Abstract
Acute graft-versus-host disease (aGVHD) is a major complication after allogeneic hematopoietic stem cell transplantation (HSCT). Corticosteroid is the first-line treatment for aGVHD, but its response rate is only approximately 50%. At present, no uniformly accepted treatment for steroid-refractory aGVHD (SR-aGVHD) is available. Blocking interleukin-2 receptors (IL-2Rs) on donor T cells using pharmaceutical antagonists alleviates SR-aGVHD. This meta-analysis aimed to compare the efficacy and safety of four commercially available IL-2R antagonists (IL-2RAs) in SR-aGVHD treatment. A total of 31 studies met the following inclusion criteria (1): patients of any race, any sex, and all ages (2); those diagnosed with SR-aGVHD after HSCT; and (3) those using IL-2RA-based therapy as the treatment for SR-aGVHD. The overall response rate (ORR) at any time after treatment with basiliximab and daclizumab was 0.81 [95% confidence interval (CI): 0.74–0.87)] and 0.71 (95% CI: 0.56–0.82), respectively, which was better than that of inolimomab 0.54 (95% CI: 0.39–0.68) and denileukin diftitox 0.56 (95% CI: 0.35–0.76). The complete response rate (CRR) at any time after treatment with basiliximab and daclizumab was 0.55 (95% CI: 0.42–0.68) and 0.42 (95%CI: 0.29–0.56), respectively, which was better than that of inolimomab 0.30 (95% CI: 0.16–0.51) and denileukin diftitox 0.37 (95% CI: 0.24–0.52). The ORR and CRR were better after 1-month treatment with basiliximab and daclizumab than after treatment with inolimomab and denileukin diftitox. The incidence of the infection was higher after inolimomab treatment than after treatment with the other IL-2RAs. In conclusion, the efficacy and safety of different IL-2RAs varied. The response rate of basiliximab was the highest, followed by that of daclizumab. Prospective, randomized controlled trials are needed to compare the efficacy and safety of different IL-2RAs.
Keywords: acute graft-versus-host disease, interleukin-2 receptor antagonist, second-line treatment, steroid-refractory, meta-analysis
Introduction
Hematopoietic stem cell transplantation (HSCT) is a curative measure for hematopoietic malignancies (1). However, its outcome has been compromised by acute graft-versus-host disease (aGVHD), which is a major complication that occurs early post-HSCT. Although many efforts have been made to prevent aGVHD, it is still responsible for early mortality post-transplantation (2). Corticosteroid is the first-line treatment of aGVHD. However, its response rate is only approximately 50% (3). Thus far, no universally accepted treatment for steroid-refractory aGVHD (SR-aGVHD) is available, and survival is poor (4).
One of the critical pathophysiological mechanisms of aGVHD is mediated by T-lymphocyte activation, which exclusively expresses the interleukin-2 receptor (IL-2R) alpha chain (5). Blocking IL-2R on donor T cells using pharmaceutical antagonists alleviates aGVHD, especially SR-aGVHD (6). Some commercially available IL-2R antagonists (IL-2RAs) are basiliximab, daclizumab, inolimomab, and denileukin diftitox. The first three are monoclonal antibodies, which can directly interrupt subsequent T-cell activation by binding to CD25 with high affinity. Inolimomab is a murine anti-human monoclonal antibody with a half-life of 44.5 h (7). Basiliximab is a murine chimeric monoclonal antibody with a half-life of 7 days (8). Daclizumab is a humanized monoclonal antibody with a half-life of 21–25 days (9). In addition, denileukin difititox is a recombinant fusion protein made of diphtheria toxin and human IL-2 sequence, which binds to IL-2R and poisons activated T lymphocytes afterward (10). The half-life of denileukin difititox is 70–80 min (11). Since the 1990s, emerging studies have identified the efficacy and safety of these IL-2RAs in SR-aGVHD treatment (8, 10, 12–41); however, the results varied dramatically because of the great heterogeneity in the study design. So far, no study has been designed to compare the efficacy of different IL-2RAs.Thus, this meta-analysis was conducted to compare the efficacy and safety of these four IL-2RAs in SR-aGVHD treatment.
Methods
Inclusion Criteria
The inclusion criteria were as follows (1): patients of any race, any sex, and all ages (2); those diagnosed with SR-aGVHD after HSCT; and (3) those using IL-2RA-based therapy as the treatment for SR-aGVHD. Reviews, case reports, duplicates, and conference abstracts were excluded. Multiple studies reporting the same data were considered as one.
Search Strategy
A literature search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (42). The PubMed and Embase databases were searched, published from January 2000 through December 2020, with the search strategy following the Population (patients with steroid refractory acute graft versus host disease), Intervention (interleukin-2 receptor antagonists), Comparison(between four different interleukin-2 receptor antagonists), Outcomes (overall response rate [ORR], complete response rate [CRR], chronic GVHD [cGVHD], overall survival [OS] rate, and infectious complications), and Study framework (retrospective, prospective non-randomized and randomized trials) (43): (interleukin-2 OR IL-2 OR CD25 OR Daclizumab OR Basiliximab OR Inolimomab OR Denileukin) AND (steroid refractory OR steroid-refractory OR steroid resistant OR steroid-resistant OR corticosteroid refractory OR corticosteroid-refractory) AND (acute graft versus host disease OR aGVHD) AND 2000/01/01[dp]:2020/12/31[dp].
Data Extraction and Outcomes
The ORR, CRR, cGVHD, overall survival rate, and infectious complications at any time after treatment with IL-2RAs were chosen as the primary end points. In addition, the response rate at 1 month after IL-2RA treatment was assessed. As different studies had different time points, the time frame for the evaluation of response rate at 1 month after IL-2RA treatment was prolonged. That is, the earliest studies evaluating at 3 weeks while the latest studies evaluating at 6 weeks after treatment with IL-2RAs were enrolled in this analysis. Missing data were documented as “not available (NA)”.
Statistical Analysis
The “meta” package version 4.18-0 (44) (R Project for Statistical Computing, version 4.0.5) was used to perform the meta-analysis. Statistical heterogeneity among studies was assessed using the I 2 statistics and Cochran Q-test. The random-effects model was adopted, with the heterogeneity test showing I 2 > 50% and P < 0.10. Also, the “stats” package version 4.0.5 (45) was used to perform the t test for comparison between the means of two subgroups, including the organ response rates, infection rates, cGVHD rates and OS rates. The null hypothesis was set to no difference. A P value <0.05 was considered statistically significant to reject the null hypothesis.
Results
Included Studies
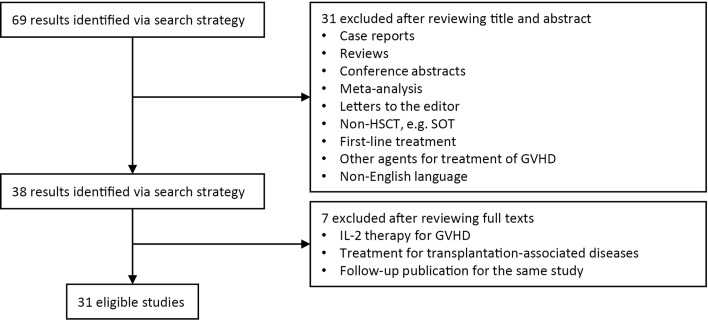
A total of 31 studies reporting on basiliximab (8, 13–20), daclizumab [(22–32); one study using the domestic generic drug (21)], inolimomab (33–40), and denileukin diftitox (10, 41) were included in this meta-analysis (Figure 1). A total of 1360 patients were enrolled, including 533, 337, 438, and 52 patients treated with basiliximab, daclizumab, inolimomab, and denileukin diftitox, respectively (Tables 1, 2, and Supplementary Table 1). Three (15–17), four (22, 23, 29, 30), and two (34, 36) studies used combined therapies of basiliximab, daclizumab, and inolimomab, respectively.
