Abstract
A valuable organocatalytic vinylogous Mannich reaction between alkylidenepyrazolones and isatin-derived ketimines has been successfully established. Squaramide organocatalyst, prepared from quinine, catalyzed the diastereo- and enantioselective vinylogous Mannich addition, affording a range of aminooxindole-pyrazolone adducts (24 examples) with excellent outcomes: up to 98% yield with complete diastereoselectivity and excellent enantioselectivity (up to 99% ee). Additionally, different synthetic transformations were performed with the chiral pyrazolone-oxindole adducts.
The direct catalytic asymmetric vinylogous reaction represents a powerful tool in synthetic organic chemistry to introduce stereocenters at the γ-position or even more remote positions of the functional groups in organic compounds in an atom-economical and efficient way.1 In this research area, the asymmetric vinylogous Mannich reaction is a powerful, direct, and straightforward C–C bond-forming reaction leading to the synthesis of optically active δ-amino-α,β-unsaturated carbonyl derivatives.1d This class of compounds is a significant building block for the synthesis of biologically active compounds and drugs.
On the contrary, pyrazolone derivatives represent one of the most important five-membered heterocycles containing nitrogen atoms, which are present in several bioactive natural products and pharmaceuticals.2 Therefore, the enantioselective synthesis of chiral pyrazolones has received the attention of the synthetic organic chemists in the last several years.3 In this context, the asymmetric vinylogous nucleophilic γ-addition of α,β-unsaturated pyrazolone bearing γ-hydrogen atoms to electrophiles has been explored for the construction of chiral pyrazolones. However, these examples are restricted to the use of α,β-unsaturated compounds4 or Morita–Baylis–Hillman carbonates5 as electrophiles. As far as we know, the corresponding asymmetric nucleophilic 1,2-addition of alkylidenepyrazolones to carbonyl compounds or imines has not yet been described in the literature (Scheme 1A).
Scheme 1. Asymmetric Vinylogous Alkylation of Alkylidenepyrazolones.
During our recent studies on the enantioselective Mannich addition of pyrazolones to imines,6 we envisioned that the corresponding asymmetric vinylogous Mannich reaction could be feasible. Using α-isopropylidenepyrazolone as a nucleophile, remote γ-exocyclic functionalization of the diazaheterocycle could be possible using isatin-derived ketimines as electrophiles and bifunctional organocatalysis. The nucleophilic addition to isatin-derived ketimines constitutes a straightforward methodology to synthesize enantioenriched amino oxindole compounds.7 Numerous natural products and pharmacologically active compounds contain in their structures the amino-oxindole scaffold, showing the importance of this structural motif in synthetic organic chemistry.8 In light of the pharmacological and biological activities of pyrazolones and amino oxindoles, the combination of both structural motifs into one molecule could result in novel and interesting chiral alkylidenepyrazolones bearing a quaternary aminooxindole stereocenter that may be useful for drug discovery (Scheme 1B).9
