Abstract
BST2/tetherin is a transmembrane protein with antiviral activity; it is synthesized following exposure to interferons, and restricts the release of budding virus particles by tethering them to the host cell membrane. We previously showed that BST2 is induced in primary neurons following measles virus (MV) infection or type I interferon; however, BST2 was dispensable for protection against challenge with neuron-restricted MV. Here, we define the contribution of BST-2 in neuronal MV infection. Surprisingly, and in contrast to its antiviral role in non-neuronal cells, murine BST2 promotes MV infection in brains of permissive mice and in primary neuron cultures. Moreover, BST2 expression was predominantly observed in the non-synaptic fraction of purified neurons. These studies highlight a cell-type dependent role of a well-characterized antiviral protein in enhancing neuronal infection.
Keywords: BST2, Tetherin, Neuron, Measles virus
Introduction
Bone marrow stromal antigen 2 (BST2; also known as tetherin, HM1.24, and CD3171) was first identified as a marker of terminally differentiated B cells2. Little attention was paid to this protein until the discovery that it could prevent the release of human immunodeficiency virus type 1 (HIV-1) particles from infected cells by tethering budding virions to the cell membrane3–7. Later, it was shown that this antiviral activity was not limited to HIV-1; BST2 restricted the release of many enveloped viruses, including herpes simplex virus type 2, vesicular stomatitis virus, and Ebola virus8–10. The importance of BST2 in controlling viral infections is highlighted by the fact that many viruses, including HIV-1, encode proteins that directly antagonize BST2’s antiviral activity6,10.
Although BST2’s ability to restrict viral budding remains extensively studied, its normal cellular function(s) are still being defined. Multiple isoforms of BST2 have been identified, each with differing signal transducing capabilities (e.g. certain BST2 isoforms activate the NF-κB pathway while others cannot)11–13. BST2 has also been implicated in modulating clathrin-mediated endocytosis11,14,15, by contributing to organization of the actin cytoskeleton16. Further, BST2 is induced in response to cellular type I and II interferon (IFN) in many cell types1, consistent with its role in antiviral defense. We previously showed that transcription of the BST2 gene is highly induced in primary mouse hippocampal neurons in response to both IFN exposure and measles virus (MV) infection, but was surprisingly dispensable for immune-mediated control in MV-infected mice17,18. Here, we explore the contribution of BST2 more fully in neuronal viral infection.
MV is not a natural mouse pathogen; to study infection of central nervous system (CNS) neurons, our laboratory uses a transgenic mouse model that expresses the MV vaccine strain receptor, CD46, under the transcriptional control of the neuron specific enolase (NSE) promoter (NSE-CD46+ mice)19. This model allows for exclusive infection of mouse CNS neurons with the MV-Edmonston vaccine strain19–21. We have shown that MV infection of neurons, both in vivo and ex vivo, does not result in cytopathicity as it does in all non-neuronal cells22, and interneuronal spread occurs in the absence of viral budding23,24. Rather, MV transmission from infected to uninfected neurons is almost exclusively trans-synaptic. In this report, we provide evidence that, contrary to BST2’s well-defined ability to restrict viral spread in non-neuronal cells, its expression enhances MV infection in hippocampal neurons, and its absence results in less accumulation of viral RNA in vivo. Furthermore, BST2 is enriched in the neuronal cytoplasm and cell body (with less abundant expression at the synapse), suggesting that it may exert its pro-viral role within the neuronal cell body23,24.
Results
Reduced viral RNA in infected brains of mice lacking BST-2
Our previous observation that the absence of BST2 had no overt pathogenic consequence following neuronal MV infection in mice25, coupled with a reduction in the amount of recoverable infectious progeny in BST2-deficient neurons, drove us to determine if the presence of BST2 had any impact on the ability of MV to productively infect CNS neurons in vivo. Viral RNA levels were assessed in whole brains of NSE-CD46+ and NSE-CD46+/BST2 KO mice throughout a two-week time course. No significant changes were observed in MV levels during the early phase of infection. However, contrary to our expectations, at later timepoints, we observed a trend towards less viral RNA in the brains of NSE-CD46+/BST2 KO mice, reaching statistical significance by 14 dpi (Figure 1A). MV nucleoprotein levels were also reduced in the brains of CD46+/BST2 KO mice 14 and 30 dpi (Figure 1B).
Figure 1: Deletion of BST2 leads to decreased MV in vivo.
