Abstract
A plethora of both acute and chronic conditions, including traumatic, degenerative, malignant, or congenital disorders, commonly induce bone disorders often associated with severe persisting pain and limited mobility. Over 1 million surgical procedures involving bone excision, bone grafting, and fracture repair are performed each year in the U.S. alone, resulting in immense levels of public health challenges and corresponding financial burdens. Unfortunately, the innate self‐healing capacity of bone is often inadequate for larger defects over a critical size. Moreover, as direct transplantation of committed osteoblasts is hindered by deficient cell availability, limited cell spreading, and poor survivability, an urgent need for novel cell sources for bone regeneration is concurrent. Thanks to the development in stem cell biology and cell reprogramming technology, many multipotent and pluripotent cells that manifest promising osteogenic potential are considered the regenerative remedy for bone defects. Considering these cells' investigation is still in its relative infancy, each of them offers their own particular challenges that must be conquered before the large‐scale clinical application.
Keywords: bone regeneration, multipotent stem cells, pluripotent stem cells
A plethora of both acute and chronic conditions, including traumatic, degenerative, malignant, and congenital varieties, often play key roles in reducing the quality of life for many people. This is particularly true in the case of critical‐size defects where the innate self‐healing capacity of bone is inadequate for a reunion. To date, a diversity of novel multipotent/pluripotent cell sources are regarded as regenerative medicine, particularly for bone regeneration, in virtue of continued worldwide collaboration. Although their potential is irrefutable, each of the cell sources mentioned has its own drawbacks, which must be entirely understood and overcome before they are released for human clinical application.

1. INTRODUCTION
The typical human skeleton is composed of 206 bones; however, individuals may have varying numbers of bones present, including various small unnamed bones that generally form in high‐friction areas. As the hardest and most rigid structures (aside from the teeth) in the body, bone provides mechanical support, mobility, and load‐bearing capacity. In addition, bones play a critical role in the production of blood cells, the storage of minerals, and the regulation of the endocrine system.
Bone injuries comprise 25‐30% of all musculoskeletal pathologies. 1 Fortunately, bone is one of the few tissues with a robust innate self‐healing repair mechanism of spontaneous resorption and reformation, allowing small bone injuries to heal in most cases. 2 However, bone regeneration capacity is not sufficient in regard to reestablishing the skeletal system's integrity and functionality when the damage reaches a critical size, such as those resulting from severe injuries, maxillofacial surgeries of cleft palates, or salvage excision of tumors. A variety of musculoskeletal diseases and congenital conditions also significantly impair bone development and regeneration. In particular, osteoporosis and osteoarthritis are two prevalent diseases within the geriatric population that present major challenges for orthopedic reconstruction. Prevalence of these diseases is shown in the fact that 33‐50% of women and 20‐35% of men over the age of 50 are at risk of experiencing osteoporotic fractures, generating an estimated cost of $25 billion in medical expenses by 2025. 3 , 4 , 5 , 6 , 7
Various efforts have been made to improve bone regeneration for decades, including restricted or modified activity, immobilization of injured structure, acupuncture, physical therapy, administration of anti‐inflammatory medication, application of corticosteroids, and revision surgeries. However, these therapies are palliative for bone defect management, as they do not directly stimulate the proliferation, differentiation, and maturation of osteogenic progenitor cells to reestablish the tissue; they merely aleviate the symptoms therein. Due to the advent of tissue engineering and regenerative medicine, new strategies to restore homeostasis in bone deficiency, such as bone graft usage, have been recently proposed. 8 , 9 , 10 , 11 To date, the autogenous bone graft is still considered the gold standard for reconstructing large skeletal defects, as it not only provides a construction composed of a natural osteoconductive scaffold accompanied by supportive growth factors but also supplies immunotolerated osteogenic‐committed cells. 12 Although this may be true, the limited resources for autogenous bone grafts and the harvesting procedure's morbidity have fueled the search for alternative bone regeneration approach(s).
In order to be comparable to autogenous bone graft as the ideal blueprint for efficacious bone regeneration, any candidate therapies should provide one or more of the following: (1) a supportive osteoconductive or osteoinductive scaffold, (2) a suitable microenvironment to stimulate cellular osteogenesis differentiation and maturation, and (3) immune‐tolerant osteogenic‐committed cells or progenitor cells harboring the osteogenic potential in vivo.
In regard to the scaffold, functional material design has accelerated the application of biodegradable biomaterials in bone tissue engineering. Several diverse natural proteins (such as collagen, 13 , 14 , 15 , 16 , 17 fibrin, 18 , 19 and silk 20 , 21 , 22 ), polysaccharides (such as hyaluronic acid 23 , 24 , 25 and chitosan 26 , 27 , 28 , 29 , 30 ), bioceramics, 31 , 32 , 33 demineralized bone matrix, 34 synthesized polymers (such as saturated aliphatic polyesters as presented by poly(D,L‐lactic acid‐co‐glycolic acid), 35 , 36 poly[(amino acid ester) phosphazenes] 37 , 38 ), and their composites (such as collagen‐hydroxyapatite‐tricalcium phosphate complex 39 and polyhydroxyalkanoate/ceramic complex 40 ) have been used to construct bone graft substitutes. 41 Meanwhile, bone graft infection is one of the most serious complications in orthopedic surgery, as they are extremely difficult to treat and typically lead to signficantly worse outcomes. 42 , 43 , 44 , 45 , 46 , 47 Thus, antibiotics, such as gentamicin, 48 and antiseptics, such as silver nanoparticles, 36 , 49 , 50 are incorporated into the bone grafts to eliminate the contamination/infection and prohibit biofilm formation. These modified scaffolds provide a new avenue for winning the “race” between the infectious organisms that seek to contaminate, colonize, and ultimately infect the graft, and the body's endogenous tissues or embedded exogenous osteogenic progenitors that seek to grow into the graft to attain a functional reunion. Meanwhile, numerous efforts have been devoted to scaffold optimization for accelerating the on growth cells proliferate and differentiate into the mature osseous tissue and deposit bone extracellular matrix (ECM). 51 , 52
Multiple growth factors have also been investigated to promote bone formation, among which bone morphogenetic protein 2 (BMP2; InfuseTM Bone Graft) has been approved for use in sinus augmentation and localized alveolar ridge augmentation. Although the osteogenic activity of BMP2 is well documented, its off‐target effects such as postoperative inflammation, 53 , 54 osteoclastogenesis, 55 , 56 adipogenesis, 54 , 55 , 57 , 58 and ectopic bone formation 56 have also been recognized and raised concerns for clinical application of BMP2. Another intensively investigated group factor is BMP7 (also known as osteogenic protein‐1 [OP‐1]). Accumulating evidence demonstrates the efficacy of BMP7 on bone regenration. 59 , 60 , 61 , 62 , 63 Unfortunately, the application of BMP2 and BMP7 in the clinical settings faces an array of obstacles, including the high costs, lingering safety concerns (vertebral osteolysis, ectopic bone formation, radiculitis, or cervical soft tissue swelling), considerable failure rates, and controversies. Consequently, recombinant human BMP7 was withdrawn from the market, and restrictions were imposed in the clinical use of recombinant human BMP2. 64 , 65 , 66 , 67 Aside from the BMP family members, other growth factors also step onto the arena of bone regeneration. For example, in 1999, Ting's group first identified the osteogenic activity of another secretory protein, neural EGFL like 1 (NELL1), in active bone formation sites of human craniosynostosis patients. 68 Since then, NELL1's osteogenic potency has been verified in several small and large animal models consisting of rodents, sheep, and nonhuman primates 69 , 70 , 71 , 72 without detecting the adverse effects seen in BMP2 administration. 71 , 73 , 74 , 75 , 76 These studies suggest that NELL1 may be an alternative therapeutic option for local or systemic bone regeneration.
In addition to osteogenic growth factors, ultrasound and electrical stimulation are also used to promote bone regeneration. 77 , 78 However, a recent systematic review of randomized controlled trials reveals that low‐intensity pulsed ultrasound stimulation did not reduce time to return to work or the number of subsequent operations of patients with fractures, and its benefits on pain management, days to weight‐bearing, and radiographic healing were also to be insignificant. 79 Although the mechanism is not entirely understood, collagen's piezoelectric property is able to generate a built‐in electric field in the organic bone matrix, 80 which may activate the membrane receptors on osteoprogenitor cells to subsequently induce osteogenesis. 81 Beyond this inherent property, faradic products generated around cathodic sites during electrical stimulation also appear to contribute to bone regeneration. 82 The cations, such as Ca2+, can rapidly deposit around the cathode, and anions, such as PO4 3−, HPO4 2−, and OH−, subsequently aggregate around the cations. 83 These depositions result in hydroxyapatite formation at the cathode, which, in turn, promotes bone formation. 83 In attempts to induce osteogenesis with electric forces, various methods such as direct electrical current, 84 capacitive coupling, 85 and inductive coupling, 86 have been used. Recently, through the engineering of a nanoscale galvanic redox system between silver nanoparticles and 316L stainless steel alloy, novel osteogenic stimulation properties and the bactericidal activities have been introduced into the composite material system, 87 which offers an innovative strategy to design multifunctional biomaterials for bone formation.
Despite the development in support materials and stimulating methods, cells hold the predominant role in bone regeneration. It is without a doubt that resident stromal resident stem cells, such as perivascular hosted CD146+ skeletal stem cells (SSCs) 88 and newly identified epiphyseal located CD146− SSCs 89 response to damage on the front line and play essential immunomodulatory and pro‐osteogenic roles in the local environment (as reviewed in Refs. 90, 91, 92, 93). Nonetheless, for critical‐size defects, the local osteoprogenitors are insufficient in restoring tissue continuity or function. Several disadvantages, such as the donor site morbidity, inadequate cell availability, poor survivability, restricted proliferation, limited spreading, and dedifferentiation, significantly hinder the clinical use of mature cells, such as osteoblasts. 94 , 95 , 96 Therefore, isolation or generation of safer and more readily available regenerative cell sources remains a major challenge, demanding alternative cell‐based regenerative therapies.
Since Becker, McCulloch, and Till first defined the stem cells functionally in 1963, 97 stem cells’ pluri‐ or multipotent properties have been a hot investigating topic among the scientific community. This broad excitement has led to continuous improvements in the understanding of stem cell biology, accompanied by worldwide competition for employing stem cell techniques in clinical applications. 98 This is particularly evident in skeletal regenerative medicine, 99 , 100 although inevitable clinical implementation barriers remain intact. A growing diversity of pluri‐ or multipotent cell sources have been investigated for bone regeneration (Figure 1), each of which present unique advantages and, on the other hand, challenges.
FIGURE 1.
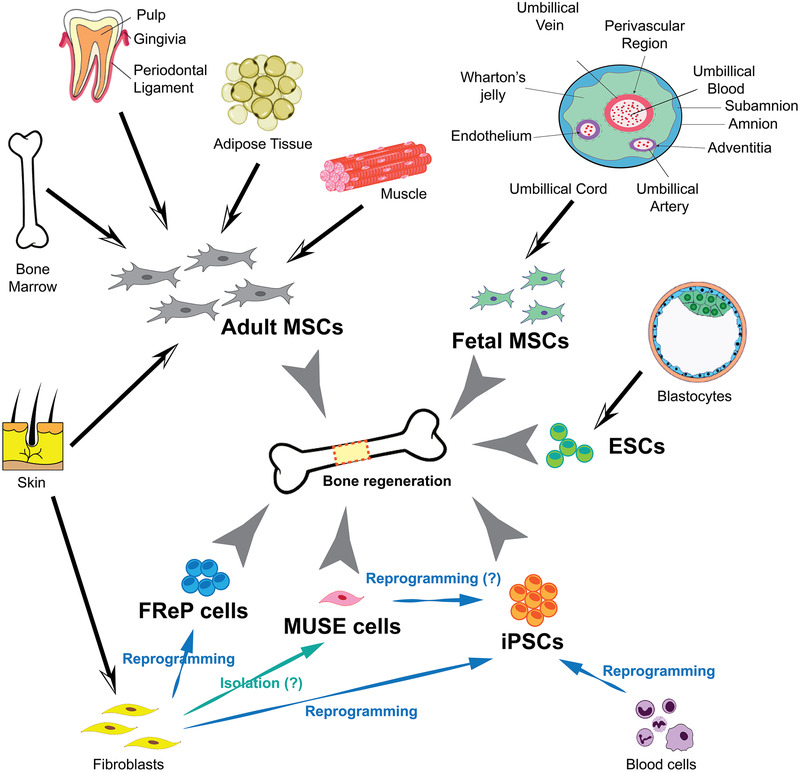
A plethora of both acute and chronic conditions, including traumatic, degenerative, malignant, and congenital varieties, often play key roles in reducing the quality of life for many people. This is particularly true in the case of critical‐size defects where the innate self‐healing capacity of bone is inadequate for a reunion. To date, a diversity of novel multipotent/pluripotent cell sources is regarded as regenerative medicine, particularly for bone regeneration, in virtue of continued worldwide collaboration. Although their potential is irrefutable, each of the cell sources mentioned has its own drawbacks, which must be entirely understood and overcome before they are released for human clinical application
2. ADULT MESENCHYMAL STEM CELLS (MSCs)
2.1. Adult MSCs were first isolated from bone marrow
In 1966, A. J. Friedenstein and colleagues detected the ectopic bone formation by a population of cells isolated from mature mouse bone marrow, 101 which provided that the first evidence endorsing Cohnheim's hypothesis nonhematopoietic regenerative cells exist in the bone marrow. 102 Under the appropriate in vitro culture condition, these colony forming unit‐fibroblasts (CFU‐F) can differentiate toward a wide range of cell types, including osteoblasts, chondrocytes, myocytes, and adipocytes. This being the case, these cells were retermed and are now commonly known as bone marrow‐derived stromal cells (BMSCs). On account of their relationship with mesenchymal tissue development and regeneration, BMSCs are recognized as the prototype of MSCs. 103 Since BMSCs enhanced bone healing in numerous small and large animal models, 104 , 105 , 106 , 107 , 108 , 109 human BMSCs are considered the current gold‐standard cell source for bone regeneration. For example, applying BMSCs to stimulate posterolateral spinal fusion was transferred from the preclinical investigation into the clinical assessment in 2008. 110 However, the invasive and painful harvesting procedure presents a significant obstacle for BMSCs’ application. Also, the percentage of BMSCs among bone marrow nucleated cells obtained from bone marrow aspiration is typically 0.001‐0.01%. 111 , 112 Therefore, large quantities of bone marrow must be procured as starting material to obtain a substantial amount of BMSCs, causing additional donor site morbidity, and thus, remains extremely challenging for BMSCs’ clinical translation. Meanwhile, purification and expansion of BMSCs via passaging are generally necessary to eliminate other cell types. 113 Unfortunately, BMSCs purified by the conventional plastic adherence method and assessed by the fibroblastic morphological criteria are heterogeneous populations containing a diversity of single stem cell‐like and progenitor cells with different lineage commitment. 114 As a result, even in the seemingly pure preparation of BMSCs, only a subpopulation of BMSCs will be susceptible to osteogenesis. 115 Further, BMSCs have a relatively low proliferative ability, while the growth factors used to help BMSC expansion can jeopardize their differentiation potential. 116 , 117 , 118
In 2006, Aslan et al isolated a population of CD105 (endoglin)‐expressing cells from bone marrow aspirate. 119 Since these CD105+ cells can be culture‐expanded, express CD90 but not CD14, CD34, CD45, or CD31, and are able to differentiate into osteogenic, chondrogenic, and adipogenic lineages, 119 they could be considered a subsection of BMSCs according to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) criteria. 120 Excitingly, freshly immunoisolated CD105+ cells can differentiate into chondrocytes and osteoblasts in vivo with the stimulation with BMP2. 119 A follow‐up randomized and prospective preliminary study demonstrates the efficacy of these noncultured, immunoisolated CD105+ cells on fracture reunion in a clinical setting. 121 However, these CD105+ cells only represent 2.3 ± 0.45% of the mononuclear cells in bone marrow aspirate. 119 Therefore, novel technologies that easily and safely isolate sufficient highly osteogenesis‐potential BMSCs are still urgently demanded.
2.2. Adult MSCs can also be isolated from other tissues
To overcome the aforementioned disadvantages of BMSCs, great efforts have been made to collect MSCs from other tissues. Until now, in addition to bone marrow, MSCs were isolated from a broad range of tissues, such as perichondrium, 122 cartilage, 123 , 124 tendon, 125 muscle, 126 , 127 , 128 skin, 129 dental pulp, 130 , 131 , 132 gut, 133 liver, 134 and salivary glands. 135 , 136 However, isolating MSCs from these tissues also involves invasive and painful harvesting procedures and is limited by the insufficient supply for cell harvesting.
2.2.1. Oral‐derived MSCs
One typical example is the dental pulp. Although studies on a laboratory‐scale demonstrated the benefits of dental‐pulp‐derived MSCs (DMSCs) in the regeneration of craniofacial bone defects, 137 , 138 , 139 , 140 , 141 , 142 the small yield of DMSCs from a single tooth leads to the long‐term cultivation of DMSCs, accompanied with the escalated costs and risks to acquire sufficient DMSCs for clinical implementation. As a result, the regulatory bar for DMSCs seems extremely high. In addition to DMSCs, the oral cavity hosts several other stem cell populations capable of bone regeneration (as reviewed in Ref. 92), while similar cell availability limitation and regulatory concerns faced by DMSCs also hindered these cells’ clinical applications.
