Version Changes
Revised. Amendments from Version 1
This manuscript has been updated to address comments from Reviewers 1 and 2. The main changes are: The introduction has been expanded as requested by both reviewers, which now includes the malaria epidemiology in Malawi, vaccine efficacy data and the RTS,S vaccine introduction process in Malawi. We have provided a clearer model Figure 1
Abstract
Background: The RTS,S/AS01 E malaria vaccine is being assessed in Malawi, Ghana and Kenya as part of a large-scale pilot implementation programme. Even if impactful, its incorporation into immunisation programmes will depend on demonstrating cost-effectiveness. We analysed the cost-effectiveness and public health impact of the RTS,S/AS01 E malaria vaccine use in Malawi.
Methods: We calculated the Incremental Cost Effectiveness Ratio (ICER) per disability-adjusted life year (DALY) averted by vaccination and compared it to Malawi’s mean per capita Gross Domestic Product. We used a previously validated Markov model, which simulated malaria progression in a 2017 Malawian birth cohort for 15 years. We used a 46% vaccine efficacy, 75% vaccine coverage, USD5 estimated cost per vaccine dose, published local treatment costs for clinical malaria and Malawi specific malaria indicators for interventions such as bed net and antimalarial use. We took a healthcare provider, household and societal perspective. Costs were discounted at 3% per year, no discounting was applied to DALYs. For public health impact, we calculated the DALYs, and malaria events averted.
Results: The ICER/DALY averted was USD115 and USD109 for the health system perspective and societal perspective respectively, lower than GDP per capita of USD398.6 for Malawi. Sensitivity analyses exploring the impact of variation in vaccine costs, vaccine coverage rate and coverage of four doses showed vaccine implementation would be cost-effective across a wide range of different outcomes. RTS,S/AS01 was predicted to avert a median of 93,940 (range 20,490–126,540) clinical cases and 394 (127–708) deaths for the three-dose schedule, or 116,480 (31,450–160,410) clinical cases and 484 (189–859) deaths for the four-dose schedule, per 100 000 fully vaccinated children.
Conclusions: We predict the introduction of the RTS,S/AS01 vaccine in the Malawian expanded programme of immunisation (EPI) likely to be highly cost effective.
Keywords: Malaria, Malawi, cost-effectiveness, RTS, S, vaccine, Markov Chain, Modelling
Introduction
Malaria is one of the most important causes of under-five morbidity and mortality in Malawi. Over the past 10 years, Malawi has substantially scaled up available malaria control tools, such as insecticide treated bed nets (ITN) and artemisinin-based combination (ACTs) treatments. During this period, the national parasite prevalence in young children has reduced by 44% (from 43% to 24% in 2010 and 2017 respectively) and mortality due to malaria has halved 1– 3 . In 2017, the National Malaria Control Programme laid out a five-year Malaria strategic plan (2018–2022). The strategy has two main aims; to reduce malaria incidence by at least 50% from a 2016 baseline of 386 per 1000 population to 193 per 1000 and reduce malaria deaths by at least 50% from 23 per 100,000 population to 12 per 100,000 population by 2022. As of 2019, there were over 286, 000 malaria cases per 1,000 people and 13 malaria attributable deaths per 100,000 people 4. With the current trajectory, there is still need of additional malaria control measures to meet these goals and to eventually eliminate malaria. There is a need to further enhance the interventions already in place but it is also critical that we explore additional tools in the battle against malaria. One of this is the introduction of prophylactic vaccination against P. falciparum parasite.
The RTS,S/AS01 E(henceforth RTS,S) is the first malaria vaccine to receive a conditional approval for use in under-five children living in moderate-to-high malaria burden settings following a large-scale Phase III study in Sub-Saharan Africa. RTS,S vaccine. The vaccine’s clinical efficacy against all clinical episodes of malaria was 51% (95% CI, 47- 55) in the 5–17 month age group after 12 months following the first 3 doses across trial all sites. The efficacy decreased to 46% (95% CI, 41.7–49.5) after 18 months follow up for the same group and dosage. The vaccine efficacy for the trial period of 48 months median follow up (after the first dose) was 26% (95% CI, 21–31) among subjects who received a 3-dose schedule and 39% (95% CI, 34–43) among those who received a 4 dose schedule 5.
