Abstract
Dry eye disease (DED) affects hundreds of millions of people worldwide. It is characterized by the production of inflammatory cytokines and chemokines as well as damaging matrix metalloproteinases (MMPs) at the ocular surface. While proteoglycan 4 (PRG4), a mucin-like glycoprotein present at the ocular surface, is most well known as a boundary lubricant that contributes to ocular surface integrity, it has been shown to blunt inflammation in various cell types, suggesting a dual mechanism of action. Recently, full-length recombinant human PRG4 (rhPRG4) has been shown to improve signs and symptoms of DED in humans. However, there remains a significant need for basic science research on rhPRG4’s biological properties and its potential therapeutic mechanisms of action in treating DED. Therefore, the objectives of this study were to characterize endogenous PRG4 expression by telomerase-immortalized human corneal epithelial (hTCEpi) cells, examine whether exogenous rhPRG4 modulates cytokine and chemokine secretion in response to dry eye associated inflammation (TNFα and IL-1β), explore interactions between rhPRG4 and MMP-9, and understand how experimental dry eye (EDE) in mice affects PRG4 expression in mice. PRG4 secretion from hTCEpi cells was quantified by Western blotting and expression visualized by immunocytochemistry. Cytokine/chemokine production was measured by ELISA and Luminex, while rhPRG4’s effect on MMP-9 activity, binding, and expression was quantified using an MMP-9 inhibitor kit, surface plasmon resonance, and RT-PCR, respectively. Finally, EDE was induced in mice, and PRG4 was visualized by immunohistochemistry in the cornea and Western blotting in lacrimal gland lysate. In vitro results demonstrate that hTCEpi cells synthesize and secrete PRG4, and PRG4 secretion is inhibited by TNFα and IL-1β. In response to these pro-inflammatory stresses, exogenous rhPRG4 significantly reduced the stimulated production of IP-10, RANTES, ENA-78, GROα, MIP-3α, and MIG, and trended towards a reduction of MIP-1α and MIP-1β. The hTCEpi cells were also able to internalize fluorescently-labelled rhPRG4, consistent with a mechanism of action that includes downstream biological signaling pathways. rhPRG4 was not digested by MMP-9 and it did not modulate MMP-9 gene expression in hTCEpi cells, but it was able to bind to MMP-9 and inhibited in vitro activity of exogenous MMP-9 in the presence of human tears. Finally, in vivo results demonstrated EDE significantly decreased immunolocalization of PRG4 on the corneal epithelium and trended towards a reduction of PRG4 in the lacrimal gland lysate. Collectively these results demonstrate rhPRG4 has anti-inflammatory properties on corneal epithelial cells, particularly as it relates to mitigating chemokine production, and is an inhibitor of MMP-9 activity, as well as that in vivo expression of PRG4 can be altered in preclinical models of DED. In conclusion, these findings contribute to our understanding of PRG4’s immunomodulatory properties in the context of DED inflammation and provide the foundation and motivation for further mechanistic research of PRG4’s properties on the ocular surface as well as expanding clinical evaluation of its ability as a multifunctional therapeutic agent to effectively provide relief to those who suffer from DED.
1. Introduction
Dry eye disease (DED) affects hundreds of millions of people worldwide, and effective treatment options are lacking. It is associated with significant pain, limitations in daily activities, diminished vitality, poor health in general, and often depression.(Miljanović et al., 2007; Smith et al., 2007; Stapleton et al., 2017). DED is highly prevalent, affecting 10–20% of the population between 20 and 40 years of age and more than 30% of those above 70 years of age (Stapleton et al., 2017). While treatment options do exist for DED, they are often misaligned with the fundamental mechanisms of the disease and do not fully address patient symptoms (Holland et al., 2019). Indeed, a recent study demonstrated more than 60% of DED patients using either of the two FDA-approved drugs had discontinued treatment within 12 months, thus indicating effective treatment options are lacking (White et al., 2019).
Recently, topical administration of a full length recombinant human proteoglycan 4 (rhPRG4) was shown to be clinically effective in improving signs and symptoms of patients with DED (Lambiase et al., 2017). This small, single site, clinical trial (NCT02507934) on 39 subjects with moderate DED assessed the safety and efficacy of rhPRG4 at 150 μg/ml as compared to a 0.18% sodium hyaluronate (HA) eye drop in subjects with moderate DED. The rhPRG4 solution demonstrated statistically significant effects compared to HA in: a) symptomatic improvements in foreign body sensation, sticky feeling, blurred vision, and photophobia in at least one eye; b) improvement in objective signs of DED in corneal fluorescein staining, tear film break-up time, eyelid and conjunctival erythema, and daily mean instillations; and c) no treatment-related adverse events. While PRG4 is most well-known for its boundary lubricating properties on biointerfaces, including cornea-conjunctiva as well as conjunctiva/cornea –contact lens or contact lens biomaterials (Korogiannaki et al., 2021, 2018; Samsom et al., 2018b, 2018a, 2015, 2014; Schmidt et al., 2013), which may contribute to maintaining ocular surface integrity, recent studies increasingly demonstrate it also has biological activity. Specifically, PRG4 has shown the ability to function as an anti-inflammatory agent on various cell types (Al-Sharif et al., 2015; Alquraini et al., 2017, 2015; Das et al., 2019; Iqbal et al., 2016; Qadri et al., 2018; Richendrfer et al., 2020; Sarkar et al., 2019). However, within the context of the ocular surface, there remains a lack of knowledge on, and significant need for basic science research on, rhPRG4’s biological properties and its potential therapeutic mechanisms of action in treating DED. Given the complex and multi-factorial pathogenesis of this disease, understanding the potential mechanisms of action at a molecular level should help identify which patients would best align with those mechanisms and stand to benefit from a course of therapy.
Tear film instability and tear hyper-osmolarity contribute to a positive feedforward loop in DED, leading to ocular surface damage and inflammation. Indeed, DED is characterized by the production of inflammatory cytokines and chemokines, as well as damaging matrix metalloproteinases (MMPs), at the ocular surface. Environmental stress, aging, bacterial infection, and contact lens wear can cause damage in the eye, specifically in the corneal epithelium and conjunctiva, which can lead to changes in tear film stability and osmolarity (Stevenson et al., 2012). These changes can increase the production of pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α, from corneal epithelial cells, which further degrades the tear film and contributing to a vicious inflammatory cycle (Massingale et al., 2009; Stern and Pflugfelder, 2004). Elevated levels of IL-1β, IL-6, and TNFα have also been reported in tears of patients with DED (Lam et al., 2009; Pflugfelder et al., 1999). Indeed, 1L-1β and TNFα are two critical innate cytokines involved in DED (Bron et al., 2017; Roda et al., 2020). Changes in tear film stability and osmolarity also cause increased release of chemokines, including CCL5 (RANTES) (El-Annan et al., 2010; Lam et al., 2009), CXCL8 (IL-8) (Enríquez-de-Salamanca et al., 2010; Pflugfelder et al., 1999), CXCL1 (GROα) (Reins et al., 2018), and CXCL10 (IP-10) (Enríquez-de-Salamanca et al., 2010), which attract immune cells to the corneal epithelium and further stimulate the proinflammatory cytokine production and matrix metalloproteinase (MMP) activity (El-Annan et al., 2010; Enríquez-de-Salamanca et al., 2010; Stern and Pflugfelder, 2004; Stevenson et al., 2012). MMP-9 levels also increase in the tears of patients with DED, where they increase with DED severity, as well as in mice with experimental DED, and MMP-9 is used as a biomarker for DED (Kaufman, 2013; Luo et al., 2004; Pinto-fraga et al., 2018). T-cell dysregulation has also been implicated in the development and progression of DED. Specifically, upregulation of T-helper cell 17 (Th17) cell activity plays a critical role in DED (Chauhan et al., 2009). The expansion of Th17 cells with desiccating stress, and their reduced response to regulatory T cells, can lead to an increase in the secretion of MMPs and inflammatory cytokines in the corneal epithelium (De Paiva et al., 2009). Several Th1-related chemokines have been shown to be upregulated in DED in both murine models and human patients, including CCL20 (MIP-3α), CXCL9 (MIG), CCL3 (MIP-1α), and CCL4 (MIP-1β) (Choi et al., 2012; De Paiva et al., 2009; Dohlman et al., 2013; Yoon et al., 2007). Given the role inflammation plays in the pathogenesis of DED, molecules that are able to appropriately modulate inflammatory activity in the cornea could lead to potential, more effective, future treatment options.
PRG4, also known as lubricin, is a mucin like glycoprotein that is present on the ocular surface where it plays a critical protective role in maintaining ocular surface integrity. PRG4 is present at the epithelial surface of human cornea and conjunctiva, where it helps maintain the tear film (Rabiah et al., 2020). Lack of PRG4 in mice results in increased ocular surface damage (Samsom et al., 2014; Schmidt et al., 2013), suggesting PRG4 contributes to the maintenance of ocular surface integrity. Mechanically, PRG4 functions as an ocular surface boundary lubricant, reducing friction at the interface between the eyelid, cornea, and contact lenses (Samsom et al., 2015). Full length rhPRG4 exhibits similar in vitro boundary lubricating properties to native PRG4, on both the ocular surface (Samsom et al., 2014) and articular cartilage (Abubacker et al., 2016). Biologically, recent studies in the context of synovial joint health and disease demonstrated that rhPRG4 has anti-inflammatory properties, including the ability to bind to and antagonize toll-like receptors, reducing inflammation by dampening nuclear factor kappa B (NF-κB) activation and inflammatory cytokine expression (Alquraini et al., 2015), as well as the ability to inhibit fibroblast-like synoviocyte proliferation (Al-Sharif et al., 2015; Alquraini et al., 2017). However, despite rhPRG4’s promising potential as a treatment for DED, rhPRG4’s anti-inflammatory properties and endogenous PRG4 expression in the context of ocular surface health and DED have yet to be examined.
