Abstract
Background and Purpose
To determine the diagnostic value of straight head hanging (SHH) in benign paroxysmal positional vertigo involving the posterior semicircular canal (PC-BPPV).
Methods
We retrospectively included 62 patients (age=56.2±15.0 years, 47 female) with unilateral PC-BPPV who underwent both the Dix-Hallpike maneuver and SHH before receiving canalith repositioning therapy (CRT) between September 2017 and July 2020 at the Dizziness Center of Seoul National University Bundang Hospital in South Korea (16 patients, 25.8%) or the Neurology Outpatient Clinic of Aerospace Central Hospital in China (46 patients, 74.2%). SHH was performed before (n=29, group A) or after (n=33, group B) the Dix-Hallpike maneuver.
Results
Torsional upbeat nystagmus typical of PC-BPPV was induced during SHH in 52 (83.9%) patients, and the incidence of this type of positional nystagmus did not differ between the groups A and B (79.3% vs. 87.9%, p=0.569). The maximum slow-phase velocity of the induced upbeat nystagmus was higher during SHH than during the Dix-Hallpike maneuver toward the lesion side [range=2.0–60.0°/s (median=18.5°/s) vs. range=2.7–40.0°/s (median=13.4°/s), p<0.001]. Reversal of the positional nystagmus was observed upon resuming the sitting position after SHH in 47 (75.8%) patients and after the Dix-Hallpike maneuver in 54 (87.7%) patients, with no significant difference between the groups (p=0.082).
Conclusions
SHH is effective for diagnosing PC-BPPV. Given its simplicity, SHH may be performed before the Dix-Hallpike maneuver, and CRT may be attempted thereafter when the typical positional nystagmus for unilateral PC-BPPV is induced during SHH.
Keywords: dizziness, vertigo, nystagmus, benign paroxysmal positional vertigo
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is characterized by recurrent vertigo triggered by head position changes with respect to gravity.1,2,3,4 BPPV is caused by the migration of otoconial debris that have dislodged from the utricular macule into the semicircular canals.4,5,6 The posterior semicircular canal (PC) is most commonly involved.7,8,9 BPPV involving the PC (PC-BPPV) is diagnosed based on the relevant history and positional nystagmus provoked by positioning maneuvers.10 The evoked positional nystagmus mostly beats upward, with a torsional component beating toward the affected ear.
Since its introduction, the Dix-Hallpike maneuver remains the gold-standard positioning maneuver for diagnosing PC-BPPV. However, there have been attempts to develop alternative maneuvers to the Dix-Hallpike maneuver or to modify the original Dix-Hallpike maneuver with the aim of making it easier to apply, including the side-lying test,11 a chair-based version of the Dix-Hallpike maneuver,12 and placing a pillow under the shoulders during the Dix-Hallpike maneuver.13 These attempts have expanded the applicability of the positioning maneuvers for diagnosing PC-BPPV, especially in restricted clinical circumstances or in patients with limitations in neck motion or difficulty relaxing their neck.14
Only one previous study (performed in China)15 has determined the role of straight head hanging (SHH) in inducing the positional nystagmus typical of PC-BPPV, and this was done in only a relatively small number of patients. The present study was also prompted by our frequent observation of torsional upbeat nystagmus typical of PC-BPPV during SHH, which is mostly included in the positioning maneuvers used to elicit central positional nystagmus. Our expectation was that patients with PC-BPPV may benefit from avoiding undergoing the Dix-Hallpike maneuver in either direction if SHH is effective in evoking the positional nystagmus for PC-BPPV, with PC-BPPV being treated using canalith repositioning therapy from the head-hanging position.
This study, therefore, aimed to define the diagnostic value of SHH by determining the incidence of positional nystagmus typical of PC-BPPV being observed during SHH in patients with a confirmed diagnosis of unilateral PC-BPPV using the Dix-Hallpike maneuver.
METHODS
Patients
We retrospectively included 62 patients (47 female, age=56.2±15.0 years, age range=30–84 years) with pure unilateral PC-BPPV who also underwent SHH before (n=29, 46.8%, group A) or after (n=33, 53.2%, group B) the Dix-Hallpike maneuver at the Dizziness Center of Seoul National University Bundang Hospital in South Korea (16 patients, 25.8%) or the Neurology Outpatient Clinic of Aerospace Central Hospital in China (46 patients, 74.2%) between September 2017 and July 2020.
