Abstract
Background
Congenital heart disease (ConHD) affects approximately 1% of all live births. People with ConHD are living longer due to improved medical intervention and are at risk of developing non‐communicable diseases. Cardiorespiratory fitness (CRF) is reduced in people with ConHD, who deteriorate faster compared to healthy people. CRF is known to be prognostic of future mortality and morbidity: it is therefore important to assess the evidence base on physical activity interventions in this population to inform decision making.
Objectives
To assess the effectiveness and safety of all types of physical activity interventions versus standard care in individuals with congenital heart disease.
Search methods
We undertook a systematic search on 23 September 2019 of the following databases: CENTRAL, MEDLINE, Embase, CINAHL, AMED, BIOSIS Citation Index, Web of Science Core Collection, LILACS and DARE. We also searched ClinicalTrials.gov and we reviewed the reference lists of relevant systematic reviews.
Selection criteria
We included randomised controlled trials (RCT) that compared any type of physical activity intervention against a 'no physical activity' (usual care) control. We included all individuals with a diagnosis of congenital heart disease, regardless of age or previous medical interventions.
Data collection and analysis
Two review authors (CAW and CW) independently screened all the identified references for inclusion. We retrieved and read all full papers; and we contacted study authors if we needed any further information. The same two independent reviewers who extracted the data then processed the included papers, assessed their risk of bias using RoB 2 and assessed the certainty of the evidence using the GRADE approach. The primary outcomes were: maximal cardiorespiratory fitness (CRF) assessed by peak oxygen consumption; health‐related quality of life (HRQoL) determined by a validated questionnaire; and device‐worn ‘objective’ measures of physical activity.
Main results
We included 15 RCTs with 924 participants in the review. The median intervention length/follow‐up length was 12 weeks (12 to 26 interquartile range (IQR)). There were five RCTs of children and adolescents (n = 500) and 10 adult RCTs (n = 424). We identified three types of intervention: physical activity promotion; exercise training; and inspiratory muscle training. We assessed the risk of bias of results for CRF as either being of some concern (n = 12) or at a high risk of bias (n = 2), due to a failure to blind intervention staff. One study did not report this outcome. Using the GRADE method, we assessed the certainty of evidence as moderate to very low across measured outcomes.
When we pooled all types of interventions (physical activity promotion, exercise training and inspiratory muscle training), compared to a 'no exercise' control CRF may slightly increase, with a mean difference (MD) of 1.89 mL.kg−1.min−1 (95% CI −0.22 to 3.99; n = 732; moderate‐certainty evidence). The evidence is very uncertain about the effect of physical activity and exercise interventions on HRQoL. There was a standardised mean difference (SMD) of 0.76 (95% CI −0.13 to 1.65; n = 163; very low certainty evidence) in HRQoL. However, we could pool only three studies in a meta‐analysis, due to different ways of reporting. Only one study out of eight showed a positive effect on HRQoL. There may be a small improvement in mean daily physical activity (PA) (SMD 0.38, 95% CI −0.15 to 0.92; n = 328; low‐certainty evidence), which equates to approximately an additional 10 minutes of physical activity daily (95% CI −2.50 to 22.20).
Physical activity and exercise interventions likely result in an increase in submaximal cardiorespiratory fitness (assessed with VO2 mL.kg‐1.min‐1 at the gas exchange threshold; MD 2.05, 95% CI 0.05 to 4.05; n = 179; moderate‐certainty evidence). Physical activity and exercise interventions likely increase muscular strength (measured by maximal voluntary contraction of knee extensions; MD 17.13, 95% CI 3.45 to 30.81; n = 18; moderate‐certainty evidence). Eleven studies (n = 501) reported on the outcome of adverse events (73% of total studies). Of the 11 studies, six studies reported zero adverse events. Five studies reported a total of 11 adverse events; 36% of adverse events were cardiac related (n = 4); there were, however, no serious adverse events related to the interventions or reported fatalities (moderate‐certainty evidence). No studies reported hospital admissions.
Authors' conclusions
This review summarises the latest evidence on CRF, HRQoL and PA. Although there were only small improvements in CRF and PA, and small to no improvements in HRQoL, there were no reported serious adverse events related to the interventions. Although these data are promising, there is currently insufficient evidence to definitively determine the impact of physical activity interventions in ConHD. Further high‐quality randomised controlled trials are therefore needed, utilising a longer duration of follow‐up.
Plain language summary
Physical activity interventions for people with congenital heart disease
Review question
This review aimed to gather evidence for the use of any physical activity intervention for people with congenital heart disease. We aimed to compare interventions including exercise training, physical activity promotion or lung training with no intervention (usual care).
Background
Congenital heart disease is the term used for a range of birth defects that affect how the heart works. People with congenital heart disease have reduced life expectancy, physical fitness and quality of life. However, due to better prenatal diagnoses, surgical procedures (often performed in the early years of life) and earlier interventions, the survival rate for those born with this disease has improved dramatically, such that most people will now live into adulthood. Exercise training and physical activity interventions are known to improve fitness, physical activity, survival and quality of life in healthy people, but it is not clear how effective these programmes are for people with long‐term medical conditions.
Study characteristics
We searched for studies in September 2019 and identified 15 studies involving 924 participants. The studies used three main types of interventions, including programmes designed to increase physical activity, aerobic fitness and health‐related quality of life and compared physical activity intervention and control interventions in people with congenital heart disease.
Key results
We included 15 trials with 924 participants. Half of the participants were female. Of the 15 trials, 5 used a total of 500 young people (less than 18 years of age) and 10 trials used a total of 424 adult participants. We found that physical fitness and physical activity may slightly increase but we are very uncertain about quality of life. There is currently no data to say if this small increase in fitness will result in fewer visits to the hospital. But there were no recorded deaths or serious events that were related to participation in physical activity.
Quality of evidence
Using a validated scientific approach (GRADE), the certainty in the evidence base was moderate for fitness, low for physical activity and very low for quality of life. Most outcomes were limited due to small study participant numbers and poor reporting of study details.
Summary of findings
Summary of findings 1. Summary of findings for the main comparison. Physical activity and exercise interventions compared to usual care for people with congenital heart disease.
| Patient or population: people with congenital heart disease. Setting: hospital‐based and home‐based settings. Intervention: physical activity promotion, inspiratory muscle training and exercise training interventions. Comparison: usual care. | |||||
|
Outcomes Length of intervention in weeks (Median, (IQR)) |
Anticipated absolute effects* (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with all interventions | ||||
| Maximal cardiorespiratory fitness (CRF) Assessed with: treadmill or cycle ergometry (12 (12, 26)) |
Mean 22 to 46 | MD 1.89 higher (0.22 lower to 3.99 higher) | 732 (14 RCTs (15 training arms)) |
⊕⊕⊕⊝ab MODERATE | Physical activity and exercise interventions may increase cardiorespiratory fitness slightly. Sensitivity analyses did not change the inference to a clinically important MD. |
| Health‐related quality of life (HRQoL) Assessed with: Questionnaires (12 (12, 12)) |
‐ | SMD 0.76 higher (0.13 lower to 1.65 higher) | 163 (3 RCTs) | ⊕⊝⊝⊝cde VERY LOW | The evidence is very uncertain about the effect of physical activity and exercise interventions on health‐related quality of life. |
| Physical activity (PA) Assessed with: Accelerometer (19 (12, 46)) |
Mean 11 to 41 | SMD 0.38 (0.15 lower to 0.92 higher) | 328 (4 RCTs) | ⊕⊕⊝⊝ce LOW | Physical activity and exercise interventions may increase physical activity slightly. |
| Submaximal cardiorespiratory fitness Assessed with: treadmill or cycle ergometry (VO2 mL.kg‐1.min‐1 at the gas exchange threshold). (12 (12, 15)) |
Mean 18 to 22 | MD 2.05 (0.05 higher to 4.05 higher) | 179 (5 RCTs (6 training arms)) | ⊕⊕⊕⊝e MODERATE | Physical activity and exercise interventions likely results in an increase in submaximal cardiorespiratory fitness. |
| Muscular strength (12) |
Mean 103 | MD 17.13 (3.45 higher to 30.81 higher) | 18 (1 RCT) | ⊕⊕⊕⊝e MODERATE | Physical activity and exercise interventions likely increases muscular strength. |
| Adverse events (12 (12, 26)) |
A total of 11 adverse events were reported in a population of 501 (2.2%), 7 of which were mild (1.4%). Mild adverse events included dizziness, discomfort, musculoskeletal (strains/ sprains) and a minor head injury. The 4 (0.8%) moderate adverse events were cardiac. There were no major adverse events reported. | 501 (11 RCTs) | ⊕⊕⊕⊝f MODERATE | Physical activity promotion and exercise training interventions did not lead to any serious adverse events. | |
| Hospital admissions | No data available | ||||
| CI: confidence interval; IQR: 25th and 75th quartiles; MD: mean difference; SMD: standardised mean difference; RCT: randomised controlled trial; CRF: Cardiorespiratory fitness; HRQoL: Health‐related quality of life. The mean SMD effect size was interpreted using Cohen effect sizes, i.e. 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
a Statistical heterogeneity was present in CRF (I2 = 75%). But it was explained using a sensitivity analysis by excluding high risk of bias studies (I2 = 18%). Therefore, we chose not to downgrade by 1 level evidence due to inconsistency.
bThe confidence interval includes both appreciable harm and appreciable benefit (i.e. 95% CI spans 0). Therefore, the certainty of evidence was downgraded by 1 level due to imprecision.
cInconsistent directions of effect and considerable heterogeneity (HRQoL, I2 = 82%; PA, I2 = 77%). Therefore, the certainty of evidence was downgraded by 1 level due to inconsistency.
dTotal high risk of bias in all studies included within the analysis, hence bias highly likely. Therefore, the certainty of evidence was downgraded by 2 levels due to the methodological limitations (risk of bias).
eImprecise due to small numbers of events (< 400) (Ryan 2016). Therefore, the certainty of evidence was downgraded by 1 level due to imprecision.
fOver 25% of studies did not report data on adverse events. Therefore, the certainty of evidence was downgraded by 1 level due to publication bias.
Background
Description of the condition
Congenital heart disease (ConHD) is a term for a range of developmental abnormalities of the heart or intrathoracic vessels, or both (Mitchell 1971). There are over 18 distinct types of ConHD, ranging in complexity (Rhodes 2008; Sommer 2008a; Sommer 2008b), that can be broadly classified as mild, moderate or severe (Hoffman 2002). During the period 2010 to 2017 the birth prevalence of ConHD is approximately 1% of all live births (9.41 per 1000 births; 95% confidence interval (CI) 8.60 to 10.25), with the mild conditions representing 65% (95% CI 58.7% to 71.7%) of the total ConHD births (Liu 2019).
Long‐term survival is reduced in ConHD, but due to improved medical care this has improved dramatically over previous decades. The most recent meta‐analysis reported that survival up to the age of 10 years was 81.4% (95% CI 73.80 to 87.90); however, 87.0% of the between‐article variance can be accounted for by the year of the study (Best 2016). Consequently, survival up to the age of 10 years of age has increased from 75% to 90% over the past two decades due to improvements in surgical correction, prenatal diagnosis and earlier interventions (Best 2016). ConHD is therefore a changing chronic medical condition with the highest proportion of deaths now occurring in geriatrics (Khairy 2010).
In both healthy and clinical (coronary heart disease) populations, impaired physical activity (PA) and cardiorespiratory fitness (CRF) are associated with the development of non‐communicable disease, morbidity and mortality (Franklin 2013; Lee 2012; Letnes 2019). People with ConHD have reduced levels of fitness (Amedro 2017); reduced health‐related quality of life (HRQoL) (Amedro 2015); and are less physically active (Brudy 2020; Dua 2007; McCrindle 2007; Sandberg 2016). Fitness has also been heavily associated with future health outcomes in this population (Dimopoulos 2006; Giardini 2009; Müller 2015; Udholm 2018). It is crucial, therefore, that people with ConHD lead an active lifestyle, but there is currently no consensus on how best to improve PA, CRF and HRQoL in people with ConHD.
Description of the intervention
Physical activity consists of any bodily movement involving skeletal muscles that results in increased energy expenditure, whereas exercise training is a planned and structured period of PA with the intention of maintaining or improving physical fitness components (Caspersen 1985). The current guidelines for PA are an average of 60 minutes of moderate to vigorous physical activity (MVPA) a day for young people (< 18 years old) and 150 minutes of MVPA a week in adults (Department of Health 2011). Currently within specialist paediatric cardiac clinics physical activity recommendations are not adequately discussed due to a lack of training, time and knowledge of the current exercise recommendations for people with ConHD (Williams 2017).
Interventions that aim to improve CRF, PA and HRQoL typically consist of a PA promotion (goal setting, motivational interviews etc.), or exercise training (aerobic/resistance training, sports participation etc.), or a combination of both. PA promotion interventions aim to increase habitual PA behaviours by using psychological conceptual frameworks in order to promote self‐efficacy, goal‐setting and intrinsic motivation. Exercise training interventions, on the other hand, usually prescribe a set 'dose' of exercise either within a hospital, centre or at home. Inspiratory muscle training (IMT) is a new method of intervention, recently gaining popularity in people with chronic cardiorespiratory conditions and aiming to improve ventilatory power and efficiency. IMT is distinctly different from PA promotion and exercise training as it involves training the inspiratory chest muscles against a breathing resistance by using a handheld device.
How the intervention might work
The 'gold standard' measure of cardiorespiratory fitness is maximal oxygen consumption, which is explained by the Fick equation where oxygen uptake is the product of the cardiac output and the arteriovenous oxygen difference (V̇O2 = Q * a‐V̇O2 diff). Improving cardiorespiratory fitness must target improving oxygen delivery (Q) and/or oxygen extraction at the peripheral sites (a‐V̇O2 diff) of the body, namely the muscles. PA and exercise training are known to improve both cardiac output and oxygen extraction through complex molecular interactions improving myocardial contractility, mitochondrial activity, stem cell proliferation, nitric oxide bioavailability and muscle fibre adaptations (Adams 2017; Gielen 2010). There is evidence that supports inspiratory muscle training to improve ventilatory efficiency and fitness in people with chronic cardiorespiratory pathologies and in healthy people, but the adaptation mechanisms are not well understood (Shei 2018; Wong 2011). It is proposed that the increase in ventilatory efficiency is due to a combination of factors, inclusive of, but not limited to, changing motor recruitment 'diaphragm sparing' and the release of inflammatory cytokines (Shei 2018).
Why it is important to do this review
Cardiorespiratory fitness is lower in people with ConHD and deteriorates faster compared to healthy people (Amedro 2017). This has significant implications as CRF has been associated with future mortality and morbidity in several ConHD conditions (Dimopoulos 2006; Giardini 2009; Müller 2015; Udholm 2018). Currently, there is a dearth of evidence to adequately inform the effectiveness of interventions to improve CRF and consequently long‐term outcomes.
Recent reviews have included both non‐randomised and randomised controlled trials (RCTs), focused on specific types of interventions (e.g. home‐based) or have restricted to particular age groups (Gomes‐Neto 2016; Li 2019; Meyer 2020). Therefore, we present the first Cochrane Review to assess the effectiveness of all types of physical activity interventions and inclusive of all age groups, using only RCT data in people with ConHD. We hope by conducting this review that we can provide clarity on the effectiveness of physical activity interventions, highlight future avenues for research and inform future healthcare policy.
Objectives
To assess the effectiveness and safety of all types of physical activity interventions versus standard care in individuals with congenital heart disease.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all types of randomised controlled trial (RCT), inclusive of but not limited to parallel, cross‐over and cluster designs. We included only parallel and randomised cross‐over designs: these trials compared all types of physical activity interventions to a 'no physical activity/no exercise' comparator. We included trials irrespective of their duration of follow‐up.
Types of participants
We included all individuals with a diagnosis of ConHD, who were deemed suitable for participation in a physical activity or exercise training intervention. We included all types of congenital heart disease, regardless of previous medical care and categorised them as mild, moderate or severe (Hoffman 2002). We also included paediatric (5 to 18 years old) and adult populations (> 18 years old).
Types of interventions
We identified and included three types of intervention: physical activity (PA) promotion; exercise training; and inspiratory muscle training (IMT). PA promotion studies incorporated psychological components to promote and educate participants on the benefits of exercise, whereas exercise training studies 'prescribed' exercise at a set dose either in a hospital or in a home‐based setting. IMT was not an anticipated intervention type at the protocol phase; we included it due to its increasing use in clinical care (Pufulete 2019). Furthermore, we included interventions whether they were structured versus unstructured, supervised versus unsupervised, home versus hospital and single versus multicomponent. All interventions were compared to 'no physical activity/physical activity as usual' control; and both the intervention and control group received usual medical care.
Types of outcome measures
Studies should have intended to assess any of the outcomes in both the intervention and the control groups. At the protocol phase we intended to extract outcomes at two time points: at the end of intervention and at long‐term follow‐up (> 12 months). Due to the lack of long‐term follow‐up data, we only extracted the data at the end of the intervention. We sought to report the following primary and secondary outcomes, but they did not form the basis of our inclusion/exclusion criteria.
Primary outcomes
Maximal cardiorespiratory fitness (CRF)
Health‐related quality of life determined by a validated questionnaire
Device‐worn ‘objective’ measures of physical activity
Secondary outcomes
Submaximal CRF
Validated questionnaire‐based ‘subjective’ measures of physical activity
Return to work or full‐time education
Hospital admissions
-
Muscular strength determined by:
grip strength
isokinetic testing
muscular endurance capacity
Adverse events
We anticipated there would be substantial variability in the reported outcome measures and we approached the primary outcomes as follows.
Cardiorespiratory fitness (CRF)
We pooled peak oxygen consumption (peak V̇O₂) measured in millilitres per kilogram per minute (mL.kg−1.min−1) as our measure of maximal CRF. Peak V̇O2 was assessed by validated cardiopulmonary exercise test protocols, measuring oxygen consumption directly or indirectly (by estimated oxygen consumption), using either a treadmill or cycle ergometer. The submaximal CRF outcome was oxygen consumption per kilogram of body mass (V̇O2 mL.kg−1.min−1) at the gas exchange threshold (GET).
Health‐related quality of life (HRQoL)
As anticipated there was large variability in the HRQoL scales used; we pooled HRQoL data in a meta‐analysis where appropriate and reported all HRQoL in Table 2 and summarised in text.
1. Health‐related quality of life.
| Author | Sample size | Type of intervention | Questionnaire | Domain |
Intervention vs. control at follow up Mean (Standard deviation) & between group P value |
Direction of effect | Overall risk of bias |
| Duppen 2015 | 73 | Exercise training | SF‐36 (8 domains) + | Physical functioning | 94.6 (10.9) 95.0 (8.5) P = 0.71 | Exercise = control | High |
| SF‐36 (8 domains) + | Bodily pain | 96.6 (8.1) 93.2 (17.3) P = 0.96 | Exercise = control | High | |||
| SF‐36 (8 domains) + | General health | 68.3 (27.9) 67.5 (19.1) P = 0.94 | Exercise = control | High | |||
| SF‐36 (8 domains) + | Vitality | 71.7 (18.0) 70 (17.0) P = 0.56 | Exercise = control | High | |||
| SF‐36 (8 domains) + | Role limitations due to physical limitations | 100 (0.0) 91.7 (21.2) P = 0.16 | Exercise = control | High | |||
| SF‐36 (8 domains) + | Social functioning | 95.8 (10.0) 100.0 (0.0)P = 0.09 | Exercise = control | High | |||
| SF‐36 (8 domains) + | Role limitations due to emotional problems | 100 (0.0) 100 (0.0) P = 0.72 | Exercise = control | High | |||
| SF‐36 (8 domains) + | Mental health | 85.0 (16.8) 81.3 (10.2) P = 0.65 | Exercise = control | High | |||
| TACQOL + | Symptoms | 95.6 (7.90) 97.8 (3.7) P = 0.16 | Exercise = control | High | |||
| TACQOL + | Impact of cardiac surveillance | 85 (6.30) 85.7 (4.80) P = 0.07 | Exercise = control | High | |||
| TACQOL + | Worries | 95.7 (8.70) 88.6 (270) P = 0.67 | Exercise = control | High | |||
| TACQOL‐CF + | Pain and physical symptoms | 26.3 (6.2) 24 (7.15) P = 0.21 | Exercise = control | High | |||
| TACQOL‐CF + | Motor functioning | 30.1 (1.9) 29.5 (4.37) P = 0.51 | Exercise = control | High | |||
| TACQOL‐CF + | Cognitive functioning | 28.6 (6.2) 29.7 (6.9) P = 0.05 | Exercise = control | High | |||
| TACQOL‐CF + | Social functioning | 31.5 (1.2) 32 (0) P = 0.45 | Exercise = control | High | |||
| TACQOL‐CF + | Positive emotional functional | 14.2 (3.5) 14.43 (2.9) P = 0.39 | Exercise = control | High | |||
| TACQOL‐CF + | Negative emotional functioning | 12.7 (2.12) 14.2 (2.2) P = 0.34 | Exercise = control | High | |||
| Madhavi 2011 | 112 | Exercise training | SF‐36 + | SF36 total score | 23.3 (13.2) 11.3 (14.3) P < 0.001 | Exercise > control | High |
| Klausen 2016 | 158 | Physical activity promotion | Danish Paediatric QoL Inventory + | Generic | NR | Exercise = control | High |
| Danish Paediatric QoL Inventory + | Disease specific | NR | Exercise = control | High | |||
| Opotowsky 2018 | 28 | Exercise training | MLHFQ ‐ | MLHFQ | 20.1 (11.4) 27.7 (10.9) P = 0.13 | Exercise = control | High |
| Sandberg 2018 | 23 | Exercise training | EQ5D VAS + | EQ5D VAS | 76.2 (15.2) 76.3 (20.7) P = 0.31 | Exercise = control | High |
| Novakovic 2018 | 14 | Exercise training (Interval) | SF36 + | Physical component | 86.7 (40.2) 101.0 (16.6) P > 0.05 | Exercise = control | High |
| SF36 + | Mental component | 80.0 (21.0) 87.3 (21.9) P > 0.05 | Exercise = control | High | |||
| Novakovic 2018 | 13 | Exercise training (Continuous) | SF36 + | Physical component | 103 (5.2) 101.0 (16.6) P > 0.05 | Exercise = control | High |
| SF36 + | Mental component | 89.3 (18.4) 87.3 (21.9) P > 0.05 | Exercise = control | High | |||
| Westhoff-Bleck 2013 | 40 | Exercise training | KCCQ + | KCCQ | NR | Exercise = control | High |
| Winter 2012
|
46 | Exercise training
|
SF‐36 + | Mental component | P = 0.17 | Exercise = control | High |
| SF‐36 + | Physical component | P = 0.20 | Exercise = control | High | |||
| ConHD‐TAAQOL + | Symptoms | 85 (10.79) 83 (10.47) P = 0.31 | Exercise = control | High | |||
| ConHD‐TAAQOL + | Worries | 77 (15.4) 84.5 (9.21) P = 0.30 | Exercise = control | High | |||
| ConHD‐TAAQOL + | Impact | 84 (7.19) 85.25 (6.20) P = 0.91 | Exercise = control | High |
Each study's outcome of health related quality of life was individually assessed using risk of bias 2; all studies were judged as a high risk of bias under the domain 'Measurement of the outcome'. '+' = a higher score represents better health; '‐' = a lower score represents better health; HRQoL, Health related quality of life; SF‐36, 36‐Item Short Form Health Survey; MLHFQ, Minnesota living with heart failure questionnaire; TACQOL, TNO/AZL child quality of life questionnaire; ConHD TAAQOL, The congenital heart disease ‐ TNO/AZL adult quality of life questionnaire; EQ5D VAS, EuroQol Vertical Visual Analogue Scale; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Device‐worn measures of physical activity
Physical activity was measured by accelerometry only. We pooled data regardless of device (Actigraph GT3X, Actigraph GT1M), device settings (epoch ranged 5 to 60 seconds) and activity parameter measured (time spent in MVPA per day as a percentage (n = 1) and minutes spent in MVPA per day) as per the protocol. There were no heart rate data to analyse. and activity parameter measured (time spent in MVPA per day as a percentage (n = 1) and minutes spent in MVPA per day) as per the protocol. There were no heart rate data to analyse.
Search methods for identification of studies
Electronic searches
We undertook a systematic search of the following databases on 23 September 2019.
