Abstract
U2 snRNP auxiliary factor (U2AF) promotes U2 snRNP binding to pre-mRNAs and consists of two subunits of 65 and 35 kDa, U2AF65 and U2AF35. U2AF65 binds to the polypyrimidine (Py) tract upstream from the 3′ splice site and plays a key role in assisting U2 snRNP recruitment. It has been proposed that U2AF35 facilitates U2AF65 binding through a network of protein-protein interactions with other splicing factors, but the requirement and function of U2AF35 remain controversial. Here we show that recombinant U2AF65 is sufficient to activate the splicing of two constitutively spliced pre-mRNAs in extracts that were chromatographically depleted of U2AF. In contrast, U2AF65, U2AF35, and the interaction between them are required for splicing of an immunoglobulin μ pre-RNA containing an intron with a weak Py tract and a purine-rich exonic splicing enhancer. Remarkably, splicing activation by U2AF35 occurs without changes in U2AF65 cross-linking to the Py tract. These results reveal substrate-specific requirements for U2AF35 and a novel function for this factor in pre-mRNA splicing.
The first ATP-dependent step in the assembly of splicing complexes is the stable association of U2 snRNP with the 3′ part of the intron (reviewed in reference 12), which includes the branch point region, the polypyrimidine (Py) tract, and the conserved dinucleotide AG at the 3′ splice site. The branch point region establishes base-pairing interactions with U2 snRNA that are critical for catalysis of the splicing reaction. The Py tract, particularly important in higher eukaryotes, is a pyrimidine-rich sequence located between the branch point and the AG dinucleotide that serves as the binding site for the U2 snRNP auxiliary factor (U2AF).
Human U2AF is an essential splicing factor purified as a heterodimer composed of 65-kDa (U2AF65) and 35-kDa (U2AF35) subunits (39). U2AF65 binds directly to Py tracts (41), while U2AF35 is tethered to the pre-mRNA through its interaction with U2AF65 (42). When bound to the Py tract, the amino-terminal arginine-serine-rich (RS) domain of U2AF65 contacts the branch point region, and it has been proposed that its positively charged surface can promote the otherwise unstable base pairing between U2 snRNA and the poorly conserved branch point sequence (7, 34). Other mechanisms, including the recruitment of splicing factors involved in prespliceosome formation (5) as well as direct protein-protein interactions with components of U2 snRNP (8), are likely to contribute to U2AF activity.
The role of U2AF35 remains controversial. In vivo analyses of Drosophila U2AF have shown that both subunits, as well as the interaction between them, are essential for viability (14, 22, 23). In contrast, biochemical complementation experiments performed with extracts chromatographically depleted of U2AF have indicated that U2AF65 alone is able to provide U2AF activity when tested with model splicing substrates (40, 41). Similar results were obtained with nuclear extracts immunodepleted with a monoclonal antibody against U2AF65 (6, 13). Results obtained with a U2AF65 mutant protein deficient in its interaction with U2AF35 also indicated that U2AF35 is dispensable for in vitro splicing of various pre-mRNAs, including substrates whose splicing depends on the presence of exonic splicing enhancers (13). These sequences are often found downstream of weak 3′ splice sites (31, 37) and stimulate early events in spliceosome assembly (16, 28), including U2AF65 binding (36, 43).
In contrast with these results, immunodepletion with antibodies against U2AF35 resulted in nuclear extracts that required addition of both U2AF65 and U2AF35 for efficient splicing of constitutive and exon enhancer-dependent substrates (43). Also supporting an essential role for U2AF35 were results obtained with a U2AF65 mutant defective in interaction with U2AF35, which was inactive in those assays (43). Based on results obtained in a reconstituted system using limiting amounts of recombinant proteins, and also based on previous data reporting protein-protein interactions mediated by the RS domain of splicing factors (38), it was proposed that U2AF35 stabilizes U2AF65 binding to weak Py tracts by interacting simultaneously with U2AF65 and SR proteins (43), the latter bound to the enhancer sequence (16, 29, 31).
In this study, we used extracts chromatographically depleted of U2AF to show that although U2AF35 is dispensable for in vitro splicing of some pre-mRNAs (adenovirus major late promoter [AdML] and human β-globin pre-mRNAs) its presence and interaction with U2AF65 are essential for prespliceosome assembly and splicing of a regulated mouse immunoglobulin μ (IgM) substrate. The splicing-stimulatory activity of U2AF35 does not influence cross-linking of U2AF65 to the Py tract, thus revealing a substrate-specific function for U2AF35 after U2AF65 binding.
MATERIALS AND METHODS
Plasmids.
pIgM ΔPy was as described by Kan and Green (13). pIgM mutPy was obtained by replacing the thymidine residues contained in the Py tract of IgM by adenosines via PCR-based site-directed mutagenesis of plasmid pμM (37) as described elsewhere (10). PCR was performed with VENT DNA polymerase (New England Biolabs). Mutant clones were confirmed by sequencing.
Expression and purification of recombinant proteins.
U2AF65 and U2AF65Δ35 were expressed as glutathione S-transferase (GST) fusion proteins in Escherichia coli and purified as described by Lin and Green (17). The plasmids used for protein expression were described previously (5, 41). The purified proteins were dialyzed against 100 mM KCl buffer D (20 mM HEPES [pH 8.0], 0.5 mM EDTA, 20% glycerol, 1 mM dithiothreitol [DTT], 0.05% NP-40).
U2AF35 was purified from baculovirus-infected insect cells as previously described (43) and dialyzed against 100 mM KCl buffer D.
Protein concentrations were estimated by comparing dilutions of the preparations to serial dilutions of a bovine serum albumin standard in sodium dodecyl sulfate (SDS)-containing denaturing gels.
Preparation of HeLa nuclear extract.
HeLa nuclear extract was prepared as described by Dignam et al. (4).
Depletion of U2AF by oligo(dT) (odT)-cellulose chromatography.
HeLa nuclear extract was depleted of U2AF exactly as described elsewhere (35). The column flowthrough of this depletion procedure yields the depleted nuclear extract (odTΔNE). The proteins eluted with 2 M guanidine-HCl are a source of partially purified U2AF after dialysis against 100 mM KCl buffer D.
Antibodies and immunoblots.
