Key Points
CXCL2 on macrophage EVs recruited neutrophils.
CXCL2 EVs activated the CXCR2/PKC/NOX4 pathway.
Targeting EVs CXCL2 is a viable strategy for sepsis treatment.
Visual Abstract
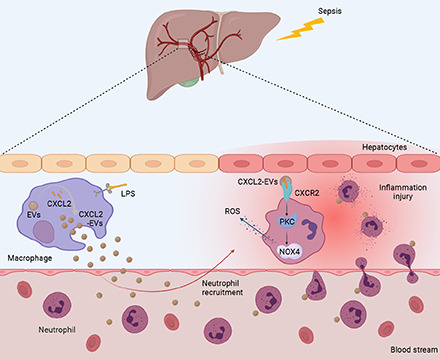
Abstract
Sepsis is a life-threatening organ dysfunction caused by a dysfunctional host response to infection. Neutrophils play a protective role by releasing antibacterial proteins or by phagocytizing bacteria. However, excess neutrophils can induce tissue damage. Recently, a novel intercellular communication pathway involving extracellular vesicles (EVs) has garnered considerable attention. However, whether EVs secreted by macrophages mediate neutrophil recruitment to infected sites has yet to be studied. In this study, we assessed the chemotactic effect of EVs isolated from mouse Raw264.7 macrophages on mouse neutrophils and found that CXCL2 was highly expressed in these EVs. By regulating CXCL2 in Raw264.7 macrophages, we found that CXCL2 on macrophage EVs recruited neutrophils in vitro and in vivo. The CXCL2 EVs activated the CXCR2/PKC/NOX4 pathway and induced tissue damage. This study provides information regarding the mechanisms underlying neutrophil recruitment to tissues and proposes innovative strategies and targets for the treatment of sepsis.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysfunctional host response to infection (1). Peripheral neutrophils play an important role in the innate immune response by rapidly accumulating in injured and infected sites (2). Bone marrow neutrophils can rapidly enter the circulation under inflammatory conditions (3). Neutrophils play a protective role by releasing antibacterial proteins or by phagocytizing bacteria (4). However, excess neutrophils can induce tissue damage (5). The classic neutrophil recruitment process is made up of the following five steps: tethering, rolling, adhesion, crawling, and transmigration. Proinflammatory mediators rapidly activate the translocation of E- and P-selectins onto the plasma membrane in endothelial cells, and these selectins act as receptors for glycosylated ligands on neutrophils, which recognize and adhere to endothelial cells in blood vessels (6). Concurrently, several chemokines bind to heparan sulfate proteoglycans on endothelial cells and establish an intravascular chemotactic gradient that is recognized by neutrophils (7). The above interactions induce a signaling cascade in neutrophils to activate β2 integrin (8), which binds to its ligands ICAM1 and ICAM2 on the endothelial cell surface, thereby enabling neutrophils to tightly adhere to the vascular endothelium (9).
Apart from the interaction between neutrophils and vascular endothelial cells, macrophages play a key role in neutrophil recruitment to tissues by recognizing pathogen- and damage-associated molecules. Proinflammatory mediators released from macrophages recognize several receptors on neutrophils and direct neutrophil recruitment by chemokines (10). Recently, a novel intercellular communication pathway that uses extracellular vesicles (EVs) has garnered considerable attention. However, whether EVs, such as those secreted by macrophages, mediate neutrophil recruitment to infected sites remains to be studied.
EVs are structures surrounded by lipid membranes. They are involved in various cellular functions that transmit information between cells. For instance, EVs transfer protective or proinflammatory information (11). Exosomes, the most studied EV type, are protected against degradation in body fluids owing to their membrane structure (12). Chemokines are enclosed in EVs, and they can play chemotactic roles. Apoptotic cells release microparticles carrying C-X3-C motif chemokine ligand 1 (CX3CL1), thereby mediating the alveolar migration of macrophages (13). Monocytes/macrophages may be recruited for rolling and migration in atherosclerosis by monocyte-derived exosomes containing inflammatory factors (14). Hepatocytes release CXCL10-rich lipotoxic EVs that promote macrophage chemotaxis to boost the development of nonalcoholic steatohepatitis (15). The above evidence implies that EVs play an important role in cellular chemotaxis. It is apparent that the important functions EVs play in sepsis mostly depend on their content.
CXCL2 plays an important role in neutrophil recruitment by binding to CXCR2 on neutrophils (16). Free CXCL1/CXCL2 released by macrophages controls early neutrophil recruitment into tissues (17). Once neutrophils enter the tissue, they begin to exert antibacterial activities by secreting proteolytic enzymes and reactive oxygen species. This mechanism involves the activation of the protein kinase C (PKC)/NADPH pathway downstream of CXCL2 binding to CXCR2 (16). Excessive neutrophil recruitment directly damages the tissue because of increased oxidative stress.
Taken together, we propose that CXCL2-containing EVs released from LPS-induced macrophages promote tissue damage by inducing neutrophil chemotaxis and activating the CXCR2/PKC/NOX4 pathway in these cells. In this study, we assessed the chemotactic effect of EVs isolated from Raw264.7 macrophages or the serum of patients with sepsis on neutrophils and observed, using chemokine chip assays, that CXCL2 is highly expressed in these EVs. By downregulating CXCL2 in Raw264.7 cells, we found that macrophagic EV CXCL2 recruits neutrophils in vitro and in vivo. EVs containing CXCL2 activate the CXCR2/PKC/NOX4 pathway and induce tissue damage. We investigated the effect of CXCL2 enclosed in LPS-induced macrophagic EVs on the chemotaxis of neutrophils ex vivo and in vivo. This study provides information regarding the mechanisms underlying neutrophil recruitment into tissues and presents innovative strategies and targets for the treatment of sepsis.
Materials and Methods
Patient samples and ethics statement
All blood samples were collected after obtaining patient consent. All experiments were performed in accordance with the ethics committee of Nanfang Hospital and clinical experiment guidelines of Southern Medical University.
Materials, reagents, and Abs
LPS and GW4869 were purchased from Sigma-Aldrich (St. Louis, MO). Clodronate liposomes were obtained from Vrije Universiteit (40337ES10; Amsterdam, the Netherlands). Primary Abs against Ly-6G (ab25377; Abcam, Cambridge, U.K.), CD63 (ab10895; Abcam), CD9 (ab92726; Abcam), CD81 (18250-1-AP; Proteintech), CXCL2 (26791-1-AP; Proteintech), CXCR2 (ab14935; Proteintech), PKC (ab19301; Abcam), and NOX4 (14347-1-AP; Proteintech) were obtained from the indicated sources.
Animal models
For the animal model, 6–8-wk-old C57BL/6 mice were purchased from the Experimental Animal Center of Southern Medical University (Guangzhou, China). LPS (20 mg/kg) was administered i.p. to induce acute inflammation, and sepsis was induced by cecal ligation and puncture (CLP) as described previously (18). In brief, mice were anesthetized, and a 2-cm midline laparotomy was performed to expose the cecum. A single puncture was performed using a 21-gauge needle between the ligation site and end of the cecum. A small amount of fecal material was extruded through the puncture. The cecum was repositioned into the peritoneal cavity, and the laparotomy wound was closed. Sham-operated animals underwent laparotomy and bowel manipulation without ligation and puncture. All mice were resuscitated with 1 ml of saline. Preoperatively and postoperatively, all mice had unlimited access to food and water. Clodronate liposomes were injected via the tail vein for 72 h to clear out macrophages before LPS injection and CLP procedure. GW4869 (2.5 mg/g) dissolved in DMSO was injected i.p. at one dose for 1 h before LPS injection and CLP procedure. The mice were anesthetized, and blood and liver samples were collected at different time points. All animal experiments were approved by the Animal Care and Use Committee at Southern Medical University.
