Abstract
The threat of global warming makes uncovering mechanisms of plant tolerance to long-term moderate heat stress particularly important. We previously reported that Arabidopsis (Arabidopsis thaliana) plants lacking mitochondrial proteases FTSH4 or OMA1 suffer phenotypic changes under long-term stress of 30°C, while their growth at 22°C is not affected. Here we found that these morphological and developmental changes are associated with increased accumulation of insoluble mitochondrial protein aggregates that consist mainly of small heat-shock proteins (sHSPs). Greater accumulation of sHSPs in ftsh4 than oma1 corresponds with more severe phenotypic abnormalities. We showed that the proteolytic activity of FTSH4, and to a lesser extent of OMA1, as well as the chaperone function of FTSH4, is crucial for protecting mitochondrial proteins against aggregation. We demonstrated that HSP23.6 and NADH dehydrogenase subunit 9 present in aggregates are proteolytic substrates of FTSH4, and this form of HSP23.6 is also a substrate of OMA1 protease. In addition, we found that the activity of FTSH4 plays an important role during recovery from elevated to optimal temperatures. Isobaric tags for relative and absolute quantification (iTRAQ)-based proteomic analyses, along with identification of aggregation-prone proteins, implicated mitochondrial pathways affected by protein aggregation (e.g. assembly of complex I) and revealed that the mitochondrial proteomes of ftsh4 and oma1 plants are similarly adapted to long-term moderate heat stress. Overall, our data indicate that both FTSH4 and OMA1 increase the tolerance of plants to long-term moderate heat stress by reducing detergent-tolerant mitochondrial protein aggregation.
Mitochondrial proteases prevent accumulation of insoluble protein aggregates and protect Arabidopsis plants against long-term moderate heat stress.
Introduction
Over the last few years, plant tolerance to heat stress became one of the most intensively studied concepts, defined as the ability of plants to survive under elevated temperatures. The growing interest in this phenomenon results from progressive global warming. Research on the response of plants to heat stress has so far focused mainly on the effects of short-term acute heat stress, while global warming is rather related to long-term stress of moderately high temperature. Our previous results documented that Arabidopsis (Arabidopsis thaliana) plants lacking mitochondrial proteases FTSH4 or OMA1 suffer morphological and developmental alterations under continuous moderately high temperature, while their post-germination growth at normal temperature is not affected (Dolzblasz et al., 2016; Smakowska et al., 2016; Migdal et al., 2017; Heidorn-Czarna et al., 2018). The phenotypic abnormalities seen in the ftsh4 mutant under slightly higher than normal temperature (30°C) included delayed germination, substantially reduced rosette size and length of roots, delay in the appearance of true leaves, and irregular serration of leaf blades. The first two features, reduction of the rosette size and root length, were also observed in oma1 under 30°C, but to a lesser extent than in ftsh4. A common alteration was also a substantially shorter inflorescence, mostly evident for the ftsh4 plants (Dolzblasz et al., 2016). FTSH4 and OMA1 are both metalloproteases anchored in the mitochondrial inner membrane, but they differ in their response to ATP: while OMA1 does not require ATP to function, FTSH4 is an ATP-dependent protease, which exhibits not only proteolytic but also chaperone-like activity (Urantowka et al., 2005; Migdal et al., 2017). Using blue-nativepolyacrylamide gel electrophoresis (BN-PAGE) we showed that both proteases significantly affect the abundance and functionality of complexes I and V of the oxidative phosphorylation system (OXPHOS) when plants are grown at elevated temperature (30°C; Smakowska et al., 2016; Migdal et al., 2017). Further studies revealed the accumulation of reactive oxygen species (ROS) and carbonylated proteins, increased number of giant mitochondria, and reduced cardiolipin content in plants devoid of FTSH4 under long-term stress of 30°C (Smakowska et al., 2016). It was postulated that the elevated level of ROS in ftsh4 induces the production of peroxidases, which in turn disturb auxin signaling leading to defects in plant growth (Zhang et al., 2014). Moreover, by modulating the extent of internal oxidative stress, FTSH4 protease may also regulate the expression of the WRKY transcription factors that control synthesis of salicylic acid and signaling pathways in autophagy and senescence (Zhang et al., 2017). So far, three membrane-bound mitochondrial proteins were identified as proteolytic substrates of FTSH4: mitochondrial import inner membrane translocase (TIM) subunit 17-2 (TIM17-2), the Pam18-2 subunit of the pre-sequence translocase-associated motor (PAM) complex, and mitochondrial pyruvate carrier 4 (MPC4; Opalinska et al., 2017, 2018), indicating the FTSH4-dependent proteolytic control of proteins and pyruvate uptake in plant mitochondria. It was also demonstrated that FTSH4 degrades oxidatively damaged proteins in Arabidopsis mitochondria (Opalinska et al., 2017). The proteolytic substrates for OMA1 protease have not been described yet.
Elevated temperature may result in protein unfolding, misfolding, or formation of insoluble aggregates by hydrophobic interactions between the affected polypeptides (Balchin et al., 2016). In consequence, the concentration of functional proteins is reduced, and generated aggregates could be cytotoxic. Recent evidence, however, indicates that formation of large aggregates might represent a cell-protective mechanism to sequester potentially toxic proteins (Bruderek et al., 2018). In response to the accumulation of unfolded/misfolded proteins, cells activate a compartment-specific unfolded protein response (UPR), which is the transcriptional induction of nuclear genes encoding proteins involved in removal of proteotoxic stress and restoring compartment-specific homeostasis (Santiago et al., 2020).
The small heat-shock proteins (sHSPs) are ATP-independent molecular chaperones, and they are believed to constitute the first line of defense against protein unfolding stress (Haslbeck and Vierling, 2015). It is documented that sHSPs are rapidly synthesized in response to heat stress and other abiotic stresses (Siddique et al., 2008; Waters et al., 2008). Additionally, their participation in plant development in the absence of stress has also been documented (Wang et al., 2004; Ma et al., 2019). The size of sHSPs ranges from 12 to 25 kDa, when they exist as monomers, but the majority of sHSPs assemble into large oligomers comprised of a minimum of 12 monomers (Waters and Vierling, 2020). All sHSPs share a conserved core domain of about 100 amino acids, which is homologous to alpha-crystallin from the vertebrate. Current models propose that sHSPs bind stress-denatured proteins to prevent their irreversible aggregation and maintain the denatured proteins in a folding-competent state to allow their subsequent refolding with the help of ATP-dependent chaperones (Nakamoto and Vigh, 2007). Moreover, sHSPs may also contribute to membrane protection upon stress by binding to the membranes and preserving their fluidity (Nakamoto and Vigh 2007; Horvath et al., 2008). In plants, sHSPs are present in the cytosol, nucleus, endoplasmic reticulum (ER), chloroplasts, mitochondria, and peroxisomes (Waters and Vierling, 2020). The presence of an organelle-targeted sHSPs appears to be unique to plants except for a mitochondrion-targeted sHSP22 in Drosophila melanogaster (Dabbaghizadeh et al., 2018). Among 19 sHSPs identified in A. thaliana, there is one mitochondria-localized sHSP (HSP26.5) and two dual-targeted to mitochondria and chloroplasts (HSP23.5 and HSP23.6; Van Aken et al., 2009). Simultaneous downregulation of these three sHSPs leads to drastic changes in vegetative and reproductive growth without external stress, suggesting that plant sHSPs are important also under nonstress conditions (Escobar et al., 2021). Almost all the studies reported so far have shown that sHSPs positively affect plant response to abiotic stress. Interestingly, the opposite effect, reduced tolerance to heat and salt stress, was observed for transgenic Arabidopsis plants constitutively expressing AsHSP17 or AsHSP26.8a from creeping bentgrass (Agrostis stolonifera; Sun et al., 2016; 2020). These findings underline the fact that the model describing sHSPs as general chaperones may not apply to all sHSPs.
The purpose of this study was to understand the role of two Arabidopsis mitochondrial proteases, FTSH4 and OMA1, during chronic moderate temperature stress and in the recovery period. We showed that the loss of FTSH4 or OMA1 resulted in an increased abundance of insoluble aggregated mitochondrial proteins when plants were grown at 30°C and that mitochondrial sHSPs are the main components of the protein aggregates. We demonstrated that the proteolytic activity of FTSH4 and OMA1 as well as the chaperone activity of FTSH4 are important in preventing protein aggregation. We found that HSP23.6 and NADH dehydrogenase subunit 9 (NAD9) are proteolytic substrates of FTSH4 under temperature stress. Our iTRAQ-based proteomic analysis highlighted potential targets of FTSH4 and/or OMA1 and revealed that mitochondrial proteomes lacking FTSH4 or OMA1 are quite similarly reprogrammed in response to chronic moderate temperature stress.