Figure 1.
Selection scheme of studies. GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; SOT, solid organ transplant.
Table 1.
Main characteristics of 31 included studies.
| Studies | Study design | N | Response/event (n) | cGVHD incidence (%) | Overall survival rate | median follow-up (months) | |||
|---|---|---|---|---|---|---|---|---|---|
| ORR | ORR at 1 month | CR | CR at 1 month | ||||||
| Basiliximab-based treatment | |||||||||
| Liu, (13) | retrospective | 230 | 151 | 151 | 140 | 140 | 44.80 | 0.617 | 41.8 |
| Tang, (14) | retrospective | 100 | 85 | 85 | 74 | 74 | 43.75 | 0.762 | 25.3 |
| Tan, (15) | prospective, unrandomized | 65 | 59 | 59 | 49 | 49 | 50.00 | 0.547 | 18.5 |
| Chakupurakal, (8) | prospective, unrandomized | 14 | 13 | NA | 7 | NA | NA | NA | NA |
| Nadeau, (16) | retrospective | 21 | 16 | 16 | 9 | 9 | 71.43 | 0.24 | 34.5 |
| Jaiswal, (17) | prospective, unrandomized | 10 | 7 | 7 | 3 | 3 | 16.67 | NA | 4.2 |
| Wang, (18) | retrospective | 53 | 46 | NA | 37 | NA | 69.39 | 0.514 | 0.2 |
| Schmidt-Hieber, (19) | prospective, unrandomized | 23 | 19 | NA | 4 | NA | 62.50 | NA | 6.1 |
| Massenkeil, (20) | retrospective | 17 | 12 | NA | 9 | NA | 61.54 | NA | 4.1 |
| Daclizumab-based treatment | |||||||||
| Tao, (21) | retrospective | 64 | 53 | 53 | 37 | 37 | 34.38 | 0.729 | 0.1 |
| Rager, (22) | retrospective | 17 | 8 | NA | 4 | NA | NA | NA | 1.5 |
| Rao, (23) | retrospective | 22 | 19 | NA | 12 | NA | 50.00 | NA | 16.2 |
| Miano, (24) | retrospective | 13 | 12 | 12 | 6 | 6 | 66.67 | NA | 14.0 |
| Hui, (25) | retrospective | 12 | 2 | 2 | 1 | 1 | NA | NA | 4.0 |
| Perales, (26) | retrospective | 57 | 31 | 31 | NA | NA | NA | NA | 98.0 |
| Teachey, (27) | retrospective | 11 | 7 | NA | 5 | NA | NA | NA | NA |
| Bordigoni, (28) | prospective, unrandomized | 62 | 56 | 56 | 43 | 43 | 67.80 | NA | 1.5 |
| Wolff, (29) | prospective, unrandomized | 21* | 14 | NA | 8 | NA | 66.67 | NA | 19.5 |
| Srinivansan, (30) | retrospective | 3 | 3 | NA | 3 | NA | 100.00 | NA | 4.0 |
| Willenbacher, (31) | prospective, unrandomized | 12 | 8 | 8 | 1 | 1 | NA | NA | 15.3 |
| Preziprka, (32) | prospective, unrandomized | 43 | 22 | 22 | 16 | 16 | NA | 0.4 | 2.6 |
| Inolimomab-based treatment | |||||||||
| Girerd, (34) | prospective, randomized | 49 | NA | NA | NA | NA | NA | 0.469 | 58.4 |
| Garcia-Cadenas, (35) | retrospective | 98 | 38 | 38 | NA | NA | NA | 0.454 | 19.4 |
| van Groningen, (36) | prospective, unrandomized | 21 | 10 | 10 | 6 | 6 | NA | 0.1 | 1.8 |
| Girerd, (37) | retrospective | 33 | 30 | 30 | 24 | 24 | 63.33 | 0.79 | NA |
| Garcia-Cadenas, (35) | retrospective | 92 | 39 | 39 | 13 | 13 | NA | 0.22 | 60.0 |
| Xhaard, (38) | retrospective | 20 | 7 | NA | NA | NA | NA | 0.3 | 74.0 |
| Pinana, (39) | retrospective | 40 | 20 | 20 | 8 | 8 | 78.26 | 0.3 | 1.9 |
| Bay, (40) | retrospective | 85 | 54 | NA | 25 | NA | NA | 0.26 | 20.0 |
| Denileukin diftitox treatment | |||||||||
| Shaughnessy, (10) | prospective, unrandomized | 22 | 9 | 9 | 9 | 9 | 60.00 | NA | 4.0 |
| Ho, (41) | prospective, unrandomized | 30 | 17 | 17 | 8 | 8 | NA | NA | 7.2 |
NA, not available; ORR, overall response rate; CR, complete response rate; cGVHD, chronic graft-versus-host disease.
*Only 20 patients were eligible for evaluation because 1 patient died 5 days after treatment due to rapid progression of pre-existing invasive aspergillosis.
Table 2.
Other characteristics of 31 included studies.
