Abstract
Aims
While myocardial ischaemia plays a major role in the pathogenesis of heart failure (HF), the indications for coronary angiography during acute HF are not established. We determined the association of early coronary angiography during acute HF hospitalization with 2-year mortality, cardiovascular death, HF readmissions, and coronary revascularization.
Methods and results
In a two-stage sampling process, we identified acute HF patients who presented to 70 emergency departments in Ontario (April 2010 to March 2013) and determined whether they underwent early coronary angiography within 14 days after presentation using administrative databases. After clinical record review, we defined a cohort with acute ischaemic HF as patients with at least one factor suggesting underlying ischaemic heart disease, including previous myocardial infarction, troponin elevation, or angina on presentation. We oversampled patients undergoing angiography. We used inverse-probability-of-treatment weighting (IPTW) to adjust for baseline differences. Of 7239 patients with acute HF, 2994 met inclusion criteria [median age 75 (interquartile range 65–83) years; 40.9% women]. Early angiography was performed in 1567 patients (52.3%) and was associated with lower all-cause mortality [hazard ratio (HR) 0.74, 95% confidence interval (CI) 0.61–0.90, P = 0.002], cardiovascular death (HR 0.72, 95% CI 0.56–0.93, P = 0.012), and HF readmissions (HR 0.84, 95% CI 0.71–0.99, P = 0.042) after IPTW. Those undergoing early angiography experienced higher rates of percutaneous coronary intervention (HR 2.58, 95% CI 1.73–3.86, P < 0.001) and coronary artery bypass grafting (HR 2.94, 95% CI 1.75–4.93, P < 0.001) within 2 years.
Conclusions
Early coronary angiography was associated with lower all-cause mortality, cardiovascular death, HF readmissions, and higher rates of coronary revascularization in acute HF patients with possible ischaemia.
Keywords: Heart failure, Ischaemia, Coronary artery disease, Coronary revascularization, Death, Outcomes
Graphical Abstract
See page 3767 for the editorial comment on this article (doi:10.1093/eurheartj/ehab513)
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
Introduction
Heart failure (HF) is a major global health burden, impacting 26 million people worldwide and rising in prevalence.1 A substantial component of this burden is due to acute HF hospitalizations, which have a 1-year mortality of 25–40%.2 Coronary artery disease (CAD) is a key risk factor for the development of HF and is identified as the primary aetiology in 66% of cases.3,4 The presence of CAD in HF portends a worse prognosis, which can be stratified by the severity of the underlying CAD.5–7 While guidelines suggest considering an ischaemic aetiology in any episode of acute HF, there is no standard approach for identifying patients requiring investigation.8,9
The decision to pursue diagnostic coronary angiography is a considerable challenge in acute HF. This decision is generally guided by the presence of ischaemic symptoms and signs, including chest pain and troponin elevation, but these features are common in patients hospitalized with HF, and their presence has been shown to be poorly predictive of subsequent outcomes.10,11 There is wide variability in the rates of invasive angiography, highlighting the clinical equipoise in its use in acute HF.12,13
We constructed a cohort of patients presenting with HF and at least one feature suggestive of potential underlying CAD, which included any of prior myocardial infarction (MI), troponin elevation, or angina on presentation. Previously, we designated these patients as presenting with syndromic acute ischaemic HF (AIHF).14 The objectives of our study were to determine the association of early coronary angiography with mortality, readmissions, and revascularization in patients with AIHF.
Methods
Data sources
The cohort and study design have been published previously.14 We initially identified patients who presented to an emergency department with HF in Ontario, Canada, from 1 April 2010 to 31 March 2013, using the National Ambulatory Care Reporting System (NACRS). Hospitalized patients were identified using the Canadian Institute of Health Information Discharge Abstract Database (CIHI-DAD). The CIHI-DAD and CIHI Same-Day Surgery (CIHI-SDS) database were used to identify in-hospital cardiac procedures.15 Patients were selected for detailed clinical chart review from academic, medium-sized (51–150 annual HF emergency visits), and large (>150 annual HF emergency visits) community hospitals from 1 April 2010 to 31 March 2013 using stratified cluster sampling.14 If there were multiple visits during the period, the first visit was chosen for chart abstraction. Both the CIHI-DAD and NACRS use the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10-CA), with the diagnostic code I50 indicating a primary diagnosis of HF. The cohort accrual period from 2010 to 2013 was utilized in order to capture long-term outcomes and cardiovascular causes of death, which became available in 2017. Primary data collection for the study began in 2015 and was completed in 2017, when the analysis was initiated on a series of projects.14
Highly trained specialized nurse or physician abstractors collected data on 140 patients from each of 13 academic and 30 large community hospitals and 50 patients from each of 27 medium-sized community hospitals.14 Clinical data were collected from hospital records using electronic case report forms with automated range checks, double-data entry for key variables, and preloaded medical record numbers to optimize administrative database linkage. Information was retrieved on demographics, clinical characteristics, medications, laboratory tests, and cardiac imaging as detailed previously.14 Coronary angiogram reports were retrieved and reviewed for the presence and location of coronary stenoses.
The Ontario Health Insurance Plan (OHIP) database was used to extract physician claims. The OHIP database, interRAI home care data, home care database, and continuing care reporting system were used to identify patients receiving palliative care. All data sources were compiled, uniquely encoded, and analysed at ICES. The Registered Persons Database (RPDB) was used to determine deaths, and cardiovascular deaths were determined using the Office of the Registrar General database. Research ethics board approval was obtained from all hospitals prior to data abstraction.