Initially, we selected the enantioselective vinylogous Mannich reaction of α-isopropylidenepyrazolone 2a, which was easily prepared from the commercially available edaravone and acetone, and isatin-derived N-Boc ketimine 1a in CH2Cl2 at room temperature. With these conditions, several bifunctional organocatalysts were tested, and the results are summarized in Table 1. We selected bifunctional organocatalysts10 with a tertiary amine responsible for the activation of the nucleophile (deprotonation of the γ-hydrogens of the α,β-unsaturated pyrazolone) and a hydrogen-bonding donor moiety with the purpose of activating the electrophile (the isatin-derived N-Boc ketimine). When quinine (I) was used as catalyst, the yield of the Mannich product 3aa was very low (6%), but the enantioselectivity was moderate (50% ee). We observed large amounts of N-methylisatine from the hydrolysis of ketimine 1a. Takemoto’s thiourea II and quinine-derived thiourea III exhibited high stereocontrol (90% ee); however, the yield of product 3aa was still low (∼20%). Delightfully, quinine-derived squaramide IV gave excellent enantiomeric excess (98%), and the yield increased to 42% after 3 days of reaction (entry 4). When benzylic (V)- and tert-butyl (VI)-substituted squaramides were used as catalysts, product 3aa was obtained in similar yield but with somewhat lower enantioselectivity. The squaramide VII, prepared from dihydroquinine, displayed similar reactivity and stereoselectivity, and product 3aa was obtained in 41% yield with 97% ee after 3 days. Squaramide VIII, prepared from quinidine, gave a similar yield (39%) and enantiomeric excess (96% ee) as quinine-based IV, but the opposite enantiomer was obtained. We chose IV to continue the optimization process of the reaction conditions. Different solvents were tested, achieving high enantioselectivities but lower yields than CH2Cl2 (entries 9–13). To improve the yield of the reaction, we studied the variation of the equivalents of the electrophile (entry 14) or nucleophile (entry 15); however, the yields were lower. We observed in all cases the formation of N-methyl isatin, the corresponding hydrolysis product of 1a. To avoid the hydrolysis of the ketimine and increase the yield, we performed the reaction under an anhydrous nitrogen atmosphere (entry 16). In this case, the yield of the Mannich product 3aa increased to 57%, maintaining the enantioselectivity (98%). Finally, we increased the reaction scale to 0.2 mmol and obtained similar results (entry 17).
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst | solvent | t (days) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | I (5%) | CH2Cl2 | 4 | 6 | 50 |
| 2 | II (5%) | CH2Cl2 | 4 | 16 | 91 |
| 3 | III (5%) | CH2Cl2 | 3 | 19 | 89 |
| 4 | IV (5%) | CH2Cl2 | 3 | 42 | 98 |
| 5 | V (5%) | CH2Cl2 | 3 | 41 | 92 |
| 6 | VI (5%) | CH2Cl2 | 3 | 44 | 94 |
| 7 | VII (5%) | CH2Cl2 | 3 | 41 | 97 |
| 8 | VIII (5%) | CH2Cl2 | 3 | 39 | 96d |
| 9 | IV (5%) | ClCH2CH2Cl | 3 | 40 | 96 |
| 10 | IV (5%) | CHCl3 | 3 | 28 | 97 |
| 11 | IV (5%) | EtOAc | 3 | 33 | 91 |
| 12 | IV (5%) | Et2O | 3 | 55 | 92 |
| 13 | IV (5%) | toluene | 3 | 42 | 95 |
| 14e | IV (5%) | CH2Cl2 | 3 | 38 | 97 |
| 15f | IV (5%) | CH2Cl2 | 3 | 42 | 97 |
| 16g | IV (5%) | CH2Cl2 | 3 | 57 | 98 |
| 17g,h | IV (5%) | CH2Cl2 | 3 | 52 | 98 |
Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), and 5 mol % of organocatalyst in 1 mL of solvent at rt under an air atmosphere.
Isolated yield of 3aa after column chromatography.
Determined by chiral HPLC.
Opposite enantiomer was obtained.
0.12 mmol of 1a was used.
0.12 mmol of 2a was used.
Reaction was performed under a N2 atmosphere.
Reaction was performed on a 0.2 mmol scale.
