Abstract
Clinical genetic testing readily detects germline genetic variants. Yet, the rarity of individual variants limits the evidence available for variant classification, leading to many variants of uncertain significance (VUS). VUS cannot guide clinical decisions, complicating counseling and management. In hereditary breast cancer gene PALB2, approximately 50% of clinically identified germline variants are VUS and approximately 90% of VUS are missense. Truncating PALB2 variants have homologous recombination (HR) defects and rely on error-prone nonhomologous end-joining for DNA damage repair (DDR). Recent reports show that some missense PALB2 variants may also be damaging, but most functional studies have lacked benchmarking controls required for sufficient predictive power for clinical use. Here, variant-level DDR capacity in hereditary breast cancer genes was assessed using the Traffic Light Reporter (TLR) to quantify cellular HR/nonhomologous end-joining with fluorescent markers. First, using BRCA2 missense variants of known significance as benchmarks, the TLR distinguished between normal/abnormal HR function. The TLR was then validated for PALB2 and used to test 37 PALB2 variants. Based on the TLR's ability to correctly classify PALB2 validation controls, these functional data where applied in subsequent germline variant interpretations at a moderate level of evidence toward a pathogenic interpretation (PS3_moderate) for 8 variants with abnormal DDR, or a supporting level of evidence toward a benign interpretation (BS3_supporting) for 13 variants with normal DDR.
Detection of germline genetic variants has increased with the rise of clinical genetic testing.1, 2, 3 Yet, many variants still lack sufficient evidence to determine whether the variant is pathogenic or benign, leading to many variants of uncertain significance (VUS) classifications. VUS cannot guide clinical decisions, complicating post-test patient counseling and management.4,5 The rarity of most individual missense VUS limits the collection of patient and family data. Laboratory tests of a variant's functional impact can potentially aid reclassification,4,6 because available clinical data is not a prerequisite for testing, but benchmarking against variants with definitive interpretations is required to have sufficient predictive power for clinical use.7
Even among genes with clear links to disease and well-understood functions, VUS account for a large portion of total identified variation. For example, many of the genes implicated in hereditary breast cancer (Mendelian Inheritance in Man [MIM] number 114480) have known roles in homology-directed repair (HR) of DNA double-strand breaks (DSBs) under normal conditions, but the clinical interpretation of missense variation in these genes remains a challenge. In one such gene, PALB2 (Partner and Localizer of BRCA2 [MIM number 610355]), approximately 50% of all clinically identified variants in the Clinical Variant database (ClinVar, National Center for Biotechnology Information; https://www.ncbi.nlm.nih.gov/clinvar; accession number NM_024675.3) are VUS, and approximately 90% of VUS are missense variants.8
For high-fidelity repair of DSBs via HR, the coiled-coil (CC) regions in BRCA1 and PALB2 must first interact to stably localize PALB2 to the sites of DNA DSBs.9,10 In turn, the PALB2 WD40 domain interacts with BRCA2 and RAD51 to form a complex directing the use of a homologous template strand to correctly repair DSBs.10, 11, 12, 13 As such, PALB2 acts as the molecular scaffold in the BRCA1–PALB2–BRCA2 complex.9 PALB2 homodimers can also form through self-interaction of the CC domain, preventing heterodimerization with BRCA1 and potentially regulating HR efficiency.14,15 Defects in HR can lead to repair via alternative nonhomologous end-joining (alt-NHEJ), an error-prone mechanism that leads to small insertions and deletions.16, 17, 18
Truncating (nonsense and frameshift) variants comprise the majority of pathogenic variation in PALB2 and demonstrate HR defects, relying instead on error-prone alt-NHEJ for DNA damage repair (DDR). As such, tumors with germline PALB2, BRCA1 (MIM number 113705), and BRCA2 (MIM number 600185) defects demonstrate a pattern of genome-wide instability and a higher mutational burden.19,20 Recent reports have indicated that some missense PALB2 variants may also be damaging, but none of these variants have been definitively classified as pathogenic. Thus far, functional studies have lacked sufficient benchmarking controls for validated use in clinical variant interpretation.21, 22, 23, 24 Other hereditary cancer genes in the HR pathway have been sufficiently well-studied and have good benchmarking controls. For example, BRCA2 missense variants have been characterized previously by their capacity for HR using the direct repeat green fluorescent protein (DR-GFP) reporter assay, where GFP serves as a marker for HR following an induced DNA DSB.5,25 Benign variants retain HR capacity and thus strongly express GFP, whereas pathogenic variants defective for HR do not. The established BRCA2 benign and pathogenic variants used in developing this assay constitute a gold standard panel for functional assay validation.5,26 These same BRCA2 gold standard missense variants were used to evaluate the Traffic Light Reporter (TLR) as an assay of DDR and to develop a robust analytical process. The TLR was then calibrated with PALB2 variants of known significance to establish a DDR assay for PALB2 missense variants.
The American College of Medical Genetics and Genomics (ACMG)/Association for Molecular Pathology (AMP) sequence variant interpretation guidelines outline each evidence criterion and the assigned weight (supporting, moderate, strong, very strong) at which the evidence can be applied.1 These categorical strengths have also been modeled as Bayesian odds of pathogenicity (OddsPath).27 Together with the ClinGen Sequence Variant Interpretation Working Group, the authors recently described recommendations for using statistical analyses to determine the strength at which the ACMG/AMP functional evidence criteria can be applied in support of a pathogenic or benign classification (PS3 or BS3 codes, respectively).7 Adhering to these recommendations, this study sought to derive the strength of evidence that could be applied for PALB2 clinical variant interpretation, based on the performance of the assay in PALB2 variants with known clinical relevance.
Materials and Methods
Plasmids, Cloning, and siRNA
Human N-terminal, FLAG-tagged PALB2 was a gift from Daniel Durocher (pDEST-FRT/T0-FLAG-PALB2, plasmid #71114; Addgene, Watertown, MA).28 Human wild-type (WT) BRCA2 was obtained from pcDNA3 236HSC WT (BRCA2), a gift from Mien-Chie Hung (#16246; Addgene).29 BRCA2 cDNA was then Gateway cloned into pDONR221 (kind gift of Jeff Sekelsky). The attB sites were introduced by touchdown, gradient PCR using the following primers sequences: forward, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCCACCATGCCTATTGGATCCAAA-3′; reverse, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTATTAGATATATTTTTTAGT-3′. This was followed by stitching PCR and restriction digest/ligation using Acl-I and SphI-HF sites to generate a complete attB-PCR product. BP recombination was performed according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA). To standardize plasmid delivery across genes, pDONR221-BRCA2 was then subcloned into pDEST-FRT/T0-FLAG by LR reaction, according to the manufacturer’s protocol (Thermo Fisher Scientific).
The BRCA2 (https://www.ncbi.nlm.nih.gov/genbank; GenBank accession number NM_000059.3) and PALB2 (GenBank accession number NM_024675.3) constructs were made siRNA resistant by introducing three silent mutations with the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent, Cedar Creek, TX), according to the manufacturer's protocol. For BRCA2, siRNA resistance was conferred with the following silent mutations: c.4773T>C, c.4779A>G, c.4785 G>A. For PALB2, siRNA resistance was achieved with the following silent mutations: c.1635A>G, c.1641C>A, c.1647C>T. The sequences of the site-directed mutagenesis primers used to confer siRNA resistance are as follows: BRCA2 forward, 5′-GCTGCCCCAAAGTGCAAAGAGATGCAAAATTCTCTCAATAATG-3′; BRCA2 reverse, 5′-CATTATTGAGAGAATTTTGCATCTCTTTGCACTTTGGGGCAG-3′; PALB2 forward, 5′-GGTCCAAGGAAGAGGTCACATCACATAAATATCAGCACG-3′; PALB2 reverse, 5′-CGTGCTGATATTTATGTGATGTGACCTCTTCCTTGGACC-3′. All variants were generated in the siRNA-resistant constructs using the same site-directed mutagenesis kit as above.
Constructs were confirmed by Sanger sequencing (Genewiz, Morrisville, NC). siRNA target sequences and sequences of primers used for site-directed mutagenesis and Sanger confirmation are provided in Table 1, Table 2, Table 3 and 4.
Table 1.
siRNA Oligonucleotide Target Sequences
| siRNA | Target sequences |
|---|---|
| siUBB control pool | 5′-GCCGUACUCUUUCUGACUA-3′ 5′-GUAUGCAGAUCUUCGUGAA-3′ 5′-GACCAUCACUCUGGAGGUG-3′ 5′-CCCAGUGACACCAUCGAAA-3′ |
| Non-targeting control pool | 5′-UAGCGACUAAACACAUCAA-3′ 5′-UAAGGCUAUGAAGAGAUAC-3′ 5′-AUGUAUUGGCCUGUAUUAG-3′ 5′-AUGAACGUGAAUUGCUCAA-3′ |
| siBRCA2 | 5′-GUAAAGAAAUGCAGAAUUC-3′ |
| siPALB2 | 5′-GAAGUCACCUCACACAAAU-3′ |
Table 2.
Sanger Sequencing Primers
| Gene | Primer name∗ | Sequence | Corresponding nucleotides (CCDS)† |
|---|---|---|---|
| BRCA2 | BRCA2_1F | 5′-ATGCCTATTGGATCCAAAG-3′ | 1-19 |
| BRCA2 | BRCA2_1342R | 5′-GTGGCAAAGAATTCTCTG-3′ | 1342-1325 |
| BRCA2 | BRCA2_1207F | 5′-CTAAATGGAGCCCAGATG-3′ | 1207-1224 |
| BRCA2 | BRCA2_2541R | 5′-TCTTGAAGGTGATGCTAC-3′ | 2541-2524 |
| BRCA2 | BRCA2_2406F | 5′-TTATGAATCTGATGTTGA-3′ | 2406-2423 |
| BRCA2 | BRCA2_3748R | 5′-CCTCACTAATATTCTCAA-3′ | 3748-3731 |
| BRCA2 | BRCA2_3610F | 5′-GCTTCTGGTTATTTAACA-3′ | 3610-3627 |
| BRCA2 | BRCA2_5059R | 5′-GTAATGAAGTCTGACTCACAG-3′ | 5059-5039 |
| BRCA2 | BRCA2_4831F | 5′-GTGCCACCTAAGCTCTTA-3′ | 4831-4848 |
| BRCA2 | BRCA2_6193R | 5′-GCTTTCCACTTGCTGTAC-3′ | 6193-6176 |
| BRCA2 | BRCA2_6058F | 5′-GAACATTCAGACCAGCTC-3′ | 6058-6075 |
| BRCA2 | BRCA2_7400R | 5′-GCTACTGCTTGATTGGAG-3′ | 7400-7383 |
| BRCA2 | BRCA2_7251F | 5′-CAGAGTTGAACAGTGTGT-3′ | 7251-7268 |
| BRCA2 | BRCA2_8580R | 5′-CTTTTGTTGGGCCTCCAC-3′ | 8580-8563 |
| BRCA2 | BRCA2_8434F | 5′-GGAGGAAATGTTGGTTGT-3′ | 8434-8451 |
| BRCA2 | BRCA2_9769R | 5′-TCTCCCCTTTACAAGACT-3′ | 9769-9752 |
| BRCA2 | BRCA2_9634F | 5′-GGAAACAAGCTTCTGATG-3′ | 9634-9651 |
| BRCA2 | BRCA2_10257R | 5′-TTAGATATATTTTTTAGTTG-3′ | 10,257-10,238 |
| PALB2 | P2_1F | 5′-ATGGACGAGCCTCCCGGG-3′ | 0-18 |
| PALB2 | P2_1305R | 5′-GACATCCAAATGACTCTG-3′ | 1305-1288 |
| PALB2 | P2_1147F | 5′-CTGGAAGCAACCTCTCCT-3′ | 1147-1164 |
| PALB2 | P2_2505R | 5′-GGAATGTTTATGCAGCTC-3′ | 2505-2488 |
| PALB2 | P2_2370F | 5′-AGTGTCAGGCAGGCAAGG-3′ | 2370-2387 |
| PALB2 | P2_3561R | 5′-TTATGAATAGTGGTATAC-3′ | 3561-3544 |
Sanger sequencing primers used to confirm BRCA2 and PALB2 construct and variant sequences.
Primer name ends with F for forward or R for reverse primer direction.
Reference sequences used were: CCDS9344.1 (https://www.ncbi.nlm.nih.gov/genbank; GenBank accession number NM_000059.3) for BRCA2, CCDS32406.1 (GenBank accession number NM_024675.3) for PALB2.
Table 3.
