spread of microglia, and limited infiltration of other effector immune cells, resulting in a reduction in EAE morbidity from 100 to 30%. It was due to the immunomodulatory impact of curcumin-loaded high-density lipoprotein-mimicking peptide-phospholipid scaffold (Cur-HPPS) on inflammatory monocytes, which inhibited the activation of NF-κB and decreased the expression of adhesion-and migration-related molecules. | (88) |
| Serum biochemical parameters assay | 196.11–788.52 mg/kg diet | Curcumin up-regulated the mRNA levels of LYZ, C3, and antimicrobial peptides [hepcidin, liver-expressed antimicrobial peptide-2 (LEAP-2), β-defensin]; anti-inflammatory cytokines of interleukin-10 (IL-10); an inhibitor of κBα (IκBα); and transforming growth factor β1 (TGF-β1); whereas, down-regulated pro-inflammatory cytokines of tumor necrosis factor-α (TNF-α), IL-6, IL-8, and IL-1β; IκB kinases (IKKα, IKKβ, and IKKγ) and nuclear factor kappa B p65 (NF-κB p65) mRNA levels in the liver and blood. | (89) |
| Serum biochemistry assay | 100–200 mg/kg diet | A substantial reduction in total leukocytes as a result of the reduction in lymphocytes was observed in animals receiving curcumin and was observed for total serum protein and globulin levels. | (75) |
| Serum inflammatory cytokines analysis | 100-300 mg/kg diet | The curcumin treatment group had reduced inflammatory responses (TNF-α, IL-1β, and IL-6,) as compared to the control group. | (92) |
| Western blot analysis | 100–300 mg/kg diet | TLR4, PCNA, and its downstream gene expression, as well as protein expression (NFκB, TLR4, and PCNA), were significantly downregulated in the heat stress curcumin supplemented group as compared to the control group. | (92) |
| ELISA assay | 200 μg/mouse | Curcumin suppressed the development of antigen-specific IgE and IgG1, inhibited CD4+ T function, and decreased ovalbumin-sensitized B-cell memory. | (100) |
| Immunofluorescence assay | - | Expression of p-STAT3Y705 and PD-L1 was similarly decreased in vivo. | (101) |
| Flow cytometry | - | After curcumin treatment, the anti-tumor immune response was remarkably improved by rising CD8 positive T cells and decreasing Tregs and MDSCs. | (101) |
| Hemagglutination assay | 5–10 mg/kg diet | Curcumin nanoparticle significantly induced primary humoral immune response with 9.00 ± 1.00 antibody titer (P < 0.05), free curcumin suppressed immunity with 3.33 ± 0.67 antibody titer compared to control. Similar findings were found with secondary humoral antibody titers. | (102) |
| Intracellular staining | 10,000 mg/kg diet | Curcumin diet reduced all populations of Th1/Th2/Th17 cells and attenuated various symptoms such as splenomegaly in scurf mice. | (91) |
| Cytokine measurement assay | 10,000 mg/kg diet | In vitro studies showed that curcumin treatment directly decreased the development of Th1/Th2 /Th17 cytokines in CD4 + T cells from IL-4, IL-17A, and IFN-γ. | (91) |
| Oxidative stress and immunological assay | 50–200 mg/kg diet | Total IgM and IgG levels increased significantly, in particular. | (85) |
Figure 4.
Schematic presentation on immunomodulatory effects of curcumin. Naïve CD4+ T cells are capable of differentiating into different T cell subsets like the Th1, Th2, Th17, Treg cells, etc. The possible effects of curcumin on some of these specific types of T cells that differentiate out of the naïve T cells have been shown here. Curcumin is seen to exert an inhibitory role in Th1 and Th17 cells which are known to be key players in pro-inflammatory T cell-mediated responses. It has also been found to inhibit Treg, Myeloid-derived suppressor T cells, overall total leucocyte counts. Curcumin has also been found to inhibit pro-inflammatory cytokine response like production of TNF-α, IL-6, IL-8, IL-1β, and Ik β kinase and promote the upregulation of anti-inflammatory cytokines like IL10, TGF-β1. Curcumin has also been seen to increase B-cells and the production of immunoglobulins like IgA and IgG.
The key pathway of entry for SARS-CoV-2 is through our respiratory system, and it has been particularly found to enter cells by binding to the ACE2 receptor (angiotensin-converting enzyme 2) (78). The spike protein present on the COVID-19 surface is pinched within the host cell that binds to the ACE2 receptor (103). TMPRSS-2 is another active site for the entry of this coronavirus (104). Curcumin has been found to have a stronger binding capacity to the ACE2 receptor, and thus may potentially block the entry of SARS-CoV-2 (105). It also has been seen to decrease the expression of TMPRSS-2, which demonstrated the ability of curcumin against SARS-CoV-2 (87).
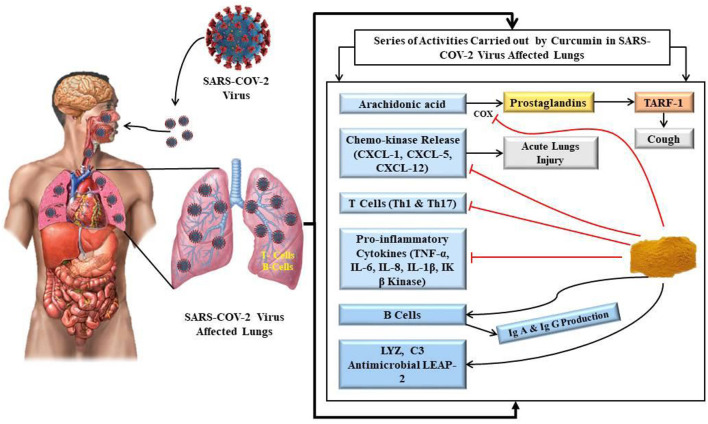
As mentioned above, the SARS-CoV-2 virus enters the human body through the upper respiratory tract. While the majority of the patients are seen to manifest mild to moderate symptoms, some are seen to have a severe pathological immune response requiring hospitalization, life support and may eventually turn out to be fatal. Many patients have the typical symptoms of upper respiratory tract infection like cough, sore throat, running nose, fever and others (106–111). Patients with severe respiratory illness are seen to have hyper-immune response diffuse alveolar damage, necrosis of the epithelium, epithelial necrosis, fibrin deposition and hyaline membrane formation. At the cellular and molecular level, a poor neutrophil to lymphocyte ratio is considered a poor prognostic indicator for COVID-19 (112, 113). An increased pro-inflammatory response may be an eventual harbinger of a cytokine storm. Curcumin has been found to have beneficial effects on many of these pathological processes (3, 4, 15, 16, 68, 93, 111, 114, 115). Curcumin can reduce cough induced by bradykinin (115, 116). It has an inhibitory effect on chemokine release and thus may prevent acute lung injury (84, 94, 115, 117). The inhibitory role of curcumin on Th1 and Th17 cells is also expected to have a beneficial role. Besides these, as has been mentioned earlier, curcumin has an inhibitory role on the production of pro-inflammatory cytokines like IL-6, IL-8, IL-1 β, Ik β kinase etc. The stimulatory role of curcumin on B cells may be beneficial in antibody production. It is also expected to prevent an eventual acute respiratory distress syndrome. A schematic diagram with the possible mechanisms of curcumin action in COVID-19 has been shown in Figure 5. While it is difficult to confirm the optimal dosage of curcumin in COVID-19 (owing to the paucity of data available), it is important to note that curcumin supplementation is considered relatively safe (3, 4, 10, 15, 16, 110, 111, 115, 116) and well-tolerated even at high doses up to 8 g/day as shown in different clinical trials (118). Generally, curcumin doses in the range of 0.5–1.5 g/day are seen to be helpful in clinical improvement in conditions like inflammatory conditions, and hence such a dose may be explored in COVID-19 as well (118).
Figure 5.
Schematic presentation on molecular mechanisms describing effects of curcumin on lungs infected with SARS-COV-2 virus.
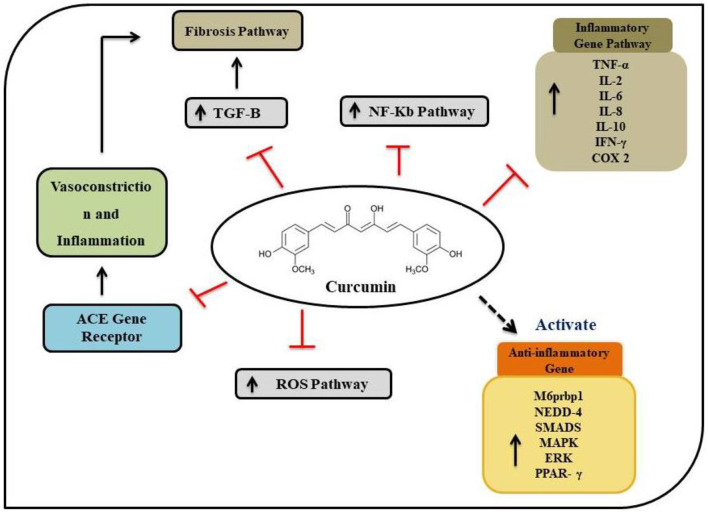
Recently, an in-silico molecular-docking study of curcumin has found a strong potential antagonist to the human ACE2 receptor and the SARS-CoV-2 spike S protein (119). In addition to these curcumins, virus replication can also be prevented by reducing the number of plaques (15). Curcumin is known to be a potent proteasome inhibitor that raises the p-53 level, and the epigenetic regulatory function of curcumin against viruses arises from its ability to interact with various biological targets to trigger molecular signaling pathways, such as apoptosis and inflammation (120). The future goal of curcumin to accomplish potential molecular targets in the different gene receptor to block replication of SARS-CoV-2 has been shown in Figure 6.
Figure 6.
Potential molecular targets for curcumin in the different gene receptors and block the SARS-CoV-2 replication signaling pathway.
In severe cases of COVID-19, acute respiratory distress syndrome (ARDS) is seen to occur due to the release of a large number of pro-inflammatory cytokines. Curcumin can potentially reduce the pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-1β, IL-4, tumor necrosis factor-α (TNF-α), and MCP-1 by blocking the nuclear factor-κB (NF-κB) and TNF-α (16). Collective information on the impact of curcumin on the human immune system at different levels and the possible efficacy of curcumin against COVID-19 has been addressed in this section (Table 3). Therefore, their incorporation into the food system to develop functional foods can be a novel method of providing adequate curcumin to the human body so that an individual can fight against coronavirus during or before the case. The detailed approach has been shown in Figure 7. In an open-label non-randomized clinical trial, Saber-Moghaddam et al. (110) revealed that oral nano-curcumin formulation was efficient in managing coronavirus infection in patients. It was observed that major symptoms including cough, fever and chills, tachypnea, and myalgia resolved significantly faster in the curcumin group. Similar results were also noticed by other researchers (Table 3).
Table 3.
