Abstract
Immunotherapy using checkpoint blockers (antibodies) has been a major advance in recent years in the management of various types of solid cancers including lung cancer. One target of checkpoint blockers is programmed death ligand 1 (PD-L1) expressed by cancer cells, which engages programmed death 1 on T cells and Natural Killer (NK) cells resulting in suppression of their activation and cancer-killing function, respectively. Apart from antibodies, other clinically relevant agents that can inhibit PD-L1 are limited. PD-L1 protein stability depends on its glycosylation. Here we show that l-glutamine:d-fructose-6-phosphate amidotransferase 1 (GFAT1), a rate-limiting enzyme of the hexosamine biosynthesis pathway, which produces uridine diphosphate-N-acetyl-β-glucosamine, a precursor for glycosylation, is required for the stability of PD-L1 protein. Inhibition of GFAT1 activity markedly reduced interferon gamma (IFNγ)-induced PD-L1 levels in various lung cancer cell lines. GFAT1 inhibition suppressed glycosylation of PD-L1 and accelerated its proteasomal degradation. Importantly, inhibition of GFAT1 in IFNγ-treated cancer cells enhanced the activation of T cells and the cancer-killing activity of NK cells. These findings support using GFAT1 inhibitors to manipulate PD-L1 protein level that could augment the efficacy of immunotherapy for lung cancer.
Introduction
Cancer immunotherapy using immune checkpoint blockers reinvigorates dysfunctional cytotoxic T cells in cancer tissues to eliminate cancer cells. Current checkpoint blockers, i.e. monoclonal antibodies against cytotoxic T lymphocyte antigen 4, programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), disrupt the inhibitory signaling that limits T-cell activity (1). Checkpoint blockers provide a vital option for treatment of many types of solid cancers including lung cancer (2,3). Despite the exciting clinical outcome, the therapeutic benefits have been limited to a minority of patients, and the response rate remains moderate and relapse is common. Understanding the mechanisms of resistance and the options to overcome them is critical to bringing immunotherapy with better efficacy to more patients.
Although a high level of PD-L1 in cancer cells is used as an indicator of a ‘hot’ immune status that predicts likely response to PD-1/PD-L1 antibodies, the protein suppresses the activities of cytotoxic T cells and Natural Killer (NK) cells that express PD-1 to reduce their effectiveness against cancer, thus contributing to resistance to immune and conventional therapies (4–6). In general, a high level of PD-L1 in cancer cells is prognostic for worse survival of patients with non-small cell lung cancer (7,8).
PD-L1 expression is controlled at transcriptional and posttranscriptional levels in cancer cells. The protein can be induced by various growth factors and cytokines (9). For example, interferon gamma (IFNγ), released by T cells upon activation to attack cancer cells, strongly increases PD-L1 transcription (4,5). As a membrane protein, PD-L1 level in a cell is affected by posttranslational modification such as glycosylation and ubiquitination, trafficking, and recycling. Glycosylation of PD-L1 was recently shown to control its degradation via proteasomal degradation pathway (9).
Hexosamine biosynthesis pathway (HBP) uses glucose, glutamine and nucleotide to produce uridine diphosphate-N-acetyl-glucosamine (uridine diphosphate-GlcNAc), a building block for glycosylation of a variety of proteins (10). In this study, we show that l-glutamine:d-fructose-6-phosphate amidotransferase 1 (GFAT1), the rate-limiting enzyme of HBP, regulates IFNγ-induced PD-L1 expression in lung cancer cells. Inhibition of GFAT1 reduced PD-L1 by accelerating proteasomal degradation of the protein. Importantly, inhibition of GFAT1 in cancer cells improves T-cell response and killing of cancer cells by NK cells. Thus, our findings support GFAT1/HBP as a potential target for improving cancer immunotherapy.
Materials and methods
Reagents and antibodies
Phorbol 12-myristate 13-acetate (P8139), ionomycin (I9657) and tunicamycin (T7765) were purchased from Sigma (St Louis, MO) and prepared in dimethyl sulfoxide. Azaserine (A4142), 6-diazo-5-oxo-l-norleucine (DON, D2141), 3-methyladenine (M9281) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, M5655] were also Sigma products and prepared in distilled water or phosphate-buffered saline. CB-839 (1439399-58-2) was obtained from Cayman Chemical (Ann Arbor, MI). VPS34 inhibitor (532628) was from Millipore (Billerica, MA). Recombinant human IFNγ (285-IF) was purchased from R&D Systems (Minneapolis, MN) and mouse IFNγ (BMS326) from eBioscience (Thermo Fisher Scientific, Waltham, MA). The following antibodies were used for western blot: GFAT1 (ab125069), GFAT2 (ab190966) and RL2 (ab2739, recognizing O-GlcNAc) from Abcam (Cambridge, MA); human interferon gamma response factor 1 (sc-74530), O-GlcNAc transferase (sc-32921) and β-actin (sc-4778) from Santa Cruz Biotechnology (Santa Cruz, CA); human PD-L1 (AF156, goat) and mouse PD-L1 (AF1019, goat) from R&D Systems; GFAT1 (5322), GFAT2 (6917) and PD-L1 (13684, rabbit) from Cell Signaling Technology (Danvers, MA); β-actin (A2103) and β-tubulin (T6074) from Sigma.
