Abstract
Members of the genus Paradileptus are apex predators in microbial food webs. They are often encountered in freshwater biotopes and have been used in research on water quality monitoring and ecology. Nevertheless, our understanding of the biodiversity of Paradileptus, especially its ecological and genetic diversities, is very poor which hinders our ability to understand the ecosystem services it provides. The present study gives a detailed account of two Chinese populations of Paradileptus elephantinus and P. conicus including their living morphology, infraciliature, and molecular phylogenies based on 18S, 5.8S, and ITS ribosomal DNA sequences. The phylogenetic relationships between these two species and other rhynchostomatians are investigated. We also explore the potential contribution of differentiation of the proboscis (e.g., extrusomes, dorsal brush, and differentiated kineties) to niche partitioning and speciation in Paradileptus. The global distribution of Paradileptus is summarized based on published data. Finally, a key to the identification of the valid species of Paradileptus is provided.
Keywords: morphology, Paradileptus conicus, Paradileptus elephantinus, Rhynchostomatia, ribosomal DNA
Introduction
Ciliated protists (ciliates) are a diverse group of morphologically differentiated eukaryotic microorganisms that play critical roles in aquatic and terrestrial ecosystems by maintaining energy flow and nutrient cycles (Lynn, 2008; Song et al., 2009; Gao et al., 2016; Hu et al., 2019). Rhynchostomatians are a large group of raptorial ciliates with a conspicuous proboscis that bears well-developed extrusomes and a dorsal brush (Vd’ačný and Rajter, 2015; Vd’ačný et al., 2017). They occur in marine, limnetic, terrestrial, and anaerobic environments including both benthic and planktonic habitats (Lynn, 2008; Vd’ačný and Foissner, 2012). Nevertheless, our understanding of their biodiversity, especially their ecological and genetic diversities, is very poor which hinders our ability to understand the ecosystem services they provide.
According to the most recent classification of rhynchostomatians (Vd’ačný and Foissner, 2012), the subclass Rhynchostomatia Jankowski, 1980 contains three families and 12 genera. Paradileptus Wenrich, 1929 is a typically planktonic genus which is characterized by the obliquely truncated anterior end of the body with a broad peristomial field and a spiral proboscis that extends anteriorly (Wenrich, 1929; Foissner et al., 1999). Ten nominal species of Paradileptus have been reported but only four are valid, namely, P. flagellatus (Rousselet, 1890) Wenrich, 1929, P. elephantinus (Šveç, 1897) Kahl, 1931, P. conicus Wenrich, 1929, and P. moniliger (Ehrenberg, 1835) Vd’ačný & Foissner, 2012. Among these, only P. conicus has been studied using observations in vivo, protargol staining, and electron microscopy, and documented using photomicrographs (Foissner et al., 1995, 1999). However, there are still some characters that have not been investigated in detail, such as the shape and arrangement of extrusomes in vivo. Furthermore, the evolutionary relationships of Paradileptus remain unknown due to a lack of molecular data.
In the present study, we isolated two Paradileptus species (P. elephantinus and P. conicus) from freshwater habitats in Lake Weishan, northern China. The two species were investigated using observations in vivo and after protargol staining. Their molecular phylogenies inferred from 18S and ITS-5.8S rDNA sequences have been reconstructed. The global distribution pattern of Paradileptus is summarized based on previous and present studies. Finally, the classification of Paradileptus is updated and a key to the identification of the four valid species is supplied.
Materials and Methods
Sample Collection, Observation, and Identification
Paradileptus elephantinus was collected from the Pontoon Dock of Lake Weishan Wetland Park (Figure 1B; N34°46′12″, E117°09′36″), Jining, China, on 24th April 2020. The physicochemical parameters of the sampling site were as follows: water temperature 17.8°C, atmospheric pressure 763.7 mm Hg, dissolved oxygen concentration 11.65 mg/L, salinity 0.63 ppt, and pH 9.39. Paradileptus conicus was isolated from an aquaculture pond of Weishan Special Aquaculture Base (Figure 1C; N34°46′18″, E117°09′54″), Jining, China, on 4th May 2020. The physicochemical parameters of the sampling site were as follows: water temperature 22.6°C, atmospheric pressure 754.8 mm Hg, dissolved oxygen concentration 7.11 mg/L, salinity 0.28 ppt, and pH 8.46. The two samples were collected from the water surface using a 20 μm mesh-sized plankton net and transferred into several Petri dishes for processing in the laboratory as soon as possible after collection (Bai et al., 2020).
FIGURE 1.
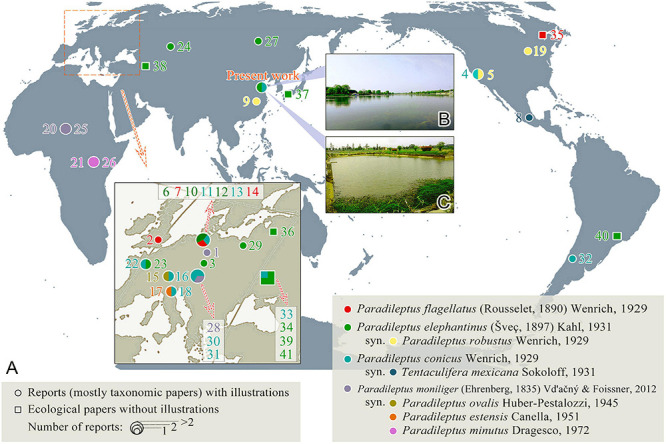
(A) The global geographic distribution of four valid Paradileptus species (see Table 1 for details). (B) The sampling location of P. elephantinus (Chinese population). (C) The sampling location of P. conicus (Chinese population).
Living cells were isolated with micropipettes and observed at 100–1000× magnifications using bright field and differential interference contrast microscopy (Olympus BX53) (Wu et al., 2020; Zhang et al., 2020). The infraciliature was revealed using the protargol staining method according to Wilbert (1975). Measurements and counts of stained specimens were conducted at magnifications of 100× and 1000×. Drawings of live cells were based on photomicrographs and free-hand sketches, while those of stained cells were accomplished with the help of a camera lucida at a magnification of 1000× (Lu et al., 2019). Terminology and systematics are mainly according to Vd’ačný et al. (2011a) and Vd’ačný and Foissner (2012).
Geographical Distribution Analyses
The global distribution patterns of the Paradileptus species were mainly derived from previous reports that include morphological descriptions and recognizable illustrations. In addition, we selected several ecological reports in geographic regions not covered by morphological studies to show the range of Paradileptus distribution (Figure 1A and Table 1). In order to display the distribution of Paradileptus more accurately, we supply a list of the nominal species names as originally reported and the names by which they are now known based on the findings of the present study (Table 1). The literature used for this analysis was mainly derived from Vd’ačný and Foissner (2012).
TABLE 1.
Global geographic distribution information of Paradileptus species.
| Number | Species name in original report | Current species name | Collection site | References |
| 1 | Amphileptus moniliger | P. moniliger | Berlin, Germany | Ehrenberg, 1835 |
| 2 | Amphileptus flagellatus Rousselet, 1890 | P. flagellatus | London, United Kingdom | Rousselet, 1890 |
| 3 | Dileptus elephantinus Šveç, 1897 | P. elephantinus | Bohemia, Czechia | Šveç, 1897 |
|
| ||||
| 4 | Paradileptus conicus Wenrich, 1929 | P. conicus | San Francisco, United States | Wenrich, 1929 |
| 5 | Paradileptus robustus Wenrich, 1929 | P. elephantinus | ||
|
| ||||
| 6 | Paradileptus (Dileptus) elephantinus (Šveç, 1897) Kahl, 1931 | P. elephantinus | Germany | Kahl, 1931 |
| 7 | Paradileptus (Amphileptus) flagellatus (Rousselet, 1890) Kahl, 1931 | P. flagellatus | ||
|
| ||||
| 8 | Tentaculifera mexicana Sokoloff, 1931 | P. conicus | Mexico City, Mexico | Sokoloff, 1931 |
| 9 | Paradileptus robustus Wenrich, 1929 | P. elephantinus | Nanjing, China | Wang and Nie, 1933 |
|
| ||||
| 10 | Paradileptus elephantinus Šveç, 1897 | P. elephantinus | Hamburg, Germany | Kahl, 1935 |
| 11 | Paradileptus conicus Wenrich, 1929 | P. conicus | ||
|
| ||||
| 12 | Paradileptus elephantinus Šveç | P. elephantinus | Germany | Kahl, 1943 |
| 13 | Paradileptus conicus Wenrich | P. conicus | ||
| 14 | Paradileptus flagellatus Rousselet | P. flagellatus | ||
|
| ||||
| 15 | Paradileptus ovalis Huber-Pestalozzi, 1945 | P. moniliger | Lake Zurich, Switzerland | Huber-Pestalozzi, 1945 |
| 16 | Paradileptus conicus | P. conicus | ||
|
| ||||
| 17 | Paradileptus estensis Canella, 1951 | P. moniliger | Ferrara, Italy | Canella, 1951 |
| 18 | Paradileptus conicus Wenrich | P. conicus | ||
|
| ||||
| 19 | Paradileptus robustus Wenrich | P. elephantinus | Michigan, United States | Lundin and West, 1963 |
| 20 | Paradileptus elephantinus Šveç | P. moniliger | Chari River, Chad | Dragesco, 1972a |
| 21 | Paradileptus minutus Dragesco, 1972 | P. moniliger | Kasinga Channel, Uganda | Dragesco, 1972b |
|
| ||||
| 22 | Paradileptus conicus Wenrich, 1929 | P. conicus | France | Fryd-Versavel et al., 1975 |
| 23 | Paradileptus elephantinus Šveç, 1897 | P. elephantinus | ||
|
| ||||
| 24 | Paradileptus elephantinus Šveç, 1897 | P. elephantinus | Volga River, Russia | Mamaeva, 1979 |
|
| ||||
| 25 | Paradileptus elephantinus Šveç | P. moniliger | Chari River, Chad | Dragesco and Dragesco-Kernéis, 1986 |
| 26 | Paradileptus minutus Dragesco, 1972 | P. moniliger | Kasinga Channel, Uganda | |
|
| ||||
| 27 | Paradileptus elephantinus Šveç, 1897 | P. elephantinus | Baikal Lake area, Russia | Lokot’, 1987 |
| 28 | Paradileptus elephantinus Šveç, 1897 | P. moniliger | Styria, Austria | Krainer, 1988 |
| 29 | Paradileptus elephantinus Šveç, 1897 | P. elephantinus | Wigry National Park, Poland | Czapik and Fyda, 1995 |
| 30 | Paradileptus elephantinus (Šveç, 1897) Kahl, 1931 | P. conicus | Salzburg, Austria | Foissner et al., 1995 |
| 31 | Paradileptus elephantinus (Šveç, 1897) Kahl, 1931 | P. conicus | Salzburg, Austria | Foissner et al., 1999 |
| 32 | Paradileptus elephantinus (Šveç) | P. conicus | Patagonia, Argentina | Modenutti and Pérez, 2001 |
|
| ||||
| 33 | Paradileptus conicus Wenrich | P. conicus* | Ukraine | Kravchenko, 1969 |
| 34 | Paradileptus elephantinus | P. elephantinus* | ||
|
| ||||
| 35 | Paradileptus flagellatus (Rousselet) | P. flagellatus* | Quebec, Canada | Puytorac et al., 1972 |
| 36 | Paradileptus elephantinus (Šveç) | P. elephantinus* | Latvia | Liepa, 1983 |
| 37 | Paradileptus elephantinus | P. elephantinus* | Hiroshima and Higashi-Hiroshima, Japan | Matsuoka et al., 1983 |
| 38 | Paradileptus elephantinus Šveç | P. elephantinus* | Azerbaijan | Alekperov, 1984 |
| 39 | Paradileptus elephantinus (Šveç) | P. elephantinus* | Ukraine | Oleksiv, 1985 |
| 40 | Paradileptus elephantinus | P. elephantinus* | Sao Paulo, Brazil | Barbieri and Godinho-Orlandi, 1989 |
| 41 | Paradileptus elephantinus Šveç | P. elephantinus* | Ukraine | Nebrat, 1992 |
*Since there is no illustration, the name in the original report is used.
DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
For each species, a single cell was isolated from the original sample and washed five times with 0.22 μm filtered in situ water to remove potential contaminants. Genomic DNA was extracted from the cleaned cells using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions but modified by using 1/4 of the suggested volume for each solution. Q5® Hot Start High-Fidelity 2× Master Mix DNA polymerase (New England BioLabs) was used to amplify the 18S and ITS-5.8S rDNA using universal eukaryotic primers 82F (5′-GAAACTGCGAATGGCTC-3′) and ITS-R (5′-TACTGATATGCTTAAGTTCAGCGG-3′) (Sogin, 1989; Chi et al., 2021). PCR amplifications were performed according to the following procedure: initial denaturation at 98°C for 30 s, followed by 18 cycles of amplification (98°C, 10 s; 69–51°C touchdown, 30 s; 72°C, 1 min), and another 18 cycles (98°C, 10 s; 51°C, 30 s; 72°C, 1 min), with a final extension of 72°C for 5 min (Lian et al., 2020). PCR products were sequenced bidirectionally in Tsingke Biological Technology Company (Qingdao, China) and assembled by SeqMan (DNAStar).
Phylogenetic Analyses
All available 18S, 5.8S, and ITS rDNA sequences of free-living litostomateans from known morphospecies were downloaded from the GenBank database and were compiled into four datasets each of which was used for separate phylogenetic analyses. The first dataset included 83 18S rDNA sequences of P. elephantinus and P. conicus, their related rhynchostomatians, other litostomateans, and armophoreans (outgroup taxa) and was used to construct the 18S tree of the Litostomatea. The second dataset contained 40 18S rDNA sequences of rhynchostomatians and spathidiids and was used to generate the phylogenetic tree focusing on the subclass Rhynchostomatia. The third dataset comprising 26 5.8S and ITS rDNA sequences of the two Paradileptus species, all available rhynchostomatians, and five spathidiids (outgroup taxa) was used to construct the ITS-5.8S tree focusing on the subclass Rhynchostomatia. The 18S, 5.8S, and ITS rDNA sequences of the rhynchostomatians and spathidiids were concatenated by SeaView v4 (Gouy et al., 2009) to form the fourth dataset that was used to generate the concatenated tree. See Supplementary Table 1 for sequence sources of these datasets.
Initial alignments were performed using the MAFFT algorithm with default parameters on the web server GUIDANCE21 (Zhang et al., 2019). Columns with scores less than 95% and residues with scores less than 90% were filtered, thus rendering the alignment suitably refined for the phylogenetic analysis. Maximum likelihood (ML) analysis was performed with RAxML-HPC2 on XSEDE v.8.2.12 (Stamatakis, 2014) on the CIPRES Science Gateway (Miller et al., 2010). Bayesian inference (BI) analysis was performed with MrBayes on XSEDE v.3.2.7a (Ronquist et al., 2012; Wang et al., 2019) on the CIPRES Science Gateway using the GTR + I + G evolutionary model as the best-fit model selected by MrModeltest v.2.2 (Nylander, 2004) according to the Akaike Information Criterion (AIC). Markov chain Monte Carlo (MCMC) simulations were then run with two sets of four chains using the default settings for 10,000,000 generations, with a sample frequency of 100 generations. The first 10% of trees were discarded as burn-in. All remaining trees were used to calculate the posterior probabilities. TreeView v.1.6.6 (Page, 1996) and MEGA v.5.2 (Tamura et al., 2011) were used to visualize tree topologies. BioEdit (Hall, 1999) was used to analyze the genetic difference between the two Paradileptus sequences.
Results
Class Litostomatea Small & Lynn, 1981
Subclass Rhynchostomatia Jankowski, 1980
Order Dileptida Jankowski, 1978
Family Dileptidae Jankowski, 1980
Genus Paradileptus Wenrich, 1929
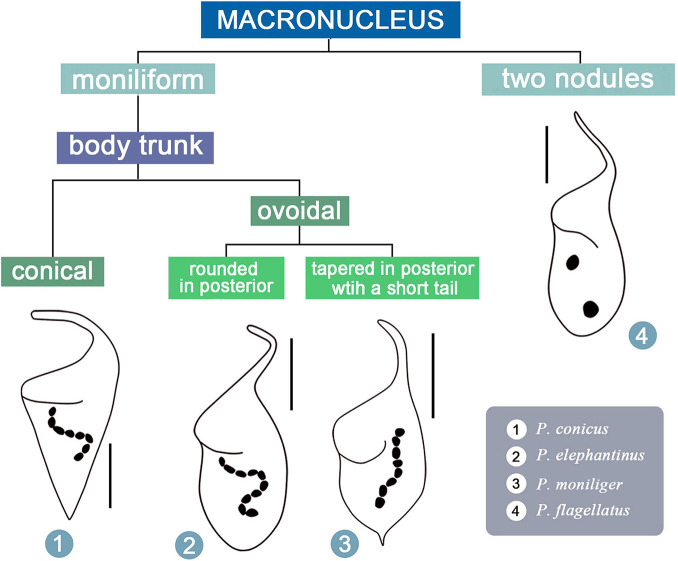
Wenrich (1929) established the genus Paradileptus with descriptions of two new species (P. conicus and P. robustus) and transferred Amphileptus flagellatus Rousselet, 1890 into it as the type species. However, Amphileptus moniliger Ehrenberg, 1835 was the first reported species, although it was only recently transferred into Paradileptus (Vd’ačný and Foissner, 2012). To date, there have been more than 30 taxonomic reports on Paradileptus with 10 nominal species established, namely, P. flagellatus (Rousselet, 1890) Wenrich, 1929, P. elephantinus (Šveç, 1897) Kahl, 1931, P. robustus Wenrich, 1929, P. conicus Wenrich, 1929, P. ovalis Huber-Pestalozzi, 1945, P. estensis Canella, 1951, P. minutus Dragesco, 1972, P. moniliger (Ehrenberg, 1835) Vd’ačný & Foissner, 2012, P. caducus Kahl, 1935, and P. canellai Dragesco, 1966. According to Vd’ačný and Foissner (2012), only one species is valid, i.e., P. elephantinus, whereas the findings of the present study support the validity of two species, i.e., P. elephantinus and P. conicus. Furthermore, our analysis of the literature suggests that P. flagellatus and P. moniliger are also probably valid. Consequently, we recognize four species, i.e., P. flagellatus, P. elephantinus, P. conicus, and P. moniliger. Traditionally, the establishment of species in this genus is based on living characters, the infraciliature having been rarely reported (Fryd-Versavel et al., 1975; Foissner et al., 1999). These species therefore need to be redefined based on a combination of in vivo morphological and infraciliature data. Furthermore, evolutionary relationships within Paradileptus remain unresolved due to the lack of molecular information.
Improved Diagnosis of the Genus Paradileptus
Flexible but non-contractile planktonic dileptids; body trunk usually ovoidal or conical; oral field broad, dish-like, and prolonged anteriorly into a spiral proboscis; contractile vacuoles small and numerous, distributed throughout body; two types of extrusomes attached to proboscis oral bulge; somatic kineties difficult to recognize, somatic kinetosomes of body trunk loosely arranged; dorsal brush diffuse and staggered; right side of circumoral kinety accompanied by a perioral kinety, left side by numerous oblique preoral kineties; freshwater habitat.
Type Species
Amphileptus flagellatus Rousselet, 1890.
Species Distributions
Paradileptus is seemingly cosmopolitan having been recorded from 21 countries representing five continents (Africa, Asia, Europe, North America, and South America). It has been reported most frequently in Europe but has not been found in Antarctica or Oceania. It mainly occurs in freshwater habitats such as lakes, reservoirs, ponds, and rivers.
The abundances of the two species reported here differed significantly, i.e., there were about 20 cells of Paradileptus conicus per 10 ml but only about two cells of P. elephantinus per 10 ml. Nevertheless, both species show a wide distribution and have been reported from four continents (Asia, Europe, North America, and South America). Paradileptus moniliger, the earliest reported species in the genus, has only been recorded in Africa and Europe, and P. flagellatus only in Europe and North America (Figure 1A and Table 1).
Paradileptus elephantinus (Šveç, 1897) Kahl, 1931
Synonyms
This list is adapted from that originally compiled by Vd’ačný and Foissner (2012).
1897 Dileptus elephantinus n. sp.–Šveç, Bulletin International de I’Academie des Sciènces 4: 13, 14 [Figures 13, 14] (original description).
1929 Paradileptus robustus n. sp.–Wenrich, Transactions of the American Microscopical Society 48: 357–359 [Figure 5] (detailed description based on living cells, synonymy proposed by Kahl, 1931).
1931 Paradileptus (Dileptus) elephantinus (Šveç, 1897)–Kahl, Die Tierwelt Deutschlands 21: 210 [Figure 24 on page 206] (short revision with author combination).
1933 Paradileptus robustus Wenrich, 1929–Wang and Nie, Contributions from the Biological Laboratory of the Science Society of China 10: 31–33 [Figure 27] (redescription of morphology based on living cells).