Various in-vivo studies conducted in humans against the Covid-19 virus.
| Number of patients included in the trial | Age (year) of patients | Male/female ratio | Location of trial | Treatment protocols followed during the treatment | Major finding(s) | References |
|---|---|---|---|---|---|---|
| 60 | – | – | Mashhad, Iran | Sinacurcumin soft gel containing 40 mg curcuminoids as nanomicelles, two soft gels twice a day | A significant difference was observed in the curcumin-treated group for symptoms like cough, chills, and disturbances in smell and taste. The lymphocyte count was significantly higher while CRP serum level was lower in the treatment group at the end of 2 weeks. | (116) |
| 99 | 39–70 | 67/32 | Wuhan, China | Oxygen therapy, antibiotic treatment, Antifungal and antiviral treatment, Glucocorticoids and intravenous immunoglobulin therapy | Older males with comorbidities were found to be more affected by the virus; which can lead to severe or in some cases even fatal respiratory infections like acute respiratory distress syndrome. | (121) |
| 145 | 47.5 (Average) | 79/66 | Zhejiang, Chin | Antiviral and corticosteroid therapy, respiratory support | It was noticed that older patients with comorbidities like diabetes mellitus or obesity were more prone to have a severe condition | (106) |
| > 100 | – | – | Wuhan, Jingzhou, China | Treatment with chloroquine phosphate | Treatment with the compound resulted in the prevention of infection with the virus; it was interfering with the glycosylation of cellular receptors of SARS-CoV-2. | (122) |
| 20 | > 12 | – | Marseille, France | Treatment with hydroxychloroquine (600 mg/day) and azithromycin | As compared to controls, there was a reduction of the viral carriage at D6-post inclusion suggesting that hydroxychloroquine is highly efficient in reducing viral load and further its effect is reinforced by azithromycin. | (123) |
| 41 | 25–64 | 30/11 | Wuhan, China | Oxygen support, antibiotics*, antiviral (oseltamivir; 75 mg twice daily), corticosteroid (methylprednisolone), renal replacement therapy | The Covid-19 infection caused clusters of severe respiratory illness similar to severe acute respiratory syndrome coronavirus and was associated with ICU admission and high mortality. | (107) |
| 155 | 42–66 | 86/69 | Wuhan, China | Oxygen support, corticosteroid*, treatment with expectorant, antiviral: arbidol, lopinavir, ritonavir, interferon inhalation, and immune enhancer (thymalfasin, immunoglobulin) | It was observed that older males with the refractory disease were more prone to infection with the virus. | (124) |
| 21 | 18–57 | 5/16 | Mashhad, Iran | Treated with Sinacurcumin® soft gel (two capsules twice a day containing 40 mg curcuminoids as nanomicelles) | Compared to the control group, the treatment of curcumin nanoformulation fastened the resolution time of COVID-19-induced symptoms, improved oxygenation, and also reduced hospital stay time. | (110) |
| 120 | – | – | Mashhad, Iran | Nanocurcumin (160 mg/day for 21 days) | Compared to the placebo group, in mild and severe COVID-19 patients who received nanocurcumin, a significant reduction in Th17 cells and Th17 cell-related cytokines levels were found. | (111) |
| 135 | 36–55 | 72/63 | Northeast Chongqing, China | Oxygen support, treatment with antibiotics*, antiviral agents (Kaletra), corticosteroid*, traditional Chinese medicine (TCM) therapy, and renal replacement therapy | It was revealed that antiviral agent Kaletra, as well as TCM, could play a significant role in viral pneumonia treatment. | (108) |
| 69 | 35–62 | 32/37 | Wuhan, China | Oxygen support, treatment with antibiotics*, antiviral*, corticosteroid*, antifungal*, arbidol, moxifloxacin, plus interferon therapy* | It was observed that more deaths occurred in the SpO2 < 90% group. The patients of this group were older with comorbidities. They had a higher plasma level of lactate dehydrogenase, C reactive protein, interleukin (IL) 6, and IL10 than the SpO2 ≥ 90% group. | (125) |
| 200 | 55 (Average) | 98/102 | Yichang, Hubei Province, China | Medical treatment, respiratory support, and renal replacement therapy | The COVID-19 infection was of clustering onset; It was resulted in the cause of severe respiratory disease and in few cases even death. | (109) |
Doses were not reported.
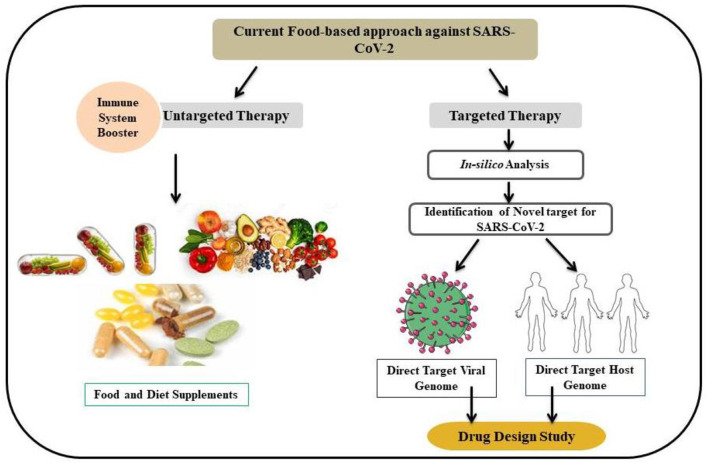
Figure 7.
A pattern of the current approach to research in food components and diets used against SARS-CoV-2. The untargeted approach is focused on improving the immune system by food nutrients and the targeted approach focuses on the interaction of protein compounds with the host and virus systems.
Extraction, Isolation and Quantification of Curcumin
Various processes have been followed in order to increase the availability of bioactive compounds, such as the selection of plant material and its component, cleaning, and subsequent drying followed by extraction and purification of the desired compound (126, 127). The extraction method can be categorized as traditional and modern extraction techniques. The most common traditional extraction methods are Soxhlet extraction (66, 127–129) and maceration (130, 131). Whereas, ultrasound-assisted extraction (UAE) (132, 133), microwave-assisted extraction (MAE) (134), enzyme-assisted extraction (128) are the most common methods in modern extraction process. Despite many drawbacks such as high temperatures, high operating times, and high organic solvent use, the traditional extraction method is commonly used due to its simple procedures and low operating costs (127, 135, 136). Identification and quantification of bioactive compounds are accompanied by the use of one or more chromatography techniques such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and ultra-HPLC with a mass spectrometer (MS) (66, 136–140).
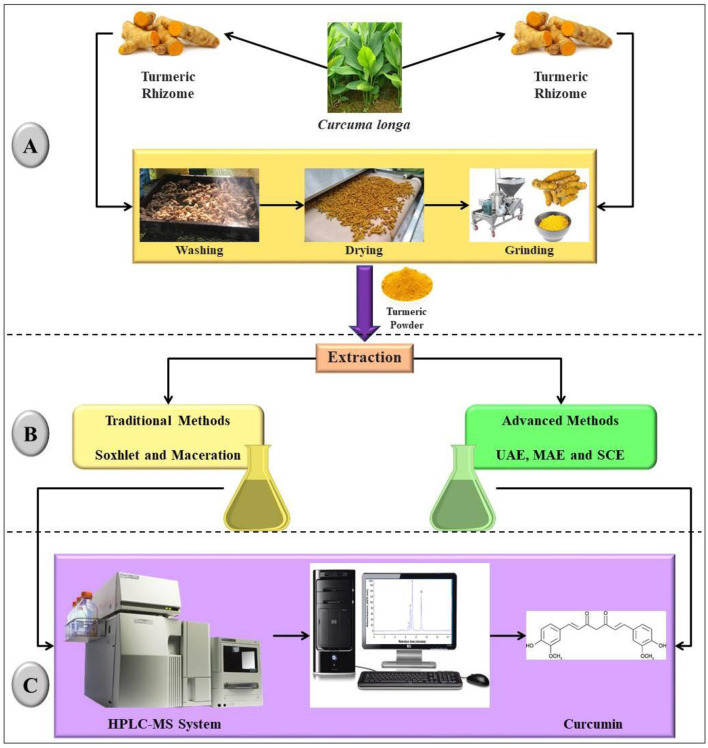
Sample preparation is a basic step followed prior to the extraction of the desired bioactive compound. The primary step is the selection of the proper plant variety and the part of the plant that will produce the targeted phytochemical (136, 141). In our case, therefore, the plant is turmeric and the rhizome of the turmeric plant has been used to extract curcumin (Figure 8A). The findings show that all researchers had extracted the powdered sample as it increases the extraction yield (Table 4). Yulianto et al. (145) obtained the higher extraction yield on using high temperature (140 and 150°C) for extraction and a lower solid:liquid ratio (1:10) also offered a higher concentration of the curcumin. Apart from these, it is also important to choose the correct solvent, as it affects the extraction yield as well as increases the toxicity of the extract. Shirsath et al. (74) found that ethanol as a solvent led to the maximum curcumin extraction yield as 72% in 1 h at 35°C, compared to the methanol, acetone, and ethyl acetate. Therefore, the extraction process should be optimized based on different extraction parameters such as time, temperature, pressure, the form of solvent, and the ratio of solvent to feed. Gökdemir et al. (139) optimized the ionic liquid bath extraction method by taking independent variables such as time (10–60 min), temperature (25–55°C), and volume of solvent (10–30 mL) and concluded that optimized conditions (60 min, 55°C, and 30 mL) with solvent as the most influential parameter had a higher extraction yield of 2.94%. The extraction of curcumin was optimized by Pan et al. (146) utilizing 80 percent ethanol, 70°C extraction temperature, a liquid-to-material ratio of 20, and a 3 h extraction time to get the highest yield (56.8 mg/g) of curcumin.
Figure 8.
Schematic presentation of extraction and isolation of curcumin with different extraction techniques coupled with an analytical technique for identification and quantification. (A) Sample Preparation: In this step, turmeric rhizomes were collected and appropriately washed. Then drying and grinding were done. Drying and size reduction are essential for processing, as size plays an important role (smaller the size, higher the diffusion of bioactive compounds from source to solvent) in the extraction process (136, 141, 142). (B) Extraction and Purification: The ground samples are then subject to an extraction procedure. The most commonly used extraction process for curcumin is the traditional method due to its low cost of operation and simple handling. Among the different extraction processes, the reflux method of powdered turmeric with dichloromethane achieved higher extraction yields ranging from 81.81 to 86.36% (138). However, as a green extraction technology, the subcritical water extraction method also raises extraction yields to 76% compared to other modern extraction techniques (137, 143). (C) Identification and Quantification: The most precise technique used to characterize curcumin was HPLC equipped with a column C18 of several lengths (100–250 mm), inner diameters (2.1–4.6 mm), and particle sizes (0.45–5 μm) (128, 137, 139, 140). The HPLC is an advanced method of liquid chromatography with a high separation capability. Further, the samples were scanned at different modes. Comparing the observed MS/MS spectra with those found in the literature was the primary tool for identifying the bioactive compounds.
Table 4.
Some recent studies on extraction, isolation, and quantifications of curcumin.
| Sample types | Extraction methods | Extraction conditions | Extraction yield (%) | Identification and quantification methods | Column type | Conditions for identification and quantification | Concentration of compound (mg/g dry weight) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent type | Time (min) | Temperature (°C) | Column/plate dimension (mm × mm) | Particle size (μm) | Injected volume (μL) | |||||||
| Powder | Surfactant-free microemulsion (SFME) extraction | Triacetin: Ethanol: water (36:24:40) | - | - | 0.921% | HPLC | C18 | 150 × 2.1 | 0.03 | 10 | 9.21 ± 0.32 | (140) |
| Powder | Refluxing | Dichloromethane | 60 | - | 81.81–86.36 | Thin Layer Chromatography (TLC) | - | 100 × 200 | - | - | 818.1–863.6 | (138) |
| Powder | Ionic liquid bath | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide | 10–60 | 25–55 | 0.76–2.94 | HPLC (Agilent 1100) | C18 | 150 × 4.6 | 5 | 20 | 29.4 | (139) |
| Powder | Dissolving | Ethanol (95%) | 10,080 | Room temperature | 15 | Column Chromatography | - | - | - | - | 150 | (144) |
| Powder | Soxhlet method | Ethanol | 480 | 70 | 12.67 | Column Chromatography | - | - | - | - | 788 | (129) |
| Powder | Soxhlet method | Petroleum ether | 60 | - | 1.55–5.163% | UHPLC | C 18 | 100 × 2.1 | 1.7 | 10 | 6.58 ± 0.023–21.31 ± 0.301 | (66) |
| Powder | Enzyme-assisted ionic liquid extraction | N,N-dipropyl ammonium and N, N-dipropylcarbamate | 120 | Room temperature | 1.48–3.95 | HPLC (Smartline, Knauer, Germany) | C 18 | 250 × 4.6 | 0.45 | - | 57.3 | (128) |
| Powder | Subcritical water extraction | Water | 60 | 140 | 76 | HPLC | C 18 | 250 × 4.6 | 0.45 | - | 38 | (137) |
| Powder | Subcritical solvent extraction | Water | 120 | 60 | 10.49–13.96 | HPLC Agilent system (1200 series, Agilent Technologies, Santa Clara, CA, USA) | C 18 | 150 × 4.6 | 5 | 20 | 49.4 | (143) |
The most commonly used extraction process is the traditional method (Figure 8B) due to its low cost of operation and simple handling, although it has many disadvantages, such as high-temperature operation, longer extraction time, and extensive solvent usage. Modern extraction techniques (Figure 8B) such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), and subcritical extraction methods can be used to improve the efficiency and yield of the extraction process (67, 127, 141–143, 147, 148). Most curcumin extraction adopted the traditional method with few exceptions, as shown in Table 4. Kwon and Chung (143) showed that subcritical solvent extraction provided a maximum yield of 13.58% at 135°C/5 min with water/ethanol mixture (50:50, v/v) as a solvent whereas the Zhou et al. (149) reported the optimization of microwave-assisted extraction of curcuminoids from Curcuma longa using 69% ethanol, 21:1 liquid:solid ratio and microwave time of 55 s with a yield of 28.97 mg/g rhizomes powder. Among different modern extraction processes, enzyme-assisted ionic liquid extraction and surfactant-free microemulsion (SFME) extraction achieved a yield of 5.73 and 0.76–2.94%, respectively (128, 140). Water used as a solvent in the subcritical extraction process has reduced the toxicity of the organic solvent and serves as a strong substitute for the organic solvent since it is safe and easily available (137, 143). This method also increases the safety of the extract as no organic solvent has been used and can be applied to the food system for use. Subcritical water extraction can also be used as a safer alternative to traditional extraction techniques.
