Abstract
RORγt is the master transcription factor for the Th17 cells. Paradoxically, in the intestine, RORγt is coexpressed in peripherally induced regulatory T cells (pTregs) together with Foxp3, the master transcription factor for Tregs. Unexpectedly, by an unknown mechanism, colonic RORγt+ Tregs show an enhanced suppressor function and prevent intestinal inflammation more efficiently than RORγt-nonexpressing pTregs. Although studies have elucidated the function of RORγt in Th17 cells, how RORγt regulates pTreg function is not understood. In our attempt to understand the role of RORγt in controlling Treg function, we discovered a RORγt-driven pathway that modulates the regulatory (suppressor) function of colonic Tregs. We found that RORγt plays an essential role in maintaining Foxp3 expression. RORγt-deficient Tregs failed to sustain Foxp3 expression with concomitant upregulation of T-bet and IFN-γ expressions. During colitis induced by adoptive transfer of CD45RBhi cells in Rag1−/− mice, RORγt-deficient colonic Tregs transitioned to a Th1-like effector phenotype and lost their suppressor function, leading to severe colitis with significant mortality. Accordingly, Foxp3-expressing, RORγt-deficient Tregs showed impaired therapeutic efficacy in ameliorating colitis that is not due to their reduced survival. Moreover, using the Treg-specific RORγt and T-bet double-deficient gene knockout mouse, we demonstrate that deletion of T-bet from RORγt-deficient Tregs restored Foxp3 expression and suppression function as well as prevented onset of severe colitis. Mechanistically, our study suggests that RORγt-mediated repression of T-bet is critical to regulating the immunosuppressive function of colonic Tregs during the inflammatory condition.
Key Points
In the absence of RORγt, colonic Tregs lose Foxp3 expression and suppressor function.
RORγt promotes Foxp3 expression by antagonizing effector fate of colonic Tregs.
RORγt represses T-bet to control the suppressor function of Tregs.
Introduction
Regulatory T cells (Tregs) suppress inflammation and play a key role in the pathogenesis of inflammatory bowel disease (IBD). During inflammation, the function of Tregs is frequently altered, diverting them from “regulatory or suppressor” to “effector” phenotypes, thereby compromising or altering their suppressor function (1–3). Additionally, experimental and clinical data from IBD patients suggest that functional adaptation of Tregs contributes to the development of IBD (3–5). Moreover, responsiveness to biological therapy in IBD patients is associated with increased frequency of FOXP3+ Tregs (6, 7). Therefore, targeting new immune pathways that control Treg function is necessary for devising novel therapeutic strategies against IBD.
RORγt is the master transcription factor (TF) for the Th17 subset of CD4+ T cells (8). Unexpectedly, in the colon, RORγt is coexpressed in Tregs with Foxp3, the master TF for Tregs (9, 10). Colonic RORγt+ Tregs show an enhanced immunosuppressive function and restrain intestinal inflammation more effectively than RORγt-nonexpressing Tregs (9, 10). RORγt+ Tregs constitute 40% of colonic Tregs in humans and are implicated in protection against IBD (9–12). How RORγt regulates colonic Treg function is poorly understood. In this study, we have addressed two fundamental questions: 1) How does RORγt promote the suppressor function of colonic Tregs? 2) How do antagonistic TFs like RORγt and Foxp3 interact to modulate Treg function? In our attempt to understand the role of RORγt in controlling colonic Treg function, we discovered a RORγt-driven signaling pathway that regulates the suppressor function of Tregs. In the absence of RORγt, Tregs failed to maintain Foxp3 expression both in vitro and during colitis. Treg-specific deletion of RORγt worsened colitis with significantly higher mortality. Moreover, in absence of RORγt, Tregs transitioned from a regulatory to a Th1-like effector phenotype with compromised suppressor function associated with downregulation of the immune checkpoint molecule, programmed cell death-1 (PD-1), which is known to be critical for the sustenance of Foxp3 expression and maintenance of Tregs. Combined deletion of RORγt and T-bet restored Foxp3 expression and rescued Rag1−/− mice from severe colitis and mortality. Our findings suggest RORγt antagonizes T-bet for promoting the suppressor function of colonic Tregs. We found that RORγt modulates Tregs by promoting their regulatory function while antagonizing alternative effector fate during colitis. Thus RORγt is a critical switch for modulating regulatory versus effector programs in colonic Tregs than can be effectively exploited in the therapy of IBD.
Materials and Methods
Mice and reagents
The following mice strains used were purchased from Jackson Laboratory: C57BL/6J (B6), Rag1−/−, B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr/J (Foxp3YFP-Cre), B6(Cg)-Rorctm3Litt/J, and B6.129-Tbx21tm2Srnr/J. The mice strains Foxp3YFP-Cre.Rorcfl/fl and Foxp3YFP-Cre Rorcfl/fl Tbx21fl/fl were generated in house. Mice were bred and maintained according to University of Alabama at Birmingham Institutional Animal Care and Use Committee. For all experiments, we used 6- to 10-wk-old male or female mice. All of the Abs were purchased from either eBioscience, BD Biosciences, BioLegend, or Fisher Scientific. For example, CD3, CD4, CD25, CD62L, CD44, CD45RB, CD45.1, CD45.2, PD1 (29F.1A12 or J43), CTLA-4 (UC10-4B9), GITR (DTA-1), T-bet (eBio4B10), Foxp3 (FJK-16S), RORγt (AFKJS-9), Gata-3 (16E10A23), IL-17A (TC11-18H10 or eBio17B7), IFN-γ (XMG1.2), IL-6 (MP5-20F3), and IL-23 (G23-8) were used.
In vitro Treg and Th17 differentiation
Naive CD4+ T cells from spleens or lymph nodes of 8- to 10-wk-old male or female mice were purified by flow cytometric sorting on a FACS Aria II instrument (BD Bioscience) by gating on the CD4+CD25−CD62LhiCD44lo fraction. Sorted naive CD4+ T cells from wild type (WT) B6, Foxp3YFP-Cre, Foxp3YFP-Cre.Rorcfl/fl, or Foxp3YFP-Cre.Rorcfl/flTbx21fl/fl mice were stimulated polyclonally in the presence of plate-bound anti-CD3 (2C11; 5 μg/ml) and soluble anti-CD28 (37.51; 5μg/ml) along with the presence or absence of 1 nM all-trans retinoic acid (RA; Sigma) and rhTGF-β (5 ng/ml; R&D Systems) in RPMI-1640 containing 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, NEAA, 50 μM 2-ME, and 2 mM l-glutamine (R10). For Th17 culture, naive cells were stimulated with rhTGF-β, recombinant mouse IL-6 (20 ng/ml; R&D Systems), and neutralizing Abs to IFN-γ (10 μg/ml) and IL-4 (10 μg/ml). For Ag-specific Treg differentiation, sorted naive OT-II TCR transgenic CD4+ T cells were activated with 5 μg/ml OVA peptide in presence of irradiated, T cell–depleted splenic feeder cells obtained from CD45.1 congenic mouse under Treg differentiation condition.
Suppression assay
Following in vitro Treg polarization of naive CD4+ T cells from either CD45.2 congenic Foxp3YFP-Cre, Foxp3YFP-Cre.Rorcfl/fl, or Foxp3YFP-Cre.Rorcfl/flTbx21fl/fl mice, cells were differentiated under Treg conditions. On day 5, live yellow fluorescent protein+ (YFP+) cells (expressing Foxp3) from three different groups were purified by sorting. Next, CFSE-labeled CD45.1 congenic naive CD4+ T responder cells were incubated with Tregs from a different group at a ratio of 1:1 for 72 h and analyzed for proliferation of responder cells by FACS.
Chronic colitis induction and Treg therapy
For the adoptive T cell transfer model, CD25−CD45RBhi CD4+ T cells (4 × 105 per mouse) from either Foxp3YFP-Cre, Foxp3YFP-Cre.Rorcfl/fl, or Foxp3YFP-Cre.Rorcfl/fl Tbx21fl/fl mice were i.p. injected into age- and sex-matched Rag1−/− mice (8–10 wk old, males or females) for induction of colitis, and all recipient mice were monitored for 10 wk. For the Treg therapy experiment, in vitro–differentiated Tregs (CD45.2+) from two different groups of mice (Foxp3YFP-Cre and Foxp3YFP-Cre.Rorcfl/fl) were adoptively transferred (5 × 105 cells per mouse) to CD45.1 naive CD4+ T cell–recipient Rag1−/− mice 2 wk after first transfer. At 7–8 wk post-transfer, all Rag1−/− recipient mice were sacrificed for determination of their colonic inflammation score and isolation of lamina propria (LP) CD4+ T cells for analysis of Foxp3, PD-1, CTLA-4, and GITR expression in the CD45.2 compartment.