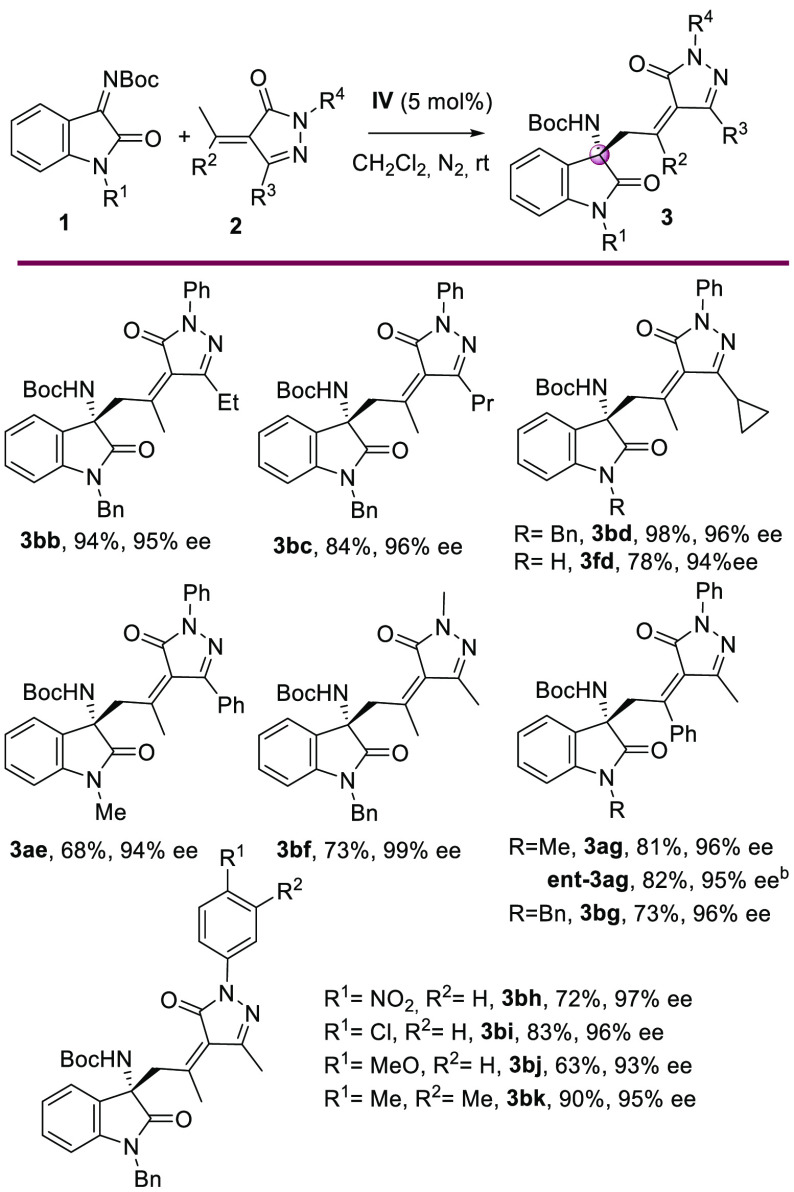
With the optimized reaction conditions in hand (entry 16, Table 1), we evaluated the scope of the vinylogous Mannich reaction with an assortment of isatin-derived ketimines 1 with several substituents in different positions (Scheme 2). Initially, substitution with different groups such as methyl, benzyl, or allyl at the N-1 of the oxindole was evaluated (3aa–3fa), providing the corresponding products in good yields (52–68%) with high enantioselectivities (97–98% ee). We also tested isatin-derived ketimines with Ph, −CH2OMe, and H substituents at the N-1 and obtained excellent enantioselectivities (92–96% ee) but lower yields (30–42%). Because the best yield was obtained with N-benzyl isatin-derived ketimines, we evaluated the effect of the substitution pattern of several N-benzylisatines. Electron-withdrawing (Br or Cl) or electron-donating (MeO) groups were tolerated at the five-position of the isatin-derived ketimine, affording the corresponding products 3ha and 3ia in good yields with excellent enantioselectivities in all cases. However, with the ketimine prepared from 5-bromoisatin, the yield was low (28%). Furthermore, isatin-derived ketimines with substituents at the six- or seven-positions reacted efficiently, providing the Mannich products 3ja and 3ka in good yields (67–75%) with excellent stereoselectivities. Also, the disubstituted ketimine 1l could be used, affording the corresponding product 3la with excellent enantioselectivity (98% ee) in 66% yield. The reaction could be accomplished on a 1 mmol scale, improving the yield of product 3ba (83%) and maintaining the enantioselectivity of the reaction (96% ee). We also tested the reaction on a 1 mmol scale lowering the catalyst amount to 2 mol % of IV. In this case, we observed a similar enantioselectivity (95% ee) but a lower yield (54%).