The anti-U2AF65 monoclonal antibody MC3 was described by Gama-Carvalho et al. (6). The anti-U2AF35 polyclonal serum was described by Zuo and Maniatis (43). Immunoblots were developed by using horseradish peroxidase-coupled secondary antibodies and detected by enhanced chemoluminescence (ECL; Amersham).
Immunodepletion of the 2 M guanidine fraction.
A 200-μl aliquot of MC3 hybridoma supernatant was incubated with 50 μl of protein A-Sepharose 4 Fast Flow (Pharmacia Biotech). This resin was incubated with 50 μl of the 2 M guanidine-HCl eluate at 4°C with agitation, and the depleted fraction was separated from the beads by centrifugation.
In vitro splicing assays and spliceosome assembly reactions.
Splicing reactions and splicing complementation assays were performed as described elsewhere (35). In brief, 13 fmol of RNA was incubated in 9-μl reaction mixtures containing 33% nuclear extract or odTΔNE and recombinant proteins at the indicated concentrations. For analysis of splicing products, the mixtures were incubated for 2 h at 30°C and deproteinized, the RNAs were precipitated and loaded on 8 or 13% denaturing polyacrylamide gels.
For spliceosome assembly analysis, reactions were incubated at 30°C for 20 min and then stopped with heparin (5 mg/ml; Sigma H-2149). Aliquots of 5 μl were separated on 4% acrylamide:bisacrylamide (80:1)–0.5% agarose gels in 50 mM Tris base–50 mM glycine buffer. The gels were exposed to film (Kodak X-Omat AR) and/or a phosphorimager screen (Fuji BAS-MP).
Specific labeling of IgM pre-mRNA.
IgM pre-mRNA specifically labeled at the 3′ splice site region was synthesized as depicted in Fig. 5C. Transcription templates for the 5′ and 3′ portions of IgM RNA were generated by PCR; 10 μg of template DNA was used in 250-μl transcription reactions containing 40 mM Tris-HCl (pH 7.9), 10 mM NaCl, 6 mM MgCl2, 2 mM spermidine, 0.8 mM DTT, 0.4 mM ATP and CTP, 0.08 mM GTP, and 400 U of SP6 or T7 RNA polymerase. Capped unlabeled 5′-half RNA was generated with SP6 RNA polymerase adding 0.4 mM UTP and 1.6 mM CAP analog [m7G(5′)ppp(5′)G; New England Biolabs] to the above reaction mixture. Internally labeled 3′-half RNA was generated with T7 RNA polymerase by supplementing the above reaction mixture with 1.6 mM uridylyl (3′-5′) guanosine (Sigma), 0.08 mM UTP, and 50 μl [α-32P]UTP (20 mCi/ml; Amersham). Both transcripts were gel purified. 3′-half RNA (100 pmol) was 5′ phosphorylated with T4 polynucleotide kinase (New England Biolabs); 100 pmol of each RNA and 200 pmol of IgM DNA bridging oligonucleotide in 12 μl of H2O were heated at 90°C for 2 min, 1 μl of 10× ligation buffer (500 mM Tris-HCl pH 7.5, 100 mM MgCl2, 100 mM DTT, 10 mM ATP, 250 μg of bovine serum albumin per ml) was added, and the reaction was incubated at room temperature for 2 min to anneal the RNA to the DNA bridging oligonucleotide. The RNA molecules were ligated as described by Moore and Sharp (19) in a 20-μl reaction by adding 2 μl of 100 mM DTT, 20 U of RNasin, 1 μl of 10× ligation buffer, and 2 μl of T4 DNA ligase (2,000 U/μl; New England Biolabs). The ligated RNA was gel purified and resuspended in 10 μl of H2O.
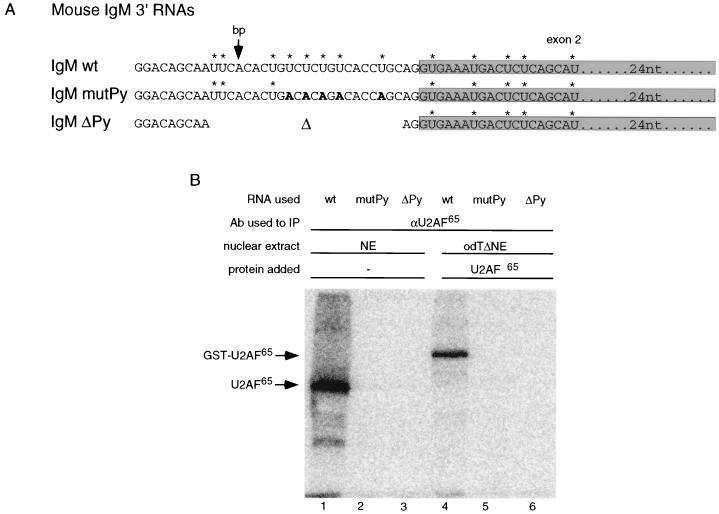
FIG. 5.
Py tract-specific binding of U2AF65 is not stimulated by U2AF35. (A) Sequence of the 3′ one-fourth of IgM pre-mRNA substrate (IgM wt) and two mutants used to document the cross-linking specificity of U2AF65. In one mutant (IgM mutPy), the U’s within the Py tract were replaced by A’s (shown in bold); in the second mutant (IgM ΔPy), the complete Py tract and part of the branch point region (indicated by an arrow) were deleted. Labeled U residues are indicated by asterisks. The shaded region represents exon 2. (B) Detection of Py tract-dependent U2AF65 cross-linking. The indicated radioactively labeled RNAs were incubated with nuclear extract (NE) or with OdTΔNE in the presence of 90 nM recombinant GST-U2AF65 (lanes 4 to 6); the mixtures were irradiated with UV light, and U2AF65 was immunoprecipitated (IP) with anti-U2AF65 antibody (Ab). The precipitates were fractionated in a SDS-polyacrylamide gel which was exposed to a phosphorimager screen. The positions of U2AF65 and GST-U2AF65 are indicated on the left. (C) Synthesis of an IgM RNA substrate labeled only at the 3′ one-fourth of the molecule. Transcription templates for the 5′ three-fourths and the 3′ one-fourth of the molecule were generated by PCR. Unlabeled capped 5′ RNA and labeled uncapped 3′ RNA were transcribed and subsequently ligated (19). NTPs, nucleoside triphosphates. (D) U2AF35 does not stimulate cross-linking of U2AF65 to the Py tract under splicing conditions. The RNA substrate used for panel B was incubated under splicing conditions with nuclear extract (NE) or odTΔNE without or with recombinant GST-U2AF65 and His-U2AF35 (concentrations indicated above the lanes). Samples were processed as for panel B. The positions corresponding to endogeneous U2AF65 and GST-U2AF65 are indicated on the left. Lane 1 shows the mock immunoprecipitation with an unrelated monoclonal antibody. (E) Quantification of the phosphorimager signals corresponding to cross-linking of GST-U2AF65 in lanes 3 to 7 of panel D (black columns). Empty columns represent the splicing efficiency of the IgM pre-mRNA under identical experimental conditions. The percentage of fully spliced mRNA is indicated on the right.
