Abstract
IL-1β is a key mediator of the cytokine storm linked to high morbidity and mortality from COVID-19 and IL-1β blockade with Anakinra and Canakinumab during COVID-19 infection has entered clinical trials. Using mass cytometry of human peripheral blood mononuclear cells, we identified effector memory CD4+ T cells and CD4-CD8low/-CD161+ T cells, specifically those positive for the chemokine receptor CCR6, as the circulating immune subtypes with the greatest response to IL-1β. This response manifested as increased phosphorylation and thus activation of the proinflammatory transcription factor NF-κB and was also seen in other subsets, including CD11c+ myeloid dendritic cells, classical monocytes, two subsets of natural killer cells (CD16-CD56brightCD161- and CD16-CD56dimCD161+), and lineage- (Lin-) cells expressing CD161 and CD25. IL-1β also induced a rapid but less robust increase in the phosphorylation of the kinase p38 as compared to that of NF-κB in most of these immune cell subsets. Prolonged IL-1β stimulation increased the phosphorylation of the transcription factor STAT3 and to a lesser extent that of STAT1 and STAT5 across various immune cell types. IL-1β-induced production of IL-6 likely led to the activation of STAT1 and STAT3 at later time points. Interindividual heterogeneity and inhibition of STAT activation by Anakinra raises the possibility that assays measuring NF-κB phosphorylation in response to IL-1β in CCR6+ T cell subtypes could identify those patients at higher risk of cytokine storm and most likely to benefit from IL-1β-neutralizing therapies.
INTRODUCTION
The cytokine storm is suspected of producing the excessive immune response driving the severe cardiopulmonary complications that are fueling the high morbidity and mortality rates in certain COVID-19-infected individuals (1, 2). The cytokine storm, as seen in the macrophage activation syndrome or secondary haemophagocytic lyphohistocytosis, has previously been described as a serious complication in other clinical contexts including individuals with rheumatologic disorders such as systemic onset juvenile inflammatory arthritis (also known as Still’s disease) (3), sepsis (4), and CAR-T cell therapy (5). IL-1β is a key inflammatory cytokine implicated in the cytokine storm syndrome (4, 6, 7). Inhibition of IL-1β-induced cellular signaling with the IL-1 receptor (IL-1R) antagonist Anakinra and the IL-1β neutralizing antibody Canakinumab has shown promise in treating cytokine storm syndrome. These treatments have been proposed as a potential therapy for subjects with this severe complication of COVID-19 (8–14). However, little is known about the immune cell subtypes that are most responsive to IL-1β-induced inflammatory signals in humans. New technologies, such as mass cytometry (CyTOF), have emerged as powerful analytic tools to identify unique cell phenotypes associated with specific inflammatory stimuli and their inhibitors (15–17). CyTOF uses antibodies conjugated to isotopes of rare-earth metals not found in human cells to eliminate background signal and minimize signal overlap, allowing for the identification of a greater number of proteins that more precisely define cellular identity and activation states. These approaches hold promise for the discovery of the cellular mechanisms mediating IL-1β-induced pathologies and the identification of sensitive and specific biomarkers that could be used to assist in the early identification of the cytokine storm and individuals who may respond to specific therapies such as IL-1β or IL-1R inhibitors.
The activation of NF-κB and the MAPKs p38, ERK and JNK has been implicated in IL-1R1-mediated immune cell activation in mostly murine studies (18–26). Based on these findings, we used a 35 antibody IL-1β-response CyTOF panel to generate an atlas of IL-1β-induced signaling in human peripheral blood mononuclear cells (PBMCs). IL-1β rapidly induced increases in the phosphorylated (p)-NF-κB and p-p38 in distinct effector memory (EM) T subsets, monocytes, dendritic cells (DCs), natural killer (NK) cells, and lineage-(Lin-) CD161+CD25+ cells. The IL-1β-induced increase in p-p38 was less robust than that of p-NF-κB. In contrast to the rapid increase in p-NF-κB with only 15 minutes of IL-1β stimulation, prolonged stimulation for two hours greatly increased p-STAT3 and to a lesser extent p-STAT1 and p-STAT5 in subsets of T cells, monocytes, DCs, and Lin-CD161+CD25+ cells. We identified CCR6 as a marker for T cells most responsive to IL-1β. Anakinra substantially inhibited IL-1β-induced p-NF-κB and p-p38 in CCR6+ T cells, CD14+ monocytes, CD11c+ myeloid DCs (mDCs), Lin-CD161+CD25+ cells and CD56bright NK cells. This knowledge of cell types, unique markers enriched on responsive cells, and temporal expression of inflammatory signaling molecules in response to IL-1β stimulation in humans may enable the discovery of new approaches to limit IL-1β-induced cytokine storm and other pathologies. Additionally, the heterogeneity in subject responses to IL-1β suggests that findings could be utilized to identify those most at risk for cytokine storm and those most likely to benefit from IL-1β inhibition.
RESULTS
IL-1β induces early increases in p-NF-κB and p-p38 in distinct immune cell subtypes in humans
To identify unique immune signatures in response to IL-1β, a 35 antibody panel was developed based on a review of the literature on IL-1β-induced effects on immune cells (18–26) (Fig. 1A and table S1) and optimized (figs. S1, and S2, A and B). The panel included antibodies to major immune cell types, IL-1Rs, and the activated form of signaling proteins including p-NF-κB, p-p38, p-ERK, p-STAT1, p-STAT3 and p-STAT5. Cryopreserved PBMCs were stimulated with vehicle or IL-1β for 5, 15, 30, 60, 120, and 240 minutes, fixed, barcoded, and analyzed by CyTOF, and subsequent computational data analysis was performed (Fig. 1B). PBMCs stimulated with varying doses of IL-1β (10, 25, and 100 ng/ml) showed similar responses in terms of the amounts of p-NF-κB and p-p38 (fig. S3, A to D), so the 10 ng/ml dose was used for subsequent experiments. To map IL-1β-induced signaling responses across major immune cell types, SPADE (spanning-tree progression analysis of density-normalized events) analysis was applied on vehicle and IL-1β-stimulated samples. SPADE analysis identified 9 major immune cell populations including CD4+, CD8+, and CD4-CD8low T cells; CD19+ B cells; HLA-DR+CD14+ and HLA-DR+CD16+ monocytes; HLA-DR+CD11c+ (mDCs) and HLA-DR+CD123+ plasmacytoid DCs (pDCs); and HLA-DR-CD56+CD16+ NK cells (Fig. 1, C and D). CD4+ T cells are the most abundant cell type within PBMCs (Fig. 1D). The representative SPADE tree shows IL-1β-induced increases in p-NF-κB, p-p38, and p-ERK across different immune cell types with 15 minutes of stimulation (Fig. 1E). This time point was chosen because time course analysis demonstrated IL-1β-induced direct signaling activation peaked at this time point (fig. S4, A and B). Although T cells, monocytes, and DCs in the vehicle-treated samples showed basal amounts of p-NF-κB, p-p38 and p-ERK, IL-1β stimulation led to an increase in p-NF-κB in the CD4+ and CD4-CD8low T cell subsets and to a lesser extent in CD11c+ mDCs and NK cells (Fig. 1E). IL-1β increased p-p38 amounts primarily in CD14+ monocytes and to a lesser extent in CD11c+ mDCs and CD4+ T cells. IL-1β did not increase p-ERK amounts in any immune cell type, and B cells were relatively unresponsive to IL-1β stimulation (Fig. 1E). As expected, short-time stimulation of PBMCs with IL-1β did not induce STAT activation (figs. S5, A-H).
Fig 1. IL-1β induces rapid increases in p-NF-κB and p-p38 in distinct immune cell subtypes.
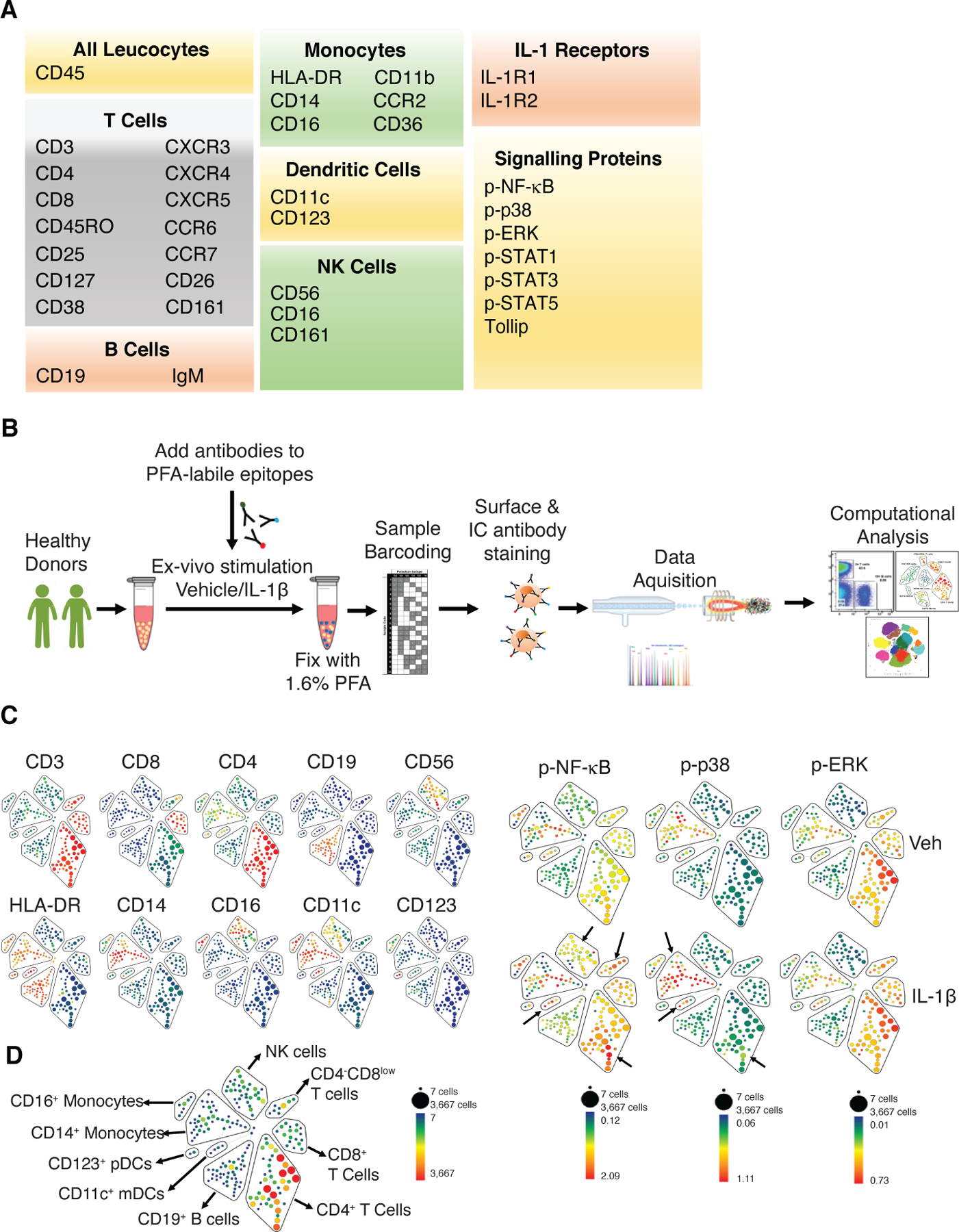
(A) CyTOF antibody panel. Note that many markers, including chemokine receptors, CD161, CD11b, and CD11c, are expressed on more than one cell type. (B) Experimental setup. Cryopreserved PBMCs from 3 healthy donors were thawed and rested for an hour in a culture incubator before being stimulated with IL-1β. (C) SPADE analysis was performed using cell surface markers to identify 200 cell clusters using 10% down-sampling. SPADE trees show median expression (blue to red depicting low to high) of the indicated lineage markers. The size of each node is proportional to the number of cells in that population. (D) 9 immune cell types were identified. Total T cells were identified as CD3+ and subsets were identified as CD4+, CD8+ and CD4-CD8low. B cells were identified as CD19+. NK cells were identified as CD56+CD16+. Monocyte subsets were identified as HLA-DR+CD14+ and HLA-DR+CD16+. DCs were identified as HLA-DR+CD11c+ (mDCs) and HLA-DR+CD123+ (pDCs). The blue to red color within each cluster corresponds to low to high cell count, with CD4+ T cells being the most abundant. (E) Representative SPADE tree from a donor showing median expression (blue to red depicting low to high) of p-NF-κB, p-p38 and p-ERK in vehicle-treated (Veh) and IL-1β-stimulated PBMC samples. The minimum and maximum for each scale depicting median expression differ for each phosphorylated protein.