Cell culture
All cell lines were obtained from American Type Culture Collection (Manassas, VA). Lung cancer cell lines (A549, H1975 and H2009, adenocarcinoma; SK-MES-1 and SW900, squamous cell carcinoma) were authenticated using short tandem repeat DNA profiling (Genetica Cell Line Testing, Burlington, NC) in 2017 (A549 and H1975) or 2018 (H2009, SK-MES-1 and SW900). Murine Lewis lung cancer cell line (LLC1) was purchased in 2016 and NK-92 NK cell line in 2020. Authenticated and recently purchased cell lines were expanded and frozen as stocks for subsequent recoveries and use. Jurkat T-cell line was not authenticated. Cancer cell lines and Jurkat T cells were maintained in RPMI1640 with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml) (Thermo Fisher Scientific). NK-92 cells were cultured in American Type Culture Collection formulated medium (Alpha Minimum Essential medium without ribonucleosides and deoxyribonucleosides, with 2 mM l-glutamine, 1.5 g/l sodium bicarbonate, 0.2 mM inositol, 0.1 mM 2-mercaptoethanol, 0.02 mM folic acid, 12.5% horse serum and 12.5% fetal bovine serum) and 15 ng/ml human recombinant interleukin 2 (IL-2, R&D Systems).
RNA interference
Silencer Select small interfering RNA (siRNA) negative control (4390844) and those targeting GFAT1 (human, s5708; mouse, s66610) or GFAT2 (human, s19305) were purchased from Thermo Fisher Scientific. siRNA was transfected with INTERFERin (PolyPlus-transfection, New York, NY) at 10 nM according to the instruction from the manufacturer.
Coculture and enzyme-linked immunoabsorbing assay of IL-2
Cancer cells were seeded at a density of 5 × 104 cells/well on a 24-well plate in triplicate overnight with 0.5 ml medium. Cells were then transfected with control or GFAT1 siRNA. Twenty-four hours post-transfection, cells were incubated with fresh medium with IFNγ (10 ng/ml) for 6 h. The medium was removed, and Jurkat T cells collected and resuspended in medium with 20 ng/ml phorbol 12-myristate 13-acetate and 200 μg/ml ionomycin were added to cancer cells according to the ratios indicated in the figure legends. After 18 h (for H2009) or 24 h (for SK-MES-1), IL-2 concentration in the culture medium was determined by an enzyme-linked immunoabsorbing assay kit from Thermo Fisher (Cat. 88-7025-88) according to the instruction of the manufacturer. T cells were then washed off and cancer cells were lysed to confirm the knockdown of GFAT1 and its effect on PD-L1 expression with western blot. For DON inhibition, DON (10 µM) was added overnight prior to the treatment of IFNγ. The ratio of cancer cell to T cell was 1:3 and IL-2 enzyme-linked immunoabsorbing assay was done 24 h later for both cell lines.
NK cell cytotoxicity assay
Cancer cells were seeded at a density of 2 × 104 cells/well on a 48-well plate in triplicate for 24 h, treated with DON (10 µM) for 30 min and then incubated with IFNγ (10 ng/ml) overnight. The media were changed and NK-92 cells were added to the monolayer culture at the indicated ratios. After 4 h of coculture, the media were refreshed to remove NK cells. Cell viability was carried out 24 h later.
Cell viability assay
Cells were incubated with 40 µg/ml MTT for 2 h. After three washes with phosphate-buffered saline, dimethyl sulfoxide was added to the wells. The color intensity (OD570) of the solutions was determined with a plate reader. Cell viability was expressed as percentages with the reading of the control set as 100.
Reverse transcription and polymerase chain reaction
Total RNA of cell lines was extracted using RNeasy RNA extraction kit from Qiagen (Valencia, CA). Reverse transcription was carried out with GoScript Reverse Transcription System (A5001; Promega, Madison, WI). The primers with the following sequences were synthesized by Integrated DNA Technologies (San Diego, CA): PD-L1, 5′-CCA TCA AGT CCT GAG TGG TAA G-3′ (forward) and 5′-ATT TGG AGG ATG TGC CAG AG-3′ (reverse); β-actin, 5′-CCA GCC TTC CTT CCT GGG CAT-3′ (forward) and 5′-AGG AGC AAT GAT CTT GAT CTT CAT T-3′ (reverse). The amplification conditions were 95°C, 20 s; 58°C, 20 s; 72°C, 20 s for 28 cycles for PD-L1 and 20 cycles for β-actin. The PCR products were run in 2.5% agarose with 0.5 μg/ml ethidium bromide, visualized and photographed using Bio-Rad ChemiDoc Imaging System (Hercules, CA). The band density was quantified with NIH ImageJ software, normalized to respective loading control and then fold change was calculated against that of control cells as 1. Real-time PCR for the enzymes of HBP was carried out using respective stock or custom TagMan assay primers and ABI PRISM 7900HT (Applied Biosystems). Amplification was done in duplicate for each gene. Results were normalized to corresponding glyceraldehyde 3-phosphate dehydrogenase simultaneously amplified and expressed as fold change using standard method as described previously (11).
