Abstract
Plant peptide hormones are important players that control various aspects of the lives of plants. RAPID ALKALINIZATION FACTOR (RALF) peptides have recently emerged as important players in multiple physiological processes. Numerous studies have increased our understanding of the evolutionary processes that shaped the RALF family of peptides. Nevertheless, to date, there is no comprehensive, family-wide functional study on RALF peptides. Here, we analyzed the phylogeny of the proposed multigenic RALF peptide family in the model plant Arabidopsis (Arabidopsis thaliana), ecotype Col-0, and tested a variety of physiological responses triggered by RALFs. Our phylogenetic analysis reveals that two of the previously proposed RALF peptides are not genuine RALF peptides, which leads us to propose a revision to the consensus AtRALF peptide family annotation. We show that the majority of AtRALF peptides, when applied exogenously as synthetic peptides, induce seedling or root growth inhibition and modulate reactive oxygen species (ROS) production in Arabidopsis. Moreover, our findings suggest that alkalinization and growth inhibition are, generally, coupled characteristics of RALF peptides. Additionally, we show that for the majority of the peptides, these responses are genetically dependent on FERONIA, suggesting a pivotal role for this receptor kinase in the perception of multiple RALF peptides.
Synthetic RAPID ALKALINIZATION FACTOR peptides induced a variety of physiological responses, many of which depend on FERONIA, a receptor kinase with functions in development and defense
Introduction
Cell-to-cell communication is crucial for plants, which, as sessile organisms, are constantly exposed to an ever-changing environment. In this scenario, plant peptide hormones are key to rapidly initiate, coordinate, and integrate responses, thanks to their large diversity in structure, function, and expression patterns (Matsubayashi, 2014; Olsson et al., 2019).
RAPID ALKALINIZATION FACTOR (RALF) peptides belong to a family of cysteine-rich plant peptide hormones that are involved in multiple physiological and developmental processes, ranging from pollen tube growth to modulation of immune responses (Murphy and De Smet, 2014; Blackburn et al., 2020). They were discovered in a peptide hormone screen due to their ability to cause medium alkalinization of tobacco (Nicotiana tabacum) cell cultures (Pearce et al., 2001). Later, RALF peptides with several conserved motifs were found to be ubiquitous in terrestrial plants, highlighting their functional importance (Cao and Shi, 2012; Murphy and De Smet, 2014; Campbell and Turner, 2017).
In Arabidopsis (Arabidopsis thaliana), more than 30 RALF peptides have been predicted. In the ecotype Col-0, between 34 and 39 members have been proposed, depending on the study and criteria considered (Olsen et al., 2002; Cao and Shi, 2012; Haruta et al., 2014; Morato do Canto et al., 2014; Sharma et al., 2016; Campbell and Turner, 2017; Stegmann et al., 2017). This discrepancy calls for a careful examination of the RALF family annotation.
The majority of plant peptide hormones are perceived by plasma-membrane localized leucine-rich repeat receptor kinases (LRR-RKs) or receptor proteins (LRR-RPs), which unlike RKs lack an intracellular domain (Hohmann et al., 2017; Olsson et al., 2019). In contrast, RALF peptides have recently been shown to be ligands of protein complexes involving Catharanthus roseus RLK1-LIKE (CrRLK1L) receptor kinases, named after the species in which its first member was identified (Schulze-Muth et al., 1996). CrRLK1L proteins are characterized by malectin-like domains in their extracellular domain (Franck et al., 2018). For example, RALF1, RALF22, and RALF23 bind to the CrRLK1L FERONIA (FER) to regulate root growth, abiotic, and biotic stress responses, respectively (Haruta et al., 2014; Stegmann et al., 2017; Zhao et al., 2018). RALF4 and RALF19 were shown to be ligands for the CrRLK1Ls ANXUR1 (ANX1), ANX2, and BUDDHA’S PAPER SEAL (BUPS) 1 and BUPS2 in the context of pollen tube growth and cell wall integrity maintenance (Ge et al., 2017). RALF34 binds the CrRLK1L THESEUS1 (THE1) to regulate growth upon cellulose biosynthesis inhibition (Gonneau et al., 2018). Notably, CrRLK1Ls, such as FER, ANX1/ANX2, and BUPS1/2, have been shown to work together with the glycosylphosphatidylinositol-anchored protein (GPI-AP) LORELEI or related (LRE)-like-GPI-anchored proteins (LLGs) to perceive RALF peptides (Feng et al., 2019; Ge et al., 2019; Xiao et al. 2019). RALF peptides can also bind LEUCINE-RICH REPEAT EXTENSINS (LRX) proteins with high affinity (Mecchia et al., 2017; Zhao et al., 2018; Moussu et al., 2020). The biochemical relationship between RALF perception by CrRLK1L/LLG complexes and LRXs remains, however, still mostly unclear.
There are 17 CrRLK1Ls, 11 LRXs, and 4 LRE/LLGs in Arabidopsis (Li et al., 2015; Franck et al., 2018; Herger et al., 2020). As such, the diversity of potential assembly modules of the RALF-perception/signaling axis could explain the functional plasticity of this family of peptides. For instance, different RALF peptides might be secreted in response to diverse stimuli in different tissues and cell types and this, in turn, would trigger the formation of receptor complexes with a combination of the above-mentioned assembly modules. There are numerous studies analyzing individual aspects of this complex signaling network (Blackburn et al., 2020). However, the functional role of the majority of RALF peptides and their bioactivity is largely unknown. In this study, we performed a family-wide physiological analysis of AtRALF peptides using seedling growth inhibition, root growth inhibition, extracellular alkalinization, and ROS production assays upon exogenous treatment with synthetic peptides. In addition to defining the core Arabidopsis Col-0 RALF family, we show that FER is required for full responsiveness to multiple RALF peptides, suggesting a pivotal role of this receptor, potentially in multiple RALF sensory complexes.
Results
Re-annotation of the Arabidopsis Col-0 RALF family
The AtRALF peptide family consists of more than 30 members, ranging from 34 to 39, depending on the studies considered (Blackburn et al., 2020). In order to define the core AtRALF family in the Col-0 ecotype, we compared the AtRALF peptide annotation used in different publications and those available in the TAIR10 and UniProt databases (Supplemental Table S1). Notably, we found some inconsistency both in the number and identity of the proteins that comprise the family.
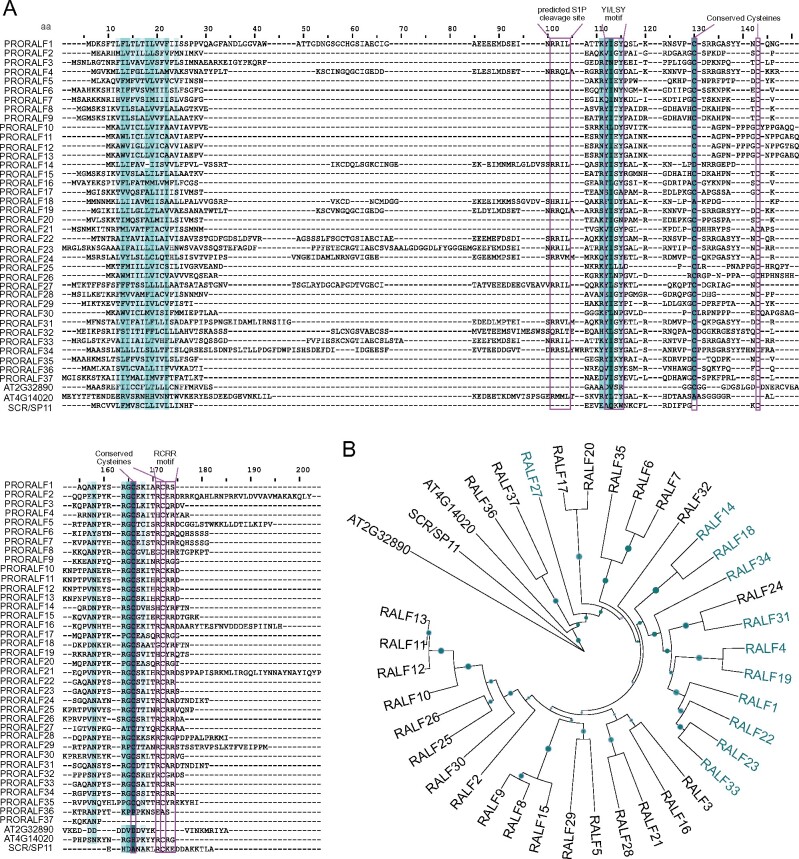
Despite having low level of amino acid sequence similarity, RALF peptides have diverse conserved motifs that have been shown to be important for diverse functions (Pearce et al., 2001, 2010; Matos et al., 2008; Srivastava et al., 2009; Stegmann et al., 2017). One such motif is the dibasic RR site in the canonical AtS1P recognition site that is important for processing of the peptide precursor (residues 101–102; Figure 1, A; Matos et al., 2008; Srivastava et al., 2009). Another conserved motif is the N-terminal YI/LSY motif (residues 112–115 of the alignment shown in Figure 1, A) of the mature peptide that is important for binding to the receptor and alkalinization activity (Pearce et al., 2010; Xiao et al., 2019). Additionally, other parts of the sequences show high conservation, such as the C-terminal RCRR(S) motif or the four disulfide bond-forming cysteine residues (residues 171–174, 130, 143, 166, and 172, respectively; Figure 1, A) that are important for bioactivity of the peptide (Pearce et al., 2001; Haruta et al., 2008). We validated the importance of the YI/LSY motif and the four conserved cysteine residues by treating seedlings with corresponding alanine substitution mutant RALF peptides (Supplemental Table S2) that did not inhibit (or weakly inhibited) seedling growth, in contrast to what observed for wild-type peptides (Supplemental Figure S1), in agreement with previous studies (Pearce et al., 2001; Haruta et al., 2008; Moussu et al., 2020). Using the domain conservation as the main criteria, we re-analyzed the published RALF family members and propose the following updates to the annotation.
