Abstract
Photosensory adaptation, which can be classified as sensor or effector adaptation, optimizes the light sensing of living organisms by tuning their sensitivity to changing light conditions. During the phototropic response in Arabidopsis (Arabidopsis thaliana), the light-dependent expression controls of blue-light (BL) photoreceptor phototropin 1 (phot1) and its modulator ROOT PHOTOTROPISM2 (RPT2) are known as the molecular mechanisms underlying sensor adaptation. However, little is known about effector adaption in plant phototropism. Here, we show that control of the phosphorylation status of NONPHOTOTROPIC HYPOCOTYL3 (NPH3) leads to effector adaptation in hypocotyl phototropism. We generated unphosphorable and phosphomimetic NPH3 proteins on seven phosphorylation sites in the etiolated seedlings of Arabidopsis. Unphosphorable NPH3 showed a shortening of its retention time in the cytosol and caused an inability to adapt to very low fluence rates of BL (∼10−5 µmol m−2 s−1) during the phototropic response. In contrast, the phosphomimetic NPH3 proteins had a lengthened retention time in the cytosol and could not enable the adaptation to BL at fluence rates of 10−3 µmol m−2 s−1 or more. Our results indicate that the activation level of phot1 and the corresponding phosphorylation level of NPH3 determine the dissociation rate and the reassociation rate of NPH3 on the plasma membrane, respectively. These mechanisms may moderately maintain the active state of phot1 signaling across a broad range of BL intensities and contribute to the photosensory adaptation of phot1 signaling during the phototropic response in hypocotyls.
The phosphorylation status of NONPHOTOTROPIC HYPOCOTYL3 pr
Introduction
Light is one of the most important environmental recognition cues among living organisms. In addition to plants and animals, primitive eukaryotes and prokaryotes perceive light and manifest suitable responses to their light environments. The light responses of organisms, as well as other environmental responses, show “adaptation” to a given stimulus following transient activation, which enables them to react to the next stimulus and to intensity changes to the stimuli in their environment (Galland, 1991). Photosensory adaptation can be classified into two types (Galland, 1991). One is sensor adaptation that shows sensitivity recovery or sensitivity loss. Another is effector adaptation which controls the degree of responsiveness after light perception. Sensor and effector adaption can be considered to operate at the photoreceptor level and signal transduction level downstream of the photoreceptor, respectively. Photosensory adaptation has been well-studied in several model systems, including the transcriptional responses to light in Neurospores (Neurospora crassa; Schwerdtfeger and Linden, 2001; Chen et al., 2010; Dasgupta et al. 2015), the phototropic responses of the sporangiophores of Phycomyces (Phycomyces blakesleeanus; Corrochano and Galland, 2019), and vision sensing in animals (Koch and Dell’orco, 2015; Honkanen et al., 2017; Chaya et al. 2019).
Phototropism in angiosperms is also well-documented in terms of photosensory adaptations (Poff et al., 1994). The hypocotyls and coleoptiles of dark-grown etiolated seedlings of dicots and monocots show a phototropic fluence-response pattern that can be depicted as a bell-shaped curve under conditions of blue-light (BL) pulse irradiation, referred to as the first positive phototropism (Iino, 2001). A decrease in the first positive phototropic response may be caused by the transient saturation of photoproducts involved in light perception, and the signal transduction between the irradiated and shaded sides of the plant (Iino, 2001; Christie and Murphy, 2013). When the duration of BL irradiation exceeds 10–30 min, the seedlings adapt to their light environment and the phototropic response recovers after this refractory state (Poff et al., 1994). This is referred to as the time-dependent, second positive phototropism.
Previous studies have revealed three molecular mechanisms of sensor adaptation in phototropism by analyzing the model plant Arabidopsis (Arabidopsis thaliana). The first of these involves two kinds of BL photoreceptors, phototropin 1 (phot1) and phot2, which behave with different photosensitivity levels (Sakai et al., 2001). In this process, the active form of phot1 excited by BL irradiation is more stable than that of phot2. Phot2 shows a faster photocycle than phot1, and thus phot1 functions as the highly photosensitive photoreceptor whereas phot2 functions as the less photosensitive photoreceptor (Sakai et al., 2001; Kasahara et al., 2002; Okajima et al., 2012). The second method of sensor adaptation involves the regulation of phot1 and phot2 expression. Light irradiation decreases and increases the phot1 and phot2 levels in seedlings, respectively (Kagawa et al., 2001; Christie and Murphy, 2013; Sullivan et al., 2019). Hence, the highly photosensitive phot1 can mediate both first and second positive phototropism under BL at a low fluence (10−1 µmol m−2) and a very low fluence rate (10−5 µmol m−2 s−1), respectively (Haga et al., 2015), and the less photosensitive phot2 mediates the second positive phototropism under high fluence rate BL (1 µmol m−2 s−1 or higher fluence rate; Sakai et al., 2001). The third method involves the control of phot1 autophosphorylation activity through the light induction of ROOT PHOTOTROPISM2 (RPT2; Sakai et al., 2000; Haga et al., 2015; Kimura et al., 2020). Light-inducible RPT2 proteins bind to phot1 and suppress its autophosphorylation activity to maintain a moderate activation of phot1 under any light intensity conditions. Hence, phot1 can mediate the phototropic responses in etiolated hypocotyls over a broad dynamic range of BL intensities between 10−5 and 102 µmol m−2 s−1 (Sakai et al., 2001; Haga et al., 2015).
The RPT2 homolog, NONPHOTOTROPIC HYPOCOTYL3 (NPH3), is an essential signaling factor for both the phot1- and phot2-mediated phototropic responses (Liscum and Briggs, 1996; Motchoulski and Liscum, 1999; Inada et al., 2004). NPH3 belongs to the NPH3/RPT2-Like (NRL) family and contains broad complex, tramtrack and bric-à-brac (BTB) domains in its N-terminus, the NPH3 domain in its mid-region, and a coiled-coil domain in its C-terminus (Christie et al., 2018). NPH3 proteins are predominantly localized at the plasma membrane and form complexes with phot1 and phot2 in planta (Motchoulski and Liscum, 1999; de Carbonnel et al., 2010; Haga et al., 2015). Although NPH3 proteins show some ubiquitin E3 ligase properties (Roberts et al., 2011), their biochemical roles in phototropism remain unclear (Christie et al., 2018). On the other hand, it has been well-established that NPH3 proteins are phosphorylated under darkness and dephosphorylated in response to phot1 activation (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008), suggesting that the dephosphorylated form is an active factor in phot1 signaling (Pedmale and Liscum, 2007). Three phosphorylation sites have been identified on the NPH3 protein at serine 213 (S213), S223, and S237, and alanine substitutions at these sites had no effect on hypocotyl phototropism [S212, S222, and S236 in Tsuchida-Mayama et al. (2008); the residues were re-numbered according to a TAIR annotation (https://www.arabidopsis.org/)]. Phosphorylation thus appears not to be required for NPH3 function whereas NPH3 dephosphorylation produces its active form. Recent studies, however, have reported that the dephosphorylation of NPH3 proteins is well-correlated with their internalization and aggregation in the cytosol, and with a desensitization of hypocotyl phototropism (Haga et al., 2015; Sullivan et al., 2019). This suggests that the phot1-induced dephosphorylation of NPH3 leads to the suppression of phot1 signaling. In this context, Haga et al. (2015) reported that there are unidentified phosphorylation sites on NPH3 in addition to S213, S223, and S237.
In our current study, we evaluated the role of NPH3 phosphorylation on phot1 signaling using molecular genetic approaches. We performed mass spectrometric analysis and identified seven serine residues as phosphorylation sites of the NPH3 protein in etiolated Arabidopsis seedlings. These serine residues were substituted for glutamic acid residues to mimic a phosphorylated state (NPH3SE), or with alanine residues to block their phosphorylation (NPH3SA). We then examined the functions of NPH3SE and NPH3SA on hypocotyl phototropism. Our findings indicated that the phosphorylation status of NPH3 proteins determines its relocalization from the cytosol to the plasma membrane under BL conditions. The duration of NPH3 cytosol localization appeared to affect the photosensitivity of etiolated hypocotyls during phototropism and the dephosphorylated form of NPH3 was necessary for the second positive phototropism under BL at a high fluence rate. We propose therefore that the phosphorylation status of NPH3 affects the effector adaptation of phot1 signaling pathways and also that the control of its status contributes to the robustness of hypocotyl phototropism in plants under various light conditions.
Results
Identification of phosphorylation sites on the NPH3 protein
The PhosPhAt 4.0 database lists 18 phosphorylation sites within the NPH3 proteins in Arabidopsis (Heazlewood et al., 2008). We performed mass spectrometric analysis to identify the phosphorylation sites for these proteins in etiolated seedlings. YFP-tagged NPH3 wild-type (NPH3WT) proteins were immunoprecipitated from extracts of etiolated seedlings of 35Spro:YFP-NPH3WT nph3 transgenic lines (Haga et al., 2015), which were irradiated with or without unilateral BL at 1.7 × 10−1 µmol m−2 s−1 for 1 h. These immunoprecipitates were then used in mass spectrometric analysis. This analysis identified four phosphoserine (pS) residues (Figure 1 and Supplemental Figure S1, A and B), pS7 (or pS5), pS467, pS474 (or pS476), and pS722 (or pS723), in addition to three serine residues which had been identified previously in etiolated seedlings (pS213, pS223, and pS237; Tsuchida-Mayama et al., 2008). Several phospho-serine residues were detected in both seedlings (Supplemental Figure S1, A and B) but this study did not examine each dephosphorylation of pS7 (or pS5), pS467, pS474 (or pS476), and pS722 (or pS723) by BL irradiation. The dephosphorylation of pS213, pS223, and pS237 had been determined using anti-phospho-peptide antibodies in the previous study (Tsuchida-Mayama et al., 2008).
Figure 1.
Phosphorylation sites in the NPH3 protein. The individual positions of the phosphorylated amino acids in the NPH3 protein are indicated. Residues denoted by a small sized font are registered as phosphorylation sites in the PhosPhAt 4.0 database. Residues denoted in a bold font were included in the phosphopeptides detected by our mass spectrometry analysis and mutated to alanine (NPH3SA) or glutamic acid (NPH3SE) for subsequent molecular genetic analysis. BTB, broad complex, tramtrack and bric-à-brac domain; NPH3, NPH3 domain; and CC, coiled-coil domain.