Effector memory T cells show the greatest increase in p-NF-κB and p-p38 in response to IL-1β stimulation
To identify the specific T cell subsets that showed IL-1β-induced increases in p-NF-κB and p-p38, we performed UMAP (uniform manifold approximation and projection) and Leiden clustering on gated live CD3+ T cells (fig. S6, A-D). Unsupervised clustering of CD3+ T cells resulted in 12 clusters (Fig. 2, A and B), which were annotated based on the expression of lineage markers (Fig. 2C). The frequency of cells positive for p-NF-κB and p-p38 within T cell clusters were then quantitated by manual gating in Flowjo (fig. S6F). The frequency of p-NF-κB+ cells increased in Th17 cells (cluster 3; CD4+CD45RO+CCR6+CD161+CD26+), T follicular helper cells (Tfh) (cluster 4; CD4+CD45RO+CXCR5+), CD8low/-CD161+ T cells (cluster 8) and memory T regulatory cells (Mtregs) (cluster 12; CD4+CD45RO+CD25+CD127low/-) (Fig. 2D). Cluster 8 was heterogenous and contained CD8- and CD8+ cell populations (fig. S7, A and B). Both CD8- and CD8+ cell populations had comparable amounts of CD161, and showed IL-1β-induced increase in p-NF-κB (fig. S7, A and C). Similar to Th17 cells (cluster 3), CD8- and CD8+ subsets within cluster 8 expressed CD26 (fig. S7D), a marker that is associated with cells that can produce IL-17 (27). Moreover, both of these subsets had comparable expression of CD45RO, CD127, CCR2 and CCR6, while did not express CCR7 (fig. S7E). Notably, the CD8+ subset in cluster 8 colocalized with the CD8lowCD161high subset as identified earlier by Billerbeck et al. (28), when overlaid on total CD8 T cells (fig. S7, F and G).
Fig 2. Effector-memory T cell subsets are the predominant subsets that show IL-1β-induced increases in p-NF-κB and p-p38.

UMAP and Leiden clustering using the cell surface markers shown in C were performed on vehicle- and IL-1β-treated CD3+ T cells. (A) The 12 T cell clusters identified were superimposed on the UMAP. (B) Frequency of T cell clusters and inter-individual variations between donors. (C) Heatmap showing the median expression of surface markers across all 12 T cell clusters. The color in the heatmap represents the median of the arcsinh for each subset with 0–1 transformed marker expression. The dendrogram represents the hierarchical similarity for subsets. (D and E) Frequency of p-NF-κB+ cells and p-p38+ cells within each cluster in the vehicle-treated (–) and IL-1β-stimulated (+) samples. Data shown are mean ± SEM. N=3 donors for A-E.
All four IL-1β responsive T cell clusters were negative for CCR7 (Fig. 2C), indicating that IL-1β specifically targets the EM T cells in humans. Similar to p-NF-κB, IL-1β induced increases in p-p38 in Th17 cells (cluster 3), Tfh cells (cluster 4), and CD8low/-CD161high cells (cluster 8) (Fig. 2E). However, there was a lower frequency of cells with p-p38 as compared to p-NF-κB (1.5–3.5% compared to 5–20%, respectively). Specifically, there was wide variation (5–20%) in IL-1β-induced expression of p-NF-κB in these T cell subtypes between individuals, suggesting that this assay could identify individual differences in susceptibility to IL-1β-induced downstream effects.
CCR6 expression distinctly identifies T cells most responsive to IL-1β
Because IL-1β signals through binding to its surface receptor IL-1R1, we reasoned that the memory T cells that show induced expression of p-NF-κB or p-p38 upon IL-1β stimulation express IL-1R1 whereas the remaining memory T cells do not. However, p-NF-κB+ memory T cells showed lower mean expression of IL-1R1 in comparison to p-NF-κB- memory T cells (Fig. 3A). IL-1β signaling can induce receptor internalization (29) yet, there was no reduction in the mean expression of surface IL-1R1 after 15 minutes of IL-1β stimulation in both the p-NF-κB- and p-NF-κB+ memory T cell subsets (Fig. 3A). Cell surface IL-1R2 binds to IL-1β but acts as a decoy receptor (30). Thus, the ratio of the levels of IL-1R1 and IL-1R2 on the cell surface may determine the signaling outcome induced by IL-1β. Therefore, we also analyzed expression of IL-1R2 on the p-NF-κB+ and p-NF-κB- memory T cells. IL-1R2 expression was not detected on T cells or any other immune cell type in either vehicle-treated or IL-1β-stimulated PBMCs. Notably, the same IL-1R2 antibody clone detected IL-1R2 expression on CD4+ memory T cells activated with anti-CD3, anti-CD28, and IL-2 for 48 hours. Unstimulated cells in culture also showed moderate levels of IL-1R2 expression, yet it was undetectable on unstimulated, freshly purified PBMCs (fig. S8).
Fig 3. CCR6 expression marks T cells that are most responsive to IL-1β.
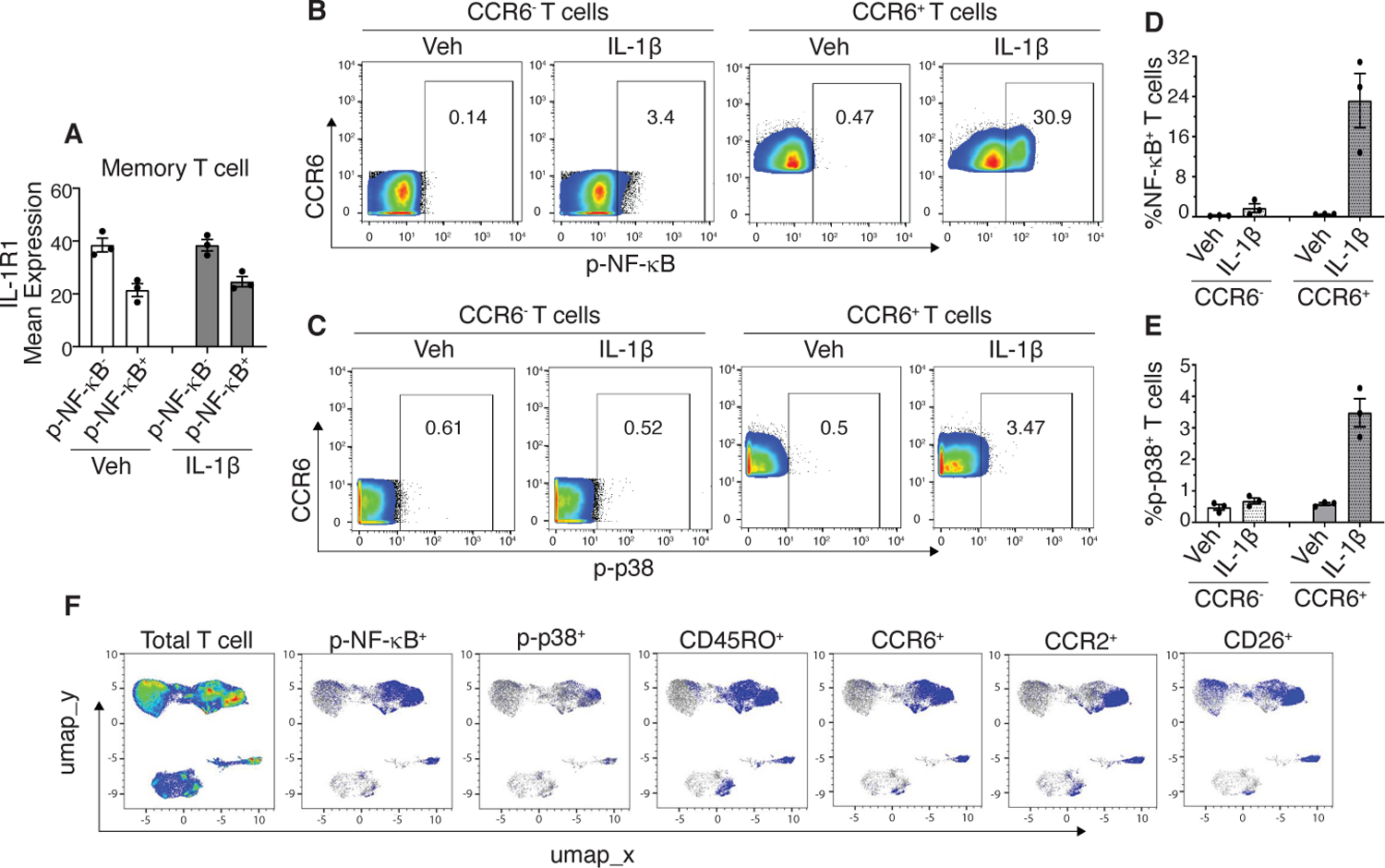
(A) Vehicle (Veh)-treated and IL-1β-stimulated CD3+ memory T cells were separated into p-NF-κB- and p-NF-κB+ subpopulations and mean expression of IL-1R1 for each subpopulation was plotted. (B and C) Representative flow plots from a donor showing frequency of p-NF-κB+ and p-p38+ cells within CCR6- and CCR6+ T cell subsets in the vehicle-treated and IL-1β-stimulated samples. (D and E) Quantified frequency of p-NF-κB+ and p-p38+ cells as shown in B and C. (F) Representative UMAP overlays of p-NF-κB+ and p-p38+ cells with the indicated cell surface markers. Data shown are mean ± SEM. N=3 donors for A-F.
CCR6 was the only marker that was present in the four T cell clusters (3, 4, 8, and 12) that showed IL-1β-induced increases in p-NF-κB (Fig. 2C). However, unlike lineage markers such as CD4, CD8 and CD45RO, CCR6 median abundance was moderate, raising the possibility that there may be a range of CCR6 abundance in these T cell subtypes and that CCR6 may mark those most prone to activation in response to IL-1β. To test this notion, we gated total CD3+ T cells into CCR6- and CCR6+ populations and analyzed p-NF-κB and p-p38 in these subsets. Indeed, higher frequencies of p-NF-κB+ and p-p38+ T cells were present within the T cells that expressed CCR6 as compared to those that do not (Fig. 3, B-E). Moreover, the majority (61.5 ± 2.75%) of the CCR6+ memory T cells that showed IL-1β-induced increases in p-NF-κB or p-p38 co-expressed CCR2 and CD26 (Fig. 3F).
