Abstract
Objective
This study aimed to evaluate the utilization of aspirin for preeclampsia prevention before and after implementation of a screening tool during nuchal translucency (NT) ultrasound.
Study Design
One-year prospective cohort study of patients at high risk for preeclampsia after the implementation of a screening tool (post screen) administered to all patients at check in for NT (11–13 weeks) ultrasound. Prospective cohort was compared with one-year retrospective cohort (prescreen) the year prior (2017). All patients who presented for NT ultrasound in both cohorts were evaluated for the presence of one or more risk factor for preeclampsia with screening tool collected prospectively and chart review retrospectively. Provider recommendation for aspirin determined by documentation in prenatal record. Primary outcome was rate of provider recommendation for aspirin pre versus post screening tool, compared by Chi-square test and adjusted for potential confounders with multiple regression analysis.
Results:
Pre- (n = 156) and post screen (n = 136) cohorts were similar except for race and multifetal gestation. Prescreen, rate of provider recommendation for aspirin was 74%. Of those with prior preeclampsia, 96% were recommended aspirin, compared with 64% of patients with other risk factors (p < 0.001). Post screen, provider recommendation of aspirin improved to 95% (p < 0.001). Rate of preeclampsia/gestational hypertension were similar between cohorts; however, there was a reduced adjusted risk in overall preterm birth <37 weeks (adjusted odds ratio [aOR] = 0.50 [0.25–0.99]) and preterm birth <34 weeks (aOR = 0.33 [0.13–0.88]) post screening tool implementation.
Conclusion:
Prior to implementation of a simple screening questionnaire, approximately 25% of high risk patients did not receive the recommendation of aspirin for preeclampsia prevention. High-risk patients who lack a history of preeclampsia were less likely to be advised of aspirin prophylaxis. Use of a simple universal screening tool at time of NT ultrasound significantly improved utilization of aspirin for preeclampsia prevention and may improve patient outcomes.
Keywords: Aspirin, preeclampsia, implementation research
Hypertensive disorders of pregnancy including gestational hypertension and preeclampsia are among the most common medical complications of pregnancy affecting 6 to 10% of pregnancies and are responsible for 15 to 43% of preterm births.1 Daily low-dose aspirin therapy initiated prior to 16 weeks has been shown to reduce the risk of preterm delivery due to preeclampsia in high-risk women.2–4 Reducing preeclampsia would help reduce the main cause of iatrogenic preterm birth and a major source of both maternal and neonatal morbidity. In September 2014, the U.S. Preventative Services Task Force (USPSTF) released an initial recommendation regarding use of low dose aspirin for preeclampsia prevention. We note that, like many medications used in pregnancy, aspirin is not Food and Drug Administration (FDA) approved for this specific indication.4 In August 2016, American College of Obstetrics and Gynecology (ACOG) released a practice update endorsing the USPSTF guidelines regarding aspirin use in high-risk groups specified by USPSTF,5 pregnant patients with at least one of the following high-risk factors for preeclampsia: history of preeclampsia, chronic hypertension, pregestational diabetes, chronic kidney disease, autoimmune disease such as lupus or antiphospholipid anti- body syndrome, and multifetal gestation.3,4
Implementation science seeks to assess the feasibility, acceptability, adoption, cost, and sustainability of interven- tions demonstrated to be effective in clinical trials.6 The disconnect between therapy development/validation in a research setting to implementation in clinical practice is the so called “second translation gap” and directly impacts whether patient health and population health improves following new evidence-based health care guidelines.6,7 There has been little published on the implementation of the recent ACOG and USPSTF recommendations regarding aspirin prophylaxis for preeclampsia prevention.8,9 One recent study from our institution found that only approximately 70% of pregnant patents with a history of chronic hypertension were recommended aspirin following the ACOG recommendations.10 The objective of this study is how use of a screening tool may improve implementation of ACOG/USPSTF guidelines regarding aspirin use for pre-eclampsia prevention.