The next step after extraction is the isolation and characterization of bioactive compounds (Figure 8C). Usually, this process is followed by many chromatographic techniques such as column chromatography, TLC, and HPLC coupled with diode array detection, etc. These techniques have been used separately or in combination to characterize unique bioactive compounds (141). Very few scientists have used column chromatography techniques to identify and quantify curcumin. The HPLC system developed by Agilent Technologies is being used by researchers for identification purposes. It is commonly used for the isolation and quantification of bioactive compounds contained in the biological extract (136, 141). Mass spectrometry (MS/MS) techniques can be used to characterize a wide variety of bioactive compounds. However, several analytical methods can be used to improve purity. These novel technologies are ultra-performance liquid chromatography and NMR spectroscopy which can be used to improve the purification and characterization process. Furthermore, mass spectrometry with electrospray ionization can be used to gain further insight into the structure and nature of bioactive compounds to characterize them.
The knowledge mentioned above will potentially benefit researchers, scientists, and industrial people employed in the field of food science. The information addressed will provide them with detailed ideas on the preparation of the sample, the selection of the extraction method, or the selection of suitable solvents to achieve the highest yield. In addition, this section would also be useful for researchers of the above area of interest in selecting the required purification technology as well as the proper identification of the desired bioactive compounds with better quantification. The method that demonstrated the maximum yield to be recommended for the extraction of curcumin from the turmeric and should be used to fortify the food product with improved immunological activity. For the people who are at risk of coronavirus when no drugs are available worldwide, these developed foods for well-recognized immunological functions in humans that have already been clinically demonstrated can be highly recommended for this issue.
Developed Functional Food Associated With Curcumin and Its Relation With the Immune System and COVID-19
Today, modern consumers have become more conscious and aware of their well-being. As a result, consumers pay more attention to those foods which contain specific ingredients that are capable of affecting their health as well as the different physiological functions. Thus, with this approach in mind, functional foods are successfully formulated using nutraceuticals and bioactive compounds to fulfill the nutritional as well as physiological needs of consumers (25, 150). During the past 20 years, studies have reported many bioactive compounds as ingredients for the production of functional food, such as polyphenols, phytosterols, vitamins, and minerals (22–25, 151, 152). Curcumin is also regarded as an important bioactive compound among these bioactive compounds, which has been identified and extracted from C. longa plants (128). The potential health benefits of this polyphenolic compound have also been clinically demonstrated. Based on suitability, curcumin was used for the development of functional food in its free form or encapsulated form, as shown in Table 5. Curcumin is used as a natural ingredient that offers a distinctive color and flavor profile, in addition to potential health benefits during the development of functional foods. Such functional foods can help to boost immunity in humans due to the immunomodulatory properties of curcumin, which has already been addressed in previous sections and thus can help people fight against the COVID-19 virus if such foods are recommended for oral consumption. The following sections addressed some of the important functional foods that have been developed using curcumin as functional ingredients.
Table 5.
Some recent works on the development of different functional foods using curcumin.
| Source | Employed method | Amount of curcumin used | Developed food | Remark | References |
|---|---|---|---|---|---|
| (A) Cereal-based functional foods | |||||
| Pure curcumin powder | Direct | 114 mg/bread | Bread | Curcumin added bread significantly reduced the low-density cholesterol and risk of cardiovascular diseases. | (153) |
| Turmeric powder | Direct | 100 mg/g custard powder | Corn starch custard powder | The microbial contamination of custard was reduced more than the control sample. | (154) |
| Turmeric powder | Direct | 50–75 mg/g wheat flour | Crackers | By adding turmeric powders the taste of the crackers improved because they had normally a pleasant turmeric taste. Overall findings indicate that turmeric powders were functional food additives with high phenolic content, antioxidant capacity, and bio-accessibility. | (155) |
| Turmeric powder | Direct | 10 mg/g maize flour | Kokoro (Nigerian snacks) | The sensory properties of Kokoro in terms of color, aroma, taste, texture, and overall acceptability were increased. | (156) |
| Pure curcumin powder | Encapsulation (Liposome) | 730 mg/g lyophilized liposome | Cake | The liposomes were lyophilized by pro-liposome hydration and the curcumin encapsulated remained stable after 70 days of storage. The results indicate that hardening and chewing were reduced and the color of the agglomerated cornstarch cakes was intensive and uniform. | (157) |
| Turmeric powder | Direct | 5 mg/g flour mixture | Biscuit | Turmeric powder had the highest positive effect on the antioxidant properties of the biscuits. | (158) |
| Turmeric powder | Direct | 50 g/kg | Whole grain wheat flour (WGF) pasta | A formulation, TP3 observed the highest total phenolics content (534.46 ± 1.93 mg kg−1), DPPH∙ (5.70 ± 0.10 g kg−1 of Trolox Equivalent (TE), and ABTS (9.02 ± 0.58 g kg−1 of TE), with high retention of antioxidant capacity after cooking; it is suggestive that wheat fiber and bioactive compounds from turmeric may be conjugated as a natural ingredient in pasta. | (159) |
| Turmeric extract | Encapsulation | 10–80 mg encapsulated curcumin/g flour mixture | Extrudate product | Extruded cereals containing encapsulated turmeric extract also demonstrated strong antioxidant activity when examined using ABTS and DPPH scavenging methods. | (160) |
| Pure curcumin powder | Encapsulation | - | Bread | The findings indicate that, when its concentration was >0.035%, curcumin microcapsules had preservative effects on food even though it was boiled. In comparison with free curcumin, not only were microcapsules better soluble and hot, but mold spores decreased by 34.5 × 2.5% to 52.3 × 4.1%. | (80) |
| (B) Dairy-based functional foods | |||||
| Turmeric powder | Direct | 1–2 mg/g butter | Cow milk Butter | Turmeric extract powder had a major effect on mold, coliform, and total microbial counts in all doses (p ≤ 0.05) and reached 17, 18, and 960 CFU/mL at the maximum dosage level, respectively. | (161) |
| Turmeric powder | Direct | 0.1–0.5 mg/mg dairy product | Cow milk-based dairy product | Turmeric extract powder had a major effect on mold, coliform, and total microbial counts in all doses (p ≤ 0.05) and reached 17, 18, and 960 CFU/mL at the maximum dosage level, respectively. | (162) |
| Turmeric powder | Direct | 0.1–0.3% | Soft cheese | Results indicated that as the concentration of the turmeric powder increased, the total bacterial count as well as coliform count decreased compared with control treatment which showed the highest total count after 9 days of storage at 5 ± 2°C. | (163) |
| Pure curcumin powder | Nano-emulsion | 25 mg/g coating mixture | Skim milk | When it was applied to milk, the lipid oxidation could be prevented by either nanostructure, as demonstrated by an insignificant change in the color of fortified milk after 5 days. | (82) |
| Turmeric extract | Nano-emulsion | 1–10 mg/mL milk | Milk | Turmeric nano-emulsion in milk was able to preserve curcuminoids during gastric digestion and to effectively release them during intestinal digestion as compared to unencapsulated turmeric extract, possibly due to the low solubility of the latter and the degradation of curcumin in the neutral-alkaline medium. | (83) |
| Pure curcumin powder | Direct | 230.8 ± 6.5–232 ± 1 μg/mL milk | Milk | The bioaccessibility of curcumin evaluated using the in vitro gastrointestinal (GI) tract was ~40%, which was due to some chemical degradation and binding of curcumin which decreased its stability and solubilization. | (73) |
| Pure curcumin powder | Nano-emulsion | 10–20 mg/g ice cream | Ice cream | The incorporation of turmeric nano-emulsion was a feasible option for reducing the use of artificial dyes, as ice creams demonstrated similar physicochemical and rheological properties. | (164) |
| Pure curcumin powder | Nano-emulsion | 3 mg/g nano-emulsion | Milk | Cut-NEs-fortified milk was demonstrated substantially lower lipid oxidation than control (unfortified) milk and milk containing curcumin-free nano-emulsions. | (81) |
| Pure curcumin powder | Nano-emulsion | 2.4 mg/g ice cream | Ice cream | Release kinetics results indicated that in simulated gastrointestinal digestion, nano-emulsion was stable against pepsin digestion (5.25% release of curcumin), while pancreatic activity resulted in 16.12% release of curcumin from nano-emulsion. No major difference was found in the scores of the sensory attributes between the control and the ice cream prepared with the nano-emulsion of curcumin. | (165) |
| (C) Miscellaneous developed functional foods | |||||
| Pure curcumin powder | Direct | 100–300 mg/kg meat | Lamb meat | Total fat in meat was substantially lower in the T200 and T300 groups than in the control group. Total SFAs were slightly lower in the T300 group than in the control group, while total PUFAs were higher. No significant differences were observed between groups with respect to total monounsaturated fatty acids (MUFAs). | (166) |
| Pure curcumin powder | Gel Form | 3.4 mg/g gel | Meat pâté | When pork backfat was partially or fully replaced by curcumin-loaded gel in pâtés, a noticeable protective effect of curcumin against lipid oxidation was observed during refrigerated storage. | (167) |
| Turmeric powder | Direct | 20–60 mg/g Hibiscus sabdariffa powder | Zobo (Traditional H. sabdariffa beverage) | The addition of turmeric to zobo had increased the nutritional quality of zobo. | (168) |
| Turmeric powder | Direct | 5–15 mg/g coating flour | Chicken nugget | Curcuma flour fortification did not affect the water-holding ability, tenderness, protein, and fat content of chicken nuggets (P > 0.05), but increased the vitamin E and curcumin content of chicken nuggets (P < 0.05). Sensory test results showed that the fortification of curcuma flour did not affect the acceptability of the sensory characteristics of the chicken nugget. | (169) |
| Turmeric powder | Direct | 7–22 g/smoothie | Fruit smoothie | The development of a functional beverage with 14 grams of turmeric was deemed significantly more appropriate with the provision of health information and resulted in a substantial increase in antioxidant ability and polyphenol content. | (170) |
| Turmeric extract | Nano-emulsion | 10 mg/mL prepared emulsion | Canned Ham | The canned ham incorporating the turmeric nano-emulsion powder obtained the same overall acceptability score as the control and demonstrated only mild yellowing. | (171) |
Cereal-Based Product
Cereal-based products, such as bread, pasta, cookies, and cakes, are used by most people around the world as their main source of energy and nutrients. While fungal growth is a major problem in cereal-based food products, the interest in bakery products continues to rise day by day due to their nutritional properties (157, 158, 172, 173). In addition, natural polyphenols such as rice or wheat bran, grape seed extract, fruit pomace powder, ginger, and turmeric are used in bakery products to improve the antioxidant function of the food. The addition of these ingredients to the dough greatly improves the content and antioxidant potential of breads and biscuits (153, 155, 174).
Since curcumin has a variety of other activities that promote health, it is mainly used in bakery products (Table 5A). In one of the earlier approaches, the highest percentage of turmeric powder (8%) observed the highest curcumin (203 mg/kg) and RSA-DPPH activity (45%) in the cake, but it showed the worst results in terms of the rheological properties (83). The authors concluded that 6% addition of turmeric gave the best results in regard to the rheological and sensory properties of the cake. Turmeric flowers are reported to be a rich source of essential oils like p-cymen8-ol, hence in this context Azmi et al. (175) employed aqueous extracts of fresh turmeric flowers (5–20%) in cookies to enhance its functional value. Lower antioxidant activity (DPPH assay) was observed in cookies prepared with higher extraction levels of turmeric flowers (20%) and it was attributed to the baking process that led to the degradation of heat-sensitive antioxidant compounds. The authors recommended that 5% and 10% incorporation levels of flower extracts can be the best formulations for bakery products. Ferguson et al. (153) developed two phytosterols-enriched breads in an experiment: (1) with curcumin, and (2) without the addition of curcumin. They found a substantial reduction of low-density cholesterol and risk of cardiovascular disease in the daily intake of those two breads with or without the addition of curcumin (114 mg curcumin/bread). After adding curcumin to crackers enhanced the antioxidant activity and phenolic contents of crackers, as well as the bio-accessibility, flavor, and taste (155). Curcumin can also be added in encapsulated form. The encapsulated curcumin reduced the hardness and chewiness as well as the homogeneous yellow color of the cakes developed with the agglomerated cornstarch (157). It was also found that encapsulated curcumin increased the shelf life of the breads by reducing the growth of the mold spores (80). Adegoke et al. (158) added 5 mg of curcumin per gram of wheat flour to make cookies. The results of their research indicated that curcumin had the strongest positive effect on the antioxidant properties of biscuits. These results indicate that the addition of curcumin to the composite flour would enhance the functionality and nutritional composition of the baked food products. Curcumin-rich turmeric (Curcuma longa) powder extracts were used as a natural antioxidant in the preparation of biscuits by Hefnawy et al. (176) along with carrot (Daucus carota) and grape (Vitis vinifera) leaf. Besides this, turmeric from 1 to 5% was used to improve the color, increase the fiber content and increase the antioxidant properties of pasta while retaining the technological attributes (159).
