Summary
Background:
Literature on cancer in adolescents and young adults living with HIV (AYALWH) is scarce. We studied cancer incidence in AYALWH in South Africa between 2004-2014.
Methods:
We included individuals aged 15-24 years from the South African HIV Cancer Match study, a large cohort resulting from a linkage between HIV-related laboratory measurements from the National Health Laboratory Services and cancer records from the National Cancer Registry. We computed incidence rates for the most common cancers. We assessed associations between these cancers and sex, age, calendar year, and CD4 cell count using Cox proportional hazards models and adjusted hazard ratios (aHR).
Findings:
We included 782,454 AYALWH (89% female) with 1,428,114 person-years of follow-up. Of those, 867 developed incident cancer (incidence rate: 61/100,000 person-years), including 429 who developed Kaposi sarcoma (30/100,000 person-years), 107 non-Hodgkin lymphoma (7.5/100,000 person-years), 48 Hodgkin lymphoma (3.4/100,000 person-years), 45 cervical cancer (3.4/100,000 woman-years), and 32 leukaemia (2.2/100,000 person-years). Kaposi sarcoma was more common in the 20-24 year age group than the 15-19 year age group (aHR 1.39, 95% CI 1.03-1.86). Male sex was associated with higher rates of Kaposi sarcoma (aHR 2.06, 95% CI 1.61-2.63), non-Hodgkin lymphoma (aHR 3.17, 95% CI 2.06-4.89), Hodgkin lymphoma (aHR 4.83, 95% 2.61-8.93), and leukaemia (unadjusted hazard ratio 5.90, 95% CI 2.87-12.1). Cancer rates decreased over the study period, driven by declining Kaposi sarcoma rates. Lower baseline CD4 cell counts were associated with higher rates of Kaposi sarcoma, cervical cancer, non-Hodgkin and Hodgkin lymphoma, but not leukaemia.
Interpretation:
Infection-related cancers were the most common cancer types among AYALWH in South Africa, and their incidence rates increased with lower CD4 cell counts. Therefore, innovative strategies to maintaining high CD4 cell counts are needed to reduce the cancer burden in this vulnerable population.
Funding:
US National Institutes of Health and Swiss National Science Foundation.
Introduction
Adolescents and young adults (AYA) in South Africa are disproportionally affected by HIV. In 2019, one in three new HIV infections in South Africa occurred among people aged 15-24 years.1 Girls and young women are particularly vulnerable, accounting for nearly 80% of new HIV infections in this age group.1 Compared to other age groups, adolescents living with HIV are less likely to initiate antiretroviral therapy (ART) and more likely to drop out of care and have poorly controlled HIV infection with higher rates of virologic failure.2,3
HIV-induced immunodeficiency combined with a higher susceptibility to oncogenic viruses increases the risk of cancer among people living with HIV.4 Cancer is rare among AYA, but an elevated cancer risk has been documented among those living with HIV, with the AIDS-defining cancers, Kaposi sarcoma and non-Hodgkin lymphoma, being the most frequently diagnosed cancers.5 However, analyses of cancer incidence and associated risk factors in this age group are often limited by small numbers of cancer cases.5 Furthermore, data from sub-Saharan Africa, where most young people living with HIV reside, are scarce.
The South African HIV Cancer Match (SAM) study is a nationwide cohort of people living with HIV in South Africa. We linked HIV-related laboratory records from the National Health Laboratory Services (NHLS) to cancer records from the National Cancer Registry (NCR) to create this cohort.6 The SAM study provided us with a unique data source to assess the incidence and spectrum of cancers among young people living with HIV in South Africa.
Methods
Study design and population
As described in detail elsewhere,6 the SAM study was constructed using data from the NHLS, the largest diagnostic pathology service in South Africa, and the pathology-based NCR for the years 2004 to 2014. The NHLS serves the public sector through laboratories in all nine South African provinces and stores data at a corporate data warehouse. The NHLS is estimated to provide diagnostic pathology services to approximately 80% of the South African population. The 1986 established NCR and collects data on histologically or cytologically confirmed cancer cases from public and private laboratories across South Africa.7 Initially, the NCR relied on laboratories voluntarily sending pathology reports of confirmed cancer cases, but in 2011, the South African government introduced legislation to make cancer a notifiable disease. We used privacy-preserving probabilistic record linkage methods to, first, identify HIV-related laboratory records which most likely belonged to the same individual and, second, link these indiviudals to cancer diagnoses from the NCR.8 HIV-related laboratory measurements included HIV tests, CD4 cell counts and percentages, and HIV RNA viral loads. Ethical approval was obtained from the Human Research Ethics Committee of the University of the Witwatersrand (M190594), Johannesburg, South Africa, and the Cantonal Ethics committee (2016–00589) in Bern, Switzerland. No written informed consent was obtained for this secondary analysis of routinely collected data.
Inclusion criteria and definitions
We included individuals aged between 15 and 24 years at baseline who had at least two HIV-related laboratory measurements on separate days between the 1st January 2004 and the 31st December 2014. We defined an individual’s baseline date as the date of their first positive HIV test or HIV-related measurement (CD4 cell count or HIV RNA viral load). We excluded individuals with missing information on sex or age. AYA diagnosed with cancer before their baseline date were excluded from the analysis of that specific cancer.
