Summary
Traumatic brain injury (TBI) is the largest non-genetic, non-aging related risk factor for Alzheimers disease (AD). We report here that TBI induces tau acetylation (ac-tau) at sites acetylated also in human AD brain. This is mediated by S-nitrosylated-GAPDH, which simultaneously inactivates Sirtuinl deacetylase and activates p300/CBP acetyltransferase, increasing neuronal ac-tau. Subsequent tau mislocalization causes neurodegeneration and neurobehavioral impairment, and ac-tau accumulates in the blood. Blocking GAPDH S-nitrosylation, inhibiting p300/CBP, or stimulating Sirtuinl all protect mice from neurodegeneration, neurobehavioral impairment, and blood and brain accumulation of ac-tau after TBI. Ac-tau is thus a therapeutic target and potential blood biomarker of TBI that may represent pathologic convergence between TBI and AD. Increased ac-tau in human AD brain is further augmented in AD patients with history of TBI, and patients receiving the p300/CBP inhibitors salsalate or diflunisal exhibit decreased incidence of AD and clinically diagnosed TBI.
Keywords: neuroprotection, traumatic brain injury, Alzheimer’s disease, acetylation, tau, neurodegeneration, omigapil, congenital muscular dystrophy, salsalate, diflunisal, P7C3
Graphical Abstract

eToc Blurb
Reducing brain injury-induced neuronal tau acetylation is neuroprotective in traumatic brain injury and has a role in Alzheimer’s disease pathogenesis.
Introduction
Traumatic brain injury (TBI) is typically caused by motor vehicle crashes, falls, contact sports, or assaults. The annual incidence of TBI in the United States alone is ~3.5 million, with ~5 million people currently living with TBI-related disabilities at an annual cost of ~$80 billion (Centers for Disease Control and Prevention, 2015; Ma et al., 2014). At present, treatments for TBI focus on patient stabilization and mitigation of symptoms, and there are no medicines that specifically target the pathophysiological processes that drive neurodegeneration after brain injury. TBI also significantly increases the risk of later developing Alzheimer’s disease (AD) (Johnson et al., 2010; Li et al., 2017). This suggests common pathologic mechanisms, and emerging evidence points to S-nitrosylation and acetylation (Uehara et al., 2006; Nakamura and Lipton, 2020; Sen et al., 2018). Indeed, a small post-mortem study recently reported increased acetylation of tau at lysine (K) 280 in the brains of three patients with AD and three patients with chronic traumatic encephalopathy (Lucke-Wold et al., 2017). In a separate study, the same rise in ac-tau at K280 was documented in the brains of ten additional patients with AD, five patients with corticobasal degeneration, and five patients with progressive supranuclear palsy (Irwin et al., 2012). Tau acetylation at both K274 and K281 was additionally reported in upwards of a dozen patients with severe AD (Tracy et al., 2016). However, these studies did not establish the driving forces or pathologic significance of the findings. Given the interrelationship between TBI and AD, we sought to determine whether elevated ac-tau was a causal pathophysiologic factor in TBI, and thus a potential locus of convergence, and if so, then whether this might provide an experimental platform to understand in vivo pathophysiology related to ac-tau in the brain.
Results
Brain injury induces neuronal tau acetylation
To begin, we employed a mouse model of multimodal TBI incorporating global concussive impact, acceleration/deceleration, and early-phase blast wave exposure (Shin et al., 2021). This model produces a complex brain injury with neurodegeneration and neurobehavioral impairment, beginning with acute axonal degeneration that persists chronically and eventually leads to blood-brain barrier degradation and nerve cell death (Yin et al., 2014, 2016; Dutca etal., 2014; Vázquez-Rosa et al., 2019, 2020; Wattiez et al., 2021). Already an established preclinical model of TBI, its clinical relevance is further strengthened here through nonbiased whole metabolomic analysis. Specifically, I week after injury, mice displayed altered plasma levels of fatty acids, amino acids, 5-oxoproline, and tricarboxylic acid cycle metabolites (Figure S1A), resembling published data from nonbiased plasma metabolomic analysis of human TBI (Orešič et al., 2016).
