Abstract
L-2-halocid dehalogenases (L-2-HADs) have been mainly characterized from terrestrial polluted environments. By contrast, knowledge is still scarce about their role in detoxification of predominant halocarbons in marine environments. Here, phylogenetic analyses showed a wide diversity of homologous L-2-HADs, especially among those belonging to marine bacteria. Previously characterized terrestrial L-2-HADs were part of a monophyletic group (named group A) including proteins of terrestrial and marine origin. Another branch (named group B) contained mostly marine L-2-HADs, with two distinct clades of Bacteroidetes homologs, closely linked to Proteobacteria ones. This study further focused on the characterization of the only L-2-HAD from the flavobacterium Zobellia galactanivorans DsijT (ZgHAD), belonging to one of these Group B clades. The recombinant ZgHAD was shown to dehalogenate bromo- and iodoacetic acids, and gene knockout in Z. galactanivorans revealed a direct role of ZgHAD in tolerance against both haloacetic acids. Analyses of metagenomic and metatranscriptomic datasets confirmed that L-2-HADs from group A were well-represented in terrestrial and marine bacteria, whereas ZgHAD homologs (group B L-2-HADs) were mainly present in marine bacteria, and particularly in host-associated species. Our results suggest that ZgHAD homologs could be key enzymes for marine Bacteroidetes, by conferring selective advantage for the recycling of toxic halogen compounds produced in particular marine habitats, and especially during interactions with macroalgae.
Keywords: L-2-haloacid dehalogenase, Bacteroidetes, haloacid catabolism, evolution, knockout mutant
Introduction
Halogenated metabolites are widely distributed on Earth. They are defined as organic compounds that covalently bind one or several halogen atom(s) such as fluorine, chlorine, bromine or iodine. Over 5,000 different natural organohalogens have been described, mainly from the marine environment (Gribble, 2015). They can be produced abiotically (Dong et al., 2021), but also derived from many different biogenic sources. For example, some chlorinated metabolites are found in land plants and fungi, while brominated metabolites have been mainly related to marine sources such as red and brown algae, or sponges (Amsler, 2008; Bialonska and Zjawiony, 2009; Cabrita et al., 2010; El-Demerdash et al., 2019). Furthermore, during the last century and with industrial developments, man-made polyhalogenated organic products were synthesized and are now persistent organic environmental pollutants (Agarwal et al., 2017; Ang et al., 2018). To mitigate this environmental issue, nature-based solutions are a promising alternative for bioremediation. During the last four decades, research efforts have identified microorganisms that can catabolize diverse mono or polyhalogenated compounds, and characterized a broad range of dehalogenases (Hardman and Slater, 1981; Janssen et al., 1994; Copley, 1998). These enzymes are highly diverse in terms of structure and biochemistry, but they all share the enzymatic capacity of cleaving carbon-halogen bonds. Depending on their catalytic mechanisms and substrates, they are classified in large superfamilies (Janssen et al., 2005; Ang et al., 2018).
The haloacid dehalogenase (HAD) superfamily includes mostly enzymes that hydrolyze carbon-phosphorus or oxygen-phosphorus bonds, such as phosphoesterases, phosphonatases, P-type ATPases, or nucleotidases. Widely conserved among living organisms, they are involved in a variety of cellular processes ranging from amino acid biosynthesis to detoxification (Burroughs et al., 2006). In plant pathogen bacteria, HADs might also be involved in biofilm formation during biotic interactions (Li and Wang, 2011). The “true” dehalogenases of the HAD superfamily are categorized into two types. Type I contains HADs that have an enantioselective dehalogenating activity on D-2-haloacids (D-2-HADs), and those that have non-stereospecific mechanism accepting both D- or L-2-haloacids as substrates (DL-2-HADs). Enzymes from type II specifically act on L-2-haloacids (L-2-HADs) to produce the corresponding hydroxyalkanoic acids with an inverted chirality (Ang et al., 2018). The L-2-HADs are phylogenetically unrelated to D-2-HADs and DL-2-HADs exhibiting a distinct protein fold and catalytic mechanism (Hill et al., 1999).
Many genes encoding L-2-HADs have previously been identified in the genomes of bacteria from contaminated soils or freshwater environments (Copley, 1997; Kurihara and Esaki, 2008). Biochemical and structure/function characterizations of L-2-HAD enzymes have been conducted during the past 30 years to identify new enzymatic or biotechnological processes for detoxification of halogenated pollutants. The large majority of characterized L-2-HADs originate from terrestrial Proteobacteria such as DehIVa from Burkholderia cepacia MBA4 (Schmidberger et al., 2007), DhlB from Xanthobacter autotrophicus GJ10 (Van der Ploeg et al., 1991), or L-DEX from Pseudomonas sp. strain YL (Nardi-Dei et al., 1994). The dhlB gene has been engineered into land plants by transformation, allowing effective treatment of soil and groundwater contaminated with halogenated solvents (Mena-Benitez et al., 2008). By contrast little is known about L-2-HAD phylogeny, gene abundance and evolution and only one partial phylogenetic tree has been reported to date (Marchesi and Weightman, 2003).
The biological roles of these HADs in terrestrial and/or freshwater bacteria are mostly related to their adaptations to degrade xenobiotic compounds, derived from industrial activities and released in the environment (Copley, 1998; Hill et al., 1999; Janssen et al., 2005). However, sampling efforts for HAD-containing microorganisms mostly focused on polluted matrices, potentially biasing the overall representation of these enzymes. Therefore, the phylogenetic diversity and biological roles of HADs might be underestimated in non-polluted environments (Janssen et al., 2005).
The marine environment contains a large range of natural halogenated compounds, some of them being highly specific and/or potentially toxic, as parts of chemical defenses of marine invertebrates or algae. For instance, while iodinated compounds are relatively rare on Earth, brown macroalgae are main producers of volatile iodocarbons that play a notable role in atmospheric chemistry (Carpenter et al., 2012). Other natural sources of marine halogenated compounds could include volcanoes and organic matter efflux in estuaries (Jordan et al., 2000; Gribble, 2003; Gordon et al., 2015). Very little is currently known about marine dehalogenases, and even less about the metabolic pathways involved in the catabolism of halogenated compounds by marine microorganisms. A recent overview on marine haloalkane dehalogenases related the discovery of 12 new enzymes leading to increase the amount of knowledge on this kind of dehalogenation (Kunka et al., 2018). To date concerning haloalkanoic dehalogenases, only four marine L-2-HADs have been biochemically characterized: DehRhb from a Rhodobacteraceae bacterium associated to marine Polychaeta (Novak et al., 2013a); two enzymes from the sponge-associated Paracoccus sp. DEH99 and Pseudomonas stutzeri DEH130 (Huang et al., 2011; Zhang et al., 2013); and one from the psychrophilic strain Psychromonas ingrahamii DehPsi (Novak et al., 2013b). The best-studied marine L-2-HAD so far is DehRhb, whose structure and catalytic properties notably differ from soil enzymes (Novak et al., 2013a).
In this context, this paper reports first on the occurrence of L-2-HAD homologous proteins in microorganisms, i.e., marine bacteria and archaea, through large in silico genomic surveys and on their phylogeny in the Bacteroidetes phylum. It then described the biochemical and functional characterization of the only haloacid dehalogenase identified in the genome of the marine flavobacterium Zobellia galactanivorans DsijT. This bacterium has been developed as a laboratory model for marine Bacteroidetes, because of its capacities to degrade algal polysaccharides. Its genome also features several genes related to halogen metabolism (Fournier et al., 2014; Barbeyron et al., 2016). Thanks to a recent protocol development (Zhu et al., 2017), a genetic knockout approach was conducted, demonstrating the potential in vivo protective role of this L-2-HAD when Z. galactanivorans was exposed to toxic halogenated substrates. In addition, the distribution and expression of phylogenetically distinct L-2-HADs were analyzed using metagenomic and metatranscriptomic datasets, highlighting an underexplored marine biodiversity. Finally, the laboratory functional characterization of Z. galactanivorans L-2-HAD and environmental survey results on homologous proteins are discussed in light of the biological and ecological roles of these enzymes in marine Bacteroidetes in their natural coastal habitats.