We classified cancers based on the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), recoded for AYA.9 We categorised cancers into the following groups10: leukaemia (AYA recode 01-04), non-Hodgkin lymphoma (05), Hodgkin lymphoma (06), central nervous system (CNS) and other intracranial and intraspinal neoplasms (07-16), osseous & chondromatous neoplasms (17-20), Kaposi sarcoma (24), soft tissue sarcomas other than Kaposi sarcoma (21-23,25), germ cell and trophoblastic neoplasms (26-28), melanoma and skin carcinomas (29, 30), carcinomas (31-48), and miscellaneous specified and unspecified neoplasms and unclassified cancers (49-56, 99). We further divided the non-Hodgkin lymphomas into their most common subtypes: Burkitt lymphomas (ICD-0-3 morphology code 9687) and diffuse large B-cell lymphomas (ICD-0-3 morphology code 9680 or 9684), with a separate category for other non-Hodgkin lymphomas. We distinguished between the following carcinomas based on the AYA recode10: thyroid carcinoma, other carcinomas of the head and neck, carcinoma of the genitourinary tract other than cervix, carcinoma of the cervix (ICD-O-3 topography code C53), carcinoma of the gastrointestinal tract, and carcinoma of other and ill-defined sites.
Statistical analysis
We produced descriptive statistics of AYA living with HIV (AYALWH), stratified by baseline age (15 to 19 years versus 20 to 24 years), stratified by sex, and overall. Calendar year, CD4 cell count (cells/μL), and HIV RNA viral load (copies/ml) were assessed at baseline. CD4 cell count and HIV RNA viral load were labelled as missing if no corresponding measurement was available for a given individual within the first two weeks of the baseline date. We used two-sided corrected tests to compare the proportion of individuals who developed a given type of cancer between ages 15 to 19 years to the proportion of individuals who developed that type of cancer from ages 20 to 24 years, among AYALWH who developed cancer of any type.
Individuals contributed person-years starting from their baseline date to six months after their last HIV-related laboratory measurement, their 25th birthday, database closure (1st January 2015), or the first diagnosis date of the cancer(s) under consideration, whichever came first. We allowed for a time window of six months after the last HIV-related laboratory measurement to capture cancer diagnoses occurring between regular HIV monitoring visits. We performed survival analyses for the most common cancer groups: cancer of any type, Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma, Hodgkin lymphoma, and leukaemia. We computed crude incidence rates for these cancers by dividing the number of cases by the number of person-years at risk. The rates were computed for ages 15 to 19 years, 20 to 24 years, and overall, treating age as a time-updated variable. We produced 95% confidence intervals (CI) for these rates assuming a Poisson distribution.
We calculated unadjusted and adjusted hazard ratios (aHR) using multivariable Cox proportional hazards models, with the origin set to the time of the baseline test for each individual. These models were adjusted for age (time-updated categorical variable: 15-19 years, 20-24 years), sex, baseline calendar period (categorical variable: 2004-2007, 2008-2011, 2012-2014), and baseline CD4 cell count (categorical variable: <200 cells/μL, 200-499 cells/μL, ≥500 cells/μL, missing). We assessed the proportional hazards assumption using Schoenfeld residuals. The proportional hazards assumption for the CD4 cell count was violated when analysing the 'any cancer' category. We, therefore, stratified the corresponding Cox model for that variable and used the Nelson-Aalen method to estimate cumulative hazard functions with 95% CIs for each CD4 cell count group. For cervical cancer, the proportional hazards assumption was violated for age group, and we stratified the corresponding Cox model with respect to that variable. Due to the limited number of cases, we only present unadjusted hazard ratios for leukaemia. In a separate analysis, we modelled the relationship between baseline CD4 cell count (treated as a continuous variable) and the log-hazard using a penalised spline transformation. We excluded individuals with missing baseline CD4 cell count from this analysis. We produced aHR curves with 95% CIs over a grid of CD4 values, with a reference CD4 cell count of 200 cells/μL. In a similar analysis, we used penalized splines to model the relationship between calendar period (as a continuous, time-updated variable) and the log-hazard. We produced aHR curves with 95% CIs from 2004 to 2014, with a reference calendar year of 2009. All analyses were done in R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Role of funding source
The funders of this study had no role in the design, data collection, data analysis, data interpretation, or writing of the report.
Results
Study population and cancer cases
We included 782,454 AYALWH over a study period of 11 years (appendix p.1): 176,019 were diagnosed with HIV between the ages of 15 to 19 years and 606,435 between the ages of 20 to 24 years. The majority (89.2%; n = 698,066) of the included AYALWH were women (Table 1). The median time between the first and second HIV-related laboratory measurement was 230 days (interquartile range [IQR] 99-533). The median CD4 cell count at baseline was 386 cells/μL (IQR 253-548) in the 15-19 age group and 349 cells/μL (IQR 216-511) in the 20-24 age group.
Table 1:
Baseline characteristics of adolescents and young adults living with HIV and included in the analysis, stratified by age.