Malawi is one of three countries participating in a large-scale pilot implementation programme of the RTS,S AS01 E (GSK) malaria vaccine (henceforth RTS,S) 6. Even if impactful, its cost-effectiveness will be a crucial determinant of subsequent introduction 7. Malawi is supported by Gavi, the global vaccine alliance, for funding existing vaccines and for introduction of any new vaccines. Gavi eligibility is based upon a World Bank determined inflation-adjusted Gross National Income per capita (GNI pc) below a US$1,580 threshold 8, Malawi’s current GNI pc is $380 9. Malawi is required to finance a proportion of vaccine cost, equivalent to US$0.20 per dose.
RTS,S has been predicted to be highly cost-effective in areas in sub-Saharan Africa with moderate-to-high malaria transmission across different model approaches 10. However, health care programmes, vaccination schedules and related cost assumptions vary considerably between LMIC countries. Cognisant of this, national policy makers increasingly seek in-country evidence to inform their decisions. There are no published RTS,S national level cost-effectiveness data for Malawi or for regional countries.
An intervention is considered cost-effective if the incremental cost effectiveness ratio (ICER) per disability adjusted life years (DALYs) averted is less than three times the GDP per capita and is highly cost effective if the ICER per DALY averted is less than the per capita GDP 11.
We sought to predict the RTS,S cost-effectiveness and public health impact in Malawi.
Methods
An intervention is considered cost-effective if the ICER per disability-adjusted life year (DALY) averted is less than three times the GDP per capita and is highly cost effective if the ICER per DALY averted is less than the per capita GDP 12.
Model description
We used a Markov static cohort model developed by GSK for the RTS,S vaccine that has been validated for sub-Saharan Africa; the model is described in depth by Sauboin et al. 13. The model simulates a birth cohort followed over 15 years under fixed-exposure levels of malaria transmission, taking into account parameters reflecting healthcare provider and societal perspective to calculate the incremental cost effectiveness ratio per DALY averted (ICER) of the RTS,S vaccine 13.
Figure 1 is a diagrammatic representation of the model. The model has compartments susceptible (S), infected (I), clinical disease (C) and severe disease (F) divided into six successive immunity levels following each infection levels.
Figure 1. Model structure.
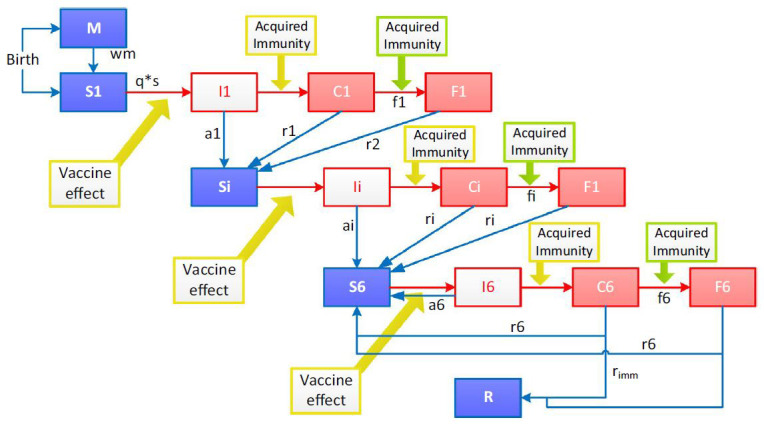
The model assumes two processes for acquisition of immunity, one process that protects against clinical malaria of any severity and a faster process that protects against severe malaria. M = maternal protection; S = susceptible; I = infected (parasites emerging from the liver); C = clinical disease episode; F = severe disease episode. There are six levels of immunity with compartments S, I, C and F divided into six levels. R = resistant; wm = waning of maternal immunity; q = probability of infection; s = susceptibility to infection as a function of age; a = probability of asymptomatic infection; r = recovery rate from clinical disease; w = waning rate of acquired immunity; r imm = probability of developing full immunity.
The model assumes initial protection against malaria from maternal antibodies (M) 14. Neonates are considered either protected from (M) or are susceptible to (S1) malaria infection. Initial immunity is presumed to wane exponentially over three months, leaving the child susceptible to infection. An infected (I1) child will have asymptomatic parasitaemia which clears and susceptibility returns (Si), or the child will develop clinical disease (C1). From clinical disease a child may recover (r1) or develop severe disease (F1) where they could either survive returning to a susceptible state or they could die. Immunity is enhanced every level from an asymptomatic state to clinical malaria and to severe disease. The model permits up to six repeated infections to cumulatively increase immunity. Beyond six infections, a fixed proportion of children is assumed to develop a state of resistance (R).