Primary neutrophil isolation and cell culture
Mouse bone marrow–derived neutrophils (BMDNs) were isolated using an optimized gradient centrifugation technique. The mouse femurs and tibias were used to prepare BMDNs. Bone marrow was flushed out with 5 ml of DMEM. RBCs were lysed, and the remaining cells were resuspended in 65% Percoll (P1644; Sigma-Aldrich), overlaid onto 70% Percoll, and centrifuged at 750 × g for 30 min. The BMDNs were collected from the interface and centrifuged at 500 × g for 10 min. Human polymorphonuclear neutrophils (PMNs) were purified using Histopaque (Sigma-Aldrich) centrifugation, followed by dextran (Pharmacosmos, Holbaek, Denmark) sedimentation as previously described (19). PMNs were suspended in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 5% FBS. Raw264.7 cells were cultured in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Lentivirus transfection and small interfering RNA synthesis
Lentivirus constructs directed against CXCL2 in Raw264.7 cells were manufactured by Hanbio Biotechnology Co. (Shanghai, China). All lentiviral vectors were purified to a titer of 1 × 109 transducing units/ml. At a 50:1 multiplicity of infection 4 h after transfection, the medium was removed, and fresh medium was added. CXCL2 small interfering RNA (siRNA) duplexes were designed and synthesized by RiboBio (Guangzhou, China). Transfection was performed using riboFECT CP (R10035.4; RiboBio, Guangzhou, China). Briefly, 1 d before transfection, Raw264.7 cells were plated in six-well plates at 30‒50% confluence and transfected with 100 nM CXCL2 siRNA for 72 h. Western blotting was used to determine CXCL2 expression levels.
EV isolation and injection
Raw264.7 cells were incubated in DMEM for 24 h in the presence of 100 ng/ml LPS or vehicle. The conditioned medium was collected and centrifuged at 1,000 × g for 20 min to remove cell debris, followed by centrifugation at 2,000 × g for 20 min and 10,000 × g for 30 min at 4°C. The supernatant was subsequently collected and filtered passed through 0.22-μm filters (MilliporeSigma) and centrifuged in Beckman Coulter Optima L-80XP at 100,000 × g for 70 min at 4°C to pellet EVs. Next, the EV pellets were washed with sterile PBS and subjected to another cycle of ultracentrifugation at 100,000 × g for 70 min at 4°C. Finally, the pelleted EVs were carefully reconstituted in sterile PBS or lysed in radioimmunoprecipitation assay buffer. For EV injection, 100 μg of isolated EVs was injected once into mice through the tail vein.
Nanoparticle tracking analysis
Particle size and concentration distribution of the isolated EVs were measured using the nanoparticle tracking analysis (NS3000). Briefly, the exosome samples were diluted 1:5000 in filtered sterile PBS. Each sample analysis was conducted for 60 s and measured three times using the automatic analysis settings of the NanoSight instrument.
Transmission electron microscopy
Pelleted EVs were mixed and prepared with paraformaldehyde and glutaraldehyde, fixed with osmium tetroxide, and dehydrated. Samples were embedded in resin. Ultrathin sections were cut using a diamond knife, and sections were then placed on either copper or nickel mesh grids. The sections were stained with the heavy metal uranyl acetate for contrast and viewed using a JEM-2100F transmission electron microscope.
Western blotting
The total protein was extracted from isolated EVs or BMDNs using ice-cold radioimmunoprecipitation assay lysis buffer (Dalian Meilun Biotechnology) containing a protease inhibitor mixture. Protein concentration was determined using the BCA method. Equal concentrations of protein were separated on 12% SDS-PAGE gels and transferred onto PVDF membranes. The blots were subsequently blocked for 1 h with 5% BSA. The EV protein blots were incubated with primary Abs (1:1000) against CD63, CD9, CD81, CXCL2, and GAPDH. The BMDN protein blots were incubated with primary Abs against CXCR2, PKC, and NOX4 (1:1000). After three washes, the membranes were incubated with fluorescence-conjugated anti-rabbit or anti-mouse Abs (LI-COR Biosciences) for 1 h at room temperature. Proteins were visualized using an ECL kit (Beyotime Biotechnology) and quantified using a Tanon 5500 imaging system (Tanon, Shanghai, China).
Immunofluorescence
The tissues were frozen in liquid nitrogen and embedded in Tissue-TEK O.C.T. compound (4583; Sakura). For immunofluorescence staining, the tissues were fixed overnight in formalin. After washing with PBS, the tissue sections were blocked with 3% BSA for 30 min and stained with an anti–Ly-6G Ab at 4°C overnight. The sections were washed three times with PBS and incubated with goat anti-mouse Cy3 Ab. Extensive washing with PBS was performed before incubating the sections with DAPI for nuclear staining. Images were observed using a Zeiss LSM 700 laser scanning confocal microscope and analyzed using ZEN software.
Quantitative reverse transcription PCR
The total RNA was isolated from mouse liver using TRIzol. cDNA was synthesized using the ReverTra Ace qPCR RT Kit (FSQ-101; Toyobo, Tokyo, Japan). Quantitative PCR was performed using SYBR Green PCR Master Mix (QPK-201; Toyobo) with a Roche Diagnostics LightCycler 480 System. Primers for TNF-α, IL-1β, IL-6, IL-4, IL-10, and GAPDH were used. The relative fold change was calculated using the comparative CT method. Each experiment was performed in triplicate.
Chemokine protein arrays
Custom human cytokine arrays (AAH-CHE-1-2; RayBio; RayBiotech, Norcross, GA) and mouse cytokine arrays (AAM-CHE-1-2; RayBio; RayBiotech) were used. Human serum EV (sEV) lysates (or Raw264.7 EV lysates) were incubated with membranes coated with anti-human chemokine Abs (or anti-mouse chemokine Abs). Briefly, the membranes were incubated with blocking buffer at room temperature for 30 min and incubated with each sample at room temperature for 90 min. The membranes were washed three times with Wash Buffer I and twice with Wash Buffer II at room temperature for 5 min per wash. The membranes were then incubated with biotin-conjugated Abs at room temperature for 90 min. Finally, the membranes were washed and incubated with HRP-conjugated streptavidin at room temperature for 2 h and with detection buffer for 2 min. We used a luminescence detector (LAS-1000; Fujifilm) for detection, and the data were digitized and subjected to image analysis using AIDA 3.28 software (Elysia-Raytest). We obtained relative protein concentrations by subtracting background staining and normalizing to the positive controls on the same membrane.
ELISA
The quantification of CXCL2 (KE10022; Proteintech), CXCL7 (DY393; R&D Systems, Minneapolis, MN), CXCL17 (AD7670Hu; Andy Gene, Beijing, China), CCL2 (KE00091; Proteintech), CCL3 (KE00092; Proteintech), and CCL5 (KE00093; Proteintech) was performed using quantitative ELISA. Optical densities were measured at 450 nm using an ELISA-dedicated instrument. Cytokine or LPS concentrations were calculated using a standard curve. All measurements were performed in duplicate, and arithmetic averages were calculated.
Transwell assay
Isolated BMDNs or PMNs (5000 cells/cm2) were seeded in transwell inserts (Invitrogen). Ten micrograms of isolated EVs was placed in the upper chamber as a chemoattractant. After incubation for 2 h, cells in the lower chamber were counted under a microscope.