Materials and Methods
Study Design
This is a 1-year prospective cohort study of patients at high risk for preeclampsia after the implementation of a screening tool (postscreen, May 14, 2018–May 11, 2019) administered to all patients at check in for nuchal translucency (NT; 11–13 weeks) ultrasound. Prospective cohort was compared with 1-year retrospective cohort (prescreen) the year prior (January 1, 2017–December 31, 2017). This study was approved by the institutional review board (IRB) of Thomas Jefferson University. There was an initial provider education for the entire Department of Obstetrics and Gynecology of Thomas Jefferson University via e-mail, and distribution of a guideline following ACOG and USPSTF initial recommendations in 2016; no additional provider education was conducted since then except in April 2018 to inform maternal-fetal medicine (MFM) physicians of the screening tool that they would be seeing on patients coming for first trimester scan. The routinely recommended dose for preeclampsia prevention at our institution is 81-mg daily based on ACOG and USPSTF recommendations. This prospective cohort was compared with a 1-year retrospective cohort from January to December 2017. The prenatal charts of all patients who presented for NT ultrasound were reviewed for the presence of at least one high-risk factor for preeclampsia to include in the cohort. Patients who received prenatal care at an outside institution were excluded. The charts of the final cohort were then reviewed manually for whether any provider recommended aspirin therapy for preeclampsia prevention and to evaluate delivery outcomes, including gestational age, antenatal complications, and indication for delivery. Notes reviewed included ultrasound reports, MFM consultation, and prenatal chart. Demographic information, obstetric (OB) history, and pregnancy outcomes were collected. Dose of aspirin recommended by all providers at our institution was 81 mg at the time of these cohort studies. Because the variables selected are all standard in any medical or prenatal records and we only included patients with complete prenatal care at our institution and missing data were not expected.
Patient Selection
Patients were considered high risk if they had any one of the following: history of preeclampsia, chronic hypertension, pregestational diabetes, chronic kidney disease, lupus, anti- phospholipid antibody syndrome, or multiple gestation. Although a combination of moderate risk factors may also be considered for recommending aspirin, 3 we chose to focus on high-risk factors for consistency in diagnosis. Because the recommendation for maximal efficacy relates to initiation prior to 16 weeks’ gestation, we selected a cohort of patients who presented for first trimester NT ultrasound. Patients who were included presented for NT ultrasound and received prenatal care at our institution.
In May 2018, we implemented the use of a single screening tool completed by the patient at check in for their first trimester ultrasound (►Supplementary Material, available in the online version). Completed forms were given to the reviewing physician who saw and counseled the patient regarding recommendation for aspirin use if any high-risk factor was checked as “yes.” Patients were eligible for inclusion if they had checked-off at least one high-risk factor. Patients were excluded if they had a nonviable pregnancy, major anomaly, care at outside center, or aspirin contraindication. Patients were then asked whether they wanted to enroll in a prospective cohort study with follow-up during pregnancy for adherence and outcomes and then verbally consented. Patients who were not approached regarding study participation or declined survey but agreed to chart review were followed observationally, patients who declined to have any part in research were excluded. Follow- up visits were done either during routinely scheduled prenatal care or if they did not present for their routinely scheduled appointment, then over the phone at 16 to 20 weeks to assess initiation of aspirin therapy and again at 24 to 28 weeks and 34 to 37 weeks to assess adherence. All completed screening forms were collected, regardless of whether patient was approached regarding study participation. This study was approved by Thomas Jefferson University IRB.
Statistical Analysis
Provider recommendation for aspirin was assessed by review of prenatal records and ultrasound report if any OB provider (MFM or primary OB) documented recommendation for aspirin. Primary outcome was the overall rate of provider recommendation for aspirin pre- versus post-screening tool, compared with Chi- square test. Secondary outcomes included the rate of provider recommendation pre- versus post-screening tool by preeclampsia risk factor and factors associated with aspirin recommendation in the prescreening tool implementation. Comparison were done using Chi-square test or Fisher’s exact test as appropriate for categorical variables, and two-sample t-test and/or Mann– Whitney U-test for continuous variables. A multivariablelogistic regression analysis with backward stepwise variable selection was performed; and the odds ratios (ORs) for the model- selected risk factors were given in ►Table 1. Rate of provider recommendation for aspirin over time was assessed by annual quarter with Pearson’s correlation coefficient. Pregnancy outcomes were also analyzed. Preeclampsia/ gestational hypertension, preterm preeclampsia/gestational hypertension, overall rates of preterm birth, and iatrogenic preterm birth (<37 and <34 weeks) in the pre- versus post-screening tool cohorts were compared with Chi-square test or Fisher’s exact test as appropriate for categorical variables. The adjusted risk for these outcomes in the post- versus prescreening cohort was assessed using multivariable logistic regression analysis with backward stepwise variable selection to adjust for potential confounders including age, race, Medicaid insurance, nulliparity, body mass index (BMI), and preeclampsia risk factors. SPSS v. 26 was used for these analyses.
Table 1.