NPH3 dephosphorylation reduces the photosensitivity of seedlings and enhances their phototropic response to high fluence rate BL
To elucidate the physiological functions of phosphorylation on the identified NPH3 serine residues, we created NPH3SA and NPH3SE mutants in which 10 serine residues including S7 (and S5, as a potential phosphorylation site), S213, S223, S237, S467, S474 (and S476, as a potential phosphorylation site), and S722 (and S723, as a potential phosphorylation site) were substituted for alanine residues that cannot be phosphorylated and for glutamate residues that mimic a phosphorylated state, respectively (Figure 1). The NPH3WT gene and NPH3SA and NPH3SE mutant genes were driven by the cauliflower mosaic virus 35S promoter and expressed in the nph3 mutant (i.e. 35Spro:NPH3WT nph3, 35Spro:NPH3SA nph3, and 35Spro:NPH3SE nph3 lines, respectively). Two independent lines were chosen for each construct in accordance with their expression levels (Supplemental Figure S2, A) and used for subsequent analyses. Modifications of phosphorylation on NPH3SA and NPH3SE were examined by immunoblotting (Supplemental Figure S2, B). BL irradiation induced the dephosphorylation and enhanced the mobility of NPH3WT proteins in the SDS–PAGE gel, as described previously (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008). The mobilities of NPH3SA and NPH3SE proteins were not altered by BL irradiation and were similar to those of dephosphorylated and phosphorylated NPH3WT, respectively (Supplemental Figure S2, B). Thus, the seven pS residues identified here were found to be major phosphorylation sites of NPH3 in the etiolated seedlings.
We next observed the phototropic responses in 35Spro:NPH3WT nph3, 35Spro:NPH3SA nph3, and 35Spro:NPH3SE nph3 lines. Two-day-old etiolated seedlings for each line were irradiated with unilateral BL at 1.7 × 10−1 and 1.7 × 10−3 µmol m−2 s−1. The nph3 mutant did not show any phototropic responses under either set of conditions, and NPH3WT expression successfully complemented the hypocotyl phototropism phenotypes of the nph3 mutant as these transgenic lines showed an equivalent response to their wild-type counterparts under both sets of BL conditions (Figure 2 and Supplemental Figure S3, A). Our previous studies had indicated that the phosphorylation status of the NPH3 protein is well-correlated with its localization at the plasma membrane and the initiation of phototropic bending of the hypocotyl, suggesting that this phosphorylation is necessary for NPH3 function (Haga et al., 2015; Christie et al., 2018). Unexpectedly, however, the NPH3SA lines also showed rescue of the nph3 mutant phenotypes (Figure 2, A and B). This suggested that the phosphorylation of the seven serine residues identified in etiolated seedlings is not required for NPH3 to function in hypocotyl phototropism. On the other hand, the 35Spro:NPH3SE nph3 seedlings showed slight and moderate responses, respectively, to BL irradiation at 1.7 × 10−1 (Figure 2, A) and 1.7 × 10−3 µmol m−2 s−1 (Figure 2, B). This phenotype was similar to that reported in our prior study for the rpt2 mutant (Haga et al., 2015).
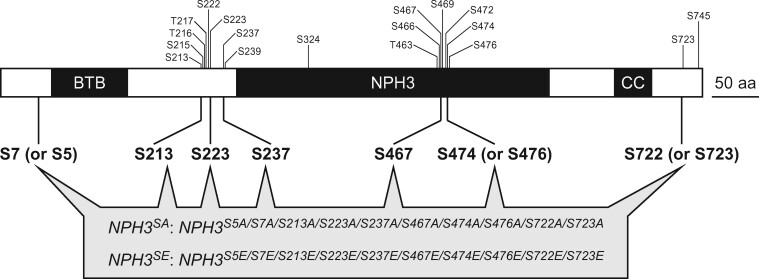
Figure 2.
Time course analysis of continuous light-induced second positive phototropism. Two-day-old etiolated seedlings of the nph3 mutant and those transformed with 35Spro:NPH3WT, 35Spro:NPH3SA, or 35Spro:NPH3SE constructs were irradiated with unilateral BL for 6 h, during which time the hypocotyl curvatures were determined at 10 min intervals. A, Time course analysis of the continuous light-induced second positive phototropism at 1.7 × 10−1 µmol m−2 s−1. The data shown are the mean values ± SE from 9 to 23 seedlings. B, Time course analysis of the continuous light-induced second positive phototropism at 1.7 × 10−3 µmol m−2 s−1. The data shown are the mean values ± SE from 11 to 18 seedlings.
We next analyzed the fluence rate–response patterns of phototropic responses in our NPH3WT and mutant seedlings (Figure 3, A, and Supplemental Figure S4, A). When the nph3 mutants were irradiated with continuous BL for 3 h, these hypocotyls did not show the phototropic response at any of the fluence rates examined (Figure 3, A and Supplemental Figure S3, B). On the other hand, 35Spro:NPH3WT nph3 seedlings showed a hypocotyl curvature plateau that was similar to that of wild type under BL conditions at 1.7 × 10−3 µmol m−2 s−1 or more (Figure 3, A and Supplemental Figure S3, B; Haga et al., 2015). The 35Spro:NPH3SA nph3 seedlings also showed almost normal phototropic curvatures, but displayed a somewhat weaker response than the 35Spro:NPH3WT nph3 line at a fluence rate of 1.7 × 10−5 µmol m−2 s−1 (Figure 3, A and Supplemental Figure S4, A). In contrast, the 35Spro:NPH3SE nph3 seedlings showed normal phototropic responses at 1.7 × 10−5 and 1.7 × 10−4 µmol m−2 s−1, and their phototropic curvatures were decreased at 1.7 × 10−3 µmol m−2 s−1 or more (Figure 3, A and Supplemental Figure S4, A). The fluence rate-response patterns in the 35Spro:NPH3SE nph3 seedlings were similar to those reported previously for the rpt2 mutant (Haga et al., 2015). These results indicated that both the NPH3SA and NPH3SE are functional with regard to the induction of a second positive phototropic response in a fluence-rate dependent manner.
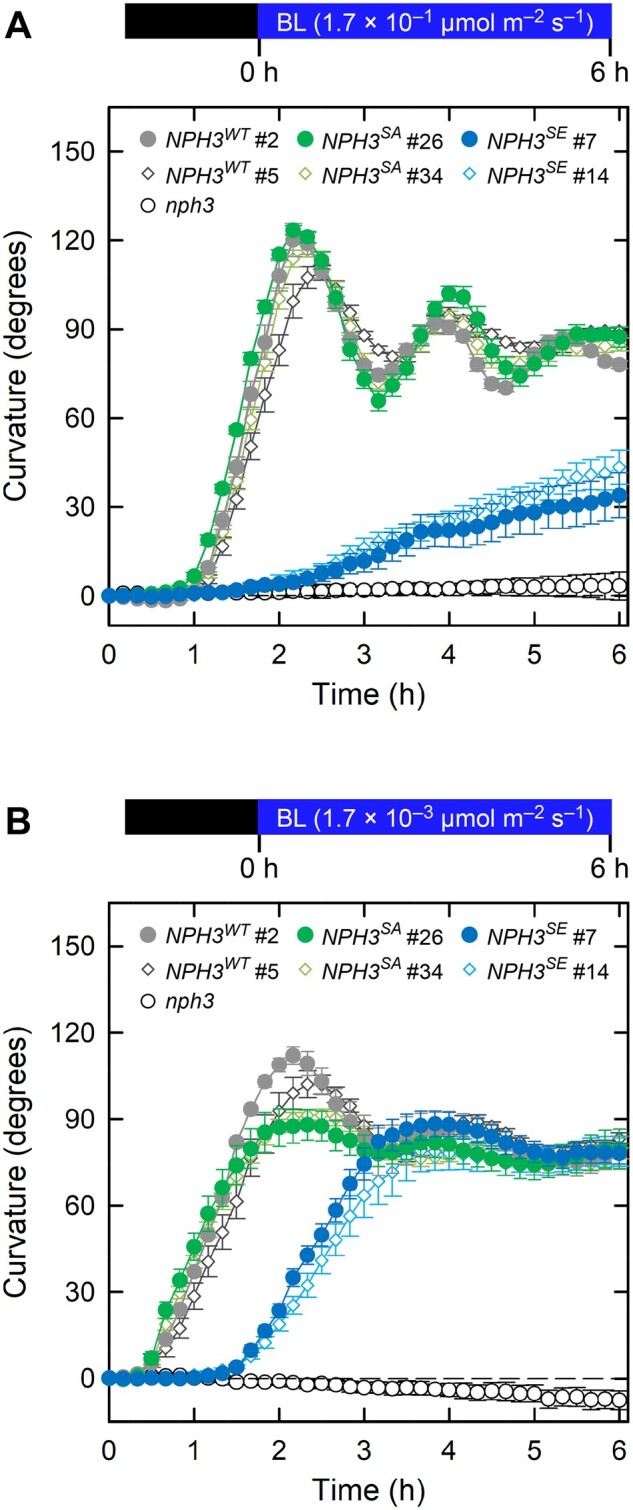
Figure 3.
Effects of the NPH3SA and NPH3SE mutations on the fluence rate-response curves for the continuous light-induced second positive phototropism and fluence-response curves for the pulse-induced first positive phototropism. A, Fluence rate-response curves for the continuous light-induced second positive phototropism. Two-day-old etiolated seedlings of the nph3 mutant and those transformed with 35Spro:NPH3WT (NPH3WT #2), 35Spro:NPH3SA (NPH3SA #26), or 35Spro:NPH3SE (NPH3SE #7) constructs were irradiated with unilateral BL at various fluence rates for 3 h. The data shown are the mean values ± SE from 17 to 26 seedlings. B, Fluence-response curves for the pulse-induced first positive phototropism. Two-day-old etiolated seedlings of the nph3 mutants and those transformed with 35Spro:NPH3WT (NPH3WT #2), 35Spro:NPH3SA (NPH3SA #26), or 35Spro:NPH3SE (NPH3SE #7) constructs were stimulated with unilateral BL at various fluence rates for 1 min. The hypocotyl curvatures were determined at 3 h after the onset of BL stimulation. The data shown are the mean values ± SE from 19 to 31 seedlings. Asterisks indicate a statistically significant difference from the curvatures at 1.0 × 10−1 µmol m−2 for NPH3WT #2 and NPH3SE #7 and those at 1.0 × 10−0.5 µmol m−2 for NPH3SA #26 within respective lines (Dunnett’s test, P < 0.05).