Results
Phylogenetic Analysis of Microbial L-2-HADs
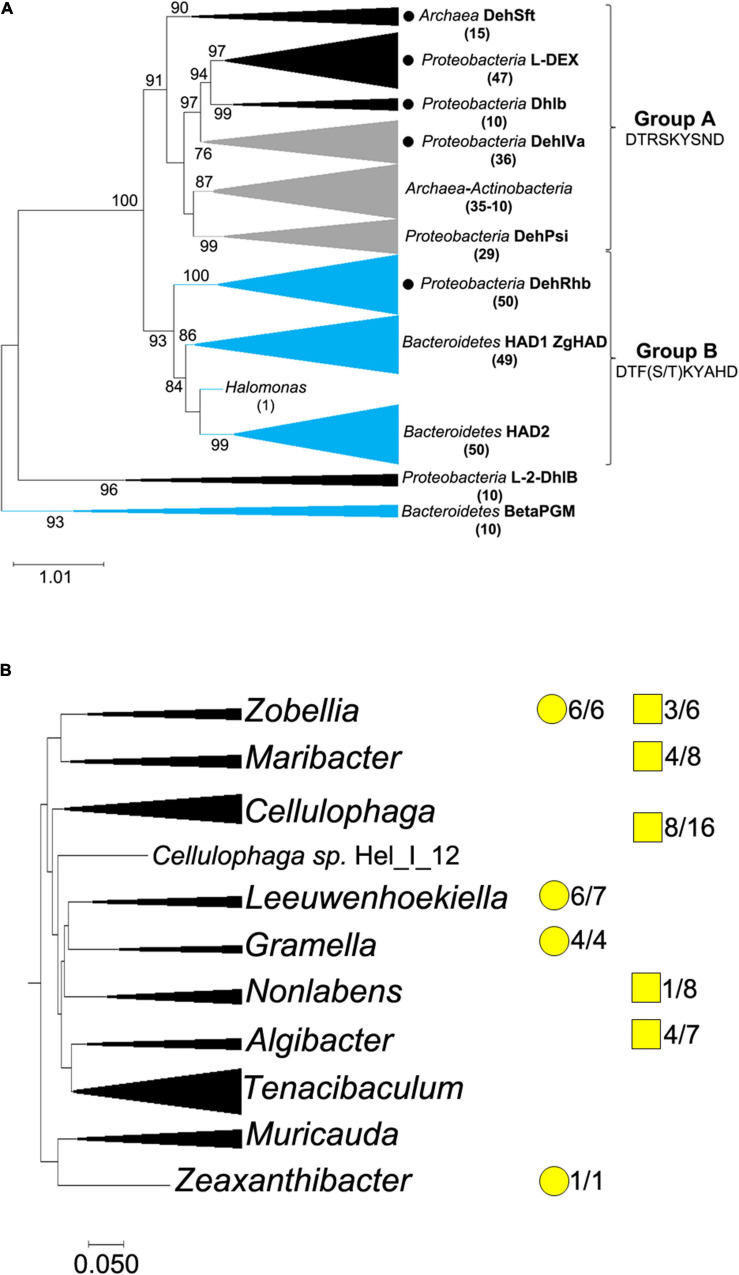
The phylogenetic analysis of microbial L-2-HAD proteins was based on a subset of 352 protein sequences, including the biochemically and structurally characterized enzymes (Supplementary Table 1) and their closest counterparts, in addition to the closest relatives of ZgHAD, identified by BlastP search on protein databases (Supplementary Table 2). The L-2-HAD proteins mainly clustered into two well-supported groups (Figure 1A), with an additional small clade of proteobacterial sequences related to the L-2-DhlB of Ancylobacter aquaticus UV5. The first group (group A) mostly comprises proteobacterial L-2-HAD sequences (122/182 sequences) that are divided into four clades, respectively containing the biochemically characterized L-2-HADs L-DEX, DhlB, DehIVa and DehPsi. Two other clades in the group A contained archaeal proteins: one features terrestrial extremophile L-2-HADs together with the DehSft protein; the second clade contains a mix of Euryarchaea and Actinobacteria putative L-2-HADs. Most L-2-HAD homologs from the group A have been identified in species of terrestrial or freshwater origins, but also few in marine species (Supplementary Table 2). By contrast, the second phylogenetic group (group B) contains, almost exclusively (138/150 sequences), L-2-HAD protein sequences belonging to microorganisms either from saline or from marine origins (Supplementary Table 2). The group B is constituted of three well-supported clades and an isolated proteobacterial putative L-2-HAD from Halomonas (Figure 1A). A clade groups all other proteobacterial L-2-HAD proteins, including DehRhb. Interestingly, the Bacteroidetes L-2-HAD homologs are separated into two phylogenetic groups, named HAD1 and HAD2 clades. No Bacteroidetes L-2-HAD was yet biochemically characterized before this study. The two phylogenetic types of Bacteroidetes L-2-HADs share between 40 and 45% amino acid identity. The phylogenetic group A (Figure 1A) features most of the biochemically characterized L-2-HADs (Supplementary Table 1), and these enzymes share around 30% of identity at the protein sequence level. Furthermore, a motif of nine amino acids (DTRSKYSND) essential for enzyme activity and substrate binding (Adamu et al., 2016) was strictly conserved between all characterized L-2-HADs of the group A (Supplementary Figure 1). In the group B, DehRhb is the closest biochemically characterized protein homolog of ZgHAD, but they only share 31% of amino acid sequence identity (Supplementary Table 1). However, a motif of nine conserved amino acids [DTF(S/T)KYAHD] is present in all group B L-2-HAD sequences and is equivalent to that found for the group A (Supplementary Figure 1). Eight of those amino acids are strictly conserved and only one amino acid is variable in this pattern corresponding to T124 in DehRhb replaced by S131 in ZgHAD (Supplementary Figure 1).
FIGURE 1.
Phylogenetic distribution of L-2-HADs. (A) Maximum likelihood phylogenetic tree based on L-2-HAD protein sequences. Group A and group B define the two major phylogenetic groups, for which nine specifically conserved amino acids of the catalytic site are shown. Black circles indicate phylogenetic clades with at least one available 3D structure of L-2-HAD. Black branches represent putative L-2-HAD proteins from terrestrial or freshwater organisms, blue branches represent those from a large majority of marine organisms and gray ones are those for mixed terrestrial/marine groups. The number of sequences in each group is indicated in brackets. DehSft, L-DEX, DhlB, DehIVa, DehPsi, DehRhb, ZgHAD, L-2-DhlB and BetaPGM proteins are indicated next to the clade where they belong. The two distinct clades of Bacteroidetes L-2-HADs are named HAD1 and HAD2. The beta-phosphoglucomutase proteins were used as outgroup to root the tree. (B) Distribution of the two types of haloacid dehalogenases identified in the family of Flavobacteriaceae. The phylogenetic tree of 10 genus clades was constructed using the comparative genomics tool for genome clustering of MaGe website. The ratio numbers correspond to the number of genomes among the total analyzed genomes with homolog genes belonging to HAD1 phylogenetic group (yellow round) and/or to HAD2 phylogenetic group (yellow square). When yellow round and square are not displayed, it means that the corresponding had genes were absent.
This phylogenetic analysis showed that most Bacteroidetes featured a single L-2-HAD belonging to either the HAD1 or HAD2 phylogenetic clades, but some of them exhibited one L-2-HAD sequence from each clade (Supplementary Table 2). When comparing the complete genomes of Zobellia species and closely related members of the Flavobacteriaceae family (Figure 1B), some inter- and intra-species variations occur, independently of the phylogenetic relationships: for example, Zobellia amurskyensis MAR_2009_138 (formerly Zobellia uliginosa; Chernysheva et al., 2019) possesses two different L-2-HAD-encoding genes whereas the Z. galactanivorans DsijT genome only encodes for ZgHAD, belonging to the HAD1 phylogenetic clade. The surrounding genes of the two types of L-2-HAD-encoded genes were also very different (Supplementary Figure 2), in agreement with their distinct phylogenetic clades. The HAD1-type homologous genes were present in the genome of all the six Zobellia strains as well as in those of Leeuwenhoekiella, Gramella and Zeaxanthinibacter (Figure 1B). The genomes of other genera (Maribacter, Cellulophaga, Algibacter and Non-labens) featured one or two HAD2-type homologous genes, but never a HAD1-type gene. Furthermore, this comparative genome study showed that some genera of Flavobacteriaceae like Tenacibaculum or Muricauda had no L-2-HAD-encoding gene.
Production, Purification and Enzymatic Characterization of the Recombinant ZgHAD
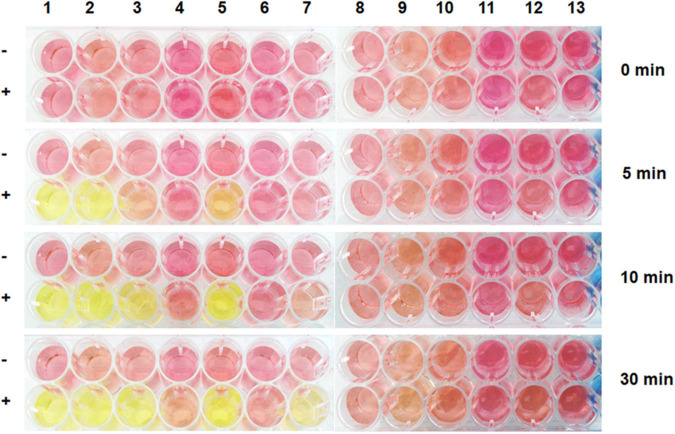
To characterize the biochemical activity of ZgHAD, the recombinant ZgHAD enzyme was produced in Escherichia coli and purified to homogeneity (Supplementary Figure 3), yielding 300 mg L–1 culture. SDS-PAGE confirmed the purity and correct size of the protein that appeared as a unique band at the expected 26.3 kDa molecular mass (Supplementary Figure 3). Based on size exclusion chromatography, the protein eluted as a homodimer in solution. A standard colorimetric assay was used to first determine the substrate specificity of the recombinant ZgHAD. Thirteen substrates with different carbon chain lengths (C2 to C4) and halogen atom nature, position and number were tested (Figure 2). Within 30 min, the recombinant ZgHAD showed activity only toward L-enantiomer substrates with short carbon chains (C2 and C3). The enzyme was active on substrates that were monoiodinated, monobrominated or monochlorinated on the α-carbon position, namely iodoacetic acid (IAA), bromoacetic acid (BAA), chloroacetic acid (CAA), L-2-bromopropionic acid and L-2-chloropropionic acid (Figure 2). The fastest activity was observed with IAA and BAA, leading to an acidic yellow coloration within 5 min. The chloroacetic acid (CAA) and the L-2-bromopropionic acid were turned partially into their corresponding alcohols at the same time, giving an orange coloration. They were totally transformed between 10 and 30 min (Figure 2). The conversion of the L-2-chloropropionic acid was achieved after more than 30 min. Longer incubations up to 16 h did not show activity on the eight other substrates (data not shown), confirming that the D-2-haloacids, multi-chlorinated or brominated acetic acids, and the halobutyric acids cannot be processed by the recombinant ZgHAD. To further compare the catalytic properties on iodinated and brominated compounds, IAA and BAA were used as substrates to calculate kinetic parameters of ZgHAD. The Vmax and Km value with IAA were 1.12 μM s–1 and 0.31 mM, respectively and with BAA they were 1.70 μM s–1 and 0.46 mM, respectively.
FIGURE 2.
Screening of ZgHAD specificity toward different halogenated substrates based on colorimetric assays. Pictures were taken after 0, 5, 10 and 30 min of enzymatic reaction. Substrates are: (1) iodoacetic acid; (2) bromoacetic acid; (3) chloroacetic acid; (4) D-2-bromopropionic acid; (5) L-2-bromopropionic acid; (6) D-2-chloropropionic acid; (7) L-2-chloropropionic acid; (8) trichloroacetic acid; (9) 3-iodopropionic acid; (10) 3-bromopropionic acid; (11) 2-chlorobutyric acid; (12) iodoacetamide; (13) dibromoacetic acid; (-) no enzyme; (+) with enzyme.