1935 Paradileptus elephantinus Šveç, 1897–Kahl, Die Tierwelt Deutschlands 30: 823 [Figures 18, 19 on page 808] (brief review and description).
1943 Paradileptus elephantinus Šveç–Kahl, Infusorien: 32 [Figure 3 on page 30] (short review).
1963 Paradileptus elephantinus–Lundin and West, Northern Michigan College Press: 1–175 (a Michigan population with illustration).
1975 Paradileptus elephantinus Šveç, 1897–Fryd-Versavel et al., Protistologica 11: 520–521 [Figures 18A,B] (morphological redescription, including infraciliature information).
1979 Paradileptus elephantinus Šveç, 1897–Mamaeva, Nauka: 31 (brief review and ecology, with illustration).
1987 Paradileptus elephantinus Šveç, 1897–Lokot’, Nauka: 35 (ecological report with illustration).
1995 Paradileptus elephantinus Šveç, 1897–Czapik and Fyda, Przegląd Zoologiczny 39: 65–72 (ecological report with illustration).
Paradileptus elephantinus was originally reported by Šveç (1897) under the name Dileptus elephantinus. Subsequently, this organism was reported numerous times, especially in ecological works but, with the exception of the study by Fryd-Versavel et al. (1975), details of its living characters and infraciliature were not provided. Based on both previous and present studies, an improved diagnosis is supplied.
Improved Diagnosis
Body about 180–600 × 100–350 μm in vivo; trunk oval in outline, with anterior spiral proboscis; macronucleus moniliform, micronuclei closely associated with macronuclear nodules; numerous contractile vacuoles distributed throughout body; two size-types of rod-shaped extrusomes attached to proboscis oral bulge; cortical granules colorless, oblong and densely scattered throughout cortex; dorsal brush diffuse and staggered; about 83–112 preoral kineties; freshwater habitat.
Voucher Slides
Three voucher slides with protargol-stained specimens are deposited in the Laboratory of Protozoology, Ocean University of China (OUC) with registration numbers: CY2020042401-01, 02, 03.
Morphological Description of Chinese Population
Body about 265–420 × 85–200 μm in vivo when swimming spirally (natural form), about 315 × 120 μm on average, length to width ratio about 2.1–3.6:1; 320–534 × 107–220 μm in protargol-stained specimens; flexible and non-contractile, trunk usually oval in outline, obliquely truncated anteriorly with a disk-like oral field and a prolonged helical proboscis, rounded or slightly pointed posteriorly; proboscis conspicuous and highly variable in length, easily damaged due to its fragility (Figures 2A,B, 3A–D, and Table 2). Macronucleus moniliform with 8–14 nodules, about 11 nodules on average, each nodule about 23 × 18 μm in size, located in trunk; micronuclei not detected in vivo and inconspicuous in protargol-stained specimens, ovoidal or globular (about 3.5 μm in diameter, n = 8), closely associated with macronuclear nodules (Figures 2A,B, 3E,H,K). Contractile vacuoles small and numerous, about 6.6–10.0 μm in diameter, distributed throughout proboscis and trunk (Figures 2A, 3C,D). Two types of rod-shaped extrusomes regularly distributed in proboscis oral bulge: type I, 11.6–13.4 μm long in vivo, on average about 12.3 μm; type II, 3.1–3.7 μm long in vivo, on average about 3.4 μm; developing argentophilic extrusomes scattered in cytoplasm, 9.2–12.6 μm long in vivo, on average about 10.5 μm (Figures 2D,E, 3E,I,K,N). Pellicle flexible and thin with numerous oblong, colorless cortical granules, about 2.6 × 0.7 μm in size, scattered throughout cortex and thus not forming oblique rows, as usual of other dileptids (Figures 2C,F, 3F,G,J). Cytoplasm brownish at low magnifications, with numerous cytoplasmic granules and food vacuoles, without symbiotic green algae (Figures 2A, 3A–E). Locomotion by swimming while rotating about main body axis.
FIGURE 2.
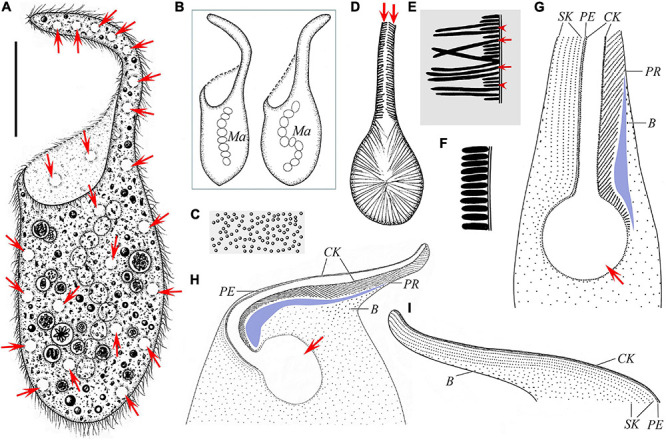
Schematic drawings of Paradileptus elephantinus from life (A–F) and after protargol staining (G–I). (A) Ventral view of a typical individual; arrows mark the contractile vacuoles. (B) Two individuals to show the different body shapes and distribution of macronuclear nodules. (C) Cortical granules underneath the pellicle. (D) Detail of oral apparatus, showing the oral opening, the basket supported by fibers, and the rod-shaped type II extrusomes (arrows) attached to the proboscis oral bulge. (E) Schematic drawing of a tangential optical section of the proboscis; arrows mark the elongated rod-shaped type I extrusomes; arrowheads indicate the short rod-shaped type II extrusomes. (F) Schematic drawing of a cross-section of the cortex showing the densely arranged oblong cortical granules. (G) Detail of oral region showing the circular oral opening (arrow), glabrous area (blue block), recognizable somatic kineties, perioral kinety, circumoral kinety, dorsal brush, and obliquely oriented preoral kineties. (H,I) Ciliary pattern in ventral view (H) and dorsal view (I) of anterior body portion of the same individual; arrow in panel (H) marks the oral opening. B, dorsal brush; CK, circumoral kinety; Ma, macronucleus; PE, perioral kinety; PR, preoral kineties; SK, somatic kineties. Scale bar = 70 μm (A).
FIGURE 3.
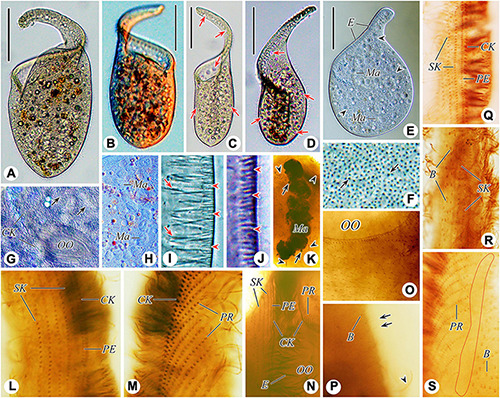
Photomicrographs of Paradileptus elephantinus from life (A–J) and after protargol staining (K–S). (A–D) Various individuals to show different body shapes; arrows indicate the contractile vacuoles. (E) To show the rod-shaped extrusomes regularly arranged in the proboscis and developing extrusomes scattered in the cytoplasm (arrowheads). (F) Cortical granules (arrows) underneath the cortex. (G) Detail of oral area showing the densely arranged cortical granules (arrows). (H) Detail of the moniliform macronucleus. (I) Tangential optical section of proboscis to show the elongated rod-shaped type I extrusomes (arrows) and short rod-shaped type II extrusomes (arrowheads). (J) Tangential optical section of cortex to show the oblong cortical granules (arrowheads). (K) Details of the macronucleus, micronuclei (arrows), and developing extrusomes (arrowheads). (L,M) Right (L) and left (M) views of the anterior regions of the proboscis of the same individual showing the perioral kinety, circumoral kinety, and recognizable somatic kineties. (N) Detail of proximal portion of oral apparatus. (O) Showing the loosely arranged somatic kinetosomes. (P) Left view of posterior portion of proboscis; arrows indicate the monokinetidal tails of dorsal brush with bristles; arrowhead marks a somatic cilium. (Q,R) Right view of the anterior (Q) and middle (R) regions of the proboscis. (S) Detail of the posterior portion of the proboscis; red circle indicates the glabrous area. B, dorsal brush; CK, circumoral kinety; E, extrusomes; Ma, macronucleus; OO, oral opening; PE, perioral kinety; PR, preoral kineties; SK, somatic kineties. Scale bars = 90 μm (A,B,D,E), 130 μm (C).
TABLE 2.
Morphometric data of Chinese populations of Paradileptus elephantinus (upper line) and P. conicus (lower line).