The primary objectives of this study were therefore to characterize endogenous PRG4 expression by human corneal epithelial cells, and to examine the ability of exogenous rhPRG4 to modulate cytokine and chemokine secretion in response to dry eye associated inflammatory stimuli, TNFα and IL-1β, in human corneal epithelial cells. Secondary objectives were to explore rhPRG4 as a potential substrate and/or inhibitor of MMP-9 and to determine whether EDE modulates corneal and lacrimal gland PRG4 expression.
2. Methods
2.1. Human Corneal Epithelial Cell Culture
2.1.1. Expansion & Differentiation:
hTCEpi cells (kindly provided by Dr. James Jester) (Robertson et al., 2005) were expanded with Keratinocyte Serum-Free Medium (KSFM) supplemented with 5 ng/mL epidermal growth factor (EGF) and 25 μg/mL bovine pituitary extract (BPE) (Gibco, Grand Island, NY) and seeded in 12 well plates. Each well received 100,000 cells in 2 mL of KSFM media. Cells were allowed to expand, to 80–90% confluency, for 48 hours. Cells in each well were then differentiated using DMEM/F12 (Gibco, Grand Island, NY) + 10% FBS (R&D Systems, Minneapolis, MN) + 10 ng/mL EGF (Gibco), as described previously (Ding et al., 2015).
2.1.2. Inflammatory Stimuli & rhPRG4 Treatment:
After 4 days of differentiation, each well received 0.45mL of stock rhPRG4 (1.33 mg/mL, Lubris Biopharma, Framingham, MA) or sterile PBS containing 0.01% Tween-20 immediately followed by 1.55 mL of treatment media (DMEM/F12 + 12.9% FBS + 12.9 ng/mL EGF). The treatment media was spiked with IL-1β (Peprotech, Cranbury, NJ), TNFα (Peprotech) or left alone as a control. The final concentrations were 10 ng/mL for IL-1β, 100 ng/mL for TNFα, and 0 or 300 μg/mL for rhPRG4. After 48 hours of treatment, the conditioned media was collected, aliquoted, and stored at −20°C. Each treatment was tested in triplicate in each experiment, and the experiment was performed three or four times (N=3–4).
2.2. Western blot
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 3–8% Tris-Acetate gels (Invitrogen, Carlsbad, CA), as described previously (Steele et al., 2013). Briefly, samples (15 μL of conditioned media per well) were electrophoresed, followed by electroblotting to a PVDF membrane and blocked in 5% non-fat dry milk (Biorad, Hercules, CA) in Tris-buffered saline + 0.05% Tween-20 (TBST) for 1h at room temperature. Membranes were then probed with anti-PRG4 Ab LPN (1:1000, Invitrogen, PA3–118) in 3% non-fat dry milk in TBST overnight at 4°C. After washing with TBST, membranes were incubated with HRP-conjugated anti-rabbit secondary antibody (1:2000, MilliporeSigma, Burlington, MA) for 1 hour and imaged on a G:Box Chemi XX9 imager (Syngene, Frederick, MD) using SuperSignal West Femto (Thermo Fisher Scientific, Waltham, MA). Resulting intensity of bands were quantified by densitometry with the Genetools software (Syngene).
2.3. Chemokine & cytokine analysis
2.3.1. ELISA analysis
Conditioned media samples were analyzed using commercially available ELISAs as per manufacturer guidelines (BioLegend, San Diego, CA), at 2X and 8X dilutions for IL-6, IP-10, RANTES, ENA-78, and MIP-3α and at 8X and 32X dilution of IL-8. Conditioned media was also analyzed using a GROα ELISA kit following manufacturer guidelines (R&D Systems, Minneapolis, MN) at 2X dilution. Resulting data was collected on a SpectraMax i3x plate reader (Molecular Devices, San Jose, CA), and concentrations were calculated using OD values within the standard curve range.
2.3.2. Luminex analysis
Conditioned media samples were analyzed using a custom MILLIPLEX® Human Cytokine/Chemokine/Growth Factor Panel A - Immunology Multiplex Assay as per manufacturer guidelines (EMD Millipore, Burlington, MA), at 1X and 10X dilutions for MIG, MIP-1α, and MIP-1β. Analysis was completed in the CLIA certified Clinical Research Center Core Lab (UConn Health) on Luminex 200 instrumentation.
2.4. Immunofluorescence Imaging
hTCEpi cells were seeded at a density of 30,000 cells per well on sterile glass coverslips in 4-well dishes (Thermo Fisher Scientific). Cells were differentiated and treated using FITC-tagged rhPRG4 as described above (2.1.2). After 2 days, conditioned media was collected, and cells were fixed with 0.5 mL of 4% paraformaldehyde for 20 minutes at room temperature, permeabilized with 0.5 mL of 0.1% Triton X-100 in PBS for 5 minutes at room temperature, and blocked with 5% normal goat serum (Rockland, Pottstown, PA) and 5% BSA (MilliporeSigma) in PBS. Wells without FITC-rhPRG4 were probed with anti PRG4 Ab LPN (1:500, Invitrogen) in blocking solution overnight at 4°C. The next day, wells were appropriately treated with anti-rabbit 594 antibody (1:1200, MilliporeSigma) for 1 hour at room temperature and then DAPI (1:1000, Thermo Fisher Scientific) for 15 minutes at room temperature. The cover slips were then mounted with gold anti-fade mounting media (Thermo Fisher Scientific) and imaged on a Zeiss Axioskop2 microscope with an AxioCam digital camera (Zeiss, Oberkochen, Germany) using 63x oil objective. Slides with samples containing FITC-PRG4 were also imaged on a Zeiss LSM 880 confocal microscope with a 63x oil objective and a slice height of 0.36 μm.
2.5. Interaction between MMP-9 and rhPRG4
2.5.1. rhPRG4 degradation by MMP-9
rhPRG4 degradation from exogenous MMP-9 was assessed using gel electrophoresis and SimplyBlue protein stain. Briefly, rhPRG4 at 0.77 mg/mL was incubated with or without 9 mU of MMP-9 enzyme for 60 minutes at 37°C. These samples were then electrophoresed on a 3–8% Tris-Acetate gel and stained with SimplyBlue, following manufacturer guidelines (Thermo Fisher Scientific).
2.5.2. Inhibition of in vitro MMP-9 activity by rhPRG4
To assess the extent to which rhPRG4 inhibited in vitro MMP-9 activity, an MMP-9 inhibitor testing kit was used following manufacturer guidelines (Abcam, Cambridge, MA). Briefly, MMP-9 enzyme, N-Isobutyl-N-(4-methoxyphenylsulfonyl) glycyl Hydroxamic Acid (NNGH) inhibitor, and rhPRG4 were warmed to 37°C, and the enzyme was incubated with either NNGH at 150 μg/mL or rhPRG4 at 150 μg/mL, 300 μg/mL, or 450 μg/mL for 60 minutes. Then, the fluorogenic substrate was added, and fluorescence was measured using an excitation wavelength of 328 nm and an emission wavelength of 420 nm. To assess any potential effect of tear matrix on the results from the experiments above, this experiment was repeated using the same concentrations of rhPRG4 and MMP-9 in the presence of human tears. The tears were warmed to 37°C before being incubated with the MMP-9 and rhPRG4 for 60 minutes. All measurements were performed on a SpectraMax i3x microplate reader.
2.5.3. Binding between rhPRG4 and MMP-9
To assess the strength of binding between MMP-9 and rhPRG4, surface plasmon resonance (SPR) was performed using a Biacore T200 (GE Healthcare Life Sciences, Marlborough, MA). rhMMP-9 (RD17439100, Biovender, Asheville, NC) at 1607 nM in 10 mM sodium acetate buffer (pH 4.5) was flowed at 20 ul/min for 20 minutes and immobilized on a CM5 Series S chip using EDC/NHS chemistry, producing a signal of 14200 RU. Next, rhPRG4 was flowed over the chip at 0, 100, 250, 500, 750, 1000, and 1500 nM in 10 mM sodium HEPES (pH 7.4, 175 mM NaCl, 0.05% polysorbate 20) at a flow rate of 30 ul/min, contact time of 180s, and dissociation time of 180s. Bound rhPRG4 was removed using 100 mM glycine (pH 2.0) at a flow rate of 50 ul/min with a contact time of 30s. The dissociation constant was calculated using Biacore T200 Evaluation software and fitted using 1:1 binding.
2.5.4. Effect of rhPRG4 on MMP-9 gene expression
To assess the effect of rhPRG4 on MMP-9 gene expression in hTCEpi cells, RNA was isolated from the cells after inflammatory stimulus treatment (as described in 2.12) using TRIzol according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). DNA synthesis was performed using Invitrogen Superscript III Reverse Transcriptase according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Quantitative PCR was performed as described previously (Ghosh et al., 2018). Primer sequences were determined using established GenBank primer sequences (http://pga.mgh.harvard.edu/primerbank/). The sequences of PCR primers are as follows: Matrix Metalloproteinase 9 (MMP9), forward primer 5’- GGGACGCAGACATCGTCATC −3’, reverse primer 5’- TCGTCATCGTCGAAATGGGC-3’; 18s, forward primer 5’- CGT TCA GCC ACC CGA GAT T −3’, reverse primer 5’- GAC CCG CAC TTA CTG GGA ATT-3’. The gene 18s was used as an internal control. The relative fold increase in MMP9 expression was normalized to 18s expression and to MMP9 expression in the control sample without rhPRG4 using the ΔΔCt method. All data was analyzed using CFX Manager (Biorad).