Western blot and immunoprecipitation
Cells were lysed in M2 buffer (20 mM Tris–HCl, pH 7.6; 0.5% NP-40, 250 mM NaCl, 3 mM ethylene glycol-bis(aminoethyl ether)-tetraacetic acid, 3 mM ethylenediaminetetraacetic acid, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerophosphate, 1 mM sodium vanadate and 1 µg/ml leupeptin) and subjected to a round of freeze–thaw cycle to obtain whole cell lysate. Protein concentration was determined with Bio-Rad protein quantification reagent (Cat. 500-0006). For immunoprecipitation, cell lysate containing ~500 µg protein was cleared with 30 µl Protein A (Millipore) for 4 h prior to incubation with anti-PD-L1 antibody (Cell Signaling, 7 µl used for H2009 and 5 µl used for SK-MES-1) and 25 μl Protein A agarose overnight. The beads were collected with centrifugation and washed seven times with M2 buffer before addition of Laemmli loading buffer. Protein samples were run on 12% sodium dodecyl sulfate–polyacrylamide gel and transferred to polyvinylidene difluoride membrane, which was blocked with 5% skim milk for 2 h at room temperature, incubated with primary antibody overnight at 4°C and then with secondary antibodies at room temperature for 1 h. Signal was developed with enhanced chemiluminescence reagent (Millipore), visualized and recorded using Bio-Rad ChemiDoc Imaging System. Quantification of the bands was done with ImageJ similar to that for conventional PCR bands as described above.
Removal of N-glycosylation from cellular proteins
Cells were treated with IFNγ (10 ng/ml) for 24 h and lysed with M2 buffer. The lysates (50 µg for A549 and 40 µg for H2009) were denatured at 100°C for 10 min followed by incubation with recombinant PNGase F (P0708S, New England Biolabs, Ipswich, MA) in 37°C water bath for 1 h according to the protocol of the manufacturer. The enzymatically treated lysates were then subjected to western blot analysis with same amounts of untreated lysates.
Statistics
Quantitative data were expressed as mean ± standard deviation. Statistics was done with GraphPad Prism 5.0 software using two-tailed unpaired t-test to compare two means. P < 0.05 was considered statistically significant.
Results
IFNγ induces PD-L1 expression in lung cancer cells without activating HBP
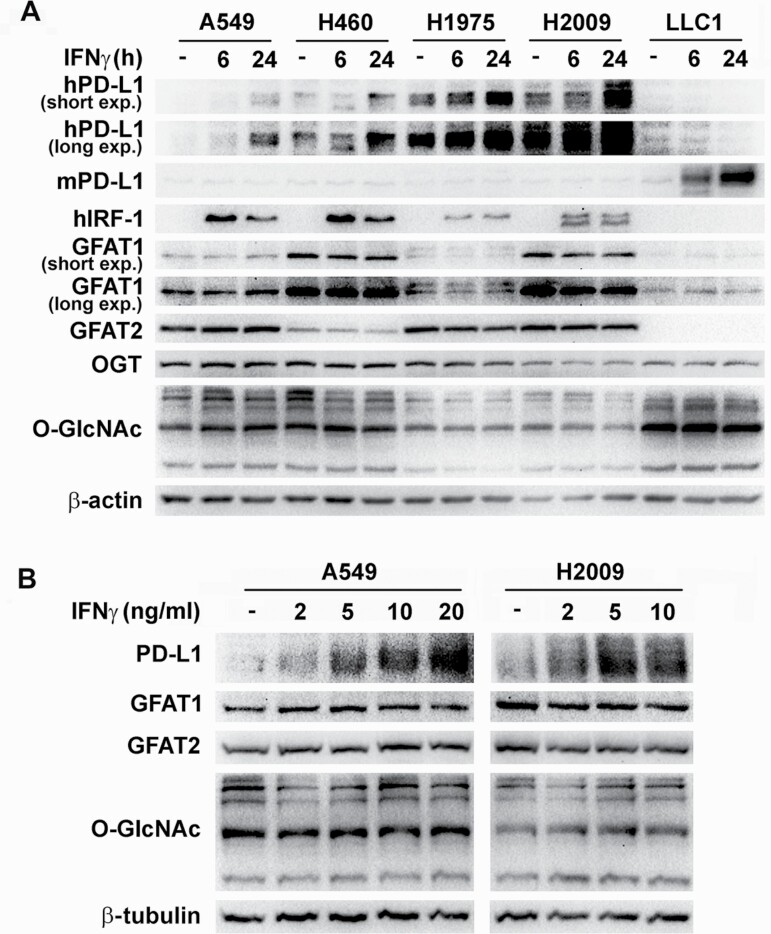
The effects of IFNγ stimulation on the expression of PD-L1 and the activation of the HBP were investigated. Consistent with previous studies (12,13), IFNγ increased PD-L1 protein level in human lung cancer cells regardless of common gene mutation status (wild-type or mutant p53, K-ras or epidermal growth factor receptor) and basal levels of the protein and in murine Lewis lung cancer cells (LLC1). All human lung cancer cell lines expressed GFAT isozymes (GFAT1 and GFAT2) at various abundances. LLC1 expressed GFAT1, but GFAT2 was undetectable in the cells. No substantial or consistent induction of the protein and mRNA of the isozymes or mRNA of other enzymes of HBP by IFNγ was observed (Figure 1A and B and Supplementary Figure S1A and B). O-GlcNAc transferase (OGT), the enzyme that catalyzes the attachment of O-GlcNAc, also did not change after IFNγ stimulation. Accordingly, protein O-GlcNAcylation levels indicative of HBP activity remained largely the same in treated and control cells. The activity of IFNγ was confirmed by the induction of interferon gamma response factor 1 (IRF-1), one of its major target genes (Figure 1A).