Study design and participants
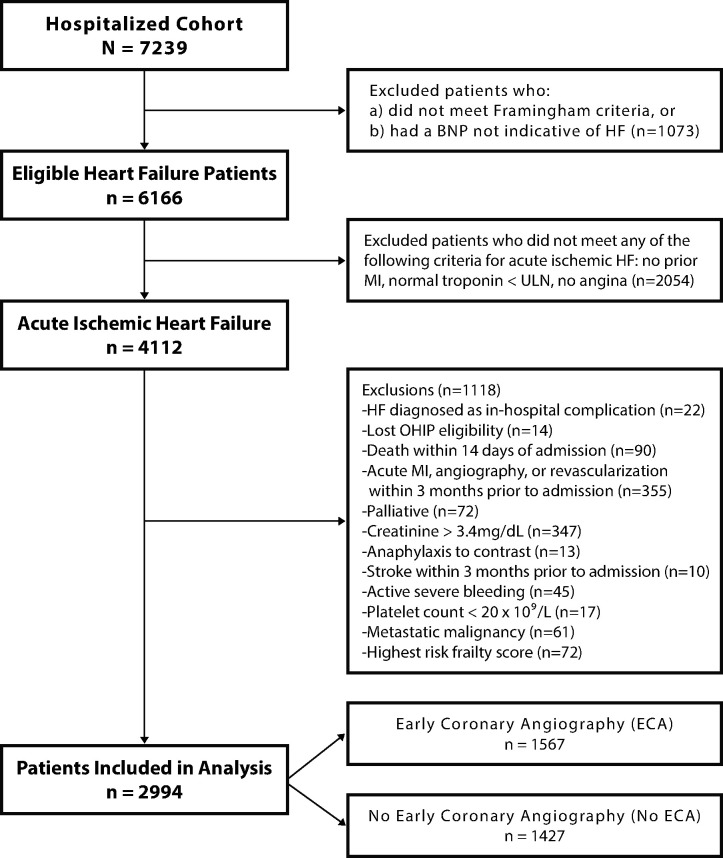
We identified patients with acute HF using the CIHI database (using the ICD-10-CA code I50) and oversampled those undergoing early coronary angiography to allow for a well-powered comparison. Patients were included only if they met Framingham criteria for HF on chart review, and were excluded if brain natriuretic peptide (BNP) or N-terminal pro BNP (NT-proBNP) levels were not indicative of HF as per the Canadian Cardiovascular Society guidelines.16 Patients with a most responsible diagnosis of an acute coronary syndrome were not included. Early coronary angiography was defined as invasive angiography performed within 14 days of presentation, identified by Canadian Classification of Interventions procedure code 3IP10 in the CIHI-DAD or CIHI-SDS, and confirmed by clinical chart review.15 The 14-day period was selected to allow for the inclusion of patients receiving either inpatient or short-term post-discharge angiography. To remove individuals for whom angiography would have a lower yield during the acute HF episode, we excluded patients with a hospitalization for acute MI, coronary angiography, or revascularization up to 3 months prior to admission. All included patients were admitted acutely via the emergency department; those admitted for elective cardiac catheterization were excluded. We also excluded patients with invalid health insurance numbers, non-residents of Ontario, HF as a secondary diagnosis, or palliative patients (Figure 1). We excluded patients with contraindications to angiography, including creatinine ≥3.4 mg/dL (300 µmol/L), anaphylactic contrast allergy, stroke within 3 months, active severe bleeding, platelet count <20 × 109/L, metastatic malignancy, or the highest degree of frailty by the Hospital Frailty Risk Score.17
Figure 1.
Cohort creation diagram. Inclusion and exclusion criteria are displayed.
Definitions
Troponin values were categorized as normal, greater than the upper limit of normal (ULN), or in the MI range, as per hospital-specific assays. Heart failure was categorized as preserved ejection fraction (HFpEF, ejection fraction ≥50%), mid-range ejection fraction (HFmrEF, ejection fraction 40–49%), or reduced ejection fraction (HFrEF, ejection fraction <40%). Any significant coronary stenosis was defined as luminal stenoses of ≥50% in the left main or ≥70% in either the left anterior descending, circumflex, or right coronary arteries. Multivessel disease was defined as significant stenoses in any two of the three arteries or significant left main disease.
Outcomes
The co-primary outcomes were all-cause and cardiovascular mortality. Secondary outcomes included readmissions for all causes, acute MI, and HF. We also examined revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery. Using the ICD-10-CA coding system we identified hospitalizations for acute MI (codes I21 and I22) and readmissions for HF (code I50), which have been validated previously.18 All outcomes were assessed at 2 years, and revascularization outcomes were further examined at 90 days. To ensure that deaths occurring before angiography did not influence the results (while also allowing enough time for angiography to occur), we used a landmark design such that subjects who died within 14 days of the index admission were excluded. Subjects were followed for outcome ascertainment from this 14-day landmark forward. Similarly, for the readmission analyses, subjects who died or were readmitted within 14 days post-admission were excluded. Subjects were then followed forward for outcome ascertainment from this 14-day landmark. For the analysis of revascularization events after angiography, procedures occurring on or after the date of angiography were considered to account for ad hoc PCI procedures.
Variable selection
Variables with the potential to impact outcomes in HF or predict coronary angiography in acute coronary syndromes were used to derive weights for propensity score analyses.19,20 We adjusted for demographics (age, sex, race), clinical features on arrival [transport by emergency medical services, systolic blood pressure, heart rate, oxygen saturation, cardiogenic shock (defined as a systolic blood pressure <90 mmHg for > 30 min accompanied by signs of hypoperfusion requiring inotropic support), severity of dyspnoea at presentation, angina within 48 h of hospital presentation], the validated EHMRG score for acute HF mortality risk,21–23 comorbidities [prior MI, diabetes, hypertension, previous stroke, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), dementia, malignancy, smoking, obesity, family history of CAD, hypercholesterolaemia, prior PCI, and prior CABG], investigations on arrival [haemoglobin, sodium, creatinine, troponin (>ULN and MI range)], BNP, atrial fibrillation/flutter, QRS duration, ST-depression on electrocardiogram, performance of echocardiography, and left ventricular ejection fraction as assessed by echocardiography or other modalities (e.g. cardiac magnetic resonance imaging, radionuclide angiography, or ventriculography) during the index hospitalization.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges and were compared using the Kruskal–Wallis test. We used the χ2 test for the comparison of categorical variables. We used multiple logistic regression analysis to determine the predictors of early angiography, selecting those with a P-value < 0.25 on univariate analysis for entry into the multivariable model.
We used inverse-probability-of-treatment weighting (IPTW) to determine a weight for each individual representing the inverse of the probability of receiving the treatment actually received (angiography or control).24 Multiple imputation was used to impute missing values via the creation of six imputed datasets. In the imputation models for missing data, we used each variable from the IPTW models (Supplementary material online, Table S1), as well as the outcomes in the analysis models. Within each imputed dataset, standardized differences were used to compare baseline variables between the two exposure groups before and after weighting. The maximum standardized differences across the six imputed datasets are presented.
Primary and secondary outcomes were compared before and after IPTW. Weighted Cox proportional hazard regression models were used to estimate hazard ratios (HRs) for each outcome at each timepoint of interest, and the proportional hazards assumption was tested for each model. Outcomes with competing risks were evaluated using cause-specific hazard models with death as a competing event for hospitalizations, and non-cardiovascular death as a competing event for the cardiovascular death outcome. Robust variance estimators were used when fitting the weighted Cox regression models or the cause-specific hazard models.25 All analyses were repeated within the three overlapping cohort-defining subgroups (prior MI, troponin elevation >ULN, and angina). Additionally, we conducted subgroup analyses to determine potential effect modification between incident or non-incident HF and early angiography using Wald’s test for interaction. If an interaction existed, we stratified the analyses by the presence of new or known HF.
Weighted Kaplan–Meier survival curves and the adjusted log-rank test were used to compare all-cause mortality between groups after IPTW. Weighted cumulative incidence functions were used to compare the cumulative incidence of cardiovascular death between groups, treating non-cardiovascular death as a competing risk. Weighted cumulative incidence functions were also used to compare incidence of PCI, CABG, and any revascularization (either PCI or CABG) over time between the early angiography and no angiography cohorts; in these analyses, time zero was defined as (i) the date of diagnostic angiography procedure for the early angiography cohort and (ii) day 14 for the no angiography cohort. P-values were derived from weighted univariate Fine-Gray models. All analyses were performed with SAS version 9.4 statistical software (SAS Institute, Cary, NC, USA). Prior to analysis, the protocol of this study, the Invasive Coronary Angiography Early in Ischaemic Heart Failure (ICE-HF) study, was registered on ClinicalTrials.gov (NCT04245605).