Isolation and intracellular staining of colonic CD4+ T cells
LP lymphocytes were isolated as described previously (13). Briefly, the large intestine was removed, cleared of luminal contents and fat, cut into small pieces, and washed in chilled HBSS without Ca2+ or Mg2+. Minced tissue pieces were incubated in the presence of EDTA for 30 min and vortexed thoroughly to remove epithelial cells, and then incubated in RPMI-1640 containing collagenase IV (1 mg/ml; Sigma-Aldrich), dispase (0.5 mg/ml; Life Technologies, Invitrogen), and DNaseI (0.25 mg/ml; Sigma-Aldrich). LP lymphocytes were collected. For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml; Sigma) and ionomycin (750 ng/ml; Calbiochem) for 4 h in the presence of GolgiPlug (BD PharMingen). For the detection of intracellular cytokines and TF expression, cells were fixed and permeabilized, either in Foxp3 staining buffer (eBioscience) or BD Permeabilization Buffer. In all cases, LIVE/DEAD Fixable Near-IR Dead Cell Stain (Invitrogen) was included prior to surface staining to exclude dead cells in flow cytometric analyses.
Real-time RT-PCR
Total RNA from colonic LP CD4+ T cells was isolated as per the manufacturer’s instructions (Qiagen). cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System (Invitrogen) and real-time PCR was performed on QuantStudio3 (Applied Biosystems) using PowerUp SYBR Green Supermix along with the following primers: for Tbx21, 5′-ACCAGAGCGGCAAGTGGG-3′ (forward) 5′-TGGACATATAAGCGGTTCCCTG-3′ (reverse); for Gata-3, 5′-TTTACCCTCCGGCTTCATCCTCCT-3′ (forward) and 5′-TGCACCTGATACTTGAGGCACTCT-3′ (reverse); for 18S rRNA, 5′-GCCGCTAGAGGTGAAATTCTTG-3′ (forward) and 5′-CATTCTTGGCAAATGCTTTCG-3′ (reverse). Reactions were run in triplicate and samples were normalized to 18S as a fold induction over controls.
Histology
Tissue samples obtained from proximal, middle, and distal portions of large intestines were fixed in 10% neutral buffered formalin, embedded in paraffin to prepare 5 μm sections, and then stained with H&E. The tissue sections were examined and scored to evaluate tissue pathology, as previously described (14). In all scoring, the identity of the specimens was concealed from the pathologist.
Statistical analysis
All statistical analyses were done using GraphPad Prism software. Two-way ANOVA with Bonferroni post hoc test was used to analyze body weight loss data. Mann–Whitney U test or Kruskal–Wallis test was used to determine the significance of the histopathological score from the colon sections. For the rest of the data, p values were calculated using the two-tailed, unpaired Student t test with one-way ANOVA, followed by the Tukey post hoc test, as described in the figure legends. All p values ≤ 0.05 were considered significant.
Results
Synergistic signaling by TGF-β and RA stabilizes RORγt expression during Treg differentiation
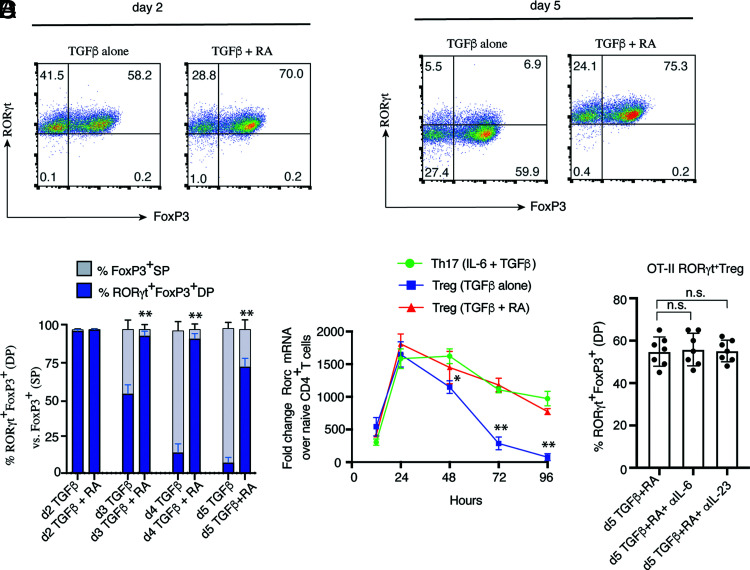
In the colon under homeostasis, RA, IL-6, and IL-23 are important for the induction or maintenance of RORγt+ Tregs (9, 10). Because the signaling requirements for in vitro RORγt+ Treg differentiation have not been established, we wanted to examine how these signaling pathways contribute to the primary differentiation of RORγt+ Tregs, as opposed to Tregs developing in the presence of TGF-β alone (9, 10). To decipher the optimal signaling requirement for the generation RORγt+ Tregs in vitro, we found TGF-β alone induced RORγt at an early time point, which is consistent with published reports (15, 16). However, TGF-β alone failed to sustain RORγt expression in Tregs during late stages of Treg differentiation (Fig. 1A, 1B). Although, during the early stages of differentiation, >90% of Tregs coexpressed RORγt and Foxp3 in the presence of TGF-β alone, expression of RORγt was diminished significantly from day 2 onwards, with only ∼5–15% retaining coexpression of RORγt and Foxp3 at day 5. However, in the presence of RA, which is abundantly present in the colon, a large population of Foxp3+ Tregs (>90%) maintained RORγt until late stages of Treg differentiation (Fig. 1A, 1B). At the transcriptional level, there was a rapid downregulation of Rorc transcripts after 24 h postdifferentiation in Tregs growing in the presence of TGF-β alone (Fig. 1C). Between 72 and 96 h, Rorc mRNA expression in Tregs grown in the presence of TGF-β alone was completely abrogated. In contrast, addition of RA along with TGF-β maintained a high level of Rorc transcripts throughout the period of observation and was comparable to Rorc mRNA expression in Th17 cells grown in the presence of IL-6 and TGF-β. Therefore, this suggests that synergistic effects of RA and TGF-β signaling is a critical signaling requirement for in vitro differentiation of RORγt+ Tregs. Because APC-derived IL-6 and IL-23 affect in vitro T cell differentiation (14), we further examined the roles of IL-6 and IL-23 during the priming phase of RORγt+ Treg differentiation. To do this, we used OVA-specific naive CD4+ T cells from OT-II TCR transgenic mice that were differentiated with TGF-β and RA in the absence or presence of neutralizing IL-6 and IL-23 Abs to block the effect of the cytokines secreted from APC. Blocking of IL-6 or IL-23 did not have any impact on the primary differentiation of OVA-specific RORγt+ Treg generation, where presence of both RA and TGF-β were sufficient to stabilize RORγt expression along with Foxp3 at day 5, irrespective of blocking IL-6 or IL-23 signaling from APCs (Fig. 1D). We also obtained the same result using Il6−/− and Il23a−/− APCs (data not shown). The results indicated that, although RORγt+ Treg differentiation is critically reliant on combined TGF-β and RA signaling, IL-6 and IL-23 are not essential during the priming phase of their development.
FIGURE 1.
Dual signaling by TGF-β and RA is necessary for the generation of RORγt+ Tregs in vitro. (A) Sorted naive WT CD4+ T cells were differentiated under Treg conditions either with TGF-β alone or TGF-β plus all-trans RA in presence of plate-bound αin pCD3 and soluble anti-CD28 and analyzed for Foxp3 and RORγt expression on day 2 (left) and day 5 (right) by flow cytometry. (B) Bar diagram represents percentage RORγt+Foxp3+ double-positive (DP) versus Foxp3+ single-positive (SP) cells in Tregs differentiated in presence of TGF-β with or without RA. Expression of RORγt and Foxp3 were observed from day 2 until day 5 of Treg culture by flow cytometry (n = 6). (C) RORγt transcripts were analyzed by real-time RT-PCR from Tregs differentiated under Treg conditions (TGF-β alone or TGF-β with RA) and under Th17 conditions (n = 6). (D) Sorted naive CD4+ T cells from OT-II transgenic mice were differentiated under Treg conditions with TGF-β plus RA in presence of irradiated APCs, mixed at a ratio of 1:5 (T cells/APCs), and OVA peptide (323-339) with or without anti–IL-6 and anti–IL-23 neutralizing Ab (10 μg/ml) and analyzed for Foxp3 and RORγt expression at day 5 by flow cytometry (n = 7). Bar diagrams represent percentage of Foxp3 and RORγt coexpressing Tregs. For FACS analysis in (A) and (B), cells were gated on live CD4+ T cells. Data in (B)–(D) are shown as mean ± SEM. Data are representative (or pooled results) of two or more independent experiments. For p values, two-tailed unpaired Student t test was used in (B); one-way ANOVA followed by Tukey post hoc test was used for (C) and (D). *p < 0.01, **p < 0.001. n.s, not significant.