Scheme 2. Scope of the Catalytic Enantioselective Vinylogous Addition of Alkylidenepyrazolone 2a to Isatin-Derived Ketimines 1.
Reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), and IV (5 mol %) in 1 mL of CH2Cl2 at rt under a N2 atmosphere. Isolated yield of 3 after column chromatography. Determined by chiral HPLC.
1 mmol scale reaction using 5 mol % of IV.
1 mmol scale reaction using 2 mol % of IV.
Next, we turned our attention to further explore the substrate scope with respect to the alkylidenepyrazolones 2 (Scheme 3). Alkyl groups (Et, Pr, and cyclopropyl) other than Me were well tolerated at the five-position of the pyrazolones (3bb–3bd), providing excellent yields (84–98%) and enantioselectivities (95–96% ee). 2,5-Diphenyl and 2,5-dimethyl alkylidenepyrazolone were also examined under the optimized reaction conditions, providing products 3ae in 68% yield with 94% ee and 3bf in 73% yield with 99% ee. Moreover, when alkylidenepyrazolone derived from acetophenone was tested with ketimines 1a and 1b, the corresponding Mannich products 3ag and 3bg were obtained with excellent results. Finally, the reaction proceeded efficiently with pyrazolones with different substituents (NO2, Cl, Me, and MeO) on the N-aryl group, providing the corresponding products in high yields (63–90%) with high enantiomeric excesses (93–97% ee).
Scheme 3. Scope of the Catalytic Enantioselective Vinylogous Addition of Alkylidenepyrazolone 2 to Isatin-Derived Ketimines 1.
Reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), and IV (5 mol %) in 1 mL of CH2Cl2 at rt under a N2 atmosphere. Isolated yield of 3 after column chromatography. Determined by chiral HPLC.
VIII was used.
The double-bond configuration and absolute configuration of the stereogenic center present in compound 3ea were ascertained to be (S, Z) by X-ray crystallographic analysis (Scheme 4); the configuration of the remaining Mannich products 3 was assigned on the assumption of a uniform mechanistic reaction pathway. A plausible transition-state model is illustrated in Scheme 4, where the squaramide catalyst IV is responsible for the preorientation and the activation of the substrates of the reaction. Whereas the methyl group of alkylidenepyrazolones is first deprotonated by the quinuclidine moiety of the organocatalyst to form the corresponding diene enolate, the isatin-derived N-Boc-ketimine moiety is activated upon the formation of hydrogen bonds between the N-Boc group and the squaramide moiety of the organocatalyst. The pyrazolone enolate will be directed to the Re-face of the ketimine, thus accounting for the observed enantioselectivity.
Scheme 4. Proposed Reaction Mechanism for the Asymmetric Vinylogous Mannich Reaction and X-ray Crystal Structure of 3ea.

To demonstrate the versatility and usefulness of our organocatalytic vinylogous methodology, we performed several chemical modifications of the Mannich products (Scheme 5). A relevant structural feature of the obtained vinylogous Mannich adducts is that they preserve the exocyclic unsaturation of the initial pyrazolone substrate. This olefinic group could be used to further functionalize the compound, thus increasing the molecular complexity of the pyrazolone products. For example, compound 3ba was stereoselectively epoxidated with meta-chloroperoxybenzoic acid (mCPBA), affording the spirooxirane 4 (Scheme 5A) with three quaternary stereocenters in 90% yield with good diastereoselectivity (84:16 dr) and maintaining the enantiomeric excess.12 We could obtain crystals of the major diastereoisomer 4, which allowed us to determine the configuration of the epoxide. Moreover, compound 3ba could be subjected to a conjugate addition of NaCN,14 providing the corresponding product 5 as a single diastereoisomer in good yield (73%) and maintaining the enantiomeric excess (Scheme 5B). Finally, the reaction of compound 3ba with methyl bromoacetate in the presence of NaH15 afforded the highly functionalized chiral pyrazole 6 in 89% yield and preserved the optical purity of the starting material (Scheme 5C).