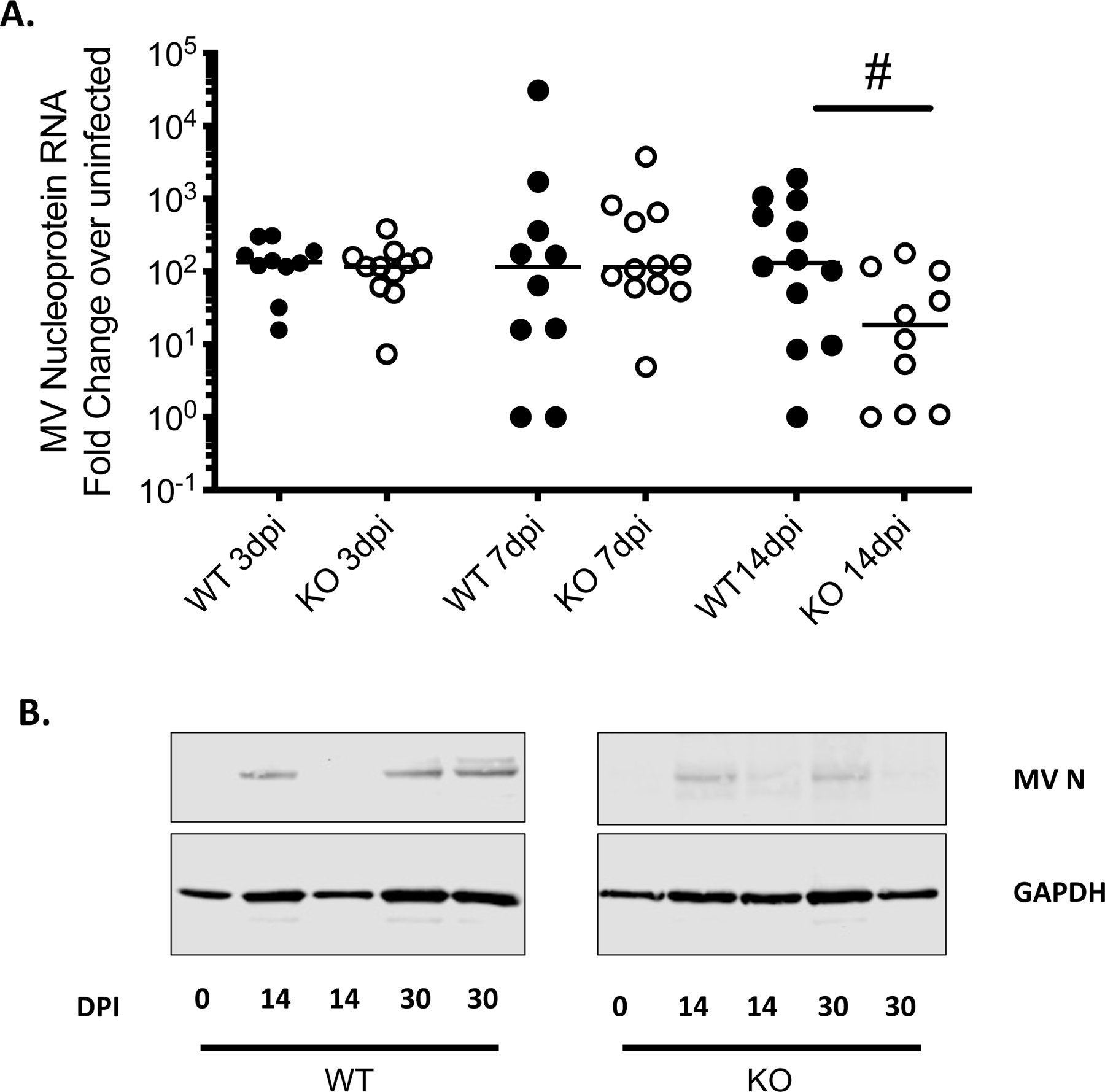
NSE-CD46+ (WT; black dots) and NSE-CD46+/BST2 KO (KO; white dots) mice were infected intracranially with 1×104 PFU MV-Edmonston. Whole brains were collected, and analyzed for MV nucleoprotein RNA and protein by RT-qPCR (A) and Western blot (B), at the indicated days post infection (dpi). n=10–12/group. Data are represented using the ΔΔCT method. # p <0.05 Mann-Whitney U Test.
Reduced measles virus RNA in infected BST-2 deficient neurons
These differences were reproducible, but subtle. Thus, we sought to determine if a similar result was seen in a more simplified setting, in which we evaluated viral levels in susceptible primary hippocampal neurons. Primary neurons were derived from E14 embryos of the indicated genotype; as we have previously shown, these neurons are non-dividing, and form functional synaptic contacts26. Using these cultures, we assessed the kinetics of MV replication in primary NSE-CD46+ and NSE-CD46+/BST2 KO neurons by analyzing MV nucleoprotein RNA and protein levels at varying times post infection (Figure 2). Consistent with our in vivo data, both viral RNA and protein levels were significantly lower in BST2 KO neuronal cultures (Figure 2A/B). Immunofluorescence staining showed significant reductions in the number of MV-infected, BST2 KO neuronal foci (Figure 2C/D). These data indicate that there is a direct correlation between the presence of BST2 and robust MV replication.