BRCA2 Site-Directed Mutagenesis Primers
| Nucleotide change | Amino acid change | Forward mutagenesis primer | Reverse mutagenesis primer |
|---|---|---|---|
| c.3055C>G | p.(L1019V) | 5′-CAGCTTCAAATAAGGAAATCAAGGTCTCTGAACATAACATTAAGAAG-3′ | 5′-CTTCTTAATGTTATGTTCAGAGACCTTGATTTCCTTATTTGAAGCTG-3′ |
| c.4570T>G | p.(F1524V) | 5′-AACCTACTCTGTTGGGTGTTCATACAGCTAGCGGG-3′ | 5′-CCCGCTAGCTGTATGAACACCCAACAGAGTAGGTT-3′ |
| c.6220C>A | p.(H2074N) | 5′-AGCAAGTTTCCATTTTAGAAAGTTCCTTAAACAAAGTTAAGGGAGTG-3′ | 5′-CACTCCCTTAACTTTGTTTAAGGAACTTTCTAAAATGGAAACTTGCT-3′ |
| c.7415A>C | p.(K2472T) | 5′-TCAAGCAGCAGCTGTAACTTTCACAACGTGTGAAGAAGAAC-3′ | 5′-GTTCTTCTTCACACGTTGTGAAAGTTACAGCTGCTGCTTGA-3′ |
| c.7544C>T | p.(T2515I) | 5′-GGCAGTCTGTATCTTGCAAAAATATCCACTCTGCC-3′ | 5′-GGCAGAGTGGATATTTTTGCAAGATACAGACTGCC-3′ |
| c.7879A>T | p.(I2627F) | 5′-GGTTTATAATCACTATAGATGGTTCATATGGAAACTGGCAGCTAT-3′ | 5′-ATAGCTGCCAGTTTCCATATGAACCATCTATAGTGATTATAAACC-3′ |
| c.7940T>C | p.(L2647P) | 5′-ATTTGCTAATAGATGCCCAAGCCCAGAAAGGGTGC-3′ | 5′-GCACCCTTTCTGGGCTTGGGCATCTATTAGCAAAT-3′ |
| c.7958T>C | p.(L2653P) | 5′-CTAAGCCCAGAAAGGGTGCCTCTTCAACTAAAATACAGAT-3′ | 5′-ATCTGTATTTTAGTTGAAGAGGCACCCTTTCTGGGCTTAG-3′ |
| c.8063T>C | p.(L2688P) | 5′-CAGCTGCAAAAACACTTGTTCCCTGTGTTTCTGACATAATTTC-3′ | 5′-GAAATTATGTCAGAAACACAGGGAACAAGTGTTTTTGCAGCTG-3′ |
| c.8165C>G | p.(T2722R) | 5′-CCCAAAAAGTGGCCATTATTGAACTTAGAGATGGGTGGTATG-3′ | 5′-CATACCACCCATCTCTAAGTTCAATAATGGCCACTTTTTGGG-3′ |
| c.8167G>C | p.(D2723H) | 5′-AGTGGCCATTATTGAACTTACACATGGGTGGTATGCTG-3′ | 5′-CAGCATACCACCCATGTGTAAGTTCAATAATGGCCACT-3′ |
| c.8187G>T | p.(K2729N) | 5′-AGATGGGTGGTATGCTGTTAATGCCCAGTTAGATC-3′ | 5′-GATCTAACTGGGCATTAACAGCATACCACCCATCT-3′ |
| c.8243G>A | p.(G2748D) | 5′-GAATGGCAGACTGACAGTTGATCAGAAGATTATTCTTCATG-3′ | 5′-CATGAAGAATAATCTTCTGATCAACTGTCAGTCTGCCATTC-3′ |
| c.8525G>A | p.(R2842H) | 5′-ATCATCTGGATTATACATATTTCACAATGAAAGAGAGGAAGAAAAGG-3′ | 5′-CCTTTTCTTCCTCTCTTTCATTGTGAAATATGTATAATCCAGATGAT-3′ |
| c.8567A>C | p.(E2856A) | 5′-AGCAGCAAAATATGTGGCGGCCCAACAAAAGAGAC-3′ | 5′-GTCTCTTTTGTTGGGCCGCCACATATTTTGCTGCT-3′ |
| c.9371A>T | p.(N3124I) | 5′-CATATGTTAATTGCTGCAAGCATCCTCCAGTGGCG-3′ | 5′-CGCCACTGGAGGATGCTTGCAGCAATTAACATATG-3′ |
Complete list of all BRCA2 mutagenesis primers used in this study. Human Genome Variant Nomenclature (HGVS) nucleotide and protein numbering shown for BRCA2 reference sequence (https://www.ncbi.nlm.nih.gov/genbank; GenBank accession number NM_000059.3).
Table 4.
PALB2 Site-Directed Mutagenesis Primers
| Nucleotide change | Amino acid change | Forward mutagenesis primer | Reverse mutagenesis primer |
|---|---|---|---|
| c.49_50delinsCC | p.(L17P) | 5′-CAGCTGTGAGGAGAAGGAAAAGCCAAAGGAGAAATTAGCATTCTTG-3′ | 5′-CAAGAATGCTAATTTCTCCTTTGGCTTTTCCTTCTCCTCACAGCTG-3′ |
| c.53A>G | p.(K18R) | 5′-AGCTGTGAGGAGAAGGAAAAGTTAAGGGAGAAATTAGCATTC-3′ | 5′-GAATGCTAATTTCTCCCTTAACTTTTCCTTCTCCTCACAGCT-3′ |
| c.61_62delinsCC | p.(L21P) | 5′-GCTGTGAGGAGAAGGAAAAGTTAAAGGAGAAACCAGCATTCTTGAAAAGGG-3′ | 5′-CCCTTTTCAAGAATGCTGGTTTCTCCTTTAACTTTTCCTTCTCCTCACAGC-3′ |
| c.70_71delinsCC | p.(L24P) | 5′-AAAGTTAAAGGAGAAATTAGCATTCCCGAAAAGGGAATACAGCAAGACACTA-3′ | 5′-TAGTGTCTTGCTGTATTCCCTTTTCGGGAATGCTAATTTCTCCTTTAACTTT-3′ |
| c.71T>C | p.(L24S) | 5′-GTTAAAGGAGAAATTAGCATTCTCGAAAAGGGAATACAGCAAGACAC-3′ | 5′-GTGTCTTGCTGTATTCCCTTTTCGAGAATGCTAATTTCTCCTTTAAC-3′ |
| c.83A>G | p.(Y28C) | 5′-AGCATTCTTGAAAAGGGAATGCAGCAAGACACTAGCCC-3′ | 5′-GGGCTAGTGTCTTGCTGCATTCCCTTTTCAAGAATGCT-3′ |
| c.82_83delinsAC | p.(Y28P) | 5′-GGAGAAATTAGCATTCTTGAAAAGGGAAACCAGCAAGACACTAGC-3′ | 5′-GCTAGTGTCTTGCTGGTTTCCCTTTTCAAGAATGCTAATTTCTCC-3′ |
| c.90G>T | p.(K30N) | 5′-CTTGAAAAGGGAATACAGCAATACACTAGCCCGCCT-3′ | 5′-AGGCGGGCTAGTGTATTGCTGTATTCCCTTTTCAAG-3′ |
| c.104T>C | p.(L35P) | 5′-CACTAGCCCGCCCTCAGCGTGCCCA-3′ | 5′-TGGGCACGCTGAGGGCGGGCTAGTG-3′ |
| c.110G>A | p.(R37H) | 5′-GCCCGCCTTCAGCATGCCCAAAGAGCT-3′ | 5′-AGCTCTTTGGGCATGCTGAAGGCGGGC-3′ |
| c.110G>C | p.(R37P) | 5′-CCCGCCTTCAGCCTGCCCAAAGAGC-3′ | 5′-GCTCTTTGGGCAGGCTGAAGGCGGG-3′ |
| c.109C>A | p.(R37S) | 5′-AGCCCGCCTTCAGAGTGCCCAAAGAGC-3′ | 5′-GCTCTTTGGGCACTCTGAAGGCGGGCT-3′ |
| c.229delT | p.(C77Vfs∗100) | 5′-GCTAAAACACTCAGAACCTAAAAATAAAATAGTGTTTATGACAAGTTACACAT-3′ | 5′-ATGTGTAACTTGTCATAAACACTATTTTATTTTTAGGTTCTGAGTGTTTTAGC-3′ |
| c.557A>T | p.(N186I) | 5′-AGGAACAGGAAGAAATCAGTAGCAAAATTCCTGCTAGATCAC-3′ | 5′-GTGATCTAGCAGGAATTTTGCTACTGATTTCTTCCTGTTCCT-3′ |
| c.721A>G | p.(N241D) | 5′-CATTCCTAAGAAGACCTGATTTCACCAGGGCGACT-3′ | 5′-AGTCGCCCTGGTGAAATCAGGTCTTCTTAGGAATG-3′ |
| c.925A>G | p.(I309V) | 5′-AACCTCCTTGTAAATAAAGCTGTAAGTAAAAGTGGCCAACTGC-3′ | 5′-GCAGTTGGCCACTTTTACTTACAGCTTTATTTACAAGGAGGTT-3′ |
| c.991G>C | p.(E331Q) | 5′-TTAGAGGCAAATATTTCATGTTCTCTAAATCAACTCACCTACAATAAC-3′ | 5′-GTTATTGTAGGTGAGTTGATTTAGAGAACATGAAATATTTGCCTCTAA-3′ |
| c.1212T>A | p.(F404L) | 5′-GCCTGAAGGCCTTCTGTTACCTGCAGAATATTATG-3′ | 5′-CATAATATTCTGCAGGTAACAGAAGGCCTTCAGGC-3′ |
| c.1468C>G | p.(P490A) | 5′-TCATTAACTAAAGTCAGCTCTGCCGCTGGGCCC-3′ | 5′-GGGCCCAGCGGCAGAGCTGACTTTAGTTAATGA-3′ |
| c.1490delA | p.(N497Mfs∗64) | 5′-GCTGGGCCCACTGAAGATATGACTTGTCTAGGA-3′ | 5′-TCCTAGACAAGTCATATCTTCAGTGGGCCCAGC-3′ |
| c.1592delT | p.(L531Cfs∗30) | 5′-GCATCAGATCATTGTGAACCACTTTGCCAACTTCTAGC-3′ | 5′-GCTAGAAGTTGGCAAAGTGGTTCACAATGATCTGATGC-3′ |
| c.1676A>G | p.(Q559R) | 5′-ATATCAGCACGAAAAATTATTTATTCGAGTGAAAGGGAAGAAAAGTCG-3′ | 5′-CGACTTTTCTTCCCTTTCACTCGAATAAATAATTTTTCGTGCTGATAT-3′ |
| c.2014G>C | p.(E672Q) | 5′-GATACAGAAATGGAGGACTTACAAGAGGACCTTATTGTTCTA-3′ | 5′-TAGAACAATAAGGTCCTCTTGTAAGTCCTCCATTTCTGTATC-3′ |
| c.2027T>C | p.(I676T) | 5′-AGGACTTAGAAGAGGACCTTACTGTTCTACCAGGAAAATC-3′ | 5′-GATTTTCCTGGTAGAACAGTAAGGTCCTCTTCTAAGTCCT-3′ |
| c.2134G>C | p.(A712P) | 5′-GTCATTATCATCAGGCGGAACCGTATTTAAAGGAGTATAAAGTA-3′ | 5′-TACTTTATACTCCTTTAAATACGGTTCCGCCTGATGATAATGAC-3′ |
| c.2135C>T | p.(A712V) | 5′-ACTCCTTTAAATACGGTTGTGCCTGATGATAATGACAGG-3′ | 5′-CCTGTCATTATCATCAGGCACAACCGTATTTAAAGGAGT-3′ |
| c.2461A>T | p.(N821Y) | 5′-CCACCCATTGAGTCATTCACTTTTAAAGAATATCAGCTCTGTAGAAA-3′ | 5′-TTTCTACAGAGCTGATATTCTTTAAAAGTGAATGACTCAATGGGTGG-3′ |
| c.2590C>T | p.(P864S) | 5′-ACCTACAATTGGTTTCAGAGTTAAAGAATTCTTCAGGTTCCTGTTC-3′ | 5′-GAACAGGAACCTGAAGAATTCTTTAACTCTGAAACCAATTGTAGGT-3′ |
| c.2794G>A | p.(V932M) | 5′-GCCTGATGTGTATAATCTCATGTGTGTAGCTTTGGGAAA-3′ | 5′-TTTCCCAAAGCTACACACATGAGATTATACACATCAGGC-3′ |
| c.2816T>G | p.(L939W) | 5′-CTCGTGTGTGTAGCTTTGGGAAATTGGGAAATCAGAGAG-3′ | 5′-CTCTCTGATTTCCCAATTTCCCAAAGCTACACACACGAG-3′ |
| c.2993G>A | p.(G998E) | 5′-GACGTTTGCAGAAGATGAAGGAGGCAAAGAAAACC-3′ | 5′-GGTTTTCTTTGCCTCCTTCATCTTCTGCAAACGTC-3′ |
| c.3073_3074delinsCG | p.(A1025R) | 5′-AGGTCCAAGGGATGCAAGAACGTCTGCTTGGTACTAC-3′ | 5′-GTAGTACCAAGCAGACGTTCTTGCATCCCTTGGACCT-3′ |
| c.3073G>A | p.(A1025T) | 5′-GAGGTCCAAGGGATGCAAGAAACTCTGCTTGGTA-3′ | 5′-TACCAAGCAGAGTTTCTTGCATCCCTTGGACCTC-3′ |
| c.3089C>T | p.(T1030I) | 5′-GCAAGAAGCTCTGCTTGGTACTATTATTATGAACAACATTGTTATTT-3′ | 5′-AAATAACAATGTTGTTCATAATAATAGTACCAAGCAGAGCTTCTTGC-3′ |
| c.3110T>C | p.(I1037T) | 5′-GCTGAGGTCCAAGGGACGCAAGAAGCTCTG-3′ | 5′-CAGAGCTTCTTGCGTCCCTTGGACCTCAGC-3′ |
| c.3209T>C | p.(L1070P) | 5′-CTATTCTGAAATGGGGCTTCCCTTTATTGTCCTGAGTCATC-3′ | 5′-GATGACTCAGGACAATAAAGGGAAGCCCCATTTCAGAATAG-3′ |
| c.3290C>G | p.(P1097R) | 5′-GCTCATTGTGATTAACCGTAAGACGACTCTCAGCG-3′ | 5′-CGCTGAGAGTCGTCTTACGGTTAATCACAATGAGC-3′ |
Complete list of all PALB2 mutagenesis primers used in this study. Human Genome Variant Nomenclature (HGVS) nucleotide and protein numbering shown for PALB2 reference (https://www.ncbi.nlm.nih.gov/genbank; GenBank accession number NM_024675.3).