2.6. Experimental Dry Eye Model
2.6.1. Sample Collection and Preparation:
Mice with experimental dry eye (EDE) were obtained as previously described (Lema et al., 2018; Reins et al., 2018). Briefly, 8 to 12-week old C57BL/6 mice (equal number male and female) were housed in a controlled room in which humidity was maintained at approximately 20% and temperature was maintained at 21°C to promote ocular surface desiccation. Additionally, mice were given subcutaneous scopolamine hydrobromide injections three times daily for five consecutive days to reduce tear production. After treatment, whole eyes were collected, snap frozen in optimal cutting temperature compound to obtain frozen tissue sections, and stored at −80°C until PRG4 immunohistochemistry analysis. Lacrimal glands were also harvested and homogenized as described previously. Briefly, a small incision was made in the epidermis at the base of the ear with the mouse laid mouse was laid lateral side up (Finley et al., 2014; Redfern et al., 2013). The orbital gland was exposed, using small dissection scissors and forceps, then gently separated from the surrounding tissue. The excised gland was then lysed in 0.2% Triton X-100 (MilliporeSigma) containing a protease inhibitor cocktail (Roche) and stored at −80°C until PRG4 western blotting (2.2). Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Houston and adhered to the standards of the Association for Research in Vision and Ophthalmology Statement for the use of animals in ophthalmic and visual research.
2.6.2. PRG4 Immunohistochemistry:
Ten μm thick frozen mouse eyeball sections were obtained on glass slides, fixed in cold acetone and then permeabilized with 0.1% Triton X-100/PBS. The sections were blocked with 15% goat serum (Abcam, Cambridge, United Kingdom) and then incubated overnight with antiPRG4 Ab LPN primary antibody (Invitrogen) at 4°C. The sections were then incubated for 1h with Alexa Fluor 488 antibody (Abcam) and counterstained with DAPI. Images were captured by using the Delta Vision microscope (GE Healthcare, Chicago, IL). Fluorescence intensity was quantitated from captured images using ImageJ.
2.7. Statistical Analysis
Data are expressed as the mean ± SEM. The effect of inflammatory stimuli on endogenous PRG4 secretion by hTCEpi cells was assessed by one-way ANOVA followed by Dunnett’s post hoc testing. The effect of inflammatory stimuli on cytokine and chemokine secretion by hTCEpi cells was also assessed by one-way ANOVA followed by Dunnett’s post hoc testing. The effect of rhPRG4 treatment on cytokine and chemokine secretion within each inflammatory stimulus condition was assessed by two-tailed t-test. The effect of rhPRG4 on MMP-9 activity was assessed by one-way ANOVA followed by Tukey post-hoc testing. The effect of inflammatory stimuli on MMP-9 expression was assessed by one-way ANOVA followed by Dunnett’s post hoc testing. The effect of rhPRG4 treatment on MMP-9 expression within each inflammatory stimulus condition was assessed by two-tailed t-test. The effect of EDE on PRG4 localization in the cornea was assessed by two-tailed t-test. Finally, the effect of EDE on PRG4 expression in lacrimal gland lysate was assessed by two-tailed t-test.
3. Results
3.1. PRG4 secretion
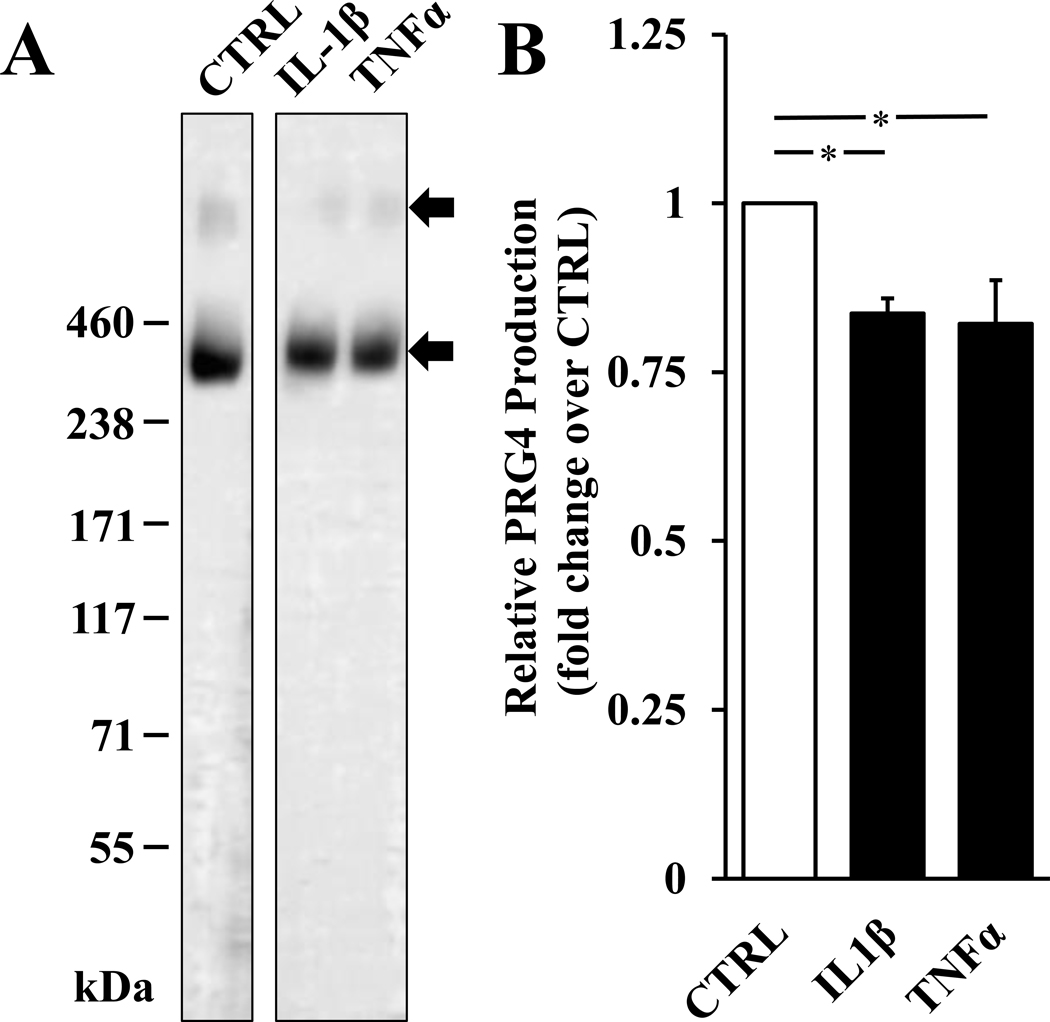
First, to determine endogenous secretion of PRG4 as well as any changes with the addition of inflammatory stimuli, conditioned media from cells treated for 48h was collected and analysed using Western blotting. hTCEpi cells secreted PRG4, and this was reduced by treatment with inflammatory stimuli IL-1β and TNFα. Conditioned media from hTCEpi cells contained PRG4, in both monomeric and dimeric form, as assessed by western blotting (Fig. 1A). The addition of either IL-1β or TNFα reduced levels of PRG4 secretion to 0.84 ± 0.02 (p < 0.05) and 0.82 ± 0.06 (p < 0.05) fold of control, respectively (Fig. 1B).
Figure 1.
Effect of inflammatory stimuli on PRG4 secretion in hTCEpi cells. Western blot analysis of conditioned media samples from control, 100 ng/mL TNFα, or 10 ng/mL IL-1β conditions with anti-PRG4 Ab (A). Densitometry values of relative secretion compared to control (B). * p < 0.05. Data are mean ± SEM (n=4).
3.2. Cytokine & chemokine secretion
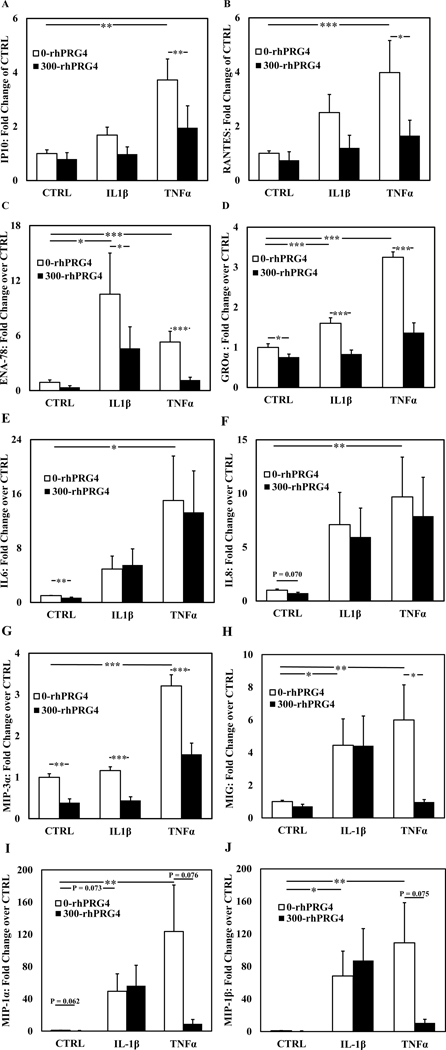
To determine how cell secretion of cytokines and chemokines is influenced by inflammatory stimuli and rhPRG4, cells were treated with IL-1β or TNFα with or without the addition of rhPRG4 for 48h, and conditioned media was collected and analysed by ELISA and by Luminex. IL-1β and TNFα increased inflammatory cytokine and chemokine production by hTCEpi cells (Fig. 2). Specifically, TNFα significantly increased production by hTCEpi cells of all detectable cytokines and chemokines analysed (IP10, RANTES, ENA-78, GROα, IL6, IL8, MIP-3α, MIG, MIP-1α, and MIP-1β). IL-1β significantly increased production of ENA-78, GROα, MIG, and MIP-1β, and trended towards an increase for MIP-1α (p=0.073). Basal expression levels and fold changes relative to CTRL are summarized in Table 1.