| Studies | Median age/year (range) | HLA matching (n) | aGVHD grade (n) | Median time from SR-aGVHD diagnosis to the application of IL-2RAs/day (range) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRD | mMRD | MUD | mMUD | I | II | III | IV | |||
| Basiliximab-based treatment | ||||||||||
| Liu, (13) | NA | 17 | 208 | 5 | 0 | 191 | 25 | 14 | 5 (3–20) | |
| Tang, (14) | 10 (1–17) | NA | NA | NA | NA | 0 | 57 | 27 | 16 | NA |
| Tan, (15) | 13 (9–55) | 13 | 40 | 12 | 0 | 0 | 0 | 21 | 44 | 8 (3–49) |
| Chakupurakal, (8) | 41 (20–69) | 0 | 1 | 6 | 7 | 1 | 1 | 5 | 7 | NA |
| Nadeau, (16) | 57 (20–71) | 7 | 1 | 10 | 3 | 0 | 0 | 13 | 8 | 5(NA) |
| Jaiswal, (17) | 7 (2–20) | 0 | 10 | 0 | 0 | 0 | 0 | 10 | NA | |
| Wang, (18) | 25 (8–52) | NA | NA | NA | NA | 0 | 10 | 27 | 16 | NA |
| Schmidt-Hieber, (19) | 51 (31–63) | 7 | 1 | 12 | 3 | 0 | 11 | 12 | 0 | NA |
| Massenkeil, (20) | 39 (23–50) | 6 | 0 | 11 | 0 | 0 | 3 | 12 | 2 | 7 (3–25) |
| Daclizumab-based treatment | ||||||||||
| Tao, (21) | 35 (13–57) | 45 | 19 | 0 | 3 | 28 | 33 | NA | ||
| Rager, (22) | 47 (35–63) | 5 | 0 | 9 | 2 | 0 | 3 | 10 | 4 | 7 (2–26) |
| Rao, (23) | NA | 4 | 0 | 12 | 6 | 0 | 0 | 7 | 15 | NA |
| Miano, (24) | NA | 3 | 10 | 0 | 4 | 4 | 5 | 48 (12–201) | ||
| Hui, (25) | 38.5 (25–55) | 9 | 0 | 2 | 1 | 0 | 0 | 12 | 0 | 8.5 (3–28) |
| Perales, (26) | 28.9 (0.7–57.7) | 21 | 12 | 13 | 11 | 5 | 23 | 14 | 15 | NA |
| Teachey, (27) | NA | NA | NA | NA | NA | 0 | 6 | 3 | 2 | NA |
| Bordigoni, (28) | 25.4 (1.5–53) | 32 | 1 | 11 | 18 | 0 | 41 | 21 | NA | |
| Wolff, (29) | 44 (15–61) | 6 | 0 | 14 | 1 | 0 | 1 | 17 | 3 | 17 (3–66) |
| Srinivansan, (30) | 33 (33–46) | NA | NA | NA | NA | 0 | 0 | 1 | 2 | NA |
| Willenbacher, (31) | 46 (28–56) | 3 | 1 | 5 | 3 | 0 | 0 | 1 | 11 | 5 (3–13) |
| Preziprka, (32) | 31 (1–53) | 14 | 15 | 14 | 0 | 1 | 22 | 12 | 8 | NA |
| Inolimomab-based treatment | ||||||||||
| Girerd, (34) | NA | 15 | 0 | 31 | 3 | 0 | 0 | 100 | 0 | 6 (3–9) |
| Garcia-Cadenas, (36) | 50 (17–70) | 54 | 44 | 0 | 6 | 51 | 41 | 15 (4–91) | ||
| van Groningen, (36) | 54 (24–66) | 11 | 0 | 7 | 3 | 0 | 0 | 17 | 4 | NA |
| Girerd, (34) | 44 (17–65) | 6 | 9 | 11 | 7 | 0 | 7 | 19 | 7 | 15 (3–36) |
| Garcia-Cadenas, (35) | 50 (17–68) | 52 | 40 | 0 | 66 | 48 | 38 | 17 (2–204) | ||
| Xhaard, (38) | 42 (5–64) | 4 | 0 | 11 | 5 | 0 | 13 | 7 | 0 | 12 (NA) |
| Pinana, (39) | 47 (17–63) | 27 | 1 | 5 | 7 | 0 | 2 | 22 | 16 | 21 (4–91) |
| Bay, (40) | 29.5 (0.2–61) | 41 | 8 | 27 | 9 | 0 | 26 | 26 | 33 | 13 (8–23) |
| Denileukin diftitox treatment | ||||||||||
| Shaughnessy, (10) | 44 (9–59) | 12 | 0 | 8 | 2 | 0 | 7 | 7 | 8 | NA |
| Ho, (41) | 43 (20–63) | 2 | 26 | 1 | 1 | 0 | 11 | 13 | 6 | NA |
NA, not available; HLA, human leukocyte antigen; MRD, matched related donor; MUD, matched unrelated donor; mMRD, mismatched related donor; mMUD, mismatched unrelated donor; aGVHD, acute graft-versus-host disease; IL-2RAs, interleukin-2 receptor antagonists.
ORR After Treatment With IL-2RAs
The results showed that ORR of basiliximab and daclizumab was 0.81 [95% confidence interval (CI): 0.74–0.87] and 0.71 (95% CI: 0.56–0.82), respectively, which seemed to be better than that of inolimomab 0.54 (95% CI: 0.39–0.68) and denileukin diftitox 0.56 (95% CI: 0.35–0.76) (Figure 2A).
Figure 2.
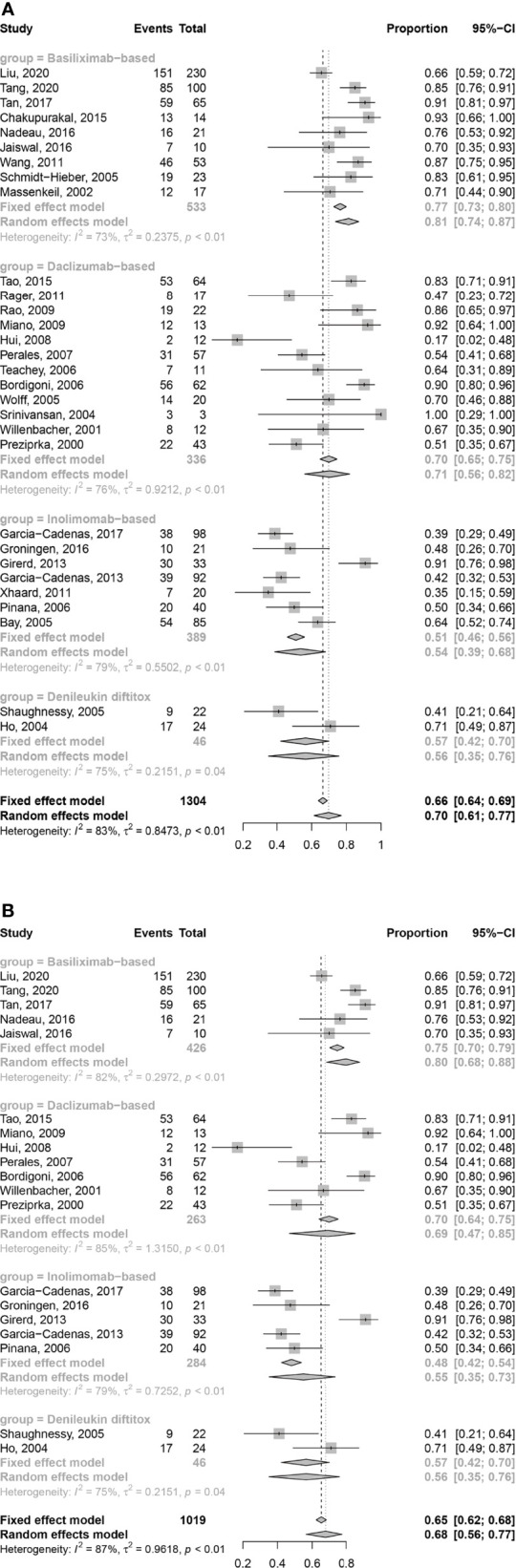
Forest plots of ORR at any time (A) and 1 month (B) after treatment with IL-2RAs.
In retrospective studies, ORR of basiliximab, daclizumab and inolimomab was 0.78 (95% CI: 0.68–0.85), 0.70 (95% CI: 0.48–0.85) and 0.55 (95% CI: 0.37–0.71), respectively. In prospective unrandomized studies, ORR of basiliximab, daclizumab, inolimomab, and denileukin diftitox was 0.87 (95% CI: 0.80–0.92), 0.73 (95% CI: 0.53–0.86), 0.48 (95% CI: 0.28–0.68), and 0.56 (95% CI: 0.35–0.76), respectively (Supplementary Figure S1). Only 1 randomized controlled trial (RCT) identifying the efficacy of inolimomab about SR-aGVHD did not provide the data about ORR (33).
In the analysis of ORR at 1 month after treatment, 4 (8, 18–20), 5 (22, 23, 27, 29, 30), and 2 (38, 40) studies on basiliximab, daclizumab, and inolimomab were excluded due to insufficient data. The ORR at 1 month after treatment with IL-2RA of basiliximab and daclizumab was 0.80 (95% CI: 0.68–0.88) and 0.69 (95% CI: 0.47–0.85), respectively, which seemed to be better than that of inolimomab 0.55 (95% CI: 0.35–0.73) and denileukin diftitox 0.56 (95% CI: 0.35–0.76) (Figure 2B).
