Abstract
Background and Purpose
Previous studies have revealed various risk factors for carpal tunnel syndrome (CTS), but few large-scale studies have been conducted. We used data from the 11-year, longitudinal, nationwide population-based National Health Insurance Service–National Health Screening cohort to identify the actual risk factors for CTS.
Methods
We collected patients with CTS newly diagnosed using electrodiagnostic studies while excluding radiculopathy, plexopathy, or polyneuropathy, which can be confused with CTS. The crude and standardized incidence rates of CTS were calculated. Univariate and multivariate Cox analyses and the incidence of CTS were used to identify the risk factors for newly diagnosed CTS.
Results
The standardized incidence was 130.8/100,000 person-years based on the World Health Organization World Standard Population as a reference. Multivariate Cox analysis identified that the risk factors for CTS were being middle-aged, female, and obese, and having rheumatoid arthritis and Raynaud's syndrome, whereas gout and hypothyroidism were not risk factors. Diabetes and end-stage renal disease did not show a significant hazard ratio, although it is implicit that the durations of these diseases affect the development of CTS.
Conclusions
This study calculated the incidence of CTS and reappraised the associated risk factors found in previous studies. This information will be helpful for determining the pathophysiology of CTS, and hence aid the establishment of effective new public health policies.
Keywords: carpal tunnel syndrome, risk factors, cohort studies, big data, epidemiology
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common compressive neuropathy of the upper extremities.1,2 The prevalence of CTS in the general population has been estimated to be range from 1% to 6%.2,3,4,5 This variation in the prevalence may be attributable to differences in diagnostic criteria, study designs, and population groups.1,5 Although CTS has been studied extensively, its pathophysiology is still not fully understood.6 Several previous studies have revealed associations between CTS and various risk factors such as being middle-aged and female1,3 and having a high body mass index (BMI), diabetes mellitus (DM), rheumatoid arthritis (RA), gout, end-stage renal disease (ESRD), hypothyroidism, Raynaud's syndrome (RS), certain occupations, trigger finger, computer use, acromegaly, excessive alcohol consumption, and smoking.1,7,8 However, studies that have analyzed the risk factors for CTS have all involved small numbers of participants.
This study used the National Health Insurance Service (NHIS) system of Korea, which covers the entire Korean population, to assess a national-scale population cohort without selective bias. The purpose of this study was to identify the actual risk factors for CTS from among various known risk factors using data obtained from an 11-year, longitudinal, population-based cohort.
METHODS
Study cohort and database
We analyzed data that had been added to the NHIS-National Health Screening (NHIS-HealS) cohort9 between 2002 and 2013. The NHIS-HealS cohort comprised 514,866 health-screening participants who represented a random selection of 10% of all health-screening participants who were aged from 40 years to 79 years in 2002 and 2003. The results of the health screenings were used as baseline data. The NHIS-HealS database contains demographic factors such as age, sex, and income-based insurance contributions (a proxy for income), as well as data on the use of medical facilities, including disease classification codes of the Korean Standard Classification of Diseases as modified from those in the tenth revision of the International Classification of Diseases (ICD-10), medical treatment, medical history, and prescriptions. The participants were followed up for 11 years until the end of 2013, unless health services ended owing to death or emigration.
Study participants
We formed a subcohort of the NHIS-HealS cohort comprising those who did not die during the study period and thus were fully followed. From the NHIS-HealS cohort we collected patients with newly diagnosed CTS based on the following ICD-10 codes from January 2002 to December 2013: carpal tunnel syndrome (G56.00), carpal tunnel syndrome in an unspecified upper limb (G56.01), carpal tunnel syndrome in the right upper limb (G56.02), and carpal tunnel syndrome in the left upper limb (G56.03). We then established a 1-year washout period by excluding cases identified within the first year in order to confirm newly identified cases of CTS. To enhance the diagnostic validity, we defined that electrodiagnostic studies were performed when there were upper limb electrodiagnostic study codes (F6111, F6121, F6122, and FA111) in NHIS claims data. We only included patients who were newly registered with CTS diagnostic codes within 6 months of conducting electrodiagnostic studies. This approach meant that all of the subjects included in this study had been electrodiagnostically confirmed as CTS. Also, other diseases with a possibility of being confused with CTS or combined diseases with CTS were excluded based on diagnostic codes such as cervical radiculopathy (M50.1), plexopathy (G55.1), and polyneuropathy (G60–G64).
Selection and measurement of the risk factors for CTS
We reappraised the known risk factors for CTS by selecting the following variables: sex, age, BMI, and the ICD-10 codes for DM (E.10, E.11, E.12, E.13, and E.14), RA (M.05 and M.06), gout (M.10), ESRD (N.18), hypothyroidism (E.031, E.032, E.038, and E.039), and RS (I.730). BMI was calculated as the weight in kilograms divided by the square of the height in meters, and classified the values into the following five categories based on the Asian standard: <18.5 kg/m2 (underweight), 18.5–22.9 kg/m2 (normal), 23.0–24.9 kg/m2 (overweight), 25.0–29.9 kg/m2 (moderate obesity), and 30.0–35.0 kg/m2 (severe obesity). To avoid confusion, we only included cases where medical comorbidities had already occurred before a CTS diagnosis.
Statistical analysis
The baseline characteristics of the group identified as having CTS (CTS group) vs. the group without CTS (non-CTS control group) are expressed as numbers and percentages in Table 1. Fisher's exact chi-square test was used to compare the distributions of baseline demographic characteristics and comorbidities between the groups. Associations between a CTS diagnosis and potential risk factors were assessed using univariate analyses. Variables for which p<0.05 in the univariate analyses were included in the subsequent multivariate analysis with a Cox proportional-hazards regression model. A significance cutoff of 0.05 was set.