Figure 2.
Effect of rhPRG4 on proinflammatory cytokine and chemokine secretion with inflammatory stimuli. Relative fold change in production of IP-10 (A), RANTES (B), ENA-78 (C), GROα (D), IL-6 (E), IL-8 (F), MIP-3α (G), MIG (H), MIP-1α (I), and MIP-1β (J) by differentiated hTCEpi cells treated with 100 ng/mL TNFα or 10 ng/mL IL-1β with or without 300 μg/mL of rhPRG4, measured by ELISA (A-G, n=10) or Luminex (H-J, n=6). * p < 0.05, **p < 0.01, *** p < 0.001. Data are mean ± SEM.
Table 1.
Effect of rhPRG4 on proinflammatory cytokine and chemokine secretion with inflammatory stimuli. The data is presented as fold change from control, with inflammatory stimuli (100 ng/mL TNFα, or 10 ng/mL IL-1β) with or without 300 μg/mL of rhPRG4. For comparing TNFα or IL-1β to CTRL (no rhPRG4)
| Cytokine / Chemokine | Control (pg/ml) | Fold Change in Cytokine/Chemokine Production over Control | ||||
|---|---|---|---|---|---|---|
| Control + rhPRG4 | IL-1β | IL-1β + rhPRG4 | TNFα | TNFα + rhPRG4 | ||
| IP10 | 329 ± 74.3 | 0.797 ± 0.237 | 1.68 ± 0.29 | 0.980 ± 0.267 | 3.72 ± 0.775## | 1.96 ± 0.810** |
| RANTES | 28.2 ± 3.05 | 0.744 ± 0.314 | 2.50 ± 0.67 | 1.201 ± 0.466 | 3.90 ± 1.18### | 1.65 ± 0.566* |
| ENA-78 | 44.8 ± 18.1 | 0.368 ± 0.169 | 10.8 ± 4.43# | 4.611 ± 2.353* | 5.99 ± 1.31### | 1.18 ± 0.303*** |
| GROα | 339 ± 80.9 | 0.559 ± 0.117* | 1.60 ± 0.137### | 0.836 ± 0.098*** | 3.25 ± 0.136### | 1.37 ± 0.24*** |
| IL-6 | 1320 ± 327 | 0.705 ± 0.078** | 4.92 ± 1.92 | 5.52 ± 2.37 | 15.0 ± 6.56# | 13.3 ± 6.11 |
| IL-8 | (2.33 ± 1.14) x 104 | 0.731 ± 0.082[*] | 7.11 ± 3.00 | 5.96 ± 2.69 | 9.69 ± 3.70## | 7.90 ± 3.62 |
| MIP-3α | 57.8 ± 9.05 | 0.388 ± 0.092** | 1.16 ± 0.09 | 0.442 ± 0.088*** | 3.21 ± 0.27### | 1.56 ± 0.270*** |
| MIG | 5.42 ± 1.17 | 0.707 ± 0.131 | 4.45 ± 1.62# | 4.42 ± 1.82 | 6.00 ± 2.15## | 0.974 ± 0.149* |
| MIP-1α | 56.3 ± 25.5 | 0.540 ± 0.206[*] | 49.4 ± 21.7[#] | 56.3 ± 25.3 | 123.6 ± 57.7## | 9.05 ± 5.35[*] |
| MIP-1β | 9.13 ± 4.71 | 0.632 ± 0.211 | 68.3 ± 30.5# | 68.3 ± 30.5 | 109.1 ± 49.3## | 10.6 ± 4.23[*] |
p < 0.05
p < 0.01
p < 0.001, and
0.05 < p < 0.08. For comparing with rhPRG4 to without rhPRG4 within each group (CTRL, IL-1β or TNFα)
p < 0.05
p < 0.01
p < 0.001, and
0.05 < p < 0.08. Data are mean ± SEM (n=6–10).
Treatment with rhPRG4 decreased the production of several inflammatory cytokines/chemokines (Fig 2). In the absence of inflammatory stimuli, exogenous rhPRG4 significantly reduced levels of GROα, IL-6, MIP-3α, and MIP-1β in the conditioned media, and trended towards a reduction for IL8 (p=0.070) and MIP-1α (p=0.062). For samples treated with TNFα, exogenous rhPRG4 significantly reduced levels of IP-10, RANTES, ENA-78, GROα, and MIP-3α, and trended towards a reduction for MIP-1α (p=0.076) and MIP-1β (p=0.075). For samples treated with IL-1β, exogenous rhPRG4 significantly reduced levels of ENA-78, GROα, and MIP-3α. The fold changes with or without rhPRG4 relative to control are summarized in Table 1.
3.3. PRG4 visualization and internalization
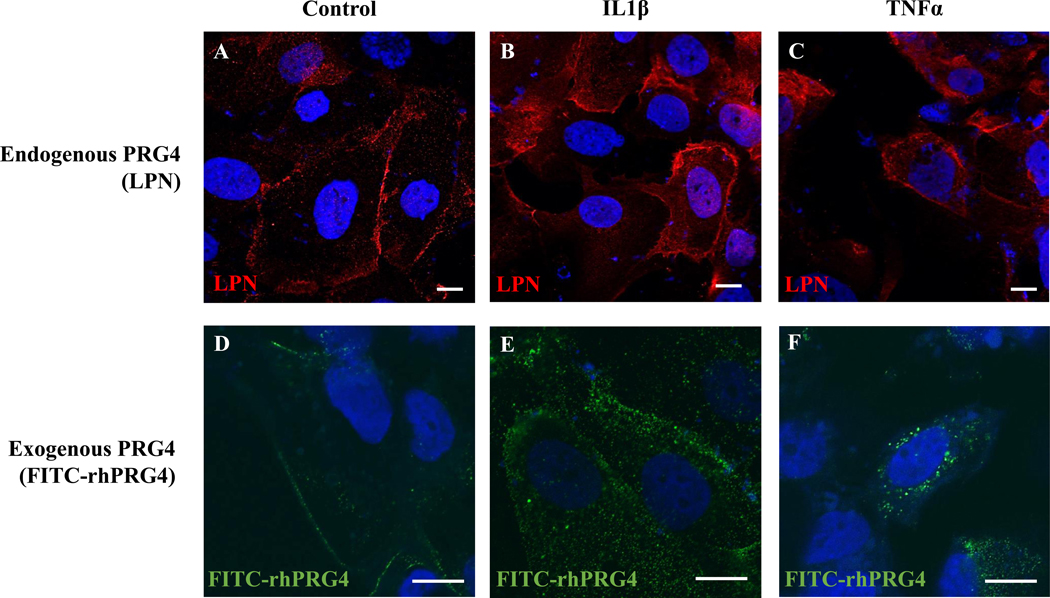
To visualize endogenous PRG4 expression and exogenous rhPRG4 internalization under normal and inflammatory conditions, hTCEpi cells were seeded onto glass coverslips and received IL-1β or TNFα with or without FITC-tagged rhPRG4 for 48h. Endogenous expression and rhPRG4 internalization was assessed by immunofluorescence and confocal microscopy, respectively. hTCEpi cells expressed PRG4 endogenously and were able to internalize exogenous rhPRG4 in response to inflammatory stimuli. Endogenous PRG4 was immunolocalized within and at the cell surface of hTCEpi cells both with and without the addition of inflammatory stimuli (Fig. 3A–C). Confocal imaging demonstrated slices within the cell contained exogenous FITC-tagged rhPRG4 as punctate staining (supplemental data), confirming that it can be internalized by hTCEpi cells in both control and IL-1β stimulation (Fig 3D–F). Interestingly, TNFα stimulation led to internalization of rhPRG4 with apparent loss of surface staining.
Figure 3.
Effect of inflammatory stimuli on endogenous and exogenous PRG4 immunolocalization. Immunolocalization of PRG4 in hTCEpi cells with anti-PRG4 Ab LPN (A), and those treated with 10 ng/ml IL-1β (B) or 100 ng/ml TNFα (C). Confocal microscopy visualization of hTCEpi cells treated with FITC-tagged PRG4 (D), along with IL-1β (E), or TNFα (F). Nuclei are stained with DAPI. Scale bar is 10 μm, 63X magnification for A-C, 63X (x2) magnification for D-F.
