Abstract
Background: In December 2019, a cluster of unknown etiology pneumonia cases occurred in Wuhan, China leading to identification of the responsible pathogen as SARS-coV-2. Since then, the coronavirus disease 2019 (COVID-19) has spread to the entire world. Computed Tomography (CT) is frequently used to assess severity and complications of COVID-19 pneumonia. The purpose of this study is to compare the CT patterns and clinical characteristics in intensive care unit (ICU) and non- ICU patients with COVID-19 pneumonia.
Design and Methods: This retrospective study included 218 consecutive patients (136 males; 82 females; mean age 63±15 years) with laboratory-confirmed SARS-coV-2. Patients were categorized in two different groups: (a) ICU patients and (b) non-ICU inpatients. We assessed the type and extent of pulmonary opacities on chest CT exams and recorded the information on comorbidities and laboratory values for all patients.
Results: Of the 218 patients, 23 (20 males: 3 females; mean age 60 years) required ICU admission, 195 (118 males: 77 females, mean age 64 years) were admitted to a clinical ward. Compared with non-ICU patients, ICU patients were predominantly males (60% versus 83% p=0.03), had more comorbidities, a positive CRP (p=0.04) and higher LDH values (p=0.008). ICU patients’ chest CT demonstrated higher incidence of consolidation (p=0.03), mixed lesions (p=0.01), bilateral opacities (p<0.01) and overall greater lung involvement by consolidation (p=0.02) and GGO (p=0.001).
Conclusions: CT imaging features of ICU patients affected by COVID-19 are significantly different compared with non-ICU patients. Identification of CT features could assist in a stratification of the disease severity and supportive treatment.
Significance for public health.
The major implication of our study is the differences in CT and laboratory findings in COVID-19 patients in ICU and non-ICU settings. Although the prospective impact of these findings was not assessed, recognition of these findings may help physicians anticipate the disease course and triage the patient to early ICU management. Our study highlights the importance of structured and quantitative reporting format where radiologists explicitly describe the specific pattern of pulmonary opacities (such as GGO, consolidation, and mixed) as well as lung areas and the number of lung lobes involved with COVID-19 pneumonia. To
Key words: CT, COVID-19, severity score, ICU
Introduction
In December 2019, several viral pneumonia cases of unknown origin occurred in Wuhan, Hubei Province, China.1,2 The virus was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the subsequent disease COVID-19. In the following weeks, the virus spread quickly all over the world with dramatic consequences for global health. Different reports provide descriptions of the clinical signs associated with COVID-19 with disease expression ranging from mild infection to severe acute respiratory distress.2-5 In most of the cases, symptoms are mild, as reported by Wu et al.; however, in older patients and patients with comorbidities, COVID-19 tends to progress more severely.6,7
The reference standard test in the diagnosis of COVID-19 is the reverse-transcription polymerase chain reaction (RT-PCR) to detect viral nucleotides from specimens obtained by bronchoalveolar lavage, nasopharyngeal swab, oropharyngeal swab, or tracheal aspirate.8 Computed tomography (CT) is considered as an important tool to diagnose COVID-19 infection in patients with false negative RT-PCR and in patients from high prevalence sites with limited RT-PCR as well as to assess disease severity and complications in those with confirmed COVID-19 pneumonia.9–11
A key point in the management of inpatients with COVID-19 pneumonia is deciding whether patients require ICU admission and/or mechanical ventilation.12 Although this decision relies on multiple factors such as clinical conditions, co-morbidities, and disease severity, a delay in ICU admission and/or mechanical ventilation can affect the disease outcome.12 For this reason, better strategies in decision-making-process are searched and CT imaging could play a role in this scenario.
The purpose of this study is to compare the CT patterns and clinical characteristics in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia in order to better define the CT features of patients requiring ICU and non-ICU management.
