Abstract
The therapeutic potential of venom-derived drugs is evident today. Currently, several significant drugs are FDA approved for human use that descend directly from animal venom products, with others having undergone, or progressing through, clinical trials. In addition, there is growing awareness of the important cosmeceutical application of venom-derived products. The success of venom-derived compounds is linked to their increased bioactivity, specificity and stability when compared to synthetically engineered compounds. This review highlights advancements in venom-derived compounds for the treatment of diabetes and related disorders. Exendin-4, originating from the saliva of Gila monster lizard, represents proof-of-concept for this drug discovery pathway in diabetes. More recent evidence emphasises the potential of venom-derived compounds from bees, cone snails, sea anemones, scorpions, snakes and spiders to effectively manage glycaemic control. Such compounds could represent exciting exploitable scaffolds for future drug discovery in diabetes, as well as providing tools to allow for a better understanding of cell signalling pathways linked to insulin secretion and metabolism.
Keywords: Clinical trials, diabetes, exendin-4, venom therapeutics
Introduction
Crude animal venom contains a diverse mixture of bioactive compounds that target a variety of receptors to support survival of venomous animals.1 However, venoms and their metabolites are now being recognised as potential exploitable tools in medicine.2,3 As such, the term ‘venomics’ was first used by Juárez et al4 to describe characterisation of the complete protein profile of snake venom. Following on from this, more recent advances in analytical techniques including incorporation of genomics, mass spectrometry and proteomics have assisted scientists to more easily explore venom profiles.5 Together with modern-day ability to rapidly screen venom compounds using high-throughput assays, this represents a step-change in realising the full therapeutic potential of animal venoms.5 This review summarises current clinically approved venom-based drugs, and briefly considers venom-derived drugs in clinical trials as well as use of venom products as cosmeceuticals before finally highlighting the therapeutic promise of such compounds for obesity-diabetes.
Clinically Approved Venom-Derived Drugs
Captopril and enalapril
Ferreira et al6 purified and characterised bradykinin-potentiating factors (BPFs) from the Brazilian viper snake venom, which the Squibb Institute of Medical Research was then able to utilise teprotide, which acted as an ACE inhibitor.7 After studying structure/function aspects of teprotide, the orally active compound named SQ 14 225 (D-2-methyl-3-mercaptopropanoly-L-proline), now better known as captopril was generated (Table 1), and was the first ACE inhibitor that effectively lowers blood-pressure in humans.7-9 Following captopril, enalapril was synthesised by the substitution of the mercapto group present on the captopril structure, to an alkyl group.10 Despite limited oral bioavailability, the prodrug displays good potency and was clinically approved in 1985 as Vasotec, for the treatment of hypertension and congestive heart failure.11-13
Table 1.
Origin, treatment indication and current methods for production of currently clinically approved venom-derived drugs.
| Drug | Species | Treatment indication | Production |
|---|---|---|---|
| Captopril and Enalapril (Capoten, Vasotec) | Brazilian viper, Bothrops jararaca | Hypertension and related cardiovascular disorders | Synthetic |
| Ziconotide (Prialt) | Cone snail, Conus magus | Chronic pain | Synthetic |
| Eptifibatide and Tirofiban (Integrilin, Aggrastat) | Saw-scaled viper, Echis carinatus | Thrombotic cerebrovascular or cardiovascular disease | Synthetic |
| Pygmy rattle snake, Sistrurus miliarius | |||
| Lepirudin and Bivalirudin (Refludan, Angiomax) | Blood-sucking leech, Hirudo medicinalis | Stroke, deep vein thrombosis and pulmonary embolism | Synthetic |
| Batroxobin (Defibrase) | Brazilian lancehead snake, Bothrops moojeni | Stroke and ischemic attack and sudden deafness | Purified from venom |
| Bee venom therapy (Apitox) | Bee, Apis mellifera | Osteoarthritis | Whole venom |
| Exenatide (Byetta, Bydureon) | Gila Monster lizard, Heloderma suspectum | Type 2 diabetes | Synthetic |
| Cobratoxin (Cobratid) | Chinese cobra, Naja naja atra | Pain | Purified from venom |
Ziconotide
Ziconotide is an intrathecal analgesic drug that was clinically approved in 2004 for the treatment of chronic pain14 (Table 1). Marketed as Prialt, this 25 amino acid peptide is a synthetic version of ω-conotoxin MVIIA (ω-MVIIA), extracted from the venomous cone snail Conus magus.14-16 It has been demonstrated that ω-conotoxin blocks N-type voltage gated calcium channels and prevents the release of pro-nociceptive neurochemicals including glutamate, calcitonin gene-related peptide and substance P.17-19 Prialt is also used to treat chronic pain in those with intolerances to morphine or other systemic analgesics.20
Eptifibatide and tirofiban
Tirofiban was the first approved venom-derived antiplatelet drug based on the structure of echistatin, a peptide extracted from venom of the saw-scaled viper, Echis carinatus21 (Table 1). Echistatin, isolated in 1988, is a glycoprotein (GP) IIb/IIIa receptor antagonist leading to effective inhibition of fibrinogen-induced platelet aggregation.21-25 Furthermore, Scarborough et al26 screened 62 snake venoms, leading to the discovery of barbourin from the pygmy rattle snake, Sistrurus miliarius, that specifically inhibited GP IIb/IIIa receptors. These findings ultimately led to the synthesis of eptifibatide (Table 1), a highly effective antiplatelet drug.27,28
Lepirudin and bivalirudin
Lepirudin and bivalirudin (Table 1) are specific thrombin inhibitors, with structures based on hirudin, a peptide with strong anticoagulant properties found in blood-sucking leeches, Hirudo medicinalis.29,30 Lepirudin was clinically approved in 1998, and bivalirudin followed in 2000.31 In 2012, lepirudin was discontinued, although this was not due to inefficacy or adverse effects, but rather manufacturing issues.32 Bivalirudin, on the other hand is still routinely prescribed to treat unstable angina and percutaneous coronary intervention.33-35
Batroxobin
Batroxobin (Table 1), isolated from the venom of the Bothrops moojeni venomous snake is a thrombin-like serine protease which is a defibrinogenating agent with anti-inflammatory effects.36,37 Batroxobin is a 231 amino acid protein that helps to form non-cross-linked fibrin clots and subsequently release fibrinopeptides A.38,39 Purified from B. Moojeni venom, it is marketed as Defibrase and currently available for use in China for the treatment of stroke and ischemic attack.40,41
Apitox
Bee venom extracted from Apis mellifera and marketed as Apitox has been clinically approved in South Korea for the treatment of osteoarthritis42,43 (Table 1). This approval came after a phase III clinical trial with 363 patients with knee osteoarthritis.44 Furthermore, Apimeds are sponsoring a clinical trial for use of apitox in the treatment of Multiple Sclerosis (MS), as the drug is believed to be effective in reducing pain and swelling associated with the disease but there are no clinical data currently available in this regard.45
Cobratid
A short-chain α-neurotoxin isolated from cobra venom Naja naja atra, is known to act as an antagonist of nicotinic acetylcholine receptors (nAChRs) and bring about analgesic effects.46 As such, in a rodent model of pain, cobratid exerted dose-dependent analgesic effects that were independent of muscarinic ACh and opioid receptor modulation.47 Furthermore, cobratid, now promoted as a tablet named Keluoqo that also contains tramadol hydrochloride and ibuprofen, has undergone clinical trials for the treatment of moderate to severe pain associated with cancer.48 Whilst results of this study were promising, issues around development of tolerance still need to be addressed. However, it should be noted that studies are ongoing in a bid to help minimise adverse effects associated with use of this drug.49-51
Exenatide
Oral glucose administration results in a greater insulinotropic response compared to similar intravenous glucose delivery in humans.52 This phenomenon, known as the ‘incretin effect’, is related to the secretion of the gut-derived hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).53 Both hormones act on specific pancreatic beta cell G-protein-coupled receptors (GPCRs), that stimulate adenylyl-cyclase activity, cAMP accumulation and ultimately insulin secretion.54 In type 2 diabetes, secretion of GLP-1 from the GI tract is blunted following nutrient intake highlighting an ideal drug target for the disease.55 However, native GLP-1 has a short biological half-life due to degradation by the ubiquitous enzyme dipeptidyl peptidase-4 (DPP-4) upon secretion into the bloodstream, limiting therapeutic application of this peptide. DPP-4 has a wide substrate specificity and acts on various regulatory peptides, chiefly cleaving small peptides with proline or alanine as the penultimate N-terminal residue.56 As such, although DPP-4 inhibitor drugs are now clinically approved for the treatment of type 2 diabetes with an excellent adverse effect profile, lack of substrate specificity was an initial concern.56 In this respect, the first clinically approved GLP-1 peptide mimetic with inherent stability against DPP-4 was derived from the saliva of the venomous Gila monster lizard, Heloderma suspectum.57,58 The peptide, first isolated by Eng et al57 was named exendin-4 and shown to have 53% homology to human GLP-1. Importantly, exendin-4 possesses an amino acid substitution at the penultimate N-terminal residue that masks the DPP-4 binding site and dramatically extends the duration of biological action of the peptide58 (Figure 1; Table 1). Exendin-4 was initially marketed as Byetta (Figure 2), and then later as an extended release preparation known as Bydureon, which were approved for clinical use in 2005 and 2017, respectively, for the treatment of type 2 diabetes.
Figure 1.
The amino acid sequence of exenatide in comparison to native GLP-1. The key amino acid substitution at position 2 that confers full resistance to DPP-4 in exenatide is highlighted.
Figure 2.