Table 1. Characteristics of the CTS and non-CTS control populations.
| CTS (n=7,258) |
Non-CTS (n=469,328) |
p | ||
|---|---|---|---|---|
| Age at the start of the study (in 2003), years | <0.0001* | |||
| 40–49 | 3,570 (49.19) | 207,467 (44.21) | ||
| 50–59 | 2,334 (32.16) | 130,960 (27.90) | ||
| 60–69 | 1,156 (15.93) | 95,445 (20.34) | ||
| ≥70 | 198 (2.73) | 35,456 (7.55) | ||
| Sex | <0.0001* | |||
| Male | 1,668 (22.98) | 263,018 (56.04) | ||
| Female | 5,590 (77.02) | 206,310 (43.96) | ||
| Body mass index at the start of the study (in 2003), kg/m2 | <0.0001* | |||
| <18.5 | 75 (1.03) | 11,390 (2.43) | ||
| 18.5–22.9 | 2,166 (29.84) | 168,177 (35.83) | ||
| 23.0–24.9 | 2,003 (27.60) | 127,586 (27.18) | ||
| 25.0–29.9 | 2,698 (37.17) | 149,293 (31.81) | ||
| 30.0–35.0 | 316 (4.35) | 12,882 (2.74) | ||
| Comorbidities | ||||
| Diabetes mellitus | 963 (13.27) | 60,516 (12.89) | 0.3456 | |
| Rheumatoid arthritis | 647 (8.91) | 22,671 (4.83) | <0.0001* | |
| Gout | 88 (1.21) | 6,408 (1.37) | 0.2649 | |
| End-stage renal disease | 14 (0.19) | 1,271 (0.27) | 0.2039 | |
| Hypothyroidism | 121 (1.67) | 4,918 (1.05) | <0.0001* | |
| Raynaud's syndrome | 12 (0.17) | 284 (0.06) | 0.0004* | |
Data are presented as n (%).
*p<0.05.
CTS: carpal tunnel syndrome.
The standardized incidence of CTS was calculated from the crude incidence using the following formula after correcting for demographic bias in this cohort relative to the national population structure [using the 2005 Korean census population and the World Health Organization (WHO) World Standard Population as a reference]:
where Pi is the proportion in each age/sex group relative to the national population, and Ii is the incidence in each age/sex group for the cohort population.
The statistical package SAS for Windows (version 9.2, SAS Institute, Cary, NC, USA) and R software (version 3.6.0, the R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) were used to create the figures in this study.
Statement of ethics
This study was approved by the Institutional Review Board of the NHIS, Ilsan Hospital, Goyang, Korea (IRB number 2015-10-001). This research project was approved by the KNHIS (The research management number is NHIS-2017-2-536). The requirement for informed consent was waived. The study protocol was consistent with the Declaration of Helsinki.
RESULTS
Study population and incidence rate of CTS
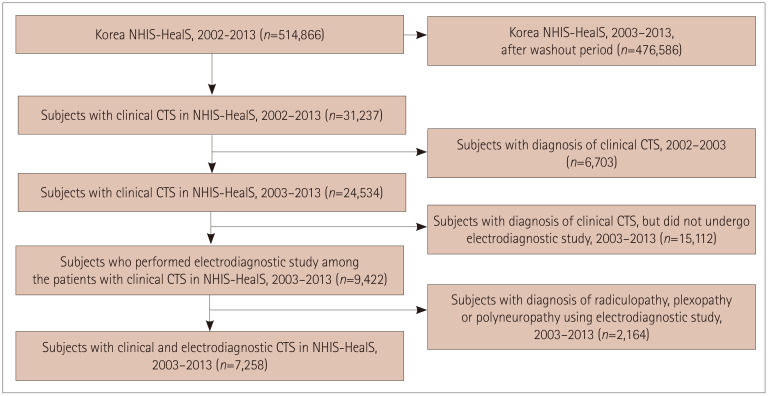
After the 1-year washout period, the cohort comprised 476,586 participants from 2003 to 2013. There were 31,237 patients with CTS codes from 2002 to 2003, and we excluded 6,703 subjects who were diagnosed with CTS during the washout period, and also 15,112 who did not undergo electrodiagnostic investigations. Moreover, 2,164 subjects with a diagnosis of radiculopathy, plexopathy, or polyneuropathy were also excluded. Finally, 7,258 subjects with a CTS diagnosis and electrodiagnostic data were enrolled in the study (Fig. 1).
Fig. 1. Flowchart of the study design including newly diagnosed clinical and electrodiagnostic CTS from 2003 to 2013. CTS: carpal tunnel syndrome, NHIS-HealS: National Health Insurance Service-National Health Screening.
The crude incidence of CTS in those aged >40 years was 138.4/100,000 person-years [95% confidence interval (CI)=135.3–141.7/100,000 person-years]. The standardized incidence was 142.9/100,000 person-years based on the national census population in 2005, and 130.8/100,000 person-years based on the WHO World Standard Population as a reference.