2.2.2. Adipose‐derived stem cells (ADSCs)
On the other hand, adipose tissue may provide an alternative avenue for MSCs isolation. 143 , 144 , 145 , 146 , 147 , 148 The human adipose tissue is relatively abundant and can generally be obtained through a liposuction procedure. 143 More importantly, adipose tissue yields a higher amount of MSCs than bone marrow: ADSCs can be harvested at the ratio of 5000 CFU‐F per gram of adipose tissue; in other words, approximately 2% of nucleated cells in processed lipoaspirate are ADSCs. 149 , 150 In comparison with BMSCs, ADSCs also have a great proliferation capability 151 , 152 and are generally considered stable throughout long‐term in vitro expansion, 153 exhibiting their potential to be a practical regenerative medicine. Unfortunately, although it has become the second most common cosmetic surgical procedures, 154 , 155 lipoaspiration/liposuction is still an invasive and painful surgery performed with anesthesia. 156 , 157 Consequently, reports of severely secondary complications of lipoaspiration, such as blood clots, negative reactions to anesthesia, pulmonary complications, infections, and venous thromboembolism, have been significantly increased. 156 , 158 Moreover, deaths secondary to lipoaspiration procedures are as high as one death in 5000 surgeries. 155 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 Thus, lipoaspiration alone may not be safe for patients with heart problems or blood clotting disorders, women who are pregnant, 156 or patients with a body mass greater than 35 kg/m2 and thus is associated with a very high risk of secondary complications. 155 , 171 , 172 , 173 More importantly, combined procedures of lipoaspiration for ADSC harvesting and implantation for bone regeneration, particularly with obese or geratiric individuals, will significantly increase the complication rates and often lead to critical safety concerns. 158
2.3. Defination of MSCs is still an open question under investigation
ADSCs are present in a stromal vascular fraction (SVF) that constitutes less than 10% of adipose tissue. 174 , 175 SVF is a heterogeneous cell population, including preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident monocytes/macrophages, lymphocytes, and putative ADSCs. 174 , 175 , 176 , 177 Because the presence of mixed stromal and endothelial cells in SVF may dilute and interact with the ADSCs, the benefit of SVF or unpurified ADSCs on bone regeneration is minimal. 178 , 179 , 180 Consequently, a method to purify ADSCs from the SVF is vital, which turns out to post a question in regard to the characterization of MSCs.
Interestingly, MSCs, including BMSCs, DMSCs, ADSCs, and muscle‐derived stem cells, 126 , 127 , 128 are identified in cultures of its dissociated original tissues instead of their native character, frequency, and anatomic location. In 2006, the Mesenchymal and Tissue Stem Cell Committee of the ISCT established a four‐point minimal criterium to define MSCs 120 :
Are plastic‐adherent when kept in standard culture conditions?
Are phenotypically positive for CD73, CD90, and CD105?
Lack of expression of CD45, CD34, CD14 or CD11b, CD19 or CD79α, and human leukocyte antigen‐antigen D related (HLA‐DR).
Hold the so‐called “tri‐lineage” differentiation potential towards osteoblasts, adipocytes, and chondroblasts in vitro.
It is worth noticing that CD44, a previously identified essential MSC‐expression cell surface antigen, 181 , 182 , 183 is not admitted to the ISCT standard. These criteria may be suitable for purifying ADSCs from SVF on account of the more homogenous CD9+/CD44+/CD73+/CD90+ phenotype presented in the SVF after cultivation. 184 Nonetheless, accumulating evidence suggests that these criteria may not be completely applicable for identifying ADSCs in vivo or isolating ADSCs from the bulk adipose tissue directly without additional in vitro cultivation steps. For instance, it is known that fibroblasts and stromal cells share the CD73+/CD105+/CD90+/CD44+ phenotype, and this quadruple‐positive panel is only sufficient to discriminate these cells from hematopoietic counterparts. 185 , 186 , 187 , 188
The expression of CD34 is another important issue for characterizing MSCs in vivo. Traditionally, CD34 was considered a unique endothelial and hematopoietic marker, which should not be expressed by MSCs. 189 Consequently, CD34 expression was used to identify and isolate hematopoietic stem cells (HSCs), 190 and it was a common misconception that CD34+ cells represented the hematopoietic contamination in nonhematopoietic samples. Nevertheless, when Simmons and Torok‐Storb originally generated the monoclonal antibody Stro‐1, which has been extensively used as a selection tool of MSCs, CD34+ bone marrow cells were employed as the immunogen. 191 Surprisingly enough, CD34+ cells constitute the majority of SVF cells (60‐80%), 192 , 193 and ADSCs harvested from lipoaspirate before in vitro cultivation exhibited some degree of CD34 expression, 194 , 195 , 196 , 197 , 198 suggesting that CD34 may not be a real negative marker of MSCs in vivo. 199 , 200 Meanwhile, CD34 expression is also present in various stem/progenitor cells, including muscle satellite cells, 201 hair follicle stem cells, 202 , 203 and keratinocyte stem cells. 204 Further studies show that the standard passaging of ADSCs gradually declines the expression of CD34, 198 accompanied by the downregulation of other MSC‐associated markers such as CD106, CD146, and CD271. 196 , 205 , 206 , 207 Be this as it may, the expression of ISTC classified MSC markers CD73, CD90, and CD105 is increased upon cell expansion in vitro. 196 The similar disappearance of CD34 expression was duplicated in muscle satellite cells when the cells were propagated and differentiated into adipogenic cells. 208 Additionally, upon activation, the quiescent CD34+ keratocytes lost the CD34 expression and acquired a fibroblastic phenotype. 209 , 210 , 211 Further, the high expression level of Stro‐1 antigens in ADSCs is also diminished in response to passaging or induced differentiation. 212 When considered together, these phenotypical shifts represent a response of multipotent cells to the environmental changes that induce an activation/differentiation from their in vivo quiescent state and indicate that CD34 could be treated as a common marker of quiescent multipotent stem/progenitor population, including ADSCs in vivo. 213
2.4. Adult MSCs are tightly related to pericytes and adventitial cells
Through their emperrical work, Tintut et al demonstrated the multilineage differentiation potency of a subpopulation of vascular cells. 214 Together with the distribution of Stro‐1 antigens in adipose tissue predominantly located in the endothelium of arterioles, capillaries, and some veins, 215 this report inspired an enthusiastic investigation to reveal the relationship between MSCs and perivascular cells. In mice, ADSCs have been shown to reside in the adipose vasculature 216 with the expression of CD34 and stem cell antigen‐1 (Sca‐1; a marker for tissue‐resident stem/progenitor cells 217 ) as well as three mura cell markers: α‐smooth muscle actin (α‐SMA), β‐type platelet‐derived growth factor receptor (PDGFRβ), and neural/glial antigen 2 (NG2). In 2008, Péault's group demonstrated that pericytes derived from multiple human organs (including white adipose tissue, skeletal muscle, pancreas, placenta, heart, skin, lung, brain, eye, gut, bone marrow, and umbilical cord) display a CD34−/CD45−/HLA‐DR−/CD44+/CD73+/CD90+/CD105+ phenotype after in vitro cultivation. 218 Moreover, when cultured in suitable conditions, pericytes exhibit the capability for colonial formation as well as osteogenic, chondrogenic, and adipogenic differentiation, which qualified them as MSCs based on the ISCT standard. 218 This astonishing equivalency of pericytes with MSCs soon after drew the scientific community's attention and led to the hypothesis that “all” MSCs are derived from pericytes. 219
The comparison between ADSCs and pericytes further emphasizes this theory. Intrestingly, like ADSCs, intact pericytes in their native origin are positive for α‐SMA, PGDFRβ, and NG2 expression, which is not diminished during in vitro expending. 218 However, unlike mouse ADSCs that are CD34+ in vivo and early‐passage in vitro, 198 , 216 lack of CD34 expression in pericytes led to confusion regarding their natural affiliation.
In addition to the pericytes that closely associate with microvessel endothelial cells surrounding capillaries and microvessels, multipotent progenitor cells with MSC characters have also been isolated from the bovine artery wall 214 and the tunica adventitia of the human pulmonary artery, 220 which suggests the existence of nonpericyte perivascular cells as alternative originators of MSCs. 221 After exclusion of myogenic (CD56) and hematopoietic (CD45) populations, two distinct populations derived from human white adipose tissue without the expression of endothelium‐specific antigen CD31 could give rise to MSCs: CD146+/CD34− pericytes and a second CD146−/CD34+ population, 222 because the clones developed from the CD146−/CD34+ cells displayed the MSC hallmark molecules CD44, CD73, CD90, and CD105, and can undergo osteogenic, adipogenic, and chondrogenic differentiation. 222 Besides, flow cytometry analysis similarly confirmed that these CD146−/CD34+ adventitial cells homogeneously express the typical MSC‐associated markers CD44, CD73, CD90, and CD105 in their native origin. 222 Compared with pericytes, adventitial cells surround the largest vessels and are not closely associated with endothelial cells. 222 , 223 Because adventitial cells hold the capability to differentiate into pericyte‐like cells under inductive conditions in vitro, they are proposed to be the precursors of pericytes. 222 , 223 A study evaluated human lipoaspirate from 70 donors to reveal that pericytes and adventitial cells comprise an average of 17.1% and 22.5% of SVF, respectively, which, in turn, accounts for approximately 39.6% of total nonadipocyte lipoaspirate cells or 3.96% of total adipose‐nucleated cells. 178
Although distinguishing these two populations provides significant impacts for stem cell biology research, combining pericytes and adventitial cells together—collectively termed perivascular stem cells (PSCs)—may maximize the MSC source in a clinical setting, especially when autologous cells are used to avoid immunogenic rejection. To bypass the in vitro cultivation steps for the plastic‐adherent cells, which generally takes weeks and increases the risk of spontaneous cellular transformation, Péault's group established a simple sorting procedure to safely and effectively obtain PSCs from routine liposuction. 224 In this procedure, adipose tissues obtained from liposuction or lipectomy are first digested with collagenase and centrifuged to remove adipocytes. Then, the CD31−/CD45−/CD34−/CD146+ pericytes and CD31−/CD45−/CD34+/CD146− adventitial cells are collected from the yielded SVF by fluorescent‐activated cell sorting (FACS). 178 Thanks to the effects of a multidisciplinary research group led by Drs. Péault and Soo, the entire procedure has been optimized to be completed in a few hours, making the direct sorting‐based PSC application feasible in a clinical setting. As expected, the purified PSCs enhanced bone formation in animal models and were superior to SVF in forming bones. 178 , 225 , 226 , 227 Thus, purified PSCs seem to be an alternative MSCs source to confer bone formation.
2.5. Adult MSCs may benefit tissue repair via bioactive soluble factor production and secreation
In the last few decades, there has been a debate regarding the way in which MSCs ameliorate tissue damages. One possibility is that MSCs directly differentiate or transdifferentiate into parenchymal cells. 228 Yet, previous studies showed a surprisingly low (less than 1%) and transient engraftment of MSCs in newly formed tissue given the associated therapeutic efficacy. 229 , 230 A recent study that tracked the fate of pericytes in vivo in injured skeletal muscle or brain suggested that pericytes did not transdifferentiate as progenitor cells in these two circumstances, further questioning the direct engraftment of MSCs in tissue regeneration in vivo. 231 Currently, it is believed that the long‐lasting therapeutic benefits of MSCs rely on their bioactive soluble factor production and secretion. 232 , 233 , 234 Particularly, by secreting trophic factors (growth factors, cytokines, and specific proteins), MSCs present their regenerative potency in neurovascular and musculoskeletal therapies. 235 , 236 MSCs also produce multiple inflammatory cytokines to modulate the interaction between osteoblast‐lineage and monocyte‐macrophage‐osteoclast lineage; both of which are essential for bone remodeling. 2 , 237 Viewing MSCs as “an injury drugstore,” 176 , 232 , 238 , 239 , 240 Dr. Arnorld I. Caplan, a pioneer of MSCs research, 103 suggested renaming the MSCs as “the Medicinal Signaling Cells” to more accurately depict their function in nature. 232 , 234 , 241
2.6. Application of adult MSCs in tissue regeneration faces multiple obstacles
2.6.1. Risk of rejection
Similarly to the way in which autologous cell source is the best choice for clinical application, allogeneic MSCs must be considered in some scenarios such as the geriatric population, who are the primary targets for stem cell therapy since the therapeutic effectiveness of MSCs is dependent upon the age of the donor. 242 , 243 , 244 , 245 MSCs were previously considered immunoprivileged 246 because undifferentiated MSCs express low to intermediate levels of HLA class I and negligible to low HLA class II. 120 , 247 However, MSCs exposed to interferon (IFN)‐γ or differentiated into mature cell types can significantly express more HLA class I and HLA class II. 248 , 249 Besides, long‐term in vitro culture was reported to impair the immunosuppressive activity of MSCs. 250 Moreover, animal model studies displayed a trend of the early death of allogeneic MSCs, 251 , 252 , 253 confirming in the human autopsy of patients who received allogeneic MSCs within a year. 254 Furthermore, rejection and chronic immune responses of allogeneic MSCs have also been reported in animal studies and human clinical trials. 255 , 256 , 257 Therefore, the current view of MSCs is “immunoevasive” instead of “immune‐privileged.” 258 , 259 Accurately measuring immune responses following MSCs treatment in a timely manner is necessary to assess the safety of allogeneic MSCs application. Also, developing novel technologies to prolong the “escaping” status of allogeneic MSCs from the donor's immune system may prolong their persistence in vivo and improve their clinical outcomes.
2.6.2. Risk of tumor formation
Another prudence of MSCs’ usage is their association with tumorigenesis. In 2005, Rubio et al reported the malignant transformation of human ADSCs that had passaged more than 4 months in vitro. 260 In the same year, Wang et al identified an outgrowth transformed subpopulation of cultured human BMSCs with a round, cuboidal to short spindle shape, distinguished from the typical MSCs. 261 These cells later termed transformed mesenchymal cells (TMCs), also displayed contact‐independent growth and anchorage‐independent growth when released into the suspension 261 —typical phenotypes seen in tumorigenic cells with metastatic potential. 262 Rosland et al reported that the ratio of spontaneous malignant transformation of human BMSCs to be as high as 45.8%. 263 TMCs were also obtained in cultured mouse and monkey BMSCs, 264 , 265 while injection of mouse and monkey TMCs resulted in tumor formation in recipient animals. 265 , 266 Follow‐up studies argued that spontaneous transformation of MSCs might be false, and the so‐called TMCs may arise from the cross‐contamination with malignant cells that were residents in origin, such as fibrosarcoma and osteosarcoma. 267 , 268 , 269 This claim, whether correct or incorrect, highly emphasizes the drawback of the MSC expansion procedure. Nevertheless, this explanation may not be sufficient for lowering the tumorigenic caution for in vivo MSC implementation, especially for bone regeneration, as bone provides one of the most congenial metastatic microenvironment for tumor progression. 270 , 271 Recent studies have shown that purified human MSCs developed chromosomal aberrations during cultivation 272 and underwent spontaneous tumorigenic transformation, 273 which cannot be explained by the cross‐contamination theory. Recently, a significant amount of efforts have been devoted to optimizing the cultivation of MSCs, 274 , 275 which may eventually result in a practical strategy to control the spontaneous tumorigenic transformation of MSCs.
Certainly, accumulating data have also clearly demonstrated the unbalanced signal transduction in MSCs directly lead to sarcoma formation in vivo. 276 Besides, the immune suppression potential of MSCs may diminish T‐cell proliferation, thus weakening the antineoplastic response. 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 More importantly, various cytokine, chemokines, and growth factors secreted by MSCs have been shown to increase the proliferation, migration, and angiogenesis of tumor cells. 276 Akimoto et al revealed that ADMCs promote the growth of cocultured glioblastoma multiforme (GBM) cells in vitro and support GBM development in vivo by at least two distinct mechanisms‐enhancing angiogenesis and inhibiting apoptosis. 285 Meanwhile, Ren et al reported that similar to tumor‐derived MSCs, tumor necrosis factor α (TNFα)‐pretreated BMSCs enhanced tumor progression by recruiting more macrophages into tumor. 286 This discovery highlights the possibility that, with an inflammatory stimulation, normal MSCs can convert into a more tumor‐promising phenotype usually found in the tumor microenvironment. These direct and/or indirect involvements of MSCs in tumorigenesis suggest that MSCs hold a high risk for bone reconstruction patients with a history of malignancies.
Taken together, as the current gold standard cell source for bone engineering therapies, adult MSCs from different tissue were intensively investigated (Table 1). The identity, function, safety, and efficacy of these cells are still debatable. Aiming to answer these questions and optimize the clinical application of adult MSCs, a large‐scale, expensive, and time‐consuming investigation is inevitable, which will require a global collaboration of academia and pharmaceutical companies. In addition, the regulation of MSC‐based therapies must be fully and accurately implemented, and most importantly, the long‐term follow‐ups that define the associated risks must be carried out in the clinical trials.
TABLE 1.