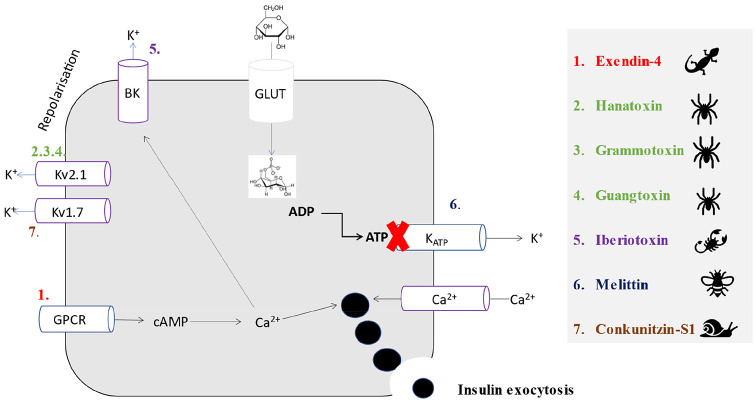
A schematic showing proposed insulin secretory pathways for the venom-derived peptides hanatoxin grammotoxin, guangtoxin, exendin-4, conkunitzin-S1, iberiotoxin and melittin within pancreatic beta cells. A simplified pathway for the secretion of insulin via the primary beta cell stimulus, namely glucose, is depicted for reference via conversion to glucose-6-phospahate, with subsequent generation of ATP and closure of KATP channels. Colour coded numbers correspond to proposed beta-cell membrane target of each venom-derived peptide, with the specific K+ channel that melittin binds to still not known. Indeed, the mechanisms of all peptides, barring exendin-4, still needs to be fully characterised. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; BK, calcium-activated (big) potassium channel; cAMP; cyclic adenosine monophosphate; GLUT, glucose transporter 2; GPCR, G-protein coupled receptor; Kv, voltage-gated potassium channel.
Venom and Cosmeceuticals
Cosmeceuticals represent an attractive avenue for commercial use of venom-derived products. This is typified by the global success of Botulinum toxin (Botox) based therapies for medical and particularly cosmetic purposes, where Botox represents a type A toxin isolated from Clostridium botulinum bacteria.59 The toxin acts on neuromuscular junctions to prevent acetylcholine (ACh) release from presynaptic neurons, subsequently causing mild muscle paralysis.60 Indeed, since first use of Botox for a cosmeceutical procedure to decrease the appearance of wrinkles in 1989, the drug now generates around $3 billion per annum.61,62 In a separate venture, snake venom has also entered the cosmeceutical industry for use in facial serums to reduce wrinkles. An example of this being the use of synthetic tripeptide (Tripeptide [SYN®-AKE]) from Tropidolaemus wagleri snake venom. Tripeptide represents a synthetic version of waglerin 1, which also blocks the action of ACh to subsequently inhibit muscle contraction.63 Additionally, venom purified from the Apis mellifera L. honeybee is also currently being exploited in facial serums as it is believed to reduce total wrinkle size in individuals with UV damage.64 Most recently, argiotoxine-636 (ArgTX-636) isolated from the Argiope lobata spider venom has been patented for its use in skin and teeth whitening.65 Although the mechanism of action of ArgTX-636 remains to be fully elucidated, inhibitory effects on melanogenesis is believed to be key in these cosmeceutical applications.66 In terms of approval processes, it is important to note the differences in regulatory authorisation for a drug and a cosmeceutical. As such, a cosmeceutical is considered to exert a pharmaceutical therapeutic benefit but not necessarily linked to an established biological benefit.67 Therefore, unlike drugs, the FDA does not necessarily review or approve cosmeceuticals prior to sale, representing a much simpler approval process than with drugs. However, there is still some controversy around the exact boundaries of what constitutes a cosmeceutical and a drug,68 that requires further detailed clarification.
Venom-Derived Drugs in Clinical Trials
Early clinical trials
There has been much effort made to utilise natriuretic peptides, namely atrial natriuretic peptide (ANP), ventricular natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) for the treatment of heart failure.69-71 The first characterised reptilian natriuretic peptide, dendroaspis natriuretic peptide (DNP), was extracted from the Eastern green mamba snake Dendroaspis angusticeps.72 DNP, a 38 amino acid peptide that has structural similarities to human natriuretic peptides, is a potent activator of cardiomyocyte guanylate cyclase A, which aids in cardiac unloading and therefore may have benefits in heart failure.73,74 Cenderitide, a derivative of DNP, was synthesised and developed by the Mayo Clinic through fusion of the 22 amino acid peptide CNP with the 15 amino acid C-terminal of DNP.74 A clinical trial was conducted in 2015 by Capricor, to assess efficacy and safety of subcutaneous infusion of cenderitide in subjects with stable, chronic heart failure.75 Although the drug was well tolerated, further clinical assessment of cenderitide by Capricor has not been carried out. However, a phase I clinical trial by Mayo Clinic with cenderitide in 30 participants with myocardial infarction was completed in 2019.76
Another approach for utilising snake venom involved the extraction of the enzyme metalloproteinase fibrolase from the southern copperhead snake Agkistrodon contortrix contortrix.77,78 The protein consisting of 203 amino acids, underwent DNA recombination to create alfimeprase, a compound which directly degrades fibrin to result in thrombolysis.79-81 Alfimeprase successfully underwent a phase I clinical trial in patients with acute peripheral arterial occlusion (PAO) and was well tolerated with no reports of bleeding or systemic thrombolysis.82 Although alfimeprase progressed to phase II and III trials, these were terminated as there was ultimately no significant improvement when compared to placebo.82,83
The discovery and success of ziconotide from Conus magus has brought much attention to the potential of marine venom-derived compounds. Therefore, it is not surprising there has been the extraction and characterisation of such peptides, progressing into clinical trials. Firstly, Vc1.1 a 16 residue α-conotoxin (ACV1) from Conus victoriae is a potent antagonist of nAChRs, where modulation of nAChR’s is associated with clear analgesic actions.84,85 Metabolic Pharmaceuticals conducted a phase I human clinical trial with ACV1 in healthy males, where it was well tolerated, and no adverse effects reported.86 However, lack of efficacy in phase IIa trials for diabetic peripheral neuropathic pain led to further clinical research with ACV1 being discontinued, but α-conotoxins are still being explored as future drugs for the treatment of neuropathic pain.87-89
In another attempt to exploit a conotoxin with analgesic properties, the χ-conotoxin χ-MrIA, was extracted from the Conus marmoreus.90 The 13-residue peptide was found to non-competitively inhibit the norepinephrine transporter (NET) in humans,91 whereby inhibiting the reuptake of norepinephrine has demonstrated analgesic properties.91 Thus, Xen2174, a synthetic version of χ-MrIA with the addition of a pyroglutamyl residue to the N terminus, demonstrated analgesic effects in a rat model of neuropathic pain.92 Whilst Xen2174 displayed promising results in a phase II clinical trial for cancer patients with chronic pain, it ultimately failed to pass into phase IIb due to dose-limiting toxicity.2 Lastly, contulakin-G is a 16 amino acid peptide extracted from the Conus geographus and was shown to inhibit neurotensin receptors (NTRs).93,94 Neurotensin is a peptide found in the CNS with an influence on the internal analgesic system. 95In this regard, contulakin-G, later named CGX-1160, received orphan drug status in 2005, after the success of a phase Ib clinical trial for the treatment of neuropathic pain associated with spinal cord injury, but has since been discontinued.96
Another venomous animal with perceived therapeutic value is the vampire bat Desmodus rotundus, specifically through isolation of a 441 amino acid fibrin-dependent plasminogen activator from its venom.97,98 Later named desmoteplase, this thrombolytic agent effectively breaks down blood clots by converting plasminogen to plasmin.97 Desmoteplase was investigated as a potential therapeutic agent for ischemic stroke,99 however, despite promising initial observations, desmoteplase was subsequently terminated following lack of prominent efficacy in a phase III clinical trial.100-102
Current clinical trials
Cancer
Chlorotoxin is a 36 amino acid peptide extracted from the venom of the Deathstalker scorpion, Leiurus quinquestratius,103 which has shown specificity towards various cancerous cell lines including glioma, melanoma and carcinoma.104 It is theorised that chlorotoxin can specifically target chloride ion channels on glioma tumour cells to prevent malignancy and decrease tissue invasion potency.105,106 As of January 2020, the City of Hope Medical Centre in California began recruiting participants for phase I testing of the safety and efficacy of chlorotoxin for recurrent glioblastoma cancer.107 The trial will involve the delivery of chimeric antigen receptor (CAR-T lymphocytes), with chlorotoxin as the tumour targeting domain. CAR-T lymphocytes are synthetic T cell receptors that can recognise tumours and activate an immune response specific to the cancerous cells.108 An additional use of chlorotoxin is with tozuleristide (Table 2), a tumour targeting agent that comprises chlorotoxin and the fluorescent dye, indocyanine green that provides intraoperative visualisation of tumours.109,110 Tozuleristide successfully underwent a phase I clinical trial in 17 patients diagnosed with glioma, treatable by surgical excision. The trial revealed no adverse effects and good tolerability of tozuleristide, as well as being highly effective in glioma imaging.111 More recently, Blaze Bioscience Inc. is recruiting paediatric patients with CNS tumours undergoing surgery for use of tozuleristide in phase II and III trials.112
Table 2.