Figure 1.
IFNγ induces PD-L1 expression in lung cancer cells without activation of HBP. (A) Lung cancer cell lines were treated with 10 ng/ml IFNγ for 6 and 24 h. (B) H2009 and A549 cells were treated with increasing concentrations of IFNγ for 24 h. Protein levels were determined by western blot in total cell lysates with respective antibodies. β-Actin or β-tubulin was used as a loading control.
Basal GFAT1 activity is indispensable for IFNγ-induced PD-L1 expression
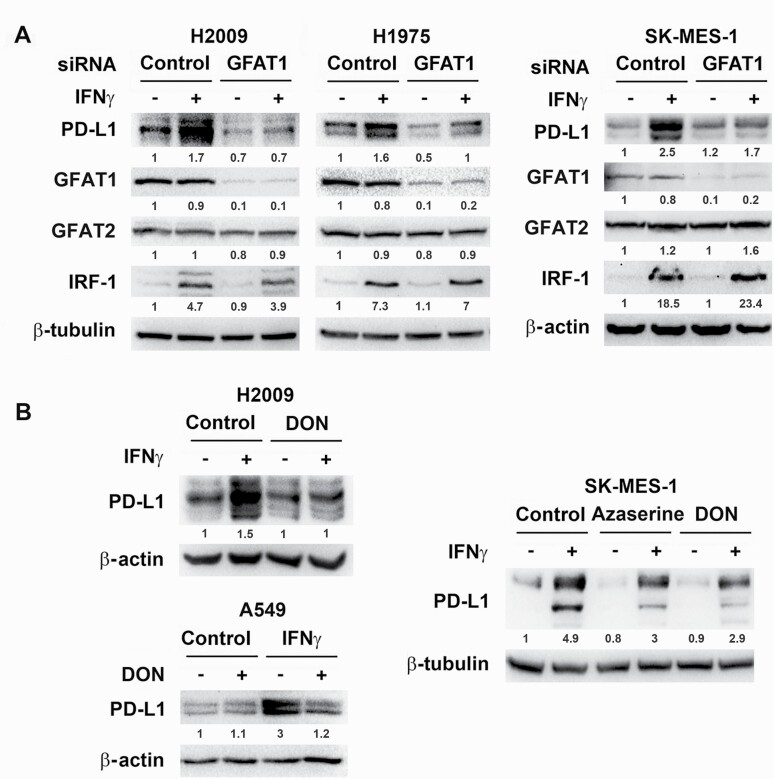
Because lung cancer cells generally have higher HBP activity in comparison with normal bronchial epithelial cells (14), whether basal GFAT activity affects IFNγ-induced PD-L1 expression was examined. siRNA knockdown of GFAT1 in all the human lung cancer cell lines tested (A549, H2009, H1975, SK-MES-1 and SW900) decreased PD-L1 levels induced by IFNγ, whereas GFAT2 knockdown exerted mild effects and no further reduction to GFAT1 knockdown (Figure 2A and Supplementary Figure S2A). In LLC1 cells with undetectable GFAT2, the effect of IFNγ on PD-L1 expression was suppressed by knocking down GFAT1 (Supplementary Figure S2B). Further, DON as well as azaserine, both glutamine analogs and inhibitors of GFAT, recapitulated the effect of GFAT1 siRNA in suppression of IFNγ-induced PD-L1 expression in cancer cells (Figure 2B and Supplementary Figure S2B). In contrast, CB-839, which inhibits glutaminase, also a target of glutamine analogs, did not reduce PD-L1 expression (Supplementary Figure S3). These results suggest that IFNγ-induced PD-L1 expression mainly depends on GFAT1 but not GFAT2.
Figure 2.
Knockdown of GFAT1 or inhibition of its activity decreases IFNγ-induced PD-L1 protein level in cancer cells. Cancer cells were transfected with control or GFAT1 siRNA for 24 h (A) or incubated with DON (10 µM for H2009 and SK-MES-1, 5 µM for A549) or azaserine (10 µM) for 30 min (B) prior to treatment with IFNγ (10 ng/ml) for 24 h. Western blot was carried out to detect the expression of proteins in total cell lysates using β-actin or β-tubulin as a loading control. The density of the bands was quantified with ImageJ software, normalized to loading control and then fold changes were calculated and marked below the bands.