Comparison among adult MSCs derived from different tissues
| Cell types | Pros | Cons |
|---|---|---|
| BMSCs |
|
|
| Oral‐derived MSCs (including DMSCs) |
|
|
| ADSCs |
|
|
3. FETAL MSCs
In addition to adult MSCs mentioned above, less mature MSCs isolated from the umbilical cord have been considered for bone regeneration. These umbilical cord‐derived MSCs (UCMSCs) have similar surface marker expression, high differentiation potential, and low immunogenicity compared with BMSCs. 287 , 288 , 289 , 290 , 291 , 292 Likewise, UCMSCs and BMSCs share the mitogen‐activated protein kinase (MAPK) signal pathway for osteogenic commitment and differentiation. 293 , 294 UCMSCs are isolated from umbilical cords, a generally discarded tissue, without ethical concerns, 295 , 296 , 297 potential pain, and medical or surgical risks such as bleeding and anesthetic adminstration. 298 Moreover, in comparison with adult MSCs, UCMSCs share a high expansion capability with other fetal‐derived stem cells 183 , 299 but rarely transform into tumor‐associated fibroblasts. 300 These advantages support the potential of UCMSCs for bone regeneration. 301
The human umbilical cord consists of an outer amniotic membrane (amniotic epithelium) that envelops a mucoid connective tissue, which can be characterized as three regions lacking clearly visible structural boundaries: subamnion, Wharton's jelly, and adventitia (a strong, elastic muscle‐like tissue layer), along with three blood vessels (a vein and two arteries). 302 Among them, Wharton's jelly contains the most abundant source of UCMSCs. 303 A population of plastic‐adherence, spindle‐shaped cells that express CD44, CD73, CD90, and CD105, but not the hematopoietic markers CD14, CD34, or CD35, can also be isolated from the amniotic membrane, 304 , 305 , 306 although the amniotic membrane was previously considered as solely epithelium and not a source of MSCs. 307 These amniotic membrane‐derived cells also have the tri‐lineage differentiation potential and can be recognized by Stro‐1. Thus, according to ISCT, they are qualified as MSCs and termed as amniotic membrane‐derived MSCs (AMMSCs). A recent study showed that the proliferation and self‐renewal capacity of AMMSCs are significantly lower than Wharton's jelly‐derived UCMSCs, 308 indicating the disadvantages of AMMSCs to fulfill the clinical scale requirement in comparison with UCMSCs. Although recent comparison studies suggest that Wharton's jelly‐derived UCMSCs offer the best clinical utility (mainly due to the high isolation percentage and less nonstem cell contaminants to avoid excessive in vitro purification and expansion 309 ), the time‐consuming and labor‐intensive dissection of the cord into discrete regions may not be necessary to obtain a valuable population of cells for clinical application, 310 particularly when FACS‐based purification is employed.
Kargozer et al revealed that implantation of human UCMSCs with three‐dimensional bioactive glass/gelatin scaffold in critical‐sized calvarial defects resulted in a similar degree of bone formation compared to those implanted with unpurified human ADSCs, which is statistically lower than that of the human BMSC‐implanted group. 179 Interestingly, neovascularization was significantly increased in the human UCMSC group, leading to better healing than using the unpurified human ADSCs. 179 In another study using RGD (Arg‐Gly‐Asp)‐peptide‐coated macroporous tetracalcium phosphate and dicalcium phosphate anhydrous as a scaffold, human BMSCs and UCMSCs resulted in similar levels of bone formation and vascularization in critical‐sized calvarial defects. 311 In addition to the influences of the different scaffold materials, another explanation of these paradoxical results could be the known fact that the yield and the differentiation potential of USMSCs highly depend on the method of cell isolation. 292 , 294 , 312 Therefore, a standard isolation/purification methodology should be established and validated before the clinical application of UCMSCs.
Although UCMSCs hold the ability to modulate natural killer (NK) cells and promote regulatory T (Treg) cell expansion and thus present a lower rejection risk, 313 , 314 immunogenic concern remains the main obstacle for their allogeneic usage. 311 Meanwhile, the proper cryopreservation of the umbilical cord from childbirth for an extended time is an essential step for autologous usage, which is accompanied by nonnegligible financial and resource inputs.
Umbilical cord blood (UCB) was also recognized as a source of MSCs, 315 , 316 although UCB has been considered a reliable source of HSCs for a long time. 317 These UCB‐derived MSCs (UCBMSCs) were also explored as an alternative cell source for bone tissue engineering and regeneration. 318 , 319 Contrary to ADSCs that support GBM development, UCBMSCs inhibit GBM growth while simultaneously inducing apoptosis, 285 suggesting that UCBMSCs are much safer than adult MSCs for clinical usage. However, UCBMSCs shared a similar immunogenic concern for allogeneic usage and costly storage difficulty for autologous application with UCMSCs. In addition, the yield of MSCs from UCB is extremely low, and the isolation of UCBMSCs is not guaranteed as UCMSCs, while UCMSCs also exhibit a great advantage in terms of proliferation. 320 , 321 , 322 Amniotic fluid is another potential MSCs source 323 , 324 , 325 ; however, due to the invasive procedure, limited availability, and ethical concern, the yielded amniotic fluid MSCs (AFMSCs) will not be further discussed in this chapter, as there are no superior clinical benefits to use AFMSCs than to use aforementioned fetal MSCs based on the current understanding.
Generally, using fetal MSCs for tissue regeneration seems to be associated with higher technological and regulatory standards, as well as a significant financial burden. Making the fetal MSC‐based therapies to be standard care available for everyone is tremendously challenging in reality, although it holds remarkable scientific interest and may be practical for some specific circumstances.
4. EMBRYONIC STEM CELLS (ESCs)
In 1998, Thomson et al first derived the human ESCs from blastocysts. 326 Briefly, the inner cell mass from the blastocyst stage of embryos is separated from the trophectoderm and plated onto mouse embryonic fibroblast feeder cells to form human ESC colonies. 326 These human ESCs have normal karyotypes, exhibit high levels of telomerase activity, and display prolonged undifferentiated proliferation. They also express cell surface markers that characterize primate ESCs, present the ability to generate embryoid body (EB) in vitro, maintain the developmental potential to form trophoblast and derivatives of all three embryonic germ layers, and produce teratomas after injection into severe combined immunodeficient (SCID)‐beige mice.
Since their discovery, human ESCs have been broadly used for drug discovery and development. 327 , 328 , 329 , 330 , 331 As holding the pluripotent differentiation potential to any tissues, human ESCs have also been investigated as regenerative medicine, including the osteogenic aspect. 332 , 333 , 334 , 335 , 336 Human ESC‐based therapies develop very quickly, ie, from Thomson's discovery of human ESCs to their clinical trial for spinal cord injury repair in 2009 (ClinicalTrials.gov Identifier: NCT01217008), it took merely 12 years.
Nevertheless, three inevitable impediments must be surmounted prior to the broad application of ESCs in humans. First, vigorous debates over ESC research and application ethics are continuing, as the human embryos have to be destroyed during the isolation of ESCs. 337 , 338 To those who believe that “human life begins at conception” and an embryo is a person with the same moral status as an adult or a live‐born child, taking a blastocyst and removing the inner cell mass to derive an ESC is equivalent to murder. 339 On the contrary, many others believe that an embryo becomes a person in a moral sense at a later stage of development than fertilization. Taking this into account, the International Society for Stem Cell Research (ISSCR) issued a regulatory guideline that mandates the research on human embryos should be limited to the first two weeks after fertilization. 340 Although this “14‐day rule” reflects the laws of at least 12 countries, 340 different administrations may hold varying positions in regard to the creation of new human ESCs, as seen in the Bush administration versus the Obama administration. 341
Second, the immunogenicity of ESCs should be disclosed, as their clinical usage is clearly allogeneic. To date, reports about the immunological properties of ESCs are still controversial, consisting of those that claim ESCs are uniquely immunoprivileged, those reveal that ESCs hold negligible immunogenicity, and those suggest ESCs can trigger an immune response. 342 , 343 , 344 , 345 , 346 , 347
Last but not least, the genomic instability 348 , 349 , 350 , 351 , 352 , 353 and tumorigenic nature 354 , 355 , 356 of ESCs raise a credible concern for their implantation in human bodies. Therefore, although the investigation of ESCs may pave the path for the investigation of reprogrammed pluripotent or multipotent cells, as discussed below, the clinical usage of ESCs seems impractical in the present stage.
5. INDUCED PLURIPOTENT STEM CELLS (iPSCs)
5.1. Generation iPSCs from diverse somatic cell origins in vitro starts a new era of cellular biology and regeneration medicine
Since circa 2006, somatic cells are now able to be reprogrammed into an ESC‐like state with pluripotency utilizing viral‐mediated genomic integration of a panel of transcriptional factors essential for embryonic development. 357 , 358 The potential to use a patient's own cells to create iPSCs provides a promising new venue for personalized cell therapies by overcoming the ethical paradox and potential immunogenicity of ESCs and fetal MSCs mentioned above. In addition, iPSCs can be directly derived from easily accessible and expandable dermal fibroblasts 359 and blood cells, 360 which places iPSCs above adult MSCs by avoiding the invasive harvest pressures to generate sufficient cell source. In acknowledgement of this breakthrough discovery, Dr. Shinya Yamanaka at Kyoko University was awarded the 2012 Nobel Prize in Physiology or Medicine.
Recently, there has been great enthusiasm for applying iPSCs for bone regeneration, with or without an MSC‐intermediate stage. Initially, creating EBs from iPSCs before MSC generation was involved in gaining iPSC‐derived MSCs (iPSC‐MSCs), 311 , 361 , 362 , 363 , 364 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , 374 , 375 , 376 , 377 , 378 , 379 , 380 , 381 , 382 , 383 , 384 , 385 , 386 while soon after, an alternative strategy that obained iPSC‐MSCs from dissociated iPSC colonies without the EB formation step was employed. 387 , 388 , 389 , 390 , 391 , 392 , 393 , 394 , 395 , 396 , 397 In comparison with adult MSCs, iPSC‐MSCs not only have the advance in regard to proliferation but also present higher telomerase activity leading to less senescence, which is favorable for clinical application. 385 , 396 Meanwhile, directly inducing osteogenic commitment of iPSCs without an MSC intermediate step was also reported by several independent groups around at the same time. 180 , 386 , 398 , 399 , 400 , 401 , 402 , 403 , 404 , 405
It has been demonstrated that the donor's age has no effects on the differentiation potential of iPSCs, 403 while some studies have revealed the influence of gender on the epigenetic stability of iPSCs. 406 From scientific perspective, further investigations are warranted to elucidate the impotence of iPSCs’ epigenetic memory on their osteogenic commitment due to the current conflicting evidence and numerous variables in these studies 369 , 403 ; however, based on the clinical consideration of minimizing the risk of the cell harvesting procedure, dermal fibroblasts may be the top, if not the only, cell choice for iPSC generation.
Due to the fact that complex spatiotemporal signals and molecular interactions regulate the in vivo osteogenic commitment of pluripotent cells, a diversity of stimulation is applied to promote the direct osteogenic differentiation of iPSCs, including electronic stimulation, 379 chemical inducers, 180 , 363 , 365 , 367 , 374 , 375 , 379 , 380 , 382 , 389 , 400 , 402 , 403 small molecules, 362 , 380 , 404 growth factors in Refs. 366, 374, 376, 380, 382, 389, 399, 403, gene modification, 361 , 365 , 370 , 377 as well as modified two‐dimensional 405 and three‐dimensional microenvironment in Refs. 364, 365, 366, 370, 371, 372, 373, 375, 377, 378, 381, 382, 383, 387, 388, 391, 399, 400, 401, 402, 403. Among which, osteogenic medium containing ascorbic acid, β‐glycerophosphate, and dexamethasone formulation is most commonly used in vitro, 363 , 364 , 367 , 374 , 375 , 376 , 379 , 382 , 389 while the three‐dimensional porous scaffold or hydrogel is popular in vivo. Although some studies suggested that iPSC‐MSCs may not completely differentiate into mature osteoblasts in vitro as evidenced by relatively lower or postponed expression of osteogenic markers, especially those indicating the late‐stage osteogenic development, 375 , 386 , 397 , 398 the performance of human iPSC‐based therapies on bone regeneration was not worse, and maybe even better, than human BMSCs, ADMCs, and UCMSCs at the same circumstances in vivo. 180 , 311 , 375
5.2. Tumorigenesis is a significant drawback for iPSCs’ application in humans
“Above all, do no harm.” 407 Tumor formation associated with cell transplantation must always be avoided in human use. The widely accepted procedure for iPSC generation, in which transcriptional factors essential for embryonic development (such as Yamanaka factors or Thomson factors) are introduced into the genome of target somatic cells, may induce unwanted gene activation and genomic alterations. 408 As a result, iPSCs are likely to carry a higher tumorigenicity risk than ESCs. 409 , 410 , 411 , 412 , 413 Teratoma formation was confirmed in about 20% of SCID mice that had received osteogenic‐induced iPSC for bone defect regeneration. 367 iPSCs also possess a potential risk for somatic tumor development, which is not present when using ESCs. 414 iPSCs’ tumorigenic nature was considered an inevitable subsequence of retroviral or lentiviral transduction, resuling in genetic dysfunction, insertional mutagenesis, and tumor formation with genome integration. Thus, different integration‐free techniques were explored for iPSC generation, including adenovirus, 415 , 416 Sendai virus, 417 expressing plasmid vector, 418 , 419 , 420 episomal vector, 421 , 422 , 423 single mini‐circle vector, 424 piggyBac transposon‐based vector, 425 RNA, 426 , 427 , 428 , 429 and cell‐penetrating protein. 430 , 431 However, retroviral‐derived and transgene‐free human iPSCs exhibit similar tumorigenicity with no appreciable difference in teratoma formation capability or teratoma microvascular density. 414 , 432 Meanwhile, great efforts are also being devoted to replacing Yamanaka factors (OCT4, SOX2, KLF4, and c‐MYC) or Thomson factors (OCT4, SOX2, NANOG, and LIN28) that tightly associate with tumor progression by defined small molecules to achieve chemical induction of pluripotency (CIP). 433 , 434 , 435 , 436 , 437 , 438 , 439 , 440 , 441 , 442 , 443 , 444 , 445 , 446 , 447 Nonetheless, current research indicates that bromodeoxyuridine (BrdU), a mutation inducer that can incorporate into the newly synthesized DNA by replacing thymidine during DNA replication, is required for CIP. 447 In addition, iPSCs generated through CIP still possess a tumorigenic nature. 433 , 434 , 435 , 436 , 437 , 438 , 439 , 440 , 441 , 442 , 443 , 444 , 445 , 446 , 447 Some reports suggested that using iPSC‐MSCs may be safer than using iPSCs directly, as MSCs provide a relatively lower risk of tumorigenic deviation; 392 , 448 however, the tumor‐supporting potency of MSCs, as mentioned above, will also jeopardize the clinical application of iPSC‐MSCs. In the interim, in order to prevent potential tumor formation from undifferentiated iPSCs, cell purification, such as flow cytometry‐ and magnetic bead‐based sorting, is empolyed before transplantation and after differentiation to ensure that only well‐differentiated cells will be transplanted in a generally adopted approach. 449 Another strategy is to use iPSCs harboring a chemical‐inducible suicide gene such that they will have to self‐destruct when tumors are created. 450 , 451 Moreover, resveratrol and irradiation were investigated to prohibit tumor formation from iPSCs and their derivatives during in vivo bone repair, 362 , 367 which paves a new avenue to battle the “evil side” of iPSCs. Unfortunately, even only a small portion of undifferentiated iPSC contamination is still sufficient to induce tumor formation. 410 , 450 , 451 Thus, purification and selective induction of cell death of undifferentiated iPSCs are inefficient and inadequate to eliminate the risk of teratoma and malignant tumors upon transplantation. 449
Taken together, while many challenges still exist before the bench‐to‐bedside translation of iPSC techniques, the high capacity of osteogenic differentiation of iPSCs grants the cautious optimism of iPSCs’ promising potential to become a clinical reality in personalized bone tissue engineering and cell therapy.
6. FIBROMODULIN (FMOD)‐REPROGRAMMED CELLS
6.1. Initial inspiration of fibromodulin reprogramming
Inspired by the pioneer exploration that transferred a somatic cell nucleus to an oocyte 452 , 453 , 454 , 455 , 456 , 457 , 458 or fused a somatic cell with an ESC 459 , 460 to gain pluripotency, Xenopus egg extracts, 461 fish oocyte extracts, 462 ESC extracts, 463 and even carcinoma extracts 463 are used to successfully obtain induced multipotent stem cells (iMSCs) from somatic cells. From a regulatory aspect, the undefined component of these extracts makes it almost impossible to use these iMSCs in humans. However, these studies strongly support the hypothesis that pluripotent cells’ surrounding microenvironment may play an important role in cell fate determination, including maintaining and/or inducing pluripotency. 464
As mentioned above, UCMSCs are predominantly harvested from Wharton's jelly, a proteoglycan‐rich connective tissue. 292 , 319 Interestingly, like other fetal MSCs, 465 , 466 UCMSCs seem to lie between the adult MSCs and ESCs on the development map as they present specific markers of ESCs in addition to those of adult MSCs. 313 , 467 These observations provoke a question: can we reprogram connective tissue somatic cells to some degree of multipotent/pluripotent by reestablishing a proteoglycan‐rich microenvironment?
Bi et al reported that FMOD, an ECM proteoglycan broadly distributed in connective tissues, is a critical component for maintaining endogenous stem cell niches by modulating the bioactivities of growth factors. 468 As a 59‐KD small leucine‐rich proteoglycan (SLRP) member, FMOD contains a central region composed of leucine‐rich repeats, with four keratan sulfate chains flanked by disulfide‐bonded terminal domains. 469 , 470 , 471 Holding high conservation among the mammalian species, FMOD core protein binds to an array of molecules including collagen, 469 , 472 transforming growth factor β (TGFβ), 473 and lysyl oxidase. 474 Accumulating evidence provides that, in addition to its originally described roles in ECM structural support, FMOD also serves as a key regulator of intracellular signaling cascades that govern multiple biological processes, 470 , 475 such as angiogenesis. 476 , 477 , 478 During our long‐time investigation into fetal scarless wound healing, we demonstrated that the single loss of FMOD is adequate to induce scar formation in early‐gestation fetal animals, which normally heal without scarring. On the other hand, exogenous administration of FMOD is sufficient in restoring scarless fetal repair to late‐gestation animals. 479 This evidence not only highlights the essential role of FMOD plays in scarless fetal wound healing, but we also demonstrate that FMOD reduces scar formation in adult cutaneous wounds by eliciting a fetal‐like phenotype of adult dermal fibroblasts. 480 These studies suggest the potential of FMOD in cell rejuvenation and maybe even reprogramming.