We additionally investigated pulse-induced phototropism (Figure 3, B and Supplemental Figure S4, B), which can be induced by 1 min of BL irradiation followed by a 3-h incubation under darkness (Haga et al., 2015). When the fluence-response pattern was investigated, a bell-shaped responsive curve with a peak at 1.0 × 10−1 µmol m−2 was observed in the 35Spro:NPH3WT nph3 and 35Spro:NPH3SE nph3 seedlings, but not in the nph3 mutants (Figure 3, B and Supplemental Figure S4, B). On the other hand, the 35Spro:NPH3SA nph3 seedlings showed a bell-shaped response curve with a peak at 1.0 × 10−0.5 µmol m−2 but not at 1.0 × 10−1 µmol m−2 (Figure 3, B and Supplemental Figure S4, B). We had already reported previously that the 35Spro:RPT2 rpt2 transgenic seedlings show such a peak shift in their fluence responsive curve (Haga et al., 2015). These results suggest that the phosphorylation status of the seven phosphoserine residues we identified in the NPH3 proteins plays a role in the fluence/fluence-rate-dependent phototropic responses in hypocotyls, namely the photosensory adaptation mechanism of hypocotyl phototropism.
NPH3 functions in the photosensory adaptation mechanism of hypocotyl phototropism in an RPT2-independent manner
We examined whether the effects of the NPH3SA and NPH3SE mutations depended on the function of RPT2. Our recent studies have indicated that the light induction of RPT2 is an underlying mechanism for the photosensory adaptation of phot1 to high fluence rate BL irradiation through a suppression of its autophosphorylation activity (Haga et al., 2015; Kimura et al., 2020). A loss-of-function mutation in RPT2 increased the responsiveness of the first positive phototropism and that of the second positive phototropism to continuous BL at 1.7 × 10−5 µmol m−2 s−1 and abolished the sensitivity of the second positive phototropism to the stimulus at 1.7 × 10−1 µmol m−2 s−1 (Haga et al., 2015). As the rpt2 mutant, the 35Spro:NPH3WT rpt2 nph3 seedlings showed an increase in their hypocotyl curvatures during the first positive phototropic response (Figure 4, A), and the second positive phototropic response at 1.7 × 10−5 µmol m−2 s−1 (Figure 4, B), but a decrease in hypocotyl curvatures during the second positive phototropic response at 1.7 × 10−2 µmol m−2 s−1 or more (Figure 4, B).
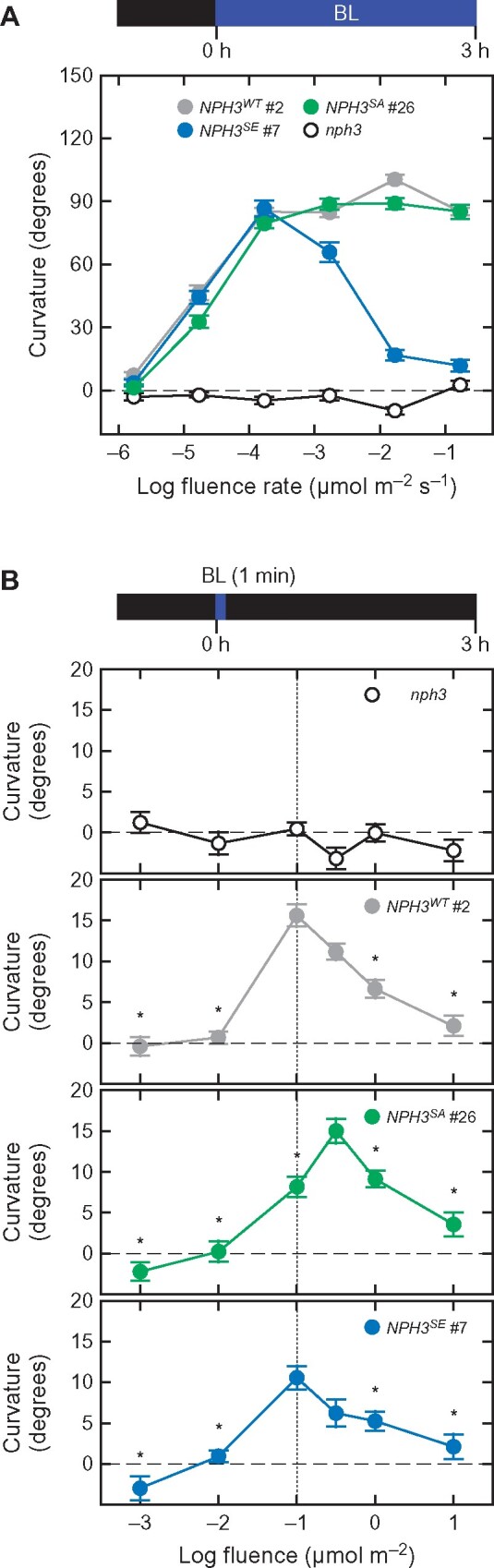
Figure 4.
Effects of the rpt2 mutation on the phototropic responses in the 35Spro:NPH3WT nph3, 35Spro:NPH3SA nph3, and 35Spro:NPH3SE nph3 transgenic lines. A, Effects of the rpt2 mutation on the fluence-response pattern of the first positive phototropism. Two-day-old etiolated seedlings of the rpt2 nph3 mutants transformed with 35Spro:NPH3WT (NPH3WT #2 rpt2), 35Spro:NPH3SA (NPH3SA #26 rpt2), or 35Spro:NPH3SE (NPH3SE #7 rpt2) constructs were stimulated with unilateral BL at various fluence rates for 1 min. The hypocotyl curvatures were determined at 3 h after the onset of BL stimulation. The data shown are the mean values ± SE from 23 to 55 seedlings. Asterisks indicate a statistically significant difference from the curvatures at 1.0 × 10−1 µmol m−2 for NPH3WT #2 rpt2 and NPH3SE #7 rpt2 and at 1.0 × 10−0.5 µmol m−2 for NPH3SA #26 rpt2 within respective lines (Dunnett’s test, P < 0.05). The curves obtained for the nph3 mutants transformed with the same three constructs, and presented in Figure 3, B, are reproduced here using dotted lines for comparison. B, Effects of the rpt2 mutation on the fluence rate-response pattern of the continuous light-induced second positive phototropism. Two-day-old etiolated seedlings of the rpt2 nph3 double mutants transformed with 35Spro:NPH3WT (NPH3WT #2 rpt2), 35Spro:NPH3SA (NPH3SA #26 rpt2), or 35Spro:NPH3SE (NPH3SE #7 rpt2) constructs were irradiated with unilateral BL at various fluence rates for 3 h. The data shown are the mean values ± SE from 17 to 31 seedlings. The curves obtained for the nph3 mutants transformed with these same three constructs, and presented in Figure 3, A, are reproduced here using dotted lines for comparison.
The 35Spro:NPH3SA rpt2 nph3 seedlings showed a peak shift in the bell-shaped fluence-response in the first positive phototropism, similar to the 35Spro:NPH3SA nph3 line, with an increase of hypocotyl curvatures (Figure 4, A), suggesting that the NPH3SA mutation causes a decrease in the photosensitivity of seedlings and facilitates an adaptation to high fluence independently of RPT2. The 35Spro:NPH3SA rpt2 nph3 seedlings showed continuous light-induced phototropic responses under BL at 1.7 × 10−5–10−2 µmol m−2 s−1, but lacked a response at 1.7 × 10−1 µmol m−2 s−1 (Figure 4, B). On the other hand, the 35Spro:NPH3SE rpt2 nph3 seedlings showed an enhanced phenotype for its second positive phototropism compared with 35Spro:NPH3SA rpt2 nph3 and 35Spro:NPH3WT rpt2 nph3, that is they lacked the response at 1.7 × 10−3 µmol m−2 s−1 or more and showed a small response under BL at 1.7 × 10−4 µmol m−2 s−1 (Figure 4, B). These findings suggested that the effects of the NPH3SE mutation are also independent of RPT2.
The effects of red-light pretreatment were also examined. This pretreatment induces RPT2 expression prior to the phototropic stimulus and reduces the lag time to the induction of phototropic responses in Arabidopsis hypocotyls (Hangarter, 1997; Haga and Sakai, 2012; Haga et al., 2015). When the 35Spro:NPH3WT nph3 and the 35Spro:NPH3SA nph3 seedlings were pretreated with red light for 2 min at 2 h prior to phototropic stimulation, bending initiation was shortened for about 30 min under unilateral BL irradiation at 1.7 × 10−1 µmol m−2 s−1 (Supplemental Figure S5, A–C), as shown previously in wild-type seedlings (Haga and Sakai, 2012). This BL condition only minimally induced the phototropic responses in 35Spro:NPH3SE nph3 (Figure 2, A and Supplemental Figure S5, B and C), but red-light pretreatment slightly shortened the bending initiation in 35Spro:NPH3SE nph3 #7 seedlings (Supplemental Figure S5, B). When 35Spro:NPH3SE nph3 seedlings were irradiated with unilateral BL at 1.7 × 10−3 µmol m−2 s−1, the red-light pretreatment reduced the lag time to the initiation of bending from ∼1.5 h to 30 min (Figure 5, A and Supplemental Figure S6). The lengths of these lag times correlated with the expression levels of RPT2 in the 35Spro:NPH3SE nph3 line (Figure 5, B and C). These results suggested that the light induction of RPT2 affects photosensory adaptation independently of the NPH3 phosphorylation status.
Figure 5.