UV cross-linking and immunoprecipitation of U2AF65.
Pre-mRNA substrates were incubated under the same conditions as described for in vitro splicing assays except that the reaction volume was increased to 36 μl and the amount of RNA was increased to 100 fmol. After a 15-min incubation at 30°C, samples were UV cross-linked on ice (Stratalinker; 254 nm, 0.6 J, 4-cm distance to light source) and then treated with RNase A (final concentration, 1 mg/ml; Boehringer Mannheim) at 37°C for 20 min. To immunoprecipitate U2AF65, 42 μl of anti-U2AF65 monoclonal antibody was added to each tube, and samples were incubated on ice for 1.5 h. Then 10 μl of anti-mouse IgG-agarose (Sigma A-6531) was added, and incubation was continued at 4°C with rotation. Beads were sedimented by centrifugation at 6,000 rpm for 10 s and washed four times with 600 μl of high-salt buffer (500 mM NaCl, 50 mM Tris-HCl [pH 8.0], 1% NP-40) and once with 50 mM Tris-HCl (pH 8.0)–1% NP-40. The beads were resuspended in 20 μl of SDS-loading dye and boiled for 5 min. After sedimentation of the beads by centrifugation, the supernatant was loaded on an SDS–10% polyacrylamide gel. The gel was fixed, dried, and exposed to a phosphorimager screen.
RESULTS
Substrate-specific differences in U2AF65 activity.
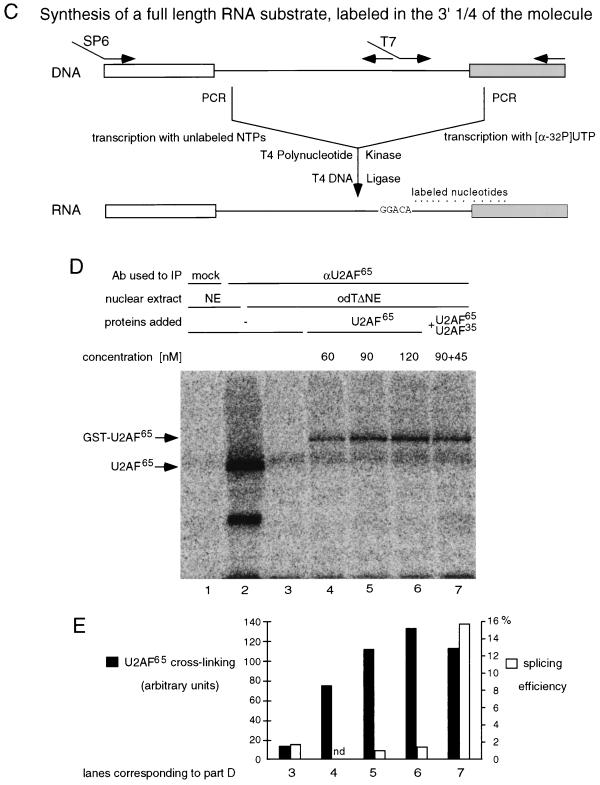
Nuclear extracts depleted of U2AF can be prepared by passing them over poly(U) (40) or odT (35) columns at high salt concentrations (1 M KCl) to minimize nonspecific and low-affinity interactions. Flowthrough fractions are depleted of U2AF and therefore cannot support pre-mRNA splicing reactions. The activity can be restored by addition of purified or recombinant U2AF65 (40, 41). Figures 1A and B illustrate this for two model pre-mRNAs: an AdML transcript and a human β-globin minigene. Both RNAs are constitutively spliced and contain strong 3′ splice site signals. Upon incubation with normal extracts, splicing intermediates and products accumulated and could be separated from pre-mRNAs by electrophoresis on denaturing polyacrylamide gels (Fig. 1A and B, lanes 1). No splicing-related products could be detected upon incubation with odTΔNE (Fig. 1A and B, lanes 2). Addition of purified recombinant GST-U2AF65 to odTΔNE resulted in efficient complementation of the depleted extracts (Fig. 1A and B, lanes 3).
FIG. 1.
Substrate-specific differences in U2AF65 activity. After incubation of radioactively labeled pre-mRNAs under splicing conditions, RNAs were isolated and fractionated in denaturing polyacrylamide gels, and the gels were exposed to film or phosphorimager screens. pre-mRNAs tested correspond to an AdML transcript (A), a human β-globin minigene (B), a mouse IgM minigene (C), or the same transcript without a splicing-inhibitory sequence (INH) (13) (D) Pre-mRNAs were incubated in HeLa nuclear extract (NE) or odTΔNE in the absence or presence of recombinant GST-U2AF65 (260 nM in panels A to C and at the indicated concentrations in panel D). The different RNA species were fractionated in 8% (A to C) or 13% (D) polyacrylamide gels. Bands corresponding in size or in mobility to splicing products and intermediates are indicated at the left. Boxes indicate exons; thin lines indicate introns.
However, when the experiment was performed under identical conditions using a mouse IgM pre-mRNA, addition of GST-U2AF65 did not restore splicing, even after prolonged incubation of the reaction mixtures or when an excess of the purified RNA was loaded on the gel (Fig. 1C; compare lanes 2 and 3). This transcript contains weak 3′ splice site signals, and the 3′ exon contains the founding member of a family of purine-rich splicing enhancer sequences (37) as well as a splicing inhibitory sequence further downstream (13). Figure 1D shows that even in the absence of the splicing-inhibitory sequence, the presence of recombinant U2AF65 at a wide range of concentrations did not result in accumulation of splicing intermediates or products.