Results
Loss of FTSH4 and OMA1 causes an increased abundance of aggregated mitochondrial proteins at 30°C
Elevated temperature can lead to protein aggregation, and proteases are able to counteract formation of such protein aggregates (Voos, 2013). Therefore, we tested whether the loss of FTSH4 and/or OMA1 affects the aggregation status of the mitochondrial proteins under prolonged moderately high temperatures. To achieve this, we compared the abundance of the Triton X-100-insoluble mitochondrial protein aggregates between ftsh4-1, oma1, and wild-type (WT) plants. Mitochondria isolated from 2-week-old seedlings grown at 22°C or 30°C were lysed with 0.5% (v/v) Triton X-100 and subjected to ultracentrifugation at 125,000 × g to separate detergent-insoluble proteins, which go to the pellet fraction, from soluble proteins, which remain in the supernatant (Figure 1A; Bender et al., 2011). Almost no protein aggregates were observed in WT and mutant mitochondria at 22°C (Figure 1, B and D). However, a clear accumulation of insoluble mitochondrial proteins was found at 30°C, mostly in the ftsh4-1 and, to a lesser extent, in oma1 mutants and the least in the WT plants (Figure 1, C and E). Thus, FTSH4 and, to a lower degree, OMA1 prevent the formation of aggregates of thermally denatured mitochondrial proteins.
Figure 1.
Accumulation of the Triton X-100-insoluble proteins in the ftsh4-1 and oma1 mitochondria under moderate heat stress (30°C). A, A schematic workflow for the studies of heat-prone protein aggregates. More details of the experiment are given in “Materials and methods”. B and C, Coomassie-stained 12% SDS–PAGE gels with Triton X-100-treated lysates of isolated mitochondria from 2-week-old WT, ftsh4-1, and oma1 plants grown either at optimal conditions (22°C) (B) or moderate heat stress (30°C) (C). D and E, show quantification of the abundance of proteins in the pellet versus total protein fraction using ImageJ (Fiji) and Coomassie-stained gels. The abundance values are given as relative to the value obtained for WT (set as 1). Mean (n = 3) ± sem. *P ≤ 0.05; **P ≤ 0.03, ***P ≤ 0.01. Statistical significance was assessed using two-tailed Student’s t test. sem, standard error of the mean.
The mitochondrial unfolded protein response is activated in ftsh4 and oma1
Multiple forms of mitochondrial dysfunction, including the accumulation of insoluble protein aggregates, activates a transcriptional response defined as the mitochondrial UPR (UPRmt; Naresh and Haynes, 2019). We examined the UPRmt response in the WT, ftsh4-1, ftsh4-2, and oma1 plants under moderate heat stress (30°C) by studying the transcripts of marker genes for plant UPRmt based on Wang and Auwerx (2017) and Tran and van Aken (2020). The obtained results presented in the Supplemental Figure S1 show that in the WT we did not observe strong changes in the transcript level for any of the tested genes while a clear transcriptional upregulation (10- to 20-fold increase) was observed for the AOX1a, HSP23.5, and UPOX genes in all three mutant plants. From the tested markers for plant UPRmt, only the expression of HSP60 was almost unaltered in all the mutants.
We also examined the induction of marker genes associated with the chloroplast (cpHsc70-1 and ClpB3; Llamas et al., 2017), cytosolic (HSP70T-1 and HSP70-15; Rana et al., 2018), and ER (BIP1/2, PDI9, and SDF2; Kim et al., 2018) UPR response (Supplemental Figure S1). There were no significant differences in the transcript levels of the tested genes with only slight upregulation found for PDI9 and HSP70-15 in WT and the mutants.
sHSPs are the main components of protein aggregates in ftsh4 and oma1
To identify moderate heat-labile, aggregation-prone mitochondrial polypeptides, the Triton X-100-insoluble pellet fractions from the WT and both mutant mitochondria were analyzed directly by liquid chromatography–mass spectrometry (LC–MS). The list of identified aggregation-prone mitochondrial proteins accumulated in the ftsh4-1 and/or oma1 in comparison to WT is presented in Supplemental Table S1A. In addition, proteins from the pellet fractions were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE), and protein bands substantially enriched in the ftsh4-1 compared to WT were excised from the gel and investigated by the MS analysis (Supplemental Figure S2). In this approach, we used only ftsh4-1 as the abundance of aggregated proteins was higher in this mutant than in oma1 (Figure 1, C and E). The list of identified proteins is provided in Supplemental Table S1B. In further analysis, we focused on the mitochondrial sHSPs, HSP23.5 and HSP23.6, whose aggregation in ftsh4-1 and oma1 compared to WT is the most striking (Supplemental Table S1, A and B). Additionally, we examined the third mitochondrial sHSP, HSP26.5, although that protein was not identified in the protein aggregates by MS analyses. First, we determined the transcript abundance of sHSPs at 22°C and 30°C (Figure 2A). The relative abundance of transcripts in plants grown at 30°C in comparison to the control plants (22°C) shows that the transcriptional response of HSP23.5 and HSP23.6 was rather similar between ftsh4-1, ftsh4-2, and oma1, but significantly higher when compared with WT (Figure 2A). There was no upregulation of HSP26.5 transcripts caused by the moderately increased temperature in both WT and the mutants. This differentiated behavior of mitochondrial sHSPs is not surprising considering that HSP23.5 and HSP23.6 are closely related and belong to a subfamily different from HSP26.5 (Waters and Vierling, 2020).
Figure 2.
Accumulation of the mitochondrial sHSP, NAD9, and RPS10 aggregates in the ftsh4-1 and oma1 plants grown under moderate heat stress (30°C). A, Relative abundance of the HSP23.5, HSP23.6, and HSP26.5 transcripts in plants grown under 30°C in comparison to the control plants (22°C) expressed as the Log2 ratio. The dashed lines indicate cutoff values ±1 (log2) of the ratio corresponding to the threshold levels for significant up- and downregulation of the transcripts. Data are the mean of minimum three independent biological replicates ± sem. ***P ≤ 0.01. Statistical significance was assessed using two-tailed Student’s t test. B, Immunodetection of HSP23.6, HSP23.5, HSP26.5, NAD9, and RPS10 in the Triton X-100 insoluble (aggregated) protein fractions at 30°C. C, Immunodetection of HSP26.5, NAD9, and RPS10 in the soluble fraction of mitochondrial proteins at 22°C. The HSP23.6 and HSP23.5 proteins were undetectable under these conditions. Representative immunoblots of detected proteins are shown. Coomassie-stained membranes show equal protein loading. sem, standard error of the mean.
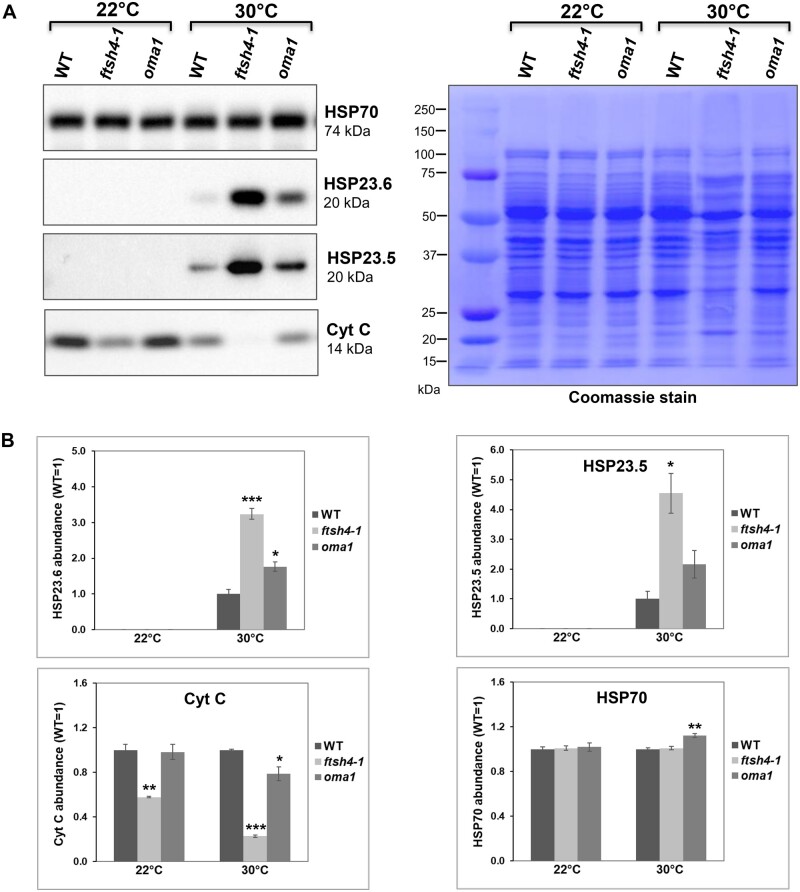
Next, using immunoblotting we demonstrated that all three mitochondrial sHSPs and two other proteins found by MS in aggregates: the NAD9 of complex I and the RPS10 protein of the mitoribosomal small subunit are present in the Triton X-100-insoluble fraction in mitochondria of plants cultivated at 30°C (Figure 2B; Supplemental Figure S3). The amount of detergent-insoluble sHSPs (HSP23.5, HSP23.6, and HSP26.5), NAD9, RPS10 was substantially increased in ftsh4-1 compared with WT plants. The increase in abundance was also observed for HSP23.5, HSP23.6, and RPS10 in oma1 mitochondria, but not to that extent like in ftsh4-1 (Figure 2B). Unexpectedly, there was no difference in the abundance for the HSP26.5 protein while NAD9 was lower in oma1 compared to WT. It should be emphasized that NAD9, RPS10, and HSP26.5 were mostly present in the soluble fraction in plants grown at 22°C (Figure 2C), while HSP23.5 and HSP23.6 were undetectable as they are heat-inducible proteins (Figure 3).