Figure 1.
Re-evaluation of the Arabidopsis RALF family. A, Alignment of AtPRORALFs, AT2G32890, and AT4G14020. Color-code based on sequence conservation: the darker the color, the more conserved the residue. Pink boxes indicate conserved motifs. B, Rooted phylogenetic tree of the AtRALF peptides, AT2G32890 and AT4G14020. UPGMA tree inferred from the MUSCLE alignment displayed in Figure 1, A. S-locus protein 11 or S-locus Cys-rich (SCR/SP11) sequence was used to root the tree. RALFs highlighted in teal indicate those predicted to be cleaved by the protease S1P. Bootstrap values (1,000 repetitions) above 50% are represented by green circles in the corresponding branches. The higher the bootstrap value for a particular branch, the higher the size of the circle.
RALF17 is described as AT2G32890 in the aforementioned databases, while it corresponds to AT2G32885 in some publications (Cao and Shi, 2012; Morato do Canto et al., 2014; Stegmann et al., 2017). Protein sequence analysis, however, revealed that AT2G32890 lacks the YI/LSY motif, including the conserved internal I/L residues (Figure 1, A). AT2G32890 additionally lacks the conserved RCRR motif (Figure 1, A), and notably also misses three out of four cysteine residues that are positionally conserved across the RALF family (Figure 1, A). The phylogenetic tree inferred from the alignment of RALF amino acid sequences places AT2G32890 as an outgroup (Figure 1, B). Accordingly, the synthetic peptide derived from AT2G32890 did not exhibit bioactivity in seedling growth inhibition assays even at 10 µM (Supplemental Figure S2). Altogether, we conclude that AT2G32890 is most likely not a RALF peptide.
When searching for AT2G32885 (proposed in some publications as RALF17) in TAIR10 and UniProt databases, we observed that this protein is annotated as RALF36. However, in other publications, RALF36 corresponds to AT2G32785 (Morato do Canto et al., 2014; Stegmann et al., 2017; Gjetting et al., 2020). If AT2G32890 is not a RALF peptide as argued above, based on our phylogeny, we propose that AT2G32885 actually corresponds to RALF17. This agrees with previous studies (Morato do Canto et al., 2014; Stegmann et al., 2017). In contrast, we propose to assign AT2G32785 to RALF36 (Supplemental Table S1). It has part of the YI/LSY motif and the first cysteine bridge is conserved, although it lacks the C-terminal RCRR motif (Figure 1, A). Phylogenetic analysis indicates that it is, however, a distant RALF, clustering with RALF37 (Figure 1, B). Furthermore, based on the information inferred from the alignment and the phylogenetic tree, it is difficult to definitively conclude whether RALF36 (AT2G32785) and RALF37 (AT2G32788) are genuine RALF peptides. As they present some of the important conserved residues, we nevertheless included them in our list of core RALF family members (Supplemental Table S1 and Supplemental Figure S3).
RALF35 has two gene identifiers associated with it: AT4G14020 and AT1G60913 (Haruta et al., 2014; Morato do Canto et al., 2014; Stegmann et al., 2017). Both have the highly conserved I/L amino acid within the YI/LSY motif, as well as the two flanking tyrosine residues (Figure 1, A). However, only AT1G60913 (as RALF35 in Figure 1, A) contains the four cysteine residues in the conserved positions, while AT4G14020 has only one cysteine at a conserved position. Moreover, both AT4G14020 and AT1G60913 have part of the conserved RCRR motif. Additionally, AT4G14020 lacks a predicted signal peptide, and is thus likely non-secreted; it also does not cluster together with any other RALF in phylogenetic analysis (Figure 1, B). Together, this indicates that AT4G14020 is not a bona fide RALF peptide (Supplemental Table S1). Unfortunately, AT4G14020 could never be successfully synthesized to be further tested in physiological assays, probably due to its long sequence.
Like other plant genes encoding polypeptide hormones, PRORALF genes encode pre-pro-peptides of approximately 60–140 amino acids, which are predicted to undergo proteolytic processing to release bioactive RALF peptides (Supplemental Figure S3, C; Olsson et al., 2019). PRORALF proteins have a N-terminal signal peptide for entry into the secretory pathway, and the mature active peptide is located at the C-terminal part (Matos et al., 2008). Only 11 PRORALF proteins actually have a predicted subtilase cleavage site (RRXL, residues 101–104; Figure 1, A), and, so far, only PRORALF23 and PRORALF22 have been experimentally shown to be cleaved by the subtilase SITE-1 PROTEASE (S1P) (Srivastava et al., 2009; Stegmann et al., 2017; Zhao et al., 2018). The protein domain organization of the PRORALF peptides that do not have the cleavage site suggests that they might not need to undergo subtilase-mediated proteolytic cleavage in order to release bioactive RALF peptides. Based on our proposed re-annotation of the AtRALF family (Supplemental Figure S3, A and B), we depict the protein domain organization of the corresponding PRORALF proteins in Supplemental Figure S3, C.
The majority of AtRALF peptides have growth inhibitory properties
One of the described functions of RALF peptides is their ability to inhibit cell expansion and growth (Blackburn et al., 2020), but this is based on the testing of only a few family members (Morato do Canto et al., 2014). Here, we screened 34 AtRALF peptides for their bioactivity using seedling and root growth inhibition as read-outs (Figure 2). RALF11 and RALF12 were not synthesized because of their high sequence similarity with RALF13 (identical mature peptide), while RALF37 could never be successfully synthesized, despite several attempts.
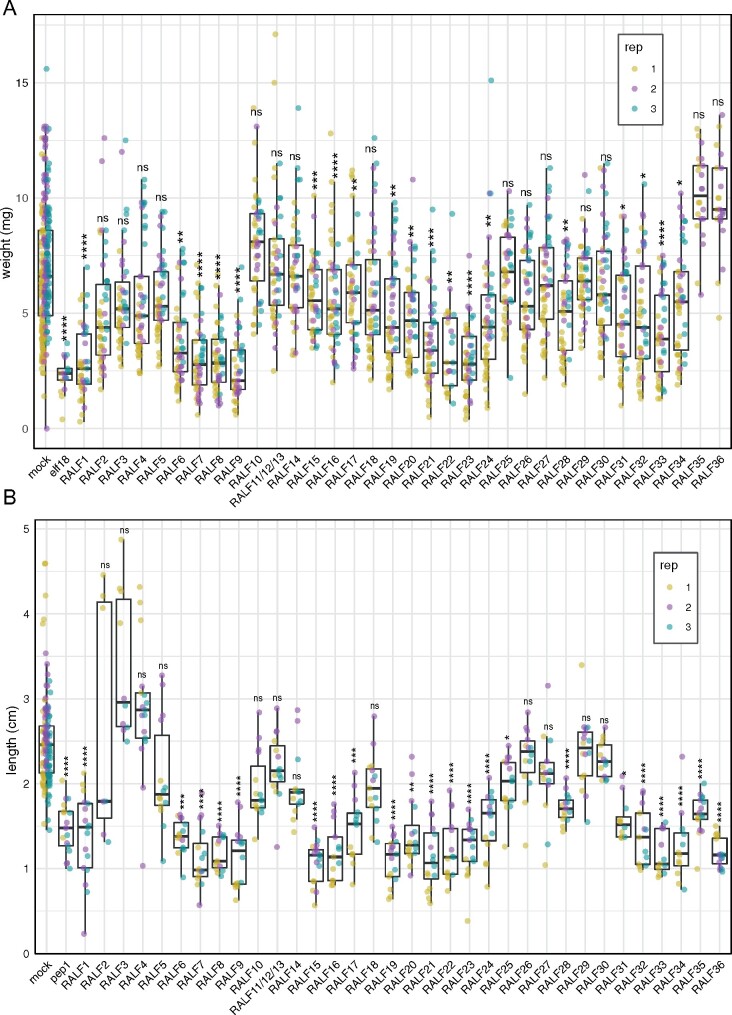
Figure 2.