6.2. Generation and characterization of FMOD‐reprogrammed (FReP) cells
In 2012, we first reported a strategy to generate multipotent cells from human dermal fibroblasts by continuously stimulating with recombinant human FMOD under a serum‐free condition. 481 Through using this technology, dermal fibroblasts isolated from donors of different ages and genders have been successfully reprogrammed into a multipotent stage. 481 , 482 The yield dome‐shaped, clustered FReP cells can be easily separated from the surrounding spindle‐shaped, monolayer FReP‐basal cells with a Xeno‐free and enzyme‐free reagent developed for passage of human ESCs and iPSCs. 482 These FReP cells express the ESC/iPSC markers, such as NANOG, SOX2, SSEA4, TRA‐1‐60, and TRA‐1‐81, while their OCT4 expression is lower than that of iPSCs generated from the traditional viral‐mediated method. 481 The activation of these essential transcriptional factors for cell reprogramming, accompanied by a specific, biphasic Smad3 phosphorylation, was also validated by multiple methods. 481 Similar to ESCs and iPSCs, FReP cells can form EBs in suspension culture and are capable of differentiating into neuron (ectoderm derivative), pancreatic lineage (endoderm derivative), and multiple mesoderm derivatives, such as osteoblasts, cardiomyocytes, skeletal myocytes, and adipocytes in vitro. 481 , 482 , 483 , 484
6.3. FReP cells exhibit superior potential for bone regeneration than iPSCs
Not only did FReP cell exhibit a similar capability of triploblastic differentiation in vitro as ESCs and iPSCs, the in vivo myogenesis and osteogenesis of FReP cells were also documented in SCID mouse models. 481 , 482 , 483 , 484 Notably, in the broadly accepted critical‐sized calvarial defect model, radiograph analysis revealed significantly more bone formation of the FReP cell implanted group than that of the empty scaffold, parent fibroblasts, and even iPSCs‐implanted groups at eight weeks posttransplantation (Figure 2A). 482 This observation was further supported by the quantification of bone volume density and bone mineral density (Figure 2B‐2C). 482 Histological staining also identified a mineralized bony bridge connecting the two defect ends without ectopic bone formation in the FReP cell group. In contrast, the bone formation of the iPSC group was limited at the defect edges. 482 Additionally, engraftment and differentiation of both iPSCs and FRePs were demonstrated by the colocalization of human markers and osteogenic markers in the newly formed bone, 482 confirming that both iPSCs and FReP cells are directly involved in the new bone formation in vivo (Figure 3). Considering the significantly higher bone formation correlated with higher density of the FReP cell group when compared to those of the iPSC group, 482 FReP may be the superior option in bone regeneration efficacy.
FIGURE 2.
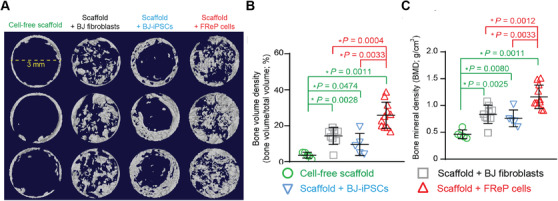
Radiographic analysis of bone regeneration in critical‐sized SCID mouse calvarial defects at week eight postimplantation. Three days prior to implantation, 5 × 105 tested cells were seeded on porous poly(D,L‐lactic acid‐co‐glycolic acid)/hydroxyapatite scaffold and culture in an osteogenic medium containing ascorbic acid, β‐glycerophosphate, and dexamethasone for in vitro induction. (A) MicroCT images of bone regeneration in critical‐sized calvarial defects implanted with cell‐free scaffold (N = 5), scaffold + parental BJ fibroblasts (N = 9), scaffold + BJ fibroblast‐derived iPSC through conventional c retrovirus‐mediated method (BJ‐iPSCs; N = 5), and scaffold + BJ‐FReP cells (N = 11). Images were documented at a resolution of 20.0 μm. Quantification of bone volume density (B) and bone mineral density (C) revealed that implantation of FReP cells resulted in significantly more new bone formation than other groups. *, statistical significance revealed by Mann‐Whitney analysis; green stars indicate the difference from the cell‐free scaffold group; red stars indicate the difference in comparison with the group of scaffold + FReP cells. Modified from Li et al 482 with permission from Elsevier
FIGURE 3.
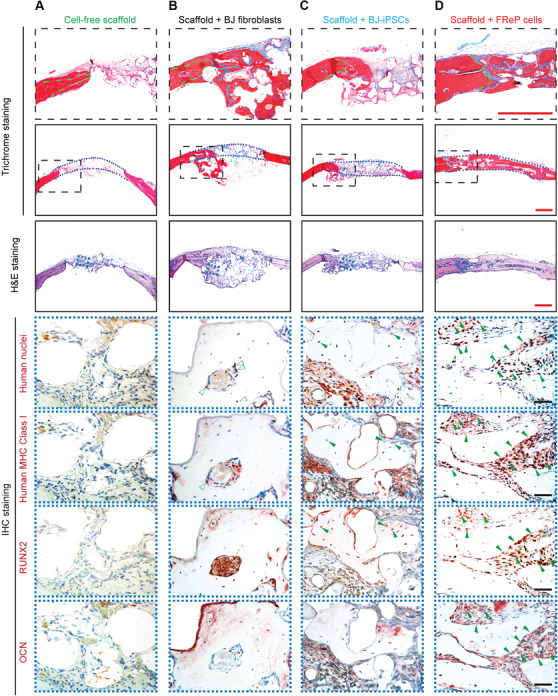
Engraftment, persistence, and osteogenesis of FReP cells in critical‐sized SCID mouse calvarial defects at week eight postimplantation. Hematoxylin and eosin (H&E) and Masson's trichrome staining confirmed that only minimal new bone regeneration occurs in the group implanted with the cell‐free scaffold (A), while implantation of BJ‐fibroblasts resulted in bone formation underneath the calvarial defect with obvious “cyst‐like bone voids” in the newly generated bone area (B). The newly formed bone tissue was predominantly observed at the edge of the defects in the group implanted with iPSCs (C). On the contrary, implantation with FReP cells led to a mineralized bony bridge connecting the two defect ends without ectopic bone formation (D). In Masson Trichrome staining, the mature bone is stained in red, and the osteoid is stained in blue. Green dotted lines outlined the initial edges of the calvarial defects, while blue dotted lines outlined the implantation area, respectively. Furthermore, immunostaining of human nuclei and human major histocompatibility complex (MHC) Class I as well as osteogenic markers runt‐related transcription factor 2 (RUNX2) and osteocalcin (OCN) confirmed the osteogenic differentiation of iPSCs and FReP cells in active osteogenic regions of the defects, while BJ fibroblasts were only detected in the fibrotic area instead of the newly formed bone tissue. Bar = 500 μm (red) or 50 μm (black). Reprinted from Li et al 482 with permission from Elsevier
6.4. FReP cells carry significantly less tumorigenic risk than iPSCs
Like iPSCs, FReP cell generation is unfettered by the ethical and logistical constraints that overshadow the generation of ESCs. Another advantage of FReP cells is that they are generated from a protein‐based technology without genome integration or oncogene activation. Importantly, unlike iPSCs that form teratomas as a consequence of the uncontrolled cellular proliferation, 414 FReP cells have low proliferative capabilities under undifferentiated circumstances, which can be disrupted by osteogenic or myogenic stimulation. 481 Under an intramuscular microenvironment, iPSCs implantation led to 25% tumor formation, while no teratoma or other kinds of tumors were generated from FReP cells in SCID mice. 484 Likewise, when FReP cells were intratesticuarly implanted in Fox Chase SCID‐Beige mouse with Matrigel® carrier, no teratoma was observed in a 4‐month experimental period, while 100% of the animals with iPSC implantation developed teratoma with progressive growth. 484 Because teratoma formation of FReP cells was not found in the kidney capsule of Fox Chase SCID‐Beige mouse either, 481 FReP cells are considered to be a safer cell source for regenerative medicine than iPSCs. Albeit, as FReP cells' investigation is still in its infancy, an abundance of in‐depth investigations must be conducted before translating FReP cells to a clinical setting, including but not limited to the optimization of productivity and the long‐time safety and efficacy assessment.
6.5. FReP cells and multilineage differentiating stress enduring (MUSE) cells present a group of multipotent cell sources for regenerative medicine
Interestingly, FReP cells bear several critical characteristics of MUSE cells: 481 , 483 , 485 (a) express pluripotent markers, albeit at lower levels than ESCs and iPSCs, (b) hold the capability to differentiate into all three germline cells under specific inductions, (c) have low levels of proto‐oncogenes, such as LIN28 and c‐MYC, (d) retain a stable karyotype, and most importantly, (e) do not form teratomas. Although FReP cells and MUSE cells are both excluded from being considered pluripotent due to the stringent mandatory criteria of teratoma formation when introduced to an in vivo environment, they may represent a different group of cells processing triploblastic differentiation capability that holds tremendous potential in regenerative medicine.
Nevertheless, FReP cell generation is distinct from MUSE cell collection. Activation and isolation of MUSE cells require severe cellular stress conditions, such as lengthy incubation and digestion, hypoxia, and low temperatures, 485 which assist in killing off all of the other viable cells. FMOD reprogramming does not require hypoxia or low temperatures, and the resultant FReP cells and FReP‐basal cells are both viable. FReP cells resemble quiescent stem cells in multiple ways, 481 and as such, the mechanism by which FMOD assists in reprogramming demands a thorough exploration. Bearing in mind the multiple striking similarities shared by FReP and MUSE cells, the relationship between these two populations should also be further investigated. MUSE cells are considered a primary source of iPSCs in human fibroblasts in the elite model for iPSC generation. 486 , 487 However, the mechanism governing the transition from nontumorigenic MUSE cells to tumorigenic iPSCs remains an enigma. Further exploration into the mechanism of MUSE cell generation, as well as FMOD reprogramming, may also benefit to clarify the molecular roadmap of somatic cell reprogramming in general.
7. CONCLUSION
A diversity of novel multipotent/pluripotent cell sources is recruited as regenerative medicine outlets (Figure 1), particularly for bone regeneration in virtue of continued worldwide collaboration. Although their potential is irrefutable, and the opportunity to develop personalized cell therapy (in the cases of iPSCs and FReP cells) is extremely enticing, each of these cell sources has its own obstacles (Table 2) that must be understood entirely and overcome before they may be used on a large scale in a clinical setting. Despite the preliminary efficacy and safety assessment in a laboratory setting, further clinical data are necessary to determine their therapeutic benefits and safety, as well as to optimize their use as a part of the novel regenerative medicine strategy. Furthermore, ways in which we can promote seeding cells survive, growth, and differentiation into desired tissues via the implantation vehicle or scaffolds is also an open question for global collaboration, although previous studies may already point out some fundamental directions. 51 , 52 We believe that in light of the currently existing evidence, a new era of cell‐based bone regeneration is becoming a reality with the continued collaborative efforts of scientists, physicians, industry, and regulatory agencies.
TABLE 2.
Summary of novel cell sources for bone regeneration discussed in this review
| Cell types | Pros | Cons |
|---|---|---|
| Adult MSCs |
|
|
| Fetal MSCs |
|
|
| ESCs |
|
|
| iPSCs |
|
|
| FReP cells |
|
|
| MUSE cells |
|
|
CONFLICT OF INTERESTS
Dr. Zhong Zheng is an inventor on FMOD‐related patents assigned to UCLA, and a founder and an office of Scarless Laboratories Inc., which sublicenses fibromodulin‐related patents from the UC Regents. Dr. Zheng also holds equity in the company.
CONTRIBUTIONS
Dr. Chenshuang Li drafted the manuscript, Mr. Zane Milles conducted the literature collection and proofreading, and Dr. Zhong Zheng conceived the ideas of the manuscript and provided revisions to the scientific concept. All authors read and approved the final manuscript.
ETHICS APPROVAL STATEMENT
Ethical approval not applicable to this review article. They will have been obtained from the original authors of the cited references.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Kang Ting and Chia Soo from the University of California, Los Angeles, for their enthusiastic support.
Li C, Mills Z, Zheng Z. Novel cell sources for bone regeneration. MedComm. 2021;2:145–174. 10.1002/mco2.51
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no new datasets were generated for this review article.