Model assumptions and inputs
The model uses an estimated 2017 annual birth cohort for Malawi and followed for 15 years 15. This birth cohort was the mean of four prior birth cohorts using the United Nations population data 15. The model accounts for heterogeneity of individual level exposure and a fixed probability of infection within each transmission category. The model assumes the vaccine efficacy wanes over time. Malaria transmission intensity in the model was defined categorically as low, medium or high based on Plasmodium falciparum parasite prevalence (PfPR) in children aged 2–10 years old of <5%, 5–40% and >40% was respectively, using the Malaria Atlas Project 16.
Table 1 shows the input parameters used in the model. The inputs were point estimates extracted from published literature or reasonable assumptions. The vaccine price was based on previously published assumptions since the product has not yet been priced by GSK. The cost of RTS,S vaccine delivery per dose was assumed equal to DTP3 (given as part of pentavalent) in Malawi 17. Service delivery make up the bulk (63%) of vaccine delivery costs whilst supply chain and logistics constitute the remainder of vaccine delivery costs 18. Vaccine delivery costs mainly comprise of cold chain management, transportation of vaccines to health facilities, waste disposal and additional training for health workers. We sought to calculate cost savings from a healthcare and household perspective. Societal costs are a combination of healthcare and household costs.
Table 1. Model inputs.
| Parameter | Description and value |
|---|---|
| Transmission
intensity |
More than half of the population (54.5%) fell in the moderate intensity category, 42.2% in the high intensity
category and only 3.4% were in the low intensity category. Assumed fixed seasonal 23 |
| Access to case
management |
38% 24 |
| Bed net coverage | 82% own at least one bed net 24 |
| Vaccine efficacy | Clinical malaria 46% (95% CI 42% to 50%)
Severe malaria 34% (95% CI 15% to 48%) |
| Vaccine schedule | In Malawi, the first dose of the RTS,S/AS01 vaccine is expected to be administered to children at 5 months with
the second and third at one month intervals; the fourth dose is to be given 15–18 months after the third dose. |
| Vaccine coverage | The coverage of dose 3 of RTS,S was estimated at 75% of DTP3 coverage to account for the challenge in
reaching older children and also for the difference in schedule of the RTS,S with traditional vaccines such as the pentavalent vaccine. Dose 4 coverage of RTS,S was 80% of dose 3. |
| Cost of vaccination | Vaccine delivery costs USD 2.50
25 were estimated from the cost of delivering a single dose of the injectable DTP
vaccine. |
| Clinical malaria estimated costs USD | |
| Healthcare system | USD 8.02
20,
21. These include drugs, laboratory investigations, staff salaries and facility costs such as laundry,
kitchen, sanitation and security. |
| Household- direct | USD 1.21 20, 21. These include transportation costs, costs of consultations, drugs and diagnostics. |
| Household- indirect | USD 0.50 26. These are lost income by the carer attributable to the episode of disease |
| Severe malaria costs USD | |
| Healthcare system | USD 12.8
27 These include drugs, laboratory investigations, staff salaries and facility costs such as laundry,
kitchen, sanitation and security. |
| Household- direct | USD 14.1 21 These include transportation costs, costs of consultations, drugs and diagnostics. |
| Household- indirect | USD 4.13 21 These are lost income by the carer attributable to the episode of disease |
| Sequelae costs USD | |
| Healthcare system | USD 40.1
22 The neurological sequelae was adapted by the cost of treatment of malaria sequelae for the
Tanzanian health system |
The Phase III RTS,S/AS01 trial vaccine schedule of 6, 7, 8 and 26 months of age and 18-month follow-up results, following the third dose, were fitted in the model. Vaccine efficacy against clinical and severe malaria in children was 46% (95% CI 42–50%) and 34% (95% CI 15–48%) respectively 5. Third and fourth dose RTS,S coverage were assumed to be 75% and 60% of the DTP3 dose 1 coverage respectively. The fourth dose was assumed to boost the waning efficacy. Access to artemisinin combination therapy (ACT) or private dispensaries was extracted from the 2014 Malawi Malaria Indicator Survey 19.