| Character | Min | Max | Mean | M | SD | CV | N |
| Body, length* (μm) | 265 | 420 | 317.0 | 305 | 53.9 | 17.0 | 5 |
| 165 | 230 | 192.9 | 185 | 21.5 | 11.2 | 7 | |
| Body, width* (μm) | 85 | 200 | 122.0 | 100 | 41.1 | 33.7 | 5 |
| 65 | 100 | 78.6 | 75 | 11.9 | 15.1 | 7 | |
| Oral apparatus, length* (μm) | 130 | 210 | 164.0 | 165 | 26.3 | 16.1 | 5 |
| 70 | 125 | 98.6 | 105 | 16.8 | 17.1 | 7 | |
| Body, length** (μm) | 320 | 534 | 426.3 | 431 | 62.0 | 14.5 | 19 |
| 118 | 273 | 207.3 | 202 | 39.1 | 18.8 | 21 | |
| Body, width** (μm) | 107 | 220 | 162.8 | 172 | 35.2 | 21.6 | 19 |
| 45 | 116 | 73.5 | 71 | 20.2 | 27.4 | 21 | |
| Oral opening, length** (μm) | 49 | 87 | 64.4 | 59 | 14.1 | 21.9 | 7 |
| 20 | 29 | 23.7 | 24 | 2.9 | 12.2 | 11 | |
| Oral opening, width** (μm) | 38 | 71 | 48.6 | 44 | 10.8 | 22.3 | 7 |
| 12 | 23 | 17.5 | 16 | 3.7 | 21.2 | 11 | |
| Preoral kineties, number | 83 | 112 | 95.3 | 96 | 9.7 | 10.1 | 7 |
| 60 | 85 | 66.7 | 65 | 6.8 | 10.2 | 11 | |
| Ma nodules, number | 8 | 14 | 10.7 | 11 | 2.0 | 18.8 | 9 |
| 6 | 17 | 10.2 | 10 | 2.4 | 23.1 | 21 | |
| Ma nodule, length** (μm) | 16 | 30 | 22.9 | 22 | 4.6 | 20.1 | 9 |
| 11 | 25 | 16.7 | 16 | 3.6 | 21.6 | 21 | |
| Ma nodule, width** (μm) | 13 | 26 | 18.2 | 19 | 4.1 | 22.5 | 9 |
| 7 | 14 | 10.7 | 10 | 2.0 | 19.0 | 21 | |
| Oral fibers, length*** (μm) | 9.7 | 15.0 | 12.3 | 12.2 | 1.4 | 11.2 | 19 |
| 7.4 | 13.6 | 10.2 | 9.8 | 1.8 | 17.5 | 19 | |
| Type I extrusomes in proboscis oral bulge, length*** (μm) | 11.6 | 13.4 | 12.3 | 12.3 | 0.5 | 4.4 | 8 |
| 4.0 | 5.7 | 5.0 | 5.0 | 0.5 | 9.0 | 19 | |
| Type II extrusomes in proboscis oral bulge, length*** (μm) | 3.1 | 3.7 | 3.4 | 3.4 | 0.2 | 5.2 | 13 |
| 1.7 | 2.3 | 2.0 | 2.0 | 0.2 | 8.8 | 9 | |
| Developing extrusomes in cytoplasm, length*** (μm) | 9.2 | 12.6 | 10.5 | 10.4 | 0.9 | 8.4 | 11 |
| 3.2 | 4.4 | 3.7 | 3.6 | 0.4 | 9.6 | 9 | |
| Cortical granules, length*** (μm) | 2.2 | 2.9 | 2.6 | 2.7 | 0.2 | 8.7 | 9 |
| 1.3 | 1.7 | 1.5 | 1.5 | 0.1 | 6.4 | 13 | |
| Cortical granules, width*** (μm) | 0.5 | 0.9 | 0.7 | 0.7 | 0.1 | 19.7 | 12 |
| 0.6 | 0.8 | 0.7 | 0.7 | 0.1 | 8.0 | 11 | |
| Contractile vacuoles, diameter*** (μm) | 6.6 | 10.0 | 8.3 | 8.1 | 1.1 | 12.9 | 19 |
| 6.9 | 9.5 | 8.4 | 8.8 | 0.7 | 8.8 | 19 | |
| Bristles, length** (μm) | 3.0 | 4.0 | 3.3 | 3.2 | 0.2 | 7.3 | 15 |
| 1.9 | 3.4 | 2.5 | 2.4 | 0.5 | 20.2 | 12 |
CV, coefficient of variation in %; M, Median; Ma, macronucleus; Max, maximum; Mean, arithmetic mean; Min, minimum; N, number of specimens investigated; SD, standard deviation.
*Data based on living cells while swimming.
**Data based on protargol-stained specimens.
***Data based on squashed living cells. Ma nodules for measurement were selected randomly in each individual.
Somatic cilia 10–14 μm long in vivo and widely spaced. Somatic kineties difficult to recognize due to somatic kinetosomes (monokinetids) loosely arranged in body trunk; only about 4–7 recognizable somatic kineties on right side of perioral kinety, starting at anterior of proboscis, extending posteriad parallel to perioral kinety and terminating at trunk; somatic kinetosomes progressively loosely arranged from anterior to posterior, becoming unrecognizable near oral opening (Figures 2G–I, 3L,N,O,Q). Circumoral kinety with closely spaced kinetosomes, distributed along contour of oral bulge in a basically U-shaped pattern, composed of dikinetids in proboscis and monokinetids around oral opening (Figures 2G–I, 3L–N,Q). Perioral kinety (first kinety on right side of circumoral kinety) with closely spaced monokinetids, commencing at anterior end of proboscis and extending to proximal part of oral opening; perioral kinety closer to circumoral kinety than to first right somatic kinety (Figures 2G–I, 3L,N,Q). Eighty-three to 112 oblique preoral kineties on left side of circumoral kinety, progressively shortened from middle of proboscis to both ends, that is, middle kineties composed of about 30–35 narrowly spaced monokinetids, fewer kinetosomes in each successive kinety (Figures 2G,H, 3M,N). Kinetosomes of dorsal brush diffuse and scattered throughout proboscis (Figures 2G–I, 3P,R,S). Dorsal brush also containing difficult-to-recognize monokinetidal tails with bristles about 3.0–4.0 μm long and extending to base of proboscis (Figure 3P). Type of dorsal brush bristles not discernable in protargol-stained specimens. Glabrous area on left of preoral kineties, extending to proximal part of oral opening (Figures 2G,H, 3S).
Oral apparatus large, consisting of a helical proboscis and a dish-like field at anterior end of trunk; oral region occupies about 45–55% of body length, distance from anterior end of proboscis to oral opening about 130–210 μm (Figures 2A,B, 3A–D). Oral opening elliptical to circular, about 49–87 × 38–71 μm in protargol-stained specimens, located laterally and inverted; oral region surrounded by circumoral kinety; perioral kinety on right side of circumoral kinety, preoral kineties on left side (Figures 2D,G,H, 3G). Pharyngeal basket conspicuous, composed of numerous fibers, about 9.7–15.0 μm long in vivo (Figures 2D, 3G).
Paradileptus conicus Wenrich, 1929
Synonyms
This list is adapted from that originally compiled by Vd’ačný and Foissner (2012).
1929 Paradileptus conicus n. sp.–Wenrich, Transactions of the American Microscopical Society 48: 353–357 [Figures 1–4, 6–9] (original description based on living cells).
1931 Tentaculifera mexicana n. sp.–Sokoloff, Anales del Instituto de Biologia, Universidad Nacional Autonoma de Mexico 2: 165–166 [Figures 1, 2] (synonymy proposed by Kahl, 1935).
1935 Paradileptus conicus Wenrich 1929–Kahl, Die Tierwelt Deutschlands 30: 823 [Figure 20 on page 808] (brief review and description).
1943 Paradileptus conicus Wenrich–Kahl, Infusorien: 32 [Figure 4 on page 30] (short review).
1945 Paradileptus conicus–Huber-Pestalozzi, Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich 90: 120–123 [Figures 1–8 on page 125] (morphological redescription based on living cells).
1951 Paradileptus conicus Wenrich–Canella, Annali dell’Universita Di Ferrara (Nuova Serie) Sezione III: Biologia Animale 1: 142–148 [Figures X, 45–49 on TAV. IX] (detailed morphological redescription based on living cells).
1975 Paradileptus conicus Wenrich, 1929–Fryd-Versavel et al., Protistologica 11: 520–521 [Figures 16, 18C,D] (morphological redescription, including infraciliature information).
1995 Paradileptus elephantinus (Šveç, 1897) Kahl, 1931–Foissner et al., Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft 1/95: 203–207 [Figures 1–21] (detailed redescription based on living cells and scanning electron micrographs).
1999 Paradileptus elephantinus (Šveç, 1897) Kahl, 1931–Foissner et al., Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft 3/99: 221–231 [Figures 1–52] (detailed review based on living morphological characters, protargol staining, and scanning electron micrographs of a Salzburg population).
2001 Paradileptus elephantinus (Šveç)–Modenutti and Pérez, Brazilian Journal of Biology 61: 391 [Figure 7 on page 393] (simple redescription).
2012 Paradileptus elephantinus (Šveç, 1897) Kahl, 1931–Vd’ačný and Foissner, Denisia 31: 437–451 [Figures 135a–w, 136a–z, 137a–z] (valuable summary based on previous reports).
Paradileptus conicus has been reported many times, but some important morphological characters were still unknown prior to this study. We here present an improved diagnosis based on previous and present descriptions.
Improved Diagnosis
Cell size in vivo about 100–230 × 65–115 μm; body trunk inverted-conical; oral area with a spiral proboscis; macronucleus moniliform, micronuclei closely associated with macronuclear nodules; numerous contractile vacuoles distributed throughout body; two types of extrusomes attached to proboscis oral bulge; numerous oblong, colorless cortical granules, irregularly scattered throughout cortex; dorsal brush diffuse and staggered; about 60–85 preoral kineties; freshwater habitat.
Voucher Slides
Three voucher slides with protargol-stained specimens are deposited in the Laboratory of Protozoology, Ocean University of China (OUC) with registration numbers: CY2020050401-01, 02, 03.
Morphological Description of Chinese Population
Body size about 165–230 × 65–100 μm in vivo, on average about 195 × 80 μm, with length to width ratio about 2.1–2.8:1; 118–273 × 45–116 μm in protargol-stained specimens; body trunk usually inverted-conical, that is, gradually tapering from anterior end to posterior end, with large oral area at anterior trunk that extends into a helical proboscis (Figures 4A,B, 5A–E, and Table 2). Nuclear apparatus usually located in trunk; macronucleus moniliform with 6–17 nodules, about 10 nodules on average, each nodule about 17 × 11 μm in size; micronuclei not detected in vivo and inconspicuous in protargol-stained specimens, ovoidal or globular (about 2.6 μm in diameter, n = 9), closely associated with macronuclear nodules (Figures 4A,B,H, 5L,N,O). Contractile vacuoles small and numerous, about 6.9–9.5 μm in diameter, scattered beneath cell surface of trunk and proboscis (Figures 4A, 5A,D,F). Two types of extrusomes regularly arranged in proboscis oral bulge: type I rod-shaped, 4.0–5.7 μm long in vivo, on average about 5.0 μm; type II oblong, 1.7–2.3 μm long in vivo, on average about 2.0 μm; developing argentophilic extrusomes scattered throughout cytoplasm, 3.2–4.4 μm long in vivo, on average about 3.7 μm (Figures 4D,F, 5J,M,O). Cortex flexible with numerous oblong, colorless cortical granules (about 1.5 × 0.7 μm in size), scattered throughout cortex and thus not forming oblique rows, as usual of other dileptids (Figures 4C,E, 5G,H,K). Cytoplasm brownish at low magnifications due to cytoplasmic inclusions and dense granulation, usually with several food vacuoles containing ingested algae, without symbiotic green algae (Figures 4A, 5A–E,L). Swims moderately fast while rotating about main body axis; when disturbed, swims rapidly backward.
FIGURE 4.