Cell Culture and TLR Cell Line Generation
HEK293T/17 cells were purchased from American Type Culture Collection (ATCC, Old Town Manassas, VA) and tested negative for mycoplasma. For TLR lentivirus production, HEK293T/17 cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA), the pCVL TLR construct (a gift from Andrew Scharenberg; Addgene #31482),30 and lentiviral packaging plasmids pMD2.G and pCMV delta R8.2 (gifts of Didier Trono; Addgene plasmids #12259 and 12,263).31 HEK293T/17 cells were transduced at a low multiplicity of infection (100 colony-forming units) with TLR lentivirus to generate a stable, polyclonal cell line (293T/TLR) with a high likelihood of single integration, as previously described.31
293T/TLR cells were maintained in Dulbecco's modified Eagle's medium – high glucose (Sigma-Aldrich, St. Louis, MO) with 10% fetal bovine serum (VWR, Radnor, PA), 1% antibiotic-antimycotic (Gibco, Grand Island, NY), 4 mmol/L l-glutamine (Corning, Manassas, VA), and 10 μg/mL puromycin (Corning). Cells were grown at 37°C, 5% CO2 in a humidified incubator. All siRNA and DNA transfections were performed in antibiotic-free Dulbecco's modified Eagle's medium – high glucose with 10% fetal bovine serum and 4 mmol/L l-glutamine.
Preliminary Assay Validation
After the generation of the stable 293T/TLR cell line, a preliminary test of the system was performed as previously described.30 On day 1, 6 × 105 293T/TLR cells were seeded per well of a 12-well plate and transiently transfected with 1.0 μg of a homologous donor template pGFPdonor–blue fluorescent protein (BFP) (pRRL SFFV d20GFP.T2A.mTagBFP Donor, Addgene plasmid #31485),30 I-Sce1 nuclease pI-SceI–infrared protein (IFP) (pRRL sEF1a HA.NLS.Sce(opt).T2A.IFP, Addgene plasmid #31484),30 or both using Lipofectamine 2000 (Invitrogen). Antibiotic-free medium was replaced the next day. On day 3, cells were transferred to a 6-well plate. On day 4, 1 × 106 cells were analyzed by flow cytometry using an LSRFortessa (BD Biosciences, San Jose, CA).
TLR Assay Optimization—DSB Repair Timing
In order to determine the optimal timing for DNA transfection and flow cytometry analysis, 5.0 × 105 cells were reverse transfected in a 12-well plate with 0.5 μg of I-SceI-IFP and 0.5 μg of GFPdonor-BFP using Lipofectamine 2000 (Invitrogen) at 12, 24, 48, or 72 hours before flow cytometry. Controls included untreated cells and cells transfected with 0.5 μg of empty vector (EV) only. To track DNA transfection efficiency and timing of plasmid expression, IFP+/BFP+ cells were analyzed as a percentage of single live cells. DSB repair timing was measured by the percentage of cells that were GFP+ (%HR) or mCherry+ (%NHEJ). At least 100,000 single live cells were analyzed in duplicate for each condition (Supplemental Figure S1A).
Timing siRNA-Mediated Knockdown
siRNA against the human ubiquitin B (UBB) gene (MIM number 191339), which results in cell death upon knockdown, was used to verify siRNA knockdown during assay development (M-013382-01-005; Dharmacon, Lafayette, CO). A nontargeting siRNA (siNT) pool (D-001206-13-05; Dharmacon) was used as a control in all knockdown experiments. Individual siRNAs were used for PALB232 and BRCA2 silencing (D-012928-04 and D-003462-02, respectively; Dharmacon) (Table 1).
In order to determine the timing of siRNA-mediated knockdown, 3.0 × 105 293T/TLR cells were reverse transfected with 75 nmol/L of siRNA (either siNT, siPALB2, or siUBB) per well of a 6-well plate using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. Cells were harvested by enzymatic release after 24, 48, 72, or 96 hours. Pellets were stored at −20°C until RNA or protein extraction was performed. mRNA expression was analyzed by real-time PCR for three independent experiments (Supplemental Figure S1B). For protein analysis, Western blots were performed using 15 μg of whole-cell lysate per well, with siNT-treated cells serving as a control for each time point (Supplemental Figure S1C).
Assessing Transient Re-Expression of PALB2 Variants
To evaluate transient PALB2 re-expression, 3.0 × 105 293T/TLR cells were treated with 75 nmol/L siPALB2, followed by cotransfection 24 hours later with 1.25 μg of I-SceI, 1.25 μg of GFPdonor, and 0.71 μg of the indicated pDEST-FRT/T0-FLAG-PALB2 construct. Cells were harvested by enzymatic release 48 hours after the siRNA transfection, and pellets were stored at −20°C until protein extraction. For protein analysis, Western blot probing for FLAG-tagged PALB2 was performed using 25 μg of whole-cell lysate per run. WT PALB2 served as a control on each blot (Supplemental Figure S1C).
TLR Assay Analysis of BRCA2 and PALB2 Variants
For analysis of DDR outcomes with BRCA2 or PALB2 variants, experiments were performed as follows. On day 1, approximately 3.0 × 105 to 5.0 × 105 293T/TLR cells were reverse transfected with 75 nmol/L of siRNA per well of a 6-well plate using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. Twenty-four hours later, medium was changed, and Lipofectamine 2000 was used to forward transfect cells with 1.25 μg of the IFP-tagged I-SceI nuclease construct, 1.25 μg of pGFPdonor-BFP, and either 1.25 μg of EV, 1.25 μg of the indicated siRNA-resistant pDEST-FLAG/BRCA2, or 0.71 μg of the indicated siRNA-resistant pDEST-FLAG/PALB2 construct. Seventy-two hours after DNA transfection, cells were harvested by enzymatic release, resuspended in cold 1% bovine serum albumin (Sigma-Aldrich) in Dulbecco's phosphate-buffered saline (Gibco) to 1 × 106 cells/mL, and 1.0 to 3.0 × 106 cells were filtered for analysis by flow cytometry. Remaining cells were pelleted and stored at −20°C for subsequent RNA analysis.
Flow cytometry analysis was performed using a LSRFortessa cell analyzer (BD Biosciences). Live cells were gated based on forward scatter area versus side scatter area. Single cells were then gated based on forward scatter area versus forward scatter height. GFP was excited by a 488-nm laser and detected by a 530/30 filter. mCherry was excited by a 561-nm laser and detected by a 610/20 filter. IFP was excited by a 640-nm laser and detected by a 710/50 filter. BFP was excited by a 405-nm laser and detected by a 450/50 filter. Data were analyzed using FACS Diva version 8.0.1 (BD Biosciences) and FCS Express version 6 (De Novo Software, Pasadena, CA) software.
At least 10,000 doubly transfected (IFP+/BFP+), single live cells were analyzed per condition. From this population of cells, readouts of GFP+/mCherry+ ratio (HR/NHEJ) and the percentage of cells that were GFP+ (an indicator of %HR) were obtained. Each variant was tested in at least three independent experiments. Every 6-well plate included the following conditions as controls: siNT-treated cells rescued with EV, siBRCA2- or siPALB2-treated cells rescued with EV, and siBRCA2- or siPALB2-treated cells rescued with siRNA-resistant WT BRCA2 or PALB2.
Western Blots
Cell pellets were resuspended in Pierce radioimmunoprecipitation assay buffer (Thermo Scientific, Rockford, IL), containing 1 mmol/L phenylmethylsulfonyl fluoride (Thermo Scientific), 1× complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), 0.1% 2-mercaptoethanol (Gibco), 0.1% dithiothreitol (Thermo Scientific), and 1 tablet of Pierce phosphatase inhibitor (Thermo Scientific) per 10 mL of buffer. Resuspended pellets were shaken for 10 minutes at 4°C and lysed by sonication in a 4°C water bath (Diagenode Biorupter, Denville, NJ) for 5 minutes (30 seconds on/30 seconds off). Lysate was then centrifuged for 10 minutes at 14,000 × g at 4°C and supernatant transferred to a new tube. Protein concentration was measured by reducing-agent–compatible microplate BCA Assay (Thermo Scientific) according to the kit protocol. Plates were read using a GloMax Multi-Detection System (Promega, Madison, WI).
For Western blotting, reduced whole-cell lysates were electrophoresed on a NuPAGE 7% Tris-Acetate pre-cast 1.0-mm gels (Invitrogen) for 50 minutes at 150 V and transferred to polyvinylidene difluoride membrane (Merck Millipore, Cork, Ireland) at 30 V for 1 hour. Membranes were blocked in 5% bovine serum albumin in Tris-buffered saline (Santa Cruz Biotechnology, Dallas, TX) for 1 hour at room temperature. Primary antibodies used were a mouse monoclonal anti-FLAG antibody (1:1000; #F1804; Sigma-Aldrich), a polyclonal rabbit antibody against an epitope between residues 200 to 250 of PALB2 (1:2000; #A301-247A; Bethyl Laboratories, Montgomery, TX), and a rabbit monoclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2500; #AB9485; Abcam, Cambridge, MA). Primary antibody incubation was performed for 1 hour at room temperature or overnight at 4°C. Secondary antibodies used were IRDye 800CW goat anti-mouse or anti-rabbit IgG (1:20,000 to 1:25,000; #925-32210 and 925-32,211, respectively) (LI-COR, Lincoln, NE). Membranes were incubated with secondary antibodies for 1 hour at room temperature, protected from light. After primary and secondary antibody incubation, membranes were washed three times for 10 minutes at room temperature with 0.1% Tween 20 (Sigma-Aldrich) in 1× Tris-buffered saline. Membranes were then rinsed with 1× Tris-buffered saline, and blots were visualized using a LI-COR Odyssey CLx Imager.
Real-Time PCR
RNA was isolated and purified from pelleted 293T/TLR cells using a RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). RNA (100 ng) was used as template with a TaqMan RNA-to-CT 1-Step Kit (Applied Biosystems, Carlsbad, CA) for gene expression analysis of PALB2 or BRCA2 relative to the beta-2-microglobulin (B2M) gene (MIM number 109700) in 25-mL triplicate reactions run on an Applied Biosystems StepOne Real-Time PCR System. The TaqMan Gene Expression Assay IDs (Applied Biosystems) used were Hs00609073_m1 (BRCA2), Hs00954121_m1 (PALB2), and Hs00187842_m1 (B2M).