Classical monocyte subsets and CD11c+ myeloid dendritic cells show IL-1β-induced increases in p-NF-κB and p-p38
To identify monocyte subsets and DCs that respond to IL-1β-induced signaling, we used CD3-CD19-CD56-HLA-DR+ cells (fig. S6E) for UMAP and Leiden clustering. Twelve clusters were identified (Fig. 4, A-C). Based on expression of lineage markers, seven clusters (1–6 and 9) belonged to the CM subtypes. Monocyte clustering with markers did not separate non-classical (NCM) and intermediate monocytes (IMM) (Fig. 4C). Both NCM and IMM and some CD14-CD16- cells were present within cluster 7 as confirmed by biaxial gating (fig. S9). Cluster 11 shared a similar phenotype to cluster 7 but expressed high levels of CD123 and was present in only one of the donors (Fig. 4B). Cluster 8 was CD11c+CD123- mDCs whereas cluster 10 was CD11c-CD123+ pDCs. Notably, cluster 12 was Lin- and only expressed CD38 and a low level of CXCR4. The frequency of cells positive for p-NF-κB within DC and monocyte clusters quantitated by manual gating in Flowjo (fig. S6, G and H) demonstrated most robust increase in p-NF-κB by IL-1β (~13-fold higher as compared to vehicle control) in the CM cluster 1 (Fig. 4D). In addition, a modest 2.5 to 5-fold induction in the frequency of p-NF-κB+ cells was observed in the other CM clusters including 2, 6 and 9. Among the CM clusters, cluster 1 showed the highest median expression of CCR2 and Tollip, and was distinct from the other CM clusters in terms of lower expression of CD11b and CD45RO (Fig. 4C). Of the two DC clusters, IL-1β-induced an increase in the frequency of p-NF-κB+ cells (13 to 30-fold) within the CD11c+ mDCs (cluster 8) (Fig. 4D). A modest increase in the frequency of p-p38+ cells was observed within the same CM clusters (1, 2, 6 and 9) and CD11c+ mDCs (Fig. 4E). In addition to monocyte subsets and CD11c+ mDCs, IL-1β also induced expression of p-NF-κB and p-p38 in the HLA-DR+Lin- cells (cluster 12).
Fig 4. Classical monocytes (CMs) and CD11c+ mDCs show IL-1β-induced increases in p-NF-κB and p-p38.
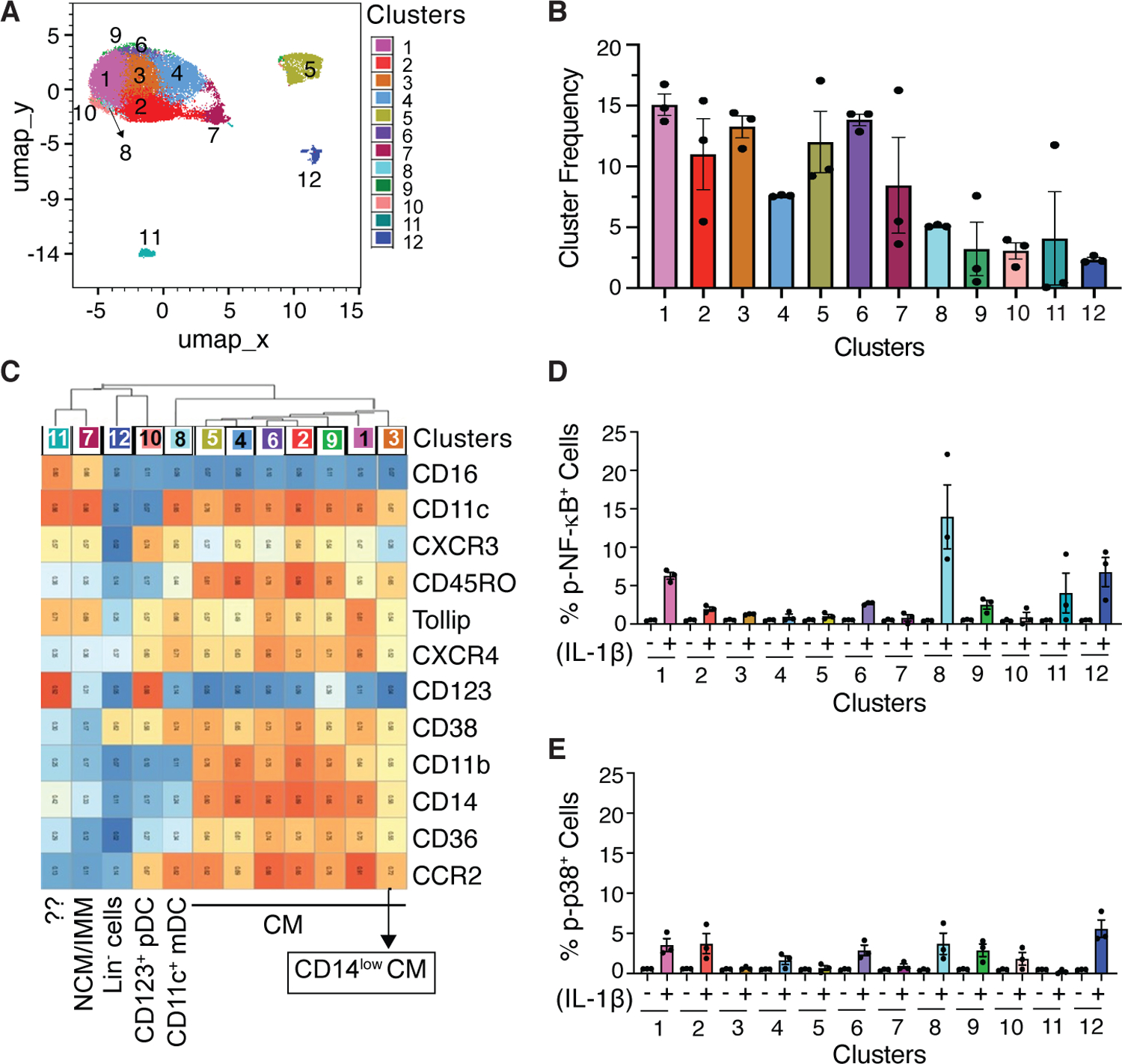
UMAP and Leiden clustering using the cell surface markers shown in C were performed on both vehicle and IL-1β-stimulated cells gated for CD3-CD19-CD56-HLA-DR+. (A) 12 cell clusters that included CMs and DCs were identified as shown superimposed on the UMAP. (B) Frequency of the 12 clusters and inter-individual variations. (C) Heatmap showing median expression of surface markers across all 12 cell clusters. (D and E) Frequency of p-NF-κB+ cells and p-p38+ cells within each cluster in the vehicle-treated (−) and IL-1β-stimulated (+) samples. Data shown are mean ± SEM. N=3 donors for A-E.
CD16-CD56-CD161+ cells expressing CD25 show a greater increase in p-NF-κB in response to IL-1β
CD3-CD19-HLA-DR- cells (fig. S6E) were used for NK cell clustering to identify IL-1β responsive subsets. These CD3-CD19-HLA-DR- cells included CD56bright, CD56dim, and CD56- populations. In addition to the prototypical NK cell markers such as CD16, CD56, and CD161, other cell surface markers expressed on NK cells, including CXCR3, CXCR4, CD38, CD25, CD11b, and CD11c were used for the clustering. A total of 17 distinct clusters were identified (Fig. 5, A-C). Analysis of IL-1β-induced signaling within cell clusters (fig. S6, I and J) demonstrated a 10 to 15-fold induction in the frequency of p-NF-κB+ cells in the CD16-CD56dimCD161+ NK cells (cluster 2) and CD16-CD56brightCD161- (cluster 3) (Fig. 5D). Cluster 17, another CD161+ cluster that expressed CD25 but was negative for both CD16 and CD56, showed the highest expression of p-NF-κB (>70-fold compared to vehicle control) (Fig. 5D). Moreover, IL-1β-induced increases in p-p38 was observed only in this cluster (Fig. 5E). Given that CD161 is also expressed on CD4+ and CD8+ T cell subsets and cluster 17 expressed CD25, we further confirmed that cluster 17 was not contaminating T cells. Although cluster 17 cells expressed similar levels of CD25 as the T cells, this cluster was negative for CD3, CD4, and CD8, therefore excluding the possibility that these are T cells (fig. S10A). Moreover, cluster 17 cells did not express other lineage markers including CD19, HLA-DR, CD14, CD16 and CD123, and the majority of the cells were CD56- (fig. S10, B and C). Human innate lymphoid cells (ILCs) also express CD161 (31) and Ohne et al. (25) demonstrated that IL-1β plays a critical role in inducing proliferation, cytokine production, maturation, and plasticity of human ILC2. IL-1β mediates these effects in combination with IL-2 and/or IL-12, and NF-κB inhibition blocks IL-1β-induced responses (25). p-NF-κB+ cells within cluster 17 exhibited a phenotype similar to that of ILC2. Consistent with the higher expression of CD127 in ILC2 as compared to CD56bright NK cells as shown by Ohne et al. (25), the p-NF-κB+ cells within cluster 17 expressed greater CD127 than the p-NF-κB+ cells within the CD56bright NK cells (cluster 3) (fig. S10D). However, in contrast to ILC2, around 20–30% of the p-NF-κB+ cells within cluster 17 were CD56dim (fig. S10, E and F).
Fig 5. CD16-CD56-CD161+ cells that express CD25 shows remarkable induction of p-NF-κB and p-p38 expression upon IL-1β stimulation.