CENTRAL in the Cochrane Library (Issue 9 of 12, 2019)
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 19 September 2019)
Embase (Ovid, 1980 to 2019 week 38)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOHost, 1937 to 23 September 2019)
Allied and Complementary Medicine Database (AMED) (Ovid, 1985 to September 2019)
BIOSIS Citation Index (Clarivate Analytics, 1926 to 23 September 2019)
Web of Science Core Collection (Clarivate Analytics, 1900 to 23 September 2019)
Latin American and Caribbean Health Sciences Literature (LILACS) (Bireme, 1982 to 23 September 2019)
DARE (NIHR Centre for Reviews and Dissemination www.crd.york.ac.uk, from inception to 31 March 2015 (when this database stopped adding records)).
We applied the Cochrane sensitivity‐maximising RCT filter for MEDLINE and for Embase terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011; Higgins 2011). For CINAHL, we used the Cochrane CINAHL RCT filter (Glanville 2019). For all other databases, except CENTRAL, LILACS and DARE, we applied an adaptation of the Cochrane RCT filter. See Appendix 1 for the search strategies.
Searching other resources
We searched ClinicalTrials.gov on 11 September 2020 for ongoing or unpublished trials.
We also searched by hand the reference list of relevant reviews, randomised and non‐randomised studies, and editorials for additional studies. We contacted authors of studies and experts in the field to ask for any missed, unreported or ongoing trials. We also searched for any retraction statements and errata for included studies.
Data collection and analysis
Selection of studies
Two review authors (CAW and CW) independently screened titles and abstracts for inclusion from all the potential trials we identified from the searches. We then sourced full texts and both review authors (CAW and CW) independently read them to confirm eligibility; in the event of exclusion, we documented the reasons. This was facilitated by Covidence systematic review software. Two authors (LL and RST) arbitrated if any disagreements arose that could not be rectified through discussion,. We have recorded the selection process with a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies (Liberati 2009).
1.
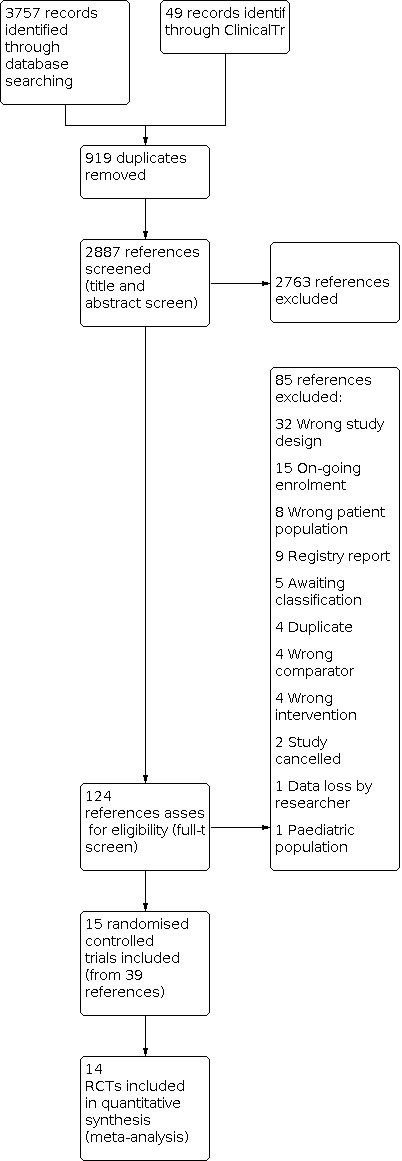
Study flow diagram
Data extraction and management
Two authors (CAW and CW) independently piloted a data collection form and independently extracted outcome data from included studies. One review author (CW) transferred data into RevMan Web 2019; and CAW checked that the data was entered correctly.
We extracted the following study characteristics.
Participants: N randomised, N lost to follow‐up, N analysed, mean age (± standard deviation), gender, severity of condition*, inclusion criteria, and exclusion criteria.
Methods: study design, total duration of study, study setting, date of study, withdrawals, number of study centres and location.
Interventions: intervention description (including the frequency, intensity, duration and modality of the intervention), comparison, and co‐interventions.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
*As ConHD is an incredibly varied and complex disease we have classified the severity of the condition using the Hoffman 2002 criteria as ‘mild’, ‘moderate’ or ‘severe’ (see Appendix 2 for further information). We have chosen the Hoffman classification as it is very inclusive and does not bias against individual intra‐diagnosis differences; it has since been adopted in the most recent guidelines from the US Task Force for adult congenital heart disease (Warnes 2008). We have used these criteria to describe the study data (to aid the reader); we have not used it for subgroup analyses.
Assessment of risk of bias in included studies
Two review authors (CAW and CW) independently assessed risk of bias for each study using the recently revised 'Risk of bias in randomised trials (RoB 2)' tool (accessed: 28 January 2020) (Higgins 2019).
We assessed risk of bias for each study outcome using the following Cochrane RoB 2 criteria (Higgins 2019).
Bias arising from the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
For each domain a series of signalling questions with the answers (yes, probably yes, no information, probably no, no) determine the risk of bias (low risk, some concerns and high risk). We included text alongside the judgements to provide supporting information for our decisions (see Risk of bias in included studies). We decided the risk of bias for an outcome (e.g. health‐related quality of life (HRQoL)) by its performance in each domain: if we judged one domain 'some concerns' or 'high risk' this judgement was taken for the whole outcome. We assessed the risk of bias of maximal and submaximal cardiorespiratory fitness (CRF), HRQoL, physical activity (PA) and muscular strength at follow‐up. The effect of assignment or 'intention to treat' was our effect of interest and we have summarised the risk of bias in traffic lights on the forest plots, in Table 2 for HRQoL and in text.
Measures of treatment effect
We analysed continuous data as mean difference with 95% confidence intervals (CIs). Where an outcome was measured and reported in more than one way, we report a standardised mean difference (SMD) with 95% CIs. We interpreted the SMD using the two approaches recommended in the Handbook (Schünemann 2017). First, we interpreted the mean SMD effect size using the following rule of thumb based on Cohen effect sizes (i.e. 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect) (Faraone 2008). In addition, for physical activity data we have converted the SMD back to the original scale units (minutes of moderate to vigorous activity) by multiplying the pooled mean SMD by an among‐person standard deviation for a particular trial (Opotowsky 2018). We have corrected for any differences in the direction of the scales for HRQoL (i.e. when some scales have a lower score for a better QoL, a reduction in score would indicate an improvement, whereas a scale that awards higher scores for better QoL would see an increase in score indicating a positive outcome). We included HRQoL data in the meta‐analysis only if it was the overall or total HRQoL score. For the outcome 'adverse events' where there was a dichotomous variable (event or no event), we analysed this using count data and summarised in text. Where data were skewed and reported as medians and interquartile ranges, we converted them to means and standard deviations using validated equations (Wan 2014).
Unit of analysis issues
We only identified studies with individual randomisation. Only one study presented long‐term follow‐up data (Winter 2012); in this instance we extracted data at the end of the intervention to keep consistency with all the other trials. One trial contained three arm: continuous exercise training; interval exercise training; and a control group (Novakovic 2018). In this case we divided the number randomised to the control group in half to obtain the denominator for data analysis; the means and standard deviation for the control group remained unchanged for both comparisons. One study had a randomised cross‐over design, but only contributed data prior to the cross‐over (Fritz 2020); it was therefore not necessary to consider a washout period.
Dealing with missing data
We contacted multiple authors to verify key study characteristics (such as randomisation), clarify data queries and obtain missing numerical outcome data. Where data were presented graphically and we were unable to obtain numerical data from the authors, we used WebPlotDigitizer to extract this information.
Assessment of heterogeneity
We explored heterogeneity amongst included studies qualitatively (through visual inspection of forest plots and by comparing the characteristics of included studies), and quantitatively (using the Chi² test of heterogeneity and the I² statistic). We used a threshold of I² greater than 50% to represent substantial heterogeneity for continuous outcomes (Deeks 2017).
Assessment of reporting biases
We were able to pool more than 10 studies in our primary outcome 'maximal cardiorespiratory fitness'. We subsequently created and examined a funnel plot and used the Egger test to explore possible small‐study biases for this primary outcome (Egger 1997).
Data synthesis
We performed meta‐analyses with 95% CIs including all available studies where appropriate (i.e. when treatments, participants, and the underlying clinical question were similar enough for pooling to be appropriate). We used random‐effects meta‐analyses for all analyses due to the qualitative (types of interventions and severities of ConHD) and quantitative (statistical) heterogeneity present. Evidence of substantial heterogeneity was confirmed using the I2 statistic of more than 50%, giving further justification for a random‐effects analysis. Random effects provides a more conservative statistical approach, as the confidence interval around a random‐effects estimate is wider than a confidence interval around a fixed‐effect estimate (Heran 2008a; Heran 2008b). Where an outcome was measured and reported in more than one way, we report a standardised mean difference (SMD) with 95% CI.
We processed data in accordance with guidance in the Handbook (Higgins 2011). We completed data synthesis and analyses using RevMan Web software (RevMan Web 2019); and we conducted meta‐regression analysis using the “metareg” command in Stata version 14.2 (Stata 2015 [Computer program]). We created additional figures using GraphPad (GraphPad Prism).
We could not pool some HRQoL. In this instance we adopted a modified version of a vote counting table, allowing us to summarise descriptive data, risk of bias and the direction of effect. Whilst this synthesis without meta‐analysis (SWiM) method has significant limitations, we believe it to be the only SWiM method that allows us to communicate the results in a transparent and concise format (Campbell 2020).
Subgroup analysis and investigation of heterogeneity
We split the outcome 'maximal cardiorespiratory fitness' into two subgroup analyses: Analysis 1.1 reports the effect of the type of physical activity intervention (i.e. PA promotion, exercise training and inspiratory muscle training (IMT); and Analysis 1.6 reports the effect of the intervention in each group of ConHD (i.e. single ventricle, tetralogy of Fallot and other/mixed ConHD).
1.1. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 1: Maximal cardiorespiratory fitness
1.6. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 6: Maximal cardiorespiratory fitness (type of ConHD subgroup analysis)
We used meta‐regression to assess the potential treatment effect modifiers from all interventions (PA promotion, exercise training and IMT) on maximal cardiorespiratory fitness. Due to the limited number of studies to co‐variate ratio we limited the meta‐regression to univariate analysis only (Higgins 2011). The meta‐regression included the following co‐variates.
Type of intervention (PA promotion or exercise training or IMT (categorical variable)).
‘Dose’ of exercise intervention (dose = number of weeks of exercise training × average number of sessions/week × average duration of session in minutes) (continuous variable).
Length of intervention/follow‐up period (continuous variable).
Sample size (continuous variable).
Setting (home‐ or centre‐based) (categorical variable).
Study location (North America or Europe or Asia) (categorical variable).
Age of participants (paediatrics or adults) (categorical variable).
Percentage of male participants (continuous variable).
Baseline cardiorespiratory fitness (continuous variable).
Risk of bias (categorical variable).
Due to the lack of data, we pooled all individual ConHD lesions (Table 3). This has limitations due to the within‐condition and between‐condition heterogeneity in clinical status (Amedro 2017; Kempny 2012). We therefore used pre‐intervention (baseline) cardiorespiratory fitness as a meta‐regression covariate to account for the heterogeneity.
2. Individual ConHD lesions pooled into the meta‐analyses.
| Study | Age | Type of ConHD and (sample size, %) | Classification |
| Avila 2016 | 35 ± 11 | Repaired ToF (17, 100%) | Severe |
| Duppen 2015 | 15 ± 3 | Fontan circulation (43, 48%) [Intra‐atrial lateral tunnel 47%; extracardiac conduit 44%; other 9%] |
Severe |
| Repaired ToF (47, 52%) | |||
| Fritz 2020 | 30 ± 9 | Fontan circulations (42, 100%) [Atrioventricular Anastomosis 19%; Atriopulmonary Anastomosis 21%; Total Cavopulmonary Connection 60%] |
Severe |
| Klausen 2016 | 15 ± 1 | Coarctation of the aorta (52, 33%) | Severe |
| TGA (35, 22%) | |||
| Steno–Fallot tetralogy (21, 13%) | |||
| Double outlet right ventricle (7, 4%) | |||
| Truncus arteriosus (4, 3%) | |||
| Atrioventricular septal defect (9, 6%) | |||
| TCPC (6, 4%) | |||
| Other (24, 15%) | |||
| Madhavi 2011 | 29 ± 11 | Acyanotic Congenital Heart Disease (112, 100%) | Mild |
| Moalla 2006 | 13 ± 1 | Fontan (4, 22%) | Mild to Severe |
| TGA (Senning/Mustard procedure) (5, 28%) | |||
| Repaired ToF (5, 28%) | |||
| Repaired ASD (4, 22%) | |||
| Morrison 2013 | 15 ± 2 | Minor CHD (no surgical intervention) (39, 27%) | Mild to Severe |
| Acyanotic corrected ConHD (61, 43%) | |||
| Cyanotic corrected (30, 21%) | |||
| Cyanotic palliated (13, 9%) | |||
| Novakovic 2018 | 38 ± 8 | Repaired ToF (30, 100%) | Severe |
| Opotowsky 2018 | 41 ±12 | ToF with pulmonary stenosis or atresia or, DORV (13, 46%) | Severe |
| TGA with a systemic RV, (9, 32%) | |||
| Fontan (2, 7%) | |||
| Pulmonary atresia with intact ventricular septum with biventricular repair (2, 7%) | |||
| Truncus arteriosus (1, 4%) | |||
| Ebstein anomaly (1, 4%) | |||
| Sandberg 2018 | 30 ± 11 | ToF (5, 22%) | Severe |
| ccTGA & d‐TGA (8, 35%) | |||
| TCPC (5, 22%) | |||
| PA (2, 9%) | |||
| Complete AV‐septal defect (1, 4%) | |||
| Ebstein anomaly (1, 4%) | |||
| Miscellaneous (1, 4%) | |||
| Therrien 2003 | 35 ± 9 | Repaired ToF (18, 100%) | Severe |
| Westhoff‐Bleck 2013 | 29 ± 3 | Systemic right ventricle ‐ TGA [Mustard] (48, 100%) | Severe |
| Winter 2012 | 32 + 11 | Systemic right ventricle ‐ TGA and ccTGA (46, 100%) | Severe |
|
van Dissel 2019 |
40 ± 9 |
ToF (12, 30%) | Severe |
| TGA (13, 33%) | |||
| Fontan circulation (9, 22%) | |||
| Pulmonary atresia (3, 7.5%) | |||
| Other (3, 7.5%) |
ToF, tetralogy of Fallot; TGA, transposition of the great arteries; ccTGA, congenitally corrected transposition of the great arteries; d‐TGA, dextro‐transposition of the great arteries; ASD, atrial septal defect; TCPC, Total cavopulmonary connection; DORV, double‐outlet right ventricle; RV, right ventricle.
Sensitivity analysis
We performed the following sensitivity analyses for the outcome of maximal cardiorespiratory fitness: removal of high risk of bias studies; direct versus indirect methods of measuring/estimating peak V̇O2; the use of a fixed‐effect model; insertion of all available change scores; and the removal of computed outcome scores (converting medians and interquartile ranges to means ± standard deviations). We did not perform sensitivity analyses for the other outcomes within the review, due to the lack of studies included in the respective outcomes and the similarity of the studies pooled.
Summary of findings and assessment of the certainty of the evidence
We created the ‘Table 1' using RevMan Web 2019 and reported the following outcomes: maximal cardiorespiratory fitness (CRF), health‐related quality of life (HRQoL), device‐worn ‘objective’ measures of physical activity (PA), submaximal CRF, muscular strength, and adverse events.
Two reviewers (CAW & CW) independently conducted GRADE analysis using GRADEpro GDT. Where disagreements arose, we asked co‐authors (LL & RST) to arbitrate. We used GRADE to assess the certainty of the available evidence, helping to inform decisions based on this evidence (Schünemann 2017). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence, as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We justified all decisions to downgrade the quality of studies using footnotes.
We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (Higgins 2011). Long‐term follow‐up (> 12 months) post intervention was our follow‐up period of most interest. However, as only one study reported long‐term follow‐up, we only report short‐term follow‐up (immediately post intervention) in the 'Summary of findings' table.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
We identified 3806 references through our electronic and manual searches. After de‐duplication and title and abstract screening, we retrieved 124 references. After screening the full text, we identified 15 RCTs from 39 references (see Figure 1). Searching of the reference lists of eligible publications did not reveal additional publications for inclusion.
We contacted 18 corresponding authors for further information regarding study inclusion. When we could not reach the authors, we included these studies (n = 5) in the Studies awaiting classification table.
Included studies
Population
We included 15 RCTs with 924 participants (50% ± 12% male) in the review. There were five paediatric RCTs and 10 adult RCTs with 500 participants and 424 participants respectively. All paediatric RCTs were based in Europe, whereas adult trials were based in Europe (n = 6), North America (n = 3) and Asia (n = 1). There were 11 RCTs that included severe classification participants (n = 559); three RCTs that pooled mild, moderate and severe classifications (n = 254); and one RCT that included mild classification participants only (n = 111). Table 3 reports the individual ConHD lesions that we pooled into the meta‐analyses.
Intervention
We identified three distinct types of interventions: exercise training (n = 11); physical activity promotion (n = 3); and inspiratory muscle training (IMT) (n = 1). See Table 4 for the characteristics of exercise training trials. Physical activity promotion aims were varied: Morrison 2013 and Klausen 2016 used motivational techniques (interviewing and goal setting vs. text 'e‐based' encouragement) to improve physical activity and fitness in children and adolescents; whereas another intervention used a family‐based psychological intervention with a subcomponent of physical activity promotion with the aim of improving HRQoL, time/behaviour in school and sports enjoyment in young children (van der Mheen 2019). The only IMT study included within the review aimed to assess the efficacy of IMT in adults with severe ConHD (Fontan circulations). The intervention was a randomised cross‐over design using a commercially available inspiratory muscle trainer. The participants completing three sets of 10 to 30 repetitions every day for six months, the intensity could be adjusted from 10 cm H2O to 90 cm H2O and was individualised for every training session to maintain an optimal training effect (Fritz 2020).
3. Characteristics of exercise training interventions.
| Study | Age group & severity | Location & supervision | Frequencya (sessions per week) | Intensity | Timeb (minutes) | Type | Durationc (Weeks) | Dose (a*b*c) |
| Avila 2016 | Adult & severe (ToF) | Hospital & supervised | 1 to 2 | 70% to 80% of the maximum HR (increased throughout intervention) | 60 | Combination of resistance and aerobic dynamic (running, rowing etc.) exercise | 12 | 1080 |
| Duppen 2015 | Paediatric & severe (ToF and Fontan) | Hospital & supervised | 2 to 3 | Resting heart rate plus 60% to 70% of the HR reserve | 60 | Aerobic dynamic | 12 | 1800 |
| Madhavi 2011 | Adult & mild | NR | NR | Individualised (NR) | NR | NR | 12 | N/A |
| Moalla 2006 | Paediatric & mild/severe | Home & semi‐supervised | 3 | HR at the gas exchange threshold | 60 | Cycling | 12 | 2160 |
| Novakovic 2018 | Adult & severe (ToF) | NR | 2 to 3 | 80% of HRpeak in high intensity exercise | 42 | Cycling or speed walking | 12 | 1260 |
| 70% of HRpeak in continuous intensity exercise | ||||||||
| Opotowsky 2018 | Adult & severe | Hospital & supervised | 2 | HR at Gas Exchange Threshold | 60 | Combination of resistance and aerobic dynamic | 12 | 1440 |
| Sandberg 2018 | Adult & severe | Home & semi‐supervised | 3 | HR was calculated according to the Karvonen method and to achieve BORG 15 to 16 | 31 | Cycling | 12 | 1116 |
| Therrien 2003 | Adult & severe (ToF) | Hospital and home (1:2 ratio) & supervised | 3 | 60% to 85% of pre training peak VO2 | 50 | Cycling and walking | 12 | 1800 |
| Westhoff‐Bleck 2013 | Adult & severe (Mustard procedure) | Home & semi‐supervised | 3‐5 | HR corresponding to 50% of peak VO2 | 10 to 30 | Cycling | 24 | 2550d |
| Winter 2012 | Adult & severe | Home & semi‐supervised | 3 | 75% to 90% of max heart rate (increased throughout intervention) | 42 | Step aerobics | 10 | 1260 |
| van Dissel 2019 | Adult & Moderate and Severe | Home & semi‐supervised | 3 | 80% of the maximum HR | 45 | Self‐selected | 26 | 3510 |
ToF, Tetralogy of Fallot; HR, Heart Rate; NR, Not Reported; d See dose calculations in Characteristics of included studies
Comparison
All studies compared to usual care for their region. Only one study had three arms: two intervention arms (interval and continuous training) and a control arm (Novakovic 2018).
Primary Outcomes
Maximal cardiorespiratory fitness (CRF) was measured in 14 out of 15 (93.3%) studies. Health‐related quality of life (HRQoL) was reported in 8 out of 15 (53.3%) studies, using a variety of validated questionnaires summarised in Table 2. Device‐worn measures of physical activity was reported by four (26.6%) studies, using a range of accelerometers, cut points and parameters such as time spent as a percentage in moderate to very vigorous activity, average minutes of moderate to vigorous activity (MVPA) and total minutes per day spent in MVPA assessed using accelerometer cut‐points greater than 2000 counts (Duppen 2015; Klausen 2016; Morrison 2013; Opotowsky 2018). No study used disease‐specific cut points.
Secondary Outcomes
Only one study numerically reported questionnaire‐based physical activity (Duppen 2015). Klausen 2016 used questionnaires in combination with device‐worn measures but did not report the questionnaire data as it reported similar results. No study measured return to work or full‐time education. One study reported episodes off school for one or more days, however (van der Mheen 2019). No study reported on hospital admissions. Submaximal CRF was reported in a variety of ways: the most commonly reported was the oxygen consumption at the gas exchange threshold (GET) scaled to body mass (mL.kg−1.min−1) (n = 5 studies) and the ventilatory equivalents (V̇E) over volume of carbon dioxide production (V̇E/V̇CO2 slope) (n = 4 studies) (Avila 2016; Duppen 2015; Fritz 2020; Moalla 2006; Novakovic 2018; Opotowsky 2018; van Dissel 2019; Westhoff‐Bleck 2013). Absolute oxygen consumption at the GET (mL.min−1), power output in watts at the GET, VE at the GET, heart rate at the GET and the oxygen uptake efficiency slope were all reported once. Muscular strength was only reported by one study using isokinetic testing (Moalla 2006); and adverse events were reported by 11 studies, independent of whether an adverse event actually took place (Avila 2016; Duppen 2015; Fritz 2020; Klausen 2016; Novakovic 2018; Opotowsky 2018; Sandberg 2018; Therrien 2003; van Dissel 2019; Westhoff‐Bleck 2013; Winter 2012).
Excluded studies
We excluded 85 references during the full‐text review, amongst which were 32 due to wrong study design, 15 because they are ongoing trials and 8 because they included the wrong patient population. For more regarding exclusions see Figure 1 and Characteristics of excluded studies.
Risk of bias in included studies
Risk of bias assessments for each outcome, including all domain judgements and support for judgement, is located in the Risk of bias section (located after the Characteristics of included studies), at the side of all forest plots and in Table 2 for HRQoL. To access further detailed risk of bias assessment data, please use the following link (doi.org/10.24378/exe.2363).
Risk of bias of outcomes across all studies was similar and predominately of 'some concerns'. Study authors reported poorly the details of blinding outcome assessors (patient‐facing members of staff conducting the outcome assessments, i.e. the person conducting the exercise test or questionnaire) and pre‐agreed statistical analysis plans with sufficient detail.
Across most outcomes risk of bias was similar: we judged it as 'some concerns'. The only exception was HRQoL which we judged to be at high risk of bias due to the nature of self‐reported questionnaires, the lack of blinding of the participants and other outcome assessors.
Effects of interventions
See: Table 1
See Table 1 and forest plots (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6).
2.

Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease. Outcome: Maximal cardiorespiratory fitness (V̇O2 mL.kg‐1.min‐1 at maximal exercise).
3.

Exercise training versus no activity (usual care) in people with congenital heart disease. Outcome: Health related quality of life.
4.