| Age at baseline | |||
|---|---|---|---|
| 15-19 years | 20-24 years | Overall | |
| N = 176,019 | N = 606,435 | N = 782,454 | |
| Sex | |||
| Female | 160,937 (91.4) | 537,129 (88.6) | 698,066 (89.2) |
| Male | 15,082 (8.6) | 69,306 (11.4) | 84,388 (10.8) |
| Calendar period | |||
| 2004 – 2007 | 31,916 (18.1) | 136,298 (22.5) | 168,214 (21.5) |
| 2008 – 2011 | 90,375 (51.3) | 307,037 (50.6) | 397,412 (50.8) |
| 2012 – 2014 | 53,728 (30.5) | 163,100 (26.9) | 216,828 (27.7) |
| Median baseline CD4 cell count [IQR] [cells/μL] | 386 [253-548] | 349 [216-511] | 357 [224-520] |
| Baseline CD4 cell count [cells/μL] | |||
| <200 | 26,392 (15.0) | 121,553 (20.0) | 147,945 (18.9) |
| 200-499 | 82,462 (46.8) | 282,455 (46.6) | 364,917 (46.6) |
| ≥500 | 49,690 (28.2) | 144,968 (23.9) | 194,658 (24.9) |
| Missing | 17,475 (9.9) | 57,459 (9.5) | 74,934 (9.6) |
| Baseline HIV RNA viral load [copies/ml] | |||
| <1,000 | 8,579 (4.9) | 32,453 (5.4) | 41,032 (5.2) |
| ≥1,000 | 10,496 (6.0) | 34,333 (5.7) | 44,829 (5.7) |
| Missing | 156,944 (89.2) | 539,649 (89.0) | 696,593 (89.0) |
Results are presented as counts and percentages if not otherwise stated.
Over 1,428,114 person-years, 867 AYALWH developed incident cancer between the age of 15 to 24 years, of which 700 (81%) were female (Table 2). The median follow-up time was 1.42 years (IQR 0.70-2.61). The most common cancer types were Kaposi sarcoma (429 cases) and non-Hodgkin lymphoma (107 cases), followed by Hodgkin lymphoma (48 cases), cervical cancer (45 cases), and leukaemia (32 cases). Among AYALWH who developed cancer, the proportion of individuals with a non-cervical carcinoma was higher in the 20-24 age group than in the 15-19 age group (13% vs. 5%, p=0.031).
Table 2:
Incident cancer cases in adolescents and young adults living with HIV, stratified by age at cancer diagnosis or sex.
| Age at cancer diagnosis | Sex | ||||
|---|---|---|---|---|---|
| 15-19 years | 20-24 years | Female | Male | Overall | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Cancer type | |||||
| Any cancer | 112 (100%) | 755 (100%) | 700 (100%) | 167 (100%) | 867 (100%) |
| Kaposi sarcoma (KS) | 52 (46%) | 377 (50%) | 347 (50%) | 82 (49%) | 429 (49%) |
| Non-Hodgkin lymphoma (NHL) | 18 (16%) | 89 (12%) | 78 (11%) | 29 (17%) | 107 (12%) |
| Burkitt lymphoma | 2 | 25 | 16 | 11 | 27 |
| Diffuse large B-cell lymphoma | 9 | 33 | 32 | 10 | 42 |
| Other NHL | 8 | 32 | 32 | 8 | 40 |
| Cervical cancer | 3 (3%) | 42 (6%) | 45 (6%) | 0 (0%) | 45 (5%) |
| Leukaemia | 5 (4%) | 27 (4%) | 20 (3%) | 12 (7%) | 32 (4%) |
| Hodgkin lymphoma | 10 (9%) | 38 (5%) | 32 (5%) | 16 (10%) | 48 (6%) |
| CNS and other intracranial and intraspinal neoplasms | 0 (0%) | 2 (<1%) | 1 (<1%) | 1 (1%) | 2(<1%) |
| Osseous and chondromatous neoplasms | 4 (4%) | 10 (1%) | 10 (1%) | 4 (2%) | 14 (2%) |
| Soft tissue sarcomas other than KS | 6 (5%) | 17 (2%) | 18 (3%) | 5 (3%) | 23 (3%) |
| Germ cell and trophoblastic neoplasms | 5 (4%) | 14 (2%) | 17 (2%) | 2 (1%) | 19 (2%) |
| Melanoma and skin carcinoma | 2 (2%) | 28 (4%) | 26 (4%) | 4 (2%) | 30 (4%) |
| Melanoma | 1 | 4 | 4 | 1 | 5 |
| Skin carcinoma | 1 | 24 | 22 | 3 | 25 |
| Carcinoma excluding cervical cancer | 6 (5%) | 98 (13%) | 93 (13%) | 11 (7%) | 104 (12%) |
| Thyroid cancer | 0 | 2 | 2 | 0 | 2 |
| Other head and neck cancer | 0 | 9 | 8 | 1 | 9 |
| Breast cancer | 1 | 12 | 13 | 0 | 13 |
| Genitourinary cancer other than cervix | 3 | 25 | 26 | 2 | 28 |
| Gastrointestinal carcinoma | 1 | 22 | 20 | 3 | 23 |
| Any other carcinoma | 1 | 28 | 24 | 5 | 29 |
| Miscellaneous specified and unspecified neoplasms | 3 (3%) | 20 (3%) | 19 (3%) | 4 (2%) | 23 (3%) |
The percentages represent the proportion of individuals who developed the specific type of cancer among those who developed any cancer, within each age group or for each sex.
The characteristics of AYALWH who developed cancer and those who remained free of cancer are shown in Table 3. Among AYALWH who remained free of cancer, only 11% were male, but >30% of those with Hodgkin lymphoma or leukaemia were male. AYALWH who developed Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma or Hodgkin lymphoma had median baseline CD4 cell counts <300 cells/μL. Median baseline CD4 cell counts were higher in AYALWH who developed leukaemia (385 cells/μL, IQR 208-806) than those who remained free of cancer (357 cells/μL, IQR 224-520).