Figure 2: Deletion of BST2 leads to decreased MV a in primary neurons.
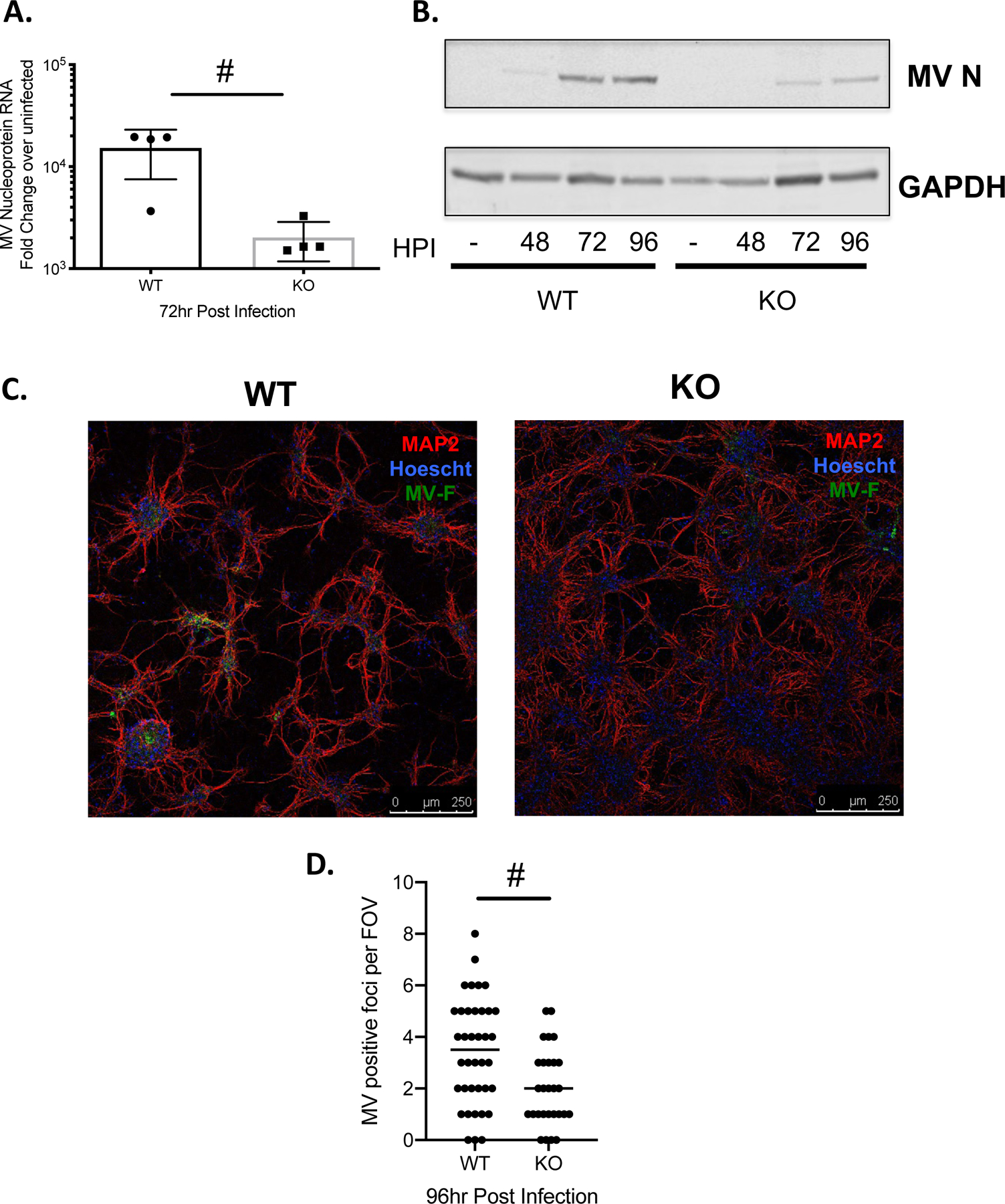
Primary neurons of the indicated genotype (WT: NSE-CD46+; KO: NSE-CD46+/BST2 KO) were infected with MV-Edmonston at an MOI=1. A) RNA was collected at the indicated time point (hours post-infection; hpi) and analyzed by RT-qPCR for MV nucleoprotein RNA. Results from at least 3 independent experiments are represented using the ΔΔCT method. B) Western blot analysis of protein collected at the indicated times post infection. Blots were probed with a polyclonal MV nucleoprotein antibody and an antibody to GAPDH as a loading control. C) Immunofluorescence staining of WT and KO primary neuronal cultures. Red- MAP2; neuronal marker. Blue-Hoescht; nuclei marker. Green- MV fusion protein. Representative images from each genotype are shown. D) MV positive foci were scored across multiple fields of view (FOV). # p <0.05 Unpaired T test.