Physical activity promotion and exercise training interventions versus no activity (usual care) in people with congenital heart disease. Outcome: Physical activity (device‐worn).
5.

Exercise training interventions versus no activity (usual care) in people with congenital heart disease. . Outcome: Sub‐maximal cardiorespiratory fitness (V̇O2 mL.kg‐1.min‐1 at the gas exchange threshold).
6.

Exercise training interventions versus no activity (usual care) in people with congenital heart disease. Outcome: Muscular strength.
Maximal cardiorespiratory fitness
A total of 14 studies (15 training arms, 732 participants) reported maximal CRF using peak oxygen consumption (peak V̇O2) scaled to body mass (mL.kg−1.min−1). One study had a long‐term follow‐up at 36 months post intervention (Winter 2012); all other studies' follow‐up was at the cessation of the intervention (median 12, IQR 12 to 26 weeks). Most studies reported the post‐score mean and standard deviation. However, van Dissel 2019 reported both a post score and a change score from baseline and Opotowsky 2018 only reported change score from baseline. To ensure consistency, change scores were included only when no post score was reported.
We pooled all available studies into a random‐effects meta‐analysis, with a subgroup analysis comparing the different types of intervention; we did not consider the result of the subgroup analysis to be significant (Chi² = 5.34, df = 2, P = 0.07, I² = 62.5%). In the pooled analysis there was a mean difference (MD) of 1.89 mL.kg−1.min−1 (95% CI −0.22 to 3.99; 14 studies (15 training arms), 732 participants; I2 = 75%). The subgroup exercise training consisted of 11 studies (435 participants) and there was a mean difference of 2.74 mL.kg−1.min−1 (95% CI 0.36 to 5.12; I2 = 73%) versus a mean difference of −1.71 mL.kg−1.min−1 (95% CI −4.64 to 1.22, I2 = 37%) and 0.70 mL.kg−1.min−1 (95% CI −4.83 to 6.23) in physical activity promotion and inspiratory muscle training respectively (Figure 2).
We performed a further subgroup analysis for the type of congenital heart disease, which reported a pooled mean difference of 1.90 mL.kg−1.min−1 (95% CI −0.14 to 3.95; 14 studies (15 training arms), 732 participants; I2 =73%). The test for subgroup differences revealed no differences between subgroups (P = 1.00); single ventricle (MD 2.06, 95% CI −0.25 to 4.38; n = 153), tetralogy of Fallot (MD 1.97, 95% CI −1.11 to 5.05; n = 104) and other or mixed populations (MD 1.98, 95% CI −1.67 to 5.62; n = 474) all had a similar response to a physical activity intervention (Analysis 1.6).
We performed several separate sensitivity analyses removing high risk of bias studies (MD 0.92, 95% CI −0.27 to 2.11; 12 studies (13 training arms), 603 participants; I2 = 18%) (Madhavi 2011; Therrien 2003); and studies that estimated peak V̇O2 using validated protocols (MD 1.07, 95% CI −0.14 to 2.28; 12 studies (13 training arms), 519 participants) (Madhavi 2011; Morrison 2013). We also report the use of fixed‐effect meta‐analyses (MD 2.00, 95% CI 1.09 to 2.91; 14 studies, 732 participants (15 training arms)); the insertion of all available change scores (MD 1.98, 95% CI 0.09 to 3.86; 14 studies (15 training arms), 732 participants) (Sandberg 2018); and the removal of computed outcome scores (converting medians and interquartile ranges to means ± standard deviations from Avila 2016, Fritz 2020, Klausen 2016, Novakovic 2018, Sandberg 2018 and Winter 2012) (MD 2.84, 95% CI −0.21 to 5.88; 8 studies, 423 participants).
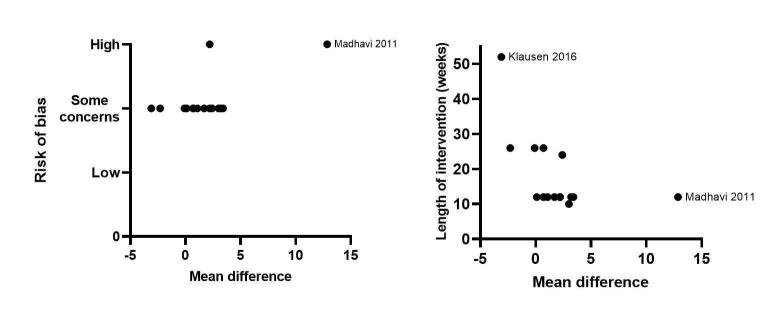
We used univariate meta‐regression to assess individual predictors of peak V̇O2. We regressed 10 predictors and the risk of bias and the intervention length produced significant associations (for regression coefficients and P values see Table 5 and Figure 7). This indicates that the shorter the intervention and the higher the risk of bias then the greater the effect on peak V̇O2. There was no evidence of publication bias (P = 0.268) (Figure 8). Using GRADE, we assessed the evidence to be of moderate certainty because of imprecision.
4. Univariate meta‐regression analysis .
| Potential effect modifiers | Regression coefficient and (Standard error) | P value |
| Age (adult vs. paediatric) | 2.5 (2.2) | 0.262 |
| Baseline CRF (peak V̇O2 mL.kg‐1.min‐1) | −0.2 (0.1) | 0.186 |
| Dose (intervention length*no. sessions per week*session length) | −0.2 (0.3) | 0.614 |
| Follow‐up period/intervention length (weeks) | −0.18 (0.1) | 0.031 |
| Percentage of male | −0.5 (0.3) | 0.083 |
| Risk of bias | 9.1 (2.2) | < 0.01 |
| Sample size | −0.001 (0.02) | 0.963 |
| Setting (home or hospital based) | −2.3 (1.6) | 0.171 |
| Study location (Continent) | −3.5 (1.8) | 0.070 |
| Type of intervention (exercise training, PA promotion, IMT) | −2.3 (1.7) | 0.208 |
Bold = statistically significant; CRF, Cardiorespiratory fitness;
7.
Meta‐regression analyses investigating the effect of the 'overall risk of bias' and the 'length of intervention'. Outcome: Maximal cardiorespiratory fitness (see Table 5).
8.

Funnel plot investigating publication bias. Outcome: Maximal cardiorespiratory fitness (Egger 1997 test, P=0.268).
Health‐related quality of life (HRQoL)
HRQoL was reported by eight studies using a variety of validated questionnaires and a median follow‐up of 12 weeks (Table 2). The '36‐item short form health survey' (SF‐36) was reported most frequently (n = 5), followed by the 'Congenital heart disease ‐ TNO/AZL adult quality of life questionnaire' (ConHD TAAQoL) which was reported twice. All other questionnaires were reported once. Where possible we pooled HRQoL scores into a random‐effects meta‐analysis; we could enter only three studies into the analyses due to the variety of measurements reported. The result of the analysis was a standardised mean difference of 0.76 (95% CI −0.13 to 1.65; I2 = 82%), which suggests a moderate effect size indicating a possibly beneficial effect of interventions on HRQoL (Figure 3). When we summarised all the evidence on HRQoL presented in the vote count table, however, this is not supported (Table 2). The vote count table aims to summarise all studies and instruments used to report HRQoL. Out of the 12 HRQoL questionnaires reported by the eight studies, only one questionnaire found a significant improvement in HRQoL (Madhavi 2011). Using GRADE, we judged the certainty of the evidence to be 'very low' due to serious to very serious concerns regarding risk of bias, inconsistency and imprecision.
Device‐worn 'objective' measures of physical activity
Four studies (328 participants) used device‐worn measures of physical activity and we entered their data into a random‐effects meta‐analysis (Figure 4). The median follow‐up was 19 weeks (IQR 12 to 39 weeks). There is weak evidence of a small effect on physical activity levels with a standardised mean difference of 0.38 (95% CI −0.15 to 0.92; I2 = 78%). The small effect size indicates a possibly beneficial albeit small effect of moderate to vigorous physical activity levels. Re‐expressing these values into the original scales we can report an approximate 10 minute increase per day in moderate to vigorous physical activity (95% CI −2.50 to 22.20). Using GRADE, we downgraded the certainty of evidence by two levels to low, due to concerns over inconsistency and imprecision.
Validated questionnaire‐based ‘subjective’ measures of physical activity
No study measured physical activity using only questionnaire measures of physical activity; two studies used them in combination with device‐worn measures (Duppen 2015; Klausen 2016). Active leisure time (sports, walking and cycling) was not different after an exercise intervention; passive leisure time (television and computer) reduced significantly in both the intervention and control group, making its attribution to the exercise intervention challenging (Duppen 2015). Klausen 2016 did not report their questionnaire results as it did not differ from their device‐worn measures.
Return to work or full‐time education
van der Mheen 2019 reported days off school for children participating in a multicomponent (physical activity promotion and psychological) intervention. The intervention group had 11 episodes of one or more days off school versus 13 episodes in one month in the control group, reported by school teachers. Interestingly when this was reported by mothers there was no effect (15 vs. 15) and the direction of effect was the other direction when reported by fathers (13 vs. 11).
Hospital admissions
No study reported this outcome.
Submaximal cardiorespiratory fitness
A total of nine studies (10 training arms) reported a measure of submaximal CRF, with a median follow‐up of 12 weeks. As previously described in the Characteristics of included studies there was a large variety of submaximal CRF parameters. Oxygen consumption scaled to body mass (mL.kg−1.min−1) at the gas exchange threshold (GET) was reported most often and was subsequently entered into a random‐effects meta‐analysis, showing a likely increase in favour of the intervention with a mean difference of 2.05 (95% CI 0.05 to 4.05; 5 studies (6 training arms), 179 participants; I2 = 33%) mL.kg−1.min−1 (Figure 5). All of the studies that contributed data to this meta‐analysis were exercise training interventions (i.e. not PA promotion or IMT). Using GRADE, we judged the certainty of evidence as moderate—we downgraded the certainty of the evidence one level due to concerns over imprecision (< 200 participants).
Muscular strength
One study (18 participants) reported muscular strength measured by maximal voluntary contraction (N·m) of knee extensions in paediatrics with congenital heart disease. At the end of the exercise (cycling) intervention (12 weeks) there was a mean difference of 17.13 (95% CI 3.45 to 30.81) N·m in favour of exercise training (Figure 6). Using GRADE, we downgraded the certainty of evidence one level to moderate due to imprecision (only 18 participants).
Adverse events (AEs)
Eleven studies (501 participants) reported on the outcome of adverse events, over a median follow‐up period of 12 weeks (IQR 12 to 26 weeks) (Avila 2016; Duppen 2015; Fritz 2020; Klausen 2016; Novakovic 2018; Opotowsky 2018; Sandberg 2018; Therrien 2003; van Dissel 2019; Westhoff‐Bleck 2013; Winter 2012). Of the eleven studies, six studies reported zero adverse events and five studies reported a total of eleven adverse events. Of the 11 AEs, seven were non‐cardiac (63%), characterised by dizziness, discomfort, minor musculoskeletal and minor head injuries. The remaining four cardiac AEs were inclusive of one suspected arrhythmia, one self‐limiting supraventricular arrhythmia (beta‐blocker administered), one episode of ventricular premature complexes (managed conservatively) and one episode of non‐sustained atrial tachycardia that could be related to exercise. There were no reported serious adverse events or fatality.
Eight studies (377 participants) reported no adverse myocardial changes; seven studies reported no adverse changes to cardiac biomarker B‐type natriuretic peptide (NT‐proBNP), with a further four studies reporting no structural or functional cardiac effects using medical imaging (cardiac magnetic resonance and echocardiography) post intervention. There were no major adverse events reported. Our judgement of the certainty of evidence using the GRADE approach was moderate due to concerns over inconsistency.
Discussion
Summary of main results
We identified 15 studies (with 924 participants) that were eligible for inclusion in this review. This review shows that based on moderate to very low certainty of evidence that all types of physical activity interventions (physical activity promotion, exercise training and inspiratory muscle training) when compared to usual care may have a small effect on cardiorespiratory fitness and physical activity level but little or no effect on HRQoL. It should be noted that there was high statistical heterogeneity amongst studies assessing cardiorespiratory fitness, physical activity and HRQoL. Seventy‐three per cent of studies reported adverse events (six studies reported zero adverse events and five studies reported a total of eleven adverse events), of which seven of 11 events were of a non‐cardiac nature, and there were no reported serious adverse events or fatalities related to the physical activity interventions. We were unable to find any data related to secondary outcomes on return to work or hospital admissions. The risk of bias under the outcomes of cardiorespiratory fitness and physical activity was predominantly of 'some concerns', for the outcome of health‐related quality of life it was judged to be a high risk of bias.
Overall completeness and applicability of evidence
The generalisability of previous systematic reviews was either limited to only adults (Li 2019), to a specific type of intervention (Meyer 2020), or to a specific population (Scheffers 2020). This review is the first to include only randomised controlled trial data, of all age groups, types of ConHD and types of physical activity intervention. The findings of this review have potentially better external and ecological validity. Many studies have small sample sizes and all studies were published in the last 17 years. We also report 15 ongoing studies, which indicates there is continuing interest in this area. The quality of the evidence was moderate to very low for all outcomes, indicating further research is very likely to have an important impact on our confidence in the estimate of effect.
Quality of the evidence
Overall, there was a general lack of reporting details of the actual intervention. Using GRADE we assessed the quality of evidence to range from moderate to very low across all outcomes.
We downgraded the certainty of evidence for cardiorespiratory fitness to moderate using GRADE, as the confidence interval includes both appreciable harm and appreciable benefit (i.e. 95% CI spans 0). Therefore, we downgraded the certainty of evidence by one level due to imprecision.
We downgraded the certainty of evidence for health‐related quality of life included in the meta‐analysis to very low using GRADE. This was due to an inconsistent directions of effect (i.e. 95% CI spans 0), considerable heterogeneity (HRQoL, I2 = 82%), a high risk of bias across all studies and impression due to the low numbers of participants (< 400). We therefore downgraded the certainty of evidence by three levels due to inconsistency, methodological limitations (risk of bias) and imprecision.
We downgraded the certainty of evidence for physical activity to low using GRADE. This was due to an inconsistent direction of effect and considerable heterogeneity (i.e. 95% CI spans 0; I2 = 77%); there was also a low number of participants (< 400). We therefore downgraded the certainty of evidence by two levels due to inconsistency and imprecision.
We downgraded the certainty of evidence for submaximal cardiorespiratory fitness and muscular strength to moderate using GRADE. This was due to the small numbers of events/participants (< 400). We therefore downgraded the certainty of evidence by one level due to imprecision.
The certainty of evidence for adverse events was downgraded to moderate using GRADE. This is due to over 25% of studies not reporting data on adverse events. We therefore downgraded the certainty of evidence by one level due to publication bias.
Potential biases in the review process
We have documented and justified alterations to our methods from the published protocol in the Differences between protocol and review section (Williams 2019).
We believe this is the most comprehensive systematic review to date of RCTs in people with ConHD. However, it has some limitations as the overall risk of bias for the included studies was predominately of 'some concerns'. Specifically, blinding of outcome assessors and statistical analysis plans were poorly reported. It is impossible to blind a physical activity/exercise intervention; there were, however, very few reported attempts to blind trial staff to the allocation of participants during randomisation, assessing the outcomes and statistical analysis of the outcomes.
All included studies reported a 'no formal exercise training' intervention comparator. However, there were three active types of intervention and the amount of data is unequally distributed between these types (PA promotion n = 3; exercise training n = 11; and IMT n = 1). This reduces the certainty of evidence in the less well represented types of interventions; they may have a significant potential for improving primary and secondary outcomes, but it could not be assessed with the limited data.
A limitation of the current data is that studies group patients using their individual ConHD lesion (diagnosis) or group multiple different types of ConHD together in a single cohort. Previous studies have reported large variations in fitness and health status between patients who have the same condition. Future studies should adopt a function‐based assessments/interventions approach, which will enable scientists to observe which types of patients respond better to interventions, improving the evidence base for individualising physical activity interventions (Budts 2013; Budts 2020; Cedars 2020; Moons 2020).
Agreements and disagreements with other studies or reviews
Cardiorespiratory fitness
Both maximal and submaximal measures of CRF have been shown to be prognostic of future mortality and morbidity in congenital heart disease (Dimopoulos 2006; Giardini 2009; Müller 2015; Udholm 2018). In the current review maximal cardiorespiratory fitness increased by a mean difference of 1.89 mL.kg−1.min−1 (95% CI −0.22 to 3.99). In a healthy population an increase of 3.5 mL.kg−1.min−1 (one MET) reduces the chance of cardiovascular diagnosis or event by approximately 15% (Letnes 2019); and in patients with cardiovascular disease a one MET increase is associated with a 8% to 35% (median 16%) reduction in mortality (Franklin 2013). Currently, in ConHD there is no consensus regarding what the prognostic implication is of an increase of 1.89 mL.kg−1.min−1. A recent systematic review in exercise training in patients with Fontan circulations reported a similar estimate of effect to the current study of 1.73 mL.kg−1.min−1 although this was not conducted using a meta‐analysis (Scheffers 2020).
Our ConHD subgroup analysis reported no difference in the response to the intervention between single ventricle, tetralogy of Fallot and other/mixed ConHD populations (P = 1.0). All subgroups responded similarly to the intervention; this may suggest that a functional‐based classification (over the traditional diagnosis/lesion‐based approach), may help to identify groups who respond better to interventions.
This was the first systematic review and meta‐analysis that assessed submaximal fitness parameters. The oxygen consumption at the gas exchange threshold (GET) improved modestly (MD 2.05, 95% CI 0.05 to 4.05); this has also been accompanied with an increase in power output (watts) at the GET (Moalla 2006; Westhoff‐Bleck 2013). Participants therefore had a greater period of time where they could operate in a predominantly aerobic state, which is an indicator of improved fitness.
Health‐related quality of life
Health‐related quality of life was reported in a variety of ways making pooling difficult: we pooled only three studies and there was a standardised mean difference indicating a moderate effect size (SMD 0.76, 95% CI −0.13 to 1.65), which we judged as very low certainty of evidence. However, using a modified vote‐counting table (Table 2), only one study out of eight showed a significant and positive effect on health‐related quality of life (Madhavi 2011). Gratz 2009 and Amedro 2015 reported that people with ConHD had a significantly poorer health‐related quality of life in the domains of physical functioning/physical well‐being and general health. Gratz 2009 also stated that the ConHD population dangerously overestimate their exercise capacity and this could explain the small to no increase in HRQoL within this review.
Physical activity
Re‐calculating the effect estimate into the original scales (minutes of moderate to vigorous physical activity (MVPA)), we can report an approximate 10‐minute increase per day in MVPA (95% CI −2.50 to 22.20). Whilst this is a small increase of MVPA, accumulatively over the course of a week more participants will be achieving the physical activity guidelines. To our knowledge this is the first review to quantitatively analyse the effects of physical activity interventions on physical activity in people with ConHD. However, were unable to perform a meta‐regression on this outcome, due to the lack of studies contributing to the analyses.
This review summarises the latest evidence on CRF, HRQoL and PA. Although there were only small improvements in CRF and PA and small to no improvements in HRQoL, there were no serious adverse events related to the interventions or adverse cardiac remodelling. These observations support the proposition that physical activity and exercise is safe and the benefits outweigh the potential risks (Koyak 2012). Although these data are promising, there is currently insufficient evidence to definitively determine the impact of physical activity interventions in ConHD. Therefore, further high‐quality randomised control trials are needed utilising a longer duration of follow‐up.
Authors' conclusions
Implications for practice.
Currently there are no guidelines outlined by the National Institute for Health and Care Excellence (NICE) for physical activity and exercise training in congenital heart disease. Moreover, in the UK there is no provision for cardiac rehabilitation (inclusive of physical activity interventions) for children and adolescents with congenital heart disease and clinical teams are encouraged to develop pathways to increase exercise and physical activity habits. By targeting young people it is suggested that good health and health behaviours will track into adulthood, subsequently reducing hospital admissions, reducing future morbidity and contributing to increasing survival rates.
Implications for research.
This review reports small and modest improvements in maximal and submaximal cardiorespiratory fitness, but there is uncertainty in the prognostic implications of this improvement over a long‐term follow‐up. We require an international effort to produce a large and long‐term randomised multicentre trial of physical activity and exercise interventions with long‐term outcomes of mortality, morbidity, cost effectiveness, cardiorespiratory fitness and health‐related quality of life. Future interventions should classify their patients (and modify the interventions) based on their functional capacity over their lesion‐specific diagnoses—this should help define what types of populations respond to interventions the best (Budts 2013; Budts 2020; Cedars 2020; Moons 2020). A prognostic factors systematic review is also required to assess the current evidence of the prognostic power of cardiorespiratory fitness for patients with congenital heart disease, as it will enable physical activity and exercise interventions to be individualised and evaluated more effectively.