Histology and immunohistochemistry
Immediately after euthanizing the mice using chloral hydrate, the livers were excised, fixed in 10% neutral buffered formalin, and embedded in paraffin. The paraffin blocks were cut into 4-µm tissue sections. Following deparaffinization and rehydration, the tissue sections were stained with H&E. For immunohistochemistry, the embedded tissue sections were treated with 3% H2O2 for 15 min and then subjected to microwave Ag retrieval. The slides were blocked with 3% BSA for 30 min and incubated with rabbit anti-mouse anti–Ly-6G Ab or rabbit anti-mouse anti–myeloperoxidase (MPO) overnight at 4°C. After washing three times with PBS, the samples were incubated with HRP-conjugated goat anti-rabbit Ab (PV9000; Zhongshan Golden Bridge Biotechnology, Beijing, China). The sections were then incubated with diaminobenzidine (Boster Bio, Wuhan, China) as a substrate and counterstained with hematoxylin. Images were captured using an Olympus microscope (Olympus, Tokyo, Japan) and analyzed using ZEN software. We randomly selected six fields per slide from a single mouse, and they were evaluated by an experienced pathologist blinded to the assignment of mice to treatments.
Systemic circulating neutrophils
Systemic circulating neutrophils were detected using MC6200VET instrument (Maxcom Technologies, Shenzhen, China).
Serum assay
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were analyzed using commercial kits purchased from Jiancheng Bioengineering Institute (Nanjing, China) according to the instruction of the manufacturer.
Statistical analysis
All results are expressed as mean ± SD. One-way ANOVA was applied for multigroup comparisons. Student t test was performed to compare the experimental and control groups. Statistical significance was set at p < 0.05.
Results
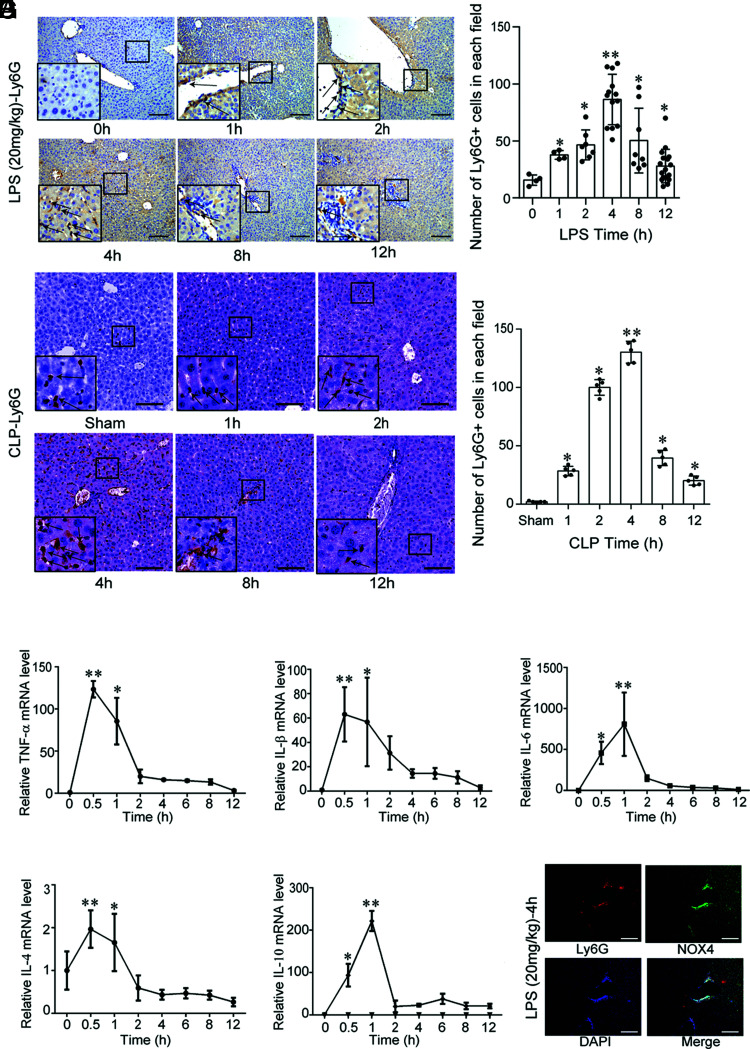
Both LPS and CLP induce early recruitment of neutrophils to the liver
LPS (20 mg/kg) was i.p. injected into wild-type C57BL/6 mice to induce acute inflammation, and sepsis was induced by CLP. The mice were sacrificed at varying time points (0, 1, 2, 4, 8, and 12 h) to observe the recruitment of neutrophils to the liver. The number of neutrophils in the liver increased over time, with the highest recruitment at 4 h. However, after 4 h, the number of neutrophils in the liver began to decline (Fig. 1A). The recruitment of neutrophils to the liver is an early phenomenon in the sepsis model. Additionally, we quantified the levels of inflammatory factors TNF-α, IL-1β, IL-6, IL-4, and IL-10 and found that IL-1β, IL-4, and TNF-α were significantly upregulated at 0.5 h. Thereafter, their levels decreased, especially at 2 h. The IL-6 and IL-10 levels increased to a maximum at 1 h and then significantly decreased at 2 h (Fig. 1B–F). Significant colocalization of Ly-6G with NOX4 was observed at 4 h after LPS exposure, indicating increased oxidative stress in the recruited neutrophils (Fig. 1G). Whether EVs participate in this recruitment required further study.
FIGURE 1.
Both LPS and CLP induce early recruitment of neutrophils to the liver. (A) Ly-6G expression in the liver after the i.p. injection of LPS (20 mg/kg) into wild-type C57BL/6 mice (n = 6 per group) or CLP (n = 5 per group) at different time points (0, 1, 2, 4, 8, and 12 h). Scale bar, 100 μm. *p < 0.05 versus the control (or sham) group, **p < 0.01 versus the 0-h (or sham) group. (B–F) TNF-α, IL-1β, IL-6, IL-4, and IL-10 mRNA levels (mean ± SD) in the liver after LPS (20 mg/kg) injection into wild-type C57BL/6 mice (n = 6 per group) at different time points (0, 0.5, 1, 2, 4, 6, 8, and 12 h). *p < 0.05 versus the 0-h group, **p < 0.01 versus the 0-h group. (G) Ly-6G and NOX4 colocalization in the liver after the i.p. injection of LPS (20 mg/kg) into wild-type C57BL/6 mice at 4 h. Scale bar, 100 μm.