RORγt maintains Foxp3 expression and prevents Th1-like effector program in Tregs
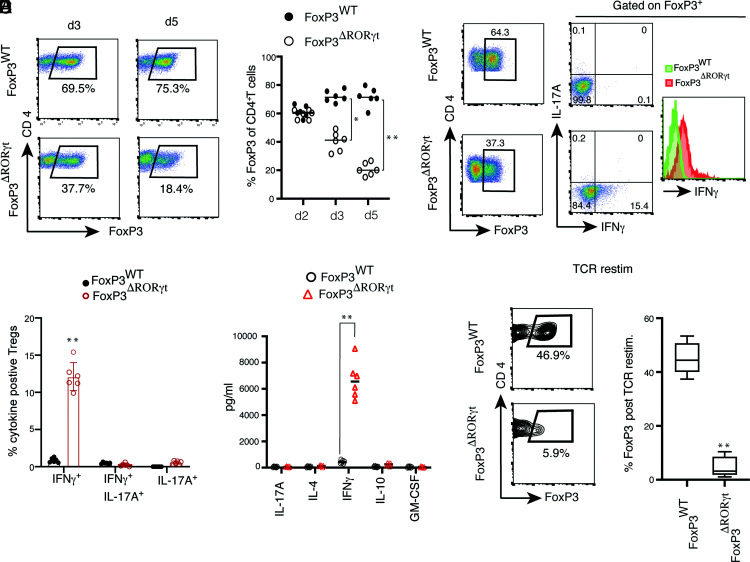
After establishing the in vitro condition for RORγt+ Treg differentiation, we next determined if deletion of RORγt from Foxp3-expressing cells has an effect on Treg differentiation. We sorted naive CD4+ T cells from Foxp3YFP-Cre and Foxp3YFP-Cre.Rorcfl/fl mice, where, in the latter, RORγt is selectively deleted from Foxp3-expressing cells. Although there was a significant difference in Foxp3 expression between the two groups at 72 h of the Treg differentiation condition, during early Treg differentiation (48 h) there was a comparable frequency of Foxp3+ cells in RORγt-deficient Tregs (Fig. 2A, 2B). By day 5, there was a severe reduction in Foxp3 frequency in RORγt-deficient Tregs, where >60% of the Tregs lost Foxp3 expression. Because RORγt is a potent inducer of IL-17, we examined IL-17 induction along with IFN-γ from Foxp3WT and Foxp3ΔRORγt Tregs. Whereas Foxp3WT Tregs did not express either IL-17 or IFN-γ, Foxp3ΔRORγt Tregs expressed IFN-γ without expression of IL-17A (Fig. 2C, 2D). Strikingly, >10% of Foxp3ΔRORγt Tregs were IFN-γ producers and there was an overall shift in IFN-γ intensity from RORγt-deficient Foxp3+ Tregs. Analysis of the cytokines obtained from the culture supernatant of Foxp3WT and Foxp3ΔRORγt Tregs by multiplex ELISA revealed ∼500-fold enhanced IFN-γ secretion from RORγt-deficient Tregs, whereas IL-4, GM-CSF, IL-10, and IL-17 levels were comparably low in both groups (Fig. 2E). Among all cytokines tested, IFN-γ was the most upregulated cytokine in RORγt-deficient Tregs. Together, this indicated that RORγt stabilizes Foxp3 expression during Treg development, and Foxp3ΔRORγt Tregs acquire a Th1-like effector phenotype. Furthermore, to examine the stability of Tregs, YFP+ (Foxp3+) cells were sorted from two groups of Tregs differentiated from Foxp3WT and Foxp3ΔRORγt naive CD4+ T cells in the presence of TGF-β plus RA and were subjected to TCR restimulation. TCR restimulation of Foxp3ΔRORγt Tregs completely abrogated Foxp3 expression (>90%) compared with Foxp3WT Tregs (Fig. 2F). Furthermore, to verify that the cytokines are indeed secreted from Foxp3+ Tregs and not from non-Foxp3+ cells present in the culture, following in vitro Treg stimulation, sorted YFP+ (Foxp3+) cells from the two groups were subjected to TCR restimulation and supernatants were assayed to detect an array of differentially regulated cytokines and chemokines (44-plex ELISA). Among all of the cytokines and chemokines analyzed, IFN-γ was the only cytokine that was highly upregulated, although there was a slight but significant upregulation of IL-5 from YFP+ΔRORγt cells (Supplemental Fig. 1A). Next, we analyzed the expression of both Tbx21 and Gata-3 transcripts in sorted live CD4+ T cells from YFPWT and YFPΔRORγt groups following TCR restimulation. Although Tbx21 transcript was ∼50-fold upregulated in YFPΔRORγt cells, there was comparably low expression of Gata-3 transcript in both groups of Tregs (Supplemental Fig. 1B and 1C). Together, the results indicate that, in absence of RORγt, Tregs fail to maintain Foxp3 and transition to a Th1-like effector fate.
FIGURE 2.
RORγt represses IFN-γ induction and is critical for maintenance of Foxp3 during Treg differentiation. (A) Naive CD4+ T cells from Foxp3YFP-Cre (Foxp3WT) and Foxp3YFP-Cre.Rorcfl/fl (Foxp3ΔRORγt) mice were differentiated in vitro under Treg conditions in presence of plate-bound anti-CD3 and soluble anti-CD28 stimulation and analyzed for Foxp3 and CD4 expressions on day 3 and day 5. For flow cytometric analysis, cells were gated on live CD4+ T cells. (B) Kinetics of maintenance of Foxp3+ cells in Treg culture from the two above-mentioned groups (n = 6). (C and D) Expression of IL-17A and IFN-γ from gated Foxp3-expressing cells of WT and RORγt-deficient Tregs. Naive CD4+ T cells from Foxp3YFP-Cre and Foxp3YFP-Cre.Rorcfl/fl mice were differentiated under Treg conditions and analyzed for CD4, IL-17A, IFN-γ, and Foxp3 following PMA and ionomycin stimulation on day 3, as shown in representative FACS plots (C) and bar diagram showing frequencies of IL-17A+, IFN-γ+, and IL-17A+ IFN-γ+ cells from two above-mentioned groups of Tregs (D). (E) Cytokine ELISA from culture supernatants of RORγt-sufficient and -deficient in vitro–differentiated Tregs at day 4 (n = 6). (F) TCR restimulation of in vitro–generated Foxp3+ Tregs analyzed for retention of Foxp3 expression, as shown in representative contour FACS plots and bar diagrams (n = 7). Data in (B) and (D)–(F) are shown as mean ± SEM. Data are representative of, or pooled from, two or more independent experiments. For p values, the two-tailed unpaired Student t test was used in (D)–(F). *p < 0.01, **p < 0.001.
Deletion of T-bet restores Foxp3 expression and suppressor function of RORγt-deficient Tregs
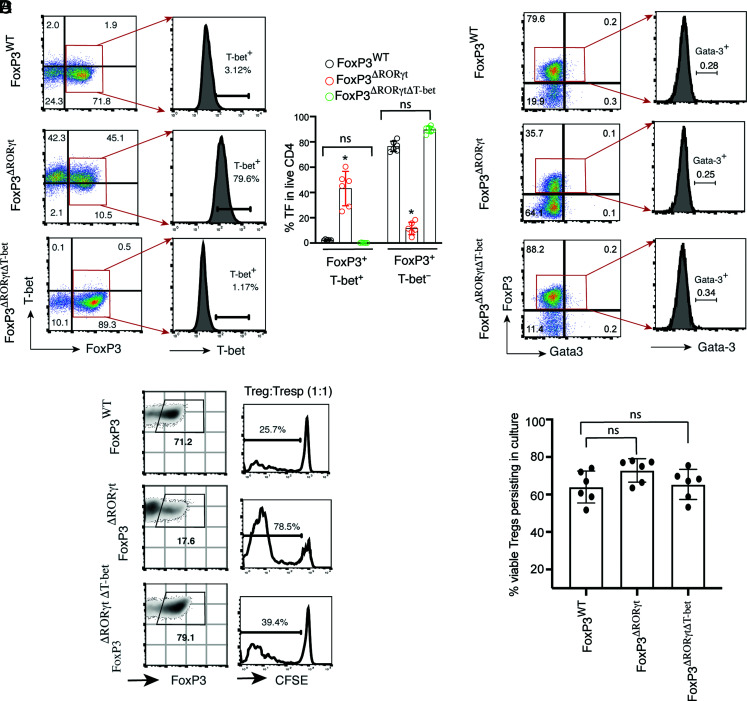
To understand whether the failure to maintain Foxp3 with concomitant high IFN-γ production in Foxp3ΔRORγt Tregs leads to the loss of suppressor function and acquisition of effector potential, we initially compared T-bet expression between Foxp3WT and Foxp3ΔRORγt Tregs. T-bet was highly induced in RORγt-deficient Tregs, with an accumulation of a high frequency (>75%) of T-bet–coexpressing Foxp3+ cells (Fig. 3A). To understand whether the acquisition of T-bet in the absence of RORγt leads to the loss of Foxp3 expression in RORγt-deficient Tregs, we generated the Foxp3YFP-Cre.Rorcfl/fl.Tbx21fl/fl double-deficient conditional gene knockout mouse (Foxp3ΔRORγtΔT-bet), where both Rorc and Tbx21 genes are simultaneously deleted upon expression of Foxp3. Surprisingly, additional deletion of T-bet in Foxp3ΔRORγt Tregs completely restored Foxp3 expression, which was comparable to that of Foxp3WT Tregs (Fig. 3A). However, we could not detect Gata-3 expression from in vitro–differentiated Foxp3WT, Foxp3ΔRORγt, and Foxp3ΔRORγtΔT-bet Tregs, which was comparably low in all of the groups, supporting previous reports that, unlike RORγt+ Tregs, Gata-3 is expressed in Helios-expressing Tregs and are mostly of thymic origin (Fig. 3B) (9, 10, 17). We then assessed whether deletion of RORγt impairs the suppressor function of Foxp3+ Tregs. Foxp3ΔRORγt Tregs showed compromised suppressor function compared with Foxp3WT Tregs as CFSE-labeled responder cells showed >3-fold higher proliferation when coincubated with RORγt-deficient Foxp3+ Tregs (Fig. 3C). Deletion of T-bet from Foxp3ΔRORγt Tregs restored their impaired suppressor function. Because all Tregs included in the culture for the suppression assay were initially 100% positive for Foxp3 expression, the defective suppressor function of Foxp3ΔRORγt Tregs is likely due to the failure to maintain Foxp3 expression. Moreover, the percentage of viable Tregs persisting in culture did not vary among the three groups, suggesting the impaired suppressor function of Foxp3ΔRORγt Tregs is not due to their compromised viability over time (Fig. 3D).