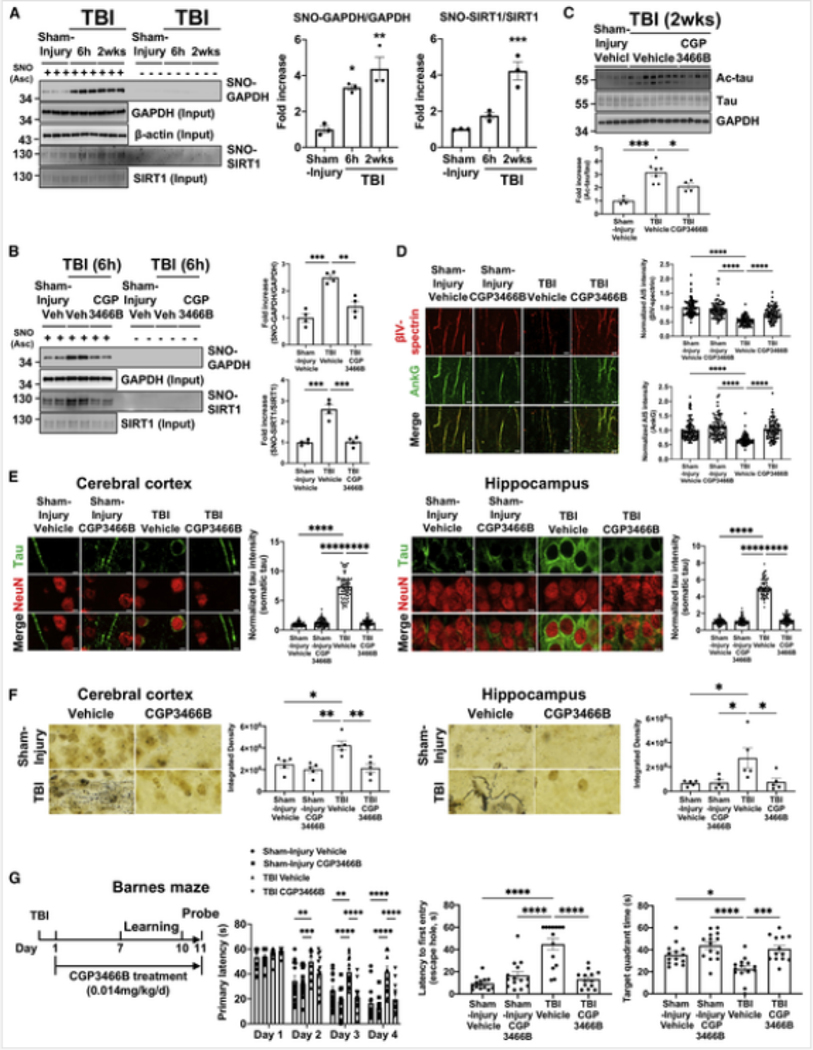
Using antibodies to mouse acetylated-tau (ac-tau) at K263 and K270 (Min et al., 2010; Figure S1B), which correspond respectively to human tau K274 and K281, we observed rapid and specific elevation of ac-tau in both the cerebral cortex and hippocampus of the brain after injury (Figure 1A). Importantly, the increased ac-tau was observed selectively in neurons of the brain (Figure 1B) and correlated with injury intensity (Figure 1C), while total tau levels remained unchanged (Figures 1A–1C). Our observation of the low-level presence of tau in non-neuronal cell types is consistent with previous reports (http://www.brainmaseq.org; Perea et al., 2019; Bolós et al., 2017), and no change in non-neuronal tau was noted as a function of injury. The injury-dependent increase in neuronal ac-tau was observed in both males and females (Figure S1C) and also generalized to multiple forms and stages of TBI. For example, we determined that this is not only an acute, but also a chronic response to injury, as ac-tau remained elevated 17 months after injury in mice (Figure S1D). Ac-tau was also elevated 7 months after acoustic blast overpressure TBI in rats (Figure S1E), and at a range of times after controlled cortical impact TBI in mice (Figure S1F). In addition, ac-tau was acutely elevated in cerebellum after multimodal TBI (Figure S1G), but not in hypothalamus (Figure S1H), consistent with the previous observation of hypothalamic resistance to neurodegeneration in this model (Yin et al., 2014).
Figure 1. Neuronal tau acetylation after TBI induces axon initial segment degradation and pathologic tau mislocalization.
(A) Quantified western blot shows increased ac-tau 6 h-2 weeks after TBI in cerebral cortex and hippocampus. Each group n = 3, **p <0.01, ***p <0.001, ****p < 0.0001 versus sham-injury group, one-way ANOVA and Dunnett multiple comparisons test.
(B) Quantified western blot shows increased ac-tau in neurons (NeuN+, GFAP−), but not glia (NeuN−, GFAP+), of the cerebral cortex, with greater tau expression in neurons. Each lane consists of pooled brain tissue from 3 animals, **p <0.01 versus sham-injury group, Student’s t test.
(C) Quantified western blot shows TBI intensity-dependent increase in ac-tau. Each group n = 3–4, **p < 0.01, ****p < 0.0001 versus sham-injury group, one-way ANOVA and Dunnett multiple comparisons test. PSI, pounds per square inch of explosive pressure.
(D) Quantified western blot shows reduced AIS proteins AnkG and βIV-spectrin after TBI, consistent with AIS degradation. Each group n = 3, *p < 0.05, **P < 0.01, *** p < 0.001 versus sham-injury group, one-way ANOVA and Dunnett multiple comparisons test.