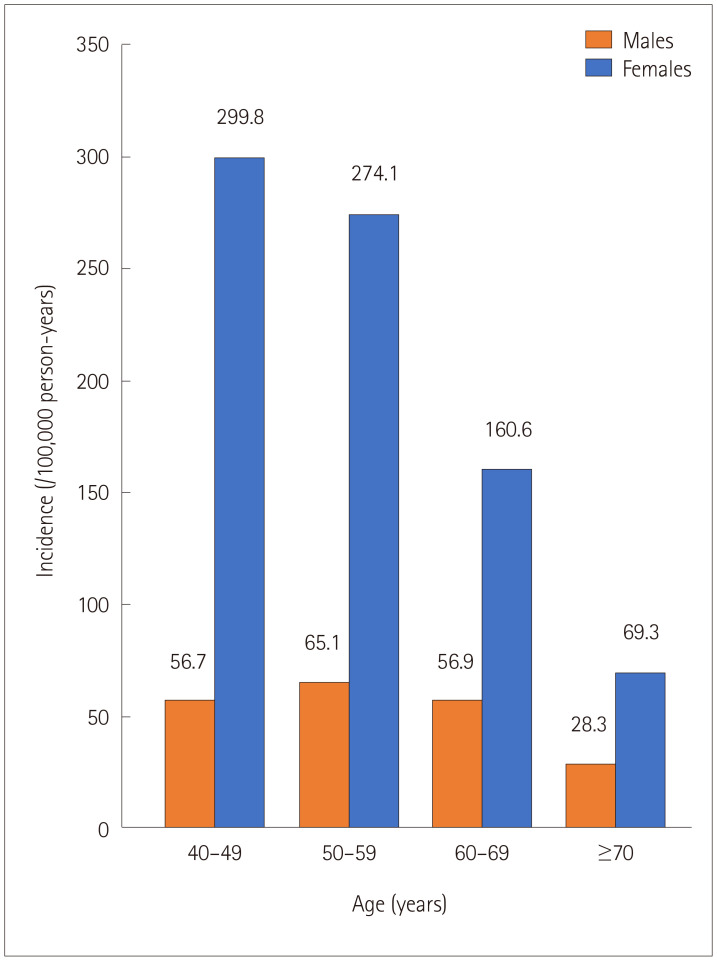
The CTS incidence rates according to sex and age are presented in Fig. 2. The incidence of CTS was higher in females than in males across all age groups. The incidence rate of CTS was highest in females aged 40–49 years and males aged 50–59 years.
Fig. 2. Incidence rates of carpal tunnel syndrome according to sex and age. The incidence was higher in females, and peaked at ages of 40–49 and 50–59 years in females and males, respectively.
Factors associated with CTS
The CTS group had significantly higher proportions of females (77.02% vs. 43.96%, p<0.0001) and middle-aged subjects (40–49 years: 49.19% vs. 44.21%, p<0.0001; 50–59 years: 32.16% vs. 27.90%, p<0.0001) than the control group. Moreover, the CTS group had significantly higher proportions of overweight (BMI=23.0–24.9 kg/m2: 27.60% vs. 27.18%, p<0.0001), moderately obese (BMI=25.0–29.9 kg/m2: 37.17% vs. 31.81%, p<0.0001), and severely obese (BMI ≥30.0 kg/m2: 4.35% vs. 2.74%, p<0.0001) subjects. Furthermore, subjects with RA (8.91% vs. 4.83%, p<0.0001), hypothyroidism (1.67% vs. 1.05%, p<0.0001), and RS (0.17% vs. 0.06%, p=0.0004) were more prevalent in the CTS group than in the non-CTS control group. However, the proportions of subjects with DM, gout, and ESRD did not differ significantly between the CTS and control groups (Table 1).
Hazard ratios of known risk factors for CTS
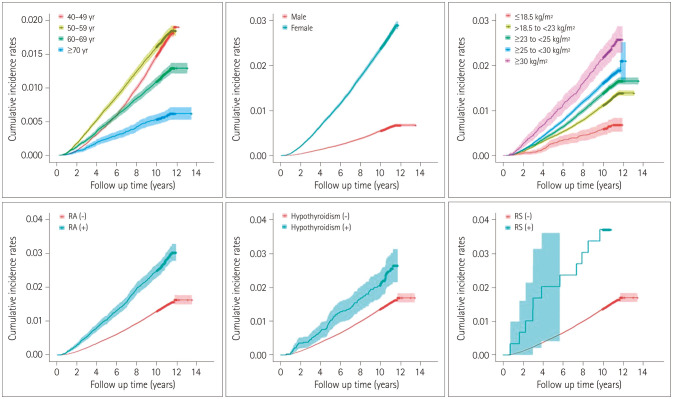
Univariate and multivariate Cox analyses of clinical variables and the incidence of CTS were used to investigate the risk factors for newly diagnosed CTS. In univariate analyses, being aged ≥60 years lowered the risk of CTS [hazard ratio (HR)=0.722, 95% CI=0.675–0.771, p<0.0001 for 60–69 years; HR=0.348, 95% CI=0.302–0.402, p<0.0001 for ≥70 years]. In contrast, the risk of CTS was higher in females (HR=4.313, 95% CI=4.083–4.555, p<0.0001) and in subjects with higher BMI (HR=1.994, 95% CI=1.544–2.447, p<0.0001 for BMI=18.5–22.9 kg/m2; HR=2.367, 95% CI=1.880–2.981, p<0.0001 for BMI=23.0–24.9 kg/m2; HR=2.725, 95% CI=2.167–3.428, p<0.0001 for BMI=25.0–29.9 kg/m2; HR=3.710, 95% CI=2.884–4.771, p<0.0001 for BMI ≥30.0 kg/m2), RA (HR=1.932, 95% CI=1.783–2.095, p<0.0001), hypothyroidism (HR=1.611, 95% CI=1.346–1.928, p<0.0001), and RS (HR=2.718, 95% CI=1.543–4.787, p=0.0005) (Table 2). Fig. 3 shows the cumulative incidence rates of CTS based on age, sex, BMI, and each risk factor including RA, hypothyroidism, and RS that showed significant intergroup differences in the univariate analyses.