FIGURE 3.
Deletion of T-bet restores Foxp3 expression and suppressor function of RORγt-deficient Foxp3+ Tregs. (A) Naive CD4+ T cells from Foxp3YFP-Cre, Foxp3YFP-Cre.Rorcfl/fl, and Foxp3YFP-Cre.Rorcfl/fl.Tbx21fl/fl (Foxp3ΔRORγtΔT-bet) mice were differentiated under Treg conditions and analyzed for expression of Foxp3 and T-bet in gated live CD4+ T cells, as shown by representative FACS plots (left); T-bet expression in Foxp3+ cells, as shown in the histograms (middle); and percentage Foxp3+T-bet+ versus Foxp3+Tbet− cells in Tregs from the three indicated groups (right; n = 6). (B) Analysis of Foxp3 and Gata-3 expressions in gated live CD4+ T cells from the three indicated Treg groups, as shown by representative FACS plots (left), and expression of Gata-3 in gated Foxp3+ cells shown by histograms (right). (C) For suppression assay, YFP+ (Foxp3+) cells from in vitro–differentiated Tregs of the three groups were sorted on day 5 (left), incubated with CFSE-labeled responder cells (CD45.1+) at a 1:1 ratio for 72 h in presence of plate-bound anti-CD3 and soluble anti-CD28 stimulation, and assayed for proliferation of CFSE-labeled responder cells by flow cytometry (right). (D) Measurement of percentage viable Tregs present in the culture when incubated with CFSE-labeled CD45.1+ responder cells (1:1) for suppression assay after 72 h (n = 6). Data in (A) and (D) are shown as mean ± SEM. Data are representative of at least two independent experiments. For p values, two-tailed unpaired Student t test used in (A), and one-way ANOVA followed by Tukey post hoc test used in (D). *p < 0.001. n.s, not significant.
We also examined how the frequency of colonic Foxp3+ Tregs is affected in the absence of RORγt and in the combined absence of RORγt and T-bet at homeostasis. We noted a significant reduction in the frequency of colonic Foxp3+ Tregs in B6 Foxp3YFP-Cre.Rorcfl/fl mice compared with B6 WT Foxp3YFP-Cre mice that was restored in Foxp3YFP-Cre.Rorcfl/fl.Tbx21fl/fl mice at homeostasis. There was also a significant reduction in the amount of Foxp3 expression from colonic Tregs of Foxp3YFP-Cre.Rorcfl/fl mice (Supplemental Fig. 2A–C). The reduction in the expression of Foxp3 in the absence of RORγt was also restored in the colonic Tregs of Foxp3YFP-Cre.Rorcfl/fl.Tbx21fl/fl mice. Moreover, expression of T-bet in the RORγt-deficient, Foxp3-expressing colonic Tregs was elevated compared with RORγt-sufficient colonic Tregs. Similar to our in vitro findings, this indicates that Foxp3ΔRORγt Tregs, even at homeostasis, express higher amounts of T-bet, suggesting RORγt is critical for repressing T-bet even in homeostasis (Supplemental Fig. 2D). In agreement with a previously published study (9), RORγt deficiency resulted in an increased frequency of Gata-3+Foxp3+ cells from colonic Tregs. However, the frequency of Gata-3+Foxp3+ cells was significantly downregulated in colonic Tregs of Foxp3YFP-Cre.Rorcfl/fl.Tbx21fl/fl mice and was comparable with the WT mouse (Supplemental Fig. 2E). Thus, when T-bet was additionally deleted from Foxp3 Tregs, the compensatory increase in colonic Gata-3+ Tregs reverted to normal levels, suggesting combined absence of RORγt and T-bet restores normal tolerogenic responses at homeostasis. Together, the findings suggest that, in Tregs, RORγt represses T-bet to sustain Foxp3 expression to promote their suppressor function.
Treg-specific deletion of RORγt causes aggressive chronic colitis with high mortality
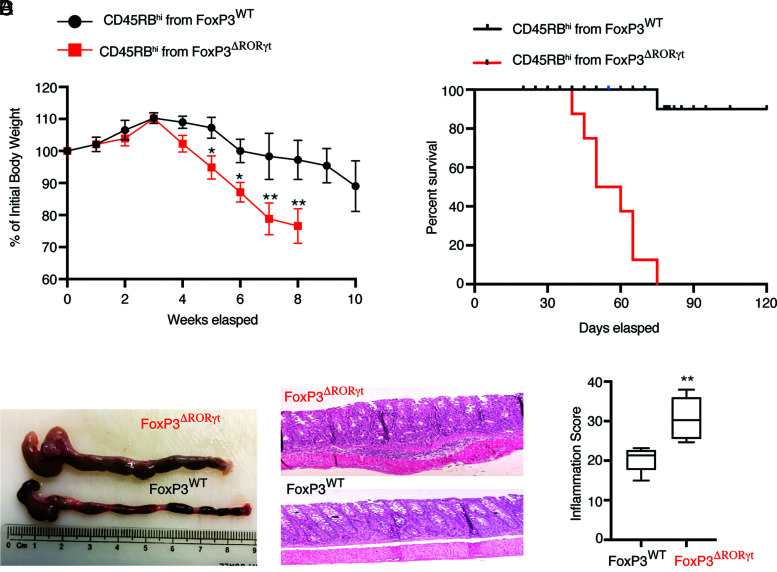
Peripherally induced Foxp3+ Tregs play a central role in the chronic intestinal inflammation induced by adoptive transfer of CD4+ T cells into lymphopenic hosts (5, 18, 19). In this T cell transfer model of colitis, endogenous Foxp3+ Tregs generated “in situ” play a critical role to control disease severity because functional inactivation of Foxp3 causes accelerated death due to colitis (5). To specifically investigate the role of RORγt in peripheral Treg function during autoimmune colitis, naive CD45RBhi CD4+ T cells isolated from Foxp3YFP-Cre and Foxp3YFP-Cre.Rorcfl/fl mice were adoptively transferred to two Rag1−/− recipient groups. The Rag1−/− recipient group that received naive Foxp3ΔRORγt cells, where RORγt is deleted from Foxp3+ Tregs, showed signs of early weight loss, severe colonic inflammation with shortened colons, and damaged epithelial layers (Fig. 4). Importantly, Foxp3ΔRORγt CD45RBhi recipient Rag1−/− mice started succumbing to colitis by 45 d post-transfer and they exhibited 100% mortality by 70 d post-transfer, whereas 90% of Foxp3WT CD45RBhi recipient Rag1−/− mice continued to survive beyond 120 d post-transfer. Foxp3ΔRORγt CD45RBhi recipient Rag1−/− mice showed severe colonic inflammation compared with the WT CD45RBhi recipient group (Fig. 4C, 4D). This suggested that endogenous RORγt-deficient colonic Tregs fail to rescue the mice from the onset of severe colonic inflammation.
FIGURE 4.