Effect of AtRALF peptides on seedling and root growth inhibition. A, Fresh weight of 12-d-old seedlings grown in the absence (mock) or presence of 1 µM RALF peptides (n = 12) using elf18 (100 nM) as control. B, Primary root length of 8-d-old seedlings grown in the absence (mock) or presence of 2 µM RALF peptides (n = 6) using Atpep1 (10 nM) as positive control. A and B, Data from three independent experiments are shown (colors indicate different replicates). Upper and lower whiskers represent 1.5 times and −1.5 times interquartile range; upper and lower hinges represent 25% and 75% quartiles; middle represents median or 50% quartile. Asterisks indicate significance levels of a Kruskal–Wallis’ multiple comparison test, each treatment was compared with its corresponding mock: ns (P-value > 0.05), *(P-value ≤ 0.05), **(P-value ≤ 0.01), ***(P-value ≤ 0.001), and ****(P-value ≤ 0.0001).
We treated 5-d-old seedlings with different synthetic RALF peptides (Supplemental Table S2) for 7 d before measuring seedling fresh weight or root length. We used the EF-Tu-derived peptide elf18 and the plant-derived peptide AtPep1 as positive controls for the seedling and root growth inhibition assays, respectively (Zipfel et al., 2006; Krol et al., 2010). Nineteen out of 34 (approximately 56%) tested RALF peptides showed a significant seedling growth inhibition in three independent biological experiments (Figure 2, A).
AtPRORALF genes and genes encoding proposed receptor modules have diverse expression patterns (Cao and Shi, 2012; Lindner et al., 2012; Murphy and De Smet, 2014), and seedling fresh weight is primarily determined by shoot biomass. It is therefore possible that no inhibition in the overall weight of the seedling may be observed if the receptor of a specific RALF peptide is only expressed in roots. For this reason, we also measured the primary root length after treatment with the different peptides. Strikingly, 22 out of 34 (approximately 65%) of the tested RALF peptides were also able to induce root growth inhibition (Figure 2, B). The 19 RALF peptides that inhibited whole seedling growth were also able to inhibit root growth, while RALF25, RALF35, and RALF36 inhibited only root growth. These data show that the majority of exogenously applied RALF peptides have the ability to inhibit growth under the conditions tested.
The majority of growth-inhibitory AtRALF peptides are FER-dependent
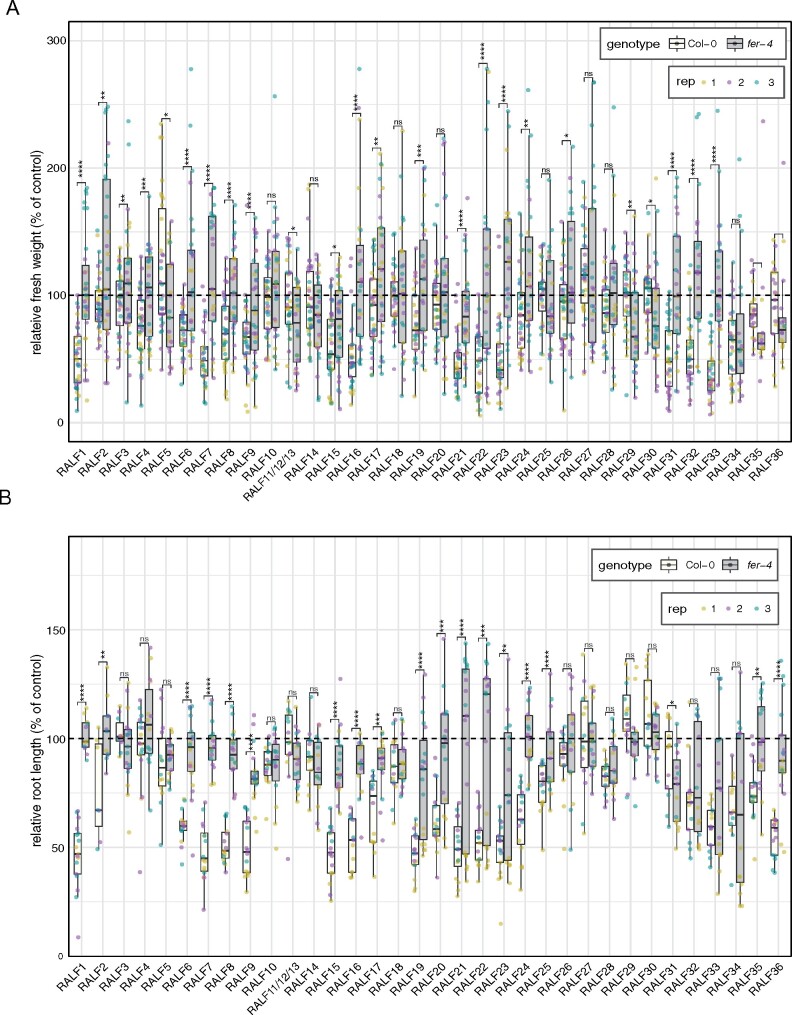
There are 17 CrRLK1L members in Arabidopsis playing multiple and diverse roles, including in cell growth, reproduction, and responses to the environment (Franck et al., 2018; Blackburn et al., 2020). FER, the best characterized member of the family, is expressed throughout the plant, and has already been shown to mediate recognition of RALF1, RALF22, RALF23, and RALF33 in diverse physiological contexts (Haruta et al., 2014; Stegmann et al., 2017; Zhao et al., 2018; Liu et al., 2021). As such, we performed seedling and root growth inhibition assays in the knock-out mutant fer-4 in comparison with Col-0 (Figure 3). Surprisingly, fer-4 mutant seedlings were insensitive to 16 out of 19 (84%) RALF peptides that inhibited seedling growth in Col-0 (Figures 2, A, 3, A). In the case of root growth inhibition, fer-4 mutant seedlings were insensitive to 18 out of 22 (approximately 82%) RALF peptides that inhibited root length in Col-0 (Figures 2, B, 3, B). The FER-independent RALF peptides that coincide between both assays are RALF28 and RALF34, which have the same effect in Col-0 and in the mutant line fer-4 (Figure 3). In comparison, RALF20 is still able to mildly inhibit seedling growth in fer-4 background, but its root growth inhibitory effect depends on FER (Figure 3). Interestingly, RALF32 and RALF33 are FER-dependent in the seedling growth inhibition assay, but are still able to inhibit root length in the mutant line fer-4 (Figure 3).
Figure 3.
FER dependency of AtRALF peptides in inducing growth inhibition. A, Fresh weight of 12-d-old seedlings grown in the presence of 1 µM RALF peptides relative to mock treatment. B, Primary root length of 8-d-old seedlings grown in the presence of 2 µM RALF peptides relative to mock treatment. Mock-treated primary root length of Col-0 (white) and fer-4 (grey) correspond to 100% on the y-axis. A and B, Data from three independent repetitions are shown (colors indicate different replicates). Upper and lower whiskers represent 1.5 times and −1.5 times interquartile range; upper and lower hinges represent 25% and 75% quartiles; and middle represents median or 50% quartile. Asterisks indicate significance levels of a two-tailed t test comparing each treatment in fer-4 to the corresponding in Col-0: ns (P-value >0.05), *(P-value ≤ 0.05), **(P-value ≤ 0.01), ***(P-value ≤ 0.001), and ****(P-value ≤ 0.0001).
It has been previously shown that fer-4 is still able to respond to RALF1 when applied at higher concentrations (Campos et al., 2018). To test that the loss of responsiveness that we observed for the majority of the RALF peptides in fer-4 still occurs at higher concentrations, we repeated the assay with RALF peptides that were active in Col-0 at 1 µM and whose response was lost in the fer-4 mutant in seedling growth inhibition assays (Figure 3, A), using 10 µM instead. Although fer-4 seedlings show sensitivity to most of the RALF tested at 10 µM, there was a significant reduction in the activity of all RALF peptides tested when compared with Col-0 (Supplemental Figure S4, A).