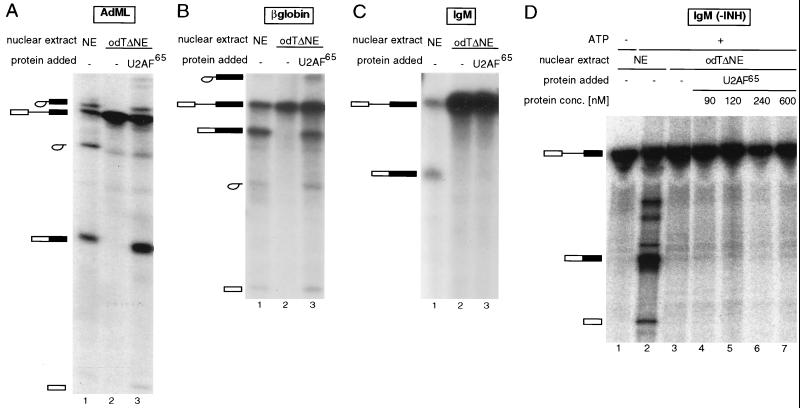
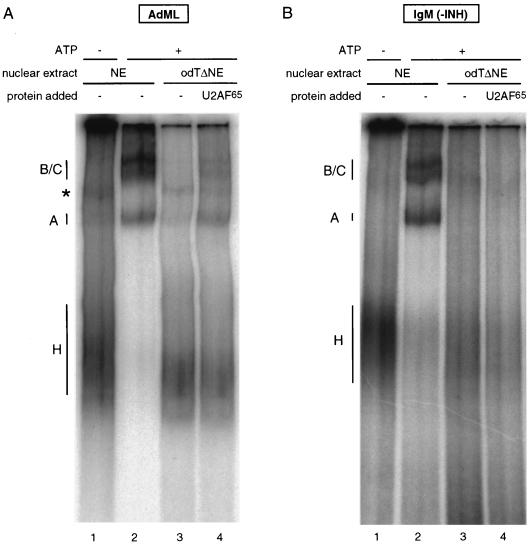
To analyze the step in spliceosome assembly at which IgM splicing was stalled, the splicing mixtures were electrophoresed on native polyacrylamide-agarose composite gels allowing the separation of ATP-independent hnRNP complexes from three ATP-dependent, splicing-related complexes: complex A, corresponding to addition of U2 snRNP to the branch point region; complex B, the fully assembled spliceosome formed by addition of the U4/5/6 tri-snRNP; and complex C, the rearranged, catalytically active spliceosome.
The result shown in Fig. 2 indicates that none of the spliceosomal complexes could form on the AdML or the IgM substrate in odTΔNE in the absence of U2AF65 (lanes 3). Recombinant U2AF65 could rescue complex formation on the AdML substrate (Fig. 2A, lane 4) but was unable to restore spliceosome assembly on the IgM substrate (Fig. 2B, lane 4). This indicates defects in spliceosome assembly before or at the stage of U2 snRNP binding, ruling out that IgM splicing is stalled in odTΔNE at steps subsequent to those in which U2AF function has been implicated.
FIG. 2.
Spliceosome assembly of IgM in odTΔNE is stalled before U2 snRNP recruitment. Radioactively labeled AdML (A) or IgM (B) pre-mRNA was incubated in HeLa nuclear extracts (NE) in the absence or presence of ATP or in odTΔNE in the absence or presence of 120 nM recombinant GST-U2AF65. The mixtures were loaded onto native polyacrylamide-agarose composite gels, allowing the separation of ATP-independent hnRNP complexes (complex H), ATP-dependent pre-spliceosomes (complex A), and two conformations of the fully assembled spliceosome (complexes B and C). The complex formed on AdML RNA in the absence of ATP is unrelated to splicing (indicated by an asterisk). INH, inhibitory sequence.
An additional activity is required for splicing of IgM in odTΔNE.
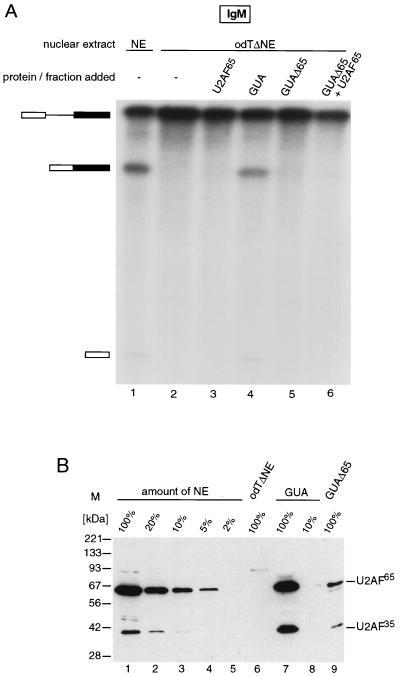
To rule out that the depletion procedure inactivated some components of the extract required for IgM splicing, the material bound to the odT column was eluted with 2 M guanidine-HCl and tested for complementation. Figure 3A shows that in contrast to GST-U2AF65 (lane 3), the eluate did complement IgM splicing in odTΔNE (lane 4).
FIG. 3.
An activity distinct from U2AF65 is required in addition to U2AF65 for splicing of IgM in odTΔNE. (A) IgM splicing reconstitution experiment in odTΔNE. pre-mRNA and mRNA are indicated on the left. Nuclear extracts (NE) and proteins used are listed above the lanes. The concentration of GST-U2AF65 used in lanes 3 and 6 was 240 nM. GUA indicates the 2 M guanidine-HCl eluate from the odTΔNE column; GUAΔ65 indicates guanidine eluate fraction immunodepleted with a monoclonal α-U2AF65 antibody. RNA was fractionated on a 8% polyacrylamide gel. (B) Codepletion of U2AF65 and U2AF35. Nuclear extracts (NE), serial dilutions of NE, odTΔNE, the 2 M guanidine-HCl eluate of the odT column (GUA), a 10-fold dilution of this eluate, and the same eluate after immunodepletion with anti-U2AF65 monoclonal antibody (GUAΔ65) were fractionated in a SDS-polyacrylamide gel and blotted onto a nitrocellulose membrane. The blot was probed with antibodies against U2AF65 and U2AF35. The positions of U2AF65 and U2AF35 are marked at the right. M, size markers.