GFAT1 affects PD-L1 protein stability
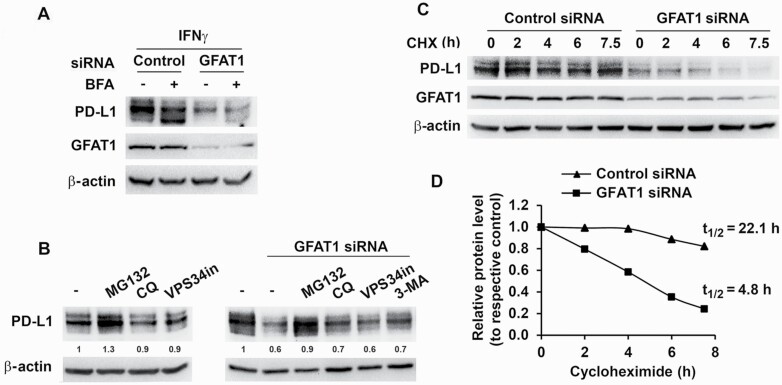
The mechanism of suppression of PD-L1 expression by inhibiting GFAT1 was investigated. Phosphorylation of STAT1 and STAT3 and the induction of interferon gamma response factor 1 (target gene of STAT1) by IFNγ (15) were largely comparable in GFAT1 knockdown cells and control cells (Figure 2A and Supplementary Figures S2A and S4A). Furthermore, GFAT1 knockdown did not decrease IFNγ-induced PD-L1 mRNA expression in various cancer cells (Supplementary Figure S4B). These results suggest that GFAT1 does not directly modulate IFNγ signaling. In addition, GFAT1 knockdown did not impede the cycling of PD-L1 (16) as assessed by treating cells with brefeldin A, which interrupts protein transport from endoplasmic reticulum to Golgi apparatus. Treatment of knockdown cells with inhibitors that suppress autophagy and lysosome activity (chloroquine, VPS34 inhibitor and 3-methyladenine) was also not able to recover PD-L1 level (Figure 3A and B). On the other hand, proteasome inhibitor MG132 increased basal level of PD-L1 and partially reversed the effect of GFAT1 knockdown on PD-L1 expression (Figure 3B). In GFAT1 knockdown H2009 cells, the basal PD-L1 level was lower (Figures 2A and 3A and C), and there was a 4-fold increase in the rate of degradation of the protein (half-life 4.8 h compared with over 22 h projected in control cells; Figure 3C and D). Therefore, inhibition of GFAT1 accelerated degradation of PD-L1 through the proteasome pathway (13).
Figure 3.
Knockdown of GFAT1 accelerates PD-L1 degradation by proteasomal pathway. (A) H2009 cells were transfected with control or GFAT1 siRNA for 24 h and then incubated with IFNγ for 6 h with or without prior brefeldin A (BFA, 5 µM) treatment for 30 min. (B) H2009 cells were treated with inhibitors [MG132, 1 µM; chloroquine (CQ), 20 µM; and 3-methyladenine (3-MA), 5 mM; left panel] or transfected with control or GFAT1 siRNA. Six hours post-transfection, media were refreshed and inhibitors (MG132, 1 µM; CQ, 20 µM; VPS34 inhibitor, 10 µM; and 3-MA, 5 mM) added (right panel). Total cell lysate was prepared 24 h later for western blot. Low concentration (1 µM) of MG132 was used to minimize cell death in this setting. (C and D) H2009 cells were transfected with control or GFAT1 siRNA for 24 h. Cycloheximide (CHX, 10 µM) was added to the cells at different time points. PD-L1 levels were detected by western blot. Band densities were quantified and normalized to loading control and the half-life (t1/2) of PD-L1 was calculated using Microsoft Excel equation with corresponding untreated control set as 1.
GFAT1 activity is important for glycosylation of PD-L1
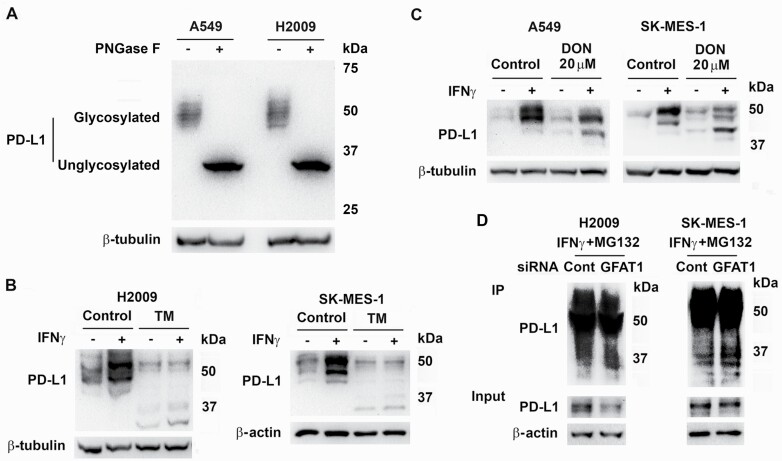
Recent studies indicate that N-linked glycosylation is essential for PD-L1 protein stability (13). The molecular weight of native human PD-L1 is 33.275 kDa (UniProt Q9NZQ7). Glycosylation results in species with higher molecular weights that accumulate at ~50 kDa. This is evident by completely removing the N-glycan from the protein with PNGase F (Figure 4A) and consistent with previous reports (13,16). Treatment with tunicamycin, which inhibits N-linked glycosylation before nascent proteins entering the endoplasmic reticulum (17), strongly reduced the total induced PD-L1 protein with the presence of lower molecular weight bands representing unglycosylated and lower glycosylated species of PD-L1 (Figure 4B and Supplementary Figure S3) (13). Since HBP product GlcNAc is the major building block for protein glycosylation, we investigated if inhibition of GFAT1 interfered the glycosylation of PD-L1. Similar to the results from tunicamycin treatment, administration of high concentration of DON decreased total PD-L1 with resultant low-molecular-weight proteins (Figure 4C). However, the 33 kDa band was not detected in DON treated or GFAT1 knockdown cells probably because of low abundance through continuous degradation in these conditions. Supporting this hypothesis, in GFAT1 knockdown cells treated with IFNγ and incubated with proteasome inhibitor MG132, there was an increase of PD-L1 species with molecular weight ~37 kDa and the 33 kDa band was detectable (Figure 4D), suggesting newly synthesized protein had less glycosylation. Inhibition of GFAT1 can reduce global protein O-GlcNAcylation, which appeared to not affect PD-L1 stability (Supplementary Figure S5).