(E) Immunohistochemical staining for tau and the neuronal marker NeuN shows normal axonal localization of tau 2 weeks after sham-injury, and pathological tau mislocalization into the somatodendritic companment 2 weeks after TBI (scale bar, 5 μm). Images are representative of 3 animals per group.
(F) Blast-injury induces increased cleaved caspase-3 levels and LDH release in TauKQ (mimicking acetylated lysine) overexpressing cells compared to TauWT (Wild type) and TauKR (nonacetylatable tau mutant) transfected cells (***P < 0.001, ****p < 0.0001 versus TauWT, TauKR transfected cells, one-way ANOVA; and Tukey’s post hoc analysis).
(G) TauKQhigh mice have axonal degeneration in cerebral cortex, hippocampus, and hypothalamus, which is absent from nontransgenic littermates (*p < 0.05, Student’s t test).
See also Figures S1 and S2.
By contrast, tau phosphorylation after brain injury was unchanged at S202 (Figures S2A and S2B), which is part of the basis for AD Braak staging (Neddens et al., 2018), and reduced at S396 and S404, sites that mediate tau’s ability to polymerize tubulin (Evans et al., 2000; Figures S2A and S2B). Tau phosphorylation was also somewhat reduced at S262 (Figures S2A and S2B). Although phosphorylation of S262 has been linked to tau acetylation in other contexts ( Cook et al., 2014), inhibiting tau acetylation after brain injury had no effect on S262 phosphorylation (Figure S2C). Taken together, the data suggest a prominent role for tau acetylation and a lesser role for tau phosphorylation in the acute period following brain injury. Notably, the post-injury increase in ac-tau was also not associated with tau seeding (Figures S2D and S2E), nor with reduced expression of postsynaptic kidney/brain (KIBBRA) protein (Figures S2F-S21), a previously suggested consequence of tau acetylation (Tracy et al., 2016).
Injury-induced neuronal tau acetylation leads to axon initial segment degradation and pathologic tau mislocalization
Clues to the possible function of ac-tau in brain injury were uncovered from published post-mortem studies of AD patients’ brains, in which the magnitude of K274 and K281 acetylation correlated inversely with amounts of ankyrin-G (AnkG) and βIV-spectrin (Sohn et al., 2016), principle components of the axon initial segment (AIS). The AIS normally maintains neuronal health and limits tau distribution predominantly to axons (Schafer et al., 2009). After injury, we observed a rapid reduction in the levels of AnkG and βIV-spectrin (Figure 1D) that correlated with amounts and time course of increased tau acetylation (Figure 1A). AIS degradation also led to pathologic tau mislocalization into the somatodendritic compartment of neurons throughout the brain (Figure 1E).
To determine whether ac-tau was directly toxic to neurons, both in vitro and in vivo genetic tests were conducted. To begin, transfection of cultured human neuroblasts with either human tau acetylation mimetic or acetylation-resistant human tau showed that tau acetylation specifically increased neuronal cell death in an in vitro model of TBI, as measured by both cleaved caspase 3 and lactate dehydrogenase (LDH) release assay (Figure 1F). For in vivo investigation, a mouse correlate of this model, known as tauKQhigh mice, was used. These transgenic mice express human tau with lysine-to-glutamine mutations that mimic acetylation at K274 and K281, which correspond to mouse K263 and K270, respectively. TauKQhigh mice have been previously shown to exhibit memory deficits and impaired long-term potentiation (Tracy et al., 2016). Here, ~1-year-old tauKQhigh mice displayed a significant degree of axonal degeneration, which was not observed in either nontransgenic wild-type littermates or human wild-type tau transgenic mice (Figures 1G, S2J, and S2K). The interesting observation that the hypothalamus of tauKQhigh mice showed prominently increased axonal neurodegeneration contrasts with previously noted protection of this region in TBI (Yin et al., 2014). This suggests that the hypothalamus in mice, may be physically protected from injury relative to other regions of the brain, rather than composed of a subpopulation of neurons endowed with unique protective properties. Synaptic integrity in these animals was also assessed, through immunohistochemical staining for synaptic vesicle protein 2 (SV2). As shown in Figure S2L, tauKQhigh mice displayed reduced SV2 in the hippocampus relative to nontransgenic wild-type littermates. Taken together, these results indicate that human ac-tau is directly toxic to neurons, both in vitro and in vivo in the brain.