Haloacetic Acid Effects on the Growth of Different Bacterial Strains
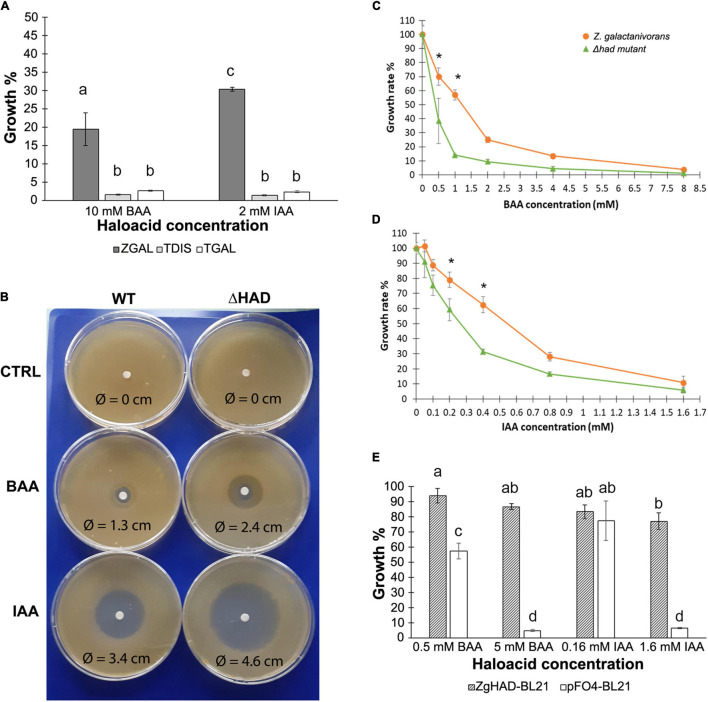
The growth of Z. galactanivorans in the presence of BAA and IAA was compared to that of two Tenacibaculum bacterial species (T. discolor and T. gallaicum), which are phylogenetically closely-related to Z. galactanivorans (85.7 and 86.35% 16S rRNA identity respectively) but do not possess any L-2-HAD homolog gene. Bacterial cultures were grown in liquid ZoBell medium with the highest concentrations of BAA (10 mM) or IAA (2 mM) tolerated by Z. galactanivorans. After 16 h, cell densities of both Tenacibaculum strains were <5% in presence of BAA and IAA compared to unamended ZoBell medium. By contrast, the cell densities of Z. galactanivorans WT were significantly higher, and still 20% with BAA and 30% with IAA compared to the unamended ZoBell condition (Figure 3A). This increased tolerance to BAA and IAA in Z. galactanivorans compared to the HAD-devoid Tenacibaculum strains suggested a potential role of ZgHAD for haloacid metabolism.
FIGURE 3.
Comparison of bacterial growth of different marine strains, Z. galactanivorans HAD knockout mutant and E. coli recombinant clones in presence of haloacetic acids (A) Growth percentage of Tenacibaculum strains (T. discolor: TDIS and T. gallaicum: TGAL) compared to Z. galactanivorans wild type (ZGAL) in liquid cultures supplemented with 10 mM bromoacetic acid (BAA) and 2 mM iodoacetic acid (IAA) (n = 3). (B) Z. galactanivorans WT and Δhad growth on solid medium with BAA and IAA solutions deposited on a filter at the center of the plate. The diameter of inhibition around the disk was measured for each plate after 3 days of incubation at 20°C. CTRL: negative control (see also Supplementary Figure 4). (C,D) Growth rates of Z. galactanivorans wild-type and Δhad mutant in liquid cultures with different concentrations of BAA (C) and IAA (D). Z. galactanivorans (orange line, round dots) and Δhad mutant (green line, triangle dots) (n = 3). (E) Growth percentage in liquid cultures supplemented with either 0.5 or 5 mM BAA and 0.16 or 1.6 mM of IAA compared to the unamended medium (100%) of the ZgHAD-expressing recombinant E. coli strain (ZgHAD-BL21) and the E. coli control strain (pFO4-BL21) (n = 3). Letters and stars indicate statistically significant differences between treatments (A,E) and between WT and Δhad mutant strains (C,D) for the same culture condition (two-way ANOVA, Tukey’s post hoc tests, p < 0.05).
To test this hypothesis, we investigated the effect of the zghad gene deletion on the Z. galactanivorans tolerance to haloacids. On solid medium, BAA or IAA induced clear growth inhibition zones for the wild-type strain (WT), with apparent diameters of 1.3 and 3.4 cm, respectively (Figure 3B). No inhibition zone was observed with the buffer alone. Compared to the WT, the inhibition zones observed for the zghad-deleted strain, Δhad, were larger, showing an increase of 85% for the BAA (apparent diameter 2.4 cm) and 35% for the IAA (4.6 cm). Consequently, the deletion effect appeared to be more pronounced with BAA than IAA. To confirm these qualitative observations, suggesting a more pronounced had deletion effect with BAA than IAA, we compared the growth of the WT and Δhad strains in liquid ZoBell medium supplemented with increasing concentrations of BAA or IAA, until reaching the upper toxic limit. For both halogenated compounds, all the culture conditions showed an apparent reduced growth rate for the Δhad mutant compared to the WT but only two concentrations are statistically significative (0.5 and 1 mM BAA; 0.2 and 0.4 mM IAA, respectively, in Figures 3C,D). The most significant effect of the zghad deletion was obtained with 1 mM BAA where the growth of the WT strain was still 60% and that of the Δhad strain was only 10% compared to the control culture conditions without haloacid. Above 2 mM of BAA, the growth rate of the WT strain dropped at 25%, and reached 15% for 4 mM of BAA, whereas that of the Δhad strain remained under 10% of the corresponding control growth rate. The growth rates of both WT and Δhad strains were very low after incubation with 8 mM of BAA. More than 2 mM of IAA was lethal for both strains. Similarly, upon IAA incubation, the WT strain growth was significantly higher than the Δhad mutant between 0.2 and 0.4 mM of IAA (Figure 3D).
In order to further test the role of ZgHAD in haloacid tolerance, the recombinant E. coli expression strain (ZgHAD-BL21) was grown in parallel with a control E. coli strain transformed with an empty pFO4 plasmid (pFO4-BL21) (Figure 3E). The control pFO4-BL21 strain did not grow with the upper BAA and IAA concentrations (4.9 and 6.4% relative growth compared to that of an unamended LB medium) while the ZgHAD-BL21 strain was able to reach 86 and 77%, respectively. The pFO4-BL21 strain was also significantly inhibited in the presence of 0.5 mM of BAA with 57% relative growth, compared to the ZgHAD-pFO4 strain, which showed no difference compared to the control (94% relative growth).
Distribution of the Two L-2-HAD Major Phylogenetic Groups in the Environment
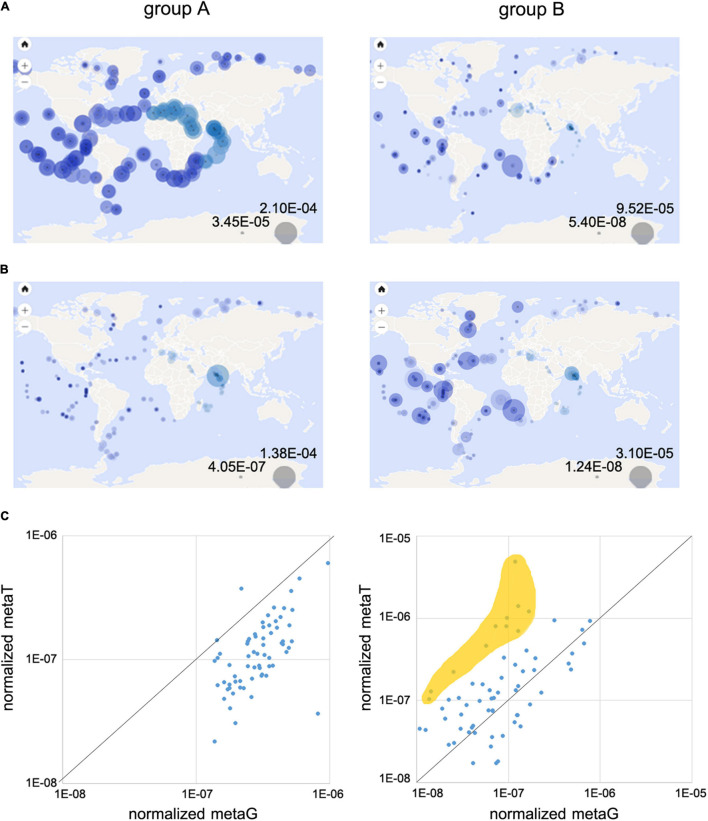
Our phylogenetic analysis suggested that the two different phylogenetic groups A and B, showing different conserved amino-acid signatures, could be differently distributed in the terrestrial and marine environments (Figure 1). To test this hypothesis, we investigated (i) the overall metagenomic abundance distribution of L-2-HAD homologs in contrasted ecosystems using the IMG/MER database (IMG/MER) and (ii) the specific marine distribution of the L-2-HADs through their gene number and gene expression using the Ocean Gene Atlas database (OGA). Among the 25 analyzed ecosystems corresponding to 2,500 metagenomes of IMG/MER (Supplementary Table 3), a total of 4,265 hits were found. The group A L-2-HAD sequences, represented by the DehIVa homologs, were ten times more frequent (3,800 hits) than the group B L-2-HAD sequences (365 hits), represented by the ZgHAD homologs. This preponderance of group A L-2-HADs was verified for all the five major ecosystems of terrestrial, aquatic non-marine and marine habitats with 75–95% of the overall hit identification represented by the group A L-2-HAD sequences (Figure 4A). Nevertheless, the environmental distribution differed in the five datasets (Figures 4B,C). The group A L-2-HAD hits, were almost equally distributed between the non-marine and marine fractions (43.9 and 56%, Figure 4B). By contrast, the majority of the group B hits originated from marine biotopes (72.6% altogether, Figure 4C). As shown in Supplementary Table 3, the group A L-2-HADs are encountered mostly in marine intertidal (764 hits) and coastal (554 hits) zones and in forest soils (560 hits). Moreover, they seemed to be more present in free-living environments (1,768 sequences representing 46.5% and 1,195 sequences representing 31.4% for marine and terrestrial environments, respectively) than in host-associations (9.5 and 7.8% for marine and terrestrial hosts only). By contrast, the group B L-2-HADs were found in bacteria closely linked to marine hosts (28.8% of all the group B hits) compared to terrestrial hosts (6% of hits, Figure 4C). Furthermore, they were particularly found in association with marine macroalgae (76 hits, representing 72.6% of the marine host-associated subset and 20.8% of all the group B hits). Other characteristics of this group B L-2-HADs revealed that these sequences are also principally present in the marine coastal and in the non-marine saline/alkaline environments (51 and 39 hits respectively, 31.9 and 90.7% of their subset, 14 and 10.7% of all the group B hits) (Supplementary Table 3).