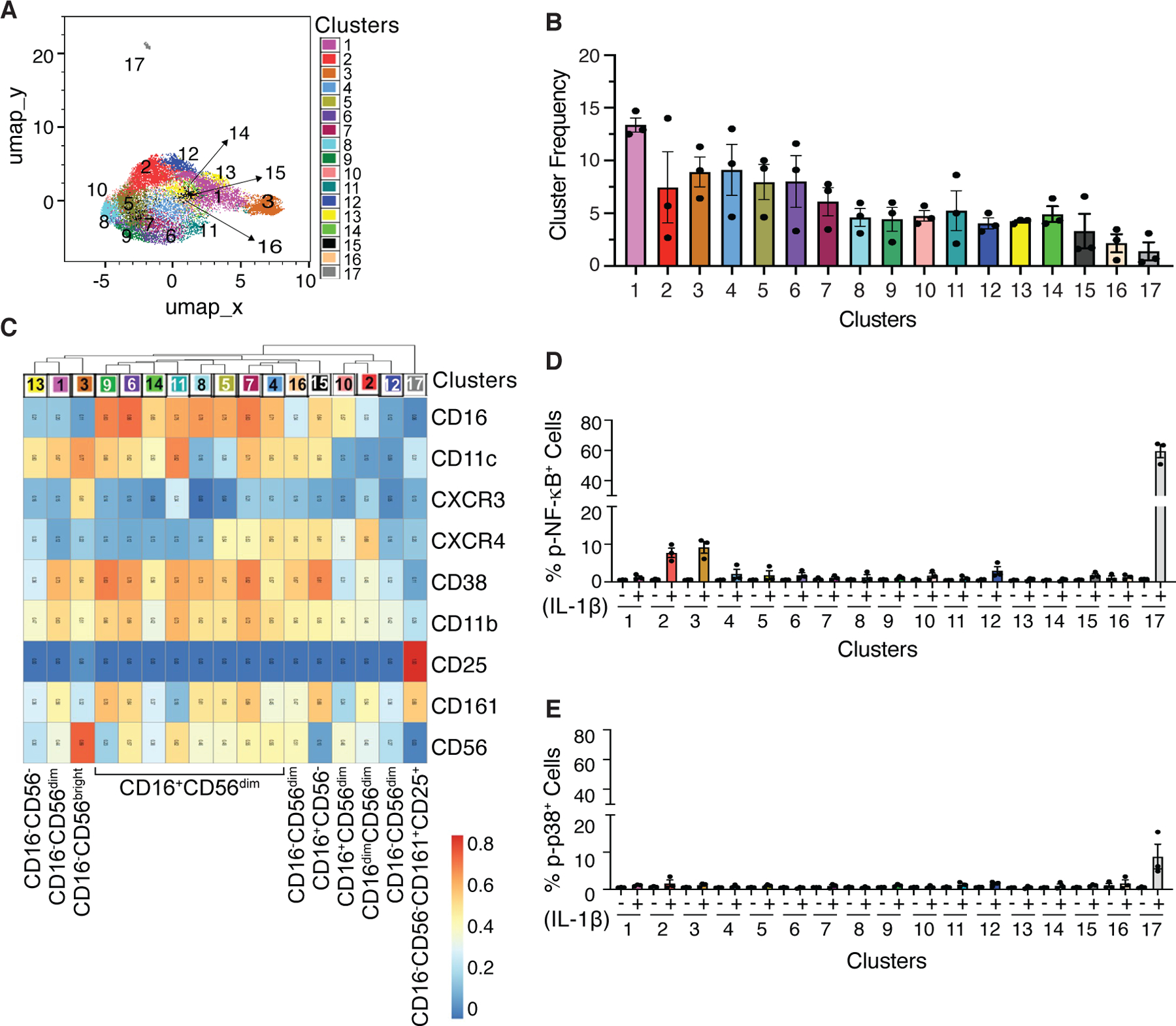
UMAP and Leiden clustering using the cell surface markers shown in C were performed on vehicle and IL-1β-stimulated cells gated for CD3-CD19-HLA-DR-. (A) 17 clusters were identified as shown superimposed on the UMAP. (B) Frequency of clusters and inter-individual variations. (C) Heatmap showing median intensity of surface markers across all 17 clusters. (D and E) Frequency of p-NF-κB+ cells and p-p38+ cells within each cluster in the vehicle-treated (−) and IL-1β-stimulated (+) samples. Data shown are mean ± SEM. N=3 donors for panel A-E.
Prolonged IL-1β stimulation induces increases in p-STAT3 and to a lesser extent in p-STAT1 and p-STAT5
To identify direct and indirect signaling mediated by IL-1β, time-dependent changes in the IL-1β-induced increases in phosphorylation were determined. IL-1β rapidly increased p-NF-κB and p-p38 in Th17 cells, Tfh cells, CD8low/-CD161+ T cells, and Mtregs (clusters 3, 4, 8, and 12), CD16-CD56dimCD161+ and CD16-CD56brightCD161- NK cells (clusters 2 and 3) and Lin-CD161+CD25+ cells (cluster 17) within 15 minutes, which reduced to baseline at later time points (figs. S4, A and B). Unlike the transient increase in p-NF-κB in T and NK clusters, the IL-1β-induced increase in p-NF-κB was sustained in the CD11c+ mDCs and CM clusters (fig. S4A). The kinetics of changes in p-p38 in CD11c+ mDCs and CM clusters was similar to those of T and NK cells (fig. S4B). Prolonged stimulation with IL-1β led to activation of STATs in T cells, monocytes, DCs and Lin-CD161+CD25+ cells (Fig. 6A and fig. S5, A-H). Compared to p-STAT1 or p-STAT5, p-STAT3 was more robustly induced by IL-1β, particularly in the CD4+ naïve and memory T cell clusters and CCR7+ CD8+ naïve T cells (Fig. 6A and fig. S5, A and B). Additionally, IL-1β-induced increases in p-STAT3 was also observed in CM (clusters 1, 2, 4, 5 and 6), HLA-DR+Lin- cells (cluster 12, Fig. 4C), CD11c+ mDCs, CD123+ pDCs, and Lin-CD161+CD25+ cells (cluster 17, Fig. 5C) (figs. S5, C and F). Notably, p-STAT3 in CD123+ pDCs was greater than in the CD11c+ mDCs and CM clusters (fig. S5C). IL-1β-induced increases in p-STAT1 was observed across all monocyte clusters (CM and NC/IMM), CD11c+ mDCs, CD123+ pDCs and Lin-CD161+CD25+ cells (figs. S5, D and G), and mostly in the CD4+ and CD8+ naïve T clusters (fig. S5A). As compared to p-STAT3 and p-STAT1, IL-1β induced low levels of p-STAT5. In contrast to the detection of p-STAT5 across monocyte and DC clusters and Lin-CD161+CD25+ cells (figs. S5, E and H), p-STAT5 in T cells was primarily detected within the Mtregs (cluster 12) and CD4+ naïve T cells expressing CD25 (cluster 6) (fig. S5B).
Fig 6. Prolonged IL-1β stimulation induces increases in p-STAT3.
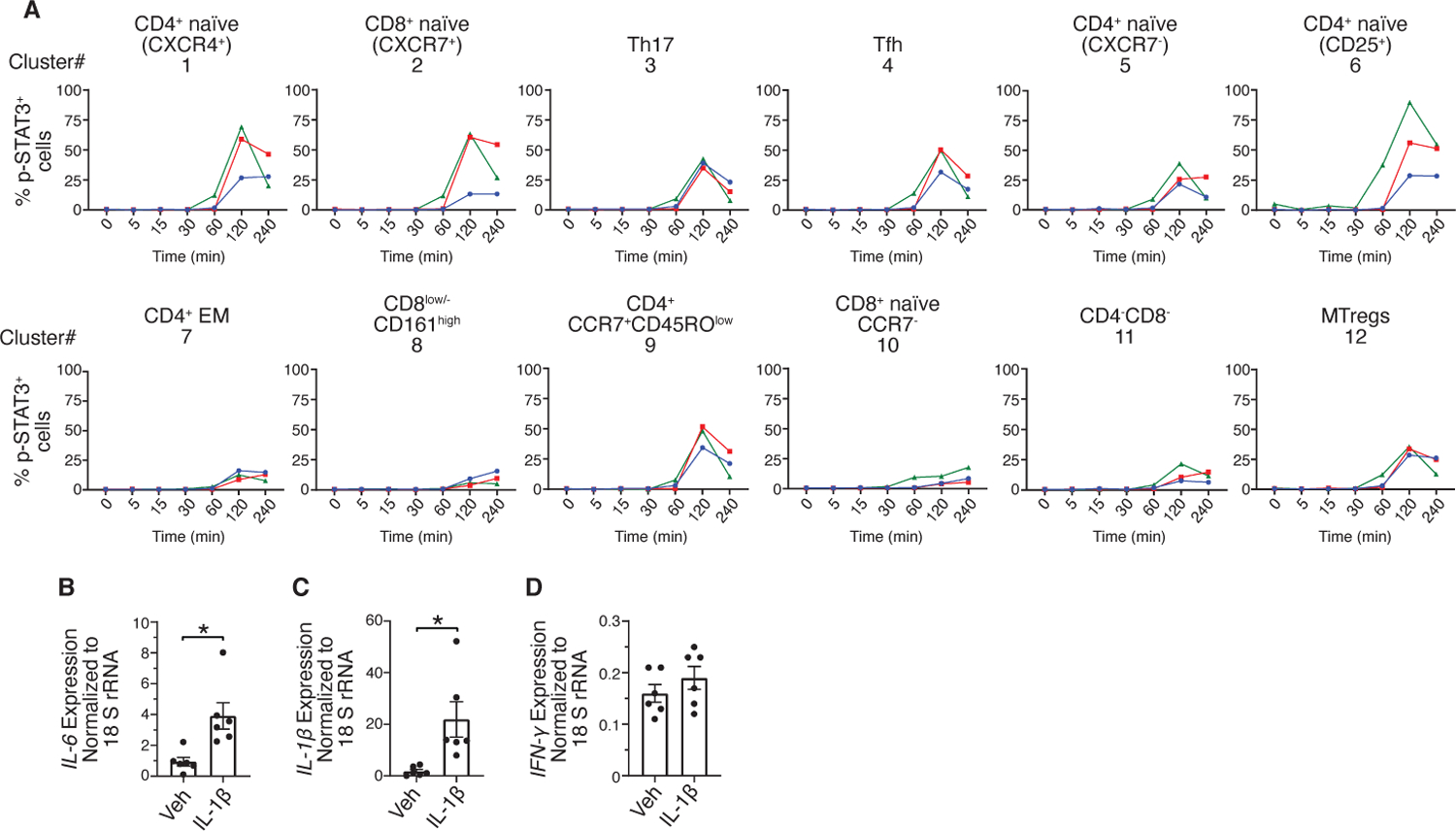
(A) Time-dependent changes in the frequency of p-STAT3+ cells within the 12 T cell clusters as identified by UMAP and Leiden clustering. Red, blue and green lines indicate 3 donors. (B to D) Rested PBMCs were incubated with vehicle (Veh) or IL-1β for 1.5 hours. Transcript levels of IL-6 (B), IL-1β (C) and IFN-γ (D) normalized to 18s rRNA based on RT-qPCR. Data shown are mean ± SEM. Wilcoxon signed-rank test; * p<0.05. N=6 donors for B-D.
IL-1β signaling leads to production of downstream cytokines including IL-6, IFN-γ and IL-1β that activate STAT and NF-κB. Thus, it is likely that these cytokines led to sustained increase in p-NF-κB in the CM and CD11c+ mDCs, and STAT activation at later time points. Indeed, transcript expression analysis demonstrated significant induction of IL-6 and IL-1β in PBMCs stimulated with IL-1β as compared to those treated with vehicle control. However, there was no change in the expression of IFN-γ (Fig. 6, B-D).
Anakinra abrogates IL-1β-induced signaling activation
Anakinra is a recombinant non-glycosylated IL-1R antagonist, which competitively binds to IL-1R1 and prevents IL-1β binding and downstream signaling. To determine the dependence of IL-1β-induced increases in p-NF-κB and p-p38 on IL-1R1, PBMCs were incubated with Anakinra prior to IL-1β stimulation. Anakinra substantially inhibited IL-1β-induced increases in p-NF-κB and p-p38 in CCR6+ EM T cells, CM, CD11c+ mDCs, Lin-CD161+CD25+ cells and CD56bright NK cells (Fig. 7, A-J).
Fig 7. Abrogation of IL-1β-induced signaling by Anakinra.