Treg-specific deletion of RORγt causes severe colitis with high mortality. Sorted naive CD45RBhiCD4+ T cells (4 × 105 per mouse i.p.) from Foxp3WT and Foxp3ΔRORγt mice were adoptively transferred to Rag1−/− recipient mice for colitis induction. (A) Kinetics of loss of body weight expressed as a percentage of starting weight (left) and survival kinetics (right) of the two indicated groups of Rag1−/− recipients. (B, C, and D) Dissected colons (B), representative histopathology of H&E-stained colonic sections (original magnification ×40) (C), and inflammation score (D) at 6 wk post–adoptive transfer in two indicated groups of Rag1−/− recipient mice. Data in (A) and (D) are shown as mean ± SEM. Data are representative of three independent experiments (n = 8–10 per group). For p values, two-way ANOVA with Bonferroni post hoc test used in (A), Mann–Whitney U test used in (D). *p < 0.01, **p < 0.001.
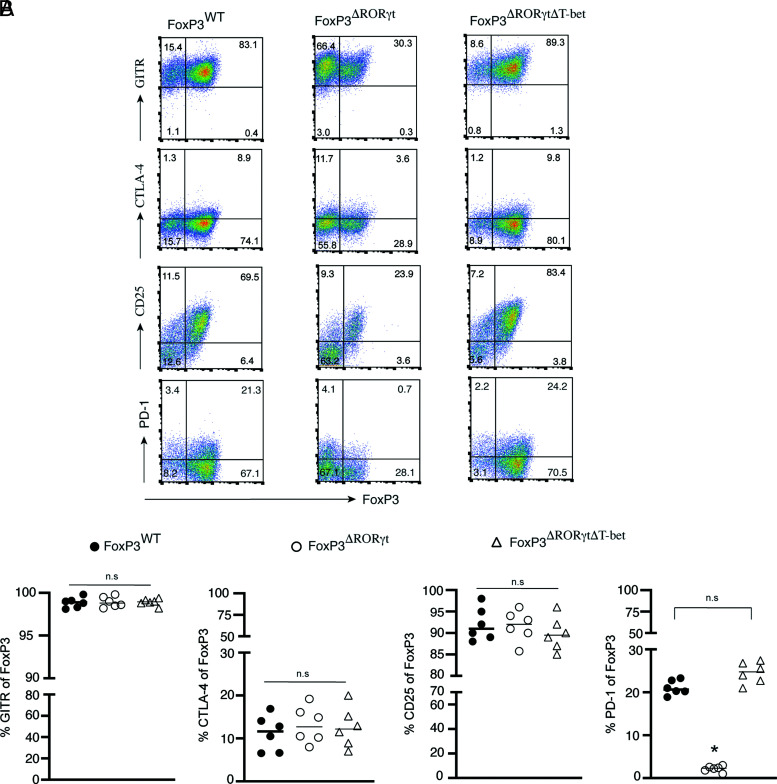
PD-1 is downregulated in RORγt-deficient Foxp3+ Tregs in a T-bet–dependent manner
T-bet is a direct repressor of the Pdcd1 gene encoding PD-1, a checkpoint protein expressed on T lymphocytes (20, 21). PD-1 is critical for the maintenance and function of peripherally induced Foxp3+ Tregs (22–26). PD-1 signaling specifically maintains Foxp3 expression in peripherally induced Tregs (25). Accordingly, inhibition of PD-1 signaling causes several autoimmune diseases, including IBD (27, 28). Because RORγt-deleted Tregs induce high T-bet, we analyzed PD-1 expression along with other activation markers of Tregs (CTLA-4, GITR, and CD25) that play a crucial role in Treg suppressor function (23, 24, 29). Along with in vitro–differentiated Foxp3WT and Foxp3ΔRORγt Tregs, we also included Foxp3ΔRORγtΔT-bet Tregs to determine the additional impact of T-bet deletion from Foxp3ΔRORγt Tregs on the expression of the Treg activation markers. Although the frequency of GITR, CTLA-4, and CD25 in Foxp3+ cells did not vary among the three groups, PD-1 expression was highly reduced in Foxp3ΔRORγt Tregs, but completely restored in Foxp3ΔRORγtΔT-bet Tregs, which was comparable to WT Tregs (Fig. 5A, 5B). This suggested the high T-bet induction in Foxp3ΔRORγt repressed PD-1 expression that was then restored in Tregs with combined deletion of T-bet and RORγt. In contrast, the expression of GITR, CTLA-4, and CD25 remained comparable in both Foxp3ΔRORγt and Foxp3ΔRORγtΔT-bet Tregs, suggesting RORγt had a minimal impact in modulating their expression. Therefore, repression of T-bet by RORγt is one of the critical mechanisms for upregulating PD-1 expression in Foxp3+ Tregs.
FIGURE 5.
PD-1 is downregulated in RORγt-deficient Tregs in a T-bet–dependent manner. Naive CD4+ T cells sorted from Foxp3WT, Foxp3ΔRORγt, and Foxp3ΔRORγtΔT-bet mice, were differentiated under Treg conditions and analyzed for surface expression of GITR, CD25, PD1, and both surface and intracellular expression of CTLA-4 along with Foxp3 gated on live CD4+ T cells at day 4 by flow cytometry, as shown in representative FACS plots (A) and scatterplots showing the percentage expression of GITR, CTLA-4, CD25, and PD-1 in gated Foxp3-expressing cells of the indicated groups (B). Data in (B) are shown as mean ± SEM (n = 6). Data are representative of two independent experiments. For p values, one-way ANOVA followed by Tukey post hoc test used in (B). *p < 0.001. n.s, not significant.
RORγt is essential to maintain Foxp3 expression in colonic Tregs during colitis
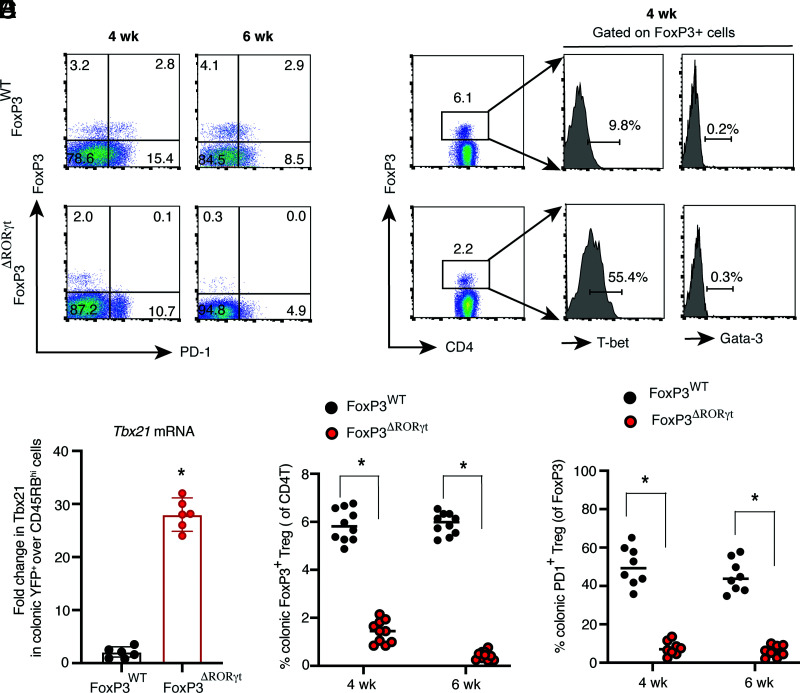
Because we observed that Treg-specific deletion of RORγt causes severe colitis with high mortality, we wanted to understand whether peripherally induced colonic RORγt-deficient Tregs lose Foxp3 and transition to Th1-like effector Tregs during chronic colitis. The Rag1−/− group, which received naive CD4+ T cells from Foxp3YFP-Cre.Rorcfl/fl mice, showed a highly reduced frequency of colonic Foxp3+ cells, both at 4 and 6 wk post-transfer. During the peak of the disease (6 wk), there was >90% reduction in the frequency of colonic Foxp3+ Tregs in Rag1−/− recipient groups that received Foxp3ΔRORγt CD45RBhi cells, compared with the group that received naive Foxp3WT CD4+ T cells (Fig. 6A, 6D). In support of our in vitro finding, RORγt-deleted colonic endogenous Tregs showed significantly higher T-bet expression at both the protein and RNA levels (>5-fold), suggesting a critical role of the RORγt-driven T-bet–PD-1 pathway in colonic Treg function during colitis (Fig. 6B, 6C). Similar to our in vitro finding, Gata-3 levels were comparably low in endogenous Foxp3+ Tregs in both Rag1−/− recipient groups that received either naive Foxp3WT or Foxp3ΔRORγt CD4+ T cells. Whereas ∼50% of colonic Foxp3+ Tregs from naive Foxp3WT cell recipient Rag1−/− mice expressed PD-1, its expression was nearly abrogated in Tregs from the Foxp3ΔRORγt recipient group (Fig. 6A, 6E). Together, the results show that RORγt-deficient Tregs fail to maintain Foxp3 and upregulate T-bet during colitis, indicating a critical role of RORγt in the maintenance of Foxp3+ Tregs during inflammation.