Figure 3.
Steady-state levels of the HSP23.6, HSP23.5, CytC, and HSP70 proteins in mitochondria isolated from 2-week-old WT, ftsh4-1, and oma1 plants grown under optimal conditions (22°C) and moderate heat stress (30°C). A, Mitochondrial proteins were separated on SDS–PAGE gel and the abundance of HSP23.6, HSP23.5, CytC, and HSP70 proteins was assessed by immunoblotting. B, The abundance of the analyzed proteins was quantified densitometrically in ImageJ (Fiji) based on immunodetection of selected proteins and Coomassie-stained membrane as a loading control. The abundance values are given as relative to the value obtained for WT set as 1. Mean (n = 3) ± sem. *P ≤ 0.05; **P ≤ 0.03, ***P ≤ 0.01. Statistical significance was assessed using two-tailed Student’s t test.
Furthermore, we tested the presence of other proteins from various mitochondrial compartments in the Triton X-100-insoluble fraction using specific antibodies (Supplemental Figure S4). Two scaffold proteins, prohibitins (PHBs), and stomatin-like protein 1 (SLP1), behave similarly and were found in both the soluble and insoluble fractions at both tested temperatures although the proportion of aggregated versus soluble has increased under 30°C. It should be emphasized that the LC–MS analysis detected PHBs and SLPs (SLP1 and SLP2) in the aggregates, but these proteins were not included in the Supplemental Table S1A because their abundance was not higher in the mutants, as it was also confirmed by the immunological analysis. Other tested proteins: mitochondrial HSP70, PREP1 protease, GrpE protein homolog 2 (MGE2), TIM17-2, and FTSH4 protease were mostly present in the soluble fraction at both 22°C and 30°C (Supplemental Figure S4).
HSP23.6 is degraded by FTSH4 and OMA1, while NAD9 only by FTSH4
To verify our hypothesis that the elevated aggregation of HSP23.6 in ftsh4-1 and oma1, as well as NAD9 in ftsh4-1, is due to a lack of proteolytic activity of respective proteases, we monitored the level of HSP23.6 and NAD9 proteins after their incubation with FTSH4 or OMA1 proteases produced in vitro in an insect cell lysate. The ftsh4-1 mitochondria isolated from seedlings grown at 30°C were resuspended in the digitonin-containing buffer (1%) and incubated with a protease or the control cell-lysate for an indicated time at 30°C (Figure 4). We found that the level of HSP23.6 remained stable in the absence of proteases, but its abundance declined moderately in the presence of externally added FTSH4 or OMA1. Furthermore, we observed the decrease in NAD9 abundance only in the presence of externally added FTSH4 but not OMA1. Degradation by FTSH4 was also observed for TIM17-2 protein, which is known as a proteolytic substrate of FTSH4 protease (Opalinska et al., 2018). No degradation by either protease was observed for mitochondrial HSP70 (Figure 4).
Figure 4.
In vitro degradation of HSP23.6, NAD9, TIM17-2, and HSP70 in the ftsh4-1 mitochondria in the absence or presence of in vitro-expressed FTSH4 or OMA1 proteases. Mitochondria were incubated in the digitonin-containing buffer with the addition of equal amount (4 µL) of insect-cell lysate containing FTSH4 or OMA1 or control lysate for the indicated time points. Representative immunoblots and Coomassie-stained membrane are presented. (Right) shows quantification of the abundance of the analyzed proteins. Protein amount was quantified densitometrically in ImageJ (Fiji) based on immunodetection with antibodies and Coomassie-stained membrane as a loading control. The abundance values are given as relative to the value obtained for time 0 h (set as 1). Mean (n = 5) ± sem. *P < 0.05; **P < 0.03; ***P < 0.01. Statistical significance was assessed using two-tailed Student’s t test. The arrowhead indicates specific signal of NAD9.
The question arises about the aggregation status of the studied proteins during the degradation assay. To verify whether the detergent used in this assay (1% digitonin) does not breakdown aggregated proteins, we performed immunoblotting after separation of digitonin-solubilized mitochondrial proteins via BN-PAGE using antibodies against HSP23.6 (Supplemental Figure S5). The obtained data document that HSP23.6 was present in the insoluble material (pellet) obtained after the solubilization and centrifugation of the mitochondrial protein sample (Supplemental Figure S5, A and B). Based on these findings, we can conclude that both FTSH4 and OMA1 are able to degrade HSP23.6 trapped in the detergent-tolerant protein aggregates.
Since FTSH4 and OMA1 are the inner membrane proteases, we decided to determine the submitochondrial localization (soluble, peripheral, and membrane) of HSP23.6 performing the alkaline treatment of mitochondria isolated from plants grown at 30°C. In this experiment, HSP23.6 was found mainly in the membrane fraction along with the inner membrane proteins, FTSH4 and TIM17-2, which served as markers (Supplemental Figure S6). Thus, HSP23.6 interaction with a membrane is more similar to an integral than a peripheral membrane protein suggesting that the majority of this protein is strongly associated with the mitochondrial membranes.
Chaperone-like activity of FTSH4 affects the HSP23.6, NAD9, and RPS10 aggregation
Using Arabidopsis plants expressing the proteolytically inactive variant of FTSH4 protease (FTSH4(H486Y); Opalinska et al., 2018), we found that at 30°C the HSP23.6, NAD9, and RPS10 proteins are present in insoluble aggregates at a level higher than in WT, however, lower when compared to that in ftsh4-1 (Figure 5A). This finding suggests that not only the proteolytic function of FTSH4 prevents protein aggregation, but also the chaperone one. The importance of a chaperone-like activity of FTSH4 under moderate heat stress is also supported by the presence of a partially restored phenotype of Arabidopsis FTSH4(H486Y) plants grown at 30°C in comparison to the ftsh4-1 insertional mutant (Figure 5B).
Figure 5.
The importance of a chaperone-like activity of FTSH4 under moderate heat stress (30°C). A, Immunodetection of selected proteins in the Triton X-100-treated mitochondria of WT, ftsh4-1, and FTSH4H486Y plants grown at 30°C. Representative immunoblots of detected proteins are shown. Coomassie-stained membrane shows equal protein loading. B, Morphology of 10-d-old seedlings of WT, ftsh4-1, and FTSH4H486Y grown on 0.5× MS at 30°C. Medium was supplemented with 1.5% sucrose. Scale bars indicate 1 cm.
The abundance of HSP23.6 is under control of FTSH4 during the recovery period
In addition, we monitored the abundance of HSP23.6 in WT, ftsh4-1, and oma1 mitochondria during the recovery period from elevated (30°C) to optimal temperature (22°C) knowing that the HSP23.6 protein is not expressed at 22°C (Figure 6). The protein aggregation was induced by prolonged moderate heat stress (13 d at 30°C). After this time, the plants were transferred to 22°C and the amount of HSP23.6 in soluble (Figure 6C) and detergent-insoluble fractions (Figure 6B) was determined by immunoblotting. As shown in Figure 6B, the abundance of HSP23.6 in the insoluble fraction of mitochondria isolated from WT plants was substantially reduced by the third and fourth days of recovery. The decline in the HSP23.6 aggregates was also observed in oma1 but to a lower degree (Figure 6B). In contrast, the level of aggregated HSP23.6 was rather stable in ftsh4-1 during the studied period (Figure 6B). These results suggest that the release of HSP23.6 trapped in aggregates and its degradation is controlled by FTSH4 protease.