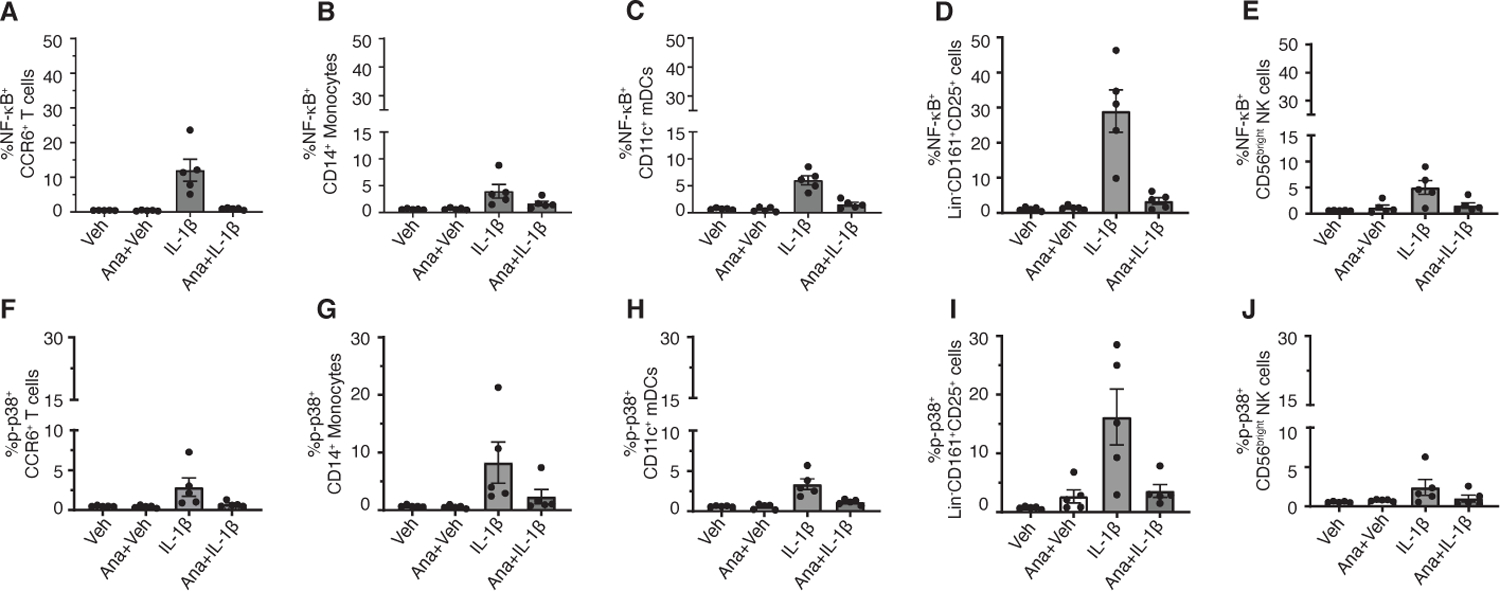
(A to J) PBMCs were incubated with 10 µg/ml Anakinra (Ana) for 30 minutes prior to treatment with vehicle (Veh) or IL-1β for 15 minutes. Cells were fixed, barcoded, and stained with cell surface and intracellular phosphoprotein antibodies. Debarcoded, normalized CyTOF data was analyzed in Flowjo. Frequency of p-NF-κB+ and p-p38+ CCR6+ T cells (A and F), p-NF-κB+ and p-p38+ CD14+ monocytes (B and G), p-NF-κB+ and p-p38+ CD11c+ mDCs (C and H) and p-NF-κB+ and p-p38+ Lin-CD161+CD25+ cells (D and I) and p-NF-κB+ and p-p38+ CD56bright NK cells (E and J). Data shown are mean ± SEM. N=5 donors for A-J.
CyTOF analysis of an independent cohort identified IL-1β induced signaling activation in the same immune cell subtypes
To validate our findings, we performed CyTOF analysis of IL-1β-stimulated PBMCs from an independent cohort of 6 healthy donors. Similar to our initial results, IL-1β-induced increase in p-NF-κB was specifically observed in CCR6+ EM T cells, CM, CD11c+ mDCs, Lin-CD161+CD25+ cells and CD56bright NK cells (Fig. 8, A-E). All these subsets, except CD56bright NK cells, also showed a significant increase in p-p38 upon IL-1β stimulation (Fig. 8, F-J). As seen in our initial CyTOF analysis, there was heterogeneity in the IL-1β-induced signaling response between donors. Differential expression of IL-1R1 and downstream signaling mediators in the IL-1β signaling pathway may contribute to the variability in the signaling response. As such, we quantitated endogenous expression of IL-R1, IL-1R2, MyD88, IRAK4 and Tollip by RT-qPCR in sort-purified CCR6+ EM T cells from these same donors. Relative values for endogeneous abundance of these transcripts were extrapolated from the standard curve generated by serial dilution of a reference sample and normalizing for the 18s rRNA. While there was some variability, especially in the expression of IL-1R2 and MyD88 between donors (fig. S11), there was no association between the abundance of transcripts encoding IL-1Rs and other signaling mediators in CCR6+ EM T cells and fold change in the frequency of p-NF-κB+ or p-p38+ CCR6+ EM T cells (table S2).
Fig 8. CyTOF analysis of an independent cohort identified IL-1β induced signaling activation in the same immune cell subtypes.
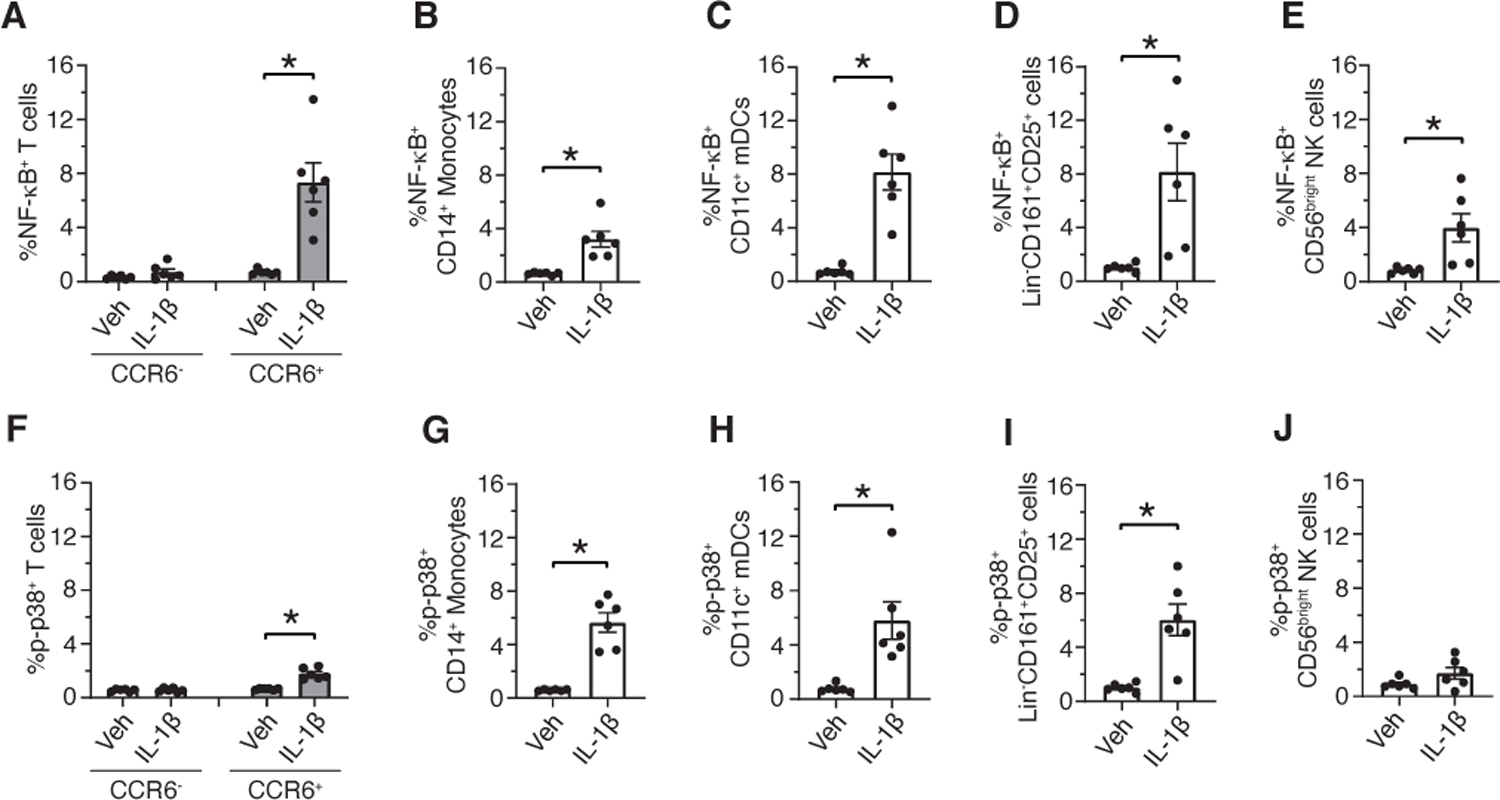
(A to J) PBMCs were incubated with vehicle (Veh) or IL-1β for 15 minutes. Cells were fixed, barcoded, and stained with cell surface and intracellular phosphoprotein antibodies. Debarcoded, normalized CyTOF data was analyzed in Flowjo. Frequency of p-NF-κB+ and p-p38+ CCR6+ T cells (A and F), p-NF-κB+ and p-p38+ CD14+ monocytes (B and G), p-NF-κB+ and p-p38+ CD11c+ mDCs (C and H), p-NF-κB+ and p-p38+ Lin-CD161+CD25+ cells (D and I) and p-NF-κB+ and p-p38+ CD56bright NK cells (E and J). Data shown are mean ± SEM. Wilcoxon signed-rank test; * p<0.05. N=6 donors for A-J.
Our future goal is to utilize this antibody panel to immunotype banked PBMCs from subjects with COVID-19 infection and other chronic inflammatory disorders in clinical trials of IL-1 blockers. We therefore confirmed if cryopreserved cells respond similarly to IL-1β stimulation as fresh PBMCs. The IL-1β-induced signaling response in cryopreserved PBMCs was comparable to that in the fresh PBMCs (fig. S12, A-H).
Discussion
Inhibition of IL-1β has emerged as a promising therapeutic approach for various inflammatory diseases (32–35) and a potential therapy for reducing the cytokine storm associated with COVID-19 (10–14). Although studies over the past decades have provided insights into pathophysiological mechanisms that lead to dysregulated production of IL-1β, knowledge about the specific human immune cell types that IL-1β targets is still limited. We identified the key human immune cell subtypes that were most responsive to IL-1β, as well as the subset-specific signaling pathways that were activated.
CD4+ EM T cells were the circulating immune cell subtype most robustly activated by IL-1β. CD4+ EM T cells display rapid effector functions as compared to their naïve counterparts and are implicated in various inflammatory diseases including atherosclerosis, rheumatoid arthritis (RA), and diabetes. Increased frequency of CD4+ EM T cells have been associated with increased disease activity and severity (36–43). T cell intrinsic IL-1R signaling is a licensing signal for cytokine production by memory CD4+ T cells and this critical cue is conserved across all EM lineages (20). IL-1β enhanced posttranscriptional stability of effector cytokines such as Th17 cytokines, IL-5, and IL-13 through the p38 pathway in the murine CD4+ EM T cells (20). Other studies, mostly mouse-based, also demonstrated that IL-1β is crucial for proinflammatory cytokine production by CD4+ EM T cells through the activation of NF-κB, p38 and JNK (18, 19, 21–24). Consistent with these studies, our CyTOF analyses in human immune cells demonstrated IL-1β-induced signaling predominantly in the CD4+ EM subsets including Th17, Tfh and MTregs. IL-1β-induced increases in p-NF-κB were more robust as compared to that of p-p38. Our data suggests that the NF-κB inflammatory pathway may play a more prominent role in IL-1β-mediated regulation of functions of the CD4+ EM T subsets in humans. Murine CD4+CXCR5hiCD25- Tfh cells express IL-1R1, and IL-1β triggers expansion of these Tfh cells in vivo and induces the production of IL-4 and IL-21, the two major B cell activating cytokines (44). Our study demonstrated that IL-1β induced NF-κB activation in the human Tfh cells, potentially providing new mechanistic insight into IL-1β-mediated dysregulated humoral response in patients with autoimmune diseases(45).