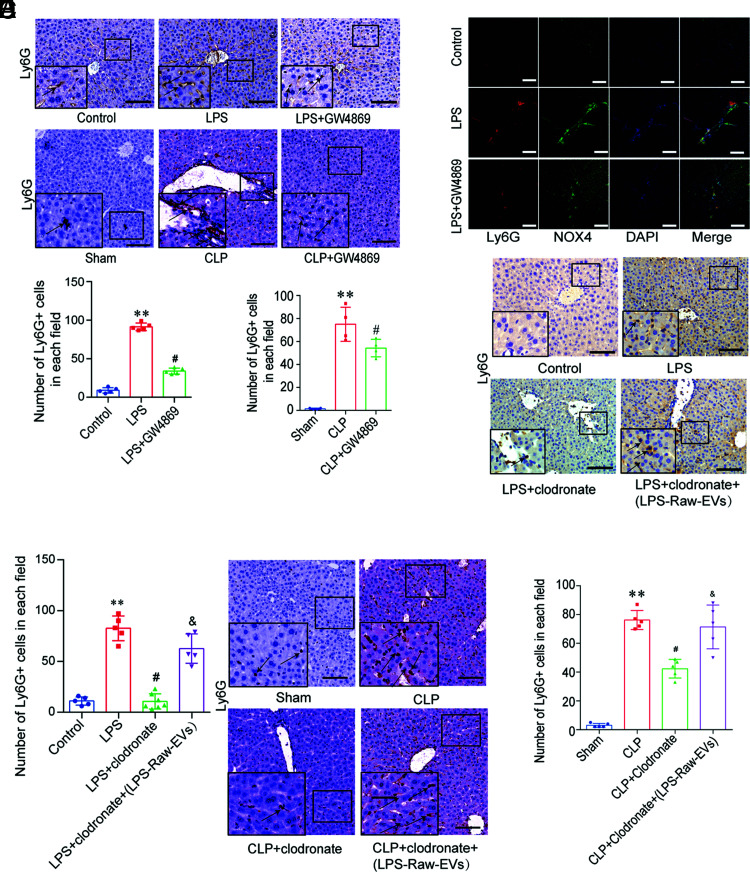
Raw264.7-derived EVs are involved in neutrophil recruitment to the liver
To study the role of EVs in the recruitment of neutrophils during sepsis, we generated an LPS acute inflammation (or CLP sepsis) model by pretreating wild-type C57BL/6 mice with GW4869 (an EV inhibitor) and found that excessive neutrophil infiltration occurred in the liver of both the LPS and CLP groups compared with that in the control or sham group, separately. The number of neutrophils in the liver of the LPS + GW4869 (or CLP + GW4869) group was reduced compared with that of the LPS (or CLP) group mice (Fig. 2A). Immunofluorescence showed that neutrophil infiltration in the LPS group significantly increased compared with that in the control group, and the infiltrated neutrophils showed high NOX4 expression. GW4869 reduced the expression of recruited neutrophils and NOX4 (Fig. 2B).
FIGURE 2.
Raw264.7 cell–derived EVs are involved in the recruitment of neutrophils to the liver. (A) Ly-6G staining of the liver in the control, LPS, LPS + GW4869 groups, sham, CLP, and CLP + GW4869 groups (n = 6 per group). Scale bar, 100 μm. **p < 0.01 versus control (or sham), #p < 0.01 versus the LPS (or CLP) group. (B) Ly-6G and NOX4 colocalization in the liver of the control, LPS, and LPS + GW4869 group mice. Scale bars, 100 μm. (C and D) EVs from Raw264.7 cells were injected into wild-type C57BL/6 mice (n = 6 per group) via the tail vein. Ly-6G+ cells (mean ± SD) in the liver of the control, LPS, LPS + clodronate, and LPS + clodronate + LPS-Raw-EV group mice were detected by immunohistochemistry. Scale bar, 100 μm. **p < 0.01 versus the control group, #p < 0.01 versus the LPS group, &p < 0.05 versus the LPS + clodronate group. (E and F) EVs from Raw264.7 cells were injected into wild-type C57BL/6 mice (n = 6 per group) via the tail vein. Ly-6G+ cells (mean ± SD) in the liver of the control group mice. Samples from the CLP, CLP + clodronate, and LPS + clodronate + CPS-Raw-EV groups were analyzed by immunohistochemistry. Scale bar, 100 μm. **p < 0.01 versus the group, #p < 0.01 versus the CLP group, &p < 0.05 versus the CLP + clodronate group.
Tissue macrophages play a key role in sepsis and are the first line of protection against bacteremia. We speculated that macrophage-derived EVs may be involved in the recruitment of neutrophils to the liver. We used clodronate (a macrophage scavenger) to clear tissue macrophages before LPS injection (or CLP surgery) to determine the function of macrophages in vivo. Liver neutrophils in the LPS + clodronate (or CLP + clodronate) group appeared reduced relative to the LPS (or CLP) group (Fig. 2C–F). We isolated EVs derived from LPS-induced Raw264.7 cells (LPS-Raw264.7-EVs) and injected them into mice after LPS + clodronate (or CLP + clodronate) injection. LPS-Raw264.7-EVs reversed the reduction in neutrophil recruitment in the liver caused by macrophage clearance (Fig. 2C–F). These results suggest that macrophage EVs play an important role in the recruitment of neutrophils.
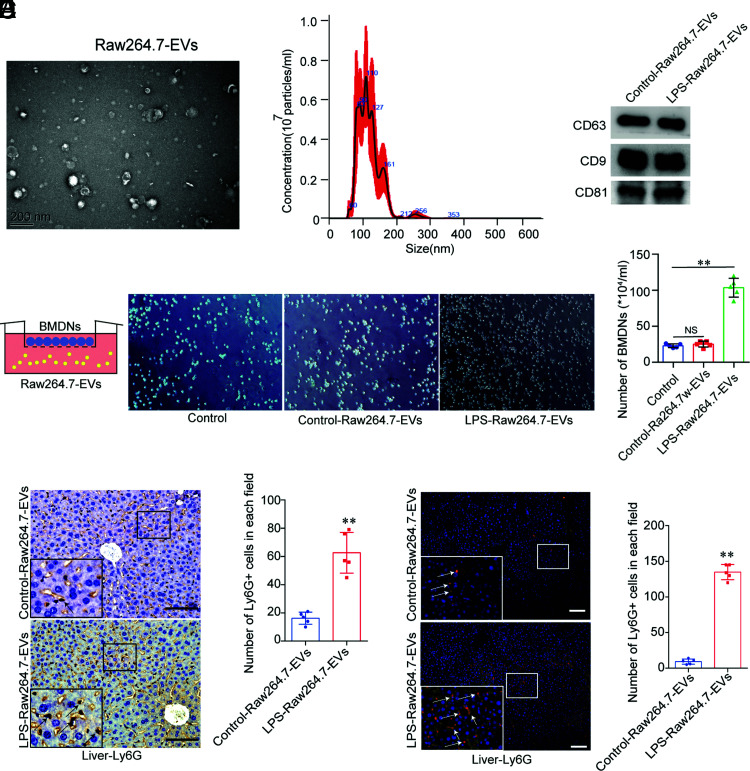
LPS-induced Raw264.7 EVs attract neutrophils in vitro and in vivo
We isolated EVs from Raw264.7 cells and examined their morphology by transmission electron microscopy (Fig. 3A). The nanoparticle tracking analysis assay revealed that the EVs were ∼110 nm in diameter (Fig. 3B). We also used Western blotting to assess EV biomarkers (CD9, CD63, and CD81) (Fig. 3C). These data showed the successful separation of EVs.
FIGURE 3.
LPS-induced Raw264.7 EVs attract neutrophils in vitro and in vivo. (A) Transmission electron microscopy of Raw264.7 EVs. Scale bar, 200 nm. (B) Nanoparticle tracking analysis to detect Raw264.7 EVs. (C) Biomarkers in the EVs from control and LPS-induced Raw264.7 EVs (CD63, CD9, and CD81), as detected by Western blotting. The experiments were repeated three times. (D) Control-Raw264.7-EVs– and LPS-Raw264.7-EVs–induced BMDN chemotaxis (mean ± SD) (original magnification × 200, n = 5 fields per group). The experiments were repeated three times. **p < 0.05 versus the control group. (E and F) Ly-6G+ cells (mean ± SD) in the liver of the control and LPS-Raw264.7-EV group mice (n = 6 per group) were detected by immunohistochemistry and immunofluorescence. **p < 0.01 versus the control-Raw264.7 group. Scale bars, 100 μm.