Normalization
For the BRCA2 and PALB2 TLR assays, flow cytometry data from each experimental condition were normalized to the in-plate mock control (siNT + EV). The assay results for EV and WT controls, which are included on each plate, were then utilized to estimate a plate effect using a linear mixed model (LMM), which assumes in-plate effects as fixed effects and between-plate effects as random effects. The lme function from the nlme package in R software version 3.5.0 (the R Project for Statistical Computing, Vienna, Austria; https://www.r-project.org)33 was used to estimate the plate effects and implement the normalization, that is, subtracting corresponding estimated plate effects from all reads. Assay results for BRCA2 variants were adjusted based on LMM with random effects normalization using BRCA2 WT and EV controls, and assay results for PALB2 variants were separately adjusted based on LMM with random effects normalization using PALB2 WT and EV controls.
Gaussian Mixture Model
The normalized HR/NHEJ readout for WT and EV controls was used to generate Gaussian mixture models (GMM) for BRCA2 and PALB2, respectively, using the normalmixEM function from the mixtools34 package in R software,33 setting k to 2. This function provides as output the mean, SD, and mixture weight for each estimated distribution, and provides the posterior probability for a given input value to belong to each distribution. The dnorm function can then calculate the probability of any arbitrary assay readout value to belong to either of the distributions (based on the mean and SD from the GMM). The ratio of these probabilities was then calculated and the threshold at which there is a 10:1 ratio in favor of belonging to either the abnormal or normal distribution was located.
Regression Analysis
Scatterplots and linear regression were generated to test the correlation between functional data and in silico predictors, using squared correlation coefficients (R2) to measure the strength of correlation in GraphPad Prism software version 8.3.0 (GraphPad Software, San Diego, CA). Three studies of the functional impact of PALB2 missense variants were recently published.22, 23, 24 To test the correlation between the TLR's HR/NHEJ readout and recently published HR functional data, regression analysis was repeated using published %HR efficiency values24 or HDR fold change22 (Supplemental Table S1). Direct comparisons of HR/NHEJ to HR readout from the third study23 could not be made because raw HR data was not published.
Results
Establishment of an in Vivo Cellular Assay to Detect HR/NHEJ Outcomes
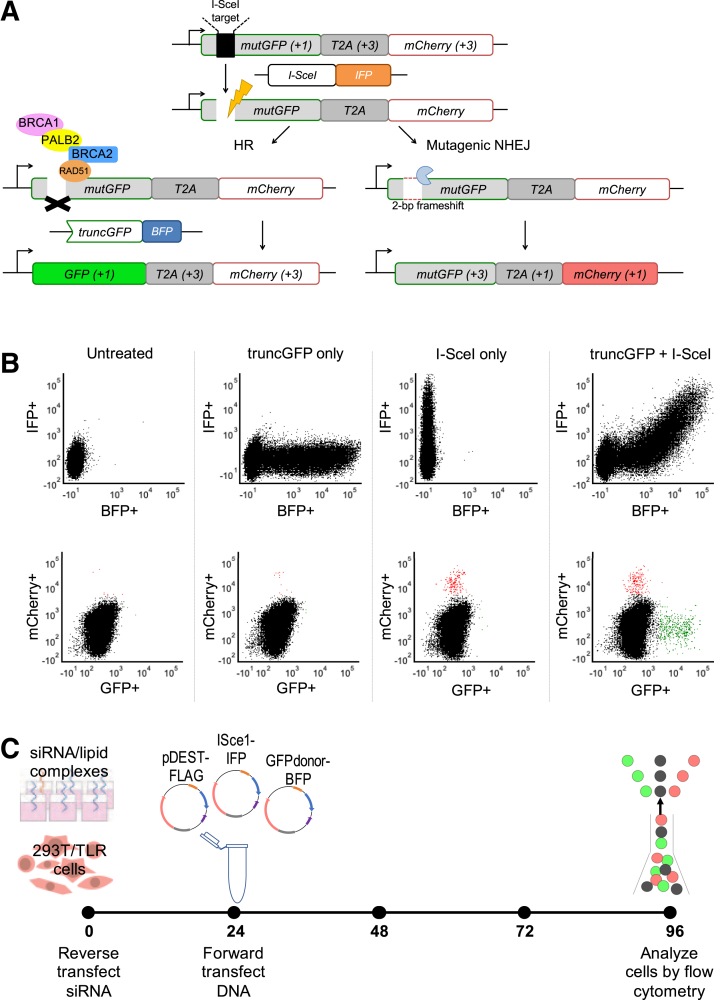
Originally developed for optimization of genome editing protocols, the TLR used cellular GFP and mCherry expression to visualize the repair of induced DNA DSBs by HR or alt-NHEJ, respectively (Figure 1A).30 Established in HEK293T/17 cells, this assay system demonstrated its utility as a reliable readout for HR and NHEJ outcomes (Figure 1B). Untreated 293T/TLR cells are nonfluorescent at baseline because the stably integrated GFP construct is disrupted by an I-SceI nuclease target site, and mCherry expression is kept out of reading frame by a T2A linker. Transfection with a plasmid containing a truncated GFPdonor (truncGFP) can be monitored by BFP expression, whereas I-SceI nuclease transfection can be monitored by IFP expression. When I-SceI is expressed, it can induce a DSB within the TLR GFP construct, and in the absence of a homologous repair template, alt-NHEJ repair events are detected by mCherry expression when a 2-bp frameshift occurs (about one-third of events).30 Cotransfection of both I-SceI and truncGFP donor template results in DSBs that are repaired by either HR or alt-NHEJ, indicated by GFP and mCherry expression, respectively, thus indicating that both repair pathways are operational in this cell line.
Figure 1.
Traffic Light Reporter assay schematic, flow cytometry output, and final assay protocol. A: The Traffic Light Reporter (TLR) assay, adapted from Certo et al,30 utilizes a cell line with a stably integrated construct encoding mutGFP [a mutant version of the GFP cDNA that does not produce green fluorescent protein (GFP) at baseline due to an engineered I-SceI nuclease target site] connected via the T2A linker to an out-of-frame mCherry cDNA. Position relative to reading frame is indicated in parentheses, where +1 leads to gene expression, whereas +3 is 2 bp out of reading frame. A DNA double-strand break in mutGFP is induced by transfection with a plasmid encoding infrared protein (IFP) and I-SceI nuclease. A plasmid encoding blue fluorescent protein (BFP) and a truncated green fluorescent protein (GFP) construct (truncGFP) provides a homologous donor sequence that can be used by the cell to repair the DNA double-strand break via the homology-directed repair (HR) pathway (left side). However, if the donor sequence is absent, or if HR is not intact (right side), the cell can undergo mutagenic nonhomologous end-joining (NHEJ) thereby restoring mCherry expression if it results in a 2-bp frameshift (about one-third of events). B: Flow cytometry plots show mean fluorescence intensity of live single cells. Expression of IFP (indicating presence of the I-SceI plasmid) versus BFP (indicating presence of the truncGFP donor plasmid) is shown in the upper panels. Expression of GFP (indicating successful HR) versus mCherry (indicating NHEJ) is shown in the lower panels. Untreated 293T/TLR cells were negative for BFP, IFP, GFP, or mCherry. Cells transfected only with truncGFP donor plasmid were reflected by a BFP+ population, otherwise negative for IFP, GFP, or mCherry. Cells transfected with I-SceI nuclease are IFP+, and some of the population also expressed mCherry, indicating DSB repair by NHEJ due to absence of the homologous donor template. Cells transfected with all components of the TLR assay exhibited presence of both truncGFP and I-SceI (double-positive BFP/IFP population), with distinct populations positive for mCherry or GFP indicating DSB repair by NHEJ or HR, respectively). C: Optimized timing of the TLR assay used for siRNA knockdown and re-expression of BRCA2 and PALB2 variants in 293T/TLR cells. Stably transfected 293T/TLR cells were reverse transfected with siRNA at time 0, followed by transient cotransfection of rescue plasmid (pDEST-FLAG), I-SceI nuclease (coexpressing IFP) and truncGFP donor construct (coexpressing BFP) 24 hours later. Flow cytometry was performed 72 hours after DNA transfection to detect DDR outcomes.
TLR assay timing and siRNA knockdown efficiency (Supplemental Figure S1) were optimized in order to determine a standardized assay protocol (Figure 1C) for use in all subsequent validation experiments. Briefly, this achieved approximately 70% siRNA-mediated knockdown of the target gene (BRCA2 or PALB2) relative to siNT-treated cells at 24 hours (Supplemental Figure S1B) and >50% knockdown at the time of flow cytometry (96-hour post-siRNA transfection) (Supplemental Figure S2), with concomitant decrease in protein expression demonstrated by Western blot (Supplemental Figure S1C). Expression of I-SceI and truncGFP plasmids peaked between 24 and 72 hours post-transfection, and GFP or mCherry repair events were detected after 48 to 72 hours (Supplemental Figure S1A). Expression of FLAG-tagged BRCA2 or PALB2 protein from the rescue plasmid was detectable by Western blot by 24 hours after transfection (Supplemental Figure S1C). Therefore, using the experimental timing as shown in Figure 1C, it is anticipated that reduction of endogenous BRCA2 or PALB2 protein expression, induction of DSB via I-SceI expression, and reconstitution via the rescue plasmid, all occur during a relevant time window for detecting the impact of missense variants on DDR outcomes as indicated by changes in the proportion of cells that are GFP or mCherry positive.
Initial Evaluation of the TLR Assay Using Gold-Standard BRCA2 Validation Controls
In order to establish that the TLR assay can discriminate between normal and abnormal DDR, a set of gold standard missense variants in BRCA2 (eight pathogenic and eight benign) were used. These missense variants can be confidently interpreted without functional evidence and have previously been used as validation controls for a similar HR assay.5,26 Cells were transfected with either EV- or siRNA-resistant FLAG-BRCA2 (encoding either WT, benign, or pathogenic BRCA2 variants) in a 6-well plate format. The ratio of GFP+/mCherry+ cells (HR/NHEJ) was measured for at least 10,000 doubly transfected (IFP+/BFP+) cells for each condition, and each BRCA2 variant was examined in at least three independent experiments.
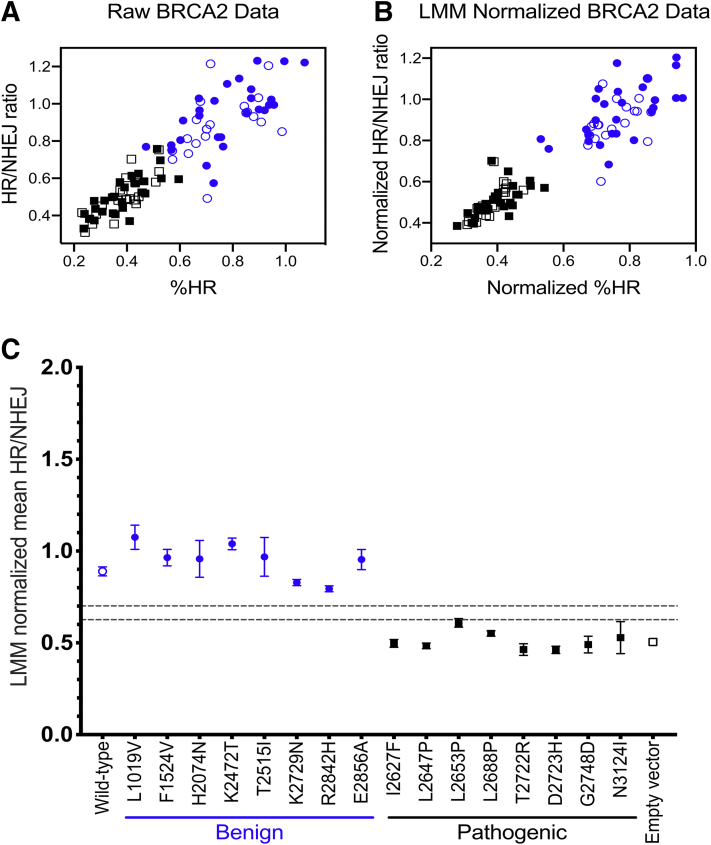
In this 6-well plate implementation of the TLR assay, three wells of each plate were dedicated to controls: mock treatment consisting of siNT-treated cells rescued with EV, siBRCA2-treated cells rescued with WT BRCA2 cDNA normal control, and siBRCA2-treated cells rescued with EV abnormal control. Therefore, experimental replicates for each of the variants were distributed across numerous batches processed over time in different plates. This setup could lead to variability between plates based on subtle factors such as the cell passage number, confluency, cell cycle phase; media and/or reagent batches; siRNA knockdown and/or transient re-expression; incubator temperature and/or CO2 concentration; variation in timing of experimental manipulations; or other unknown variables. To address this potential source of irregularity, the raw % HR and HR/NHEJ ratio within each plate was first adjusted by normalizing to the mock-treated well. Additional normalization was then performed across the plates using a LMM with random effects in order to adjust for factors that could change over time. Although the raw data already demonstrated a relatively clear separation between the classes (Figure 2A), LMM random effect normalization improved this separation dramatically (Figure 2B).