FIGURE 6.
Colonic RORγt-deficient Tregs fail to maintain Foxp3 with high T-bet and low PD-1 expression during colitis. (A) Analysis of Foxp3 and PD-1 expression in colonic CD4+ LP cells obtained from two indicated groups of Rag1−/− recipients that received CD45RBhi Foxp3WT or Foxp3ΔRORγt CD4+ T cells at 4 wk and 6 wk post-transfer by flow cytometry. (B) Expression of T-bet and Gata-3 in colonic Foxp3+ cells from the two indicated groups of Rag1−/− recipients. (C) Tbx21 mRNA expression from sorted, live colonic YFP+ CD4+ T cells from the two indicated groups of Rag1−/− mice at 6 wk post-transfer (n = 6). (D) Frequency of colonic Foxp3+ Tregs in the two indicated Rag1−/− recipient groups at 4 wk and 6 wk post-transfer (n = 10 per group). (E) Frequency of PD-1–expressing colonic Foxp3+ Tregs in the two indicated groups of Rag1−/− recipients (n = 8 per group). Data in (C)–(E) are shown as mean ± SEM. Data are representative of three independent experiments (D and E) or pooled from two independent experiments (C). For p values, two-tailed unpaired Student t test used in (C)–(E). *p < 0.0001.
RORγt-deficient Tregs show compromised therapeutic efficacy in treating colitis and transition to IFN-γ–producing Th1-like cells
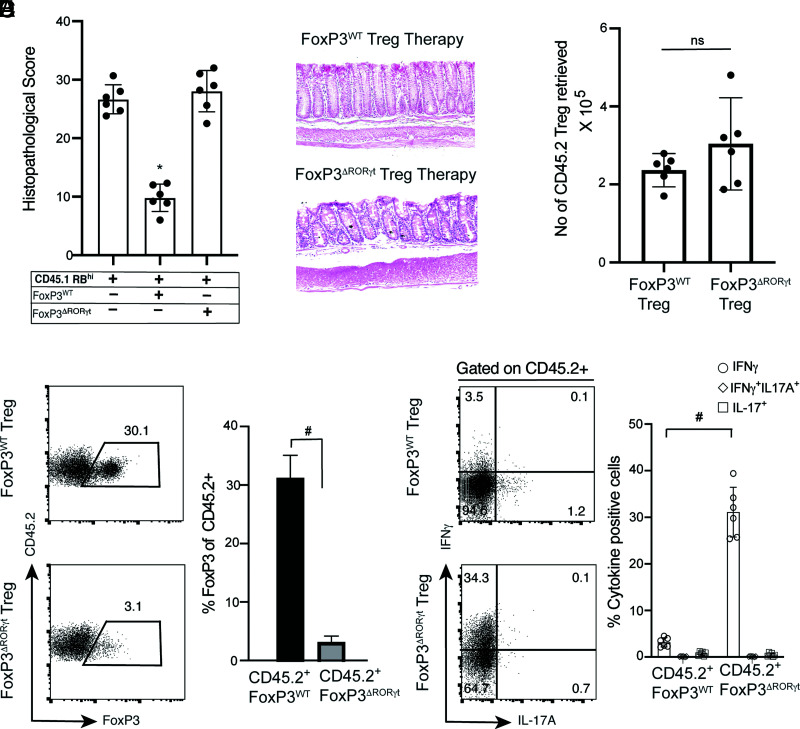
Therapy with adoptively transferred Tregs ameliorates colitis in Rag1−/− mice (5, 19). Although we found that Foxp3ΔRORγt Tregs induce high levels of T-bet associated with loss of Foxp3, a previous study has shown that T-bet expression in Tregs was required for enhancing Treg function during type-1 inflammation (30). Therefore, we examined whether RORγt-deficient Tregs, which promote T-bet expression from Foxp3-expressing cells, could retain their suppressive function in vivo. We examined the comparative therapeutic efficacy of sorted YFP+ cells from in vitro–differentiated Foxp3WT and Foxp3ΔRORγt Tregs in treating colitis by adoptively transferring CD45.2+ YFP+Foxp3WT and YFP+Foxp3ΔRORγt Tregs in Rag1−/− recipient groups 2 wk after initial CD45.1+RBhi transfer. Whereas therapy with Foxp3WT Tregs significantly reduced the severity of colitis, therapy with Foxp3ΔRORγt Tregs did not have any impact on disease amelioration (Fig. 7A, 7B). Although the number of CD45.2+ Tregs retrieved from colonic LP of Rag1−/− recipient mice 6 wk post-transfer of CD45RBhi transfer was comparable between the two groups, the frequency of Foxp3-expressing cells from transferred Foxp3ΔRORγt Tregs declined 10-fold more than Foxp3WT Tregs in the CD45.2 compartment (Fig. 7C). The result suggests that impaired suppressor function is not the result of a reduced number or survival of Foxp3ΔRORγt Tregs. Under an inflammatory condition, transferred CD45.2+Foxp3ΔRORγt Tregs not only lost suppressor function but also showed high IFN-γ expression (>35%) compared with WT Tregs, suggesting RORγt in Tregs maintains the tolerogenic response by preventing the transition to a Th1-like effector phenotype (Fig. 7D, 7E). WT Tregs not only restricted the inflammation in the colon but also showed significant retention of Foxp3, with <5% of Foxp3WT Tregs expressing IFN-γ. In contrast, IL-17A expression was comparably low in both Foxp3WT and Foxp3ΔRORγt Tregs, indicating RORγt expression in Tregs, unlike Th17 cells, does not promote IL-17 production. In conclusion, the impaired therapeutic efficacy of Foxp3ΔRORγt Tregs is attributable to their conversion to a Th1-like effector phenotype and failure to maintain Foxp3 expression rather than their failure to persist.
FIGURE 7.
Treg therapy with RORγt-deficient Tregs fails to ameliorate colitis. CD45.1+CD45RBhi CD4+ T cell recipient Rag1−/− mice (4 × 105 cells per mouse) were treated with in vitro–differentiated CD45.2+ Foxp3WT or Foxp3ΔRORγt Tregs (5 × 105 Tregs per mouse) at 2 wk after naive CD45.1+CD4+ T cell transfer. (A) Histopathological scores of colonic tissue sections at 8 wk post-transfer from the three indicated naive CD45.1+CD4+ T cell recipient Rag1−/− groups where two recipient groups received CD45.2+ Treg therapy. (B) Representative histopathology of H&E-stained colonic sections of CD45.1+CD45RBhi Rag1−/− recipients treated with either Foxp3WT or Foxp3ΔRORγt Tregs. (C) Quantitation of total number of retrieved CD45.2+ cells (original magnification ×40) from colonic LP of Foxp3WT or Foxp3ΔRORγt Treg-treated Rag1−/− recipient groups, as indicated (n = 6). (D) Representative FACS plots (left) and bar diagram (right) showing percentage Foxp3 expression in gated CD45.2 compartment from the two indicated Treg-treated Rag1−/− recipient groups. (E) Representative FACS plots (left) and bar diagram showing percentage IFN-γ– and IL-17–expressing cells in gated CD45.2 compartment from the two indicated Treg-treated Rag1−/− recipient groups. Data (C, D, and E) are shown as mean ± SEM. Data in (A)–(E) are representative of two independent experiments (n = 8–10 per group). For p values, Kruskal–Wallis test was used in (A), and the two-tailed unpaired Student t test was used in (C)–(E). *p < 0.005, #p < 0.0001. n.s, not significant.