BST2 is not present at the synapse during neuronal MV infection
To further understand how BST2 aids MV replication and spread, we purified synaptic and non-synaptic neuronal fractions from infected NSE-CD46+ mouse brains at varying times post-infection. Synaptic termini were purified using synaptosome preparations, obtained following homogenization of neuronal tissue or cultures in isotonic buffer followed by serial centrifugations. Such preparations are enriched in synaptosome-associated protein (SNAP25) and represent sealed neuronal terminals27. Using this established purification technique, we defined BST2’s cellular localization. BST2 RNA expression trended higher in the non-synaptic neuronal fraction than in the synaptic fractions purified from infected whole brain tissue (Figure 3A). We further corroborated this observation using primary NSE-CD46+ neuronal cultures. BST2 RNA expression was reduced in the synaptic fraction of infected neurons at later time points and protein expression was significantly reduced in the synaptic fraction of neurons infected for 72hr (Figure 3B/C). These data suggest that BST2 may exert its pro-viral function during viral replication in the neuronal cytoplasm, rather than by affecting neurotransmission.
Figure 3: BST2 is not concentrated at the neuronal synapse during MV infection.
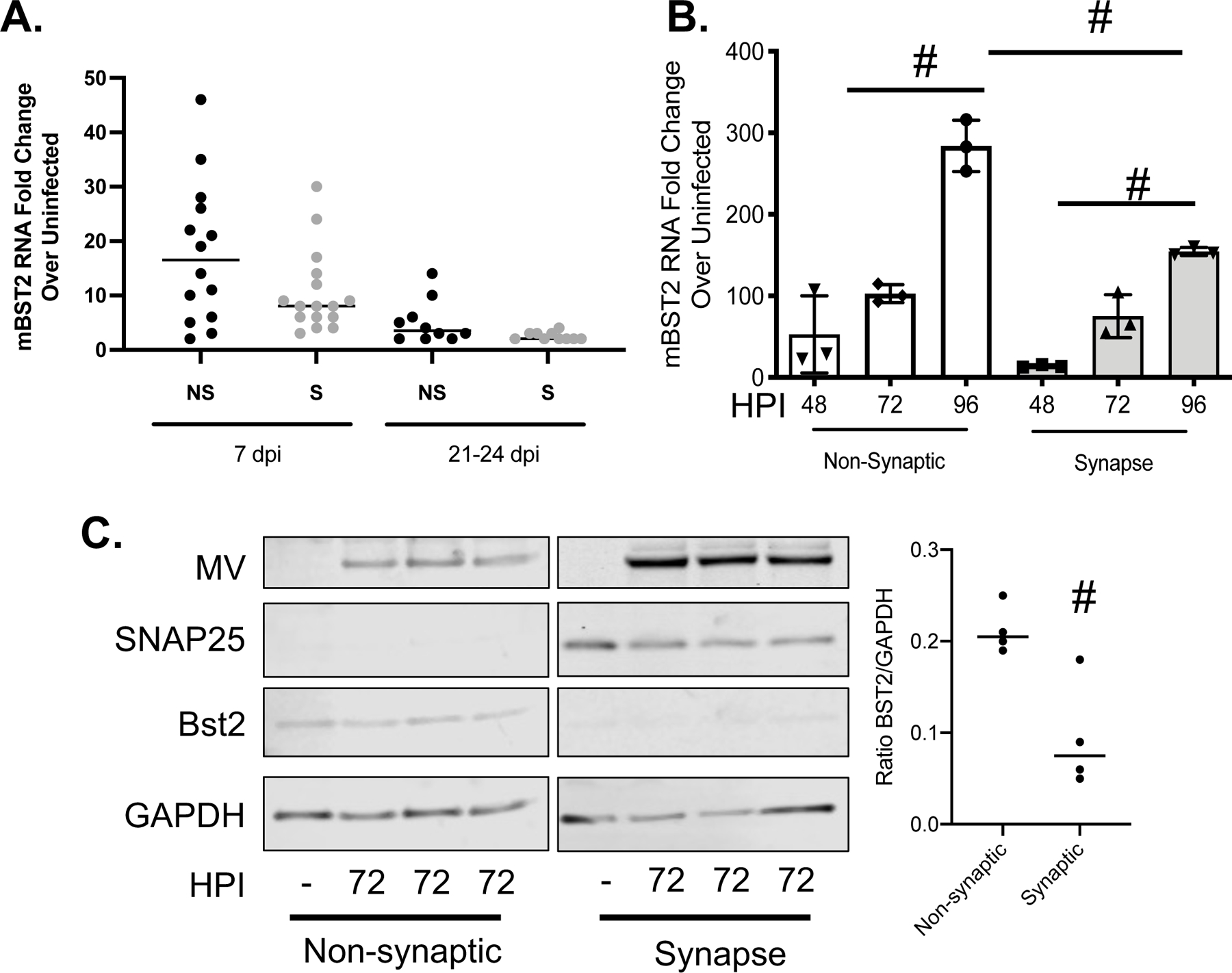
A) RNA collected from fractionated whole brain tissue was analyzed by RT-qPCR for murine BST2 (mBST2) RNA and normalized to cyclophilin. B/C) Primary NSE-CD46+ neurons were infected with MV at an MOI=1. Infected cells were collected at the indicated hours post infection followed by synaptosome purification. B) RNA was analyzed by RT-qPCR for murine BST2 (mBST2) RNA and normalized to cyclophilin B. Data represent the results of an experiment performed in triplicate and analyzed using the ΔΔCT method. C) Western blot analysis of protein collected at the indicated times post infection from either the pelleted synaptic or remaining fractions (non-synaptic). Blots were probed with antibodies against BST2, MV, SNAP25 (to indicate synaptic fraction purity) and an antibody to GAPDH (loading control). # p <0.05 Unpaired T test with equal standard deviations. Error bars represent SD.
Discussion
It is well established that BST2 can block egress of multiple enveloped viruses3,4,7,8,25,28–31. Paradoxically, some reports have demonstrated that BST2 supports cell-cell viral spread32–36. In this report, we show that the presence of BST2 in primary hippocampal neurons supports neuronal MV replication both in and ex vivo. Our data contribute to a growing literature which describes the cell type-specific functions of BST2/tetherin following viral infection.