3.4. Inhibition of MMP-9 activity by PRG4
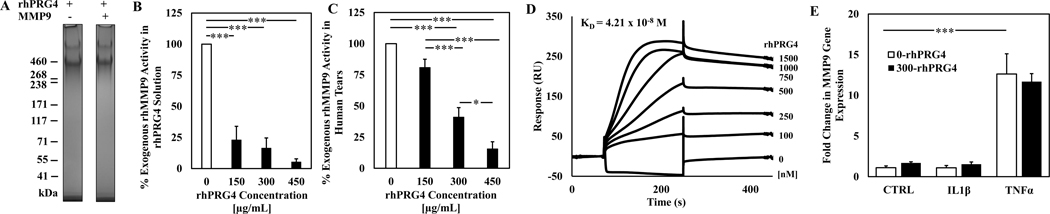
rhPRG4 is not degraded by MMP-9, and in vitro activity of exogenous MMP-9 was inhibited by rhPRG4 both in solution and in the presence of human tear. No evidence of rhPRG4 in vitro degradation by exogenous MMP-9 was observed using gel electrophoresis and protein staining after co-incubation for 1h at 37°C (Fig. 4A). Analysis with an in vitro MMP-9 specific inhibitor assay demonstrated that rhPRG4 reduced in vitro activity of exogenous MMP-9 to 23% ± 11% for 150 μg/mL (p < 0.001), 16.5% ± 8.2% for 300 μg/mL (p < 0.001), and 5.3% ± 2.6% for 450 μg/mL (p < 0.001, Fig. 4B) compared to no rhPRG4 control. rhPRG4 also reduced in vitro activity of exogenous MMP-9 in the presence of human tears, (Fig. 4C) to 80.9% ± 6.6% for 150 μg/mL (p > 0.05), 41.1% ± 7.6% for 300 μg/mL (p < 0.001), and 15.6% ± 5.7% for 450 μg/mL (p < 0.001, Fig. 4B) compared to no rhPRG4 control. Additionally, there was a dose-dependent effect in reducing exogenous MMP-9 activity in the presence of tears (p < 0.001 –p < 0.05).
Figure 4.
Effect of rhPRG4 on in vitro exogenous MMP-9 activity and endogenous gene expression, as well as binding between MMP-9 and rhPRG4. rhPRG4 incubated with or without MMP-9 for 60 minutes at 37C (A). Exogenous MMP-9 activity with 150 μg/mL, 300 μg/mL, and 450 μg/mL of rhPRG4 in solution (B) or in the presence of normal human tears (C). Surface plasmon resonance sensograms of binding between rhMMP-9 and rhPRG4 (D). Endogenous MMP-9 expression in hTCEpi cells with or without inflammatory treatment and rhPRG4. Samples normalized to housekeeping gene 18s and to control samples using ΔΔCt method (E). * p < 0.05, *** p < 0.001. Data are mean ± SEM (n=4) for (A-C) and n=3 for (E).
3.5. Protein-protein interaction between MMP-9 and rhPRG4
The strength of binding interaction between MMP-9 and rhPRG4 was assessed by SPR. MMP-9 was immobilized to a CM5 chip using EDC/NHS chemistry, rhPRG4 was flowed over the chip at various concentrations, and HEPES buffer containing 175 mM NaCl was flowed for the dissociation phase. The resulting sensograms were generated, and association and dissociation rate constants were calculated using a 1:1 Langmuir binding model (Fig. 4D). From the binding model, the Rmax value was calculated to be 293.8 RU, and the ka and kd values were calculated to be 2.23 × 104 1/Ms and 9.37 × 10−4 1/s, respectively, resulting in a KD value of 4.21 × 10−8 M.
3.6. Effect of rhPRG4 on MMP-9 gene expression
To assess the effect of rhPRG4 on MMP-9 gene expression in hTCEpi cells, quantitative RT-PCR was employed on RNA samples isolated from hTCEpi cells treated with IL1β or TNFα with or without rhPRG4. There was no significant increase in the samples treated with IL-1β compared to CTRL, while the addition of TNFα did significantly increased MMP-9 expression (12.6-fold, p < 0.001). This increase was not affected by the addition of rhPRG4, nor did rhPRG4 have an effect on MMP-9 expression in CTRL or IL-1β treated cells (Fig. 4E).
3.7. Experimental dry eye model
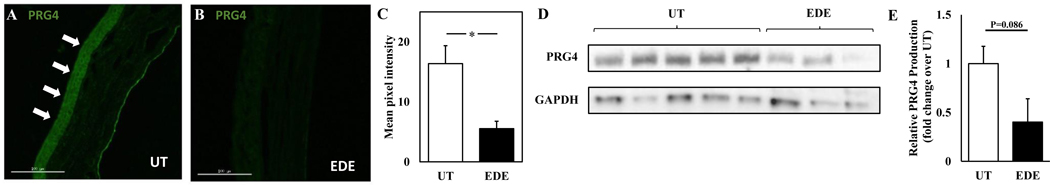
To evaluate changes in PRG4 expression in an EDE model, mouse corneas from untreated and EDE mice were evaluated by IHC and lacrimal gland lysate samples were assayed by Western blotting. PRG4 expression was significantly reduced in the cornea and tended to reduction in the lacrimal gland in EDE. PRG4 immunolocalization was clear in the cornea epithelium of untreated mice (Fig. 5A) and was reduced in the corneas of mice with EDE (Fig. 5B); there was a 2.96-fold reduction in the mean pixel intensity of PRG4 immunostaining between corneas of untreated mice and mice with EDE (Fig. 5C, p < 0.05). Lacrimal gland lysate from mice with EDE had reduced PRG4 expression of 0.40 ± 0.24-fold compared to lysate from untreated mice; however, this reduction was not statistically significant (Fig. 5D–E, p = 0.086).
Figure 5.
Effect of experimental dry eye (EDE) on PRG4 expression in the cornea and the lacrimal gland in vivo. PRG4 in mouse cornea immunolocalized with anti-PRG4 Ab LPN from untreated mice (A) and mice with EDE (B), quantified using ImageJ, * p < 0.05, n = 4 (C). Lacrimal gland lysate analyzed by western blot using anti-PRG4 Ab LPN as primary antibody (D), densitometry of relative fold change in PRG4 (E). Data are mean ± SEM (n = 3–5).
4. Discussion
The in vivo results of this study demonstrated for the first time that ocular surface PRG4 can be diminished in an experimental model of DED. In this model, which results in ocular surface damage, reduced tear production, and elevated protein levels of MMP-9 in corneal cell lysate (Reins et al., 2018), PRG4 immunolocalization was reduced at the corneal epithelium. While unchallenged PRG4-deficient mice were previously shown to have increased ocular surface damage (Schmidt et al., 2013), suggesting PRG4 deficiency may play a role in promoting corneal damage, the current data establish a relationship between DED and diminished PRG4 expression at the ocular surface. While the apparent decrease in PRG4 protein in lacrimal lysate in the experimental model of DED approached but did not reach statistical significance, these findings do align with previously reported mRNA expression in the lacrimal gland (Schmidt et al., 2013). PRG4 has multiple properties relevant to the ocular surface, including those mechanical (e.g. lubrication (Korogiannaki et al., 2021, 2018; Samsom et al., 2018b, 2018a, 2015, 2014; Schmidt et al., 2013), maintaining tear film stability (Rabiah et al., 2020)) as well as biological (e.g. anti-inflammatory properties observed here) in nature. Given these properties along with the multiple factors that can drive and/or initiate DED, the reduction of ocular surface PRG4 in DED may be a key contributing mechanism in the progression of DED, leading directly to increased evaporation, increased friction, and a pro-inflammatory environment. The epithelial glycocalyx, of which PRG4 is likely an integral part, is similarly involved in the retention of water at the ocular surface as the lipid layer. The data herein support the notion that the loss of PRG4 expression in the corneal epithelia is at least a co-equal aspect of the positive feedback loop of disease pathogenesis in DED.
In addition, the in vitro results presented here extend previous studies examining PRG4 on the ocular surface as well as the regulation of PRG4 expression by other cell types. While PRG4 has previously been immunolocalized in human corneal epithelium (Schmidt et al., 2013), the results here demonstrate that PRG4 is expressed and secreted by hTCEpi cells and is downregulated by IL-1β and TNFα. This is also consistent with previous studies examining PRG4 secretion in synoviocytes (Alquraini et al., 2017), in chondrocytes with cartilage explants (Schmidt et al., 2008), and in synovial fluid after unilateral anterior cruciate ligament injury, where an increase in the concentration of inflammatory cytokines, including IL-1β and TNFα was associated with a reduction of PRG4 (Elsaid et al., 2008). Given the parallels with other organ systems, these data suggest the addition of exogenous rhPRG4 may contribute to restoring homeostatic biological conditions (Abubacker et al., 2016; Al-Sharif et al., 2015; Alquraini et al., 2017; Iqbal et al., 2016; Ludwig et al., 2012).
A key finding of this study is that exogenous rhPRG4 can inhibit hTCEpi production of several chemokines stimulated by proinflammatory signals. Specifically, rhPRG4 treatment inhibited the RANTES, IP-10, ENA-78, GROα, MIP-3α, and MIG response, and tended toward reduction for MIP-1α and MIP-1β, to IL-1β or TNFα (Fig. 2). RANTES is known to play a key role in promoting lymphocyte migration to the corneal epithelium (Stern et al., 2004) and is upregulated in the tears of patients with DED (Pinto-fraga et al., 2018). IP-10 is a chemoattractant for Th1 lymphocytes and monocytes (Taub et al., 1993), and it is also upregulated in the tears of patients with DED (Pinto-fraga et al., 2018). ENA-78, which is involved in neutrophil activation and chemotaxis (Walz et al., 1991), is another chemokine found to be upregulated in the tears of patients with DED (Na et al., 2012), and it has not been extensively studied in the corneal epithelium. GROα is a neutrophil chemoattractant and has not been studied extensively in human patients with DED, but it has been shown to be upregulated in inflamed human corneas (Spandau et al., 2003) and in induced DED in mice (Reins et al., 2018). MIG, MIP-1α, MIP-1β, and MIP-3α levels were elevated with the addition of dry eye-associated inflammatory stimuli, which agrees with previous work on corneal epithelial cells and on human patients (Choi et al., 2012; Shirane et al., 2004; Yoon et al., 2010). While PRG4 purified from synovial fluid was previously shown to have anti-inflammatory effects in an induced-DED blinking eye model, where it reduced secretion of IL-1β, TNFα, and IL-8 (Seo et al., 2019), the present study demonstrates rhPRG4 has an in vitro anti-inflammatory effect on chemokines and long range signals to a variety of immune cell classes. These findings were observed in the hTCEpi cell line, which is commonly used (Alfuraih et al., 2020; Ding et al., 2015; McClintock and Ceresa, 2010; Reins et al., 2016; Roy et al., 2014; Shetty et al., 2015) and when compared to primary human corneal cultures give consistent results (Reins et al., 2018; Robertson et al., 2005; Roy et al., 2014). We have previously shown PRG4 expression in human corneal epithelium (Schmidt et al., 2013), as well as the clinical efficacy of rhPRG4 in improving signs and symptoms of DED (Lambiase et al., 2017); future work could examine/confirm in vitro effects of rhPRG4 observed here on primary human corneal epithelial cells in vitro.