Figure 6.
In vivo degradation of HSP23.6 in the WT, ftsh4-1, and oma1 mitochondria. Plants were grown for 13 d under moderate heat stress (30°C) and then were transferred to optimal conditions (22°C) for the next 4 d. Mitochondria isolated from the 13-d-old plants from 30°C (13 d, 30°C, red font) and after 1, 3, and 4 d of a recovery period (+1 d, +3 d, and +4 d, 22°C, respectively) were treated with Triton X-100 to separate protein aggregates from soluble fractions. A, Total fraction of treated mitochondria; (B) Protein aggregates in the pellet fraction; (C) Soluble proteins in the supernatant fraction. The abundance of HSP23.6 and FTSH4 in the detergent insoluble and soluble fractions was determined by immunoblotting. Representative immunoblots and Coomassie-stained membranes are presented.
Loss of FTSH4 or OMA1 decreases thermotolerance of Arabidopsis to moderately high temperature, but does not affect basic and acquired thermotolerance
Four major plant thermotolerance types can be categorized based on the heat-stress regimes: basal thermotolerance (BT), short-term acquired thermotolerance (SAT), long-term acquired thermotolerance (LAT), and thermotolerance to moderately high temperature (TMHT; Hu et al., 2012; Yeh et al., 2012). We performed the thermotolerance phenotyping assays for the ftsh4-1 and oma1 mutants using conditions known from literature (Figure 7; Yeh et al., 2012). An Arabidopsis hot1 mutant, mutated in the HSP 101 gene (HSP101) was chosen as the heat-sensitive control because it is defective in all types of thermotolerance except for TMHT (Larkindale et al., 2005). Additionally, the insertional mutant ftsh11, which is devoid of chloroplast AtFTSH11 protease (Adam et al., 2019), was used as a positive control for the SAT and LAT assays (Chen et al., 2006), and mge2-1, lacking mitochondrial MGE2 protein, represented a positive control for TMHT (Hu et al., 2012).
Figure 7.
Involvement of FTSH4 and OMA1 in thermotolerance to a moderately high temperature. A, Phenotypes of WT, ftsh4-1, oma1, and mge2-1 seedlings after 8 d of recovery from the TMHT assay. The mge2-1 mutant was used as a positive control for the TMHT. The TMHT assay is schematically shown on the right. B, Phenotypes of WT, ftsh4-1, oma1, mge2-1, ftsh11, and hot1 seedlings after 10 d of recovery from the BT assay or after 14 d of recovery from the SAT or LAT assays. The schematic heat stress regimes of BT, SAT, and LAT assays are presented below the photographs. The hot1 mutant was used as a positive control for the BT, SAT, and LAT assays, while ftsh11 was a positive control for the SAT and LAT assay. Scale bars indicate 1 cm.
The phenotypes of Arabidopsis seedlings after the BT, SAT, LAT, and TMHT tests are shown in Figure 7. The results indicate that FTSH4 and OMA1 proteases are not required in BT, SAT, and LAT responses. Notably, the ftsh4-1 mutant showed severely altered phenotype under TMHT conditions. The oma1 mutant displayed milder phenotypic alterations in TMHT, comparable to the mge2-1 mutant. In conclusion, the classical thermotolerance tests indicate that both FTSH4 and OMA1 proteases, similarly to the MGE2 chaperone, are important in the response of a plant to chronic moderately high heat stress.
In the context of the TMHT assay results, we decided to check whether the lack of MGE2, like the deficiency of FTSH4 and OMA1, leads to an increased aggregation of mitochondrial proteins caused by long-term stress of 30°C. A substantial increase in the abundance of HSP23.6, NAD9, and RPS10 in detergent-tolerant aggregates was observed in mge2-1 compared with WT (Supplemental Figure S7). Interestingly, the accumulation of aggregated polypeptides has been reduced in the absence of PAM16 protein, which is, similarly to MGE2, one of the components of the inner membrane PAM complex, crucial for protein import into the mitochondrial matrix (Murcha et al., 2015).
iTRAQ analysis reveals that mitochondrial proteomes lacking FTSH4 or OMA1 are similarly reprogrammed in response to chronic moderate temperature stress
To gain insight into the reprogramming of the ftsh4-1 and oma1 proteomes due to prolonged stress of moderately high temperature, quantitative comparative iTRAQ-based proteomic analyses were performed. Comparison of the mitochondrial proteomes of 2-week-old ftsh4-1 and oma1 versus WT plants was carried out at both 22°C and 30°C. The identified proteins were categorized into functional categories according to their involvement in specific biological processes (Supplemental Table S2). Using a 1.2-fold increase or a 0.8-fold decrease in protein abundance as a criterion for a physiologically significant change, 44 differentially expressed proteins were identified in ftsh4-1 and only 3 in oma1 when plants were grown at 22°C. A higher number of proteins showing differential abundances were detected in the mutants grown at 30°C, namely 157 proteins in ftsh4-1 and 131 in oma1. We have noticed a general tendency that proteins involved in the same processes behaved similarly in ftsh4-1 and oma1, that is, they were either upregulated or downregulated. All proteins involved in stress response, protein import except for MIA40 in ftsh4-1, and the majority of proteins involved in mitochondrial gene expression, including translation, were upregulated in both ftsh4-1 and oma1. Among the accumulating proteins, we noted those which were identified in increased amounts in aggregates of both mutants, such as sHSPs, RPS10, and the elongation factor encoded by At2g45030 as well as two J proteins involved in the heat-stress response, GFA2 and AtDjB1/DNAJA3 (Zhou et al., 2012) in oma1 (Supplemental Table S2). The high thermolability of the mitochondrial elongation factor was noticed also in mammalian mitochondria under mild heat-stress conditions (Wilkening et al., 2018). Conversely, all proteins of complexes III and V, the majority of complex I subunits as well as proteins involved in photorespiration, transport, amino acid metabolism, and cofactor biosynthesis were reduced in both mutants (Supplemental Table S2). One of the most surprising findings of this study was the extremely reduced level of cytochrome c (CytC) in ftsh4-1 and its decreased level in oma1 due to prolonged stress of 30°C as shown by Western blotting (Figure 3; Supplemental Figure S5). A decrease in the amount of CytC, although not as large, was also observed in the ftsh4-1 mitochondria isolated from plants grown at 22°C. The reduced abundance of two isoforms of CytC (CytC-1 and CytC-2) was confirmed by the iTRAQ analysis for ftsh4-1 at both 22°C and 30°C (Supplemental Table S2). A link between elevated temperature, sHSP proteins, and the reduced level of CytC during plant seed germination was postulated (Ma et al., 2019).
Potential targets of FTSH4 and/or OMA1 proteases based on the iTRAQ analysis
Since complex I is the most heat-sensitive among the OXPHOS complexes (Downs and Heckathorn, 1998), we took a closer look at its components identified using iTRAQ. Changes in the amount of the subunits of complex I were detected at 22°C in ftsh4-1, but not in oma1 (Supplemental Table S2; Figure 8). Four out of six affected subunits (NAD7, NAD9, PSST, and 13 kDa) are either located within the so-called Q module (120 kDa complex), which is assembled independently of the other parts of complex I, or in its proximity (Figure 8). These four subunits were found at a reduced level suggesting the involvement of FTSH4 in the assembly of the Q module at optimal growth conditions (Figure 8A). Furthermore, we found that at the elevated temperature, 15 and 6 subunits of complex I decreased in abundance, while 4 (NAD9, PSST, MNLL, and B14.7) and three (PSST, TYKY, and B15) subunits accumulated in ftsh4-1 and oma1, respectively (Supplemental Table S2). The majority of these differing proteins is localized at the junction between the hydrophilic and the membrane arms of complex I, including the Q module (Figure 8, A and B). It is conceivable that at least some of the accumulating proteins are proteolytic substrates of FTSH4 and/or OMA1. Of note, NAD9 and PSST proteins are prone to aggregation at 30°C (Supplemental Table S1, A and B). Previously, based on co-immunoprecipitation results, we found the intermembrane-space-localized l-galactono-1,4-lactone dehydrogenase (GLDH) as a potential target of FTSH4 (Opalinska et al., 2017). This protein not only catalyzes the last step of ascorbate biosynthesis pathway, but it also has a non-enzymatic function in the assembly of respiratory complex I (Schimmeyer et al., 2016). The iTRAQ analysis revealed the reduced abundance of this protein in ftsh4-1 at 30°C (Supplemental Table S2; Figure 8A). The lower GLDH expression in addition to other effects of FTSH4 on the abundance of complex I subunits certainly leads to the reduction in total complex I level.