FIGURE 4.
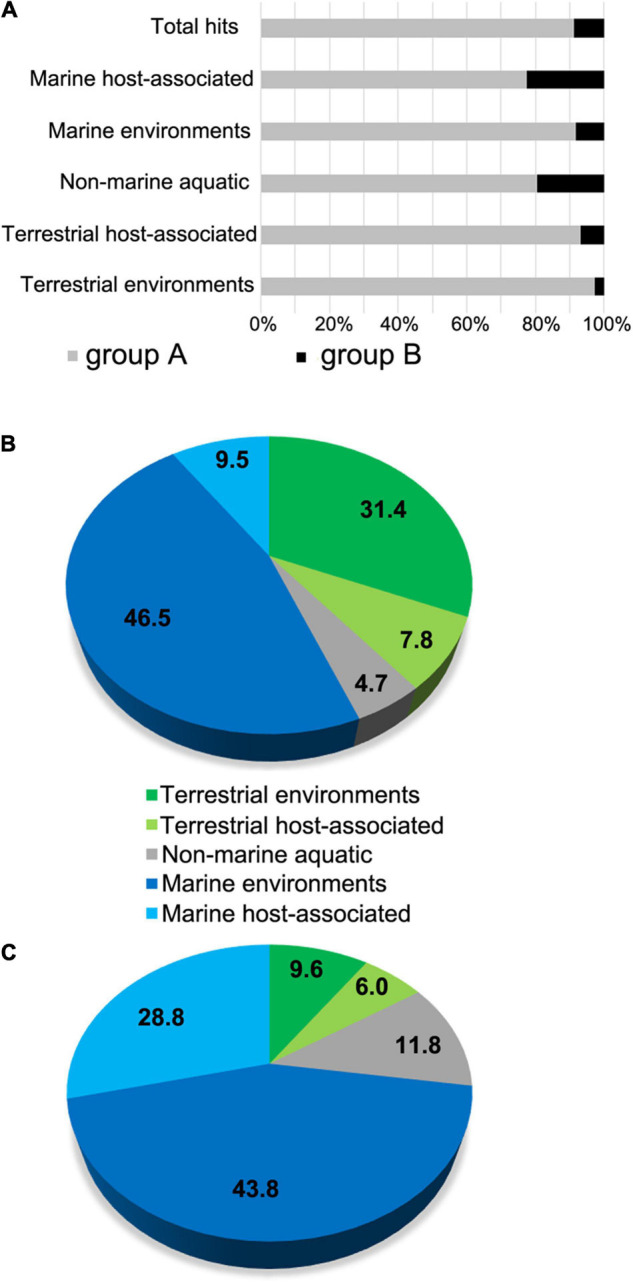
Analysis of environmental distributions of L-2-HAD homologs into IMG/MER metagenome database. (A) Global abundance representation of the DehIVa homologs (group A) and ZgHAD homologs (group B) among L-2-HADs hit number in the full set of IMG/MER metagenomes. (B,C) Distributions of the group A hits (B) and the group B hits (C) sequence hits among five IMG/MER ecosystem subsets.
The distribution of the group A and group B L-2-HADs was assessed more precisely in marine environment using Ocean Gene Atlas (OGA, Supplementary Table 4). Interestingly, a similar order of magnitude of the group A sequences was obtained when the marine DehPsi and DehDEH99 proteins were used as queries (166 and 214 hits) while a highest number of hits (about 3–7 fold) was retrieved using the 15 terrestrial sequences. The three selected L-2-HAD protein sequences from the phylogenetic group B (ZgHAD, DehRhb and WP_038235908) allowed to retrieve 204–234 hits (Supplementary Table 4), which all feature the conserved amino acid consensus DETF(S/T)KYAHD. The group A hits were detected in the metagenomes of 66 stations worldwide with an overall gene abundance of at least 10–5 (Figure 5A). The group A homologs were more abundant in temperate and tropical zones than in arctic and subarctic zones. By comparison, the group B hits were detected in the metagenomes of 64 stations also well-distributed around the globe but with lower abundances (Figure 5B). We further evaluated the expression of these L-2-HAD homologs by searching hits in the OGA metatranscriptomes. Overall, the representation of group A sequences in metatranscriptomes was low compared to their corresponding detection in metagenomes. The scatterplot of the normalized abundances in metatranscriptomes vs. metagenomes showed that all but one station fell below the 1:1 line, confirming a comparatively low expression of the group A L-2-HAD-encoding genes in most oceanic regions (Figure 5A). Conversely, the metatranscriptomic abundances of group B L-2-HADs were more heterogeneous, and often higher than the corresponding metagenomic abundances for Atlantic and Pacific stations (Figure 5B). The scatterplot demonstrated that the ratio of the group B L-2-HAD-encoding genes abundance in metatranscriptomes vs. metagenomes was ≥1 for 75% of the 64 stations, suggesting a consistent gene expression. In particular, the ratios of 11 stations fell well above the 1:1 line (orange colored zone, Figure 5C), with a metatranscriptome abundance exceeding 5–40 fold that in metagenome. The sample exhibiting the highest expression level for the group B (MetaT/MetaG ratio >40) was located at the station 137 (sample TARA_B100001964, TARA_137_DCM) in the Pacific Ocean at about 500 km off the west coast of Mexico. It corresponds to water collected during late autumn at 40 m depth in the deep chlorophyll maximum layer. The temperature of the water was 20.5°C and pigment detection indicated that chlorophyll a, fucoxanthin, lutein and zeaxanthin were present in the sample. The exploration of individual results for all group B L-2-HAD hits found in this particular sample suggests that mainly two homolog genes (corresponding to OM-RGC.v2.011753063 and OM-RGC.v2.014543634) are highly expressed. Both hits were affiliated to the gammaproteobacterium Alteromonas australica.
FIGURE 5.
Analysis of oceanic distribution of L-2-HADs into OGA databases. Geographic distribution of relative abundances of group A and group B homologs into metagenomic (A) and metatranscriptomic (B) OGA databases. Global abundance scales are represented by the two gray circles at the bottom right of the maps. (C) Scatterplots of the normalized gene abundance (normalized metaG) vs. normalized transcript abundance (normalized metaT) for each sampling station.
Discussion
A bacterial protein named ZgHAD was identified based on its amino acid sequence as the sole L-2-HAD coded in the genome of Z. galactanivorans DsijT, a marine bacterium belonging to the Bacteroidetes phylum. Although L-2-HAD protein homologs are distributed in numerous bacteria and archaea, only four enzymes have been previously described at the biochemical level from marine bacteria, and little was known about their biological functions, potential ecological role(s) and distribution in marine environment.
The biochemical properties of this newly characterized marine L-2-HAD were compared to other previously characterized L-2-HADs, mostly in Proteobacteria. The phylogenetic analysis revealed a poor conservation of amino acid sequences between L-2-HADs from group A and group B. Differences of the two amino acid patterns related to active sites suggested divergent protein folding and biochemical properties. ZgHAD exhibited highest activity toward C2 and C3 carbon-chain substrates with a preference for bromo and iodoacetate, similarly to DehRhb (Novak et al., 2013a), DehIVa (Schmidberger et al., 2007), DehPsi (Novak et al., 2013b) and H-2 (Kawasaki et al., 1981), which used BAA as best substrate. However, ZgHAD did not show any activity toward substrates with a longer carbon-chain or dihalogenated acid substrates, contrasting with other L-2-HADs like DehSft (Rye et al., 2009), DhlB (Ridder et al., 1997), and DehPsi (Novak et al., 2013b) from the phylogenetic group A, suggesting some enzymatic specificities of the marine L-2 HADs belonging to the phylogenetic group B. The Km value of ZgHAD for BAA was 0.46 mM, whereas it varied from 1.1 to 6.72 mM for other L-2-HADs. Notably its closest relative DehRhb showed a higher Km value of 6.72 mM (Novak et al., 2013a) revealing a lower affinity for BAA compared to ZgHAD. The Km of ZgHAD for IAA was lower (0.31 mM) compared to BAA suggesting a higher affinity of ZgHAD for the iodinated substrate. IAA has been previously shown to be a substrate for several L-2-HADs of the phylogenetic group A like DEH99 (Zhang et al., 2014), L-DEX (Liu et al., 1994), DhlS5I (Köhler et al., 1998), and H-2 (Kawasaki et al., 1981), but not for all as shown for DEH130 of Pseudomonas stutzeri, isolated from the marine sponge Hymeniacidon perlevis (Zhang et al., 2013). However, it is the first time that IAA was reported to be a better substrate than other haloacids for a L-2-HAD. Previous assays with the characterized L-2-HADs did not always include iodinated substrates. As iodide is a better leaving group than bromide or chloride, it is likely that other L-2-HAD enzymes are also active on IAA and therefore on both brominated and iodinated short carbon chains, especially those from marine origin, as these halocarbons are predominant in the marine environment.