Figure 4.
Knockdown of GFAT1 decreases glycosylation of PD-L1. (A) A549 and H2009 cells were treated with IFNγ for 24 h. Cell lysates were untreated or denatured and then incubated with PNGase F in 37°C water bath for 1 h. (B) H2009 and SK-MES-1 cells were treated with tunicamycin (TM, 1 µM) for 30 min before addition of IFNγ for 24 h. (C) A549 and SK-MES-1 cells were treated with DON (20 µM) 30 min prior to addition of IFNγ for 24 h. Proteins were detected with western blot (A–C). (D) H2009 and SK-MES-1 cells were transfected with control (Cont) or GFAT1 siRNA for 24 h. The cells were incubated with medium with IFNγ for 6 h before the addition of MG132 (1 µM for H2009 and 2 µM for SK-MES-1). The next day, cells were lysed for immunoprecipitation with anti-PD-L1 antibody and Protein A agarose. Western blot was carried out with 5% lysate as inputs using TrueBlot rabbit second antibody.
Inhibition of GFAT1 in lung cancer cells diminishes the suppression of T-cell activation and enhances NK cells killing of IFNγ-treated cancer cells
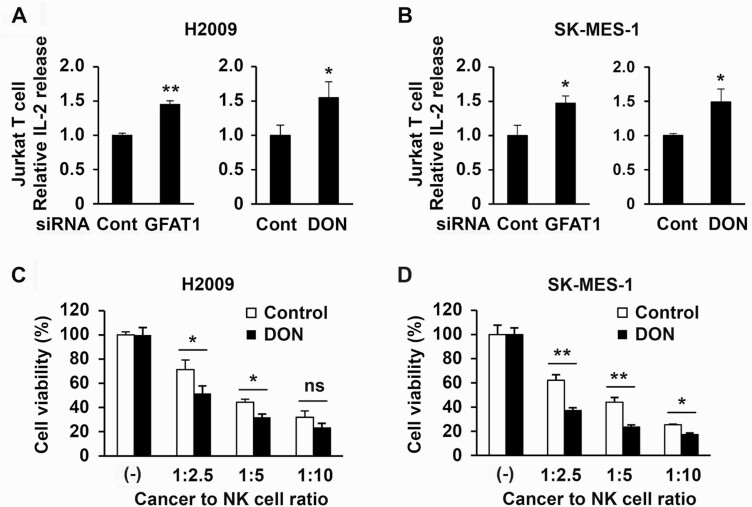
The functional consequence of inhibition of GFAT1 activity and PD-L1 expression was assessed by knocking down GFAT1 in cancer cells or treating cancer cells with DON before exposure to IFNγ followed by culturing the treated cancer cells with activated T cells or NK cells. Both immune cells express PD-1, which greatly increased in activated T cells (Supplementary Figure S6A) as reported previously (18). After addition to cancer cells in monolayer culture, the immune cells were seen to attach on cancer cells, indicating their interaction, irrespective of prior treatment with DON or knockdown of GFAT1. However, compared with cocultures with control cancer cells, activated T cells released ~50% more IL-2 in the presence of cancer cells transfected with GFAT1 siRNA or treated with DON (Figure 5A and B and Supplementary Figure S6B). Similarly, the addition of NK cells for 4 h to H2009 and SK-MES-1 cultures showed dose-dependent reduction in cancer cell viability that was greater with reduced GFAT activity (Figure 5C and D).
Figure 5.