Sensitivity analyses
We used the methods described by Lin et al.,26 to explore the potential effect of unmeasured confounders on the association between early angiography and all-cause mortality. The method infers robustness of the findings, if after varying γ (the magnitude of the HR for the unknown confounder, U), the upper limit of the confidence interval (CI) for the main exposure HR is bounded by unity for the differing prevalence of U in the two comparator arms (P1, P2).26 Additionally, utilization of non-invasive ischaemic testing (i.e. single-photon emission computed tomography myocardial perfusion, cardiac positron emission tomography imaging, stress echocardiography, coronary computed tomography angiography, exercise electrocardiogram) within 14 days was compared between the early angiography and no angiography cohorts as determined by detailed clinical chart review or physician claims data (using administrative data codes that were previously published).15 Inverse-probability-of-treatment weighting was then repeated, incorporating the performance of non-invasive testing as an additional covariate in the propensity score model.
Results
Study cohort
The study-eligible HF cohort included 6166 patients, of whom 4112 met the criteria for AIHF (Figure 1). After applying exclusion criteria, 2994 patients remained in the final study cohort, of whom 1567 (52.3%) underwent early coronary angiography and 1427 (47.7%) did not. There were no losses to follow-up.
Clinical factors associated with coronary angiography
Baseline clinical characteristics are reported in Table 1. Odds ratios representing the association of each covariate with the performance of early angiography are displayed in Figure 2. The means and standard deviations used to calculate odds ratios for continuous variables are also presented (Supplementary material online, Table S2). Factors associated with higher odds of early angiography included prior MI, angina, dyspnoea symptoms, family history of CAD, HFrEF, HFmrEF, troponin elevation (MI range), new ST-segment changes, and higher haemoglobin. Factors associated with lower odds of early coronary angiography included dementia, higher creatinine, COPD, atrial fibrillation/flutter, prior CABG, older age, and white race.
Table 1.
Baseline clinical characteristics according to early coronary angiography status
| Variable | Early coronary angiography (n = 1567) | No early coronary angiography (n = 1427) | SD | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 71 (62‒79) | 79 (70‒85) | 0.645 | <0.001 |
| Men | 973 (62.1) | 790 (55.4) | 0.137 | <0.001 |
| White race | 560 (35.7) | 572 (40.1) | 0.090 | 0.014 |
| Features on arrival | ||||
| Transport by EMS | 655 (41.8) | 684 (47.9) | 0.124 | <0.001 |
| Systolic BP (mmHg) | 146 (127‒167) | 140 (123‒160) | 0.195 | <0.001 |
| Heart rate (b.p.m.) | 99 (80‒118) | 87 (72‒106) | 0.382 | <0.001 |
| Oxygen saturation | 0.96 (0.92‒0.98) | 0.96 (0.92‒0.98) | 0.048 | 0.197 |
| Cardiogenic shock | 27 (1.7) | 9 (0.6) | 0.101 | 0.006 |
| Dyspnoea symptoms | 1,323 (84.4) | 1,089 (76.3) | 0.205 | <0.001 |
| Angina | 838 (53.5) | 594 (41.6) | 0.239 | <0.001 |
| EHMRG 7-D risk scorea | 211 (161‒256) | 231 (188‒278) | 0.332 | <0.001 |
| Comorbidities | ||||
| Prior MI | 1,079 (68.9) | 847 (59.4) | 0.199 | <0.001 |
| Diabetes | 685 (43.7) | 593 (41.6) | 0.044 | 0.233 |
| Hypertension | 1,155 (73.7) | 1,115 (78.1) | 0.104 | 0.005 |
| Cerebrovascular disease | 187 (11.9) | 236 (16.5) | 0.132 | <0.001 |
| Peripheral artery disease | 139 (8.9) | 152 (10.7) | 0.060 | 0.100 |
| COPD | 240 (15.3) | 352 (24.7) | 0.235 | <0.001 |
| Dementia | 23 (1.5) | 90 (6.3) | 0.252 | <0.001 |
| Malignancy | 163 (10.4) | 192 (13.5) | 0.094 | 0.010 |
| Smoking | 310 (19.8) | 181 (12.7) | 0.193 | <0.001 |
| Obesity | 334 (21.3) | 241 (16.9) | 0.113 | 0.002 |
| Family history of CAD | 284 (18.1) | 102 (7.1) | 0.335 | <0.001 |
| Hypercholesterolaemia | 943 (60.2) | 898 (62.9) | 0.057 | 0.122 |
| Prior PCI | 249 (15.9) | 290 (20.3) | 0.115 | 0.002 |
| Prior CABG | 224 (14.3) | 331 (23.2) | 0.230 | <0.001 |
| Laboratory features | ||||
| Haemoglobin (g/L) | 132 (117‒146) | 121 (108‒137) | 0.444 | <0.001 |
| Sodium (mEq/L) | 138 (136‒140) | 138 (135‒141) | 0.004 | 0.912 |
| Creatinine (mg/dL) | 1.09 (0.87‒1.36) | 1.23 (0.95‒1.64) | 0.366 | <0.001 |
| Troponin > ULN | 1,045 (66.7) | 922 (64.6) | 0.044 | 0.232 |
| Troponin (MI range) | 839 (53.5) | 651 (45.6) | 0.159 | <0.001 |
| ECG/Echo features | ||||
| Atrial fibrillation/flutter | 543 (34.7) | 714 (50.0) | 0.315 | <0.001 |
| QRS duration (ms) | 104 (91‒134) | 105 (88‒140) | 0.014 | 0.721 |
| New ST changes | 783 (50.0) | 558 (39.1) | 0.220 | <0.001 |
| Echocardiogram done | 1,409 (89.9) | 994 (69.7) | 0.521 | <0.001 |
| HFrEF | 826 (52.7) | 421 (29.5) | 0.485 | <0.001 |
| HFmrEF | 280 (17.9) | 191 (13.4) | 0.124 | <0.001 |
| HFpEF | 396 (25.3) | 460 (32.2) | 0.154 | <0.001 |
| LVEF not assessed | 65 (4.1) | 355 (24.9) | 0.616 | <0.001 |
Values are median (interquartile range), or n (%). Additional covariates: The presence of chronic kidney disease was evaluated and was present in <0.5% of patients and not significantly different between the two groups (P = 0.458). For the preservation of confidentiality, any variables with <6 patients present were suppressed, so these variables were not reported.
BNP, brain natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EMS, emergency medical service; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standardized difference; ULN, upper limit of normal.
EHMRG (Emergency Heart failure Mortality Risk Grade) risk score: tool used to estimate 7-day mortality of patients presenting to the emergency department with acute heart failure.
Figure 2.