To verify that U2AF65 is involved in IgM splicing, the guanidine eluate was immunodepleted with a U2AF65-specific antibody. The immunodepleted guanidine fraction (GUAΔ65) was unable to rescue splicing (Fig. 3A, lane 5). This suggests that both U2AF65 and other factors present in the guanidine eluate are necessary for IgM splicing. Splicing was also not rescued when GUAΔ65 and U2AF65 were added together (lane 6). This suggests that the additional factor required for IgM splicing binds tightly to U2AF65 because the two factors are bound to each other at the high salt concentrations used in the depletion procedure and they are immunodepleted together even after guanidine elution.
One factor that binds tightly to U2AF65 is the small subunit of U2AF, U2AF35 (42). Specific antibodies were used to detect the two subunits in the different extracts and fractions (Fig. 3B). U2AF65 and U2AF35 could be detected in nuclear extract (lane 1), and their concentration was reduced by at least 98% (U2AF65) or 90% (U2AF35) in odTΔNE (compare lanes 2 to 6). U2AF65 and U2AF35 were present in the guanidine eluate (lane 7), and the concentration of both factors was reduced by immunodepletion with the U2AF65-specific antibody (compare lanes 7 and 9). These observations are consistent with U2AF35 being the factor required for IgM splicing in odTΔNE in addition to U2AF65.
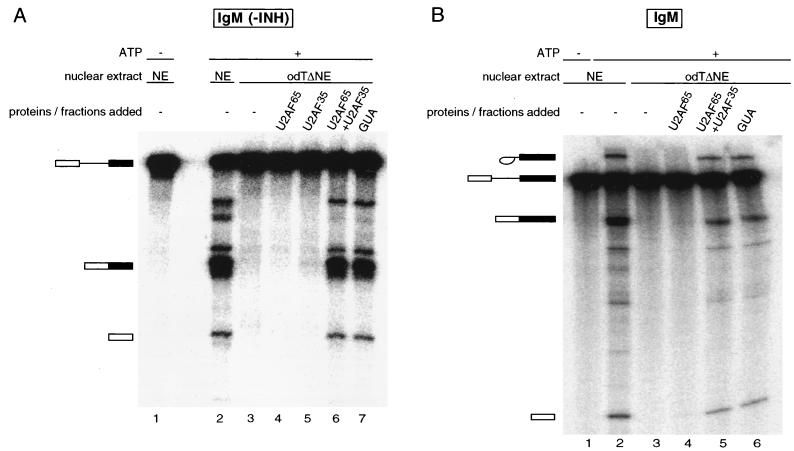
To directly test this possibility, both recombinant U2AF65 and U2AF35 were added in the IgM complementation assay (Fig. 4). Neither of the U2AF subunits alone was able to restore splicing (Fig. 4A, lanes 4 and 5). Addition of both proteins, however, restored splicing to the same level as in the 2 M guanidine eluate (Fig. 4A, lanes 6 and 7). The same result was obtained when a longer IgM substrate containing a recently described splicing-inhibitory sequence (13) was used in the assay (Fig. 4B). As expected, spliceosome assembly was also stimulated by the presence of both subunits (data not shown).
FIG. 4.
IgM splicing in odTΔNE is dependent on the presence of both U2AF subunits. In vitro splicing reconstitution assay of the IgM pre-mRNA substrate was performed without (A) or with (B) a splicing-inhibitory sequence (INH) (13). Splicing products fractionated in a 13% polyacrylamide gel are indicated on the left. Nuclear extracts (NE) used are as in previous figures; 90 nM GST-U2AF65 and 45 nM His-tagged U2AF35 were added. GUA indicates the 2 M guanidine-HCl eluate of the odT column.
Previous results have indicated that splicing factors of the SR family can complement nuclear extracts chromatographically depleted of U2AF in a substrate-specific fashion (18). This activity, however, appears to be distinct from the activity of U2AF35 in our experimental system because (i) while SC35 could substitute for both U2AF subunits in the system described by MacMillan et al. (18), both U2AF65 and U2AF35, as well as the interaction between them, are required for complementation of the IgM substrate; and (ii) SR proteins were not sufficient to complement IgM splicing in our depleted extracts either in the presence or in the absence of U2AF65 (data not shown).
The stimulatory activity of U2AF35 does not correlate with increased U2AF65 binding to the Py tract.
Zuo and Maniatis (43) demonstrated that U2AF35 can enhance U2AF65 binding to a uniformly labeled Drosophila doublesex pre-mRNA in a reconstituted system with limiting amounts of all components. We wanted to examine whether the effect of U2AF35 in our depletion/complementation system, under splicing conditions (i.e., in nuclear extract in the presence of ATP and Mg2+ at 30°C), was related to enhanced U2AF65 binding to the IgM Py tract.
To monitor U2AF65 binding specifically at the Py tract, RNAs corresponding to the 3′ end of IgM pre-mRNA or mutant derivatives were synthesized. The sequences of these RNAs (Fig. 5A) correspond to the wild-type (wt) sequence (IgM wt), an RNA containing U-to-A mutations within the Py tract (IgM mutPy), or an RNA containing a deletion of the entire Py tract and part of the branch point region (IgM ΔPy).
The binding specificity of U2AF65 to the labeled RNAs was analyzed by UV cross-linking. The different RNAs were incubated under splicing conditions in nuclear extracts or odTΔNE supplemented with GST-U2AF65; the samples were irradiated with short-wavelength UV light, treated with RNase A, and then incubated with an anti-U2AF65 monoclonal antibody. The complexes formed were precipitated with anti-mouse IgG covalently bound to agarose beads and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). The radioactive signals were quantified with a phosphorimager (Fuji).
Figure 5B shows that both endogenous U2AF65 and recombinant GST-U2AF65 could be efficiently cross-linked to the IgM wt substrate (lanes 1 and 4). No cross-linking was detected when the polypyrimidine tract was mutated (IgM mutPy; lanes 2 and 5) or deleted (IgM ΔPy; lanes 3 and 6), indicating that cross-linking required the Py tract in the wt RNA. Combined with the body of evidence indicating that U2AF65 function involves its direct binding to this region of the pre-mRNA (reviewed in reference 27), these results suggest that the cross-link observed with the wt substrate reflects functional and specific binding of U2AF65 to the Py tract.