REFERENCES
- 1. United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States. In: Chapter 6: Musculoskeletal injuries, 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeon; 2011:129‐143. [Google Scholar]
- 2. Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis. J Bone Miner Res. 1992;7(9):1005‐1010. [DOI] [PubMed] [Google Scholar]
- 4. Melton LJ, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res. 1998;13(12):1915‐1923. [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Johnell O, Oden A, et al. Long‐term risk of osteoporotic fracture in Malmo. Osteoporosis Int. 2000;11(8):669‐674. [DOI] [PubMed] [Google Scholar]
- 6.What is osteoporosis? International Osteoporosis Foundation. https://www.iofbonehealth.org/what-is-osteoporosis. [Google Scholar]
- 7.What is osteoporosis and what causes it? National Osteoporosis Foundation. https://www.nof.org/patients/what-is-osteoporosis/. [Google Scholar]
- 8. Goodman SB, Gibon E, Pajarinen J, et al. Novel biological strategies for treatment of wear particle‐induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J R Soc Interface. 2014;11(93):20130962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez‐Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93‐101. [DOI] [PubMed] [Google Scholar]
- 10. Ong JL, Guda T. Translating Biomaterials for Bone Graft: Bench‐Top to Clinical Applications. Boca Raton, FL: CRC Press, Talyor & Francis Group; 2017:1‐263. [Google Scholar]
- 11. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioactive Mater. 2017;2(4):224‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pirris SM, Nottmeier EW, Kimes S, O'Brien M, Rahmathulla G. A retrospective study of iliac crest bone grafting techniques with allograft reconstruction: do patients even know which iliac crest was harvested? Clinical article. J Neurosurg Spine. 2014;21(4):595‐600. [DOI] [PubMed] [Google Scholar]
- 13. Narotam PK, Jose S, Nathoo N, Taylon C, Vora Y. Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique. Spine. 2004;29(24):2861‐2867; discussion 2868‐2869. [DOI] [PubMed] [Google Scholar]
- 14. Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP‐2) mRNA expression. Tissue Eng. 2006;12(12):3459‐3465. [DOI] [PubMed] [Google Scholar]
- 15. Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell‐seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A. 2009;88(3):778‐786. [DOI] [PubMed] [Google Scholar]
- 16. Chan BP, Hui TY, Wong MY, Yip KH, Chan GC. Mesenchymal stem cell‐encapsulated collagen microspheres for bone tissue engineering. Tissue Eng Part C Methods. 2010;16(2):225‐235. [DOI] [PubMed] [Google Scholar]
- 17. Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Wozney JM, Wikesjo UM. Bone reconstruction following implantation of rhBMP‐2 and guided bone regeneration in canine alveolar ridge defects. Clin Oral Implants Res. 2007;18(2):224‐230. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):199‐215. [DOI] [PubMed] [Google Scholar]
- 19. de la Puente P, Ludeña D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp Cell Res. 2014;322(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk‐BMP‐2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115‐3124. [DOI] [PubMed] [Google Scholar]
- 21. Kim HJ, Kim UJ, Kim HS, et al. Bone tissue engineering with premineralized silk scaffolds. Bone. 2008;42(6):1226‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mieszawska AJ, Fourligas N, Georgakoudi I, et al. Osteoinductive silk‐silica composite biomaterials for bone regeneration. Biomaterials. 2010;31(34):8902‐8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J, Kim IS, Cho TH, et al. Bone regeneration using hyaluronic acid‐based hydrogel with bone morphogenic protein‐2 and human mesenchymal stem cells. Biomaterials. 2007;28(10):1830‐1837. [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Kim IS, Hwang SJ, Kim HC, Park Y, Sun K. Bone regeneration using MMP sensitive‐hyaluronic acid based hydrogels. 2009 35th Annual Northeast Bioengineering Conference. 2009:42. [Google Scholar]
- 25. Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31(26):6772‐6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levengood SL, Zhang M. Chitosan‐based scaffolds for bone tissue engineering. J Mater Chem B Mater Biol Med. 2014;2(21):3161‐3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cicco SR, Vona D, De Giglio E, et al. Chemically modified diatoms biosilica for bone cell growth with combined drug‐delivery and antioxidant properties. ChemPlusChem. 2015;80(7):1104‐1112. [DOI] [PubMed] [Google Scholar]
- 28. Venkatesan J, Vinodhini PA, Sudha PN, Kim SK. Chapter five ‐ chitin and chitosan composites for bone tissue regeneration. Adv Food Nutr Res. 2014;73:59‐81. [DOI] [PubMed] [Google Scholar]
- 29. Deepthi S, Venkatesan J, Kim SK, Bumgardner JD, Jayakumar R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int J Biol Macromol. 2016;93:1338‐1353. [DOI] [PubMed] [Google Scholar]
- 30. Tamburaci S, Tihminlioglu F. Diatomite reinforced chitosan composite membrane as potential scaffold for guided bone regeneration. Mater Sci Eng C. 2017;80:222‐231. [DOI] [PubMed] [Google Scholar]
- 31. Baino F, Novajra G, Vitale‐Brovarone C. Bioceramics and acaffolds: a winning combination for tissue engineering. Front Bioeng Biotech. 2015;3:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuttappan S, Mathew D, Nair MB. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering ‐ a mini review. Int J Biol Macromol. 2016;93(Pt B):1390‐1401. [DOI] [PubMed] [Google Scholar]
- 33. Denes E, Barriere G, Poli E, Leveque G. Commentary: bioceramics and scaffolds: a winning combination for tissue engineering. Front Bioeng Biotech. 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Stok J, Hartholt KA, Schoenmakers DAL, Arts JJC. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: a systematic review. Bone Joint Res. 2017;6(7):423‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic‐co‐glycolic) acid (PLGA)‐based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640‐3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng Z, Yin W, Zara JN, et al. The use of BMP‐2 coupled ‐ Nanosilver‐PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials. 2010;31(35):9293‐9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng Biotechnol. 2006; 102;47‐90. [DOI] [PubMed] [Google Scholar]
- 38. Nair LS, Lee DA, Bender JD, et al. Synthesis, characterization, and osteocompatibility evaluation of novel alanine‐based polyphosphazenes. J Biomed Mater Res A. 2006;76(1):206‐213. [DOI] [PubMed] [Google Scholar]
- 39. Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21(32‐33):3368‐3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goonoo N, Bhaw‐Luximon A, Passanha P, Esteves SR, Jhurry D. Third generation poly(hydroxyacid) composite scaffolds for tissue engineering. J Biomed Mater Res B Appl Biomater. 2017;105(6):1667‐1684. [DOI] [PubMed] [Google Scholar]
- 41. Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical application. Drug Des Devel Ther. 2018;12:3117‐3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gomez J, Rodriguez M, Banos V, et al. Orthopedic implant infection: prognostic factors and influence of long‐term antibiotic treatment on evolution. Prospective study, 1992–1999. Enferm Infecc Microbiol Clin. 2003;21(5):232‐236. [DOI] [PubMed] [Google Scholar]
- 43. Darouiche RO. Treatment of infections associated with surgical implants. N Eng J Med 2004;350(14):1422‐1429. [DOI] [PubMed] [Google Scholar]
- 44. Giavaresi G, Borsari V, Fini M, et al. Preliminary investigations on a new gentamicin and vancomycin‐coated PMMA nail for the treatment of bone and intramedullary infections: an experimental study in the rabbit. J Orthop Res. 2008;26(6):785‐792. [DOI] [PubMed] [Google Scholar]
- 45. Shirai T, Tsuchiya H, Shimizu T, Ohtani K, Zen Y, Tomita K. Prevention of pin tract infection with titanium‐copper alloys. J Biomed Mater Res B Appl Biomater. 2009;91(1):373‐380. [DOI] [PubMed] [Google Scholar]
- 46. Khosravi AD, Ahmadi F, Salmanzadeh S, Dashtbozorg A, Montazeri EA. Study of bacteria isolated from orthopedic implant infections and their antimicrobial susceptibility pattern. Res J Microbiol. 2009;4(4):158‐163. [Google Scholar]
- 47. Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331‐2339. [DOI] [PubMed] [Google Scholar]
- 48. Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty. A ten‐year survey of 1,688 hips. Clin Orthop Relat Res. 1993(292):210‐214. [PubMed] [Google Scholar]
- 49. Liu Y, Zheng Z, Zara JN, et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL‐lactic‐co‐glycolic acid)‐coated stainless steel. Biomaterials. 2012;33(34):8745‐8756. [DOI] [PubMed] [Google Scholar]
- 50. Murphy M, Ting K, Zhang XL, Soo C, Zheng Z. Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J Nanomater. 2015;696918. [Google Scholar]
- 51. Marrelli M, Maletta C, Inchingolo F, Alfano M, Tatullo M. Three‐point bending tests of zirconia core/veneer ceramics for dental restorations. Int J Dent. 2013;2013:831976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marrelli M, Pujia A, Palmieri F, et al. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with CAD‐CAM technology. Int J Immunopathol Pharmacol. 2016;29(4):778‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schultz DG. FDA Public Health Notification: Life‐Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. Health CfDaR, Food and Drug Administration; 2008. [Google Scholar]
- 54. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein‐2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471‐491. [DOI] [PubMed] [Google Scholar]
- 55. Balseiro S, Nottmeier EW. Vertebral osteolysis originating from subchondral cyst end plate defects in transforaminal lumbar interbody fusion using rhBMP‐2. Report of two cases. Spine J. 2010;10(7):e6‐e10. [DOI] [PubMed] [Google Scholar]
- 56. Kaneko H, Arakawa T, Mano H, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)‐2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27(4):479‐486. [DOI] [PubMed] [Google Scholar]
- 57. Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein‐2 as a bone‐graft substitute in a canine segmental defect model. J Orthop Res. 2000;18(2):289‐302. [DOI] [PubMed] [Google Scholar]
- 58. Zara JN, Siu RK, Zhang X, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo . Tissue Eng Part A. 2011;17(9‐10):1389‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dunn CA, Jin Q, Taba M, Jr. , Franceschi RT, Bruce Rutherford R, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11(2):294‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee YJ, Lee JH, Cho HJ, Kim HK, Yoon TR, Shin H. Electrospun fibers immobilized with bone forming peptide‐1 derived from BMP7 for guided bone regeneration. Biomaterials. 2013;34(21):5059‐5069. [DOI] [PubMed] [Google Scholar]
- 61. Zhang YF, Wu CT, Luo T, Li S, Cheng XR, Miron RJ. Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone. 2012;51(4):704‐713. [DOI] [PubMed] [Google Scholar]
- 62. Sun P, Wang JX, Zheng YN, Fan Y, Gu ZY. BMP2/7 heterodimer is a stronger inducer of bone regeneration in peri‐implant bone defects model than BMP2 or BMP7 homodimer. Dent Mater J. 2012;31(2):239‐248. [DOI] [PubMed] [Google Scholar]
- 63. Williams JC, Maitra S, Anderson MJ, Christiansen BA, Reddi AH, Lee MA. BMP‐7 and Bone regeneration: evaluation of dose‐response in a rodent segmental defect model. J Orthop Trauma. 2015;29(9):e336‐341. [DOI] [PubMed] [Google Scholar]
- 64. Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein‐2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84(12):2123‐2134. [DOI] [PubMed] [Google Scholar]
- 65. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(Suppl 3):S32‐38. [DOI] [PubMed] [Google Scholar]
- 66. Lissenberg‐Thunnissen SN, de Gorter DJJ, Sier CFM, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35(9):1271‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sreekumar V, Aspera‐Werz RH, Tendulkar G, et al. BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re‐)implementation by personalized medicine. Arch Toxicol. 2017;91(3):1353‐1366. [DOI] [PubMed] [Google Scholar]
- 68. Ting K, Vastardis H, Mulliken JB, et al. Human NELL‐1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 69. Aghaloo T, Cowan CM, Chou YF, et al. Nell‐1‐induced bone regeneration in calvarial defects. Am J Pathol. 2006;169(3):903‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li W, Zara JN, Siu RK, et al. Nell‐1 enhances bone regeneration in a rat critical‐sized femoral segmental defect model. Plast Reconstr Surg. 1995;96(6):1251‐9.discussion 1260‐1. 2011;127(2):580‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Siu RK, Lu SS, Li W, et al. Nell‐1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011;17(7‐8):1123‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. James AW, Shen J, Zhang X, et al. NELL‐1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. James AW, Pan A, Chiang M, et al. A new function of Nell‐1 protein in repressing adipogenic differentiation. Biochem Bioph Res Commun. 2011;411(1):126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xue J, Peng J, Yuan M, et al. NELL1 promotes high‐quality bone regeneration in rat femoral distraction osteogenesis model. Bone. 2011;48(3):485‐495. [DOI] [PubMed] [Google Scholar]
- 75. Shen J, James AW, Zara JN, et al. BMP2‐Induced inflammation can be suppressed by the osteoinductive growth factor NELL‐1. Tissue Eng Part A. 2013;19(21‐22):2390‐2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shen J, James AW, Zhang X, et al. Novel Wnt regulator NEL‐like molecule‐1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186(2):419‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang MH, Lim KT, Choung PH, Cho CS, Chung JH. Application of ultrasound stimulation in bone tissue engineering. Int J Stem Cells. 2010;3(2):74‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jacobs A. Bone growth stimulation: what the evidence reveals. Podiatry Today. 2016;29(5):44‐48. [Google Scholar]
- 79. Schandelmaier S, Kaushal A, Lytvyn L, et al. Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. BMJ. 2017;356:j656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nair AK, Gautieri A, Chang S‐W, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun. 2013;4:1724‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Black J, Baranowski T, Brighton C. Electrochemical aspects of dc stimulation of osteogenesis. Bioelectrochem Bioenerg. 1984;12(3):323‐327. [Google Scholar]
- 82. Bodamyali T, Kanczler J, Simon B, Blake D, Stevens C. Effect of faradic products on direct current‐stimulated calvarial organ culture calcium levels. Biochem Bioph Res Commun. 1999;264(3):657‐661. [DOI] [PubMed] [Google Scholar]
- 83. Shirkhanzadeh M. Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes. J Mater Sci Mater Medi. 1998;9(2):67‐72. [DOI] [PubMed] [Google Scholar]
- 84. Jeon H, Schmidt R, Barton JE, et al. Chemical patterning of ultrathin polymer films by direct‐write multiphoton lithography. J Am Chem Soc. 2011;133(16):6138‐6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Z, Clark CC, Brighton CT. Up‐regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am. 2006;88(5):1053‐1065. [DOI] [PubMed] [Google Scholar]
- 86. Ning C, Yu P, Zhu Y, et al. Built‐in microscale electrostatic fields induced by anatase–rutile‐phase transition in selective areas promote osteogenesis. NPG Asia Mater. 2016;8(3):e243‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Y, Zheng Z, Yu M, et al. Using an Engineered galvanic redox system to generate positive surface potentials that promote osteogenic functions. ACS Appl Mater Interfaces. 2018;10(18):15449‐15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sacchetti B, Funari A, Michienzi S, et al. Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324‐336. [DOI] [PubMed] [Google Scholar]
- 89. Chan CKF, Gulati GS, Sinha R, et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43‐56 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ballini A, Cantore S, Scacco S, Coletti D, Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine 2018. Stem Cells Int. 2018;2018:6927401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ballini A, Scacco S, Coletti D, Pluchino S, Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Stem Cells Int. 2017;2017:3292810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Spagnuolo G, Codispoti B, Marrelli M, Rengo C, Rengo S, Tatullo M. Commitment of oral‐derived stem cells in dental and maxillofacial applications. Dent J (Basel). 2018;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ambrosi TH, Longaker MT, Chan CKF. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019;7:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three‐dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 95. Yamanouchi K, Satomura K, Gotoh Y, et al. Bone formation by transplanted human osteoblasts cultured within collagen sponge with dexamethasone in vitro. J Bone Miner Res. 2001;16(5):857‐867. [DOI] [PubMed] [Google Scholar]
- 96. Park MS, Kim SS, Cho SW, Choi CY, Kim BS. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J Biomed Mater Res B. 2006;79B(2):353‐359. [DOI] [PubMed] [Google Scholar]
- 97. Becker AJ, Till JE, Mcculloch EA. Cytological demonstration of clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197(486):452‐454. [DOI] [PubMed] [Google Scholar]
- 98. Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Asatrian G, Pham D, Hardy WIR, James AW, Peault B. Stem cell technology for bone regeneration: current status and potential applications. Stem Cells Cloning. 2015;8:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Walmsley GG, Ransom RC, Zielins ER, et al. Stem cells in bone regeneration. Stem Cell Rev Rep. 2016;12(5):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Friedenstein AJ, Piatetzky S, II , Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Expe Morphol. 1966;16(3):381‐390. [PubMed] [Google Scholar]
- 102. Cohnheim J. Ueber entzundung und eiterung. Archiv für Pathol Anatomie Physiol klinische Medizin. 1867;40:1‐79. [Google Scholar]
- 103. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641‐650. [DOI] [PubMed] [Google Scholar]
- 104. Minamide A, Yoshida M, Kawakami M, et al. The use of cultured bone marrow cells in type I collagen gel and porous hydroxyapatite for posterolateral lumbar spine fusion. Spine. 2005;30(10):1134‐1138. [DOI] [PubMed] [Google Scholar]
- 105. Hutton DL, Grayson WL. Stem cell‐based approaches to engineering vascularized bone. Curr Opin Chem Eng. 2014;3:75‐82. [Google Scholar]
- 106. Zhao JJ, Yang CB, Su C, et al. Reconstruction of orbital defects by implantation of antigen‐free bovine cancellous bone scaffold combined with bone marrow mesenchymal stem cells in rats. Graef Arch Clin Exp. 2013;251(5):1325‐1333. [DOI] [PubMed] [Google Scholar]
- 107. Fernandes MBC, Guimaraes JAM, Casado PL, et al. The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet Res. 2014;10:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Velez R, Hernandez‐Fernandez A, Caminal M, et al. Treatment of femoral head osteonecrosis with advanced cell therapy in sheep. Arch Orthop Trauma Surg. 2012;132(11):1611‐1618. [DOI] [PubMed] [Google Scholar]
- 109. Shamsul BS, Tan KK, Chen HC, Aminuddin BS, Ruszymah BHI. Posterolateral spinal fusion with ostegenesis induced BMSC seeded TCP/HA in a sheep model. Tissue Cell. 2014;46(2):152‐158. [DOI] [PubMed] [Google Scholar]
- 110. Gan YK, Dai KR, Zhang P, Tang TT, Zhu ZN, Lu JX. The clinical use of enriched bone marrow stem cells combined with porous beta‐tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973‐3982. [DOI] [PubMed] [Google Scholar]
- 111. Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11(3):312‐324. [DOI] [PubMed] [Google Scholar]
- 112. Stenderup K, Justesen J, Eriksen EF, Rattan SIS, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16(6):1120‐1129. [DOI] [PubMed] [Google Scholar]
- 113. Izadpanah R, Trygg C, Kriedt C, Bunnell B. Biological comparison of mesenchymal stem cells derived from bone marrow and adipose tissue. Mol Ther. 2006;13:S134‐S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal stem or stromal cells: toward a better understanding of their biology? Transfus Med Hemoth. 2010;37(2):75‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Phinney DG. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J Cell Biochem. 2012;113(9):2806‐2812. [DOI] [PubMed] [Google Scholar]
- 116. Bianchi G, Banfi A, Mastrogiacomo M, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287(1):98‐105. [DOI] [PubMed] [Google Scholar]
- 117. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24(2):462‐471. [DOI] [PubMed] [Google Scholar]
- 118. Dhanasekaran M, Indumathi S, Lissa RP, Harikrishnan R, Rajkumar JS, Sudarsanam D. A comprehensive study on optimization of proliferation and differentiation potency of bone marrow derived mesenchymal stem cells under prolonged culture condition. Cytotechnology. 2013;65(2):187‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Aslan H, Zilberman Y, Kandel L, et al. Osteogenic differentiation of noncultured immunoisolated bone marrow‐derived CD105+ cells. Stem Cells. 2006;24(7):1728‐1737. [DOI] [PubMed] [Google Scholar]
- 120. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 121. Liebergall M, Schroeder J, Mosheiff R, et al. Stem cell‐based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21(8):1631‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. O'Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001:391:S190‐S207. [DOI] [PubMed] [Google Scholar]
- 123. Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheumat. 2004;50(5):1522‐1532. [DOI] [PubMed] [Google Scholar]
- 124. Hiraoka K, Grogan S, Olee T, Lotz M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology. 2006;43(3‐4):447‐454. [PubMed] [Google Scholar]
- 125. de Mos M, Koevoet WJ, Jahr H, et al. Intrinsic differentiation potential of adolescent human tendon tissue: an in‐vitro cell differentiation study. BMC Musculoskelet Dis. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shen HC, Peng HR, Usas A, et al. Ex vivo gene therapy‐induced endochondral bone formation: comparison of muscle‐derived stem cells and different subpopulations of primary muscle‐derived cells. Bone. 2004;34(6):982‐992. [DOI] [PubMed] [Google Scholar]
- 127. Wright VJ, Peng HR, Usas A, et al. BMP4‐expressing muscle‐derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6(2):169‐178. [DOI] [PubMed] [Google Scholar]
- 128. Peng HR, Huard J. Muscle‐derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol. 2004;12(3‐4):311‐319. [DOI] [PubMed] [Google Scholar]
- 129. Kuhl T, Mezger M, Hausser I, Handgretinger R, Bruckner‐Tuderman L, Nystrom A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Mol Ther. 2015;23(8):1368‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endodont. 2009;35(11):1536‐1542. [DOI] [PubMed] [Google Scholar]
- 131. Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191‐199. [DOI] [PubMed] [Google Scholar]
- 133. Lanzoni G, Alviano F, Marchionni C, et al. Isolation of stem cell populations with trophic and immunoregulatory functions from human intestinal tissues: potential for cell therapy in inflammatory bowel disease. Cytotherapy. 2009;11(8):1020‐1031. [DOI] [PubMed] [Google Scholar]
- 134. Wang YN, Yu XP, Chen EM, Li LN. Liver‐derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Res Ther. 2016;7:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rotter N, Oder J, Lindner U, et al. Isolation and characterisation of stem cells from different human salivary glands. Tissue Eng Part A. 2008;14(5):750‐751. [Google Scholar]
- 136. Rotter N, Oder J, Schlenke P, et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17(3):509‐518. [DOI] [PubMed] [Google Scholar]
- 137. Moshaverinia A, Chen C, Xu XT, et al. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD‐modified alginate scaffold. Tissue Eng Part A. 2014;20(3‐4):611‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Menicanin D, Mrozik KM, Wada N, et al. Periodontal‐ligament‐derived stem cells exhibit the capacity for long‐term survival, self‐renewal, and regeneration of multiple tissue types in vivo . Stem Cells Dev. 2014;23(9):1001‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zheng Y, Liu Y, Zhang CM, et al. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88(3):249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kim SH, Kim KH, Seo BM, et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri‐implant defect model: A pilot Sstudy. J Periodontol. 2009;80(11):1815‐1823. [DOI] [PubMed] [Google Scholar]
- 141. d'Aquino R, De Rosa A, Lanza V, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75‐83. [DOI] [PubMed] [Google Scholar]
- 142. Giuliani A, Manescu A, Langer M, et al. Three years after transplants in human mandibles, histological and in‐line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: Biological and clinical implications. Stem Cells Trans Med. 2013;2(4):316‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 2001;7(2):211‐228. [DOI] [PubMed] [Google Scholar]
- 144. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue‐derived stromal cells. J Cell Physiol. 2001;189(1):54‐63. [DOI] [PubMed] [Google Scholar]
- 145. Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87(1):125‐128. [DOI] [PubMed] [Google Scholar]
- 146. Lindroos B, Boucher S, Chase L, et al. Serum‐free, xeno‐free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11(7):958‐972. [DOI] [PubMed] [Google Scholar]
- 147. Schreml S, Babilas P, Fruth S, et al. Harvesting human adipose tissue‐derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11(7):947‐957. [DOI] [PubMed] [Google Scholar]
- 148. Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose‐derived stem cells in vascular growth and tissue repair. Curr Opin Organ Tran. 2010;15(1):86‐91. [DOI] [PubMed] [Google Scholar]
- 149. Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue‐derived stem cells. Keio J Med. 2005;54(3):132‐141. [DOI] [PubMed] [Google Scholar]
- 150. Boquest AC, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2(4):319‐329. [DOI] [PubMed] [Google Scholar]
- 151. Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi‐lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101‐109. [DOI] [PubMed] [Google Scholar]
- 153. Rodriguez AM, Elabd C, Delteil F, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315(2):255‐263. [DOI] [PubMed] [Google Scholar]
- 154. 2019 American Society of Plastic Surgeons. Plastic Surgery Statistics Report. 2019. https://www.plasticsurgery.org/news/plastic-surgery-statistics. Accessed June 25, 2020.