We used published treatment costs for mild-moderate and severe gastroenteritis, respectively 20, 21, since published treatment costs for malaria were outdated or unavailable. These health costs including drugs, laboratory investigations, staff salaries and facility costs. Where these specific costs were unavailable for clinical and severe malaria, we used malaria sequelae costing data from Tanzania 22, as cost data from Malawi were not available. Direct and indirect household costs incurred in care seeking were also based on those locally empirically observed in gastroenteritis 21. Direct household costs included travel, consultation fees, treatment sought before and after health facility visit and the costs of food and shelter for the carer. Indirect costs comprised income lost while caring for the child 21. Bed net use and access to and usage of ACTs and the proportion of those who seek treatment at a private dispensary were derived from the Malawi Malaria Indicator Survey 19. Vaccine price per dose has not yet been set by GSK, so we assessed a range of costs of USD1, USD5 and USD10. RTS,S delivery in the Malawi EPI was taken from the administration cost pentavalent vaccine 7. The cost of delivery includes all the necessary materials and health worker time required to administer a vaccine in the EPI. The mean Malawi GDP per capita from 2010 to 2015, as reported by the World Bank, was used to compare with the ICER per DALY averted 28.
Sensitivity analysis
Univariate analysis was conducted by running the model through different values of vaccine price and vaccine coverage, as shown in Table 3 and Table 4, whilst the other input parameters were held constant.
Results
Based on a 15-year cohort of 711,743 children, the model calculated an ICER of USD 115 and 109 per DALY averted in the health system and the societal perspective respectively compared to no vaccination. Based on a vaccine schedule of four doses, this is less than the Malawi mean GDP per capita of USD 398.6, suggesting that the introduction of RTS,S vaccine to the Malawian EPI programme would be highly cost-effective. The model predicted 721,768 (95% CI: 529,296–894,991) averted clinical malaria cases per year, 14% of current burden. The model demonstrated that 117,260 clinical cases and 700 malaria attributable deaths would be prevented per 100,000 fully vaccinated children per year. The vaccine introduction was also very cost effective at an assumed vaccine price of USD 1 and USD 10 with four doses of the RTS,S vaccine. We predicted cost savings for the society, healthcare system and household as USD 3,025,521, USD 2,433,777 and USD 591,744, respectively. Healthcare costs contributed to over two-thirds of societal costs.
Modelling findings
Table 2 shows the cumulative cost-effectiveness results for a birth cohort followed up over 15 years, using assumed vaccine prices of USD 1, USD 5, and USD 10. At USD 5, the ICER was 115 USD per DALY averted. At USD 1 vaccine price the ICER was USD 40 per DALY averted and at USD 10 the ICER was USD 209 per DALY averted. We showed that the vaccine would remain very cost-effective even at an inflated vaccine price of USD 10 per dose. However, the societal cost savings remain unchanged with a change in vaccine price.
Table 2. Discounted cost-effectiveness results over a 15-year period.
| Variable | No vaccination | 6–9m schedule plus 4 th dose | Cost savings * | ||
|---|---|---|---|---|---|
| USD 1 per dose | USD 5 per dose | USD 10 per dose | |||
| Healthcare system ICER (USD per DALY
averted) |
--- | 40 | 115 | 209 | --- |
| Societal ICER (USD per DALY averted) | --- | 34 | 109 | 202 | --- |
| DALYs | 1,237,356 | 1,176,557 | 1,176,557 | 1,176,557 | 96,799 |
| Vaccination costs | --- | 6,334,532 | 13,573,997 | 22,623,329 | --- |
| Healthcare system costs | 26,396,028 | 23,962,251 | 23,962,251 | 23,962,251 | 2,433,777 |
| Incremental costs for healthcare system | 3,900,755 | 11,140,220 | 20,189,552 | ||
| Household costs | 6,576,176 | 5,984,432 | 5,984,432 | 5,984,432 | 591,744 |
| Societal costs * | 32,972,204 | 29,946,683 | 29,946,683 | 29,946,683 | 3,025,521 |
| Incremental costs for the society | 3,309,011 | 10,548,476 | 19,597,808 | ||
DALY = disability-adjusted life year; ICER = Incremental Cost Effectiveness Ratio. Note 1. Societal costs = health care system + household level costs. * Cost savings are the difference in costs between no vaccination scenario and vaccination scenario
Table 3 shows the cumulative public health impact results over 15 years with comparison of different vaccine coverage versus the number of malaria clinical cases averted. The number of DALYs and malaria cases and deaths avoided are largely dependent on the vaccine coverage in the population. At an assumed coverage of 75% of DTP3 coverage, the model predicted 10,826,521 clinical cases averted ( Table 4). This is equal to 721,768 malaria clinical cases per year. The highest number of malaria clinical cases avoided was with a 93% vaccine coverage which is similar to current DTP3 coverage for Malawi. Table 5 shows the comparison in vaccine cost-effectiveness between a three-dose schedule and a four-dose schedule with an assumed vaccine price of USD 5 per dose. It shows that more DALYs are averted with a four-dose schedule than a three-dose schedule, but a four-dose schedule has higher societal costs because of ancillary costs associated with an additional visit.