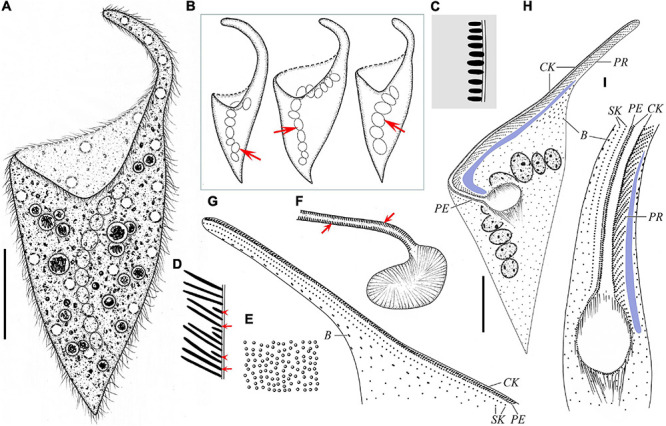
Schematic drawings of Paradileptus conicus from life (A–F) and after protargol staining (G–I). (A) Ventral view of a typical individual. (B) Three individuals to show different body shapes and the moniliform macronucleus (arrows). (C) Schematic drawing of a cross-section of the cortex showing the oblong cortical granules. (D) Schematic drawing of a tangential optical section of the proboscis; arrows mark the rod-shaped type I extrusomes; arrowheads indicate the oblong type II extrusomes. (E) Cortical granules underneath the pellicle. (F) Detail of oral apparatus, showing the oral opening, the basket supported by fibers, and the oblong type II extrusomes (arrows) attached to the proboscis oral bulge. (G,H) Ventral (H) and dorsal (G) views of the same individual showing the ciliary pattern. (I) Oral region showing the oral opening, glabrous area (blue block), recognizable somatic kineties, perioral kinety, circumoral kinety, dorsal brush, and obliquely oriented preoral kineties. B, dorsal brush; CK, circumoral kinety; PE, perioral kinety; PR, preoral kineties; SK, somatic kineties. Scale bars = 40 μm (A,H).
FIGURE 5.
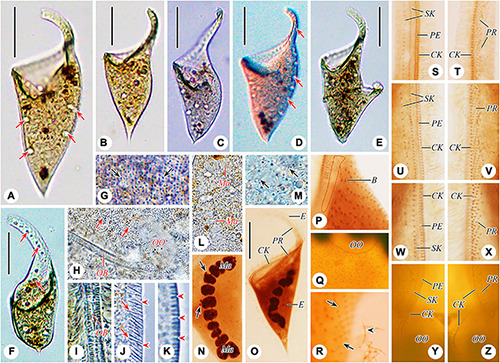
Photomicrographs of Paradileptus conicus from life (A–M) and after protargol staining (N–Z). (A–D) Various individuals to show different body shapes, arrows indicate the contractile vacuoles. (E) Cell undergoing binary fission. (F) A squashed cell to show the numerous contractile vacuoles (arrows mark three of these). (G) Cortical granules (arrows) underneath the pellicle. (H) Detail of oral region showing the densely arranged cortical granules (arrows). (I) Detail of the proboscis oral bulge. (J) Tangential optical section of the proboscis to show the rod-shaped type I extrusomes (arrows) and oblong type II extrusomes (arrowheads). (K) Tangential optical section of the cortex to show the oblong cortical granules (arrowheads). (L,N) Detail of the macronucleus and micronuclei (arrows). (M) Developing extrusomes scattered in the cytoplasm (arrows). (O) Dorsal view showing the circumoral kinety, developing extrusomes and preoral kineties. (P,R) Detail of the posterior portion of the proboscis; red circle indicates the glabrous area; arrows indicate the monokinetidal tails of dorsal brush with bristles; arrowhead marks a somatic cilium. (Q) Showing the loosely arranged somatic kinetosomes. (S–Z) Right (S,U,W,Y) and left (T,V,X,Z) views of the anterior (S,T from the same cell), middle (U,V from the same cell), and posterior (W,X from the same cell) regions of the proboscis, and proximal portion of oral apparatus (Y,Z from the same cell). B, dorsal brush; CK, circumoral kinety; E, extrusomes; Ma, macronucleus; OB, oral bulge; OO, oral opening; PE, perioral kinety; PR, preoral kineties; SK, somatic kineties. Scale bars = 60 μm (A,C–E), 50 μm (B,F,O).
Somatic cilia 6–8 μm long in vivo and widely spaced. Somatic kineties difficult to recognize due to somatic kinetosomes (monokinetids) loosely arranged in trunk; only 1–2 recognizable somatic kineties on right side of perioral kinety, commencing at anterior end of proboscis, extending posteriad parallel to perioral kinety and terminating at trunk; somatic kinetosomes progressively loosely arranged from anterior to posterior, becoming unrecognizable near oral opening (Figures 4G–I, 5Q,S,U,W). Circumoral kinety with narrowly spaced kinetosomes, distributed along contour of oral bulge in a basically U-shaped pattern, composed of dikinetids in proboscis and monokinetids around oral opening (Figures 4G–I, 5S–Z). Perioral kinety on right of circumoral kinety with closely spaced monokinetids, commencing at anterior end of proboscis and terminating near proximal part of oral opening; space between perioral kinety and circumoral kinety narrower than that between perioral kinety and first right somatic kinety (Figures 4G–I, 5S–Z). Sixty to 85 oblique preoral kineties on left of circumoral kinety, middle kineties composed of about 10–15 narrowly spaced monokinetids, other kineties progressively shortened from middle to both ends of proboscis (Figures 4H,I, 5T,V,X,Z). Kinetosomes of dorsal brush diffuse and scattered throughout proboscis (Figures 4G–I, 5P,R). Dorsal brush also containing difficult-to-recognize monokinetidal tails with bristles about 1.9–3.4 μm long and extending to base of proboscis (Figure 5R). Type of dorsal brush bristles not discernable in protargol-stained specimens. Glabrous area on left of preoral kineties extending to proximal part of oral opening (Figures 4H,I, 5P).
Oral apparatus conspicuous, composed of a helical proboscis and a dish-like field at anterior end of trunk; oral region occupies about 42–63% of body length, distance from anterior of proboscis to oral opening about 70–125 μm (Figures 4A,B, 5A–D). Oral opening elliptical to circular, located laterally and inverted, about 20–29 × 12–23 μm in protargol-stained specimens; oral region surrounded by circumoral kinety; right side of the circumoral kinety accompanied by perioral kinety, left side associated with numerous obliquely oriented preoral kineties (Figures 4G–I, 5S–Z). Pharyngeal basket obconical, composed of numerous fibers, about 7.4–13.6 μm long in vivo (Figures 4F,H,I, 5H).
Phylogenetic Analyses
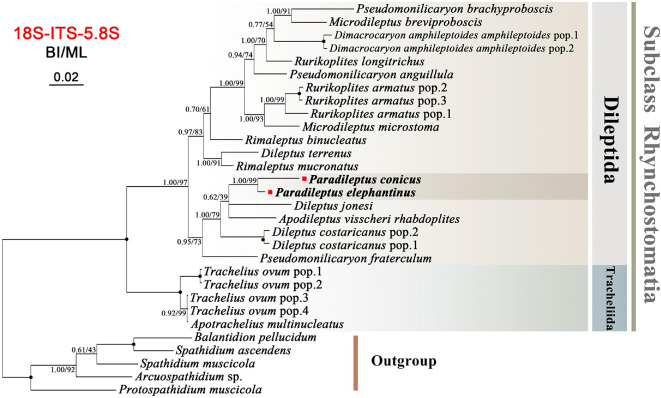
The 18S rDNA sequences of the two Chinese populations were deposited in GenBank with lengths, G + C contents, and accession numbers as follows: Paradileptus elephantinus 1518 bp, 42.23%, MZ147012; P. conicus 1518 bp, 41.83%, MZ147013. The topologies of the BI and ML trees based on 18S rDNA data were highly concordant, therefore only the BI tree is presented (Figure 6A). All dileptids grouped together in the subclass Rhynchostomatia with strong support (BI 1.00, ML 99%) and were divided into two well-supported monophyletic orders, namely, Dileptida (BI 1.00, ML 97%) and Tracheliida (BI 1.00, ML 99%). The two sequences of Paradileptus clustered together with low support (BI 0.77, ML 23%) as a sub-clade that was sister group to Dileptus margaritifer (BI 0.96, ML 28%) within Dileptida. The other 18S rDNA tree focusing on the subclass Rhynchostomatia had a very similar topology (Supplementary Figure 1).
FIGURE 6.
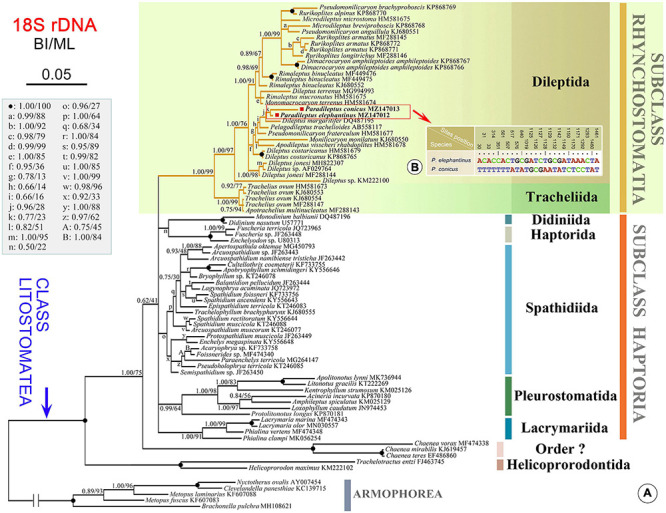
(A) Bayesian inference (BI) tree inferred from 18S rDNA sequences (78 litostomatean and five armophorean taxa). Numbers near branches show the posterior probabilities from BI and bootstrap values from maximum likelihood (ML) analyses. The newly sequenced species in this study are shown in bold. The scale bar corresponds to 5 substitutions per 100 nucleotide positions. (B) Nucleotide differences between Paradileptus elephantinus and P. conicus based on 18S rDNA sequences. The numbers in the header indicate the unmatched site positions.
The ITS-5.8S rDNA region sequences of the two Chinese populations were deposited in GenBank with lengths, G + C contents, and accession numbers as follows: Paradileptus elephantinus 392 bp, 33.42%, MZ574467; P. conicus 391 bp, 31.71%, MZ574468. The topologies of the BI and ML trees based on ITS-5.8S rDNA data were generally concordant, therefore only the BI tree is presented (Figure 7). The phylogenetic tree inferred from ITS-5.8S rDNA data had a similar overall topology to that inferred from the 18S rDNA data, i.e., all rhynchostomatians were divided into two orders. The two Paradileptus species grouped together with maximal support (BI 1.00, ML 100%).
FIGURE 7.