Table 3:
Baseline characteristics of adolescents and young adults living with HIV who developed cancer or remained free of cancer.
| Any cancer | Kaposi sarcoma | Cervical cancer | Non-Hodgkin lymphoma |
Hodgkin lymphoma |
Leukaemia | Free of cancer | |
|---|---|---|---|---|---|---|---|
| N=867 | N=429 | N=45 | N=107 | N=48 | N=32 | N=781,587 | |
| Age | |||||||
| 15-19 years | 231 (26.6) | 108 (25.2) | 13 (28.9) | 25 (23.4) | 15 (31.2) | 9 (28.1) | 175,788 (22.5) |
| 20-24 years | 636 (73.4) | 321 (74.8) | 32 (71.1) | 82 (76.6) | 33 (68.8) | 23 (71.9) | 605,799 (77.5) |
| Sex | |||||||
| Female | 700 (80.7) | 347 (80.9) | 45 (100.0) | 78 (72.9) | 32 (66.7) | 20 (62.5) | 697,366 (89.2) |
| Male | 167 (19.3) | 82 (19.1) | 0 | 29 (27.1) | 16 (33.3) | 12 (37.5) | 84,221 (10.8) |
| Calendar period | |||||||
| 2004 - 2007 | 282 (32.5) | 152 (35.4) | 13 (28.9) | 32 (29.9) | 18 (37.5) | 11 (34.4) | 167,932 (21.5) |
| 2008 - 2011 | 423 (48.8) | 206 (48.0) | 27 (60.0) | 49 (45.8) | 20 (41.7) | 17 (53.1) | 396,989 (50.8) |
| 2012 - 2014 | 162 (18.7) | 71 (16.6) | 5 (11.1) | 26 (24.3) | 10 (20.8) | 4 (12.5) | 216,666 (27.7) |
| Median CD4 cell count [IQR] [cells/μL] | 248 [121, 399] | 207 [84, 350] | 261 [168, 361] | 240 [139, 345] | 267 [147, 447] | 385 [208, 806] | 357 [224, 520] |
| CD4 cell count [cells/μL] | |||||||
| <200 | 293 (33.8) | 179 (41.7) | 15 (33.3) | 37 (34.6) | 15 (31.2) | 6 (18.8) | 147,652 (18.9) |
| 200-499 | 338 (39.0) | 158 (36.8) | 19 (42.2) | 42 (39.3) | 17 (35.4) | 11 (34.4) | 364,579 (46.6) |
| ≥500 | 127 (14.6) | 42 (9.8) | 5 (11.1) | 14 (13.1) | 10 (20.8) | 11 (34.4) | 194,531 (24.9) |
| Missing | 109 (12.6) | 50 (11.7) | 6 (13.3) | 14 (13.1) | 6 (12.5) | 4 (12.5) | 74,825 (9.6) |
| HIV RNA viral load [copies/ml] | |||||||
| <1,000 | 44 (5.1) | 15 (3.5) | 3 (6.7) | 8 (7.5) | 3 (6.2) | 3 (9.4) | 40,988 (5.2) |
| ≥1,000 | 76 (8.8) | 47 (11.0) | 3 (6.7) | 11 (10.3) | 3 (6.2) | 2 (6.2) | 44,753 (5.7) |
| Missing | 747 (86.2) | 367 (85.5) | 39 (86.7) | 88 (82.2) | 42 (87.5) | 27 (84.4) | 695,846 (89.0) |
Results are presented as counts and percentages if not otherwise stated.
Cancer incidence rates and risk factors
The overall cancer incidence rate among AYALWH aged 15-24 years was 60.7/100,000 person-years, specifically 53.6/100,000 person-years in female and 137.9/100,000 person-years in male AYALWH (Table 4). Table 5 shows adjusted hazard ratios for the risk of developing various cancer types (for results from unadjusted analyses see appendix p.2). The risk of developing any cancer was higher in 20-24-year-olds than 15-19-year-olds (aHR 1.32, 95% CI 1.08-1.61). Similarly, the risk of Kaposi sarcoma was higher in 20-24-year-olds than 15-19-year-olds (aHR 1.39, 95% CI 1.03-1.86). Because the proportional hazards assumption was violated we did not compute a hazard ratio for the association of age group with cervical cancer risk. There was no evidence of an association between age group and the incidence rates of the other types of cancers among AYALWH. The risk of developing cancer decreased in more recent baseline calendar years, mostly driven by a decrease in the risk of Kaposi sarcoma. This was confirmed in a separate analysis of the association between calendar year (time-updated) and cancer incidence (appendix p. 3). Male sex was associated with a higher risk of being diagnosed with any cancer (aHR 2.16, 95% CI 1.82-2.56), Kaposi sarcoma (aHR 2.06, 95% CI 1.61-2.63), non-Hodgkin lymphoma (aHR 3.17, 95% CI 2.06-4.89), Hodgkin lymphoma (aHR 4.83, 95% CI 2.61-8.93), and leukaemia (unadjusted hazard ratio 5.90, 95% CI 2.87-12.1).