PC-BPPV was diagnosed according to the following criteria formulated by the Bárány Society:5 1) recurrent attacks of positional vertigo or positional dizziness induced when lying down or turning over in the supine position, 2) duration of attacks <1 minute, and 3) positional nystagmus elicited by the Dix-Hallpike tests after a latency of up to a few seconds. The type of nystagmus was upbeat and ipsiversive torsional. The positional nystagmus was recorded using 2D video-oculography (n=46; Micromedical Technologies, Chatham, IL, USA) in China and 3D video-oculography (n=16; SLMED, Seoul, South Korea) in South Korea. We only included subjects with good-quality recordings that permitted accurate measurements of the maximum slow-phase velocity (SPV) of upbeat nystagmus. Nine patients were excluded from the analyses in China due to the poor-quality recordings: five of these patients showed stronger nystagmus during the SHH test than during the Dix-Hallpike maneuver, two showed a similar intensity, and two patients showed weaker nystagmus during the SHH test than during the Dix-Hallpike maneuver.
Dix-Hallpike and SHH tests
For the Dix-Hallpike maneuver,10 the patient's head was turned with the nose pointing 45° toward the side to be examined, and then the patient was moved quickly into a supine position with the head hanging about 20° over the end of the examination table. This position was maintained for at least 1 minute or until the induced nystagmus resolved. Then, while the patient's head was kept turned 45° toward the side being examined, the patient was brought back to the sitting-up position.
For SHH,16 the patient was laid down from the upright sitting position with the head extended approximately 30° below the examination table. This position was again kept at least 1 minute or until the induced nystagmus resolved before resuming the upright sitting position.
To determine the effects of testing order on the intensity of the nystagmus triggered, we further divided the patients into two groups: in group A (n=29) the patients underwent the SHH test before the Dix-Hallpike maneuver, while those in group B (n=33) underwent SHH after the Dix-Hallpike maneuver. The methods of each positioning maneuver were identical in the two groups.
Statistical analyses
Statistical analyses were performed using SPSS software (version 20.0, IBM Corp., Armonk, NY, USA). Continuous variables are expressed as a mean±SD for parametric values and as a range (median) for nonparametric ones. Count variables are expressed as percentages. The normality of the data was determined using the Shapiro-Wilk test. The Wilcoxon signed-rank test was used to identify differences in the maximum SPV of the induced upbeat nystagmus between the two maneuvers. The chi-square test was used to determine whether the incidence of nystagmus reversal upon resuming the sitting position differed after the two maneuvers. A probability value of p<0.05 was considered statistically significant.
Ethical standard
The research protocol was implemented in compliance with the tenets of the Declaration of Helsinki. Informed consent was not required because this study was retrospective and did not affect patient care in any way. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-2012-657-103).
RESULTS
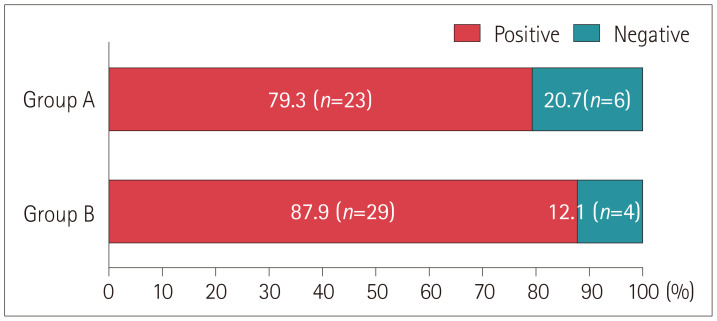
During SHH, the upbeat and ipsiversive torsional nystagmus that is typical of unilateral PC-BPPV was observed in 52 (83.9%) of the 62 patients (Supplementary Video 1 in the online-only Data Supplement). This incidence of positional nystagmus during SHH did not differ between the patients evaluated in China and South Korea [41/46 (89.1%) vs. 11/16 (68.8%), p=0.13], between PC-BPPV involving the right and left ear [27/31 (87.1%) vs. 25/31 (80.6%), p=0.49], or between the patients who underwent SHH before and after the Dix-Hallpike maneuver [23/29 (79.3%) vs. 29/33 (87.9%), p=0.569] (Fig. 1).