Next, we verified the chemotaxis of LPS-induced macrophage EVs toward neutrophils in vitro. First, we isolated BMDNs from wild-type C57BL/6 mice and seeded in the upper layer of a transwell chamber. We collected the culture supernatants from the control and LPS-stimulated Raw264.7 cells and isolated the EVs by ultracentrifugation. We added these EVs into the lower transwell chamber to detect their effect on BMDN migration. There was no significant difference in the migration of BMDNs in the control-Raw264.7-EVs (EVs derived from control Raw264.7 cells) group compared with that in the control group, whereas LPS-Raw264.7-EVs significantly increased BMDN migration compared with that in the control group (Fig. 3D). Ly-6G staining demonstrated that neutrophil infiltration in the LPS-Raw264.7-EVs group significantly increased compared with that in the control-Raw264.7-EVs group (Fig. 3E, 3F).
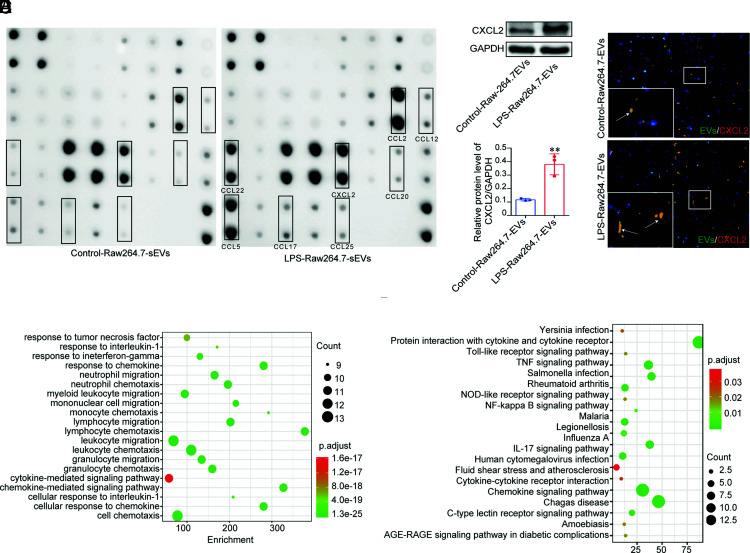
LPS-induced Raw264.7 EVs induced the recruitment of neutrophils to the liver. The mechanism is most likely associated with the content of these secreted EVs. We isolated EVs from the control and LPS-induced Raw264.7 cells. The expression of CCL2, CXCL2, CCL22, CCL5, CCL17, CCL12, CCL25, and CCL20 in the LPS-Raw264.7-EV group appeared significantly increased compared with that in the control-Raw264.7-EV group (Fig. 4A). We speculated that EV CXCL2 may be an important chemokine in the recruitment of neutrophils. Using Western blotting, we verified that LPS macrophage EVs expressed more CXCL2 than control macrophage EVs (Fig. 4B). PKH67-labeled EVs were injected into mice via the tail vein. Immunofluorescence analysis showed that PKH67-labeled EVs and CXCL2 in the liver were colocalized, indicating that LPS-induced macrophage EVs carried CXCL2 and were taken up by the liver (Fig. 4C).
FIGURE 4.
Chemokine profile of the control and LPS-induced Raw264.7 EVs. (A) The chemokine profile of EVs from control and LPS-induced Raw264.7 cells. (B) The CXCL2 level (mean ± SD) in the control-Raw-EV and LPS-Raw-EV groups. The experiments were repeated three times. **p < 0.01 versus the control-Raw264.7-EV group. (C) PKH67-labeled control-Raw264.7-EVs and LPS-Raw264.7-EVs were injected into wild-type C57BL/6 mice (n = 6 per group) via the tail vein. The colocalization of PKH67-EVs and CXCL2 in the liver was detected by immunofluorescence (original magnification × 200). (D and E) GO analysis and KEGG analysis of various chemokines expressed in EVs from control and LPS-induced Raw264.7 cells.
Gene ontology (GO) analysis indicated that the biological functions of increased chemokines in the LPS-Raw264.7-EV group are mainly enriched in response to TNF, IL-1, IFN-γ, chemokine, neutrophil migration, neutrophil chemotaxis, myeloid leukocyte migration, mononuclear cell migration, monocyte chemotaxis, lymphocyte chemotaxis, leukocyte migration, leukocyte chemotaxis, granulocyte migration, granulocyte chemotaxis, cytokine-mediated signaling pathway, chemokine-mediated signaling pathway, cell response to IL-1, and cell chemotaxis compared with LPS-EVs (Fig. 4D). The Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that the enriched signal pathways mainly include Yersinia infection, protein interaction with cytokine and cytokine receptor, TLR signaling pathway, TNF signaling pathway, Salmonella infection, rheumatoid arthritis, NOD-like receptor signaling pathway, NF-κB signaling pathway, malaria, legionellosis, influenza A, IL-17 signaling pathway, human CMV infection, fluid shear stress and atherosclerosis, cytokine–cytokine receptor interaction, chemokine signaling pathway, Chagas’ disease, C-type lectin receptor signaling pathway, amoebiasis, and AGE–RAGE signaling pathway in diabetic complications (Fig. 4E).
LPS-induced CXCL2 on Raw264.7 EVs attracts neutrophils in vitro and in vivo and activates neutrophils through the CXCR2/PKC/NOX4 pathway
Additionally, we upregulated or downregulated the macrophage CXCL2 level with lentivirus or siRNA, respectively (Fig. 5A). The CXCL2 level in EVs released by LPS + lenti-CXCL2 Raw264.7 cells appeared to be significantly higher than that of the LPS-Raw264.7–EVs, whereas the CXCL2 level in the EVs released by Raw264.7 cells in the LPS + siRNA-CXCL2 group was significantly lower than that in the LPS-Raw264.7–EVs (Fig. 5B). We added control-Raw264.7–EV, LPS-Raw264.7–EV, (LPS + lenti-CXCL2)-Raw264.7–EV, or (LPS + siRNA-CXCL2)-Raw264.7–EVs into the lower chamber to determine their chemotactic influence on BMDNs. The results showed that the LPS-Raw264.7–EVs exerted a stronger chemotactic effect on BMDNs than the control-Raw264.7–EVs. Otherwise, the (LPS-lenti-CXCL2)-Raw264.7–EVs exhibited a stronger chemotactic effect on BMDNs than the LPS-Raw264.7–EVs, and the (LPS-siRNA-CXCL2)-Raw264.7–EVs exerted reduced chemotactic effects on BMDNs (Fig. 5C). The above EVs were added onto the isolated BMDNs to detect the activation of the CXCR2/PKC/NOX4 pathway. LPS-Raw264.7-EVs treatment significantly increased the expression of CXCR2, PKC, and NOX4 in BMDNs (Fig. 5D) compared with the control-Raw264.7–EVs treatment. The (LPS + lenti-CXCL2)-Raw264.7–EVs upregulated CXCR2, PKC, and NOX4 in BMDNs compared with the LPS-Raw264.7–EVs, whereas the (LPS + siRNA-CXCL2)-Raw264.7–EVs downregulated CXCR2, PKC, and NOX4 in BMDNs compared with the LPS-Raw264.7–EVs. The above results imply that CXCL2 in LPS-induced macrophage EVs can activate the CXCR2/PKC/NOX4 pathway in neutrophils.
FIGURE 5.