In retrospective studies, the ORR at 1 month after treatment with IL-2RA of basiliximab, daclizumab, and inolimomab was 0.76 (95% CI: 0.63–0.85), 0.66 (95% CI: 0.31–0.89), and 0.57 (95% CI: 0.33–0.79), respectively. In prospective unrandomized studies, the ORR at 1 month after treatment with IL-2RA of basiliximab, daclizumab, inolimomab, and denileukin diftitox was 0.88(95% CI: 0.79-0.94), 0.73 (95% CI: 0.47–0.89), 0.48 (95% CI: 0.28–0.68), and 0.56 (95% CI: 0.35–0.76), respectively (Supplementary Figure S2). The RCT about inolimomab did not provide the data about ORR at 1 month after treatment (33).
CRR After Treatment With IL-2RAs
The CRR at any time after treatment with basiliximab and daclizumab was found to be 0.55 (95% CI: 0.42–0.68) and 0.42 (95%CI: 0.29–0.56), respectively, which was better than that of inolimomab 0.30 (95% CI: 0.16–0.51) and denileukin diftitox 0.37 (95% CI: 0.24–0.52) (Figure 3A).
Figure 3.
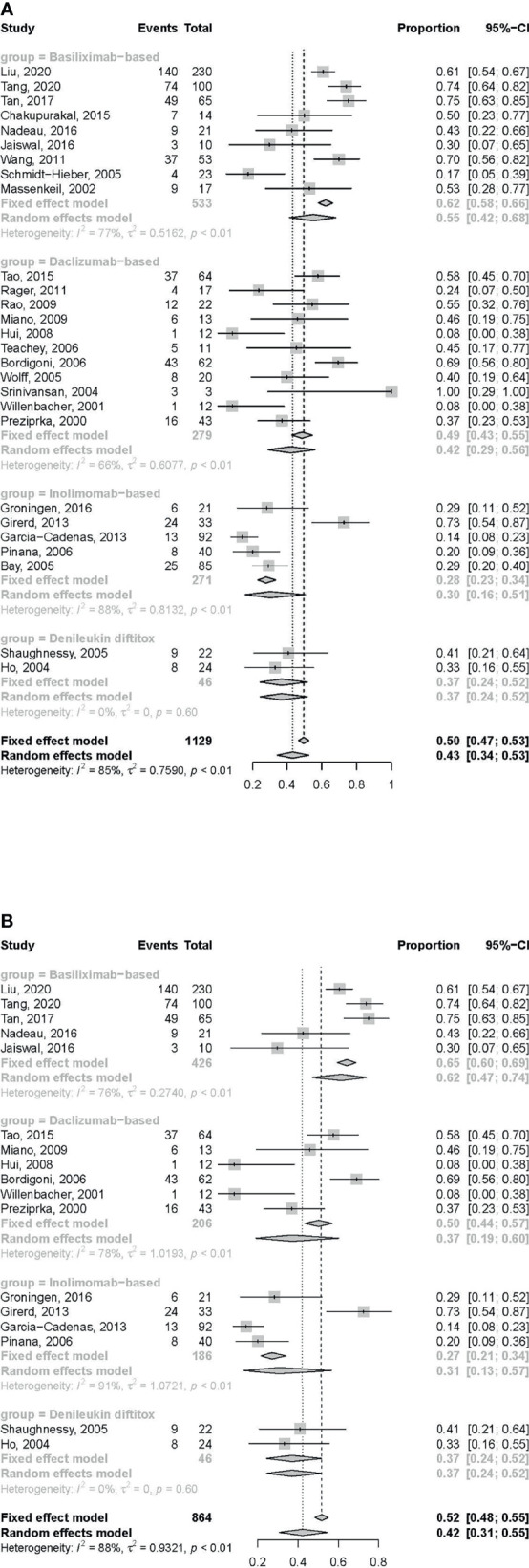
Forest plots of CRR at any time (A) and 1 month (B) after treatment with IL-2RAs.
In retrospective studies, the CRR of basiliximab, daclizumab and inolimomab was 0.64 (95% CI: 0.55–0.72), 0.44 (95% CI: 0.29–0.60), 0.31 (95% CI: 0.14–0.56), respectively. In prospective unrandomized studies, CRR of basiliximab, daclizumab, inolimomab and denileukin diftitox was 0.44 (95% CI: 0.21–0.70), 0.39 (95% CI: 0.19–0.64), 0.29 (95% CI: 0.13–0.51), and 0.37 (95% CI: 0.24–0.52), respectively (Supplementary Figure S3). The RCT about inolimomab did not provide the data about CRR (33).
In the analysis of CRR at 1 month after treatment, 4 (8, 18–20), 5 (22, 23, 27, 29, 30), and 1 (40) studies on basiliximab, daclizumab, and inolimomab, respectively, were excluded due to insufficient data. The CRR at 1 month after treatment with IL-2RAs of basiliximab and daclizumab was found to be 0.62 (95% CI: 0.47–0.74) and 0.37 (95% CI: 0.19–0.60), respectively, which was better than that of inolimomab 0.31 (95% CI: 0.13–0.57) and denileukin diftitox 0.37 (95% CI: 0.24–0.52) (Figure 3B).
In retrospective studies, the CRR at 1 month after treatment with IL-2RAs of basiliximab, daclizumab and inolimomab was 0.63 (95% CI: 0.50–0.74), 0.37 (95% CI: 0.15–0.67) and 0.32 (95% CI: 0.10–0.66), respectively. In prospective unrandomized studies, the CRR at 1 month after treatment with IL-2RAs of basiliximab, daclizumab, inolimomab and denileukin diftitox was 0.58 (95% CI: 0.25–0.85), 0.38 (95% CI: 0.13–0.71), 0.29 (95% CI: 0.13–0.51) and 0.37 (95% CI: 0.24–0.52), respectively (Supplementary Figure S4). The RCT about inolimomab did not provide the data about CRR at 1 month after treatment (33).
Response According to the Involved Organs After Treatment With IL-2RAs
The ORRs and CRRs of different organs involved for these four drugs were compared. Five (13–15, 19, 20), five (21, 24–26, 31), two (38, 40), and one (41) studies on basiliximab, daclizumab, inolimomab, and denileukin diftitox, respectively, were included in the analysis of the ORRs. The ORRs of skin, gut, and liver at any time after treatment were all comparable among these four IL-2RAs (Supplementary Figure S5A).
Two (15, 20), five (21, 24, 25, 31, 32), and two (10, 41) studies on basiliximab, daclizumab, and denileukin diftitox, respectively, were included in the analysis of CRRs. In the gut and liver aGVHD, basiliximab showed a higher CRR at any time compared with daclizumab [gut: 0.76 (95% CI: 0.34–1.19) vs 0.34 (95% CI: 0.05–0.62), P = 0.012; liver: 0.74 (95% CI: 0.67–0.82) vs 0.14 (95% CI: 0.08–0.20), P < 0.001; Supplementary Figure S5B].