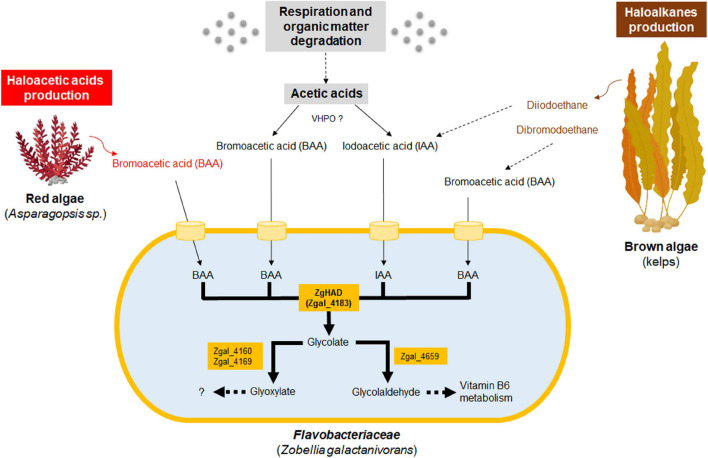
Zobellia galactanivorans was able to grow in the presence of higher concentrations of BAA and IAA than its Δhad knockout mutant and both fish pathogens T. discolor and T. gallaicum. The strong growth inhibition induced by BAA and IAA in those strains lacking a L-2-HAD gene suggested it could confer a selective advantage to Z. galactanivorans in the presence of toxic short haloacetic acids. This potential benefit was in agreement with the improved tolerance of E. coli cells to these haloacids, only when expressing ZgHAD. As ZgHAD enzyme was produced inside E. coli cells (in the cytoplasm as also predicted for ZgHAD in Z. galactanivorans), this result suggested that bacteria could import BAA and IAA into their cytoplasm for detoxification. Soil bacteria such as Burkholderia cepacia MBA4 or Xanthobacter autotrophicus GJ10 have been studied for their capacity to grow on and metabolize haloacid compounds. They possess the genetic tools to internalize and degrade a large range of organic halogenated toxic compounds (Kwok et al., 2007; Mena-Benitez et al., 2008; Horisaki et al., 2011). In B. cepacia MBA4, the dehalogenase gene dehIVa and the downstream permease gene dehIVp were shown to form an inducible operon that mediates the transformation and uptake of halogenated acids (Yu et al., 2007). No clear haloacid operon, permease gene or equivalent transport system were identified within Z. galactanivorans genome. Nevertheless, the detoxification of haloacetic acids was efficient in Z. galactanivorans suggesting that an unidentified transport system might exist. L-2-HADs potentially convert haloacetic acids to glycolate through dehalogenation. Z. galactanivorans genome encodes predicted enzymes for downstream glycolate catabolism (Zgal_4160/Zgal_4169 and Zgal_4659) as shown in its KEGG metabolic network and schematized in Figure 6. This putative pathway might link BAA/IAA catabolism either to the glyoxylate cycle or to the vitamin B6 metabolism via glycolaldehyde (Figure 6). The Δhad mutant of Z. galactanivorans resisted better to haloacetic acids than the Tenacibaculum strains lacking L-2-HAD. Other unidentified genes could therefore be involved in the resistance against haloacetic acids in Z. galactanivorans, similarly to what has been demonstrated for the BAA resistance in E. coli (Desai and Miller, 2010). Altogether, these functional studies and gene mining in Z. galactanivorans suggested a complete degradation pathway of short carbon chain haloacids, downstream of ZgHAD activity (Figure 6). Future transcriptome analysis of WT and Δhad Z. galactanivorans in the presence of BAA or IAA could help elucidate the metabolic processes involved in intracellular haloacid catabolism.
FIGURE 6.
Hypothetical scheme of production and biotransformation of haloacetates by Z. galactanivorans, in macroalgae-dominated coastal habitats. ZgHAD and Zgal_4183, L-2-Haloacid dehalogenase of Z. galactanivorans; BAA, bromoacetic acid; IAA, iodoacetic acid; Zgal_4160, glyoxylate/hydroxypyruvate reductase; Zgal_4169, glyoxylate reductase; Zgal_4659, glycolaldehyde dehydrogenase; VHPO, vanadium-haloperoxidases. Dotted arrows refer to multistep enzymatic transformations. Oceanic biomass, red algal and brown algal transformation and productions are, respectively, colored in gray, red and brown. Specific toxic compounds produced by red and brown algae are also in red and brown fonts. Orange cylinder models the putative BAA and IAA transporters.
All the available Zobellia isolates have one or two copies of L-2-HAD-encoding genes in their genome, a trait that might be a selective advantage linked to the presence of short haloacetic acids in their natural environment. Indeed, coastal ecosystems could be the production source of toxic haloacids through various natural processes (Figure 6). First, low-molecular-weight organic acids such as acetic acids are ubiquitous in seawater, produced either via respiration, microbial degradation of lipids or photochemical breakdown of dissolved organic matter (Liang et al., 2020). They might be converted to the corresponding halogenated acetic acids abiotically or biotically, as reported previously in terrestrial environments. For example, the decomposition of forest soil organic matter leads to the production of CAA (Matucha et al., 2003; Laturnus et al., 2005), potentially due to the action of fungal and bacterial chloroperoxidases (Wever and Barnett, 2017). In line with this, we hypothesize that the combination of vanadium-haloperoxidases and L-2-HADs could also contribute to the recycling of acetic acids dissolved in seawater through the production of BAA and IAA intermediates (Figure 6). Second, brominated or iodinated compounds are produced by some marine bacteria, and also by a range of marine macro-organisms living in close association with them (e.g., corals, sponges, macroalgae) (Gribble, 2010). In particular, marine macroalgae are known to release organic brominated and iodinated toxic compounds in the seawater (Belkin, 1992; Paul et al., 2006) and also haloacetic acids such as reported for the red alga Asparagopsis taxiformis (Woolard et al., 1979; Fenical, 1982). Moreover, haloalkanes like diiodoethane produced by kelps during biotic interactions could be sequentially transformed into haloacetic acids similarly to the dichloroethane degradation found in Xanthobacter autotrophicus GJ10 (Van der Ploeg et al., 1991). Since all known Zobellia species live with or close to macroalgae, including red algae such as Delesseria sanguinea (Barbeyron et al., 2001) and brown algae such as Fucales and Laminariales (Nedashkovskaya et al., 2004; Martin et al., 2015), it is likely that they regularly experience the release of haloacetic acids or haloalkanes (Figure 6). Another source of haloacetic acids in coastal seawater might also result from anthropogenic activities (e.g., agriculture, water-treatment stations). Chlorine residuals and humic acids of effluents can react with bromide-rich coastal seawaters (0.8 mM) to form BAA (Gordon et al., 2015). Similar reactions might occur for IAA formation, even if seawater contains only 0.3 μM of iodide (La Barre et al., 2010). The catabolic properties of ZgHAD toward both BAA and IAA might reflect the genetic adaptation of Zobellia strains to these halogen-rich marine ecological niches, and especially those formed by the coastal macroalgal beds (Figure 6).
As said above, the phylogenetic tree of L-2-HADs revealed two major distinct phylogenetic groups that might correlate to specific catalytic properties. They also correspond to distinct geographical and environmental distributions. The phylogenetic group A contained a majority of terrestrial but also marine sequences and the phylogenetic group B was essentially composed of marine ones. Furthermore, the group B showed a separation of two distinct Bacteroidetes L-2-HAD clades, namely HAD1 (including ZgHAD) and HAD2. Comparative genomics in the Flavobacteriaceae family showed that these two L-2-HAD types did not have the same genomic context, suggesting independent acquisition and evolution in the different strains, and a yet unexplored biochemical diversity among Bacteroidetes L-2-HAD.
This phylogenetic overview was enlarged with environmental analyses through oceanic (OGA) and multi-ecosystemic (IMG/MER) data. Group A L-2-HADs are more widespread than group B sequences, irrespective of the biotopes, being almost equally distributed between terrestrial and marine habitats. Moreover, the analyses of ocean genomic data revealed that these L-2-HADs were more frequent in the marine environment than initially thought, often exceeding group B sequence abundance. The group B L-2-HADs appeared to be less frequent but they are selectively present in marine environments, in particular in close association with macroalgae. Overall, the presence of toxic haloacids in marine environments likely drove the selection and evolution of L-2-HAD genes. Indeed, the strong prevalence of L-2-HADs in metagenomes from macroalgae or hypersaline waters suggests that these enzymes could be involved in the elimination of haloacetic acids in these particular environments. The high abundance of L-2-HAD genes in oceanic stations could reflect strong biomass production spots like microalgal blooms. Metatranscriptomic analyses of OGA data revealed that group A L-2-HADs have a homogeneously low expression level around the globe while group B expression seems to be induced only in specific zones in response to yet-unknown stimuli. For instance, the highest expression of group B L-2-HADs affiliated to the Alteromonas at station TARA_137. Chlorophyll a, fucoxanthin, lutein and zeaxanthin detection at the same point might suggest associations between these Gammaproteobacteria and microalgae.
Conclusion
Biochemical and functional characterizations of the sole L-2-HAD encoded in the genome of Z. galactanivorans (ZgHAD) have shown its key role in the catabolism of IAA and BAA in this marine Bacteroidetes. ZgHAD could confer a selective advantage to live in haloacetic acid-producing niches, such as those formed by coastal macroalgae. An extensive phylogenetic analysis has revealed the occurrence of two sister groups of L-2-HADs, namely group A and group B. When the well-represented group A contains both terrestrial and marine sequences, the group B was mainly composed of marine L-2-HADs and contained two distinct Bacteroidetes clades. The search for group A and group B L-2-HADs homologs in environmental databases further confirmed worldwide, but distinct ecological distribution and expression levels of these two L-2-HAD groups, according to geographical origins, suggesting different ecological functions. For instance, the presence of homologs of ZgHAD could be the functional signature of bacterial haloacetic acid catabolism, especially during micro- and macroalgal interactions. Our study further highlighted the abundance and diversity of marine L-2-HADs, especially of those belonging to the phylogenetic group A, but also some other Bacteroidetes L-2-HADs, which are still to be functionally characterized. All these bacterial L-2-HADs are expected to be important actors of halogenated compounds biotransformation and halogen biogeochemical cycle in open marine environments.