Figure 8.
Visualization of the changes in the protein abundance of the complex I subunits in the ftsh4-1 and oma1 mitochondria based on the iTRAQ analysis. A, Protein changes in the ftsh4-1 mutant grown under 22°C and 30°C. B, Protein changes in the oma1 mutant grown under 22°C and 30°C. The Q module subunits are shown in the box of each panel. Blue color indicates a lowered abundance, yellow = an increased abundance, and gray = no difference in the abundance of the identified protein. The dashed line indicates the protein (GLDH) that is involved in the complex I assembly but is not a permanent component of it. More details are shown in Supplemental Table S2. Complex I architecture is based on Ivanova et al. (2019).
According to iTRAQ, the TIM17-2 subunit of the TIM (TIM17:23 complex), which has been an experimentally confirmed as a proteolytic substrate of FTSH4 (Opalinska et al., 2018), accumulated in the ftsh4-1 mitochondria at both 22°C and 30°C. Like TIM17-2, other inner membrane proteins that accumulated under both tested conditions and therefore could be potential substrates of FTSH4, were as follows: the At4g16450 gene product (20.9 kDa subunit, MNLL) essential for the assembly of the membrane arm of complex I in fungi (Schulte et al., 1994), the uncharacterized import TIM subunit Tim17/Tim22/Tim23 family protein (MJH22), the membrane insertase OXA1, and adenine nucleotide transporter (ADNT1; Supplemental Table S2). Interestingly, ADNT1 was found to not only accumulate but also aggregate at 30°C in ftsh4-1 (Supplemental Table S1A).
The group of proteins that accumulated at both temperatures in ftsh4-1 also includes the MGE1 protein (homolog of GrpE protein 1), which is one of the two factors of nucleotide exchange factors for HSP70 proteins in Arabidopsis mitochondria. MGE1 is also elevated in oma1 at 30°C (Supplemental Table S2). Interestingly, the second Arabidopsis GrpE protein homolog, MGE2, which is required in thermotolerance of Arabidopsis seedlings to the chronic moderate heat stress (Figure 7A; Hu et al., 2012), did not show changes in the abundance in both mutants.
Discussion
In this study, we found that growth disorders caused by exposure of plants deficient in FTSH4 or OMA1 proteases to a long-term elevated temperature of 30°C are associated with substantially increased accumulation of detergent-tolerant protein aggregates. The insoluble aggregates contain mitochondrial sHSPs along with a specific set of mitochondrial proteins most probably prone to unfolding at 30°C. Our data suggest that higher accumulation of aggregates in ftsh4-1 and oma1 than in WT is due to a lack of proteolytic activity of FTSH4 or OMA1, respectively. The chaperone-like activity of FTSH4 also prevents protein aggregation induced by 30°C. Greater accumulation of aggregates in ftsh4-1 than in oma1 correlates with stronger severity of morphological and developmental abnormalities found in ftsh4. Moreover, we documented that activity of FTSH4, but not OMA1, is particularly important in release and degradation of HSP23.6 trapped in protein aggregates after transferring plants from stress (30°C) to optimal conditions (22°C).
Based on classical thermotolerance phenotyping assays, FTSH4 and OMA1 are involved in TMHT but not basic or acquired thermotolerance. Our experiments indicate that the lack of MGE2, a mitochondrial homolog of bacterial GrpE, which similarly to FTSH4 and OMA1 plays an important role in Arabidopsis TMHT (Hu et al., 2012), results in a strong accumulation of HSP23.6, NAD9, and RPS10 in the detergent-insoluble fraction under prolonged stress of 30°C (Supplemental Figure S7). Thus, FTSH4, OMA1, and MGE2 are crucial for TMHT by protecting mitochondria from the accumulation of insoluble protein aggregates. The protection of unfolded proteins from aggregation by MGE2 is likely related to the chaperone function of this protein. Our proteomic data indicate that at 30°C the level of heat-induced MGE2 in ftsh4-1 and oma1 was comparable with WT; however, MGE1, the second copy of GrpE which is expressed constitutively in Arabidopsis mitochondria, was found at an elevated level in both mutants (Supplemental Table S2). Excess amounts of MGE1 together with MGE2 at 30°C in ftsh4-1 and oma1 may result in a dosage imbalance leading to the accumulation of protein aggregates. This assumption is based on the finding in Escherichia coli that the overexpression of GrpE (MGE homolog) caused a defect in the DnaK chaperone system, which prevents the accumulation of unfolded proteins (Sugimoto et al., 2008).
Our previous results showed that FTSH4 degrades oxidatively damaged mitochondrial proteins (Opalinska et al., 2017); however, it was not clear if unfolding state or modification of proteins by ROS trigger the degradation. In this work, we focused on proteins that are prone to misfolding/unfolding and subsequent aggregation due to long-term stress of 30°C. To isolate such aggregates, we used a technique based on centrifugation of detergent-lysed mitochondria, which was previously used to characterize heat-induced aggregates in yeast and human (Bender et al., 2011; Wilkening et al., 2018). Our results showed that the majority of mitochondrial proteins remains soluble after prolonged stress of 30°C and only a small subset of polypeptides was found in the aggregate fraction in WT, ftsh4-1, and oma1. We clearly showed that the loss of FTSH4 or OMA1 led to more intense accumulation of some aggregation-prone proteins compared to WT (e.g. NAD9 in ftsh4-1, RPS10 in both ftsh4-1 and oma1; Figure 2). A virtual absence of protein aggregates was observed at 22°C in WT, ftsh4-1, and oma1 mitochondria. The exception was two scaffold proteins, PHB and SLP1, whose detergent tolerance probably arises from their membrane environment since they belong to the raft-associated membrane proteins (Borner et al., 2005).
Among the proteins identified in insoluble aggregates, we focused on a set of sHSP (HSP23.5, HSP23.6, and HSP26.5). They were detected almost exclusively in aggregates and a substantial increase was found in ftsh4-1, ftsh4-2, and oma1 compared to WT (Figure 2; Supplemental Figure S3). Our data showed that the transcript upregulation is at least partially responsible for accumulation of HSP23.5 and HSP23.6, but not HSP26.5 in ftsh4 and oma1 mutants. It was intriguing, however, that nearly similar upregulation of the HSP23.5 and HSP23.6 transcripts was accompanied by a significant difference in the abundance of proteins they encode suggesting an additional level of regulation. Our experimental approaches (experiments with proteolytically inactive FTSH4, incubation with FTSH4 or OMA1 expressed in insect-cell lysate) indicate that this additional level is connected with a lack of proteolytic activity of FTSH4 or OMA1. We argue that HSP23.6 is a proteolytic substrate of both proteases but chaperone-like activity of FTSH4 also affects the abundance of HSP23.6 in aggregates (Figure 5). Of note, we demonstrated that HSP23.6 is strongly associated with mitochondrial membranes at moderate heat stress (Supplemental Figure S6). Protein- and/or lipid-mediated association has been documented for many members of the sHSP family, although it is also known that the number of sHSPs is localized in the mitochondrial matrix (Horváth et al., 2008). The data show that the nature of the sHSP interaction with the membrane is highly specific and depends on the lipid composition and the extent of lipid unsaturation (Tsvetkova et al., 2002). Interestingly, it was shown that some HSPs (e.g. the 72-kDa inducible Hsp70 in human cells), after association with membrane, might translocate across the membrane (Broquet et al., 2003). At present, it is not known whether HSP23.6 is merely associated with the mitochondrial membranes or is inserted in the membrane (and in what form) under moderate heat stress. The substrate spectrum of inner membrane-anchored FTSH4 and OMA1 proteases includes membrane and membrane-associated proteins (Levytskyy et al., 2017; Opalinska and Janska, 2018). Thus, the strong association of HSP23.6 with the membranes allows it to be degraded by FTSH4 and OMA1. Furthermore, we also do not exclude the possibility that the investigated proteases require adapter proteins for delivery of a substrate to the catalytic center of the protease. The adaptor proteins are known for homologs of FTSH4 in yeast (Dunn et al., 2008). Taken together, the substantial accumulation of sHSPs in insoluble aggregates in the mutants is due to the transcriptional response (ftsh4 and oma1), no degradation by FTSH4 (ftsh4) or OMA1 (oma1), the protective effect of the FTSH4 chaperone activity (ftsh4). In addition, our results strongly favor the view that FTSH4 facilitates the dissociation and degradation of sHSPs from insoluble aggregates during recovery from prolonged moderate heat stress (Figure 6). However, more research is needed to fully understand the involvement of FTSH4 in this process.