Fig. 1. Incidence of upbeat and ipsiversive torsional nystagmus induced during SHH. Patients in group A underwent the SHH test before the Dix-Hallpike maneuver, while those in group B underwent SHH after the Dix-Hallpike maneuver. SHH, straight head hanging.
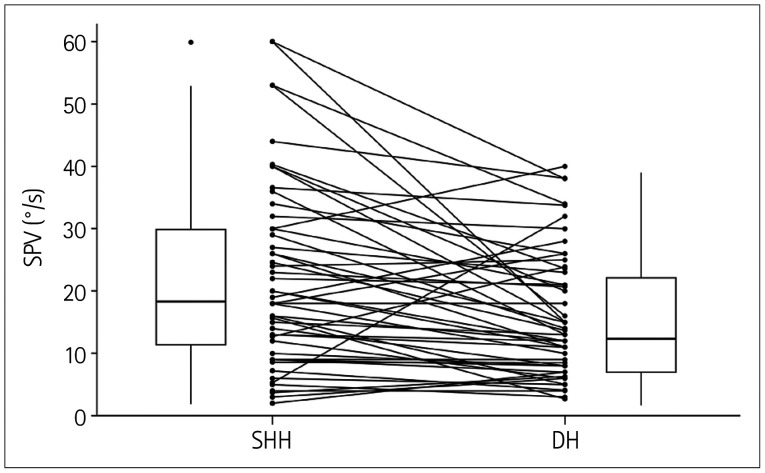
The maximum SPV of upbeat nystagmus was greater during SHH than during the Dix-Hallpike maneuver both in those who underwent SHH before (group A) [range=3.7–53.0°/s (median=18.0°/s) vs. range=2.7–38.1°/s (median=13.7°/s); Wilcoxon signed-rank test: p=0.017] and after (group B) [range=2.0–60.0°/s (median=19.0°/s) vs. range=3.0–40.0°/s (median=13.0°/s); Wilcoxon signed-rank test: p=0.002] the Dix-Hallpike maneuver. Overall, the maximum SPV of upbeat nystagmus was greater during SHH than during the Dix-Hallpike maneuver toward the lesion side [range=2.0–60.0°/s (median=18.5°/s) vs. range=2.7–40.0°/s (median=13.4°/s), Z=−3.851; Wilcoxon signed-rank test: p<0.001] (Fig. 2).
Fig. 2. Comparison of the maximum SPV of the upbeat nystagmus induced during SHH and the DH. DH, Dix-Hallpike maneuver; SHH, straight head hanging; SPV, slow-phase velocity.
The maximum SPV of upbeat nystagmus did not differ between the first and following maneuvers regardless of the testing order [median range=3–53°/s (median=14.5°/s) vs. range=2–60°/s (median=17.0°/s); Wilcoxon signed-ranks test: p=0.584].
The latency of induced torsional upbeat nystagmus was larger during the SHH test than during the Dix-Hallpike maneuver [range=0.8–16.0 s (median=3.0 s) vs. range=0–6.3 s (median=2.0 s), Z=−3.311; Wilcoxon signed rank test: p=0.001]. However, the incidence of nystagmus reversal upon resuming the sitting position did not differ between SHH and the ipsiversive Dix-Hallpike maneuver [47/62 (75.8%) vs. 54/62 (87.7%); chi-square test: p=0.082].
DISCUSSION
In this study, upbeat and ipsiversive torsional nystagmus was mostly observed during SHH in patients with unilateral PC-BPPV confirmed using the Dix-Hallpike maneuver.