IL-1β induced increases in p-NF-κB in the EM T cell cluster 8 that comprised of two distinct cell subsets, CD4-CD8+ and CD4-CD8-, which express high levels of CD161, a predominantly NK cell marker. CD4-CD8+CD161high T cells with a similar phenotype as CD4-CD8+ cells within cluster 8 are present in human peripheral blood and represent mucosal-associated invariant T (MAIT) cells (28, 46–49). These CD4-CD8+CD161high T cells express reduced levels of CD8 and share phenotypic features with Th17 cells, including expression of chemokine receptors (CCR6, CCR2 and CXCR6), cytokine receptors (IL-23R and IL-18R) and the transcription factor RORC (28, 46, 49). Moreover, CD4-CD8+CD161high cells are a subset of the conventional TCR-αβ EM (CD45RO+CCR7-) T cells (28, 46) that are not exhausted or terminally differentiated and a subset of these cells express IL-17, IFN-γ, TNF-α, and IL-22 (28, 50). When overlaid on total CD8+ T cells, CD4-CD8+CD161high cells in cluster 8 colocalized with the CD8lowCD161high subset as identified by Billerbeck et al. (28). In the present study, we did not measure expression of CXCR6, IL-23R and IL-18R. However, the expression of markers including CCR2, CCR6, CD45RO, and CD127 on CD8+CD161high T cells in cluster 8 and the absence of CCR7 expression suggest that they may be CD8+CD161high MAIT cells as reported earlier (28, 46, 49). The study by Turtle et al., which demonstrated that IL-1β in cooperation with T cell receptor signaling enhances cell proliferation and IL-17 secretion in human CD8α+CD161high cells (50), further supports this notion. Although MAIT cells play an important role in protective immunity to pathogens, emerging evidence support their pathogenic role in chronic inflammatory, metabolic, and autoimmune pathologies (51–57). MAIT cells express high levels of tissue-targeted chemokine receptors (48) and display an activated proinflammatory Th17 phenotype (57). Indeed, increased frequencies of MAIT cells have been reported at inflammation sites in patients with RA, multiple sclerosis, diabetes, obesity, inflammatory bowel disease, and other diseases (54, 55, 58, 59).
In addition to CD4-CD8+CD161high cells, the CD4-CD8- subset within cluster 8 also showed increased p-NF-κB in response to IL-1β. These CD4-CD8- cells expressed comparable levels of CD161 and displayed a phenotype similar to that of CD4-CD8+CD161high cells within cluster 8. Moreira-Teixeira et al. (60) demonstrated that co-stimulation with IL-β, TGF-β, and IL-23 induces IL-17 production in the CD4-CD161+ human invariant NKT (iNKT) cells (60). Different cell types, including Th17 cells, γδT, NKT, and MAIT cells (48, 61–63), express CD161 and confer pathogenic roles in inflammatory diseases (51, 64, 65). Further work is warranted to establish the identity of the CD4-CD8-CD161high and CD4-CD8+CD161high cells (cluster 8) that showed IL-1β-induced increases in p-NF-κB and p-p38, and investigate how IL-1β modulates effector functions of these cells.
This study showed that CCR6 is a marker that identify T cells most responsive to IL-1β stimulation. Our analysis demonstrated that the IL-1β responsive CCR6+ cells belong to the EM phenotype and most of these cells co-express CCR2 and CD26. Notably, circulating human CD4+ and CD8+ T cells that can produce IL-17 are enriched in CCR6+ memory T cells (66), and CD4+ helper T cells that produce type 17 cytokines express the greatest amounts of the catalytically active dipeptidylpeptidase IV (CD26) ectoenzyme (27). Although Tregs are largely anti-inflammatory, a proportion of the circulating MTregs in healthy humans express high levels of RORC and secrete IL-17 (67). IL-1β and IL-23 plays a critical role in human Th17 cell development and regulation of Th17 cytokines (68–71). It is possible that the cells within the CCR6+ EM T cell population that respond to IL-1β resemble Th17 cells. Th17 cells and sustained production of IL-17 are implicated in the pathogenesis of diverse inflammatory and autoimmune diseases (72–78). Increased frequency of Th17 cells and higher levels of Th17 cytokines have been reported in patients with inflammatory diseases and blocking the IL-17 pathway has shown potential benefit in some inflammatory diseases (72, 74, 76, 79, 80). IL-17 confers pathogenic effects by enhancing the production of cytokines (IL-6, IL-1β, TNF- α, and GM-CSF), chemokines (IL-8, CXCL1, CXCL2, CCL20, and MCP-1), metalloproteinases and other inflammatory molecules (81). These proinflammatory mediators promote inflammation, immune cell infiltration and tissue damage. Additionally, IL-17-induced production of IL-6 maintains the Th17-T cell population (81). The IL-1β-responsive EM T cells expressing CCR6, CCR2 and possibly other chemokine receptors may be recruited to sites of inflammation where IL-1β and other proinflammatory cytokines such as IL-23 or antigen-mediated TCR activation may activate these T cells to produce proinflammatory cytokines and cause tissue damage. Together, these findings raise the possibility that CCR6+ EM T cells and/or IL-17-producing cells play an important role in mediating IL-1β-induced inflammatory response in humans. Thus, a probable anti-inflammtory mechanism of IL-1 blockers may be through reducing proinflammatory Th17 response. Indeed, in RA patients, Anakinra therapy was associated with clinical improvement potentially through a decrease in the frequency of Th17 cells and serum levels of IL-17 and IL-21 (82).
Donor heterogeneity in IL-1β-induced signaling response in the CCR6+ EM T cells was not explained by the expression of IL-1R1. Moreover, transcript expression of downstream signaling mediators, including those encoding MyD88, IRAK-4 and Tollip, in CCR6+ EM T cells did not correlate with responsiveness to IL-1β. Another possibility for the donor heterogeneity may be at the genetic level due to single nucleotide polymorphisms (SNPs) that may influence protein expression, protein-protein interaction and functions. Indeed, SNPs in IL-1R1, IL-1R2, MyD88, IRAK-4 and Tollip have been associated with susceptibility to various diseases including diabetes, viral infections, thrombotic complications etc in humans (83–85). It would be interesting to explore the relationship between polymorphisms of IL-1 receptors and downstream signaling mediators with the signaling response of IL-1β in the future.
Monocytes and DCs are major producers of IL-1β and autocrine IL-1β signaling induces its own production in human monocytes (86). All three conventional human monocyte subsets (namely, CMs, IMMs and NCMs) produce IL-1β. However, NCMs produce less IL-1β, both under basal conditions and after LPS stimulation as compared to CMs and IMMs (87). It is unclear if all monocyte subsets respond to IL-1β stimulation. Our CyTOF analysis, which identified 8 monocyte subsets and CD11c+ mDCs and CD123+ pDCs, demonstrated that IL-1β increased p-NF-κB and p-p38 only in the CM subsets. Human monocyte subsets display remarkable heterogeneity in their surface marker expression, effector functions and differentiation potential (88). CMs exhibit a more proinflammatory phenotype as compared NCMs and IMMs. Our data showing different responsiveness to IL-1β stimulation further adds to the knowledge of the unique characteristics of the monocyte subsets. Within the two DC subsets, IL-1β-induced increase in p-NF-κB was seen only in CD11c+ mDCs. Unlike T cells and NK cells, IL-1β-induced increase in p-NF-κB, but not in p-p38 in CM and CD11c+ mDCs remained elevated for prolonged periods of time. This data suggests involvement of the NF-κB pathway in the autocrine IL-1β signaling that may lead to continued production of IL-1β under chronic inflammatory conditions.
Within the NK subsets, IL-1β induced increases in p-NF-κB in CD16-CD56dimCD161+ cells (cluster 2), CD16-CD56brightCD161- cells (cluster 3) and Lin-CD161+CD25+ cells (cluster 17). In contrast, IL-1β-induced increase in p-p38 and p-STATs was seen only within Lin-CD161+CD25+ cells that showed some phenotypic resemblance to ILC2. The expression of the C-type lectin receptor CD161 divides circulating NK cells in healthy adults into two distinct populations, with ~80% cells being CD161+ (89). Both CD56bright and CD56dim subsets comprise CD161+ cells. CD161+ NK cells within the CD56dim subset produced more IFN-γ than those in the CD161- subset, were more proliferative, and showed increased responsiveness to IL-12 and IL-18 (89). Furthermore, CD161+ NK cells are present at higher frequency in the inflamed intestinal lamina propria of patients with inflammatory bowel disease (89). Here, using the high-dimensional mass cytometry, we identified a subset of CD161+ NK cells with low CD56 expression that responded to IL-1β stimulation. It would be interesting to investigate if our cluster 2 shares phenotypic and functional properties with that of the CD161+ NK cells identified by Kurioka et al. (89) or constitute a separate subset that responds specifically to IL-1β.
A limitation of our study is the small number of subjects used for the initial CyTOF analysis to identify cell types that show IL-1β signaling response. Given the heterogeneity in IL-1β-induced signaling, limited sample size may have precluded revealing important differences. However, identification of IL-1β-induced signaling response consistently in the key immune cell types in an independent cohort and in separate experiments supports the validity of the findings.
Together, this atlas of IL-1β-induced signaling in circulating human immune cells provides new insights into the cell types most likely to be activated by IL-1β and the timing of these effects in humans. These findings provide key data for the study and design of more targeted approaches to chronic inflammatory diseases that may lessen adverse effects. Robust increases in p-NF-κB in CCR6+ EM T cells, CD11c+ mDCs, and Lin-CD161+CD25+ cells indicate that these cell types may play an important upstream role in IL-1β-mediated inflammation including cytokine storm syndromes. Anakinra and Canakinumab reduced inflammation and improved lung function in COVID-19 infected subjects (10–14) and larger trials testing their efficacy are currently ongoing. Moreover, IL-1β blockade by Canakinumab and Anakinra could reduce the residual risk of cardiovascular events beyond that achievable with lipid lowering treatments alone (34). Although effective at reducing inflammation, these therapies may cause immune suppression and fatal infections in some patients, underscoring the need for personalized therapy. Individuals in clinical trials for these drugs would likely show similar heterogeneity in IL-1β-induced increases in p-NF-κB in CCR6+ EM T cells as that seen in the subjects analyzed in this study. We hypothesize that those with the largest increase in p-NF-κB would be the most responsive to IL-1β blockade whether by blocking IL-1β itself (Canakinumab or Rilonacept) or by blocking IL-1R (Anakinra). Future studies utilizing customized panels as reported here in clinical trials of these different IL-1β inhibitors could provide insights.