Altogether, our data indicate that FER is involved in the perception and/or signaling pathway of the majority of the RALF peptides tested in the context of seedling and root growth inhibition. Additionally, it is known that RALF23 is perceived by a heteromeric complex composed of LLG1 in combination with FER (Xiao et al., 2019). The residues mediating the interaction of RALF23 with LLG1 are conserved in related RALF peptides belonging to subfamily 1, which suggests that these peptides may also interact with LLG proteins (Xiao et al., 2019). For this reason, we performed seedling growth inhibition assays in the knock-out mutant llg1-2 (Supplemental Figure S4, B). The majority of the predicted LLG1-dependent RALF peptides (indicated in bold) showed a reduced activity in llg1-2 mutant seedlings (Supplemental Figure S4, B). Nevertheless, some RALF peptides with conserved LLG1-binding residues, such as RALF32, did not show a significant reduction in fresh weight in llg1-2 (Supplemental Figure S4, B). Additionally, other RALF peptides that are not predicted to bind LLG proteins also showed a significant reduction in their activity when compared with Col-0, such as RALF8, RALF9, RALF15, and RALF17, which suggests that LLG1 also plays a role in their perception (Supplemental Figure S4, B).
Alkalinization and growth inhibition properties correspond in a FER-dependent manner
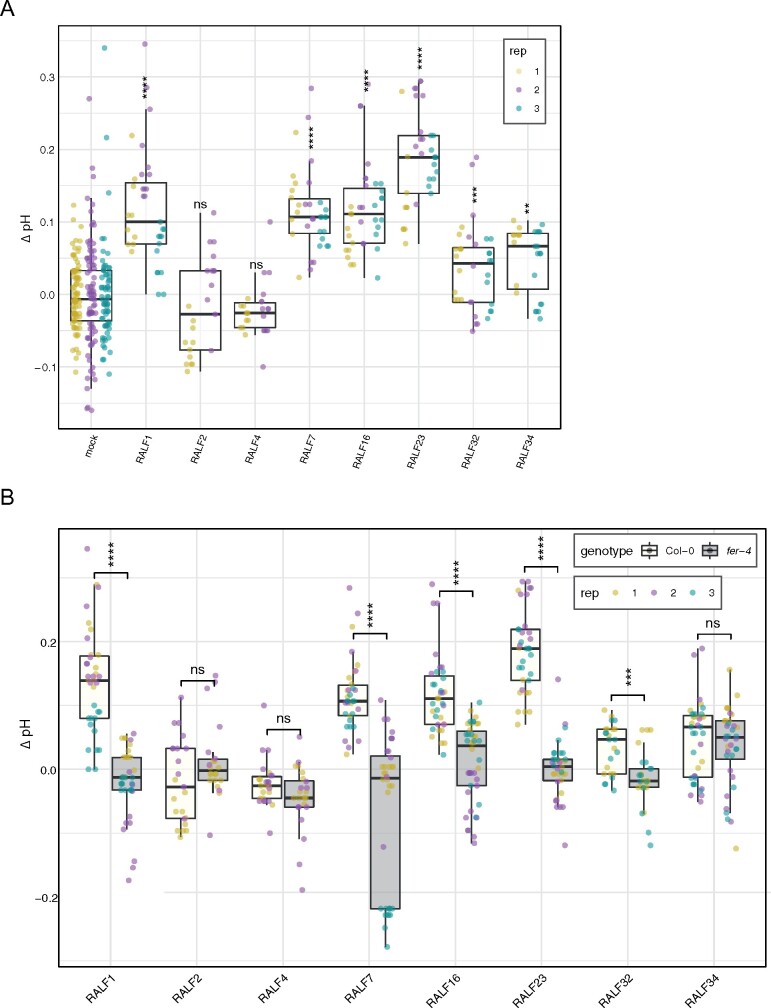
Another characteristic of RALF peptides is their ability to alkalinize the extracellular medium (Blackburn et al., 2020). For instance, it was previously shown that eight out of nine recombinant RALF peptides tested were able to alkalinize the Arabidopsis cell suspension medium (Morato do Canto et al., 2014). To test if the bioactivity we observed for the majority of RALF peptides is transferable to other typical physiological reactions induced by RALF peptides, we also performed alkalinization assays. Six out of eight RALF peptides tested were able to alkalinize the medium (Figure 4, A). These data correspond with the RALF bioactivity shown in the seedling and root growth inhibition assays in which RALF1, RALF7, RALF16, and RALF23 had a strong growth inhibition, RALF2 and RALF4 were inactive, and RALF32 and RALF34 had a weaker growth inhibition. In order to assess whether the alkalinization activity of the bioactive RALF peptides was FER-dependent, we performed the assay comparing the alkalinization of the media of fer-4 and Col-0 seedlings. Indeed, the alkalinization activity of RALF1, RALF7, RALF16, RALF23, or RALF32 was lost in fer-4 mutant seedlings, while the alkalinization activity of RALF34 seemed FER-independent, in agreement with the seedling and root growth inhibition data (Figures 3, 4, B). These results further demonstrate the importance of FER in the perception/signaling of these RALF peptides. Additionally, these findings suggest that alkalinization and growth inhibition are coupled characteristics of RALF peptides. Interestingly, media of fer-4 mutant seedlings was more alkaline than the media of Col-0 seedlings (Supplemental Figure S5).
Figure 4.
Alkalinization and growth inhibition properties correspond in a FER-dependent manner. A, Change in pH (ΔpH) measurements of 7-d-old seedlings recorded 4 h after addition of RALF peptides. Asterisks indicate significance levels of a two-tailed t test in which each treatment was compared with the corresponding mock treatment. B, Change in pH measurements of 7-d-old seedlings recorded 4 h after addition of RALF peptides relative to mock treatment. Col-0 data are shown in white and fer-4 data in gray. A and B, Data from three independent repetitions are shown (colors indicate different replicates). Upper and lower whiskers represent 1.5 times and −1.5 times interquartile range; upper and lower hinges represent 25% and 75% quartiles; and middle represents median or 50% quartile. Asterisks indicate significance levels of a two-tailed t test comparing each treatment in fer-4 to the corresponding in Col-0: ns (P-value > 0.05), **(P-value ≤ 0.01), ***(P-value ≤ 0.001), and ****(P-value ≤ 0.0001).