It was found that an increased level of temperature-induced mitochondrial sHSP during cotton seed germination led to a decline in the CytC level, inhibition of the COX pathway, and induction of ROS (Ma et al., 2019). It is believed that mitochondrial sHSPs are inhibitors of the CcmFc–CcmH complex formation, resulting in the reduced production of CytC as this protein requires the CcmFc–CcmH complex for maturation. It is conceivable that the same mechanism is behind the spectacular lower abundance of CytC in ftsh4-1 and oma1 at 30°C (Figure 3). Data showing that ftsh4 mutants suffer endogenous oxidative stress (Dolzblasz et al., 2016; Smakowska et al., 2016) are consistent with predicted ROS overproduction due to decreased CytC biogenesis. However, the decreased CytC level in ftsh4-1, although not that prominent, was also observed at 22°C, suggesting that there is an additional mechanism independent of heat-induced sHSPs leading to a reduction in CytC level in the FTSH4-deficient plants.
Numerous studies have demonstrated that the overexpression of sHSPs in homologous and heterologous plant systems, as well as in E. coli and yeast, protects the cells from stress damage and enhances heat tolerance and growth (Santhanagopalan et al., 2015; Waters and Vierling, 2020). Notably, so far there are two studies reporting negative effects of the sHSP accumulation on plant heat stress tolerance. Transgenic Arabidopsis plants constitutively expressing AsHSP17 or AsHSP26.8a from A. stolonifera displayed reduced tolerance to heat stress (Sun et al., 2016, 2020). This effect was explained by an excessive production of sHSPs, and it was proposed that an optimized level of sHSPs in stressed plants is critical to assure their protective function. The change in the sHSP abundance above the basal level has a negative impact on plant response to environmental stress. In agreement with the above, we observed that ftsh4, which had a very high amount of mitochondrial sHSPs was more heat sensitive than oma1.
We have previously reported that mitochondria lacking FTSH4 or OMA1 have reduced complex I abundance and activity under chronic stress of 30°C, and this decline is not related with transcriptional changes but defects in the complex assembly/stability (Smakowska et al., 2016; Migdal et al., 2017). Here, we showed that indeed most of the complex I subunits were downregulated in both mutants; however, four and three subunits accumulated in ftsh4-1 or oma1, respectively, after long-term stress of 30°C (Figure 8). Among proteins accumulating in ftsh4-1, but not in oma1, we identified NAD9, a mitochondrially encoded subunit of the junction region (Q-module) located between hydrophilic and membrane arms of complex I. We revealed that NAD9 is prone to aggregation at 30°C (Figure 2B) and its specific enrichment in ftsh4-1 is due to the lack of proteolytic activity of FTSH4 (Figure 4). Unlike sHSPs, which are substrates for FTSH4 and OMA1, NAD9 is under the proteolytic control of FTSH4 only.
The PSST protein is another subunit of the Q module found in insoluble aggregates in ftsh4-1 and oma1. The NAD9 and PSST proteins inactivated by heat-induced aggregation likely destabilize complex I, and in consequence, other unassembled subunits are degraded and their level is decreased. The reason for a reduced abundance of the Q module subunits including NAD9 in ftsh4-1 at optimal conditions (22°C) is different and is likely due to the lack of a chaperone-like activity of FTSH4. The problem with the protein homeostasis of complex I in ftsh4-1 is probably also caused by the lower abundance of GLDH. This enzyme is involved in the formation of complex I and its stability depends on the direct interaction with FTSH4 (Opalinska et al., 2017). Taken together, FTSH4 controls the protein homeostasis of complex I through a number of regulatory mechanisms.
Conclusions
The extensive Arabidopsis genome-wide mRNA-based analysis classified OMA1 and FTSH4 along with two other mitochondrial (DEG14 and FTSH10) and plastid (FTSH6 and EGY3) peptidases within one co-expression module associated with thermotolerance and protein unfolding (Majsec et al., 2017). Our experimental data confirm this prediction in relation to OMA1 and FTSH4, but FTSH4 is by far more important in that function. Our study revealed that the proteolytic activity of OMA1 and FTSH4 and the chaperone activity of FTSH4 are important in a special type of thermotolerance classified as thermotolerance to chronic moderate heat stress. The activity of these proteases protects the mitochondria from accumulation of insoluble protein aggregates that can cause phenotypic changes. It seems that FTSH4 is crucial not only in thermotolerance, but also during recovery from temperature stress.
Materials and methods
Basic methods (Isolation of Mitochondria, SDS–PAGE and Immunoblotting Analyses, BN Gel Electrophoresis, and Quantitative Reverse Transcription PCR Analyses), as well as MS-based Proteomics, are presented as Supplemental Methods in the Supplemental Data.
Plant materials and growth conditions
WT and mutant Arabidopsis (A. thaliana) plants were of the Columbia-0 ecotype. Plants were grown in growth chambers on agar plates or water cultures at 22°C or 30°C under long-day (LD) photoperiod (16-h light and 8-h dark) with a light intensity of 150 μmol m−2 s−1 and 70% humidity as described in Migdal et al. (2017). The transgenic knockout (KO) lines of ftsh4-1 (SALK_035107) and oma1 (SALK_088054C) were obtained from the Nottingham Arabidopsis Stock Centre (NASC), while the KO line of ftsh4-2 (GABI_103H09) from the Max Planck Institute for Breeding Research. The proteolytically inactive version of FTSH4 protease (FTSH4H486Y) was obtained by a single point mutation in the Zn2+-binding site as described by Opalinska et al. (2018). The ftsh4-1-FTSH4 (revertant) line was characterized previously by Smakowska et al. (2016). The transgenic KO lines of mge2-1 and hot1 were a kind gift from Yee-Yung Charng, Academia Sinica, Taipei, Taiwan. The transgenic KO lines of ftsh11 (SALK_033047) and pam16 (SALK_006266) were obtained from NASC.
Protein aggregation assay
The isolation of insoluble protein aggregates in nonionic detergent from mitochondrial fractions was carried out based on Bender et al. (2011) with slight modifications (Figure 1A). A total of 300 µg isolated mitochondria was centrifuged for 10 min at 21,000g and 4°C. The obtained pellet was resuspended in lysis buffer (0.5% (v/v) Triton X-100, 30 mM Tris pH 7.4, 200 mM KCl, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) to final concentration 0.6 mg/mL and incubated on ice for 30 min. Afterwards, a 125 μL total protein sample was subjected to protein precipitation with trichloroacetic acid (TCA). The remaining volume was subjected to ultracentrifugation at 125,000 × g and 4°C for 30 min. Supernatant was precipitated with TCA, whereas pellet was re-extracted by strong agitation with 500 µL of lysis buffer and then again subjected to ultracentrifugation at the same conditions. Supernatant was discarded and the pellet was resuspended in the solubilization buffer (25 mM Tris–HCl, pH 6.8, 0.8% (w/v) SDS, 2% (v/v) β-mercaptoethanol, 8 M urea, 2 M thiourea, and 0.08% (w/v) bromophenol blue).
Cell-free expression of FTSH4 and OMA1
To synthesize FTSH4 and OMA1 proteases, PCR-based generation of linear templates was performed using the RTS Linear Template Kit (Biotechrabbit, Hennigsdorf, Germany). The generated PCR products were applied in the Spodoptera frugiperda-based cell-free RTS 100 Insect Membrane Kit (Biotechrabbit, Hennigsdorf, Germany) to express mature form of FTSH4 (54-717 AA) and OMA1 (80-442 AA) proteases according to the provided manual.
In vitro degradation assay
Mitochondria isolated from ftsh4-1 grown at 30°C were resuspended in the digitonin-containing buffer (300 mM sucrose, 10 mM MOPS pH 7.2, 1% (w/v) digitonin, 100 mM KCl, 10 mM MgCl2, 25 µM ZnSO4, 8 mM ATP, 5 mM SA, 2 mM PMSF, and 10% (v/v) glycerol) in the amount 2.5 mg/mL and incubated at 30°C with FTSH4 or OMA1 protease synthesized in the cell-free expression system or the control cell-lysate for indicated time points. The reaction was stopped with Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA).