In most cases, the “pro-viral” functions of BST2 that have been reported are linked to increased entry into permissive cells. For example, in the case of cytomegalovirus (CMV) replication, in vitro studies using primary human monocytes and fibroblasts revealed that BST2 expression increases CMV cellular entry via a reverse-tethering mechanism in which BST2 expressed at the cell surface can enhance virion binding and subsequent entry to adjacent permissive cells32. BST2 KO mice infected with either vesicular stomatitis virus or influenza virus showed similar results, in that loss of BST2 results in decreased viral loads in vivo, presumably due to disruption of intracellular vesicle trafficking33,34.
We hypothesize that these seemingly opposing functions may be influenced by how a given virus spreads in distinct cell populations. For example, in the case of HIV-1 infection, BST2’s contribution in inhibiting spread of viral particles has mainly focused on the release of infectious progeny4,5,7. However, when investigators studied direct cell-to-cell spread of HIV-1, BST2 promoted spread specifically at the virological synapse, as siRNA knockdown of BST2 inhibited transmission of particles into uninfected cells35. Similar data were observed with feline immunodeficiency virus (FIV): BST2 expression could efficiently block egress, although cell-to-cell transmission was enhanced, as visualized by increased syncytia formation36.
The cell-type differences that alter BST2 biology may be of particular relevance to viral transmission in neurons: many viruses, including MV, polio, neurotropic herpesviruses, and rabies, adopt a trans-synaptic transmission strategy, in addition to, or wholly replacing, viral budding23,24,37. While one study has shown that BST2 overexpression inhibits MV induced syncytia formation in rapidly dividing HEK293T cells38, no studies to date have examined what role BST2 may play in non-dividing primary cell populations, such as CNS neurons. In the case of MV infection of primary hippocampal neurons, it is possible that BST2 aids in interneuronal MV trafficking. Supporting this hypothesis is the fact that BST2 has been identified in the trans-Golgi network and recycling endosomes, which, in neurons, may aid in transporting ribonucleoprotein complexes or viral protein-containing vesicles from the Golgi to the synapse, enabling MV spread across the synaptic cleft15. Alternatively, the normal cellular roles of BST2, which include clathrin-mediated endocytosis, induction of NF-κB, and restriction of cellular protein synthesis, could lead to the induction or activation of other cellular pathways necessary to facilitate MV replication in neurons15,16,29,39. The observation that BST2 facilitates MV reproduction in neurons underscores the importance of considering cell type when studying the functions of certain ISGs.