Scheme 5. Synthetic Transformations.

In summary, we have established an organocatalytic diastereo- and enantioselective vinylogous Mannich reaction of alkylidenepyrazolones with isatin-derived ketimines using a quinine-derived squaramide organocatalyst, obtaining the corresponding chiral Mannich adducts in moderate to high yields (up to 98%) with complete diastereoselectivities toward the Z double bond and excellent enantioselectivities (up to 99% ee) under mild reaction conditions. The reaction showed a wide substrate scope for different N-Boc-ketimines and alkylidenepyrazolones. The new compounds feature pyrazolone and amino-oxindole moieties, which are privileged structures in medicinal chemistry. Moreover, several synthetic transformations of the chiral Mannich product 3ba have been performed, showing the potential applicability of the present methodology. Studies on extending the scope of the reaction are currently ongoing in our laboratory.
Acknowledgments
Financial support from the Agencia Estatal de Investigación (AEI, Spanish Government) and Fondo Europeo de Desarrollo Regional (FEDER, European Union) (PID2020-116944GB) and from Conselleria d’Innovació, Universitat, Ciència i Societat Digital (AICO/2020/68) is acknowledged. L.C.-F. thanks the Universitat de València for a predoctoral grant. C.V. thanks the Spanish Government for a RyC contract (RYC-2016-20187). Access to the NMR, MS, and X-ray facilities from the Servei Central de Suport a la Investigació Experimental (SCSIE)-UV is also acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c02571.
Complete experimental procedures and characterization of new products and 1H and 13C NMR spectra for all compounds (PDF)
Accession Codes
CCDC 2092891–2092892 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- For the principle of vinylogy, see:; a Fuson R. C. The Principle of Vinylogy. Chem. Rev. 1935, 16, 1. 10.1021/cr60053a001. [DOI] [Google Scholar]; For selected reviews of asymmetric vinylogous reactions, see:; b Li H.; Yin L. Recent progress on direct catalytic asymmetric vinylogous reactions. Tetrahedron Lett. 2018, 59, 4121. 10.1016/j.tetlet.2018.10.012. [DOI] [Google Scholar]; c Yin Y.; Jiang Z. Organocatalytic asymmetric vinylogous Michael reactions. ChemCatChem 2017, 9, 4306. 10.1002/cctc.201700941. [DOI] [Google Scholar]; d Sánchez-Roselló M.; Del Pozo C.; Fustero S. A decade of advance in the asymmetric vinylogous Mannich reaction. Synthesis 2016, 48, 2553. 10.1055/s-0035-1561650. [DOI] [Google Scholar]; e Schneider C.; Abels F. Catalytic, enantioselective vinylogous Michael reactions. Org. Biomol. Chem. 2014, 12, 3531. 10.1039/c4ob00332b. [DOI] [PubMed] [Google Scholar]; f Bisai V. Organocatalytic asymmetric vinylogous aldol reactions. Synthesis 2012, 44, 1453. 10.1055/s-0031-1290981. [DOI] [Google Scholar]; g Casiraghi G.; Battistini L.; Curti C.; Rassu G.; Zanardi F. The vinylogous aldol and related addition reactions: ten years of progress. Chem. Rev. 2011, 111, 3076. 