Figure 2.
Validation of the Traffic Light Reporter (TLR) assay using BRCA2 gold standard controls. A: Raw flow cytometry data for %HR and homology-directed repair/nonhomologous end-joining (HR/NHEJ) ratio are shown for BRCA2 wild-type (open blue circles), empty vector (open black squares), and gold standard benign (closed blue circles) and pathogenic (closed black squares) validation controls (normalized against the internal mock-treated control for each plate). B: Batch effects due to plate-to-plate experimental variability were addressed by normalization based on a linear mixed model (LMM) with random effects, utilizing the assay results for wild-type and empty vector controls included in each plate, demonstrating improvement in separation between the different classes of variants. C: TLR assay results for the BRCA2 experiments are summarized, demonstrating HR function after siBRCA2 knockdown and rescue with plasmids containing BRCA2 variants or controls. HR function is rescued by BRCA2 wild-type cDNA and benign validation controls but is impaired with empty vector and pathogenic validation controls. Each rescue condition is depicted across the x axis. The y axis represents the LMM normalized HR/NHEJ ratio. Thresholds for normal and abnormal functional impact are indicated by gray dashed lines. Data are expressed as mean ± SEM. n ≥ 3 independent experiments; n = 21 wild-type and empty vector controls.
WT and benign BRCA2 variants restored HR capacity as indicated by a higher HR/NHEJ ratio (means ranged from 0.794 to 1.075), whereas EV and pathogenic BRCA2 variants demonstrated abnormal HR function, as indicated by a lower HR/NHEJ ratio (means ranged from 0.462 to 0.609). Although there was a clear visual separation between benign and pathogenic variant control groups (Figure 2C), the goal of this instance of the TLR assay was to provide functional evidence to be used for clinical variant interpretation according to the ACMG/AMP guidelines1 with assignment of the strength of evidence according to a Bayesian formulation of the combining rules27 supported by statistical validation based on OddsPath.7 In order to define thresholds that define normal, abnormal, and indeterminate TLR assay readouts, an unsupervised GMM was utilized to estimate the density distribution contributions for the WT and EV controls. The distribution that corresponds to WT controls had a mean HR/NHEJ of 0.894 (SD ±0.098), and the distribution that corresponds to EV controls had a mean HR/NHEJ of 0.501 (SD ±0.066). Thresholds were then defined based on a 10:1 ratio of the probability to belong to either the normal function (HR/NHEJ >0.701) or abnormal function (HR/NHEJ <0.627) distribution, respectively (Supplemental Figure S3). Using these thresholds, all eight benign variants and seven of eight pathogenic variants were correctly classified, with one pathogenic control (L2653P) classified as intermediate or indeterminate. Therefore, using the OddsPath levels promulgated by Tavtigian et al27 and further elaborated in Brnich et al,7 this functional evidence reached moderate strength for PS3 and moderate strength for BS3 (albeit with caveats about normal function in this assay not necessarily reflecting all potential functional readouts) (Supplemental Table S2). The strength of the assay can be improved with additional pathogenic and benign controls. Nevertheless, these results demonstrate that the TLR assay is able to distinguish between normal and abnormal DDR function, permitting subsequent validation of the assay for analysis of missense variants in PALB2.
TLR Assay Validation for Use in Clinical Interpretation of PALB2 Missense Variants
Previous studies in PALB2 have primarily been domain-specific.16,35,36 In order to validate an assay for PALB2 as a whole, variants across the length of the protein (Supplemental Table S1) were selected. As controls, nine benign/likely benign missense variants (N241D, I309V, Q559R, E672Q, I676T, A712V, P864S, V932M, and G998E) were selected from ClinVar8 and manually reviewed to ensure interpretation without the use of functional evidence. Because there are currently no definitive likely pathogenic/pathogenic missense variants in PALB2, three frameshift/early truncating variants (C77Vfs∗100, N497Mfs∗64, L531Cfs∗30) that could reach at least a likely pathogenic classification without functional evidence were selected as controls. It should be noted that expression of these truncating variants from cDNA may not reflect the true biological context (in which nonsense-mediated decay would be expected to occur). However, these cDNA constructs should still produce a nonfunctioning protein given that a large portion of the C-terminal end of the protein will be absent. Additionally, there is evidence that the c.1592delT variant that encodes L531Cfs∗30 escapes nonsense-mediated mRNA decay but results in truncated protein that is highly unstable with low levels of protein product and is functionally abnormal.37 In addition, 25 missense VUS were selected from ClinVar or the literature, or were synthetically designed. The available evidence was examined, and those without prior functional evidence (K18R, L24S, K30N, R37S, N186I, E331Q, F404L, P490A, A712P, N821Y, L939W, A1025T, I1037T, L1070P, P1097R) were prioritized because these VUS could stand to the most from the addition of new functional data. Other VUS selected from ClinVar included the PALB2 variants Y28C, L35P, R37H, and T1030I. Synthetic variants and those selected from the literature included L17P, L21P,38 L24P,38 Y28P, R37P, and A1025R.39 Typical flow plots for one run of the PALB2 adaptation of the TLR assay are represented in Figure 3.
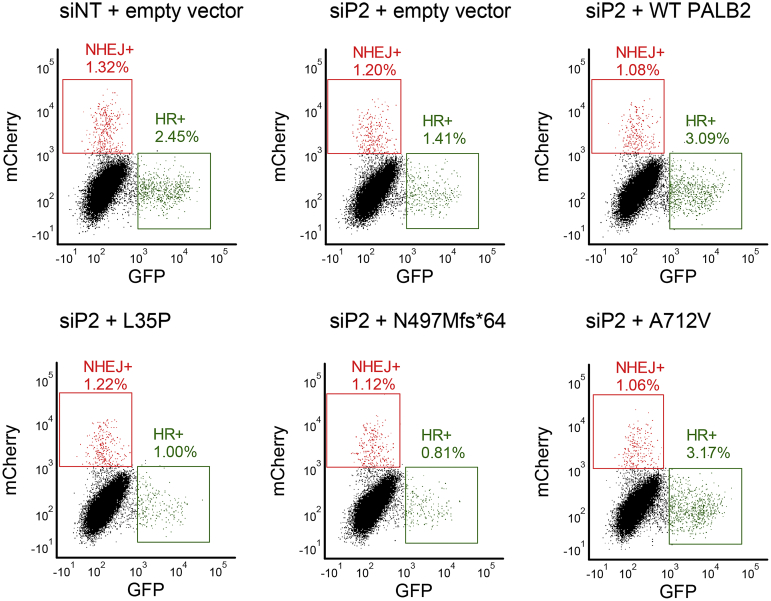
Figure 3.
Representative PALB2 Traffic Light Reporter (TL)R assay flow cytometry data. Typical flow plots for one run of the TLR assay. Row 1 (left to right): controls for siRNA/DNA transfection and batch effects. Row 2 (left to right): transient rescue with PALB2 variants L35P (potentially pathogenic missense variant),21 N497Mfs∗64 (known pathogenic), and A712V (known benign). GFP, green fluorescent protein; HR, homology-directed repair; NHEJ, nonhomologous end-joining; siP2, siRNA against PALB2.
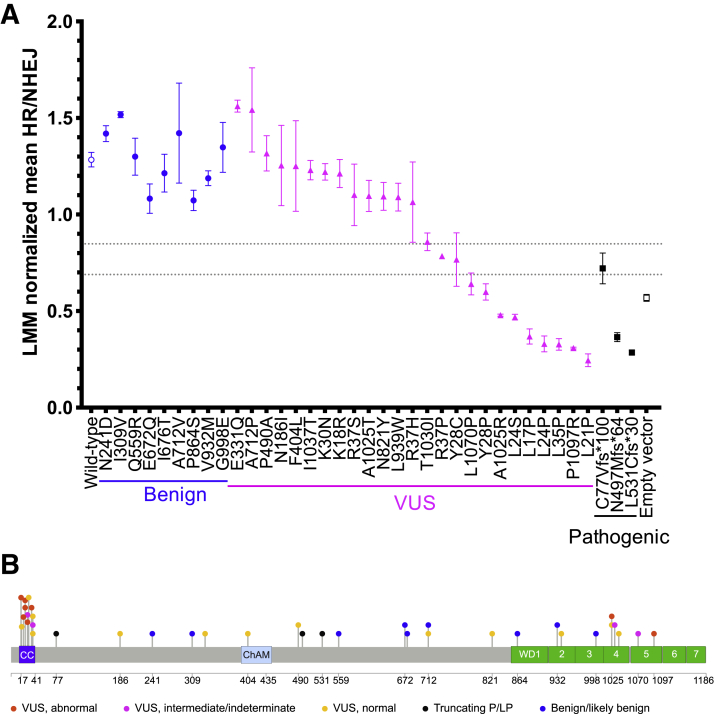
The experimental protocol was the same as for BRCA2, using PALB2 WT cDNA and EV as normal and abnormal controls, respectively. Each PALB2 variant was included in at least three independent experiments. In-plate adjustment to the mock-treated condition and LMM random effect normalization across all of the PALB2 plates were performed as described above. As shown in Figure 4A, WT PALB2 and benign PALB2 variants restored HR, indicated by a higher HR/NHEJ ratio (means ranged from 1.073 to 1.517), whereas EV and truncated PALB2 variants exhibited fewer HR events, with a lower HR/NHEJ ratio (means ranged from 0.285 to 0.721). In the PALB2 experiments, the GMM distribution corresponding to WT PALB2 controls had a mean HR/NHEJ of 1.249 (SD ±0.262), and the distribution corresponding to the EV controls had a mean HR/NHEJ of 0.554 (SD ±0.098). Again, thresholds were applied based on a 10:1 ratio of the probability to belong to either the normal (HR/NHEJ >0.848) or abnormal (HR/NHEJ <0.689) distributions (Supplemental Figure S3). Using these thresholds, the mean HR/NHEJ assay readout of all PALB2 B/LB validation controls fell in the normal range. Two of the truncating variants (N497Mfs∗64 and L531Cfs∗30) had mean HR/NHEJ assay values in the abnormal range, whereas one truncating variant (C77Vfs∗100) was considered of intermediate or indeterminate functional impact. Therefore, the TLR correctly classified all nine PALB2 B/LB controls and two of three PALB2 P/LP controls, which corresponds to an OddsPath of 6 (PS3_moderate) for an abnormal functional readout and an OddsPath of 0.333 (BS3_supporting) for a normal functional readout (Supplemental Table S2). Assay results are depicted for variants across the entire PALB2 protein (Figure 4B).
Figure 4.
Summary of PALB2 TLR assay readout for all variants tested. A: Traffic Light Reporter (TLR) assay results for the PALB2 experiments are summarized, demonstrating homology-directed repair (HR) function after siPALB2 knockdown and rescue with plasmids containing PALB2 variants or controls. HR function was rescued by PALB2 wild-type cDNA and benign validation controls but was impaired with empty vector and pathogenic validation controls. Each rescue condition is depicted across the x axis. The y axis represents the linear mixed model (LMM) normalized homology-directed repair/nonhomologous end-joining (HR/NHEJ) ratio. Thresholds for normal and abnormal functional impact are indicated by gray dashed lines. B: Lollipop diagram of PALB2 protein variants tested in the TLR assay, colored by functional impact. Benign validation controls are shown in blue, pathogenic validation controls are shown in black. Variants of uncertain significance (VUS) are coded by functional impact, based on the thresholds set for normal (yellow) or abnormal (red) assay readout. VUS with intermediate assay readout are labeled in pink. Coiled-coil (CC), chromatin association motif (ChAM), and WD40 protein domains (WD1-7, seven blades of the WD beta propeller) are indicated by colored boxes. Data are expressed as mean ± SEM. n ≥ 3 independent experiments; n = 40 wild-type and empty vector controls.