Design and Methods
Study population
In this single-center retrospective study, 218 patients (136 males; 82 females; mean age 63±15 years) with confirmed RTPCR of COVID-19 were enrolled from March 3 to April 28, 2020 at the Azienda Ospedaliero Universitaria (A.O.U.) “Maggiore d.c.”, University of Eastern Piedmont, Novara, Italy. For each of the 218 patients a chest CT and laboratory test were performed at time of admission to the hospital. Patient characteristics including age, gender, clinical symptoms, time course of the symptoms, medical history and outcomes were recorded.Patients were divided into two groups: (a) ICU and (b) non-ICU.13-15
CT technique and feature analysis
All chest CT scans were performed during a single full inspiratory breath-hold in supine position on a 128-slice multidetectorrow CT scanner (Philips Ingenuity Core, Philips Healthcare, Netherlands). No intravenous or oral contrast media were administered. The CT examinations were performed at 120 kV, 226 mAs (using automatic tube current modulation – Z-DOM, Philips), 1.08 spiral pitch factor, 0.5-second gantry rotation time and 64*0.625 detector configuration. One-mm thick images were reconstructed with soft tissue kernel using 512 × 512 matrix (mediastinal window) and with lung kernel using 768 × 768 matrix (lung window). CT images were reviewed on the Picture Archiving and Communication System (PACS) workstation equipped with two 35 × 43 cm monitors produced by Eizo, with 2048 × 1536 matrix.
Two radiologists (AC with 32 years and LS with 16 years of experience in chest radiology) assessed the type and extent of pulmonary opacities on chest CT images. Imaging was reviewed independently, and final decision reached by consensus are reported. No negative control cases were examined. All 218 chest CT examinations were evaluated for the presence of ground glass opacity (GGO), consolidation, mixed GGO and consolidation, crazy paving, reverse halo sign, bilateral lung involvement, and lymph node enlargement (lymphadenopathy).
The severity on CT was estimated by visual assessment based on the areas of pulmonary opacities.16,17 The distribution of lung changes was also classified based on visual quantification of the percentage of lung parenchyma affected by COVID-19, as follow: 0, no lung involvement; 1 1-10%; 2 11-25%; 3 26-50%; 4 51-75%; 5 >75%.18-20 Mediastinal lymph nodes were considered enlarged when their short-axis diameter was greater than 1.0 cm. Radiological lesions were categorized based on the Fleischner Society’s glossary of terms.21
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (with interquartile range) values. Categorical variables were described as frequency rates and percentages, comparisons of continuous data were performed using the independent samples t-test or Mann-Whitney U test; Normality of distribution for continuous variable was tested using Kolmogorov-Smirnov before performing t-test or Mann-Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Correlation was assessed using the Pearson r and Spearman rho coefficient as appropriate. A p-value <0.05 was considered statistically significant. All statistical analysis was performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA).
Results
Overall study cohort
The mean (±SD) age of the patients was 63±15 years, 136 males, 82 females. The most common symptoms were cough (n=130/218; 59%), followed by dyspnea (n =113/218; 52%) Among the comorbidities, hypertension was the most common (n=99/218; 45%), followed by coronary heart disease (n=45/218; 21%) and diabetes (n=35; 11%). Forty patients (n=40/218; 18,3%) had fever on admission. The most common chest CT features of both groups included GGO (202/218, 92%) consolidation (184/218, 84%), mixed of GGO and consolidation (180/218, 82%). Less frequent CT findings were crazy paving pattern (60/218, 27%), reversed halo sign (22/218, 10%). In addition, 27% of patients had hilar and/or mediastinal lymphadenopathy.
ICU versus non-ICU patients
ICU patients were 23 (20/23 [80%] were males, mean age 60 years) versus 195 who did not require ICU admission (118/195 [60%] were males, mean age 64 years) The proportion of ICU admissions represents 10.5% of the total cases (23/218).
Compared with non-ICU patients, ICU patients were predominantly males (p=0.02), had more underlying diseases, such as cancer (p=0.02). Therefore, patients with emphysema, which is more common in older patients (p=0.01), had correlation with mortality (p=0.01, r=0.33). The ICU group had a greater positivity of the creactive protein (p=0.04), LDH (p=0.008) and higher mortality (p=0.01). Baseline characteristics and laboratory findings are reported in Table 1.
Figure 1.
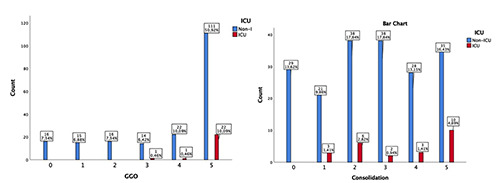
Number of lung lobes involved by GGO and by consolidation in ICU patients compared with non-ICU patients.
Patients in the ICU had higher incidence of lung consolidation (p=0.03), mixed of GGO and consolidation (p=0.01) and a bilateral lung involvement (p=0.03) with a worse CT severity score and a greater number of lung lobar involvement compared with non- ICU patients, with statistically significant differences in GGO (p=0.001) and consolidation (p=0.02) (Figure 1). Distribution of chest CT findings are summarized in Tables 2 and 3.