The majority of predicted RALF peptides are able to modulate elf18-induced ROS production
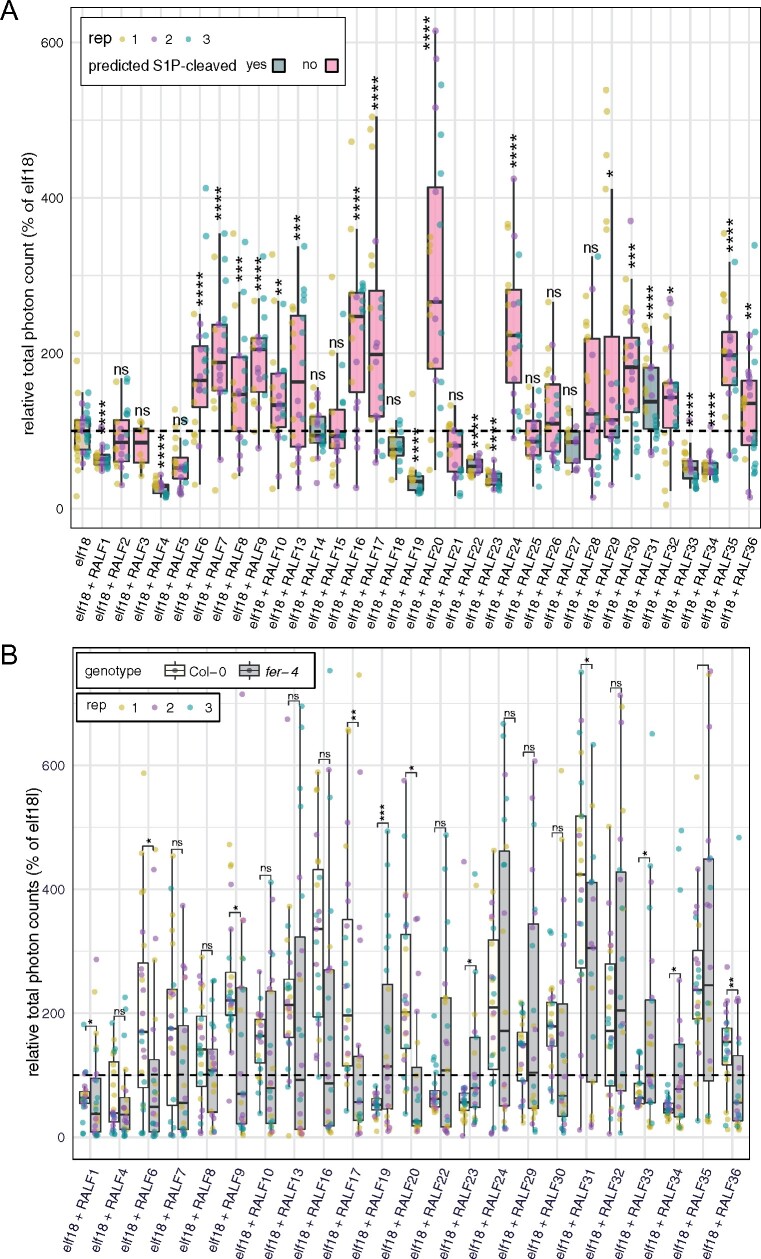
Several RALF peptides have been shown to modulate immune responses (Stegmann et al., 2017). Notably, the predicted S1P-cleaved RALF peptides RALF23, RALF33, and RALF34 inhibited elf18-induced ROS production (Stegmann et al., 2017). In contrast, treatment with a RALF peptide lacking a predicted S1P cleavage site, RALF17, induced ROS production in an additive manner to elf18 and was sufficient to induce resistance to bacterium Pseudomonas syringae pv tomato DC3000 (Stegmann et al., 2017). In order to test whether this observed dichotomy was applicable to the whole family, we co-treated Arabidopsis leaf discs with elf18 and 34 different individual RALF peptides and measured ROS production (Figure 5, A and Supplemental Figures S6, S7). In addition to the previously mentioned RALF peptides containing the predicted S1P-cleavage site, RALF1, RALF4, RALF19, and RALF22 were also able to significantly reduce elf18-induced ROS production (Figure 5, A and Supplemental Figures S6, S7, A). On the other hand, the majority of non-cleaved RALF peptides could significantly increase elf18-induced ROS production, as previously shown for RALF17 (Figure 5, A and Supplemental Figures S6, S7, A). We also investigated whether RALF peptides alone could be sufficient to trigger ROS production (Supplemental Figures S8, A, S9, S10, A). Several RALF peptides were able to induce a significant ROS production (Supplemental Figures S8, A, S9, S10, A) independent of the presence of a predicted S1P cleavage site (Figure 1). In fact, the majority of the RALF peptides that induced ROS production were also able to increase elf18-induced ROS production, with the exception of RALF14, RALF18, and RALF22, which did not increase elf18-induced ROS production (Figure 5, A).
Figure 5.
Effect of predicted AtRALF peptides on ROS production in Col-0 and fer-4. A, ROS production in Col-0 leaf discs co-treated with 100 nM elf18 and 1 µM RALF peptides. In blue, box-plots of predicted S1P-cleaved RALF peptides and in pink, box plots of non-cleaved RALF peptides. Asterisks indicate significance levels of a one-way ANOVA followed by a Dunnet’s test comparing each treatment to elf18. B, ROS production in Col-0 and fer-4 leaf discs co-treated with 100 nM elf18 and 1 µM RALF peptides. Col-0 data are shown in white and fer-4 data in gray. Asterisks indicate significance levels of a two-tailed t test comparing each treatment in fer-4 to the corresponding treatment in Col-0. A and B, Values are means of total photon counts over 45 min relative to mock data. elf18 ROS data correspond to 100% in the y-axis (dashed lines). Upper and lower whiskers represent 1.5 times and −1.5 times interquartile range; upper and lower hinges represent 25% and 75% quartiles; and middle represents median or 50% quartile. Data from three independent repetitions are shown (colors indicate different replicates). Asterisk significance: ns (P-value >0.05), *(P-value ≤ 0.05), **(P-value ≤ 0.01), ***(P-value ≤ 0.001), and ****(P-value ≤ 0.0001).
It was previously shown that the RALF23- and RALF17-induced modulation of elf18-triggered ROS production was FER-dependent (Stegmann et al., 2017). In order to test for FER dependence, we repeated the assays for those RALF peptides that induced ROS by themselves or had an effect on elf18-induced ROS production using Col-0 and fer-4 plants. The effect of approximately half of the RALF peptides that modulated elf18-induced ROS responses was significantly reduced in fer-4 plants (Figure 5, B and Supplemental Figures S7, B, S11). The effect of other RALF peptides such as RALF7, RALF10, or RALF16, although not significant, appeared reduced in fer-4 mutant plants (Figure 5, B and Supplemental Figures S7, B, S11). Additionally, fer-4 mutant plants were insensitive to treatment with the majority of RALF peptides that induced ROS responses in Col-0 on their own (Supplemental Figures S6, B, S10, B, S12). These data illustrate the putative function of some of the RALF peptides as modulators of immunity and corroborate the importance of FER in the signaling pathway of the majority of AtRALF peptides. Future genetic and biochemical investigations will allow us to further understand to which extent these peptides are involved in immunity in Arabidopsis and how this is mechanistically orchestrated.
Discussion and conclusions
Several studies on the RALF peptide family in different plant species have generated extensive knowledge about their roles and functions (Blackburn et al., 2020). Yet, even in the model plant Arabidopsis, there is no consensus about the exact AtRALF family composition (Olsen et al., 2002; Cao and Shi, 2012; Morato do Canto et al., 2014; Sharma et al., 2016; Campbell and Turner, 2017). Here, we gathered information from previous publications and databases to perform phylogenetic analyses of AtRALF isoforms and propose a revised consensus annotation (Supplemental Table S1 and Supplemental Figure S3). By focusing on the model plant Arabidopsis, we wanted to provide a more accurate annotation of the RALF peptide family rather than trying to provide evolutionary information on the family, as has been previously done (Cao and Shi, 2012; Sharma et al., 2016; Campbell and Turner, 2017). Based on previously identified conserved motifs (Figure 1 and Supplemental Figure S1; Pearce et al., 2001, 2010; Olsen et al., 2002; Matos et al., 2008; Cao and Shi, 2012; Xiao et al., 2019; Moussu et al., 2020), we conclude that AT2G32890 and AT4G14020 are not genuine RALF peptides. AT2G32890 was probably mis-annotated in the family due to its close proximity on Chromosome 2 with the genuine RALF17 (AT2G32885). In turn, AT4G14020 was probably annotated in the family as a result of some degree of sequence similarity. It has been previously shown that tandem duplications played a dominant role in the evolution of Arabidopsis RALF peptides (Cao and Shi, 2012; Campbell and Turner, 2017). Specifically, RALF peptides of the Brassicaceae family experienced a rapid expansion since the size of the genome does not correspond with the number of RALF genes (Campbell and Turner, 2017). It is possible that some of the recently duplicated genes have evolved under positive selection, causing changes in the protein sequence, which could explain why some of the proposed RALF peptides lack some of the otherwise conserved motifs. Nevertheless, it has been shown that the majority of RALF sequences have evolved under strong purifying selection and that tandem duplication has played a dominant role in the expansion of AtRALF peptides (Campbell and Turner, 2017). For example, RALF11, RALF12, and RALF13, which form the largest cluster of AtRALF peptides together with RALF10, have identical mature amino acid sequences, indicating that they maintain the same function and that they might result from recent tandem duplication events (Campbell and Turner, 2017). The RALF peptide family has been divided into four major clades depending on the variation of the mature, functional peptide sequence (Campbell and Turner, 2017). Clade IV (which represents around two-third of AtRALF dataset) was found to be the more distinct clade due to substantial differences within the peptide sequences. Notably, AT2G32890 and AT4G14020 were annotated as part of clade IV, consistent with their lack of domain conservation. Nonetheless, there are other RALF peptides, which, despite belonging to clade IV, are still bioactive, as shown for RALF8, RALF9, or RALF15 (Figures 2, 3), or are inactive despite harboring conserved domains, such as RALF2 or RALF13 (Figure 1).