What's new
| Date | Event | Description |
|---|---|---|
| 21 May 2021 | Amended | Link to Table in in Results section corrected. |
History
Protocol first published: Issue 8, 2019 Review first published: Issue 10, 2020
| Date | Event | Description |
|---|---|---|
| 8 February 2021 | Amended | Technical issue with risk of bias tables resolved |
| 26 January 2021 | Amended | Minor edits to abstract and summary of findings table. |
Notes
None
Risk of bias
Risk of bias for analysis 1.1 Maximal cardiorespiratory fitness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Exercise training | ||||||||||||
| Therrien 2003 | High risk of bias | No information on method of randomisation and significant baseline imbalance between groups. There is a 8 year age gap – the intervention group are younger and age of repair was younger. Right ventricular outflow tract (11 vs 22 mmhg) were half in the intervention group. Daily activity levels were less in the intervention group. This may suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | 17 of 18 participants (95%) were included at follow up. One person lost from the control group due to a lack of interest. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No registry of protocol available. Common CRF parameter reported. No information whether there were multiple eligible analyses. | High risk of bias | Lack of information on randomisation, baseline imbalances and blinding of outcome assessors and no pre‐registered protocol or statistical plan. |
| Moalla 2006 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All outcome data was presented | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Overall due to a lack of detail on randomisation, blinding outcome assessors and lack of information in the pre‐registered methods. |
| Madhavi 2011 | High risk of bias | Patients were randomly divided into study and control group by simple randomization using the lottery method. However there were substantial differences in patients fitness (as measured by VO2 peak) between the intervention and control group. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. There was no information to support deviations from the intended intervention. | High risk of bias | Loss to follow up not explained. All people lost to follow up were in the intervention group. No analysis to check the effect of the missing data. | High risk of bias | YMCA cycle test. This is a predictive test not a direct assessment furthermore no description of protocol. Furthermore, there was a lack of blinding outcome assessors and a high effect size seen. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | High risk of bias | Overall high due to differences at baseline, missing outcome data without explanation and a lack of blinding outcome assessors. |
| Winter 2012 | Some concerns | “Randomization was performed using sealed envelops. Each participant chose an opaque envelop from a shuffled stack which contained either ‘yes’, which allocated him to the treatment group or ‘no’ which allocated him to the control group. Randomization was stratified by participating centre.” There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | 15% of participants were lost to follow‐up. Dropping out of the study could be related to the outcome. However, the half of participants who dropped out were for reasons that are unrelated to CRF | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | There was a trial register entry. It provided details of time points, but not of analyses nor of specific means of measuring outcomes (specific scales or methods). There is a risk that outcomes were selected after analysis completed | Some concerns | Overall judged some concerns due to missing outcome data, no information on blinding of outcome assessors and the risk that outcomes were selected after analysis completed. |
| Westhoff‐Bleck 2013 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | Overall: 16% of people dropped out. Similar numbers from both groups. Reasons for dropping out for some participants were duration of intervention, but some reasons were unrelated. There was no analysis to assess the effect of missing data | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | There was a trial register entry. It provided details of time points, but not of analyses nor of specific means of measuring outcomes (specific scales or methods). There is a risk that analyses were chosen to selectively. | Some concerns | Overall some concerns due to no information about blinding of outcome assessors, pre published statistical plan and lack of information on randomisation. |
| Duppen 2015 | Low risk of bias | Participants were randomized in a 2:1 ratio to the exercise group or control group. Randomization was performed by an independent blinded researcher. Stratification was based on gender, ConHD, and age group (10‐12, 12‐15, 15‐18, and 18‐25 years). There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Most people were followed up >95% | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Avila 2016 | Low risk of bias | Randomization was performed by sealed envelopes, with a computer‐generated allocation sequence. There were no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Opotowsky 2018 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Sandberg 2018 | Low risk of bias | Computer based random allocation with allocation and no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | 12% of participants were lost to follow up 3/16 intervention and 0/13 control. There is no analysis to look at the effect of the missing data. A small study so the 3 drop outs looks like a large percentage, reasons documented for patient withdrawal. | Low risk of bias | “This study was performed at two centers. The center recruiting 5 patients (22%) was not able to keep the investigators performing the exercise tests strictly blinded for group allocation.” | Some concerns | The trial register report was registered before the trial (date 2009) It indicates that CRF would be measured using Vo2 Max. | Some concerns | Unable to locate a detailed statistical plan |
| van Dissel 2019 | Some concerns | Computer based random allocation and no baseline imbalances that would suggest a problem with randomisation, but there was no information about allocation concealment. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | Overall: 15% of people dropped out, similar numbers from both groups. Reasons for dropping out for some participants were duration of intervention, but some reasons were unrelated. There was no analysis to assess the effect of missing data | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to a lack of detail on randomisation, missing outcome data, blinding outcome assessors and lack of information in the pre‐registered methods. |
| Subgroup 1.1.2 Physical activity promotion | ||||||||||||
| Morrison 2013 | Some concerns | Participants were randomised using balanced blocks of four to intervention and control groups. Randomisation was not stratified by any factor. There were some baseline imbalances in BMI and Deprivation, which could be of importance in a physical activity promotion trial. The concerns were due to no information on the randomisation process rather than the baseline imbalances. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Some concerns | Twenty‐three participants did not attend reassessment despite being offered three separate appointments, and a number of patients withdrew at this stage. Four patients who were randomised to the intervention group did not attend the group sessions offered. These patients were analysed with the rest of the intervention group on an intention‐to‐treat basis. There was no analysis to assess the effect of missing data. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Judged some concerns over issues with randomisation, blinding outcome assessors, indirect measurements of CRF, missing outcome data, and no prior published statistical plan. |
| Klausen 2016 | Low risk of bias | The randomization was performed by the Copenhagen Trial Unit ten days after the baseline test. A trial investigator centrally randomized eligible patients 1:1. The computer‐generated allocation sequence used permuted blocks with varying sizes of 6, 8, or 10. The allocation sequence and block size were unknown to the trial investigators. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Deviations from the original protocol were approved by the Regional Ethics Committee trial protocol and applied to the ClinicalTrials.gov identifier. Participants were aware of intervention received. There were no deviations from intervention. The team was blinded to the group allocation of patients and the analysis was appropriate. | Some concerns | 39 lost to follow up (24%).Multiple imputations were used to handle missing data. However, multiple imputation based on 'missing at random' does not provide justification to say that there was evidence that results were robust. | Low risk of bias | The team was blinded to the group allocation of patients. | Low risk of bias | Protocol registered, with a pre published protocol and statistical plan. Industry standard CRF data were presented. | Some concerns | Some concerns due to 24% loss to follow up. |
| Subgroup 1.1.3 Inspiratory muscle training | ||||||||||||
| Fritz 2020 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 9.5% dropout rate in this study, however for this type of intervention this is reasonable. Authors accounted for 20% drop out in sample size calculations. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Overall due to a lack of detail on randomisation, blinding outcome assessors and lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.2 Health‐related quality of life.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Madhavi 2011 | High risk of bias | Patients were randomly divided into study and control group by simple randomization using the lottery method. However there were substantial differences in patients fitness (as measured by VO2 peak) between the intervention and control group. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. There was no information to determine whether there were deviations from the intended intervention. | High risk of bias | Loss to follow up not explained. All people lost to follow up were in the intervention group.No analysis to check the effect of the missing data. | High risk of bias | The participant assessed this outcome and was aware of the intervention they received. HRQoL is a patient reported outcome. Therefore, it is not unrealistic it could be effected by knowledge of allocation to intervention. | Some concerns | No pre‐registered method (registry or protocol) available. SF‐36 is usually reported in 2 or 8 domains, they report a 'total score' that is statistically significant. Potentially selective reporting due to multiple outcome scales. | High risk of bias | High risk across randomisation, missing outcome data and blinding of the outcome assessor. |
| Opotowsky 2018 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | All participants were included in the analysis | High risk of bias | The participant assessed this outcome and was aware of the intervention they received. HRQoL is a patient reported outcome. Therefore, it is not unrealistic it could be effected by knowledge of allocation to intervention | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard HRQoL data were presented. | High risk of bias | Overall high due to the nature of self reported questionnaires and patients being aware of their assignment. |
| Sandberg 2018 | Low risk of bias | Computer based random allocation with allocation and no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate | Low risk of bias | 12% of participants were lost to follow up 3/16 intervention and 0/13 control. There is no analysis to look at the effect of the missing data. A small study so the 3 drop outs looks like a large percentage, reasons documented for patient withdrawal. | High risk of bias | The participant assessed this outcome and was aware of the intervention they received. HRQoL is a patient reported outcome. Therefore, it is not unrealistic it could be effected by knowledge of allocation to intervention | Some concerns | The trial register report was registered before the trial (date 2009) It indicates that HRQoL was to be measured at 12 weeks using both EQ5D and SF‐36. Only EQ‐5D is presented . | High risk of bias | Overall high due to the nature of self reported questionnaires and patients being aware of their assignment. |
Risk of bias for analysis 1.3 Physical activity (device‐worn).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Duppen 2015 | Low risk of bias | Participants were randomized in a 2:1 ratio to the exercise group or control group. Randomization was performed by an independent blinded researcher. Stratification was based on gender, ConHD, and age group (10‐12, 12‐15, 15‐18, and 18‐25 years). There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Most people were followed up >95% | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard physical activity data were presented. | Some concerns | Blinding outcome assessors and potential bias in the reported result. |
| Klausen 2016 | Low risk of bias | The randomization was performed by the Copenhagen Trial Unit ten days after the baseline test. A trial investigator centrally randomized eligible patients 1:1. The computer‐generated allocation sequence used permuted blocks with varying sizes of 6, 8, or 10. The allocation sequence and block size were unknown to the trial investigators. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Participants were aware of intervention received. There were no deviations from intervention. The team was blinded to the group allocation of patients and the analysis was appropriate | Some concerns | 39 lost to follow up (24%).Multiple imputations were used to handle missing data. However, multiple imputation based on 'missing at random' does not provide justification to say that there was evidence that results were robust. | Low risk of bias | The team was blinded to the group allocation of patients. | Low risk of bias | Protocol registered, with a pre‐published protocol and statistical plan. Industry standard activity (MVPA) data were presented with an appropriate statistical approach. | Some concerns | Some concerns due to 24% loss to follow up. |
| Morrison 2013 | Some concerns | Participants were randomised using balanced blocks of four to intervention and control groups. Randomisation was not stratified by any factor. There were some baseline imbalances in BMI and Deprivation, which could be of importance in a physical activity promotion trial. The concerns were due to no information on the randomisation process rather than the baseline imbalances. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Some concerns | Twenty‐three participants did not attend reassessment despite being offered three separate appointments, and a number of patients withdrew at this stage. Four patients who were randomised to the intervention group did not attend the group sessions offered. These patients were analysed with the rest of the intervention group on an intention‐to‐treat basis. There was no analysis to assess the effect of missing data. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard physical activity data were presented. | Some concerns | Only judged low risk in one domain. Missing information on randomisation with baseline imbalances, missing outcome data, no information on whether outcome assessors were aware of the intervention received, no pre registered statistical plan. |
| Opotowsky 2018 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard physical activity data were presented. | Some concerns | Some concerns in 2 domains measurement of outcome and selection of the reported result |
Risk of bias for analysis 1.4 Submaximal cardiorespiratory fitness (gas exchange threshold).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Moalla 2006 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All/most data available | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Overall due to a lack of detail on randomisation, blinding outcome assessors and lack of information in the pre‐registered methods. |
| Westhoff‐Bleck 2013 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | Overall: 16% of people dropped out. Similar numbers from both groups. Reasons for dropping out for some participants were duration of intervention, but some reasons were unrelated. There was no analysis to assess the effect of missing data | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | There was a trial register entry. It provided details of time points, but not of analyses nor of specific means of measuring outcomes (specific scales or methods). There is a risk that analyses were chosen to selectively. | Some concerns | Overall some concerns due to the potential lack of blinding, pre published statistical plan and lack of information on randomisation. |
| Duppen 2015 | Low risk of bias | Participants were randomized in a 2:1 ratio to the exercise group or control group. Randomization was performed by an independent blinded researcher. Stratification was based on gender, ConHD, and age group (10‐12, 12‐15, 15‐18, and 18‐25 years). There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Most people were followed up >95% | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Avila 2016 | Low risk of bias | Randomization was performed by sealed envelopes, with a computer‐generated allocation sequence. There were no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 1.5 Muscular strength.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Moalla 2006 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All or most patients were included at follow up | Low risk of bias | Gold standard measure of strength and unlikely outcome assessors can influence score. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard strength data is available. | Some concerns | Due to lack of information on randomisation and lack of a pre‐registered protocol. |
Risk of bias for analysis 1.6 Maximal cardiorespiratory fitness (type of ConHD subgroup analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.6.1 Population with a single ventricle | ||||||||||||
| Winter 2012 | Some concerns | “Randomization was performed using sealed envelops. Each participant chose an opaque envelop from a shuffled stack which contained either ‘yes’, which allocated him to the treatment group or ‘no’ which allocated him to the control group. Randomization was stratified by participating centre.” There were no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | 15% of participants were lost to follow‐up. Dropping out of the study could be related to the outcome. However, the half of participants who dropped out were for reasons that are unrelated to CRF | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | There was a trial register entry. It provided details of time points, but not of analyses nor of specific means of measuring outcomes (specific scales or methods). There is a risk that outcomes were selected after analysis completed | Some concerns | Overall judged some concerns due to missing outcome data, no information about blinding of outcome assessors and the risk that outcomes were selected after analysis completed. |
| Westhoff‐Bleck 2013 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | Overall: 16% of people dropped out. Similar numbers from both groups. Reasons for dropping out for some participants were duration of intervention, but some reasons were unrelated. There was no analysis to assess the effect of missing data | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | There was a trial register entry. It provided details of time points, but not of analyses nor of specific means of measuring outcomes (specific scales or methods). There is a risk that analyses were chosen to selectively. | Some concerns | Overall some concerns due to no information on blinding of outcome assessors, pre published statistical plan and lack of information on randomisation. |
| Duppen 2015 | Low risk of bias | Participants were randomized in a 2:1 ratio to the exercise group or control group. Randomization was performed by an independent blinded researcher. Stratification was based on gender, ConHD, and age group (10‐12, 12‐15, 15‐18, and 18‐25 years). There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Most people were followed up >95% | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Fritz 2020 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 9.5% dropout rate in this study, however for this type of intervention this is reasonable. Authors accounted for 20% drop out in sample size calculations. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Overall due to a lack of detail on randomisation, blinding outcome assessors and lack of information in the pre‐registered methods. |
| Subgroup 1.6.2 Population with repaired Tetralogy of Fallot | ||||||||||||
| Therrien 2003 | High risk of bias | No information on method of randomisation and significant baseline imbalance between groups. There is a 8 year age gap – the intervention group are younger and age of repair was younger. Right ventricular outflow tract (11 vs 22 mmhg) were half in the intervention group. Daily activity levels were less in the intervention group. This may suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | 17 of 18 participants (95%) were included at follow up. One person lost from the control group due to a lack of interest. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No registry of protocol available. Common CRF parameter reported. No information whether there were multiple eligible analyses. | High risk of bias | lack of information on randomisation, baseline imbalances and of blinding outcome assessors and no pre‐registered protocol or statistical plan. |
| Duppen 2015 | Low risk of bias | Participants were randomized in a 2:1 ratio to the exercise group or control group. Randomization was performed by an independent blinded researcher. Stratification was based on gender, ConHD, and age group (10‐12, 12‐15, 15‐18, and 18‐25 years). There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | Most people were followed up >95% | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Avila 2016 | Low risk of bias | Randomization was performed by sealed envelopes, with a computer‐generated allocation sequence. There were no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Novakovic 2018 | Low risk of bias | Participants were randomized into 3 groups (2 interventional and 1 control group) with a 1:1:1 ratio using adaptive (urn) randomisation with sealed envelopes and randomisation concealment from the recruiting investigator. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | Only 2 people discontinued intervention and 1 person was lost to follow up, reasons given. 90% of randomised sample accounted for in outcome data | Some concerns | Measurements were similar across all intervention groups but there is no information in whether the outcome assessors were blinded. Outcome assessors, through encouragement during testing, could affect the outcome, therefore we have some concerns about potential bias. | Some concerns | Protocol registered (NCT02643810). Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Subgroup 1.6.3 Other or mixed ConHD populations | ||||||||||||
| Moalla 2006 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All outcome data was presented | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | Some concerns | Overall due to a lack of detail on randomisation, blinding outcome assessors and lack of information in the pre‐registered methods. |
| Madhavi 2011 | High risk of bias | Patients were randomly divided into study and control group by simple randomization using the lottery method. However there were substantial differences in patients fitness (as measured by VO2 peak) between the intervention and control group. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. There was no information to support deviations from the intended intervention. | High risk of bias | Loss to follow up not explained. All people lost to follow up were in the intervention group. No analysis to check the effect of the missing data. | High risk of bias | YMCA cycle test. This is a predictive test not a direct assessment furthermore no description of protocol. Furthermore, there was a lack of blinding outcome assessors and a high effect size seen. | Some concerns | No pre‐registered method (registry or protocol) available. However, industry standard CRF data is available. | High risk of bias | Overall high due to differences at baseline, missing outcome data without explanation and a lack of blinding outcome assessors. |
| Morrison 2013 | Some concerns | Participants were randomised using balanced blocks of four to intervention and control groups. Randomisation was not stratified by any factor. There were some baseline imbalances in BMI and Deprivation, which could be of importance in a physical activity promotion trial. The concerns were due to no information on the randomisation process rather than the baseline imbalances. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Some concerns | Twenty‐three participants did not attend reassessment despite being offered three separate appointments, and a number of patients withdrew at this stage. Four patients who were randomised to the intervention group did not attend the group sessions offered. These patients were analysed with the rest of the intervention group on an intention‐to‐treat basis. There was no analysis to assess the effect of missing data. | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Judged some concerns over issues with randomisation, blinding outcome assessors, indirect measurements of CRF, missing outcome data, and no prior published statistical plan. |
| Klausen 2016 | Low risk of bias | The randomization was performed by the Copenhagen Trial Unit ten days after the baseline test. A trial investigator centrally randomized eligible patients 1:1. The computer‐generated allocation sequence used permuted blocks with varying sizes of 6, 8, or 10. The allocation sequence and block size were unknown to the trial investigators. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Deviations from the original protocol were approved by the Regional Ethics Committee trial protocol and applied to the ClinicalTrials.gov identifier. Participants were aware of intervention received. There were no deviations from intervention. The team was blinded to the group allocation of patients and the analysis was appropriate. | Some concerns | 39 lost to follow up (24%).Multiple imputations were used to handle missing data. However, multiple imputation based on 'missing at random' does not provide justification to say that there was evidence that results were robust. | Low risk of bias | The team was blinded to the group allocation of patients. | Low risk of bias | Protocol registered, with a pre published protocol and statistical plan. Industry standard CRF data were presented. | Some concerns | Some concerns due to 24% loss to follow up. |
| Opotowsky 2018 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | All participants were included in the analysis | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to no information on blinding of outcome assessors and potential bias in the selection of the reported result. |
| Sandberg 2018 | Low risk of bias | Computer based random allocation with allocation and no baseline imbalances that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | 12% of participants were lost to follow up 3/16 intervention and 0/13 control. There is no analysis to look at the effect of the missing data. A small study so the 3 drop outs looks like a large percentage, reasons documented for patient withdrawal. | Low risk of bias | “This study was performed at two centers. The center recruiting 5 patients (22%) was not able to keep the investigators performing the exercise tests strictly blinded for group allocation.” | Some concerns | The trial register report was registered before the trial (date 2009) It indicates that CRF would be measured using Vo2 Max. | Some concerns | Unable to locate a detailed statistical plan |
| van Dissel 2019 | Some concerns | Computer based random allocation and no baseline imbalances that would suggest a problem with randomisation, but there was no information about allocation concealment. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received but there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | Overall: 15% of people dropped out, similar numbers from both groups. Reasons for dropping out for some participants were duration of intervention, but some reasons were unrelated. There was no analysis to assess the effect of missing data | Some concerns | There was no information on whether outcome assessors were aware of the intervention received. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol registered. Not enough information in the protocol on a statistical plan and no published statistical plan found. Industry standard CRF data were presented. | Some concerns | Due to a lack of detail on randomisation, missing outcome data, blinding outcome assessors and lack of information in the pre‐registered methods. |
Acknowledgements
The background and methods section of this review is based on a standard template provided by Cochrane Heart. We would like to thank the Cochrane Heart Group and in particular Nicole Martin for their support in the drafting of this paper. We would like to acknowledge Kerry Dwan from the Cochrane Editorial and Methods Department, Andrea Takeda, Charlene Bridges and Aparna Kulkarni from Cochrane Heart and peer reviewers Professor Neil A. Smart and Dr Ari Cedars M.D. Furthermore, we would also like to acknowledge the University of Exeter, Bristol Heart Institute and the University of Glasgow as host institutions.
Appendices
Appendix 1. Search strategy
CENTRAL
#1 MeSH descriptor: [Exercise] explode all trees
#2 MeSH descriptor: [Physical Fitness] this term only
#3 MeSH descriptor: [Sports] explode all trees
#4 MeSH descriptor: [Rehabilitation] this term only
#5 MeSH descriptor: [Dance Therapy] this term only
#6 MeSH descriptor: [Exercise Therapy] explode all trees
#7 MeSH descriptor: [Recreation Therapy] this term only
#8 MeSH descriptor: [Physical Exertion] this term only
#9 MeSH descriptor: [Physical Education and Training] explode all trees
#10 MeSH descriptor: [Dancing] this term only
#11 exercis*
#12 aerobic*
#13 sport*
#14 walk*
#15 bicycle*
#16 ((lifestyle or life‐style) NEAR/5 activ*)
#17 ((lifestyle or life‐style) NEAR/5 physical*).tw.
#18 (physical* NEAR/5 (fit* or train* or activ* or endur* or exert* or perform* or inact*))
#19 anaerobic
#20 rehabilitat*
#21 heart rate recovery
#22 danc*
#23 (run* or jog*)
#24 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 MeSH descriptor: [Heart Defects, Congenital] explode all trees
#26 MeSH descriptor: [Heart Diseases] explode all trees and with qualifier(s): [congenital ‐ CN]
#27 (heart NEAR/2 (defect* or abnormal* or malform*))
#28 (congenital NEAR/2 (heart or cardiac or cardio*))
#29 #25 or #26 or #27 or #28
#30 #24 and #29
MEDLINE Ovid
1 exp Exercise/
2 Physical Fitness/
3 exp Sports/
4 Rehabilitation/
5 Dance Therapy/
6 exp Exercise Therapy/
7 Recreation Therapy/
8 Physical Exertion/
9 exp "Physical Education and Training"/
10 Dancing/
11 exercis*.tw.
12 aerobic$.tw.
13 sport$.tw.
14 walk$.tw.
15 bicycle$.tw.
16 ((lifestyle or life‐style) adj5 activ$).tw.
17 ((lifestyle or life‐style) adj5 physical$).tw.
18 (physical$ adj5 (fit$ or train$ or activ$ or endur$ or exert$ or perform* or inact*)).tw.
19 anaerobic.tw.
20 rehabilitat$.tw.
21 heart rate recovery.tw.
22 danc*.tw.
23 (run* or jog*).tw.
24 or/1‐23
25 exp Heart Defects, Congenital/
26 exp Heart Diseases/cn [Congenital]
27 (heart adj2 (defect* or abnormal* or malform*)).tw.
28 (congenital adj2 (heart or cardiac or cardio*)).tw.
29 or/25‐28
30 24 and 29
31 randomized controlled trial.pt.
32 controlled clinical trial.pt.
33 randomized.ab.
34 placebo.ab.
35 drug therapy.fs.
36 randomly.ab.
37 trial.ab.
38 groups.ab.
39 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40 exp animals/ not humans.sh.
41 39 not 40
42 30 and 41
Embase Ovid
1 exp exercise/
2 fitness/
3 exp sport/
4 rehabilitation/
5 dance therapy/
6 exp kinesiotherapy/
7 recreational therapy/
8 exp physical education/
9 dancing/
10 exercis*.tw.
11 aerobic$.tw.
12 sport$.tw.
13 walk$.tw.
14 bicycle$.tw.
15 ((lifestyle or life‐style) adj5 activ$).tw.
16 ((lifestyle or life‐style) adj5 physical$).tw.
17 (physical$ adj5 (fit$ or train$ or activ$ or endur$ or exert$ or perform* or inact*)).tw.
18 anaerobic.tw.
19 rehabilitat$.tw.
20 heart rate recovery.tw.
21 danc*.tw.
22 (run* or jog*).tw.
23 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 exp congenital heart malformation/
25 heart disease/cn [Congenital Disorder]
26 (heart adj2 (defect* or abnormal* or malform*)).tw.
27 (congenital adj2 (heart or cardiac or cardio*)).tw.
28 24 or 25 or 26 or 27
29 23 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 crossover procedure/
42 double blind procedure/
43 randomized controlled trial/
44 single blind procedure/
45 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
CINAHL
S52 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45
S51 S49 not S50
S50 MH (human)
S49 S46 OR S47 OR S48
S48 TI (animal model*)
S47 MH (animal studies) S46 MH animals+
S45 AB (cluster W3 RCT)
S44 MH (crossover design) OR MH (comparative studies)
S43 AB (control W5 group)
S42 PT (randomized controlled trial)
S41 MH (placebos)
S40 MH (sample size) AND AB (assigned OR allocated OR control)
S39 TI (trial)
S38 AB (random*)
S37 TI (randomised OR randomized)
S36 MH cluster sample
S35 MH pretest‐posttest design
S34 MH random assignment
S33 MH single‐blind studies
S32 MH double‐blind studies
S31 MH randomized controlled trials
S30 S24 AND S29
S29 S25 OR S26 OR S27 OR S28
S28 TX (congenital n2 (heart or cardiac or cardio*))
S27 TX (heart n2 (defect* or abnormal* or malform*))
S26 (MH "Heart Diseases+/FG")
S25 (MH "Heart Defects, Congenital+")
S24 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 TX (run* or jog*)
S22 TX danc*
S21 TX heart rate recovery
S20 TX rehabilitat*
S19 TX anaerobic
S18 TX (physical* n5 (fit* or train* or activ* or endur* or exert* or perform* or inact*))
S17 TX ((lifestyle or life‐style) n5 physical*)
S16 TX ((lifestyle or life‐style) n5 activ*)
S15 TX bicycle*
S14 TX walk*
S13 TX sport*
S12 TX aerobic*
S11 TX exercis*
S10 (MH "Dancing")
S9 (MH "Physical Education and Training+")
S8 (MH "Exertion")
S7 (MH "Recreational Therapy")
S6 (MH "Therapeutic Exercise+")
S5 (MH "Dance Therapy")
S4 (MH "Rehabilitation")
S3 (MH "Sports+")
S2 (MH "Physical Fitness")
S1 (MH "Exercise+")
AMED
1 exp Exercise/
2 Physical fitness/
3 exp Sports/
4 Rehabilitation/
5 Dance therapy/
6 exp Exercise therapy/
7 Recreation/
8 Exertion/
9 exp Physical education/
10 Dancing/
11 exercis*.tw.
12 aerobic$.tw.
13 sport$.tw.
14 walk$.tw.
15 bicycle$.tw.
16 ((lifestyle or life‐style) adj5 activ$).tw.
17 ((lifestyle or life‐style) adj5 physical$).tw.
18 (physical$ adj5 (fit$ or train$ or activ$ or endur$ or exert$ or perform* or inact*)).tw.
19 anaerobic.tw.
20 rehabilitat$.tw.
21 heart rate recovery.tw.
22 danc*.tw.
23 (run* or jog*).tw.
24 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or
25 exp Heart defects congenital/
26 (heart adj2 (defect* or abnormal* or malform*)).tw.