In addition, the number of lung lobar involvement showed a positive correlation with duration of symptoms (p=0.002, r=0.33), cough (p=0.01, r=0.31), higher value of temperature (p=0.01, r=0.23), positive C-reactive protein (CRP) (p=0.001, r=0.41) and greater lymph node involvement in CT (p=0.01, r=0.28) (Supplementary Table 1).
Table 1.
Baseline characteristics and laboratory findings in ICU and non-ICU patients.
| ICU | Non-ICU | p | Size of effect | |
|---|---|---|---|---|
| Age | 60 ± 11 | 64 ± 15 | 0.07 | 0.24 |
| Male | 19/23, 80% | 117/195, 60% | 0.02* | 0.29 |
| Height | 172.2 ± 8 | 170 ± 9 | 0.23 | 0.21 |
| Weight | 87.2 ± 14 | 80 ± 17.7 | 0.07 | 0.04 |
| CAD | 4/23, 17% | 41/195, 20% | 0.56 | 0.07 |
| Hypertension | 11/23, 47% | 88/195, 44% | 0.56 | 0.02 |
| Diabetes mellitus | 5/23, 21% | 30/195, 15% | 0.69 | 0.05 |
| Emphysema | 2/23, 8% | 6/195, 3% | 0.66 | 0.08 |
| Lung diseases (no COPD) | 1/23, 4% | 2/195, 1% | 0.23 | 0.16 |
| Cancer | 2/23, 8% | 19/195, 10% | 0.02* | 0.21 |
| Neurovascular diseases | 1/23, 4% | 28/195, 14% | 0.45 | 0.06 |
| Chronic kidney diseases | 1/23, 4% | 17/195, 8% | 0.54 | 0.08 |
| Chronic liver diseases | 0/23, 0% | 7/195, 3% | 0.41 | 0.12 |
| Rheumatoid arthritis | 1/23, 4% | 1/195, 0,5 % | 0.14 | 0.28 |
| Smoke | 2/23, 8% | 28/195, 14% | 0.38 | 0.12 |
| Temperatures | 37.66 (36.66-38.66) | 37.56 (36.52-38.60) | 0.38 | 0.11 |
| Cough | 14/23 60% | 116/195, 58% | 0.76 | 0.04 |
| Sore Throat | 1/23 4% | 5/195, 2% | 0.65 | 0.06 |
| Dyspnea | 15/23 65% | 98/195, 49% | 0.13 | 0.20 |
| Days from symptoms onset to time of CT | 6.23 ± 3.5 | 6.89 ± 5.3 | 0.82 | 0.01 |
| CRP + | 23/23 100% | 148/195, 74% | 0.04* | 0.17 |
| LDH | 1461 ± 394 | 905 ± 590 | 0.008* | 0.85 |
| Platelets | 247.428 ±141944 | 199.516 ± 68650 | 0.66 | 0.10 |
| Mortality | 8/23 34% | 24/195 12% | 0.01 | 0.34 |
ICU, intensive care unit; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRP, c-reactive protein; LDH, lactate dehydrogenase. Mean ± SD. Data are n (%), or median (interquartile range). Size of effect: Cohen D or Was appropriate.
Table 2.
Distribution of chest CT findings in ICU and non-ICU patients.
| ICU | Non-ICU | p | Size of effect | |
|---|---|---|---|---|
| GGO | 23/23, 100% | 174/195, 89% | 0.14 | 0.19 |
| Consolidation | 23/23, 100% | 156/195, 80% | 0.03* | 0.28 |
| GGO (lobes involved) | 4.8±0.45 | 3.9±1.6 | 0.001* | 0.41 |
| Consolidation (lobes involved) | 3.4±1.5 | 2.6±1.7 | 0.02* | 0.29 |
| Mixed GGO and consolidation | 23/23 100% | 152/195, 78% | 0.01* | 0.32 |
| Bilateral involvement | 23/23 100% | 159/195, 81% | 0.03* | 0.28 |
| Nodes involvement | 6/23, 26% | 49/195, 25% | 0.79 | 0.03 |
| Crazy paving | 8/23, 35% | 54/195, 27% | 0.55 | 0.08 |
| Reversed halo sign | 2/23, 9% | 19/195, 10% | 0.73 | 0.04 |
GGO, ground-glass opacities; ICU, intensive care unit. Mean ±SD; Data are n (%). Size of effect, Cohen D or W as appropriate.