Materials and Methods
Chemicals
Iodoacetic acid (I4386), bromoacetic acid (17000), chloroacetic acid (C19627), D-2-bromopropionic acid (18165), L-2-bromopropionic acid (38551), D-2-chloropropionic acid (306800), L-2-chloropropionic acid (306797), trichloroacetic acid (91233), 3-iodopropionic acid (I10457), 3-bromopropionic acid (101281), 2-chlorobutyric acid (24008), iodoacetamide (I1149), and dibromoacetic acid (242357) were obtained from Merck.
Bacterial Strains and Plasmids
Bacterial strains and plasmids used in this study are listed in Supplementary Table 5.
Phylogenetic Analysis
The selected set of L-2-HAD proteins was derived from NCBI BlastP queries against the RefSeq database. Sequences were loaded into the NGPhylogeny.fr “A la carte” pipeline and analyzed as follows. A total of 352 archaeal and bacterial protein sequences were aligned using MAFFT under default parameters then cleaned with trimAl resulting in 165 informative positions over the 400 aligned positions (see Supplementary Table 2 for complete information). A maximum likelihood phylogenetic analysis was carried out using default parameters of the PhyML-SMS tool allowing the best substitution model selection. Bootstrap analysis with 100 replicates (>70%) was used to provide estimates for the phylogenetic tree topology and it resulted in a newick file formatted with the program MEGA v10.1.1 to obtain the corresponding simplified dendrogram tree figure.
The phylogenetic species tree of 10 genus clades was constructed using the comparative genomics tool for genome clustering of MaGe website. See Supplementary Table 5 for complete list of bacterial genomes.
Genomic Context Decryption
The had gene context in Flavobacteriaceae was analyzed by synteny reconstruction on the Genoscope Microscope (MaGE) platform. All the bacterial genomes containing a had gene, listed in Supplementary Table 5, were then aligned to allow the identification of the conserved neighboring genes (Supplementary Figure 2).
Environmental IMG/MER Data Analyses
L-2-HAD homologs were searched by BlastP in the environmental metagenomic IMG/MER database at the JGI website1, using DehIVa and ZgHAD sequences as the representative sequences of the phylogenetic group A and group B, respectively. A stringent e-value cutoff of 10–50 was selected to identify reliable hits of group A and group B with at least 38% amino acid identity in five datasets (Terrestrial environments, Terrestrial host-associated, Non-marine aquatic, Marine environments, Marine host-associated). Each dataset was constituted of five subsets comprising 100 random selected metagenomes (Supplementary Table 3). The BlastP results of group A and group B sequences were expressed as hit number abundances in each dataset and subset, and as overall percentages.
Oceanic Distribution and Expression in Ocean Gene Atlas Databases
BlastP searches were carried out with 20 representative L-2-HAD sequences (Supplementary Table 4) against the Ocean Gene Atlas (OGA) database2, comprising metagenomic OM-RGCv2 + G and metatranscriptomic OM-RGCv2 + T data collections from the Tara Oceans stations (Villar et al., 2018). A stringent e-value cutoff of 10–30 was selected in order to cover a majority of each phylogenetic clade (Supplementary Table 4). In a second step, we focused on the DehIVa and ZgHAD BlastP hits, respectively, as representative sequences of the phylogenetic group A and group B, to compare their geographic repartition, relative gene abundance (metaG) and relative gene expression (metaT) levels. All the data for BlastP searches can be downloaded and extracted at the website. Relative abundance values were calculated based on the sum of all the hits found at a precise site and showed on geographical maps. Relative abundance was further normalized by the number of hits in order to evaluate the average gene abundance and gene expression for every OGA stations. Normalized relative abundances of HAD homologs in metagenomes and metatranscriptomes were calculated for each station and depicted as log10 correlation plots of 66 and 64 spots, respectively, for DehIVa (group A hits) and ZgHAD (group B hits) homologs. Finally, environmental parameters were compared using the bubble plot tools available on the OGA website platform.
Gene Cloning
The ZgHAD-encoding gene sequence (zgal_4183) was cloned from the genomic DNA of Zobellia galactanivorans DsijT (Barbeyron et al., 2001), using primers Zgal_4183fw and Zgal_4183rv (Supplementary Table 6). The PCR product was ligated into the pFO4 vector, using BamHI and EcoRI restriction sites and the T4 DNA ligase protocol (New England Biolabs). The recombinant vector was transformed firstly into E. coli DH5α for sequence verification and secondly into E. coli BL21 (DE3) expression strain.
Overexpression and Purification
The recombinant E. coli strain expressing ZgHAD was grown in LB medium containing 100 μg ml–1 ampicillin at 37°C to an optical density of ∼1.0, then the temperature was lowered to 20°C for 1 h followed by a 20 h induction of protein production by the addition of 0.5 mM IPTG. After centrifugation (3,000 g, 30 min, 4°C), the bacterial pellet was stored at −20°C, then resuspended in 50 mM Tris (pH 7.5), 500 mM NaCl, bovine DNase I (500 U/μl), 0.1 mg/ml lysozyme, Complete ProteaseTM Inhibitor Cocktail (Merck) and 6 mM MgCl2 and lysed using a French press and the lysate was centrifuged at 23,000 g during 30 min and 4°C. The recombinant protein was purified by a two-step chromatography carried on an ÄKTA Avant purification system (GE Healthcare Life Sciences). The first step was performed on an immobilized nickel affinity HisTrapTM column (GE Healthcare Life Sciences) using an equilibration buffer composed of 50 mM Tris (pH 7.5), 500 mM NaCl and 50 mM imidazole and an elution buffer containing 500 mM imidazole instead of 50 mM. Proteins were eluted with a constant gradient of imidazole concentration. The pooled fractions of the elution peak were concentrated on Centriprep devices and the protein sample was injected on a gel filtration HiLoadTM 16/600 SuperdexTM 200 pg column (GE Healthcare Life Sciences). The purified protein was stored in a buffer containing 20 mM Tris (pH 8.0) and 150 mM NaCl. The purity of the recombinant protein was analyzed by SDS-PAGE (Bio-Rad Mini-PROTEAN® precast gels and systems).
Substrate Specificity and Kinetic Enzymatic Parameters Determination
The ZgHAD enzyme specificity was determined by adapting the colorimetric assay from Holloway et al., 1998. The detection was based on a pH decrease when the halogenated substrate was reduced to a hydroxyalkanoic acid. Upon dehalogenation, the release of halide ions X– decreased the pH inducing a color transition of the phenol red pH indicator (from pink to orange to yellow) that was monitored visually. The assay solution contained final concentrations of 0.3 mg ml–1 recombinant ZgHAD enzyme, 1 mM HEPES, 1 mM EDTA, 20 mM sodium sulfate and 56 μM of phenol red (pH 8.2). The assay was adapted for 48-well plates to test 13 different substrates at 10 mM (iodoacetic acid, bromoacetic acid, chloroacetic acid, D-2-bromopropionic acid, L-2-bromopropionic acid, D-2-chloropropionic acid, L-2-chloropropionic acid, trichloroacetic acid, 3-iodopropionic acid, 3-bromopropionic acid, 2-chlorobutyric acid, iodoacetamide, dibromoacetic acid) in 1 ml final volume. The plates were incubated at 20°C during 30 min then photographed and left 16 h at 20°C to check for long-term variations.
The colorimetric assay was further used for measuring enzymatic kinetic parameters toward IAA or BAA as substrates. All reactions were performed in triplicates at 20°C. Each assay was carried out in a 180 μl reaction mixture. The final concentration of purified ZgHAD protein was 0.3 mg ml–1. The kinetics of the reaction was followed by measuring the decrease of absorbance at 560 nm for 10 min on a Spark® multimode microplate reader (Tecan Group Ltd., Switzerland). A standard curve was produced by mixing the assay solution with 1 M HCl to final concentrations of 0–2 mM in a total volume of 200 μl. The Lineweaver-Burke plots were used to calculate the Km and Vmax steady-state kinetic parameters.
Deletion of ZgHAD Gene in Zobellia galactanivorans
A deletion mutant of the zghad gene was constructed following the previously described method from Zhu et al., 2017. A 2.2-kb fragment corresponding to the first 36 bp of the zghad gene with the region directly upstream was amplified using the primers OEG007 and OEG009 (Supplementary Table 6). The fragment was digested with XbaI and SalI and ligated into the pYT313 vector cut with the same enzymes to generate the vector pEG1. Similarly, a 2.3-kb fragment corresponding to the last 35 bp of the zghad gene and the region directly downstream was amplified with the primers OEG008 and OEG010 (Supplementary Table 6). The fragment was inserted between BamHI and XbaI sites of pEG1 to generate the suicide plasmid pEG3. Suicide plasmid was introduced into the wild type Z. galactanivorans DsijT by conjugation with E. coli S17-1 strain. Erythromycin resistance was used to select the cells having integrated the plasmid in their genome. Resulting colonies were grown overnight in Cytophaga medium (per liter: 1.0 g yeast extract, 1.0 g tryptone, 24.7 g/NaCl, 0.7 g KCl, 6.3 g MgSO4.7H2O, 4.6 g MgCl2.6H2O, 1.2 g CaCl2.2H2O, 0.2 g NaHCO3) without antibiotics at 30°C to allow the loss of the plasmid. The cells were then plated onto Cytophaga-agar containing 5% sucrose. The sucrose-resistant colonies were checked for erythromycin sensitivity. The deletion of the zghad gene was confirmed by PCR using primer pairs OEG011-OEG012 (Supplementary Table 6) and then verified by Sanger sequencing at the GENOMER platform (FR2424; Roscoff Biological Station).
Growth Inhibition Analyses of Marine Flavobacteriaceae and Phenotyping of the Zobellia galactanivorans Δhad Mutant
The ability to grow and resist to increasing haloacid concentrations was tested using an antibiogram-like method. Z. galactanivorans wild type (WT) and its deleted had gene mutant (Δhad) were grown on ZoBell 2216-agar plates with a Whatman paper disk at the center. The paper was impregnated with 25 μl solution of either 500 mM BAA or IAA (shown to be in vitro substrates of ZgHAD) or Tris-glycine 100 mM pH 8.0 as negative control. The haloacid effect was determined by monitoring the size of the inhibition zone after 72 h at 20°C. This experiment was realized two times independently.