Injury-induced neuronal tau acetylation is driven by non-canonical signaling
In cellular models of AD, protein acetylation has been linked to S-nitrosylation of GAPDH, which coordinately enhances p300/CBP acetyltransferase activity and inhibits Sirtuinl (Sirtl) deacetylase activity to increase overall amounts of acetylation. Inhibitory S-nitrosylation of Sirtl is mediated by aberrant transnitrosylase activity of GAPDH, whereas S-nitrosylated-GAPDH (SNO-GAPDH) also stimulates activation and acetylation of p300/CBP acetyltransferase (Hara and Snyder, 2006; Kornberg et al., 2010; Sen et al., 2018). After TBI, we found that both GAPDH and Sirtl were S-nitrosylated (Figure 2A). Increased protein acetylation was further confirmed by measuring acetylation of histone H2AK5, a p300/CBP and Sirtl substrate (Figures S3A and S3B). Initiation of daily treatment 15 min after injury with CGP3466B (0.014 mg/kg IP), a specific inhibitor of GAPDH nitrosylation also known as omigapil (Waldmeier et al., 2000), blocked S-nitrosylation of both GAPDH and Sirtl (Figures 2B and S3C). Furthermore, protein or activity levels of histone deacetylase 6 (HDAC6), which can deacetylate tau (Cohen et al., 2011; Cook et al., 2014), did not change at any of the time points after TBI (Figure S3D). Blocking GAPDH S-nitrosylation also reduced ac-tau and ac-p300/CBP (Figures 2C and S4A), and blocked AIS degradation (Figures 2D and S4B), redistribution of tau into the somatodendritic compartment (Figures 2E and S4C), and axonal degeneration throughout the brain (Figure 2F). Lastly. protective efficacy for cognition was assessed in the Bames maze task of learning and memoly. Daily treatment with CGP3466B (0.014 mg/kg IP), this time initiated 24 h after injury, protected mice from deficits in leaming and memory (Figure 2G), without altering motor speed (Figures S4D and S4E) or body weight (Figure S4F).
Figure 2. SNO-GAPDH mediates the post-TBI p300/CBP acetyltransferase activation and Sirtl deacetylase inhibition that leads to accumulated ac-tau, AIS degradation, tau mislocalization, neurodegeneration, and cognitive deficits.
(A) Western blot and its quantification show significantly increased S-nitrosylation (SNO) of GAPDH and Sirtl in cerebral cortex after TBI (n = 3 per group, *p < 0.05, **p < 0.01, ***p < 0.001 versus sham-injury group, one-way ANOVA; and Dunnett multiple comparisons test). “Ascorbate (Asc) - negative control” shows specificity of signal in the SNO-resin-assisted capture technique.
(B) Western blot and its quantification show that treatment of CGP3466B inhibits S-nitrosylation of GAPDH and Sirtl at 0.014 mg/kg. Each group n = 4, **p < 0.01, ***p < 0.001 versus TBI+Vehicle group, one-way ANOVA and Tukey’s post hoc analysis. Asc + represents SNO, and Asc - represents control.
(C) Western blot and its quantification show that 0.014 mg/kg CGP3466B reduces ac-tau in cerebral cortex after TBI. Each group n = 4–7,*p < 0.05, ***p < 0.001 versus TBI+vehicle group, one-way ANOVA; and Tukey’s post hoc analysis.
(D) CGP3466B protects mice from post-TBI AIS degradation in the cerebral cortex_(scale bar, 5 4μm).
(E) CGP3466B protects mice from post-TBI tau mislocalization (scale bar, 5 μm). Lower magnification pictures of the field from which these pictures were derived are shown in Figure S8C.
(F) CGP3466B protects mice from post-TBI axonal degeneration, as evidenced by silver staining of degenerating axons (scale bar, 5 μm). In (D)-(F), each group n = 3–5, *p < 0.05, **P < 0.01, ****P < 0.0001 versus TBI+vehicIe group, one-way ANOVA; and Tukey’s post hoc analysis.
(G) CGP3466B protects mice from post-TBI impaired cognition in both leaming and memory phases of the Bames maze task (*p < 0.05, **P < 0.01, ***P < 0.001, ****p < 0.0001 versus TBI-+vehicle group, repeated-measures two-way ANOVA (learning) and one-way ANOVA (memory) and Tukey ‘s post hoc analysis.
See also Figures S3 and S4.
Inhibiting p300/CBP acetyltransferase blocks injury-induced tau acetylation, neurodegeneration, and neurobehavioral impairment
Given the multiple mechanistic steps that follow injury-dependent S-nitrosylation of GAPDH to converge on elevating ac-tau, complementary therapeutic approaches were considered. Salsalate, a known inhibitor of rodent and human p300/CBP (Shirakawa et al., 2016), was tested first. Because salsalate has anti-inflammatory properties, a low dose that inhibited p300/CBP (Figures S5A-S5D) without blocking elevated levels of neuroinflammatory cytokines after injury (Figures S5E and S5F) was identified. Daily administration of this low non-anti-inflammatory dose of salsalate, beginning 24 h after TBI, reduced amounts of ac-tau in the brain without changing the total amount of tau protein (Figure 3A) or GAPDH S-nitrosylation (Figure S5G). This same low-dose salsalate treatment also preserved the AIS after injury, as evidenced by unchanged levels of AnkG and βIV-spectrin (Figure 3B). Encouragingly, compared with sham-injured animals, low-dose salsalate preserved normal axonal localization of tau (Figures 3C and S5H) and protected mice from axonula4 degeneration (Figure 3D).