- 155. Cardenas‐Camarena L, Andres Gerardo LP, Duran H, Bayter‐Marin JE. Strategies for reducing fatal complications in liposuction. Plast Reconstr Surgery Glob Open. 2017;5(10):e1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cirino E. Cool sculpting vs. liposuction: know the difference. https://www.healthline.com/health/coolsculpting-vs-liposuction. 2019. Accessed October 8, 2019. [Google Scholar]
- 157. Mowlavi A. Be prepared: liposuction pain explained. https://drlaguna.com/liposuction-pain/. 2020. Accessed December 7, 2017. [Google Scholar]
- 158. Kaoutzanis C, Gupta V, Winocour J, et al. Cosmetic liposuction: preoperative risk factors, major complication rates, and safety of combined procedures. Aesthet Surg J. 2017;37(6):680‐694. [DOI] [PubMed] [Google Scholar]
- 159. Christman KD. Death following suction lipectomy and abdominoplasty. Plast Reconstr Surg. 1995;96(6):1251‐1259; discussion 1260‐1. 1986;78(3):428. [DOI] [PubMed] [Google Scholar]
- 160. McAllister RK, Meyer TA, Bittenbinder TM. Can local anesthetic‐related deaths during liposuction be prevented? Plast Reconstr Surg. 1995;96(6):1251‐1259; discussion 1260‐1. 2008;122(6):232e‐233e. [DOI] [PubMed] [Google Scholar]
- 161. Schnur P, Penn J, Fodor PB. Deaths related to liposuction. N Eng J Med. 1999;341(13):1002‐1003. [PubMed] [Google Scholar]
- 162. Rigel DS, Wheeland RG. Deaths related to liposuction. N Eng J Med. 1999;341(13):1001‐1002; author reply 1002‐1003. [PubMed] [Google Scholar]
- 163. Talmor M, Barie PS. Deaths related to liposuction. N Eng J Med. 1999;341(13):1001; author reply 1002‐1003. [PubMed] [Google Scholar]
- 164. Klein JA. Deaths related to liposuction. N Eng J Med. 1999;341(13):1001; author reply 1002‐1003. [PubMed] [Google Scholar]
- 165. Vermeulen C, Serra M, Roujeau JC. Deaths related to liposuction. N Eng J Med. 1999;341(13):1000‐1001; author reply 1002‐1003. [PubMed] [Google Scholar]
- 166. Ginsberg MM, Gresham L. Deaths related to liposuction. N Eng J Med. 1999;341(13):1000; author reply 1002‐1003. [DOI] [PubMed] [Google Scholar]
- 167. Rao RB, Ely SF, Hoffman RS. Deaths related to liposuction. N Eng J Med. 1999;340(19):1471‐1475. [DOI] [PubMed] [Google Scholar]
- 168. Katz BE, Bruck MC, Felsenfeld L, Frew KE. Power liposuction: a report on complications. Dermatol Surg. 2003;29(9):925‐927; discussion 927. [DOI] [PubMed] [Google Scholar]
- 169. Grazer FM, de Jong RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg. 1995;96(6):1251‐1259; discussion 1260‐1. 2000;105(1):436‐446; discussion 447‐438. [DOI] [PubMed] [Google Scholar]
- 170. Ezzeddine H, Husari A, Nassar H, et al. Life threatening complications post‐liposuction. Aesthetic Plast Surg. 2018;42(2):384‐387. [DOI] [PubMed] [Google Scholar]
- 171. Haeck PC, Swanson JA, Schechter LS, et al. Evidence‐based patient safety advisory: blood dyscrasias. Plast Reconstr Surg. 1995;96(6):1251‐9; discussion 1260‐1. 2009;124(4 Suppl):82S‐95S. [DOI] [PubMed] [Google Scholar]
- 172. Horton JB, Reece EM, Broughton G, 2nd , Janis JE, Thornton JF, Rohrich RJ. Patient safety in the office‐based setting. Plast Reconstr Surg. 1995;96(6):1251‐1259; discussion 1260‐1. 2006;117(4):61e‐80e. [DOI] [PubMed] [Google Scholar]
- 173. de Jong RH. Body mass index: risk predictor for cosmetic day surgery. Plast Reconstr Surg. 1995;96(6):1251‐1259; discussion 1260‐1. 2001;108(2):556‐561; discussion 562‐553. [DOI] [PubMed] [Google Scholar]
- 174. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xu HY, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest. 2003;112(12):1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rehman J, Traktuev D, Li JL, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292‐1298. [DOI] [PubMed] [Google Scholar]
- 177. Traktuev DO, Merfeld‐Clauss S, Li J, et al. A population of multipotent CD34‐positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77‐85. [DOI] [PubMed] [Google Scholar]
- 178. James AW, Zara JN, Zhang X, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Trans Med. 2012;1(6):510‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kargozar S, Mozafari M, Hashemian SJ, et al. Osteogenic potential of stem cells‐seeded bioactive nanocomposite scaffolds: a comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton's jelly, and adipose tissue. J Biomed Mater Res B. 2018;106(1):61‐72. [DOI] [PubMed] [Google Scholar]
- 180. Ardeshirylajimi A, Soleimani M, Hosseinkhani S, Parivar K, Yaghmaei P. A comparative study of osteogenic differentiation human induced pluripotent stem cells and adipose tissue derived mesenchymal stem cells. Cell J. 2014;16(3):235‐244. [PMC free article] [PubMed] [Google Scholar]
- 181. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 182. Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first‐trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396‐2402. [DOI] [PubMed] [Google Scholar]
- 183. Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14(4‐6):311‐324. [DOI] [PubMed] [Google Scholar]
- 184. Hamid AA, Idrus RBH, Bin Saim A, Sathappan S, Chua KH. Characterization of human adipose‐derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics. 2012;67(2):99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo . Rheumatol. 2008;47(2):126‐131. [DOI] [PubMed] [Google Scholar]
- 186. Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheumat. 2004;50(3):817‐827. [DOI] [PubMed] [Google Scholar]
- 187. Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402‐1416. [DOI] [PubMed] [Google Scholar]
- 188. Ishii M, Koike C, Igarashi A, et al. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005;332(1):297‐303. [DOI] [PubMed] [Google Scholar]
- 189. Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419‐427. [DOI] [PubMed] [Google Scholar]
- 190. Huss R. Isolation of primary and immortalized CD34(‐) hematopoietic and mesenchymal stem cells from various sources. Stem Cells. 2000;18(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 191. Simmons PJ, Torokstorb B. Identification of stromal cell precursors in human bBone‐marrow by a novel monoclonal‐antibody, Stro‐1. Blood. 1991;78(1):55‐62. [PubMed] [Google Scholar]
- 192. Planat‐Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells ‐ physiological and therapeutic perspectives. Circulation. 2004;109(5):656‐663. [DOI] [PubMed] [Google Scholar]
- 193. Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three‐dimensional perfusion culture of human adipose tissue‐derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25(7):1823‐1829. [DOI] [PubMed] [Google Scholar]
- 194. Boquest AC, Shahdadfar A, Brinchmann JE, Collas P. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol. 2006;325:35‐46. [DOI] [PubMed] [Google Scholar]
- 195. Varma MJO, Breuls RGM, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue‐derived stem cells. Stem Cells Dev. 2007;16(1):91‐104. [DOI] [PubMed] [Google Scholar]
- 196. Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208(1):64‐76. [DOI] [PubMed] [Google Scholar]
- 197. Al‐Nbaheen M, Vishnubalaji R, Ali D, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep. 2013;9(1):32‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose‐derived cells: temporal changes in stromal‐associated and stem cell‐associated markers. Stem Cells. 2006;24(2):376‐385. [DOI] [PubMed] [Google Scholar]
- 199. Lin CS, Ning HX, Lin GT, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14(10):1159‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells. 2013;5(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Alfaro LAS, Dick SA, Siegel AL, et al. CD34 Promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells. 2011;29(12):2030‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self‐renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635‐648. [DOI] [PubMed] [Google Scholar]
- 203. Trempus CS, Morris RJ, Ehinger M, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67(9):4173‐4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Trempus CS, Dang H, Humble MM, et al. Comprehensive microarray transcriptome profiling of CD34‐enriched mouse keratinocyte stem cells. J Invest Dermatol. 2007;127(12):2904‐2907. [DOI] [PubMed] [Google Scholar]
- 205. Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti‐nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783‐791. [DOI] [PubMed] [Google Scholar]
- 206. Kuci S, Kuci Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment‐promoting properties. Haematologica. 2010;95(4):651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20(1):53‐66. [DOI] [PubMed] [Google Scholar]
- 208. Lecourt S, Marolleau JP, Fromigue O, et al. Characterization of distinct mesenchymal‐like cell populations from human skeletal muscle in situ and in vitro . Exp Cell Res. 2010;316(15):2513‐2526. [DOI] [PubMed] [Google Scholar]
- 209. West‐Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell B. 2006;38(10):1625‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Branch MJ, Hashmani K, Dhillon P, Jones DRE, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophth Vis Sci. 2012;53(9):5109‐5116. [DOI] [PubMed] [Google Scholar]
- 211. Hashmani K, Branch MJ, Sidney LE, et al. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Ning HX, Lin GT, Lue T, Lin CS. Mesenchymal stem cell marker Stro‐1 is a 75kd endothelial antigen. Biochem Biophys Res Commun. 2011;413(2):353‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Tintut Y, Alfonso Z, Saini T, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108(20):2505‐2510. [DOI] [PubMed] [Google Scholar]
- 215. Lin GT, Liu G, Banie L, et al. Tissue distribution of mesenchymal stem cell marker Stro‐1. Stem Cells Dev. 2011;20(10):1747‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Holmes C, Stanford WL. Concise review: stem cell antigen‐1: expression, function, and enigma. Stem Cells. 2007;25(6):1339‐1347. [DOI] [PubMed] [Google Scholar]
- 218. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301‐313. [DOI] [PubMed] [Google Scholar]
- 219. Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229‐230. [DOI] [PubMed] [Google Scholar]
- 220. Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368(2):305‐310. [DOI] [PubMed] [Google Scholar]
- 221. Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6):1104‐1109. [DOI] [PubMed] [Google Scholar]
- 222. Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Crisan M, Corselli M, Chen WCW, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851‐2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Corselli M, Crisan M, Murray IR, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytom Part A. 2013;83A(8):714‐720. [DOI] [PubMed] [Google Scholar]
- 225. James AW, Zara JN, Corselli M, et al. Use of human perivascular stem cells for bone regeneration. JoVE. 2012(63):e2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. James AW, Zara JN, Corselli M, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Trans Med. 2012;1(9):673‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Chung CG, James AW, Asatrian G, et al. Human perivascular stem cell‐based bone graft substitute induces rat spinal fusion. Stem Cells Trans Med. 2014;3(10):1231‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chavez‐Munoz C, Nguyen KT, Xu W, Hong SJ, Mustoe TA, Galiano RD. Transdifferentiation of adipose‐derived stem cells into keratinocyte‐like cells: engineering a stratified epidermis. PLoS One. 2013;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair ‐ current views. Stem Cells. 2007;25(11):2896‐2902. [DOI] [PubMed] [Google Scholar]
- 230. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell‐targeting strategies, and immune modulation. Stem Cells Int. 2013:Article ID 732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Guimaraes‐Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo . Cell Stem Cell. 2017;20(3):345‐359 e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017;2017:5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Caplan AI. New MSC: MSCs as pericytes are sentinels and gatekeepers. J Orthop Res. 2017;35(6):1151‐1159. [DOI] [PubMed] [Google Scholar]
- 235. Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kocan B, Maziarz A, Tabarkiewicz J, Ochiya T, Banas‐Zabczyk A. Trophic activity and phenotype of adipose tissue‐derived mesenchymal stem cells as a background of their regenerative potential. Stem Cells Int. 2017;2017:1653254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149(4):393‐404. [DOI] [PubMed] [Google Scholar]
- 238. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815‐1822. [DOI] [PubMed] [Google Scholar]
- 239. Bai LH, Lennon DP, Eaton V, et al. Human bone marrow‐derived mesenchymal stem cells induce Th2‐polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Caplan AI. What's in a aame? Tissue Eng Part A. 2010;16(8):2415‐2417. [DOI] [PubMed] [Google Scholar]
- 242. Muschler GF, Nitto H, Boehm CA, Easley KA. Age‐ and gender‐related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19(1):117‐125. [DOI] [PubMed] [Google Scholar]
- 243. Stolzing A, Jones E, McGonagle D, Scutt A. Age‐related changes in human bone marrow‐derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163‐173. [DOI] [PubMed] [Google Scholar]
- 244. Bruna F, Contador D, Conget P, Erranz B, Sossa CL, Arango‐Rodriguez ML. Regenerative potential of mesenchymal stromal cells: age‐related changes. Stem Cells Int. 2016;2016:1461648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Schimke MM, Marozin S, Lepperdinger G. Patient‐specific age: the other side of the coin in advanced mesenchymal stem cell therapy. Front Physiol. 2015;6:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Faiella W, Atoui R. Immunotolerant properties of mesenchymal stem cells: updated review. Stem Cells Int. 2016;1859567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(9):S45‐S49. [DOI] [PubMed] [Google Scholar]
- 248. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890‐896. [DOI] [PubMed] [Google Scholar]
- 249. Morandi F, Raffaghello L, Bianchi G, et al. Immunogenicity of human mesenchymal stem cells in HLA‐class I‐restricted T‐cell responses against viral or tumor‐associated antigens. Stem Cells. 2008;26(5):1275‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Li XY, Ding J, Zheng ZH, Li XY, Wu ZB, Zhu P. Long‐term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol Med Rep. 2012;6(5):1183‐1189. [DOI] [PubMed] [Google Scholar]
- 251. Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture‐expanded mesenchymal stem cells in the microvasculature in vivo observations of cell kinetics. Circ Res. 2009;104(3):398‐U204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell. 2009;5(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614‐2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long‐term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575‐1578. [DOI] [PubMed] [Google Scholar]
- 255. Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I‐ and class II‐mismatched recipient mice. Blood. 2005;106(13):4057‐4065. [DOI] [PubMed] [Google Scholar]
- 256. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy. The POSEIDON randomized trial. JAMA. 2012;308(22):2369‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Richardson C, Roberts E, Nelms S, Roberts NB. Optimisation of whole blood and plasma manganese assay by ICP‐MS without use of a collision cell. Clin Chem Lab Med. 2012;50(2):317‐323. [DOI] [PubMed] [Google Scholar]
- 258. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Rubio D, Garcia‐Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035‐3039. [DOI] [PubMed] [Google Scholar]
- 261. Wang Y, Huso DL, Harrington J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7(6):509‐519. [DOI] [PubMed] [Google Scholar]
- 262. Mori S, Chang JT, Andrechek ER, et al. Anchorage‐independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28(31):2796‐2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Rosland GV, Svendsen A, Torsvik A, et al. Long‐term cultures of bone marrow‐derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331‐5339. [DOI] [PubMed] [Google Scholar]
- 264. Zhou YF, Bosch‐Marce M, Okuyama H, et al. Spontaneous transformation of cultured mouse bone marrow‐derived stromal cells. Cancer Res. 2006;66(22):10849‐10854. [DOI] [PubMed] [Google Scholar]
- 265. Ren ZH, Wang JY, Zhu WW, et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro . Exp Cell Res. 2011;317(20):2950‐2957. [DOI] [PubMed] [Google Scholar]
- 266. Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371‐379. [DOI] [PubMed] [Google Scholar]
- 267. Garcia S, Bernad A, Martin MC, et al. Pitfalls in spontaneousin vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316(9):1648‐1650. [DOI] [PubMed] [Google Scholar]
- 268. Torsvik A, Rosland GV, Svendsen A, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross‐contamination: putting the research Field on track (Correction letter for Cancer Res. 2009 Jul 1, 69(13), 5331–9). Cancer Res. 2010;70(15):6393‐6396. [DOI] [PubMed] [Google Scholar]
- 269. Torsvik A, Rosland GV, Bjerkvig R. Comment to: “Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro” by Z. Ren et al. Exp. Cell Res. 317 (2011) 2950–2957, Spontaneous transformation of mesenchymal stem cells in culture: facts or fiction? Exp Cell Res. 2012;318(5):441‐443. [DOI] [PubMed] [Google Scholar]
- 270. Podolanczuk A, Psaila B, Lyden D. Role of bone microenvironment/metastatic niche in cancer progression. Bone and cancer. Topics in bone biology. London: Springer; 2010;5:89‐101. [Google Scholar]
- 271. Park C, Lee JY, Yoon YS. Role of Bone marrow‐derived lymphatic endothelial progenitor cells for lymphatic neovascularization. Trends Cardiovas Med. 2011;21(5):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Omelchenko DO, Rzhaninova AA, Gol'dshtein DV. Comparative transcriptome pairwise analysis of spontaneously transformed multipotent stromal cells from human adipose tissue. Russ J Genet. 2014;50(1):96‐104. [PubMed] [Google Scholar]
- 273. Pan Q, Fouraschen SM, de Ruiter PE, et al. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood). 2014;239(1):105‐115. [DOI] [PubMed] [Google Scholar]