Table 3. Public health impact results cumulative over a period of 15 years.
| Absolute vaccine coverage | |||||
|---|---|---|---|---|---|
| Events averted | 93% β | 85% | 75% | 65% | 55% |
| DALYs | 120,101 | 109,706 | 96,799 | 83,893 | 70,986 |
| malaria cases | 13,424,866 | 12,270,057 | 10,826,521 | 9,382,985 | 7,939,449 |
| severe malaria cases | 313,359 | 286,403 | 252,709 | 219,014 | 185,320 |
| malaria hospitalisations | 260,329 | 237,935 | 209,943 | 181,950 | 153,958 |
| malaria deaths | 81,824 | 74,786 | 65,987 | 57,189 | 48,391 |
β=coverage of DTP3 in Malawi.
Table 4. Events averted across different outcome.
| Assessed scenario | |||
|---|---|---|---|
| Events averted | Over a 15 year
follow up (% reduction compared with no vaccination) |
Average per year | per 100,000 vaccines |
| malaria cases | 10,826,521 (14%) | 721,768 | 117,260 |
| severe malaria cases | 252,709 (11%) | 16,847 | 2,737 |
| malaria hospitalisations | 209,943 (11%) | 13,996 | 2,274 |
| malaria deaths | 65,987 (11%) | 4,993 | 714.7 |
Table 5. Comparison of public health impact results over 15 years follow-up between the three- and four-dose schedules.
| Events averted | 6–9m schedule without
a 4 th dose |
6–9m schedule plus
a 4 th dose |
|---|---|---|
| DALYs | 73,361 | 96,799 |
| malaria cases | 8,504,970 | 10,826,521 |
| severe malaria cases | 200,322 | 252,709 |
| malaria hospitalisations | 166,421 | 209,943 |
| malaria deaths | 52,308 | 65,987 |
DALY = disability-adjusted life year.
Discussion
This analysis has shown that the introduction of the RTS,S vaccine in the Malawi EPI would be a highly cost-effective malaria intervention. Cost-effectiveness of interventions affects decisions to introduce and invest in their sustainable use. Additional economic analyses will further inform budget impact, domestic funding required and long-term financial sustainability of such interventions. With the Markov model, we predicted the incremental cost-effectiveness ratio and public health impact of vaccinating children with four doses of RTS,S as recommended by WHO in the pilot implementation programme.
As the vaccine price is currently unknown, we tested the model at different vaccine prices with other input parameters held constant to determine if the vaccine programme would remain cost-effective. Our results showed that even at an inflated vaccine price of USD 10 per dose, the ICER per DALY calculated was USD 209 suggesting the RTS,S vaccination programme would remain highly cost-effective. We analysed the cost-effectiveness ratio of a three-dose versus a four-dose schedule of the RTS,S vaccine programme. Despite higher vaccine and delivery costs of the four-dose than three-dose schedule, cost-effectiveness is maintained due to greater DALYs averted with the four-dose schedule.
Malawi introduced the Rotavirus vaccine (Rotarix, GSK), in 2012. Similar to RTS,S, Rotarix is a moderately (64%) efficacious vaccine against rotavirus acute gastro-enteritis in Malawi 29. A cost-effectiveness analysis in Malawi found it to be highly cost-effective with USD 5.07 ICER per DALY averted with GAVI co-financing and USD 74.73 at vaccine market price 20. Rotarix is expectedly more cost-effective than RTS,S as it is delivered in the same schedule and existing vaccine deliver infrastructure as other existing EPI vaccines. The first RTS,S dose will be at 5 months and the last dose at 24 months. This means RTS,S will require a separate immunisation schedule driving the vaccine delivery costs higher. In addition, Rotarix is an oral vaccine with only two doses priced below USD 2.3 per dose whilst RTS,S is an injectable vaccine and has a four-dose schedule with a price assumed to be USD 5 per dose.