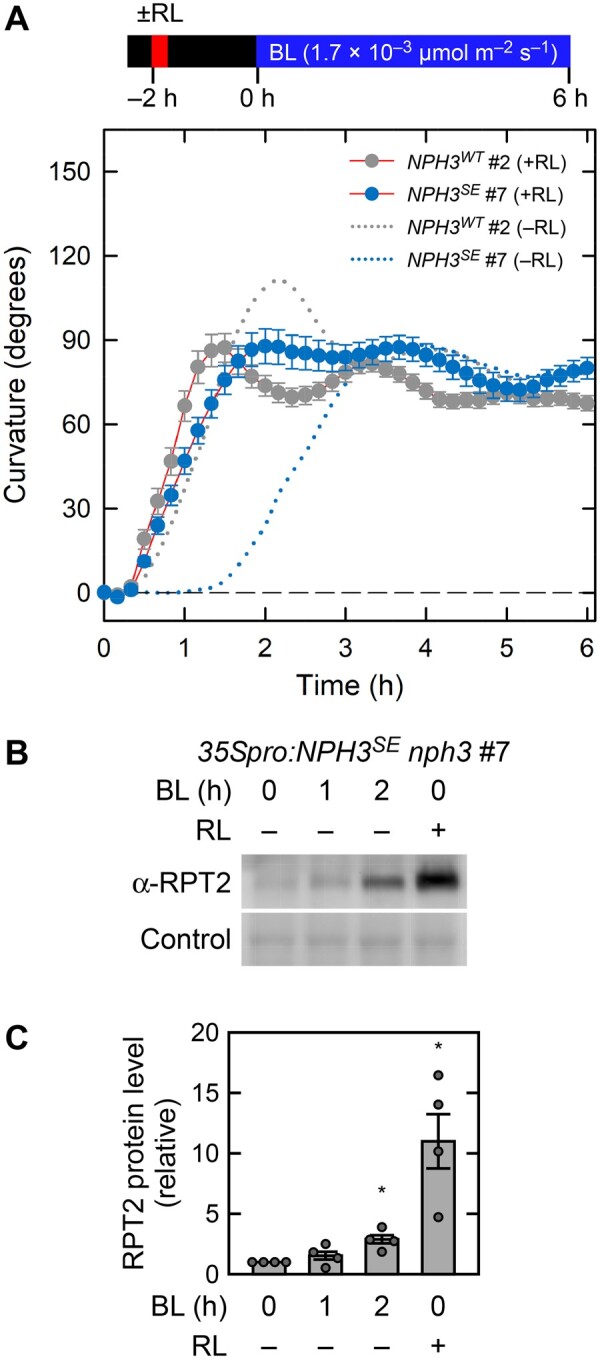
Effect of red light on the second positive phototropism in 35Spro:NPH3SE nph3 transgenic lines. A, Time course analysis of the continuous light-induced second positive phototropism in red light-pretreated seedlings. Two-day-old etiolated seedlings of the nph3 mutants transformed with 35Spro:NPH3WT or 35Spro:NPH3SE constructs were pretreated with an overhead red light at 20 µmol m−2 s−1 for 2 min (+RL) at 2 h prior to phototropic stimulation. The hypocotyls were then irradiated with unilateral BL at 1.7 × 10−3 µmol m−2 s−1 for 6 h, during which time the hypocotyl curvatures were determined at 10 min intervals. The responses of the seedlings without RL-pretreatment (–RL) are shown with dotted lines. The data shown are the mean values ± SE from 12 seedlings. B, Immunoblotting analysis of RPT2. Two-day-old etiolated seedlings of the nph3 mutant transformed with 35Spro:NPH3SE construct were pretreated with (RL+) or without (RL–) overhead red light at 20 µmol m−2 s−1 for 2 min at 2 h prior to BL irradiation. The hypocotyls were then irradiated with BL at 1.7 × 10−3 µmol m−2 s−1 for 0, 1, or 2 h. Total proteins (10 µg) were separated using a 7.5% SDS–PAGE gel, followed by immunoblotting with anti-RPT2 (α-RPT2) antibodies. The PVDF membrane was stained with the Pierce reversible protein staining kit as a loading control. C, Statistical analysis of the data shown in (B). The values were normalized with a loading control and then calculated relative to the data from the BL and red light-unirradiated seedlings. The data shown are the mean values ± SE (n = 4 experiments). Dots represent the results of individual experiments. Asterisks indicate a statistically significant difference from the RPT2 protein level in the blue and red-unirradiated seedlings (Steel test, P < 0.05).
Immunoblotting analysis using a Phos-tag acrylamide gel revealed that the BL-induced, molecular size shift of the PHOT1 proteins was very similar among seedlings of 35Spro:NPH3WT nph3, 35Spro:NPH3SA nph3, and 35Spro:NPH3SE nph3 (Figure 6). On the other hand, the PHOT1 protein in 35Spro:NPH3WT rpt2 nph3 seedlings showed an enhanced molecular size shift (Figure 6). These results suggested that the autophosphorylation activity of phot1 is enhanced by the rpt2 mutation, as described previously (Kimura et al., 2020), but not by the NPH3SA or NPH3SE mutations. Immunoblotting analysis additionally indicated that the NPH3SA and NPH3SE mutations have no effect on the BL induction of the RPT2 proteins (Figure 6). These results demonstrated that the phosphorylation status of NPH3 is involved in the photosensory adaptation of hypocotyl phototropism, and that this occurs independently of the control of either phot1 autophosphorylation activity or RPT2 light induction.
Figure 6.
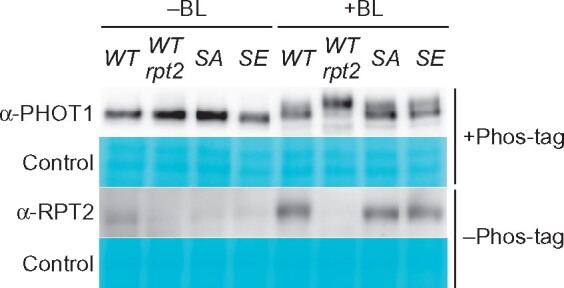
BL-induced autophosphorylation of Phot1 and the expression of RPT2 protein in 35Spro:NPH3 transgenic lines. Immunoblotting analysis of phot1 and RPT2 proteins. Two-day-old etiolated seedlings of the 35Spro:NPH3WT nph3, 35Spro:NPH3WT rpt2 nph3, 35Spro:NPH3SA nph3, or 35Spro:NPH3SE nph3 lines were irradiated with unilateral BL at 1.7 × 10−1 µmol m−2 s−1 for 2 h. Total proteins (20 µg) were separated using a 6% SDS–PAGE gel containing 2 µM Phos-tag, followed by immunoblotting with anti-PHOT1 (α-PHOT1) antibodies. Total proteins were also separated using a 7.5% SDS–PAGE gel without Phos-tag, followed by immunoblotting with anti-RPT2 (α-RPT2) antibodies. PVDF membranes were stained with the Pierce reversible protein staining kit as a loading control.
The phosphorylation status of NPH3 does not affect its membrane localization under dark conditions or its release to the cytosol by BL irradiation
Our previous study revealed that the phosphorylation and dephosphorylation of NPH3 proteins correlate well with NPH3 localization at the plasma membrane and internalization of a portion of NPH3, respectively (Haga et al., 2015). In our present study, therefore, we generated YFP-tagged NPH3WT, NPH3SA, and NPH3SE proteins and investigated their subcellular localization patterns in etiolated seedlings. These constructs were functional and complemented the nph3 hypocotyl phototropism phenotypes as effectively as NPH3WT, NPH3SA, and NPH3SE with no YFP tag (Supplemental Figure S7). Unexpectedly, however, all three YFP-tagged NPH3 variants primarily localized along the plasma membrane of the epidermal cells of etiolated hypocotyls under darkness (Figure 7, A–C). This finding indicated that the phosphorylation of the seven serine residues on NPH3 that we identified is not required for the plasma membrane localization of this protein under darkness. When the seedlings were irradiated with BL at 1.7 × 10−1 µmol m−2 s−1 for 0.25 h, a portion of the YFP-tagged NPH3WT, NPH3SA, and NPH3SE proteins were released from the plasma membrane to the cytosol and formed aggregates (Figure 7, D–F). Hence, these results suggested that neither the subcellular localization of NPH3 on the plasma membrane under darkness, nor its release to the cytosol, is dependent on the phosphorylation status of seven serine residues identified in this study.
Figure 7.
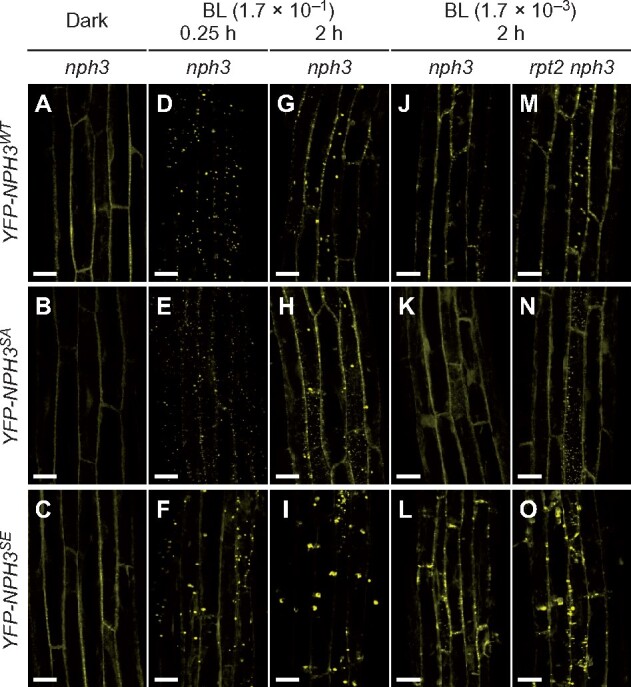
Localization pattern changes of YFP-NPH3 proteins in response to BL. A–I, Subcellular localization of YFP-tagged NPH3 proteins under BL irradiation at 1.7 × 10−1 µmol m−2 s−1. Two-day-old etiolated seedlings of the nph3 mutants transformed with 35Spro:YFP-NPH3WT (A, D, G), 35Spro:YFP-NPH3SA (B, E, H), or 35Spro:YFP-NPH3SE (C, F, I) constructs were irradiated with unilateral BL at 1.7 × 10−1 µ mol m−2 s−1 for 0.25 h (D, E, F) or 2 h (G, H, I). Localization patterns under darkness are also shown (A–C). White bar, 25 µm. J–O, Effects of the rpt2 mutation on the subcellular localization of YFP-tagged NPH3 proteins. Two-day-old etiolated seedlings of nph3 mutants transformed with 35Spro:YFP-NPH3WT (J), 35Spro:YFP-NPH3SA (K) or 35Spro:YFP-NPH3SE (L) constructs, and the rpt2 nph3 double mutants transformed with 35Spro:YFP-NPH3WT (M), 35Spro:YFP-NPH3SA (N) or 35Spro:YFP-NPH3SE (O) constructs, were irradiated with unilateral BL at 1.7 × 10−3 µmol m−2 s−1 for 2 h. White bar, 25 µm.
We confirmed the subcellular localization of our NPH3 variants by immunoblotting analysis. The majority of the NPH3WT, NPH3SA, and NPH3SE proteins were detectable in the microsomal fraction under darkness (Figure 8, A and B). BL irradiation at 1.7 × 10−1 µmol m−2 s−1 for 0.25 h resulted in detectable NPH3WT, NPH3SA, and NPH3SE proteins in the soluble fraction (Figure 8, A). Although this irradiation led to an enhanced mobility shift of NPH3WT and a portion of NPH3SE proteins (asterisk in Figure 8), the latter appeared to reflect its degradation (Supplemental Figure S8). NPH3WT, NPH3SA, and NPH3SE proteins were also detected in the soluble fraction under BL irradiation at 1.7 × 10−3 µmol m−2 s−1 for 0.25 h (Figure 8, B). Thus, these immunoblotting results supported our observations with the YFP-tagged proteins (Figure 7).
Figure 8.