The Dix-Hallpike maneuver has been considered the gold-standard for diagnosing PC-BPPV by inducing the ampullofugal migration of otolithic debris in the PC and the associated appearance of characteristic ipsiversive torsional and upbeat nystagmus when it is performed toward the lesion side.17,18,19 Even though the correlation with a pathology involving the PC was established later, the utility of this maneuver was based on aligning the orientation of the PC in the direction of maximal gravity during the positioning, thus effectively inducing ampullofugal migration of otolithic debris in the PC.10 This means that the Dix-Hallpike maneuver should be performed in both directions, and it is difficult to perform in patients with restricted cervical motion or obesity.
Even though SHH was already incorporated into the maneuvers to induce positional nystagmus by Nylén,20 its role in diagnosing PC-BPPV remains to be delineated. Instead, its role has mostly been confined to diagnosing central positional nystagmus or BPPV involving the anterior or horizontal semicircular canals,21,22,23,24 even though a study conducted in China found positional nystagmus typical of PC-BPPV in 22 (78.6%) of 28 patients with PC-BPPV during SHH, which is similar to the findings of the present study.15
This study was prompted by our frequent observations of positional nystagmus typical of PC-BPPV during SHH in patients with unilateral PC-BPPV confirmed using the Dix-Hallpike maneuver. The advantages of SHH over the Dix-Hallpike maneuver lie in its ease of application and the simplicity of requiring only one positioning procedure. If SHH is demonstrated to be effective in diagnosing PC-BPPV, it may be performed before the Dix-Hallpike maneuver, and the modified Epley maneuver may be attempted from the SHH position when the typical positional nystagmus for PC-BPPV is induced during SHH. Indeed, our preliminary results have demonstrated the effectiveness of this procedure.
In this study, the latency and intensity of induced upbeat nystagmus were larger during SHH than during the Dix-Hallpike maneuver toward the lesion side when the ipsiversive torsional and upbeat nystagmus was induced during SHH. This may be related to the differences in the changes in the spatial orientation of the PC during SHH and the Dix-Hallpike maneuver. According to temporal bone dissections25 and imaging analyses,26,27 the PC is directed laterally at from 52.5°±7.7° to 55.8°±4.0° relative to the sagittal plane. According to a previous study,28 deflection of the cupula may be induced only when the aggregates of otolithic debris are with a specific size range. Due to the lateral slanting of the PC during SHH, the gravitational force acting on the otolithic debris parallel to the PC plane would be lower during SHH initially than during the ipsiversive Dix-Hallpike maneuver. Thus, the larger latency of induced positional nystagmus observed during SHH may be explained by the delayed initiation of the migration of the otoconial debris in the PC during SHH.
The higher peak intensity of induced upbeat nystagmus during SHH than during the Dix-Hallpike maneuver was an unexpected finding. The intensity of nystagmus triggered by positional maneuvers depends on the number, size, and density of the otoconia,28 the angle between the plane of the semicircular canals and the force vector of gravity during the positional maneuvers, the moving distance of otolithic debris,29 the speed of positional maneuvers,1 and the amplitude of head motion during the positional maneuvers.29,30 It was not possible to control all of these factors in the present retrospective study, but the higher peak intensity of induced upbeat nystagmus during SHH might be explained by two factors. First, the migration distance of the otolithic debris in the PC is greater during SHH than during the Dix-Hallpike maneuver, since the angle of neck extension below the examination table is mostly larger and the dependent position in the PC is farther from the ampulla during SHH than during the Dix-Hallpike maneuver. The second factor is a higher speed of the positional maneuver, since SHH could be performed faster than the Dix-Hallpike maneuver due to its ease of application and the greater neck mobility during SHH.
Our study was subject to two main limitations. First, its retrospective design did not allow a random allocation of the positioning order or blind evaluation of the positional nystagmus. Second, we could not quantify the torsional nystagmus due to only 2D video-oculography being applied to the patients recruited in China.
Given its relative simplicity, we recommend adopting SHH before the Dix-Hallpike maneuver, which may simplify the diagnosis and treatment process for PC-BPPV. When a patient suspected of having BPPV shows normal results during SHH or the torsional component of the induced nystagmus is inconclusive, the Dix-Hallpike maneuver should be performed to determine the presence and lateralization of PC-BPPV.
Footnotes
- Conceptualization: Xia Ling, Xu Yang, Ji-Soo Kim.