Table 2. Results of univariate and multivariate regression analyses of carpal tunnel syndrome.
| Univariate | Multivariate (full model) | Multivariate (reduced model) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| Age at the start of the study (in 2003), years | ||||||||||
| 40–49 | Ref. | Ref. | Ref. | |||||||
| 50–59 | 1.047 | 0.994–1.103 | 0.0846 | - | - | - | - | |||
| 60–69 | 0.722 | 0.675–0.771 | <0.0001* | 0.590 | 0.551–0.632 | <0.0001* | 0.590 | 0.551–0.631 | <0.0001* | |
| ≥70 | 0.348 | 0.302–0.402 | <0.0001* | 0.312 | 0.270–0.360 | <0.0001* | 0.312 | 0.270–0.360 | <0.0001* | |
| Sex | ||||||||||
| Male | Ref. | Ref. | Ref. | |||||||
| Female | 4.313 | 4.083–4.555 | <0.0001* | 4.431 | 4.192–4.685 | <0.0001* | 4.429 | 4.190–4.682 | <0.0001* | |
| Body mass index at the start of the study (in 2003), kg/m2 | ||||||||||
| <18.5 | Ref. | Ref. | Ref. | |||||||
| 18.5–22.9* | 1.994 | 1.544–2.447 | <0.0001* | 1.604 | 1.274–2.019 | <0.0001* | 1.604 | 1.274–2.020 | <0.0001* | |
| 23.0–24.9* | 2.367 | 1.880–2.981 | <0.0001* | 2.088 | 1.658–2.629 | <0.0001* | 2.089 | 1.658–2.630 | <0.0001* | |
| 25.0–29.9* | 2.725 | 2.167–3.428 | <0.0001* | 2.450 | 1.947–3.083 | <0.0001* | 2.451 | 1.948–3.084 | <0.0001* | |
| 30.0–35.0* | 3.710 | 2.884–4.771 | <0.0001* | 2.726 | 2.119–3.508 | <0.0001* | 2.728 | 2.120–3.510 | <0.0001* | |
| Comorbidities | ||||||||||
| Diabetes mellitus | 1.041 | 0.972–1.114 | 0.2510 | |||||||
| Rheumatoid arthritis | 1.932 | 1.783–2.095 | <0.0001* | 1.540 | 1.418–1.673 | <0.0001* | 1.543 | 1.421–1.674 | <0.0001* | |
| Gout | 0.888 | 0.719–1.095 | 0.2665 | - | - | - | - | |||
| End-stage renal disease | 0.715 | 0.423–1.207 | 0.2090 | - | - | - | - | |||
| Hypothyroidism | 1.611* | 1.346–1.928 | <0.0001* | 1.049 | 0.876–1.256 | 0.6064 | - | - | ||
| Raynaud's syndrome | 2.718* | 1.543–4.787 | 0.0005* | 2.267* | 1.286–3.996 | 0.0047 | 2.273* | 1.289–4.007 | 0.0045* | |
*p<0.05.
CI: confidence interval, HR: hazard ratio, Ref.: reference.
Fig. 3. Cumulative incidence rates of carpal tunnel syndrome based on age, sex, body mass index, and each risk factor including RA, hypothyroidism, and RS that showed significant differences in the univariate analyses (see Table 2). RA: rheumatoid arthritis, RS: Raynaud's syndrome.
The multivariate Cox proportional-hazards model included potential risk factors that had been identified by univariate analyses. Using backward elimination, being aged ≥60 years lowered the risk of CTS (HR=0.590, 95% CI=0.551–0.631, p<0.0001 for 60–69 years; HR=0.312, 95% CI=0.270–0.360, p<0.0001 for ≥70 years), while the risk of CTS was higher in females (HR=4.429, 95% CI=4.190–4.682, p<0.0001) and in subjects with higher BMI (HR=1.604, 95% CI=1.274–2.020, p<0.0001 for BMI=18.5–22.9 kg/m2; HR=2.089, 95% CI=1.658–2.630, p<0.0001 for BMI=23.0–24.9 kg/m2; HR=2.451, 95% CI=1.948–3.084, p<0.0001 for BMI=25.0–29.9 kg/m2, HR=2.728, 95% CI=2.120–3.510, p<0.0001 for BMI ≥30.0 kg/m2), RA (HR=1.543, 95% CI=1.421–1.674, p<0.0001), and RS (HR=2.273, 95% CI=1.289–4.007, p=0.0045) (Table 2).
DISCUSSION
CTS is one of the most common entrapment neuropathies, which has prompted numerous investigators to attempt to identify its risk factors. We reappraised the known risk factors identified in previous small-scale studies by using a nationwide-population-based cohort. This study has revealed that some of the known risk factors (age, sex, BMI, RA, and RS) increase the risk of CTS.
Total incidence of CTS
The incidence of CTS in this study (130.8/100,000 person-years standardized by the WHO World Standard Population) was lower than those found in previous studies that used clinically diagnosed CTS groups (105–544.12/100,000 person-years). These discrepancies are due to differences in search settings. The reported incidence is naturally higher for clinically diagnosed than electrophysiologically diagnosed CTS. To increase the specificity of the diagnoses, we only included patients with CTS who had undergone electrodiagnostic studies within the previous 6 months, and excluded patients with concurrent radiculopathy, plexopathy, or polyneuropathy. Excluding the study conducted in Italy by Mondelli et al.10 resulted in previously reported incidence rates for electrophysiologically diagnosed CTS of 98–104/100,000 person-years, which are similar to our results (Table 3).5,10,11,12,13,14,15,16,17
Table 3. Comparisons with previously reported models, incidence rates, and peak ages of CTS5,10,11,12,13,14,15,16,17.