Figure 3. Low-dose salsalate-mediated inhibition of p300/CBP acetyltransferase protects mice from post-TBI-induced elevated ac-tau, AIS degradation, tau mislocalization, neurodegeneration, and cognitive deficits.
(A) Low-dose salsalate dose-dependently reduces post-TBI elevations in ac-tau in the brain (n = 3, *p < 0.05, **p < 0.01, ***p <0.001 versus TBI-+Vehicle group, one-way ANOVA; and Tukey’s post hoc analysis).
(B) Low-dose salsalate protects mice from post-TBI AIS degradation (scale bar, 5 μm).
(C) Low-dose salsalate protects mice from post-TBI tau mislocalization (scale bar, 5 μm).
(D) Low-dose salsalate protects mice from post-TBI axonal degeneration (scale bar, 5 μm). In (BHD), each group n = 3, *p < 0.05, **p < 0.01, ***p <0.001, ****p < 0.0001 versus TBI+Vehicle group, one-way ANOVA and Tukey’s post hoc analysis.
(E) Low-dose salsalate protects mice from post-TBI impairments in motor (foot slip assay) and cognitive (learning and memory in the Bames maze) behavioral assays. (*p < 0.05, **p < 0.01, ***p<0.001, ****p < 0.0001 versus TBI+vehicle group, repeated-measures two-way ANOVA (learning) and one-way ANOVA (memory) and Tukey’s post hoc analysis.
See also Figures S5 and S6.
As a functional measure of protection, motor and cognitive function after injury were assessed (Figure 3E). Notably, daily low-dose, non-anti-inflammatory salsalate treatment initiated 24 h after injury preserved normal motor function in the foot slip assay (Figure 3E) and also protected mice from acquiring deficits in learning (Figures 3E and S6A) and memoly (Figure 3E) in the Barnes maze task, without impacting body weight (Figure S6B) or motor speed (Figures S6C and S6D).
Elevating NAD+ enhances Sirtl activity and blocks injury-induced tau acetylation, neurodegeneration, and neurobehavioral impairment
Next, we tested the complementary approach of increasing Sirtl -mediated tau deacetylation. Sirtl activity is dependent on nicotinamide adenine dinucleotide (NAD+), and high expression of the Ube4b/nicotinamide mononucleotide adenylyltransferase 1 fusion protein in WldS mice results in constitutively elevated NAD* levels that protect these animals from axonal degeneration and neurobehavioral impairment after brain injury (Pieper and McKnight, 2018; Yin et al., 2016). Here, WldS mice were also completely protected from injury-induced increases in ac-tau (Figure 4A) and AIS degradation throughout the brain (Figure 4B), which correlated with complete protection from tau mislocalization as well (Figures 4C and S5I).
Figure 4. WldS mice are protected from post-TBI-induced elevated ac-tau, AIS degradation, and tau mislocalization.
(A) WldS mice are resistant to post-TBI elevations in ac-tau in the brain (for each group n =4, *p < 0.05, **p < 0.01 versus WT+TBI group, one-way ANOVA; and Tukey’s post hoc analysis).
(B) WldS mice are resistant to post-TBI AIS degradation (scale bar, 5 μm).
(C) WldS mice are resistant to post-TBI tau mislocalization (scale bar, 5 μm).
In (B)-(C), each group n = 3, ****p < 0.0001 versus WT+TBI group, one-way ANOVA; and Tukey’s post hoc analysis. See also Figure S5.
NAD+ levels under conditions of neuronal stress can also be preserved by treatment with the aminopropyl carbazole P7C3-A20 (Pieper et al., 2010; Wang et al., 2014; Pieper and McKnight, 2018). Here, P7C3-A20, administered daily beginning 24 h after brain injury, preserved brain NAD+ levels (Figure 5A) and prevented accumulation of ac-tau (Figure 5B). This correlated with protection from injury-induced AIS degradation (Figure 5C) and mislocalization of tau into the somatodendritic compartment (Figures 5D and S5J). Importantly, P7C3-A20-mediated protection was conferred without impacting upstream injury-induced S-nitrosylation of GAPDH (Figure 5E). All aspects of P7C3A20-mediated protection were blocked by inhibiting Sirtl with EX527 or inhibiting NAD* synthesis with FK866 (Figures 5A–5D). Thus, P7C3-A20-mediated preservation of otherwise depleted neuronal NAD+ after brain injury promoted downstream Sirtl-mediated deacetylation of tau, which blocked neurodegeneration-inducing AIS degradation and tau mislocalization.
Figure 5. P7C3-A20 treatment protects mice from post-TBI-induced elevated ac-tau, AIS degradation, and tau mislocalization.
(A) treatment rescued normal NAD+ levels after TBI, which was blocked by co-administration ofFK866 (each group n = 3, **p < 0.01, ***p < 0.001 one-way ANOVA and Tukey’s post hoc analysis).