27 (congenital adj2 (heart or cardiac or cardio*)).tw.
28 25 or 26 or 27
29 24 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 randomly.ab.
35 trial.ab.
36 groups.ab.
37 30 or 31 or 32 or 33 or 34 or 35 or 36
38 exp animals/ not humans.sh.
39 37 not 38
40 29 and 39
Web of Science and BIOSIS
# 20 #19 AND #18
# 19 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
# 18 #17 AND #14
# 17 #16 OR #15
# 16 TS=(congenital NEAR/2 (heart or cardiac or cardio*))
# 15 TS=(heart NEAR/2 (defect* or abnormal* or malform*))
# 14 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
# 13 TS=(run* or jog*)
# 12 TS=danc*
# 11 TS=heart rate recovery
# 10 TS=rehabilitat*
# 9 TS=anaerobic
# 8 TS=(physical* NEAR/5 (fit* or train* or activ* or endur* or exert* or perform* or inact*))
# 7 TS=((lifestyle or life‐style) NEAR/5 physical*)
# 6 TS=((lifestyle or life‐style) NEAR/5 activ*)
# 5 TS=bicycle*
# 4 TS=walk*
# 3 TS=sport*
# 2 TS=aerobic*
# 1 TS=exercis*
LILACS
(heart or cardiac$ or cardio$) AND (defect or congenital or malform$ or abnormal$) [Words] and Exercise$ or aerobic$ or sport$ or walk$ or bicycle$ or anaerobic$ or rehabilitat$ or heart rate recovery$ or danc$ or run$ or jog$ or active$ or train$ or fit$ [Words]
DARE
heart or cardiac* or cardio* AND defect or congenital or malform* or abnormal* AND exercise* or aerobic* or sport* or walk* or bicycle* or anaerobic* or rehabilitat* or heart rate recovery* or danc* or run* or jog* or active* or train* or fit*
ClinicalTrials.gov
Condition or disease: Congenital Heart Disease Other terms: exercise Study type: Interventional studies (Clinical Trials)
Appendix 2. Severity classification in congenital heart disease
Severity of congenital heart disease is most often classified by lesion‐specific data. While this approach is appropriate in most cases, it must be stressed that severity is highly individual and should be judged by a physician using validated criteria (Budts 2013; Budts 2020).
Mild ConHD
Mild ConHD is the least severe classification in our planned review. Patients with mild ConHD may be asymptomatic and have no significant murmur. Some example lesions of mild ConHD are as follows.
Bicuspid aortic valve (BAV)
Small atrial septal defects (ASD)
Small ventricular septal defects (VSD)
Small patent ductus arteriosus (PDA)
Moderate ConHD
Patients with moderate ConHD are likely to be symptomatic and the lesions will likely be identified in a clinical study. For example:
mild or moderate aortic stenosis (AS) or aortic incompetence;
moderate pulmonary stenosis (PS) or incompetence;
non‐critical coarctation of the aorta;
large atrial septal defect;
complex forms of ventricular septal defect.
Severe ConHD
This category includes complex conditions that usually require immediate medical intervention. Some example lesions are:
dextro‐transposition of the great arteries;
tetralogy of fallot, including pulmonary atresia and absent pulmonary valve;
hypoplastic right heart;
tricuspid atresia;
pulmonary atresia with an intact ventricular septum;
Ebstein anomaly;
-
hypoplastic left heart;
aortic atresia
mitral atresia
-
hypoplastic left heart;
aortic atresia
mitral atresia
double outlet right ventricle;
truncus arteriosus;
total anomalous pulmonary venous connection;
large atrioventicular septal defect; large VSD; large PDA;
severe AS and/or severe PS;
critical coarctation of the aorta.
This framework has been adopted from the work of Hoffman 2002 and Warnes 2008.
Data and analyses
Comparison 1. Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Maximal cardiorespiratory fitness | 14 | 732 | Mean Difference (IV, Random, 95% CI) | 1.89 [‐0.22, 3.99] |
| 1.1.1 Exercise training | 11 | 435 | Mean Difference (IV, Random, 95% CI) | 2.74 [0.36, 5.12] |
| 1.1.2 Physical activity promotion | 2 | 259 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐4.64, 1.22] |
| 1.1.3 Inspiratory muscle training | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐4.83, 6.23] |
| 1.2 Health‐related quality of life | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.76 [‐0.13, 1.65] |
| 1.3 Physical activity (device‐worn) | 4 | 328 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.15, 0.92] |
| 1.4 Submaximal cardiorespiratory fitness (gas exchange threshold) | 5 | 179 | Mean Difference (IV, Random, 95% CI) | 2.05 [0.05, 4.05] |
| 1.5 Muscular strength | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Maximal cardiorespiratory fitness (type of ConHD subgroup analysis) | 14 | 732 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐0.14, 3.95] |
| 1.6.1 Population with a single ventricle | 4 | 154 | Mean Difference (IV, Random, 95% CI) | 2.05 [‐0.25, 4.35] |
| 1.6.2 Population with repaired Tetralogy of Fallot | 4 | 104 | Mean Difference (IV, Random, 95% CI) | 1.97 [‐1.11, 5.05] |
| 1.6.3 Other or mixed ConHD populations | 7 | 474 | Mean Difference (IV, Random, 95% CI) | 1.98 [‐1.67, 5.62] |
1.2. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 2: Health‐related quality of life
1.3. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 3: Physical activity (device‐worn)
1.4. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 4: Submaximal cardiorespiratory fitness (gas exchange threshold)
1.5. Analysis.

Comparison 1: Physical activity promotion, exercise training and inspiratory muscle training interventions versus no activity (usual care) in people with congenital heart disease, Outcome 5: Muscular strength
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avila 2016.
| Study characteristics | ||
| Methods |
Aim of study: to assess the impact of exercise on ventricular arrhythmias in adults with tetralogy of Fallot Study design: parallel‐group randomised controlled trial (2:1 randomisation) No. of centres: 1 (Montreal Heart Institute Adult Congenital Centre) Country: Canada |
|
| Participants |
N randomised: 17 (exercise 13; control 4) Diagnosis (% of pts): ConHD total: 17 (100%); intervention: 13 (100%); comparator 4 (100%) Severity of condition (corrected tetralogy of Fallot): Exercise: severe = 13 (100%); Control: severe = 4 (100%) Age (mean ± SD), years: total: 35 ± 11.3; exercise: 35 ± 11.3: control: 34 ± 14.5 (converted from median and IQR). Percentage male: total 65%; exercise 69%; control 50% Percentage white: not reported Inclusion criteria: adults (≥ 18 years of age) with surgically repaired tetralogy of Fallot Exclusion criteria: non‐cardiac contraindications to exercising, prior sustained ventricular arrhythmias, aborted sudden death, or New York Heart Association (NYHA) functional class III or IV symptoms. Pregnant women and patients unable to provide informed consent. |
|
| Interventions |
Exercise:
Control group/Comparison Usual care (standard clinical care with encouragement to perform moderate‐intensity aerobic activities for at least 30 minutes a minimum of 3 days per week, in conjunction with moderate intensity muscle‐strengthening activities at least 2 days per week). |
|
| Outcomes | Maximal and submaximal CRF Adverse events |
|
| Notes | Adults randomised in a 2:1 ratio All patients randomised to the exercise program were required to wear a portable 2‐lead Holter monitor (Quark 12, Cosmed, Rome, Italy) during each exercise session. But compliance to target heart rate not reported. Country and settings: Canada; single centre Follow‐up: 3 months |
|
Duppen 2015.
| Study characteristics | ||
| Methods |
Aim of study: assess effects of an exercise training program on cardiopulmonary fitness and daily physical activity in patients with corrected tetralogy of Fallot (ToF) or Fontan circulation. Study design: RCT No. of centres: 5 Country: the Netherlands |
|
| Participants |
N randomised total: 93; intervention: 56; comparator: 37 Diagnosis of ConHD total: 93; intervention: 56; comparator: 37 Severity of condition (corrected tetralogy of Fallot or Fontan):
Age (mean ± SD) total 15 ± 3; intervention 15 ± 3; control 16 ± 3 Percentage male total: 66 (73%); intervention 40 (76%); control 26 (70) N lost to follow‐up total: intervention: 1 refused; comparator: 0 N analysed total: 90; intervention: 53; comparator: 37 Inclusion criteria: correction of ToF, using the transatrial‐transpulmonary approach, had to be performed before the age of 3.5 years. The Fontan circulation had to be completed before the age of 6 years. All participants had to be able, both mentally as well as physically, to adhere to a training programme. Exclusion criteria: excluded were patients with contraindications for exercise, mental retardation, standard contraindications for magnetic resonance imaging, or a ventricular outflow obstruction (peak Doppler gradient > 60 mm Hg). |
|
| Interventions |
Description: standardised exercise training programme, aerobic dynamic cardiovascular training Setting: hospital Supervision: supervised Detail of exercise
|
|
| Outcomes |
|
|
| Notes |
No. of centres: 5 Country: the Netherlands Comparator: Description: regular medical care Co‐interventions: none |
|
Fritz 2020.
| Study characteristics | ||
| Methods |
Aim of study: the aims of the current study were (1) to investigate the effect of a telephone supervised, daily inspiratory muscle training for six months on exercise capacity and 2) on lung volumes in adult patients with Fontan circulation. Study design: RCT No. of centres: 1 Country: Germany |
|
| Participants |
N randomised total: 42; intervention: 20; comparator: 22 Age (mean ± SD) total: 28.6 (24.7; 36.5); intervention 28.8 (25.3; 38.3); control 27.7 (23.7; 36.0) Percentage male total: 21 (50%); intervention: 11(55%); comparator: 10(45%) Severity of condition:
Inclusion criteria: 18 years and older, Fontan physiology Exclusion criteria: patients who underwent cardiac catheter examination in the last 6 months or heart surgery in the last 12 months were excluded from the study. Further exclusion criteria were a change in drug administration in the last 3 months, planned intervention in the near future, neuromuscular or mental disorders, moderate to severe ventricular dysfunction as well as an unstable general state of health. |
|
| Interventions |
Setting:home/hospital/internet delivery or combination: home Supervision: supervised/unsupervised/not reported: telephone semi‐supervised Detail of exercise:
|
|
| Outcomes |
Other outcomes measured:
|
|
| Notes | ||
Klausen 2016.
| Study characteristics | ||
| Methods |
Aim of study: to assess benefit and harms of adding an eHealth intervention to health education and individual counselling in adolescents with congenital heart disease Study design: randomised clinical trial. No. of centres: (nationwide?) Country: Denmark |
|
| Participants |
N randomised total: 158; intervention: 81; comparator: 77 N lost to follow‐up total: 39; intervention: 23; comparator: 16 N analysed total: intervention: 81; comparator: 77 Severity of condition:
(Other reported – impossible to know so assumed severe; furthermore, included criteria state complex, albeit not referencing Hoffman 2002 criteria). Inclusion criteria: age between 13 and 16 years, previous repair for a complex CHD, and assignment to lifelong medical follow‐up Exclusion criteria: residual defects significant for physical activity restrictions, assessed by the participants' regular cardiologist |
|
| Interventions |
Description: 52‐week internet, mobile application, and SMS‐based programme delivering individually tailored text messages to encourage physical activity. Patients recorded exercise duration and type in a mobile application that translated intensity into virtual points, a system designed to provide motivation. Setting: home/internet delivery Supervision: unsupervised but self‐reported adherence using an app. Detail of exercise: activity encouragement. Modality: text/technology based PA encouragement Intensity: text encouraged ‘high intensity’ but this it is probably more appropriate to state MVPA due to a loose definition of high intensity. Resistance training included? NR Impossible to assess dose |
|
| Outcomes |
|
|
| Notes | Adherence to the eHealth program was assessed by patient registration of physical activities via the eHealth application for at least two consecutive weeks during the trial. Not statistically powered (needed 216 randomised) |
|
Madhavi 2011.
| Study characteristics | ||
| Methods |
Aim of study: to find out the influence of graded aerobic exercise on post‐surgical adult acyanotic congenital heart diseases. Study design: RCT Country: India |
|
| Participants |
N randomised total: 111; intervention: 60; comparator: 51 N analysed total: NR; intervention: NR; comparator: NR Severity of condition:
Inclusion criteria: atrial septal defect (ASD) with pulmonary stenosis, ventricular septal defect (VSD), patent ductus arteriosus (PDA), left to right shunts. Exclusion criteria: cyanotic heart disease; severe pulmonary vascular disease; cardiomyopathy; severe atrioventricular valve regurgitation; exercise‐induced ventricular arrhythmia; samples with moderate to severe obstructive lesions |
|
| Interventions |
Description: individualized structured exercise protocol and were modified weekly as per the individual tolerance Setting: home/hospital/internet delivery or combination: NR assumed hospital based Supervision: supervised/unsupervised/not reported – assumed supervised Detail of exercise:
|
|
| Outcomes |
|
|
| Notes | ||
Moalla 2006.
| Study characteristics | ||
| Methods |
Aim of study: to assess exercise tolerance, and to investigate how the 6’WT could be a useful test in the follow‐up of rehabilitation programme in children with CHD Study design: RCT Country: France |
|
| Participants |
N randomised total: 18; intervention: 10; comparator: 8
Age (mean ± SD); intervention: 13.0 ± 1.4; comparator: 12.8 ± 1.3 Inclusion criteria: the left ventricle ejection fraction (LVEF) of CHD group was < 40%. All patients had undergone cardiac surgery reconstruction for complex heart disease. The CHD subjects had to be stabilized with drug treatment for at least 3 months; their medication was the same during the training period. Medical therapy included diuretics, cardiotonics, antivitamins K, and angiotensin‐converting enzyme inhibitor. Exclusion criteria: subjects with additional diagnoses of locomotor or mental disorders or other diseases that could limit muscle performance were excluded from the study. Beta‐blockers, pacemaker. |
|
| Interventions |
Description: cycling 12 week intervention Setting: home Supervision: unsupervised – pulse monitors checked weekly for compliance Detail of exercise:
|
|
| Outcomes |
|
|
| Notes | ||
Morrison 2013.
| Study characteristics | ||
| Methods |
Aim of study: to ascertain if motivational techniques and a structured exercise programme can increase activity in adolescents afflicted with congenital heart disease Study design: RCT Country: Northern Ireland |
|
| Participants |
N randomised total: 143; intervention: 72; comparator: 71 N lost to follow‐up total: 42; intervention: 10; comparator: 32 N analysed total: 101; intervention: 62; comparator: 39 Severity of condition: mild = 39; moderate = 61; severe = 43 Age (mean ± SD) intervention: 15.24; comparator: 15.89 Percentage male total: 60%; intervention: 66.7%; comparator: 53.5% Inclusion criteria: people with ConHD 12 to 20 years old Exclusion criteria: patients were excluded if they had a syndromic diagnosis, major learning difficulty, or if exercise was contraindicated (i.e. severe left ventricular outflow tract obstruction, severe aortic stenosis). |
|
| Interventions |
Description: 6 activity interventions days. Motivational interviewing techniques promoting exercise/activity. Given bespoke training plan to implement at home. Setting: home/hospital combination: home‐based Supervision: unsupervised – patients were contacted 1 month into 6‐month intervention to check problems Detail of exercise: programme suitable for their diagnosis no specifics reported Not possible to calculate exercise dosage. |
|
| Outcomes |
|
|
| Notes | ||
Novakovic 2018.
| Study characteristics | ||
| Methods |
Aim of study: the aim of the study was to compare high‐interval exercise training with moderate continuous training in terms of improving exercise capacity, vascular function, disease‐specific biomarkers, cardiac autonomic function (heart rate variability (HRV)) and post‐exercise heart rate recovery (HRR)) and HRQoL, in patients with repaired ToF. Study design: RCT with 3 parallel groups No. of centres: 1 Country: Slovenia |
|
| Participants |
N randomised total; HIE intervention: 10; CIE intervention: 10; comparator: 10 N lost to follow‐up total: 3; HIE intervention: 1; CIE intervention: 1; comparator: 1 N analysed total: 27; HIE intervention: 9; CIE intervention: 9; comparator: 9 Severity of condition:
Age (mean ± SD) total: 38.5 (8.7); HIE intervention: 36.2 (6.8); 7 CIE intervention: 40.1 (10.4); comparator: 38.4 (8.9) Percentage male total: 37; HIE intervention: 22; CIE intervention: 44; comparator: 44 Inclusion criteria: adults with surgically repaired ToF in childhood Exclusion criteria: exclusion criteria included known or symptomatic atherosclerotic disease, unstable cardiovascular disease or recent (< 3 months prior to inclusion) cardiovascular events, acute illness or recent (< 3 months prior to inclusion) non‐cardiovascular diseases requiring hospitalisations, emergency or unplanned specialist management, unstable or poorly controlled dysrhythmias, permanent atrial fibrillation, pregnancy and intellectual development disorder. |
|
| Interventions |
High intensity interval training vs. continuous intensity exercise vs. Control group Setting: home/hospital/Internet delivery or combination: not reported assumed hospital‐based Supervision: supervised/unsupervised/not reported: not reported assumed supervised Detail of exercise:
|
|
| Outcomes |
Other:
|
|
| Notes | ||
Opotowsky 2018.
| Study characteristics | ||
| Methods |
Aim of study: compare the effects of standard of care to SOC plus participant in a typical clinical CR program in ACHD. Study design: RCT No. of centres: 4 Country: USA |
|
| Participants |
N randomised total: 33; intervention: 18; comparator: 15 Diagnosis of ConHD total: 33; intervention: 18; comparator: 15 Severity of condition:
N analysed total: 28; intervention: 13; comparator: 15 (5 did not receive intervention due to geographical reasons) Age (mean ± SD) total: 41.1 (12.1); intervention: 47.5 (9.0); comparator: 35.7 (11.9) Percentage male total: 50; intervention: 53.9; comparator: 46.7 Inclusion criteria: age > 16 years, ability to provide informed consent, ability and willingness to participate in a 12‐week CR program and repeated cardiopulmonary exercise testing (CPET), impaired aerobic capacity defined as peak _VO2 < 80% predicted on baseline exercise test, baseline resting oxygen saturation (sO2%) > 92%, and CHD of at least moderate complexity. Exclusion criteria: cardiac intervention (catheterization or surgery) within the prior 6 months or planned cardiac intervention within 12 months of enrolment, formal CR within 24 months prior to enrolment, current or recent pregnancy (delivery < 90 days prior to enrolment), pregnancy planned within 12 months, active heart failure or hospitalization or other major clinical change during the 30 days prior to enrolment, and other recent or planned events expected to substantially impact exercise capacity. |
|
| Interventions |
Setting: hospital Supervision: supervised Detail of exercise
|
|
| Outcomes |
|
|
| Notes | ||
Sandberg 2018.
| Study characteristics | ||
| Methods |
Aim of study: effect of a home‐based exercise training programme on fitness and HRQoL Study design: RCT No. of centres: 1 Country: Sweden |
|
| Participants |
N randomised total: 26; intervention: 16; comparator: 10 N analysed total: 23; intervention: 13; comparator: 10 Severity of condition:
Age (median (IQR)) total: 30.1 (22.9 to 36.6); intervention: 31.3 (26.9 to 36.6); comparator: 26.3 (22.9 to 35.6) Percentage male total: 52; intervention: 62; comparator: 40 Inclusion criteria: complex ConHD e.g. Fontan, TGA ToF > 18yrs old Exclusion criteria: the exclusion criteria were present exercise training > times/week aimed at increasing or sustaining exercise capacity, arrhythmia or other adverse events (e.g. important symptoms, drop in blood pressure) at CPET, clinically relevant arrhythmia, intellectual disability or mental illness affecting independent decision making, extracardiac disease affecting physical activity, peak VO2 > 30ml/kg/min at run‐in CPET, or no internet access. |
|
| Interventions |
Description: home‐based cycling intervention Setting: home Supervision: semi‐supervised Detail of exercise:
|
|
| Outcomes |
|
|
| Notes | Compliance 79% (median 83%, 47% to 100%). Range of session completed 17 to 36. Comparator: usual care |
|
Therrien 2003.
| Study characteristics | ||
| Methods |
Aim of study: assess impact of exercise training on aerobic capacity in repaired tetralogy of Fallot (ToF) patients. Study design: RCT No. of centres: 1 Country: Canada |
|
| Participants |
Diagnosis of ConHD total: 18; intervention: 9 ; comparator: 9 Severity of condition: repaired ToF
Age (mean ± SD) intervention: 35 (9.5); comparator: 43.3 (7.3) Percentage male %: total @ randomisation: 55; intervention: 55; comparator: 55 N randomised total: 18; intervention: 9; comparator: 9 N lost to follow‐up total: 1; intervention: 0; comparator: 1 N analysed total: 17; intervention: 9; comparator: 8 Inclusion criteria: adult patients with repaired ToF Exclusion criteria: recent surgery, syncope or malignant arrhythmia |
|
| Interventions |
Description: 12‐week structured exercise programme, patients in the exercise group were evaluated by an exercise physiologist (MW) and given an individualized aerobic training programme. Exercise sessions were held once a week at the Toronto General Hospital Cardiac Rehabilitation Department under the direct supervision of the exercise physiologist. Setting: combination of home (2* weekly walking) and hospital based Supervision: both supervised and unsupervised Detail of exercise:
Comparator: Description: usual care; regular daily exercise Co‐interventions: |
|
| Outcomes |
CRF Adverse events |
|
| Notes | No deaths or morbid events occurred in either group, nor were any symptoms reported during the study period. Occasional premature ventricular and atrial beats were recorded in 4 patients during exercise testing (at baseline study in 2 patients and follow‐up study in 2 patients). None of these episodes required any intervention or discontinuation of testing. Furthermore, no significant ST segment shift was observed in any patient during the exercise or recovery period of either the baseline or follow‐up cardiopulmonary testing. Of note: patients were not on telemetry during hospital training sessions. | |
van der Mheen 2019.
| Study characteristics | ||
| Methods |
Aim: does participating in the CHIP‐Family intervention improve the psychosocial well‐being of children with CHD and their parents, family functioning, and parents’ disease‐specific knowledge? Study Design: single‐blinded parallel randomised controlled trial No. of centres: 1 Country: the Netherlands |
|
| Participants |
N randomised total: 93; intervention: 49; comparator: 44 Diagnosis of ConHD: total 90; intervention: 47; comparator: 43 Severity of condition: limited to 1: atrial septal defect, patent ductus arteriosus, pulmonary valve stenosis, total anomalous pulmonary venous connection, ventricular septal defect. Mild, moderate, severe: anomalous left coronary artery from the pulmonary artery, atrioventricular septal defect, coarctation of the aorta, complex biventricular (e.g. truncus arteriosus, aortic arch defects with ventricular septal defect), univentricular heart defects – Fontan circulation, Ebstein’s anomaly (sub)valvular aortic stenosis, tetralogy of Fallot (ToF) including main aorta to pulmonary connecting artery, transposition of the great arteries
Age (mean ± SD) intervention: 5.43 ± 1.30; comparator: 5.21 ± 1.26 Percentage male total: 50%; intervention: 53.2%; comparator: 46.5% N lost to follow‐up total: parents of three children did not complete any questionnaires after randomisation. N analysed total: 90; intervention: 45 (Baseline assessment data: n = 47 families; Follow‐up assessment data: n = 45 families; Excluded from analyses: n = 2 (no complete questionnaires)); comparator: 40 (Baseline assessment data: n = 40 families; Follow‐up assessment data: n = 40 families; Excluded from analyses: n = 1 (no complete questionnaires)). Inclusion criteria: underwent at least one invasive medical procedure for CHD (i.e. cardiac catheterisation or open heart surgery or both); and (2) were attending kindergarten or first or second year of primary school at the time of first assessment and were eligible for participation. Can speak Dutch. Exclusion criteria: children with known intellectual impairment (intelligence quotient ≤ 70) were excluded, as a sufficient level of intelligence was required to participate in the child intervention programme. Moreover, prematurely born children (i.e. gestational age at birth < 37 weeks) with no CHD other than a patent ductus arteriosus were excluded, as families of prematurely born children experience different psychosocial problems |
|
| Interventions |
Description: the CHIP‐Family is an adaptation and extension of the CHIP‐School intervention. 36 CHIP‐Family consisted of a 6‐hour group workshop (3 to 5 families per workshop) for parents and children and an individual 1‐hour follow‐up session per parent couple. The 1‐day parent workshop consisted of problem prevention therapy, psychoeducation, general parenting skills, skills specific to parenting a child with CHD (provided by 2 senior clinical psychologists for 4 hours), and medical issues (provided by a paediatric cardiologist supported by a senior clinical psychologist for 1 hour). Approximately 4 weeks after the workshop, each parent couple received an individual follow‐up session provided by a senior psychologist who was present at the parent workshop and a psychologist who was present at the child workshop. The CHIP‐Family module also comprised a specific child module. The child module consisted of a workshop that was held concurrently with the parent workshop. The child workshop consisted of cognitive behavioural exercises based on the evidence‐based 'Fun FRIENDS' protocol and focused on strengthening self‐esteem, regulating emotions, relaxation, problem‐solving skills, and positive thinking (provided by 2 junior psychologists who were supervised by 2 senior clinical psychologists for 4 hours). The children also did sport exercises based on a standardised exercise programme specifically developed for children with CHD and their siblings (provided by a physiotherapist and assistant for 1 hour). Each child was allowed to bring a 4‐ to 10‐year‐old sibling or friend to normalise participation and to stimulate practice at home. Setting: hospital Supervision: supervised Detail of exercise: as above
|
|
| Outcomes |
|
|
| Notes | Due to logistical reasons in the starting phase of the project, the first 4 families who consented to participate were allocated to the CHIP‐Family group without randomisation. | |
van Dissel 2019.