Our results show that the majority of RALF peptides induce inhibitory effects on seedling fresh weight and primary root length when exogenously applied (Figure 2). This result is consistent with a previous study that showed biological activity for nine recombinant RALF peptides (Morato do Canto et al., 2014). Strikingly, despite being closely related and varying only in seven amino acids, RALF19, but not RALF4, induced growth inhibition (Figure 2). RALF19 and RALF4 were previously tested for alkalinization activity, which RALF19 possesses but RALF4 does not (Morato do Canto et al., 2014). This indicates that the growth inhibition activity is linked to the ability of these peptides to increase the pH of the extracellular space. Our results support this hypothesis since the growth inhibition activity seems to correspond with the capacity to alkalinize the medium (Figures 2, 4). In fact, treatment with RALF1 suppresses cell elongation of the primary root by activating FER, which in turns causes the phosphorylation of the plasma membrane H+-ADENOSINE TRIPHOSPHATASE 2 (AHA2), which inhibits proton transport (Haruta et al., 2014). Whether this pathway is transferable to the rest of the family remains elusive. Interestingly, it was shown that BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1 (BAK1) and (CALMODULIN-LIKE 38) CML38 are required for the growth inhibitory ability of RALF1 but not for the alkalinization, which suggested an uncoupling of these two biological activities (Dressano et al., 2017; Campos et al., 2018). It has been suggested that BAK1-LIKE KINASE 1 (BKK1), a paralog of BAK1, could replace BAK1 function only in the ion-flux response but not in the root inhibition assay (Dressano et al., 2017), but this requires further testing. Interestingly, we observed that the bathing medium of fer-4 seedlings is more alkaline than the medium of Col-0 seedlings (Supplemental Figure S5), which does not agree with previous data showing, rather, that fer-4 seedlings acidify the medium faster than Col-0, according to the hypothesis that the H+-ATPase activity of AHA2 is constitutively up-regulated in fer-4 (Haruta et al., 2014). Overall, future work using the whole family of RALF peptides should determine the unclear and complex link between pH and growth.
Our results show that several RALF peptides have the ability to modulate elf18-induced ROS production, as shown before for the S1P-cleaved and non-cleaved peptides RALF23 and RALF17, respectively (Stegmann et al., 2017). It was previously hypothesized that predicted S1P-cleaved RALF peptides would inhibit immune responses due to the negative role of S1P in immunity (Stegmann et al., 2017), and, in turn, that non-cleaved RALF peptides could play a positive role in immunity by potentiating PAMP-induced responses. Here, we show that this dichotomy is true for the majority of the cases, although some exemptions exist, such as RALF31 (a predicted S1P-cleaved RALF), which increases elf18-induced ROS production, or RALF14 and 27 that do not have any effect despite having the predicted S1P cleavage site (Figures 1, 5).
Overall, our data reveal that most of RALF peptides that showed bioactivity in the seedling and root growth inhibition assays were also able to modulate elf18-induced ROS production in a negative or positive way (Figure 6). In addition, several RALF peptides, which were inactive in seedling and root growth inhibition assays, did not induce or modulate ROS production (Figure 6). This could be explained by the fact that these peptides might not be fully folded in our experiments—as we use synthetic peptides—and thus that a higher concentration could be required, or that these RALF peptides do not trigger any of the responses tested. Another explanation could be that the perception of these RALF peptides is confined to a specific tissue and/or cell type. For example, RALF23 and RALF33 are able to induce ROS production in the stigma papillae in a FER and ANJEA (ANJ) dependent manner (Liu et al., 2021), while RALF23 and RALF33 did not induce ROS production in leaves of 4-week-old plants (Supplemental Figure S8), contrasting activity in different tissues, which could be explained by different heteromeric receptor complexes being involved. Still, both RALF peptides suppressed elf18-induced ROS production in a FER-dependent manner (Figure 5). This example illustrates the functional complexity and specificity of the signaling of these peptides.
Figure 6.
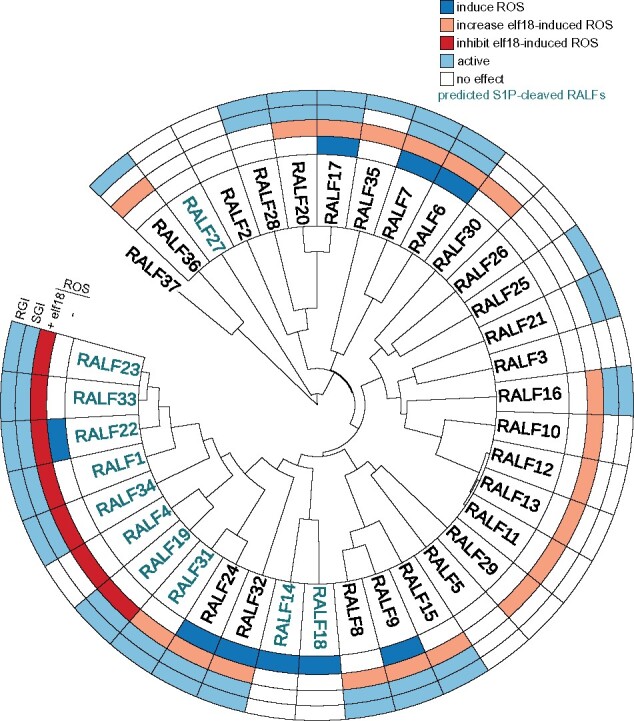
Graphical summary of AtRALF peptides activities in Col-0. Phylogenetic rooted tree of the AtRALF peptides. RALFs highlighted in blue indicate those predicted to be cleaved by the protease S1P. Each circular layer represents one assay. Colored boxes indicate effect of RALF peptides.
FER is the best studied member of the CrRLK1L family and has been shown to be involved in numerous physiological processes (Franck et al., 2018; Blackburn et al., 2020). FER was recently shown to recognize diverse RALF peptides, such as RALF1, RALF22, RALF23, and RALF33 (Haruta et al., 2014; Stegmann et al., 2017; Zhao et al., 2018; Xiao et al., 2019; Liu et al., 2021). Here, we show that FER is required for the inhibitory activity of the majority of RALF peptides in the context of growth inhibition (Figure 3) and alkalinization activity (Figure 4). Additionally, fer-4 seedlings were insensitive to 8 out of 10 RALF peptides that induced ROS production in Col-0 (Supplementary Figure S8, B). However, approximately half of the RALF peptides that were able to modulate elf18-induced ROS production did so in a FER-dependent manner. As mentioned above, RALF peptides are predicted to have distinct and specific expression patterns, and only some of them are predicted to be expressed in vegetative tissues (Cao and Shi, 2012). Therefore, future genetic investigations guided by the expression patterns are required to decipher the role of some of the RALF peptides as immunity modulators. FER is widely expressed throughout the plant (Lindner et al., 2012). As our assays rely on the exogenous treatment with synthetic RALF peptides, our results do not necessarily imply that FER is the primary receptor for the corresponding endogenous RALF peptides. Nevertheless, it is interesting to notice that not all RALF peptides that inhibit growth or modulate elf18-induced ROS production are FER dependent. Additionally, some RALF peptides, such as RALF32 and RALF33, are FER dependent for seedling growth inhibition and modulation of ROS production (in the case of RALF33), but are still able to inhibit root growth in fer-4 (Figures 3, 5). This suggests that these RALF peptides might be perceived by different heteromeric receptor complexes in the root and in the shoot. Notably, different CrRLK1Ls have recently been shown to work together as part of hetero-multimeric protein complexes to mediate RALF perception or control RALF-regulated processes. For example, RALF4 and 19 are proposed to be perceived by a complex involving ANX1/2 and BUPS1/2 to control pollen tube growth and cell wall integrity maintenance (Ge et al., 2017), while FER, ANJ, and HERCULES RECEPTOR KINASE 1 (HERK1) regulate pollen tube reception (Escobar-Restrepo et al., 2007; Galindo‐Trigo et al., 2020). More recently, FER and ANJ have been shown to perceive RALF33 in the context of pollen hydration regulation (Liu et al., 2021). Likewise, while THE1 is the receptor for RALF34, fer-4 is also insensitive to RALF34 treatment (Gonneau et al., 2018), suggesting that THE1 and FER might form a heteromeric complex to control responsiveness to cellulose biosynthesis inhibition. In our experiments, however, RALF34-induced growth inhibition was similar in Col-0 and fer-4 (Figure 3). This suggests that, while THE1 is the primary RALF34 receptor, it might form distinct heteromeric complexes with different CrRLK1L-family members depending on the context. Interestingly, Ca2+ influx and H+ efflux signatures induced by RALF36 were shown to be FER-independent (Gjetting et al., 2020). However, the kinetics of both Ca2+ and H+ signals in fer-4 upon treatment with RALF36 were altered, indicating that FER might still play a role in these responses despite not being the main receptor (Gjetting et al., 2020). In contrast, RALF36 effect in root growth inhibition is FER-dependent (Figure 3, B), which also suggests that this RALF peptide is perceived by different receptor complexes depending on the tissue considered. These results illustrate the complexity of RALF peptides perception/signaling and the limitation of whole-organism or even tissue-specific assays to elucidate the signaling pathways involved.