In general, rhPRG4 inhibited the stimulatory effect of TNFα and at times that of IL-1β. In other studies, fibroblast-like synoviocytes have shown rhPRG4 can inhibit inflammatory output simulated by both TNFα and IL-1β together (Al-Sharif et al., 2015; Alquraini et al., 2017). While both these cytokines can activate NF-κB and MAPK (ERK, JNK, p38) signaling, there are different molecular mediators in their pathways. These, in addition to further time courses and TNFα/IL-1β dose dependent studies, are of interest but beyond the scope of the current investigation. Irrespective, the anti-inflammatory effect of PRG4 may stem from its ability to normalize the microenvironment through simultaneous friction reduction, downregulation of proinflammatory cytokines, and reduction of the recruitment of new immune cells. This pleiotropic nature of the molecule is unique, and strongly motivates its use for, and study in, DED, where multiple coincident etiologies are commonly observed.
Another key finding of this work is the visualization of exogenous FITC-labelled rhPRG4 being internalized by hTCEpi cells (Fig. 3 and supplemental data). Both in basal conditions and with the addition of inflammatory stimuli, exogenous rhPRG4 was internalized and localized inside the cell, as visualized through confocal microscopy (Fig. 3), suggesting coupling of the molecule to intracellular signaling pathways, as seen in macrophages previously (Qadri et al., 2018) and not simply acting as a passive mechanical mucin-like coating or barrier on the cells that might inhibit the stimulatory effect of the inflammatory inputs. Given that rhPRG4 can modulate NF-κB activity in other systems (Al-Sharif et al., 2015; Seo et al., 2019), future studies could also examine the effect of rhPRG4 on NF-κB signaling, or other signaling pathways, as a potential mechanism by which rhPRG4 exerts its anti-inflammatory properties on corneal epithelial cells. Interestingly, and qualitatively, there appeared to be an increase in internalization with the addition of TNFα. Future work examining colocalization of rhPRG4 with endosomal markers will provide important clues to the fate of rhPRG4 post internalization. It is also worth noting rhPRG4 tagged with FITC also retained its ability to reduce levels of cytokine and chemokine secretion from hTCEpi cells (data not shown). This suggests that rhPRG4’s biological activity, at least in the context studied here, was not negatively affected by fluorescent tagging and therefore FITC-tagged rhPRG4 could be a useful reagent in the future to explore PRG4’s internalization into cells as well as its biological mechanism of action. rhPRG4 has been previously shown to be internalized in macrophages mediated, in part, through interactions with CD44 (Qadri et al., 2018). However, the precise processes by which PRG4 is internalized in hTCEpi cells (i.e., through receptor interactions, endocytosis, or a combination of the two) and the role it plays in rhPRG4’s anti-inflammatory mechanism of action remain under study.
Initial in vitro analysis here demonstrated that rhPRG4 is not a proteolytic substrate of MMP-9 and that rhPRG4 inhibits in vitro activity of exogenous MMP-9 (Fig. 4). While PRG4 is a substrate of other enzymes, including cathepsin S (Regmi et al., 2017), cathepsin G (Huang et al., 2020), MMP-1 (Jones et al., 2003), and MMP-7 (Jones et al., 2003), it is not subject to degradation by MMP-9. Therefore, the observed inhibition of in vitro activity of exogenous MMP-9 is not due to rhPRG4 functioning as an alternative substrate. Other studies have demonstrated that exogenous purified PRG4 can decrease MMP-9 levels in tears from a DED cell model (Seo et al., 2019) and MMP-9 gene expression stimulated by IL-1β in synoviocytes (Alquraini et al., 2017). Under the conditions and time point studied here, rhPRG4 did not have any inhibitory effect on MMP-9 expression in hTCEpi cells, whether under basal or stimulated conditions. However, the inhibition of MMP-9 activity by rhPRG4 observed here is an important and potentially significant discovery. An MMP inhibitor, NNGH, was used as a positive control for testing inhibition, and 300 μg/mL of rhPRG4 displayed the same inhibitory ability as 150 μg/mL of NNGH (data not shown). Additionally, bovine serum albumin was tested for inhibition at 775 μg/mL, and there was no significant reduction in MMP9 activity, supporting the specificity of inhibition observed with rhPRG4 (data not shown). This inhibition effect was also observed in solution and in the presence of human tears in a dose dependent manner. While one cannot make any claims as to potential intracellular interaction/binding of PRG4 and MMP-9, the SPR results clearly demonstrate biophysical evidence that rhPRG4 binds to MMP-9, with a relatively high affinity as demonstrated by a dissociation constant of 42 nM, which could indicate a potential mechanism of MMP-9 activity inhibition. Indeed, rhPRG4 may inhibit MMP-9 activity through binding at the hemopexin-like (PEX) domain present on both rhPRG4 and MMP-9 (Piccard et al., 2007; Roeb et al., 2002). The MMP-9 PEX domain binds to CD44, gelatin and α4β1 integrins, and its inhibition may prevent homodimerization and reduce additional inflammatory signaling, including through MMP-9 damage-associated molecular pattern related pathways, such as MyD88 and TLR4, which are strongly associated with DED severity (Dufour et al., 2010; Redfern et al., 2015; Reins et al., 2018). Future studies could examine if ocular surface MMP-9 levels and/or activity are altered (i.e. diminished) in PRG4 deficient mice (Schmidt et al., 2013), given that MMP-9 expression and activity have been shown to be elevated in Prg4 −/− superficial zone chondrocytes (Maenohara et al., 2020), as well as any potential intracellular interaction between the two. Given the role MMP-9 plays in the pathogenesis of DED, and the known correlation between MMP-9 activity and ocular surface signs (Chotikavanich et al., 2013), these data may help elucidate some of the clinical efficacy of PRG4 in treating DED.
The “vicious cycle of inflammation” has been proposed as a core driver of DED, which consists of a 4-part process including initiation (proinflammatory cytokine release), amplification (T-cell differentiation and proliferation), recruitment (T cell) and damage/self-perpetuation with the involvement of the innate and adaptive immune system (Periman et al., 2020). The ocular surface immune response occurs at the corneal surface, in ocular tissue and reginal lymph nodes, and involves various T cells. While in vitro models are inherently limited as to the insight into disease pathogenesis they provide, the commonly studied hCTEpi cells and DED relevant stimuli/output was not meant to elucidate the role of PRG4 in all of the multitude of inflammatory mechanisms that exist in various forms of DED. Indeed, it is largely limited to the innate responses of the epithelial cells with the motivation to inquire as to whether rhPRG4 can blunt the inflammatory response. Future work that explores the effect of rhPRG4 on T cell immunity and if/how rhPRG4 can stop or slow the vicious cycle of DED would further elucidate rhPRG4’s function on the various aspects of DED.
5. Conclusion
In conclusion, these results underpin the understanding of PRG4 expression and anti-inflammatory properties of rhPRG4 within the context of corneal epithelium in DED. The demonstrated clinical utility of rhPRG4 in improving signs and symptoms in a small single site clinical trial, combined with ongoing research studying rhPRG4’s anti-inflammatory properties in other systems (Al-Sharif et al., 2015; Alquraini et al., 2017, 2015; Das et al., 2019; Iqbal et al., 2016; Qadri et al., 2018; Richendrfer et al., 2020; Sarkar et al., 2019), as well as established mechanical lubricating properties (Korogiannaki et al., 2021, 2018; Samsom et al., 2018b, 2018a, 2015, 2014; Schmidt et al., 2013), strongly suggests a multimodal therapeutic mechanism of action. While primary human corneal epithelial cells were not examined in vitro here, nor were in vivo mechanisms explored in the animal model, these findings collectively provide the foundation and motivation for continued basic research in further mechanistic understanding PRG4’s properties on the ocular surface and associated glands, both in vitro and in vivo, as well as expanding clinical evaluation of its ability as a multifunctional therapeutic agent to effectively provide relief to those who suffer from DED.
Supplementary Material
Supplemental Figure: Confocal microscopy z stacks of hTCEpi cells treated with FITC-tagged PRG4 and no stimulus (A), IL-1β (B) or TNFα (C). Nuclei are stained with DAPI. Scale bar is 10 μm, 126X magnification.
Highlights.
hTCEpi cells express PRG4, which is decreased by IL-1β and TNFα
rhPRG4 reduces IL-1β and TNFα stimulated hTCEpi cell cytokine/chemokine secretion
Exogenous rhPRG4 is internalized by hTCEpi cells
rhPRG4 is not degraded by and binds to MMP-9, and can inhibit in vitro exogenous MMP-9 activity
Ocular surface PRG4 expression is decreased in experimental dry eye disease
Acknowledgments
We also gratefully acknowledge Dr. David Sullivan and Wendy Kam (Schepens Eye Research Institute, Boston MA) for aid with the culture of the hTCEpi as well as Dr. James Jester (University of California Irvine) for providing the cells. Finally, we thank Dr. Benjamin D. Sullivan (TearLab Corp) for discussions on and critique of the manuscript.