(B) P7C3-A20 treatment protects mice from post-TBI elevations in ac-tau in the brain (each group n = 3–4, *p < 0.05, **p< 0.01, ***p <0.001 ****p <0.0001, one-way ANOVA; and Tukey’s post hoc analysis). This protective effect is blocked by treatment with the NAMPT inhibitor FK866 or the Silti Inhibitor EX527.
(C) P7C3-A20 treatment protects mice from post-TBI AIS degradation (n = 3 per group, ****p < 0.0001, one-way ANOVA; and Tukey’s post hoc analysis). This protective effect is blocked by treatment with the NAMPT inhibitor FK866 or the Sirtl Inhibitor EX527.
(D) P7C3-A20 treatment protects mice from post-TBI tau mislocalization (each group n = 3, ****p < 0.0001, one-way ANOVA, and Tukey’s post hoc analysis, scale bar, 5 μm). This protective effect is blocked by treatment with the NAMPT inhibitor FK866 or the Sirtl Inhibitor EX527.
(E) SNO-GAPDH is not affected by P7C3-A20 (western blot, each group n = 3, *p < 0.05, **p < 0.01, one-way ANOVA and Tukey’s post hoc analysis). “Ascorbate (Asc) - negative control” shows specificity of signal in the SNO-resin-assisted capture technique.
See also Figure S5.
Acetylated tau is a blood biomarker of traumatic brain injury-induced neurodegeneration in mice and humans
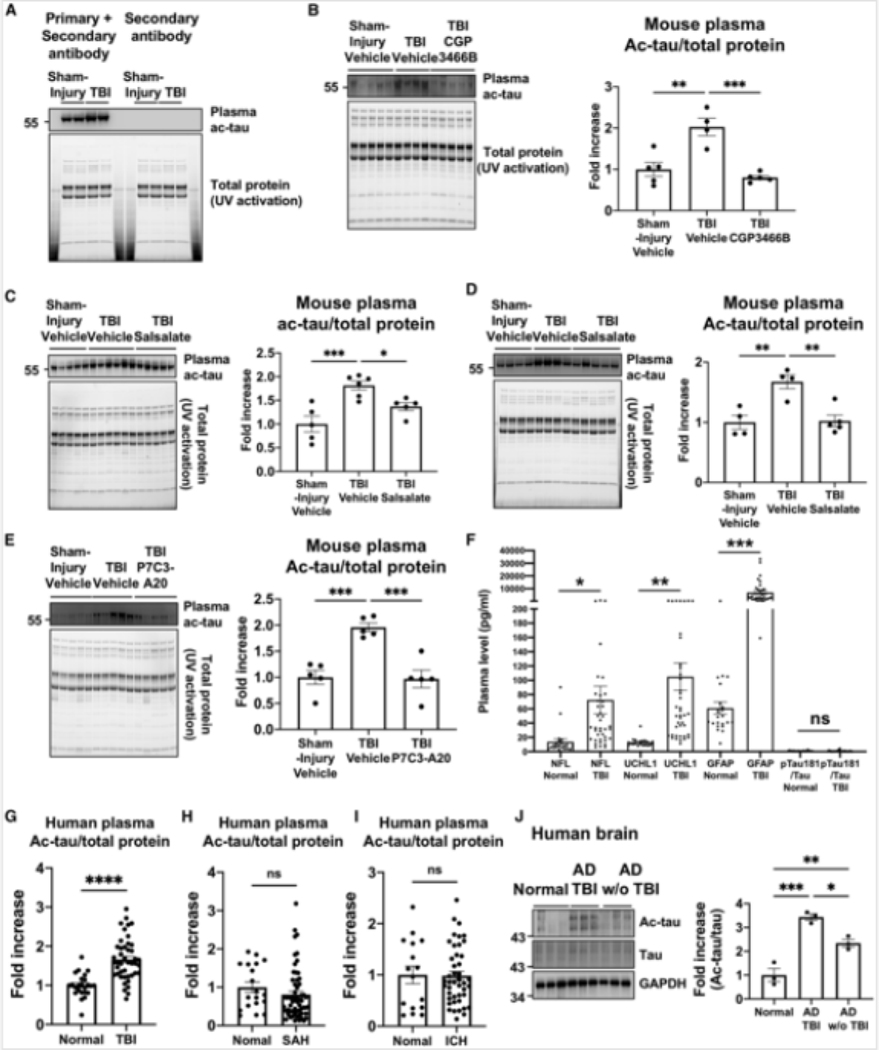
Because tau freely diffuses from brain to blood, we postulated that ac-tau might serve as a blood biomarker of post-TBI neurodegeneration. Western blot of immunoglobulin-depleted (because of its size overlap with tau) plasma revealed elevated levels of ac-tau in mouse plasma after TBI (Figure 6A). Specificity of plasma ac-tau was confirmed by using wild-type and tau knockout mice plasma samples with the same plasma immunoglobulin and albumin depletion strategy (Figure S6E) that has been previously established for assessment of rodent and human plasma tau under normal conditions and in AD or TBI (Sparks et al., 2012; Arun et al., 2013; Shekhar et al., 2016). Notably, the same protective therapies that reduced brain concentrations of ac-tau (treatment with CGP3466B, low-dose salsalate, or P7C3-A20) also decreased the concentration of ac-tau in plasma (Figures 6B–6E).