It can be thus concluded that bakery products can be the perfect medium for the delivery of curcumin to the human body. Since curcumin has anti-inflammatory, anti-oxidant, anti-cancer, immune enhancement effects, it can boost the functionality of food without affecting the sensory properties of bakery products. The immunological activity of curcumin is linked to macrophages, B, and T lymphocytes. Curcumin can enhance immunological activity by a variety of mechanisms, such as the regulation of cytokines and several transcript factors (114). These curcumins are added to bakery products, such as bread, biscuits, and cake, which can quickly boost immunity in humans after consumption due to the increased immunity of curcumin.
Dairy-Based Product
Dairy-related products were used, in particular for functional dairy products that make up 40% of the global demand for functional food. The global demand for dairy products is very competitive and this worldwide dairy demand will hit US$ 13.9 billion by 2021 (177), without considering the traditional dairy products such as buttermilk, kefir, etc. Dairy products are widely appreciated and flexible and are important for any population group regardless of height, weight, and age. The key benefit of the use of dairy products is the prevention and treatment of many diseases such as diabetes, malnutrition, cancer, and hypertension affecting the world's population (178). Functional ingredients most widely used in the development of functional dairy products include vitamins, probiotics or prebiotics, bioactive compounds, minerals, etc. (177). In bioactive compounds, polyphenols are known as functional food formulations due to their various health benefits such as antioxidant, antimicrobial, antidiabetic, anti-allergenic, anti-inflammatory operation, etc. (71, 152). Curcumin, a natural polyphenol of turmeric rhizome with anti-inflammatory, anti-oxidant, anti-cancer, and immunosuppressive effects, is used mainly in the development of dairy products. Further, it is also observed that milk and milk products are considered as the best suitable media for curcumin as the presence of fat (triglycerides) enhances the solubility of curcumin; and hence its bioavailability in the body.
Curcumin extracted from turmeric has been used in cow's milk butter to increase its functionality (161). Asadaii et al. found that the addition of curcumin in the range of 1–2 mg/g butter improved nutritional and antimicrobial activity by removing mold, coliform, and total microbial counts (161). Furthermore, the addition of curcumin to cow's milk also increased the calcium content and antioxidant activity throughout the storage period (162). Nano-emulsion is a novel technique designed to increase the bioavailability of curcumin. Adding 25 mg curcumin/g encapsulated material or 1–10 mg curcumin nano-emulsion/mL milk to milk minimized lipid oxidation and also effectively released during intestinal digestion (82, 83). In a recent investigation, Hasneen et al. (179) demonstrated that the total phenolic content of yogurt was improved by the incorporation of 1% turmeric extract. Similarly, the radical scavenging activity (RSA) % of both skim milk yogurt and cast Kariesh cheese were significantly enhanced by turmeric. Similarly, in another approach, Ricotta cheese supplemented with curcumin was observed to enhance organoleptic properties, phenolic compounds, and antioxidant activity (180). Additionally, several studies have also shown that the addition of turmeric powder led to inhibit the growth of coliforms and other bacteria in milk-based products especially, cheese during the storage period (163, 180, 181).
Curcumin has also been applied to dairy products, such as ice cream, by several researchers. In an experiment, Borrin et al. (164) added 10–20 mg of pure curcumin per g of ice cream to investigate the effect of curcumin and found that curcumin could be a better alternative to artificial dye, and prepared functional ice cream also showed similar physiological properties compare to control. The curcumin emulsion incorporated into the ice cream was stable against pepsin digestion and there was no major difference between the sensory properties of curcumin added and the control of ice cream (165). Further, the addition of curcumin (160–350 ppm) not only improved the sensory attribute of ghee but also contributed to its antioxidant potential, as found by Lodh et al. (182). On the other hand, the turmeric powder containing curcumin was used to prepare soft cheese which improved its oxidative stability and microbiological quality (163). A detailed summary of the development of some dairy functional foods using curcumin has been shown in Table 5B.
Based on the above findings, it can be concluded that the addition of curcumin could improve the consistency, nutritional value, sensory characteristics, and shelf life of functional dairy products without the addition of any artificial dye or other substances. Due to its antimicrobial and immunity-boosting properties, curcumin can be used in a variety of ways in dairy products. Dairy products are a rich source of vitamin-B complex, protein and calcium and the addition of curcumin to increase the nutritional value of dairy products and these dairy products, due to their enhanced nutritional properties and the availability of vitamins and minerals, can be eaten more and more to create a powerful protection mechanism within the body to defend against the novel coronavirus.
Other Functional Food Product
The health benefit of functional foods is derived from the bioactive compounds, such as phytochemicals, vitamins, and peptides, found naturally in them, formed during processing, or extracted from other sources and added to them (62, 151). The functional food other than cereal-based or dairy-based that are highly demanding are meat-based and fruit and vegetable-based products (Table 5C). Though meat is highly nutritious but presence of certain compounds affects negatively on the human health. Therefore, the functional food concept gives an excellent opportunity to improve the functionality of meat products (24). In functional meat production, generally synthetic and natural GRAS (generally recommended as safe) ingredients were used to improve the end products' safety and quality. Therefore, plant bioactive compounds because of their health benefits are now are of great interest. The incorporation of these bioactive compounds improves the nutrient and functionality of meat products (22). The increasing awareness regarding the nutritional compounds and health benefits of fruit juices increased consumer demand toward healthy fruit or vegetable-based beverages increased as compared to carbonated drinks. Fruit juices, which are naturally rich in bioactive compounds with health-promoting and disease-reducing properties, are important contributors to human nutrition (72). Fortification of fruit juices with natural bioactive compounds such as polyphenols (183), active peptides (184), vitamins (185) etc., has been investigated for several years.
However, there is very little literature available on the incorporation of curcumin to develop functional meat and fruit-based food products. The curcumin was added to lamb meat with an aim to reduce the fat in meat and it was observed that the addition of 100–300 mg curcumin/g meat reduced the saturated fatty acid without affecting PUFA and MUFA (166). A similar effect was observed in meat pâté where pork fat was totally or partially replaced by the curcumin hydrogel and lipid oxidation was also reduced (167). During modified atmospheric packaging of fresh lamb sausages, turmeric extract rich in curcumin slowed the lipid oxidation and generation of related volatile compounds as well as improved the antioxidant capacity (186). Moreover, except for the yellow color, none of the physico-chemical parameters of the product were greatly influenced by the addition of turmeric extract. The authors concluded that the addition of turmeric extract can replace sodium erythorbate up to 500 ppm. In another experiment, Sujarwanta et al. (169) found that direct incorporation of 5–15 mg curcumin/g flour improved the vitamin-E and curcumin content without affecting the sensory characteristics of the chicken nugget. Direct turmeric was also added 7-22 g per g of smoothie to study the effect of turmeric on smoothie by 138. In this experiment, the author found that the addition of 14 grams of turmeric to the beverage improved the functionality of the smoothie with a significant increase in polyphenol content and antioxidant capacity. A substitution of wheat flour with turmeric powder (up to 4%) observed good antioxidant activity as well as acceptable sensory scores with normal wheat bread; thus, authors concluded that breads incorporated with turmeric powder can be developed as a health-promoting functional food (187). Zenzer et al. (188) performed a randomized, single-blind study and concluded that compared to control, spice-based beverages containing turmeric increased p-PYY (plasma-peptide tyrosin-tyrosin) and lowered the “desire to eat” and “prospective consumption (quantity of food wanted to it)” in a healthy human.
Curcumin has many health promoting activities and is an outstanding compound. Increasing customer demand for food and beverages with health-promoting nutrients and immunity boosting components has been met by developing functional food and drinks. This improves the health of humans by adding turmeric to regulate the fat content of some items. Curcumin not only improves the color of meat products but also increases the content of vitamin-E in poultry nuggets, which allows the human body to receive more vitamin-E. The increased availability of functional foods and drinks has a long-term impact on human health. These rich curcumin foods can enhance immunity in the human body, reducing the possibility of an invasion of coronavirus.
Limitations and Advances in the Use of Curcumin in Rational Drug Therapy
Curcumin is extensively employed in ayurvedic herbal remedies from ancient times. Several in-vitro and in-vivo studies have shown that curcumin possesses the chemo-preventive and chemotherapeutic agents for colon, skin, oral and intestinal cancers (87, 117). However, in humans phase I/II clinical trials have revealed that curcumin exhibited the low bioavailability. Owing to poor bioavailability and rapid metabolism, the clinical efficacy of oral curcumin may be lower than in-vitro studies. It is reported that in-vitro the therapeutic potential of curcumin has been reported at micromolar range concentrations, whereas after oral intake the plasma concentration is in the nanomolar range (189). Recent technological developments including adjuvants, liposomes, phospholipid complexes, micelles, and nanoparticles are being evaluated to increase the oral bioavailability of curcumin. It is suggested that for the instability and weak pharmacokinetic profiles of curcumin, the β-diketone moiety is responsible (13). Hence, researchers have performed structural modification in curcumin to prepare the analogs without the β-diketone moiety. Predominantly, mono-carbonyl analogs of curcumin (MACs) have been reported to have enhanced stability in-vitro and an improved pharmacokinetic profile in-vivo and some of them have been intensively studied in order to develop novel drugs (13). The majority of studies often do not mention the exact amount of curcumin employed, and it is variable in commercial preparations. Additionally, it exists in different forms that also differ in biological potencies (190). In-vitro results suggested that curcuminoids inhibit collagenase, hyaluronidase, and elastase (90, 191). Curcuminoids have potential in cosmeceuticals as antioxidant and skin lightening agents.
Sundar et al. (192) developed 3-aminopropyltriethoxy silane (APTES) coated magnetite nanopowders as carriers for curcumin as an anticancer drug. It was prepared through the modified controlled chemical co-precipitation method employing oleic acid as the apt surfactant to achieve well-dispersed ultrafine spherical Fe3O4 magnetic nanoparticles. Within 1 h about 15% drug was released while 80% drug release was observed in 48 h, showing its promising application for in-vivo trials. Similarly, Mai et al. (193) fabricated polylactic acid (PLA) microcapsules as novel drug delivery systems using curcumin as a model drug by using an electrospray technique. The drug-loaded microcapsules had significant biocompatibility, low cytotoxicity, and had shown excellent anti-bacterial activities. The authors concluded that it has a potential for a wide range of applications in medical fields including drug delivery (193). To sum up, it can be stated that along with previously used technologies like liposomes, microencapsulation as well as nanoencapsulation are the newly emerging viable alternatives that have been shown to deliver therapeutic concentrations of potent chemo-preventive including curcumin and other polyphenols into the systemic circulation.
Future Prospective and Research Opportunities
The major purpose of enrichment or fortification of the food product is to achieve the desired properties such as increase nutrient composition, influence physicochemical characteristics, reduce oxidation, improve sensory characteristics, and enhance product shelf life. Based on previous research on the biological activities of curcumin, it has been found that there is a great potential for it to be used as a functional ingredient in the development of functional foods. After a thorough review, it may be suggested that encapsulated curcumin is more effective than direct incorporation. Directly added curcumin can be degraded by the digestive enzymes whereas in presence of suitable coating material encapsulated curcumin can withstand the effect of digestive enzymes and their direct release in the gastrointestinal tract can increase their bio-availability. Therefore, food scientists and researchers are recommended to work more on the encapsulation of curcumin and study the different properties of the encapsulated curcumin. Besides this, as curcumin has a positive impact on the human immunity system, it is required to do more and more studies on it. Because one of the main reasons for coronavirus spread was poor human immunity. Hence, it is required to study the effect of curcumin on humans as till now it has been done in-vitro or in an animal model. Further, the best way to consume curcumin is by incorporating it into food. From this review, it was found that many researchers have incorporated curcumin into the food matrix to develop functional food. However, none of them has done the immunological effect of curcumin added food products on human or animal models or in-vitro. Consequently, it is a great opportunity for researchers or food scientists to perform those experiments to collect more strong evidence of the effect of curcumin against COVID-19. In addition, there is a need for commercialization and awareness regarding the health beneficial activities especially the immunological effect of curcumin added food is needed. Therefore, it is humble advice from the authors to the respective country government and the concerned authorities of the World Health Organization (WHO) to spread the health benefits of curcumin and its advantages against novel coronavirus, in order to control the spread of the virus.