The WHO harmonisation exercise on RTS,S cost-effective analysis for sub-Saharan Africa involved four modelling groups: The Institute for Disease Modeling (EMOD-DTK), GSK Vaccines (GSK), Imperial College London (Imperial), and the Swiss Tropical and Public Health Institute (OpenMalaria) 30. The EMOD DTK model is a discrete, stochastic, individual-based model for malaria in either local or spatially distributed settings. The model accounts for the combined effect of an extensive set of both vector- and human-directed interventions 31, 32. The Imperial College model is a stochastic, individual-based simulation of a single population of humans linked to a stochastic compartmental model for mosquitoes 33. The model includes larval stages as well as adult female mosquitoes to capture the feedback of vector control that kills adult mosquitoes in the population dynamics 34. Swiss TPH – OpenMalaria is a stochastic, individual-based, single location simulation model of malaria in humans 35 linked to a deterministic models of malaria in mosquitoes 36. The simulation model includes sub-models of infection of humans 37, blood-stage parasite densities 38, infectiousness to mosquitoes as a lagged function of asexual parasite density 39, incidence of morbidity including severe and hospitalisation and mortality 40.
The GSK Markov Model has the advantage of considering the three categories of transmission ( PfPR2<5%, 5≤ PfPR≤40%, PfPR2-10>40%) and capacity to factor in heterogeneity in exposure among individuals for each transmission level. This model does not report confidence bounds, which is expected from a cohort model nor can it account for possible herd protection. The GSK cohort-based model was the optimal option as the other models use individual level data which are unavailable in Malawi. Our findings were within the confidence bounds of 116,480 (31,450–160,410) clinical malaria cases averted per 100,000 vaccinated children as predicted by the other three models. The GSK model is unable to capture the effect of herd immunity as compared to the three other models which do. Should herd immunity occur, cost-effectiveness would be greater than our predictions. The modest efficacy of RTS,S and its short duration of protection may limit its potential for reducing the parasite circulation capacity. Our method provides point estimates but not 95% confidence bounds, the latter which require microsimulation on individual data which were unavailable to us. The fourth dose of RTS,S was assumed to restore waning immunity but recent data has shown the efficacy to be lower after dose four.
Models are input dependent. Cost data in Africa are sparse, may be out of date or insufficiently robust. Regional data or neighbouring country data may be used when available. A malaria cost of illness study in Tanzania, Kenya and Ghana estimated clinical malaria and severe malaria costs for household and the healthcare system 41. Where Malawian data were unavailable, we used Tanzanian data rather than data from Ghana or Kenya. This is because the Tanzanian and Malawian health financing systems are similar, both provide government funded free health care through primary and referral level systems, and both lack a national insurance system or any substantial private health sector 42. Additionally, direct household cost for clinical malaria was more similar for Malawi (USD 0.5) and Tanzania (USD 0.4) than it was for Kenya (USD 0.7) and Ghana (USD 4.4) Malawi and Tanzania are geographically contiguous and share similar malaria epidemiology.
In the absence of published malaria treatment cost data from the societal perspective, we used rotavirus empirical cost data. Our data on bed net usage, an important model parameter, was taken from the 2014 Malaria Indicator Survey 19 which preceded the national wide bed net campaign that that distributed over 2.3 million bed nets from November 2014 to February 2015 24. The RTS,S vaccine is a complementary malaria intervention whose impact on the reduction malaria morbidity is also dependent on the coverage of other interventions such as bed net usage.
Population coverage is crucial to the success of any vaccine programme 43. RTS,S will be given to older children, aged 5 months, not as part of the standard EPI schedule. Additional, the fourth dose of the RTS,S will be administered to children when they are about 2 years of age. This booster dose will be outside the normal immunisation schedule whilst the first 3 doses will be before the measles vaccine which is given at 9 months. In our study we assumed the RTS,S dose 3 coverage at 75% of DTP3 and the fourth dose to be even lower at 80% of RTS,S of the third dose 44, 45. The coverage rate for the measles vaccine has been above 80% since 2010 44, 45 even though the vaccine is given to older children. Almost all Malawians have been affected by malaria, which may translate to high vaccine acceptance despite the non-standard schedule.