Figure 6. Elevated blood plasma ac-tau is a blood biomarker of TBI in mice and humans.
(A) Western blot shows that TBI increases plasma ac-tau. Because tau and immunoglobulin G (IgG) have closely similar molecular weights, secondary antibody alone served as a control to ensure there was no cross reactivity with any residual IgG after IgG depletion.
(B) Western blot and its quantification show that CGP3466B significantly reduced plasma ac-tau levels after TBI. Each group n = 4–5 with each lane representing a separated animal, **p < 0.01, ***p < 0.001 versus TBI+vehicle group, one-way ANOVA; and Tukey’s post hoc analysis.
(C) Western blot and its quantification show that salsalate reduces plasma ac-tau levels. Each group n = 54 with each lane representing a separated animal, *p < 0.05, ***< 0.001 versus TBI+vehicle group, one-way ANOVA; and Tukey’s post hoc analysis.
(D) A repeat experiment in an independent cohort of animals confirmed the results shown in (C). Each lane represents a separate animal. For both (C) and (D), **p <0.01, TBI+vehicle group, one-way ANOVA; and Tukey’s post hoc analysis.
(E) Western blot and its quantification show that P7C3-A20 significantly reduced plasma ac-tau levels after TBL Each group n = 5 with each lane representing a separated animal, ***p < 0.001 versus TBI+Nehicle group, one-way ANOVA; and Tukey’s post hoc analysis.
(F) Plasma Nil, UCHLI, and GFAP, but not pTauI 81/Tau, levels are higher in TBI cohorts at 24 h after injury, compared to controls (*p <0.05, **P <0.01,***P <0.001).
(G) The mean level of ac-tau was significantly higher in the TBI cohort at 24 h in comparison to the controls (1.8 ± 0.58 versus 1.16 ± 0.5, ****p <0.0001).
(H) The mean level of ac-tau in the subarachnoid hemorrhage (SAH) cohort at 24 h was no different from controls. (I) The mean level of ac-tau in the intracranial hemorrhage (ICH) cohort at 24 h was no different from controls.
(J) Western blot and its quantification show that AD patients with TBI history have higher ac-tau levels than AD patients without TBI exposure (*p <0.05, **p <0.01, ***p < 0.001, one-way ANOVA; and Tukey’s post hoc analysis).
See also Figures S6 and S7 and Tables S1 and S2.
Next, to evaluate for clinical relevance in human patients, plasma ac-tau levels were evaluated from TBI patients admitted to a neuroscience intensive care unit of Memorial Herman Hospital-Texas Medical Center (Houston, TX) from December 2017 to April 2019 (Figure S6F; Table S1). In these patients, plasma neurofilament light chain (NtL), ubiquitin C-terminal hydrolase Ll (UCHLI), and glial fibrillary acidic protein (GFAP), but not phosphorylated tau (pTau181)/Tau, were increased relative to control samples (Figure 6F). Plasma ac-tau was also increased by ~50% within day 1 of admission compared to controls (p < 0.0001) (Figure 6G) and remained consistently elevated across all time points (Figure S7A). Ac-tau levels showed no age- or gender-dependent differences (Figure S7B; for ac-tau female versus male 1.32 versus 1.64, p > 0.05), and there was no tau seeding activity in human TBI versus control samples (Figure S7C). Lastly. specificity for elevated levels of plasma ac-tau in TBI was observed, relative to plasma samples from human patients with either subarachnoid hemorrhage (Figures 6H and S7D) or intracerebral hemorrhage (Figures 6I and S7E).