Table 4:
Incidence rates per 100,000 person-years (95% confidence interval) for various types of cancer, stratified by current age group (time-updated) or sex.
| Age group | Sex | ||||
|---|---|---|---|---|---|
| 15-19 years | 20-24 years | Female | Male | Overall | |
| Any cancer | 50.9 (41.9-61.3) | 62.5 (58.1-67.1) | 53.6 (49.7-57.7) | 137.9 (117.8-160.5) | 60.7 (56.7-64.9) |
| Kaposi sarcoma | 23.6 (17.6-31) | 31.2 (28.1-34.5) | 26.5 (23.8-29.5) | 67.6 (53.8-83.9) | 30 (27.2-33) |
| Cervical cancer | 1.5 (0.3-4.4) | 3.8 (2.7-5.1) | 3.4 (2.5-4.6) | 0 | 3.4 (2.5-4.6) |
| Non-Hodgkin lymphoma | 8.2 (4.8-12.9) | 7.4 (5.9-9.1) | 6.0 (4.7-7.4) | 23.9 (16-34.3) | 7.5 (6.1-9) |
| Hodgkin lymphoma | 4.5 (2.2-8.4) | 3.1 (2.2-4.3) | 2.4 (1.7-3.5) | 13.2 (7.5-21.4) | 3.4 (2.5-4.5) |
| Leukaemia | 2.3 (0.7-5.3) | 2.2 (1.5-3.2) | 1.5 (0.9-2.4) | 9.9 (5.1-17.3) | 2.2 (1.5-3.2) |
Table 5:
Adjusted hazard ratios for developing different cancer types among adolescents and young adults living with HIV.
| Hazard ratios* (95% confidence intervals) | ||||||
|---|---|---|---|---|---|---|
| Any cancer | Kaposi sarcoma | Cervical cancer | Non-Hodgkin lymphoma |
Hodgkin lymphoma |
Leukaemia | |
| Age [years] | ||||||
| 15-19 | 1 | 1 | † | 1 | 1 | 1 |
| 20-24 | 1.32 (1.08-1.61) | 1.39 (1.03-1.86) | 1.05 (0.63-1.76) | 0.72 (0.35-1.47) | 1.19 (0.46-3.12) | |
| Sex | ||||||
| Female | 1 | 1 | - | 1 | 1 | 1 |
| Male | 2.16 (1.82-2.56) | 2.06 (1.61-2.63) | - | 3.17 (2.06-4.89) | 4.83 (2.61-8.93) | 5.90 (2.87-12.1) |
| Calendar period | ||||||
| 2004 – 2007 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2008 – 2011 | 0.71 (0.60-0.83) | 0.61 (0.49-0.76) | 1.25 (0.61-2.57) | 0.69 (0.43-1.09) | 0.56 (0.28-1.11) | 0.66 (0.31-1.41) |
| 2012 – 2014 | 0.69 (0.57-0.85) | 0.54 (0.41-0.73) | 0.72 (0.24-2.15) | 0.83 (0.48-1.42) | 0.61 (0.26-1.40) | 0.38 (0.12-1.20) |
| CD4 cell count [cells/μL] | ||||||
| <200 | 1 | 1 | 1 | 1 | 1 | |
| 200-499 | † | 0.34 (0.28-0.42) | 0.44 (0.22-0.86) | 0.46 (0.29-0.72) | 0.47 (0.23-0.95) | 0.67 (0.25-1.81) |
| ≥500 | 0.17 (0.12-0.24) | 0.21 (0.07-0.57) | 0.30 (0.16-0.55) | 0.53 (0.24-1.20) | 1.23 (0.81-3.33) | |
| Missing | 0.42 (0.31-0.58) | 0.59 (0.22-1.58) | 0.66 (0.35-1.24) | 0.58 (0.22-1.54) | 1.19 (0.33-4.21) | |
Adjusted for age, sex, baseline calendar period, and baseline CD4 cell count in the analysis of any cancer, Kaposi sarcoma, non-Hodgkin lymphoma, and Hodgkin lymphoma. Adjusted for age, baseline calendar period and baseline CD4 cell count in the analysis of cervical cancer. No adjustment was made in the analysis of leukaemia. Age was treated as a time-updated variable in all models.
To preserve proportional hazards, we stratified the model for any cancer with respect to CD4 cell count and the model for cervical cancer with respect to age.
The risk of developing cervical cancer decreased with increasing baseline CD4 cell count (≥500 versus <200 cells/μL, aHR 0.21, 95% CI 0.07-0.57). A similar pattern of decreasing cancer risk with higher baseline CD4 cell counts was observed for non-Hodgkin lymphoma (≥500 versus <200 cells/μL, aHR 0.30, 95% CI 0.16-0.55). Individuals with a missing CD4 cell count had a lower risk of developing Kaposi sarcoma compared to individuals with a CD4 cell count below 200 cells/μL, but a higher risk than individuals with a CD4 cell count above 500 cells/μL. In appendix p. 4 we show the cumulative hazards for developing any cancer, stratified by CD4 cell count category. The cumulative hazard was consistently highest in the group with 0-199 CD4 cells/μL and lowest in those with ≥500 CD4 cells/μL. In Figure 1, we show aHRs for the incidence of various types of cancers, comparing baseline CD4 cell count (treated as a continuous variable) to a reference value of 200 cells/μ. We excluded 74,934 AYALWH from this analysis due to missing CD4 cell counts at baseline. A lower baseline CD4 cell count was associated with higher rates of any cancer, Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma, and Hodgkin lymphoma, with the association being strongest for Kaposi sarcoma. There was no evidence for an association between lower CD4 cell counts and a higher risk of leukaemia.
Figure 1: Hazard ratios (solid lines) with 95% confidence intervals (shaded areas) for cancer incidence, comparing a grid of baseline CD4 cell counts to the reference value of 200 cells/μL.