Infections After Treatment With IL-2RAs
Seven (13–15, 17–20), nine (21–26, 28, 29, 31), five (33–35, 37, 39), and two (10, 41) studies on basiliximab, daclizumab, inolimomab, and denileukin diftitox, respectively, were enrolled to analyze the incidence of infection after IL-2RA treatment. Two of them did not have information on viral infection and were excluded from the analysis of viral infections [daclizumab (25) and denileukin diftitox (41)]. The incidence of infection after inolimomab treatment [1.65 cases per person (95% CI: 0.78–2.53 cases per person)] was the highest compared with other IL-2RAs. The infection rates were comparable between the basiliximab group [1.19 cases per person (95% CI: 0.51–1.86 cases per person)] and the daclizumab group [0.95 cases per person (95% CI: 0.58–1.32 cases per person)], which both seemed to be higher than those in the denileukin diftitox group [0.24 cases per person (95% CI: 0–1.76 cases per person)]. The frequencies of viral infection were comparable among the four IL-2RAs (Supplementary Figure S6).
cGVHD
Eight (13–20), six (21, 23, 24, 28–30), two (34, 39) and one (10) studies on basiliximab, daclizumab, inolimomab and denileukin diftitox, respectively, could be enrolled in the analysis of cGVHD. The incidence of cGVHD after basiliximab, daclizumab, inolimomab and denileukin diftitox treatment was 52.5% (95% CI: 37.5%–67.5%), 64.3% (95% CI: 41.3%–87.2%), 70.8% (95% CI: 24.0%–100.0%) and 60.0%, respectively. In retrospective studies, the incidence of basiliximab, daclizumab and inolimomab was 58.2% (95% CI: 41.8%–74.6%), 62.8% (95% CI: 18.0%–100.0%) and 70.8% (95% CI: 24.0%–100.0%), respectively. In prospective unrandomized studies, the incidence of basiliximab, daclizumab and denileukin diftitox was 43.1% (95% CI: 15.8%–100.0%), 67.2% (95% CI: 60.1%–74.4%) and 60.0%, respectively. The RCT about inolimomab did not provide the data about cGVHD (33).
OS
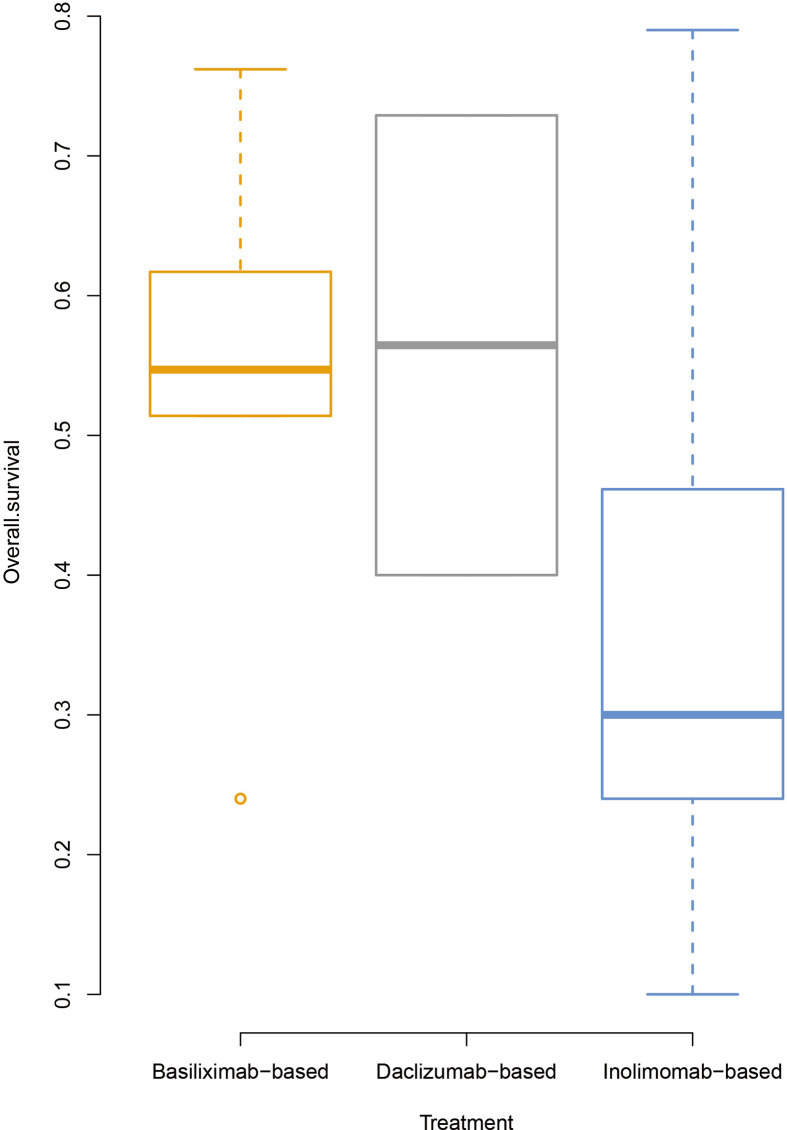
Five (13–16, 18), two (21, 32), and eight (33–40) studies on basiliximab, daclizumab, and inolimomab, respectively, were included in the survival analysis. Two studies on denileukin diftitox were excluded because they did not provide the information on OS. The OS rate for basiliximab and daclizumab was 53.6% (95% CI: 29.9%–77.31%) and 56.5% (only two studies were enrolled, ranging from 40% to 72.9%), respectively, which seemed to be higher than that in the inolimomab group [36.2% (95% CI: 18.6%–53.8%)] (Figure 4).
Figure 4.
Overall survival rates of patients after treatment with IL-2RAs.
In retrospective studies, only one study for daclizumab could be observed and its OS rate was 72.9%. The OS rate of basiliximab and inolimomab was 53.3% (95% CI: 18.3%–88.4%) and 38.7% (95% CI: 16.4%–61.0%), respectively. One study each for basiliximab, daclizumab, and inolimomab could be included in the analysis of prospective unrandomized studies, and the OS rate was 54.7%, 40.0% and 10.0%, respectively. The OS rate of the unique RCT about inolimomab was 46.9% (33).
Discussion
In this meta-analysis, basiliximab seemed to have the highest response rate, particularly in the gut and liver GVHD, and inolimomab treatment showed a higher infection rate. However, the survival seemed to be comparable among basiliximab, daclizumab, and inolimomab. This was the first meta-analysis comparing the efficacy and safety of different IL-2RAs, which provided valuable information for the treatment of SR-aGVHD.
Activation of T lymphocytes mediates one of the major pathophysiological mechanisms of aGVHD, which exclusively expresses the IL-2R alpha chain (46). IL-2RAs prohibit T-cell proliferation (47); however, in in vitro experiments, the ability of inhibiting T lymphocytes varied among different IL-2RAs. Kircher et al. (48) separated peripheral blood mononuclear cells from heparinized peripheral blood of healthy volunteers and then incubated them with 100 μg/Ml anti-CD3 monoclonal antibody. They set the level of proliferation in the absence of the compounds as 100%. At the concentrations of 0.001, 0.01, and 0.1 μg/Ml, basiliximab seemed to be stronger in terms of suppressing T-cell proliferation compared with daclizumab. Particularly, at the concentration of 0.1 μg/Ml, basiliximab could reduce T-cell proliferation from 100% to 41%, while daclizumab could reduce it only from 100% to 69%. However, at higher concentrations (e.g., 1 and 10 μg/Ml), both of them inhibited T-cell proliferation to a similar degree. Thus, T cells were more sensitive to inhibition by basiliximab in this study.
Similarly, Baan et al. (49) identified the inhibitory effect of different IL-2RAs (basiliximab, daclizumab, and inolimomab) on T-cell proliferation induced by IL-2, IL-7, and IL-15. At lower concentrations (0.1, 0.5, and 1.0 μg/Ml), basiliximab occupied the dominant position for suppressing T-cell proliferation induced by IL-2, followed by daclizumab and inolimomab ranking the last. For suppressing T-cell proliferation induced by IL-7, daclizumab seemed to be stronger than basiliximab at concentrations of 0.1 and 0.5 μg/Ml, while basiliximab seemed to be better at other concentrations (1.0, 5.0, and 10.0 μg/Ml). Irrespective of the concentration, T cells appeared to be minimally inhibited by inolimomab. In IL-15-driven T-cell proliferation, daclizumab performed better than basiliximab at concentrations higher than 0.5 μg/Ml, while the function of inolimomab was still the weakest. These results might also partly explain the fact that inolimomab was less effective than basiliximab and daclizumab in SR-aGVHD treatment. Among these three cytokines (i.e., IL-2, IL-7 and IL-15), IL-2 showed the preferential protective effects on T cells against glucocorticoid-induced apoptosis (50). Therefore, basiliximab exhibited the best efficacy in treating SR-aGVHD.