Materials and Methods
Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was reviewed and approved by the Fox Chase Cancer Center Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare assurance number A3285–01).
Cell culture and virus infections
Primary hippocampal neurons were obtained from day 14–16 mouse embryos and cultured in Neurobasal media (Invitrogen) supplemented with L-glutamine in the absence of an astrocyte feeder layer, as described previously19,23,24. Neurons were plated in 12-well plates coated with poly-L-lysine (Sigma-Aldrich) at a density of 2×105 cells/well. Neuron cultures were frequently quality-controlled, and were routinely >95% MAP2-positive. Neurons were plated and incubated for 5 days (d) prior to infection to allow for full differentiation.
MV-Edmonston (vaccine strain) was purchased from American Type Culture Collection and passaged and titered at low multiplicity in Vero cells. Passages 2 or 3 of the resulting stock were used for all infections. All infections of primary cultures were carried out at a multiplicity of infection (MOI)=1 for 1 hour before washing and replacement of conditioned medium.
Synaptosome Purification
Cytosolic and synaptic neuronal fractions were purified as previously described27. Supernatants (cytosolic fraction) and pellets (synaptosome fraction) were re-suspended in Illustra Triple Prep kit (GE Healthcare) buffer, and RNA and protein were purified according to manufacturer’s instructions.
Western Blot analyses
Protein was obtained from primary neurons using the Illustra Triple Prep kit (GE Healthcare) and stored at −80°C until analysis. Samples were electrophoresed into a 10% Bis-Tris gel (Life Technologies) in MES running buffer (Life Technologies) and transferred to an Immobilon membrane (Millipore). Membranes were blocked with Odyssey Blocking Buffer and immunoblotted for GAPDH (Millipore AB2302; 1:10,000), MV nucleoprotein (Sigma 95051114), SNAP25 (Cell Signaling 5308), or BST2 (Thermo Fisher Scientific PA5–23505). Secondary antibodies were obtained from LI-COR (IRDye® 680RD Donkey anti-Chicken IgG; IRDye® 800CW Donkey anti-Rabbit IgG). Images were captured with LI-COR Odyssey Classic Infrared Imager.
Reverse transcriptase quantitative real-time PCR (RT-qPCR)
RNA was purified from whole cell lysates using the RNeasy Mini kit (Qiagen) or the Illustra Triple Prep kit (GE Healthcare). RNA was reverse transcribed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems) with random hexamer priming. Gene-specific primers were used in combination with probes designed using the Universal Probe Library algorithm (Roche) and Universal Master mix (Roche); all reactions were run on a Mastercycler Realplex2 system (Eppendorf). Relative quantification to the control (cyclophilin B) was done using the comparative ΔΔCT method. Individual sample PCR reactions were performed in triplicate. The following gene specific primers (Integrated DNA Technologies) were used: Cyclophilin B Forward – 5’-ttcttcataaccacagtcaagacc-3’, Cyclophilin B Reverse – 5’-accttccgtaccacatccat-3’, UPL 20; MV nucleoprotein Forward - 5’-ggaaactgcaccctacatgg-3’, MV nucleoprotein Reverse- 5’-gggtatgatcctgcactgaact-3’, UPL 80; Bst2 Forward- 5’-gaagtcacgaagctgaacca-3’, Bst2 Reverse- 5’-cctgcactgtgctagaagtctc-3’, UPL 78.
Immunofluorescence Staining
Primary neurons were plated on poly lysine-coated coverslips. Coverslips were fixed at the indicated timepoint using 4% paraformaldehyde/4% sucrose in phosphate buffered saline, further fixed and permeabilized with 100% methanol and 0.2% Triton, and blocked before addition of primary antibodies. Primary antibodies for MV fusion protein (Bioss USA bs-0886R), MAP2 (Millipore MAB364) were applied. Directly conjugated secondary antibodies were used (Hoescht; 1:1000, Donkey anti-rabbit AF488; 1:5000, Goat anti-mouse AF555; 1:5000). Images were captured at 40× using an inverted TE2000 Nikon C1 confocal microscope.
Mice and viral inoculation
Homozygous NSE-CD46+ transgenic mice (line 18; H-2b)19 were maintained in the closed breeding colony of the Fox Chase Cancer Center. BST2 KO mice were obtained from Marco Colonna, Washington University, St. Louis, MO and crossed to NSE-CD46+ transgenic mice for three generations. Genotypes of mice used in these experiments were confirmed by PCR analysis of tail biopsy DNA.