- Data curation: Xia Ling, Jong-Hee Lee.
- Formal analysis: Xia Ling, Hyo-Jung Kim, Ji-Soo Kim.
- Funding acquisition: Ji-Soo Kim.
- Investigation: Xia Ling, Hyo-Jung Kim, Jong-Hee Lee.
- Methodology: Xia Ling, Hyo-Jung Kim, Jong-Hee Lee, Ji-Soo Kim.
- Project administration: Hyo-Jung Kim.
- Resources: Xu Yang, Jeong-Yoon Choi, Ji-Soo Kim.
- Software: Hyo-Jung Kim, Jong-Hee Lee.
- Supervision: Xu Yang, Ji-Soo Kim.
- Validation: Jeong-Yoon Choi, Ji-Soo Kim.
- Visualization: Xia Ling, Hyo-Jung Kim.
- Writing—original draft: Xia Ling.
- Writing—review & editing: Ji-Soo Kim.
Conflicts of Interest: Ji-Soo Kim and Jeong-Yoon Choi, a contributing editor of the Journal of Clinical Neurology, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement: This study was supported by the MYUNG-IN PHARM.CO., LTD. (no. 06-2019-224).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.4.558.
Upbeat and ipsiversive clockwise torsional nystagmus (where the upper poles of the eyes beat toward the right ear) was induced during both straight head hanging and the right Dix-Hallpike maneuver in a patient with benign paroxysmal positional vertigo involving the posterior semicircular canal.
References
- 1.Baloh RW, Sakala SM, Honrubia V. Benign paroxysmal positional nystagmus. Am J Otolaryngol. 1979;1:1–6. doi: 10.1016/s0196-0709(79)80002-2. [DOI] [PubMed] [Google Scholar]
- 2.McClure JA. Horizontal canal BPV. J Otolaryngol. 1985;14:30–35. [PubMed] [Google Scholar]
- 3.Gresty MA, Bronstein AM, Brandt T, Dieterich M. Neurology of otolith function. Peripheral and central disorders. Brain. 1992;115:647–673. doi: 10.1093/brain/115.3.647. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–1147. doi: 10.1056/NEJMcp1309481. [DOI] [PubMed] [Google Scholar]
- 5.von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update) Otolaryngol Head Neck Surg. 2017;156:S1–S47. doi: 10.1177/0194599816689667. [DOI] [PubMed] [Google Scholar]
- 7.De la Meilleure G, Dehaene I, Depondt M, Damman W, Crevits L, Vanhooren G. Benign paroxysmal positional vertigo of the horizontal canal. J Neurol Neurosurg Psychiatry. 1996;60:68–71. doi: 10.1136/jnnp.60.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honrubia V, Baloh RW, Harris MR, Jacobson KM. Paroxysmal positional vertigo syndrome. Am J Otol. 1999;20:465–470. [PubMed] [Google Scholar]
- 9.Vannucchi P, Pecci R. Pathophysiology of lateral semicircular canal paroxysmal positional vertigo. J Vestib Res. 2010;20:433–438. doi: 10.3233/VES-2010-0387. [DOI] [PubMed] [Google Scholar]
- 10.Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354. doi: 10.1177/003591575204500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen HS. Side-lying as an alternative to the Dix-Hallpike test of the posterior canal. Otol Neurotol. 2004;25:130–134. doi: 10.1097/00129492-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Michael P, Oliva CE, Nuñez M, Barraza C, Faúndez JP, Breinbauer HA. An abbreviated diagnostic maneuver for posterior benign positional paroxysmal vertigo. Front Neurol. 2016;7:115. doi: 10.3389/fneur.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon EJ, Lee DH, Park JM, Oh JH, Seo JH. The efficacy of a modified Dix-Hallpike test with a pillow under shoulders. J Vestib Res. 2019;29:197–203. doi: 10.3233/VES-190666. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Park J, Kim JS. Update on benign paroxysmal positional vertigo. J Neurol. 2021;268:1995–2000. doi: 10.1007/s00415-020-10314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong C, Shaohua C, Cuiyuan M. The use of center head hanging Dix-Hallpike in the diagnosis of benign paroxysmal positional vertigo. Journal of Audiology and Speech Pathology. 2008;6:483–485. [Google Scholar]