Conclusion
Introduction of the RTS,S/AS01 vaccine would be a highly cost-effective malaria intervention in Malawi. This holds regardless of potential changes to key variables for the vaccine programme. Following full recommendation of vaccine use by WHO, individual level cost-effective analyses will provide more accurate data that can assist other sub-Saharan African countries.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Funding Statement
LN is supported by Wellcome through the core grant to the Malawi Major Overseas Programme (grant 206545). DM is funded by the Wellcome under the Wellcome Masters Fellowship in Public Health and Tropical Medicine (grant 205324). This work is not funded by any grant; however, conception of this work started while LN was supported by the Bill and Melinda Gates Foundation to do a Masters in Vaccinology at the University of Siena Italy.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1.National Malaria Control Programme (NMCP) and ICF: Malawi Malaria Indicator Survey.Maryland UN and IM and R. Malawi. Lilongwe;2018. [Google Scholar]
- 2.National Malaria Control Programme (NMCP) [Malawi] and ICF International: 2012 Malawi Malaria Indicator Survey.2014;11–49. Reference Source [Google Scholar]
- 3.National Malaria Control Programme (NMCP) [Malawi] and ICF International: Malawi Malaria Indicator Survey 2014 Ministry of Health National Malaria Control Programme.2015. Reference Source [Google Scholar]
- 4.Programme National Malaria Control: Malaria strategic plan 2017–2022.Malawi: Lilongwe;2017. [Google Scholar]
- 5.Partnership SCT: Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11(7):e1001685. 10.1371/journal.pmed.1001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO (World Health Organisation): ghana-kenya-and-malawi-take-part-who-malaria-vaccine-pilot-programme.2017. Reference Source [Google Scholar]
- 7.EPI Comprehensive Multi-Year Plan Malawi 2016-2020.2015. Reference Source [Google Scholar]
- 8.General Guidelines for Applications for all types of Gavi support – New and underused Vaccines Support (NVS) and Health System Strengthening (HSS) – in 2016.2016. [Google Scholar]
- 9.World Bank: GNI per capita, Atlas method (current US$) - Malawi | Data.2021[cited 2021 Feb 8]. Reference Source [Google Scholar]
- 10.Penny MA, Galactionova K, Tarantino M, et al. : The public health impact of malaria vaccine RTS,S in malaria endemic Africa: country-specific predictions using 18 month follow-up Phase III data and simulation models. BMC Med. 2015;13:170. 10.1186/s12916-015-0408-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marseille E, Larson B, Kazi DS, et al. : Thresholds for the cost–effectiveness of interventions: Alternative approaches. Bull World Health Organ. 2015[cited 2021 Jun 2];93(2):118–24. 10.2471/BLT.14.138206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karp L, Traeger C: Discounting. Encycl Energy, Nat Resour Environ Econ. 2013;2–3:286–92. [Google Scholar]
- 13.Sauboin CJ, Van Bellinghen LA, Van De Velde N, et al. : Potential public health impact of RTS , S malaria candidate vaccine in sub ‑ Saharan Africa : a modelling study. Malar J. 2015;14:524. 10.1186/s12936-015-1046-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobbs KR, Dent AE: Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143(2):129–38. 10.1017/S0031182015001626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics UN: CountryProfile @ data.un.org.2017. Reference Source [Google Scholar]
- 16.Gething PW, Patil AP, Smith DL, et al. : A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. 10.1186/1475-2875-10-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO: comprehensive Multi-Year Planning (cMYP) A Tool and User Guide for cMYP Costing and Financing.World Heal Organ [Internet].2014. Reference Source [Google Scholar]
- 18.Lydon P, Gandhi G, Vandelaer J, et al. : Health system cost of delivering routine vaccination in low- and lower-middle income countries: What is needed over the next decade? Bull World Health Organ. 2014;92(5):382–4. 10.2471/BLT.13.130146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malawi Malaria Indicator Survey 2014.2014. Reference Source [Google Scholar]
- 20.Bar-Zeev N, Tate JE, Pecenka C, et al. : Cost-Effectiveness of Monovalent Rotavirus Vaccination of Infants in Malawi: A Postintroduction Analysis Using Individual Patient-Level Costing Data. Clin Infect Dis. 2016;62 Suppl 2(Suppl 2):S220–8. 10.1093/cid/civ1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix N, Bar-Zeev N, Atherly D, et al. : The economic impact of childhood acute gastroenteritis on Malawian families and the healthcare system. BMJ Open. 2017;7(9):e017347. 10.1136/bmjopen-2017-017347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicuri E, Vieta A, Lindner L, et al. : The economic costs of malaria in children in three sub-Saharan countries : Ghana , Tanzania and Kenya. Malar J. 2013;12:307. 10.1186/1475-2875-12-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.University of Oxford: Malaria Atlas Project.2015. Reference Source [Google Scholar]