Details of venom-derive drugs undergoing clinical trials including name, origin, treatment indication and clinical trial phase progression.
| Drug | Species | Treatment indication | Clinical trial phase |
|---|---|---|---|
| Chlorotoxin (CTx) | Deathstalker scorpion, Leiurus quinquestratius | Recurrent glioblastoma cancer | Phase 1 [NCT04214392] |
| Chlorotoxin with tozuleristide (BLZ-100) | Deathstalker scorpion, Leiurus quinquestratius | Central nervous system tumours | Phase I and phase II [NCT03579602] |
| SOR-C13 (Sorcidin) | Northern short-tailed shrew, Blarina brevicauda | Advanced refractory solid tumours | Phase Ib [NCT03784677] |
| ShK-186 (Dalazatide) | Caribbean sea anemone, Bunodosoma granulifera | Lupus and erythematous | Phase II |
| RPI-78M (Receptin) | Chinese cobra, Naja naja | Multiple sclerosis and adrenomyeloneuropathy | Phase II and phase III |
| RPI-MN (Pepteron) | Chinses cobra, Naja naja | Amyotrophic lateral sclerosis, herpes simplex keratitis and human immunodeficiency virus | Phase I and phase II |
In an alternative approach, cancer cell growth has been linked to increased ambient calcium levels, with the Transient Receptor Potential Vanilloid channels (TRPV6) known to play a key role in regulating normal calcium homeostasis.113 Overexpression of TRPV6 within some cancerous cell lines such as breast, pancreatic and ovarian has been detected.113,114 Interestingly, a peptide named SOR-C13 (Table 2), modelled on a 253 amino acid protein extracted from the venom of the northern short-tailed shrew, Blarina brevicauda, has been shown to prevent calcium uptake via TRPV6.115 As such, a clinical trial enrolling patient with solid tumours such as pancreatic and ovarian,116 ultimately lead to SOR-C13 being awarded orphan drug status for both ovarian and pancreatic cancer.116 More recently, the Anderson Cancer Centre in Texas is currently recruiting patients with advanced refractory solid tumours for treatment with SOR-C13. This is a phase Ib trial that will determine the most effective dose of SOR-C13 in these patients.117
Autoimmune disease
As well as cancer, there are also ongoing clinical trials with venom-derived compounds in auto-immune disease. In this regard, in 1996 a 35 amino acid peptide called Stichodactyla toxin (ShK) was extracted from a Caribbean Sea anemone Bunodosoma granulifera and shown to block Kv1.3 potassium channels118 (Table 2). ShK-186, a 37 amino acid analogue of ShK, now known as dalazatide, successfully underwent a Phase I trial consisting of 32 healthy volunteers, displaying only mild adverse effects and good tolerability.119 In addition, in a Phase Ib trial for psoriasis in 2014, dalazatide was extremely well tolerated.120 This is particularly encouraging given the current lack of effective treatment options for those with autoimmune disease, and the increasing prevalence of the disease worldwide. Furthermore, TEKv Therapeutics (Columbus, OH) are currently preparing Phase II clinical trials for the treatment of autoimmune diseases such as lupus and erythematous with dalazatide.
Additionally, cobratoxin and cobrotoxin from Naja atra cobra snake venom has entered clinical trials for the treatment of a range of diseases. Cobratoxin and cobrotoxin have been detoxified to produce chemically modified versions, namely RPI-78M (Receptin) and RPI-MN (Pepteron), respectively (Table 2). The peptides displayed analgesic effects by specifically targeting nAChRs.121-123 Additionally, RPI-78M proved safe and effective in a phase I clinical trial for the treatment of MS.2 RPI-MN has also proved effective in a preclinical study against the human immunodeficiency virus, as it has shown an ability to inhibit viral replication.124
Emerging Venom-Derived Drugs for Type 2 Diabetes
Overview of type 2 diabetes
Type 2 diabetes is a complex metabolic disorder characterised by insulin resistance, as well as a decrease in pancreatic beta cell mass and insulin secretion, that ultimately leads to overt hyperglycaemia.125,126 If the disease is poorly managed several complications can arise, including cardiovascular and kidney disorders, blindness and neurogenerative disorders.127,128 Diabetes is described as one of largest epidemics of the 21st century with the prediction that 642 million people will suffer from diabetes by 2040.129 The treatment for obesity-related diabetes is initially education and lifestyle intervention, but such strategies are often ineffective, and patients inevitably progress to pharmacological treatment.130 There is an array of drugs available to treat 2 diabetes that can improve insulin secretion (sulfonylurea and meglitinide) or sensitivity (thiazolidines and metformin), enhance the incretin effect (GLP-1 mimetics and DPP-4 inhibitors), increase glucose excretion (sodium glucose transporter 2 inhibitors) or ultimately insulin replacement therapy as the last line of treatment.131,132 Although these drugs are effective, they are often associated with adverse effects such as weight gain and hypoglycaemia.131 Additionally, drug failure over time is commonplace with type 2 diabetes therapeutics, leading to polypharmacy and ineffective blood glucose control.132,133 Therefore, it is essential that new pharmacological agents are developed to provide more effective treatment for diabetes, with less adverse effects that should improve compliance and overall clinical outcomes.
The discovery of exendin-4 from the venom of the Gila monster, as noted above, has brought much attention and acceptability to the use of venom-derived drugs in the field of diabetes therapies. Unlike other antidiabetic agents, exendin-4 has much fewer side effects such as hypoglycaemia and encourages weight loss.134 Additionally, it has been demonstrated that exendin-4 increases beta cell proliferation and protects against cardiovascular disorders.135,136 To date, products derived from the venoms of bees, cone snails, sea anemones, scorpions, snakes and spiders are being actively investigated as new and effective therapeutic approaches for diabetes,137 with exendin-4 recognised as clear proof-of-concept for this exciting drug discovery route.
Cone snail insulin
Human insulin is composed of 2 peptide chains linked by 2 disulphide bonds, chain A contains 21 amino acids and chain B which contains 30 amino acids.138 Con-Ins G is an insulin molecule derived from the Conus geographus cone snail that lacks the C-terminus of the B chain but can activate human insulin receptors.139,140 Additionally, unlike human insulin, Cons-Ins G1 has a lower affinity for the primary binding site on the human insulin receptor (hIR), with a preferential affinity for the secondary binding site, suggesting an alternative mechanistic approach to hIR activation.141 Furthermore, this insulin peptide contains post-translational modifications in the A and B chain, namely a γ-carboxylated glutamate residue and a hydroxylated proline residue, respectively, which have been hypothesised to increase biological activity.141 In this regard, the crystal structure of Con-Ins G1 in comparison to human insulin has been described.141 The discovery of Cons-Ins G1 has led to the synthesis of a new recombinant insulin analogue, with an extremely fast onset of action due to its smaller size.141 Moreover, the small size of the peptide means chemical synthesis is less complicated, making it a prime candidate for the development of a new insulin therapeutic regimen for humans141 (Table 3). Thus, Cons-Ins G1 could represent another important option within the array of clinically approved insulin analogues or could have use in premixed insulin combinations.137
Table 3.
Name, amino acid sequence and origin of venom-derived drugs with reported antidiabetic efficacy.
| Species | Toxin name | Sequence | Uniprot | Reference | |
|---|---|---|---|---|---|
| Tarantula, Grammostola rosea |

|
Hanatoxin | ECRYLFGGCKTTSDCCKHLGCKFRDKYCAWDFTFS | P56852 | Swartz and MacKinnon181 |
| Tarantula, Grammostola rosea |

|
Grammotoxin | DCVRFWGKCSQTSDCCPHLACKSKWPRNICVWDGSV | P60590 | Takeuchi et al186 |
| Tarantula, Plesiophrictus guangxiensis |

|
Guangtoxin | DEGECGGFWWKCGSGKPACCPKYVCSPKWGLCNFPMP | P84835 | Tilley et al188 |
| Cone snail, Conus geographus |

|
Cons-Insa | A chain: GVVyHCCHRPCSNAEFKKYC | A0A0B5AC95 | Safavi-Hemami et al139 |
| B chain: TFDTOHRCGSyITNSYMDLCYR | |||||
| Cone snail, Conus striatus |

|
Conkunitzin-S1 | KDRPSLCDLPADSGSGTKAEKRIYYNSARKQCLR | P0C1X2 | Finol-Urdaneta et al143 |
| FDYTGQGGNENNFRRTYDCQRTCLYT | |||||
| Social wasp, Agelaia pallipes pallipes |

|
Agelaia MP-1 | INWLKLGKAIIDAL | P69436 | Baptista-Saidemberg et al165 |
| Eastern Indian red scorpion, Buthus tamulus |

|
Iberiotoxin | PyrFTDVDCSVSKECWSVCKDLFGVDRGKCMGKKCRCYQ | P24663 | Galvez et al179 |
In Con-Ins, the y indicates a carboxyglutamic acid residue and the O indicates an hydroxyproline residue.
In a separate approach to utilise cone snail venom, there was the isolation of Conkunitzin-S1 (Conk-S1) form the cone snail Conus striatus.142 This peptide was shown to specifically inhibit Kv1.7 beta cell channels (Figure 2), which resulted in an increase in glucose-stimulated insulin secretion from rat islets. However, unlike current insulinotropic therapeutics such as sulfonylureas and meglitinides, conk-S1 is not associated with hypoglycaemia as glucose-dependent stimulation of insulin secretion has been observed.143 The NMR-derived solution structure of recombinant Conk-S1 has been revealed, exhibiting 2 disulphide bonds.142 As such, conk-S1 represents a tool to help characterise Kv1.7 channel mechanisms involved in insulin secretion and could also prove to be a valuable novel therapeutic option for type 2 diabetes.143
Caribbean sea anemone
As well as their role in the immune system, Kv1.3 channels have been implicated in the development of insulin resistance and subsequently type 2 diabetes.144 This is evident from Kv1.3 gene deletion studies in mice, resulting in enhanced peripheral insulin sensitivity and anti-satiety effects.145 Thus, Shk-186 was administered to diet-induced obese mice, resulting in normalisation of blood glucose and insulin, as well body weight reduction.146 The anti-obesity mechanism of action has not been fully established. However, it has been suggested that Shk-186 contributes to improved peripheral insulin sensitivity through activation of brown adipose tissue, or via reduction of obesity-induced inflammation of abdominal white adipose tissue.146 These results were similar to that observed with Kv1.3 gene deletion, supporting the role of Kv1.3 in insulin resistance.145 However, it should also be noted that Kv1.3 channel blockade did not reduce body weight gain in diet-induced obese rats,147 and whilst this might be related to subtle differences in dosing regimens and animal models employed, it does necessitate further study on the anti-obesity potential of Kv1.3 inhibition. Although, it has already been demonstrated in clinical trials that ShK-186 has a good safety profile, further promoting potential therapeutic promise for obesity and insulin resistance.