Concluding Remarks
The purpose of the present review is to discuss about the different extraction, isolation, and quantification methods of curcumin and its potential application in respiratory diseases such as COVID-19. Several methods were found for extraction, isolation, and quantification. But the subcritical water extraction method and HPLC is the most effective method with a higher extraction yield. In the development of functional food, curcumin was successfully added to different food matrices to develop functional food. These functional foods being rich in curcumin can enhance the immunity to fight against novel coronavirus. However, further progress is still essential, especially in terms of the development of functional foods. Additional information is required on the effects of food processing and storage conditions on the biological potential of curcumin. Further, healthcare organizations across the globe need to spread awareness regarding the immunomodulatory properties of curcumin and people should consume these functional foods in order to improve their immunity to fight against various pathogens including the SARS-CoV-2. Together, by consuming healthy nutritious food and following the WHO guidelines, we can win the fight against coronavirus.
Author Contributions
DV has conceptualized, interpreted, corrected, and technically sound final versions of the manuscript. ST has compiled literature for manuscripts. MT, SS, AP, HV, and AG have interpreted the manuscript, corrected it, and made it scientifically sound for the final version. MC-G and GU have read final versions of the manuscript. NC has provided suggestions, medical expertise, and corrections for the final version. CA has provided technical suggestions, corrections, and permissions for the finalization of the manuscript. PS has read and approved permissions for the finalization and submission of the manuscript. All the authors approved the submission of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author, ST thanks the Ministry of Education (formerly the Ministry of Human Resource Development), Government of India for an Institute Research Assistantship, and also thanks to the Agricultural and Food Engineering Department, Indian Institute of Technology Kharagpur for their assistance in this study.
References
- 1.Han B, Hoang BX. Opinions on the current pandemic of COVID-19: Use functional food to boost our immune functions. J Infect Public Health. (2020) 13:1811–7. 10.1016/j.jiph.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mu C, Sheng Y, Wang Q, Amin A, Li X, Xie Y. Potential compound from herbal food of rhizoma polygonati for treatment of COVID-19 analyzed by network pharmacology and molecular docking technology. J Funct Foods. (2020) 77:104149. 10.1016/j.jff.2020.104149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soni VK, Mehta A, Shukla D, Kumar S, Vishvakarma NK. Fight COVID-19 depression with immunity booster: Curcumin for psychoneuroimmunomodulation. Asian J Psychiatr. (2020) 53:102378. 10.1016/j.ajp.2020.102378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soni VK, Mehta A, Ratre YK, Tiwari AK, Amit A, Singh RP, et al. Curcumin, a traditional spice component, can hold the promise against COVID-19?. Eur J Pharmacol. (2020) 886:173551. 10.1016/j.ejphar.2020.173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathy S, Verma DK, Thakur M, Patel AR, Srivastav PP, Singh S, et al. Encapsulated food products as a strategy to strengthen immunity against COVID-19. Front Nutr. (2021) 8:245. 10.3389/fnut.2021.673174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YW, Yiu CPB, Wong KY. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. (2020) 9:22457. 10.12688/f1000research.22457.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. (2019) 16:1–22. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. (1997) 48:1–100. 10.1016/S0065-3527(08)60286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses-drug discovery and therapeutic options. Nat Rev Drug Discovery. (2016) 15:327–347. 10.1038/nrd.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, et al. A distinct name is needed for the new coronavirus. Lancet. (2020) 395:949. 10.1016/S0140-6736(20)30419-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. (2020) 92:595–601. 10.1002/jmv.25726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan T. Novel coronavirus: From discovery to clinical diagnostics. Infect Gene Evolut. (2020) 79:104211. 10.1016/j.meegid.2020.104211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. Am J Respir Crit Care Med. (2020) 202:756–9. 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel M, Dominguez E, Sacher D, Desai P, Chandar A, Bromberg M, et al. Etoposide as salvage therapy for cytokine storm due to COVID-19. Chest. (2020) 159:e7–11. 10.1016/j.chest.2020.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. (2020) 34:2911–20. 10.1002/ptr.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawitan JA. Curcumin as adjuvant therapy in COVID-19: friend or foe? J Int Dental Med Res. (2020) 13:824–9. [Google Scholar]
- 17.Verma HK, Farran B, Bhaskar LVKS. Convalescent plasma transfusion a promising therapy for coronavirus diseases 2019 (COVID-19): current updates. Antibody Therapeutics. (2020) 3:115–25. 10.1093/abt/tbaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma HK, Merchant N, Verma MK, Kuru CI, Singh AN, Ulucan F, et al. Current updates on the European and WHO registered clinical trials of coronavirus disease 2019 (COVID-19). Biomed J. (2020) 43:424–33. 10.1016/j.bj.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh AP. Model for prediction of death rate due to COVID-19 transmission and required precautions. Mater Today: Proc. (2020) 37:2318–20. 10.1016/j.matpr.2020.07.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumgartner S, Bruckert E, Gallo A, Plat J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis. (2020) 311:116–23. 10.1016/j.atherosclerosis.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 21.Ashaolu TJ. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed Pharmacother. (2020) 130:110625. 10.1016/j.biopha.2020.110625 [DOI] [PubMed] [Google Scholar]
- 22.Das AK, Nanda PK, Madane P, Biswas S, Das A, Zhang W, et al. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci Technol. (2020) 99:323–36. 10.1016/j.tifs.2020.03.010 [DOI] [Google Scholar]
- 23.Gong X, Li X, Xia Y, Xu J, Li Q, Zhang C, et al. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci Technol. (2020) 103:304–20. 10.1016/j.tifs.2020.07.026 [DOI] [Google Scholar]
- 24.Jiménez-Colmenero F, Cofrades S, Herrero AM, Ruiz-Capillas C. Implications of domestic food practices for the presence of bioactive components in meats with special reference to meat-based functional foods. Crit Rev Food Sci Nutr. (2018) 58:2334–45. 10.1080/10408398.2017.1322937 [DOI] [PubMed] [Google Scholar]
- 25.Verma DK, Thakur M. Phytochemicals in Food and Health: Perspectives for Research and Technological Development. CRC Press/Apple Academic Press Inc., (2021). [Google Scholar]
- 26.Trigo JP, Alexandre EM, Saraiva JA, Pintado ME. High value-added compounds from fruit and vegetable by-products-Characterization, bioactivities, and application in the development of novel food products. Crit Rev Food Sci Nutr. (2020) 60:1388–416. 10.1080/10408398.2019.1572588 [DOI] [PubMed] [Google Scholar]
- 27.Chikhale RV, Sinha SK, Khanal P, Gurav NS, Ayyanar M, Prasad SK, et al. Computational and network pharmacology studies of Phyllanthus emblica to tackle SARS-CoV-2. Phytomed Plus. (2021) 1:100095. 10.1016/j.phyplu.2021.100095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murugesan S, Kottekad S, Crasta I, Sreevathsan S, Usharani D, Kumar PM, et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytocompounds of ayurvedic medicinal plants-Emblica officinalis (Amla), Phyllanthus niruri Linn.(Bhumi Amla) and Tinospora cordifolia (Giloy)-a molecular docking and simulation study. Comput Biol Med. (2021) 136:104683. 10.1016/j.compbiomed.2021.104683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakr ESH, Naga ME. Immunomodulatory efficacy of phyllanthus emblica and costus speciosus aqueous extracts for immunosuppressive rats. Egypt J Nutr. (2020) 35:101–23. 10.21608/enj.2020.144766 [DOI] [Google Scholar]
- 30.Jantan I, Haque M, Ilangkovan M, Arshad L. An insight into the modulatory effects and mechanisms of action of phyllanthus species and their bioactive metabolites on the immune system. Front Pharmacol. (2019) 10:878. 10.3389/fphar.2019.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HMD, Fu L, Cheng CC, Gao R, Lin MY, Su HL, et al. Inhibition of LPS-induced oxidative damages and potential anti-inflammatory effects of Phyllanthus emblica extract via down-regulating NF-κB, COX-2, and iNOS in RAW 264.7 Cells. Antioxidants. (2019) 8:270. 10.3390/antiox8080270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putri DU, Rintiswati N, Soesatyo MH, Haryana SM. Immune modulation properties of herbal plant leaves: Phyllanthus niruri aqueous extract on immune cells of tuberculosis patient-in vitro study. Nat Prod Res. (2018) 32:463–7. 10.1080/14786419.2017.1311888 [DOI] [PubMed] [Google Scholar]
- 33.Kadosh Y, Muthuraman S, Yaniv K, Baruch Y, Gopas J, Kushmaro A, et al. Quorum sensing and NF-κB inhibition of synthetic coumaperine derivatives from piper nigrum. Molecules. (2021) 26:2293. 10.3390/molecules26082293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saetang J, Tedasen A, Sangkhathat S, Sangkaew N, Dokduang S, Prompat N, et al. Low piperine fractional piper nigrum extract enhanced the antitumor immunity via regulating the Th1/Th2/Treg cell subsets on NMU-induced tumorigenesis rats. Planta Med. (2021). 10.1055/a-1458-5646 [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Jia X, Yu Q, Shen S, Gao Y, Lin X, et al. Piper nigrum extract attenuates food allergy by decreasing Th2 cell response and regulating the Th17/Treg balance. Phytother Res. (2021) 35:3214–25. 10.1002/ptr.7034 [DOI] [PubMed] [Google Scholar]
- 36.Bui TT, Fan Y, Piao CH, Van Nguyen T, Shin DU, Jung SY, et al. Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell Immunol. (2020) 351:104035. 10.1016/j.cellimm.2019.104035 [DOI] [PubMed] [Google Scholar]
- 37.Choudhary P, Chakdar H, Singh D, Selvaraj C, Singh SK, Kumar S, et al. Computational studies reveal piperine, the predominant oleoresin of black pepper (Piper nigrum) as a potential inhibitor of SARS-CoV-2 (COVID-19). Curr Sci. (2020) 119:1333–42. [Google Scholar]
- 38.Pei H, Xue L, Tang M, Tang H, Kuang S, Wang L, et al. Alkaloids from black pepper (Piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-κB pathway. J Agric Food Chem. (2020) 68:2406–17. 10.1021/acs.jafc.9b07754 [DOI] [PubMed] [Google Scholar]
- 39.Abdelnour S, Alagawany M, El-Hack A, Mohamed E, Sheiha AM, Saadeldin IM, et al. Growth, carcass traits, blood hematology, serum metabolites, immunity, and oxidative indices of growing rabbits fed diets supplemented with red or black pepper oils. Animals. (2018) 8:168. 10.3390/ani8100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mousavi S, Weschka D, Bereswill S, Heimesaat MM. Immune-modulatory effects upon oral application of cumin-essential-oil to mice suffering from acute campylobacteriosis. Pathogens. (2021) 10:818. 10.3390/pathogens10070818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yediel SA, Bakir B, Yildiz SE, Kiliçle PA, Süleyman GÜL, Sari E, et al. Immunohistochemical investigation of Trk-A receptor levels in pancreatic tissue of cumin (Cuminum cyminum) Plant essential oil treated-mice. Caucasian J Sci. (2020) 7:72–82. [Google Scholar]
- 42.Kang N, Yuan R, Huang L, Liu Z, Huang D, Huang L, et al. Atypical nitrogen-containing flavonoid in the fruits of cumin (Cuminum cyminum L.) with anti-inflammatory activity. J Agric Food Chem. (2019) 67:8339–47. 10.1021/acs.jafc.9b02879 [DOI] [PubMed] [Google Scholar]