Prescreening tool cohort: baseline differences between pregnant patients at high risk for preeclampsia who presented for 12-week ultrasound who were or were not recommended aspirin therapy
| Total (n ¼ 156) | No aspirin recommended n ¼ 40 (25.6%) | Aspirin recommended n ¼ 116 (74.4%) | p-Value | Adjusted OR | |
|---|---|---|---|---|---|
| Baseline demographics | |||||
| Maternal age (y) | 31.9 ± 5.9 | 30.9 ± 5.9 | 32.2 ± 5.9 | 0.23 | NS |
| Nulliparity | 44 (28.2) | 17 (42.5) | 27 (23.3) | 0.02a | NS |
| Race | 0.04a | NS | |||
| African–American | 95 (61.3%) | 19 (47.5%) | 76 (66.1%) | ||
| Caucasian | 27 (17.4%) | 12 (30.0%) | 15 (13.0%) | ||
| Asian | 11 (7.1%) | 4 (10.0%) | 7 (6.1%) | ||
| Latino/Hispanic | 16 (10.3%) | 3 (7.5%) | 13 (11.3%) | ||
| Other/unknown | 6 (3.8%) | 2 (5.0%) | 4 (3.5%) | ||
| Body mass index (kg/m2) | 33.1 ± 8.4 | 32.4 ± 9.2 | 33.4 ± 8.1 | 0.537 | NS |
| Current smoker | 13 (8.3%) | 3 (7.5%) | 10 (8.6%) | >0.999 | |
| Alcohol use in pregnancy | 3 (1.9%) | 1 (2.6%) | 2 (1.7%) | >0.999 | |
| Illicit opiate/cocaine use | 4 (2.6%) | 1 (2.5%) | 3 (2.6%) | >0.999 | |
| Preeclampsia risk factor | |||||
| Prior mild preeclampsia | 32 (20.5%) | 2 (5.0%) | 30 (25.9%) | 0.005a | 10.15 (2.94–53.79) |
| Prior severe preeclampsia | 19 (12.2%) | 0 (0%) | 19 (16.4%) | 0.004a | 29.02 (3.62–3,768.02) |
| Chronic hypertension | 89 (57.1%) | 17 (42.5%) | 72 (62.1%) | 0.041a | 3.48 (1.57–7.96) |
| Pregestational diabetes | 43 (27.6%) | 16 (40.0%) | 27 (23.3%) | 0.063 | NS |
| Chronic kidney disease | 11 (7.1%) | 5 (12.5%) | 6 (5.2%) | 0.153 | NS |
| Systemic lupus erythematous | 5 (3.2%) | 1 (2.5%) | 4 (3.4%) | 0.999 | NS |
| Antiphospholipid antibody syndrome | 3 (1.9%) | 0 (0.0%) | 3 (2.6%) | 0.570 | NS |
| Multifetal gestation | 9 (5.8%) | 4 (10.0%) | 5 (4.3%) | 0.236 | NS |
Abbreviations: OR, odds ratio; NS, not significant.
Note: Data presented as n (%) or mean ± standard deviation. No missing data.
Indicates significance, p < 0.05. Adjusted OR represents multiple logistic regression analysis with backward stepwise variable selection for factors associated with being recommended aspirin. NS represents nonsignificant (95% confidence interval includes 1.0).
This was planned as a 1 year pre- and post-screening tool analysis. Based on a review of women with chronic hypertension January to June 2017 we had identified approximately 60% of eligible patients were being offered aspirin. We would need at least 180 women (90 in each group) to demonstrate an increase from 60 to 80% before and after screening tool implementation, with a power of 80% and two-sided α of 0.05. We estimated that with a delivery volume of 2,000/year and approximately 10% of women with at least one high-risk criterion for preeclampsia would have approximately 200 women/year included. Recruitment was based on time of study (1-year pre- and post-screening) and not on number of women enrolled.
Results
►Fig. 1 demonstrates the screening and eligibility of retrospective and prospective cohorts. Overall, approximately 12% of patients who presented for NT ultrasound in either cohort had at least one high-risk factor for preeclampsia.
Fig. 1.