T-bet deficiency in RORγt-ablated Tregs rescues colitic mice with maintenance of Foxp3+ Tregs
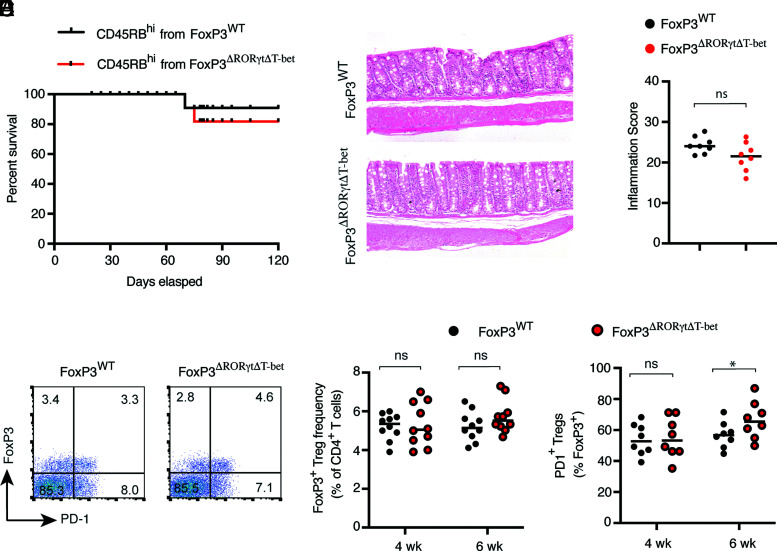
We observed that the Rag1−/− recipients that received Foxp3ΔRORγt CD45RBhi CD4+ T cells succumbed to the disease, and this was associated with a significantly reduced frequency of endogenous Foxp3+ Tregs with downregulated PD-1 expression. Because in vitro–derived Foxp3ΔRORγtΔT-bet Tregs showed normal Foxp3 levels and a comparable level of suppressor ability to WT Tregs, we investigated whether additional T-bet deficiency in Foxp3ΔRORγt Tregs could rescue the mice from severe colitis. Rag1−/− recipients that received naive Foxp3ΔRORγtΔT-bet CD4+ T cells not only showed comparable survival (80%) to that of the naive Foxp3WT CD4+ T cell recipient Rag1−/− group, histopathological analysis of colonic tissue sections showed similar extent of tissue inflammation (Fig. 8A–C). Moreover, during colitis, simultaneous ablation of RORγt and T-bet enabled the maintenance of an equivalent frequency of colonic Foxp3+ Tregs, which was similar to the naive Foxp3WT cell recipient Rag1−/− group. Accordingly, PD-1 expression on endogenous colonic Foxp3+ Tregs was restored in Foxp3ΔRORγt Tregs in the absence of T-bet. Recipients of naive Foxp3ΔRORγtΔT-bet CD4+ T cells showed slightly higher expression of PD-1 compared with naive Foxp3WT cell recipients at 6 wk post-transfer, suggesting active repression of PD-1 by T-bet (Fig. 8D–F). Supporting our in vitro finding, expressions of GITR and CTLA-4 on endogenous Tregs did not vary in presence or absence of RORγt and were comparably high in all three groups. Frequency of in vivo CTLA-4 expression on endogenous Foxp3+ Tregs was higher than in vitro–differentiated Tregs, suggesting additional inflammation-derived factors enhance CTLA-4 expression (Supplemental Fig. 3). Therefore, these results demonstrated that deletion of T-bet in Foxp3ΔRORγt Tregs not only allows for the maintenance of Foxp3 expression, which reinstates their tolerogenic function during colitis, but also rescues the mice from severe colonic inflammation and death. This suggests that repression of T-bet is a critical function of RORγt, whereby it maintains Foxp3 expression to promote tolerogenic function of peripherally induced Tregs during inflammation.
FIGURE 8.
Deletion of T-bet from RORγt-deficient Tregs ameliorates colitis with low mortality. Sorted naive CD45RBhi cells from Foxp3WT and Foxp3ΔRORγtΔT-bet mice were adoptively transferred to Rag1−/− recipient mice for colitis induction. (A) Survival kinetics of the two indicated groups of Rag1−/− recipients. (B and C) Representative histopathology of H&E-stained colonic sections (original magnification ×40) and inflammation score of colonic tissue sections at 6 wk post–adoptive transfer from two indicated groups of Rag1−/− recipient mice. (D) Representative FACS plot showing Foxp3 and PD-1 expressions in colonic CD4+ LP cells obtained from the two indicated groups of Rag1−/− recipients at 6 wk post-transfer. (E and F) Frequency of Foxp3+ cells in colonic CD4+ T cells (E) and frequency of PD-1–expressing cells in colonic Foxp3+ Tregs from the two indicated groups of Rag1−/− mice (F) at 4 and 6 wk post-transfer. Data (C, E, and F) are shown as mean ± SEM. Data are representative of three or more independent experiments (n = 8–10 per group). For p values, Mann–Whitney U test was used in (C), and two-tailed unpaired Student t test was used in (E) and (F). *p < 0.05. n.s, not significant.
Discussion
A breakthrough in the field of mucosal immunology is the recent discovery of RORγt+ Tregs: a population of peripherally induced colonic Tregs with a high immunosuppressive function that is implicated in IBD pathogenesis (9–12). To date, it is not known exactly how the master TF of Th17 cells, RORγt, cooperates with Foxp3 to enhance the suppressor function of colonic Tregs (9, 10, 31). Our findings in this study demonstrate, to our knowledge, a novel function of RORγt in the maintenance of Foxp3 expression, revealing its crucial role in Treg function.
Our data show that RORγt modulates the regulatory versus effector functions of colonic Tregs, both in vitro and during colitis. RORγt antagonizes the T-bet–induced Th1-like effector program to sustain Foxp3 expression and Treg suppressor function during colitis. Deletion of RORγt from Tregs results in worsening of autoimmune colitis with loss of Foxp3 expression, whereas additional Treg-specific deletion of T-bet maintained Foxp3 expression in RORγt-deficient Tregs and rescued colitic mice. We also established that combined TGF-β and RA signaling are necessary for the sustenance of RORγt expression in Tregs that primarily restricts T-bet induction to oppose the development of the effector program in Tregs.
Although TGF-β signaling is a known inducer of both Foxp3 and RORγt, we found TGF-β alone fails to sustain RORγt expression in Tregs. We established that stable coexpression of Foxp3 and RORγt requires dual signaling by RA and TGF-β, which are critical for the optimal generation of RORγt+ Tregs. Interestingly, despite the presence of TGF-β, which is a potent suppressor of T-bet (32, 33), RORγt is additionally required along with TGF-β for effective suppression of T-bet during Treg differentiation. However, we found that IL-6 and IL-23 are dispensable during primary differentiation of RORγt+ Tregs in vitro, suggesting IL-6 and IL-23 might play additional roles in the stabilization of RORγt expression in colonic Tregs, particularly in light of their known role in the induction of RORγt during Th17 differentiation (16). In addition, we found that RORγt+ Tregs did not significantly express IL-17 both in vitro and in vivo, supporting a previous observation that IL-17 is not significantly induced in Tregs, despite RORγt expression (9). This suggests that RORγt regulates additional signaling pathways intrinsic to Tregs which do not overlap with Th17 development.
Although the RORγt–T-bet interaction has been demonstrated during Th17 differentiation, their interaction has not been studied in Treg function. It has been shown that T-bet inhibits RORγt expression by blocking the association of Runx1 with the Rorc promoter, and ectopic T-bet expression is sufficient to repress Rorc gene expression (34). Indeed, the interaction of RORγt and T-bet in Th17 cells leads to transition to a Th1-like program (35). Whereas most studies demonstrated how T-bet suppresses RORγt, a study has shown that overexpression of RORγt suppresses T-bet induction in Th17 cells in vitro in an unknown manner (36). To our knowledge, our results demonstrate for the first time that, via RORγt-mediated suppression of T-bet, two antagonistic TFs, Foxp3 and RORγt, cooperate to enhance the suppressor function of colonic Tregs.
Intriguingly, in natural Tregs, the Tbx21 locus remains in a transcriptionally poised state and low levels of T-bet are expressed from freshly isolated natural Tregs (37). During infection, Tregs acquire T-bet and IFN-γ expression, leading to a decline in their frequency (38). These studies suggest that T-bet remains in a transcriptionally poised state in Tregs, resulting in their instability during inflammation, when T-bet is actively expressed, which leads to the downregulation of Foxp3. In support of these studies, we found that T-bet expression is increased in colonic Foxp3ΔRORγt Tregs not only during colitis, but also in the colonic Tregs of the B6 Foxp3ΔRORγt mouse at homeostasis. This suggests peripherally differentiated colonic Tregs might be developmentally poised to rapidly upregulate T-bet during inflammation, which is counteracted by RORγt, resulting in the maintenance of Foxp3. Although we demonstrate that one of RORγt’s primary functions in Tregs is to repress T-bet induction to maintain Foxp3 expression, the signaling pathway through which RORγt interacts with T-bet remains to be determined. T-bet is the first identified inhibitor of PD-1. T-bet is a direct transcriptional repressor of PD-1, where it binds to the upstream regulatory region of the Pdcd1 gene and antagonizes PD-1 expression (20, 21). The PD-l/PD-L1 pathway promotes the development and function of inducible and adaptive Tregs by induction and maintenance of Foxp3 (23, 25, 26). Although we demonstrated PD-1 expression and suppressor function were restored by deletion of T-bet from RORγt-deficient Tregs, we have not proved that downregulation of PD-1 expression is directly linked to their failure to maintain Foxp3 expression. The role of RORγt in PD-1–driven maintenance of Foxp3 expression remains to be determined. In the absence of RORγt, multiple T-bet–driven signaling pathways are likely to suppress Foxp3 expression and the suppressor function of colonic Tregs. Although we could not detect Gata-3 expression from in vitro–differentiated Foxp3ΔRORγt Tregs or endogenous Foxp3ΔRORγt Tregs during colitis, the frequency of Gata-3+ Tregs was enhanced from colonic Foxp3ΔRORγt Tregs in homeostasis, which supports a previous study (9). When T-bet is additionally deleted from colonic Foxp3ΔRORγt Tregs, not only does the compensatory increase in frequency of Gata-3+ Tregs revert to normal levels, but the frequency of Foxp3+ Tregs is also restored. This further indicates that interaction between RORγt and T-bet play a critical in maintaining normal tolerogenic responses in the colon, even in homeostasis.