Financial Support
This work was supported by the Department of Biomedical Engineering at UConn Health (TAS), National Institute of Health [grant numbers R01HL127449 (LHS and MG), R01AR067748 (GDJ), EY023628 (RLR), EY07551 (Laura Frishman)].
Footnotes
Conflict of Interest
TAS and GDJ have authored patents on rhPRG4 and hold equity in Lubris LLC, MA, USA. TAS is also a paid consultant for Lubris LLC, MA, USA. RJK also has authored patents on rhPRG4. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abubacker S, Dorosz SG, Ponjevic D, Jay GD, Matyas JR, Schmidt TA, 2016. Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Ann. Biomed. Eng 44, 1128–1137. 10.1007/s10439015-1390-8 [DOI] [PubMed] [Google Scholar]
- Al-Sharif A, Jamal M, Zhang LX, Larson K, Schmidt TA, Jay GD, Elsaid KA, 2015. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis Rheumatol. (Hoboken, N.J.) 67, 1503–1513. 10.1002/art.39087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfuraih S, Barbarino A, Ross C, Shamloo K, Jhanji V, Zhang M, Sharma A, 2020. Effect of high glucose on ocular surface epithelial cell barrier and tight junction proteins. Investig. Ophthalmol. Vis. Sci 61, 1–10. 10.1167/iovs.61.11.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquraini A, Garguilo S, Souza GD, Zhang LX, Schmidt TA, Jay GD, Elsaid KA, 2015. The interaction of lubricin / proteoglycan 4 ( PRG4 ) with toll-like receptors 2 and 4 : an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res. Ther 1–12. 10.1186/s13075-015-0877-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA, 2017. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res. Ther 19, 1–15. 10.1186/s13075-017-1301-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA, 2017. TFOS DEWS II pathophysiology report. Ocul. Surf 15, 438–510. 10.1016/j.jtos.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R, 2009. Autoimmunity in Dry Eye Is Due to Resistance of Th17 to Treg Suppression. J. Immunol 182, 1247–1252. 10.4049/jimmunol.182.3.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Li Z, Oh HJ, Im SK, Lee SH, Park SH, You IC, Yoon KC, 2012. Expression of CCR5 and its ligands CCL3, −4, and −5 in the tear film and ocular surface of patients with dry eye disease. Curr. Eye Res 37, 12–17. 10.3109/02713683.2011.622852 [DOI] [PubMed] [Google Scholar]
- Chotikavanich S, Paiva C.S. De Li DQ, Chen JJ, Bian F, 2013. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Investig. Ophthalmol. Vis. Sci 50, 3203–3209. 10.1167/iovs.082476.Production [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Schmidt TA, Krawetz RJ, Dufour A, 2019. Proteoglycan 4: From Mere Lubricant to Regulator of Tissue Homeostasis and Inflammation: Does proteoglycan 4 have the ability to buffer the inflammatory response? BioEssays 41, 1–9. 10.1002/bies.201800166 [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC, 2009. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2, 243–253. 10.1038/mi.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wirostko B, Sullivan DA, 2015. Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea 34, 686–692. 10.1097/ICO.0000000000000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, Sadrai Z, Dana R, 2013. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Investig. Ophthalmol. Vis. Sci 54, 4081–4091. 10.1167/iovs.1211216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A, Zucker S, Sampson NS, Kuscu C, Cao J, 2010. Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. J. Biol. Chem 285, 35944–35956. 10.1074/jbc.M109.091769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R, 2010. Regulation of T-cell chemotaxis by programmed Death-Ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Investig. Ophthalmol. Vis. Sci 51, 3418–3423. 10.1167/iovs.09-3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD, 2008. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 58, 1707–1715. 10.1002/art.23495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enríquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, Herreras JM, Calonge M, 2010. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis 16, 862–873. [PMC free article] [PubMed] [Google Scholar]
- Finley JK, Farmer D, Emmerson E, Pacheco NC, Knox SM, 2014. Manipulating the murine lacrimal gland. J. Vis. Exp 1–6. 10.3791/51970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Thangada S, Dasgupta O, Khanna KM, Yamase HT, Kashgarian M, Hla T, Shapiro LH, Ferrer FA, 2018. Cell-intrinsic sphingosine kinase 2 promotes macrophage polarization and renal inflammation in response to unilateral ureteral obstruction. PLoS One 13, 1–23. 10.1371/journal.pone.0194053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM, 2019. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf 17, 412–423. 10.1016/j.jtos.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Huang S, Thomsson KA, Jin C, Alweddi S, Struglics A, Rolfson O, Björkman LI, Kalamajski S, Schmidt TA, Jay GD, Krawetz R, Karlsson NG, Eisler T, 2020. Cathepsin g Degrades Both Glycosylated and Unglycosylated Regions of Lubricin, a Synovial Mucin. Sci. Rep 10, 1–12. 10.1038/s41598-020-61161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal SM, Leonard C, Regmi SC, De Rantere D, Tailor P, Ren G, Ishida H, Hsu CY, Abubacker S, Pang DSJ, Salo PT, Vogel HJ, Hart DA, Waterhouse CC, Jay GD, Schmidt TA, Krawetz RJ, 2016. Lubricin/Proteoglycan 4 binds to and regulates the activity of Toll-Like Receptors in Vitro. Sci. Rep 6, 1–12. 10.1038/srep18910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Hughes C, Wainright S, Flannery C, Little C, Caterson B, Sd W, Cr F, Cb L, Caterson B, 2003. Degradation of Prg4 / Szp By Matrix Proteases. 49th Annu. Meet. Orthop. Res. Soc. Pap. # 0133 133. [Google Scholar]
- Kaufman HE, 2013. The practical detection of MMP-9 diagnoses ocular surface disease and may help prevent its complications. Cornea 32, 211–216. 10.1097/ICO.0b013e3182541e9a [DOI] [PubMed] [Google Scholar]
- Korogiannaki M, Samsom M, Matheson A, Soliman K, Schmidt TA, Sheardown H, 2021. Investigating the Synergistic Interactions of Surface Immobilized and Free Natural Ocular Lubricants for Contact Lens Applications: A Comparative Study between Hyaluronic Acid and Proteoglycan 4 (Lubricin). Langmuir 37, 1062–1072. 10.1021/acs.langmuir.0c02796 [DOI] [PubMed] [Google Scholar]
- Korogiannaki M, Samsom M, Schmidt TA, Sheardown H, 2018. Surface-Functionalized Model Contact Lenses with a Bioinspired Proteoglycan 4 (PRG4)-Grafted Layer. ACS Appl. Mater. Interfaces 10, 30125–30136. 10.1021/acsami.8b09755 [DOI] [PubMed] [Google Scholar]
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC, 2009. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am. J. Ophthalmol 147, 198–205.e1. 10.1016/j.ajo.2008.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Sullivan BD, Schmidt TA, Sullivan DA, Jay GD, Truitt ER, Bruscolini A, Sacchetti M, Mantelli F, 2017. A Two-Week, Randomized, Double-masked Study to Evaluate Safety and Efficacy of Lubricin (150 μg/mL) Eye Drops Versus Sodium Hyaluronate (HA) 0.18% Eye Drops (Vismed®) in Patients with Moderate Dry Eye Disease. Ocul. Surf 15, 77–87. 10.1016/j.jtos.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Lema C, Reins RY, Redfern RL, 2018. High-mobility group box 1 in dry eye inflammation. Investig. Ophthalmol. Vis. Sci 59, 1741–1750. 10.1167/iovs.17-23363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA, 2012. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum. 64, 3963–3971. 10.1002/art.34674 [DOI] [PubMed] [Google Scholar]
- Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC, 2004. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci 45, 4293–4301. 10.1167/iovs.03-1145 [DOI] [PubMed] [Google Scholar]
- Maenohara Y, Chijimatsu R, Tachibana N, Uehara K, Xuan F, Mori D, Murahashi Y, Nakamoto H, Oichi T, Chang SH, Matsumoto T, Omata Y, Yano F, Tanaka S, Saito T, 2020. Lubricin Contributes to Homeostasis of Articular Cartilage by Modulating Differentiation of Superficial Zone Cells. J. Bone Miner. Res 00, 1–11. 10.1002/jbmr.4226 [DOI] [PubMed] [Google Scholar]
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA, 2009. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 28, 1023–1027. 10.1097/ICO.0b013e3181a16578 [DOI] [PubMed] [Google Scholar]
- McClintock JL, Ceresa BP, 2010. Transforming growth factor-α enhances corneal epithelial cell migration by promoting EGFR recycling. Investig. Ophthalmol. Vis. Sci 51, 3455–3461. 10.1167/iovs.09-4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljanović B, Dana R, Sullivan DA, Schaumberg DA, 2007. Impact of Dry Eye Syndrome on Vision-Related Quality of Life. Am. J. Ophthalmol 143. 10.1016/j.ajo.2006.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na K, Mok J, Kim JY, Rho CR, Joo C, 2012. Soluble Receptors and Clinical Severity of Dry Eye Disease 53, 1–4. 10.1167/iovs.11-9417 [DOI] [PubMed] [Google Scholar]
- Periman LM, Perez VL, Saban DR, Lin MC, Neri P, 2020. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther 36, 137–146. 10.1089/jop.2019.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D, 1999. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res 19, 201–211. 10.1076/ceyr.19.3.201.5309 [DOI] [PubMed] [Google Scholar]