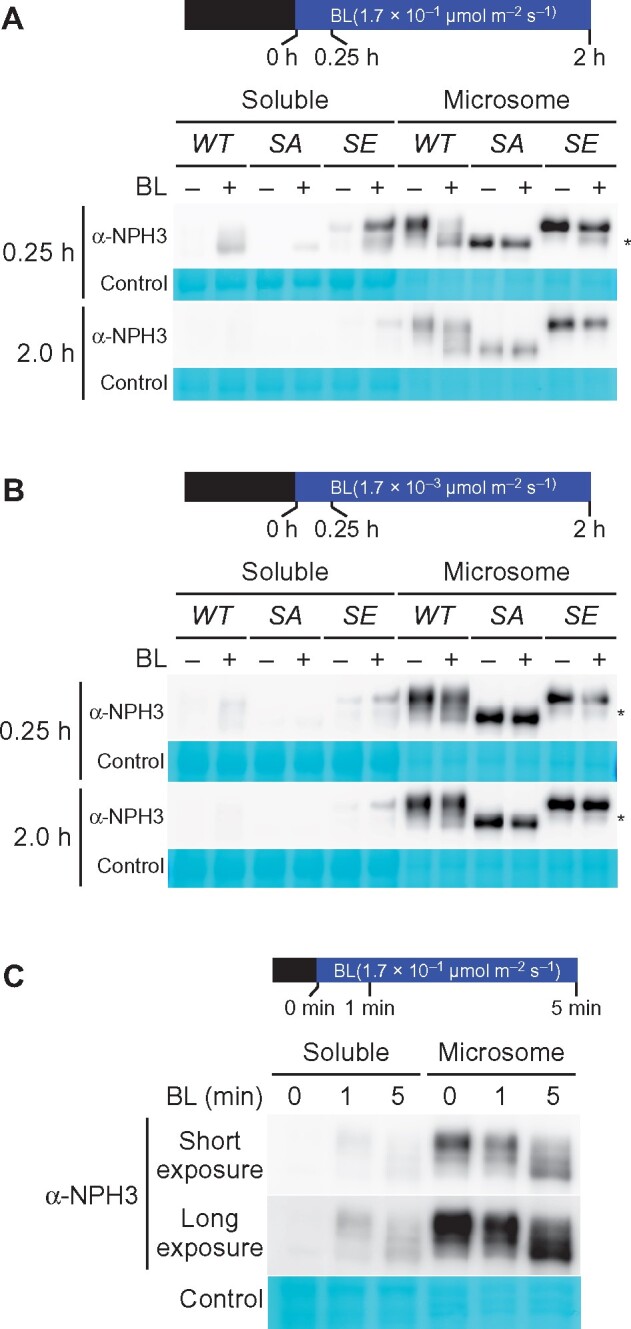
Immunochemical analysis of the subcellular localization of NPH3 proteins. A and B, Effects of the NPH3SA and NPH3SE mutations on the subcellular localization of NPH3 proteins. Two-day-old etiolated seedlings of the nph3 mutants transformed with 35Spro:NPH3WT (WT), 35Spro:NPH3SA (SA) or 35Spro:NPH3SE (SE) constructs were irradiated with unilateral BL at 1.7 × 10−1 (A) or 1.7 × 10−3 µmol m−2 s−1 (B) for the indicated times. After the light treatment, soluble proteins and crude microsomal proteins ware prepared from harvested seedlings. Proteins (5 µg) from each fraction were separated using a 7.5% SDS–PAGE gel, followed by immunoblotting with anti-NPH3 (α-NPH3) antibodies. Asterisks indicate the degradation products of NPH3SE. PVDF membranes were stained with the Pierce reversible protein staining kit as a loading control. C, Effects of short-term BL irradiation on the NPH3 protein. Two-day-old etiolated seedlings of Col wild type were irradiated with BL at 1.7 × 10−1 µmol m−2 s−1 for the indicated times. Soluble proteins and crude microsomal proteins ware prepared from harvested seedlings. Proteins (7.5 µg) from each fraction were separated using a 7.5% SDS–PAGE gel, followed by immunoblotting with anti-NPH3 (α-NPH3) antibodies. PVDF membranes were stained with the Pierce reversible protein staining kit as a loading control.
Our current results suggested that the release of NPH3 to the cytosol depends on the activation of phot1 but not the dephosphorylation of NPH3. Our previous study had shown the release of a portion of the phosphorylated NPH3 proteins to the soluble fraction of the rpt2 mutant under dark conditions (Haga et al., 2015), supporting the idea that the dephosphorylation of NPH3 is not required for its release to the cytosol. We confirmed this hypothesis here using wild-type seedlings (Figure 8, C). BL irradiation at 1.7 × 10−1 µmol m−2 s−1 for 1 min was sufficient for the detection of NPH3 proteins in the soluble fraction of the Col wild-type seedlings (Figure 8, C). Those NPH3 proteins did not show a molecular size shift reflecting their dephosphorylation (Figure 8, C), and 5 min of irradiation led to the dephosphorylation of NPH3 in both the soluble and microsomal fractions (Figure 8, C). These findings suggested that the release of phosphorylated NPH3 proteins to the cytosol is the first event after the activation of phot1 and that the dephosphorylation of NPH3 is the second event, which is independent of its release to the cytosol.
The phosphorylation status of NPH3 affects its relocalization from the cytosol to the plasma membrane
When BL was irradiated at 1.7 × 10−1 µmol m−2 s−1 for 2 h, most of YFP-NPH3SA and YFP-NPH3SE proteins seemed to localize at the plasma membrane and in the cytosol, respectively (Figure 7, G–I). Immunoblotting analysis also indicated that, in contrast to the NPH3WT and NPH3SA proteins, NPH3SE proteins remained in the soluble fractions after 2 h of BL irradiations at 1.7 × 10−1 and 1.7 × 10−3 µmol m−2 s−1 (Figure 8, A and B). Thus, we quantified the subcellular localizations of YFP-NPH3 proteins on the plasma membrane and the size and number of particles of NPH3 aggregates. First, alterations to the subcellular localizations of YFP-NPH3 proteins were quantified using colocalization analysis with the membrane-impermeable dye FM4-64 (Figure 9, A and Supplemental Figure S9), which preferentially stains the plasma membrane (Rigal et al., 2015). Under darkness, the Pearson’s correlation coefficients that show the strength of the colocalization between YFP-NPH3 and FM4-64 were very similar among the YFP-tagged proteins of NPH3WT, NPH3SA, and NPH3SE (Figure 9, A). These coefficients suggested that YFP-tagged NPH3WT proteins on the plasma membrane disappeared in response to 0.25 h of BL irradiation at 1.7 × 10−1 µmol m−2 s−1 and relocalized partially after 2 h irradiation (Figure 9, A, left panel). On the other hand, YFP-tagged NPH3SA proteins appeared to be moderately released from the plasma membrane after 0.25 h irradiation and fully relocalized to the plasma membrane after 2 h irradiation (Figure 9, A, middle panel). Conversely, YFP-NPH3SE proteins did not show recovery of the coefficient after 2 h irradiation (Figure 9, A, right panel). These results suggested that the phosphorylation status of NPH3 proteins affects their relocalization from the cytosol to the plasma membrane and that the dephosphorylated forms of NPH3 preferentially localize to the plasma membrane under continuous BL conditions.
Figure 9.
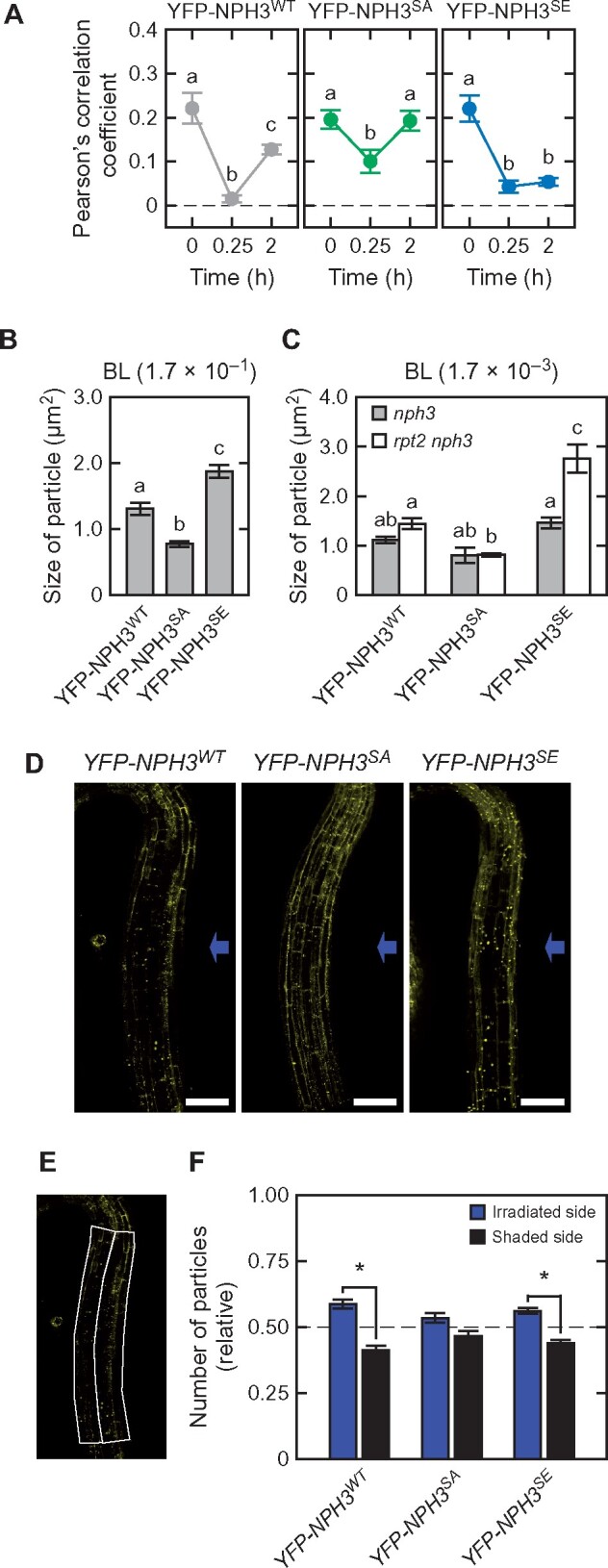
Quantitative analysis of the subcellular localization of YFP-NPH3 proteins. A, Pearson’s correlation coefficients for the colocalization of YFP-NPH3 proteins with FM4-64. Two-day-old etiolated seedlings were treated with 5 µM FM4-64 and irradiated with BL at 1.7 × 10−1 µmol m−2 s−1 for the indicated times. Pearson’s correlation coefficients between the YFP and FM4-64 signals were measured. The data shown are the mean values ± SE from 18 to 34 seedlings. Different letters on the graphs denote statistically significant differences (Tukey–Kramer multiple comparison, P < 0.05). B and C, Statistical analysis of the sizes of the YFP-NPH3 granules under BL irradiation at 1.7 × 10−1 µmol m−2 s−1 for 0.25 h (B) or 1.7 × 10−3 µmol m−2 s−1 for 2 h (C). The data shown are the mean values ± SE from 197 to 235 granules. Different letters on the graphs denote statistically significant differences (Tukey–Kramer multiple comparison, P < 0.05). D, Distribution of the aggregates of YFP-NPH3 proteins across the hypocotyl. Two-day-old etiolated seedlings of the nph3 mutants transformed with 35Spro:YFP-NPH3WT (YFP-NPH3WT: left panel), 35Spro:YFP-NPH3SA (YFP-NPH3SA: mid panel), or 35Spro:YFP-NPH3SE (YFP-NPH3SE: right panel) constructs were irradiated with unilateral BL at 1.7 × 10−1 µmol m−2 s−1 for 1.5 h. Blue arrows indicate the direction of BL. White bar, 100 µm. E and F, Quantification of the number of YFP-NPH3 aggregates in the irradiated and shaded sides of the hypocotyl. The number of YFP-NPH3 aggregates was measured by designating two regions of interest on the irradiated and shaded sides of the hypocotyl (E). The image in (E) is the same as that of YFP-NPH3WT in (D). The counted number was then fixed to those per unit area (7,200 µm2) (F). The data shown are the mean values ± SE from 23 to 31 seedlings. Asterisks indicate a statistically significant difference (paired t test, P < 0.05).