Snake venom
Poor glycaemic control in diabetes can lead to an increased risk of damage to blood vessels in the eyes known as diabetic retinopathy.148 Indeed, type 2 diabetes is now the leading cause of blindness in the UK.149 Blindness usually arises due to an angiogenesis cascade leading to neovascularisation from the retinal vessel in a process called choroidal angiogenesis.150 In this regard, integrins are receptors that have been implicated in angiogenesis.150 Lebecetin is a C-type lectin extracted from the blunt-nose viper snake, Macrovipera lebetina, which has been shown to interact with α5β1- and αv-containing integrins.150 C-type lectins represent a class of snake proteins which have been implicated in anticoagulant- and platelet-modulating activities. In one study, lebecetin is demonstrated to reduce angiogenesis in a chorioallantoic membrane assay and effectively decrease the extent of choroidal neovascularisation.150 Importantly, lebecetin was specific towards the proliferating vascular cells rather than the mature blood vessels suggesting it would be a safe and effective treatment for choroidal angiogenesis.150
Sulfonylureas and meglitinides are approved classes of type 2 diabetes drugs that target the ATP-sensitive potassium (KATP) channel on pancreatic beta cells to promote insulin secretion.151 However, a major disadvantage of these insulin secretagogues is non-glucose dependent insulin release and subsequent hypoglycaemia.152 Thus, constituents of snake venom could yield products that help give a better understanding of these beta cell signalling pathways, leading to drug modifications and a reduction in side effect profiles. As such, the venom from the monocle cobra snake, Naja kaouthia, was analysed and fractioned, leading to the isolation of Cardiotoxin-1.153 Cardiotoxins have previously demonstrated cytotoxicity and cell damage however, this peptide was demonstrated to be non-toxic to the rodent beta cell-line INS-1.153 Cardiotoxin-1 stimulates insulin release in a dose-dependent manner, but this effect is independent of glucose concentrations. Whilst the mechanism of action has yet to be fully established, an increase in beta-cell intracellular calcium is observed with cardiotoxin-1 and action at Kv channels has been hypothesised.153 Thus, it could be utilised to gain a better understanding of the important Kv channel-dependent insulin secretory pathway in pancreatic beta cells.154 In addition, Moore et al isolated insulin releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis snakes by gel filtration chromatography. The insulinotropic action appeared to be linked to serine proteinases, phospholipases A2 (PLA2) and disintegrins within the venom.155 PLA2 belong to a group of snake toxins with insulinotropic abilities, as exposure results in hydrolysis of membrane phospholipids, the production of arachidonic acid and subsequently insulin secretion.156,157 Additionally, disintegrins represent a class of snake peptides that have been implicated in anti-platelet activity but may also possess effects on beta cell insulin secretion.158
Finally, a crotamine-like protein was extracted from the venom of the south American rattlesnake Crotalus durissus cascavella that displayed glucose-dependent insulin secretory actions.159 Crotamine is one of the main toxins of the American rattlesnake and has previously be shown to be non-toxic to human endothelial, fibroblasts and muscle cells.160,161 Although the insulinotropic effects have not been fully elucidated, it may be linked to modulation of Na+ channels. The putative mechanism of action comes from it inducing membrane depolarisation-dependent muscle contractions by increasing the Na+ permeability of skeletal muscle membrane.162 The glucose-dependent nature of the peptide could represent a major therapeutic advantage over other classes of antidiabetic drugs, as already noted with exendin-4.
Wasp and bee venom
The most abundant class of peptides found within wasp venom are known as mastoparans.163 It has previously been shown that mastoparans stimulate the release of insulin in the presence or absence of glucose, however phospholipase A2 was required for this biological action.164 This suggests an interaction between beta cell GPCRs and mastoparan, leading to activation of phospholipase A2 and subsequent insulin release.164 Furthermore, agelaia MP-1 is a 14 amino acid mastoparan peptide isolated from the venom of the social wasp Agelaia pallipes pallipes (Table 3; Figure 3), with 3D structure predicted by computer software packages.165 Agelaia MP-1 was shown to induce mast cell degranulation as well as stimulate insulin secretion.165 Importantly, insulinotropic actions were not related to beta cell lysis, and interestingly persisted despite inhibition of the Ca2+ and KATP channels, suggesting GPCR interaction and activation of subsequent second messenger cell signalling pathways.165 However, there has been some concern about bee venom and its non-specific mode of action and possible adverse side effects.166 Nonetheless, melittin is recognised as a potential anti-diabetic agent and studies are underway to reduce or neutralise related toxicity, without detrimentally affecting therapeutic promise. This includes polymer nanoparticle delivery systems appear to significantly reduce, or even annul, toxicity.167,168 Further to this, melittin derived from bee venom belongs to a class of gating modifier toxins (GMTS) that modulate voltage-gated ion channels and possibly stimulate insulin secretion through interaction with beta cell K+ channels169 (Figure 2). A GMTS nanocomplex was formulated to extend duration of action and prevent possible toxicity, with strong electrostatic interactions between negatively charged polyanions, dextran sulphate (DS) and the positively charged melittin.169 The formation of this (DS)/melittin nanocomplex successfully increased the half-life of melittin and reduced its acute toxicity in concert with controlling blood-glucose levels for 48 hours in diabetic mouse models.169
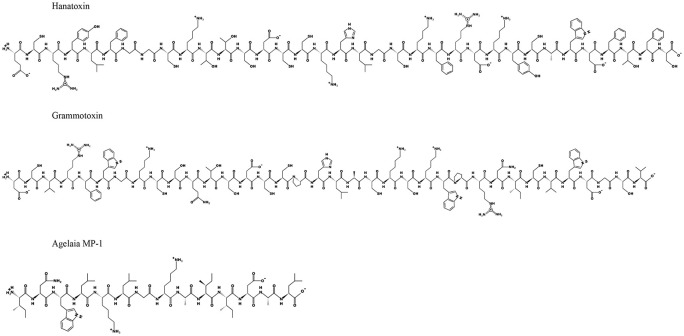
Figure 3.
2D structural information for hanatoxin, grammotoxin and agelaia MP-1 based on known amino acid sequences.
Moreover, bee venom may not only improve glycaemic control, but can also help wound healing, a classic complication of diabetes.170 Bee venom is known for its antimicrobial and anti-inflammatory qualities, suggesting potential for the improvement of this complication.171 Therefore, it is not surprising that diabetic rodents treated with bee venom display improved wound healing, in studies that not only improve our understanding of diabetic wound healing processes, but also suggests bee venom as a potential therapeutic option.171 The mechanism of anti-inflammatory effects of bee venom are still not fully understood. However, gene expression studies demonstrate ability of bee venom to inhibit lipid accumulation and downregulate adipogenic transcription factors, such as PARγ and C/EBPα.172 Furthermore, bee venom was recently shown to improve GLUT-4 expression and insulin sensitivity suggesting bee venom as a potential treatment for chronic inflammation in obesity as well as diabetes.172 Indeed, venom from the Egyptian honeybee Apis mellifera lamarckii, improved diabetic status in alloxan treated rats.173 Beneficial effects were believed to be related to the melittin and phospholipase A2 components of the venom.173 Finally, diabetes and prolonged periods of hyperglycaemia can lead to protein glycation and impairment of biological activity.174 In this respect, honeybee Apis mellifera venom reduced the extent of glycation of haemoglobin in an in vitro assay over a 5-week incubation period,175 with a mechanism linked to preventing the binding of glucose to haemoglobin, and therefore preventing glycation induced changes in the haemoglobins secondary structure.175
Scorpion venom
As noted above, The Kv channel mode of action on pancreatic beta cells includes repolarisation of the membrane and limiting Ca2+ entry into the beta cell.176,177 In this regard, there has been identification of beta cell calcium-activated potassium BK (big potassium) channels.178 This channel is activated upon Ca2+ influx into the beta cell and subsequent membrane depolarisation. In this regard, a peptide extracted from the Eastern Indian red scorpion Buthus tamulus, named iberiotoxin is an antagonist of this channel,137,179 with its 3D structure available from the uniport number provided (Table 3). Iberiotoxin has been shown to increase the duration of the beta cell action potential by delaying rectifier currents, and subsequently increase insulin secretion in human pancreatic beta cells.137,179 However, whilst iberiotoxin has helped with understanding of beta cell secretory dynamics, therapeutic promise could be compromised by lack of specificity, as BK channels also play an important role in the CNS.180 Thus, further study into the therapeutic potential and safety profile of iberiotoxin in diabetes is still required.
Spider venom
Two K+ ion channel modifier peptides, named hanatoxin and grammotoxin (Table 3; Figure 3), were successfully extracted from the venom of the Chilean rose tarantula Grammostola rosea.137,181 Hanatoxin is a 35 amino acid peptide with 6 cysteine residues181 and is described as a potent inhibitor of the Kv2.1 component of the K+ repolarisation beta cell channel181,182 (Figures 2 and 3). Interestingly, the peptide is known to insert itself into the phospholipid membrane hydrocarbon core, without the requirement of pore formation, before it then encounters and inhibits the Kv channel inside the membrane phospholipid.183,184 It has been demonstrated that the peptide can also inhibit the Kv2.2 channels, but with a lower infinity.137 In keeping with this, hanatoxin increases glucose-stimulated insulin secretion and calcium oscillators in both mouse and human islets.2 Grammotoxin exhibits 43% amino acid sequence homology to hanatoxin and is believed to have a similar mechanism (Figure 3), but it binds to the same Kv channels with lower affinity185,186 (Figure 2). However, whilst comparison of the 3D structures of hanatoxin and grammotoxin does reveal some clear conservation of structures between both peptides, slight differences in the surface shape and distribution of the charged residues may help explain the lower binding affinity of grammotoxin.186 Following on from this, guangtoxin (GxTX) has been extracted from the Plesiophrictus guangxiensis tarantula187,188 (Table 3). GxTX has been shown to broaden the length of the glucose-induced beta cell action potential and increase related Ca2+ oscillations through interaction with the Kv channel, whilst being ineffective at low glucose levels, thus limiting potential to cause hypoglycaemia.187,188
Although exciting in terms of potential therapeutic value, a disadvantage is the widespread expression of Kv channels, and it may prove challenging to specifically target beta cell Kv channels.137 However, inhibition of the Kv2.1 channel has been demonstrated to promote beta cell survival in streptozotocin-diabetic mice, demonstrating clear potential as a type 2 diabetes therapeutic.189 Furthermore, in comparison to snake venoms, the study and application of spider venom peptides as potential diabetes therapeutics is somewhat limited. This is most likely a direct reflection of the amount of venom that can be milked, and subsequently analysed and tested, from a snake as opposed to a spider. However, it should be acknowledged that the majority of current peptide discovery efforts focus more on genomics and transcriptomics to identify potentially interesting sequences, which are then synthesised or expressed, rather than relying on extraction from the animal. Nonetheless, spider venoms are conservatively predicted to contain more than 10 million bioactive peptides, many of which have never been investigated, making them a valuable resource for peptide-based drug discovery.190 Additionally, although venom-derived peptides are generally extremely enzymatically stable in the circulation, their administration will likely still require parenteral delivery to avoid proteases activity within the stomach. However, major strides have been taken in terms of oral formulation of peptides, including clinical trials with insulin, GLP-1, calcitonin, parathyroid hormone and vasopressin.191 Given the wealth of peptides present in spider venom and this prospect of oral delivery, they provide an ideal subject matter for exploration in relation to insulin secretion from pancreatic beta cells, and merit much further study in this regard.