LPS-induced CXCL2 of Raw264.7 EVs attracts neutrophils in vitro and in vivo and activates neutrophils via the CXCR2/PKC/NOX4 pathway. (A) The CXCL2 level (mean ± SD) of Raw264.7 in the control, LPS, LPS + lenti-CXCL2, and LPS + siRNA-CXCL2 groups detected by Western blotting (WB). The experiments were repeated three times. *p < 0.01 versus the control group, &p < 0.01 versus the LPS group, #p < 0.01 versus the LPS group. (B) The CXCL2 level (mean ± SD) in Raw264.7 EVs of the control, LPS, LPS + lenti-CXCL2, and LPS + siRNA-CXCL2 groups detected by WB. The experiments were repeated three times. (C) Raw264.7 EVs derived from the control, LPS, LPS + lenti-CXCL2, and LPS + siRNA-CXCL2 groups induced BMDN chemotaxis (mean ± SD) (n = 5 fields per group). (D) The expression of CXCR2, PKC, and NOX4 (mean ± SD) of BMDNs in the control EVs, LPS-EVs, (LPS + lenti-CXCL2)-EVs, and (LPS + siRNA-CXCL2)-EVs groups was detected by WB. The experiments were repeated three times. (E) H&E staining, MPO+ cells, and Ly-6G+ cells (mean ± SD) in the liver of the control EVs, LPS-EVs, (LPS + lenti-CXCL2)-EVs, and (LPS + siRNA-CXCL2)-EV group mice (n = 6 per group) detected by immunohistochemistry. Scale bars, 100 μm. (F–H) ALT, AST, and LDH levels (mean ± SD) in the serum of the control EVs, LPS-EVs, (LPS + lenti-CXCL2)-EVs, and (LPS + siRNA-CXCL2)-EV group mice (n = 6 per group). (I) The number of systemic circulating neutrophils (mean ± SD) in wild-type C57BL/6 mice treated with control EVs, LPS-EVs, (LPS + lenti-CXCL2)-EVs, and (LPS + siRNA-CXCL2)-EVs (n = 3 per group). (J) The expression of CXCR2, PKC, and NOX4 (mean ± SD) in the liver of the control EVs, LPS-EVs, (LPS + lenti-CXCL2)-EVs, and (LPS + siRNA-CXCL2)-EV group mice was detected by WB. The experiments were repeated three times. *p < 0.01 versus the control-Raw264.7-EV group, &p < 0.01 versus the LPS-Raw264.7-EV group, #p < 0.01 versus the LPS-Raw264.7-EV group.
Next, EVs were also injected into wild-type C57BL/6 mice through the tail vein, and the mice were sacrificed 4 h later to evaluate neutrophil infiltration in the liver using immunohistochemistry analyses. In contrast with the control-Raw264.7-EVs, neutrophil recruitment was significantly enhanced by LPS-Raw264.7-EVs in the liver. Relative to the LPS-Raw264.7–EVs, (LPS + lenti-CXCL2)-Raw264.7–EVs increased and (LPS + siRNA-CXCL2)-Raw264.7–EVs decreased the recruitment of neutrophils in the liver (Fig. 5E). In addition, H&E staining and MPO staining of the liver showed that the liver of the LPS-Raw264.7-EVs group mice was damaged and the MPO staining intensity increased compared with those of the control-Raw264.7-EVs group. Moreover, the (LPS + lenti-CXCL2)-Raw264.7-EVs group showed enhanced liver injury and MPO expression, whereas the (LPS + siRNA-CXCL2)-Raw264.7-EVs group showed reduced liver injury and MPO expression compared with the LPS-Raw264.7-EVs group (Fig. 5E).
Liver injury was analyzed based on the ALT, AST, and LDH levels, and we found that the levels of ALT, AST, and LDH in the LPS-Raw264.7-EVs group increased compared with those in the control-Raw264.7-EVs group. Moreover, the levels of ALT, AST, and LDH in the (LPS + lenti-CXCL2)-Raw264.7-EVs group increased, whereas their levels decreased in the (LPS + siRNA-CXCL2) group compared with those in the LPS-Raw264.7-EVs group (Fig. 5F–H). There was no significant difference in the number of systemic circulating neutrophils among the four EVs groups (Fig. 5I). The LPS-Raw264.7-EVs treatment significantly increased the expression of CXCR2, PKC, and NOX4 in the liver tissue (Fig. 5J) compared with the control-Raw264.7–EVs group. The (LPS + lenti-CXCL2)-Raw264.7–EVs upregulated CXCR2, PKC, and NOX4 expression in the liver compared with the LPS-Raw264.7–EVs, whereas the (LPS + siRNA-CXCL2)-Raw264.7–EVs downregulated CXCR2, PKC, and NOX4 expression in BMDNs compared with the LPS-Raw264.7–EVs. The above results indicate that CXCL2 in EVs released by LPS-stimulated macrophages can recruit neutrophils in vitro and in vivo and activate the CXCR2/PKC/NOX4 pathway in neutrophils.
EVs from the serum of patients with sepsis attract neutrophils and activate the neutrophil CXCR2/PKC/NOX4 pathway in vitro
We finally performed chemokine protein microarrays on sEVs and found that CXCL7, CXCL2, CCL2, CCL5, SDF-1a, CCL17, CCL3, and CCL19 were highly expressed in the sEVs of patients with sepsis (n = 26) compared with those in the healthy control EVs (n = 8) (Fig. 6A, Supplemental Fig. 1A). The GO analysis indicated that the biological functions of elevated chemokines in sepsis EVs were mainly enriched in chemokine-mediated signaling pathways, leukocyte chemotaxis, cell chemotaxis, myeloid leukocyte migration, neutrophil chemotaxis, lymphocyte migration, neutrophil migration, granulocyte chemotaxis, lymphocyte chemotaxis, and monocyte chemotaxis compared with EVs from healthy controls (Supplemental Fig. 1B). The KEGG analysis indicated that the enriched signal pathways mainly include chemokine signaling pathway, cytokine–cytokine receptor interaction, rheumatoid arthritis, IL-17 signaling pathway, NF-κB signaling pathway, TLR signaling pathway, TNF signaling pathway, human CMV infection, Salmonella infection, Chagas’ disease, intestinal immune network for IgA production, and NOD-like receptor signaling pathway (Supplemental Fig. 1C). We collected serum and separated healthy control sEVs and sepsis sEVs. After determining the chemokine levels, we found that the levels of CXCL2 (Fig. 6B), CXCL7 (Fig. 6C), CCL2 (Fig. 6E), and CCL3 (Fig. 6F) were significantly higher in sepsis EVs than in the healthy control EVs. However, the level of CXCL17 (Fig. 6D) in sepsis EVs was significantly lower than that in the healthy controls, whereas no significant difference was observed in the CCL5 level (Fig. 6G) between the healthy control EVs and sepsis EVs. Meanwhile, we observed that the number of chemotactic neutrophils in the sepsis-sEV group was significantly increased compared with that in the healthy control-sEV group (Fig. 6H). After adding these two groups of sEVs to neutrophils, the expression of CXCR2, PKC, and NOX4 in the sepsis-sEV group was increased compared with that in the healthy control-sEV group (Fig. 6I). The above results indicate that EVs from the serum of patients with sepsis attract neutrophils and activate the CXCR2/PKC/NOX4 pathway of neutrophils in vitro.
FIGURE 6.