- 43.Saleh AA, Kirrella AA, Dawood MA, Ebeid TA. Effect of dietary inclusion of cumin seed oil on the performance, egg quality, immune response and ovarian development in laying hens under high ambient temperature. J Anim Physiol Anim Nutr. (2019) 103:1810–7. 10.1111/jpn.13206 [DOI] [PubMed] [Google Scholar]
- 44.Sheikh Asadi M, Gharaei A, Mirdar Harijani J, Arshadi A. A Comparison between dietary effects of Cuminum cyminum essential oil and Cuminum cyminum essential oil, loaded with iron nanoparticles, on growth performance, immunity and antioxidant indicators of white leg shrimp (Litopenaeus vannamei). Aquaculture Nutr. (2018) 24:1466–73. 10.1111/anu.12683 [DOI] [Google Scholar]
- 45.Abdel-Tawwab M, Khalil RH, Diab AM, Khallaf MA, Abdel-Razek N, Abdel-Latif HM, et al. Dietary garlic and chitosan enhanced the antioxidant capacity, immunity, and modulated the transcription of HSP70 and Cytokine genes in Zearalenone-intoxicated European seabass. Fish Shellfish Immunol. (2021) 113:35–41. 10.1016/j.fsi.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury DK, Sahu NP, Sardar P, Deo AD, Bedekar MK, Singha KP, et al. Feeding turmeric in combination with ginger or garlic enhances the digestive enzyme activities, growth and immunity in Labeo rohita fingerlings. Anim Feed Sci Technol. (2021) 277:114964. 10.1016/j.anifeedsci.2021.114964 [DOI] [Google Scholar]
- 47.Hamza RZ, Abd El-Aziz SA, Said AA, Khairy MH, Mahmoud SH, Habib WA, et al. Improving the efficacy of garlic extract in African catfish against copper sulfate-induced immunological and histological effects. Regional Stud Marine Sci. (2021) 41:101579. 10.1016/j.rsma.2020.101579 [DOI] [Google Scholar]
- 48.Ismail IE, Alagawany M, Taha AE, Puvača N, Laudadio V, Tufarelli V. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood haematology, immunity and antioxidant status of broiler chickens. Animal Biosci. (2021) 34:363. 10.5713/ajas.20.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adineh H, Harsij M, Jafaryan H, Asadi M. The effects of microencapsulated garlic (Allium sativum) extract on growth performance, body composition, immune response and antioxidant status of rainbow trout (Oncorhynchus mykiss) juveniles. J Appl Anim Res. (2020) 48:372–8. 10.1080/09712119.2020.1808473 [DOI] [Google Scholar]
- 50.Donma MM, Donma O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med Hypotheses. (2020) 144:109934. 10.1016/j.mehy.2020.109934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkrishna A, Khandrika L, Varshney A. Giloy Ghanvati (Tinospora cordifolia (Willd.) Hook. f. and Thomson) reversed SARS-CoV-2 viral spike-protein induced disease phenotype in the xenotransplant model of humanized zebrafish. Front Pharmacol. (2021) 12:534. 10.3389/fphar.2021.635510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chowdhury P. In silico investigation of phytoconstituents from Indian medicinal herb 'Tinospora cordifolia (giloy)'against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J Biomol Struct Dyn. (2020) 7:1–18. 10.1080/07391102.2020.1803968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naliato RF, Carvalho PLPF, Vicente IST, Xavier WDS, Guimarães MG, et al. Ginger (Zingiber officinale) powder improves growth performance and immune response but shows limited antioxidant capacity for Nile tilapia infected with Aeromonas hydrophila. Aquaculture Nutr. (2021) 27:850–64. 10.1111/anu.13229 [DOI] [Google Scholar]
- 54.Fazelan Z, Vatnikov YA, Kulikov EV, Plushikov VG, Yousefi M. Effects of dietary ginger (Zingiber officinale) administration on growth performance and stress, immunological, and antioxidant responses of common carp (Cyprinus carpio) reared under high stocking density. Aquaculture. (2020) 518:734833. 10.1016/j.aquaculture.2019.734833 [DOI] [Google Scholar]
- 55.Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Van Doan H. Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol. (2020) 99:267–73. 10.1016/j.fsi.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 56.Ahmadifar E, Sheikhzadeh N, Roshanaei K, Dargahi N, Faggio C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture. (2019) 507:341–8. 10.1016/j.aquaculture.2019.04.049 [DOI] [Google Scholar]
- 57.Elmowalid GA, Abd El-Hamid MI, Abd El-Wahab AM, Atta M, Abd El-Naser G, Attia AM. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp Immunol Microbiol Infect Dis. (2019) 66:101334. 10.1016/j.cimid.2019.101334 [DOI] [PubMed] [Google Scholar]
- 58.Eftekhar N, Moghimi A, Roshan NM, Saadat S, Boskabady MH. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement Altern Med. (2019) 19:1–11. 10.1186/s12906-019-2765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheoran N, Kumar R, Kumar A, Batra K, Sihag S, Maan S, et al. Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in broilers. Vet World. (2017) 10:121. 10.14202/vetworld.2017.121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setlur AS, Naik SY, Skariyachan S. Herbal lead as ideal bioactive compounds against probable drug targets of Ebola virus in comparison with known chemical analogue: A computational drug discovery perspective. Interdiscipli Sci Comput Life Sci. (2017) 9:254–77. 10.1007/s12539-016-0149-8 [DOI] [PubMed] [Google Scholar]
- 61.Santos DI, Saraiva JMA, Vicente AA, Moldão-Martins M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In: Barba FJ, Saraiva JMA, Cravotto G, Lorenzo JM. editors. Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds. Woodhead Publishing; Elsevier; (2019). 10.1016/B978-0-12-814174-8.00002-0 [DOI] [Google Scholar]
- 62.Verma DK, Goyal K, Kumar P. Giloy (Tinospora cordifolia L.): a critical review on phytochemicals, medicinal properties and antioxidant activity. Curr Pharm Des. (2020) 26:1. 10.2174/1381612826666200625111530 [DOI] [PubMed] [Google Scholar]
- 63.Kumar P, Verma DK, Kimmy G, Srivastav PP, Sandhu KS. Phytochemicals in giloy (Tinospora cordifolia L.): structure, chemistry, health benefits. In: Verma DK. editor. Phytochemicals in Food and Health: Perspectives for Research and Technological Development. Apple Academic Press; (2021). [Google Scholar]
- 64.Bhushan I, Sharma M, Mehta M, Badyal S, Sharma V, Sharma I, et al. Bioactive compounds and probiotics-a ray of hope in COVID-19 management. Food Sci Human Wellness. (2021) 10:131–40. 10.1016/j.fshw.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy S, Rhim JW. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. Int J Biol Macromol. (2020) 162:1780–9. 10.1016/j.ijbiomac.2020.08.094 [DOI] [PubMed] [Google Scholar]
- 66.Ahmad N, Ahmad R, Naqvi AA, Alam MA, Ashafaq M, Iqbal Z, et al. Isolation, characterization, and quantification of curcuminoids and their comparative effects in cerebral ischemia. J Liq Chromatogr Relat Technol. (2017) 40:133–46. 10.1080/10826076.2017.1293549 [DOI] [Google Scholar]
- 67.Putnik P, Lorenzo JM, Barba FJ, Roohinejad S, ReŽek Jambrak A, Granato D, et al. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods. (2018) 7:106. 10.3390/foods7070106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang T, Ghosh R, Charcosset C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci Technol. (2021) 112:419–30. 10.1016/j.tifs.2021.04.015 [DOI] [Google Scholar]
- 69.Campbell MS, Fleenor BS. The emerging role of curcumin for improving vascular dysfunction: A review. Crit Rev Food Sci Nutr. (2018) 58:2790–2799. 10.1080/10408398.2017.1341865 [DOI] [PubMed] [Google Scholar]
- 70.Tønnesen HH, Másson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharmaceutics. (2002) 244:127–35. 10.1016/S0378-5173(02)00323-X [DOI] [PubMed] [Google Scholar]
- 71.Delfanian M, Sahari MA. Improving functionality, bioavailability, nutraceutical and sensory attributes of fortified foods using phenolics-loaded nanocarriers as natural ingredients. Food Res Int. (2020) 137:109555. 10.1016/j.foodres.2020.109555 [DOI] [PubMed] [Google Scholar]
- 72.Ephrem E, Najjar A, Charcosset C, Greige-Gerges H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. J Funct Foods. (2018) 48:65–84. 10.1016/j.jff.2018.06.021 [DOI] [Google Scholar]
- 73.Zheng B, Lin H, Zhang X, McClements DJ. Fabrication of curcumin-loaded dairy milks using the ph-shift method: formation, stability, and bioaccessibility. J Agric Food Chem. (2019) 67:12245–54. 10.1021/acs.jafc.9b04904 [DOI] [PubMed] [Google Scholar]
- 74.Shirsath SR, Sable SS, Gaikwad SG, Sonawane SH, Saini DR, Gogate PR. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: effect of different operating parameters. Ultrason Sonochem. (2017) 38:437–45. 10.1016/j.ultsonch.2017.03.040 [DOI] [PubMed] [Google Scholar]
- 75.Molosse V, Souza CF, Baldissera MD, Glombowsky P, Campigotto G, Cazaratto CJ, et al. Diet supplemented with curcumin for nursing lambs improves animal growth, energetic metabolism, and performance of the antioxidant and immune systems. Small Ruminant Res. (2019) 170:74–81. 10.1016/j.smallrumres.2018.11.014 [DOI] [Google Scholar]
- 76.Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab Syndr. (2020) 14:367–82. 10.1016/j.dsx.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nawab A, Tang S, Li G, An L, Wu J, Liu W, et al. Dietary curcumin supplementation effects on blood immunological profile and liver enzymatic activity of laying hens after exposure to high temperature conditions. J Therm Biol. (2020) 90:102573. 10.1016/j.jtherbio.2020.102573 [DOI] [PubMed] [Google Scholar]
- 78.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. (2020) 94:127. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain A, Saxena S, Rani V. Comprehensive Assessment of Curcumin as a Functional Food. Singapore: Springer; (2018). 10.1007/978-981-13-1123-9_6 [DOI] [Google Scholar]
- 80.Wang YF, Shao JJ, Zhou CH, Zhang DL, Bie XM, Lv FX, et al. Food preservation effects of curcumin microcapsules. Food Control. (2012) 27:113–7. 10.1016/j.foodcont.2012.03.00819775769 [DOI] [Google Scholar]
- 81.Joung HJ, Choi MJ, Kim JT, Park SH, Park HJ, Shin GH. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: antioxidant property and in vitro digestion. J Food Sci. (2016) 81:N745–53. 10.1111/1750-3841.13224 [DOI] [PubMed] [Google Scholar]
- 82.Chuacharoen T, Sabliov CM. Comparative effects of curcumin when delivered in a nanoemulsion or nanoparticle form for food applications: Study on stability and lipid oxidation inhibition. LWT - Food Sci Technol. (2019) 113:108319. 10.1016/j.lwt.2019.108319 [DOI] [Google Scholar]
- 83.Park SJ, Hong SJ, Garcia CV, Lee SB, Shin GH, Kim JT. Stability evaluation of turmeric extract nanoemulsion powder after application in milk as a food model. J Food Eng. (2019) 259:12–20. 10.1016/j.jfoodeng.2019.04.011 [DOI] [Google Scholar]
- 84.Almatroodi SA, Alrumaihi F, Alsahli MA, Alhommrani MF, Khan A, Rahmani AH. Curcumin, an active constituent of turmeric spice: implication in the prevention of lung injury induced by Benzo (a) Pyrene (BaP) in rats. Molecules. (2020) 25:724. 10.3390/molecules25030724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahmoud HK, Al-Sagheer AA, Reda FM, Mahgoub SA, Ayyat MS. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture. (2017) 475:16–23. 10.1016/j.aquaculture.2017.03.043 [DOI] [Google Scholar]
- 86.Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, future perspectives. Food Funct. (2018) 9:705–14. 10.1039/C7FO01242J [DOI] [PubMed] [Google Scholar]
- 87.Katta S, Srivastava A, Thangapazham RL, Rosner IL, Cullen J, Li H, et al. Curcumin-gene expression response in hormone dependent and independent metastatic prostate cancer cells. Int J Mol Sci. (2019) 20:4891. 10.3390/ijms20194891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu L, Qi S, Chen Y, Luo H, Huang S, Yu X, et al. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials. (2020) 245:119987. 10.1016/j.biomaterials.2020.119987 [DOI] [PubMed] [Google Scholar]