Conclusion
Advances in biological methodologies has allowed for the discovery and characterisation of more animal venom-derived compounds with potential therapeutic or cosmeceutical application. The success of clinically approved drugs in this field, as well as others undergoing clinical trials and some well-established cosmeceuticals such as Botox, only serves to strengthen this viewpoint. In particular, approval of exendin-4 as a first in class therapeutic for diabetes, highlights the possibility of future antidiabetic agents being derived directly from venom constituents. Although this is an extremely exciting area of drug discovery, further studies are needed to dissect the underlying mechanisms, safety profile and possible requirement for tissue-targeting of currently characterised compounds in this field. Indeed, off-target effects may see many of these toxin products utilised only as research tools to better understand pancreatic beta-cell function, rather than clinically approved therapeutics or useful cosmeceuticals. However, in particular, more investigation into the unquestionable untapped therapeutic potential of spider venom peptides is still required.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work on venom therapeutics within the Diabetes Research Group at Ulster University is currently supported by a Diabetes UK funded PhD studentship (AC-P).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s Note: The Editor in Chief of Clinical Medicine Insights: Endocrinology and Diabetes is an author on this paper. Therefore, the peer review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process.
Data Availability Statement: Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1.Pennington M, Czerwinski A, Norton R.Peptide therapeutics from venom: current status and potential. Bioorg Med Chem. 2018;26:2738-2758. [DOI] [PubMed] [Google Scholar]
- 2.King G.Venoms to drugs: venom as a source for the development of human therapeutics. Chem Sci. 2015;42: 80-96. [Google Scholar]
- 3.Herzig V, Cristofori-Armstrong B, Israel M, Nixon S, Vetter I, King G.Animal toxins—Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem Pharmacol. 2020;181:114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juárez P, Sanz L, Calvete J.Snake venomics: characterization of protein families in Sistrurus barbouri venom by cysteine mapping, N-terminal sequencing, and tandem mass spectrometry analysis. Proteomics. 2004;4:327-338. [DOI] [PubMed] [Google Scholar]
- 5.Ghezellou P, Garikapati V, Kazemi S, Strupat K, Ghassempour A, Spengler B.A perspective view of top-down proteomics in snake venom research. Rapid Commun Mass Spectrom. 2018;33:20-27. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira SH, Bartelt DC, Grenne LJ.Greene isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583-2593. [DOI] [PubMed] [Google Scholar]
- 7.Harris C, Smith G.Captopril (Capoten®, E.R. Squibb & Sons). Drug Intell Clin Pharm. 1981;15:932-939. [DOI] [PubMed] [Google Scholar]
- 8.Ondetti M, Williams N, Sabo E, Pluscec J, Weaver E, Kocy O.Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971;10:4033-4039. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson R, Brunner H, Turini G, Gavras H, Mckinstry D.A specific orally active inhibitor of angiotensin-converting enzyme in man. Lancet. 1977;309:775-778. [DOI] [PubMed] [Google Scholar]
- 10.Patchett A.The chemistry of enalapril. Br J Clin Pharmacol. 1984;18:201S-207S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe I.Adverse effects profile of sulfhydryl compounds in man. Am J Med. 1986;80:471-476. [DOI] [PubMed] [Google Scholar]
- 12.Ulm E, Hichens M, Gomez H.Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982;14:357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleary J, Taylor J.Enalapril: a new angiotensin converting enzyme inhibitor. Drug Intell Clin Pharm. 1986;20:177-186. [DOI] [PubMed] [Google Scholar]
- 14.Wallace M.Ziconotide: a new nonopioid intrathecal analgesic for the treatment of chronic pain. Expert Rev Neurother. 2006;6:1423-1428. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh M, Cruz L, Hunkapiller M, Gray W, Olivera B.Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch Biochem Biophys. 1982;218:329-334. [DOI] [PubMed] [Google Scholar]
- 16.Miljanich G.Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029-3040. [DOI] [PubMed] [Google Scholar]
- 17.Maggi C, Giuliani S, Santicioli I, Tramontana M, Meli A.Effect of omega conotoxin on reflex responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder and peptide release from the rat spinal cord. Neuroscience. 1990;34:243-250. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Hamid J, Doering C, Bosey G, Snutch T, Zamponi G.Residue Gly1326of the N-type calcium channel α1B subunit controls reversibility of ω-conotoxin GVIA and MVIIA block. J Biol Chem. 2001;276:15728-15735. [DOI] [PubMed] [Google Scholar]
- 19.Takasusuki T, Yaksh T.Regulation of spinal substance P release by intrathecal calcium channel blockade. Anesthesiology. 2011;115:153-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safavi-Hemami H, Brogan SE, Olivera BM.Pain therapeutics from cone snail venoms: from Ziconotide to novel non-opioid pathways. J Proteomics. 2019;190:12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan ZR, Gould RJ, Jacobs JW, Friedman PA, Polokoff MA., Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J Biol Chem. 1988;263:19827-19832. [PubMed] [Google Scholar]
- 22.Huang TF, Holt JC, Lukasiewicz H, Niewiarowski S., Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J Biol Chem. 1987;262:16157-16163. [PubMed] [Google Scholar]
- 23.Coller B, Folts J, Smith S, Scudder L, Jordan R.Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of GPIIb/IIIa receptors. Circulation. 1989;80:1766-1774. [DOI] [PubMed] [Google Scholar]
- 24.Egbertson M, Chang C, Duggan M, et al. Non-peptide fibrinogen receptor antagonists. 2. Optimization of a tyrosine template as a mimic for Arg-Gly-Asp. J Med Chem. 1994;37:2537-2551. [DOI] [PubMed] [Google Scholar]
- 25.Nachman R, Leung L.Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982;69:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362. [PubMed] [Google Scholar]
- 27.Scarborough R, Kleiman N, Phillips D.Platelet glycoprotein IIb/IIIa antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation. 1999;100:437-444. [DOI] [PubMed] [Google Scholar]
- 28.Scarborough R.Development of eptifibatide. Am Heart J. 1999;138:1093-1104. [DOI] [PubMed] [Google Scholar]
- 29.Markwardt F.Untersuchungen uber Hirudin. Die Naturwissenschaften. 1955;42:537-538. [Google Scholar]
- 30.Bagdy D, Barabas E, Gráf L, Petersen TE, Magnusson S., Hirudin. Methods Enzymol. 1976;45:669-678. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Ansell J.Direct thrombin inhibitors. Br J Clin Pharmacol. 2011;72:581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theodore E. Heparin-induced thrombocytopenia. In: Kitchens CS, Kessler CM, Konkle BA, eds. Consultative Haemostasis and Thrombosis. 3rd ed.Elsevier; 2013:442-473. [Google Scholar]
- 33.Bittl J, Chaitman B, Feit F, Kimball W, Topol E.Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J. 2001;142:952-959. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Zhao HW, Wang CF, et al. Efficacy and safety of bivalirudin during percutaneous coronary intervention in high-bleeding-risk elderly patients with chronic total occlusion: a prospective randomized controlled trial. Catheter Cardiovasc Interv. 2019;93:825-831. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Chen S, Redfors B, et al. Safety and efficacy of bivalirudin monotherapy in patients with non-ST-segment elevation acute coronary syndromes with positive biomarkers undergoing percutaneous coronary intervention. Coron Artery Dis. 2020;31:59-65. [DOI] [PubMed] [Google Scholar]
- 36.Stocker K, Barlow GH.The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol. 1976;45:214-223. [DOI] [PubMed] [Google Scholar]
- 37.Itoh N, Tanaka N, Funakoshi I, Kawasaki T.The complete nucleotide sequence of the gene for batroxobin, a thrombin-like snake venom enzyme. Nucleic Acids Res. 1988;16:10377-10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holleman WH, Weiss LJ.The thrombin-like enzyme from Bothrops atrox snake venom. Properties of the enzyme purified by affinity chromatography on p-amino benzamidine-substituted agarose. J Biochem. 1976;251:1663-1669. [PubMed] [Google Scholar]
- 39.Aronson D.Comparison of the actions of thrombin and the thrombin-like venom enzymes ancrod and batroxobin. Thromb Haemost. 1976;36:9-13. [PubMed] [Google Scholar]
- 40.Xu G, Liu X, Zhu W, Yin Q, Zhang R, Fan X.Feasibility of treating hyperfibrino-genemia with intermittently administered batroxobin in patients with ischemic stroke/transient ischemic attack for secondary prevention. Blood Coagul Fibrinolysis. 2007;18:193-197. [DOI] [PubMed] [Google Scholar]
- 41.Ding J, Zhou D, Hu Y, et al. The efficacy and safety of Batroxobin in combination with anticoagulation on cerebral venous sinus thrombosis. J Thromb Thrombolysis. 2018;46:371-378. [DOI] [PubMed] [Google Scholar]
- 42.Bastos EMAF, Heneine LGD, Pesquero JL, Merlo LA. Pharmaceutical composition containing an Apitoxin fraction and use thereof. WO/2011/041865 patent application. 2011. [Google Scholar]
- 43.Bordon K, Cologna C, Fornari-Baldo E, et al. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol. 2020;11:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ClinicalTrials.gov, Christopher MH. Apitox, honeybee toxin for pain and inflammation of osteoarthritis. National Library of Medicine (US) Identi-fier [NCT01112722]. 2016. Accessed August 12, 2020. https://clinicaltrials.gov/ct2/show/NCT01112722