10.1021/cr100304n. [DOI] [PubMed] [Google Scholar]; h Pansare S. V.; Paul E. K. The organocatalytic vinylogous aldol reaction: recent advances. Chem. - Eur. J. 2011, 17, 8770. 10.1002/chem.201101269. [DOI] [PubMed] [Google Scholar]; i Cui H.-L.; Chen Y.-C. α,α-Dicyanoalkenes: versatile vinylogous nucleophiles for organic synthesis. Chem. Commun. 2009, 4479. 10.1039/b906201g. [DOI] [PubMed] [Google Scholar]; j Denmark S. E.; Heemstra J. R.; Beutner G. L. Catalytic, enantioselective, vinylogous aldol reactions. Angew. Chem., Int. Ed. 2005, 44, 4682. 10.1002/anie.200462338. [DOI] [PubMed] [Google Scholar]
- Varvounis G. Chapter 2 Pyrazol-3-ones. Part IV: Synthesis and Applications. Adv. Heterocycl. Chem. 2009, 98, 143. 10.1016/S0065-2725(09)09802-X. [DOI] [Google Scholar]
- a Chauhan P.; Mahajan S.; Enders D. Asymmetric synthesis of pyrazoles and pyrazolones employing the reactivity of pyrazolin-5-one derivatives. Chem. Commun. 2015, 51, 12890. 10.1039/C5CC04930J. [DOI] [PubMed] [Google Scholar]; b Liu S.; Bao X.; Wang B. Pyrazolone: a powerful synthon for asymmetric diverse derivatizations. Chem. Commun. 2018, 54, 11515. 10.1039/C8CC06196C. [DOI] [PubMed] [Google Scholar]; c Carceller-Ferrer L.; Blay G.; Pedro J. R.; Vila C. Recent advances in catalytic enantioselective synthesis of pyrazolones with a tetrasubstituted stereogenic center at the 4-position. Synthesis 2021, 53, 215. 10.1055/s-0040-1707298. [DOI] [Google Scholar]
- a Rassu G.; Zambrano V.; Pinna L.; Curti C.; Battistini L.; Sartori A.; Pelosi G.; Casiraghi G.; Zanardi F. Direct and enantioselective vinylogous Michael addition of α-alkylidenepyrazolinones to nitroolefins catalyzed by dual cinchona alkaloid thioureas. Adv. Synth. Catal. 2014, 356, 2330. 10.1002/adsc.201300964. [DOI] [Google Scholar]; b Yetra S. R.; Mondal S.; Mukherjee S.; Gonnade R. G.; Biju A. T. Enantioselective synthesis of spirocyclohexadienones by NHC-catalyzed formal [3+ 3] annulation reaction of enals. Angew. Chem., Int. Ed. 2016, 55, 268. 10.1002/anie.201507802. [DOI] [PubMed] [Google Scholar]; c Liu J.-Y.; Zhao J.; Zhang J.-L.; Xu P.-F. Quaternary carbon center forming formal [3+ 3] cycloaddition reaction via bifunctional catalysis: Asymmetric synthesis of spirocyclohexene pyrazolones. Org. Lett. 2017, 19, 1846. 10.1021/acs.orglett.7b00610. [DOI] [PubMed] [Google Scholar]; d Mondal S.; Mukherjee S.; Yetra S. R.; Gonnade R. G.; Biju A. T. Organocatalytic enantioselective vinylogous Michael-aldol cascade for the synthesis of spirocyclic compounds. Org. Lett. 2017, 19, 4367. 10.1021/acs.orglett.7b02085. [DOI] [PubMed] [Google Scholar]; e Leng H.-J.; Li Q.-Z.; Zeng R.; Dai Q.-S.; Zhu H.-P.; Liu Y.; Huang W.; Han B.; Li J.-L. Asymmetric construction of spiropyrazolone skeletons via amine-catalyzed [3 + 3] annulation. Adv. Synth. Catal. 2018, 360, 229. 10.1002/adsc.201701035. [DOI] [Google Scholar]; f Xu J.; Hu L.; Hu H.; Ge S.; Liu X.; Feng X. Enantioselective vinylogous Michael-aldol reaction to synthesize spirocyclohexene pyrazolones in aqueous media. Org. Lett. 2019, 21, 1632. 10.1021/acs.orglett.9b00168. [DOI] [PubMed] [Google Scholar]; g Sun B.-B.; Zhang J.