To determine whether U2AF35 can increase cross-linking of U2AF65 to the Py tract of IgM, similar experiments were performed under splicing conditions using a full-length RNA obtained by ligation of the labeled 3′ wt substrate to the unlabeled 5′ portion of IgM pre-mRNA (Fig. 5C) (19). This specifically labeled RNA was used because in our hands approximately 50% of the signal obtained with a uniformly labeled IgM RNA was due to U2AF65 cross-linking to regions farther upstream from the Py tract (data not shown).
The RNA was incubated with nuclear extract or odTΔNE supplemented with either U2AF65 alone or both U2AF65 and U2AF35. After cross-linking and immunoprecipitation with the U2AF65-specific antibody, bound proteins were separated by SDS-PAGE and the dried gel was exposed to a phosphorimager screen. Figure 5D shows the image obtained from the phosphorimager scan. The intensity of the signal corresponding to GST-U2AF65 was quantified, and the results of the quantification are shown below each lane of Fig. 5D as black columns in Fig. 5E. For comparison, Fig. 5E also shows the splicing efficiency of parallel reactions as empty columns.
The strong signal corresponding to endogenous U2AF65 (Fig. 5D, lane 2) was absent when proteins were precipitated with an unrelated antibody (lane 1) or in odTΔNE (lane 3), consistent with U2AF65-specific precipitation. Addition of increasing amounts of U2AF65 to odTΔNE resulted in a proportional increase in signal (Figures 5D and E, lanes and columns 4 to 6), indicating that the assay can be used to quantify U2AF65 binding. Addition of U2AF35 to an intermediate concentration of U2AF65 resulted in negligible changes in U2AF65 binding (Fig. 5D and E, lane and column 7). These changes could not justify the strong stimulatory effect of U2AF35 in splicing (Fig. 5E, empty columns) because even stronger U2AF65 binding, achieved by addition of U2AF65 at a concentration of 120 nM (lane 6) or higher (data not shown), did not stimulate accumulation of splicing products (Fig. 1D, lane 5). Taken together, these results indicate that the effect of U2AF35 in promoting splicing of IgM in odTΔNE cannot be explained by an increase in U2AF65 binding to the Py tract.
The novel function of U2AF35 depends on interaction with U2AF65.
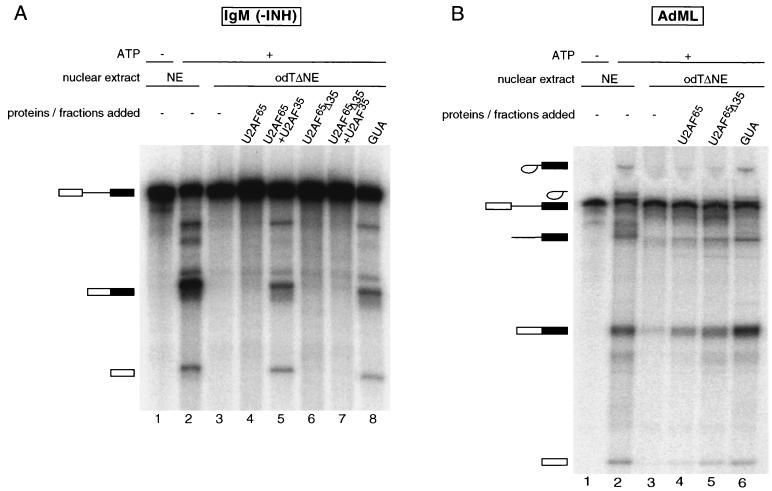
To test whether the activity of U2AF35 was independent of its interaction with U2AF65, the activity of a U2AF65 mutant lacking the interaction domain with U2AF35 (amino acid residues 95 to 138, U2AF65Δ35 [5]) was tested in complementation experiments. Wild-type U2AF65 and U2AF65Δ35 were added to odTΔNE in the presence or absence of U2AF35. IgM splicing was detectable only in the presence of wt U2AF65 and U2AF35 (Fig. 6A, lane 5), not in the presence of U2AF65Δ35 and U2AF35 (Fig. 6A, lane 7). To rule out that U2AF65Δ35 was simply inactive in all assays, we tested the activity of the same protein preparation in complementing odTΔNE for splicing of the AdML pre-mRNA. As shown in Fig. 1A, splicing could be restored by addition of U2AF65 alone (Fig. 6B, lane 4). Importantly, the mutant U2AF65Δ35 was at least as active as the wt protein in this assay (Fig. 6B, lane 5), in contrast with the lack of activity of this mutant in IgM splicing.
FIG. 6.
The function of U2AF35 depends on its interaction with U2AF65. (A) Splicing complementation assay using the IgM pre-mRNA and either 90 nM GST-U2AF65 or a mutant protein lacking the interaction domain with U2AF35 (U2AF65Δ35) and 45 nM His-U2AF35. INH, inhibitory sequence. (B) The U2AF65Δ35 mutant is active in reconstituting AdML splicing. A splicing complementation assay was performed as described for panel A with the AdML pre-mRNA substrate; 13% polyacrylamide gels were used to fractionate the RNAs.
We conclude that the interaction between the two U2AF subunits is essential for IgM splicing, even if the role of this interaction is not to promote U2AF65 binding to the Py tract.
DISCUSSION
Gene-specific requirements for U2AF35.
The gene encoding the Drosophila homologue of U2AF35 (dU2AF38) and its interaction with the large subunit (dU2AF50) are essential in vivo (22, 23). The simplest explanation for the requirement of dU2AF38 is that its absence compromises pre-mRNA processing events. However, attempts to correlate the phenotypic defects of flies containing a viable mutant allele of dU2AF38 with deficiencies in constitutive or regulated splicing of a number of RNAs have failed (22). Our results could serve to reconcile these observations assuming that the essential function of dU2AF38 is related to defects in the splicing of other vital pre-mRNAs. It is conceivable that other U2AF35-like activities (15, 33) could collaborate with U2AF65 in a substrate-specific fashion, similar to the gene-specific activation of transcription mediated by different TATA binding protein-associated factors (reviewed in reference 9).