- 45.ClinicalTrials.gov, Christopher MH. Evaluate safety and efficacy of apitox add-on therapy for improving disability and quality of life in MS patients. National Library of Medicine (US). Identifier [NCT03710655]. 2018. Accessed August 12, 2020https://clinicaltrials.gov/ct2/show/NCT03710655
- 46.Chang L, Chou Y, Lin S, et al. A novel neurotoxin, cobrotoxin b, from Naja naja atra (Taiwan cobra) venom: purification, characterization, and gene organization. J Biochem. 1997;122:1252-1259. [DOI] [PubMed] [Google Scholar]
- 47.Gazerani P, Cairns B.Venom-based biotoxins as potential analgesics. Expert Rev Neurother. 2014;14:1261-1274. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Song S, Feng F, et al. Cobrotoxin-containing analgesic compound to treat chronic moderate to severe cancer pain: results from a randomized, double-blind, cross-over study and from an open-label study. Oncol Rep. 2006;16:1077-1084. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q, Huang J, Wang S, Qin Z, Lin F.Cobrotoxin extracted from Naja atra venom relieves arthritis symptoms through anti-inflammation and immunosuppression effects in rat arthritis model. J Ethnopharmacol. 2016;194:1087-1095. [DOI] [PubMed] [Google Scholar]
- 50.China Approval no. H53022101. Yunnan Nanzhao Pharmaceutical Co., Ltd. [Google Scholar]
- 51.Chen C, Hu Y, Shi X, et al. A single-label fluorescent derivatization method for quantitative determination of neurotoxin in vivo by capillary electrophoresis coupled with laser-induced fluorescence detection. Analyst. 2016;141:4495-4501. [DOI] [PubMed] [Google Scholar]
- 52.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W.Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46-52. [DOI] [PubMed] [Google Scholar]
- 53.Irwin N, Flatt P.New perspectives on exploitation of incretin peptides for the treatment of diabetes and related disorders. World J Diabetes. 2015;6:1285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin N, Flatt P.Enteroendocrine hormone mimetics for the treatment of obesity and diabetes. Curr Opin Pharmacol. 2013;13:989-995. [DOI] [PubMed] [Google Scholar]
- 55.Kieffer TJ, McIntosh CHS, Pederson RA.Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585-3596. [DOI] [PubMed] [Google Scholar]
- 56.Andersen E, Deacon C, Holst J.Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes Metab. 2017;20:34-41. [DOI] [PubMed] [Google Scholar]
- 57.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP.Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402-7405. [PubMed] [Google Scholar]
- 58.Schepp W, Schmidtler J, Riedel T, et al. Exendin-4 and exendin-(9-39) NH2: agonist and antagonist, respectively, at the rat parietal cell receptor for glucagon-like peptide-1-(7-36) NH2. Eur J Pharmacol. 1994;269:183-191. [DOI] [PubMed] [Google Scholar]
- 59.Scott A.Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044-1049. [DOI] [PubMed] [Google Scholar]
- 60.Burgen A, Dickens F, Zatman L.The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109:10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark GC, Casewell NR, Elliott CT, et al. Friends or foes? Emerging impacts of biological toxins. Trends Biochem Sci. 2019;44:365-379. [DOI] [PubMed] [Google Scholar]
- 62.Clark R, Berris C.Botulinum toxin. Plast Reconstr Surg. 1989;84:353-355. [PubMed] [Google Scholar]
- 63.McArdle JJ, Lentz TL, Witzemann V, Schwarz H, Weinstein SA, Schmidt JJ.Wagerlin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 1999;289:543-550. [PubMed] [Google Scholar]
- 64.Han S, Hong I, Woo S, et al. The beneficial effects of honeybee-venom serum on facial wrinkles in humans. Clin Interv Aging. 2015;10:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mabrouk K, Luis J, De Pomyers H, Bertin D, Bengeloune AH, Verdoni M.Use of spider venoms for skin whitening/depigmenting and composition comprising spider venoms molecules or synthetic analogs. WO/2014/037111 patent application. 2018. [Google Scholar]
- 66.Verdoni M, Roudaut H, De Pomyers H, et al. ArgTX-636, a polyamine isolated from spider venom: a novel class of melanogenesis inhibitors. Bioorg Med Chem. 2016;24:5685-5692. [DOI] [PubMed] [Google Scholar]
- 67.Pandey A, Jatana GK, Sonthalia S.Cosmeceuticals. In: StatPearls [Internet]. StatPearls Publishing; 2020. Updated August 10, 2020. Accessed July 15, 2020. https://www.ncbi.nlm.nih.gov/books/NBK544223/
- 68.Hammes C.Cosmeceuticals: the cosmetic-drug borderline. In: Hori W, ed. Drug Discovery Approaches for Developing Cosmeceuticals: Advanced Skin Care and Cosmetic Products. IBC Library Series; 1997. [Google Scholar]
- 69.De Bold A, Borenstein H, Veress A, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89-94. [DOI] [PubMed] [Google Scholar]
- 70.Nemer M, Lavigne J, Drouin J, Thibault G, Gannon M, Antakly T.Expression of atrial natriuretic factor gene in heart ventricular tissue. Peptides. 1986;7:1147-1152. [DOI] [PubMed] [Google Scholar]
- 71.Vollmar A, Gerbes A, Nemer M, Schulz R.Detection of C-type natriuretic peptide (CNP) transcript in the rat heart and immune organs. Endocrinology. 1993;132:1872-1874. [DOI] [PubMed] [Google Scholar]
- 72.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M.A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusti-ceps). J Biol Chem. 1992;267:13928-13932. [PubMed] [Google Scholar]
- 73.Johns D, Ao Z, Heidrich B, et al. Dendroaspis natriuretic peptide binds to the natriuretic peptide clearance receptor. Biochem Biophys Res Commun. 2007;358:145-149. [DOI] [PubMed] [Google Scholar]
- 74.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr.Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ClinicalTrials.gov, Neutel J. Safety study of cenderitide in stable chronic heart failure. National Library of Medicine (US) Identifier [NCT02359227]. 2015. Accessed July 1, 2020. https://clinicaltrials.gov/ct2/show/NCT02359227?term=NCT02359227&draw=2&rank=1
- 76.ClinicalTrials.gov, Schirger J. CD-NP (cenderitide) therapy for the preservation of left ventricular function (BELIEVE III). National Library of Medicine (US) Identifier [NCT02071602]. 2019. Accessed July 1, 2020. https://clinicaltrials.gov/ct2/show/NCT02071602
- 77.Guan AL, Retzios AD, Henderson GN, Markland FS.Purification and characterization of a fibrinolytic enzyme from venom of the southern copperhead snake (Agkistrodon con-tortrix contortrix). Arch Biochem Biophys. 1991;289:197-207. [DOI] [PubMed] [Google Scholar]
- 78.Pretzer D, Schulteis BS, Smith CD, Vander Velde DG, Mitchell JW, Manning MC., Fibrolase. A fibrinolytic protein from snake venom. Pharm Biotechnol. 1993;5:287-314. [PubMed] [Google Scholar]
- 79.Randolph A, Chamberlain S, Chu H, Masiarz F, Retzios A, Marklan F.Amino acid sequence of fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon contortrix contortrix venom. Protein Sci. 1992;1:590-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toombs C.Alfimeprase: pharmacology of a novel fibrinolytic metalloproteinase for thrombolysis. Haemostasis. 2001;31:141-147. [DOI] [PubMed] [Google Scholar]
- 81.Deitcher S, Toombs C.Non-clinical and clinical characterization of a novel acting thrombolytic: alfimeprase. Pathophysiol Haemost Thromb. 2005;34:215-220. [DOI] [PubMed] [Google Scholar]
- 82.Han S, Weaver F, Comerota A, Perler B, Joing M.Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). J Vasc Surg. 2010;51:600-609. [DOI] [PubMed] [Google Scholar]
- 83.ClinicalTrials.gov, Begelman SM. Phase 2 proof-of-concept study of the safety and efficacy of alfimeprase to rapidly open arteries and restore brain function following a stroke. National Library of Medicine (US) Identifier [NCT00499902]. 2007. Accessed July 5, 2020. https://clinicaltrials.gov/ct2/show/NCT00499902?term=NCT00499902&draw=2&rank=1
- 84.Sandall D, Satkunanathan N, Keays D, et al. A novel α-conotoxin identified by gene sequencing is active in suppressing the vascular response to selective stimulation of sensory nerves in vivo. Biochemistry. 2003;42:6904-6911. [DOI] [PubMed] [Google Scholar]
- 85.Umana I, Daniele C, McGehee D.Neuronal nicotinic receptors as analgesic targets: it’s a winding road. Biochem Pharmacol. 2013;86:1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Australian New Zealand Clinical Trials Registry, Herd C. A randomised, placebo-controlled, double-blind, single and multiple ascending dose study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of subcutaneous doses of ACV1 in healthy adult male subjects. Identifier [ACTRN12605000408684]. 2005. Accessed July 3, 2020. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=468&isReview=true
- 87.Australian New Zealand Clinical Trials Registry, Herd C. A randomised, double-blind, placebo-controlled study to assess the safety, tolerability, pharmacodynamics, and pharmacokinetics of subcutaneous doses of ACV1 in patients with diabetic peripheral neuropathic pain or post-herpetic neuralgia. Identifier [ACTRN12607000201471]. 2007. Accessed July 3, 2020. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=81930&isReview=true
- 88.Kryukova E, Ivanov I, Lebedev D, et al. Orthosteric and/or allosteric binding of α-conotoxins to nicotinic acetylcholine receptors and their models. Mar Drugs. 2018;16:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kennedy A, Belgi A, Husselbee B, Spanswick D, Norton R, Robinson A.α-Conotoxin peptidomimetics: probing the minimal binding motif for effective analgesia. Toxins. 2020;12:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McIntosh J, Corpuz G, Layer R, et al. Isolation and characterization of a novel conus peptide with apparent antinociceptive activity. J Biol Chem. 2000;275:32391-32397. [DOI] [PubMed] [Google Scholar]
- 91.Bryan-Lluka L, Bönisch H, Lewis R.χ-Conopeptide MrIA partially overlaps desipramine and cocaine binding sites on the human norepinephrine transporter. J Biol Chem. 2003;278:40324-40329. [DOI] [PubMed] [Google Scholar]