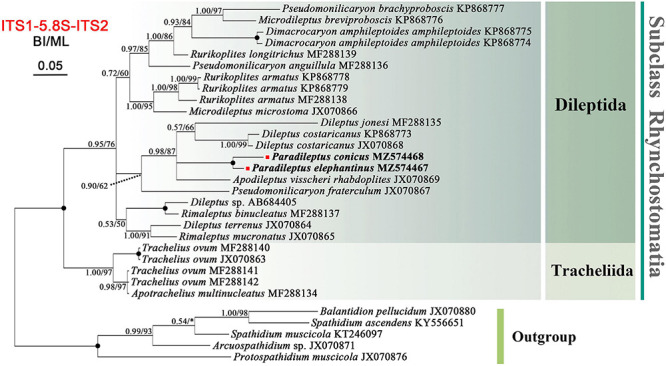
Bayesian inference (BI) tree inferred from ITS-5.8S rDNA sequences focusing on the subclass Rhynchostomatia (26 rhynchostomatian and five spathiid taxa). Numbers near branches show the posterior probabilities from BI and bootstrap values from maximum likelihood (ML) analyses. Asterisks indicate a mismatch in branching pattern between the BI and ML trees. Fully supported (1.00/100) branches are marked with solid circles. The newly sequenced species in this study are shown in bold. The scale bar corresponds to 5 substitutions per 100 nucleotide positions.
Phylogenetic reconstructions based on concatenated sequences of 18S, 5.8S, and ITS region by ML and BI methods had similar topologies; therefore, only the BI tree is presented (Figure 8). Relationships within the subclass Rhynchostomatia were generally consistent with those inferred from the single-gene analyses, the main difference being that the order Dileptida was divided into two clades instead of three, and the clustering of Paradileptus elephantinus and P. conicus was strongly supported (BI 1.00, ML 99%).
FIGURE 8.
Bayesian inference (BI) tree inferred from concatenated rDNA (concatenated with 18S rDNA and ITS1-5.8S-ITS2) sequences focusing on the subclass Rhynchostomatia (25 rhynchostomatian and five spathiid taxa). Numbers near branches show the posterior probabilities from BI and bootstrap values from maximum likelihood (ML) analyses. Fully supported (1.00/100) branches are marked with solid circles. The scale bar corresponds to 5 substitutions per 100 nucleotide positions.
Discussion
The genus Paradileptus is easily recognizable by the shape of the body, which is obliquely truncated in the oral region, and the presence of a spiral proboscis that serves to capture and manipulate prey (Wenrich, 1929; Vd’ačný and Foissner, 2012). The type species was originally reported by Rousselet (1890) as Amphileptus flagellatus, but Wenrich (1929) established a new genus (Paradileptus) for A. flagellatus based on the broad peristomial field with its raised rim and the spiral arrangement of the proboscis. It is noteworthy that A. moniliger, reported by Ehrenberg (1835), was the first species discovered in this genus, but was overlooked in subsequent revisions (Wenrich, 1929; Kahl, 1931, 1935) until it was transferred to Paradileptus by Vd’ačný and Foissner (2012). To date, ten nominal species of Paradileptus have been reported. Paradileptus caducus Kahl, 1935 and P. canellai Dragesco, 1966 are considered to be synonymous with Pelagodileptus trachelioides (Zacharias, 1894) Foissner et al., 1999 (Kahl, 1935; Dragesco, 1966; Foissner et al., 1999). Vd’ačný and Foissner (2012) reviewed the descriptions of the remaining eight species and concluded that only one, P. elephantinus, is valid. However, based on our present findings and following a reassessment of the historical studies, we consider four species to be valid, namely, P. flagellatus (Rousselet, 1890) Wenrich, 1929 [basionym: Amphileptus flagellatus Rousselet, 1890]; P. elephantinus (Šveç, 1897) Kahl, 1931 [see section “Results” for synonyms]; P. conicus Wenrich, 1929 [see section “Results” for synonyms]; and P. moniliger (Ehrenberg, 1835) Vd’ačný & Foissner, 2012 [synonyms: P. ovalis Huber-Pestalozzi, 1945; P. estensis Canella, 1951; P. minutus Dragesco, 1972; P. elephantinus sensu Dragesco, 1972; P. elephantinus sensu Dragesco and Dragesco-Kernéis, 1986; P. elephantinus sensu Krainer, 1988].
The type species Paradileptus flagellatus has only appeared in a few reports, each with inadequate illustrations (Rousselet, 1890; Wenrich, 1929; Kahl, 1931, 1943; Puytorac et al., 1972). Nevertheless, according to the original description (Rousselet, 1890), P. flagellatus can be distinguished from other species by having two macronuclear nodules (vs. moniliform macronucleus). The most recent report on P. flagellatus was that by Puytorac et al. (1972) who identified it based on observations of both living and silver-stained specimens, although no illustrations were provided. The presence of two macronuclear nodules was confirmed, thus we accept the validity of this species.
The other valid species not sampled in present work is Paradileptus moniliger, the body of which has a trunk that is oval in outline and tapers posteriorly to form a short tail (Ehrenberg, 1838). Populations with the same body shape have been reported several times (Huber-Pestalozzi, 1945; Canella, 1951; Dragesco, 1972a, b; Dragesco and Dragesco-Kernéis, 1986; Krainer, 1988). Paradileptus moniliger can be easily distinguished from its congeners by the presence (vs. absence) of a short tail (Ehrenberg, 1838; Rousselet, 1890; Šveç, 1897; Wenrich, 1929). However, according to Foissner et al. (1999), the body shape of species in this genus can quickly change in unfavorable environments with the disappearance of the tail and the body becoming more bulky. During the present study, P. elephantinus and P. conicus were starved in filtered habitat water for 3 days but there was no change in body shape. We also interchanged the living environment (filtered habitat water) of the two species, but their body shape remained unchanged after 3 days of starvation. Thus, we conclude that individuals with a short tail represent an independent species and accept the validity of P. moniliger.
The Chinese Population of Paradileptus elephantinus
Paradileptus elephantinus was originally reported by Šveç (1897) under the name Dileptus elephantinus, but it was omitted from Paradileptus when Wenrich (1929) established this genus. Wenrich (1929) also described a new species, P. robustus, but Kahl (1935) considered this to be a junior synonym of P. elephantinus. The Chinese population of P. elephantinus closely resembles the original population with respect to its body shape, number and distribution of contractile vacuoles, moniliform macronucleus, and habitat (Šveç, 1897). The main difference is the cell size (265–420 μm in Chinese population vs. 200–250 μm). Considering the size range of the body of P. elephantinus (length 180–600 μm), we consider these two forms to be conspecific.
Paradileptus flagellatus and P. moniliger remain insufficiently described since there is no detailed living or infraciliature information for either. But they can still be separated from the Chinese population of P. elephantinus by the body shape (posterior end rounded or slightly pointed in P. elephantinus vs. posteriorly end sharply tapered with a short tail in P. moniliger) and the macronucleus (moniliform in P. elephantinus vs. two macronuclear nodules in P. flagellatus) (Ehrenberg, 1838; Wenrich, 1929).
According to the present and previous studies, Paradileptus elephantinus and P. conicus differ significantly in their morphology in vivo and infraciliature. The trunk of the body is oval in outline in P. elephantinus (vs. inverted-conical in P. conicus) and the extrusomes differ in length (type I, 11.6–13.4 μm and type II, 3.1–3.7 μm in P. elephantinus vs. type I, 4.0–5.7 μm and type II, 1.7–2.3 μm in P. conicus). In terms of its infraciliature, P. elephantinus can be distinguished from P. conicus by the number of preoral kineties (83–112, on average about 95 in P. elephantinus vs. 60–85, on average about 67 in P. conicus).
The Chinese Population of Paradileptus conicus
Paradileptus conicus was first described by Wenrich (1929) who characterized it as follows: “total length usually 100–200 μm, body conical in shape, tapering posteriorly to a spike-like projection; broad anterior end occupied by a cytostome and a peristomial field, surrounded by a flange or rim from which the spirally wound proboscis arises as an extension; contractile vacuoles numerous, distributed over the body and along the posterior part of the proboscis; macronucleus beaded, composed of from four to eight segments”. The Chinese population closely resembles the original population. Paradileptus conicus can be clearly distinguished from P. flagellatus and P. moniliger by its body shape (inverted-conical trunk in P. conicus vs. ovoidal trunk with rounded posterior end in P. flagellatus vs. ovoidal trunk with posterior sharply tapered to form a short tail in P. moniliger) (Ehrenberg, 1838; Rousselet, 1890). It also can be distinguished from P. flagellatus by its moniliform macronucleus (vs. two macronuclear nodules) (Rousselet, 1890).
Key to the Identification of the Four Valid Morphospecies of Paradileptus
For illustrations of selected key characters, see Figure 9.
FIGURE 9.
Illustrated key to valid Paradileptus species.
-
(1)
Two macronuclear nodules………………… P. flagellatus
-
–
Moniliform macronucleus…………………………… 2
-
(2)
Body trunk inverted-conical. . . . . . . . . . . . . . . . . . . . . . P. conicus
-
–
Body trunk ovoidal………………………………… . .3
-
(3)
Posterior end of body trunk rounded or slightly pointed ………….…….……….……………… P. elephantinus
-
–
Posterior end of body trunk sharply tapered and with a short tail …………………………………… . . . . P. moniliger
Molecular Phylogeny of Paradileptus
Rhynchostomatia was established by Jankowski (1980) as one of three subclasses of the class Litostomatea. This subclass comprises two orders, Tracheliida and Dileptida (Vd’ačný et al., 2017). Phylogenetic analyses based on 18S, 5.8S, and ITS rDNA sequence data demonstrated that each of these two orders is monophyletic (Figures 6A, 7, 8), which is consistent with previous studies (Vd’ačný et al., 2011b, 2014; Jang et al., 2014; Vd’ačný and Rajter, 2015; Huang et al., 2018).
The topology of the 18S rDNA tree (Figure 6A) shows that the two Paradileptus species sequenced here nest within the Dileptida where they form a clade that clusters with other dileptids in the following order: Dileptus margaritifer (DQ487195) followed by Pelagodileptus trachelioides (AB558117) followed by Pseudomonilicaryon fraterculum (HM581677). Paradileptus can be clearly separated from D. margaritifer by morphological features such as the body shape (wide body with a spiral proboscis in Paradileptus vs. narrow body with a non-spiral proboscis) and the macronucleus (moniliform or as two nodules in Paradileptus vs. many scattered macronuclear nodules) (Vd’ačný and Foissner, 2012). In addition, it can be distinguished from Pseudomonilicaryon fraterculum by the mode of locomotion (free-swimming vs. gliding in P. fraterculum) and the body shape (wide vs. narrow to cylindrical in P. fraterculum) (Vd’ačný and Foissner, 2012). Pelagodileptus trachelioides is a planktonic dileptid with a moniliform macronucleus that superficially resembles Paradileptus. However, Paradileptus can be distinguished by the shape of its oral opening (roundish vs. narrowly elliptical in P. trachelioides) and the presence (vs. absence in P. trachelioides) of a strongly broadened proboscis base (Foissner et al., 1999; Vd’ačný and Foissner, 2012).