In summary, our results provide the basis for the future identification of RALF-CrRLK1L ligand–receptor pairs. It will, however, be essential to determine overlapping expression patterns of different PRORALF and CrRLK1L genes across different organs, tissues, and cell types, and during different developmental stages using either transcriptional reporters (Gonneau et al., 2018) or capitalizing on recent quantitative proteomics studies of the Arabidopsis proteome (Zhang et al., 2019; Bassal et al., 2020; Mergner et al., 2020). These approaches will guide downstream biochemical/biophysical characterizations of ligand–receptor binding and potential heteromeric CrRLK1L complexes, as well as the genetic characterization of PRORALF and CrRLK1L genes, which otherwise can suffer from functional redundancy and pleiotropic issues. For example, recently developed approaches, such as cell-specific CRISPR/Cas9-mediated genome editing, could be used to generate higher-order receptor and ligand mutants (Decaestecker et al., 2019; Wang et al., 2020). Together, these integrated approaches will be needed to decipher the complex signaling network that RALF peptides and their corresponding receptors weave in their native contexts.
Materials and methods
Plant growth and conditions
Arabidopsis thaliana seeds were surface-sterilized using 70% (v/v) and 100% ethanol for 20 min and grown on 0.5 Murashige and Skoog (MS) media with 1% (w/v) sucrose, adjusted to pH 5.8 using KOH, with or without 0.9% (w/v) agar at 20 °C and a 16-h photoperiod. The fer-4 and llg1-2 seeds were kindly provided by Alice Cheung (University of Massachusetts Amherst).
Peptides
RALF peptides were synthesized (Supplemental Table S2) by SciLight Biotechnology LLC (www.scilight-peptide.com) with a purity of >85%. All peptides were dissolved in sterile pure water for usage and stored at −20 °C at a concentration of 1 mM.
Phylogenetic analysis
Multiple sequence alignments of the full-length or mature peptide sequences were created using the MUSCLE algorithm with the MEGA X software (Kumar et al., 2018). Sequence alignment was colored according to sequence conservation and amino acid type using the software Jalview. Phylogenetic rooted trees were constructed with the MEGA X software by using the UPGMA algorithm with the default parameters. Bootstrapping was performed 1000 times. The inferred trees were visualized using iTOL (https://itol.embl.de/; Letunic and Bork, 2019).
Seedling growth inhibition assay
Seeds were surface-sterilized and grown on MS agar plates for 5 d before transferring individual seedlings in each well of a 48-well plate containing 500 µL per well of MS medium containing 1 µM RALF, 10 µM RALF, or 5 nM elf18 as control. Seedling weight was measured 7 d later. Control seedlings were grown under identical conditions in a peptide-free medium. Twelve seedlings for each treatment were measured. The experiments were repeated three times using independent biological replicates.
Root growth inhibition assay
Seeds were surface-sterilized and vertically grown on MS agar plates for 5 d before transferring six seedlings to each well of a 12-well plate containing 4 mL per well of MS medium containing 2 µM RALF; 10 nM AtPep1 as control. Seedlings were transferred 3 d later to solid MS plates. Control seedlings were grown under identical conditions in a peptide-free medium. Primary root length was measured by scanning the plates and quantified using the software Fiji (https://imagej.net/Fiji; Schindelin et al., 2012). Roots from approximately six seedlings per treatment and genotype were measured. Experiments were repeated three times using independent biological replicates.
Alkalinization assay
Seeds were surface-sterilized and vertically grown on MS agar plates for 7 d before transferring one seedling to each well of a 48-well plate containing 500 µL per well of MS medium with 1 µM RALF. Control seedlings were placed under identical conditions in a peptide-free medium. pH was measured after 4 h using an In Lab Micro & Micro Pro pH electrode (Mettler-Toledo). The media pH of 12 seedlings for each treatment and genotype was recorded. Experiments were repeated three times using independent biological replicates.
ROS production assay
Eight leaf discs (4-mm diameter) per individual treatment were collected in 96-well plates containing sterile water. After collection, leaf discs were incubated overnight. The next day, the water was replaced by 75 µL 2 mM MES-KOH pH 5.8 to mimic the apoplastic pH. Leaf discs were incubated for 4 h before adding 75 µL 40 μg/mL horseradish peroxidase (HRP), 1 μM L-O12 (Wako Chemicals, Germany), 200 nM elf18, and 2 µM RALF peptide (final concentration 20 μg/mL HRP, 0.5 μM L-O12, 100 nM elf18, and 1 µM RALF). The velocity (vmax), as shown in Supplemental Figures S7, S10, was calculated as the slope of the ROS curves (shown in Supplemental Figures S6, S9, S11): difference between the maximum and the minimum photon counts divided by the time difference at the maximal and minimal photon counts.
Statistical analysis
Statistical analysis was performed applying one-way ANOVA followed by Dunnet’s test or non-parametric Kruskal–Wallis multiple comparison test, comparing every treatment to its respective mock control using Prism 8.0 (GraphPad Software). Similar significance levels were obtained when transforming the data to normal distribution and performing a two-way ANOVA test followed by Dunnett’s post hoc test using the software R. Two-tailed t tests were performed to assess the significant differences between Col-0 and fer-4 in growth inhibition and alkalinization experiments, using Prism 8.0 (GraphPad Software).
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers provided in Supplemental Table S3.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. YISY motif and conserved cysteine residues are important for AtRALF bioactivity.
Supplemental Figure S2. Fresh weight of 12-d-old seedings grown in the absence (mock) or presence of 10 µM AT2G32890 peptide (n = 8).
Supplemental Figure S3. Phylogenetic relationship of our proposed revised consensus list of AtRALF.
Supplemental Figure S4. FER dependency of AtRALF peptides in inducing seedling growth inhibition at 10 µM.
Supplemental Figure S5. fer-4 bathing medium is more alkaline than Col-0 medium.
Supplemental Figure S6. Effect of AtRALF peptides on elf18-induced ROS production.
Supplemental Figure S7. Effect of AtRALF peptides on ROS production.
Supplemental Figure S8. Effect of AtRALF peptides on ROS production in Col-0 and fer-4.
Supplemental Figure S9. Effect of AtRALF peptides on ROS production.
Supplemental Figure S10. Effect of predicted AtRALF peptides on ROS production in Col-0 and fer-4.
Supplemental Figure S11. FER dependency of AtRALF peptide effects on elf18-induced ROS production.
Supplemental Figure S12. FER dependency of AtRALF peptide effects on ROS production.
Supplemental Table S1. Re-annotation of AtRALFs.
Supplemental Table S2. Sequences of AtRALF peptides synthesized.
Supplemental Table S3. Accession numbers of the proteins used in this study.
Supplementary Material
Acknowledgments
We thank all the members of the Zipfel group and most particularly Marta Bjornson, Julian Dindas, Julien Gronnier, and Isabel Monte for fruitful discussions and guidance. We also thank Joop Vermeer and Vinay Shekhar for providing access to the plate scanner. Finally, we thank Clara Sanchez’s group for providing technical advices for the alkalinization assay.
Funding
This work was supported by the European Research Council (ERC) under the European Union (EU)’s Horizon 2020 research and innovation programme under grant agreement No 773153 (grant “IMMUNO-PEPTALK”), the University of Zürich, and the Swiss National Science Foundation grant no. 31003A_182625. C.M.F. was supported by a post-doctoral fellowship (EMBO ALTF 512-2019) from the European Molecular Biology Organization.
Conflict of interest statement. None declared.
C.Z. conceived the original screening and research plan, supervised and completed the writing, and agrees to serve as the author responsible for contact and ensures communication. A.A. and C.M.F. performed the experiments. A.A. designed the experiments and analyzed the data, wrote the first draft of manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Cyril Zipfel (cyril.zipfel@uzh.ch).