Biochemical depletion/complementation analyses of U2AF35 function have yielded conflicting results (see the introduction). It seems likely that this is a consequence of the different methods and reagents used to deplete nuclear extracts, the precise reaction conditions, the pre-mRNA substrate used, and the source of recombinant proteins. While chromatographic methods can achieve stronger levels of depletion (data not shown), other poly(U) binding proteins and factors associated with them can be codepleted and change the requirements for complementation in this type of depleted extracts. Suboptimal concentrations of one factor, for example, are more likely to allow detection of additional activities or requirements. In fact, addition of U2AF35 can also improve the complementation of AdML or β-globin splicing when suboptimal amounts of U2AF65 are used (reference 43 and data not shown). The results in Fig. 1D, 3A, and 4, however, suggest that the presence of U2AF35 represents a qualitative rather than a quantitative requirement for splicing of IgM in odT-depleted extracts.
Possible implications for the function of exonic splicing enhancers.
Although the aim of our studies was not to address the molecular mechanism of exon enhancer function, we discuss below possible implications of our results for the function of these sequences, assuming that the specific requirements of our system are related to the presence of the purine-rich element present in exon M2 (37). The currently most detailed model for exon enhancer function proposes that enhancers promote U2AF65 binding to the Py tract by recruiting SR proteins, which can interact with U2AF35 and thus increase the local concentration of U2AF at the 3′ splice site region (43). Although our data are compatible with a requirement for U2AF35 for exon enhancer function, they do not support a U2AF35-mediated increase in U2AF65 binding to the Py tract under splicing conditions (Fig. 5). This is in contrast with results obtained in a reconstituted system containing a different exon enhancer-dependent pre-mRNA (Drosophila doublesex) and limiting amounts of U2AF65, U2AF35, and SR proteins (43). These different results may reflect the capacity of the enhancer to promote different steps in the assembly of splicing complexes as they become rate limiting under different experimental conditions. Py tract-specific U2AF65 binding under splicing conditions may no longer be limiting in our depleted extracts supplemented with significant amounts of exogenously added protein, while binding to the pre-mRNA may admit further stabilization in the reconstituted system of Zuo and Maniatis (43). The concept that combinatorial control of pre-mRNA splicing can be achieved by individual contributions of distinct splicing signals (e.g., Py tract, branch point, and enhancer sequences) is supported by a number of elegant selection experiments (2, 3, 26, 32).
Transcriptional activators have been shown to be able to promote different interactions in preinitiation complex assembly, and this multiplicity of targets may be at the basis of the synergistic effects observed when multiple activators are bound to a promoter region (reviewed in reference 20). Although synergism has not been observed at a particular multisite splicing enhancer (11), it is conceivable that multiple molecular mechanisms of enhancer function operate in different enhancers recognized by different sets of SR proteins (21, 28, 30). In this context, it is worth mentioning that in vivo results have failed to demonstrate a function for U2AF35 in a particular enhancer-dependent splicing event in Drosophila (22, 24).
Role of U2AF35 in spliceosome assembly.
Recently, U2AF35 has been shown to be able to directly assist U2AF65 binding to Py tracts (25). Figures 5C and D show that a protein of ∼35 kDa is cross-linked with the same specificity as U2AF65 to the Py tract, is immunoprecipitated by the anti-U2AF65 antibody, and is absent in odT-depleted extracts. Although this cross-linked species could correspond to U2AF35, the results of Fig. 5D argue that the stimulatory effect of U2AF35 is not related to assisting U2AF65 binding.
This function of U2AF35 after Py tract recognition by U2AF65 is related to prespliceosome formation (Fig. 2 and data not shown) and could reflect either a direct effect in U2 snRNP recruitment or effects in 5′ splice site definition or in 5′-to-3′ splice site communication, for example, through RS domain-mediated interactions with U1 70K or SR proteins like ASF/SF2 or SC35 (38).
Stimulation of U2 snRNP recruitment could also be accomplished by assisting U2AF65 to define the branch point region (34) or by assisting the function of UAP56, a DEAH-box splicing factor with homology to RNA helicases that is recruited by U2AF65 (5). One putative function of UAP56 is to mediate the extensive rearrangements of RNA-RNA duplexes occurring in U2 snRNP during its association with the pre-mRNA and U6 snRNA (reviewed in reference 1). U2AF35 could be important for the stabilization of some of those RNA conformations.
SAP155 is a component of U2 snRNP that has been shown to interact with both U2AF65 and U2AF35 (8). The contribution of the SAP155-U2AF35 interaction could be particularly critical for U2 snRNP recruitment to IgM pre-mRNA.
In any event, detailed investigation of the role of U2AF35 in pre-mRNA splicing is likely to be helped by the robust biochemical assay described in this paper.
ACKNOWLEDGMENTS
We are particularly thankful to Tom Maniatis and Brent Graveley for their generous gifts of reagents, experimental support, insightful discussions, and comments on the manuscript, to Michael Green and Julie Kan for sharing results before publication, experimental advice, the U2AF65Δ35 and IgMΔPy mutants, and comments on the manuscript, and to Iain Mattaj, Oscar Puig, Berthold Rutz, and Bertrand Séraphin for critical reading of the manuscript.