| Study characteristics | ||
| Methods |
Aim of the study: physical activity promotion and exercise training interventions for people with congenital heart disease Study design: RCT No. of centres: 1 Country: the Netherlands |
|
| Participants |
N randomised total: 40; intervention: 20; comparator: 20 Diagnosis of ConHD total: 40; intervention: 20; comparator: 20 Severity of condition: eligible patients were adults with CHD and New York Heart Association (NYHA) class II or III symptoms: tetralogy of Fallot; transposition of the great arteries; Fontan circulation; pulmonary atresia; other
Age (mean ± SD) total; intervention: 39.9 ± 8.6; comparator: 40.0 ± 15.4 Percentage male total: 55%; intervention: 9 (45%); comparator: 13 (65%) N lost to follow‐up total: 6; intervention: 3; comparator: 3 N analysed total: 34; intervention: 17; comparator: 17 Inclusion criteria: eligible patients were adults with CHD and New York Heart Association (NYHA) class II or III symptoms, included in the CONgenital CORvitia database, the Dutch nationwide registry and DNA‐bank for adults with CHD Exclusion criteria: exclusion criteria were NYHA class I or IV, presence of exercise‐induced arrhythmia, major comorbidities or limitations that could interfere with exercise training, recent (≤ 6 months) major cardiovascular events or procedures, cyanosis at rest, a resting blood pressure N200 mm Hg or diastolic blood pressure N110 mm Hg, pregnancy (wish) or mental or physical incapability to participate in a home‐based exercise training programme. |
|
| Interventions |
Description: home‐based exercise training programme, with a target training regimen of minimum 3 sessions of 45 min per week for the duration of 6 consecutive months. Setting: home Supervision: unsupervised ‐ compliance and adherence monitored. Detail of exercise:
Control group/Comparator: patients randomised to the control group did not receive any formal advice on exercise training, and were neither encouraged, nor discouraged to participate in sports. All patients were asked to continue habitual daily activities, even if these included regular physical exercise. |
|
| Outcomes |
|
|
| Notes | Patients in the exercise training group reportedly exercised for a median of 2.5 h per week. 13 of the 17 patients in the exercise training group exercised at or above the target training level of 2¼ h per week. The remaining 4 patients exercised at least 1 h per week. The main reason patients could not fully adhere to the training regimen was lack of time related to work or family commitments. The types of sports performed by patients in the exercise group are high‐dynamic sports (76%) and a small subset of those patients (23%) practised sports both at the highest static and dynamic level, such as rowing, cycling and ice‐skating. | |
Westhoff‐Bleck 2013.
| Study characteristics | ||
| Methods |
Aim of study: investigated the effect of 6‐month aerobic exercise training on cardiorespiratory and subaortic RV function in a prospective randomised trial. Study design: RCT No. of centres: 1 Country: Germany |
|
| Participants |
N randomised total: 48; intervention: 24; comparator: 24 Diagnosis of ConHD: total 48; intervention: 24; comparator: 24 Severity of condition: adult patients with previous atrial redirection surgery for D‐TGA were eligible for the study. At our institution all patients underwent the Mustard procedure.
Age (mean ± SD) total: 29.3 ± 3.4 ; intervention: 29.9 ± 3.1 ; comparator: 28.6 ± 3.1 Percentage male total: 31; intervention: 13; comparator: 18 N lost to follow‐up total: 8; intervention: 5; comparator: 3 N analysed total: 40; intervention: 19; comparator: 21 Inclusion criteria: adult patients with previous atrial redirection surgery for D‐TGA were eligible for the study (mustard procedure). Additional inclusion criteria were: stable heart failure according to classification of New York Heart association (NYHA) class I/II, unchanged medication angiotensin‐converting enzyme inhibitors, beta‐blockers) for the last 6 months, no physical training programme at inclusion, and the physical and mental ability to follow a controlled training programme. Exclusion criteria: exclusion criteria were: clinical diagnosis of NYHA functional class III–IV, known pulmonary vascular disease, significant baffle‐obstruction, recent onset or change of heart failure medication within the last 6 months, pregnancy, pacemaker or defibrillator implantation, history of ventricular arrhythmias, renal/liver insufficiency, claustrophobia, and mental retardation. |
|
| Interventions |
Description: patients randomised to training participated in a structured exercise programme with a goal of improved exercise capacity over a period of 24 weeks. As home equipment, patients received a bicycle ergometer and heart rate monitors (Polar USA Inc. New York, New York). Patients in the training group were advised to document heart rate and number and duration of training units. Setting: home Supervision: unsupervised weekly phone calls to monitor adherence. Detail of exercise:
Comparator: Description: usual care Co‐interventions: |
|
| Outcomes | Other outcomes measured:
|
|
| Notes | During the study, 2 patients in the training and 1 patient in the control group experienced an episode of supra‐ventricular tachycardia, which was terminated by cardioversion. None of these episodes were timely related to physical training or to cardiac decompensation. | |
Winter 2012.
| Study characteristics | ||
| Methods |
Aim of study: the primary aim of our study was to determine whether exercise training improves maximal exercise capacity in adult patient with a systemic RV. Additionally, we aimed to determine whether exercise training decreases serum N‐terminal pro hormone brain natriuretic peptide (NT‐proBNP) levels and improves quality of life in these patients Study design: RCT No. of centres: 4 Country: the Netherlands (3 sites) Italy (1 site) |
|
| Participants |
N randomised total: 54; intervention: 28; comparator: 26 Diagnosis of ConHD total: 54; intervention: 28; comparator: 26 Severity of condition: adults with a systemic RV due to a congenitally or surgically corrected TGA.
Age (mean ± SD) total: 32 ± 11; intervention: 31 ± 10; comparator: 34 ± 11 Percentage male total: 23; intervention: 9; comparator: 14 N lost to follow‐up total: 8; intervention: 4; comparator: 4 N analysed total: 46; intervention: 24; comparator: 22 N randomised total: 54; intervention: 28; comparator: 26 N lost to follow‐up total: 8; intervention: 6; comparator: 8 N analysed total: 40; intervention: 22; comparator: 18 Inclusion criteria: eligible participants were adults with a systemic RV due to a congenitally or surgically corrected TGA Exclusion criteria: exclusion criteria were mental or physical incapability to participate in a home‐based exercise training programme, the presence of exercise‐induced arrhythmia, symptomatic myocardial ischaemia, a resting systolic blood pressure .200 mm Hg and/or diastolic blood pressure.110 mm Hg, New York Heart Association (NYHA) class III or IV, pregnancy during the training period, and non‐cardiac co‐morbidity that could affect exercise performance or that could aggravate by exercise. |
|
| Interventions |
Description: the training protocol was home‐based, and consisted of 3 sessions of steps aerobics per week for the duration of 10 consecutive weeks. Setting: home Supervision: unsupervised but compliance followed up through email and calls Detail of exercise:
Comparator: Description: usual care Co‐interventions: none |
|
| Outcomes |
Other outcomes measured:
|
|
| Notes | Exercise tests could be performed without complications in all patients. One baseline exercise test was aborted due to nausea of the patient, but was repeated successfully 6 h later. As stated above, 1 patient developed ventricular bigeminy in the recovery phase, and was excluded from participation in the study. During the training protocol, 1 patient sustained calf injury during exercise, and had to discontinue the protocol for 2 weeks. No other complaints or complications were reported. | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali Faisal 2016 | Wrong intervention |
| Altamirano Diaz 2017 | Wrong study design |
| Amedro 2019 | Wrong study design |
| Babu 2013 | Wrong study design |
| Babu 2017 | Wrong study design |
| Becker Gruenig 2012 | Wrong study design |
| Bhasipol 2018 | Wrong study design |
| Bhasipol 2018a | Wrong study design |
| BoaSorteSilva 2017 | Wrong patient population |
| Callaghan 2018 | Duplicate |
| Camargo 2007 | Wrong comparator |
| Chen 2017 | Wrong intervention |
| Coats 1992 | Wrong patient population |
| Cordina 2013 | Wrong study design |
| Curnier 2012 | Wrong study design |
| DRKS00011363 | Registry Report |
| Du 2015 | Wrong study design |
| Du 2017 | Wrong study design |
| Dua 2010 | Wrong study design |
| Fredriksen 2000 | Wrong study design |
| Fredriksen 2000a | Wrong study design |
| Gierat Haponiuk 2014 | Wrong study design |
| Goldbeck 2011 | Wrong study design |
| Gomes Neto 2016 | Wrong study design |
| Gotink 2017 | Wrong intervention |
| Hedlund 2018 | Wrong study design |
| Hooglugt 2018a | Wrong study design |
| IlarrazaLomel 2008 | Wrong study design |
| IoannisLaoutaris 2015 | Wrong patient population |
| IRCT20180417039341N1 | Registry report |
| ISRCTN74393113 | Registry report |
| Joshi 2019 | Wrong study design |
| Kobashigawa 1999 | Data loss by researcher |
| Lalonde 2014 | Wrong study design |
| Lam 2014 | Wrong patient population |
| Longmuir 1985 | Wrong study design |
| Longmuir 2013 | Wrong study design |
| Lozada 2017 | Wrong comparator |
| Marra 2015 | Wrong study design |
| McKillop 2018 | Wrong comparator |
| NCT00930800 | Registry report |
| NCT01463800 | Study cancelled |
| NCT01671566 | Registry report |
| NCT01822769 | Registry report |
| NCT02632253 | Study cancelled |
| NCT02643810 | Registry report |
| NCT02980393 | Wrong study design |
| NCT03297918 | Registry report |
| Nehyba 2009 | Wrong patient population |
| NTR2527 | Wrong patient population |
| NTR3041 | Wrong patient population |
| Rhodes 2006 | Wrong study design |
| Rowland 2016 | Wrong study design |
| Ruttenberg 1983 | Wrong study design |
| Stefani 2014 | Wrong study design |
| Sutherland 2018 | Wrong comparator |
| Tan 1996 | Wrong intervention |
| Tikkanen 2016 | Duplicate |
| Zhou 2017a | Duplicate |
| Zhou 2017b | Paediatric population |
Characteristics of studies awaiting classification [ordered by study ID]
Ali 2018.
| Methods | |
| Participants | Fontan patients |
| Interventions | Controlled respiratory training |
| Outcomes | CPET |
| Notes |
Callaghan 2017.
| Methods | |
| Participants | ConHD |
| Interventions | Physical activity |
| Outcomes | Potentially HRQoL |
| Notes |
Morrison 2015.
| Methods | |
| Participants | ConHD |
| Interventions | PA promotion |
| Outcomes | Physical activity |
| Notes |
Neidenbach 2017.
| Methods | |
| Participants | Fontan patients |
| Interventions | Inspiratory muscle training |
| Outcomes | Oxygen saturations |
| Notes |
Nilsson 2019.
| Methods | RCT |
| Participants | Patients following aortic valve replacement |
| Interventions | Exercise training |
| Outcomes | Cardiopulmonary exercise testing |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Ganzoni 2019.
| Study name | Impact of a structural phonation training on respiratory muscle function in patients with structural heart disease ‐ HeartChoir (Study NCT03297918) |
| Methods | RCT |
| Participants | Inclusion criteria:
|
| Interventions | Active comparator: intervention group ‐ patients will be asked to participate in a structured singing and respiratory training for 12 weeks vs. no activity control. |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | December 2017 |
| Contact information | Principal Investigator: Daniel Tobler |
| Notes | Unpublished. Author team contacted and they were unable to share data. |
ISRCTN74643496.
| Study name | Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway: a single blind randomised controlled trial with four month and twelve month follow up comparing GroupMCT plus usual CR (intervention) with usual CR (control). |
| Methods | RCT. Metacognitive therapy vs. usual care control. |
| Participants | Inclusion:
|
| Interventions | Experimental: metacognitive therapy plus CR Group psychological treatment focused on reducing worry and rumination and modifying beliefs about thinking in addition to treatment as usual (standard cardiac rehabilitation). Metacognitive therapy (MCT) helps clients to identify episodes of worry and rumination in response to negative thoughts and bring these responses under control. This process is facilitated by exercises that enhance the flexibility of attention control, challenge unhelpful beliefs about thinking and enable new relationships with thoughts 2. Active Comparator: Usual group‐based cardiac rehabilitation (treatment as usual) involving stress management, exercise, education. |
| Outcomes |
Primary
Secondary
|
| Starting date | 1 June 2015 |
| Contact information | Jane Garnett, University of Manchester 5th Floor (Research) St. Mary's Hospital Oxford Road M13 9WL Manchester United Kingdom |
| Notes | Control group are exercise training. Mixed population of Non congenital heart as well as congenital heart patients. Would not be eligible for inclusion into this review unless the intervention group underwent no exercise/physical activity . |
NCT01397110.
| Study name | Influence of respiratory and exercise therapy on oxygen uptake, quality of life in patients with severe associated pulmonary arterial hypertension (apah) as part of a congenital heart defect with/without Eisenmenger's syndrome |
| Methods | Randomized parallel assignment |
| Participants | Inclusion Criteria:
|
| Interventions | Active comparator: respiratory and exercise therapy Randomized, prospective, controlled, blinded study of three‐week inpatient rehabilitation and subsequent continuing of the training at home for 12 weeks. The control group received conventional rehabilitation without a specific training program. After 15 weeks training is also offered to patients in the control group. No Intervention: Control group without exercise training Patients of the control group continue their sedentary lifestyle without given advice for exercise training. The time before start of rehabilitation (three months) serves as control group. Afterwards patients take part in the training program as well. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | January 2012 |
| Contact information | Ekkehard Gruenig, MD+49 6221 396 8053 ekkehard.gruenig@med.uni-heidelberg.de |
| Notes |
NCT02240147.
| Study name | Start‐to‐sport ‐ feasibility and efficacy of individualized, telemonitored, home‐based exercise for adolescents and adults with congenital heart disease |
| Methods | Randomized parallel assignment |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | During a 30 minute face‐to‐face motivational interview with an exercise specialist, the patient will be advised and coached about his exercise prescription, on how to implement it in his own daily life and on how to prevent relapse. Furthermore, the patients will receive instructions on how to monitor their exercise intensity and on recognising adverse signals. During the following 12 weeks, patients will be asked to exercise 4.5 hours per week within the prescribed exercise intensity range according to the guidelines |
| Outcomes | peak oxygen uptake (time frame: baseline, post‐intervention, after 1 year) physical activity (time frame: baseline, post‐intervention and after 1 year) |
| Starting date | 1 January 2015 |
| Contact information | Principal investigator: Roselien Buys, PhD KU Leuven Principal investigator: Werner Budts, PhD KU Leuven |
| Notes | Unpublished. Author team contacted and they were unable to share data. |
NCT02283255.
| Study name | Cardiovascular, Pulmonary and Skeletal Muscle Evaluation in Late Postoperative Period of the Fontan Surgery |
| Methods | A long‐term randomized clinical study that will include 60 patients between 12 and 30 years, submitted to total cavopulmonary connection for at least 5 years post operative at the Heart Institute, University of São Paulo Medical School. The patients will be divided into four groups: 1) Exercise training with aerobic exercise + lower and upper limb strength exercise (GTF‐I); 2) Respiratory training with respiratory muscle exercise (GTF‐II); 3) Exercise training with aerobic exercise + lower and upper limb strength exercise + Respiratory training with respiratory muscle exercise (GTF‐III); and A non‐exercise group as control group (GNTF‐IV). The patients will be revaluated after the 4‐month period of intervention. |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Aerobic training: Aerobic training and muscle strength exercise for upper and lower limbs, 3 times a week for 4 months. Respiratory training: muscle training using POWERbreathe device, 7 times a week, 3 series of 30 repetitions per day, for 4 months. Aerobic and respiratory training: aerobic training and muscle strength exercise for upper and lower limbs, 3 times a week for 4 months and respiratory muscle training using POWERbreathe device, 7 times a week, 3 series of 30 repetitions per day, for 4 months. No physical activity: control group (usual care) |
| Outcomes |
|
| Starting date | 2014 |
| Contact information | Contact: Aida LR Turquetto, Researcher +55 11 26615399 ext +5511981140723 aidaturquetto@me.com Contact: Marcelo B Jatene, Researcher +551126615399 ext +551126615399 marcelo.jatene@incor.usp.br |
| Notes |
NCT02658266.
| Study name | Effect of resistance training in adults with complex congenital heart disease |
| Methods | RCT Behavioural: home‐based resistance training |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Home‐based resistance training 12 weeks home based resistance training 3 times per week, 10 to 12 reps, 2 sets |
| Outcomes |
|
| Starting date | 2017 |
| Contact information | Contact: Bengt Johansson, MD, PhD +46907852782 bengt.johansson@medicin.umu.se Contact: Camilla Sandberg, RPT, PhD +46907858441 camilla.sandberg@medicin.umu.se |
| Notes |
NCT03335475.
| Study name | Congenital Heart Disease Physical Activity Lifestyle Study (CHD‐PALS) |
| Methods | Randomized. Study includes 2 possible conditions to which participants are randomized: (1) Fitbit only and (2) Fitbit + coaching sessions. |
| Participants | Inclusion Criteria:
|
| Interventions | Intervention: In the Fitbit + Coaching Sessions arm, participant will receive their exercise prescription, as devised from their baseline exercise stress test results, a Fitbit, and will have 8 sessions with a coach (interventionist) over the course of 20 weeks. They will undergo a 9 week (interim) and a 22 week assessment (follow‐up). Control: In the Fitbit Only arm, participants will receive their exercise prescription, as devised from their baseline exercise stress test results, and a Fitbit. They will undergo a 9 week (interim) and a 22 week assessment (follow‐up). |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 7 November 2017 |
| Contact information | Principal investigator: Jamie L Jackson, PhD Nationwide Children's Hospital |
| Notes |
NCT03435354.
| Study name | Impacting child physical and mental health outcomes in congenital heart disease: a randomized, controlled trial of enhanced physical activity support in clinical care |
| Methods | Cluster‐randomized trial, with randomization by within each site by week (i.e. site‐week) |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Clinician counselling about physical activity using standardised tools to promote daily physical activity. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | March 5, 2018 |
| Contact information | Principal Investigator: Patricia Longmuir, PhD Scientist |
| Notes |
NCT03479957.
| Study name | Remotely monitored and coached exercise based cardiac rehabilitation in northern Sweden |
| Methods | RCT |
| Participants |
Inclusion criteria:
|
| Interventions | Remotely monitored and coached exercise training in real time using the REMOTE‐CR system. The patients randomized to remotely monitored exercise can either work out with self‐selected activities e.g. Nordic‐walking, cycling, skiing or participate in supervised exercise session via video link. The patients randomized to be controls will receive individualized information and instructions regarding current exercise recommendations but will not be monitored or coached during their exercise sessions (usual care). |
| Outcomes |
Primary outcome: The aerobic exercise capacity (W) will be evaluated using a standardised submaximal exercise test on cycle ergometer
Secondary outcome:
|
| Starting date | 3 September 2019 |
| Contact information | Camilla Sandberg, PhD+46907858441 camilla.sandberg@umu.se |
| Notes | Most likely will not meet participant inclusion criteria |
NCT03690518.
| Study name | Impact of cardiovascular rehabilitation on the quality of life of adolescents and young adults with congenital heart disease: a randomized controlled multicentre trial |
| Methods | |
| Participants | Inclusion Criteria:
|
| Interventions | Cardiac rehabilitation: rehabilitation will have a regular follow‐up with intervention (rehabilitation program) |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 27 July 2018 |
| Contact information | Contact: Sophie GUILLAUMONT0467336632 s-guillaumont@chu-montpellier.fr Contact: Pascal AMEDRO0467336632 p-amedro@chu-montpellier.fr |
| Notes | Amedro P, Gavotto A, Legendre A, Lavastre K, Bredy C, De La Villeon G, Matecki S, Vandenberghe D, Ladeveze M, Bajolle F, Bosser G, Bouvaist H, Brosset P, Cohen L, Cohen S, Corone S, Dauphin C, Dulac Y, Hascoet S, Iriart X, Ladouceur M, Mace L, Neagu OA, Ovaert C, Picot MC, Poirette L, Sidney F, Soullier C, Thambo JB, Combes N, Bonnet D, Guillaumont S. Impact of a centre and home-based cardiac rehabilitation program on the quality of life of teenagers and young adults with congenital heart disease: The QUALI-REHAB study rationale, design and methods. Int J Cardiol. 2019 May 15;283:112-118. doi: 10.1016/j.ijcard.2018.12.050. Epub 2018 Dec 20. |
NCT03999320.
| Study name | Sophrology and congenital heart disease (SOPHRO CARE) |
| Methods | RCT |
| Participants |
Inclusion criteria:
|
| Interventions | Sophrology sessions |
| Outcomes |
Primary outcome: VO2max Variation
Secondary outcomes:
|
| Starting date | 19 July 2019 |
| Contact information | Pascal AMEDRO, MD0467336632 p-amedro@chu-montpellier.fr |
| Notes |
NCT04135859 (YACHD‐PALS).
| Study name | Young Adult Congenital Heart Disease Physical Activity Lifestyle Study (YACHD‐PALS) |
| Methods | This study will adapt a physical activity lifestyle intervention to emerging adult congenital heart disease (CHD) survivors with the primary goal of increasing physical activity levels. The study will be split into 2 phases. In Phase 1, participants will be asked to complete questionnaires, wear an accelerometer around the waist for 7 days, and undergo an exercise stress test. The accelerometer and exercise stress test will be used to determine whether participants are eligible to be randomized for the intervention study. In Phase 2, participants will be randomized to one of two conditions: 1) receiving a physical activity tracker (a Fitbit) or 2) receiving a Fitbit AND engaging in videoconferencing sessions with a physical activity coach. During Phase 2, participants will also be asked to complete 3 assessments (weeks 9 and 22, and a 6‐month follow‐up). The week 9 assessment will consist of completing questionnaires and wearing an accelerometer for 7 days. Week 22 will be similar to week 9 with the addition of a final exercise stress test. The 6‐month follow‐up will mirror the week 9 assessment. |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | In the Fitbit Only arm, participants will receive their exercise prescription, as devised from their baseline exercise stress test results, and a Fitbit. They will undergo a 9 week (interim) and a 22 week assessment (follow‐up). Intervention: Behavioral: Physical Activity Monitoring In the Fitbit + Coaching Sessions arm, participants will receive their exercise prescription, as devised from their baseline exercise stress test results, a Fitbit, and will have 8 sessions with a coach (interventionist) over the course of 20 weeks. They will undergo a 9 week (interim) and a 22 week assessment (follow‐up). Interventions:
|
| Outcomes | Number of minutes spent in moderate to vigorous physical activity as measured by an accelerometer. |
| Starting date | 2019 |
| Contact information | Jamie Jackson, Nationwide Children's Hospital |
| Notes |
NCT04208893.
| Study name | Exercise training strategies for children with repaired tetralogy of Fallot |
| Methods | RCT |
| Participants | Paediatrics with repaired tetralogy of Fallot |
| Interventions | The aerobic training intervention will include 60 minutes/session, 3 times/week for 12 weeks at an intensity of 65% to 85% of participants' heart rate reserve (HRR), as determined by the CPET. Patients will be asked to wear a fitness‐tracking device to monitor their heart rate response and in order to comply with the prescribed training intensity. All training sessions will start with a 10‐minute warm up, 40‐minute aerobic interventions, and ends with a 10‐minutes cool down. One study doctor will be on call during in‐hospital training. Onsite supervised aerobic interventions will include play‐based activities, whereas home‐based aerobic activities will include stationary bikes and exercise activities that would target desired heart rate ranges. Home exercise equipment will be provided. |
| Outcomes | Primary
Secondary
|
| Starting date | 2019 |
| Contact information | Brian McCrindle, MDThe Hospital for Sick Children, Toronto, Canada |
| Notes |
NCT04264650.
| Study name | Long‐term effectiveness of an mHealth intervention for improving the disease knowledge and physical activity of youth With congenital heart disease: a randomized controlled trial |
| Methods |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | The COOL program is a 12‐month randomized controlled trial that compared two active intervention groups to a standard‐care control group (n = 47). Participants with simple and moderate CHD aged 15‐24 years were recruited from paediatric or adult CHD outpatient departments. Participants in one active intervention group (n = 49) were provided with COOL Passport, a mobile healthcare application. Those in the other group (n = 47) were provided with access to the Health Promotion Cloud system and use of game‐based interactive platforms along with COOL Passport. Outcomes were the Leuven Knowledge Questionnaire for CHD and the International Physical Activity Questionnaire‐Taiwan Show‐Card Version. |
| Outcomes | Cardiac disease knowledge and physical activity |
| Starting date | 2020 |
| Contact information | Chi‐Wen Chen, PhD National Yang Ming University |
| Notes |
UMIN000021661.
| Study name | Evaluation test about safety and efficacy of the respiratory muscle training therapy by abdominal respiratory weight exercises in chronic cardiovascular disease patients |
| Methods | Parallel randomized |
| Participants |
Inclusion criteria:
|
| Interventions | The respiratory muscle training therapy is performed in addition to performing conventional cardiac rehabilitation. The respiratory muscle training therapy is abdominal muscle training method. Intervention period is 6 months |
| Outcomes |
|
| Starting date | 4 April 2016 |
| Contact information | Miki Biwa biwa_miki@med.kurume‐u.ac.jp |
| Notes |
Differences between protocol and review
WHO ICTRP was unavailable due to increased traffic to the site because of COVID 19, therefore it was not searched. Clinical trials.gov was searched.