- 16.Yacovino DA, Hain TC, Gualtieri F. New therapeutic maneuver for anterior canal benign paroxysmal positional vertigo. J Neurol. 2009;256:1851–1855. doi: 10.1007/s00415-009-5208-1. [DOI] [PubMed] [Google Scholar]
- 17.Schuknecht HF. Positional vertigo: clinical and experimental observations. Trans Am Acad Ophthalmol Otolaryngol. 1962;66:319–332. [PubMed] [Google Scholar]
- 18.Schuknecht HF. Cupulolithiasis. Arch Otolaryngol. 1969;90:765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- 19.Hall SF, Ruby RR, McClure JA. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8:151–158. [PubMed] [Google Scholar]
- 20.Nylén CO. Positional nystagmus; a review and future prospects. J Laryngol Otol. 1950;64:295–318. [PubMed] [Google Scholar]
- 21.Yetiser S, Ince D. Vertical nystagmus during the seated-supine positional (straight head-hanging) test in patients with benign paroxysmal positional vertigo. J Laryngol Otol. 2014;128:674–678. doi: 10.1017/S0022215114001480. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Ling X, Shen B, Hong Y, Li K, Si L, et al. Diagnosis strategy and Yacovino maneuver for anterior canal-benign paroxysmal positional vertigo. J Neurol. 2019;266:1674–1684. doi: 10.1007/s00415-019-09312-1. [DOI] [PubMed] [Google Scholar]
- 23.Choi JY, Kim JH, Kim HJ, Glasauer S, Kim JS. Central paroxysmal positional nystagmus: characteristics and possible mechanisms. Neurology. 2015;84:2238–2246. doi: 10.1212/WNL.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 24.Bertholon P, Bronstein AM, Davies RA, Rudge P, Thilo KV. Positional down beating nystagmus in 50 patients: cerebellar disorders and possible anterior semicircular canalithiasis. J Neurol Neurosurg Psychiatry. 2002;72:366–372. doi: 10.1136/jnnp.72.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanks RH, Curthoys IS, Markham CH. Planar relationships of the semicircular canals in man. Acta Otolaryngol. 1975;80:185–196. doi: 10.3109/00016487509121318. [DOI] [PubMed] [Google Scholar]
- 26.Della Santina CC, Potyagaylo V, Migliaccio AA, Minor LB, Carey JP. Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction. J Assoc Res Otolaryngol. 2005;6:191–206. doi: 10.1007/s10162-005-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DK, Kim DR, Jeong SH, Kim GJ, Chang KH, Jun BC. Analysis of the coplanarity of functional pairs of semicircular canals using three-dimensional images reconstructed from temporal bone magnetic resonance imaging. J Laryngol Otol. 2015;129:430–434. doi: 10.1017/S0022215115000201. [DOI] [PubMed] [Google Scholar]
- 28.Valli P, Botta L, Zucca G, Valli S, Buizza A. Simulation of cupulolithiasis and canalolithiasis by an animal model. J Vestib Res. 2008;18:89–96. [PubMed] [Google Scholar]
- 29.Lim HJ, Park K, Park HY, Choung YH. The significance of 180-degree head rotation in supine roll test for horizontal canal benign paroxysmal positional vertigo. Otol Neurotol. 2013;34:736–742. doi: 10.1097/MAO.0b013e31827de2d1. [DOI] [PubMed] [Google Scholar]
- 30.Ping L, Yi-Fei Z, Shu-Zhi W, Yan-Yan Z, Xiao-Kai Y. Diagnosis and treatment of the short-arm type posterior semicircular canal BPPV. Braz J Otorhinolaryngol. 2020 Nov 23; doi: 10.1016/j.bjorl.2020.10.012. [Epub]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upbeat and ipsiversive clockwise torsional nystagmus (where the upper poles of the eyes beat toward the right ear) was induced during both straight head hanging and the right Dix-Hallpike maneuver in a patient with benign paroxysmal positional vertigo involving the posterior semicircular canal.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.