Compared to the 18S tree, nodal support for the ITS-5.8S and concatenated trees was generally higher, in particular for the resolution of the Paradileptus clade (BI 1.00, ML 100%; BI 1.00, ML 99%). The concatenated alignment might amplify the phylogenetic signal of single markers resulting in highly resolved and robust trees for rhynchostomatians (Figures 7, 8).
Geographical Distribution of Paradileptus
The findings of the present study support previous reports that suggest species of Paradileptus, and in particular P. elephantinus and P. conicus, are cosmopolitan (Figure 1A and Table 1; Foissner et al., 1999; Vd’ačný and Foissner, 2012). The Chinese population of P. conicus was isolated from an aquaculture pond where food resources are rich, whereas P. elephantinus was isolated from Lake Weishan where food resources are poorer. Considering that both the morphological and the 18S rDNA sequence data (Figure 6B) of these two taxa provide reliable and robust resolution of their separation at species level, we hypothesize that they might have undergone a putative speciation process via food preference and niche differentiation. The presence of greater numbers of preoral kineties, the larger body, the longer extrusomes and the larger buccal cavity of P. elephantinus suggest that its prey probably differs significantly from that of P. conicus, which may also be an adaptation to life in resource-poor habitats.
Features of the Proboscis in Predatory Rhynchostomatians
Rhynchostomatians are raptorial feeders whose predatory lifestyle has led to the development of special structures for prey recognition and capture. The primary feature is the apical proboscis which carries a dorsal brush that may be used for sensory feedback, extrusomes (toxicysts) to paralyze and/or kill prey organisms, and differentiated kineties such as the circumoral kinety (∼paroral membrane) and preoral kineties (∼adoral zone of membranelles) to aid feeding (Visscher, 1923; Dragesco, 1962; Vd’ačný and Foissner, 2012; Vd’ačný et al., 2017; Chi et al., 2021). Furthermore, differences in hunting strategies and preferred prey among dileptids (Vd’ačný and Foissner, 2012) seem to be related to these differentiated structures. Exploring this phenotypic divergence might improve understanding of the distribution patterns of ryhnchostomatians and their adaptations to different environments.
According to Vd’ačný et al. (2017), the most common type of proboscis bears two kinds of extrusomes and a multi-rowed dorsal brush, which will influence speciation, extinction, and net diversification of rhynchostomatians. Therefore, we speculate that: (1) there are competitive advantages in having two types of extrusomes compared to a single type of extrusome; (2) a multi-rowed dorsal brush provides distinct advantages over a two-rowed dorsal brush by increasing sensory function during locomotion; and (3) the increased number of differentiated kineties on the proboscis improves the efficiency of predation and the dietary niche differentiation of Paradileptus elephantinus and P. conicus and could contribute to their separation as different species.
In addition to the dorsal brush, extrusomes, circumoral kinety, and preoral kineties, the rhynchostomatian proboscis also bears one or two perioral kineties, i.e., the first one or two kineties on the right side of the circumoral kinety. Most rhynchostomatians possess one perioral kinety whereas the planktonic genera Paradileptus and Pelagodileptus have two perioral kineties, which is thought to increase the efficiency of food acquisition (Foissner et al., 1999; Vd’ačný and Foissner, 2012). In the present study, we revealed that there are five to eight recognizable kineties on the right side of the circumoral kinety in P. elephantinus, and two to three in P. conicus. We also found that the first kinety to the right of the circumoral kinety has densely spaced kinetosomes, whereas those of the remaining right kineties are loosely arranged, and that the first right kinety and circumoral kinety are closely adjacent, whereas there is significantly larger gap to the second right kinety. This arrangement is found in almost all other rhynchostomatians in which the first right kinety on the right side of the circumoral kinety is the perioral kinety and the remaining right kineties are somatic kineties (for details, see Vd’ačný and Foissner, 2012). Therefore, we conclude that the first kinety to the right of the circumoral kinety in P. elephantinus and P. conicus is the perioral kinety. It is noteworthy that the arrangement of kineties on the right side of the circumoral kinety in schematic drawings of Pelagodileptus trachelioides is also similar to almost all other rhynchostomatians (Vd’ačný and Foissner, 2012). In particular, Packroff and Wilbert (1991) labeled to the 2nd kinety to the right of the circumoral kinety as “somatic kinety 1,” whereas the first right kinety was marked the right circumoral kinety. We suggest that this latter structure is the perioral kinety and that all rhynchostomatians are characterized by the possession of a single perioral kinety.
Finally, we speculate that the densely arranged right kineties on the proboscis (vs. loosely arranged somatic kineties in the trunk) may be a taxonomically informative character for species separation and identification in Paradileptus. Unfortunately, the lack of ultrastructural and ontogenetic information of the proboscis in Paradileptus makes the origin of these densely ciliated kineties uncertain, which should be investigated in further studies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank, MZ147012, MZ147013, MZ574467, and MZ574468.
Author Contributions
YZ and HM conceived and designed the manuscript. YC carried out the live observation, protargol staining, DNA extraction, and data analyses. ZW, BL, HM, and CM checked all the data and assisted in the interpretation of the data. YC, AW, and YZ contributed to the revision of the manuscript. All authors wrote the manuscript, read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Our special thanks are given to Professor Weibo Song (OUC) for his kind suggestions during the drafting of the manuscript. Thanks are due to Weishan Wetland Station for the institutional support. We are also grateful to the two reviewers for their constructive suggestions.
Footnotes
Funding
This work was supported by the Natural Science Foundation of China (Nos. 32070461, 32070432, and 32000300) and Beijing Natural Science Foundation (No. 5212001).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.709566/full#supplementary-material
References
- Alekperov I. K. (1984). Free-living infusoria of Khachinchay reservoir. Hydrobiol. J. 20 17–22. [Google Scholar]
- Bai Y., Wang R., Song W., Suzuki T., Hu X. Z. (2020). Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao, China. Mar. Life. Sci. Technol. 2 209–221. 10.1007/s42995-020-00034-2 [DOI] [Google Scholar]
- Barbieri S. M., Godinho-Orlandi M. J. L. (1989). Planktonic protozoa in a tropical reservoir: temporal variations in abundance and composition. Rev. Hydrobiol. Trop. 22 275–285. [Google Scholar]
- Canella M. F. (1951). Osservazioni morfologiche, biologiche e sistematiche su Paradileptus estensis sp. n. e su altri Tracheliidae (Holotricha). Annali Univ. Ferrara (N.S.) Sez. III 1 81–170. 10.1080/11263507409426349 [DOI] [Google Scholar]
- Chi Y., Chen X. R., Li Y. Q., Wang C. D., Zhang T. T., Ayoub A., et al. (2021). New contributions to the phylogeny of the ciliate class Heterotrichea (Protista, Ciliophora): analyses at family-genus level and new evolutionary hypotheses. Sci. China Life Sci. 64 606–620. 10.1007/s11427-020-1817-5 [DOI] [PubMed] [Google Scholar]
- Czapik A., Fyda J. (1995). Wstęppe badania nad mikro- i meiofauną suchárow Wigierskiego Parku Narodowego [Micro- and meiofauna of dystrophic lakes in the Wigry National Park]. Pez. Zool. 39 65–72. [Google Scholar]
- Dragesco J. (1962). Capture et ingestion des proies chez les infusoires ciliés. Bull. Biol. Fr. Belg. 96 123–167. [Google Scholar]
- Dragesco J. (1966). Ciliés libres de thonon et ses environs. Protistologica 2 59–95. [Google Scholar]
- Dragesco J. (1972a). Ciliés libres de la cuvette tchadienne. Annls Fac. Sci. Cameroun. 11 71–91. [Google Scholar]
- Dragesco J. (1972b). Ciliés libres de l’Ouganda. Annls Fac. Sci. Cameroun. 9 87–126. [Google Scholar]
- Dragesco J., Dragesco-Kernéis A. (1986). Ciliés Libres de l’Afrique Intertropicale: Introduction à la Connaissance et à L’étude des Ciliés. Paris: Faune Tropicale (Éditions de l’ORSTOM). [Google Scholar]
- Ehrenberg C. G. (1835). Zusätze zur erkenntniβ groβer organischer ausbildung in den kleinsten thierischen organismen. Abh. dt. Akad. Wiss. Berl. 1835, 151–180. 10.1002/zaac.19683600306 [DOI] [Google Scholar]
- Ehrenberg C. G. (1838). Die infusionsthierchen als vollkommene organismen. Ein blick in das tiefere organische leben der natur. Leipzig: Verlag von Leopold Voss. [Google Scholar]
- Foissner W., Berger H., Schaumburg J. (1999). Identification and Ecology of Limnetic Plankton Ciliates. Munich: Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft. [Google Scholar]
- Foissner W., Berger H., Blatterer H., Kohmann F. (1995). Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems. Band IV: Gymnostomatea, Loxodes, Suctoria. Munich: Informationsberichte des Bayer Landesamtes für Wasserwirtschaft. [Google Scholar]
- Fryd-Versavel G., Iftode F., Dragesco J. (1975). Contribution a la connaissance de quelques ciliés gymnostomes II. Prostomiens, pleurostomiens: morphologie, stomatogenèse. Protistologica 11 509–530. [Google Scholar]
- Gao F., Warren A., Zhang Q. Q., Gong J., Miao M., Sun P., et al. (2016). The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Sci. Rep. 6:24874. 10.1038/srep24874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O. (2009). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27 221–224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- Hall T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Hu X. Z., Lin X. F., Song W. B. (2019). Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press. [Google Scholar]