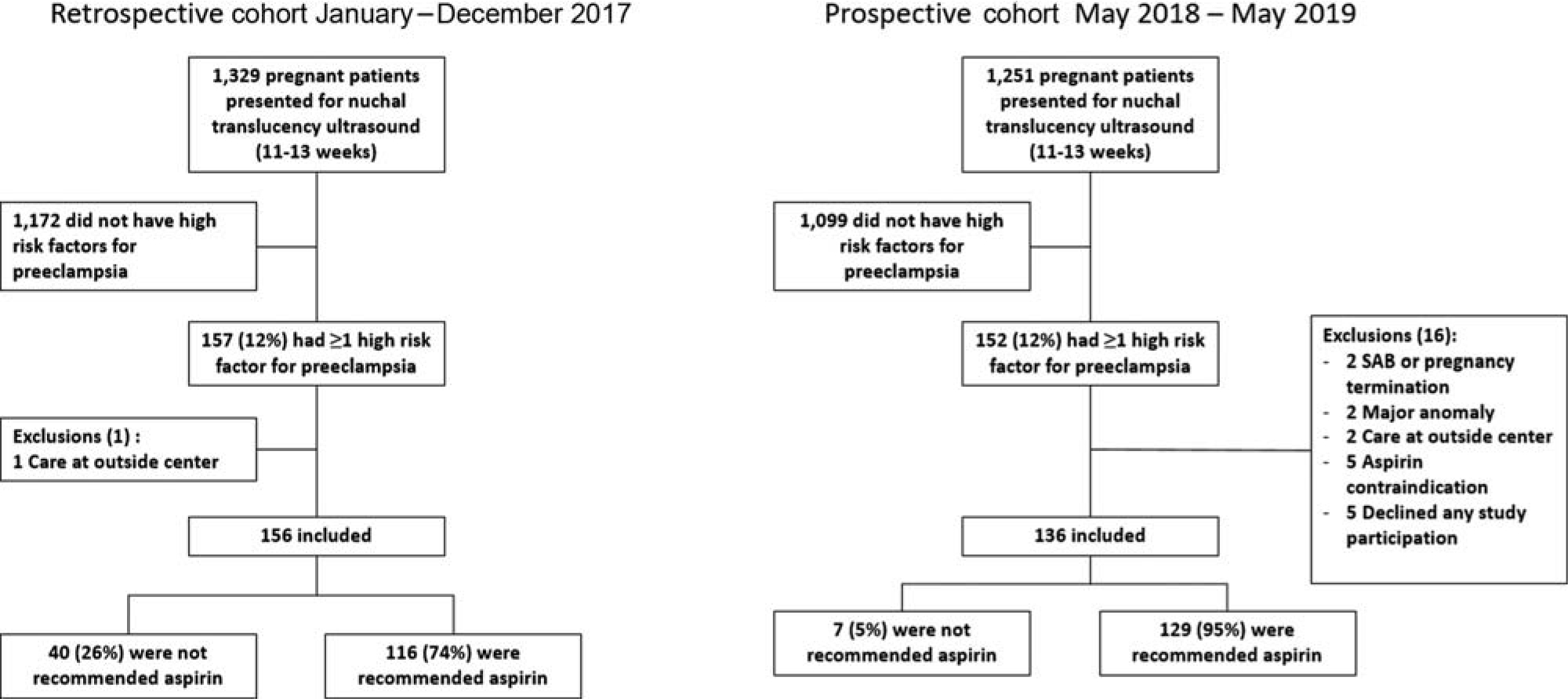
Cohort flow diagram.
Prescreening Tool (Retrospective) Cohort Outcomes During the prescreening tool cohort period, 1,329 patients presented for a first trimester NT ultrasound, 157 (12%) had at least one high risk factor for preeclampsia, one was excluded because she received her prenatal care at another institution (►Fig. 1). Of 156 remaining in retrospective cohort, 116 (74%) were recommended aspirin for prevention of preeclampsia and 40 (26%) were not. There appeared to be a trend of increasing provider recommendation with time, but there was no statistically significant correlation between annual quarter and rate of provider recommendation (Pear- son’s correlation coefficient = 0.109, p = 0.17, ►Fig. 2). In the retrospective cohort, of those with prior preeclampsia, 96% were recommended aspirin, compared with 64% of patients with other high risk factors but no history of preeclampsia (p < .001). Baseline demographics and risk factors between those who were and were not recommended aspirin therapy for preeclampsia prevention are summarized and compared in ►Table 1, using univariable analysis. Race was different with those recommended aspirin being 66 and 13% African American and Caucasian, respectively, while those not recommended aspirin were 47.5 and 30%, respectively (p = 0.04). Those not recommended aspirin were also more likely to be nulliparous (42.5 vs. 23.3%, p = 0.02). Among the preeclampsia risk factors associated with provider recommendation, history of any type of preeclampsia and chronic hypertension were significantly associated with provider recommendation of aspirin, while pregestational diabetes, chronic kidney disease, systemic lupus erythematosus or antiphospholipid antibody syndrome, and multifetal gestation were not. We conducted multivariable logistic regression with backward stepwise variable selection examining factors associated with provider recommendation for aspirin including, maternal age, BMI, insurance, parity, race, and preeclampsia risk factor ►Table 1. Only history of severe preeclampsia (OR = 29.02 [3.62–3,768.02]), history of mild preeclampsia (OR = 10.15 [2.94–53.79]), and chronic hyper- tension (OR = 3.48 [1.57–7.96]) were predictive of being prescribed aspirin for prevention of preeclampsia
Fig. 2.

Rate of provider recommendation of aspirin by quarter in retrospective prescreen and prospective postscreening tool cohorts.