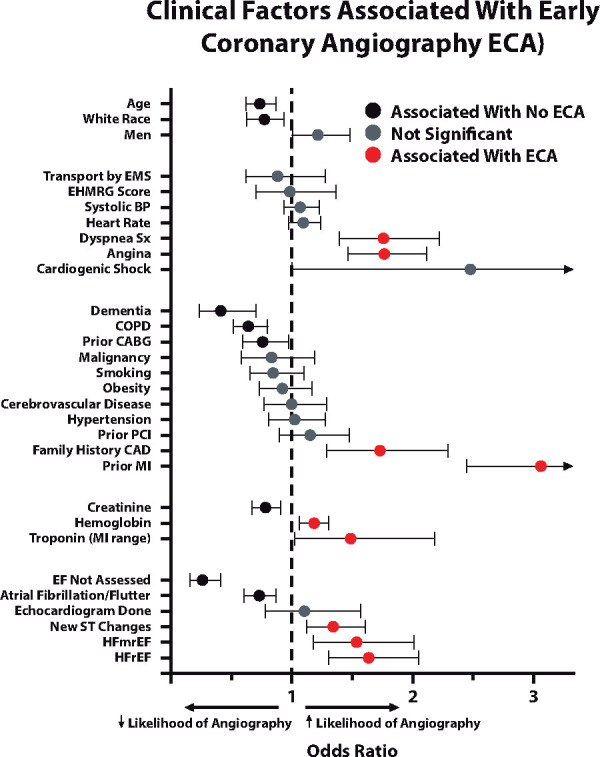
Clinical factors associated with early coronary angiography. Odds ratios (95% confidence intervals) represent the association between each characteristic and the performance of early angiography. Only characteristics that were significantly different between the two groups (Table 1) were included. Confidence intervals marked with an arrowhead were too large to include. Exact values were: cardiogenic shock: 2.47 (0.99–6.17); prior myocardial infarction (MI): 3.05 (2.44–3.80). BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; EMS, emergency medical service; HFmrEF, heart failure with mid-range ejection fraction; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention.
Inverse-probability-of-treatment weighting analysis
After imputation and IPTW analysis, there were no meaningful differences in the distribution of any measured baseline clinical characteristics between the two exposure groups; all maximal weighted standardized differences were <0.062 across the six imputed datasets (Figure 3). Notably, the creatinine concentrations were also well-matched between the two groups, with 95% of patients having values ≤2.27 mg/dL (201 µmol/L, early) and ≤2.33 mg/dL (206 µmol/L, no cath). Only 1% of patients in either of the early or no catheterization groups had creatinine values >3.0 mg/dL (265 µmol/L). After IPTW, the first presentation creatinine (Supplementary material online, Figure S1a), peak (Supplementary material online, Figure S1b), and pre-discharge (Supplementary material online, Figure S1c) creatinine distributions were very similar. Natriuretic peptide tests were performed in 16.7% of early and 15.3% of no angiography groups and were not significantly different between arms (P = 0.330); median values were 493 (304–710) and 530 (343–742) ng/L, respectively, which were also not significantly different between the two arms (P = 0.173).
Figure 3.
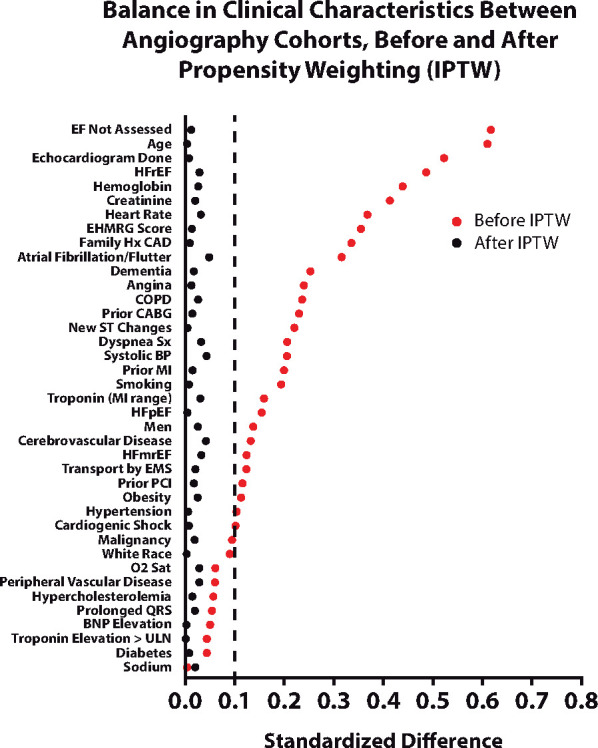
Balance in clinical characteristics before and after the inverse-probability-of-treatment weighting. After inverse-probability-of-treatment weighting, standardized differences of clinical characteristics were well-balanced between groups; for imputed values, the maximal standardized differences are shown. Higher EHMRG score indicates higher risk of 7-day mortality. BNP, brain natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; EMS, emergency medical service; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; ULN, upper limit of normal.
Primary outcomes
The unadjusted and weighted co-primary outcomes for the two exposure groups are reported in Table 2. In all, 914 all-cause deaths were observed over a total of 4853 person-years of follow-up. Before weighting, the early angiography group had lower all-cause (unadjusted HR 0.51, 95% CI; 0.42–0.63, P < 0.001) and cardiovascular mortality (unadjusted HR 0.55, 95% CI; 0.42–0.72, P < 0.001) over 2 years. After IPTW, all-cause mortality (adjusted HR 0.74, 95% CI; 0.61–0.90, P = 0.002) and cardiovascular death (adjusted HR 0.72, 95% CI; 0.56–0.93, P = 0.012), remained significantly lower in those undergoing early coronary angiography. The results were robust to disaggregation of the EHMRG score into its individual subcomponents in the propensity score model, with additional adjustment for potassium concentration and prior metolazone use. The weighted cumulative incidence functions demonstrated lower cumulative incidence of all-cause mortality in those receiving early cardiac catheterization (Figure 4) and unadjusted cumulative incidence curves are shown in Supplementary material online, Figure S2. Weighted cumulative incidence curves for cardiovascular death (Figure 5) also demonstrated a lower incidence in the early angiography cohort. Unadjusted cumulative incidence functions for cardiovascular death are shown in Supplementary material online, Figure S3.
Table 2.
Unadjusted and weighted mortality and readmission outcomes according to early coronary angiography status
| Outcomes |
Unadjusted outcomes (before IPTW) |
Weighted outcomes (after IPTW) |
||||||
|---|---|---|---|---|---|---|---|---|
| ECA | No ECA | P-value | ECA | No ECA | P-value | Hazard ratio (95% CI) | P-value | |
| Mortality | ||||||||
| All-cause | 21.6% | 40.4% | <0.001 | 26.4% | 33.8% | <0.001 | 0.74 (0.61–0.90) | 0.002 |
| Cardiovascular | 12.8% | 23.0% | <0.001 | 15.0% | 19.9% | <0.001 | 0.72 (0.56–0.93) | 0.012 |
| Readmission | ||||||||
| All-cause | 61.2% | 72.3% | <0.001 | 67.0% | 70.4% | 0.047 | 0.91 (0.80–1.02) | 0.106 |
| Heart failure | 36.4% | 48.2% | <0.001 | 40.9% | 44.8% | 0.032 | 0.84 (0.71–0.99) | 0.042 |
| Acute MI | 7.0% | 9.1% | 0.040 | 7.4% | 9.0% | 0.102 | 0.73 (0.50–1.08) | 0.112 |
Weighting performed via IPTW.
ECA, early coronary angiography; IPTW, inverse-probability-of-treatment weighting; MI, myocardial infarction.