EVs from the serum of patients with sepsis attract neutrophils and activate the neutrophil CXCR2/PKC/NOX4 pathway in vitro. (A) The chemokine profile of EVs from the serum of healthy individuals and patients with sepsis. (B–G) CXCL2, CXCL7, CXCL17, CCL2, CCL3, and CCL5 levels (mean ± SD) in EVs from the serum of healthy individuals (n = 8) and patients with sepsis (n = 26) detected using ELISA. *p < 0.05 versus the healthy-EV group, ***p < 0.01 versus the healthy-EV group. (H) sEVs derived from healthy control (HC) and patients with sepsis-induced PMN chemotaxis (mean ± SD) (n = 5 fields per group). (I) The expression of CXCR2, PKC, and NOX4 (mean ± SD) in the PMNs of the HC-sEV– and sepsis-sEV–treated groups was detected by Western blotting. The experiments were repeated three times. **p < 0.01 versus the healthy-EV group, #p < 0.05 versus the healthy-EV group, &p < 0.05 versus the healthy-EV group.
Discussion
It is well known that EVs may function as carriers, as they are made up of a lipid bilayer and contain RNA and proteins. Previous studies have illustrated that EVs can mediate cell–cell communication by transferring RNAs or proteins into target cells (20). Neutrophils are rapidly recruited to infection sites, where they mediate effective bacterial clearance via several mechanisms, including the release of lytic enzymes, production of reactive oxygen intermediates, and the formation of neutrophil extracellular traps (21). It is not clear whether macrophage EVs mediate the early recruitment of neutrophils in sepsis. In this study, the main findings are as follows: 1) LPS-induced Raw264.7 EVs induce the recruitment of neutrophils in the liver; 2) LPS-induced, Raw264.7-released EVs express CXCL2 and attract neutrophils in vitro; and 3) LPS-induced macrophage CXCL2 EVs activate neutrophils via the CXCR2/PKC/NOX4 pathway. This study describes a new mechanism through which macrophage-derived EVs are involved in the neutrophil recruitment process in sepsis. EVs are important for mediating neutrophil recruitment. Blocking EVs in vivo, especially EVs expressing CXCL2, to inhibit the excessive recruitment of neutrophils provides a new strategy for the clinical intervention of sepsis.
Consistent with the findings of previous studies, the recruitment of neutrophils during sepsis was found to occur as an early process. Chemokines play a key role in the recruitment of neutrophils. Macrophages are an important source of chemokines and the first line of defense against sepsis. Therefore, we chose macrophages as the initiating factor of neutrophil chemotaxis. Besides, previous studies have shown that tissue macrophages play a key role in the synthesis of CXCL2 and CXCL1 during neutrophil recruitment (17). As CXCL2 binding to glycosaminoglycans is substantially stronger than that of CXCL1, this binding might potentially serve a separate function in tethering and tissue-associated gradient formation (22). Our results suggest that LPS acute inflammation-induced neutrophil recruitment to the liver is reduced after the abolition of tissue macrophages. Chemokines, cytokines, and cell surface receptors are known to mediate intercellular communications. In addition to the role of chemokines, Yoshida et al. (23) revealed that neutrophils with cytoskeletal rearrangements preferentially sequester within the lungs during pneumonia, and this sequestration is not due to CD11/CD18-mediated adhesion, L-selectin expression, or platelet adhesion to neutrophils. Du et al. (24) reported that F-actin reorganization initiates lung inflammation via increased blood neutrophil adhesion and migration and by the production of inflammatory factors by pulmonary monocytes. Blocking F-actin reorganization may potentially prevent and treat systemic inflammatory response syndrome–induced acute lung injury (24). Chen et al. (25) found that the enhancement of self-organized actomyosin contributes to alveolar-capillary barrier disruption and neutrophil recruitment in inflammatory response, which is a potential therapeutic target for acute lung injury. In our research, LPS-Raw264.7-EVs induced neutrophil infiltration in the lung (Supplemental Fig. 2). Emerging evidence implies that EVs also serve as an important mediator of cell–cell communication. However, whether macrophagic EVs can carry chemokines, whether chemokine-containing EVs participate in the recruitment of neutrophils, and the underlying mechanism remained unclear. These are the three key questions that this study aimed to clarify.
Previous studies have shown that EVs carry chemokines under various disease conditions. CX3CL1 on the apo-MP (microparticles derived from apoptotic cells) surface is likely to act as an adhesion molecule to promote apo-MP adhesion to recipient NR8383 cells via the CX3CL1–CX3CR1 axis (26). Endothelial cell–derived EVs contain several proinflammatory mediators, including chemotactic mediators, such as IL-6, IL-8, CXCL10, MCP-1 (CCL2), and macrophage inflammatory proteins (CCL4 and CCL5) (27). Toxic lipid-mediated hepatocyte EVs contain an abundance of CXCL10 and are MLK3 dependent (28). We isolated EVs from control and LPS-induced Raw264.7 cells. CCL2, CXCL2, CCL22, CCL5, CCL17, CCL12, CCL25, and CCL20 in LPS-Raw264.7-EVs appeared significantly increased compared with those in the control-Raw264.7-EV group. We performed chemokine protein microarrays on sEVs and found that CXCL7, CXCL2, CCL2, CCL5, SDF-1a, CCL17, CCL3, and CCL19 were highly expressed in patients with sepsis compared with those in the healthy controls. In our previous work, we used mass spectrometry to detect protein profiles in EVs released by macrophages. We found that the levels of CXCL2, CCL22, CCL3, CXCL10, and CCL5 in the EVs were significantly higher in the LPS-Raw264.7–EVs than in the control-Raw264.7–EVs (29). These results indicate that both EVs and free-state chemokines are found in serum. Unlike free chemokines, EVs contain multiple chemokines, as well as other proteins and RNA, which further complicate the role of EVs in cell–cell communication because EVs possess better stability and targeting characteristics in vivo. EVs can effectively participate in the chemoattraction of neutrophils in tissues as carriers of chemokines. Future research should compare the number of free-state chemokines and EV chemokines, the occurrence time, and the functional differences.
Similar to these studies, we discovered that macrophage EVs could induce excessive neutrophil recruitment in vivo. Macrophage-derived CXCL2-bearing EVs chemoattract BMDNs and regulate the functional diversity of the recipient cells. Our research shows the importance of CXCL2 carried by EVs in neutrophil chemotaxis. It must be noted that we only measured the function of CXCL2 within EVs. The roles of other neutrophil chemoattractants were not considered in this study.
Our study revealed that CXCL2 in macrophagic EVs induces oxidative stress in neutrophils via the CXCR2/PKC/NOX4 pathway. Another study showed that CCR2-positive, mesenchymal stem cell–derived exosomes strongly bound to extracellular CCL2 and reduced its concentration (30). The consequences differ completely, although the exosomes and recipient cell interactions both occurred through the binding of chemokines to their receptors, largely because of different proteins within the EVs. This also suggests that free chemokines may be antagonized by EV surface receptors, whereas the chemokines in the EVs are not affected by this limitation. We describe another mechanism involving macrophages that can both modify chemotaxis and activate neutrophils by means of exosome delivery. However, it should be noted that EVs contain various substances, especially LPS-induced macrophagic EVs, which contain various proinflammatory mediators that can cause oxidative stress in target cells. Our previous studies also showed that LPS stimulates macrophages to release substances from exosomes that may be involved in the activation of multiple signaling pathways, such as the NOD-like receptor signaling pathway, the IL-17 signaling pathway, cytokine–cytokine receptor interactions, the chemokine signaling pathway, and the TLR signaling pathway (29). These pathways may be involved in increasing oxidative stress in target cells.