Discussion
In this study we aimed at characterizing chest CT features of COVID-19 patients and to compare the clinical characteristics and CT patterns of inpatients in intensive care unit (ICU) with non-ICU COVID-19 patients, in order to better define the CT features of patients requiring ICU and non-ICU treatments. In our cases of SARS-coV-2 infection, we observed a greater number of men than women, as reported in other studies22 and a gender difference in ICU patients, with a predominance of males. The gender difference could be explained, as reported by Jaillon et al., by a sexual dimorphism in the innate response with higher susceptibility to infections in males.23
Cough was the most common presenting symptom followed by dyspnea, and the mean duration of symptoms was 1 week. Although 50% of patients had fever, there were no statistically significant differences in the presence of high temperature in the two groups under analysis; this suggests that fever was not a useful criterion to determine the severity of illness in our study cohort.24 The median age of the patients admitted to the ICU was 60 ± 11 years, with no statistically significant difference in comparison to non- ICU patients, this might suggest that age alone is not a risk factor for ICU.25
The proportion of ICU admissions represents 10.5% of the total cases, this percentage is higher than what was observed in prior studies from China;26 though, these data are in agreement with other Italian studies.27 This could be explained, as suggested by Grasselli et al., by a demographic difference between the populations in two countries.27
ICU patients had more comorbidities, such as chronic obstructive pulmonary disease. In particular, the presence of emphysema is associated with higher mortality, so an underlying lung disease affects the clinical outcome.28,29
Compared with the non-ICU group, ICU patients had higher incidences of positive CRP and increased LDH values. CRP levels were positively correlated with lung lesions and disease severity, and CRP levels correlate well with the level of inflammation.30 The baseline characteristics and laboratory findings of subjects enrolled in the study are summarized in Table 1.
Table 3.
Comparison of the number of affected lung lobes with require ICU.
| Number of lobes involved | ICU | Non-ICU |
|---|---|---|
| 0 | 0, 100% | 29/195, 15% |
| 1 | 3/23, 12,5% | 21/195, 11% |
| 2 | 6/23, 25% | 38/195, 20% |
| 3 | 2/23, 8.5% | 38/195, 20% |
| 4 | 3/23, 12.5% | 28/195, 15% |
| 5 | 10/23 41% | 35/195, 18% |
Figure 2.
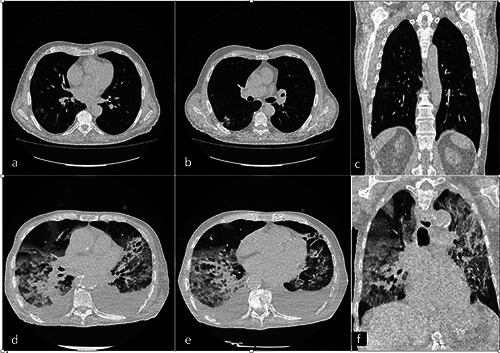
a-f ) Chest CT of an 89-year-old man with ICU COVID-19 pneumonia (a). An axial and coronal CT image showed diffuse large regions of crazy-paving pattern with partial consolidation and bilateral pleural effusion. CT findings in a 54-year-old man with non-ICU COVID-19 pneumonia (b). An axial and coronal CT image showed bilateral multiple small regions of subpleural GGO and consolidation.