Figure 4.
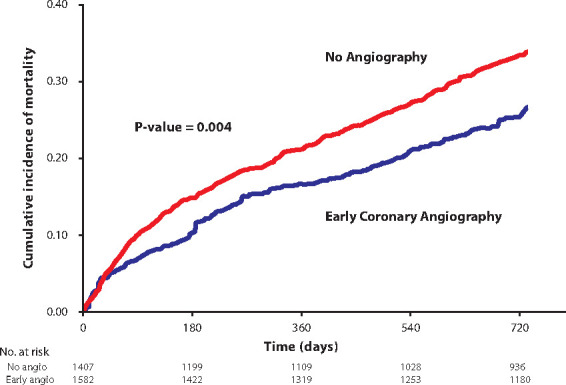
Kaplan–Meier curve of all-cause mortality post-inverse-probability-of-treatment weighting. Early coronary angiography was associated with reduced all-cause mortality.
Figure 5.
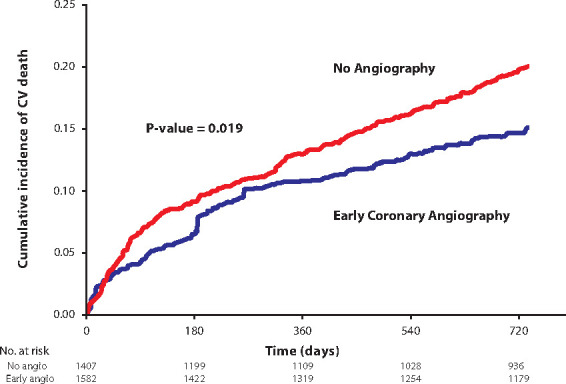
Cumulative incidence function of cardiovascular death post-inverse-probability-of-treatment weighting. Early coronary angiography was associated with reduced cardiovascular mortality.
Secondary outcomes
The unadjusted and propensity-weighted secondary readmission outcomes are also reported in Table 2. Before IPTW, the early coronary angiography group had lower rates of readmissions for all causes (unadjusted HR 0.73, 95% CI 0.67–0.80, P < 0.001), acute MI (unadjusted HR 0.69, 95% CI 0.53–0.90, P = 0.006), and HF (unadjusted HR 0.67, 95% CI 0.59–0.75, P < 0.001) over 2 years. After IPTW, only HF readmissions remained significantly lower (adjusted HR 0.84, 95% CI 0.71–0.99, P = 0.042) in the early angiography group.
Angiographic findings and revascularization outcomes
Among those who underwent early coronary angiography, 58.5% had obstructive coronary disease (Supplementary material online, Table S3a), and 17.6% had coronary revascularization within 90 days of angiography. In those who did not undergo early angiography, only 3.1% had coronary revascularization within 90 days. Unadjusted and weighted rates of revascularization at 90 days and 2 years are presented in Table 3. Unadjusted rates of PCI, CABG, and any revascularization were increased in the early angiography group at both timepoints (all P < 0.001). After IPTW, the early angiography group demonstrated higher 90-day rates of PCI (HR 4.14, 95% CI 2.53–6.79, P < 0.001), CABG (HR 4.99, 95% CI 2.43–10.24, P < 0.001), and any revascularization procedure (HR 4.69, 95% CI 3.11–7.08, P < 0.001). Higher rates of revascularization persisted over 2 years for PCI (HR 2.58, 95% CI 1.73–3.86, P < 0.001), CABG (HR 2.94, 95% CI 1.75–4.93, P < 0.001), and any revascularization (HR 2.82; 95% CI 2.06–3.86, P < 0.001). Cumulative incidence functions for revascularization procedures post-IPTW are displayed in Supplementary material online, Figures S4‒S6. In those undergoing early angiography, obstructive CAD (93.2% vs. 49.0%) and multivessel disease (69.2% vs. 29.9%) were present in significantly higher proportions of patients who underwent revascularization during follow-up, than those who did not undergo revascularization (both P < 0.001, see Supplementary material online, Table S3b). Over 2-year follow-up, coronary revascularization was performed with PCI alone in 145 (42.9%), CABG surgery in 183 (54.1%), and both procedures in 10 (3%) patients.
Table 3.
Unadjusted and weighted revascularization outcomes according to decision to pursue early coronary angiography
| Outcomes |
Unadjusted outcomes (before IPTW) |
Weighted outcomes (after IPTW) |
||||||
|---|---|---|---|---|---|---|---|---|
| ECA | No ECA | P-value | ECA | No ECA | P-value | Hazard ratio (95% CI) | P-value | |
| 90-day revascularization | ||||||||
| PCI | 7.5% | 2.2% | <0.001 | 9.6% | 2.4% | <0.001 | 4.14 (2.53–6.79) | <0.001 |
| CABG | 10.2% | 1.0% | <0.001 | 7.8% | 1.6% | <0.001 | 4.99 (2.43–10.24) | <0.001 |
| PCI or CABG | 17.6% | 3.1% | <0.001 | 17.2% | 4.0% | <0.001 | 4.69 (3.11–7.08) | <0.001 |
| 2-year revascularization | ||||||||
| PCI | 9.9% | 4.2% | <0.001 | 12.2% | 4.9% | <0.001 | 2.58 (1.73–3.86) | <0.001 |
| CABG | 12.3% | 1.8% | <0.001 | 9.4% | 3.3% | <0.001 | 2.94 (1.75–4.93) | <0.001 |
| PCI or CABG | 21.6% | 6.0% | <0.001 | 21.1% | 8.2% | <0.001 | 2.82 (2.06–3.86) | <0.001 |
Weighting performed via IPTW.
CABG, coronary artery bypass graft; CI, confidence interval; ECA, early coronary angiography; IPTW, inverse probability of treatment weighting; PCI, percutaneous coronary intervention.
Subgroup analyses
The weighted HR for each outcome in the three cohort-defining subgroups is represented in Supplementary material online, Table S4. The association between early angiography and lower all-cause mortality remained significant in the subgroups of prior MI (HR 0.75, 95% CI 0.60–0.95, P = 0.015), troponin elevation (HR 0.78, 95% CI 0.62–0.98, P = 0.034), and angina (HR 0.75, 95% CI 0.58–0.97, P = 0.029). There was a significant association of angiography with reduced cardiovascular death (HR 0.71, 95% CI 0.53–0.94, P = 0.018) and acute MI readmissions (HR 0.64, 95% CI 0.45–0.92, P = 0.017) in the subgroup with prior MI. Each subgroup retained a significant increase in all forms of revascularization with early angiography at both timepoints (all P ≤ 0.025). Additionally, these associations were consistent in those with troponin > ULN or in the MI range, with no significant interactions when all-cause mortality (P-interaction = 0.322) and cardiovascular death (P-interaction = 0.797) were examined.
To determine whether coronary angiography was associated with greater benefit during the first compared with non-incident HF hospitalizations, we examined the interaction between incident or non-incident HF. There were no significant interactions between angiography and incident or non-incident HF with any mortality or readmission outcome, with the exception of 2-year acute MI readmission (P-interaction = 0.04). In stratified analyses, patients hospitalized with their first HF episode and who underwent early angiography experienced lower risk of readmission for MI (adjusted HR 0.46, 95% CI 0.29–0.73, P = 0.001), while no association with this outcome was found for non-incident HF (adjusted HR 0.97, 95% CI; 0.59–1.57, P = 0.89).