- 24.National Malaria Control Programme (NMCP), ICF: Malawi, Malaria Indicator Survey.2017;2. Reference Source [Google Scholar]
- 25.Central Medical Stores Trust: The Central Medical Stores Trust Catalogue.2017. Reference Source [Google Scholar]
- 26.Ewing VL, Lalloo DG, Phiri KS, et al. : Seasonal and geographic differences in treatment-seeking and household cost of febrile illness among children in Malawi. Malar J. 2011;10:32. 10.1186/1475-2875-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo MK, Baker P, Ngo KNL: Cost-effectiveness analysis of vaccinating children in Malawi with RTS,S vaccines in comparison with long-lasting insecticide-treated nets. Malar J. 2014;13:66. 10.1186/1475-2875-13-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malawi Data [Internet].[cited 2020 Sep 2]. Reference Source [Google Scholar]
- 29.Bar-Zeev N, Kapanda L, Tate JE, et al. : Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect Dis. 2015;15(4):422–8. 10.1016/S1473-3099(14)71060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penny MA, Verity R, Bever CA, et al. : Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: A systematic comparison of predictions from four mathematical models. Lancet. 2016;387(10016):367–75. 10.1016/S0140-6736(15)00725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenger EA, Eckhoff PA: A mathematical model of the impact of present and future malaria vaccines. Malar J. 2013;12:126. 10.1186/1475-2875-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckhoff P: Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 2013;88(5):817–27. 10.4269/ajtmh.12-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin JT, Hollingsworth TD, Okell LC, et al. : Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS Med. 2010;7(8):e1000324. 10.1371/journal.pmed.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MT, Griffin JT, Churcher TS, et al. : Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit Vectors. 2011;4:153. 10.1186/1756-3305-4-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith T, Killeen GF, Maire N, et al. : Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: Overview. Am J Trop Med Hyg. 2006;75(2 Suppl):1–10. 10.4269/ajtmh.2006.75.2_suppl.0750001 [DOI] [PubMed] [Google Scholar]
- 36.Chitnis N, Hardy D, Smith T: A Periodically-Forced Mathematical Model for the Seasonal Dynamics of Malaria in Mosquitoes. Bull Math Biol. 2012;74(5):1098–124. 10.1007/s11538-011-9710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith T, Maire N, Dietz K, et al. : Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(2 Suppl):11–8. 10.4269/ajtmh.2006.75.2_suppl.0750011 [DOI] [PubMed] [Google Scholar]
- 38.Maire N, Smith T, Ross A, et al. : A model for natural immunity to asexual blood stages of Plasmodium falciparum malaria in endemic areas. Am J Trop Med Hyg. 2006;75(2 Suppl):19–31. 10.4269/ajtmh.2006.75.19 [DOI] [PubMed] [Google Scholar]
- 39.Ross A, Killeen G, Smith T: Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. Am J Trop Med Hyg. 2006;75(2 Suppl):32–7. 10.4269/ajtmh.2006.75.32 [DOI] [PubMed] [Google Scholar]
- 40.Smith T, Molineaux L, Maire N, et al. : An epidemiologic model of severe morbidity and mortality caused by Plasmodium falciparum. Am J Trop Med Hyg. 2006;75(2 Suppl):63–73. 10.4269/ajtmh.2006.75.63 [DOI] [PubMed] [Google Scholar]
- 41.Galactionova K, Bertram M, Lauer J, et al. : Costing RTS,S introduction in Burkina Faso, Ghana, Kenya, Senegal, Tanzania, and Uganda: A generalizable approach drawing on publicly available data. Vaccine. 2015;33(48):6710–8. 10.1016/j.vaccine.2015.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ministry of Health Zanzibar, National Bureua of Statistics Dar es Salaam, Office of Chief Government Statitician Zanzibar, ICF, USAID, UNICEF, UNFPA: Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) 2015-16. Dar es Salaam, Tanzania, Rockville, Maryl USA.2016;172–3. Reference Source [Google Scholar]
- 43.Liu F, Enanoria WTA, Zipprich J, et al. : The role of vaccination coverage, individual behaviors, and the public health response in the control of measles epidemics: an agent-based simulation for California. BMC Public Health. 2015;15:447. 10.1186/s12889-015-1766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Statistics Office: Malawi Demographic and Health Survey 2010.2011. Reference Source [Google Scholar]
- 45.National Statistical Office: Malawi MDG Endline Survey 2014.2014;684. Reference Source [Google Scholar]