- 92.Brust A, Palant E, Croker D, et al. χ-Conopeptide pharmacophore development: toward a novel class of norepinephrine transporter inhibitor (Xen2174) for pain. J Med Chem. 2009;52:6991-7002. [DOI] [PubMed] [Google Scholar]
- 93.Craig A, Norberg T, Griffin D, et al. Contulakin-G, an O-glycosylated invertebrate neurotensin. J Biol Chem. 1999;274:13752-13759. [DOI] [PubMed] [Google Scholar]
- 94.Lee H, Zhang L, Smith M, et al. A marine analgesic peptide, Contulakin-G, and neurotensin are distinct agonists for neurotensin receptors: uncovering structural determinants of desensitization properties. Front Pharmacol. 2015;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boules M, Li Z, Smith K, Fredrickson P, Richelson E.Diverse roles of neurotensin agonists in the central nervous system. Front Endocrinol. 2013;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sang CN, Barnabe KJ, Kern SE.Phase IA clinical trial evaluating the tolerability, pharmacokinetics, and analgesic efficacy of an intrathecally administered neurotensin A analogue in central neuropathic pain following spinal cord injury. Clin Pharmacol Drug Dev. 2016;5:250-258. [DOI] [PubMed] [Google Scholar]
- 97.Krätzschmar J, Haendler B, Langer G, et al. The plasminogen activator family from the salivary gland of the vampire bat Desmodus rotundas: cloning and expression. Gene. 1991;105:229-237. [DOI] [PubMed] [Google Scholar]
- 98.Reddrop C, Moldrich R, Beart P, et al. Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke. 2005;36:1241-1246. [DOI] [PubMed] [Google Scholar]
- 99.Liberatore G, Samson A, Bladin C, Schleuning W, Medcalf R.Vampire bat salivary plasminogen activator (desmoteplase). Stroke. 2003;34:537-543. [DOI] [PubMed] [Google Scholar]
- 100.Hacke W, Furlan A, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albers G, von Kummer R, Truelsen T, et al. Safety and efficacy of desmoteplase given 3-9 h after ischaemic stroke in patients with occlusion or high-grade stenosis in major cerebral arteries (DIAS-3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Neurol. 2015;14:575-584. [DOI] [PubMed] [Google Scholar]
- 102.ClinicalTrials.gov, Lundbeck H. Efficacy and safety study of desmoteplase to treat acute ischemic stroke (DIAS-4). National Library of Medicine (US) Iden-tifier [NCT00856661]. 2014. Accessed August 13, 2020. https://clinicaltrials.gov/ct2/show/NCT00856661
- 103.DeBin J, Maggio J, Strichartz G.Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol Cell Physiol. 1993;264:361-369. [DOI] [PubMed] [Google Scholar]
- 104.Dardevet L, Rani D, Aziz T, et al. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins. 2015;7:1079-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeBin J, Strichartz G.Chloride channel inhibition by the venom of the scorpion Leiurus quinquestriatus. Toxicon. 1991;29:1403-1408. [DOI] [PubMed] [Google Scholar]
- 106.Soroceanu L, Manning T, Sontheimer H.Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J Neurosci. 1999;19:5942-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.ClinicalTrials.gov, Badie B. Chimeric antigen receptor (CAR) T cells with a chlorotoxin tumor-targeting domain for the treatment of MPP2+ recurrent or progressive glioblastoma. National Library of Medicine (US) Identifier [NCT04214392]. 2020. Accessed July 2, 2020. https://clinicaltrials.gov/ct2/show/NCT04214392?term=NCT04214392&draw=2&rank=1
- 108.Schultz L, Mackall C.Driving CAR T cell translation forward. Sci Transl Med. 2019;11:2127. [DOI] [PubMed] [Google Scholar]
- 109.McGonigle S, Majumder U, Kolber-Simonds D, et al. Neuropilin-1 drives tumor-specific uptake of chlorotoxin. Cell Commun Signal. 2019;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butte P, Mamelak A, Parrish-Novak J, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus. 2014;36:E1. [DOI] [PubMed] [Google Scholar]
- 111.Patil C, Walker D, Miller D, et al. Phase 1 safety, pharmacokinetics, and fluorescence imaging study of tozuleristide (BLZ-100) in adults with newly diagnosed or recurrent gliomas. Neurosurgery. 2019;85:E641-E649. [DOI] [PubMed] [Google Scholar]
- 112.ClinicalTrials.gov, Leary S. Study of tozuleristide and the canvas imaging system in pediatric subjects with CNS tumors undergoing surgery. National Library of Medicine (US) Identifier [NCT03579602]. 2020. Accessed July 2, 2020. https://clinicaltrials.gov/ct2/show/NCT03579602?term=NCT03579602&draw=2&rank=1
- 113.Stewart J.TRPV6 as a target for cancer therapy. J Cancer. 2020;11:374-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song H, Dong M, Zhou J, Sheng W, Li X, Gao W.Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer. Oncol Rep. 2018;39:1432-1440. [DOI] [PubMed] [Google Scholar]
- 115.Fu S, Hirte H, Welch S, et al. First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2017;35:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.ClinicalTrials.gov, Ilenchuk TT. Safety and tolerability study of SOR-C13 in subjects with advanced cancers commonly known to express the TRPV6 channel. National Library of Medicine (US) Identifier [NCT01578564]. 2012. Accessed July 3, 2020. https://clinicaltrials.gov/ct2/show/NCT01578564?term=NCT01578564&draw=2&rank=1
- 117.ClinicalTrials.gov Fu S. SOR-C13 in treating patients with advanced refractory solid tumors. National Library of Medicine (US) Identifier [NCT03784677]. 2012. Accessed July 3, 2020. https://clinicaltrials.gov/ct2/show/NCT03784677?term=NCT03784677&draw=2&rank=1
- 118.Castaneda O, Rowan E, Young L, Harvey A, Karlsson E.ShK is a potassium channel blocker from the Caribbean Sea anemone Stichodactyla helianthus. Toxicon. 1993;33:603-613. [DOI] [PubMed] [Google Scholar]
- 119.ClinicalTrials.gov, Iadonato S. Multiple ascending dose safety study of ShK-186 (dalazatide) in healthy volunteers. National Library of Medicine (US) Identifier [NCT02446340]. 2013. Accessed July 5, 2020. https://clinicaltrials.gov/ct2/show/NCT02446340?term=NCT02446340&draw=2&rank=1
- 120.ClinicalTrials.gov, Iadonato S. A 4 week study of the safety, tolerability, and pharmacodynamics of ShK-186 (dalazatide) in active plaque psoriasis. National Library of Medicine (US) Identifier [NCT02435342]. 2014. Accessed July 5, 2020. https://clinicaltrials.gov/ct2/show/NCT02435342?term=NCT02435342&draw=2&rank=1
- 121.Reid P.PART II. Toxins as therapeutic agents alpha-cobratoxin as a possible therapy for multiple sclerosis: a review of the literature leading to its development for this application. Crit Rev Immunol. 2007;27:291-302. [DOI] [PubMed] [Google Scholar]
- 122.Reid PF, Raymond LN.Modified elapid venoms as stimulators of the immune reaction. 11/592896 application number. 2010. [Google Scholar]
- 123.Chen R, Robinson S.The effect of cholinergic manipulations on the analgesic response to cobrotoxin in mice. Life Sci. 1990;47:1949-1954. [DOI] [PubMed] [Google Scholar]
- 124.Harvey AL.Toxins and drug discovery. Toxicon. 2014;92:193-200. [DOI] [PubMed] [Google Scholar]
- 125.Flatt P, Conlon J.Editorial: newer peptide-based agents for treatment of patients with Type 2 diabetes. Peptides. 2018;100:1-2. [DOI] [PubMed] [Google Scholar]
- 126.Tanday N, Irwin N, Flatt P, Moffett R.Dapagliflozin exerts positive effects on beta cells, decreases glucagon and does not alter beta- to alpha-cell transdifferentiation in mouse models of diabetes and insulin resistance. Biochem Pharmacol. 2020;177:114009. [DOI] [PubMed] [Google Scholar]
- 127.Gault V, Lennox R, Flatt P.Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403-413. [DOI] [PubMed] [Google Scholar]
- 128.Harding J, Pavkov M, Magliano D, Shaw J, Gregg E.Global trends in diabetes complications: a review of current evidence. Diabetologia. 2018;62:3-16. [DOI] [PubMed] [Google Scholar]
- 129.Ogurtsova K, da Rocha Fernandes J, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [DOI] [PubMed] [Google Scholar]
- 130.Gault V, Kerr B, Harriott P, Flatt P.Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin Sci (Lond). 2011;121:107-117. [DOI] [PubMed] [Google Scholar]
- 131.Bailey C.Glucose-lowering therapies in type 2 diabetes: opportunities and challenges for peptides. Peptides. 2018;100:9-17. [DOI] [PubMed] [Google Scholar]
- 132.Pathak V, Irwin N, Flatt P. The enteroinsular axis. In: Opara E, Dagogo-Jack S eds. Nutrition and Diabetes, Pathophysiology and Management. CRC press; 2019:Chapter 3. [Google Scholar]
- 133.Dobrică EC, Găman MA, Cozma MA, Bratu OG, Pantea Stoian A, Diaconu CC.Polypharmacy in type 2 diabetes mellitus: insights from an internal medicine department. Medicina (Kaunas). 2019;55:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trautmann M, Van Gaal L, Han J, Hardy E.Three-year efficacy and safety of exenatide once weekly: a pooled analysis of three trials. J Diabetes Complications. 2017;31:1415-1422. [DOI] [PubMed] [Google Scholar]
- 135.Xu G, Stoffers DA, Habener JF, Bonner-Weir S.Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276. [DOI] [PubMed] [Google Scholar]
- 136.Ding W, Chang W, Guo X, et al. Exenatide protects against cardiac dysfunction by attenuating oxidative stress in the diabetic mouse heart. Front Endocrinol (Lausanne). 2019;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robinson S, Safavi-Hemami H.Venom peptides as pharmacological tools and therapeutics for diabetes. Neuropharmacology. 2017;127:79-86. [DOI] [PubMed] [Google Scholar]
- 138.Sanger F, Thompson E. The amino-acid sequence in the glycyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J. 1953;53:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Safavi-Hemami H, Gajewiak J, Karanth S, et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. PNAS. 2015;112:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Menting J, Gajewiak J, MacRaild C, et al. A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nat Struct Biol. 2016;23:916-920. [DOI] [PubMed] [Google Scholar]