Sensitivity analyses
Using the Lin method, our findings were robust to nearly all values of γ between 0.75 and 1, even when the differential prevalence of the confounder was as high as 50% between groups. Our findings were robust for γ = 0.7 for up to a 40% differential prevalence of the confounder. Furthermore, the potential effect of a possible but important confounder, the performance of non-invasive testing, was specifically tested. Non-invasive testing for CAD was performed in 9.7% of the non-angiographic cohort and was an adjunct to early coronary angiography in 19.5% of patients. After accounting for the performance of non-invasive testing as a covariate in the propensity model, each previously observed association remained statistically significant (Supplementary material online, Table S5). Additionally, in further sensitivity analyses, we accounted for baseline differences in pre-admission cardioprotective medications including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-adrenoreceptor antagonists, mineralocorticoid receptor antagonists, anticoagulants, antiplatelet agents, and HMG-CoA reductase inhibitors (Supplementary material online, Table S6). After accounting for these medications, we found that propensity-weighted outcomes using IPTW remained significant with adjusted HRs of 0.73 (95% CI 0.61–0.88) for all-cause mortality (P = 0.001) and 0.73 (95% CI 0.56–0.94) for cardiovascular death (P = 0.015).
Discussion
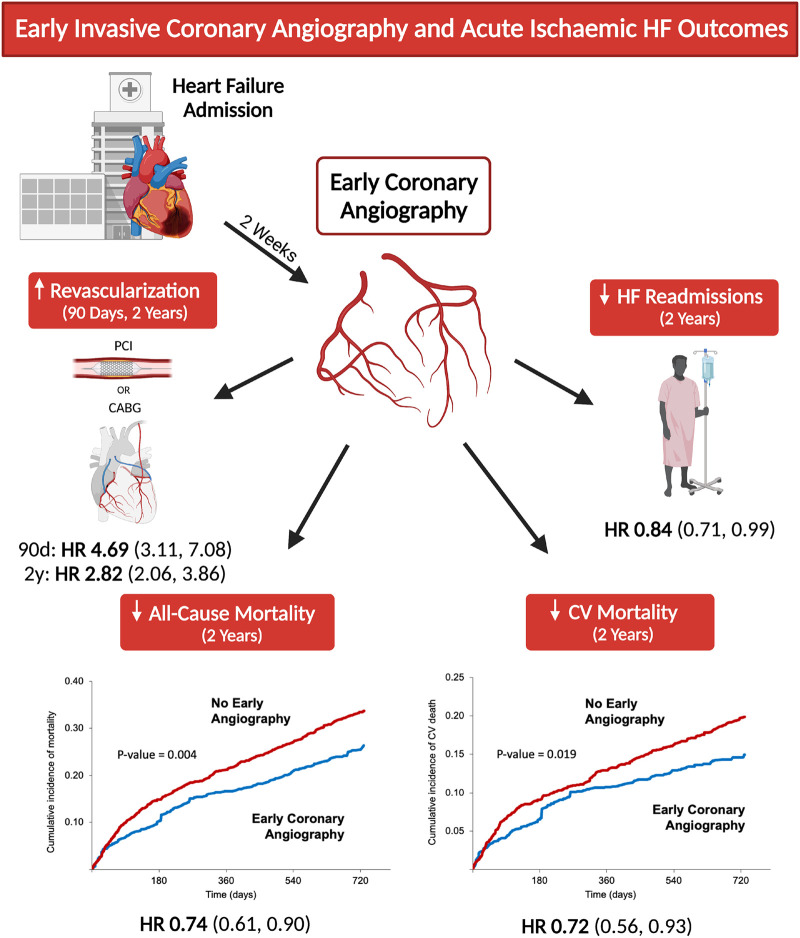
Ischaemic heart disease is a common underlying cause of HF, conferring an increased risk of death and readmission compared with non-ischaemic aetiologies.27 Ischaemia is also a trigger for the initiation and exacerbation of acute decompensated HF.3–7,28 However, there is a paucity of evidence for early invasive evaluation and revascularization of CAD in acute HF.6,28 Consequently, there are no clear guidelines regarding the selection of patients for coronary angiography, nor are there studies clearly demonstrating the benefits of early testing.8,9 In this study of hospitalized AIHF, early coronary angiography was associated with lower all-cause mortality, cardiovascular death, and HF readmissions at 2 years. There were higher rates of coronary revascularization at 90 days and 2 years when early angiography was performed, suggesting that early intervention could contribute to improved outcomes (Graphical abstract).
Early coronary angiography in patients with acute heart failure was associated with reduced all-cause mortality, cardiovascular mortality, and heart failure readmissions, and rates of coronary revascularization were increased. ICE-HF, Invasive Coronary Angiography Early in Ischaemic Heart Failure. Created and used with permission from BioRender.com.
Our study expands the current understanding of the role of angiography in acute HF. The American College of Cardiology/American Heart Association HF guidelines state that ‘left heart catheterization or coronary angiography is indicated for patients with HF and angina and may be useful for those patients without angina’.29 However, this recommendation was based on level C evidence (i.e. consensus opinion) and was not supported by published data. The European Society of Cardiology guidelines for HF suggest that angiography is a Class IIa recommendation in ‘intermediate to high pre-test probability of CAD and suggestions of ischemia on non-invasive ischemia testing’, but the level of evidence was grade C.9 Despite frequently having ready access to cardiac catheterization facilities, there is no clear guidance on whether HF patients should be considered for angiography.8,9,29
There are retrospective and prospective data to support revascularizing patients with CAD and chronic left ventricular systolic dysfunction,9 but there are comparatively little data in the setting of hospitalized acute HF. While coronary angiography has been previously associated with improved all-cause mortality within 30 days of worsening HF symptoms, the improved outcomes were only seen in non-ischaemic HF and may have been confounded by imbalanced clinical profiles favouring angiography.12 In contrast, OPTIMIZE-HF demonstrated that in-hospital angiography was associated with improved adjusted short-term (60–90 day) all-cause mortality and rehospitalization in acute HF, but only in those with known CAD.28 Finally, while the STICHES trial found that surgical revascularization reduced mortality and hospitalizations in chronic ischaemic left ventricular dysfunction, most enrolled patients were not acutely decompensated.30 In our study, over 80% of patients had symptoms at rest or with minimal exertion (thus precipitating hospitalization), and we included those with reduced and preserved left ventricular ejection fractions. We expand on these findings by demonstrating sustained improvement in mortality and readmission outcomes up to 2 years in a broad cohort of patients with AIHF, of whom only a third had prior MI. The improvement in outcomes was seen uniformly across subgroups of those with angina, prior MI, and troponin elevation, indicating that patients with acute HF and any one of these three characteristics may benefit from ischaemic testing.