Functional Assessment of PALB2 Missense VUS
PALB2 missense VUS spanned the range of functional readouts from normal to abnormal, with some of intermediate or indeterminate function. Interestingly, there were substantial effects on DDR for eight VUS with mean HR/NHEJ ratios between 0.245 and 0.599. Consistent with Foo and colleagues'21 original report, as well as subsequent investigations,22, 23, 24 L35P had a robust abnormal functional impact on HR/NHEJ in the TLR assay. Other variants displaying abnormal function included: L17P, L21P, L24P, L24S, Y28P, A1025R, and P1097R. Four PALB2 missense variants had intermediate function (Y28C, R37P, T1030I, and L1070P), with mean HR/NHEJ ratios between 0.641 and 0.859. Although T1030I and L1070P could be assessed based on mean HR/NHEJ as having normal and abnormal function, respectively, the SEM for each extended into the indeterminate range. For this reason, they were interpreted more conservatively as having indeterminate functional readout for the purposes of applying functional evidence in variant classification. It is likely that additional replicates for these variants would provide a more precise estimate of the mean HR/NHEJ value. The remaining 13 VUS performed above the threshold for normal function (mean HR/NHEJ 1.064 to 1.562). Overall, these results indicate that the TLR assay is able to distinguish missense variants with normal and abnormal repair functions. These results also suggest that some missense variation in the BRCA1 and BRCA2-interacting domains of PALB2 may have deleterious effects on DDR, as seen previously in truncating PALB2 variants.
Next, follow-up evaluation of protein re-expression by Western blotting was performed for FLAG-tagged missense PALB2 variants, including seven with abnormal HR/NHEJ, as well as EV and a benign variant control, N241D. EV control does not express FLAG-tagged PALB2. These seven VUS had detectable expression, similar to N241D and WT PALB2 (Supplemental Figure S1D). For L17P, L21P, L24P, L24S, Y28P, L35P, and P1097R, this suggests that their abnormal HR/NHEJ ratios were not due to absent expression or reduced protein stability. Consistent with the TLR results, Boonen et al24 recently demonstrated normal protein expression for L24S and L35P variants. They also found normal protein expression for K18R, Y28C, R37H, L531Cfs∗30, Q559R, E672Q, P864S, L939W, G998E, and A1025R, but decreased expression/stability for T1030I, I1037T, and L1070P.24
TLR Readout Is Concordant with Other Assays of HR in PALB2
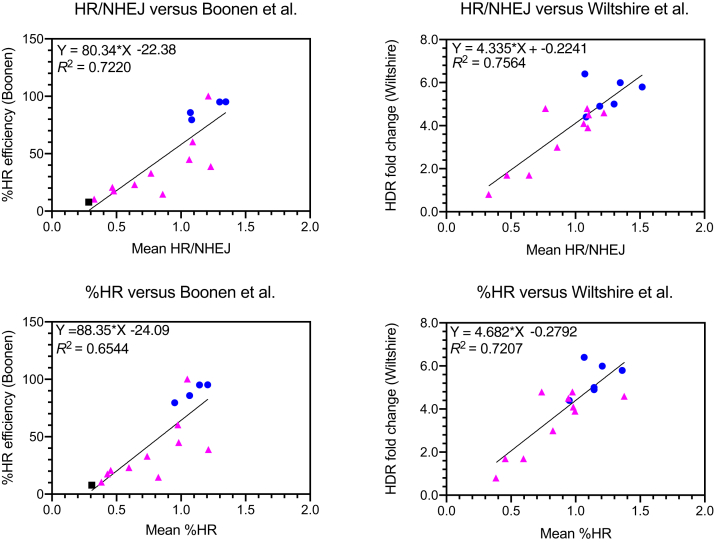
While this paper was in preparation, Boonen et al24 and Wiltshire et al22 published papers that examined HR capacity of PALB2 variants using the DR-GFP fluorescent reporter system in Trp53KO/Palb2KO mouse cell lines, either mammary tumor or mouse embryonic stem cells (Supplemental Table S1). Therefore, linear regression was used to assess how well TLR assay readouts correlated with the results for missense variants tested across these other two assays (Figure 5). When plotted against TLR, mean HR/NHEJ, R2 values from these regressions (0.7220 and 0.7564) suggested that TLR results were generally consistent with the published DR-GFP data (Figure 5). Considering that the TLR readout of % HR might make a more direct comparison, regressions were repeated with this data. Interestingly, the R2 value decreased slightly to 0.6544 and 0.7207 for the Boonen et al24 and Wiltshire et al22 datasets, respectively (Figure 5). Overall, the positive correlation of TLR HR/NHEJ readout with other data in the literature increases confidence in this metric of functional impact for PALB2 missense variants.
Figure 5.
Traffic Light Reporter (TLR) assay HR/NHEJ values correlate with published homology-directed repair (HR) functional data. Top row (left to right): linear regression of normalized mean HR/nonhomologous end-joining (NHEJ) versus %HR efficiency (Boonen et al24), or HDR fold change (Wiltshire et al22). Bottom row (left to right): linear regression as above, but using normalized mean %HR instead of HR/NHEJ ratio. Benign/likely benign variant controls are shown as blue circles, variants of uncertain significance (VUS) as magenta triangles, and truncating variants as black squares. n = 15 (Boonen et al24); n = 16 (Wiltshire et al22).
Discussion
To assess the functional impact of missense variants in PALB2 for use in clinical variant interpretation, the TLR cellular in vivo fluorescent reporter assay of HR and alt-NHEJ DDR outcomes was developed and validated. First, the TLR's performance was determined using 16 BRCA2 missense variants as benchmarks for normal/abnormal HR function. Readouts for these controls were consistent with previous studies, confirming that the TLR can distinguish between normal and abnormal DDR activity.5,40,41The assay was then validated for PALB2 and tested for a total of 37 PALB2 variants. By setting stringent thresholds for normal, abnormal, and indeterminate DDR outcomes, the assay readout accurately classified all nine benign validation controls, whereas not all of the pathogenic validation controls would have been correctly classified (one of the three had a readout in the indeterminate range). Thus, an assay readout in the abnormal range increases confidence in a variant not being in the benign category, and an assay readout in the normal range increases some confidence in a variant not being in the pathogenic category. Applying a Bayesian approach to estimate OddsPath,7,27 one arrives at a moderate strength of evidence in favor of pathogenicity (PS3_moderate) when the assay readout for a variant is below the abnormal threshold, and a supporting strength of evidence in favor of a benign classification (BS3_supporting) when the assay readout for a variant is above the normal threshold.
PALB2 missense VUS seem to cluster by normal and abnormal functional impact, with a few VUS having intermediate or indeterminate function. Of the 25 PALB2 missense VUS examined, eight demonstrated abnormal function comparable to EV and truncating variants. These variants are all located in either the N-terminal CC domain or the C-terminal WD40 domain of PALB2, suggesting that these regions are critical for normal PALB2 DDR activity and may be hot spots for pathogenic missense variation.
CC Domain and BRCA1 Interaction
The CC domain (residues 9 to 44) is made up of a heptad of supercoiled alpha helices and is the site of PALB2 homodimerization, as well as heterodimerization with BRCA1.9,42 Alpha helical structures are disrupted by proline substitutions.14 As such, it is unsurprising that changes to proline at key residues (L17, L21, L24, Y28, and L35) of the PALB2-PALB214 and PALB2-BRCA1 interface had abnormal readouts.
Recently, two other groups found abnormal HR function, decreased RAD51 foci, increased sensitivity to poly (ADP-ribose) polymerase inhibitors, and impaired interaction/complexing with BRCA1 in L24S.22,24 They found normal protein expression in L24S, consistent with our findings (Supplemental Figure S1D), as well as normal nuclear localization, and increased protein stability.22,24 This suggests that defects in DNA damage repair are likely mediated by impaired BRCA1 interaction, rather than issues of protein expression, stability, or localization.
PALB2 residues K18, K30, and R37 are also part of the CC protein-interacting domain, but are not points of direct contact.21 This may help explain why certain CC domain residues tested here may be more sensitive to alterations than others. It is important to note that for variants demonstrating a supporting level of evidence for normal DDR activity, all functions of PALB2 that may lead to disease pathogenesis, such as splicing, have not been assessed, and thus caution is still warranted in applying benign functional criteria for clinical variant interpretation.
Interestingly, pathogenic frameshift variant C77Vfs∗100 had a higher mean HR/NHEJ ratio (0.721) than EV alone (0.568), falling in the intermediate range. Tischkowitz and colleagues43 first described c.229delT (p.C77Vfs∗100) in a family of Scottish ancestry with strong history of breast cancer and found the variant had decreased HR activity and increased sensitivity to mitomycin C (MMC), a DNA cross-linking agent. This loss of normal protein function is predicted to occur through either protein truncation or nonsense-mediated decay, because the frameshift leads to the creation of a premature stop codon at position 100 in the new reading frame. The truncated protein includes the complete CC domain of PALB2, which would permit interaction with BRCA1 and PALB2-PALB2 binding. Although this is also true of the other frameshift variants examined, it is possible that the artificial system of truncating variant cDNA used here led to overexpression of an artificial protein fragment that retained enough of the C-terminal function to affect the relative HR/NHEJ. Alternatively, this result may reflect the true range of HR/NHEJ activity for pathogenic variants.
WD40 Domain and BRCA2 Interaction
Two variants in the PALB2 C-terminal WD40 domain, A1025R and P1097R, demonstrated robust abnormal HR/NHEJ functional readout in the TLR assay. The WD40 domain is a ring-like beta-propeller structure common in protein–protein interactions.39 PALB2 residues V1019, M1022, A1025, I1037, L1046, K1047, L1070, P1097, and K1098 line a hydrophobic pocket of the WD40 domain that binds BRCA239; residues A1025 and P1097 are located in the fourth and fifth blades,35,39 respectively, suggesting that the observed abnormalities in HR activity may be related to impaired BRCA2 interaction. A1025R abrogates the PALB2-BRCA2 interaction22,23,36,39,44,45 without affecting PALB2 protein stability.24,39 Truncation of PALB2 after P1097 abrogates BRCA2 binding,35 further implicating this domain in BRCA2 interactions and HR activity.
Interestingly, prediction algorithms for the impact on splicing suggest that P1097R may activate a cryptic exonic splice donor site.46 Although the TLR results suggest that the missense change itself results in abnormal HR function, splicing abnormalities cannot be assessed using the cDNA constructs used here. Therefore, it remains possible that an alternative splice-disrupting mechanism may occur in the endogenous gene context.
L1070P is also located in the hydrophobic pocket and occurred just below the threshold for abnormal functional impact with a mean HR/NHEJ of 0.641, though its SEM crossed into the indeterminate range. Other studies indicate that L1070P has defects in HR and protein expression/stability, increases sensitivity to olaparib and cisplatin, partially disrupts the BRCA2 interaction, decreases PALB2 recruitment to DSBs, and increases cytoplasmic retention.22,24 Additional replicates may further improve estimates of the mean HR/NHEJ readout for L1070P; alternatively, these conflicting results may indicate a limitation of the dynamic range of the current TLR assay to discriminate between normal and abnormal functional impact of all variants. It is also possible that L1070P and other variants with DDR activity between the abnormal or normal thresholds have intermediate function, requiring family studies and correlation with clinical phenotype to help resolve their interpretation moving forward. Currently, applying BS3 or PS3 criteria in the clinical interpretation of variants of intermediate or indeterminate function is not recommended.
Rigorous Assay Thresholding for Result Concordance
The results for PALB2 missense variants presented here are generally consistent (Figure 5) with recent HR functional assays conducted in U2OS osteosarcoma cells,22, 23, 24 as well as Trp53KO/Palb2KO mouse mammary tumor B40022 and mES cell lines.24 Given the potential confounding effects of background mutations in cancer-derived cell lines, DSB repair was studied in a relatively unrelated cell type where the DSB machinery is expected to be intact (confirmed by optimization experiments showing baseline rates of HR and NHEJ in controls). However, HEK293T cells inadequately resembling the relevant breast cancer precursor cell was expected to reduce the dynamic range of the TLR assay and reduce the ability to predict outcomes in the relevant tissue. It is unlikely that the cell type selected here overestimated the role of PALB2 variants in DDR, as results in HEK293T cells were comparable to those from other cell types.
Most discrepant interpretations between the results presented here and other published studies involve variants of intermediate function. The overall interpretation depends on each assay's thresholds for designating the readout abnormal, normal, or intermediate. Comparing results is further complicated if some assays use a binary classifier, eliminating an intermediate function category. It should be noted that whereas other assays normalize their functional readout to the WT control, the current approach masks the true variation in WT readouts across separate experimental replicates and creates potential challenges when drawing thresholds for normal versus abnormal function. In the TLR assay, PALB2 WT and EV controls were used instead to set thresholds for assay readout ranges that could be considered normal, intermediate, or abnormal, based on a GMM. This allowed the assay to be benchmarked based on the results for several validation controls, consistent with guidance for validating functional assays and setting strength thresholds for evidence application developed in collaboration with the ClinGen Sequence Variant Interpretation Working Group.7 The discrepancies in the interpretation of DDR assay readout highlight the importance of rigorous assay design to enable reproducible statistical thresholding.