Isoflurane-anesthetized mice were infected with MV-Edmonston via intracranial inoculation of 1×104 PFU in a volume of 30 μl, delivered along the midline using a 27g needle. Mice were monitored daily post-infection for signs of illness. Moribund mice were euthanized in accordance with IACUC guidelines. RNA was isolated from individual mice at indicated times post infection using TriReagent (Sigma).
Statistical analysis and figure preparation
Data representation and statistical analysis were performed using Prism GraphPad. Statistical analysis was done using the Mann-Whitney U test or unpaired T test with equal standard deviations as indicated in the legends.
Research Highlights:
BST2 promotes neuronal measles virus infection in primary neuronal cultures.
BST2 promotes neuronal measles virus infection in the brain.
BST2 is increased away from the neuronal synapse.
Acknowledgements
The authors would also like to acknowledge the Fox Chase Cancer Center Laboratory Animal Facility.
Support
This was work supported by F31 NS092307, T32 NS007180–32 (KDM), and Cancer Center Support Grant 5P30CA006927–53 (CM, RW, CJ, GR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
KDM, CM, RW, and GR conceptualized the goals and experiments in this manuscript. KDM, CM, RW, and GR wrote and reviewed this manuscript. KDM, CM, RW, and CBJ performed experiments associated with this manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. Journal of Immunology. 2006;177(5):3260–3265. [DOI] [PubMed] [Google Scholar]
- 2.Goto T, Kennel SJ, Abe M, et al. A Novel Membrane Antigen Selectively Expressed on Terminally Differentiated Human B Cells. Blood. 1994;84:1922–1930. [PubMed] [Google Scholar]
- 3.Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick K, Skasko M, Deerinck TJ, Crum J, Ellisman MH, Guatelli J. Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles. Hope TJ, ed. PLoS Pathog. 2010;6(3):e1000701–e1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Caballero D, Zang T, Ebrahimi A, et al. Tetherin Inhibits HIV-1 Release by Directly Tethering Virions to Cells. Cell. 2009;139(3):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme N, Goff D, Katsura C, et al. The Interferon-Induced Protein BST-2 Restricts HIV-1 Release and Is Downregulated from the Cell Surface by the Viral Vpu Protein. Cell Host and Microbe. 2008;3(4):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammonds J, Wang J-J, Yi H, Spearman P. Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane. Hope TJ, ed. PLoS Pathog. 2010;6(2):e1000749–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Luo S, He S, et al. Tetherin restricts HSV-2 release and is counteracted by multiple viral glycoproteins. Virology. 2015;475(c):96–109. [DOI] [PubMed] [Google Scholar]
- 9.Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. Interferon-Induced Cell Membrane Proteins, IFITM3 and Tetherin, Inhibit Vesicular Stomatitis Virus Infection via Distinct Mechanisms. Journal of Virology. 2010;84(24):12646–12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. PNAS. 2009;106:2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocka LJ, Bates P. Identification of Alternatively Translated Tetherin Isoforms with Differing Antiviral and Signaling Activities. Aiken C, ed. PLoS Pathog. 2012;8(9):e1002931–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends in Microbiology. 2010;18(9):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotter D, Sauter D, Kirchhoff F. Emerging Role of the Host Restriction Factor Tetherin in Viral Immune Sensing. Journal of Molecular Biology. 2013;425(24):4956–4964. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Serrano J, Neil SJD. Host factors involved in retroviral budding and release. Nat Rev Micro. 2011;9(7):519–531. [DOI] [PubMed] [Google Scholar]
- 15.Masuyama N, Kuronita T, Tanaka R, et al. HM1.24 Is Internalized from Lipid Rafts by Clathrin-mediated Endocytosis through Interaction with alpha-Adaptin. Journal of Biological Chemistry. 2009;284(23):15927–15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollason R, Korolchuk V, Hamilton C, Jepson M, Banting G. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. The Journal of Cell Biology. 2009;184(5):721–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren AM, Miller KD, Cavanaugh SE, Rall GF. Bone marrow stromal antigen-2 (Bst2)/tetherin is induced in neurons by type I interferon and viral infection, but is dispensable for protection against neurotropic viral challenge. Journal of Virology. August2015:JVI.01745–15–10. [DOI] [PMC free article] [PubMed]