Two studies estimated cardiorespiratory fitness (CRF) using validated CPET protocols, these were pooled with direct measures (gas analysis) of CRF
Baseline CRF and risk of bias were added to the univariate meta regression.
A vote counting table was used to synthesise all the information regarding health related quality of life.
Sensitivity analysis was changed from 'all studies versus only including those studies we judge to have overall low risk of bias (low risk in all domains)' to all studies versus exclusion of high risk studies. This was changed as only one study had a low risk of bias in all domains.
Contributions of authors
CAW and CW independently completed title and abstract screening, full text review, risk of bias assessments, data extraction and GRADE.
CAW had overall control of the design of the study and co‐wrote the manuscript.
CW assisted data analysis, produced the SoF table and co‐wrote the manuscript.
GEP gave specialist clinical insight into the literature and population with congenital heart disease.
GS gave specialist clinical insight into the literature and population with congenital heart disease
RST designed and carried out the statistical analysis and arbitrated any discrepancies.
LL was the lead author overseeing the project and arbitrated any discrepancies.
All authors contributed to the peer review and agreed on the final version of this manuscript.
Sources of support
Internal sources
-
Canon Medical UK Ltd., UK
C Wadey was funded by an industrial PhD studentship from the University of Exeter and Canon Medical Systems UK Ltd.
GE Pieles is lead researcher in a contractual research partnership between the University of Bristol and Canon Medical Systems UK Ltd. investigating cardiac function during exercise in children.
Authors had full control of the design of the study, methods used, outcome parameters, analysis of the data and production of any manuscripts.
External sources
-
NIHR, UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
CAW has received funding from Heart Research UK and Canon Medical Systems Ltd to complete research into the heart health of young people. The author had full control of the design of the study, methods used, outcome parameters, analysis of the data and production of any manuscripts. Neither of these organisations have a financial interest in this review. CW has a funded PhD scholarship from the University of Exeter and Canon Medical. Canon Medical Systems Ltd does not have a financial interest in this review. GEP is lead researcher in a contractual research partnership between the University of Bristol and Canon Medical Systems UK Ltd. Canon Medical Systems Ltd does not have a financial interest in this review. GS is Medical Director of Sports Cardiology UK. Research grant from Heart research UK to evaluate an exercise prescription programme in congenital heart patients. Fee for lecturing at scientific meetings and financial support for educational arrhythmia meeting in February 2019 from Medtronic Actelion. None of the organisations named have a financial interest in this review. LL has no known conflicts of interest. RST has no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Avila 2016 {published data only}
- Avila P, Marcotte F, Dore A, Mercier LA, Shohoudi A, Mongeon FP, et al. Exercise and ventricular arrhythmias in adults with tetralogy of fallot: a randomized pilot clinical trial. Heart Rhythm 2015:S395. [DOI] [PubMed]
- Avila P, Marcotte F, Dore A, Mercier LA, Shohoudi A, Mongeon FP, et al. The impact of exercise on ventricular arrhythmias in adults with tetralogy of Fallot. International Journal of Cardiology 2016;219:218-24. [DOI: ] [DOI] [PubMed] [Google Scholar]
Duppen 2015 {published data only}
- Dulfer K, Duppen N, Blom NA, Dijk AP, Helbing WA, Verhulst FC, et al. Effect of exercise training on sports enjoyment and leisure-time spending in adolescents with complex congenital heart disease: the moderating effect of health behavior and disease knowledge. Congenital Heart Disease 2014;9(5):415-23. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Dulfer K, Duppen N, Blom NA, Van Domburg RT, Helbing WA, Verhulst FC, et al. Effects of exercise training on behavioral and emotional problems in adolescents with tetralogy of Fallot or a Fontan circulation: a randomized controlled trial. International Journal of Cardiology 2014;172(3):e425-47. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Dulfer K, Duppen N, Kuipers IM, Schokking M, Domburg RT, Verhulst FC, et al. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. Journal of Adolescent Health 2014;55(1):65-72. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Dulfer K, Duppen N, Van Dijk AP, Kuipers IM, Van Domburg RT, Verhulst FC, et al. Parental mental health moderates the efficacy of exercise training on health-related quality of life in adolescents with congenital heart disease. Pediatric Cardiology 2015;36(1):33-40. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Duppen N, Etnel JR, Spaans L, Takken T, van den Berg-Emons RJ, Boersma E, et al. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. American Heart Journal 2015;170(3):606-14. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Duppen N, Etnel Jr G, Spaans L, Takken T, Van Den Berg-Emons, RJ, Boersma E, et al. Does exercise training improve cardio-respiratory fitness and daily physical activity in adolescents with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Cardiology in the Young 2014:S39. [DOI] [PubMed]
- Duppen N, Geerdink LM, Kuipers IM, Bossers SS, Koopman LP, Dijk AP, et al. Regional ventricular performance and exercise training in children and young adults after repair of tetralogy of Fallot: randomized controlled pilot study. Circulation: Cardiovascular Imaging 2015;8(4):e002006. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Duppen N, Kapusta L, De Rijke Y, Snoeren M, Kuipers IM, Blank AC, et al. Exercise training improves fitness without adverse cardiac remodelling in patients after repair of tetralogy of Fallot: preliminary results of the TOFFIT study. Cardiology in the Young 2013:S95.
- Duppen N, Kapusta L, De Rijke Y, Snoeren M, Kuipers IM, Koopman LP, et al. The effect of exercise training on cardiac remodelling in adolescents with corrected tetralogy of Fallot and Fontan circulation: a randomized control trial. Cardiology in the Young 2014:S104-5. [DOI] [PubMed]
- Duppen N, Kapusta L, Rijke YB, Snoeren M, Kuipers IM, Koopman LP, et al. The effect of exercise training on cardiac remodelling in children and young adults with corrected tetralogy of Fallot or Fontan circulation: a randomized controlled trial. International Journal of Cardiology 2015;179:97-104. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fritz 2020 {published data only}
- Fritz C, Müller J, Nagdyman N, Oberhoffer R, Ewert P, Hager A. Inspiratory muscle training improves oxygen saturation and hemoglobin levels in patients with fontan circulation - results from a randomized home-based training study. Cardiology in the Young 2019:S29.
- Fritz C, Müller J, Oberhoffer R, Ewert P, Hager A. Inspiratory muscle training did not improve exercise capacity and lung function in adult patients with Fontan circulation: a randomized controlled trial. International Journal of Cardiology 2020;305:50-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Fritz C, Müller J, Oberhoffer R, Ewert P, Hager A. Inspiratory muscle training did not improve physical capacity in adult patients with Fontan circulation. Cardiology in the Young 2018:S30-1.
Klausen 2016 {published data only}
- Klausen SH, Andersen LL, Søndergaard L, Jakobsen JC, Zoffmann V, Dideriksen K, et al. Effects of eHealth physical activity encouragement in adolescents with complex congenital heart disease: the PReVaiL randomized clinical trial. International Journal of Cardiology 2016;221:1100-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Klausen SH, Hwiid S, Mikkelsen UR, Hirth A, Wetterslev J, Kjaergaard H, et al. Effects on exercise capacity of eHealth encouragements in adolescents with congenital heart disease; development of a prospective and randomised complex intervention. European Journal of Cardiovascular Nursing 2012:S47-8.
Madhavi 2011 {published data only}
- Madhavi K, Abhachandra AG, Maiya G. Influence of graded aerobic exercise in post-surgical adult acyanotic congenital heart disease - a prospective randomized clinical trial. Indian Journal of Physiotherapy & Occupational Therapy 2011;5(4):87-94. [Google Scholar]
Moalla 2006 {published data only}
- Moalla W, Maingourd Y, Gauthier R, Cahalin LP, Tabka Z Ahmaidi S. Effect of exercise training on respiratory muscle oxygenation in children with congenital heart disease. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(4):604-11. [DOI] [PubMed] [Google Scholar]
- Moalla W, Elloumi M, Chamari K, Dupont G, Maingourd Y, Tabka Z, et al. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Applied Physiology, Nutrition and Metabolism 2012;37(4):621-30. [DOI] [PubMed] [Google Scholar]
- Moalla W, Gauthier R, Maingourd Y, Ahmaidi S. Six-minute walking test to assess exercise tolerance and cardiorespiratory responses during training program in children with congenital heart disease. International Journal of Sports Medicine 2005;26(9):756-62. [DOI] [PubMed] [Google Scholar]
Morrison 2013 {published data only}
- Morrison ML, Sands AJ, McCusker CG, McKeown P, McMahon M, Gordon J, et al. Exercise training improves activity and psychosocial wellbeing in adolescents with congenital heart disease. Irish Journal of Medical Science 2011:S398-9.
- Morrison ML, Sands AJ, McCusker CG, McKeown PP, McMahon M, Gordon J, et al. Exercise training improves activity in adolescents with congenital heart disease. BMJ Heart 2013;99(15):1122-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Novakovic 2018 {published data only}
- Novaković M, Prokšelj K, Rajkovič U, Cuderman TV, Trontelj KJ, Fras Z, et al. Exercise training in adults with repaired tetralogy of Fallot: a randomized controlled pilot study of continuous versus interval training. International Journal of Cardiology 2018;255:37-44. [DOI: ] [DOI] [PubMed] [Google Scholar]
Opotowsky 2018 {published data only}
- Opotowsky AR, Rhodes J, Landzberg MJ, Bhatt AB, Shafer KM, Yeh DD, et al. A randomized trial comparing cardiac rehabilitation to standard of care for adults with congenital heart disease. World Journal for Pediatric and Congenital Heart Surgery 2018;9(2):185-93. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Opotowsky AR, Rhodes J, Moko L, Bradley R, Systrom D, Waxman A, et al. A randomized trial of cardiac rehabilitation for adolescents and adults with congenital heart disease. Journal of the American College of Cardiology 2016;13(Suppl 1):987. [DOI] [PubMed]
- Tikkanen AU, Rhodes J, Landzberg M, Bhatt A, Systrom DM, Waxman A, et al. A randomized trial of cardiac rehabilitation for adolescents and adults with congenital heart disease. PM&R 2016:S169. [DOI] [PubMed]
Sandberg 2018 {published data only}
- Sandberg C, Hedstrom M, Wadell K, Dellborg M, Ahnfelt A, Zetterstrom AK, et al. Home-based interval training increases endurance capacity in adults with complex congenital heart disease. Congenital Heart Disease 2018;13(2):245-62. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Sandberg C, Hedstrom M, Dellborg M, Magnusson A, Zetterstom AK, Wadell K, et al. Increased endurance capacity in adults with complex congenital heart disease after home-based interval exercise training on ergometer cycle. European Heart Journal 2015:458.
Therrien 2003 {published data only}
- Therrien J, Fredriksen P, Walker M, Granton J, Reid G, Webb G. A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. Canadian Journal of Cardiology 2003;19(6):685-9. [PubMed] [Google Scholar]
van der Mheen 2019 {published data only}
- Van Der Mheen M, McCusker CG, Van Beynum IM, Dulfer K, Van Galen E, Bogers AJ, et al. CHIP-Family to improve psychosocial wellbeing of young children with congenital heart disease and their families. Cardiology in the Young 2018:S28. [DOI] [PubMed]
- Mheen M, Meentken MG, Beynum IM, Van Der Ende J, Van Galen E, Zirar A, et al. CHIP-Family intervention to improve the psychosocial well-being of young children with congenital heart disease and their families: results of a randomised controlled trial. Cardiology in the Young 2019;29(9):1172-82. [DOI: ] [DOI] [PubMed] [Google Scholar]
van Dissel 2019 {published data only}
- Hooglugt JQ, Van Dissel AC, De Haan FH, Blok IM, Jorstad HR, Mulder BJ, et al. Efficacy and compliance of long-term, individualised exercise training in adults with congenital heart disease and heart failure symptoms: a randomized controlled trial. European Heart Journal 2018:Suppl 1:245.
- Dissel AC, Blok IM, Hooglugt JL, Haan FH, Jørstad HT, Mulder BJ, et al. Safety and effectiveness of home-based, self-selected exercise training in symptomatic adults with congenital heart disease: A prospective, randomised, controlled trial. International Journal of Cardiology 2019;278:59-64. [DOI: ] [DOI] [PubMed] [Google Scholar]
Westhoff‐Bleck 2013 {published data only}
- Westhoff-Bleck M, Schieffer B, Tegtbur U, Meyer GP, Hoy L, Schaefer A, et al. Aerobic training in adults after atrial switch procedure for transposition of the great arteries improves exercise capacity without impairing systemic right ventricular function. International Journal of Cardiology 2013;170(1):24-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Winter 2012 {published data only}
- Balducci A, Winter MM, Fabi M, Donti A, Prandstraller D, Formigari R, et al. Exercise training is beneficial and safe in adult patients with a systemic right ventricle. Giornale Italiano di Cardiologia 2011:16S.
- Van Der Bom T, Winter MM, De Vries LCS, Bouma BJ, Van Dijk APJ, Van Der Plas MN, et al. Exercise training improves exercise capacity in adult patients with a systemic right ventricle. European Heart Journal 2010:Suppl 1:615. [DOI] [PubMed]
- Van Der Bom T, Winter MM, Knaake JL, Cervi E, De Vries LS, Balducci A, et al. Long-term benefits of exercise training in patients with a systemic right ventricle. International Journal of Cardiology 2015;179(105):105-11. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Winter MM, Bom T, Vries LC, Balducci A, Bouma BJ, Pieper PG, et al. Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. European Heart Journal 2012;33(11):1378-85. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali Faisal 2016 {published data only}
- Ali-Faisal SF, Benz Scott L, Johnston L, Grace SL. Cardiac rehabilitation referral and enrolment across an academic health sciences centre with eReferral and peer navigation: a randomised controlled pilot trial. BMJ Open 2016;6(3):e010214. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altamirano Diaz 2017 {published data only}
- Altamirano-Diaz L, Rombeek M, De Jesus S, Welisch E, Prapavessis H, Dempsey AA, et al. Remote lifestyle counseling influences cardiovascular health outcomes in youth with overweight or obesity and congenital heart disease. Front 2017;5:269. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amedro 2019 {published data only}
- Amedro P, Gavotto A, Legendre A, Lavastre K, Bredy C, De La Villeon G, et al. Impact of a centre and home-based cardiac rehabilitation program on the quality of life of teenagers and young adults with congenital heart disease: the QUALI-REHAB study rationale, design and methods. International Journal of Cardiology 2019;283:112-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Babu 2013 {published data only}
- Babu AS, Padmakumar R, Maiya AG. A review of ongoing trials in exercise based rehabilitation for pulmonary arterial hypertension. Indian Journal of Medical Research 2013;137:900-6. [PMC free article] [PubMed] [Google Scholar]
Babu 2017 {published data only}
- Babu AS, Ramachandran P, Maiya AG. Effects of home-based exercise training in Eisenmenger's syndrome: secondary analysis from a RCT. Journal of Cardiopulmonary Rehabilitation and Prevention 2017;37(5):367-8. [DOI: ] [Google Scholar]
Becker Gruenig 2012 {published data only}
- Becker-Gruenig T, Ehlken N, Gorenflo M, Hager A, Halank M, Klose H, et al. Efficacy of exercise training in congenital heart disease associated pulmonary hypertension. European Respiratory Journal 2012;40(56):P927. [Google Scholar]
Bhasipol 2018 {published data only}
- Bhasipol A, Sanjaroensuttikul N, Pornsuriyasak P, Yamwong S, Tangcharoen T. Effect of the home cardiac rehabilitation program for adult with complex congenital heart disease: the EMPOWER trial. European Heart Journal 2018;39(Suppl 1):22. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bhasipol 2018a {published data only}
- Bhasipol A, Sanjaroensuttikul N, Pornsuriyasak P, Yamwong S, Tangcharoen T. Efficiency of the home cardiac rehabilitation program for adults with complex congenital heart disease. Congenital Heart Disease 2018;13(6):952-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
BoaSorteSilva 2017 {published data only}
- Boa Sorte Silva NC, Gregory MA, Gill DP, Petrella RJ. Multiple-modality exercise and mind-motor training to improve cardiovascular health and fitness in older adults at risk for cognitive impairment: a randomized controlled trial. Archives of Gerontology and Geriatrics 2017;68:149‐60. [DOI: 10.1016/j.archger.2016.10.009] [DOI] [PubMed] [Google Scholar]
Callaghan 2018 {published data only}
- Callaghan S, Morrison ML, McCusker C, McKeown P, Casey F. A structured intervention programme can improve the biophysical wellbeing in children with congenital heart disease. Ulster Medical Journal 2018;87(2):146. [Google Scholar]
Camargo 2007 {published data only}
- Camargo DM, Campos MT, Sarmiento JM, Garzón M, Navia J, Merchán A. Hemodynamic response to training in resistance and muscular strength of upper limbs in cardiac rehabilitation [Respuesta hemodinámica con el entrenamiento en resistencia y fuerza muscular de miembros superiores en rehabilitación cardiaca]. Revista Colombiana de Cardiología 2007;14(4):198-206. [Google Scholar]
Chen 2017 {published data only}
- Chen CW, Fanjiang YY, Chiang YT, Ho CL. Mobile health program to promote self management in youths with congenital heart disease design and development of the cool randomized controlled trial. Cardiology in the Young 2017;27(4):S89. [DOI: ] [Google Scholar]
Coats 1992 {published data only}
- Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992;85(6):2119-31. [DOI: 10.1161/01.Cir.85.6.2119] [DOI] [PubMed] [Google Scholar]
Cordina 2013 {published data only}
- Cordina RL, O'Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. International Journal of Cardiology 2013;168(2):780-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Curnier 2012 {published data only}
- Curnier D, Lalonde F, Mathieu ME, Fournier A, Bigras JL, Miro J, et al. Children with congenital heart disease and exercise rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(4):232. [DOI: ] [Google Scholar]
DRKS00011363 {published data only}
- DRKS00011363. Breathing training in children, adolescents and young adults with Tetralogy of Fallot after total surgical repair. www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00011363 (accesed prior to 21 October 2020).
Du 2015 {published data only}
- Du Q, Zhou X, Wang X, Chen S, Yang X, Chen N, et al. Passive movement and active exercise for very young infants with congenital heart disease: a study protocol for a randomized controlled trial. Trials 2015;16:288. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2017 {published data only}
- Du Q, Salem Y, Liu HH, Zhou X, Chen S, Chen N, et al. A home-based exercise program for children with congenital heart disease following interventional cardiac catheterization: study protocol for a randomized controlled trial. Trials 2017;18(1):38. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dua 2010 {published data only}
- Dua JS, Cooper AR, Fox KR, Graham Stuart A. Exercise training in adults with congenital heart disease: feasibility and benefits. International Journal of Cardiology 2010;138(2):196-205. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fredriksen 2000 {published data only}
- Fredriksen PM, Walker M, Granton J, Webb G, Reid G, Therrien J. A controlled trial of exercise training in adult patients with repaired tetralogy of Fallot. Canadian Journal of Cardiology 2000;16(Suppl F):137F. [PubMed] [Google Scholar]
Fredriksen 2000a {published data only}
- Fredriksen PM, Kahrs N, Blaasvaer S, Sigurdsen E, Gundersen O, Roeksund O, et al. Effect of physical training in children and adolescents with congenital heart disease. Cardiology in the Young 2000;10(2):107-14. [DOI] [PubMed] [Google Scholar]
Gierat Haponiuk 2014 {published data only}
- Gierat-Haponiuk K, Haponiuk I, Jaworski R, Chojnicki M, Szalewska D, Leszczynska K, et al. Physical activity in patients with grown-up congenital heart defects after comprehensive cardiac rehabilitation. Polish Journal of Thoracic and Cardiovascular Surgery 2014;11(4):452-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldbeck 2011 {published data only}
- Goldbeck L, Holling I, Schlack R, West C, Besier T. The impact of an inpatient family-oriented rehabilitation program on parent-reported psychological symptoms of chronically ill children. Klinische Pädiatrie 2011;223(2):79-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gomes Neto 2016 {published data only}
- Gomes-Neto M, Saquetto, MB, da Silva e Silva CM, Conceicao CS, Carvalho VO. Impact of exercise training in aerobic capacity and pulmonary function in children and adolescents after congenital heart disease surgery: a systematic review with meta-analysis. Pediatric Cardiology 2016;37(2):217-24. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gotink 2017 {published data only}
- Gotink RA, Younge JO, Wery MF, Utens E, Michels M, Rizopoulos D, et al. Online mindfulness as a promising method to improve exercise capacity in heart disease: 12-month follow-up of a randomized controlled trial. PloS One 2017;12(5):e0175923. [DOI: 10.1371/journal.pone.0175923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedlund 2018 {published data only}
- Hedlund ER, Lundell B, Soderstrom L, Sjoberg G. Can endurance training improve physical capacity and quality of life in young Fontan patients? Cardiology in the Young 2018;28(3):438-46. [DOI: 10.1017/s1047951117002360] [DOI] [PubMed] [Google Scholar]
Hooglugt 2018a {published data only}
- Hooglugt JQ, van Dissel AC, Blok IM, de Haan FH, Jorstad HT, Bouma BJ, et al. The effect of exercise training in symptomatic patients with grown-up congenital heart disease: a review. Expert Review of Cardiovascular Therapy 2018;16(6):379-86. [DOI: ] [DOI] [PubMed] [Google Scholar]
IlarrazaLomel 2008 {published data only}
- Ilarraza Lomelí H, Quiroga P, Rius Suárez MD. Cardiac rehabilitation in children [Rehabilitación cardíaca en población pediátrica. Más allá que ayudar a un niño a readaptar su corazón]. Archivos de Cardiología de México 2008;78(2):129-33. [PubMed] [Google Scholar]
IoannisLaoutaris 2015 {published data only}
- Ioannis Laoutaris ID, Dritsas A, Kariofyllis P, Manginas A. Benefits of inspiratory muscle training in patients with pulmonary arterial hypertension. European Journal of Preventive Cardiology 2015;22(Suppl 1):S78. [DOI: ] [DOI] [PubMed] [Google Scholar]
IRCT20180417039341N1 {published data only}
- IRCT20180417039341N1. Evaluation of self-care education on self-efficacy and quality of life in adolescents with congenital heart disease. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180417039341N1 (accessed prior to 21 October 2020).