- 274. Marrazzo P, Paduano F, Palmieri F, Marrelli M, Tatullo M. Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem Cells Int. 2016;2016:7230987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Tatullo M, Codispoti B, Paduano F, Nuzzolese M, Makeeva I. Strategic tools in regenerative and translational dentistry. Int J Mol Sci. 2019;20(8):1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Lye KL, Nordin N, Vidyadaran S, Thilakavathy K. Mesenchymal stem cells: from stem cells to sarcomas. Cell Biol Int. 2016;40(6):610‐618. [DOI] [PubMed] [Google Scholar]
- 277. Djouad F, Noel D, Noel D, et al. Immunosuppressive effect of mesenchymal stem cells in collagen‐induced arthritis. Arthritis Rheumat. 2003;48(9):S550‐S550. [Google Scholar]
- 278. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837‐3844. [DOI] [PubMed] [Google Scholar]
- 279. Karnoub AE, Weinberg RA. Chemokine networks and breast cancer metastasis. Breast Dis. 2006;26:75‐85. [DOI] [PubMed] [Google Scholar]
- 280. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557‐U554. [DOI] [PubMed] [Google Scholar]
- 281. Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adhesion Migr. 2012;6(3):220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kim JH, Lee YT, Hong JM, Hwang YI. Suppression of in vitro murine T cell proliferation by human adipose tissue‐derived mesenchymal stem cells is dependent mainly on cyclooxygenase‐2 expression. Anatomy Cell Biol. 2013;46(4):262‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, Hashemi SM, Moradi SLA, Soleimani M. Immunomodulatory effects of adipose‐derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int Immunopharmacol. 2014;20(2):316‐321. [DOI] [PubMed] [Google Scholar]
- 284. Holt DDC, Wood JA, Granick JL, Walker NJ, Clark KC, Borjesson DL. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue Ssource. Stem Cells Dev. 2014;23(11):1258‐1265. [DOI] [PubMed] [Google Scholar]
- 285. Akimoto K, Kimura K, Nagano M, et al. Umbilical cord blood‐derived mesenchymal stem cells inhibit, but adipose tissue‐derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013;22(9):1370‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Ren GW, Zhao X, Wang Y, et al. CCR2‐dependent recruitment of macrophages by tumor‐educated mesenchymal stromal cells promotes tumor development and is mimicked by TNF alpha. Cell Stem Cell. 2012;11(6):812‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24(3):781‐792. [DOI] [PubMed] [Google Scholar]
- 288. Conconi MT, Burra P, Di Liddo R, et al. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18(6):1089‐1096. [PubMed] [Google Scholar]
- 289. Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis‐supportive function and other potentials. Haematologica. 2006;91(8):1017‐1026. [PubMed] [Google Scholar]
- 290. Zhou C, Yang B, Tian Y, et al. Immunomodulatory effect of human umbilical cord Wharton's jelly‐derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. He H, Nagamura‐Inoue T, Takahashi A, et al. Immunosuppressive properties of Wharton's jelly‐derived mesenchymal stromal cells in vitro . Int J Hematol. 2015;102:368‐378. [DOI] [PubMed] [Google Scholar]
- 292. Davies JE, Walker JT, Keating A. Concise review: Wharton's jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Trans Med. 2017;6(7):1620‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J Biol Chem. 2000;275(13):9645‐9652. [DOI] [PubMed] [Google Scholar]
- 294. Li CS, Zheng Z, Su XX, et al. Activation of the extracellular signal‐regulated kinase signaling is critical for human umbilical cord mesenchymal stem cell osteogenic differentiation. Biomed Res Int. 2016;2016:2764372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus‐derived stem cells. Stem Cells. 2007;25(11):2886‐2895. [DOI] [PubMed] [Google Scholar]
- 296. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384‐1392. [DOI] [PubMed] [Google Scholar]
- 297. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220‐229. [DOI] [PubMed] [Google Scholar]
- 298. Hjortholm N, Jaddini E, Halaburda K, Snarski E. Strategies of pain reduction during the bone marrow biopsy. Ann Hematol. 2013;92(2):145‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Bosch J, Houben AP, Radke TF, et al. Distinct differentiation potential of “MSC” derived from cord blood and umbilical cord: are cord‐derived cells true mesenchymal stromal cells? Stem Cells Dev. 2012;21(11):1977‐1988. [DOI] [PubMed] [Google Scholar]
- 300. Subramanian A, Shu‐Uin G, Kae‐Siang N, et al. Human umbilical cord Wharton's jelly mesenchymal stem cells do not transform to tumor‐associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J Cell Biochem. 2012;113(6):1886‐1895. [DOI] [PubMed] [Google Scholar]
- 301. Klontzas ME, Kenanidis EI, Heliotis M, Tsiridis E, Mantalaris A. Bone and cartilage regeneration with the use of umbilical cord mesenchymal stem cells. Expert Opin Biol Ther. 2015:15:1541‐1552. [DOI] [PubMed] [Google Scholar]
- 302. Nanaev AK, Kohnen G, Milovanov AP, Domogatsky SP, Kaufman P. Stromal differentiation and architecture of the human umbilical cord. Placenta. 1997;18(1):53‐64. [DOI] [PubMed] [Google Scholar]
- 303. Schugar RC, Chirieleison SM, Wescoe KE, et al. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol. 2009;2019:789526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Whittle WL, Gibb W, Challis JRG. The characterization of human amnion epithelial and mesenchymal cells: the cellular expression, activity and glucocorticoid regulation of prostaglandin output. Placenta. 2000;21(4):394‐401. [DOI] [PubMed] [Google Scholar]
- 305. Insausti CL, Blanquer M, Bleda P, et al. The amniotic membrane as a source of stem cells. Histol Histopathol. 2010;25(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 306. Parolini O, Alviano F, Bagnara GP, et al. Concise review: Isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300‐311. [DOI] [PubMed] [Google Scholar]
- 307. Jeschke MG, Gauglitz GG, Phan TT, Herndon DN, Kita K. Umbilical cord lining membrane and Wharton's jelly‐derived mesenchymal stem cells: the similarities and differences. Open Tissue Eng Regen Med J. 2011;11(4):21‐27. [Google Scholar]
- 308. Pu L, Meng MY, Wu J, et al. Compared to the amniotic membrane, Wharton's jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Res Ther. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Subramanian A, Fong CY, Biswas A, Bongso A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton's jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS One. 2015;10(6):e0127992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Wang P, Liu X, Zhao L, et al. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015;18:236‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Salehinejad P, Alitheen NB, Ali AM, et al. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Develop Biol Anim. 2012;48(2):75‐83. [DOI] [PubMed] [Google Scholar]
- 313. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly‐derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14(6):11692‐11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Anzalone R, Lo Iacono M, Corrao S, et al. New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte‐like differentiative capacity. Stem Cells Dev. 2010;19(4):423‐438. [DOI] [PubMed] [Google Scholar]
- 315. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Bri J Haematol. 2000;109(1):235‐242. [DOI] [PubMed] [Google Scholar]
- 316. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669‐1675. [DOI] [PubMed] [Google Scholar]
- 317. Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical‐cord blood as a potential source of transplantable hematopoietic stem progenitor Cells. PNAS. 1989;86(10):3828‐3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Liu GP, Li YL, Sun J, et al. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood‐derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16(3):971‐982. [DOI] [PubMed] [Google Scholar]
- 319. Marmotti A, Mattia S, Castoldi F, et al. Allogeneic umbilical cord‐eerived mesenchymal stem cells as a potential source for cartilage and bone regeneration: an in vitro study. Stem Cells Int. 2017:Article ID 1732094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26(1):146‐150. [DOI] [PubMed] [Google Scholar]
- 321. Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010;34(7):693‐701. [DOI] [PubMed] [Google Scholar]
- 322. Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67(5):793‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Renda MC, Fecarotta E, Schillaci G, et al. Mesenchymal fetal stem cells (fMSC) from amniotic fluid (AF): expansion and phenotypic characterization. Blood. 2015;126(23):4758. [Google Scholar]
- 324. Moraghebi R, Kirkeby A, Chaves P, et al. Term amniotic fluid: an unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res Ther. 2017;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Dziadosz M, Young BK, Basch RS. Human amniotic fluid: a source of stem cells for possible therapeutic use Reply. Am J Obstet Gynecol. 2016;215(3):401. [DOI] [PubMed] [Google Scholar]
- 326. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145‐1147. [DOI] [PubMed] [Google Scholar]
- 327. Prelle K, Zink N, Wolf E. Pluripotent stem cells ‐ model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31(3):169‐186. [DOI] [PubMed] [Google Scholar]
- 328. Winkler J, Sotiriadou I, Chen S, Hescheler J, Sachinidis A. The potential of embryonic stem cells combined with ‐omics technologies as model systems for toxicology. Curr Med Chem. 2009;16(36):4814‐4827. [DOI] [PubMed] [Google Scholar]
- 329. Pouton CW, Haynes JM. Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov. 2007;6(8):605‐616. [DOI] [PubMed] [Google Scholar]
- 330. Liu WW, Deng YG, Liu Y, Gong WR, Deng WB. Stem cell models for drug discovery and toxicology studies. J Biochem Mol Toxic. 2013;27(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 331. Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Bio. 2016;17(3):170‐182. [DOI] [PubMed] [Google Scholar]
- 332. Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell‐derived mesenchymal stem cells. Stem Cells Dev. 2009;18(7):955‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Kuhn LT, Liu Y, Boyd NL, et al. Developmental‐like bone regeneration by human embryonic stem cell‐derived mesenchymal cells. Tissue Eng Part A. 2014;20(1‐2):365‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Buttery LD, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7(1):89‐99. [DOI] [PubMed] [Google Scholar]
- 335. Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10(9‐10):1518‐1525. [DOI] [PubMed] [Google Scholar]
- 336. Liu X, Wang P, Chen W, Weir MD, Bao C, Xu HH. Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater. 2014;10(10):4484‐4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Sugarman J. Human stem cell ethics: beyond the embryo. Cell Stem Cell. 2008;2(6):529‐533. [DOI] [PubMed] [Google Scholar]
- 338. Sugarman J, Siegel A. How to determine whether existing human embryonic stem cell lines can be used ethically. Cell Stem Cell. 2008;3(3):238‐239. [DOI] [PubMed] [Google Scholar]
- 339. Monitoring Stem Cell Research. Washington, DC: The President's Council on Bioethics; 2004. [Google Scholar]
- 340. Hyun I, Wilkerson A, Johnston J. Embryology policy: revisit the 14‐day rule. Nature. 2016;533(7602):169‐171. [DOI] [PubMed] [Google Scholar]
- 341. Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Zavazava N. Embryonic stem cells and potency to induce transplantation tolerance. Expert Opin Biol Ther. 2003;3(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 343. Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune‐privileged properties. Stem Cells. 2004;22(4):448‐456. [DOI] [PubMed] [Google Scholar]
- 344. Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26(1):89‐98. [DOI] [PubMed] [Google Scholar]
- 345. Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100‐104. [DOI] [PubMed] [Google Scholar]
- 346. Swijnenburg RJ, Tanaka M, Vogel H, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112(9):I166‐I172. [DOI] [PubMed] [Google Scholar]
- 347. Grinnemo KH, Kumagai‐Braesch M, Mansson‐Broberg A, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13(5):712‐724. [DOI] [PubMed] [Google Scholar]
- 348. Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53‐54. [DOI] [PubMed] [Google Scholar]
- 349. Enver T, Soneji S, Joshi C, et al. Cellular differentiation hierarchies in normal and culture‐adapted human embryonic stem cells. Hum Mol Genet. 2005;14(21):3129‐3140. [DOI] [PubMed] [Google Scholar]
- 350. Mitalipova MM, Rao RR, Hoyer DM, et al. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol. 2005;23(1):19‐20. [DOI] [PubMed] [Google Scholar]
- 351. Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem. 2014;289(8):4578‐4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Narva E, Autio R, Rahkonen N, et al. High‐resolution DNA analysis of human embryonic stem cell lines reveals culture‐induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28(4):371‐U103. [DOI] [PubMed] [Google Scholar]
- 353. Amps K, Andrews PW, Anyfantis G, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29(12):1132‐U1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31(3):146‐153. [DOI] [PubMed] [Google Scholar]
- 355. Werbowetski‐Ogilvie TE, Bosse M, Stewart M, et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. 2009;27(1):91‐97. [DOI] [PubMed] [Google Scholar]
- 356. Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663‐676. [DOI] [PubMed] [Google Scholar]
- 358. Yu JY, Vodyanik MA, Smuga‐Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917‐1920. [DOI] [PubMed] [Google Scholar]
- 359. Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. PNAS. 2008;105(8):2883‐2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Liu J, Brzeszczynska J, Samuel K, et al. Efficient episomal reprogramming of blood mononuclear cells and differentiation to hepatocytes with functional drug metabolism. Exp Cell Res. 2015;338(2):203‐213. [DOI] [PubMed] [Google Scholar]
- 361. Tashiro K, Inamura M, Kawabata K, et al. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009;27(8):1802‐1811. [DOI] [PubMed] [Google Scholar]
- 362. Kao CL, Tai LK, Chiou SH, et al. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010;19(2):247‐258. [DOI] [PubMed] [Google Scholar]
- 363. Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109(4):643‐652. [DOI] [PubMed] [Google Scholar]
- 364. Bilousova G, Jun DH, King KB, et al. Osteoblasts derived from induced pluripotent stem cells Form calcified structures in scaffolds both in vitro and in vivo . Stem Cells. 2011;29(2):206‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Ye JH, Xu YJ, Gao J, et al. Critical‐size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2‐modified iPSCs. Biomaterials. 2011;32(22):5065‐5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Li F, Niyibizi C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF‐beta family give rise to osteoblasts differentiation and form bone in vivo . BMC Cell Biol. 2012;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Hayashi T, Misawa H, Nakahara H, et al. Transplantation of osteogenically differentiated mouse iPS cells for bone repair. Cell Transplant. 2012;21(2‐3):591‐600. [DOI] [PubMed] [Google Scholar]
- 368. Villa‐Diaz LG, Brown SE, Liu Y, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30(6):1174‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Nasu A, Ikeya M, Yamamoto T, et al. Genetically matched human iPS cells reveal that propensity for cartilage and bone differentiation differs with clones, not cell Ttype of origin. PLoS One. 2013;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Liu J, Chen WC, Zhao ZH, Xu HHK. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials. 2013;34(32):7862‐7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. TheinHan WW, Liu J, Tang MH, Chen WC, Cheng LZ, Xu HHK. Induced pluripotent stem cell‐derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res. 2013;1:371‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Ardeshirylajimi A, Hosseinkhani S, Parivar K, Yaghmaie P, Soleimani M. Nanofiber‐based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep. 2013;40(7):4287‐4294. [DOI] [PubMed] [Google Scholar]
- 373. Tang MH, Chen WC, Liu J, Weir MD, Cheng LZ, Xu HHK. Human induced dluripotent stem cell‐derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20(7‐8):1295‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Ochiai‐Shino H, Kato H, Sawada T, et al. A novel strategy for enrichment and isolation of osteoprogenitor cells from induced pluripotent stem cells based on surface marker combination. PLoS One. 2014;9(6):e99534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Ko JY, Park S, Im GI. Osteogenesis from human induced pluripotent stem cells: an in vitro and in vivo comparison with mesenchymal stem cells. Stem Cells Dev. 2014;23(15):1788‐1797. [DOI] [PubMed] [Google Scholar]
- 376. Egusa H, Kayashima H, Miura J, et al. Comparative analysis of mouse‐induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro . Stem Cells Dev. 2014;23(18):2156‐2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Liu J, Chen WC, Zhao ZH, Xu HHK. Effect of NELL1 gene overexpression in iPSC‐MSCs seeded on calcium phosphate cement. Acta Biomater. 2014;10(12):5128‐5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Ji J, Tong X, Huang X, Zhang J, Qin H, Hu Q. Patient‐derived human induced pluripotent stem cells from gingival fibroblasts composited with defined nanohydroxyapatite/chitosan/gelatin porous scaffolds as potential bone graft substitutes. Stem Cells Trans Med. 2016;5(1):95‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Ardeshirylajimi A, Soleimani M. Enhanced growth and osteogenic differentiation of induced pluripotent stem cells by extremely low‐frequency electromagnetic field. Cell Mol Biol. 2015;61(1):36‐41. [PubMed] [Google Scholar]