- 89.Ming J, Ye J, Zhang Y, Xu Q, Yang X, Shao X, et al. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. (2020) 97:540–53. 10.1016/j.fsi.2019.12.074 [DOI] [PubMed] [Google Scholar]
- 90.Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives-A review. J Traditional Complement Med. (2017) 7:205–33. 10.1016/j.jtcme.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee G, Chung HS, Lee K, Lee H, Kim M, Bae H. Curcumin attenuates the scurfy-induced immune disorder, a model of IPEX syndrome, with inhibiting Th1/Th2/Th17 responses in mice. Phytomedicine. (2017) 33:1–6. 10.1016/j.phymed.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 92.Nawab A, Li G, An L, Chao L, Xiao M, Zhao Y, et al. Effect of curcumin supplementation on TLR4 mediated non-specific immune responses in liver of laying hens under high-temperature conditions. J Therm Biol. (2019) 84:384–97. 10.1016/j.jtherbio.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 93.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. (2017) 174:1325–48. 10.1111/bph.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Y, Liu L. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway. Influenza Other Respi Viruses. (2017) 11:457–63. 10.1111/irv.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai J, Gu L, Su Y, Wang Q, Zhao Y, Chen X, et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol. (2018) 54:177–87. 10.1016/j.intimp.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 96.Hongtao C, Youling F, Fang H, Huihua P, Jiying Z, Jun Z. Curcumin alleviates ischemia reperfusion-induced late kidney fibrosis through the APPL1/Akt signaling pathway. J Cell Physiol. (2018) 233:8588–96. 10.1002/jcp.26536 [DOI] [PubMed] [Google Scholar]
- 97.Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. (2019) 10:912. 10.3389/fmicb.2019.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Titto M, Ankit T, Saumya B, Gausal AK, Sarada SKS. Curcumin prophylaxis refurbishes alveolar epithelial barrier integrity and alveolar fluid clearance under hypoxia. Respir Physiol Neurobiol. (2020) 274:103336. 10.1016/j.resp.2019.103336 [DOI] [PubMed] [Google Scholar]
- 99.Liu M, Lu Y, Gao P, Xie X, Li D, Yu D, et al. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult Sci. (2020) 99:2196–202. 10.1016/j.psj.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang J, Dong X, Yang A, Zhu D, Kong D, Lv F. A dual fluorescent reverse targeting drug delivery system based on curcumin-loaded ovalbumin nanoparticles for allergy treatment. Nanomedicine. (2019) 16:56–68. 10.1016/j.nano.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 101.Liao F, Liu L, Luo E, Hu J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. (2018) 92:32–37. 10.1016/j.archoralbio.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 102.Afolayan FI, Erinwusi B, Oyeyemi OT. Immunomodulatory activity of curcumin-entrapped poly d, l-lactic-co-glycolic acid nanoparticles in mice. Integrative Med Res. (2018) 7:168–75. 10.1016/j.imr.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. (2020) 181:281–92. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maurya VK, Kumar S, Prasad AK, Bhatt ML, Saxena SK. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease. (2020) 31:179–93. 10.1007/s13337-020-00598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q, Zheng Z, Zhang C, Zhang X, Wu H, Wang J, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. (2020) 48:543–51. 10.1007/s15010-020-01432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan S, Xiang YI, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. (2020) 92:797–806. 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang L, Liu J, Zhang R, Li M, Li Z, Zhou X, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. (2020) 129:104475. 10.1016/j.jcv.2020.104475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saber-Moghaddam N, Salari S, Hejazi S, Amini M, Taherzadeh Z, Eslami S, et al. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial. Phytother Res. (2021) 35:2616–23. 10.1002/ptr.7004 [DOI] [PubMed] [Google Scholar]
- 111.Tahmasebi S, El-Esawi MA, Mahmoud ZH, Timoshin A, Valizadeh H, Roshangar L, et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J Cell Physiol. (2021) 236:5325–38. 10.1002/jcp.30233 [DOI] [PubMed] [Google Scholar]
- 112.Jimeno S, Ventura PS, Castellano JM, García-Adasme SI, Miranda M, Touza P, et al. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur J Clin Invest. (2021) 51:e13404. 10.1111/eci.13404 [DOI] [PubMed] [Google Scholar]
- 113.Ulloque-Badaracco JR, Salas-Tello WI, Al-kassab-Córdova A, Alarcón-Braga EA, Benites-Zapata VA, Maguiña JL, et al. Prognostic value of Neutrophil-to-lymphocyte ratio in COVID-19 patients: A systematic review and meta-analysis. Int J Clin Pract. (2021) 00:e14596. 10.1111/ijcp.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, Johnston TP, Abdollahi E, Sahebkar A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev. (2018) 17:125–35. 10.1016/j.autrev.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 115.Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Sci Nutr. (2020) 8:5215–27. 10.1002/fsn3.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmadi R, Salari S, Sharifi MD, Reihani H, Rostamiani MB, Behmadi M, et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci Nutr. (2021) 9:4068–75. 10.1002/fsn3.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jyoti K, Pandey RS, Kush P, Kaushik D, Jain UK, Madan J. Inhalable bioresponsive chitosan microspheres of doxorubicin and soluble curcumin augmented drug delivery in lung cancer cells. Int J Biol Macromol. (2017) 98:50–8. 10.1016/j.ijbiomac.2017.01.109 [DOI] [PubMed] [Google Scholar]
- 118.Thimmulappa RK, Kumar MNK, Shivamallu C, Subramaniam KT, Radhakrishnan A, Suresh B, et al. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon. (2021) 7:e06350. 10.1016/j.heliyon.2021.e06350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jena AB, Kanungo N, Nayak V, Chainy GBN, Dandapat J. Catechin and Curcumin interact with corona (2019-nCoV/SARS-CoV2) viral S protein and ACE2 of human cell membrane: insights from Computational study and implication for intervention. Sci Rep. (2020) 11:2043. 10.1038/s41598-021-81462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hassan FU, Rehman MSU, Khan MS, Ali MA, Javed A, Nawaz A, et al. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet. (2019) 10:514. 10.3389/fgene.2019.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–3. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- 123.Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. (2020) 56:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mo P, Xing Y, Xiao YU, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. (2020) 16:ciaa270. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) 71:769–77. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belwal T, Devkota HP, Ramola S, Andola HC, Bhatt ID. Optimization of Extraction Methodologies and Purification Technologies to Recover Phytonutrients From Food. Woodhead Publishing; (2020). 10.1016/B978-0-12-815354-3.00007-1 [DOI] [Google Scholar]
- 127.Chávez-González ML, Sepúlveda L, Verma DK, Luna-García HA, Rodríguez-Durán LV, Ilina A, et al. Conventional and emerging extraction processes of flavonoids. Processes. (2020) 8:434. 10.3390/pr8040434 [DOI] [Google Scholar]
- 128.Sahne F, Mohammadi M, Najafpour GD, Moghadamnia AA. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind Crops Prod. (2017) 95:686–94. 10.1016/j.indcrop.2016.11.037 [DOI] [Google Scholar]
- 129.Nurjanah N, Saepudin E. Curcumin isolation, synthesis and characterization of curcumin isoxazole derivative compound. In: AIP Conference Proceedings. AIP Publishing LLC; (2019). 10.1063/1.5132492 [DOI] [Google Scholar]
- 130.Monton C, Settharaksa S, Luprasong C, Songsak T. An optimization approach of dynamic maceration of Centella asiatica to obtain the highest content of four centelloids by response surface methodology. Rev Brasil Farmacognosia. (2019) 29:254–61. 10.1016/j.bjp.2019.01.001 [DOI] [Google Scholar]
- 131.Zakaria F, Ibrahim WNW, Ismail IS, Ahmad H, Manshoor N, Ismail N. LCMS/MS metabolite profling and analysis of acute toxicity effect of the ethanolic extract of centella asiatica on zebrafsh model. Pertanika J Sci Technol. (2019) 27:2. [Google Scholar]
- 132.Rafi M, Handayani F, Darusman LK, Rohaeti E, Wahyu Y, Honda K, et al. A combination of simultaneous quantification of four triterpenes and fingerprint analysis using HPLC for rapid identification of Centella asiatica from its related plants and classification based on cultivation ages. Ind Crops Prod. (2018) 122:93–7. 10.1016/j.indcrop.2018.05.062 [DOI] [Google Scholar]
- 133.Charoenchaitrakool M, Niamnuy C, Dittanet P, Chantes O, Chuangyang P. Statistical optimization for precipitation of bioactive compounds from extracted Centella asiatica using gas anti-solvent technique. J Food Process Eng. (2020) 43:e13318. 10.1111/jfpe.13318 [DOI] [Google Scholar]
- 134.Yingngam B, Chiangsom A, Brantner A. Modeling and optimization of microwave-assisted extraction of pentacyclic triterpenes from Centella asiatica leaves using response surface methodology. Ind Crops Prod. (2020) 147:112231. 10.1016/j.indcrop.2020.112231 [DOI] [Google Scholar]
- 135.BanoŽić M, Babić J, Jokić S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind Crops Prod. (2020) 144:112009. 10.1016/j.indcrop.2019.112009 [DOI] [Google Scholar]
- 136.Verma DK, Srivastav PP. Bioactive compounds of Rice (Oryza sativa L.): review on paradigm and its potential benefit in human health. Trends Food Sci Technol. (2020) 97:355–65. 10.1016/j.tifs.2020.01.007 [DOI] [Google Scholar]
- 137.Kiamahalleh MV, Najafpour-Darzi G, Rahimnejad M, Moghadamnia AA, Kiamahalleh MV. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J Chromatogr B. (2016) 1022:191–8. 10.1016/j.jchromb.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 138.Eltoum MSA, Elfaki AAM. Extraction, characterization, and usage of turmeric curcumin for color coating of metronidazole tablets. Highlights BioSci. (2020) 3:20206. 10.36462/H.BioSci.20206 [DOI] [Google Scholar]
- 139.Gökdemir B, Baylan N, Çehreli S. Application of a novel ionic liquid as an alternative green solvent for the extraction of curcumin from turmeric with response surface methodology: determination and optimization study. Analy Lett. (2020) 53:2111–21. 10.1080/00032719.2020.1730394 [DOI] [Google Scholar]
- 140.Degot P, Huber V, Hofmann E, Hahn M, Touraud D, Kunz W. Solubilization and extraction of curcumin from Curcuma longa using green, sustainable, and food-approved surfactant-free microemulsions. Food Chem. (2020) 336:127660. 10.1016/j.foodchem.2020.127660 [DOI] [PubMed] [Google Scholar]
- 141.Patra JK, Das G, Lee S, Kang SS, Shin HS. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci Technol. (2018) 82:89–109. 10.1016/j.tifs.2018.10.001 [DOI] [Google Scholar]
- 142.Verma DK, Srivastav PP. A paradigm of volatile aroma compounds in rice and their product with extraction and identification methods: a comprehensive review. Food Res Int. (2020) 130:1–33. 10.1016/j.foodres.2019.108924 [DOI] [PubMed] [Google Scholar]
- 143.Kwon HL, Chung MS. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. (2015) 185:58–64. 10.1016/j.foodchem.2015.03.114 [DOI] [PubMed] [Google Scholar]
- 144.Win TT, Thandar S. Identification of Isolated Curcumin from Rhizomes of Curcuma longa (Na-nwin) L. and investigation of antimicrobial activity of the various crude extracts. 3rd Myanmar Korea Conference Res J. (2020) 3:1953–60. [Google Scholar]
- 145.Yulianto ME, Amalia R, Paramita V, Hartati I, Maulinda NA, Shulthoni MA. The effect of operating conditions on curcumin extracted from turmeric by hydrothermal extraction. In: E3S Web of Conferences. EDP Sciences; (2019). 10.1051/e3sconf/201912519001 [DOI] [Google Scholar]