Zobellia galactanivorans WT and both Tenacibaculum species (T. discolor DSM 18842T and T. gallaicum DSM 18841T) were grown in 3 ml ZoBell 2216 liquid medium with high concentrations of BAA and IAA (2 mM) buffered by 20 mM HEPES buffer pH 7.0 and during 16 h at 20°C. Each medium was initially inoculated at 1/100 dilution with a two-day preculture. Z. galactanivorans WT strain and the mutant Δhad strain were also grown in ZoBell liquid medium in the same conditions with different concentration ranges of IAA or BAA dissolved in 200 mM HEPES buffer pH 7.0. The final concentrations were defined from 0 to 1.6 mM and 0 to 8 mM, respectively, for IAA and BAA. Each concentration was tested in biological triplicates. Bacterial growth was measured as the mean of OD600 in technical duplicates. Growth rates were calculated from the slopes of the growth curves between 15 and 20 h, during the exponential phase. They were expressed as a percentage of the standard growth rate in ZoBell medium for each corresponding strain.
Phenotyping of Recombinant Escherichia coli Tolerance to Bromoacetic Acid and Iodoacetic Acid
The E. coli BL21 expression strains containing the pFO4 vector alone (pFO4-BL21 strain) or recombined with the zghad gene (ZgHAD-BL21 strain) were grown in LB medium containing 100 μg ml–1 ampicillin and IPTG overnight at 20°C. These precultures were used to inoculate a fresh LB/Ampicillin medium supplemented with 0.5 mM IPTG at 1/100 dilution. Two concentrations distant of 10 folds were tested in triplicates as follows, 0.5 and 5 mM for BAA and 0.16 and 1.6 mM for IAA. After 15 h of incubation at 20°C, the final OD600 was measured and reported to that of an equivalent culture in standard conditions, without haloacids. Growth was expressed as a percentage of the corresponding culture in standard LB/ampicillin medium without addition of haloacid.
Statistical Analyses
Growth rate values obtained under the different experiments were analyzed using two-way analysis of variance (two-way ANOVA p < 0.05). Mean comparisons between conditions and treatments were made using multiple comparisons by Tukey’s post hoc tests at p < 0.05 using the PAST software version 4.06b (Hammer et al., 2001).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
EG and LD conceived and performed the experiments. EG contributed to the majority of the experiments, data collection, and analyses. AG performed the zghad gene cloning and the preliminary expression results. EG wrote the manuscript with the support from LD, FT, and CL. FT contributed in the zghad gene deletion experiments, IMG/MER experimental design and general discussion. All authors corrected and approved of the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Tristan Barbeyron for giving us access to Tenacibaculum strains.
Footnotes
Funding
This work benefited from the support of the French Government via the National Research Agency investment expenditure program IDEALG (ANR-10-BTBR-04) and via the Centre National de la Recherche Scientifique (CNRS). The Ph. D. project of EG was also supported by Region Bretagne (ARED 2017, project MHALIN).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.725997/full#supplementary-material
References
- Adamu A., Wahab R. A., Huyop F. (2016). l-2-Haloacid dehalogenase (DehL) from Rhizobium sp. RC1. Springerplus 5:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal V., Miles Z., Winter J., Eustaquio A., El Gamal A., Moore B. (2017). Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem. Rev. 117 5619–5674. 10.1021/acs.chemrev.6b00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsler C. D. (2008). Algal Chemical Ecology. Berlin: Springer. [Google Scholar]
- Ang T. F., Maiangwa J., Bakar Salleh A., Normi Y., Leow T. C. (2018). Dehalogenases: from improved performance to potential microbial dehalogenation applications. Molecules 23 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeyron T., L’Haridon S., Corre E., Kloareg B., Potin P. (2001). Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 51 985–997. 10.1099/00207713-51-3-985 [DOI] [PubMed] [Google Scholar]
- Barbeyron T., Thomas F., Barbe V., Teeling H., Schenowitz C., Dossat C., et al. (2016). Habitat and taxon as driving forces of carbohydrate catabolism in marine heterotrophic bacteria: example of the model algae-associated bacterium Zobellia galactanivorans DsijT. Environ. Microbiol. 18 4610–4627. 10.1111/1462-2920.13584 [DOI] [PubMed] [Google Scholar]
- Belkin S. (1992). Biodegradation of haloalkanes. Biodegradation 3 299–313. 10.1007/bf00129090 [DOI] [Google Scholar]
- Bialonska D., Zjawiony J. K. (2009). Aplysinopsins – marine indole alkaloids: chemistry, bioactivity and ecological significance. Mar. Drugs 7 166–183. 10.3390/md7020166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs A. M., Allen K. N., Dunaway-Mariano D., Aravind L. (2006). Evolutionary Genomics of the HAD Superfamily: understanding the Structural Adaptations and Catalytic Diversity in a Superfamily of Phosphoesterases and Allied Enzymes. J. Mol. Biol. 361 1003–1034. 10.1016/j.jmb.2006.06.049 [DOI] [PubMed] [Google Scholar]
- Cabrita M. T., Vale C., Rauter A. P. (2010). Halogenated compounds from marine algae. Mar. Drugs 8 2301–2317. 10.3390/md8082301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L. J., Archer S. D., Beale R. (2012). Ocean-atmosphere trace gas exchange. Chem. Soc. Rev. 41 6473–6506. 10.1039/c2cs35121h [DOI] [PubMed] [Google Scholar]
- Chernysheva N., Bystritskaya E., Stenkova A., Golovkin I., Nedashkovskaya O., Isaeva M. (2019). Comparative genomics and CAZyme genome repertoires of marine Zobellia amurskyensis KMM 3526T and Zobellia laminariae KMM 3676T. Mar. Drugs 17:661. 10.3390/md17120661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley S. D. (1997). Diverse mechanistic approaches to difficult chemical transformations: microbial dehalogenation of chlorinated aromatic compounds. Chem. Biol. 4 169–174. 10.1016/s1074-5521(97)90285-4 [DOI] [PubMed] [Google Scholar]
- Copley S. D. (1998). Microbial dehalogenases: enzymes recruited to convert xenobiotic substrates. Curr. Opin. Chem. Biol. 2 613–617. 10.1016/s1367-5931(98)80092-6 [DOI] [PubMed] [Google Scholar]
- Desai K. K., Miller B. G. (2010). Recruitment of genes and enzymes conferring resistance to the non-natural toxin bromoacetate. Proc. Natl. Acad. Sci. U. S. A. 107 17968–17973. 10.1073/pnas.1007559107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Peng W., Liu Y., Wang Z. (2021). Photochemical origin of reactive radicals and halogenated organic substances in natural waters: a review. J. Hazard Mater. 401:123884. 10.1016/j.jhazmat.2020.123884 [DOI] [PubMed] [Google Scholar]
- El-Demerdash A., Atanasov A., Horbanczuk O., Tammam M., Abdel-Mogib M., Hooper J., et al. (2019). Chemical diversity and biological activities of marine sponges of the genus Suberea: a systematic review. Mar. Drugs 17:115. 10.3390/md17020115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenical W. (1982). Natural products chemistry in the marine environment. Science 215 923–928. [DOI] [PubMed] [Google Scholar]
- Fournier J.-B., Rebuffet E., Delage L., Grijol R., Mesclet-Cladière L., Rzonca J., et al. (2014). The vanadium iodoperoxidase from the marine Flavobacteriaceae species Zobellia galactanivorans reveals novel molecular and evolutionary features of halide specificity in the vanadium haloperoxidase enzyme family. Appl. Environ. Microbiol. 80 7561–7573. 10.1128/aem.02430-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. R., Richardson T. L., Pinckney J. L. (2015). Ecotoxicology of bromoacetic acid on estuarine phytoplankton. Environ. Pollut. 206 369–375. 10.1016/j.envpol.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Gribble. (2003). The diversity of naturally produced organohalogens. Chemosphere 52 289–297. 10.1016/s0045-6535(03)00207-8 [DOI] [PubMed] [Google Scholar]
- Gribble G. W. (2010). Naturally Occurring Organohalogen Compounds - A Comprehensive Update. Berlin: Springer. [Google Scholar]
- Gribble G. W. (2015). A recent survey of naturally occurring organohalogen compounds. Environ. Chem. 12 396–405. 10.1071/en15002 [DOI] [Google Scholar]
- Hammer O., Harper D. A. T., Ryan P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Paleontol. Electron. 4:9. [Google Scholar]
- Hardman D. J., Slater J. H. (1981). Dehalogenases in soil bacteria. J. Gen. Microbiol. 123 117–128. 10.1099/00221287-123-1-117 [DOI] [Google Scholar]
- Hill K. E., Marchesi J. R., Weightman A. J. (1999). Investigation of two evolutionarily unrelated halocarboxylic acid dehalogenase gene families. J. Bacteriol. 181 2535–2547. 10.1128/jb.181.8.2535-2547.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P., Trevors J. T., Lee H. (1998). A colorimetric assay for detecting haloalkane dehalogenase activity. J. Microbiol. Methods 32 31–36. 10.1016/s0167-7012(98)00008-6 [DOI] [Google Scholar]
- Horisaki T., Yoshida E., Sumiya K., Takemura T., Yamane H., Nojiri H. (2011). Isolation and characterization of monochloroacetic acid-degrading bacteria. J. Gen. Appl. Microbiol. 57 277–284. 10.2323/jgam.57.277 [DOI] [PubMed] [Google Scholar]