Nuances and Caveats of Functional Assays for Use in Clinical Variant Interpretation
In the setting of few gold standard control variants, one previously described approach to variant interpretation used personal and family history data to predict clinical significance.47 However, this depends on many observations of each variant, limiting applicability for genes like PALB2 that do not have substantial clinical data available for type of analysis. Instead, the authors propose that a pathway-based approach can be an efficient way of developing assays that can be used for genes with shared biochemical functions. It is worth emphasizing that using the BRCA2 gold standard variants in this context was intended to demonstrate that this version of the TLR assay can effectively distinguish between DSB repair outcomes (HR and NHEJ) and can provide functional evidence toward a pathogenic or benign clinical interpretation. The subsequent validation of the assay for use with PALB2 still required validation controls (P/LP and B/LP variants) in PALB2 itself. However, this approach can generally be a useful starting point for genes such as PALB2 in which few gold standard variants are available.
Functional evidence can provide valuable information for clinical variant interpretation. However, the performance characteristics and inherent limitations of these assays need to be well understood. In the ACMG/AMP standards,1 any piece of evidence that can be used in the classification of a variant is assigned a particular strength, which then defines the weight given to that evidence in the combining rules leading to a final classification. In this framework, only a minor allele frequency >5% is considered standalone evidence (BA1). All other evidence codes, including functional evidence, must be used in conjunction with other types of evidence in order to achieve a classification other than VUS. Therefore, it is important to consider how the validation of any functional assay leads to an assertion regarding the strength of evidence that can be applied. With this requirement firmly in mind, a set of validation controls were utilized to define the performance of this assay according to thresholds established by ClinGen for assessing the strength of evidence that can be applied in clinical variant interpretation. As demonstrated, when applied to PALB2 variants under the conditions described in this particular instance, the TLR assay can provide moderate evidence in favor of pathogenicity when the assay readout is below the abnormal threshold, but only supporting evidence in favor of a benign classification when the assay readout is above the normal threshold. Given that this level of evidence is insufficient to classify a variant independently, it will be necessary for additional categories of evidence (such as clinical observations, minor allele frequency in control populations, family segregation data, etc.) to be applied according to the ACMG/AMP framework. However, this additional functional evidence may help to nudge the classification of some missense variants out of the VUS range into a more clinically useful likely pathogenic or likely benign category. Furthermore, as additional validation controls are evaluated, the strength of evidence that can be applied in this assay may be reassessed.
It is important to note that these assays are calibrated to differentiate between variants that have normal function versus abnormal function, but that these distinctions do not predict the penetrance or expressivity in an individual who harbors those variants. Although cancer predisposition syndromes are often inherited in a dominant fashion at an organismal level, the cellular phenotype of carcinogenesis occurs via a more complex multi-step process with additional somatic mutations (eg, a second hit in the affected tumor suppressor gene, loss of other critical genes such as TP53 [MIM number 191170], chromosomal aberrations, epigenetic alterations, etc.). Therefore, DDR outcomes were used as a proxy for the clinical impact of individual missense variants, with the assumption that decreased PALB2 function in 293T cells correlates with pathogenicity (and therefore increased cancer risk) as it does for BRCA2. However, due to differences between the assay conditions (degree of siRNA knockdown or cDNA re-expression levels) and the relative importance of each of these components of the DDR pathway in 293T cells, the TLR assay HDR outcomes were not directly comparable between PALB2 and BRCA2. It is possible that differences in penetrance at the organismal level, where BRCA2 is considered a high-risk gene and PALB2 is considered a moderate-risk gene, could be related to their degree of importance to the repair event, but the TLR assay does not address this hypothesis. Furthermore, many other factors influence expressivity and penetrance at the individual level, including the burden of genetic and nongenetic modifiers in any given person. Importantly, we do not suggest making comparisons between BRCA2 and PALB2 variants with respect to repair outcomes or clinical implications. Indeed, the relative dependence of different cell types (eg, breast tissue versus fallopian tube epithelium) for certain components of the DSB repair pathway may underlie differences in the spectrum of cancers for which people with these variants are at risk, and their lifetime risk to develop any of those cancers.
Conclusions
The work presented here represents a validated functional assay for PALB2 with statistically determined thresholds of strength for application of PS3/BS3 criteria in clinical variant interpretation. The approach to initial assay development using 16 known benign and pathogenic variants in BRCA2 to establish the protocol for this assay demonstrates that pathway-based approaches for assay development are promising when benchmarking controls are limited. Nine B/LB PALB2 missense controls and three truncating P/LP variants were used to further benchmark the TLR assay for PALB2. Although results for variants with normal readout can be used at a supporting level of evidence in favor of a benign classification (BS3_supporting) and abnormal readouts as moderate evidence supporting pathogenicity (PS3_moderate), including additional variants of known significance (as they become available), would further increase the strength of evidence that can be applied. This limitation is unfortunately an inherent problem for all assays of PALB2 function, given the paucity of pathogenic missense variants that can be used for clinical validation.
It is now becoming clear that L35P is not the only PALB2 missense variant with abnormal HR function—others in the BRCA1- and BRCA2-interacting domains have now demonstrated abnormal functional impact on DDR outcomes in multiple different assays. As such, further exploration of missense variation in these domains is merited. However, additional clinical observations and family segregation evidence, in addition to this moderate strength functional evidence, will be needed in order to conclude that these variants are pathogenic. Adapting the TLR assay for multiplexed assessment of PALB2 VUS would permit evaluation of larger numbers of variants before they are seen in a patient, pre-empting delays in conclusive variant interpretation while functional evidence is generated. Overall, functional data presented here is expected to contribute to the additional evidence required to help reclassify these VUS within the ACMG/AMP variant interpretation framework for direct clinical benefit.
Acknowledgment
We would like to thank Stephanie B. Crowley, PhD, for her assistance in molecular cloning and preliminary validation experiments.
Footnotes
J.S.B. is a recipient of the Yang Family Biomedical Scholars Award. S.E.B. was supported in part by the National Institute of General Medical Sciences grants 5T32 GM007092 and 5T32 GM008719-6. E.C.A. received support in part from the Chancellor's Science Scholars fund. The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center, as well as Center for AIDS Research award number 5P30AI050410.
Disclosures: None declared.
Portions of this manuscript were previously presented as an abstract at the 2019 Association for Molecular Pathology annual meeting, November 7-9, 2019 in Baltimore, MD.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2021.04.010.
Supplemental Data
Development of TLR assay protocol. A: Timing DSB repair by flow cytometry. 293T/TLR cells were transfected with I-SceI-IFP and truncGFP donor-BFP at 12, 24, 48, or 72 hours before flow cytometry was performed. Infrared protein-positive/blue fluorescent protein-positive (IFP+/BFP+) cells (doubly transfected with both nuclease and truncGFP) are shown as percentage of single live cells. mCherry and GFP expression serve as markers for alt-NHEJ and HR events, respectively; B: Time course of siRNA-mediated knockdown of PALB2. Expression of PALB2 mRNA after siRNA-mediated knockdown relative to nontargeting control siRNA (siNT)-treated cells. 293T/TLR cells were transfected with 75 nmol/L siRNA, and cells were pelleted after the indicated number of hours and frozen for later RNA extraction and analysis. Real-time PCR was performed using primers for PALB2 and B2M. C: Western blot analysis of whole-cell lysates from 293T/TLR cells 48, 72, or 96 hours (h) after transfection with siNT or siRNA against PALB2 (siPALB2). Whole-cell lysate (15 μg) was loaded per lane and endogenous PALB2 expression was probed with 0.5 μg/mL anti-PALB2 antibody. GAPDH serves as a loading control. Lanes reordered here for ease of comparison against siNT control; D: PALB2 protein re-expression. Western blot analysis of whole-cell lysates from 293T/TLR cells treated with 75 nmol/L siPALB2 and transiently transfected 24-hour later with empty vector (empty v.) or pDEST-FRT/T0-FLAG-PALB2 encoding wild-type (WT) or variant PALB2. Forty-eight hours after siRNA transfection, exogenous PALB2 expression was probed with 1.0 μg/mL anti-FLAG antibody. GAPDH serves as a loading control. Data are expressed as means ± SD. n = 3 independent experiments.
Monitoring siRNA knockdown in flow cytometry experiments. Results of real-time PCR performed in triplicate on 293T/TLR cells treated with either nontargeting control siRNA (siNT), siBRCA2, or siPALB2 and rescued with empty vector and pelleted at the time of flow cytometry, 96 hours after siRNA transfection. Probes used: B2M, BRCA2, PALB2. All experiments demonstrated >50% knockdown of the target gene relative to siNT-treated cells at the time of flow cytometry. The only exception was the 3/8/2019 PALB2 flow sample, which appears to have had poor RNA integrity, because this real-time PCR result does not reflect the level of knockdown observed by flow cytometry [linear mixed model (LMM) normalized HR/NHEJ of 0.569 for empty vector rescue)].
Gaussian mixture models. Each circle represents the TLR assay readout for wild-type (WT, blue) or empty vector (EV, red) controls, with corresponding curves based on an unconstrained Gaussian mixture model that provides distributions for normal and abnormal assay readout. Dashed lines indicate readout values where there is a 10:1 ratio of the probability to belong to one curve versus the other. HR, homologous recombination; NHEJ, nonhomologous end-joining.