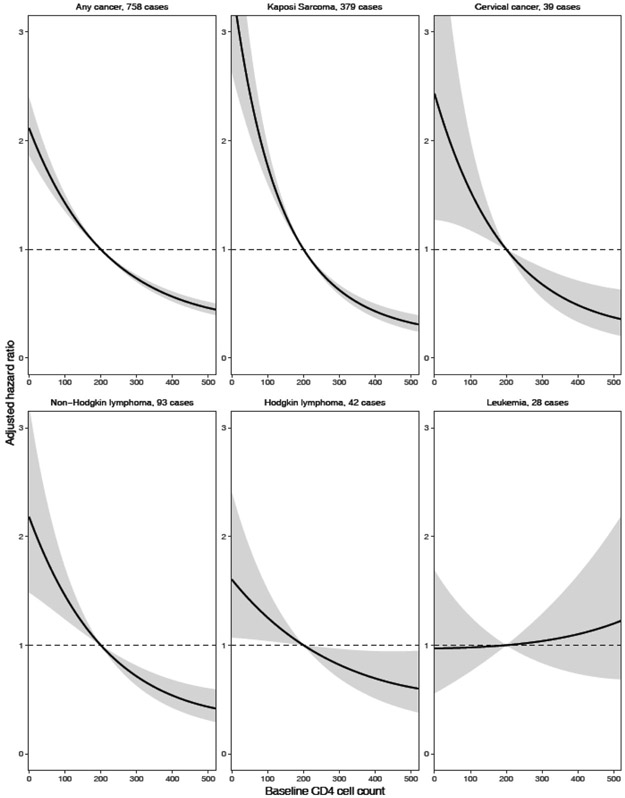
The models for any cancer, Kaposi sarcoma, non-Hodgkin lymphoma and Hodgkin lymphoma are adjusted for age, sex, and baseline calendar period. The model for cervical cancer is adjusted for age and baseline calendar period. The model for leukaemia is not adjusted for other variables.
Discussion
Kaposi sarcoma and non-Hodgkin lymphoma were the most frequent incident cancers among AYALWH in South Africa. The overall risk of developing cancer increased slightly with age and was higher in male than female AYALWH. Lower CD4 cell counts at baseline were associated with an elevated risk of Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma, and Hodgkin lymphoma after adjusting for sex and baseline calendar period.
In 2020, the most common cancer types among AYA worldwide were leukaemia, thyroid cancer and testicular cancer followed by non-Hodgkin lymphoma, CNS tumours, Hodgkin lymphoma, and breast cancer.11 However, in South Africa, where close to 1 in 10 AYA are living with HIV,12 the most frequently diagnosed cancers among AYA were the AIDS-defining cancers, Kaposi sarcoma (crude incidence rate of 4.6/100,000 person-years), cervical cancer (2.6/100,000 female person-years), and non-Hodgkin lymphoma (2.2/100,000 person-years).11 With an incidence rate of 30/100,000 person-years, Kaposi sarcoma was also by far the most common cancer in our study of AYALWH. This is likely due to the low proportion of ART exposure among AYALWH in South Africa,3 as ART reduces the risk of developing Kaposi sarcoma substantially.13 It has been estimated that between 2005 and 2012, less than one in five AYALWH aged 15-19 years who entered HIV care received ART in South Africa, but between 2013 and 2016 ART coverage in this population had increased to 79%.3 The improved access to ART may partly explain why we observed declining Kaposi sarcoma rates over the study period. Furthermore, the prevalence of human herpesvirus 8 (HHV-8), the underlying infectious cause of Kaposi sarcoma, shows a distinct geographic pattern across and within continents.14 In parts of sub-Saharan Africa, where HHV-8 prevalence is high, the virus is typically transmitted horizontally during childhood.14 In Europe and North America, however, HHV-8 seems to be predominantly sexually transmitted and is particularly common among men who have sex with men.14 In line with the geographic variation of HHV-8 prevalence, crude Kaposi sarcoma incidence rates have been reported to be approximately three times higher among AYALWH (age 16-25 years) who initiated ART in South Africa compared with those who initiated ART in Europe or North America.15
Hematologic malignancies are among the most frequent cancers in AYA globally.11 The risk of developing non-Hodgkin and Hodgkin lymphoma is elevated among people living with HIV, whereas leukaemia is not linked to HIV infection.16 However, studies on hematologic malignancies among AYALWH are scarce. A British study of 290 AYA aged 10-24 years who had been perinatally infected with HIV found that 6 out of 8 malignancies diagnosed in this group were lymphomas (3 Hodgkin lymphomas, 1 Burkitt lymphoma, 2 B-cell lymphomas).17 In adults, cumulative HIV viremia is a strong, independent risk factor for the development of non-Hodgkin lymphoma, particularly Burkitt lymphoma.18,19 For AYALWH, this is anecdotally supported by a report on two vertically infected 15-year-olds in Italy who were diagnosed with Burkitt lymphoma after prolonged exposure to HIV viremia.20 Compared to other age groups, AYALWH have higher rates of non-adherence to antiretroviral treatment and, thus, of virologic failure.2 This may put AYALWH at particularly high risk of developing HIV-related hematologic malignancies.
Cervical cancer is one of the most preventable malignancies but still appears among the five most common cancer types in our analysis. In the general population, cervical cancer incidence rates among AYA are very low, and infections with human papillomavirus virus (HPV) in this age group are often transient.21 Therefore, cervical cancer screening is generally not recommended for HIV-negative AYA below the age of 25 years.22 However, HIV increases the risk of developing cervical cancer by a factor of six,23 and, thus, South African guidelines recommend that sexually active AYALWH should be screened for cervical cancer irrespective of age.22 In contrast, in its recently released cervical cancer screening guidelines the World Health Organization suggest not to screen AYALWH below the age of 25 years, but acknowledges that this suggestion is based on low-certainty evidence.24 We found cervical cancer incidence in AYALWH to increase with lower CD4 cell counts. This is in line with what has previously been reported for adult women living with HIV.25 Timely initiation of ART and maintained adherence is likely to reduce the risk of developing cervical cancer.26 Another pillar in the prevention of cervical cancer is vaccination against high-risk HPV genotypes. In South Africa, a national HPV vaccination program targeting school girls aged 9-13 years, regardless of HIV status, was launched in 2014.27 Given the closing date of our analysis, most AYALWH included in the present study would not have been vaccinated against HPV yet.