| Country, authors | Study location, period | Study design | Method of CTS diagnosis | Incidence of CTS (per 100,000 person-years) | Peak age of CTS |
|---|---|---|---|---|---|
| United States, Stevens et al.11 | Rochester, 1961–1980 | Rochester Epidemiology Project | Clinical or electrodiagnostic CTS (diagnostic codes in database) | 105 | Males: increased with age |
| Females: 45–54 years | |||||
| United States, Nordstrom et al.12 | Marshfield, 1991–1993 | Wisconsin HMO database | Clinical or electrodiagnostic CTS (diagnostic codes in database and chart review) | 346 | Males: >65 years |
| Females: 50–64 years | |||||
| Italy, Mondelli et al.10 | Siena, 1991–1998 | Data from local neurophysiological center | Electrodiagnostic CTS | Crude: 329 (WHO Standardized: 276) | Males: 50–59 and 70–79 years (bimodal distribution) |
| Males: 139 | Females: 50–59 years | ||||
| Females: 506 | |||||
| Italy, Mattioli et al.13 | Tuscany, 1997–2000 | Discharge records from all hospitals (hospital data) | Clinical CTS | WHO Standardized: 106 | Males: 70–79 years |
| Males: 44 | Females: 50–59 years | ||||
| Females: 166 | |||||
| Netherlands, Bongers et al.14 | Whole population, 1987–2001 | Dutch national survey of general practice | Clinical or electrodiagnostic CTS | 1987: 130 | 1987: 25–44 years |
| 2001: 180 | 2001: 45–64 years | ||||
| United Kingdom, Bland and Rudolfer15 | East Kent and Huddersfield, 1991–2001 | Data from local neurophysiological center | Electrodiagnostic CTS | 104 | 50–54 and 75–84 years (bimodal distribution) |
| Males: 67 | |||||
| Females: 139 | |||||
| United States, Gelfman et al.16 | Olmsted County, 1981–2005 | Rochester Epidemiology Project | Clinical or electrodiagnostic CTS (diagnostic codes in database) | 376 | Males: 70–79 years |
| Females: 50–59 years | |||||
| Sweden, Atroshi et al.5 | Skane County, 2003–2008 | Skane Health Care Register (covers all public health care providers) | Clinical or electrodiagnostic CTS | Males: 125 | Males: 50–59 years |
| Females: 324 | Females: 50–59 years | ||||
| Taiwan, Tsai et al.17 | Whole population, 2003–2012 | Taiwan National Health Insurance Research Database | Clinical or electrodiagnostic CTS | 544.12 | Males: 50–59 years |
| Females: 50–59 years | |||||
| Korea, current study | Whole population, 2003–2013 | Korea National Health Insurance Service–Health Screening database (age >39 years) | Clinical and electrodiagnostic CTS | WHO Standardized: 130.8 | Males: 50–59 years |
| Females: 40–49 years |
CTS: carpal tunnel syndrome, HMO: Health Maintenance Organization, WHO: World Health Organization.
Risk factors consistent with previous studies
We found that known risk factors, including being female, being aged 40–59 years, and having a higher BMI, a diagnosis of RA, and a diagnosis of RS were related to the onset of CTS.
It is well known that CTS is more common in females, whose incidence is reportedly two- to fourfold higher than in males.5,18 This is consistent with our result of HR=4.429 for the incidence among females of all ages. Also, this study found that the incidence of CTS was highest in females aged 40–49 years and males aged 50–59 years. This sex-related difference could be due to the relative vulnerability of females to CTS, whose incidence peaks at perimenopausal age.10,15,19 It is consistent with the hypothesis that in females there is a hormonal component in developing CTS, possibly involving long-term hormonal effects of pregnancy or a cumulative exposure to female sex hormones.20,21 In addition, the physical activity associated with employment, exercise, and housework is usually the most vigorous before middle age.
However, some of the data in Table 3 suggest different age-specific CTS distributions among males. One study conducted in Italy10 and another in the United Kingdom15 suggested a bimodal age distribution, with peaks at ages of 50–59 and 70–79 years. Other studies in the United States12,16 and Italy13 showed that the CTS incidence rate gradually increases with age, peaking at >65 or 70–79 years. CTS is also related to the physical workload, and several previous studies have shown that the CTS incidence is higher in rural and industrial areas than in the urban areas.22,23,24 This means that there could be an effect of occupation and physical work intensity on the incidence of CTS, especially in males. In Korea, there has been a constant movement of people from rural to urban areas over the past few decades, and urban knowledge-based service industries need less physical labor. Furthermore, because accessibility to health care is better in Korea than in most other countries, the associated earlier diagnoses of CTS could affect the results. Also, the relevant socioeconomic and demographic factors may vary with the time, country, or study population, and so further studies of the association between occupation parameters and CTS incidence are necessary.
Our study found that a higher BMI was associated with a higher HR, which is also consistent with previous reports of a higher BMI being one of the main risk factors for CTS.18,25,26,27,28 The proposed hypothesis is that increased fat tissue inside the carpal tunnel increases hydrostatic pressure or that water accumulation is accelerated in connective tissues and therefore causes compression of the median nerve.6,25,27,28
RA is a chronic inflammatory disease accompanied by various extra-articular manifestations and progressive articular damage.29 It frequently causes tenosynovitis and can anatomically alter the carpal tunnel.30 RA is a known risk factor for CTS because the tenosynovitis caused by RA increases the intracarpal pressure and injures the median nerve.30,31,32 The results of our study support the conclusion that RA is one of the risk factors for CTS.
RS is caused by vasculitis associated with systemic inflammatory disorders and results in impaired microcirculation.33,34 Autonomic dysfunction that may occur in RS or CTS can produce symptoms associated with Raynaud's phenomenon,35 or both conditions may be present concurrently.33,34 A metaanalysis found that CTS and RS were statistically related to each other.36 In our study, the HR for RS (at 2.273) was the highest among all CTS risk factors.