References
- 1.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., Voelkerding K., Rehm H.L. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrie A., Hemphill S.E., Ruiz-Schultz N., Cushman B., DiStefano M.T., Azzariti D., Harrison S.M., Rehm H.L., Eilbeck K. ClinVar Miner: demonstrating utility of a Web-based tool for viewing and filtering ClinVar data. Hum Mutat. 2018;39:1051–1060. doi: 10.1002/humu.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans J.P., Powell B.C., Berg J.S. Finding the rare pathogenic variants in a human genome. JAMA. 2017;317:1904–1905. doi: 10.1001/jama.2017.0432. [DOI] [PubMed] [Google Scholar]
- 4.Starita L.M., Ahituv N., Dunham M.J., Kitzman J.O., Roth F.P., Seelig G., Shendure J., Fowler D.M. Variant interpretation: functional assays to the rescue. Am J Hum Genet. 2017;101:315–325. doi: 10.1016/j.ajhg.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidugli L., Pankratz V.S., Singh N., Thompson J., Erding C.A., Engel C., Schmutzler R., Domchek S., Nathanson K., Radice P., Singer C., Tonin P.N., Lindor N.M., Goldgar D.E., Couch F.J. A classification model for BRCA2 DNA binding domain missense variants based on homology-directed repair activity. Cancer Res. 2013;73:265–275. doi: 10.1158/0008-5472.CAN-12-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brnich S.E., Rivera-Muñoz E.A., Berg J.S. Quantifying the potential of functional evidence to reclassify variants of uncertain significance in the categorical and Bayesian interpretation frameworks. Hum Mutat. 2018;39:1531–1541. doi: 10.1002/humu.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brnich S.E., Abou Tayoun A.N., Couch F.J., Cutting G.R., Greenblatt M.S., Heinen C.D., Kanavy D.M., Luo X., McNulty S.M., Starita L.M., Tavtigian S.V., Wright M.W., Harrison S.M., Biesecker L.G., Berg JS, Clinical Genome Resource Sequence Variant Interpretation Working Group. Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med. 2020;12:3. doi: 10.1186/s13073-019-0690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landrum M.J., Lee J.M., Benson M., Brown G.R., Chao C., Chitipiralla S., Gu B., Hart J., Hoffman D., Jang W., Karapetyan K., Katz K., Liu C., Maddipatla Z., Malheiro A., McDaniel K., Ovetsky M., Riley G., Zhou G., Holmes J.B., Kattman B.L., Maglott D.R. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sy S.M.H., Huen M.S.Y., Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci. 2009;106:7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F., Ma J., Wu J., Ye L., Cai H., Xia B., Yu X. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009;19:524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakash R., Zhang Y., Feng W., Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7:a016600. doi: 10.1101/cshperspect.a016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C.-C., Feng W., Lim P.X., Kass E.M., Jasin M. Homology-directed repair and the role of BRCA1, BRCA2, and related proteins in genome integrity and cancer. Annu Rev Cancer Biol. 2018;2:313–336. doi: 10.1146/annurev-cancerbio-030617-050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord C.J., Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 14.Song F., Li M., Liu G., Swapna G.V.T., Daigham N.S., Xia B., Montelione G.T., Bunting S.F. Antiparallel coiled-coil interactions mediate the homodimerization of the DNA damage-repair protein PALB2. Biochemistry. 2018;57:6581–6591. doi: 10.1021/acs.biochem.8b00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buisson R., Masson J.Y. PALB2 self-interaction controls homologous recombination. Nucleic Acids Res. 2012;40:10312–10323. doi: 10.1093/nar/gks807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauty J., Couturier A.M., Rodrigue A., Caron M.C., Coulombe Y., Dellaire G., Masson J.Y. Cancer-causing mutations in the tumor suppressor PALB2 reveal a novel cancer mechanism using a hidden nuclear export signal in the WD40 repeat motif. Nucleic Acids Res. 2017;45:2644–2657. doi: 10.1093/nar/gkx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haber J.E. Alternative endings. Proc Natl Acad Sci U S A. 2008;105:405–406. doi: 10.1073/pnas.0711334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salles B., Calsou P., Mirey G. DNA-PK, a pharmacological target in cancer chemotherapy and radiotherapy? J Cancer Sci Ther. 2013;5:1–11. doi: 10.1016/j.patbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Li A., Geyer F.C., Blecua P., Lee J.Y., Selenica P., Brown D.N. Homologous recombination DNA repair defects in PALB2-associated breast cancers. NPJ Breast Cancer. 2019;5:23. doi: 10.1038/s41523-019-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polak P., Kim J., Braunstein L.Z., Karlic R., Haradhavala N.J., Tiao G., Rosebrock D., Livitz D., Kübler K., Mouw K.W., Kamburov A., Maruvka Y.E., Leshchiner I., Lander E.S., Golub T.R., Zick A., Orthwein A., Lawrence M.S., Batra R.N., Caldas C., Haber D.A., Laird P.W., Shen H., Ellisen L.W., D’Andrea A.D., Chanock S.J., Foulkes W.D., Getz G. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476–1486. doi: 10.1038/ng.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foo T.K., Tischkowitz M., Simhadri S., Boshari T., Zayed N., Burke K.A., Berman S.H., Blecua P., Riaz N., Huo Y., Ding Y.C., Neuhausen S.L., Weigelt B., Reis-Filho J.S., Foulkes W.D., Xia B. Compromised BRCA1-PALB2 interaction is associated with breast cancer risk. Oncogene. 2017;36:4161–4170. doi: 10.1038/onc.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiltshire T., Ducy M., Foo T.K., Hu C., Lee K.Y., Belur Nagaraj A., Rodrigue A., Gomes T.T., Simard J., Monteiro A.N.A., Xia B., Carvalho M.A., Masson J.-Y., Couch F.J. Functional characterization of 84 PALB2 variants of uncertain significance. Genet Med. 2020;22:622–632. doi: 10.1038/s41436-019-0682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigue A., Margaillan G., Torres Gomes T., Coulombe Y., Montalban G., da Costa E Silva Carvalho S., Milano L., Ducy M., De-Gregoriis G., Dellaire G., Araújo da Silva W., Monteiro A.N., Carvalho M.A., Simard J., Masson J.-Y. A global functional analysis of missense mutations reveals two major hotspots in the PALB2 tumor suppressor. Nucleic Acids Res. 2019;47:10662–10677. doi: 10.1093/nar/gkz780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boonen R.A.C.M., Rodrigue A., Stoepker C., Wiegant W.W., Vroling B., Sharma M., Rother M.B., Celosse N., Vreeswijk M.P.G., Couch F., Simard J., Devilee P., Masson J., van Attikum H. Functional analysis of genetic variants in the high-risk breast cancer susceptibility gene PALB2. Nat Commun. 2019;10:5296. doi: 10.1038/s41467-019-13194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moynahan M.E., Pierce A.J., Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 26.Guidugli L., Carreira A., Caputo S.M., Ehlen A., Galli A., Monteiro A.N.A., Neuhausen S.L., Hansen T.V.O., Couch F.J., Vreeswijk M.P.G. Functional assays for analysis of variants of uncertain significance in BRCA2. Hum Mutat. 2014;35:151–164. doi: 10.1002/humu.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavtigian S.V., Greenblatt M.S., Harrison S.M., Nussbaum R.L., Prabhu S.A., Boucher K.M., Biesecker L.G. Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet Med. 2018;20:1054–1060. doi: 10.1038/gim.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orthwein A., Noordermeer S.M., Wilson M.D., Landry S., Enchev R.I., Sherker A., Munro M., Pinder J., Salsman J., Dellaire G., Xia B., Peter M., Durocher D. A mechanism for the suppression of homologous recombination in G1 cells. Nature. 2015;528:422–426. doi: 10.1038/nature16142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Wang S.-C., Shao R., Pao A.Y., Zhang S., Hung M.-C., Su L.-K. Inhibition of cancer cell growth by BRCA2. Cancer Res. 2002;62:1311–1314. [PubMed] [Google Scholar]
- 30.Certo M.T., Ryu B.Y., Annis J.E., Garibov M., Jarjour J., Rawlings D.J., Scharenberg A.M. Tracking genome engineering outcome at individual DNA breakpoints. Nat Methods. 2011;8:671–676. doi: 10.1038/nmeth.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger M.J., Certo M.T. In: Gene Correction: Methods and Protocols. Storici F., editor. Humana Press; Totowa, NJ: 2014. Design and analysis of site-specific single-strand nicking endonucleases for gene correction; pp. 237–244. [DOI] [PubMed] [Google Scholar]
- 32.Adamson B., Smogorzewska A., Sigoillot F.D., King R.W., Elledge S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat Cell Biol. 2012;14:318–328. doi: 10.1038/ncb2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A language and environment for statistical computing. [Google Scholar]
- 34.Benaglia T., Chauveau D., Hunter D.R., Young D.S. mixtools: an R package for analyzing finite mixture models. J Stat Softw. 2009;32:1–29. [Google Scholar]
- 35.Park J.-Y., Singh T.R., Nassar N., Zhang F., Freund M., Hanenberg H., Meetei A.R., Andreassen P.R. Breast cancer-associated missense mutants of the PALB2 WD40 domain, which directly binds RAD51C, RAD51 and BRCA2, disrupt DNA repair. Oncogene. 2014;33:4803–4812. doi: 10.1038/onc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma J., Cai H., Wu T., Sobhian B., Huo Y., Alcivar A., Mehta M., Cheung K.L., Ganesan S., Kong A.-N.T., Zhang D.D., Xia B. PALB2 interacts with KEAP1 to promote NRF2 nuclear accumulation and function. Mol Cell Biol. 2012;32:1506–1517. doi: 10.1128/MCB.06271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikkilä J., Christin Parplys A., Pylkäs K., Bose M., Huo Y., Borgmann K., Rapakko K., Nieminen P., Xia B., Pospiech H., Winqvist R. Heterozygous mutations in PALB2 cause DNA replication and damage response defects. Nat Commun. 2013;4:2578. doi: 10.1038/ncomms3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F., Fan Q., Ren K., Andreassen P.R. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol Cancer Res. 2009;7:1110–1118. doi: 10.1158/1541-7786.MCR-09-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliver A.W., Swift S., Lord C.J., Ashworth A., Pearl L.H. Structural basis for recruitment of BRCA2 by PALB2. EMBO Rep. 2009;10:990–996. doi: 10.1038/embor.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guidugli L., Shimelis H., Masica D.L., Pankratz V.S., Lipton G.B., Singh N., Hu C., Monteiro A.N.A., Lindor N.M., Goldgar D.E., Karchin R., Iversen E.S., Couch F.J. Assessment of the clinical relevance of BRCA2 missense variants by functional and computational approaches. Am J Hum Genet. 2018;102:233–248. doi: 10.1016/j.ajhg.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesman R.L.S., Calléja F.M.G.R., Hendriks G., Morolli B., Misovic B., Devilee P., van Asperen C.J., Vrieling H., Vreeswijk M.P.G. The functional impact of variants of uncertain significance in BRCA2. Genet Med. 2019;21:293–302. doi: 10.1038/s41436-018-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nepomuceno T., De Gregoriis G., de Oliveira F.M.B., Suarez-Kurtz G., Monteiro A., Carvalho M. The role of PALB2 in the DNA damage response and cancer predisposition. Int J Mol Sci. 2017;18:1886. doi: 10.3390/ijms18091886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tischkowitz M., Xia B., Sabbaghian N., Reis-Filho J.S., Hamel N., Li G., van Beers E.H., Li L., Khalil T., Quenneville L.A., Omeroglu A., Poll A., Lepage P., Wong N., Nederlof P.M., Ashworth A., Tonin P.N., Narod S.A., Livingston D.M., Foulkes W.D. Analysis of PALB2/FANCN-associated breast cancer families. Proc Natl Acad Sci U S A. 2007;104:6788–6793. doi: 10.1073/pnas.0701724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caleca L., Catucci I., Figlioli G., De Cecco L., Pesaran T., Ward M., Volorio S., Falanga A., Marchetti M., Iascone M., Tondini C., Zambelli A., Azzollini J., Manoukian S., Radice P., Peterlongo P. Two missense variants detected in breast cancer probands preventing BRCA2-PALB2 protein interaction. Front Oncol. 2018;8:480. doi: 10.3389/fonc.2018.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anantha R.W., Simhadri S., Foo T.K., Miao S., Liu J., Shen Z., Ganesan S., Xia B. Functional and mutational landscapes of BRCA1 for homology-directed repair and therapy resistance. Elife. 2017;6:1–21. doi: 10.7554/eLife.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desmet F.O., Hamroun D., Lalande M., Collod-Bëroud G., Claustres M., Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:1–14. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Easton D.F., Deffenbaugh A.M., Pruss D., Frye C., Wenstrup R.J., Allen-Brady K., Tavtigian S.V., Monteiro A.N.A., Iversen E.S., Couch F.J., Goldgar D.E. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer-predisposition genes. Am J Hum Genet. 2007;81:873–883. doi: 10.1086/521032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Development of TLR assay protocol. A: Timing DSB repair by flow cytometry. 293T/TLR cells were transfected with I-SceI-IFP and truncGFP donor-BFP at 12, 24, 48, or 72 hours before flow cytometry was performed. Infrared protein-positive/blue fluorescent protein-positive (IFP+/BFP+) cells (doubly transfected with both nuclease and truncGFP) are shown as percentage of single live cells. mCherry and GFP expression serve as markers for alt-NHEJ and HR events, respectively; B: Time course of siRNA-mediated knockdown of PALB2. Expression of PALB2 mRNA after siRNA-mediated knockdown relative to nontargeting control siRNA (siNT)-treated cells. 293T/TLR cells were transfected with 75 nmol/L siRNA, and cells were pelleted after the indicated number of hours and frozen for later RNA extraction and analysis. Real-time PCR was performed using primers for PALB2 and B2M. C: Western blot analysis of whole-cell lysates from 293T/TLR cells 48, 72, or 96 hours (h) after transfection with siNT or siRNA against PALB2 (siPALB2). Whole-cell lysate (15 μg) was loaded per lane and endogenous PALB2 expression was probed with 0.5 μg/mL anti-PALB2 antibody. GAPDH serves as a loading control. Lanes reordered here for ease of comparison against siNT control; D: PALB2 protein re-expression. Western blot analysis of whole-cell lysates from 293T/TLR cells treated with 75 nmol/L siPALB2 and transiently transfected 24-hour later with empty vector (empty v.) or pDEST-FRT/T0-FLAG-PALB2 encoding wild-type (WT) or variant PALB2. Forty-eight hours after siRNA transfection, exogenous PALB2 expression was probed with 1.0 μg/mL anti-FLAG antibody. GAPDH serves as a loading control. Data are expressed as means ± SD. n = 3 independent experiments.
Monitoring siRNA knockdown in flow cytometry experiments. Results of real-time PCR performed in triplicate on 293T/TLR cells treated with either nontargeting control siRNA (siNT), siBRCA2, or siPALB2 and rescued with empty vector and pelleted at the time of flow cytometry, 96 hours after siRNA transfection. Probes used: B2M, BRCA2, PALB2. All experiments demonstrated >50% knockdown of the target gene relative to siNT-treated cells at the time of flow cytometry. The only exception was the 3/8/2019 PALB2 flow sample, which appears to have had poor RNA integrity, because this real-time PCR result does not reflect the level of knockdown observed by flow cytometry [linear mixed model (LMM) normalized HR/NHEJ of 0.569 for empty vector rescue)].
Gaussian mixture models. Each circle represents the TLR assay readout for wild-type (WT, blue) or empty vector (EV, red) controls, with corresponding curves based on an unconstrained Gaussian mixture model that provides distributions for normal and abnormal assay readout. Dashed lines indicate readout values where there is a 10:1 ratio of the probability to belong to one curve versus the other. HR, homologous recombination; NHEJ, nonhomologous end-joining.