Thermotolerance assays
The thermotolerance assays were performed according to Hu et al. (2012) with slight modifications. For the BT assay, 4-d-old seedlings grown at 22°C were subjected to heat stress of 45°C for 30 min. For the SAT test, 5-d-old seedlings grown at 22°C were first acclimated to 38°C heat treatment for 1-h and after 2-h recovery at 22°C the seedlings were challenged with acute heat shock of 45°C for 150 min. For the LAT assay, 9-d-old seedlings grown at 22°C were first acclimated to the heat treatment (38°C, 1 h), subjected to recovery for 2 d at 22°C and then treated with heat stress of 45°C for 50 min. For TMHT assay, 5-d-old seedlings grown at 22°C were subjected to moderately high temperature of 33°C for 6 d. For the BT, SAT, and LAT assays, Arabidopsis seedlings were grown on agar plates with 0.5 × MS (Murashige and Skoog) medium and 1% (w/v) sucrose. For the TMHT assay, 0.1% (w/v) sucrose was used.
Fractionation of mitochondria
Fractionation of mitochondria isolated from plants grown at 30°C was performed according to Millar and Heazlewood (2003) with some modifications. Mitochondrial proteins were resuspended in 10 mM TES-KOH (pH 7.5) and subjected to three cycles of freeze/thaw stress to rupture membranes. After ultracentrifugation (135,000g, 15 min, 4°C), the pellet was resuspended in 100 mM Na2CO3 (pH 11.5) and incubated on ice for 30 min. The samples were ultracentrifuged for 30 min at 135,000g and 4°C. The pellet contained integral membrane proteins, while supernatant—the membrane-associated proteins. The samples were resuspended in denaturing solution (1% (w/v) SDS, 0.1 M NaOH).
Accession numbers
Sequence data of FTSH4 and OMA1 genes can be found in the GenBank/EMBL data libraries under accession numbers AT2G26140 and AT5G51740, respectively. Accession numbers for genes used in RT-qPCR experiments are listed in Supplemental Table S4. Accession numbers for proteins identified in MS-based experiments are listed in Supplemental Tables S1, A and B and S2.
Supplemental data
The following supplemental materials are available in the online version of this article.
Supplemental Figure S1. Transcript level of selected marker genes of the UPR in the 2-week-old WT, ftsh4-1, ftsh4-2, and oma1 plants.
Supplemental Figure S2. Coomassie-stained 12% SDS–PAGE gel with Triton X-100-insoluble pellet fractions obtained from mitochondria of WT and ftsh4-1 plants grown under moderate heat stress (30°C).
Supplemental Figure S3. Accumulation of the HSP23.6, HSP23.5, NAD9, and RPS10 aggregates in the ftsh4-1, ftsh4-2, and ftsh4-1-FTSH4 (revertant) plants grown under moderate heat stress (30°C).
Supplemental Figure S4. Immunodetection of selected proteins in the Triton X-100-treated mitochondria of WT, ftsh4-1, and oma1 plants grown under moderate heat stress (30°C) (A) and optimal growth conditions (22°C) (B).
Supplemental Figure S5. Immunodetection of HSP23.6 and CytC in protein fractions after solubilization of mitochondria in the 5% digitonin buffer in the WT, ftsh4-1, FTSH4OE, and oma1 plants.
Supplemental Figure S6. Intramitochondrial localization of HSP23.6 in Arabidopsis.
Supplemental Figure S7. The influence of moderate heat stress of 30°C on morphological changes and protein aggregation in the mitochondria of indicated plants.
Supplemental Table S1A. List of mitochondrial proteins enriched in the Triton X-100-insoluble pellet fractions of the WT, ftsh4-1, and oma1 mitochondria from 30°C, identified by LC–MS.
Supplemental Table S1B. List of mitochondrial proteins identified by MS in bands cut out of the SDS–PAGE gel with Triton X-100-insoluble protein pellet fraction of the ftsh4-1 mitochondria from 30°C.
Supplemental Table S2. Quantitative comparative iTRAQ analyses of the mitochondrial proteomes of ftsh4-1 and oma1 mutants in comparison to the WT Arabidopsis plants grown under optimal (LD, 22°C) and moderate heat stress conditions (LD, 30°C).
Supplemental Table S3. List of antibodies used in this study.
Supplemental Table S4. Primer pairs used for RT-qPCR.
Supplementary Material
Acknowledgments
We would like to thank Magdalena Opalinska for her scientific help at the beginning of the project. We thank Jose M. Gualberto and Geraldine Bonnard (CNRS, Strasbourg, France), Elzbieta Glaser (University of Stockholm, Sweden), Lee Sweetlove (University of Oxford, UK), Monika Murcha (University of Western Australia, Australia), and Yee-Yung Charng (Academia Sinica, Taipei, Taiwan) for providing antibodies. We are also grateful Yee-Yung Charng for providing the transgenic KO lines of mge2-1 and hot1.
Funding
This work was supported by grant 2017/27/B/NZ2/00558 from the National Science Centre, Poland. Publication of this article in open access was financially supported by the Excellence Initiative - Research University (IDUB) programme for the University of Wroclaw.
Conflict of interest statement. None declared.
A.M. performed the experiments based on the protein aggregation assay and mitochondria fractionation with assistance of M.H.C. A.M. performed the thermotolerance assays. A.M. and M.H.C. performed the immunoblotting assays. M.H.C. performed cell-free expression of proteases, in vitro degradation assays, and BN-PAGE-based experiments. A.M., M.H.C., and H.J. analyzed the MS-based proteomics data. A.W. and A.M. conducted the RT-qPCR analysis. H.J. designed the study, supervised the experiments, and wrote the manuscript with contribution from M.H.C. All authors analyzed the data.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Hanna Janska (hanna.janska@uwr.edu.pl).
References
- Adam Z, Aviv-Sharon E, Keren-Paz A, Naveh L, Rozenberg M, Savidor A, Chen J (2019) The chloroplast envelope protease FTSH11 - interaction with CPN60 and identification of potential substrates. Front Plant Sci 10: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353: aac4354. [DOI] [PubMed] [Google Scholar]
- Bender T, Lewrenz I, Franken S, Baitzel C, Voos W (2011) Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim/LON protease. Mol Biol Cell 22: 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 137: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem 278: 21601–21606 [DOI] [PubMed] [Google Scholar]
- Bruderek M, Jaworek W, Wilkening A, Rüb C, Cenini G, Förtsch A, Sylvester M, Voos W (2018) IMiQ: a novel protein quality control compartment protecting mitochondrial functional integrity. Mol Biol Cell 29: 235–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Burke JJ, Velten J, Xin Z (2006) FtsH11 protease plays a critical role in Arabidopsis thermotolerance. Plant J 48: 73–84 [DOI] [PubMed] [Google Scholar]
- Dabbaghizadeh A, Morrow G, Amer YO, Chatelain EH, Pichaud N (2018) Identification of proteins interacting with the mitochondrial small heat shock protein Hsp22 of Drosophila melanogaster: implication in mitochondrial homeostasis. PLoS One 13: e0193771 doi.org/10.1371/journal.pone.0193771, 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzblasz A, Smakowska E, Gola E, Sokolowska K, Kicia M, Janska H (2016) The mitochondrial protease AtFTSH4 safeguards Arabidopsis shoot apical meristem function. Sci Rep 6: 28315–28329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CA, Heckathorn SA (1998) The mitochondrial small heat-shock protein protects NADH:ubiquinone oxidoreductase of the electron transport chain during heat stress in plants. FEBS Lett 430: 246–250 [DOI] [PubMed] [Google Scholar]
- Dunn CD, Tamura Y, Sesaki H, Jensen RE (2008) Mgr3p and Mgr1p are adaptors for the mitochondrial i-AAA protease complex. Mol Biol Cell 19: 5387–5397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyballa N, Metzger S (2009) Fast and sensitive colloidal Coomassie G-250 staining for proteins in polyacrylamide gels. J Vis Exp 30:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar MR, Feussner I, Valle EM (2021) Mitochondrial small heat shock proteins are essential for normal growth of Arabidopsis thaliana. Front Plant Sci 12: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E (2015) A first line of stress defence: small heat shock proteins and their function in protein homeostasis. J Mol Biol 427: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidorn-Czarna M, Domanski D, Kwasniak-Owczarek M, Janska H (2018) Targeted proteomics approach toward understanding the role of the mitochondrial protease FTSH4 in the biogenesis of OXPHOS during Arabidopsis seed germination. Front Plant Sci 9: 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath I, Multhoff G, Sonnleitner A, Vigh L (2008) Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta 1778: 1653–1664 [DOI] [PubMed] [Google Scholar]