Second, the particle sizes of the NPH3 aggregates in the cytosol were measured. These also differed among the YFP-NPH3WT, YFP-NPH3SA, and YFP-NPH3SE proteins in the seedlings when irradiated with BL at 1.7 × 10−1 µmol m−2 s−1 for 0.25 h (Figure 7, D–F and Figure 9, B). Particles were smaller for YFP-NPH3SA and larger for YFP-NPH3SE than YFP-NPH3WT (Figure 9, B). When the seedlings were irradiated with BL at 1.7 × 10−3 µmol m−2 s−1 for 2 h, particle structures of YFP-NPH3WT were observed in the 35Spro:YFP-NPH3WT rpt2 nph3 line (Figure 7, M), but were barely detectable in the 35Spro:YFP-NPH3WT nph3 seedlings (Figure 7, J). Thus, low phot1 activity under a low fluence rate at 1.7 × 10−3 µmol m−2 s−1 did not effectively induce the formation of NPH3 aggregates, whereas high phot1 activity with the rpt2 mutation caused their formation, as described previously (Haga et al., 2015; Kimura et al., 2020). On the other hand, at 1.7 × 10−3 µmol m−2 s−1, the particles that formed for YFP-NPH3SA and YFP-NPH3SE were smaller and larger than those of YFP-NPH3WT, respectively, in the rpt2 nph3 mutants (Figures 7, J–O, 9, C). These results suggested that both the activation level of phot1 and the phosphorylation status of NPH3 affect the particle size of NPH3 aggregates, resulting in the alteration of the retention time of NPH3 in the cytosol. It should be noted however that another possibility is that a difference of the expression levels of YFP-NPH3WT, -NPH3SE, and -NPH3SA also affects their particle sizes in the cytosol (Supplemental Figure S7, A).
Third, the numbers of particles were determined in the upper part of the etiolated hypocotyls of the YFP-NPH3 transgenic lines. As described previously (Sullivan et al., 2019), the number of YFP-NPH3WT aggregates in the irradiated side was larger than that in the shaded side of hypocotyls of 35Spro:YFP-NPH3WT nph3 seedlings after 1.5 h of unilateral BL irradiation at 1.7 × 10−1 µmol m−2 s−1. The hypocotyls of 35Spro:YFP-NPH3SE nph3 also showed a similar difference with 35Spro:YFP-NPH3WT nph3 but not the 35Spro:YFP-NPH3SA nph3 seedlings (Figure 9, D–F), suggesting that both the activation level of phot1 and the phosphorylation status of NPH3 in response to the fluence rate of BL irradiation affect the numbers of NPH3 aggregates. As a normal phototropic response was induced in 35Spro:NPH3SA nph3 but not 35Spro:NPH3SE nph3 under this condition (Figure 2, A), the difference in the number of NPH3 aggregates across the hypocotyl is probably not a cause of the differential growth of the phototropic response.
Discussion
We reveal in our current study that the phosphorylation status of the NPH3 proteins affects the photosensory adaptation of phot1 signaling to induce the phototropic responses in the etiolated hypocotyl of Arabidopsis. NPH3SE and NPH3SA variants were suitable for hypocotyl phototropism under BL conditions at very low fluence rates (∼10−5 µmol m−2 s−1) and at fluence rates of 10−3 µmol m−2 s−1 or more, respectively. These results are meaningful in terms of further understanding the physiological functions of NPH3, because this protein is phosphorylated under darkness and dephosphorylated by BL irradiation. We reported in our recent study that RPT2 contributes to sensor adaptation through the suppression of phot1 activity (Kimura et al., 2020). Our current study findings have indicated that RPT2 and NPH3 independently contribute to the photosensory adaptation of phot1 signaling, and that the phosphorylation status of NPH3 has no effect on the expression or autophosphorylation activity of phot1 (Figure 6). These data collectively indicate that the control of the NPH3 phosphorylation status contributes to effector adaptation downstream of phot1 activation. The phototropic responses of Arabidopsis etiolated hypocotyls are induced by unilateral BL from 10−5 to 102 µmol m−2 s−1 or more, and this dynamic range of photosensitivity can be achieved with phot1 alone because our previous study showed that the phot2 single mutant underwent normal hypocotyl phototropism under BL irradiation at all of the fluence rates examined (Inada et al., 2004). Such a wide dynamic range for phot1 signaling would be caused by not only the control of phot1 activity through the expression regulation of RPT2 and PHOT1, but also via the control of phot1 signaling through the phosphorylation status of NPH3.
An important question that arises from our present analyses is how the phosphorylation status of NPH3 proteins affects the photosensory adaptation in phot1 signaling. Imaging analyses of YFP-NPH3 proteins indicated that phosphomimetic NPH3SE proteins and unphosphorable NPH3SA proteins showed a lengthening and shortening of their subcellular localization in the cytosol, respectively (Figure 7). One possible hypothesis is that NPH3 functions on the plasma membrane as a positive regulator of phot signaling and that a short retention time of NPH3SA in the cytosol is suitable for the phototropic responses, especially under high fluence rate BL conditions. This hypothesis, however, does not explain why the 35Spro:NPH3SA nph3 seedlings showed weaker responses than the 35Spro:NPH3WT nph3 line in the second positive phototropism at the very low fluence rate (1.7 × 10−5 µmol m−2 s−1; Figure 3, A) and in the first positive phototropism (Figure 3, B).
Previous studies have indicated that a lengthening and shortening of the lifetime of the phototropin active state leads to a photosensory adaptation to BL at a low and high fluence rate, respectively, during the phototropic response (Christie et al., 2002; Kasahara et al., 2002; Aihara et al., 2008; Okajima et al., 2012; Kimura et al., 2020). It means that the suppression of phot1 signaling is suitable for the photosensory adaptation to BL irradiation at high fluence rates. Therefore, we propose another hypothesis for the function of NPH3 in hypocotyl phototropism, which is that the formation of a complex of phot1 and NPH3 on the plasma membrane is a steady state of the phot1 signaling and the dissociation of this complex represents an active state of the phot1 signaling in hypocotyl phototropism (Supplemental Figure S10). This hypothesis suitably explains the 35Spro:NPH3SA nph3 and 35Spro:NPH3SE nph3 phenotypes. The lengthening of the retention time of NPH3 proteins in the cytosol via its phosphorylation may enhance phot1 signaling to induce the phototropic response under very low fluence rate BL conditions, and the shortening of the retention time of NPH3 proteins in the cytosol via its dephosphorylation may suppress phot1 signaling to adapt to high fluence rate BL conditions. Further studies will be necessary to reveal whether NPH3 binding to phot1 on the plasma membrane acts as a negative or positive regulator of the phot1 signaling.
Our present results indicated that the activation of phot1 separately induces a release of NPH3 proteins from the plasma membrane to the cytosol and their dephosphorylation (Figures 7, 8). Imaging analysis of YFP-NPH3SA suggested that the dephosphorylation of NPH3 enhances its relocalization to the plasma membrane (Figures 7, 9), indicating that phot1 controls both the dissociation and reassociation rates of NPH3 at the plasma membrane (Supplemental Figure S10). When the activation level of phot1 is low under very low fluence rate BL conditions, the extent of the release of NPH3 proteins from the plasma membrane to the cytosol and their relocalization to the plasma membrane is low in both cases. When the activation level of phot1 is high under high fluence rate BL conditions, both this release from and relocalization to the plasma membrane are high. When this balance is lost by the NPH3SE mutation or the rpt2 mutation under BL conditions, the hypocotyls of Arabidopsis-etiolated seedlings fail to undergo phototropic responses under high fluence rate BL conditions. This release and relocalization balance may therefore be required for a formation of a suitable gradient of phot1 signaling activity between the irradiated and shaded sides, in accordance with the fluence rate of unilateral BL. Thus, the activation level of phot1 and the corresponding phosphorylation level of NPH3, respectively, determine the dissociation and reassociation rates of phot1 and NPH3 at the plasma membrane, which may moderately maintain the active state of phot1 signaling across a broad range of BL intensities and contribute to the photosensory adaptation of phot1 signaling during the phototropic response in hypocotyls (Supplemental Figure S10).
We have here identified four phosphorylation sites, S7 (or S5), S467, S474 (or S476), and S722 (or S723) on the NPH3 protein in Arabidopsis-etiolated seedlings, in addition to the previously described S213, S223, and S237 sites (Tsuchida-Mayama et al., 2008). We examined the effects of both an alanine-substitution (NPH3SA) and glutamate-substitution (NPH3SE) at all of these serine residues, and thereby identified a role of the phosphorylation status of NPH3 proteins in photosensory adaptation during hypocotyl phototropism. However, it is debatable whether those mutated NPH3 proteins truly function as respective dephosphorylated and phosphorylated forms in planta. First, we did not determine whether four phosphoserine residues, S7 (or S5), S467, S474 (or S476), and S722 (or S723), are dephosphorylated by BL irradiation in this current study. Second, we could detect only seven phosphoserine residues on this protein in etiolated seedlings, although phosphoproteome analyses of Arabidopsis has predicted ∼18 phosphorylation sites on NPH3 (PhosPhAt 4.0). We also found in our current analyses that neither the NPH3SA nor NPH3SE proteins showed any obvious SDS–PAGE mobility shift under BL irradiation (Figure 8 and Supplemental Figure S2), and our mass spectrometry analysis of immunoprecipitated NPH3 proteins detected peptides that included 16 of the 18 phosphorable amino acid residues in the PhosPhAt 4.0 database. These observations suggest that our seven identified serine residues are the main phosphorylation sites of NPH3 in etiolated seedlings, but that verification of this will be necessary in future studies. PSIPRED analysis (Supplemental Materials and Methods) predicted that all of these NPH3 phosphorylation events occur within the intrinsically disordered regions of the protein (Supplemental Figure S11). Modifications of phosphorylation events in intrinsically disordered regions of NPH3 may cause their aggregation in the cytosol, resulting in a lengthening of the NPH3 protein retention time in the cytosol and/or an alteration of its E3 ligase activity (Roberts et al., 2011). The effects of phosphorylation on the biochemical functions of NPH3 will be also the subject of future studies.