REFERENCES
- 1.Ares M J, Weiser B. Rearrangement of snRNA structure during assembly and function of the spliceosome. Prog Nucleic Acid Res Mol Biol. 1995;50:131–159. doi: 10.1016/s0079-6603(08)60813-2. [DOI] [PubMed] [Google Scholar]
- 2.Buvoli M, Mayer S A, Patton J G. Functional crosstalk between exon enhancers, polypyrimidine tracts and branchpoint sequences. EMBO J. 1997;16:7174–7183. doi: 10.1093/emboj/16.23.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulter L R, Landree M A, Cooper T A. Identification of a new class of exonic splicing enhancers by in vivo selection. Mol Cell Biol. 1995;17:2143–2150. doi: 10.1128/mcb.17.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dignam J, Lebovitz R, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleckner J, Zhang M, Valcárcel J, Green M. U2AF65 recruits a novel human DEAD-box protein required for the U2 snRNP-branchpoint interaction. Genes Dev. 1997;11:1864–1872. doi: 10.1101/gad.11.14.1864. [DOI] [PubMed] [Google Scholar]
- 6.Gama-Carvalho M, Krauss R, Chiang L, Valcárcel J, Green M, Carmo-Fonseca M. Targeting of U2AF65 to sites of active splicing in the nucleus. J Cell Biol. 1997;137:975–987. doi: 10.1083/jcb.137.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur R, Valcárcel J, Green M. Sequential recognition of the pre-mRNA branch point by U2AF65 and a novel spliceosome-associated 28 kDa polypeptide. RNA. 1995;1:407–417. [PMC free article] [PubMed] [Google Scholar]
- 8.Gozani O, Potashkin J, Reed R. A potential role for U2AF-SAP 155 interaction in recruiting U2 snRNP to the branch site. Mol Cell Biol. 1998;18:4752–4760. doi: 10.1128/mcb.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn S. The role of TAFs in RNA polymerase II transcription. Cell. 1998;95:579–582. doi: 10.1016/s0092-8674(00)81625-6. [DOI] [PubMed] [Google Scholar]
- 10.Hemsley A, Arnheim N, Toney M D, Cortopassi G, Galas D J. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989;17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hertel K, Maniatis T. The function of multisite splicing enhancers. Mol Cell. 1998;1:449–455. doi: 10.1016/s1097-2765(00)80045-3. [DOI] [PubMed] [Google Scholar]
- 12.Hodges P, Beggs J. U2 fulfills a commitment. Curr Biol. 1994;4:264–267. doi: 10.1016/s0960-9822(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 13.Kan J, Green M. Pre-mRNA splicing of IgM exons M1 and M2 is directed by a juxtaposed splicing enhancer and inhibitor. Genes Dev. 1999;13:462–471. doi: 10.1101/gad.13.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaar R, Roche S, Beall E, Green M, Rio D. The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science. 1993;262:569–573. doi: 10.1126/science.7692602. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa K, Wang X, Hatada I, Yamaoka T, Nojima H, Inazawa J, Abe T, Mitsuya K, Oshimura M, Murata A, et al. Isolation and mapping of human homologues of an imprinted mouse gene U2af1-rs1. Genomics. 1995;30:257–263. doi: 10.1006/geno.1995.9879. [DOI] [PubMed] [Google Scholar]
- 16.Lavigueur A, LaBranche H, Kornblihtt A, Chabot B. A splicing enhancer in the human fibronectin alternate ED1 exon interacts with SR proteins and stimulates U2 snRNP binding. Genes Dev. 1993;7:2405–2417. doi: 10.1101/gad.7.12a.2405. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y-S, Green M R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991;64:971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- 18.MacMillan A M, McCaw P S, Crispino J D, Sharp P A. SC35-mediated reconstitution of splicing in U2AF-depleted nuclear extract. Proc Natl Acad Sci USA. 1997;94:133–136. doi: 10.1073/pnas.94.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore M, Sharp P. Site-specific modification of pre-mRNA: the 2′ hydroxyl groups at the splice sites. Science. 1992;256:992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- 20.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 21.Ramchatesingh J, Zahler A, Neugebauer K, Roth M, Cooper T. A subset of SR proteins activates splicing of the cardiac troponin T alternative exon by direct interactions with an exonic enhancer. Mol Cell Biol. 1995;15:4898–4907. doi: 10.1128/mcb.15.9.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudner D, Kanaar R, Breger K, Rio D. Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc Natl Acad Sci USA. 1996;93:10333–10337. doi: 10.1073/pnas.93.19.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudner D, Kanaar R, Breger K, Rio D. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol Cell Biol. 1998;18:1765–1773. doi: 10.1128/mcb.18.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudner D, Breger K, Rio D. Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or the small Drosophila subunit is dispensible in vivo. Genes Dev. 1998;12:1010–1021. doi: 10.1101/gad.12.7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudner D, Breger K, Kanaar R, Adams M, Rio D. RNA binding activity of heterodimeric splicing factor U2AF: at least one RS domain is required for high-affinity binding. Mol Cell Biol. 1998c;18:4004–4011. doi: 10.1128/mcb.18.7.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaal T D, Maniatis T. Selection and characterization of pre-mRNA splicing enhancers: identification of novel SR protein-specific enhancer sequences. Mol Cell Biol. 1999;19:1705–1719. doi: 10.1128/mcb.19.3.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh R, Valcárcel J, Green M. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 28.Staknis D, Reed R. SR proteins promote the first specific recognition of pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol Cell Biol. 1994;14:7670–7682. doi: 10.1128/mcb.14.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q, Mayeda A, Hampson R, Krainer A, Rottman F. General splicing factor SF2/ASF promotes alternative splicing by binding to an exonic splicing enhancer. Genes Dev. 1993;7:2598–2608. doi: 10.1101/gad.7.12b.2598. [DOI] [PubMed] [Google Scholar]
- 30.Tacke R, Chen Y, Manley J L. Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: creation of an SRp40-specific splicing enhancer. Proc Natl Acad Sci USA. 1997;94:1148–1153. doi: 10.1073/pnas.94.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 32.Tian H, Kole R. Selection of novel exon recognition elements from a pool of random sequences. Mol Cell Biol. 1995;15:6291–6298. doi: 10.1128/mcb.15.11.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tronchere H, Wang J, Fu X. A protein related to splicing factor U2AF35 that interacts with U2AF65 and SR proteins in splicing of pre-mRNA. Nature. 1997;388:397–400. doi: 10.1038/41137. [DOI] [PubMed] [Google Scholar]
- 34.Valcárcel J, Gaur R, Singh R, Green M. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science. 1996;273:1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 35.Valcárcel J, Martínez C, Green M. Functional analysis of splicing factors and regulators. In: Richter J D, editor. mRNA formation and function. New York, N.Y: Academic Press; 1997. pp. 31–53. [Google Scholar]
- 36.Wang Z, Hoffmann H, Grabowski P. Intrinsic U2AF binding is modulated by exon enhancer signals in parallel with changes in splicing activity. RNA. 1995;1:21–35. [PMC free article] [PubMed] [Google Scholar]
- 37.Watakabe A, Tanaka K, Shimura Y. The role of exon sequences in splice site selection. Genes Dev. 1993;7:407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 39.Zamore P, Green M. Identification, purification and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamore P, Green M. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991;10:207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamore P, Patton J, Green M. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Zamore P, Carmo-Fonseca M, Lamond A, Green M. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc Natl Acad Sci USA. 1992;89:8769–8773. doi: 10.1073/pnas.89.18.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]