To our knowledge, this is the first report of Italian patients that compared CT findings of ICU patients with non-ICU patients with COVID-19 pneumonia. In this study, we found that the most common CT imaging features were GGO, consolidation, mixed of both and crazy-paving pattern. The CT findings are closely related to the physiological and pathological basis of the disease. According to Xu et al. pathological findings in COVID-19 patients resemble those seen in SARS and Middle Eastern respiratory syndrome (MERS) coronavirus infection.31 Moreover, COVID-19 shows CT similarities to SARS and MERS pneumonia32,33 and other lung disease. 34. Chest CT undoubtedly may play a key role in differential diagnosis. Specific CT features can help in the proper diagnosis, such as centrilobular nodules, mucoid impaction or unilateral involvement to diagnostic a bacterial or superinfection pneumonia. On the other hand, septal lines, large pulmonary vein suggest a pulmonary edema.34 Li et al. assessed in their research the rate of misdiagnosis of COVID-19 at the CT admission. The authors reported a low rate of missed diagnosis (3.9%) based on CT features.35 Furthermore, Fang et al. compared the sensitivity of chest CT with that of RT-PCR at the initial patient presentation, The authors reported that the sensitivity of chest CT was greater compared to RT-PCR (98 % vs 71%, p<0.001).36
GGO were defined as hazy increased opacity of lung without obscuration of bronchial and vascular margins, its formation results in incomplete filling of the alveolar cavity and mild interstitial thickening.37,38 Consolidation was defined as an exudate or other product of disease that replaces alveolar air. In this scenario, it could be related to diffuse alveolar damage.38 These findings are common in patients with COVID-19.18,39,40 Whereas crazy paving has not often been observed as GGO and consolidation,39 and may be related to thickening of interlobular and intralobular interstitial.18,39,41 According to a recent study comprising 21 patients with COVID-19, crazy paving pattern can be a marker of COVID-19 peak stage.41 Another CT finding present in our cohort of patients was reverse halo sign, this was described in several COVID-19 patients,42 which could be related to disease progression43 and may reflect an organizing pneumonia pattern in COVID-19 cases.44 Compared with non-ICU patients, ICU group has shown higher frequency of consolidation and mixed lesions. The pathological bases of these changes are not currently clear. Consolidation indicates that alveolar air is being replaced by inflammatory exudation, and in COVID-19 patients histological examination showed that consolidation may be referred to cellular fibromyxoid exudates.31 Moreover, ICU patients showed a greater bilateral lung involvement (Figure 2). The CT findings between the two groups are summarized in Table 2.
In our study, laboratory parameters and clinical characteristics, such as PCR, cough, fever, duration of symptoms and nodes involvement showed significant positive correlation with the number of lobes involved by pneumonia. These findings may be related to a gradual increase in lung involvement over time with the occurrence of severe inflammation and it could be considered as a marker of disease progression.18,43
In addition, CT reports showed lymphadenopathy in 27% of the patients analyzed, a higher prevalence of nodal involved than in previous studies. This observation is not concordant with studies in Chinese populations.3,13 Lymphadenopathy could be considered as an uncommon feature of COVID-19, such as in viral pneumonia. Recent research of ICU patients reported increased lymph node involvement compared with the Chinese populations. 18,40,43,45
As reported by Sardanelli et al. lymphadenopathy may be considered a predictor of a worse outcome, and the differences in disease severity could probably explain this discrepancy in different studies.18,45 Furthermore, ICU-patients showed greater number of lung lobar involvement (Table 3) by consolidation and GGO, as reported in previous studies,41,46,47 and may help us make an early identification of patients that require ICU from patients who do not. Our results are in line with the current literature.20,48 Ruch et al. reported that quantification of CT lung lesion involvement is related to ICU admission, especially in patients with lung involvement >50%.20
This study has several limitations. First, the retrospective nature of our analysis. Secondly, the data of the two groups were not balanced, because too few ICU patients were included in this study, which decreased the reliability of the statistical results. Further studies with more patients, especially patients who require ICU, are warranted. Third, the quantitative method for measuring the pulmonary involvement may have subjectivity and we cannot rule out the possibility of concomitant superinfection due to bacterial pneumonias, aspiration, and pulmonary edema. In conclusion, the results of this study confirm that chest CT is important in the diagnosis and management of the SARS-coV-2 infection. The CT features of the COVID-19 pneumonia were different in ICU patients compared with non-ICU patients, we found more consolidative lesions in critically ill patients and a greater pulmonary involvement. Our data suggest CT could be a useful tool for stratify patients who require ICU.
Funding Statement
Funding: The authors state that this work has not received any funding.