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) Col-0 was used as the wild-type control in the current experiments. rpt2-2, nph3-102, and 35Spro:YFP-NPH3WT nph3 have been described previously (Inada et al., 2004; Tsuchida-Mayama et al., 2008; Haga et al., 2015).
35Spro:NPH3WT nph3-102 transgenic lines were generated as follows. The NPH3 coding sequence (CDS) in a pENTR vector (Haga et al., 2015) was transferred to a pH35GS binary vector using an LR recombination reaction (Invitrogen). 35Spro:NPH3WT in the binary vector was transformed into the nph3-102 mutant via the floral dip method using Agrobacterium tumefaciens (Strain C58C1)-mediated transformation, as described previously (Clough and Bent, 1998).
35Spro:NPH3SA nph3-102, 35Spro:NPH3SE nph3-102, 35Spro:YFP-NPH3SA nph3-102, and 35Spro:YFP-NPH3SE nph3-102 transgenic Arabidopsis lines were generated as follows. The 5′, middle and 3′-fragments of the NPH3 CDS were PCR amplified using primers harboring point mutations and BtsI-restriction enzyme sites, and subsequently subcloned into the pENTR vector via a D-TOPO reaction (Invitrogen). The primers used are listed in Supplemental Table S1. pENTR-NPH3 3′, pENTR-NPH3 middle, and pENTR-NPH3 5′ were digested with BtsI (New England BioLabs) and the resulting fragments were ligated using a DNA Ligation kit (Takara). The cDNAs of NPH3SA and NPH3SE within the pENTR vectors were transferred to both pH35GS and pH35YG binary vectors (Yamaguchi et al., 2008) using an LR recombinant reaction (Invitrogen). 35Spro:NPH3SA, 35Spro:NPH3SE, 35Spro:YFP-NPH3SA, and 35Spro:YFP-NPH3SE inserts in the binary vectors were transformed into the nph3-102 mutant via the floral dip method using A. tumefaciens (Strain C58C1)-mediated transformation, as described previously (Clough and Bent, 1998). The 35Spro:NPH3WT rpt2 nph3, 35Spro:NPH3SA rpt2 nph3, 35Spro:NPH3SE rpt2 nph3, 35Spro:YFP-NPH3WT rpt2 nph3, 35Spro:YFP-NPH3SA rpt2 nph3, and 35Spro:YFP-NPH3SE rpt2 nph3 lines were generated by crossings with rpt2-2-mutant lines.
For physiological experiments, etiolated Arabidopsis seedlings were prepared according to the tube method (Haga and Kimura, 2019). Briefly, Arabidopsis seeds were sown in 0.2-mL plastic tubes filled with 1.5% (w/v) agar medium, placed in a black plastic box, and kept at 4°C for 3–5 d. Following the induction of germination, the prepared seeds were incubated for 2 d under complete darkness. Seedlings were then selected based on the length of the hypocotyls (3–5 mm). During the experiments, seedlings were kept in a black plastic box under high humidity until needed. For immunoblotting and microscopy analyses, the etiolated seedlings were grown along the surface of vertically oriented agar medium (Haga and Kimura, 2019). Experimental manipulations were performed under dim green light.
MS analysis
The collection of microsomal fractions and immunoprecipitation of YFP-NPH3WT were performed as described previously (Inada et al., 2004; Haga et al., 2015). Briefly, microsomal fractions were obtained from ∼1,000 two-day-old etiolated seedlings of 35Spro:YFP-NPH3WT nph3 irradiated with unilateral BL at 1.7 × 10−1 µmol m−2 s−1 for 2 h by centrifuging the extracts of seedlings at 100,000g for 75 min. Subsequently, YFP-NPH3WT proteins were immunoprecipitated with GFP-Trap-A agarose beads (Chromotech) for 1 h at 4°C. The collected YFP-NPH3WT proteins were separated on 7.5% SDS–PAGE gels which were then stained with CBB Stain One (Nacalai Tesque).
In-gel digestions were performed as described previously (Shevchenko et al., 2006). Briefly, digested peptides in the gel pieces were recovered by adding 5% (v/v) formic acid/acetonitrile, desalted using StageTips with C18 disk membranes (EMPORE, 3M; Rappsilber et al., 2003), dried in a vacuum evaporator, and dissolved in 9 μL of 5% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid. An LTQ-Orbitrap XL (Thermo Fisher Scientific) coupled with an EASY-nLC 1000 (Thermo Fisher Scientific) was subsequently used for nano-LC–MS/MS analyses. A self-pulled needle (150 mm length × 100 µm i.d., 6 µm opening) packed with ReproSil C18 resin (3 μm; Dr. Maisch GmbH) was used as an analytical column with a “stone-arch” frit (Ishihama et al., 2002). A spray voltage of 2,400 V was applied. The injection volume was 6 μL and the flow rate was 500 nL min−1. The mobile phase consisted of 0.5% acetic acid (A) and 0.5% (v/v) acetic acid and 80% (v/v) acetonitrile (B). A two-step linear gradient of 0%–40% B in 30 min, 40%–100% B in 5 min, and 100% B for 10 min was employed. The MS scan range was m/z 300–1,400. The top 10 precursor ions were selected in the MS scan by Orbitrap at a 100,000 resolution and for subsequent MS/MS scans by ion trap in the automated gain control mode, where the automated gain control values of 5.00e + 05 and 1.00e + 04 were set for full MS and MS/MS, respectively. The normalized collision-induced dissociation was set to 35.0. A lock mass function was used for the LTQ-Orbitrap XL to obtain constant mass accuracy during gradient analysis (Olsen et al., 2005). Multi-stage activation was enabled upon detection of a neutral loss of phosphoric acid (98.00, 49.00, or 32.66 a.m.u.; Schroeder et al., 2004) for further ion fragmentation. Selected sequenced ions were dynamically excluded for 60 s after sequencing.
Mass Navigator version 1.3 (Mitsui Knowledge Industry) with default parameters for the LTQ-Orbitrap XL was used to create peak lists on the basis of the recorded fragmentation spectra. The m/z values of the isotope peaks were converted to the corresponding monoisotopic peaks when the isotope peaks were selected as the precursor ions. To improve the quality of the MS/MS spectra, Mass Navigator discarded all peaks with an absolute intensity below 10% and of <0.1% of the most intense peak in the MS/MS spectra (Ravichandran et al., 2009). Peptides and proteins were identified by means of automated database searching using Mascot version 2.4.1 (Matrix Science) in The Arabidopsis Information Resource database (TAIR10_pep_20101214, ftp://ftp.arabidopsis.org/home/tair/Sequences/blast_datasets/TAIR10_blastsets/) which contains protein sequence information for YFP-tagged NPH3WT with a precursor mass tolerance of 3 ppm, a fragment ion mass tolerance of 0.8 Da, and strict trypsin specificity (Olsen et al., 2004), allowing for up to two missed cleavages. The carbamidomethylation of Cys was set as a fixed modification and the oxidation of Met and phosphorylation of Ser, Thr, and Tyr were allowed as variable modifications. The accession numbers for the mass spectrometry data generated in this study are PXD017975 for ProteomeXchange and JPST000761 for jPOST (Okuda et al., 2017).
Induction of phototropism and measurement of the curvature
Phototropic stimulation was performed (470 ± 30 nm, LED-B; Eyela) through two layers of blue filter (no. 72 film; Tokyo Butai Shomei). The fluence rate was controlled with neutral-density plastic filters (Fujifilm). The direction of the phototropic stimulation was perpendicular to the plane of the hook (Haga and Sakai, 2012). For pretreatment with red light, the seedlings were irradiated with overhead red light (660 ± 20 nm, LED-R; Eyela) at 20 μmol m−2 s−1 for 2 min. Images of dark-grown seedlings were recorded just before phototropic stimulation and at 3 h after the onset of the stimulation with a digital camera (D5000; Nikon), from which a UV/infrared light cut filter was removed (IDAS Division, ICAS Enterprises) under infrared illumination (IRDR-110; Nissen Electronics). For time-course experiments, images of the seedlings were captured at 10-min intervals using the same equipment. The phototropic curvatures of the hypocotyls were measured using CANVAS11 software (ACD Systems International Inc.).
Immunoblotting analysis
Protein preparations were made as described in our previous study (Haga et al., 2015; Kimura et al., 2020). Briefly, for total protein extractions, seedlings were homogenized in 2× SDS buffer. For Phos-tag immunoblotting analysis, seedlings were homogenized in extraction buffer optimized for Phos-tag containing 50 mM Tris–MES, pH 7.5, 300 mM sucrose, 150 mM NaCl, 10 mM potassium acetate, 0.2% (v/v) Triton X-100, and a protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics). Homogenates were centrifuged twice at 10,000g at 4°C for 10 min. For the collection of soluble and microsomal fractions, seedlings were homogenized with extraction buffer containing 50 mM Tris–MES, pH 7.5, 300 mM sucrose, 150 mM NaCl, 10 mM potassium acetate, 5 mM EDTA, and a protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics) using a mortar and pestle. Homogenates were centrifuged twice at 10,000g at 4°C for 10 min and subsequently at 100,000g at 4°C for 75 min.
For Phos-tag SDS–PAGE, denatured samples were separated on a 6% SDS–PAGE gel containing 2 µM of Phos-tag (FUJIFILM Wako Pure Chemical Corporation). For other SDS–PAGE analyses, denatured samples were separated on a 7.5% of SDS–PAGE gel (SuperSep Ace; FUJIFILM Wako Pure Chemical Corporation). The following antibodies were used for immunoblotting: anti-PHOT1 (Cat#KR095, TransGenic Inc., 1:4,000 dilution), anti-RPT2 (Inada et al., 2004), anti-NPH3 (Tsuchida-Mayama et al., 2008), and HRP-conjugated anti-rabbit IgG (Cat#NA934-1ML, GE Healthcare, 1:50,000 dilution). As a loading control, PVDF membranes were stained with the Pierce reversible protein staining kit (Thermo Scientific). The results were confirmed using independent samples.