From the perspective of structure, inolimomab was a murine anti-human monoclonal antibody (7), basiliximab was a murine chimeric monoclonal antibody (8), and daclizumab was a humanized monoclonal antibody (9). The human immune system can produce its own antibodies to clear rodent antibodies rapidly because they are foreign proteins, leading to reduced efficacy (51, 52). An increased risk of an infusion reaction may exist as well (53). Moreover, a longer half-life may help to inhibit T cells more effectively. The half-lives of basiliximab and daclizumab are 7 days and 21–25 days (8, 9), respectively, while the half-life of inolimomab is only 44.5 h (7). It might partially explain the higher ORRs of basiliximab (81%) and daclizumab (71%), while that of inolimomab was only 50%.
It is reported that IL-2RA may suppress regulatory T cells (54) which possibly leads to chronic GVHD after treatment (55, 56). However, aGVHD was a significant risk factor of cGVHD, and more than 65% of patients with grades II to IV aGVHD would develop cGVHD (57–59). This was similar to the rate of cGVHD in the present study. Thus, it was suggested that the suppression of regulatory T cells caused by IL-2RA might have an influence on cGVHD, however, the incidence of cGVHD could also be driven by the prior SR-aGVHD, which needs to be further explored by prospective RCTs.
In this study, basiliximab, daclizumab, inolimomab, and denileukin diftitox were found to have similar efficacy in aGVHD of the skin, while basiliximab seemed to be better in aGVHD of the gut and liver. Clinically, aGVHD of the gut and liver significantly increased the risk of transplant-related mortality (60). Therefore, basiliximab could improve the prognosis to a much greater extent, especially in severe SR-aGVHD.
This study had several limitations. First, the generalizability of this meta-analysis was limited by various circumstances due to the heterogeneity originating from different study designs and the process of conducting and analyzing, which might influence the accuracy of our results. Second, the time frame for the evaluation of the response rate at 1 month after IL-2RA treatment was prolonged because different studies had different time points. That is, the earliest studies evaluating at 3 weeks while the latest studies evaluating at 6 weeks after treatment with IL-2RAs were enrolled in this analysis, which might have influenced the study outcomes. Third, one more drawback was the lack of prospective RCTs to compare distinct categories of IL-2RA directly. Instead, most studies that could be used for analysis were retrospective studies with relatively limited sample sizes. Finally, several biases might have been introduced into this meta-analysis, including unbalanced medical resources from different time points or areas. Thus, the superiority of basiliximab over other IL-2RAs in patients with SR-aGVHD needs to be validated by further studies.
Conclusion
In conclusion, the efficacy and safety of different IL-2RAs varied. The response rate of basiliximab seemed to be the highest, followed by daclizumab. More prospective RCTs are needed to compare the efficacy and safety of different IL-2RAs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Two reviewers J-XL and M-ZS conducted the study selection and data extraction independently. A third reviewer X-DM arbitrated when the former two reviewers had diverse opinions X-DM and X-JH designed the study. J-XL, M-ZS, L-PX, X-HZ, YW, and K-YL collected the data. J-XL, M-ZS, and S-DH analyzed the data and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2017YFA0104500), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-5-034), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the Key Program of the National Natural Science Foundation of China (grant number 81930004), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.749266/full#supplementary-material
References
- 1.Storb R. Allogeneic Hematopoietic Stem Cell Transplantation–Yesterday, Today, and Tomorrow. Exp Hematol (2003) 31(1):1–10. doi: 10.1016/s0301-472x(02)01020-2 [DOI] [PubMed] [Google Scholar]
- 2.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of Moderate/Severe Acute Graft-Versus-Host Disease After Allogeneic Bone Marrow Transplantation: An Analysis of Clinical Risk Features and Outcome. Blood (1990) 75(4):1024–30. doi: 10.1182/blood.V75.4.1024.1024 [DOI] [PubMed] [Google Scholar]
- 3.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and Second-Line Systemic Treatment of Acute Graft-Versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeg HJ. How I Treat Refractory Acute GVHD. Blood (2007) 109(10):4119–26. doi: 10.1182/blood-2006-12-041889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara JL, Deeg HJ. Graft-Versus-Host Disease. N Engl J Med (1991) 324(10):667–74. doi: 10.1056/NEJM199103073241005 [DOI] [PubMed] [Google Scholar]
- 6.Anasetti C, Hansen JA, Waldmann TA, Appelbaum FR, Davis J, Deeg HJ, et al. Treatment of Acute Graft-Versus-Host Disease With Humanized Anti-Tac: An Antibody That Binds to the Interleukin-2 Receptor. Blood (1994) 84(4):1320–7. doi: 10.1182/blood.V84.4.1320.1320 [DOI] [PubMed] [Google Scholar]
- 7.Dartois C, Freyer G, Michallet M, Henin E, You B, Darlavoix I, et al. Exposure-Effect Population Model of Inolimomab, a Monoclonal Antibody Administered in First-Line Treatment for Acute Graft-Versus-Host Disease. Clin Pharmacokinet (2007) 46(5):417–32. doi: 10.2165/00003088-200746050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakupurakal G, Garcia-Marquez MA, Shimabukuro-Vornhagen A, Theurich S, Holtick U, Hallek M, et al. Immunological Effects in Patients With Steroid-Refractory Graft-Versus-Host Disease Following Treatment With Basiliximab, a CD25 Monoclonal Antibody. Eur J Haematol (2016) 97(2):121–7. doi: 10.1111/ejh.12691 [DOI] [PubMed] [Google Scholar]
- 9.Othman AA, Tran JQ, Tang MT, Dutta S. Population Pharmacokinetics of Daclizumab High-Yield Process in Healthy Volunteers: Integrated Analysis of Intravenous and Subcutaneous, Single- and Multiple-Dose Administration. Clin Pharmacokinet (2014) 53(10):907–18. doi: 10.1007/s40262-014-0159-9 [DOI] [PubMed] [Google Scholar]
- 10.Shaughnessy PJ, Bachier C, Grimley M, Freytes CO, Callander NS, Essell JH, et al. Denileukin Diftitox for the Treatment of Steroid-Resistant Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2005) 11(3):188–93. doi: 10.1016/j.bbmt.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 11.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a Fusion Protein of IL-2 and Diphtheria Toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to Eliminate Regulatory T Lymphocytes in Patients With Melanoma. J Immunother (2005) 28(6):582–92. doi: 10.1097/01.cji.0000175468.19742.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hervé P, Wijdenes J, Bergerat JP, Bordigoni P, Milpied N, Cahn JY, et al. Treatment of Corticosteroid Resistant Acute Graft-Versus-Host Disease by In Vivo Administration of Anti-Interleukin-2 Receptor Monoclonal Antibody (B-B10). Blood (1990) 75(4):1017–23. doi: 10.1182/blood.V75.4.1017.1017 [DOI] [PubMed] [Google Scholar]