- 18.O’Donnell LA, Conway S, Rose RW, et al. STAT1-Independent Control of a Neurotropic Measles Virus Challenge in Primary Neurons and Infected Mice. Journal of Immunology. 2012;188(4):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rall GF, Manchester M, Daniels LR, Callahan EM, Belman AR, Oldstone MBA. A transgenic mouse model for measles virus infection of the brain. PNAS. 1997;94:4659–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. Journal of Virology. 1993;67(10):6025–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorig RE, Marcil A, Chopra A, Richardson CD. The Human CD46 Molecule Is a Receptor for Measles Virus (Edmonston Strain). Cell. 1993;75:295–305. [DOI] [PubMed] [Google Scholar]
- 22.Patterson CE, Lawrence DMP, Echols LA, Rall GF. Immune-Mediated Protection from Measles Virus-Induced Central Nervous System Disease Is Noncytolytic and Gamma Interferon Dependent. Journal of Virology. 2002;76(9):4497–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makhortova NR, Askovich P, Patterson CE, Gechman LA, Gerard NP, Rall GF. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology. 2007;362(1):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence DMP, Patterson CE, Gales TL, D’Orazio JL, Vaughn MM, Rall GF. Measles Virus Spread between Neurons Requires Cell Contact but Not CD46 Expression, Syncytium Formation, or Extracellular Virus Production. Journal of Virology. 2000;74(4):1908–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmgren AM, Miller KD, Cavanaugh SE, Rall GF. Bone marrow stromal antigen-2 (Bst2)/tetherin is induced in neurons by type I interferon and viral infection, but is dispensable for protection against neurotropic viral challenge. Journal of Virology. August2015:JVI.01745–15–34. [DOI] [PMC free article] [PubMed]
- 26.Lawrence DMP, Vaughn MM, Belman AR, Cole JS, Rall GF. Immune Response-Mediated Protection of Adult but Not Neonatal Mice from Neuron-Restricted Measles Virus Infection and Central Nervous System Disease. Journal of Virology. 1999;73(3):1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokolow S, Henkins KM, Williams IA, et al. Isolation of synaptic terminals from Alzheimer’s disease cortex. Cytometry. 2011;81A(3):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooi Y, Dubé M, Kielian M. BST2/Tetherin Inhibition of Alphavirus Exit. Viruses. 2015;7(4):2147–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Wang J, Qu M, et al. Viral Restriction Activity of Feline BST2 Is Independent of Its N-Glycosylation and Induction of NF-κB Activation. Schindler M, ed. PLoS ONE. 2015;10(9):e0138190–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahauad-Fernandez WD, Jones PH, Okeoma CM. Critical role for bone marrow stromal antigen 2 in acute Chikungunya virus infection. Journal of General Virology. 2014;95(Pt_11):2450–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg Virus Production by Tetherin. Journal of Virology. 2009;83(5):2382–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan K, Smith MS, Malouli D, Mansouri M, Nelson JA, Früh K. BST2/Tetherin Enhances Entry of Human Cytomegalovirus. Britt WJ, ed. PLoS Pathog. 2011;7(11):e1002332–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Londrigan SL, Tate MD, Job ER, et al. Endogenous Murine BST-2/Tetherin Is Not a Major Restriction Factor of Influenza A Virus Infection. Schindler M, ed. PLoS ONE. 2015;10(11):e0142925–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swiecki M, Wang Y, Gilfillan S, Lenschow DJ, Colonna M. Cutting Edge: Paradoxical Roles of BST2/Tetherin in Promoting Type I IFN Response and Viral Infection. Journal of Immunology. 2012;188(6):2488–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly C, Booth NJ, Neil SJD. Cell-Cell Spread of Human Immunodeficiency Virus Type 1 Overcomes Tetherin/BST-2-Mediated Restriction in T cells. Journal of Virology. 2010;84(23):12185–12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich I, McMonagle EL, Petit SJ, et al. Feline Tetherin Efficiently Restricts Release of Feline Immunodeficiency Virus but Not Spreading of Infection. Journal of Virology. 2011;85(12):5840–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomme EA, Wirblich C, Addya S, Rall GF, Schnell MJ. Immune Clearance of Attenuated Rabies Virus Results in Neuronal Survival with Altered Gene Expression. PLoS Pathog. 2012;8(10):e1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly JT, Human S, Alderman J, et al. BST2/Tetherin Overexpression Modulates Morbillivirus Glycoprotein Production to Inhibit Cell-Cell Fusionn. Viruses. July2019:1–15. [DOI] [PMC free article] [PubMed]
- 39.Narkpuk J, Wanitchang A, Kramyu J, Frantz PN, Jongkaewwattana A, Teeravechyan S. An unconventional BST-2 function: Down-regulation of transient protein expression. Biochemical and Biophysical Research Communications. 2014;450(4):1469–1474. [DOI] [PubMed] [Google Scholar]