Post screening Tool (Prospective) Cohort Outcomes
In the post screening tool cohort, 1,251 patients presented for NT ultrasound, and 136 were included (►Fig. 1). Pre- (n = 156) and post screen (n ¼ 136) cohorts were similar except race and multifetal gestation (►Table 2). Regarding the primary outcome, provider recommendation of aspirin improved from 74% prescreen to 95% post screen (p < 0.001), and aspirin recommendation for those high- risk women without prior preeclampsia also improved (64 vs. 92%, p < 0.001). Rates of aspirin recommendation also specifically improved in patients with a history of chronic hypertension (81 vs. 98%, p = 0.001) and diabetes (63 vs. 97%, p = 0.001) (►Fig. 3). After adjusting for baseline characteristics (►Table 2) using multivariable logistic regression with backward stepwise variable selection, post- screening tool cohort had an increased adjusted odds of aspirin recommendation (adjusted OR [aOR] = 8.2 [3.3– 20.5]). The rate of recommendation for aspirin by quarter ranged from 91 to 100% and there was no correlation between quarter and rate of aspirin recommendation in the post screen cohort (Pearson’s correlation coefficient, r = 0.068, p = 0.435).
Table 2.
Differences in baseline characteristics between retrospective prescreening tool and prospective postscreening tool implementation cohorts
| Prescreening tool (n ¼ 156) | Postscreening tool (n ¼ 136) | p-Value | |
|---|---|---|---|
| Baseline demographics | |||
| Race | 0.01a | ||
| African American | 95 (61) | 80 (59) | |
| Caucasian | 27 (17) | 43 (32) | |
| Asian | 11 (7) | 6 (4) | |
| Latino/Hispanic | 16 (10) | 5 (4) | |
| Other/unknown | 6 (4) | 2 (2) | |
| Maternal age (y) | 31.9 ± 5.9 | 31.7 ± 5.7 | 0.83 |
| Nulliparity | 44 (28) | 35 (25) | 0.72 |
| BMI (kg/m2) | 33.1 ± 8.4 | 32.7 ± 8.7 | 0.60 |
| Medicaid | 99 (64) | 85 (63) | 0.96 |
| Current smoker | 13 (8) | 15 (11) | 0.42 |
| Preeclampsia risk factor | |||
| Prior preeclampsia (any) | 51 (33) | 48 (35) | 0.64 |
| Prior severe preeclampsia | 19 (12) | 17 (13) | 0.93 |
| Chronic hypertension | 89 (57) | 65 (48) | 0.11 |
| Pregestational diabetes | 43 (28) | 29 (21) | 0.22 |
| Chronic kidney disease | 11 (7) | 3 (2) | 0.06 |
| Lupus | 5 (3) | 5 (3) | 0.83 |
| Antiphospholipid ab syndrome | 3 (2) | 0 (0) | 0.25 |
| Multifetal gestation | 9 (6) | 19 (14) | 0.02a |
Abbreviation: BMI, body mass index.
Note: Data presented as n (%) or mean ± standard deviation. No missing data.
Indicates significance, p < 0.05.
Fig. 3.

Rate of provider recommendation of aspirin for prevention of preeclampsia based on risk factor in pregnant women at high risk of preeclampsia. DM, diabetes mellitus. * indicates significant difference between pre and postscreening tool cohorts, p < 0.05 considered significant.
Survey Outcomes
Of the 136 women in the prospective cohort, 125 agreed to survey follow-up and 109 completed at least one survey. Regarding aspirin initiation, 88% of 109 respondents reported having initiated aspirin when surveyed at 16 to 20 weeks (mean gestational age at survey 17.9 ± 2.2 weeks). The most common reasons for not initiating aspirin were concerns about efficacy (31%) and not remembering to start (31%). At 24 to 28 weeks of follow-up, 91% of 104 respondents reported still taking aspirin. Of those who reported contin- ued aspirin use, 75% reported never or rarely missing a dose. At 34 to 37 weeks of follow-up, 87% of 87 respondents reported still taking aspirin.
Pregnancy Outcomes
Regarding delivery outcomes, in the prospective cohort, 124 had delivery data available and in the retrospective cohort, 139 had delivery data available. Patients without delivery data available had received prenatal care at our institution but ultimately delivered elsewhere. The overall rate of pre- eclampsia or gestational hypertension and adjusted risk were similar between pre- and post-screening cohorts (33 vs. 35%, aOR = 1.09 [0.65–1.83]) as was the rate of preterm preeclampsia/gestational hypertension (15 vs. 11%, aOR = 0.54 [0.24–1.21]). After adjusting for the baseline characteristics in ►Table 2, there was a significant reduction in overall preterm birth at <37 weeks (aOR = 0.50 [0.25– 0.99]), preterm birth at <34 weeks (aOR = 0.33 [0.13–0.88]), and indicated preterm birth at <37 weeks (aOR = 0.34 [0.15–1.76 ]) in the post screening tool cohort compared with the prescreening tool cohort (►Table 3). The indications for iatrogenic preterm birth are described in ►Table 4.
Table 3.