Knockdown of GFAT1 or DON treatment in cancer cells increases T-cell activation and NK cell killing. (A and B) Cancer cells were seeded in triplicate on 24-well plates at a density of 5 × 104 cells/well. The next day, cells were transfected with control or GFAT1 siRNA for another 24 h. The cells were then incubated with media containing IFNγ (10 ng/ml) for 6 h. The medium was then removed and Jurkat T cells in medium with 20 ng/ml phorbol 12-myristate 13-acetate and 200 μg/ml ionomycin were added to cancer cells. The ratio of cancer cell to T cell was 1:2 for H2009 and 1:4 for SK-MES-1. The next day (18 h for H2009 and 24 h for SK-MES-1), the media were collected (100 µl/well) for enzyme-linked immunoabsorbing assay for IL-2 concentration. For DON inhibition, 10 µM DON was added overnight before the treatment of IFNγ, and the ratio of cancer cell to T cell was 1:3 and incubation time was 24 h for both cell lines. (C and D) Cancer cells were seeded at a density of 2 × 104 cells/well on a 48-well plate in triplicate for 24 h, treated with DON (10 µM) for 30 min followed by incubated with IFNγ (10 ng/ml) overnight. The media were changed and NK-92 cells were added to the monolayer at the indicated ratios for 4 h. The media were then refreshed to remove NK cells. MTT assay was carried out 24 h later. *P < 0.05; **P < 0.01; ns, not significant.
Discussion
Currently PD-L1 is targeted by monoclonal antibodies in clinical practice. Other compounds that can modulate PD-L1 expression could provide alternatives to antibodies with different pharmacological properties and toxicity profiles, which may be used in combinational therapy. For example, metformin induces degradation of PD-L1 through endoplasmic reticulum-associated (19) or other signaling pathways (20) and was shown to improve overall response and disease control rate in combination with checkpoint inhibitors in small clinical trials in non-small cell lung cancer (21) and melanoma (22). Many natural compounds (e.g. Triptolide (23), Gallic acid (24) and Resveratrol (25)) were also able to inhibit the expression of PD-L1 in cancer cells through various mechanisms. Like metformin, these compounds are multifunctional rather than just affecting the expression of PD-L1. There are also efforts to screen small molecules (26), peptides (27) and kinase inhibitors (28) for PD-L1 inhibitors.
Modulating turnover is a novel approach to control PD-L1 protein level. PD-L1 is heavily N-glycosylated, which increases its stabilization. Several proteins are involved in posttranslational modification, trafficking and degradation of PD-L1. Protease COP9 signalosome 5 was shown to inhibit the ubiquitination and proteasomal degradation of PD-L1 (29). Further, CKLF-like MARVEL transmembrane domain-containing protein 4 and 6 are proteins maintaining the cell surface expression of PD-L1 and reducing its endocytosis and degradation in lysosome (16,30). Targeting these proteins as well as glycosylation to increase the degradation of PD-L1 is in development.
This work provides compelling evidence for the regulation of PD-L1 by GFAT1, a rate-limiting enzyme of the HBP. We show that inhibition of GFAT1 activity can increase the degradation of PD-L1 in lung cancer cells. The increasing degradation of the protein is attributed to decreased N-linked glycosylation when GFAT1 activity is impaired, although additional mechanisms cannot be ruled out. In addition, if inhibition of other enzymes of HBP has similar effect remains to be determined. Importantly, inhibiting GFAT1 in cancer cells significantly alleviates the inhibitory effects of cancer cells on the function of T cells and NK cells. Inhibition of GFAT can suppress cancer cell proliferation that could confound the analysis of the immune response, particularly IL-2 secretion from Jurkat T cells. As detailed in the Materials and methods, the coculture experiments were carried out in relative short times with other implementations to minimize the impact of treatments on cell proliferation, thus negated any significant contribution of differences in proliferation on the increased immune response.
The advantage of inhibiting GFAT1 is that DON is available as a prototypic GFAT inhibitor and a research tool. As a glutamine analog, DON is not specific to GFAT, but it has gone through numerous clinical trials in the past; therefore, its toxicity is known and efforts to minimize the side effects are ongoing (31,32). New inhibitors for GFAT are also in development (33–35). Another advantage of targeting GFAT/HBP is that inhibition of the pathway itself has anticancer properties, as we (14) and others have demonstrated (36–39). HBP is frequently hyperactive in cancer cells. Inhibition of HBP can thus have much broader influences besides PD-L1 degradation in cancer cells in systemic therapy, which may cause side effects but increase efficacy against cancer. Therefore, our study supports developing therapeutics to modulate HBP/GFAT1 activity to augment the effectiveness of immunotherapy using checkpoint blockers.