References
- 1.Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 2020;92:401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cau R, Bassareo P, Saba L.Cardiac involvement in COVID-19 - Assessment with echocardiography and cardiac magnetic resonance imaging. SN Compr Clin Med 2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cau R, Bassareo PP, Mannelli L, et al. Imaging in COVID-19- related myocardial injury. Int J Cardiovasc Imaging 2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [DOI] [PubMed] [Google Scholar]
- 7.Saba L, Gerosa C, Fanni D, et al. Molecular pathways triggered by COVID-19 in different organs: ACE2 receptorexpressing cells under attack? A review. Eur Rev Med Pharmacol Sci 2020;24:12609-22. [DOI] [PubMed] [Google Scholar]
- 8.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng M-Y, Lee EYP, Yang J, et al. Imaging profile of the COVID-19 infection: Radiologic findings and literature review. Radiol Cardiothorac Imaging 2020;2:e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology 2020;295:22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: Relationship to negative RT-PCR testing. Radiology 2020;296:E41-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8:506-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlos WG, Dela Cruz CS, Cao B, et al. Novel Wuhan (2019- nCoV) coronavirus. Am J Respir Crit Care Med 2020;201:P7-8. [DOI] [PubMed] [Google Scholar]
- 14.Vandroux D, Allyn J, Ferdynus C, et al. Mortality of critically ill patients with severe influenza starting four years after the 2009 pandemic. Infect Dis (Lond) 2019;51:831-7. [DOI] [PubMed] [Google Scholar]
- 15.Gouda W, Yasin R.COVID-19 disease: CT pneumonia analysis prototype by using artificial intelligence, predicting the disease severity. Egypt J Radiol Nucl Med 2020;51:196. [Google Scholar]
- 16.Lyu P, Liu X, Zhang R, et al. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: Identifying critical cases based on CT characteristics. Invest Radiol 2020;55:412-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasilewski PG, Mruk B, Mazur S, et al. COVID-19 severity scoring systems in radiological imaging - a review. Polish J Radiol 2020;85:e361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;55:327-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessmann N, Sánchez CI, Beenen L, et al. Automated assessment of COVID-19 reporting and data system and chest CT severity scores in patients suspected of having COVID-19 using artificial intelligence. Radiology 2021;298:e18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruch Y, Kaeuffer C, Ohana M, et al. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect 2020;26:1417.e5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [DOI] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaillon S, Berthenet K, Garlanda C.Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol 2019;56:308-21. [DOI] [PubMed] [Google Scholar]
- 24.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - Case series. N Engl J Med 2020;382:2012-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G, Pesenti A, Cecconi M.Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 2020;323:1545-6. [DOI] [PubMed] [Google Scholar]
- 28.Team CDCC-19 R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020;69:382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tal-Singer R, Crapo JD. COPD at the time of COVID-19: A COPD foundation perspective. Chronic Obstr Pulm Dis 2020;7:73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L. C-reactive protein levels in the early stage of COVID- 19. Med Mal Infect 2020;50:332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID- 19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajlan AM, Ahyad RA, Jamjoom LG, et al. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: Chest CT findings. Am J Roentgenol 2014;203:782-7. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y-L, Tsay P-K, Cheung Y-C, et al. A correlation between the severity of lung lesions on radiographs and clinical findings in patients with severe acute respiratory syndrome. Korean J Radiol 2007;8:466-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hani C, Trieu NH, Saab I, et al. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn Interv Imaging 2020;101:263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Xia L.Coronavirus disease 2019 (COVID-19): Role of chest CT in diagnosis and management. Am J Roentgenol 2020;214:1280-6. [DOI] [PubMed] [Google Scholar]
- 36.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: Comparison to RT- PCR. Radiology 2020;296:e115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engeler CE, Tashjian JH, Trenkner SW, Walsh JW. Groundglass opacity of the lung parenchyma: a guide to analysis with high-resolution CT. Am J Roentgenol 1993;160:249-51. [DOI] [PubMed] [Google Scholar]
- 38.Guan CS, Lv Z Bin, Yan S, et al. Imaging features of coronavirus disease 2019 (COVID-19): Evaluation on thin-section CT. Acad Radiol 2020;27:609-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 2020;30:4381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID- 19). Radiology 2020;295:715-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Feng LC, Xian XY, et al. [Novel coronavirus pneumonia (COVID-19) CT distribution and sign features].[Article in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:321-6. [DOI] [PubMed] [Google Scholar]
- 43.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID- 19): Relationship to duration of infection. Radiology 2020;295:200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): Analysis of nine patients treated in Korea. Korean J Radiol 2020;21:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sardanelli F, Cozzi A, Monfardini L, et al. Association of mediastinal lymphadenopathy with COVID-19 prognosis. Lancet Infect Dis 2020;20:1230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faghihi Langroudi T, Khazaei M.Common imaging patterns of COVID-19 on spiral chest CT scan: a diagnostic approach for pulmonary involvement in ICU patients. J Cell Mol Anesth 2020;5:6-15. [Google Scholar]
- 47.Xiong Y, Sun D, Liu Y, et al. Clinical and high-resolution CT features of the COVID-19 infection: Comparison of the initial and follow-up changes. Invest Radiol 2020;55:332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emara DM, Naguib NN, Moustafa MA, et al. Typical and atypical CT chest imaging findings of novel coronavirus 19 (COVID-19) in correlation with clinical data: impact on the need to ICU admission, ventilation and mortality. Egypt J Radiol Nucl Med 2020;51:227. [Google Scholar]