Among adolescents aged 15-19 years living with HIV in the United States, 49% of cancer cases were attributable to infections.28 Many of the most common cancer types among AYALWH included in our study were also infection-related: together, Kaposi sarcoma (HHV-8), non-Hodgkin and Hodgkin lymphomas (Epstein-Barr virus), and cervical cancer (HPV) made up 70% of the cancer cases. These cancer types showed a clear association with lower CD4 cell counts at baseline. Increasing HPV vaccination coverage is a vital step towards decreasing cervical cancer incidence, whereas further studies are needed to better understand whether cervical cancer screening programs should include AYALWH below the age of 25 years. Additionally, the burden of infection-related cancers among AYALWH may be reduced by preventing HIV-induced immunodeficiency through improved HIV testing and early initiation of ART. Mental health and HIV-related stigma are key issues in ALYALWH that may negatively impact treatment adherence and retention in care.29
Our analysis is one of the first large-scale studies to assess cancer risk among AYALWH and included data of more than 700,000 AYALWH and 867 incident cancer cases across South Africa. Our large sample size enabled us to separately assess incidence rates and risk factors for the most common cancer types. We censored AYALWH at their 25th birthday and treated age as a time-updated variable to describe the burden of cancers diagnosed between age 15 to 24 years. We did not include cancer diagnoses that AYALWH developed later in life. Several limitations must be noted. Even though our study of cancer risk among AYALWH may be one of the largest to date, case numbers for many specific cancer types were still small. The number of cancer cases among AYALWH is likely to be underestimated in our study, as cancer diagnoses were provided by the pathology-based NCR, and clinically diagnosed cases, particularly of cancers at internal anatomic sites or terminal stage, may have been missed. Furthermore, obligatory cancer registration in South Africa started in 2011, and some private laboratories withheld their data before government legislation required and protected the sharing of data. However, as our study focused on AYALWH receiving care in the South African public sector, missing cancer diagnoses from the private sector should not bias our results substantially. Of note, our results may not be generalizable to the minority of AYALWH obtaining HIV care in the South African private sector. Almost 90% of AYALWH included in our study were female which is in line with higher HIV incidence rates among female compared with male AYA in South Africa.30 Additionally, men are less likely to access health services and test for HIV than women and, therefore, tend to be sicker when they present for care.30 This may partly explain the much higher cancer risk we observed among male compared with female AYALWH. About 90% of the included AYALWH did not have an HIV RNA viral load measurement recorded at their baseline visit. Thus, we did not assess the association between HIV viremia and cancer risk in this population. Furthermore, as the SAM study is based on laboratory records from the NHLS and cancer diagnoses from the NCR, we did not have access to other relevant patient-level information such as socio-economic and behavioural data, mode of HIV infection, ART status, HPV status, and cancer treatment. Within the SAM study, we did not have information on the death or migration of individuals. Hence, we censored time-at-risk for all individuals six months after their last HIV-related laboratory measurement. We defined incident cancer cases as cancers diagnosed after the first HIV-related laboratory measurement, with no lag time. However, estimates of cancer incidence rates and hazard ratios are sensitive to the definition of incident cancers and person-time-at-risk. Therefore, our estimates may not necessarily be comparable with estimates from other studies using different definitions, including cancer incidence rates reported for the general population.
In conclusion, infection-related cancers are the most common types of cancer occurring in AYALWH in South Africa. HPV vaccination is essential to reduce the incidence of cervical cancer and other HPV-related cancers. Additionally, innovative strategies to maintaining high CD4 cell counts through early ART initiation and enhanced treatment adherence in AYALWH are needed to reduce the risk of developing cancer and other co-morbidities in this vulnerable population.
Supplementary Material
Research in context.
Evidence before this study:
We searched PubMed for articles with combinations of the terms “HIV”, “immunodeficiency”, “cancer incidence”, “adolescents”, and “young adults” with no restriction on publication date. Compared to other age groups, adolescents and young adults living with HIV (AYALWH) are more likely to drop out of HIV care and have poorly controlled HIV infection, which may put them at increased risk of developing cancer. Studies of cancer incidence in AYALWH are scarce, particularly in sub-Saharan Africa, where the majority of AYALWH reside. According to the International Agency for Research on Cancer, the most frequently diagnosed cancers among adolescents and young adults in South Africa are Kaposi sarcoma, cervical cancer, and non-Hodgkin lymphoma. A multiregional study found that Kaposi sarcoma incidence rates were about three times higher among AYALWH in South Africa than those in Europe or North America. We found no studies examining risk factors for incident cancer specifically among AYALWH.
Added value of this study:
Our analysis is one of the largest studies of cancer risk in AYALWH to date, focusing on a region of the world where HIV prevalence is particularly high in adolescents and young adults. We found that the most common cancers among AYALWH in South Africa were Kaposi sarcoma and non-Hodgkin lymphoma. The overall risk of cancer increased slightly with age and was higher in male than female AYALWH. Lower CD4 cell counts were associated with an increased risk of Kaposi sarcoma, cervical cancer, non-Hodgkin lymphoma, and Hodgkin lymphoma.