- 13.Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic Factors and Long-Term Follow-Up of Basiliximab for Steroid-Refractory Acute Graft-Versus-Host Disease: Updated Experience From a Large-Scale Study. Am J Hematol (2020) 95(8):927–36. doi: 10.1002/ajh.25839 [DOI] [PubMed] [Google Scholar]
- 14.Tang FF, Cheng YF, Xu LP, Zhang XH, Yan CH, Han W, et al. Basiliximab as Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease in Pediatric Patients After Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(2):351–7. doi: 10.1016/j.bbmt.2019.10.031 [DOI] [PubMed] [Google Scholar]
- 15.Tan Y, Xiao H, Wu D, Luo Y, Lan J, Liu Q, et al. Combining Therapeutic Antibodies Using Basiliximab and Etanercept for Severe Steroid-Refractory Acute Graft-Versus-Host Disease: A Multi-Center Prospective Study. Oncoimmunology (2017) 6(3):e1277307. doi: 10.1080/2162402X.2016.1277307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadeau M, Perreault S, Seropian S, Foss F, Isufi I, Cooper DL. The Use of Basiliximab-Infliximab Combination for the Treatment of Severe Gastrointestinal Acute GvHD. Bone Marrow Transplant (2016) 51(2):273–6. doi: 10.1038/bmt.2015.247 [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal SR, Zaman S, Chakrabarti A, Sehrawat A, Bansal S, Gupta M, et al. T Cell Costimulation Blockade for Hyperacute Steroid Refractory Graft Versus-Host Disease in Children Undergoing Haploidentical Transplantation. Transpl Immunol (2016) 39:46–51. doi: 10.1016/j.trim.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Wang JZ, Liu KY, Xu LP, Liu DH, Han W, Chen H, et al. Basiliximab for the Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease After Unmanipulated HLA-Mismatched/Haploidentical Hematopoietic Stem Cell Transplantation. Transplant Proc (2011) 43(5):1928–33. doi: 10.1016/j.transproceed.2011.03.044 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Hieber M, Fietz T, Knauf W, Uharek L, Hopfenmuller W, Thiel E, et al. Efficacy of the Interleukin-2 Receptor Antagonist Basiliximab in Steroid-Refractory Acute Graft-Versus-Host Disease. Br J Haematol (2005) 130(4):568–74. doi: 10.1111/j.1365-2141.2005.05631.x [DOI] [PubMed] [Google Scholar]
- 20.Massenkeil G, Rackwitz S, Genvresse I, Rosen O, Dorken B, Arnold R. Basiliximab is Well Tolerated and Effective in the Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2002) 30(12):899–903. doi: 10.1038/sj.bmt.1703737 [DOI] [PubMed] [Google Scholar]
- 21.Tao T, Ma X, Yang J, Zou JY, Ji SM, Tan YS, et al. Humanized Anti-CD25 Monoclonal Antibody Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease: A Chinese Single-Center Experience in a Group of 64 Patients. Blood Cancer J (2015) 5:e308. doi: 10.1038/bcj.2015.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, et al. Inflammatory Cytokine Inhibition With Combination Daclizumab and Infliximab for Steroid-Refractory Acute GVHD. Bone Marrow Transplant (2011) 46(3):430–5. doi: 10.1038/bmt.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao K, Rao A, Karlsson H, Jagani M, Veys P, Amrolia PJ. Improved Survival and Preserved Antiviral Responses After Combination Therapy With Daclizumab and Infliximab in Steroid-Refractory Graft-Versus-Host Disease. J Pediatr Hematol Oncol (2009) 31(6):456–61. doi: 10.1097/MPH.0b013e31819daf60 [DOI] [PubMed] [Google Scholar]
- 24.Miano M, Cuzzubbo D, Terranova P, Giardino S, Lanino E, Morreale G, et al. Daclizumab as Useful Treatment in Refractory Acute GVHD: A Paediatric Experience. Bone Marrow Transplant (2009) 43(5):423–7. doi: 10.1038/bmt.2008.331 [DOI] [PubMed] [Google Scholar]
- 25.Hui CH, Sia H, Mangos H, Horvath N, Lee H, Lewis I, et al. Daclizumab has Poor Efficacy in Steroid-Refractory Severe Acute Graft-Versus-Host Disease: A Single Centre Experience With 12 Allograft Patients. Bone Marrow Transplant (2008) 41(4):409–10. doi: 10.1038/sj.bmt.1705927 [DOI] [PubMed] [Google Scholar]
- 26.Perales MA, Ishill N, Lomazow WA, Weinstock DM, Papadopoulos EB, Dastigir H, et al. Long-Term Follow-Up of Patients Treated With Daclizumab for Steroid-Refractory Acute Graft-vs-Host Disease. Bone Marrow Transplant (2007) 40(5):481–6. doi: 10.1038/sj.bmt.1705762 [DOI] [PubMed] [Google Scholar]
- 27.Teachey DT, Bickert B, Bunin N. Daclizumab for Children With Corticosteroid Refractory Graft-Versus-Host Disease. Bone Marrow Transplant (2006) 37(1):95–9. doi: 10.1038/sj.bmt.1705199 [DOI] [PubMed] [Google Scholar]
- 28.Bordigoni P, Dimicoli S, Clement L, Baumann C, Salmon A, Witz F, et al. Daclizumab, an Efficient Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease. Br J Haematol (2006) 135(3):382–5. doi: 10.1111/j.1365-2141.2006.06321.x [DOI] [PubMed] [Google Scholar]
- 29.Wolff D, Roessler V, Steiner B, Wilhelm S, Weirich V, Brenmoehl J, et al. Treatment of Steroid-Resistant Acute Graft-Versus-Host Disease With Daclizumab and Etanercept. Bone Marrow Transplant (2005) 35(10):1003–10. doi: 10.1038/sj.bmt.1704929 [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan R, Chakrabarti S, Walsh T, Igarashi T, Takahashi Y, Kleiner D, et al. Improved Survival in Steroid-Refractory Acute Graft Versus Host Disease After Non-Myeloablative Allogeneic Transplantation Using a Daclizumab-Based Strategy With Comprehensive Infection Prophylaxis. Br J Haematol (2004) 124(6):777–86. doi: 10.1111/j.1365-2141.2004.04856.x [DOI] [PubMed] [Google Scholar]
- 31.Willenbacher W, Basara N, Blau IW, Fauser AA, Kiehl MG. Treatment of Steroid Refractory Acute and Chronic Graft-Versus-Host Disease With Daclizumab. Br J Haematol (2001) 112(3):820–3. doi: 10.1046/j.1365-2141.2001.02582.x [DOI] [PubMed] [Google Scholar]
- 32.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I, et al. Daclizumab, a Humanized Anti-Interleukin-2 Receptor Alpha Chain Antibody, for Treatment of Acute Graft-Versus-Host Disease. Blood (2000) 95(1):83–9. doi: 10.1182/blood.V95.1.83 [DOI] [PubMed] [Google Scholar]
- 33.Socie G, Vigouroux S, Yakoub-Agha I, Bay JO, Furst S, Bilger K, et al. A Phase 3 Randomized Trial Comparing Inolimomab vs Usual Care in Steroid-Resistant Acute GVHD. Blood (2017) 129(5):643–9. doi: 10.1182/blood-2016-09-738625 [DOI] [PubMed] [Google Scholar]