MATERIALS AND METHODS
PBMC Isolation
Healthy volunteers provided written informed consent under the guidelines of the Institutional Review Board (IRB protocol # 16017). Blood from healthy donors was drawn into BD K2 EDTA vacutainer tubes and processed at room temperature within one hour of collection. Whole blood in vacutainers were centrifuged at 400 x g for 10 minutes at room temperature to remove platelet rich plasma. PBMCs were separated by Ficoll-Paque density-gradient centrifugation (Ficoll-Paque PLUS (GE Healthcare Biosciences AB) and SepMate-50 (Stemcell Technologies Inc) following the manufacturer’s protocol. Trypan blue staining was performed to obtain live cell counts. PBMCs were cryopreserved in freezing solution (90% FBS/10% DMSO). PBMC vials were stored in Mr. Frosty (Thermo Fisher) for 48 hours at −80°C and were then stored in liquid nitrogen until used.
CyTOF Panel Optimization
All metal-conjugated antibodies were purchased from Fluidigm. Purified unlabeled antibodies were purchased from the vendors as shown in table S1. Unlabeled antibodies were conjugated in-house using the MaxPAR antibody labeling kit (Fluidigm) according to the manufacturer’s protocol. After determining the percent yield by measurement of absorbance at 280 nm, metal-labeled antibodies were diluted in Candor PBS Antibody Stabilization solution (Candor Bioscience GmbH) for long-term storage at 4°C.
Cryopreserved PBMCs were used for CyTOF panel optimization. Antibody titrations were perfomed to determine optimal antibody concentrations. For titration of phospho signaling antibodies, PBMCs were stimulated with phorbol myrstate acetate (PMA) (50 nM, Sigma Aldrich) and ionomycin (1 µg/ml, Thermo Fischer) for 15 minutes. To capture IL-1β-induced transient signaling, PBMCs were fixed immediately with paraformaldehyde (PFA, 1.6% final concentration) after stimulation. PFA fixation led to reduced immunostaining of CXCR4, CXCR5, CCR6, CCR7 and CD127 staining while increased non-specific immunostaining of CXCR3 (fig. S1). Therefore, an alternative approach was utilized to label cells with these PFA-labile antibodes. A small volume of a cocktail of these PFA-labile antibodies was added simultaneously during IL-1β stimulation of PBMCs prior to PFA fixation. Antibody addition during PBMC stimulation did not interfere with IL-1β-mediated signaling (fig. S2, A and B), while allowing for specific staining.
PBMC Stimulation, Barcoding and CyTOF Staining
Fresh or thawed cryopreserved PBMCs were washed twice with warm complete media (RPMI supplemented with 5% FBS, 1 mM sodium pyruvate and Pen-Strep). All PBMC samples had viability in the range of 85–98%. 1–2 × 106 cells per stimulation were used. Prior to IL-1β (Peprotech) stimulation, PBMCs were rested in 1.8 ml low retention eppendorf tubes (Fisherbrand) for 1 hour at 37°C in 5% CO2 incubator. At the end of the stimulation period, PFA (1.6% final concentration) methanol free, Ultra Pure EM grade, Polysciences, Inc) was immediately added to the PBMC suspension, mixed by vortexing, and cells were incubated at room temperature for 10 minutes. PFA was then diluted at least to 0.8% or lower with ice-cold Maxpar Cell Staining Buffer (CSB) (Fluidigm), and fixed cells were centrifuged at 800 × g for 10 minutes at room temperature. PBMC samples were barcoded using the palladium-based 20-Plex Pd Barcoding Kit (Fluidigm) according to the manufacturer’s protocol. Because PBMCs were fixed with PFA, which is similar to the Maxpar Fix I Buffer, this step was excluded and cells were washed twice with 1 ml of 1x Maxpar Barcode Perm Buffer. Barcoded cells were then combined into a single tube prior to Fc receptor blocking (BD Biosciences) and staining with a cocktail of metal-conjugated antibodies against cell-surface markers for 30 minutes at room temperature with frequent gentle shaking. After washing once with CSB, cells were resuspended in residual CSB and chilled on ice for 5 minutes. For cell permeabilization, 0.5 ml of 100% ice-cold methanol was added to the cells, and samples were vortexed and incubated on ice for 10 minutes. Eppendorf tubes were filled up with the CSB and samples were centrifuged at 800 × g for 10 minutes at room temperature. An additional wash with the CSB was performed to remove residual methanol prior to staining with antibodies against intracellular phospho markers for 45 minutes at room temperature. After washing, cell were fixed with 2% PFA for 10 minutes at room temperature and washed again and cell pellets were stored overnight at 4°C. Stained cells were incubated with iridium DNA intercalator (Fluidigm) in Maxpar Fix and Perm Buffer (Fluidigm) for 20 minutes at room temperature. Prior to acquisition, cells were washed once with CSB and then twice with the Maxpar Cell Acquisition Solution (Fluidigm). Cells were filtered through a 40 μm membrane just before being acquired on a Helios mass cytometer (Fluidigm). EQ Four Element Calibration Beads from Fluidigm were added as per the manufacturer’s directions prior to running.
CyTOF data pre-processing and subsequent analysis
Data obtained from the Helios instrument were in the form of .fcs files format. Samples were normalized using the Nolan lab MATLAB normalizer (http://github.com/nolanlab/bead-normalization/releases) and debarcoded using Zunder’s lab debarcoder (90) (https://github.com/zunderlab/single-cell-debarcoder). Normalized and debarcoded .fcs files were then analyzed in Flowjo version 10.3 for Mac (Flowjo LLC, Ashland, OR). Arcsinh transformation was performed on all measurements (16). Clean-up gating was done on barcode stringency parameters and iridium DNA intercalator to remove non-cell debris and cellular aggregates (fig. S6A and B). The .fcs files were uploaded to Cytobank for Spanning-tree Progression Analysis of Density-normalized Events (SPADE). For SPADE tree construction, clean CD45+ cells and cell surface markers as shown in table S1 were used for clustering using the default settings in Cytobank.
Cell types were identified by first manually gating total CD3+ (for T cells), CD3-CD19-CD56-HLA-DR+ cells (for monocytes and DCs) and CD3-CD19-HLA-DR- cells (for NK cells) (fig. S6, C-E), and then passing through an R pipeline similar to Nowicka et al. (91). Dimensionality reduction by UMAP (92, 93) was performed in R using the “UMAP” package. All parameters were used at default values except for min_dist, which was changed to 0.01. Leiden clustering (94) was performed on the UMAP graph using Modularity Vertex Partition in the Python Leidenalg package. Cell surface markers used for UMAP and Leiden clustering for each individual immune cell subsets (T cells, monocytes/DCs and NK cells) are described in the Results. Cells were gated in Flowjo to quantitate frequency of cells expressing phospho markers within immune cell clusters (fig. S6, F-J).
Fluorescence-activated cell sorting
To sort purify CCR6+ memory T cells, total PBMCs from 6 healthy donors were stained with fluorophore-labeled antibodies; anti-CD3- APC-H7 (clone SK7; BD Biosciences; Cat# 560176), CD4- FITC (clone RPA-T4; Biolegend; Cat# 300506), CD8- APC (clone HIT8a; BD Biosciences; Cat# 566852), CD45RO- BV421 (clone UCHL1; BD Biolegend; Cat# 304224), CCR6- PE (clone 11A9; BD Biosciences; Cat# 559562), CD19- PE-CF594 (clone HIB19; BD Biosciences; Cat# 562294), CD14- BUV737 (clone M5E2; BD Biosciences; Cat# 612763) and CD56-PE-Cy7 (clone CMSSB; BD eBioscience; Cat# 25–0567-42) and 7AAD live dead stain (eBioscience; Cat# 00–6993-50). Sorting was performed on an Influxor FACS Vantagecell sorter (BD Bioscience).
Real time quantitative polymerase chain reaction
RNA was extracted from total PBMCs and sort-purified CCR6+ memory T cells using RNeasy Plus mini kit (Qiagen). RNA was quantified spectrophotometrically (Nanodrop, Thermo Scientific). 1 μg of RNA was then treated with DNase (Invitrogen), reverse transcribed to cDNA using an iScript cDNA synthesis kit (BioRad) and diluted 1:10 in water. mRNA expression was measured by RT-pPCR using Taqman probes from Thermo Fisher. 15 ng of the cDNA per 10 μL of the Taqman RT-qPCR reaction mix (Applied Biosystems) was used. Taqman components included 5 μL of the Taqman universal PCR master mix and 0.5 μL of the TaqMan gene expression assay probe. Results were normalized to 18S ribosomal RNA (rRNA), and gene expression levels were calculated using the ΔΔCt method or from a standard curve of serial dilutions of pooled cDNA from total PBMCs.
Statistics
GraphPad Prism Version 8.2.0 (GraphPad Software, Inc) was used for data analysis, graphing and statistics. All data are expressed as mean ± SEM and analyzed with Wilcoxon signed-rank test. A p<0.05 was considered statistically significant.
Supplementary Material
Fig. S1. PFA fixation affects cell surface marker epitopes and antibody reactivity.
Fig. S2. Antibody staining during IL-1β stimulation does not interfere with IL-1β-induced signaling.
Fig. S3. IL-1β dose response relationships.
Fig. S4. IL-1β induces a sustained increase in p-NF-κB in CMs and CD11c+ mDCs but a transient increase in memory T cells, NK cells, and Lin-CD161+CD25+ cells.
Fig. S5. Time-dependent changes in the frequency of p-STAT1+, p-STAT3+ and p-STAT5+ cells within different clusters of T cells, monocytes, DCs and Lin-CD161-CD25+ cells.
Fig. S6. Gating scheme.
Fig. S7 T cell cluster 8 contains CD8- and CD8+ populations that both express high levels of CD161.
Fig. S8. Stimulation with anti-CD3, anti-CD28 and IL-2 increases the expression of IL-1R2 on memory CD4+ T cells.
Fig. S9. Monocyte cluster 7 was heterogeneous and contained both intermediate and non-classical monocytes.
Fig. S10. Cluster 17 (Lin-CD161+CD25+) cells do not represent T cells and do not express lineage markers.
Fig. S11. Endogenous transcript expression of IL-1Rs and downstream signaling mediators in CCR6+ EM T cells.
Fig. S12. Comparison of IL-1β-induced signaling response in fresh and cryopreserved PBMCs.
Table S1. CyTOF antibodies.
Table S2. Spearman correlation analysis between endogenous transcript levels of IL-1Rs and downstream signaling mediators in CCR6+ EM T cells and fold change in frequency of p-NF-κB+ and p-p38+ CCR6+ EM T cells.
Acknowledgments
We thank Mike Solga and Claude Chew from the UVA Flow Cytometry Core for their excellent technical assistance and acquisition of CyTOF data.
Funding
This work was supported by the American Heart Association Innovative Project Award (18IPA34170443), an NIH grant (R01HL 136098 to CAM), and an NIH NHLBI grant (T32HL007284 to CMW).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Mehta P et al. , COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y et al. , Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome 2020.2003.2002.20029975 (2020).
- 3.Schulert GS et al. , Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors. Arthritis Rheumatol 71, 1943–1954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shakoory B et al. , Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 44, 275–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia Borrega J et al. , In the Eye of the Storm: Immune-mediated Toxicities Associated With CAR-T Cell Therapy. Hemasphere 3, e191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KS, Jung H, Shin IK, Choi BR, Kim DH, Induction of interleukin-1 beta (IL-1beta) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J Med Virol 87, 1104–1112 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Wen Wen WS, Tang Hao, Le Wenqing, Zhang Xiaopeng, Zheng Yingfeng, Liu XiuXing, Xie Lihui, Li Jianmin, Ye Jinguo, Cui Xiuliang, Miao Yushan, Wang Depeng, Dong Jiantao, Xiao Chuan-Le, Chen Wei, Wang Hongyang, Immune Cell Profiling of COVID-19 Patients in the Recovery Stage by Single-Cell Sequencing. medRxiv, (2020). [DOI] [PMC free article] [PubMed]
- 8.Sonmez HE, Demir S, Bilginer Y, Ozen S, Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol 37, 3329–3335 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Adam Monteagudo L, Boothby A, Gertner E, Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol, (2020). [DOI] [PMC free article] [PubMed]
- 10.Nicastri E, Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection, (2020).