Implications of all the available evidence:
Infection-related cancers are the most common types of cancer occurring in AYALWH in South Africa. Innovative strategies to encourage early antiretroviral therapy initiation and enhance treatment adherence in AYALWH are needed to achieve sustained viral suppression and reduce the risk of developing cancer and other co-morbidities in this vulnerable population.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under Award Number U01AI069924. The work was also supported by the NIH administrative supplement to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp) (U01AI069924-09, to ME, JB), PEPFAR supplement (to ME), the Swiss National Science Foundation (SNSF, 320030_169967, to JB, ME), and the U.S. CRDF Global (HIV_DAA3-16-62705-1, to MM). ME was supported by special project funding (17481189498) from the SNSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors state that they have no conflicts of interest to declare.
Data sharing: The data underlying this article will be shared on reasonable request to the corresponding author, subject to approval by the SAM cohort scientific committee.
References
- 1.AIDSinfo ∣ UNAIDS. Available from: https://aidsinfo.unaids.org/ (accessed March 5, 2021).
- 2.Slogrove AL, Mahy M, Armstrong A, Davies MA. Living and dying to be counted: What we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc 2017; 20: 21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maskew M, Bor J, Macleod W, Carmona S, Sherman GG, Fox MP. Adolescent HIV treatment in South Africa ’ s national HIV programme : a retrospective cohort study. Lancet HIV 2019; 6: e760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva Neto MM, Brites C, Borges ÁH. Cancer during HIV infection. Apmis 2020; 128: 121–8. [DOI] [PubMed] [Google Scholar]
- 5.Bohlius J, Foster C, Naidu G, Sengayi M, Turkova A. Cancer in adolescents and young adults living with HIV. Curr Opin HIV AIDS 2018; 13: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muchengeti M, Bartels L, Olago V, et al. Cohort Profile: The South African HIV Cancer Match Study (SAM) [Prepint] OSF Preprints. August11, 2020. doi: 10.31219/osf.io/w52sb. [DOI] [Google Scholar]
- 7.Singh E, Sengayi M, Urban M, Babb C, Kellett P, Ruff P. The South African National Cancer Registry: an update. Lancet Oncol 2014; 15: e363. [DOI] [PubMed] [Google Scholar]
- 8.Schmidlin K, Clough-Gorr KM, Spoerri A, Group for SNC study. Privacy Preserving Probabilistic Record Linkage (P3RL): a novel method for linking existing health-related data and maintaining participant confidentiality. BMC Med Res Methodol 2015; 15: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer 2006; 106: 1425–30. [DOI] [PubMed] [Google Scholar]
- 10.AYA Site Recode/WHO 2008 Definition. Available from: https://seer.cancer.gov/ayarecode/aya-who2008.html (accessed April 8, 2021). [Google Scholar]
- 11.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. Available from: https://gco.iarc.fr/today (accessed March 6, 2021). [Google Scholar]
- 12.Zanoni BC, Archary M, Buchan S, Katz IT, Haberer JE. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: The Cresting Wave. BMJ Glob Heal 2016; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr Opin Oncol 2012; 24: 522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohner E, Wyss N, Heg Z, et al. HIV and human herpesvirus 8 co-infection across the globe: Systematic review and meta-analysis. Int J Cancer 2016; 138: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohner E, Bütikofer L, Schmidlin K, et al. Comparison of Kaposi Sarcoma risk in human immunodeficiency virus-positive adults across 5 continents: A multiregional multicohort study. Clin Infect Dis 2017; 65: 1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimani SM, Painschab MS, Horner MJ, et al. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV 2020; 7: e641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhabra S, Fidler S, Ayers S, Bower M, Lyall H, Foster C. Malignancy and all-cause mortality; incidence in adolescents and young adults living with perinatally acquired HIV. J Virus Erad 2020; 6: 30–3. [PMC free article] [PubMed] [Google Scholar]
- 18.Zoufaly A, Stellbrink HJ, an der Heiden M, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis 2009; 200: 79–87. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Ramírez RU, Qin L, Lin H, et al. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV 2019; 6: e240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zangari P, Santilli V, Cotugno N, et al. Raising Awareness of Non-Hodgkin Lymphoma in HIV-infected Adolescents. J Pediatr Hematol Oncol 2013; 35: e134–7. [DOI] [PubMed] [Google Scholar]
- 21.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural History of Cervicovaginal Papillomavirus Infection in Young Women. N Engl J Med 1998; 338: 423–8. [DOI] [PubMed] [Google Scholar]
- 22.Botha M, Dreyer G. Guidelines for cervical cancer screening in South Africa. South Afr J Gynaecol Oncol 2017; 9: 8–12. [Google Scholar]
- 23.Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Heal 2021; 9: e161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. Geneva: World Health Organization; 2021. Available from: https://www.who.int/publications/i/item/9789240030824 (accessed July 7, 2021). [PubMed] [Google Scholar]
- 25.Abraham AG, Strickler HD, D’Souza G. Invasive cervical cancer risk among HIV-infected women is a function of CD4 count and screening. J Acquir Immune Defic Syndr 2013; 63: e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5: e45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dlamini SK, Madhi SA, Muloiwa R, et al. Guidelines for the vaccination of HIV-infected adolescents and adults in South Africa. South Afr J HIV Med 2018; 19: a839. [Google Scholar]
- 28.De Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS 2015; 29: 2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc 2017; 20: 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UNAIDS. A snapshot of men and HIV in South Africa. Geneva, Switzerland, 2017. Available from: https://www.thembisa.org/downloads (accessed June 29, 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