Risk factors differing from previous studies
DM is a well-known risk factor for CTS.25,37,38 Previous studies have found that CTS is present in up to one-third of patients with DM and is three times more prevalent in diabetic than healthy populations.31,37 Also, a nationwide populationbased cohort study conducted in Taiwan involving patients with DM revealed that females and younger patients with DM had the highest risk of diabetic hand syndromes, including CTS.39 However, the research method used in our study meant that it did not directly address the relationship between DM and the occurrence of CTS. We only included participants with CTS diagnosed electrodiagnostically and without radiculopathy, plexopathy, and polyneuropathy. The prevalence of diabetic polyneuropathy increases with the duration and severity of DM.40,41 In other words, the present study was highly likely to have excluded many patients with a longer DM duration, and the findings suggest that DM requires some time to affect the occurrence of CTS. This is consistent with a previous study of the NHIS National Sample Cohort finding that patients with DM polyneuropathy had an increased risk of developing CTS over time compared with those without DM polyneuropathy.42
Similarly, ESRD, a known risk factor for CTS,8,17,43,44 was less relevant to CTS in the present study. Previous studies have shown that patients with ESRD receiving renal dialysis are likely to develop CTS due to beta-2 microglobulin, which is an amyloid-like deposit in the soft tissue that is similar to gouty tophi.45,46 However, uremic polyneuropathy is common in patients with ESRD, although the polyneuropathy is often subclinical and detectable only by electrophysiological studies. Patients with CTS would have been excluded from our study due to those with polyneuropathy being eliminated.
There have been some reports that tophus deposition in the carpal tunnel can lead to CTS.47,48 However, our study showed that gout does not increase the risk of CTS development. Despite the space-occupying nature of gouty tophi, a review of the literature showed that CTS secondary to gout is uncommon.49 This is because gout rarely affects the wrist, and is even more rare when gout is managed.50
There is controversy about the effect of hypothyroidism on CTS. One meta-analysis found a modest association between hypothyroidism and CTS, but this may have been due partly to publication bias, as evidenced by an asymmetric funnel plot.51 An investigation of one million people in Taiwan found that the occurrence of CTS in patients younger than 39 years was related to hypothyroidism, whereas CTS in those older than 40 years was not.52 Our analysis targeted people older than 40 years, and found no significant relationship between hypothyroidism and CTS in the multivariate analysis.
Strengths
This study had several strengths. First, the NHIS-HealS data could be highly representative of the general population aged from 40 years to 79 years in South Korea. Second, the sample was very large, which increased the statistical power of our study. Third, the analyzed database contains nationwide follow-up data with a high coverage rate of the general population, and almost all data in the database are available.
Limitations
Our study was subject to some inherent limitations. First, the diagnoses of CTS in our database constituted administrative data rather than clinically ascertained data, and there is a possibility of misdiagnoses of CTS in health insurance claims data. To mitigate this vulnerable aspect of the database, we used the codes for electrodiagnostic studies to complement the disease codes. This enhanced the specificity of the diagnoses and so improved their robustness. Second, we assumed that factors such as the BMI, age groups (40–49, 50–59, 60–69, or ≥70 years), and comorbidities (DM, RA, gout, ESRD, hypothyroidism, and RS) remained unchanged over the time period of this study. It is, therefore, possible that bias from unknown or changed confounders and errors related to inadequate claims data affected our results. However, this could have been offset by the strength of this big-data study involving a nationwide database based on NHIS claims data. Furthermore, a well-designed prospective randomized controlled study would be necessary to determine the causal relationship. The third limitation is that this study did not include participants younger than 40 years, because national periodic health screening is only offered to people aged ≥40 years. However, it is already known that CTS usually does not occur in people younger than 40 years, and so our study cohort included the more-affected age groups. Fourth, by excluding CTS combined with cervical radiculopathy, plexopathy, or polyneuropathy, the incidence of electrodiagnostic CTS in our study could have been underestimated and thereby have affected the risk factor analysis. However, one of the strengths of this study was its robustness in diagnosing CTS based on electrodiagnostic confirmation. Lastly, the increasing use of ultrasound in diagnosing CTS supplementary to electrodiagnosis might have resulted in underestimation of the incidence of CTS in this study. Future studies that include ultrasound-diagnosed CTS might therefore be necessary.
Conclusion
In this study we identified the following risk factors for CTS: being female, being 40–59 years old, and having a high BMI, RA, or RS. However, DM, gout, hypothyroidism, and ESRD were not associated with CTS in this study. We suggest that the results of our study will be helpful in determining the pathophysiology of CTS and the early-stage prevention of CTS.
Footnotes
- Conceptualization: Jong Hun Kim, Hyoung Seop Kim
- Data curation: Han Eol Cho, Hyoung Seop Kim
- Formal analysis: all authors
- Investigation: Seung Yeon Rhee
- Methodology: Jong Hun Kim, Hyoung Seop Kim
- Software: Jong Hun Kim, Hyoung Seop Kim
- Supervision: Hyoung Seop Kim.
- Writing—original draft: Seung Yeon Rhee, Han Eol Cho.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This study was supported by the National Health Insurance Service Ilsan Hospital research fund.
Availability of Data and Material
The datasets generated or analyzed during the current study are available in the NHIS-HealS repository (https://nhiss.nhis.or.kr/bd/ab/bdaba022Heng.do).