References
- Bassal M, Abukhalaf M, Majovsky P, Thieme D, Herr T, Ayash M, Hoehenwarter W (2020) Reshaping of the Arabidopsis thaliana proteome landscape and co-regulation of proteins in development and immunity. Mol Plant 13: 1709–1732 ( 10.1016/j.molp.2020.09.024) [DOI] [PubMed] [Google Scholar]
- Blackburn MR, Haruta M, Moura DS (2020) Twenty years of progress in physiological and biochemical investigation of RALF peptides. Plant Physiol 182: 1657–1666 ( 10.1104/pp.19.01310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Turner SR (2017) A comprehensive analysis of RALF proteins in green plants suggests there are two distinct functional groups. Front Plant Sci 8: 37 ( 10.3389/fpls.2017.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos WF, Dressano K, Ceciliato PHO, Guerrero-Abad JC, Silva AL, Fiori CS, Moura DS (2018) Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J Biol Chem 293: 2159–2171 ( 10.1074/jbc.M117.808881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Shi F (2012) Evolution of the RALF gene family in plants: Gene duplication and selection patterns. Evol Bioinform 2012: 271–292 ( 10.4137/EBO.S9652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker W, Buono RA, Pfeiffer ML, Vangheluwe N, Jourquin J, Karimi M, Jacobs TB (2019) CRISPR-Tsko: A technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell 31: 2868–2887 ( 10.1105/tpc.19.00454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressano K, Ceciliato PHO, Silva AL, Guerrero-Abad JC, Bergonci T, Ortiz-Morea FA, Moura DS (2017) BAK1 is involved in AtRALF1-induced inhibition of root cell expansion. PLOS Genet 13: e1007053 ( 10.1371/journal.pgen.1007053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The Feronia receptor-like kinase mediates male–female interactions during pollen tube reception. Science 317: 656–660 ( 10.1126/science.1143562) [DOI] [PubMed] [Google Scholar]
- Feng H, Liu C, Fu R, Zhang M, Li H, Shen L, Li C (2019) LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis. Mol Plant 12: 1612–1623 ( 10.1016/j.molp.2019.09.004) [DOI] [PubMed] [Google Scholar]
- Franck CM, Westermann J, Boisson-Dernier A (2018) Plant malectin-like receptor kinases: From cell wall integrity to immunity and beyond. Annu Rev Plant Biol 69: 301–328 ( 10.1146/annurev-arplant-042817-040557) [DOI] [PubMed] [Google Scholar]
- Galindo‐Trigo S, Blanco‐Touriñán N, DeFalco TA, Wells ES, Gray JE, Zipfel C, Smith LM (2020) Cr RLK 1L receptor‐like kinases HERK 1 and ANJEA are female determinants of pollen tube reception. EMBO Rep 21: 1–18 ( 10.15252/embr.201948466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Qu LJ (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358: 1596–1600 ( 10.1126/science.aao3642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Zhao Y, Liu MC, Zhou LZ, Wang L, Zhong S, Qu LJ (2019) LLG2/3 are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Curr Biol 29: 3256–3265 ( 10.1016/j.cub.2019.08.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjetting SK, Mahmood K, Shabala L, Kristensen A, Shabala S, Palmgren M, Fuglsang AT (2020) Evidence for multiple receptors mediating RALF‐triggered Ca2+ signaling and proton pump inhibition. Plant J 104: 433–446 ( 10.1111/tpj.14935) [DOI] [PubMed] [Google Scholar]
- Gonneau M, Desprez T, Martin M, Doblas VG, Bacete L, Miart F, Höfte H (2018) Receptor kinase THESEUS1 is a rapid alkalinisation factor 34 receptor in Arabidopsis. Curr Biol 28: 2452–2458.e4 ( 10.1016/j.cub.2018.05.075) [DOI] [PubMed] [Google Scholar]
- Haruta M, Monshausen G, Gilroy S, Sussman MR (2008) A cytoplasmic Ca2+ functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: identification of AtRALF1 peptide. Biochemistry 47: 6311–6321 ( 10.1021/bi8001488) [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science (New York, NY) 343: 408–411 ( 10.1126/science.1244454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herger A, Gupta S, Kadler G, Franck CM, Boisson-Dernier A, Ringli C (2020) Overlapping functions and protein–protein interactions of LRR-extensins in Arabidopsis. PLoS Genet 16: e1008847 ( 10.1371/journal.pgen.1008847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann U, Lau K, Hothorn M (2017) The structural basis of ligand perception and signal activation by receptor kinases. Annu Rev Plant Biol 68: 109–137 ( 10.1146/annurev-arplant-042916-040957) [DOI] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Hedrich R (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285: 13471–13479 ( 10.1074/jbc.M109.097394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 ( 10.1093/molbev/msy096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P (2019) Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res 47: 256–259 ( 10.1093/nar/gkz239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, Wu HM (2015) Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4: 1–21 ( 10.7554/eLife.06587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U (2012) CrRLK1L receptor-like kinases: Not just another brick in the wall. Curr Opin Plant Biol 15: 659–669 ( 10.1016/j.pbi.2012.07.003) [DOI] [PubMed] [Google Scholar]
- Liu C, Shen L, Xiao Y, Vyshedsky D, Peng C, Sun X, Liu Z, Cheng L, Chao L (2021) Pollen PCP-B peptides unlock a stigma peptide–receptor kinase gating mechanism for pollination. Science 375: 171–175 [DOI] [PubMed] [Google Scholar]
- Matos JL, Fiori CS, Silva-Filho MC, Moura DS (2008) A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett 582: 3343–3347 ( 10.1016/j.febslet.2008.08.025) [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y (2014) Posttranslationally modified small-peptide signals in plants. Annu Rev Plant Biol 65: 385–413 ( 10.1146/annurev-arplant-050312-120122) [DOI] [PubMed] [Google Scholar]
- Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Grossniklaus U (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358: 1600–1603 ( 10.1126/science.aao5467) [DOI] [PubMed] [Google Scholar]
- Mergner J, Frejno M, List M, Papacek M, Chen X, Chaudhary A, Kuster B (2020) Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 579: 409–414 ( 10.1038/s41586-020-2094-2) [DOI] [PubMed] [Google Scholar]
- Morato do Canto A, Ceciliato PHO, Ribeiro B, Ortiz Morea FA, Franco Garcia AA, Silva-Filho MC, Moura DS (2014) Biological activity of nine recombinant AtRALF peptides: Implications for their perception and function in Arabidopsis. Plant Physiol Biochem 75: 45–54 ( 10.1016/j.plaphy.2013.12.005) [DOI] [PubMed] [Google Scholar]
- Moussu S, Broyart C, Santos-Fernandez G, Augustin S, Wehrle S, Grossniklaus U, Santiago J (2020) Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc Natl Acad Sci USA 117: 7494–7503 ( 10.1073/pnas.2000100117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, De Smet I (2014) Understanding the RALF family: A tale of many species. Trends Plant Sci 19: 664–671 ( 10.1016/j.tplants.2014.06.005) [DOI] [PubMed] [Google Scholar]
- Olsen AN, Mundy J, Skriver K (2002) Peptomics, identification of novel cationic Arabidopsis peptides with conserved sequence motifs. In Silico Biol 2: 441–451 [PubMed] [Google Scholar]
- Olsson V, Joos L, Zhu S, Gevaert K, Butenko MA, De Smet I (2019) Look closely, the beautiful may be small: Precursor-derived peptides in plants. Annu Rev Plant Biol 70: 153–186 ( 10.1146/annurev-arplant-042817-040413) [DOI] [PubMed] [Google Scholar]
- Pearce G, Moura DS, Stratmann J, Ryan CA (2001) RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci USA 98: 12843–12847 ( 10.1073/pnas.201416998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Yamaguchi Y, Munske G, Ryan CA (2010) Structure-activity studies of RALF, rapid alkalinization factor, reveal an essential—YISY—motif. Peptides 31: 1973–1977 ( 10.1016/j.peptides.2010.08.012) [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Cardona A (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682 ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Muth P, Irmler S, Schroder G, Schroder J (1996) Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus). cDNA, gene, intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J Biol Chem 271: 26684–26689 (doi:10.1074/jbc.271.43.26684) [DOI] [PubMed] [Google Scholar]
- Sharma A, Hussain A, Mun BG, Imran QM, Falak N, Lee SU, Yun BW (2016) Comprehensive analysis of plant rapid alkalization factor (RALF) genes. Plant Physiol Biochem 106: 82–90 ( 10.1016/j.plaphy.2016.03.037) [DOI] [PubMed] [Google Scholar]
- Srivastava R, Liu J-X, Guo H, Yin Y, Howell SH (2009) Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J 59: 930–939 ( 10.1111/j.1365-313X.2009.03926.x) [DOI] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-luzan E, Rovenich H, Lehner A, Holton N, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signalling. Sience 355: 287–289 [DOI] [PubMed] [Google Scholar]
- Wang X, Ye L, Lyu M, Ursache R, Löytynoja A, Mähönen AP (2020) An inducible genome editing system for plants. Nat Plants 6: 766–772 ( 10.1038/s41477-020-0695-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Stegmann M, Han Z, DeFalco TA, Parys K, Xu L, Chai J (2019) Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 572: 270–274 ( 10.1038/s41586-019-1409-7) [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu P, Guo T, Zhao H, Bensaddek D, Aebersold R, Xiong L (2019) Arabidopsis proteome and the mass spectral assay library. Sci Data 6: 278 ( 10.1038/s41597-019-0294-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu CC, Zhu JK (2018) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci U S A 115: 13123–13128 ( 10.1073/pnas.1816991115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts agrobacterium-mediated transformation. Cell 125: 749–760 ( 10.1016/j.cell.2006.03.037) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.