The hypothesized potential benefit of early angiography in acute HF is via coronary revascularization or the diagnostic certainty arising from the knowledge that critical CAD is not present, thus obviating the need for unneeded antiplatelet agents, which can cause bleeding, and anti-anginal drugs that may lower blood pressure and limit the ability to uptitrate evidence-based drugs. The increased rates of coronary revascularization observed in the early angiography cohort lend strength to the hypothesis that angiography was associated with improved outcomes, in part, by limiting the impact of ischaemic disease on the progression of HF and its associated adverse cardiovascular outcomes. Another possible mechanism of benefit is medical therapy optimization directed at reducing underlying CAD-related risk. Although this study did not examine detailed coronary anatomic findings, the higher rates of surgical revascularization likely indicate that our HF cohort had a broad range of CAD severity with a considerable proportion of complex disease. Our findings were not explained by the use of non-invasive cardiac testing, as the significant associations persisted after weighting for non-invasive testing within the 14-day observation period.
Our study informs decision-making about coronary angiography in acute HF, suggesting that early angiography should be strongly considered for those with HF and suspicion of underlying ischaemic heart disease. The lack of interaction between incident and non-incident HF refutes the notion that ischaemic evaluation primarily should be considered at the first presentation of HF; rather, this observation supports the ongoing re-evaluation of potential underlying ischaemia. At each hospital presentation, the contribution of ischaemia should be considered and, sometimes, repeat physiologic or anatomic evaluation may be required even when the coronary anatomy was defined years before. While the presence of clinical features consistent with ischaemia, such as angina and troponin elevation, could be a manifestation of ventricular dysfunction and hypertrophy in HF, they could also be indicators of significant underlying coronary disease as well.7,11 Additionally, the presence of angina or troponin elevation are also poor prognostic indicators for adverse cardiovascular events in both acute and chronic HF.31–34 Our study has many implications for those with underlying severe coronary disease, as early angiography may lead to subsequent coronary revascularization. Conversely, the absence of significant coronary disease (and diagnostic certainty provided by angiography) may lead to discontinuation of non-beneficial therapies, since all pharmacotherapies have the potential to cause adverse effects. Importantly, the decision to perform coronary angiography should be coupled with good clinical judgement regarding precipitants of acute HF, which may or may not be related to coronary ischaemia. Our definition of AIHF was not inclusive of all potential patients with HF with suspected underlying CAD, and further studies with expanded definitions may be useful in clarifying the breadth of the benefit of early ischaemic investigation. Finally, patients undergoing early catheterization and coronary revascularization exhibited high rates of multivessel disease, which could be amenable to either percutaneous intervention or CABG surgery. Contemporary changes in the frequency and distribution of revascularization procedures, with increasing use of PCI procedures over time,35 and recommendations for a heart team approach,36 may have an impact on outcomes after coronary angiography, which can be assessed in future studies.
Limitations
Our study has several potential limitations. There was likely heterogeneity in the institution-specific definitions of troponin elevation; however, earlier studies suggest that the ULN is a good landmark to define accentuated risk in acute HF.14,22 Our patients were sampled from an earlier timeframe in order to allow for detailed chart review and complete outcome capture and could have been impacted by secular trends. However, recent studies have demonstrated a stabilization of utilization of both invasive and non-invasive ischaemic testing since 2011, indicating that the observed associations remain relevant.15 We did not examine changes in the relative proportion of revascularization procedures performed by PCI or CABG during more recent time periods, which was beyond the scope of the current study. Second, the use of the landmark design to exclude patients who died within 14 days of admission may have led to the exclusion of more severely ill patients, a typical characteristic of this design.37 Third, we did not have data describing the aetiology of HF exacerbation. Fourth, although IPTW analysis accounts for measured clinical features relevant to both the performance of angiography and subsequent outcomes, unmeasured confounding may exist. However, our findings were robust to potential confounders with equivalent and greater magnitudes of effect that could have, hypothetically, large differences in prevalence between the two study arms. Fifth, BNP was not available for most patients, and while there is a potential for misclassification, the clinical diagnosis utilized both validated criteria and a final diagnosis of HF in the discharge record. Sixth, some patients may not be suitable or agreeable to the performance of invasive coronary angiography; for instance, patients in acute HF may have been limited by the requirement for supine positioning due to pulmonary oedema. However, the median time to angiography in the early catheterization group was 4 (3–7) days, and a 14-day window, employed in our study, should be adequate time for decongestion of most patients to be able to undergo the procedure. Finally, some patients may have possessed other contraindications that were not captured by our study. In these cases, non-invasive cardiac testing may be preferred, but comparing the utility of imaging vs. conservative management was outside the scope of the present study and will require future study.
Conclusions
In conclusion, patients with AIHF who underwent early coronary angiography exhibited reduced all-cause mortality, cardiovascular mortality, and HF readmissions over 2 years, as well as higher rates of revascularization. While randomized trial evidence is required to validate these results, this study suggests that early coronary angiography may benefit patients with acute HF and potential underlying ischaemia, supporting the need for an evidence-based clinical framework for the diagnosis and assessment of CAD in the acute HF population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
ICES is supported in part by a grant from the Ontario Ministry of Health and Long Term Care. The opinions, results, and conclusions are those of the authors and no endorsement by the Ministry of Health and Long-Term Care or by ICES is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI and the Ontario Registrar General, the original source of which is Service Ontario. However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI, ORG, or the Ministry of Government Services. This study was supported by a Grant-in-Aid from the Heart and Stroke Foundation and a Foundation Grant from the Canadian Institutes of Health Research (grant # FDN 148446). Dr D.S.L. is the Ted Rogers Chair in Heart Function Outcomes, University Health Network, University of Toronto. Dr P.C.A. is supported by a Mid-Career investigator award from the Heart and Stroke Foundation (grant G-13-0002620).
Conflict of interest: H.A.Q. has received consulting honoraria from the Canadian Vigour Centre, not relevant to the current work. S.G.G. has received funding from Amgen, AstraZeneca, Bayer, CSL Behring, Daiichi-Sankyo/American Regent, Merck, Regeneron, and Sanofi, has received additional consulting honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Novartis, Pendopharm, Pfizer, Servier, and Valeo Pharma, participates in data safety monitoring with Daiichi-Sankyo/American Regent, GlaxoSmithKline, and Novo Nordisk, and receives salary support/honoraria from the Heart and Stroke Foundation of Ontario, Canadian Heart Research Centre/MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Center for Clinical Research, Duke Clinical Research Institute, and the New York University Clinical Coordinating Centre; none are relevant to the current work. M.E.F. received funding from Amgen, Novo Nordisk, and Novartis, not relevant to the current work. J.A.S. received funding from Abbott Vascular and Janssen, consulting honoraria from Myokardia, Novartis, Merck, Amgen, and Janssen, has leadership positions with Blue Cross Blue Shield of Kansas City and the United Healthcare Scientific Advisory Board, and has copyrights to the Seattle Angina Questionnaire and Kansas City Cardiomyopathy Questionnaire; none of these are relevant to the current work. L.B.K., P.C.A., H.J.R., X.W., R.C., P.R.L., and D.S.L. declare no conflicts of interest.
Data availability
Data used in this study are not made freely available due to privacy laws. Requests for access to data for aggregate analysis should be addressed to Dr. Douglas Lee.