References
- 1.Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15:1273–1284. doi: 10.1016/S1474-4422(16)30231-9. [DOI] [PubMed] [Google Scholar]
- 2.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 3.de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol. 1992;45:373–376. doi: 10.1016/0895-4356(92)90038-o. [DOI] [PubMed] [Google Scholar]
- 4.Mediouni Z, Bodin J, Dale AM, Herquelot E, Carton M, Leclerc A, et al. Carpal tunnel syndrome and computer exposure at work in two large complementary cohorts. BMJ Open. 2015;5:e008156. doi: 10.1136/bmjopen-2015-008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atroshi I, Englund M, Turkiewicz A, Tägil M, Petersson IF. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch Intern Med. 2011;171:943–944. doi: 10.1001/archinternmed.2011.203. [DOI] [PubMed] [Google Scholar]
- 6.Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol. 2002;113:1373–1381. doi: 10.1016/s1388-2457(02)00169-4. [DOI] [PubMed] [Google Scholar]
- 7.Oktayoglu P, Nas K, Kilinç F, Tasdemir N, Bozkurt M, Yildiz I. Assessment of the presence of carpal tunnel syndrome in patients with diabetes mellitus, hypothyroidism and acromegaly. J Clin Diagn Res. 2015;9:OC14–OC18. doi: 10.7860/JCDR/2015/13149.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourmemari MH, Viikari-Juntura E, Shiri R. Smoking and carpal tunnel syndrome: a meta-analysis. Muscle Nerve. 2014;49:345–350. doi: 10.1002/mus.23922. [DOI] [PubMed] [Google Scholar]
- 9.Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the National Health Insurance Service-National Health Screening cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640. doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondelli M, Giannini F, Giacchi M. Carpal tunnel syndrome incidence in a general population. Neurology. 2002;58:289–294. doi: 10.1212/wnl.58.2.289. [DOI] [PubMed] [Google Scholar]
- 11.Stevens JC, Sun S, Beard CM, O'Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134–138. doi: 10.1212/wnl.38.1.134. [DOI] [PubMed] [Google Scholar]
- 12.Nordstrom DL, DeStefano F, Vierkant RA, Layde PM. Incidence of diagnosed carpal tunnel syndrome in a general population. Epidemiology. 1998;9:342–345. [PubMed] [Google Scholar]
- 13.Mattioli S, Baldasseroni A, Curti S, Cooke RM, Bena A, de Giacomi G, et al. Incidence rates of in-hospital carpal tunnel syndrome in the general population and possible associations with marital status. BMC Public Health. 2008;8:374. doi: 10.1186/1471-2458-8-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bongers FJ, Schellevis FG, van den Bosch WJ, van der Zee J. Carpal tunnel syndrome in general practice (1987 and 2001): incidence and the role of occupational and non-occupational factors. Br J Gen Pract. 2007;57:36–39. [PMC free article] [PubMed] [Google Scholar]
- 15.Bland JD, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991-2001. J Neurol Neurosurg Psychiatry. 2003;74:1674–1679. doi: 10.1136/jnnp.74.12.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelfman R, Melton LJ, 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CH, Hsu HH, Chen SH, Chien L, Lin JA, Chang CJ, et al. Incidence and risk of carpal tunnel syndrome in end-stage renal disease patients on dialysis: a nationwide population-based matched cohort study. Ann Plast Surg. 2020;84:S100–S106. doi: 10.1097/SAP.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 18.Lam N, Thurston A. Association of obesity, gender, age and occupation with carpal tunnel syndrome. Aust N Z J Surg. 1998;68:190–193. doi: 10.1111/j.1445-2197.1998.tb04743.x. [DOI] [PubMed] [Google Scholar]
- 19.Liss GM, Armstrong C, Kusiak RA, Gailitis MM. Use of provincial health insurance plan billing data to estimate carpal tunnel syndrome morbidity and surgery rates. Am J Ind Med. 1992;22:395–409. doi: 10.1002/ajim.4700220312. [DOI] [PubMed] [Google Scholar]
- 20.Ferry S, Hannaford P, Warskyj M, Lewis M, Croft P. Carpal tunnel syndrome: a nested case-control study of risk factors in women. Am J Epidemiol. 2000;151:566–574. doi: 10.1093/oxfordjournals.aje.a010244. [DOI] [PubMed] [Google Scholar]
- 21.Toesca A, Pagnotta A, Zumbo A, Sadun R. Estrogen and progesterone receptors in carpal tunnel syndrome. Cell Biol Int. 2008;32:75–79. doi: 10.1016/j.cellbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Wild DK, Cameron LL, Freund E. Association of occupational and non-occupational risk factors with the prevalence of self-reported carpal tunnel syndrome in a national survey of the working population. Am J Ind Med. 1997;32:550–556. doi: 10.1002/(sici)1097-0274(199711)32:5<550::aid-ajim18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anderson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27:451–470. doi: 10.1002/ajim.4700270402. [DOI] [PubMed] [Google Scholar]
- 24.Roquelaure Y, Mechali S, Dano C, Fanello S, Benetti F, Bureau D, et al. Occupational and personal risk factors for carpal tunnel syndrome in industrial workers. Scand J Work Environ Health. 1997;23:364–369. doi: 10.5271/sjweh.233. [DOI] [PubMed] [Google Scholar]
- 25.Becker J, Nora DB, Gomes I, Stringari FF, Seitensus R, Panosso JS, et al. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113:1429–1434. doi: 10.1016/s1388-2457(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 26.Hlebs S, Majhenic K, Vidmar G. Body mass index and anthropometric characteristics of the hand as risk factors for carpal tunnel syndrome. Coll Antropol. 2014;38:219–226. [PubMed] [Google Scholar]