- 380. Kato H, Ochiai‐Shino H, Onodera S, Saito A, Shibahara T, Azuma T. Promoting effect of 1,25(OH)(2) vitamin D‐3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte‐like cells. Open Biol. 2015;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Okawa H, Kayashima H, Sasaki JI, et al. Scaffold‐free fabrication of osteoinductive cellular constructs using mouse gingiva‐derived induced pluripotent stem cells. Stem Cells Int. 2016;2016:6240794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Wang P, Song Y, Weir MD, et al. A self‐setting iPSMSC‐alginate‐calcium phosphate paste for bone tissue engineering. Dent Mater. 2016;32(2):252‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Xie J, Peng C, Zhao QH, et al. Osteogenic differentiation and bone regeneration of iPSC‐MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016;29:365‐379. [DOI] [PubMed] [Google Scholar]
- 384. Hynes K, Menicanin D, Han J, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833‐839. [DOI] [PubMed] [Google Scholar]
- 385. Tsuji O, Miura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe‐induced pluripotent stem cells for spinal cord injury. PNAS. 2010;107(28):12704‐12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Diederichs S, Tuan RS. Functional comparison of human‐induced pluripotent stem cell‐derived mesenchymal cells and bone marrow‐derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014;23(14):1594‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. de Peppo GM, Marcos‐Campos I, Kahler DJ, et al. Engineering bone tissue substitutes from human induced pluripotent stem cells. PNAS. 2013;110(21):8680‐8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Zou LJ, Luo YL, Chen MW, et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci Rep. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Dogaki Y, Lee SY, Niikura T, et al. Efficient derivation of osteoprogenitor cells from induced pluripotent stem cells for bone regeneration. Int Orthop. 2014;38(9):1779‐1785. [DOI] [PubMed] [Google Scholar]
- 390. Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014;23(10):1084‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Kang R, Luo YL, Zou LJ, et al. Osteogenesis of human induced pluripotent stem cells derived mesenchymal stem cells on hydroxyapatite contained nanofibers. RSC Adv. 2014;4(11):5734‐5739. [Google Scholar]
- 392. Hong SG, Winkler T, Wu CF, et al. Path to the clinic: Assessment of iPSC‐based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014;7(4):1298‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Ishiy FAA, Fanganiello RD, Griesi‐Oliveira K, et al. Improvement of in vitro osteogenic potential through differentiation of induced pluripotent stem cells from human exfoliated dental tissue towards mesenchymal‐like stem cells. Stem Cells Int. 2015;2015:249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Lepage SI, Nagy K, Sung HK, Kandel RA, Nagy A, Koch TG. Generation, characterization, and multilineage potency of mesenchymal‐like progenitors derived from equine induced pluripotent stem cells. Stem Cells Dev. 2016;25(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 395. Kang R, Zhou Y, Tan S, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res Ther. 2015;6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Lian QZ, Zhang YL, Zhang JQ, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113‐U1191. [DOI] [PubMed] [Google Scholar]
- 397. Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One‐step derivation of mesenchymal stem cell (MSC)‐like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7(3):e33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Duan X, Tu Q, Zhang J, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226(1):150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Levi B, Hyun JS, Montoro DT, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. PNAS. 2012;109(50):20379‐20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Jin GZ, Kim TH, Kim JH, et al. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J Biomed Mater Res A. 2013;101(5):1283‐1291. [DOI] [PubMed] [Google Scholar]
- 401. Ardeshirylajimi A, Dinarvand P, Seyedjafari E, Langroudi L, Adegani FJ, Soleimani M. Enhanced reconstruction of rat calvarial defects achieved by plasma‐treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013;354(3):849‐860. [DOI] [PubMed] [Google Scholar]
- 402. Kang HM, Shih YRV, Hwang Y, et al. Mineralized gelatin methacrylate‐based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta Biomater. 2014;10(12):4961‐4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Phillips MD, Kuznetsov SA, Cherman N, et al. Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: In vitro versus in vivo Assays. Stem Cells Trans Med. 2014;3(7):867‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Kanke K, Masaki H, Saito T, et al. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum‐free and feeder‐free conditions. Stem Cell Rep. 2014;2(6):751‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Wang MK, Deng Y, Zhou P, et al. In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides‐decorated two‐dimensional microenvironment. ACS Appl Mater Interfaces. 2015;7(8):4560‐4572. [DOI] [PubMed] [Google Scholar]
- 406. Anguera MC, Sadreyev R, Zhang ZQ, et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell. 2012;11(1):75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Malecki M. ‘Above all, do no harm’: safeguarding pluripotent stem cell therapy against iatrogenic tumorigenesis. Stem Cell Res Ther. 2014;5(3):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Furno EL, Svd Laan, Maiorano D. Genomic instability of pluripotent stem cells: origin and consequences. In: Tomizawa M, ed. Pluripotent Stem Cells ‐ From the Bench to the Clinic. Cham: Springer International Publishing; 2016. [Google Scholar]
- 409. Yamanaka S. Strategies and new developments in the generation of patient‐specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39‐49. [DOI] [PubMed] [Google Scholar]
- 410. Yamanaka S. A fresh look at iPS cells. Cell. 2009;137(1):13‐17. [DOI] [PubMed] [Google Scholar]
- 411. Gutierrez‐Aranda I, Ramos‐Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28(9):1568‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743‐745. [DOI] [PubMed] [Google Scholar]
- 413. Walia B, Satija N, Tripathi RP, Gangenahalli GU. Induced pluripotent stem cells: fundamentals and applications of the reprogramming process and its ramifications on regenerative medicine. Stem Cell Rev. 2012;8(1):100‐115. [DOI] [PubMed] [Google Scholar]
- 414. Ben‐David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nay Rev Cancer. 2011;11(4):268‐277. [DOI] [PubMed] [Google Scholar]
- 415. Zhou WB, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667‐2674. [DOI] [PubMed] [Google Scholar]
- 416. Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene‐free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949‐953. [DOI] [PubMed] [Google Scholar]
- 419. Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus‐free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Si‐Tayeb K, Noto FK, Sepac A, et al. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev Biol. 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Yu JY, Hu KJ, Smuga‐Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration‐free human iPS cells. Nat Methods. 2011;8(5):409‐U452. [DOI] [PubMed] [Google Scholar]
- 423. Chou BK, Mali P, Huang XS, et al. Efficient human iPS cell derivation by a non‐integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21(3):518‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Jia FJ, Wilson KD, Sun N, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197‐U146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Nagy K, Sung HK, Zhang PZ, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep. 2011;7(3):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Yoshioka N, Gros E, Li HR, et al. Efficient generation of human iPSCs by a synthetic self‐replicative RNA. Cell Stem Cell. 2013;13(2):246‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Johannesson B, Sagi I, Gore A, et al. Comparable frequencies of coding mutations and loss of imprinting in human pluripotent cells derived by nuclear transfer and defined factors. Cell Stem Cell. 2014;15(5):634‐642. [DOI] [PubMed] [Google Scholar]
- 428. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633‐638. [DOI] [PubMed] [Google Scholar]
- 430. Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Moriguchi H, Chung RT, Sato C. Tumorigenicity of human induced pluripotent stem cells depends on the balance of gene expression between p21 and p53. Hepatology. 2010;51(3):1088‐1089. [DOI] [PubMed] [Google Scholar]
- 433. Pei DQ. The magic continues for the iPS strategy. Cell Res. 2008;18(2):221‐223. [DOI] [PubMed] [Google Scholar]
- 434. Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269‐1275. [DOI] [PubMed] [Google Scholar]
- 435. Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525‐528. [DOI] [PubMed] [Google Scholar]
- 436. Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small‐molecule compounds. Cell Stem Cell. 2008;3(5):568‐574. [DOI] [PubMed] [Google Scholar]
- 437. Xu Y, Shi Y, Ding S. A chemical approach to stem‐cell biology and regenerative medicine. Nature. 2008;453(7193):338‐344. [DOI] [PubMed] [Google Scholar]
- 438. Pei DQ. Regulation of pluripotency and reprogramming by transcription factors. J Biol Chem. 2009;284(6):3365‐3369. [DOI] [PubMed] [Google Scholar]
- 439. Ichida JK, Blanchard J, Lam K, et al. A small‐molecule inhibitor of tgf‐Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5(5):491‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Zhu SY, Li WL, Zhou HY, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7(6):651‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Chen JK, Liu J, Yang JQ, et al. BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell Res. 2011;21(1):205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Chen JK, Liu J, Chen Y, et al. Rational optimization of reprogramming culture conditions for the generation of induced pluripotent stem cells with ultra‐high efficiency and fast kinetics. Cell Res. 2011;21(6):884‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Li YQ, Zhang QA, Yin XL, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21(1):196‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Hou PP, Li YQ, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small‐molecule compounds. Science. 2013;341(6146):651‐654. [DOI] [PubMed] [Google Scholar]
- 445. Zhao Y, Zhao T, Guan JY, et al. A XEN‐like state bridges somatic cells to pluripotency during chemical reprogramming. Cell. 2015;163(7):1678‐1691. [DOI] [PubMed] [Google Scholar]
- 446. Long Y, Wang M, Gu HF, Xie X. Bromodeoxyuridine promotes full‐chemical induction of mouse pluripotent stem cells. Cell Res. 2015;25(10):1171‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Cao ST, Yu SY, Li DW, et al. Chromatin accessibility dynamics during chemical induction of pluripotency. Cell Stem Cell. 2018;22(4):529‐542. [DOI] [PubMed] [Google Scholar]
- 448. Kikuchi K, Masuda T, Fujiwara N, et al. Craniofacial bone regeneration using iPS cell‐derived neural crest like cells. J Hard Tissue Biol. 2018;27(1):1‐10. [Google Scholar]
- 449. Ortuno‐Costela MD, Garcia‐Lopez M, Cerrada V, Gallardo ME. iPSCs: a powerful tool for skeletal muscle tissue engineering. J Cell Mol Med. 2019;23(6):3784‐3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Wu C, Hong SG, Winkler T, et al. Development of an inducible caspase‐9 safety switch for pluripotent stem cell‐based therapies. Mol Ther Methods Clin Dev. 2014;1:14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Kimura Y, Shofuda T, Higuchi Y, et al. Human genomic safe harbors and the suicide gene‐based safeguard system for iPSC‐based cell therapy. Stem Cells Trans Med. 2019;8(7):627‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622‐640. [PubMed] [Google Scholar]
- 453. Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256‐273. [DOI] [PubMed] [Google Scholar]
- 454. Gurdon JB, Uehlinger V. “Fertile” intestine nuclei. Nature. 1966;210(5042):1240‐1241. [DOI] [PubMed] [Google Scholar]
- 455. Laskey RA, Gurdon JB. Genetic content of adult somatic cells tested by nuclear transplantation from cultured cells. Nature. 1970;228(5278):1332‐1334. [DOI] [PubMed] [Google Scholar]
- 456. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810‐813. [DOI] [PubMed] [Google Scholar]
- 457. Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full‐term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394(6691):369‐374. [DOI] [PubMed] [Google Scholar]
- 458. Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450(7169):497‐502. [DOI] [PubMed] [Google Scholar]
- 459. Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11(19):1553‐1558. [DOI] [PubMed] [Google Scholar]
- 460. Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309(5739):1369‐1373. [DOI] [PubMed] [Google Scholar]
- 461. Hansis C, Barreto G, Maltry N, Niehrs C. Nuclear reprogramming of human somatic cells by xenopus egg extract requires BRG1. Curr Biol. 2004;14(16):1475‐1480. [DOI] [PubMed] [Google Scholar]
- 462. Zhu XQ, Pan XH, Wang W, et al. Transient in vitro epigenetic reprogramming of skin fibroblasts into multipotent cells. Biomaterials. 2010;31(10):2779‐2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Taranger CK, Noer A, Sorensen AL, Hakelien AM, Boquest AC, Collas P. Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol Biol Cell. 2005;16(12):5719‐5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880‐1885. [DOI] [PubMed] [Google Scholar]
- 465. Mauro A, Turriani M, Ioannoni A, et al. Isolation, characterization, and in vitro differentiation of ovine amniotic stem cells. Vet Res Commun. 2010;34:S25‐S28. [DOI] [PubMed] [Google Scholar]
- 466. Antonucci I, Stuppia L, Kaneko Y, et al. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011;20(6):789‐795. [DOI] [PubMed] [Google Scholar]
- 467. Kalaszczynska I, Ferdyn K. Wharton's jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int. 2015;2015:430847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219‐1227. [DOI] [PubMed] [Google Scholar]
- 469. Oldberg A, Antonsson P, Lindblom K, Heinegard D. A collagen‐binding 59‐kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG‐S1 and PG‐S2 (decorin). EMBO J. 1989;8(9):2601‐2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Iozzo RV, Goldoni S, Berendsen AD, Young MF. Small leucine‐rich proteoglycans: the extracellular matrix: an overview. In: Mecham RP, ed. Biology of Extracellular Matrix. Berlin, Heidelberg: Springer; 2011:197‐231. [Google Scholar]
- 471. Paracuellos P, Kalamajski S, Bonna A, Bihan D, Farndale RW, Hohenester E. Structural and functional analysis of two small leucine‐rich repeat proteoglycans, fibromodulin and chondroadherin. Matrix Biol. 2017;63:106‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Viola M, Bartolini B, Sonaggere M, Giudici C, Tenni R, Tira ME. Fibromodulin interactions with type I and II collagens. Connect Tissue Res. 2007;48(3):141‐148. [DOI] [PubMed] [Google Scholar]
- 473. Hildebrand A, Romaris M, Rasmussen L, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Kalamajski S, Bihan D, Bonna A, Rubin K, Farndale RW. Fibromodulin interacts with collagen cross‐linking sites and activates lysyl oxidase. J Biol Chem. 2016;291(15):7951‐7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine‐rich proteoglycans (SLRPs). J Cell Commun Signal. 2009;3(3‐4):323‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Jian J, Zheng Z, Zhang K, et al. Fibromodulin promoted in vitro and in vivo angiogenesis. Biochem Biophys Res Commun. 2013;436:537‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Zheng Z, Jian J, Velasco O, et al. Fibromodulin enhances angiogenesis during cutaneous wound healing. Plast Reconstr Surg Glob Open. 2014;2(12):e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Adini I, Ghosh K, Adini A, et al. Melanocyte‐secreted fibromodulin promotes an angiogenic microenvironment. J Clin Invest. 2014;124(1):425‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Zheng Z, Zhang X, Dang C, et al. Fibromodulin is essential for fetal‐type scarless cutaneous wound healing. Am J Pathol. 2016;186(11):2824‐2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Zheng Z, James AW, Li C, et al. Fibromodulin reduces scar formation in adult cutaneous wounds by eliciting a fetal‐like phenotype. Signal Transduct Target Ther. 2017;2:17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481. Zheng Z, Jian J, Zhang X, et al. Reprogramming of human fibroblasts into multipotent cells with a single ECM proteoglycan, fibromodulin. Biomaterials. 2012;33(24):5821‐5831. [DOI] [PubMed] [Google Scholar]
- 482. Li CS, Yang P, Ting K, et al. Fibromodulin reprogrammed cells: a novel cell source for bone regeneration. Biomaterials. 2016;83:194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Zheng Z, Li C, Ha P, et al. CDKN2B upregulation prevents teratoma formation in multipotent fibromodulin reprogrammed cells. J Clin Invest. 2019;129(8):3236‐3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Yang P, Li C, Lee M, et al. Photopolymerizable hydrogel‐encapsulated fibromodulin‐reprogrammed cells for muscle regeneration. Tissue Eng Part A. 2020;26(19‐20):1112‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Fisch SC, Gimeno ML, Phan JD, et al. Pluripotent nontumorigenic multilineage differentiating stress enduring cells (Muse cells): a seven‐year retrospective. Stem Cell Res Ther. 2017;8:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Wakao S, Kitada M, Kuroda Y, et al. Multilineage‐differentiating stress‐enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. PNAS. 2011;108(24):9875‐9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Wakao S, Kitada M, Dezawa M. The elite and stochastic model for iPS cell generation: multilineage‐differentiating stress enduring (Muse) cells are readily reprogrammable into iPS cells. Cytom Part A. 2013;83A(1):18‐26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no new datasets were generated for this review article.