Supplementary Material
Tweet: Coronary angiography within 14 days of acute HF admission was associated with ↓ mortality, CV death, and HF readmissions, and ↑ revascularization at 2 years.
References
- 1.Savarese G, Lund LH.. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikh KS, Sheng S, Hammill BG, Yancy CW, Fonarow GC, Hernandez AF, DeVore AD.. Characteristics of acute heart failure hospitalizations based on presenting severity. Circ Heart Fail 2019;12:e005171. [DOI] [PubMed] [Google Scholar]
- 3.Lala A, Desai AS.. The role of coronary artery disease in heart failure. Heart Fail Clin 2014;10:353–365. [DOI] [PubMed] [Google Scholar]
- 4.Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC.. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001;22:228–236. [DOI] [PubMed] [Google Scholar]
- 5.Bart BA, Shaw LK, McCants CB, Fortin DF, Lee KL, Califf RM, O’Connor CM.. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 1997;30:1002–1008. [DOI] [PubMed] [Google Scholar]
- 6.Purek L, Laule-Kilian K, Christ A, Klima T, Pfisterer ME, Perruchoud AP, Mueller C.. Coronary artery disease and outcome in acute congestive heart failure. Heart 2006;92:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, Shaw LK, O’Connor CM.. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002;39:210–218. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C.. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 10.Lettman NA, Sites FD, Shofer FS, Hollander JE.. Congestive heart failure patients with chest pain: incidence and predictors of acute coronary syndrome. Acad Emerg Med 2002;9:903–909. [DOI] [PubMed] [Google Scholar]
- 11.Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM.. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira JP, Rossignol P, Demissei B, Sharma A, Girerd N, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Voors A, Zannad F.. Coronary angiography in worsening heart failure: determinants, findings and prognostic implications. Heart 2018;104:606–613. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L; International Working Group on Acute Heart Failure Syndromes. Acute heart failure syndromes: current state and framework for future research. Circulation 2005;112:3958–3968. [DOI] [PubMed] [Google Scholar]
- 14.Freitas C, Wang X, Ge Y, Ross HJ, Austin PC, Pang PS, Ko DT, Farkouh ME, Stukel TA, McMurray JJV, Lee DS.. Comparison of troponin elevation, prior myocardial infarction, and chest pain in acute ischemic heart failure. CJC Open 2020;2:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braga JR, Leong-Poi H, Rac VE, Austin PC, Ross HJ, Lee DS.. Trends in the use of cardiac imaging for patients with heart failure in Canada. JAMA Netw Open 2019;2:e198766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, Howlett JG, Koshman SL, Lepage S, McKelvie RS, Moe GW, Rajda M, Swiggum E, Virani SA, Zieroth S, Al-Hesayen A, Cohen-Solal A, D'Astous M, De S, Estrella-Holder E, Fremes S, Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, LeBlanc MH, Masoudi FA, Ross HJ, Roussin A, Sussex B.. 2017 comprehensive update of the Canadian Cardiovascular Society Guidelines for the management of heart failure. Can J Cardiol 2017;33:1342–1433. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, Bardsley M, Conroy S.. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko DT, Khera R, Lau G, Qiu F, Wang Y, Austin PC, Koh M, Lin Z, Lee DS, Wijeysundera HC, Krumholz HM.. Readmission and mortality after hospitalization for myocardial infarction and heart failure. J Am Coll Cardiol 2020;75:736–746. [DOI] [PubMed] [Google Scholar]
- 19.Bauer T, Koeth O, Junger C, Heer T, Wienbergen H, Gitt A, Zahn R, Senges J, Zeymer U; for the Acute Coronary Syndromes Registry (ACOS) Investigators. Effect of an invasive strategy on in-hospital outcome in elderly patients with non-ST-elevation myocardial infarction. Eur Heart J 2007;28:2873–2878. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Harrington RA, Greenbaum AB, Berger PB, Cannon CP, Cohen DJ, Gibson CM, Saucedo JF, Kleiman NS, Hochman JS, Boden WE, Brindis RG, Peacock WF, Smith SC, Pollack CV, Gibler WB, Ohman EM; Crusade Investigators. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004;292:2096–2104. [DOI] [PubMed] [Google Scholar]
- 21.Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, Newton GE, Lee JS, Tu JV.. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med 2012;156:767–775. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Lee JS, Schull MJ, Borgundvaag B, Edmonds ML, Ivankovic M, McLeod SL, Dreyer JF, Sabbah S, Levy PD, O'Neill T, Chong A, Stukel TA, Austin PC, Tu JV.. Prospective validation of the emergency heart failure mortality risk grade for acute heart failure. Circulation 2019;139:1146–1156. [DOI] [PubMed] [Google Scholar]
- 23.Greig D, Austin PC, Zhou L, Tu JV, Pang PS, Ross HJ, Lee DS.. Ischemic electrocardiographic abnormalities and prognosis in decompensated heart failure. Circ Heart Fail 2014;7:986–993. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC.The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin DY, Psaty BM, Kronmal RA.. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948–963. [PubMed] [Google Scholar]
- 27.Chun S, Tu JV, Wijeysundera HC, Austin PC, Wang X, Levy D, Lee DS.. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 2012;5:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaherty JD, Rossi JS, Fonarow GC, Nunez E, Stough WG, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, Yancy CW, Young JB, Davidson CJ, Gheorghiade M.. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2009;157:1018–1025. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL.. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 30.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL, Stiches I.. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mentz RJ, Broderick S, Shaw LK, Fiuzat M, O'Connor CM.. Heart failure with preserved ejection fraction: comparison of patients with and without angina pectoris (from the Duke Databank for Cardiovascular Disease). J Am Coll Cardiol 2014;63:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braga JR, Tu JV, Austin PC, Chong A, You JJ, Farkouh ME, Ross HJ, Lee DS.. Outcomes and care of patients with acute heart failure syndromes and cardiac troponin elevation. Circ Heart Fail 2013;6:193–202. [DOI] [PubMed] [Google Scholar]
- 33.Peacock WF, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AHB; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]
- 34.Badar AA, Perez-Moreno AC, Jhund PS, Wong CM, Hawkins NM, Cleland JG, van Veldhuisen DJ, Wikstrand J, Kjekshus J, Wedel H, Watkins S, Gardner RS, Petrie MC, McMurray JJ.. Relationship between angina pectoris and outcomes in patients with heart failure and reduced ejection fraction: an analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J 2014;35:3426–3433. [DOI] [PubMed] [Google Scholar]
- 35.Kataruka A, Maynard CC, Kearney KE, Mahmoud A, Bell S, Doll JA, McCabe JM, Bryson C, Gurm HS, Jneid H, Virani SS, Lehr E, Ring ME, Hira RS.. Temporal trends in percutaneous coronary intervention and coronary artery bypass grafting: insights from the Washington Cardiac Care Outcomes Assessment Program. J Am Heart Assoc 2020;9:e015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 37.Lee DS, Schull MJ, Alter DA, Austin PC, Laupacis A, Chong A, Tu JV, Stukel TA.. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circ Heart Fail 2010;3:228–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are not made freely available due to privacy laws. Requests for access to data for aggregate analysis should be addressed to Dr. Douglas Lee.