- 34.Girerd S, Renaud M, Guilhot J, Giraud C, Larchee R, Jollet I, et al. Long-Term Follow-Up of Corticosteroid Refractory Acute GVHD Treated With an Inolimomab-Based Algorithm: A Single Center Experience. Bone Marrow Transplant (2013) 48(9):1243–8. doi: 10.1038/bmt.2013.16 [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Cadenas I, Rivera I, Martino R, Esquirol A, Barba P, Novelli S, et al. Patterns of Infection and Infection-Related Mortality in Patients With Steroid-Refractory Acute Graft Versus Host Disease. Bone Marrow Transplant (2017) 52(1):107–13. doi: 10.1038/bmt.2016.225 [DOI] [PubMed] [Google Scholar]
- 36.van Groningen LF, Liefferink AM, de Haan AF, Schaap NP, Donnelly JP, Blijlevens NM, et al. Combination Therapy With Inolimomab and Etanercept for Severe Steroid-Refractory Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2016) 22(1):179–82. doi: 10.1016/j.bbmt.2015.08.039 [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Cadenas I, Valcarcel D, Martino R, Pinana JL, Novelli S, Esquirol A, et al. Updated Experience With Inolimomab as Treatment for Corticosteroid-Refractory Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2013) 19(3):435–9. doi: 10.1016/j.bbmt.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 38.Xhaard A, Rocha V, Bueno B, de Latour RP, Lenglet J, Petropoulou A, et al. Steroid-Refractory Acute GVHD: Lack of Long-Term Improved Survival Using New Generation Anticytokine Treatment. Biol Blood Marrow Transplant (2012) 18(3):406–13. doi: 10.1016/j.bbmt.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Pinana JL, Valcarcel D, Martino R, Moreno ME, Sureda A, Briones J, et al. Encouraging Results With Inolimomab (Anti-IL-2 Receptor) as Treatment for Refractory Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2006) 12(11):1135–41. doi: 10.1016/j.bbmt.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 40.Bay JO, Dhedin N, Goerner M, Vannier JP, Marie-Cardine A, Stamatoullas A, et al. Inolimomab in Steroid-Refractory Acute Graft-Versus-Host Disease Following Allogeneic Hematopoietic Stem Cell Transplantation: Retrospective Analysis and Comparison With Other Interleukin-2 Receptor Antibodies. Transplantation (2005) 80(6):782–8. doi: 10.1097/01.tp.0000173995.18826.de [DOI] [PubMed] [Google Scholar]
- 41.Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C, et al. Safety and Efficacy of Denileukin Diftitox in Patients With Steroid-Refractory Acute Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation. Blood (2004) 104(4):1224–6. doi: 10.1182/blood-2004-01-0028 [DOI] [PubMed] [Google Scholar]
- 42.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 43.Miller SA, Forrest JL. Enhancing Your Practice Through Evidence-Based Decision Making: PICO, Learning How to Ask Good Questions. J Evid Base Dent Pract (2001) 1:136–41. doi: 10.1016/S1532-3382(01)70024-3 [DOI] [Google Scholar]
- 44.Balduzzi S, Rucker G, Schwarzer G. How to Perform a Meta-Analysis With R: A Practical Tutorial. Evid Based Ment Health (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austrias: R Foundation for Statistical Computing; (2020). Available at: https://www.R-project.org/. [Google Scholar]
- 46.Ferrara JL, Levy R, Chao NJ. Pathophysiologic Mechanisms of Acute Graft-vs.-Host Disease. Biol Blood Marrow Transplant (1999) 5(6):347–56. doi: 10.1016/s1083-8791(99)70011-x [DOI] [PubMed] [Google Scholar]
- 47.Ferrara JL, Holler E, Blazar B. Monoclonal Antibody and Receptor Antagonist Therapy for GVHD. Cancer Treat Res (1999) 101:331–68. doi: 10.1007/978-1-4615-4987-1_15 [DOI] [PubMed] [Google Scholar]
- 48.Kircher B, Latzer K, Gastl G, Nachbaur D. Comparative In Vitro Study of the Immunomodulatory Activity of Humanized and Chimeric Anti-CD25 Monoclonal Antibodies. Clin Exp Immunol (2003) 134(3):426–30. doi: 10.1111/j.1365-2249.2003.02324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baan CC, Boelaars-van Haperen MJ, van Riemsdijk IC, van der Plas AJ, Weimar W. IL-7 and IL-15 Bypass the Immunosuppressive Action of Anti-CD25 Monoclonal Antibodies. Transplant Proc (2001) 33(3):2244–6. doi: 10.1016/s0041-1345(01)01954-6 [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Murakami T, Oppenheim JJ, Howard OMZ. Differential Response of Murine CD4+CD25+ and CD4+CD25- T Cells to Dexamethasone-Induced Cell Death. Eur J Immunol (2004) 34(3):859–69. doi: 10.1002/eji.200324506 [DOI] [PubMed] [Google Scholar]
- 51.Group OMTS . A Randomized Clinical Trial of OKT3 Monoclonal Antibody for Acute Rejection of Cadaveric Renal Transplants. N Engl J Med (1985) 313(6):337–42. doi: 10.1056/NEJM198508083130601 [DOI] [PubMed] [Google Scholar]
- 52.Kuus-Reichel K, Grauer LS, Karavodin LM, Knott C, Krusemeier M, Kay NE. Will Immunogenicity Limit the Use, Efficacy, and Future Development of Therapeutic Monoclonal Antibodies? Clin Diagn Lab Immunol (1994) 1(4):365–72. doi: 10.1128/cdli.1.4.365-372.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baert F, Noman M, Vermeire S, Van Assche G, DH G, Carbonez A, et al. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s Disease. N Engl J Med (2003) 348(7):601–8. doi: 10.1056/NEJMoa020888 [DOI] [PubMed] [Google Scholar]
- 54.Game DS, Hernandez-Fuentes MP, Lechler RI. Everolimus and Basiliximab Permit Suppression by Human CD4+CD25+ Cells In Vitro . Am J Transplant (2005) 5(3):454–64. doi: 10.1111/j.1600-6143.2005.00758.x [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered Regulatory T Cell Homeostasis in Patients With CD4+ Lymphopenia Following Allogeneic Hematopoietic Stem Cell Transplantation. J Clin Invest (2010) 120(5):1479–93. doi: 10.1172/JCI41072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckner JH. Mechanisms of Impaired Regulation by CD4(+)CD25(+)FOXP3(+) Regulatory T Cells in Human Autoimmune Diseases. Nat Rev Immunol (2010) 10(12):849–59. doi: 10.1038/nri2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochs LA, Blazar BR, Roy J, Rest EB, Weisdorf DJ. Cytokine Expression in Human Cutaneous Chronic Graft-Versus-Host Disease. Bone Marrow Transplant (1996) 17(6):1085–92. [PubMed] [Google Scholar]
- 58.Loughran TP, Jr, Sullivan K, Morton T, Beckham C, Schubert M, Witherspoon R, et al. Value of Day 100 Screening Studies for Predicting the Development of Chronic Graft-Versus-Host Disease After Allogeneic Bone Marrow Transplantation. Blood (1990) 76(1):228–34. doi: 10.1182/blood.V76.1.228.228 [DOI] [PubMed] [Google Scholar]
- 59.Afram G, Simon JAP, Remberger M, Caballero-Velazquez T, Martino R, Pinana JL, et al. Reduced Intensity Conditioning Increases Risk of Severe cGVHD: Identification of Risk Factors for cGVHD in a Multicenter Setting. Med Oncol (2018) 35(6):79. doi: 10.1007/s12032-018-1127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE, et al. Plasma Biomarkers of Lower Gastrointestinal and Liver Acute GVHD. Blood (2012) 119(12):2960–3. doi: 10.1182/blood-2011-10-387357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.