- Piccard H, Van den Steen PE, Opdenakker G, 2007. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J. Leukoc. Biol 81, 870–892. 10.1189/jlb.1006629 [DOI] [PubMed] [Google Scholar]
- Pinto-fraga J, Enríquez-de-salamanca A, Calonge M, González-garcía MJ, López-miguel A, López-de A, García-vázquez C, Calder V, Stern ME, Fernández I, 2018. The Ocular Surface Severity, therapeutic, and activity tear biomarkers in dry eye disease : An analysis from a phase III clinical trial ☆. Ocul. Surf 16, 368–376. 10.1016/j.jtos.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Qadri M, Jay GD, Zhang LX, Wong W, Reginato AM, Sun C, Schmidt TA, Elsaid KA, 2018. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages. Arthritis Res. Ther 20, 1–16. 10.1186/s13075-018-1693-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiah NI, Sato Y, Kannan A, Kress W, Straube F, Fuller GG, 2020. Understanding the adsorption and potential tear film stability properties of recombinant human lubricin and bovine submaxillary mucins in an in vitro tear film model. Colloids Surfaces B Biointerfaces 195, 111257. 10.1016/j.colsurfb.2020.111257 [DOI] [PubMed] [Google Scholar]
- Redfern RL, Barabino S, Baxter J, Lema C, McDermott AM, 2015. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp. Eye Res 134, 80–89. 10.1016/j.exer.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RL, Patel N, Hanlon S, Farley W, Gondo M, Pflugfelder SC, McDermott AM, 2013. Toll-like receptor expression and activation in mice with experimental dry eye. Investig. Ophthalmol. Vis. Sci 54, 1554–1563. 10.1167/iovs.12-10739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi SC, Samsom ML, Heynen ML, Jay GD, Sullivan BD, Srinivasan S, Caffery B, Jones L, Schmidt TA, 2017. Degradation of proteoglycan 4/lubricin by cathepsin S: Potential mechanism for diminished ocular surface lubrication in Sjögren’s syndrome. Exp. Eye Res 161, 1–9. 10.1016/j.exer.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Reins RY, Lema C, Courson J, Kunnen CME, Redfern RL, 2018. MyD88 deficiency protects against dry eye–induced damage. Investig. Ophthalmol. Vis. Sci 59, 2967–2976. 10.1167/iovs.17-23397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reins RY, Mesmar F, Williams C, McDermott AM, 2016. Vitamin D induces global gene transcription in human corneal epithelial cells: Implications for corneal inflammation. Investig. Ophthalmol. Vis. Sci 57, 2689–2698. 10.1167/iovs.16-19237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer HA, Levy MM, Elsaid KA, Schmidt TA, Zhang L, Cabezas R, Jay GD, 2020. Recombinant Human Proteoglycan-4 Mediates Interleukin-6 Response in Both Human and Mouse Endothelial Cells Induced Into a Sepsis Phenotype. Crit. Care Explor 2, e0126. 10.1097/cce.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV, 2005. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci 46, 470–478. 10.1167/iovs.04-0528 [DOI] [PubMed] [Google Scholar]
- Roda M, Corazza I, Reggiani MLB, Pellegrini M, Taroni L, Giannaccare G, Versura P, 2020. Dry eye disease and tear cytokine levels— a meta-analysis. Int. J. Mol. Sci 21, 1–17. 10.3390/ijms21093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeb E, Schleinkofer K, Kernebeck T, Pötsch S, Jansen B, Behrmann I, Matern S, Grötzinger J, 2002. The matrix metalloproteinase 9 (MMP-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J. Biol. Chem 277, 50326–50332. 10.1074/jbc.M207446200 [DOI] [PubMed] [Google Scholar]
- Roy S, Karmakar M, Pearlman E, 2014. CD14 mediates Toll-like Receptor 4 (TLR4) endocytosis and Spleen Tyrosine Kinase (Syk) and Interferon Regulatory Transcription Factor 3 (IRF3) activation in epithelial cells and impairs neutrophil infiltration and pseudomonas aeruginosa killing in vivo. J. Biol. Chem 289, 1174–1182. 10.1074/jbc.M113.523167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsom M, Chan A, Iwabuchi Y, Subbaraman L, Jones L, Schmidt TA, 2015. In vitro friction testing of contact lenses and human ocular tissues: Effect of proteoglycan 4 (PRG4). Tribol. Int 89, 27–33. 10.1016/j.triboint.2014.11.022 [DOI] [Google Scholar]
- Samsom M, Iwabuchi Y, Sheardown H, Schmidt TA, 2018a. Proteoglycan 4 and hyaluronan as boundary lubricants for model contact lens hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater 106, 1329–1338. 10.1002/jbm.b.33895 [DOI] [PubMed] [Google Scholar]
- Samsom M, Korogiannaki M, Subbaraman LN, Sheardown H, Schmidt TA, 2018b. Hyaluronan incorporation into model contact lens hydrogels as a built-in lubricant: Effect of hydrogel composition and proteoglycan 4 as a lubricant in solution. J. Biomed. Mater. Res. Part B Appl. Biomater 106, 1818–1826. 10.1002/jbm.b.33989 [DOI] [PubMed] [Google Scholar]
- Samsom ML, Morrison S, Masala N, Sullivan BD, Sullivan DA, Sheardown H, Schmidt TA, 2014. Characterization of full-length recombinant human Proteoglycan 4 as an ocular surface boundary lubricant. Exp. Eye Res 127, 14–19. 10.1016/j.exer.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Sarkar A, Chanda A, Regmi SC, Karve K, Deng L, Jay GD, Jirik FR, Schmidt TA, Bonni S, 2019. Recombinant human PRG4 (rhPRG4) suppresses breast cancer cell invasion by inhibiting TGFβ-Hyaluronan-CD44 signalling pathway. PLoS One 14, 1–29. 10.1371/journal.pone.0219697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL, 2008. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1α, IGF-I, and TGF-β1. Osteoarthr. Cartil 16, 90–97. 10.1016/j.joca.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Schmidt TA, Sullivan DA, Knop E, Richards SM, Knop N, Liu S, Sahin A, Darabad RR, Morrison S, Kam WR, Sullivan BD, 2013. Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 131, 766–776. 10.1001/jamaophthalmol.2013.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Byun WY, Alisafaei F, Georgescu A, Yi Y-S, Massaro-Giordano M, Shenoy VB, Lee V, Bunya VY, Huh D, 2019. Multiscale reverse engineering of the human ocular surface. Nat. Med 25, 1310–1318. 10.1038/s41591-019-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty Rohit, Ghosh Anuprita, Lim RR, Subramani M, Mihir K, Reshma AR, Ranganath A, Nagaraj S, Nuijts RMMA, Beuerman R, Shetty Reshma, Das D., Chaurasia SS, Sinha-Roy A, Ghosh Arkasubhra, 2015. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Investig. Ophthalmol. Vis. Sci 56, 738–750. 10.1167/iovs.14-14831 [DOI] [PubMed] [Google Scholar]
- Shirane J, Nakayama T, Nagakubo D, Izawa D, Hieshima K, Shimomura Y, Yoshie O, 2004. Corneal epithelial cells and stromal keratocytes efficently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Curr. Eye Res 28, 297–306. 10.1076/ceyr.28.5.297.28682 [DOI] [PubMed] [Google Scholar]
- Smith JA, Albenz J, Begley C, Caffery B, Nichols K, Schaumberg D, Schein O, 2007. The epidemiology of dry eye disease: Report of the epidemiology subcommittee of the international Dry Eye WorkShop (2007). Ocul. Surf 5, 93–107. 10.1016/s1542-0124(12)70082-4 [DOI] [PubMed] [Google Scholar]
- Spandau UHM, Toksoy A, Verhaart S, 2003. High Expression of Chemokines Gro-α (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in Inflamed Human Corneas In Vivo. Lab. Sci 826–831. 10.1001/archopht.121.6.825 [DOI] [PubMed] [Google Scholar]
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L, 2017. TFOS DEWS II Epidemiology Report. Ocul. Surf 15, 334–365. 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Steele BL, Alvarez-Veronesi MC, Schmidt TA, 2013. Molecular weight characterization of PRG4 proteins using multi-angle laser light scattering (MALLS). Osteoarthr. Cartil 21, 498–504. 10.1016/j.joca.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC, 2004. The role of the lacrimal functional unit in the pathophysiology of dry eye 78, 409–416. 10.1016/j.exer.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Stern ME, Pflugfelder SC, 2004. Inflammation in dry eye. Ocul. Surf 2, 124–130. 10.1016/S1542-0124(12)70148-9 [DOI] [PubMed] [Google Scholar]
- Stevenson W, Chauhan SK, Dana R, 2012. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol 130, 90–100. 10.1001/archophthalmol.2011.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ, 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med 177, 1809–1814. 10.1084/jem.177.6.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz BA, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM, 1991. Structure and Neutrophil-activating Properties of a Novel Inflammatory Peptide (ENA-78) with Homology to Interleukin 8. J. Exp. Med 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DE, Zhao Y, Ogundele A, Fulcher N, Acs A, Moore-Schiltz L, Karpecki PM, 2019. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin. Ophthalmol 13, 2285–2292. 10.2147/OPTH.S226168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC, 2007. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: Effects of desiccating stress. Investig. Ophthalmol. Vis. Sci 48, 2561–2569. 10.1167/iovs.07-0002 [DOI] [PubMed] [Google Scholar]
- Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC, 2010. Expression of CXCL9, −10, −11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Investig. Ophthalmol. Vis. Sci 51, 643–650. 10.1167/iovs.09-3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Confocal microscopy z stacks of hTCEpi cells treated with FITC-tagged PRG4 and no stimulus (A), IL-1β (B) or TNFα (C). Nuclei are stained with DAPI. Scale bar is 10 μm, 126X magnification.