Comparison of pregnancy outcomes between pre- and postscreening tool implementation cohorts
| Prescreen (n ¼ 139) | Postscreen (n ¼ 134) | Odds ratio (95%CI) | Adjusted odds ratio (95% CI) | |
|---|---|---|---|---|
| Preeclampsia/Ghtn | 46 (33%) | 43 (35%) | 1.07 (0.66–1.79) | 1.10 (0.64–1.88) |
| Preeclampsia/Ghtn <37 wk | 21 (15%) | 13 (11%) | 0.66 (0.31–1.38) | 0.54 (0.24–1.21) |
| Preeclampsia/Ghtn <34 wk | 10 (7%) | 5 (4%) | 0.54 (0.18–1.63) | 0.41 (0.12–1.35) |
| Indicated PTB <37 wk | 29 (21%) | 13 (11%)a | 0.44 (0.21–0.90)a | 0.34 (0.15–0.76)a |
| Indicated PTB <34 wk | 13 (9%) | 5 (4%) | 0.41 (0.14–1.17) | 0.35 (0.11–1.10) |
| Overall PTB <37 wk | 39 (28%) | 27 (22%) | 0.71 (0.41–1.26) | 0.50 (0.25–0.99)a |
| Overall PTB <34 wk | 19 (14%) | 9 (7%) | 0.49 (0.22–1.14) | 0.33 (0.13–0.88)a |
Abbreviations: CI, confidence interval; Ghtn: gestational hypertension; PTB: preterm birth.
Note: The postscreen cohort 124 had delivery data available and in the prescreen cohort 139 had delivery data available. OR adjusted for baseline characteristics in ►Table 1 using multivariable logistic regression analysis with backward selection.
Indicates significance, p < 0.05.
Table 4.
Description of indicated preterm births in pre- and postscreening cohorts
| PTB indication | Prescreen (n ¼ 29) | Postscreen (n ¼ 13) | p-Value |
|---|---|---|---|
| Hypertensive disorder | 19 (66) | 9 (69) | 0.81 |
| Fetal growth restriction | 8 (28) | 3 (18) | 0.46 |
| Maternal diabetes | 0 (0) | 1 (8) | 0.31 |
| Multiple gestation | 2 (7) | 0 (4) | 0.33 |
Abbreviation: PTB: preterm birth.
Note: Data presented as n (%) and compared with Chi-square analysis or Fisher’s exact test as appropriate.
Combining the cohorts to examine predictors of pre- eclampsia or gestational hypertension overall, African American race remained the only significant predictor of preeclampsia/gestational hypertension. Predictors evaluated included baseline characteristics from ►Table 2 in addition to cohort and provider recommendation for aspirin using multivariable logistic regression with backward selection. Those who identified as black or African American had an almost two-fold increased risk of preeclampsia or gesta- tional hypertension (40 vs. 25%, aOR = 1.95 [1.14–3.36]) compared with non–African Americans.
Discussion
Principal Findings
This study is unique in its description of the implementation of ACOG and USPSTF guidelines for utilization of aspirin for prevention of preeclampsia, assessing both provider and patient barriers. Given the low risk, low cost, and simple medical history screen necessary for aspirin use in preeclampsia prevention, there should be few barriers to its use. However, we have identified approximately 25% of pregnant women with appropriate risk factors who were not told of the recommendation of aspirin for preeclampsia prevention. Furthermore, we have identified which pre- eclampsia risk factors qualifying for prophylaxis are often overlooked. It appears that providers have made a strong association between history of preeclampsia and history of hypertension with the need for aspirin prophylaxis, but in the setting of diabetes, or other high-risk factor without a history of preeclampsia, the recommendation for aspirin prophylaxis is more often neglected. The use of a simple screening tool to remind providers of these other risk factors, at the time that aspirin should be started, was an effective method to improve provider compliance with recommendations for aspirin use in preeclampsia prevention and may have contributed to improved patient out- comes (►Fig. 3).
Additional Findings
There are interesting findings regarding pregnancy outcome. It is important to note that comparison of pregnancy outcome was a secondary outcome and not primary. A cohort study, such as this, cannot establish causation. However, the pre- and post-screening cohorts were contemporaneous cohorts, we adjusted for any multiple potential confounders and practice management, aside from utilizing the screening tool, did not change. There was an initial provider education for the entire Department of Obstetrics and Gynecology of Thomas Jefferson University via e-mail and distribution of a guideline following ACOG and USPSTF initial recommendations in 2016; no additional provider education was conducted since then except in May 2018 to inform MFM physicians of the screening tool that they would be seeing on patients coming for first trimester scan. The improvement in outcome is congruent with another study in Texas looking only at patients with prior preeclampsia that identified improved outcome after USPSTF recommendations, although they did not evaluate actual aspirin prescription, use, or adherence.9 Our finding that African American race remained the only significant predictor of preeclampsia or gestational hypertension after evaluating all potential predictors of outcome including provider prescription of aspirin and cohort timing, certainly warrants further research into how race and aspirin efficacy may interact. This data on African American race was limited in the Texas study due to significantly different population demographics. Our results high- light the need for evaluating implementation of new guidelines and assessing contributors to variation in response, whether it is provider compliance, patient adherence, genetics, race, or comorbidities. In this case, using a simple tool to aid in provider compliance significantly improved provider adherence to recommended guidelines, and may have contributed to improved patient outcomes.