- Huang J., Xin Y., Zhang W. (2011). Isolation, characterization and identification of a Paracoccus sp. 2-haloacid-degrading bacterium from the marine sponge Hymeniacidon perlevis. J. Basic Microbiol. 51 318–324. 10.1002/jobm.201000205 [DOI] [PubMed] [Google Scholar]
- Janssen D., Dinkla I., Poelarends G., Terpstra P. (2005). Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities. Environ. Microbiol. 7 1868–1882. 10.1111/j.1462-2920.2005.00966.x [DOI] [PubMed] [Google Scholar]
- Janssen D., Pries F., Van der Ploeg J. (1994). Genetics and biochemistry of dehalogenating enzymes. Annu. Rev. Microbiol. 48 163–191. 10.1146/annurev.mi.48.100194.001115 [DOI] [PubMed] [Google Scholar]
- Jordan A., Harnisch J., Borchers R., Le Guern F., Shinohara H. (2000). Volcanogenic Halocarbons. Environ. Sci. Technol. 34:1122–1124. 10.1021/es990838q [DOI] [Google Scholar]
- Kawasaki H., Yahara H., Tonomura K. (1981). Isolation and Characterization of Plasmid pUOl Mediating Dehalogenation of Haloacetate and Mercury Resistance in Moraxella sp. B. Agric. Biol. Chem. 45 1477–1481. 10.1080/00021369.1981.10864721 [DOI] [Google Scholar]
- Köhler R., Brokamp A., Schwarze R., Reiting R. H., Schmidt F. R. (1998). Characteristics and DNA-sequence of a cryptic haloalkanoic acid dehalogenase from Agrobacterium tumefaciens RS5. Curr. Microbiol. 36 96–101. 10.1007/s002849900286 [DOI] [PubMed] [Google Scholar]
- Kunka A., Damborsky J., Prokop Z. (2018). Haloalkane Dehalogenases From Marine Organisms. Methods Enzymol. 605 203–251. 10.1016/bs.mie.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Kurihara T., Esaki N. (2008). Bacterial hydrolytic dehalogenases and related enzymes: occurrences, reaction mechanisms, and applications. Chem. Rec. 8 67–74. 10.1002/tcr.20141 [DOI] [PubMed] [Google Scholar]
- Kwok S.-Y., Siu A., Ngai S.-M., Che C.-M., Tsang J. (2007). Proteomic analysis of Burkholderia cepacia MBA4 in the degradation of monochloroacetate. Proteomics 7 1107–1116. 10.1002/pmic.200600660 [DOI] [PubMed] [Google Scholar]
- La Barre S., Potin P., Leblanc C., Delage L. (2010). The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs 8 988–1010. 10.3390/md8040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laturnus F., Fahimi I., Gryndel M., Hartmann A., Heal M. R., Matucha M., et al. (2005). Natural Formation and Degradation of Chloroacetic Acids and Volatile Organochlorines in Forest Soil. Environ. Sci. Pollut. Res. 12 233–244. 10.1065/espr2005.06.262 [DOI] [PubMed] [Google Scholar]
- Li J., Wang N. (2011). Genome-wide mutagenesis of Xanthomonas axonopodis pv. citri reveals novel genetic determinants and regulation mechanisms of biofilm formation. PLoS One 6:e21804. 10.1371/journal.pone.0021804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Lyu L.-N., Sun C., Ding H., Wurgaft E., Yang G.-P. (2020). Low-molecular-weight organic acids as important factors impacting seawater acidification: a case study in the Jiaozhou Bay, China. Sci. Total Environ. 727:138458. 10.1016/j.scitotenv.2020.138458 [DOI] [PubMed] [Google Scholar]
- Liu J. Q., Kurihara T., Hasan A. K., Nardi-Dei V., Koshikawa H., Esaki N., et al. (1994). Purification and characterization of thermostable and nonthermostable 2-haloacid dehalogenases with different stereospecificities from Pseudomonas sp. strain YL. Appl. Environ. Microbiol. 60 2389–2393. 10.1128/aem.60.7.2389-2393.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J. R., Weightman A. J. (2003). Comparing the Dehalogenase Gene Pool in Cultivated-Halocarboxylic Acid-Degrading Bacteria with the Environmental Metagene Pool. Appl. Environ. Microbiol. 69 4375–4382. 10.1128/aem.69.8.4375-4382.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Barbeyron T., Martin R., Portetelle D., Michel G., Vandenbol M. (2015). The cultivable surface microbiota of the brown alga Ascophyllum nodosum is enriched in macroalgal-polysaccharide-degrading bacteria. Front. Microbiol. 6:1487. 10.3389/fmicb.2015.01487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matucha M., Gryndler M., Forczek S. T., Uhlirova H., Fuksova K., Schröder P. (2003). Chloroacetic acids in environmental processes. Environ. Chem. Lett. 1 127–130. 10.1007/s10311-003-0030-y [DOI] [Google Scholar]
- Mena-Benitez G., Gandia-Herrero F., Graham S., Larson T., McQueen-Mason S., French C., et al. (2008). Engineering a catabolic pathway in plants for the degradation of 1,2-dichloroethane. Plant Physiol. 147 1192–1198. 10.1104/pp.108.119008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi-Dei V., Kurihara T., Okamura T., Liu J.-Q., Koshikawa H., Ozaki H., et al. (1994). Comparative studies of genes encoding thermostable L-2-haloacid dehalogenase from Pseudomonas sp. strain YL, other dehalogenases, and two related hypothetical proteins from Escherichia coli. Appl. Environ. Microbiol. 60 3375–3380. 10.1128/aem.60.9.3375-3380.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedashkovskaya O. I., Suzuki M., Vancanneyt M., Cleenwerck I., Lysenko A. M., Mikhailov V. V., et al. (2004). Zobellia amurskyensis sp. nov., Zobellia laminariae sp. nov. and Zobellia russellii sp. nov., novel marine bacteria of the family Flavobacteriaceae. Int. J. Syst. Evol. Microbiol. 54 1643–1648. 10.1099/ijs.0.63091-0 [DOI] [PubMed] [Google Scholar]
- Novak H. R., Sayer C., Isupov M. N., Paszkiewicz K., Gotz D., Spragg A. M., et al. (2013a). Marine Rhodobacteraceae L -haloacid dehalogenase contains a novel His / Glu dyad that could activate the catalytic water. FEBS J. 280 1664–1680. 10.1111/febs.12177 [DOI] [PubMed] [Google Scholar]
- Novak H. R., Sayer C., Panning J., Littlechild J. A. (2013b). Characterisation of an L-haloacid dehalogenase from the marine psychrophile Psychromonas ingrahamii with potential industrial application. Mar. Biotechnol. 15 695–705. 10.1007/s10126-013-9522-3 [DOI] [PubMed] [Google Scholar]
- Paul N. A., de Nys R., Steinberg P. D. (2006). Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Marine Ecology Progress Series 306 87–101. 10.3354/meps306087 [DOI] [Google Scholar]
- Ridder I. S., Rozeboom H. J., Kalk K. H., Janssen D. B., Dijkstra B. W. (1997). Three-dimensional structure of L-2-haloacid dehalogenase from Xanthobacter autotrophicus GJ10 complexed with the substrate-analogue formate. J. Biol. Chem. 272 33015–33022. 10.1074/jbc.272.52.33015 [DOI] [PubMed] [Google Scholar]
- Rye C. A., Isupov M. N., Lebedev A. A., Littlechild J. A. (2009). Biochemical and structural studies of a l-haloacid dehalogenase from the thermophilic archaeon Sulfolobus tokodaii. Extremophiles 13 179–190. 10.1007/s00792-008-0208-0 [DOI] [PubMed] [Google Scholar]
- Schmidberger J. W., Wilce J. A., Tsang J. S., Wilce M. C. (2007). Crystal structures of the substrate free-enzyme, and reaction intermediate of the HAD superfamily member, haloacid dehalogenase DehIVa from Burkholderia cepacia MBA4. J. Mol. Biol. 368 706–717. 10.1016/j.jmb.2007.02.015 [DOI] [PubMed] [Google Scholar]
- Van der Ploeg J., Van Hall G., Janssen D. (1991). Characterization of the haloacid dehalogenase from Xanthobacter autotrophicus GJ10 and sequencing of the dhlB gene. J. Bacteriol. 173 7925–7933. 10.1128/jb.173.24.7925-7933.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar E., Vannier T., Vernette C., Lescot M., Cuenca M., Alexandre A., et al. (2018). The Ocean Gene Atlas: exploring the biogeography of plankton genes online. Nucleic Acids Res. 46 W289–W295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever R., Barnett P. (2017). Vanadium Chloroperoxidases: the Missing Link in the Formation of Chlorinated Compounds and Chloroform in the Terrestrial Environment? Chem. Asian J. 12 1997–2007. 10.1002/asia.201700420 [DOI] [PubMed] [Google Scholar]
- Woolard F. X., Moore R. E., Roller P. P. (1979). Halogenated acetic and acrylic acids from the red alga Asparagopsis taxiformis. Phytochemistry 18 617–620. 10.1016/s0031-9422(00)84271-5 [DOI] [Google Scholar]
- Yu M., Faan Y.-W., Chung W., Tsang J. (2007). Isolation and characterization of a novel haloacid permease from Burkholderia cepacia MBA4. Appl. Environ. Microbiol. 73 4874–4880. 10.1128/aem.00576-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cao X., Xin Y., Xue S., Zhang W. (2013). Purification and characterization of a dehalogenase from Pseudomonas stutzeri DEH130 isolated from the marine sponge Hymeniacidon perlevis. World J. Microbiol. Biotechnol. 29 1791–1799. 10.1007/s11274-013-1340-2 [DOI] [PubMed] [Google Scholar]
- Zhang J., Xin Y., Cao X., Xue S., Zhang W. (2014). Purification and characterization of 2-haloacid dehalogenase from marine bacterium Paracoccus sp. DEH99, isolated from marine sponge Hymeniacidon perlevis. J. Ocean Univ. China 13 91–96. 10.1007/s11802-014-2357-3 [DOI] [Google Scholar]
- Zhu Y., Thomas F., Larocque R., Li N., Duffieux D., Cladière L., et al. (2017). Genetic analyses unravel the crucial role of a horizontally acquired alginate lyase for brown algal biomass degradation by Zobellia galactanivorans. Environ. Microbiol. 19 2164–2181. 10.1111/1462-2920.13699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.