- 146.Pan Y, Ju R, Cao X, Pei H, Zheng T, Wang W. Optimization extraction and purification of biological activity curcumin from Curcuma longa L by high-performance counter-current chromatography. J Sep Sci. (2020) 43:1586–92. 10.1002/jssc.201901174 [DOI] [PubMed] [Google Scholar]
- 147.Gullón B, Gagaoua M, Barba FJ, Gullón P, Zhang W, Lorenzo JM. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci Technol. (2020) 100:1–18. 10.1016/j.tifs.2020.03.039 [DOI] [Google Scholar]
- 148.Verma DK, Srivastav PP. Extraction, identification and quantification methods of rice aroma compounds with emphasis on 2-Acetyl-1-Pyrroline (2-AP) and its relation with rice quality: a comprehensive review. Food Rev Int. (2020). 10.1080/87559129.2020.1720231 [DOI] [Google Scholar]
- 149.Zhou PP, Shan JF, Jiang JL. Extraction optimization of rhizome of curcuma longa by response surface methodology and support vector regression. J Chin Med Mater. (2015) 38:2611–5. [PubMed] [Google Scholar]
- 150.Jamshidi A, Cao H, Xiao J, Simal-Gandara J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res Int. (2020) 137:109353. 10.1016/j.foodres.2020.109353 [DOI] [PubMed] [Google Scholar]
- 151.Tadesse SA, Emire SA. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon. (2020) 6:e04765. 10.1016/j.heliyon.2020.e04765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaur K, Asthir B, Verma DK. Biosynthesis, bioavailability, and metabolism of plant polyphenols: biological activities and their potential benefits in human health. In: Verma DK. editor. Phytochemicals in Food and Health: Perspectives for Research and Technological Development. Apple Academic Press; (2021). [Google Scholar]
- 153.Ferguson JJ, Wolska A, Remaley AT, Stojanovski E, MacDonald-Wicks L, Garg ML. Bread enriched with phytosterols with or without curcumin modulates lipoprotein profiles in hypercholesterolaemic individuals. A randomised controlled trial. Food Funct. (2019) 10:2515–27. 10.1039/C8FO02512F [DOI] [PubMed] [Google Scholar]
- 154.Odimegwu NE, Ubbaonu CN, Ofoedu CE, Akajiaku LO, Njoku NE, Agunwah IM, et al. Comparative study on the proximate composition, functional and sensory properties of turmeric (Curcuma longa) and Pawpaw (Carica papaya) custard products. Curr J Appl Sci Technol. (2019) 34:1–11. 10.9734/cjast/2019/v33i430090 [DOI] [Google Scholar]
- 155.Yildiz E, Gungor G, Yilmaz H, Gocmen D. Changes in bioaccessibility, phenolic content and antioxidant capacity of novel crackers with turmeric (Curcuma longa L.) and mahaleb (Prunus mahaleb L.) powders. Q Assurance Safety Crops Foods. (2019) 11:107–16. 10.3920/QAS2018.1334 [DOI] [Google Scholar]
- 156.Fadairo O, Diósi G, Mironescu I, Máthé E. Development of fortified bakery products based on kokoro, a traditional Nigerian snack. Acta Universitatis Sapientiae Alimentaria. (2018) 11:145–60. 10.2478/ausal-2018-0009 [DOI] [Google Scholar]
- 157.Ferreira LS, Chaves MA, Dacanal GC, Pinho SC. Wet agglomeration by high shear of binary mixtures of curcumin-loaded lyophilized liposomes and cornstarch: Powder characterization and incorporation in cakes. Food Biosci. (2018) 25:74–82. 10.1016/j.fbio.2018.08.003 [DOI] [Google Scholar]
- 158.Adegoke GO, Oyekunle AO, Afolabi MO. Functional biscuits from wheat, soya bean and turmeric (Curcuma longa): optimization of ingredients levels using response surface methodology. Res J Food Nutr. (2017) 1:13–22. [Google Scholar]
- 159.Wahanik AL, Neri-Numa IA, Pastore GM, Kil Chang Y, Pedrosa Silva Clerici MT. Turmeric (Curcuma longa L.): new application as source of fiber and antioxidants in pasta with whole wheat flour. Rev Facult Nacional Agronomía Medellín. (2018) 71:8423–35. 10.15446/rfna.v71n1.66210 [DOI] [Google Scholar]
- 160.Laokuldilok N, Thakeow P, Kopermsub P, Utama-ang N. Quality and antioxidant properties of extruded breakfast cereal containing encapsulated turmeric extract. Chiang Mai J Sci. (2017) 44:946–55. Available online at: http://cmuir.cmu.ac.th/jspui/handle/6653943832/63922 [Google Scholar]
- 161.Asadaii H, Sani AM, Arianfar A, Salehi EA. Effect of tomato lycopene, turmeric and beetroot extract on microbial and chemical properties of cow's milk butter. J BioSci Biotechnol. (2020) 9:59–64. [Google Scholar]
- 162.Britto GCSD, Bécker G, Soares WP, Nascimento E, Rodrigues EC, Picanço NFM, et al. Bioactive compounds and physicochemical properties of dairy products supplemented with plantain and turmeric. J Food Process Preserv. (2020) 44:e14720. 10.1111/jfpp.14720 [DOI] [Google Scholar]
- 163.Al-Obaidi LFH. Effect of adding different concentrations of turmeric powder on the chemical composition, oxidative stability and microbiology of the soft cheese. Plant Arch. (2019) 19:317–21. [Google Scholar]
- 164.Borrin TR, Georges EL, Brito-Oliveira TC, Moraes IC, Pinho SC. Technological and sensory evaluation of pineapple ice creams incorporating curcumin-loaded nanoemulsions obtained by the emulsion inversion point method. Int J Dairy Technol. (2018) 71:491–500. 10.1111/1471-0307.12451 [DOI] [Google Scholar]
- 165.Kumar DD, Mann B, Pothuraju R, Sharma R, Bajaj R. Formulation and characterization of nanoencapsulated curcumin using sodium caseinate and its incorporation in ice cream. Food Funct. (2016) 7:417–24. 10.1039/C5FO00924C [DOI] [PubMed] [Google Scholar]
- 166.Marcon H, Baldissera MD, Furlan VJ, Wagner R, Alba DF, Molosse VL, et al. Curcumin supplementation positively modulates fatty acid profiles in lamb meat. Small Ruminant Res. (2020) 190:106141. 10.1016/j.smallrumres.2020.106141 [DOI] [Google Scholar]
- 167.Ramírez-Carrasco P, Paredes-Toledo J, Romero-Hasler P, Soto-Bustamante E, Díaz-Calderón P, Robert P, et al. Effect of adding curcumin on the properties of linseed oil organogels used as fat replacers in pâtés. Antioxidants. (2020) 9:735. 10.3390/antiox9080735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koopman E. The Nutritional Quality of Turmeric Fortified Zobo (Hibiscus sabdariffa). (2019). Availanle online at: https://edepot.wur.nl/496703 (accessed October 20, 2020).
- 169.Sujarwanta RO, Suryanto E, Yuliatmo R, Prayitno AH. Physicochemical and sensory characteristics of chicken nugget with curcuma (Curcuma zanthorrhiza) flour fortification. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing; (2019). 10.1088/1755-1315/387/1/012091 [DOI] [Google Scholar]
- 170.Grasso SM. The Effect of Health Information on the Acceptability of a Functional Beverage with Fresh Turmeric (Doctoral dissertation, Virginia Tech) (2018). [Google Scholar]
- 171.Kim SW, Garcia CV, Lee BN, Kwon HJ, Kim JT. Development of turmeric extract nanoemulsions and their incorporation into canned ham. Korean J Food Sci Animal Res. (2017) 37:889. 10.5851/kosfa.2017.37.6.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arepally D, Reddy RS, Goswami TK, Datta AK. Biscuit baking: A review. LWT - Food Sci Technol. (2020) 131:109726. 10.1016/j.lwt.2020.109726 [DOI] [Google Scholar]
- 173.Khaneghah AM, Farhadi A, Nematollahi A, Vasseghian Y, Fakhri Y. A systematic review and meta-analysis to investigate the concentration and prevalence of trichothecenes in the cereal-based food. Trends Food Sci Technol. (2020) 102:193–202. 10.1016/j.tifs.2020.05.026 [DOI] [Google Scholar]
- 174.Ou J, Wang M, Zheng J, Ou S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. (2019) 284:90–9. 10.1016/j.foodchem.2019.01.096 [DOI] [PubMed] [Google Scholar]
- 175.Azmi NS, Bhat R, Yeoh TK. Quality evaluation of novel cookies prepared by supplementing with fresh turmeric flower (Curcuma longa L.) extracts as a value added functional ingredient. Int Food Res J. (2016) 23:1514–22. [Google Scholar]
- 176.Hefnawy HT, El-Shourbagy GA, Ramadan MF. Phenolic extracts of carrot, grape leaf and turmeric powder: antioxidant potential and application in biscuits. J Food Measure Character. (2016) 10:576–83. 10.1007/s11694-016-9339-7 [DOI] [Google Scholar]
- 177.Turkmen N, Akal C, Özer B. Probiotic dairy-based beverages: A review. J Funct Foods. (2019) 53:62–75. 10.1016/j.jff.2018.12.004 [DOI] [Google Scholar]
- 178.Monteiro SH, Silva EK, Guimarães JT, Freitas MQ, Meireles MAA, Cruz AG. High-intensity ultrasound energy density: How different modes of application influence the quality parameters of a dairy beverage. Ultrason Sonochem. (2020) 63:104928. 10.1016/j.ultsonch.2019.104928 [DOI] [PubMed] [Google Scholar]
- 179.Hasneen DF, Zaki NL, Abbas MS, Soliman AS, Ashoush IS, Fayed AE. Comparative evaluation of some herbs and their suitability for skimmed milk yoghurt and cast Kariesh cheese fortification as functional foods. Ann Agricult Sci. (2020) 65:6–12. 10.1016/j.aoas.2020.05.001 [DOI] [Google Scholar]
- 180.El-Den EM. The chemical and microbiological properties of ricotta cheese supplemented with curcumin and bifidobacteria. Egypt J Food Sci. (2020) 48:65–72. 10.21608/ejfs.2020.21792.1037 [DOI] [Google Scholar]
- 181.Hosny IM, El Kholy WI, Murad HA, El Dairouty RK. Antimicrobial activity of Curcumin upon pathogenic microorganisms during manufacture and storage of a novel style cheese 'Karishcum'. J Am Sci. (2011) 7:611–8. [Google Scholar]
- 182.Lodh J, Khamrui K, Prasad WG. Optimization of heat treatment and curcumin level for the preparation of anti-oxidant rich ghee from fermented buffalo cream by Central Composite Rotatable Design. J Food Sci Technol. (2018) 55:1832–9. 10.1007/s13197-018-3098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shao P, Zhang J, Fang Z, Sun P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. (2014) 41:132–9. 10.1016/j.foodhyd.2014.04.003 [DOI] [Google Scholar]
- 184.Alessa F, Hettiarachchy N, Rayaprolu S, Benamara M, Greathouse D, Singh S. Stability of nano encapsulated rice bran derived bioactive pentapeptide in apple juice. J Food Process Technol. (2014) 5:356. 10.4172/2157-7110.1000356 [DOI] [Google Scholar]
- 185.de Lourdes Samaniego-Vaesken M, Alonso-Aperte E, Varela-Moreiras G. Vitamin food fortification today. Food Nutr Res. (2012) 56:5459. 10.3402/fnr.v56i0.5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Carvalho FAL, Munekata PE, de Oliveira AL, Pateiro M, Domínguez R, Trindade MA, et al. Turmeric (Curcuma longa L.) extract on oxidative stability, physicochemical and sensory properties of fresh lamb sausage with fat replacement by tiger nut (Cyperus esculentus L.) oil. Food Res Int. (2020) 136:109487. 10.1016/j.foodres.2020.109487 [DOI] [PubMed] [Google Scholar]
- 187.Lim HS, Park SH, Ghafoor K, Hwang SY, Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. (2011) 124:1577–82. 10.1016/j.foodchem.2010.08.016 [DOI] [Google Scholar]
- 188.Zanzer YC, Batista ÂG, Dougkas A, Tovar J, Granfeldt Y, Östman E. Difficulties in translating appetite sensations effect of turmeric-based beverage when given prior to isoenergetic medium-or high-fat meals in healthy subjects. Nutrients. (2019) 11:736. 10.3390/nu11040736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Burgos-Morón E, Calderón-Montaño JM, Salvador J, Robles A, López-Lázaro M. The dark side of curcumin. Int J Cancer. (2010) 126:1771–5. 10.1002/ijc.24967 [DOI] [PubMed] [Google Scholar]
- 190.Hatcher HC, Torti FM, Torti SV. Curcumin, oxidative Stress, Cancer Therapy. Oxidative Stress in Cancer Biology and Therapy. Totowa, NJ: Humana Press; (2012). 10.1007/978-1-61779-397-4_12 [DOI] [Google Scholar]
- 191.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Alternative Complement Med. (2003) 9:161–8. 10.1089/107555303321223035 [DOI] [PubMed] [Google Scholar]
- 192.Sundar S, Mariappan R, Piraman S. Synthesis and characterization of amine modified magnetite nanoparticles as carriers of curcumin-anticancer drug. Powder Technol. (2014) 266:321–8. 10.1016/j.powtec.2014.06.033 [DOI] [Google Scholar]
- 193.Mai Z, Chen J, He T, Hu Y, Dong X, Zhang H, et al. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. (2017) 7:1724–34. 10.1039/C6RA25314H [DOI] [Google Scholar]