A unique aspect of this study is the prospective patient survey to assess for common patient barriers to aspirin use. For those that continue aspirin, reported adherence is good; however, our survey result highlights areas that providers may focus on preemptively to address common barriers, including more detailed conversation and/or supplemental patient focused educational material explaining the recom- mendation for aspirin and techniques to assist in taking a pill every day (pill box, timer, etc).
Clinical Implications
A recent expert commentary highlighted the historically poor rate of provider follow through with risk-based screening.8 Research should not end with positive randomized trials demonstrating clinical benefit. Our study underscores the importance of evaluating the implementation of evidence- based guidelines to ensure patients are actually benefiting from the research conducted. Historically, study of implementation of guidelines has identified a significant percentage of providers who do not adhere to recommended practices.6,8 Specifically in OBs, studies have evaluated risk-based screening for gestational diabetes,11 utilization of progesterone for preterm birth prevention,12–14 and cervical length screening.15–17 These studies have prompted a change in clinical practice to improve adherence (i.e., opt out instead of opt in, as with cervical length screening),15,18 and removing barriers to progesterone access.14 This study should prompt individual institutions to evaluate their own practice, identify which patients are being missed, and identify solutions to improve implementation of this important intervention. A paper screening tool worked the best within our ultrasound office work flow; however, electronic medical record systems, such as Epic, are poised to incorporate these screening forms as part of prenatal medical record documentation. Given this success, the next step would be to incorporate moderate risk factors as well. Such an electronic tool could be implemented at the initial OB visit.
Strengths and Limitations
Our study has several strengths. There is a paucity of data regarding the implementation of this recent guideline, and thus it is unclear how many patients are truly benefitting from this change in practice guideline. One recent study9 demonstrated reduced rate of preeclampsia in pre- or post-guideline cohort comparison at a specific institution. In contrast to our study, that study did not evaluate the rate of provider adherence to recommended screening/treatment and only examined the cohort of patients with a history of preeclampsia.9 We have noted that the high-risk subgroup of women with a history of preeclampsia were most likely to receive appropriate counseling while other high-risk sub-groups may be neglected. Another strength is that each chart was manually reviewed for accuracy thus our results are not limited by the accuracy of diagnosis codes, as in other population-based studies. We were able to identify not only the rate of provider compliance with the new guidelines but also which patients were likely to be missed. We were able to demonstrate implementation of a simple screening tool that was associated not just with a significant improvement in provider compliance with guidelines but possibly also with improved pregnancy outcomes. Finally, we were able to prospectively evaluate patient-related barriers to adherence.
Our study is not without limitations. This is a single- institution study at an urban academic center. The number of patients in subgroups, for example, lupus and multifetal gestation, were limited. On chart review, we were able to identify documentation of discussion of aspirin use for preeclampsia prevention, and in the prospective cohort, we surveyed patient-reported adherence; however, we did not objectively quantify patient adherence throughout pregnancy. Adherence to aspirin is an important factor in its efficacy in prevention of preeclampsia.19 We focused on patients presenting in the first trimester because early initiation of aspirin (<16 weeks) is encouraged; thus we do not have data on the number of high-risk patients presenting late to care who miss this window of intervention. Additionally, our assessment of adherence was solely by patient self-report without any objective verification. By nature of the study, we were able to collect more information prospectively than retrospectively, including contraindications to aspirin expressed verbally that were not specifically documented in record. Our retrospective study comparison is limited in this way, as it was exclusively chart review. As this is a cohort study, we can only describe an association with outcomes and not causation; this limitation applies to assessment of both provider recommendation of aspirin, as well as any conclusions, regarding pregnancy outcomes.
Conclusion
Our results demonstrate that almost one quarter of high-risk patients may not be getting appropriate counseling regarding use of aspirin in prevention of preeclampsia. Particularly at risk are those without a history of prior preeclampsia or hypertension. The ACOG and USPSTF have outlined seven key high-risk factors that should always prompt the recommendation for aspirin but most may be missed by clinical providers.3 Use of a universal screening tool in the first trimester, either with use of electronic medical record prompts or otherwise, should be considered to maximize OB providers’ ability to offer the best care. Our results are from an urban tertiary care academic medical center; com- munity health centers and areas with limited OB access may be at even higher risk of not executing these guidelines. Prevention of hypertensive disorders of pregnancy reduces a key source of both maternal and neonatal morbidity and mortality. An abundance of data support the benefit of aspirin in prevention of preterm preeclampsia,2,20 now it is our duty to ensure that we are implementing the results of these trials.