- Hu C, Lin S, Chi W, Charng Y (2012) Recent gene duplication and subfunctionalization produced a mitochondrial GrpE, the nucleotide exchange factor of the Hsp70 complex, specialized in thermotolerance to chronic heat stress. Plant Physiol 158: 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Gill-Hille M, Huang S, Branca R, Kmiec B, Teixeira PF, Lehtiö J, Whelan J, Murcha MW (2019) A mitochondrial LYR protein is re-quired for Complex I assembly. Plant Physiol 181: 1632–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Yamaguchi-Shinozaki K, Shinozaki K (2018) ER-anchored transcription factors bZIP17 and bZIP28 regulate root elongation. Plant Physiol 176: 2221–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138: 882–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levytskyy RM, Bohovych I, Khalimonchuk O (2017) Metalloproteases of the inner mitochondrial membrane. Biochemistry 56: 4737–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas E, Pulido P, Rodríguez-Concepción M (2017) Interference with plastome gene expression and Clp protease activityin Arabidopsis triggers a chloroplast unfolded protein response to restore protein homeostasis. PLoS Genet 13: e1007022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu W, Selinski J, Li L, Day DA, Murcha MW, Whelan J, Wang Y (2018) Isolation and respiratory measurements of mitochondria from Arabidopsis thaliana. J Vis Exp 131: e56627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Guan X, Li J, Pan R, Wang L, Liu F, Ma H, Zhu S, Hu J, Ruan YL et al. (2019) Mitochondrial small heat shock protein mediates seed germination via thermal sensing. Proc Natl Acad Sci USA 116: 4716–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majsec K, Bhuiyan NH, Sun Q, Kumari S, Kumar V, Ware D, Van Wijk KJ (2017) The plastid and mitochondrial peptidase network in Arabidopsis thaliana: a foundation for testing genetic interactions and functions in organellar proteostasis. Plant Cell 29: 2687–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdal I, Skibior-Blaszczyk R, Heidorn-Czarna M, Kolodziejczak M, Garbiec A, Janska H (2017) AtOMA1 affects the OXPHOS system and plant growth in contrast to other newly identified ATP-independent proteases in Arabidopsis mitochondria. Front Plant Sci 8: 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Heazlewood JL (2003). Genomic and proteomic analysis of mitochondrial carrier proteins in Arabidopsis. Plant Physiol 131: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcha M, Narsai R, Devenish J, Kubiszewski-Jakubiak S, Whelan J (2015) MPIC: a mitochondrial protein import components database for plant and non-plant species. Plant Cell Physiol 56: e10(1–12). [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Vígh L (2007) The small heat shock proteins and their clients. Cell Mol Life Sci 64: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh NU, Haynes CM (2019) Signaling and regulation of the mitochondrial unfolded protein response. Cold Spring Harb Perspect Biol 11: a03394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalinska M, Parys K, Janska H (2017) Identification of physiological substrates and binding partners of the plant mitochondrial protease FTSH4 by the trapping approach. Int J Mol Sci 18: 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opalinska M, Parys K, Murcha MW, Janska H (2018) The plant i-AAA protease controls the turnover of an essential mitochondrial protein import component. J Cell Sci 131: 1–6 [DOI] [PubMed] [Google Scholar]
- Opalinska M, Janska H (2018) AAA proteases: guardians of mitochondrial function. Cells 7: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana RM, Iqbal A, Wattoo FM, Khan MA, Zhang H (2018) HSP70 mediated stress modulation in plants. In Asea A, Kaur P, eds, Heat Shock Proteins and Stress. Heat Shock Proteins, Vol 15. Springer, Cham, Switzerland, pp 281–290 [Google Scholar]
- Santhanagopalan I, Basha E, Ballard KN, Bopp NE, Vierling E (2015) Model chaperones: small heat shock proteins from plants. In Tanguay R, Hightower L, eds, The Big Book on Small Heat Shock Proteins. Heat Shock Proteins, Vol 8. Springer, Cham, Switzerland, pp 119–153 [Google Scholar]
- Santiago AM, Gonçalves DL, Morano KA (2020) Mechanisms of sensing and response to proteotoxic stress. Exp Cell Res 395: 112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowsky C, Thal B, Braun H-P, Eubel H (2018) Sample preparation for analysis of the plant mitochondrial membrane proteome. Methods Mol Biol 1696: 163–183 [DOI] [PubMed] [Google Scholar]
- Schimmeyer J, Bock R, Meyer EH (2016) L-Galactono-1,4-lactone dehydrogenase is an assembly factor of the membrane arm of mitochondrial complex I in Arabidopsis. Plant Mol Biol 90: 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte U, Fecke W, Krüll C, Nehls U, Schmiede A, Schneider R, Ohnishi T, Weiss H (1994) In vivo dissection of the mitochondrial respiratory NADH:ubiquinone oxidoreductase (complex I). Biochim Biophys Acta 1187: 121–124 [DOI] [PubMed] [Google Scholar]
- Siddique M, Gernhard S, von Koskull-Doring P, Vierling E, Scharf KD (2008) The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 13: 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smakowska E, Skibior-Blaszczyk R, Czarna M, Kolodziejczak M, Kwasniak-Owczarek M, Parys K, Funk C, Janska H (2016) Lack of FTSH4 protease affects protein carbonylation, mitochondrial morphology and phospholipid content in mitochondria of Arabidopsis: new insights into a complex interplay. Plant Physiol 171: 2516–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto S, Saruwatari K, Higashi C, Sonomoto K (2008) The proper ratio of GrpE to DnaK is important for protein quality control by the DnaK/DnaJ/GrpE chaperone system and for cell division. Microbiology 154: 1876–1885 [DOI] [PubMed] [Google Scholar]
- Sun X, Sun C, Li Z, Hu Q, Han L, Luo H (2016) AsHSP17, a creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signaling to attenuate plant response to abiotic stress. Plant Cell Environ 39: 1320–1337 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhu J, Li X, Li Z, Han L (2020) AsHSP26.8a, a creeping bentgrass small heat shock protein integrates different signaling pathways to modulate plant abiotic stress response. BMC Plant Biol 20: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HC, van Aken O (2020) Mitochondrial unfolded protein-related responses across kingdoms: similar problems, different regulators. Mitochondrion 53: 166–177 [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Horvath I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, et al. (2002) Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA 99: 13504–13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urantowka A, Knorpp C, Olczak T, Kolodziejczak M, Janska H (2005) Plant mitochondria contain at least two i-AAA-like complexes. Plant Mol Biol 59: 239–252 [DOI] [PubMed] [Google Scholar]
- Van Aken O, Zhang B, Carrie C, Ugalla V, Paynter E, Giraud E, Whelan J (2009) Defining the mitochondrial stress response in Arabidopsis thaliana. Mol Plant 2: 1310–1324 [DOI] [PubMed] [Google Scholar]
- Voos W (2013) Chaperone-protease networks in mitochondrial protein homeostasis. Biochim Biophys Acta 1833: 388–399 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9: 244–252 [DOI] [PubMed] [Google Scholar]
- Wang X, Auwerx J (2017) Systems phytohormone responses to mitochondrial proteotoxic stress. Mol Cell 68: 540–551 [DOI] [PubMed] [Google Scholar]
- Waters ER, Nguyen SL, Eskandar R, Behan J, Sanders-Reed Z (2008) The recent evolution of a pseudogene: diversity and divergence of a mitochondria-localized small heat shock protein in Arabidopsis thaliana. Genome 51: 177–186 [DOI] [PubMed] [Google Scholar]
- Waters E, Vierling E (2020) Plant small heat shock proteins-evolutionary and functional diversity. New Phytol 1: 25–37 [DOI] [PubMed] [Google Scholar]
- Wilkening A, Rüb C, Sylvester M, Voos W (2018) Analysis of heat-induced protein aggregation in human mitochondria. J Biol Chem 293: 11537–11552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Kaplinsky NJ, Hu C, Charng YY (2012) Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Sci 195: 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wu J, Yuan D, Zhang D, Huang Z, Xiao L, Yang C (2014) Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates Arabidopsis architecture. Mol Plant 7: 856–873 [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C, Wang R, Chen Y, Shu S, Huang R, Zhang D, Li J, Xiao S, Yao N et al. (2017) The Arabidopsis mitochondrial protease FtSH4 is involved in leaf senescence via regulation of WRKY-dependent salicylic acid accumulation and signaling. Plant Physiol 173: 2294–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhou T, Li MX, Zhao CL, Jia N, Wang XX, Sun YZ, Li GL, Xu M, Zhou RG, et al. (2012) The Arabidopsis J-protein AtDjB1 facilitates thermotolerance by protecting cells against heat-induced oxidative damage. New Phytol 194: 364–378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.