ISRCTN74393113 {published data only}
- ISRCTN74393113. Biophysical & psychosocial wellbeing in children with congenital heart disease. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN74393113 (accessed prior to 21 October 2020).
Joshi 2019 {published data only}
- Joshi VL, Tang LH, Zwisler AD, Taylor RS. Rehabilitation for people with cardiac conditions: an overview of systematic reviews. Physiotherapy (United Kingdom) 2019;105(Suppl 1):e11-2. [DOI: ] [Google Scholar]
Kobashigawa 1999 {published data only}
- Kobashigawa JA, Leaf DA, Lee, N, Gleeson MP, Liu H, Hamilton MA, et al. A controlled trial of exercise rehabilitation after heart transplantation. New England Journal of Medicine 1999;340(4):272-7. [DOI] [PubMed] [Google Scholar]
Lalonde 2014 {published data only}
- Lalonde D, Welisch E, Altamirano-Diaz L, De Jesus S, Prapavessis H, Rombeek M, et al. The impact of a structured lifestyle intervention on body composition and exercise capacity in obese children with congenital heart defect (Smart Heart Trial). Journal of Physical Activity and Health 2014;11:S163-4. [Google Scholar]
Lam 2014 {published data only}
- Lam K, Dias P, Green G, Maiorana A. Echocardiographic Findings of chronic heart failure patients following exercise training. Journal of the American Society of Echocardiography 2014;27(6):B119. [Google Scholar]
Longmuir 1985 {published data only}
- Longmuir PE, Turner JA, Rowe RD, Olley PM. Postoperative exercise rehabilitation benefits children with congenital heart disease. Clinical and Investigative Medicine 1985;8(3):232-8. [PubMed] [Google Scholar]
Longmuir 2013 {published data only}
- Longmuir PE, Tyrrell PN, Corey M, Faulkner G, Russell JL, McCrindle BW. Home-based rehabilitation enhances daily physical activity and motor skill in children who have undergone the Fontan procedure. Pediatric Cardiology 2013;34(5):1130-51. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lozada 2017 {published data only}
- Lozada MA, Balderas J, Bunyi MI, Suarez C. Comparison of the effectiveness of four(4) session play module versus the conventional cardiovascular rehabilitation therapy among post operative congenital heart disease pediatric patients ages 5 12 years old. Cardiology in the Young 2017;27(4):S144. [DOI: ] [Google Scholar]
Marra 2015 {published data only}
- Marra AM, Egenlauf B, Bossone E, Eichstaedt C, Grunig E, Ehlken N. Principles of rehabilitation and reactivation: pulmonary hypertension. Respiration 2015;89(4):265-73. [DOI: 10.1159/000371855] [DOI] [PubMed] [Google Scholar]
McKillop 2018 {published data only}
- McKillop A, Grace SL, Ghisi GL, Allison KR, Banks L, Kovacs AH, et al. Adapted motivational interviewing to promote exercise in adolescents with congenital heart disease: a pilot trial. Pediatric Physical Therapy 2018;30(4):326-34. [DOI: ] [DOI] [PubMed] [Google Scholar]
NCT00930800 {published data only}
- NCT00930800. Exercise training in children with congenital heart defect. clinicaltrials.gov/show/nct00930800 (first posted 2 July 2009).
NCT01463800 {published data only}
- NCT01463800. Rehabilitation in patients with congenital heart disease. clinicaltrials.gov/show/nct01463800 (first posted 2 November 2011).
NCT01671566 {published data only}
- NCT01671566. Interval training in adults with congenital heart disease a randomized trial. clinicaltrials.gov/show/nct01671566 (first posted 23 August 2012).
NCT01822769 {published data only}
- NCT01822769. Cardiopulmonary rehabilitation for adolescents and adults with congenital heart disease. clinicaltrials.gov/show/nct01822769 (first posted 2 April 2013).
NCT02632253 {published data only}
- NCT02632253. Effects of high-intensity interval training on exercise capacity in patients with grown-up congenital heart disease. clinicaltrials.gov/show/nct02632253 (first posted 16 December 2015).
NCT02643810 {published data only}
- NCT02643810. Exercise training in adults with corrected Tetralogy of Fallot. clinicaltrials.gov/show/nct02643810 (first posted 31 December 2015).
NCT02980393 {published data only}
- NCT02980393. Smart Heart Trial: structured lifestyle intervention for overweight and obese youth with operated heart defects. clinicaltrials.gov/show/nct02980393 (first posted 2 December 2016).
NCT03297918 {published data only}
- NCT03297918. Impact of a structural phonation training on respiratory muscle function in patients with structural heart disease. clinicaltrials.gov/show/nct03297918 (first posted 29 September 2017).
Nehyba 2009 {published data only}
- Nehyba S, Chaloupka V, Soucek R, Chaloupkova S, Vysoky R, Stetka F, et al. The programme of managed ambulatory rehabilitation for patients after heart valve defect surgery. Vnitr̆ní Lékar̆ství 2009;55(12):1118-25. [PubMed] [Google Scholar]
NTR2527 {published data only}
- NTR2527. High intensity interval training after cardiac resynchronization therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR2527 (date of registration 25 September 2010).
NTR3041 {published data only}
- NTR3041. Effects of high intensity interval training on cardiac function at rest and during exercise. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR3041 (date of registration 19 August 2011).
Rhodes 2006 {published data only}
- Rhodes J, Curran TJ, Camil L, Rabideau N, Fulton DR, Gauthier NS, et al. Sustained effects of cardiac rehabilitation in children with serious congenital heart disease. Pediatrics 2006;118(3):e586-93. [DOI] [PubMed] [Google Scholar]
Rowland 2016 {published data only}
- Rowland T. Cardiovascular physiology and disease in youth. Pediatric Exercise Science 2016;28(1):44-7. [DOI: 10.1123/pes.2016-0013] [DOI] [PubMed] [Google Scholar]
Ruttenberg 1983 {published data only}
- Ruttenberg HD, Adams TD, Orsmond GS, Conlee RK, Fisher AG. Effects of exercise training on aerobic fitness in children after open heart surgery. Pediatric Cardiology 1983;4(1):19-24. [DOI] [PubMed] [Google Scholar]
Stefani 2014 {published data only}
- Stefani L, Galanti G, Innocenti G, Mercuri R, Maffulli N. Exercise training in athletes with bicuspid aortic valve does not result in increased dimensions and impaired performance of the left ventricle. Cardiology Research and Practice 2014;2014:238694. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 2018 {published data only}
- Sutherland N, Jones B, Westcamp Aguero S, Melchiori T, du Plessis K, Konstantinov IE, et al. Home- and hospital-based exercise training programme after Fontan surgery. Cardiology in the Young 2018;28(11):1299-305. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tan 1996 {published data only}
- Tan LL, Huang JF, Wang H. The effect of rehabilitation training on postoperative recovery of children with congenital heart disease. Chinese Journal of Nursing 1996;31(6):314-5. [PubMed] [Google Scholar]
Tikkanen 2016 {published data only}
- Tikkanen AU, Rhodes J, Landzberg M, Bhatt A, Systrom DM, Waxman A, et al. Poster 26A: randomized trial of cardiac rehabilitation for adolescents and adults with congenital heart disease. PM&R 2016;8(9):S169. [DOI: 10.1016/j.pmrj.2016.07.069] [DOI] [PubMed] [Google Scholar]
Zhou 2017a {published data only}
- Zhou X, Duqing, Sun K. GW28-e0722 randomized controlled trial of a home-based exercise program for children with congenital heart disease following interventional cardiac catheterization: a preliminary study. Journal of the American College of Cardiology (JACC) 2017;70:C165. [DOI: 10.1016/j.jacc.2017.07.601] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2017b {published data only}
- Zhou X, Duqing, Sun K. Randomized controlled trial of a home-based exercise program for children with congenital heart disease following interventional cardiac catheterization: a preliminary study. Journal of the American College of Cardiology 2017;70(16 Supplement 1):C165. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ali 2018 {published data only}
- Ali LA, Pingitore A, Piaggi P, Brucini F, Passera M, Marotta M, et al. Respiratory training late after Fontan intervention: impact on cardiorespiratory performance. Pediatric Cardiology 2018;39(4):695-704. [DOI: 10.1007/s00246-018-1808-9] [DOI] [PubMed] [Google Scholar]
Callaghan 2017 {published data only}
- Callaghan S, Morrison ML, McCusker C, McKeown P, Casey F. A structured intervention programme can improve the biophysical wellbeing in children with congenital heart disease. Cardiology in the Young 2017;27(4):S228-9. [DOI: ] [Google Scholar]
Morrison 2015 {published data only}
- Morrison BN, DeSouza AM, Voss C, Potts JE, Sandor GG, Harris KC. The use of individualized exercise prescription and activity trackers to promote physical activity in children with congenital heart disease. Canadian Journal of Cardiology 2015;31(10 Suppl 1):S123-4. [Google Scholar]
Neidenbach 2017 {published data only}
- Neidenbach RC, Oberhoffer R, Nagdyman N, Seitz U, Ewert P, Kaemmerer H, et al. Inspiratory muscle training in children after fontan operation increases oxygen saturation. Cogent Medicine 2017;4(1):18. [DOI: ] [Google Scholar]
Nilsson 2019 {published data only}
- Nilsson H, Nylander E, Borg S, Tamas E, Hedman K. Cardiopulmonary exercise testing for evaluation of a randomized exercise training intervention following aortic valve replacement. Clinical Physiology and Functional Imaging 2019;39(1):103-10. [DOI: 10.1111/cpf.12545] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Ganzoni 2019 {published data only}
- Ganzoni C, Arslani K, Pfister O, Freese M, Strobel W, Muller C, et al. Regular phonation and respiratory muscle training improve respiratory muscle strength and quality of life in patients with structural heart disease-the HeartChoir randomized clinical controlled trial. Cardiovascular Medicine 2019;22:P29. [DOI: ] [DOI] [PubMed] [Google Scholar]
ISRCTN74643496 {published data only}
- ISRCTN74643496. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway: a single-blind randomised controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN74643496 (date assigned 8 April 2015).
NCT01397110 {published data only}
- NCT01397110. Respiratory and physical therapy in patients with associated pulmonary arterial hypertension (APAH) with congenital heart defects. clinicaltrials.gov/show/nct01397110 (first posted 19 July 2011).
NCT02240147 {published data only}
- NCT02240147. Start-to-Sport - home-based exercise for adolescents and adults with congenital heart disease. clinicaltrials.gov/show/nct02240147 (first posted 15 September 2014).
NCT02283255 {unpublished data only}
- NCT02283255. Cardiovascular, pulmonary and skeletal muscle evaluation in late postoperative period of the Fontan surgery. clinicaltrials.gov/ct2/show/NCT02283255 (first posted 5 November 2014).
NCT02658266 {unpublished data only}
- NCT02658266. Effect of resistance training in adults with complex congenital heart disease. clinicaltrials.gov/ct2/show/NCT02658266 (first posted 18 January 2016).
NCT03335475 {published data only}
- NCT03335475. Congenital heart disease physical activity lifestyle study. clinicaltrials.gov/show/nct03335475 (first posted 7 November 2017).
NCT03435354 {published data only}
- NCT03435354. Enhanced physical activity support in congenital heart disease clinical care. clinicaltrials.gov/show/nct03435354 (first posted 19 February 2018).
NCT03479957 {published data only}
- NCT03479957. Remotely monitored and coached cardiac rehabilitation northern Sweden. clinicaltrials.gov/show/nct03479957 (first posted 27 March 2018).
NCT03690518 {published data only}
- NCT03690518. Rehabilitation of adolescents and young adults with congenital heart diseases. clinicaltrials.gov/show/nct03690518 (first posted 1 October 2018).
NCT03999320 {published data only}
- NCT03999320. Sophrology and congenital heart disease. clinicaltrials.gov/show/nct03999320 (first posted 26 June 2019).
NCT04135859 (YACHD‐PALS) {published data only}
- NCT04135859. Young adult congenital heart disease physical activity lifestyle study (YACHD-PALS). clinicaltrials.gov/ct2/show/NCT04135859 (first posted 23 October 2019).
NCT04208893 {published and unpublished data}
- NCT04208893. Exercise training strategies for children with repaired Tetralogy of Fallot. clinicaltrials.gov/ct2/show/NCT04208893 (first posted 23 December 2019).
NCT04264650 {published data only}
- NCT04264650. Effectiveness of an mHealth intervention for youth with congenital heart disease. clinicaltrials.gov/ct2/show/NCT04264650 (first posted 11 February 2020).
UMIN000021661 {published data only}
- UMIN000021661. Evaluation test about safety and efficacy of the respiratory muscle training therapy by abdominal respiratory weight exercises in chronic cardiovascular disease patients. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000021661 (date of disclosure 1 April 2016).
Additional references
Adams 2017
- Adams V, Reich B, Uhlemann M, Niebauer J. Molecular effects of exercise training in patients with cardiovascular disease: focus on skeletal muscle, endothelium, and myocardium. American Journal of Physiology. Heart and Circulatory Physiology 2017;5(313):72-88. [DOI] [PubMed] [Google Scholar]
Amedro 2015
- Amedro P, Dorka R, Moniotte S, Guillaumont S, Fraisse A, Kreitmann B, et al. Quality of life of children with congenital heart diseases: a multicenter controlled cross-sectional study. Pediatric Cardiology 2015;36(8):1588-601. [DOI] [PubMed] [Google Scholar]
Amedro 2017
- Amedro P, Gavotto A, Guillaumont S, Bertet H, Vincenti M, De La Villeon G, et al. Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart (British Cardiac Society) 2017;104(12):1026-36. [DOI] [PubMed] [Google Scholar]
Best 2016
- Best KE, Rankin J. Long-term survival of individuals born with congenital heart disease: A systematic review and meta-analysis. Journal of the American Heart Association 2016;5(6):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brudy 2020
- Brudy L, Hock J, Häcker AL, Meyer M, Oberhoffer R, Hager A, et al. Children with congenital heart disease are active but need to keep moving: a cross-sectional study using wrist-worn physical activity trackers. Journal of Pediatrics 2020;217:13-19. [DOI] [PubMed] [Google Scholar]
Budts 2013
- Budts W, Börjesson M, Chessa M, Van Buuren F, Trigo Trindade P, Corrado D, et al. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. European Heart Journal 2013;34(47):3669-74. [DOI] [PubMed] [Google Scholar]
Budts 2020
- Budts W, Pieles GE, Roos-Hesselink JW, Sanz de la Garza M, D’Ascenzi F, Giannakoulas G, et al. Recommendations for participation in competitive sport in adolescent and adult athletes with Congenital Heart Disease (CHD): position statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), the European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease and the Sports Cardiology, Physical Activity and Prevention Working Group of the Association for European Paediatric and Congenital Cardiology (AEPC). European Heart Journal 2020 Aug 26 [E-pub ahead of print]. [DOI: 10.1093/eurheartj/ehaa501] [DOI] [PubMed]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;16:368. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports (Washington, D.C. : 1974) 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
Cedars 2020
- Cedars AM, Kutty S. The way forward in congenital heart disease research. JAMA Cardiology 2020;5(9):979-80. [DOI: 10.1001/jamacardio.2020.2034] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, accessed before 2 October 2020. Available at www.covidence.org.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Department of Health 2011
- Department of Health, Physical Activity, Health Improvement and Protection. Start active, stay active: a report on physical activity from the four home countries. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216370/dh_128210.pdf (accessed 13 August 2019).
Dimopoulos 2006
- Dimopoulos K, Okonko DO, Diller GP, Broberg CS, Salukhe TV, Babu-Narayan SV, et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006;113(24):2796-802. [DOI] [PubMed] [Google Scholar]
Dua 2007
- Dua J, Cooper AR, Fox KR, Stuart AG. Physical activity levels in adults with congenital heart disease. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14(2):287–93. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Christoph M. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faraone 2008
- Faraone, S. Interpreting Estimates of Treatment Effects. Pharmacy and Therapeutics 2018;33(12):700. [PMC free article] [PubMed] [Google Scholar]
Franklin 2013
- Franklin BA, Lavie CJ, Squires RW, Milani RV. Exercise-based cardiac rehabilitation and improvements in cardiorespiratory fitness: implications regarding patient benefit. Mayo Clinic Proceedings 2013;88(5):431-7. [DOI] [PubMed] [Google Scholar]
Giardini 2009
- Giardini A, Hager A, Lammers AE, Derrick G, Müller J, Diller GP, et al. Ventilatory efficiency and aerobic capacity predict event-free survival in adults with atrial repair for complete transposition of the great arteries. Journal of the American College of Cardiology 2009;53(17):1548-55. [DOI] [PubMed] [Google Scholar]
Gielen 2010
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training molecular mechanisms. Circulation 2010;122(12):1221-38. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel‐Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information & Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gomes‐Neto 2016
- Gomes-Neto M, Saquetto MB, Silva CM, Conceição CS, Carvalho VO. Impact of exercise training in aerobic capacity and pulmonary function in children and adolescents after congenital heart disease surgery: a systematic review with meta-analysis. Pediatric Cardiology 2016;37(2):217-24. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc), 2015. Available at gradepro.org.
GraphPad Prism [Computer program]
- GraphPad Prism. Version 8.1.0. California USA: GraphPad Software Inc., 2019. Available at www.graphpad.com/scientific-software/prism. [WEBSITE: www.graphpad.com]
Gratz 2009
- Gratz A, Hess J, Hager A. Self-estimated physical functioning poorly predicts actual exercise capacity in adolescents and adults with congenital heart disease. European Heart Journal 2009;30(4):497-504. [DOI] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003823. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003822. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Savovic J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. Draft version (29 January 2019) for inclusion in: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch Va (editors). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane. Available from www.training.cochrane.org/handbook.
Hoffman 2002
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology 2002;39(12):1890-900. [DOI] [PubMed] [Google Scholar]
Kempny 2012
- Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—single centre experience and review of published data. European Heart Journal 2012;33(11):1386–96. [DOI] [PubMed] [Google Scholar]
Khairy 2010
- Khairy P, Ionescu-Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. Journal of the American College of Cardiology 2010;56(14):1149-57. [DOI] [PubMed] [Google Scholar]
Koyak 2012
- Koyak Z, Harris L, Groot JR, Silversides CK, Oechslin EN, Bouma BJ, et al. Sudden cardiac death in adult congenital heart disease. Circulation 2012;126(16):1944-54. [DOI] [PubMed] [Google Scholar]
Lee 2012
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380(9838):219-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Letnes 2019
- Letnes JM, Dalen H, Vesterbekkmo EK, Wisløff U, Nes BM. Peak oxygen uptake and incident coronary heart disease in a healthy population: the HUNT Fitness Study. European Heart Journal 2019;40(20):1633–9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Li 2019
- Li X, Chen N, Zhou X, Yang Y, Chen S, Song Y, et al. Exercise training in adults with congenital heart disease: a systematic review and meta-analysis. Journal of Cardiopulmonary Rehabilitation and Prevention 2019;39(5):299-307. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine 2009;6(7):e1000100. [10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019
- Liu Y, Chen S, Zü L, Black GC, Choy M, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology 2019;48(2):455-63. [DOI: 10.1093/ije/dyz009] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCrindle 2007
- McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Archives of Disease in Childhood 2007;92(6):509-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2020
- Meyer M, Brudy L, García-Cuenllas L, Hager A, Ewert P, Oberhoffer R, et al. Current state of home-based exercise interventions in patients with congenital heart disease: A systematic review. Heart 2020;106(5):333-41. [DOI] [PubMed] [Google Scholar]
Mitchell 1971
- Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation 1971;43(3):323-32. [DOI] [PubMed] [Google Scholar]
Moons 2020
- Moons P, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, et al. Physical functioning, mental health, and quality of life in different congenital heart defects: comparative analysis in 3538 patients from 15 countries. Canadian Journal of Cardiology 2020 Apr 6 [Epub ahead of print]. [DOI: 10.1016/j.cjca.2020.03.044] [DOI] [PubMed]
Müller 2015
- Müller J, Hager A, Diller GP, Derrick G, Buys R, Dubowy KO, et al. Peak oxygen uptake, ventilatory efficiency and QRS-duration predict event free survival in patients late after surgical repair of tetralogy of Fallot. International Journal of Cardiology 2015;196:158-64. [DOI] [PubMed] [Google Scholar]
Pufulete 2019
- Pufulete, M. Effectiveness and cost-effectiveness of INSPIRatory musclE training (IMT) for reducing postoperative pulmonary complications (PPC): a sham-controlled randomised controlled trial (RCT) (INSPIRE). Available at europepmc.org/grantfinder/grantdetails?query=pi%3A%22Pufulete%2BM%22%2Bgid%3A%2216%2F140%2F07%22%2Bga%3A%22National%20Institute%20for%20Health%20Research%20%28Department%20of%20Health%29%22.
RevMan Web 2019 [Computer program]
- Review Manager Web (RevMan Web). Version 5.3. The Cochrane Collaboration, 2019. Available at: revman.cochrane.org.
Rhodes 2008
- Rhodes JF, Hijazi ZM, Sommer RJ. Pathophysiology of congenital heart disease in the adult, part II: simple obstructive lesions. Circulation 2008;117(9):1228-37. [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence, version 3. Cochrane Consumers and Communication Group 2016;3:.. [http://cccrg.cochrane.org/author-resources.] [Google Scholar]
Sandberg 2016
- Sandberg C, Pomeroy J, Thilén U, Gradmark A, Wadell K, Johansson B. Habitual physical activity in adults with congenital heart disease compared with age- and sex-matched controls. Canadian Journal of Cardiology 2016;32(4):547-53. [DOI] [PubMed] [Google Scholar]
Scheffers 2020
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors) Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook..
Shei 2018
- Shei RJ. Recent advancements in our understanding of the ergogenic effect of respiratory muscle training in healthy humans: a systematic review. Journal of Strength and Conditioning Research 2018;32(9):2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sommer 2008a
- Sommer RJ, Hijazi ZM, Rhodes JF. Pathophysiology of congenital heart disease in the adult: part III: Complex congenital heart disease. Circulation 2008;117(10):1340-50. [DOI] [PubMed] [Google Scholar]
Sommer 2008b
- Sommer RJ, Hijazi ZM, Rhodes JF. Pathophysiology of congenital heart disease in the adult: part I: Shunt lesions. Circulation 2008;117(8):1090-9. [DOI] [PubMed] [Google Scholar]
Stata 2015 [Computer program] [Computer program]
- Stata. StataCorp L, Version 15. College Station, TX, USA: StataCorp, 2015. Available at www.stata.com.
Udholm 2018
- Udholm, S Aldweib, N Hjortdal, VE Veldtman, GR. Prognostic power of cardiopulmonary exercise testing in Fontan patients: a systematic review. Open Heart 2018;5(1):e000812.. [DOI: doi:10.1136/ openhrt-2018-000812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warnes 2008
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Writing Committee to develop guidelines on the management of adults with congenital heart disease). Journal of the American College of Cardiology 2008;52(23):e143-e263. [DOI] [PubMed] [Google Scholar]
WebPlotDigitizer [Computer program]
- Web Plot Digitizer. Version 4.2. San Francisco: Ankit Rohatgi, 2019. Available at automeris.io/WebPlotDigitizer.
Williams 2017
- Williams CA, Gowing L, Horn R, Stuart AG. A survey of exercise advice and recommendations in United Kingdom paediatric cardiac clinics. Cardiology in the Young 2017;27(5):951-6. [DOI] [PubMed] [Google Scholar]
Wong 2011
- Wong E, Selig S, Hare DL. Respiratory muscle dysfunction and training in chronic heart failure. Heart, Lung and Circulation 2011;20(5):289-94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Williams 2019
- Williams CA, Wadey C, Pieles G, Stuart G, Taylor RS, Long L. Physical activity interventions for people with congenital heart disease (Protocol). Cochrane Database of Systematic Reviews 2019;8:1-14. [DOI: 10.1002/14651858.CD013400.] [DOI] [PMC free article] [PubMed] [Google Scholar]