Supplementary Material
Key Points:
Despite recommendations, aspirin use for preeclampsia prevention is suboptimal.
High-risk patients who lack a history preeclampsia were less likely to be advised of aspirin use.
A simple universal screening tool can significantly improve aspirin utilization.
Acknowledgments
Funding
This study was funded by March of Dimes Foundation, Community Grant 2018 (PI: R.C.B.), U.S. Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences, grant no.: T32GM008562; and Pharmaceutical Research and Manufacturers of America Foundation, Faculty Development Award.
Conflict of Interest
R.C.B. reports grants from March of Dimes Community Grant, grants from NIH (grant no.: T32GM008562), grants from PhRMA Foundation Faculty Development Award, during the conduct of the study. The other authors report no conflict of interest.
References
- 1.Wong AE, Grobman WA. Medically indicated–iatrogenic prematurity. Clin Perinatol 2011;38(03):423–439 [DOI] [PubMed] [Google Scholar]
- 2.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377(07):613–622. Doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 3.ACOG Practice Advisory on Low-Dose Aspirin and Prevention of Preeclampsia: Updated Recommendations. Available at: http://www.losolivos-obgyn.com/info/md/acog/Low-dose%20aspirin,%20ACOG%20Practice%20Advisory%202016.pdf.Accessed December 28, 2017
- 4.US Preventive Services Task Force Final recommendation statement: low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication.Accessed December 28, 2017
- 5.ACOG Practice advisory on low dose aspirin and prevention of preeclampsia: updated recommendations. Available at: http://www.losolivos-obgyn.com/info/md/acog/Low-dose%20aspirin,%20ACOG%20Practice%20Advisory%202016.pdf.Accessed September 22, 2020
- 6.Neta G, Brownson RC, Chambers DA. Opportunities for epidemiologists in implementation science: a primer. Am J Epidemiol 2018;187(05):899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hull L, Athanasiou T, Russ S. Implementation science. Ann Surg 2017;265(06):1104–1112 [DOI] [PubMed] [Google Scholar]
- 8.Ayala NK, Rouse DJ. A nudge toward universal aspirin for preeclampsia prevention. Obstet Gynecol 2019;133(04): 725–728 [DOI] [PubMed] [Google Scholar]
- 9.Tolcher MC, Chu DM, Hollier LM, et al. Impact of USPSTF recommendations for aspirin for prevention of recurrent preeclampsia. Am J Obstet Gynecol 2017;217(03):365.e1–365.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banala C, Moreno S, Cruz Y, et al. Impact of the ACOG guideline regarding low-dose aspirin for prevention of superimposed pre- eclampsia in women with chronic hypertension. Am J Obstet Gynecol 2020;223(03):419.e1–419.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson M, Winkvist A, Mogren I. Surprisingly low compliance to local guidelines for risk factor based screening for gestational diabetes mellitus - a population-based study. BMC Pregnancy Childbirth 2009;9(01):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boelig RC, Jiang E, Scheidemantle B, Villani M, Berghella V. Utilization of progesterone and cervical length screening for prevention of recurrent preterm birth. J Matern Fetal Neonatal Med 2018;32(24):4146–4153 [DOI] [PubMed] [Google Scholar]
- 13.Orsulak MK, Block-Abraham D, Gee RE. 17α-hydroxyprogesterone caproate access in the Louisiana Medicaid population. Clin Ther 2015;37(04):727–732 [DOI] [PubMed] [Google Scholar]
- 14.Iams JD, Applegate MS, Marcotte MP, et al. A statewide progestogen promotion program in Ohio. Obstet Gynecol 2017;129(02): 337–346 [DOI] [PubMed] [Google Scholar]
- 15.Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol 2016;214(04):523.e1–523.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orzechowski KM, Nicholas SS, Baxter JK, Weiner S, Berghella V. Implementation of a universal cervical length screening program for the prevention of preterm birth. Am J Perinatol 2014;31(12): 1057–1062 [DOI] [PubMed] [Google Scholar]
- 17.Son M, Grobman WA, Ayala NK, Miller ES. A universal mid- trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol 2016;214(03):365.e1–365.e5 [DOI] [PubMed] [Google Scholar]
- 18.Orzechowski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol 2014;124(03):520–525 [DOI] [PubMed] [Google Scholar]
- 19.Wright D, Poon LC, Rolnik DL, et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol 2017;217(06):685.e1–685.e5 [DOI] [PubMed] [Google Scholar]
- 20.Roberge S, Bujold E, Nicolaides KH. Meta-analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol 2018;218(05): 483–489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