Supplementary Material
Acknowledgements
This paper is dedicated to Dr Yong Lin, a highly valued member of the Lovelace Lung Cancer Program who lost his battle to cancer in April 2020. Yong was a productive molecular biologist who made many important contributions toward understanding the mechanisms of programmed cell death and deregulation of signal transduction pathways in cancer.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- DON
6-diazo-5-oxo-l-norleucine
- GFAT
l-glutamine:d-fructose-6-phosphate amidotransferase
- GlcNAc
N-acetyl-glucosamine
- HBP
hexosamine biosynthesis pathway
- IFNγ
interferon gamma
- IL-2
interleukin 2
- NK
Natural Killer
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- siRNA
small interfering RNA
Funding
National Cancer Institute, National Institutes of Health (R03CA223637 to W.C. and P30CA11800 to S.A.B.).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Thommen, D.S., et al. (2018) T cell dysfunction in cancer. Cancer Cell, 33, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettinger, D.S., et al. (2017) Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw., 15, 504–535. [DOI] [PubMed] [Google Scholar]
- 3.Abdel Karim, N., et al. (2019) Role of targeted therapy and immune checkpoint blockers in advanced non-small cell lung cancer: a review. Oncologist, 24, 1270–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma, P., et al. (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell, 168, 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun, C., et al. (2018) Regulation and function of the PD-L1 checkpoint. Immunity, 48, 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mojic, M., et al. (2017) The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int. J. Mol. Sci., 19, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepesi, B., et al. (2017) Programmed death cell ligand 1 (PD-L1) is associated with survival in stage I non-small cell lung cancer. Semin. Thorac. Cardiovasc. Surg., 29, 408–415. [DOI] [PubMed] [Google Scholar]
- 8.Handa, Y., et al. (2020) Prognostic impact of programmed death-ligand 1 and surrounding immune status on stage I lung cancer. Clin. Lung Cancer, 21, e302–e314. [DOI] [PubMed] [Google Scholar]
- 9.Cha, J.H., et al. (2019) Mechanisms controlling PD-L1 expression in cancer. Mol. Cell, 76, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akella, N.M., et al. (2019) Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol., 17, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tellez, C.S., et al. (2011) EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res., 71, 3087–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lastwika, K.J., et al. (2016) Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res., 76, 227–238. [DOI] [PubMed] [Google Scholar]
- 13.Li, C.W., et al. (2016) Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun., 7, 12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, W., et al. (2019) Inhibition of the hexosamine biosynthesis pathway potentiates cisplatin cytotoxicity by decreasing BiP expression in non-small-cell lung cancer cells. Mol. Carcinog., 58, 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, D.S., et al. (2017) Current prospects of type II interferon γ signaling and autoimmunity. J. Biol. Chem., 292, 13925–13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burr, M.L., et al. (2017) CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature, 549, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasconcelos-Dos-Santos, A., et al. (2015) Biosynthetic machinery involved in aberrant glycosylation: promising targets for developing of drugs against cancer. Front. Oncol., 5, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vibhakar, R., et al. (1997) Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp. Cell Res., 232, 25–28. [DOI] [PubMed] [Google Scholar]
- 19.Cha, J.H., et al. (2018) Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell, 71, 606–620.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, J.J., et al. (2019) Metformin attenuates PD-L1 expression through activating Hippo signaling pathway in colorectal cancer cells. Am. J. Transl. Res., 11, 6965–6976. [PMC free article] [PubMed] [Google Scholar]
- 21.Afzal, M.Z., et al. (2019) Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag., 8, LMT11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afzal, M.Z., et al. (2018) Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J. Immunother. Cancer, 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, M., et al. (2008) Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett., 270, 337–341. [DOI] [PubMed] [Google Scholar]
- 24.Kang, D.Y., et al. (2020) The inhibitory mechanisms of tumor PD-L1 expression by natural bioactive gallic acid in non-small-cell lung cancer (NSCLC) cells. Cancers (Basel), 12, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdura, S., et al. (2020) Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging (Albany, NY), 12, 8–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han, Y., et al. (2018) PD-1/PD-L1 inhibitor screening of caffeoylquinic acid compounds using surface plasmon resonance spectroscopy. Anal. Biochem., 547, 52–56. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y., et al. (2019) PD-1-targeted discovery of peptide inhibitors by virtual screening, molecular dynamics simulation, and surface plasmon resonance. Molecules, 24, 3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie, Y., et al. (2020) Screening of kinase inhibitors downregulating PD-L1 expression via on/in cell quantitative immunoblots. Eur. J. Pharm. Sci., 142, 105088. [DOI] [PubMed] [Google Scholar]
- 29.Lim, S.O., et al. (2016) Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell, 30, 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezzadra, R., et al. (2017) Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature, 549, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemberg, K.M., et al. (2018) We’re not “DON” yet: optimal dosing and prodrug delivery of 6-diazo-5-oxo-l-norleucine. Mol. Cancer Ther., 17, 1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leone, R.D., et al. (2019) Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science, 366, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian, Y., et al. (2011) Discovery of 1-arylcarbonyl-6,7-dimethoxyisoquinoline derivatives as glutamine fructose-6-phosphate amidotransferase (GFAT) inhibitors. Bioorg. Med. Chem. Lett., 21, 6264–6269. [DOI] [PubMed] [Google Scholar]
- 34.Vyas, B., et al. (2013) Glutamine: fructose-6-phosphate amidotransferase (GFAT): homology modelling and designing of new inhibitors using pharmacophore and docking based hierarchical virtual screening protocol. SAR QSAR Environ. Res., 24, 733–752. [DOI] [PubMed] [Google Scholar]
- 35.Kertmen, A., et al. (2019) Emerging anticancer activity of candidal glucoseamine-6-phosphate synthase inhibitors upon nanoparticle-mediated delivery. Langmuir, 35, 5281–5293. [DOI] [PubMed] [Google Scholar]
- 36.Onodera, Y., et al. (2014) Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J. Clin. Invest., 124, 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying, H., et al. (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chokchaitaweesuk, C., et al. (2019) Enhanced hexosamine metabolism drives metabolic and signaling networks involving hyaluronan production and O-GlcNAcylation to exacerbate breast cancer. Cell Death Dis., 10, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma, N.S., et al. (2020) Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Invest., 130, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.