Confocal laser scanning microscopy
YFP signals were detected with a TCS-SP5 confocal laser scanning microscope (Leica Microsystems). The fluorescent signals were excited with an argon laser at 514 nm and the spectral detector was set at 525–560 nm. The same settings (laser power and HyD photon counting mode) were used for direct comparison of fluorescence intensities. Scans were performed at a 2048 × 2048-pixel resolution with repeated scanning of two lines. The sizes and numbers of the YFP-NPH3 aggregates were measured using the Analyze Particles tool in the Fiji software (Schindelin et al., 2012), with a diameter of 0.2–50 µm and circularity of 0.25–1. The fluorescent intensity of YFP-NPH3 proteins on the plasma membranes was also measured using Fiji software.
For colocalization analysis between YFP-NPH3 and FM4-64, 2-d-old etiolated seedlings were incubated in half strength OS medium containing 5 µM FM4-64 (Thermo Fisher) for 1 h with BL treatment. Both YFP-NPH3 and FM4-64 were excited with an argon laser at 514 nm and the spectral detector was set at 525–560 nm and 640–752 nm, respectively, to capture the Z-stack of 9–11 images at a 2048 × 512 pixel resolution. YFP and FM4-64 signals whose luminosities exceeded 3 and 60, respectively, were measured to calculate the Pearson’s correlation coefficients using Imaris software (Zeiss).
Accession numbers
Sequence data for this article can be found in the Arabidopsis Genome Initiative or the EMBL/GenBank data libraries under the following accession numbers: NPH3 (AT5G64330), and RPT2 (AT2G30520). The germplasm used included nph3-102 (Salk_110039). The accession numbers for the mass spectrometry data generated in this study are PXD017975 for ProteomeXchange and JPST000761 for jPOST.
Supplemental data
Supplemental Figure S1. MS analysis of YFP-NPH3WT.
Supplemental Figure S2. NPH3 protein expression in 35Spro:NPH3 transgenic lines and their SDS–PAGE mobilities.
Supplemental Figure S3. Complementation of the aphototropic phenotype of the nph3 mutant with 35Spro:NPH3WT.
Supplemental Figure S4. Continuous light-induced second positive phototropism and pulse-induced first positive phototropism in other 35Spro:NPH3 transgenic lines.
Supplemental Figure S5. Red light enhancement of the second positive phototropism under BL at 1.7 × 10−1 µmol m−2 s−1.
Supplemental Figure S6. Red light enhancement of the second positive phototropism under BL at 1.7 × 10−3 µmol m−2 s−1 in other 35Spro:NPH3 transgenic lines.
Supplemental Figure S7. Phototropic responses of the 35Spro:YFP-NPH3WT, 35Spro:YFP-NPH3SA, and 35Spro:YFP-NPH3SE transgenic lines.
Supplemental Figure S8. The mobility shift of NPH3SE protein on SDS–PAGE reflects its degradation.
Supplemental Figure S9. Colocalization of YFP-NPH3 proteins with FM4-64.
Supplemental Figure S10. Hypothetical model of effector adaption via the NPH3 phosphorylation status during a phototropic response.
Supplemental Figure S11. Spatial relationships between the position of the phosphorylation sites and the disordered regions within the NPH3 protein.
Supplemental Table S1. Gene-specific primers used for construction.
Supplementary Material
Acknowledgments
We thank T. Demura and M. Yamaguchi (Nara Institute of Science and Technology) for providing the pH35GS and pH35YG binary vectors. We also thank the Platforms for Advanced Technologies and Research Resources “Advanced Bioimaging Support” (JSPS: KAKENHI JP16H06280) for their technical support.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS: KAKENHI 25120710, 16H01231, 17H03694 to T.S.; 16J01942 to T. K.; 17K07451 to K.H.; and 16H06280, 17K19380, 18H05492 to T.H.), the Max-Planck-Gesellschaft to H.N., the Sasaki Environment Technology Foundation (to T.S.), Takeda Science Foundation (to T.S.), Ohsumi Frontier Science Foundation (to T.S.), and the NAGAI NS Promotion Foundation for Science of Perception (to T.S.).
Conflict of interest statement. None declared.
T.K., K.H., and T.S. conceived and designed the experiments. Y.N. and H.N. performed the mass spectrometry analysis. T.H. analyzed the microscopic data. T.S. generated the transgenic lines. T.K. and K.H. performed the experiments and analyzed the data. T.K. and T.S. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Tatsuya Sakai (tsakai@gs.niigata-u.ac.jp).
References
- Aihara Y, Tabata R, Suzuki T, Shimazaki K, Nagatani A (2008) Molecular basis of the functional specificities of phototropin 1 and 2. Plant J 56: 364–375 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Chaya T, Tsutsumi R, Varner LR, Maeda Y, Yoshida S, Furukawa T (2019) Cul3-Klhl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation. EMBO J 38: e101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, DeMay BS, Gladfelter AS, Dunlap JC, Loros JJ (2010) Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. Proc Natl Acad Sci USA 107: 16715–16720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: new light through old concepts. Am J Bot 100: 35–46 [DOI] [PubMed] [Google Scholar]
- Christie JM, Suetsugu N, Sullivan S, Wada M (2018) Shining light on the function of NPH3/RPT2-like proteins in phototropin signaling. Plant Physiol 176: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Swartz TE, Bogomolni RA, Briggs WR (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Corrochano LM, Galland P (2019) Measurement of phototropism of the sporangiophore of Phycomyces blakesleeanus. Methods Mol Biol 1924: 63–81 [DOI] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MRG, Inoue S, Schepens I, Lariguet P, Geisler M, Shimazaki K-i, Hangarter R, Fankhauser C (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Chen CH, Lee C, Gladfelter AS, Dunlap JC, Loros JJ (2015) Biological significance of photoreceptor photocycle length: VIVID photocycle governs the dynamic VIVID-White Collar complex pool mediating photo-adaptation and response to changes in light intensity. PLoS Genet 11: e1005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P (1991) Photosensory adaptation in aneural organisms. Photochem Photobiol 54: 1119–1134 [Google Scholar]
- Haga K, Kimura T (2019) Physiological characterization of phototropism in Arabidopsis seedlings. Methods Mol Biol 1924: 3–17 [DOI] [PubMed] [Google Scholar]
- Haga K, Sakai T (2012) PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol 160: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Tsuchida-Mayama T, Yamada M, Sakai T (2015) Arabidopsis ROOT PHOTOTROPISM2 contributes to the adaptation to high-intensity light in phototropic responses. Plant Cell 27: 1098–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20: 796–800 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Durek P, Hummel J, Selbig J, Weckwerth W, Walther D, Schulze WX (2008) PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res 36: D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen A, Immonen EV, Salmela I, Heimonen K, Weckström M (2017) Insect photoreceptor adaptations to night vision. Phil Trans R Soc B 372: 20160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin1 in Arabidopsis thaliana. Plant Cell 16: 887–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M (2001) Phototropism in higher plants. In D Häder M Lebert, eds, Photomovement, Elsevier, Amsterdam, The Netherlands, pp 659–811 [Google Scholar]
- Ishihama Y, Rappsilber J, Andersen JS, Mann M (2002) Microcolumns with self-assembled particle frits for proteomics. J Chromatogr A 979: 233–239 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kasahara M, Swartz TE, Olney MA, Onodera A, Mochizuki N, Fukuzawa H, Asamizu E, Tabata S, Kanegae H, Takano M, et al. (2002) Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol 129: 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tsuchida-Mayama T, Imai H, Okajima K, Ito K, Sakai T (2020) Arabidopsis ROOT PHOTOTROPISM2 is a light-dependent dynamic modulator of phototropin1. Plant Cell 32: 2004–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KW, Dell’orco D (2015) Protein and signaling networks in vertebrate photoreceptor cells. Front Mol Neurosci 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1996) Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 112: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Okajima K, Kashojiya S, Tokutomi S (2012) Photosensitivity of kinase activation by blue-light involves the lifetime of a cysteinyl-flavin adduct intermediate, S390, in the photoreaction cycle of the LOV2 domain in phototropin, a plant blue-light receptor. J Biol Chem 287: 40972–40981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Watanabe Y, Moriya Y, Kawano S, Yamamoto T, Matsumoto M, Takami T, Kobayashi D, Araki N, Yoshizawa AC, et al. (2017) jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res 45: D1107–D1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, de Godoy LMF, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 4: 2010–2021 [DOI] [PubMed] [Google Scholar]
- Olsen JV, Ong SE, Mann M (2004) Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol Cell Proteomics 3: 608–614 [DOI] [PubMed] [Google Scholar]
- Pedmale UV, Liscum E (2007) Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J Biol Chem 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Poff KL, Janoudi A-K, Rosen ES, Orbovi V, Konjevic R, Fortin M-C, Scott TK (1994) The physiology of tropisms. In Meyerowitz EM, Somerville CR, eds, Arabidopsis, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 639–664 [Google Scholar]
- Rappsilber J, Ishihama Y, Mann M (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem 75: 663–670 [DOI] [PubMed] [Google Scholar]
- Ravichandran A, Sugiyama N, Tomita M, Swarup S, Ishihama Y (2009) Ser/Thr/Tyr phosphoproteome analysis of pathogenic and non-pathogenic Pseudomonas species. Proteomics 9: 2764–2775 [DOI] [PubMed] [Google Scholar]
- Rigal A, Doyle SM, Robert S (2015) Live cell imaging of FM4-64, a tool for tracing the endocytic pathways in Arabidopsis root cells. Methods Mol Biol 1242: 93–103 [DOI] [PubMed] [Google Scholar]
- Roberts D, Pedmale UV, Morrow J, Sachdev S, Lechner E, Tang X, Zheng N, Hannink M, Genschik P, Liscum E (2011) Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3 (NPH3). Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MJ, Shabanowitz J, Schwartz JC, Hunt DF, Coon JJ (2004) A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal Chem 76: 3590–3598 [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H (2001) Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol Microbiol 39: 1080–1087 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1: 2856–2860 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Kharshiing E, Laird J, Sakai T, Christie JM (2019) Deetiolation enhances phototropism by modulating NON-PHOTOTROPIC HYPOCOTYL3 phosphorylation status. Plant Physiol 180: 1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T, Nakano M, Uehara U, Sano M, Fujisawa N, Okada K, Sakai T (2008) Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci 174: 626–633 [Google Scholar]
- Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55: 652–664 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.