- Huang J. B., Zhang T. T., Zhang Q. Q., Li Y., Warren A., Pan H. B., et al. (2018). Further insights into the highly derived haptorids (Ciliophora, Litostomatea): phylogeny based on multigene data. Zool. Scr. 47 231–242. 10.1111/zsc.12269 [DOI] [Google Scholar]
- Huber-Pestalozzi G. (1945). Neue planktonorganismen im Zürichsee. Paradileptus conicus Wenrich und Paradileptus ovalis nova spec. (3. Mitteilung). Vjschr. Naturf. Ges. Zürich 90 120–126. [Google Scholar]
- Jang S. W., Vd’ačný P., Shazib S. U. A., Shin M. K. (2014). Morphological and molecular characterization of the name-bearing type species Rimaleptus binucleatus (Kahl, 1931), with a phylogenetic re-analysis of dileptid evolutionary history (Ciliophora: Litostomatea: Rhynchostomatia). Eur. J. Protistol. 50 456–471. 10.1016/j.ejop.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Jankowski A. W. (1980). Konspekt novoj sistemy tipa Ciliophora [Conspectus of a new system of the phylum Ciliophora]. Trudy Zool. Inst. Leningr. 94 103–121. [Google Scholar]
- Kahl A. (1931). Urtiere oder Protozoa I: wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt. Dtl. 21 181–398. [Google Scholar]
- Kahl A. (1935). Urtiere oder protozoa I: wimpertiere oder ciliata (Infusoria) 4. Peritricha Chonotricha Tierwelt. Dtl. 30 651–886. [Google Scholar]
- Kahl A. (1943). Infusorien (1. Teil). Handbücher Für Die Praktische Wissenschaftliche Arbeit 31/32. Stuttgart: Franckh’sche Verlagsbuchhandlung. [Google Scholar]
- Krainer K. H. (1988). Alpha-Taxonomie und Ökologie Neuer Sowie Mehrerer Wenig Bekannter Pelagischer Ciliaten (Protozoa: Ciliophora aus den Klassen Kinetofragminophora, Oligohymenophora, Polyhymenophora) Einiger Grundwasserbaggerteiche des Nördlichen Leibnitzer Feldes (Steiermark, Österreich). Ph.D. Thesis. Graz: University of Graz. [Google Scholar]
- Kravchenko V. M. (1969). O faune infuzorij vodoemov bassejna Severskogo Donca [On fauna of infusoria in the reservoirs of the Seversky Donets Basin]. Vestn. Akad. Nauk Ukr. 3 69–75. [Google Scholar]
- Lian C. Y., Luo X. T., Warren A., Zhao Y., Jiang J. M. (2020). Morphology and phylogeny of four marine or brackish water spirotrich ciliates (Protozoa, Ciliophora) from China, with descriptions of two new species. Eur. J. Protistol. 72:125663. 10.1016/j.ejop.2019.125663 [DOI] [PubMed] [Google Scholar]
- Liepa R. A. (1983). Èkologo-faunističeskaâ harakteristika infuzorij vodoemov s povyšennoj saprobiost’û [Ecologo-faunistic characteristics of ciliates in water bodies of higher saprobity]. Protozoologiâ 8 134–141. [Google Scholar]
- Lokot’ L. I. (1987). Èkologiâ Resniènyh Prostejših v Ozerah Central’nogo Zabajkal’â [Ecology of Ciliated Protozoa In Lakes Of The Central Baikal Region]. Novosibirsk: Nauka. [Google Scholar]
- Lu B. R., Li L. F., Hu X. Z., Ji D. D., Al-Rasheid K. A. S., Song W. B. (2019). Novel contributions to the peritrich family Vaginicolidae (Protista: Ciliophora), with morphological and phylogenetic analyses of poorly known species of Pyxicola, Cothurnia and Vaginicola. Zool. J. Linn. Soc. 187 1–30. 10.1093/zoolinnean/zlz009 [DOI] [Google Scholar]
- Lundin F. C., West L. S. (1963). The Free-Living Protozoa of the Upper Peninsula of Michigan. Marquette, WI: Northern Michigan College Press. [Google Scholar]
- Lynn D. H. (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature, 3rd Edn. Dordrecht: Springer. [Google Scholar]
- Mamaeva N. V. (1979). Infuzorii Bassejna Volgi. Èkologièeskij Oèerk [Infusoria of the Volga basin. Ecological survey]. Leningrad: Nauka. [Google Scholar]
- Matsuoka T., Matsuo N., Maesako J., Shigenaka Y. (1983). Distribution of fresh-water protozoa I. Municipal and suburban districts of Hiroshima and Higashi-Hiroshima. Bull. biol. Soc. Hiroshima Univ. 49 13–18. [Google Scholar]
- Miller M. A., Pfeiffer W., Schwartz T. (2010). “Creating the CIPRES Science Gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop, New Orleans, LA. [Google Scholar]
- Modenutti B. E., Pérez G. L. (2001). Planktonic ciliates from an oligotrophic South Andean lake, Morenito Lake (Patagonia, Argentina). Braz. J. Biol. 61 389–395. 10.1590/S1519-69842001000300007 [DOI] [PubMed] [Google Scholar]
- Nebrat A. A. (1992). Planktonnye infuzorii nižnego tečeniâ r. Pripâti [Planktonic infusoria in the lower flow of the Pripyat River]. Gidrobiol. Ž. 28 27–31. [Google Scholar]
- Nylander J. A. A. (2004). MrModeltest ver. 2: Evolutionary Biology Centre. Uppsala: Uppsala University. [Google Scholar]
- Oleksiv I. T. (1985). Vidovoj sostav i čislennost’ planktonnyh infuzorij v prudah [Species composition and quantity of the plankton infusoria in ponds]. Gidrobiol. Ž. 21 89–93. [Google Scholar]
- Packroff G., Wilbert N. (1991). Taxonomische studien über die ciliatenfauna (Protozoa, Ciliophora) der Eifelmaare. Arch. Protistenk 140 121–139. 10.1016/s0003-9365(11)80180-6 [DOI] [Google Scholar]
- Page R. D. (1996). TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12 357–378. 10.1093/bioinformatics/12.4.357 [DOI] [PubMed] [Google Scholar]
- Puytorac P., Mignot J. P., Grain J., Grolière C. A., Bonnet L., Couillard P. (1972). Premier relevé de certains groupes de protozoaires libres sur le territoire de la station de biologie de l’université de Montréal (Saint-Hippolyte, Comté de Terrebonne, Québec). Nat. Can. 99 417–440. [Google Scholar]
- Ronquist F., Teslenko M., van Der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet C. (1890). On “Amphileptus flagellatus,” sp. n. a new infusorian. J. Quekett. Microsc. Club 4 114–115. [Google Scholar]
- Sogin M. L. (1989). Evolution of eukaryotic microorganisms and their small subunit ribosomal RNAs. Am. Zool. 29 487–499. 10.1093/icb/29.2.487 31919651 [DOI] [Google Scholar]
- Sokoloff D. (1931). Un nuevo infusorio ciliado de agua dulce. Nota Preliminar. An. Inst. Biol. Univ. Nac. Auton. Mex. 2 165–166. [Google Scholar]
- Song W. B., Warren A., Hu X. Z. (2009). Free-living Ciliates in the Bohai and Yellow Seas, China. Beijing: Science Press. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šveç F. (1897). Beiträge zur kenntnis der infusorien Böhmens. I. Die ciliaten infusorien des Unterpočernitzer teiches. Bull. Int. Acad. Sci. 4 29–47. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vd’ačný P., Foissner W. (2012). Monograph of the dileptids (Protista, Ciliophora, Rhynchostomatia). Denisia 31 1–529. [Google Scholar]
- Vd’ačný P., Rajter L. (2015). Reconciling morphological and molecular classification of predatory ciliates: evolutionary taxonomy of dileptids (Ciliophora, Litostomatea, Rhynchostomatia). Mol. Phylogen. Evol. 90 112–128. 10.1016/j.ympev.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Vd’ačný P., Bourland W. A., Orsi W., Epstein S. S., Foissner W. (2011a). Phylogeny and classification of the Litostomatea (Protista, Ciliophora), with emphasis on free-living taxa and the 18S rRNA gene. Mol. Phylogen. Evol. 59 510–522. 10.1016/j.ympev.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Vd’ačný P., Breiner H.-W., Yashchenko V., Dunthorn M., Stoeck T., Foissner W. (2014). The chaos prevails: molecular phylogeny of the Haptoria (Ciliophora, Litostomatea). Protist 165 93–111. 10.1016/j.protis.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Vd’ačný P., Orsi W., Bourland W. A., Shimano S., Epstein S. S., Foissner W. (2011b). Morphological and molecular phylogeny of dileptid and tracheliid ciliates: resolution at the base of the class Litostomatea (Ciliophora, Rhynchostomatia). Eur. J. Protistol. 47 295–313. 10.1016/j.ejop.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vd’ačný P., Rajter L., Shazib S. U. A., Jang S. W., Shin M. K. (2017). Diversification dynamics of rhynchostomatian ciliates: the impact of seven intrinsic traits on speciation and extinction in a microbial group. Sci. Rep. 7:9918. 10.1038/s41598-017-09472-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher J. P. (1923). Feeding reactions in the ciliate, Dileptus gigas, with special reference to the function of trichocysts. Biol. Bull. Mar. Biol. Lab. Woods Hole 45 113–143. 10.2307/1536640 [DOI] [Google Scholar]
- Wang C. C., Nie D. (1933). Report on the rare and new species of fresh water infusoria, part 1. Contr. Biol. Lab. Sci. Soc. China 10 1–99. [Google Scholar]
- Wang Y. R., Wang C. D., Jiang Y. H., Katz L. A., Gao F., Yan Y. (2019). Further analyses of variation of ribosome DNA copy number and polymorphism in ciliates provide insights relevant to studies of both molecular ecology and phylogeny. Sci. China Life Sci. 62 203–214. 10.1007/s11427-018-9422-5 [DOI] [PubMed] [Google Scholar]
- Wenrich D. H. (1929). Observations on some freshwater ciliates. (Protozoa) II. Paradileptus, n. gen. Trans. Am. Microsc. Soc. 48 352–365. 10.2307/3222055 [DOI] [Google Scholar]
- Wilbert N. (1975). Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64 171–179. [Google Scholar]
- Wu T., Li Y. Q., Lu B. R., Shen Z., Song W. B., Warren A. (2020). Morphology, taxonomy and molecular phylogeny of three marine peritrich ciliates, including two new species: Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. (Protozoa, Ciliophora, Peritrichia). Mar. Life. Sci. Technol. 2 334–348. 10.1007/s42995-020-00046-y [DOI] [Google Scholar]
- Zhang T. T., Fan X. P., Gao F., Al-Farraj S. A., El-Serehy H. A., Song W. B. (2019). Further analyses on the phylogeny of the subclass Scuticociliatia (Protozoa, Ciliophora) based on both nuclear and mitochondrial data. Mol. Phylogen. Evol. 139:106565. 10.1016/j.ympev.2019.106565 [DOI] [PubMed] [Google Scholar]
- Zhang T. Y., Dong J. Y., Cheng T., Duan L. L., Shao C. (2020). Reconsideration of the taxonomy of the marine ciliate Neobakuella aenigmatica Moon et al., 2019 (Protozoa, Ciliophora, Hypotrichia). Mar. Life. Sci. Technol. 2 97–108. 10.1007/s42995-020-00032-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank, MZ147012, MZ147013, MZ574467, and MZ574468.