-Q.; Chen J.-B.; Fan W.-T.; Yu J.-Q.; Hu J.-M.; Wang X.-W. Stereoselective synthesis of spirocyclohexadiene-pyrazolones via organic base and/or hydrogen bonding assisted [3 + 3] annulation reactions. Org. Chem. Front. 2019, 6, 1842. 10.1039/C8QO01391H. [DOI] [Google Scholar]; h Ji Y.-L.; Li H.-P.; Ai Y.-Y.; Li G.; He X.-H.; Huang W.; Huang R.-Z.; Han B. Enantio-and diastereoselective synthesis of spiropyrazolones via an organocatalytic [1+ 2+ 3] multicomponent reaction. Org. Biomol. Chem. 2019, 17, 9217. 10.1039/C9OB01927H. [DOI] [PubMed] [Google Scholar]; i Sun B.-B.; Chen J.-B.; Zhang J.-Q.; Yang X.-P.; Lv H.-P.; Wang Z.; Wang X.-W. Organo-catalyzed asymmetric cascade annulation reaction for the construction of bi-spirocyclic pyrazolone and oxindole derivatives. Org. Chem. Front. 2020, 7, 796. 10.1039/D0QO00001A. [DOI] [Google Scholar]; j Krishna A. V.; Reddy G. S.; Gorachand B.; Ramachary D. B. Organocatalytic asymmetric formal [3 + 3]-cycloaddition to access 2,3-diazaspiro[4.5]deca-3,6-dien-1-ones. Eur. J. Org. Chem. 2020, 2020, 6623. 10.1002/ejoc.202001175. [DOI] [Google Scholar]
- Yang W.; Sun W.; Zhang C.; Wang Q.; Guo Z.; Mao B.; Liao J.; Guo H. Lewis-base-catalyzed asymmetric [3+ 3] annulation reaction of Morita–Baylis–Hillman carbonates: Enantioselective synthesis of spirocyclohexenes. ACS Catal. 2017, 7, 3142. 10.1021/acscatal.7b00320. [DOI] [Google Scholar]
- a Amr F. I.; Vila C.; Blay G.; Muñoz M. C.; Pedro J. R. Organocatalytic enantioselective alkylation of pyrazol-3-ones with isatin-derived ketimines: stereocontrolled construction of vicinal tetrasubstituted stereocenters. Adv. Synth. Catal. 2016, 358, 1583. 10.1002/adsc.201600036. [DOI] [Google Scholar]; b Vila C.; Amr F. I.; Blay G.; Muñoz M. C.; Pedro J. R. Organocatalytic enantioselective synthesis of pyrazoles Bearing a quaternary stereocenter. Chem. - Asian J. 2016, 11, 1532. 10.1002/asia.201600325. [DOI] [PubMed] [Google Scholar]; c Carceller-Ferrer L.; Vila C.; Blay G.; Fernández I.; Muñoz M. C.; Pedro J. R. Organocatalytic enantioselective aminoalkylation of pyrazol-3-ones with aldimines generated in situ from α-amido sulfones. Org. Biomol. Chem. 2019, 17, 9859. 10.1039/C9OB02252J. [DOI] [PubMed] [Google Scholar]; d Carceller-Ferrer L.; González del Campo A.; Vila C.; Blay G.; Muñoz M. C.; Pedro J. R. Organocatalytic enantioselective aminoalkylation of 5-aminopyrazole derivatives with cyclic imines. Eur. J. Org. Chem. 2020, 2020, 7450. 10.1002/ejoc.202001314. [DOI] [Google Scholar]; e Carceller-Ferrer L.; Blay G.; Pedro J. R.; Vila C. Squaramide-catalyzed enantioselective Michael addition of pyrazol-3-ones to ortho-quinone methides. Lett. Org. Chem. 2020, 17, 837. 10.2174/1876402911666190806105543. [DOI] [Google Scholar]