- 27.Sharifi-Mollayousefi A, Yazdchi-Marandi M, Ayramlou H, Heidari P, Salavati A, Zarrintan S, et al. Assessment of body mass index and hand anthropometric measurements as independent risk factors for carpal tunnel syndrome. Folia Morphol (Warsz) 2008;67:36–42. [PubMed] [Google Scholar]
- 28.Werner RA, Albers JW, Franzblau A, Armstrong TJ. The relationship between body mass index and the diagnosis of carpal tunnel syndrome. Muscle Nerve. 1994;17:632–636. doi: 10.1002/mus.880170610. [DOI] [PubMed] [Google Scholar]
- 29.Kobak S. Demographic, clinical, and serological features of Turkish patients with rheumatoid arthritis: evaluation of 165 patients. Clin Rheumatol. 2011;30:843–847. doi: 10.1007/s10067-011-1678-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee KH, Lee CH, Lee BG, Park JS, Choi WS. The incidence of carpal tunnel syndrome in patients with rheumatoid arthritis. Int J Rheum Dis. 2015;18:52–57. doi: 10.1111/1756-185X.12445. [DOI] [PubMed] [Google Scholar]
- 31.Vinik A, Mehrabyan A, Colen L, Boulton A. Focal entrapment neuropathies in diabetes. Diabetes Care. 2004;27:1783–1788. doi: 10.2337/diacare.27.7.1783. [DOI] [PubMed] [Google Scholar]
- 32.Shiri R. Arthritis as a risk factor for carpal tunnel syndrome: a meta-analysis. Scand J Rheumatol. 2016;45:339–346. doi: 10.3109/03009742.2015.1114141. [DOI] [PubMed] [Google Scholar]
- 33.Chung MS, Gong HS, Baek GH. Raynaud's phenomenon in idiopathic carpal tunnel syndrome: postoperative alteration in its prevalence. J Bone Joint Surg Br. 2000;82:818–819. doi: 10.1302/0301-620x.82b6.10991. [DOI] [PubMed] [Google Scholar]
- 34.Waller DG, Dathan JR. Raynaud's syndrome and carpal tunnel syndrome. Postgrad Med J. 1985;61:161–162. doi: 10.1136/pgmj.61.712.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verghese J, Galanopoulou AS, Herskovitz S. Autonomic dysfunction in idiopathic carpal tunnel syndrome. Muscle Nerve. 2000;23:1209–1213. doi: 10.1002/1097-4598(200008)23:8<1209::aid-mus8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann P, Mohokum M, Schlattmann P. The association of Raynaud's syndrome with carpal tunnel syndrome: a meta-analysis. Rheumatol Int. 2012;32:569–574. doi: 10.1007/s00296-011-2122-5. [DOI] [PubMed] [Google Scholar]
- 37.Karpitskaya Y, Novak CB, Mackinnon SE. Prevalence of smoking, obesity, diabetes mellitus, and thyroid disease in patients with carpal tunnel syndrome. Ann Plast Surg. 2002;48:269–273. doi: 10.1097/00000637-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care. 2002;25:565–569. doi: 10.2337/diacare.25.3.565. [DOI] [PubMed] [Google Scholar]
- 39.Chen LH, Li CY, Kuo LC, Wang LY, Kuo KN, Jou IM, et al. Risk of hand syndromes in patients with diabetes mellitus: a population-based cohort study in Taiwan. Medicine (Baltimore) 2015;94:e1575. doi: 10.1097/MD.0000000000001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. 2019;14:e0212574. doi: 10.1371/journal.pone.0212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabezas-Cerrato J. The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS) Diabetologia. 1998;41:1263–1269. doi: 10.1007/s001250051063. [DOI] [PubMed] [Google Scholar]
- 42.Moon HI, Shin J, Kim YW, Chang JS, Yoon S. Diabetic polyneuropathy and the risk of developing carpal tunnel syndrome: a nationwide, population-based study. Muscle Nerve. 2020;62:208–213. doi: 10.1002/mus.26901. [DOI] [PubMed] [Google Scholar]
- 43.Kwon HK, Pyun SB, Cho WY, Boo CS. Carpal tunnel syndrome and peripheral polyneuropathy in patients with end stage kidney disease. J Korean Med Sci. 2011;26:1227–1230. doi: 10.3346/jkms.2011.26.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soyupek F, Demir M, Süslü FE, Baykal B, Sezer MT, Yesildag A. The upper extremity musculoskeletal complications in dialysis patients: comparison between hemodialysis and peritoneal dialysis. J Back Musculoskelet Rehabil. 2013;26:267–371. doi: 10.3233/BMR-130375. [DOI] [PubMed] [Google Scholar]
- 45.Niwa T. Dialysis-related amyloidosis: pathogenesis focusing on AGE modification. Semin Dial. 2001;14:123–126. doi: 10.1046/j.1525-139x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 46.Hauglustaine D, Waer M, Michielsen P, Goebels J, Vandeputte M. Haemodialysis membranes, serum beta 2-microglobulin, and dialysis amyloidosis. Lancet. 1986;1:1211–1212. doi: 10.1016/s0140-6736(86)91189-x. [DOI] [PubMed] [Google Scholar]
- 47.Chen CK, Chung CB, Yeh L, Pan HB, Yang CF, Lai PH, et al. Carpal tunnel syndrome caused by tophaceous gout: CT and MR imaging features in 20 patients. AJR Am J Roentgenol. 2000;175:655–659. doi: 10.2214/ajr.175.3.1750655. [DOI] [PubMed] [Google Scholar]
- 48.Kang HJ, Jung SH, Yoon HK, Hahn SB, Kim SJ. Carpal tunnel syndrome caused by space occupying lesions. Yonsei Med J. 2009;50:257–261. doi: 10.3349/ymj.2009.50.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HS. Carpal tunnel syndrome caused by tophaceous gout. Korean J Intern Med. 2014;29:544–545. doi: 10.3904/kjim.2014.29.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raimbeau G, Fouque PA, Cesari B, Le Bourg M, Saint-Cast Y. Gouty arthritis of the wrist. Five case reports. Chir Main. 2001;20:325–331. doi: 10.1016/s1297-3203(01)00054-3. [DOI] [PubMed] [Google Scholar]
- 51.Shiri R. Hypothyroidism and carpal tunnel syndrome: a meta-analysis. Muscle Nerve. 2014;50:879–883. doi: 10.1002/mus.24453. [DOI] [PubMed] [Google Scholar]
- 52.Tseng CH, Liao CC, Kuo CM, Sung FC, Hsieh DP, Tsai CH. Medical and non-medical correlates of carpal tunnel syndrome in a Taiwan cohort of one million. Eur J Neurol. 2012;19:91–97. doi: 10.1111/j.1468-1331.2011.03440.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available in the NHIS-HealS repository (https://nhiss.nhis.or.kr/bd/ab/bdaba022Heng.do).