To our knowledge, our findings demonstrate a novel role for RORγt in controlling Treg function in colonic Tregs, where RORγt inhibits T-bet–induced Th1-like effector programs to maintain Foxp3 expression. Our data support the notion that the master TF of an effector T cell lineage can play a key role in controlling the tolerogenic function of Tregs. In both IBD and colitis-associated cancer, a functional adaptation of Tregs, rather than altered frequency, determines disease progression (4, 39–41) where RORγt+ Tregs may play a critical role in modulating the suppressor versus effector function of colonic Tregs. In light of these findings, further insight into RORγt-driven pathways in Tregs might lead to therapeutic reprogramming of Tregs to switch between regulatory and effector functions.
Supplementary Material
This work was supported by the Crohn’s and Colitis Foundation of America Career Development Award 347717 (to R.B.). This work was supported by a Career Development Award grant to R.B. from the Crohn’s and Colitis Foundation of America (identifier 347717) and by startup funds from the University of Alabama School of Medicine (to R.B.).
The online version of this article contains supplemental material.
- IBD
- inflammatory bowel disease
- LP
- lamina propria
- PD-1
- programmed cell death-1
- RA
- retinoic acid
- TF
- transcription factor
- Treg
- regulatory T cell
- WT
- wild type
- YFP
- yellow fluorescent protein
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Dominguez-Villar M., Hafler D. A.. 2018. Regulatory T cells in autoimmune disease. Nat. Immunol. 19: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S., Vignali D. A., Rudensky A. Y., Niec R. E., Waldmann H.. 2013. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13: 461–467. [DOI] [PubMed] [Google Scholar]
- 3.Himmel M. E., Yao Y., Orban P. C., Steiner T. S., Levings M. K.. 2012. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology 136: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaser A., Zeissig S., Blumberg R. S.. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28: 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haribhai D., Lin W., Edwards B., Ziegelbauer J., Salzman N. H., Carlson M. R., Li S. H., Simpson P. M., Chatila T. A., Williams C. B.. 2009. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J. Immunol. 182: 3461–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschetti G., Nancey S., Sardi F., Roblin X., Flourié B., Kaiserlian D.. 2011. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm. Bowel Dis. 17: 160–170. [DOI] [PubMed] [Google Scholar]
- 7.Veltkamp C., Anstaett M., Wahl K., Möller S., Gangl S., Bachmann O., Hardtke-Wolenski M., Länger F., Stremmel W., Manns M. P., et al. 2011. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut 60: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 8.Mickael M. E., Bhaumik S., Basu R.. 2020. Retinoid-related orphan receptor RORγt in CD4+ T-cell-mediated intestinal homeostasis and inflammation. Am. J. Pathol. 190: 1984–1999. [DOI] [PubMed] [Google Scholar]
- 9.Sefik E., Geva-Zatorsky N., Oh S., Konnikova L., Zemmour D., McGuire A. M., Burzyn D., Ortiz-Lopez A., Lobera M., Yang J., et al. 2015. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnmacht C., Park J. H., Cording S., Wing J. B., Atarashi K., Obata Y., Gaboriau-Routhiau V., Marques R., Dulauroy S., Fedoseeva M., et al. 2015. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 349: 989–993. [DOI] [PubMed] [Google Scholar]
- 11.Yang B. H., Hagemann S., Mamareli P., Lauer U., Hoffmann U., Beckstette M., Föhse L., Prinz I., Pezoldt J., Suerbaum S., et al. 2016. Foxp3(+) T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 9: 444–457. [DOI] [PubMed] [Google Scholar]
- 12.Britton G. J., Contijoch E. J., Mogno I., Vennaro O. H., Llewellyn S. R., Ng R., Li Z., Mortha A., Merad M., Das A., et al. 2019. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory t cells and exacerbate colitis in mice. Immunity 50: 212–224.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu R., Whitley S. K., Bhaumik S., Zindl C. L., Schoeb T. R., Benveniste E. N., Pear W. S., Hatton R. D., Weaver C. T.. 2015. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat. Immunol. 16: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu R., O’Quinn D. B., Silberger D. J., Schoeb T. R., Fouser L., Ouyang W., Hatton R. D., Weaver C. T.. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manel N., Unutmaz D., Littman D. R.. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L., Ivanov I. I., Spolski R., Min R., Shenderov K., Egawa T., Levy D. E., Leonard W. J., Littman D. R.. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8: 967–974. [DOI] [PubMed] [Google Scholar]
- 17.Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E. A., Pott J., Griseri T., Bollrath J., Hegazy A. N., et al. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izcue A., Coombes J. L., Powrie F.. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27: 313–338. [DOI] [PubMed] [Google Scholar]
- 19.Mottet C., Uhlig H. H., Powrie F.. 2003. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170: 3939–3943. [DOI] [PubMed] [Google Scholar]
- 20.Kao C., Oestreich K. J., Paley M. A., Crawford A., Angelosanto J. M., Ali M. A., Intlekofer A. M., Boss J. M., Reiner S. L., Weinmann A. S., Wherry E. J.. 2011. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bally A. P., Austin J. W., Boss J. M.. 2016. Genetic and epigenetic regulation of PD-1 expression. J. Immunol. 196: 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francisco L. M., Sage P. T., Sharpe A. H.. 2010. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236: 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francisco L. M., Salinas V. H., Brown K. E., Vanguri V. K., Freeman G. J., Kuchroo V. K., Sharpe A. H.. 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206: 3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Fosco D., Kline D. E., Meng L., Nishi S., Savage P. A., Kline J.. 2014. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur. J. Immunol. 44: 2603–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stathopoulou C., Gangaplara A., Mallett G., Flomerfelt F. A., Liniany L. P., Knight D., Samsel L. A., Berlinguer-Palmini R., Yim J. J., Felizardo T. C., et al. 2018. PD-1 inhibitory receptor downregulates asparaginyl endopeptidase and maintains Foxp3 transcription factor stability in induced regulatory T cells. Immunity 49: 247–263.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Pino-Lagos K., de Vries V. C., Guleria I., Sayegh M. H., Noelle R. J.. 2008. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. USA 105: 9331–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Som A., Mandaliya R., Alsaadi D., Farshidpour M., Charabaty A., Malhotra N., Mattar M. C.. 2019. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J. Clin. Cases 7: 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celli R., Kluger H. M., Zhang X.. 2018. Anti-PD-1 therapy-associated perforating colitis. Case Rep. Gastrointest. Med. 2018: 3406437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohr J., Knoechel B., Abbas A. K.. 2006. Regulatory T cells in the periphery. Immunol. Rev. 212: 149–162. [DOI] [PubMed] [Google Scholar]
- 30.Koch M. A., Tucker-Heard G., Perdue N. R., Killebrew J. R., Urdahl K. B., Campbell D. J.. 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R.. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 32.Gorelik L., Constant S., Flavell R. A.. 2002. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park I. K., Shultz L. D., Letterio J. J., Gorham J. D.. 2005. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J. Immunol. 175: 5666–5674. [DOI] [PubMed] [Google Scholar]
- 34.Lazarevic V., Chen X., Shim J. H., Hwang E. S., Jang E., Bolm A. N., Oukka M., Kuchroo V. K., Glimcher L. H.. 2011. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Godec J., Ben-Aissa K., Cui K., Zhao K., Pucsek A. B., Lee Y. K., Weaver C. T., Yagi R., Lazarevic V.. 2014. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukasa R., Balasubramani A., Lee Y. K., Whitley S. K., Weaver B. T., Shibata Y., Crawford G. E., Hatton R. D., Weaver C. T.. 2010. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32: 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T. Y., Watford W. T., et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldenhove G., Bouladoux N., Wohlfert E. A., Hall J. A., Chou D., Dos Santos L., O’Brien S., Blank R., Lamb E., Natarajan S., et al. 2009. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31: 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geem D., Harusato A., Flannigan K., Denning T. L.. 2015. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 21: 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito T., Nishikawa H., Wada H., Nagano Y., Sugiyama D., Atarashi K., Maeda Y., Hamaguchi M., Ohkura N., Sato E., et al. 2016. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 22: 679–684. [DOI] [PubMed] [Google Scholar]
- 41.Salama P., Phillips M., Grieu F., Morris M., Zeps N., Joseph D., Platell C., Iacopetta B.. 2009. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 27: 186–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.