Overall, we found that LPS-induced macrophage CXCL2 EVs play an important role in the recruitment and CXCR2/PKC/NOX4-driven activation of neutrophils. Although EVs contain many other substances, our results suggest that targeting EV CXCL2 in vivo may reduce excessive recruitment of neutrophils and provide an explanation for EV-mediated neutrophil recruitment. Targeting EV CXCL2 is a viable strategy for the treatment of sepsis.
Supplementary Material
Acknowledgments
We thank Prof. Pingsheng Wu, Prof. Zhenshu Zhang, and Prof. Ying Meng for kind help in this study.
This work was supported by the National Natural Science Foundation of China (Grants 81873583 and 81670556 [to X.L.]), the Youth Fund of National Natural Science Foundation of China (Grant 81900581 [to G.W.]), Science and Technology Projects of Guangzhou (201904010482 [to X.L.]), and China postdoctoral Science Foundation on the 66th grant program (F119NF0124 [to G.W.]).
The online version of this article contains supplemental material.
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- BMDN
- bone marrow–derived neutrophil
- CLP
- cecal ligation and puncture
- CX3CL1
- C-X3-C motif chemokine ligand 1
- EV
- extracellular vesicle
- GO
- gene ontology
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- LDH
- lactate dehydrogenase
- LPS-Raw264.7-EVs
- EVs derived from LPS-induced Raw264.7 cells
- MPO
- myeloperoxidase
- NTA
- nanoparticle tracking analysis
- PKC
- protein kinase C
- PMN
- polymorphonuclear neutrophil
- sEV
- serum EV
- siRNA
- small interfering RNA
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolás-Ávila J. A., Adrover J. M., Hidalgo A.. 2017. Neutrophils in homeostasis, immunity, and cancer. Immunity 46: 15–28. [DOI] [PubMed] [Google Scholar]
- 3.Eash K. J., Greenbaum A. M., Gopalan P. K., Link D. C.. 2010. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120: 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann F., Tacke F.. 2016. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13: 88–110. [DOI] [PubMed] [Google Scholar]
- 5.Huebener P., Pradere J. P., Hernandez C., Gwak G. Y., Caviglia J. M., Mu X., Loike J. D., Schwabe R. F.. 2015. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. [Published errata appear in 2019 J. Clin. Invest. 130: 1802 and 2019 J. Clin. Invest. 130: 1802] J. Clin. Invest. 125: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nourshargh S., Alon R.. 2014. Leukocyte migration into inflamed tissues. Immunity 41: 694–707. [DOI] [PubMed] [Google Scholar]
- 7.Massena S., Christoffersson G., Hjertström E., Zcharia E., Vlodavsky I., Ausmees N., Rolny C., Li J. P., Phillipson M.. 2010. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood 116: 1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarbock A., Ley K., McEver R. P., Hidalgo A.. 2011. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118: 6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borregaard N.2010. Neutrophils, from marrow to microbes. Immunity 33: 657–670. [DOI] [PubMed] [Google Scholar]
- 10.Futosi K., Fodor S., Mócsai A.. 2013. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 17: 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrasini N., Lionetti V.. 2017. Exosomes in critical illness. Crit. Care Med. 45: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 12.Shah R., Patel T., Freedman J. E.. 2018. Circulating extracellular vesicles in human disease. N. Engl. J. Med. 379: 958–966. [DOI] [PubMed] [Google Scholar]
- 13.Truman L. A., Ford C. A., Pasikowska M., Pound J. D., Wilkinson S. J., Dumitriu I. E., Melville L., Melrose L. A., Ogden C. A., Nibbs R., et al. 2008. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112: 5026–5036. [DOI] [PubMed] [Google Scholar]
- 14.Tang N., Sun B., Gupta A., Rempel H., Pulliam L.. 2016. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 30: 3097–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita K., Kabashima A., Freeman B. L., Bronk S. F., Hirsova P., Ibrahim S. H.. 2017. Mixed lineage kinase 3 mediates the induction of CXCL10 by a STAT1-dependent mechanism during hepatocyte lipotoxicity. J. Cell. Biochem. 118: 3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marra F., Tacke F.. 2014. Roles for chemokines in liver disease. Gastroenterology 147: 577–594.e1. [DOI] [PubMed] [Google Scholar]
- 17.De Filippo K., Dudeck A., Hasenberg M., Nye E., van Rooijen N., Hartmann K., Gunzer M., Roers A., Hogg N.. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937. [DOI] [PubMed] [Google Scholar]
- 18.Gong S., Yan Z., Liu Z., Niu M., Fang H., Li N., Huang C., Li L., Chen G., Luo H., et al. 2019. Intestinal microbiota mediates the susceptibility to polymicrobial sepsis-induced liver injury by granisetron generation in mice. Hepatology 69: 1751–1767. [DOI] [PubMed] [Google Scholar]
- 19.Hong C. W., Kim T. K., Ham H. Y., Nam J. S., Kim Y. H., Zheng H., Pang B., Min T. K., Jung J. S., Lee S. N., et al. 2010. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J. Immunol. 184: 4401–4413. [DOI] [PubMed] [Google Scholar]
- 20.Sahoo S., Losordo D. W.. 2014. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 114: 333–344. [DOI] [PubMed] [Google Scholar]
- 21.Kolaczkowska E., Kubes P.. 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 22.Tanino Y., Coombe D. R., Gill S. E., Kett W. C., Kajikawa O., Proudfoot A. E., Wells T. N., Parks W. C., Wight T. N., Martin T. R., Frevert C. W.. 2010. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J. Immunol. 184: 2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida K., Kondo R., Wang Q., Doerschuk C. M.. 2006. Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats. Am. J. Respir. Crit. Care Med. 174: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du L., Zhou J., Zhang J., Yan M., Gong L., Liu X., Chen M., Tao K., Luo N., Liu J.. 2012. Actin filament reorganization is a key step in lung inflammation induced by systemic inflammatory response syndrome. Am. J. Respir. Cell Mol. Biol. 47: 597–603. [DOI] [PubMed] [Google Scholar]
- 25.Chen B., Yang Z., Yang C., Qin W., Gu J., Hu C., Chen A., Ning J., Yi B., Lu K.. 2018. A self-organized actomyosin drives multiple intercellular junction disruption and directly promotes neutrophil recruitment in lipopolysaccharide-induced acute lung injury. FASEB J. 32: 6197–6211. [DOI] [PubMed] [Google Scholar]
- 26.Tsai W. H., Shih C. H., Feng S. Y., Li I. T., Chang S. C., Lin Y. C., Hsu H. C.. 2014. CX3CL1(+) microparticles mediate the chemoattraction of alveolar macrophages toward apoptotic acute promyelocytic leukemic cells. Cell. Physiol. Biochem. 33: 594–604. [DOI] [PubMed] [Google Scholar]
- 27.Hosseinkhani B., Kuypers S., van den Akker N. M. S., Molin D. G. M., Michiels L.. 2018. Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front. Immunol. 9: 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim S. H., Hirsova P., Tomita K., Bronk S. F., Werneburg N. W., Harrison S. A., Goodfellow V. S., Malhi H., Gores G. J.. 2016. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 63: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G., Jin S., Ling X., Li Y., Hu Y., Zhang Y., Huang Y., Chen T., Lin J., Ning Z., et al. 2019. Proteomic profiling of lps-induced macrophage-derived exosomes indicates their involvement in acute liver injury. Proteomics 19: 1800274. [DOI] [PubMed] [Google Scholar]
- 30.Shen B., Liu J., Zhang F., Wang Y., Qin Y., Zhou Z., Qiu J., Fan Y.. 2016. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016: 1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.