- a Chauhan P.; Chimni S. S. Organocatalytic asymmetric synthesis of 3-amino-2-oxindole derivatives bearing a tetra-substituted stereocenter. Tetrahedron: Asymmetry 2013, 24, 343. 10.1016/j.tetasy.2013.03.002. [DOI] [Google Scholar]; b Kaur J.; Chimni S. S.; Mahajan S.; Kumar A. Stereoselective synthesis of 3-amino-2-oxindoles from isatin imines: new scaffolds for bioactivity evaluation. RSC Adv. 2015, 5, 52481. 10.1039/C5RA06969F. [DOI] [Google Scholar]; c Yu J.-S.; Zhou F.; Liu Y.-L.; Zhou J. A journey in the catalytic synthesis of 3-substituted 3-aminooxindoles. Synlett 2015, 26, 2491. 10.1055/s-0034-1378873. [DOI] [Google Scholar]; d Mohammadi Ziarani G.; Moradi R.; Lashgari N. Asymmetric synthesis of chiral oxindoles using isatin as starting material. Tetrahedron 2018, 74, 1323. 10.1016/j.tet.2018.01.025. [DOI] [Google Scholar]; e Kaur J.; Chimni S. S. Catalytic synthesis of 3-aminooxindoles via addition to isatin imine: an update. Org. Biomol. Chem. 2018, 16, 3328. 10.1039/C7OB03002A. [DOI] [PubMed] [Google Scholar]
- a Dounay A. B.; Overman L. E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945. 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]; b Lin H.; Danishefsky S. J. Gelsemine: A thought-provoking target for total synthesis. Angew. Chem., Int. Ed. 2003, 42, 36. 10.1002/anie.200390048. [DOI] [PubMed] [Google Scholar]; c Galliford C. V.; Scheidt K. A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem., Int. Ed. 2007, 46, 8748. 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]
- For reviews of hybrid-based drugs, see:; a Tsogoeva S. B. Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini-Rev. Med. Chem. 2010, 10, 773. 10.2174/138955710791608280. [DOI] [PubMed] [Google Scholar]; b Decker M. Hybrid molecules incorporating natural products: applications in cancer therapy, neurodegenerative disorders and beyond. Curr. Med. Chem. 2011, 18, 1464. 10.2174/092986711795328355. [DOI] [PubMed] [Google Scholar]; c Sandhu S.; Bansal Y.; Silakari O.; Bansal G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014, 22, 3806. 10.1016/j.bmc.2014.05.032. [DOI] [PubMed] [Google Scholar]; d Vandekerckhove S.; D’hooghe M. Quinoline-based antimalarial hybrid compounds. Bioorg. Med. Chem. 2015, 23, 5098. 10.1016/j.bmc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- a Doyle A. G.; Jacobsen E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; b Connon S. Organocatalysis mediated by (thio)urea derivatives. Chem. - Eur. J. 2006, 12, 5418. 10.1002/chem.200501076. [DOI] [PubMed] [Google Scholar]; c Alemán J.; Parra A.; Jiang H.; Jørgensen K. A. Squaramides: bridging from molecular recognition to bifunctional organocatalysis. Chem. - Eur. J. 2011, 17, 6890. 10.1002/chem.201003694. [DOI] [PubMed] [Google Scholar]
- The two diastereoisomers could be separated after column chromatography.
- Deruiter J.; Carter D. A.; Arledge W. S.; Sullivan P. J. Synthesis and Reactions of 4-isopropylidene-1-aryl-3-methyl-2-pyrazolin-5-ones. J. Heterocycl. Chem. 1987, 24, 149. 10.1002/jhet.5570240128. [DOI] [Google Scholar]
- Maruoka H.; Hokao M.; Masumoto E.; Okabe F.; Tanaka R.; Miake F.; Fujioka T.; Yamagata K. Chemical Reactivity and Application of 4-Alkylidene-3H-pyrazol-3-ones: Synthesis and Antifungal Activity of Polysubstituted Pyrazoles. Heterocycles 2016, 93, 362. 10.3987/COM-15-S(T)13. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.