- 11.Ucciferri C et al. , Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol 2, e457–ee458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caracciolo M et al. , Case Report: Canakinumab for the Treatment of a Patient With COVID-19 Acute Respiratory Distress Syndrome 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huet T et al. , Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2, e393–e400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalli G et al. , Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2, e325–e331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodenmiller B et al. , Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol 30, 858–867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendall SC et al. , Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter RM, Kong DS, Garcia-Perez JE, O’Gorman WE, Hsieh EWY, Single-cell Analysis of Immunophenotype and Cytokine Production in Peripheral Whole Blood via Mass Cytometry. J Vis Exp, (2018). [DOI] [PMC free article] [PubMed]
- 18.Li L, Kim J, Boussiotis VA, IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol 185, 4148–4153 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HG et al. , Pathogenic function of bystander-activated memory-like CD4(+) T cells in autoimmune encephalomyelitis. Nat Commun 10, 709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain A, Song R, Wakeland EK, Pasare C, T cell-intrinsic IL-1R signaling licenses effector cytokine production by memory CD4 T cells. Nat Commun 9, 3185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu R et al. , IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat Immunol 16, 286–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitley SK et al. , IL-1R signaling promotes STAT3 and NF-kappaB factor recruitment to distal cis-regulatory elements that regulate Il17a/f transcription. J Biol Chem 293, 15790–15800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarreberg LD et al. , Interleukin-1beta Signaling in Dendritic Cells Induces Antiviral Interferon Responses. MBio 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss DI et al. , IL-1beta Induces the Rapid Secretion of the Antimicrobial Protein IL-26 from Th17 Cells. J Immunol 203, 911–921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohne Y et al. , IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol 17, 646–655 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Cooper MA et al. , Interleukin-1beta costimulates interferon-gamma production by human natural killer cells. Eur J Immunol 31, 792–801 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Bengsch B et al. , Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J Immunol 188, 5438–5447 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Billerbeck E et al. , Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A 107, 3006–3011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizel SB, Kilian PL, Lewis JC, Paganelli KA, Chizzonite RA, The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol 138, 2906–2912 (1987). [PubMed] [Google Scholar]
- 30.Peters VA, Joesting JJ, Freund GG, IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav Immun 32, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spits H et al. , Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13, 145–149 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA, Simon A, van der Meer JW, Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11, 633–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arranz L, Arriero MDM, Villatoro A, Interleukin-1beta as emerging therapeutic target in hematological malignancies and potentially in their complications. Blood Rev 31, 306–317 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM et al. , Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM et al. , Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Ammirati E et al. , Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J Am Heart Assoc 1, 27–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson NC et al. , Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 8, e71498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson NC et al. , Associations of Circulating Lymphocyte Subpopulations with Type 2 Diabetes: Cross-Sectional Results from the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One 10, e0139962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rattik S et al. , Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res 16, 270–280 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Spanier JA et al. , Increased Effector Memory Insulin-Specific CD4(+) T Cells Correlate With Insulin Autoantibodies in Patients With Recent-Onset Type 1 Diabetes. Diabetes 66, 3051–3060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeshita M et al. , Multi-dimensional analysis identified rheumatoid arthritis-driving pathway in human T cell. Ann Rheum Dis 78, 1346–1356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neidhart M, Fehr K, Pataki F, Michel BA, The levels of memory (CD45RA-, RO+) CD4+ and CD8+ peripheral blood T-lymphocytes correlate with IgM rheumatoid factors in rheumatoid arthritis. Rheumatol Int 15, 201–209 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Guo N et al. , A critical epitope in CD147 facilitates memory CD4(+) T-cell hyper-activation in rheumatoid arthritis. Cell Mol Immunol 16, 568–579 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritvo PG et al. , Tfr cells lack IL-2Ralpha but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Sci Immunol 2, (2017). [DOI] [PubMed] [Google Scholar]
- 45.Ritvo PG, Klatzmann D, Interleukin-1 in the Response of Follicular Helper and Follicular Regulatory T Cells. Front Immunol 10, 250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Dejbakhsh-Jones S, Strober S, Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol 176, 211–216 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Ussher JE et al. , CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 44, 195–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dusseaux M et al. , Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Walker LJ et al. , Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119, 422–433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turtle CJ et al. , Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161(hi) CD8alpha(+) semi-invariant T cells. Blood 118, 2752–2762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar V, Ahmad A, Role of MAIT cells in the immunopathogenesis of inflammatory diseases: New players in old game. Int Rev Immunol 37, 90–110 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Willing A, Jager J, Reinhardt S, Kursawe N, Friese MA, Production of IL-17 by MAIT Cells Is Increased in Multiple Sclerosis and Is Associated with IL-7 Receptor Expression. J Immunol 200, 974–982 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Touch S et al. , Mucosal-associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. FASEB J, fj201800052RR (2018). [DOI] [PubMed]
- 54.Cho YN et al. , Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol 193, 3891–3901 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Rouxel O, Lehuen A, Mucosal-associated invariant T cells in autoimmune and immune-mediated diseases. Immunol Cell Biol 96, 618–629 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Bertrand L, Lehuen A, MAIT cells in metabolic diseases. Mol Metab 27S, S114–S121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiba A, Murayama G, Miyake S, Mucosal-Associated Invariant T Cells in Autoimmune Diseases. Front Immunol 9, 1333 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haga K et al. , MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol 31, 965–972 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Willing A et al. , CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol 44, 3119–3128 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Moreira-Teixeira L et al. , Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J Immunol 186, 5758–5765 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Battistini L et al. , Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. J Immunol 159, 3723–3730 (1997). [PubMed] [Google Scholar]
- 62.Maggi L et al. , CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol 40, 2174–2181 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Loza MJ, Metelitsa LS, Perussia B, NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol 32, 3453–3462 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Papotto PH, Reinhardt A, Prinz I, Silva-Santos B, Innately versatile: gammadelta17 T cells in inflammatory and autoimmune diseases. J Autoimmun 87, 26–37 (2018). [DOI] [PubMed] [Google Scholar]
- 65.van Puijvelde GHM, Kuiper J, NKT cells in cardiovascular diseases. Eur J Pharmacol 816, 47–57 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM, Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 180, 214–221 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Ayyoub M et al. , Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A 106, 8635–8640 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lasiglie D et al. , Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One 6, e20014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Revu S et al. , IL-23 and IL-1beta Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep 22, 2642–2653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee WW et al. , Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115, 530–540 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mailer RK et al. , IL-1beta promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep 5, 14674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Stebut E et al. , IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front Immunol 10, 3096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hot A, Miossec P, Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis 70, 727–732 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Robert M, Miossec P, IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front Med (Lausanne) 5, 364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang SL et al. , Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J Mol Cell Cardiol 122, 69–79 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Rahmati Z et al. , Association of levels of interleukin 17 and T-helper 17 count with symptom severity and etiology of chronic heart failure: a case-control study. Croat Med J 59, 139–148 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taleb S, Tedgui A, Mallat Z, IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol 35, 258–264 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Miossec P, Kolls JK, Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11, 763–776 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Lee YH, Bae SC, Associations between circulating IL-17 levels and rheumatoid arthritis and between IL-17 gene polymorphisms and disease susceptibility: a meta-analysis. Postgrad Med J 93, 465–471 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Chen DY et al. , Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther 13, R126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Onishi RM, Gaffen SL, Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niu X et al. , Regulatory immune responses induced by IL-1 receptor antagonist in rheumatoid arthritis. Mol Immunol 49, 290–296 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Xie M et al. , Association of genetic polymorphisms in IL-1R1 and IL-1R2 genes with IgA nephropathy in the Han Chinese population. Oncotarget 8, 50673–50679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo C et al. , Association of polymorphisms in the MyD88, IRAK4 and TRAF6 genes and susceptibility to type 2 diabetes mellitus and diabetic nephropathy in a southern Han Chinese population. Mol Cell Endocrinol 429, 114–119 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Huang C et al. , Tollip SNP rs5743899 modulates human airway epithelial responses to rhinovirus infection. Clin Exp Allergy 46, 1549–1563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toda Y et al. , Autocrine induction of the human pro-IL-1beta gene promoter by IL-1beta in monocytes. J Immunol 168, 1984–1991 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Hadadi E et al. , Differential IL-1beta secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability. Sci Rep 6, 39035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapellos TS et al. , Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol 10, 2035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurioka A et al. , CD161 Defines a Functionally Distinct Subset of Pro-Inflammatory Natural Killer Cells. Front Immunol 9, 486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fread KI, Strickland WD, Nolan GP, Zunder ER, An Updated Debarcoding Tool for Mass Cytometry with Cell Type-Specific and Cell Sample-Specific Stringency Adjustment. Pac Symp Biocomput 22, 588–598 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Nowicka M et al. , CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 6, 748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McInnes L, Healy J, Saul N, & Grossberger L, UMAP: uniform manifold approximation and projection. The Journal of Open Source Software, (2018).
- 93.Becht E et al. , Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol, (2018). [DOI] [PubMed]
- 94.Traag VA, Waltman L, van Eck NJ, From Louvain to Leiden: guaranteeing well-connected communities. Sci Rep 9, 5233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. PFA fixation affects cell surface marker epitopes and antibody reactivity.
Fig. S2. Antibody staining during IL-1β stimulation does not interfere with IL-1β-induced signaling.
Fig. S3. IL-1β dose response relationships.
Fig. S4. IL-1β induces a sustained increase in p-NF-κB in CMs and CD11c+ mDCs but a transient increase in memory T cells, NK cells, and Lin-CD161+CD25+ cells.
Fig. S5. Time-dependent changes in the frequency of p-STAT1+, p-STAT3+ and p-STAT5+ cells within different clusters of T cells, monocytes, DCs and Lin-CD161-CD25+ cells.
Fig. S6. Gating scheme.
Fig. S7 T cell cluster 8 contains CD8- and CD8+ populations that both express high levels of CD161.
Fig. S8. Stimulation with anti-CD3, anti-CD28 and IL-2 increases the expression of IL-1R2 on memory CD4+ T cells.
Fig. S9. Monocyte cluster 7 was heterogeneous and contained both intermediate and non-classical monocytes.
Fig. S10. Cluster 17 (Lin-CD161+CD25+) cells do not represent T cells and do not express lineage markers.
Fig. S11. Endogenous transcript expression of IL-1Rs and downstream signaling mediators in CCR6+ EM T cells.
Fig. S12. Comparison of IL-1β-induced signaling response in fresh and cryopreserved PBMCs.
Table S1. CyTOF antibodies.
Table S2. Spearman correlation analysis between endogenous transcript levels of IL-1Rs and downstream signaling mediators in CCR6+ EM T cells and fold change in frequency of p-NF-κB+ and p-p38+ CCR6+ EM T cells.