- 141.Xiong X, Menting J, Disotuar M, et al. A structurally minimized yet fully active insulin based on cone-snail venom insulin principles. Nat Struct Biol. 2020;27:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bayrhuber M, Vijayan V, Ferber M, et al. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family. Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family: structural and functional characterization. J Biol Chem. 2005;280:23766-23770. [DOI] [PubMed] [Google Scholar]
- 143.Finol-Urdaneta RK, Remedi MS, Raasch W, et al. Block of Kv1.7 potassium currents increases glucose-stimulated insulin secretion. EMBO Mol Med. 2012;4:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tucker K, Overton J, Fadool D.Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int J Obes (Lond). 2008;32:1222-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu J, Wang P, Li Y, et al. The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. PNAS. 2004;101:3112-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Upadhyay S, Eckel-Mahan K, Mirbolooki M, et al. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. PNAS. 2013;110:2239-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jaimes-Hoy L, Gurrola G, Cisneros M, Joseph-Bravo P, Possani L, Charli J. The Kv1.3 channel blocker Vm24 enhances muscle glucose transporter 4 mobilization but does not reduce body-weight gain in diet-induced obese male rats. Life Sci. 2017;181:23-30. [DOI] [PubMed] [Google Scholar]
- 148.Van der Heijden A, Nijpels G, Badloe F, et al. Prediction models for development of retinopathy in people with type 2 diabetes: systematic review and external validation in a Dutch primary care setting. Diabetologia. 2020;63:1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rübsam A, Parikh S, Fort P.Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Montassar F, Darche M, Blaizo A, et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. FASEB J. 2016;31:1107-1119. [DOI] [PubMed] [Google Scholar]
- 151.Sola D, Rossi L, Schianca G, et al. State of the art paper Sulfonylureas and their use in clinical practice. Arch Med Res. 2015;4:840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Douros A, Dell’Aniello S, Yu O, Filion K, Azoulay L, Suissa S.Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population-based cohort study. BMJ. 2018;362:2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nguyen T, Folch B, Létourneau M, et al. Cardiotoxin-I: an unexpectedly potent insulinotropic agent. Chembiochem. 2012;13:1805-1812. [DOI] [PubMed] [Google Scholar]
- 154.Nguyen T, Folch B, Létourneau D, et al. Design of a truncated cardiotoxin-I analogue with potent insulinotropic activity. J Med Chem. 2014;57:2623-2633. [DOI] [PubMed] [Google Scholar]
- 155.Moore S, Bhat V, Flatt P, Gault V, McClean S.Isolation and characterisation of insulin-releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis venom. Toxicon. 2015;101:48-54. [DOI] [PubMed] [Google Scholar]
- 156.Yamamoto S, Nakaki T, Nakadate T, Kato R.Insulinotropic effects of exogenous phospholipase A2 and C in isolated pancreatic islets. Eur J Pharmacol. 1982;86:121-124. [DOI] [PubMed] [Google Scholar]
- 157.Nogueira T, Ferreira F, Toyama M, et al. Characterization of the insulinotropic action of a phospholipase A2 isolated from Crotalus durissus collilineatus rattlesnake venom on rat pancreatic islets. Toxicon. 2005;45:243-248. [DOI] [PubMed] [Google Scholar]
- 158.Huang TF, Holt JC, Lukasiewicz H, Niewiarowski S., Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J Biol Chem. 1987;262:16157-16163. [PubMed] [Google Scholar]
- 159.Toyama D, Boschero A, Martins M, Fonteles M, Monteiro H, Toyama M.Structure? Function relationship of new crotamine isoform from the Crotalus durissus cascavella. Protein J. 2005;24:9-19. [DOI] [PubMed] [Google Scholar]
- 160.Rádis-Baptista G, Oguiura N, Hayashi MAF, et al. Nucleotide sequence of crotamine isoform precursors from a single South American rattlesnake (Crotalus durissus terrificus). Toxicon. 1999;37:973-984. [DOI] [PubMed] [Google Scholar]
- 161.Kerkis A, Kerkis I, Rádis-Baptista G, et al. Crotamine is a novel cell-penetrating protein from the venom of rattlesnake Crotalus durissus terrificus. FASEB J. 2004;18:1407-1409. [DOI] [PubMed] [Google Scholar]
- 162.Nicastro G, Franzoni L, De Chiara C, Mancin A, Giglio J, Spisni A.Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur J Biochem. 2003;270:1969-1979. [DOI] [PubMed] [Google Scholar]
- 163.Hirai Y, Yasuhara T, Yoshida H, Nakajima T, Fujino M, Kitada C.A new mast cell degranulating peptide ‘mastoparan’ in the venom of Vespula lewisii. Chem Pharm Bull (Tokyo). 1979;27:1942-1944. [DOI] [PubMed] [Google Scholar]
- 164.Yokokawa N, Komatsu M, Takeda T, Aizawa T, Tamada T.Mastoparan, a wasp venom, stimulates insulin release by pancreatic islets through pertussis toxin sensitive GTP-binding protein. Biochem Biophys Res Commun. 1989;158:712-716. [DOI] [PubMed] [Google Scholar]
- 165.Baptista-Saidemberg N, Saidemberg D, Ribeiro R, Arcuri H, Palma M, Carneiro E.Agelaia MP-I: a peptide isolated from the venom of the social wasp, Agelaia pallipes pallipes, enhances insulin secretion in mice pancreatic islets. Toxicon. 2012;60:596-602. [DOI] [PubMed] [Google Scholar]
- 166.Mitchell H, Lowy P, Sarmiento L, Dickson L.Melittin: toxicity to Drosophila and inhibition of acetylcholinesterase. Arch Biochem. 1971;145:344-348. [DOI] [PubMed] [Google Scholar]
- 167.Hoshino Y, Koide H, Furuya K, et al. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. Proc Natl Acad Sci USA. 2011;109:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jallouk A, Palekar R, Marsh J, et al. Delivery of a protease-activated cytolytic peptide prodrug by perfluorocarbon nanoparticles. Bioconjugate Chem. 2015;26:1640-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gui Z, Zhu J, Ye S, et al. Prolonged melittin release from polyelectrolyte-based nanocomplexes decreases acute toxicity and improves blood glycaemic control in a mouse model of type II diabetes. Int J Pharm. 2020;577:119071. [DOI] [PubMed] [Google Scholar]
- 170.Brem H, Tomic-Canic M.Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hozzein W, Badr G, Badr B, et al. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signalling. Mol Immunol. 2018;103:322-335. [DOI] [PubMed] [Google Scholar]
- 172.Kim H, Jo M, Nam S, Kim K, Choi M, Lee Y. Evaluating the effects of honeybee (Apis mellifera L.) venom on the expression of insulin sensitivity and inflammation-related genes in co-culture of adipocytes and macrophages. Entomol Res. 2020;50:236-244. [Google Scholar]
- 173.Hassan A, El-kotby D, Tawfik M, Badr R, Bahgat I.Antidiabetic effect of the Egyptian honeybee (Apis mellifera) venom in alloxan-induced diabetic rats. JOBAZ. 2019;80:58. [Google Scholar]
- 174.Nowotny K, Jung T, Höhn A, Weber D, Grune T.Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Behroozi J, Divsalar A, Saboury A.Honeybee venom decreases the complications of diabetes by preventing haemoglobin glycation. J Mol Liq. 2014;199:371-375. [Google Scholar]
- 176.Smith P, Bokvist K, Arkhammar P, Berggren P, Rorsman P.Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990;95:1041-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.MacDonald P, Sewing S, Wang J, et al. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic β-cells enhances glucose-dependent insulin secretion. J Biol Chem. 2002;277:44938-44945. [DOI] [PubMed] [Google Scholar]
- 178.Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618-1628. [DOI] [PubMed] [Google Scholar]
- 179.Galvez A, Gimenez-Gallego G, Reuben JP.Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083-11090. [PubMed] [Google Scholar]
- 180.Hu H, Shao L, Chavoshy S, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585-9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Swartz K, MacKinnon R.Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997;18:665-673. [DOI] [PubMed] [Google Scholar]
- 182.Ashcroft FM, Rorsman P.Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87-143. [DOI] [PubMed] [Google Scholar]
- 183.Lou K, Hsieh M, Chen W, et al. Hanatoxin inserts into phospholipid membranes without pore formation. Biochim Biophys Acta Biomembr. 2017;1859:917-923. [DOI] [PubMed] [Google Scholar]
- 184.Hsieh M, Huang P, Liou H.The penetration depth for hanatoxin partitioning into the membrane hydrocarbon core measured with neutron reflectivity. Langmuir. 2018;34:9036-9046. [DOI] [PubMed] [Google Scholar]
- 185.Jacobson D, Kuznetsov A, Lopez J, Kash S, Ämmälä C, Philipson L.Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007;6:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takeuchi K, Park E, Lee C, et al. Solution structure of ω-grammotoxin SIA, a gating modifier of P/Q and N-type Ca2+ channel. J Mol Biol. 2002;321:517-526. [DOI] [PubMed] [Google Scholar]
- 187.Herrington J, Zhou YP, Bugianesi RM, et al. Blockers of the delayed-rectifier potassium current in pancreatic beta-cells enhance glucose-dependent insulin secretion. Diabetes. 2006;55:1034-1042. [DOI] [PubMed] [Google Scholar]
- 188.Tilley D, Angueyra J, Eum K, et al. The tarantula toxin GxTx detains K+ channel gating charges in their resting conformation. J Gen Physiol. 2018;151:292-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou T, Quan L, Chen L, et al. SP6616 as a new Kv2.1 channel inhibitor efficiently promotes β-cell survival involving both PKC/Erk1/2 and CaM/PI3K/Akt signaling pathways. Cell Death Dis. 2016;7:e2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saez N, Senff S, Jensen J, et al. Spider-venom peptides as therapeutics. Toxins. 2010;2:2851-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Drucker DJ.Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19:277-289. [DOI] [PubMed] [Google Scholar]