Accumulation of ac-tau in the brains of patients with AD is significantly increased by a prior history of TBI
We next investigated whether increased ac-tau in AD would be further augmented by a previous history of TBI in people. Here, nine human frontal cortex brain specimens from the Northwestern University Alzheimers Disease Research Center brain bank were analyzed. Cases were selected based on their cognitive status during life, history of TBI, and degree of Alzheimer’s disease neuropathologic change (ADNC) at the time of brain autopsy (Montine et al., 2012; Hyman et al., 2012). There were three cases per study group, with ages ranging from 75–89 years old at the time of death. Group 1 consisted of normal controls with no cognitive impairment (NCI) and a maximum ADNC score of Low. Groups 2 and 3 consisted of subjects that had been diagnosed with dementia of the Alzheimer’s type (DAT), with ADNC score of High. Group 2 had an additional history of TBI, whereas group 3 did not. Within group 2, TBI was required to have occurred prior to the onset of cognitive symptoms. Specifically, subject 1 in group 2 sustained a TBI from an unknown cause at age 23 with loss of consciousness (LOC). Subject 2 in group 2 sustained a TBI with LOC while playing football in high school and an additional TBI with LOC at age 72 after driving their tractor over a cliff. The duration of LOC for subject I or 2 was not known. Lastly. subject 3 in group 2 sustained a severe TBI of unknown cause at age 54 that resulted in coma for 4 days. Importantly, none of the selected cases had co-existing confounding lesions involving the neocortex, such as neocortical Lewy body disease, and all cases were matched by age, sex, and post-mortem interval times (Table S2). Consistent with the hypothesis, ac-tau levels in AD patients in group 3 were significantly higher than ac-tau levels in group I NCI controls, and ac-tau levels in AD patients in group 2 with history of TBI were significantly higher than ac-tau levels in group 3 AD patients without a history of TBI (Figure 6J).
NSAIDs that inhibit p300/CBP are associated with decreased incidence of clinically diagnosed TBI and AD in people
In addition to the NSAID salsalate, the related salicylate diflunisal also inhibits p300/CBP (Shirakawa et al., 2016). Although diflunisal has much lower blood-brain barrier penetration than salsalate, along the order of 1:100 (Merck & Co.), it also inhibits p300/CBP acetyltransferase at much lower doses than what is typically administered for anti-inflammatory effect. Thus, we examined whether salsalate or diflunisal usage in people might be neuroprotective, relative to the commonly used NSAID aspirin that does not inhibit p300/CBP (Shirakawa et al., 2016). Notably, a recent randomized placebo-controlled trial in ~19,000 subjects showed that aspirin does not significantly reduce the incidence of dementia, minor cognitive impairment, or cognitive decline (Ryan et al., 2020).
The relationship between salsalate or diflunisal use and incidence of clinically diagnosed TBI or AD was investigated by analyzing 7.23 million United States (US) commercially insured individuals (MarketScan Medicare Supplemental database). Two cohort analyses were conducted to evaluate the predicted association, based on individual level of longitudinal patient data and pharmacoepidemiologic methods, as previously established (Cheng et al., 2018). The two cohorts included: (l) salsalate versus a matched aspirin population, and (2) diflunisal versus a matched aspirin population (Table S3). For each comparison, the un-stratified Kaplan-Meier curves and conducted propensity score (PS) stratified (n strata = 10) log-rank tests and a Cox regression model after adjusting age, race, sex, and disease comorbidities (including diabetes, hypertension, and coronary artery disease) were estimated using a PS-matching method. The International Classification of Diseases codes used to define the presence of each condition are listed in Table S4. After 6 years of follow-up, salsalate usage was associated with a significantly reduced risk of clinically diagnosed TBI (hazard ratio [HR] = 0.70, 95% confidence interval [CI] = 0.55–0.89, p < 0.001, Figure 7A) and AD (HR = 0.57, 95% CI = 0.42–0.77, p < 0.001, Figure 7B), compared with a PS-matched aspirin cohort by Cox regression model. Strikingly, diflunisal usage was associated with an even greater reduced incidence of clinically diagnosed TBI (HR = 0.61, 95% Cl = 0.43–0.86, p = 0.003, Figure 7A) and AD (HR = 0.17, 95% Cl 0.08–0.36, p < 0.001, Figure 7B), compared with a PS-matched aspirin cohort. The greater efficacy of diflunisal relative to salsalate correlates with the greater potency of diflunisal to inhibit p300/CBP. Lastly. to more specifically control for co-morbid factors associated with AD, additional subgroup analysis was performed by removing all subjects with diabetes, hypertension, and coronary artery disease. This confirmed that usage of either salsalate or diflunisal was associated with a reduced incidence of AD, with much stronger effect seen in patients prescribed diflunisal (Figure 7C).
Figure 7. Diflunisal usage is associated with decreased incidence of TBI and AD in people and with inhibition of ac-tau after TBI in mice.
(A) Longitudinal analyses reveal that salsalate and diflunisal usages reduce risk of traumatic brain injury (TBI) in all patient data from the IBM MarketScan Medicare Supplemental Database. The un-stratified Kaplan-Meier curves, conducted propensity score stratified (n strata = 10) log-rank test and Cox model, and hazard ratio and 95% confidence interval for two cohort studies, were illustrated for both (A) and (B). Two cohott studies were conducted including: (I) salsalate users and aspirin users, and (2) diflunisal users and aspirin users. Using propensity score stratified survival analyses by adjusting the initiation time of drugs, enrollment history, age and gender, and disease comorbidities (diabetes, or hypertension, or coronary anery disease). Propensity score stratified Cox-proportional hazards models were used to conduct statistical inference for the hazard ratios.
(B) Longitudinal analyses reveal that salsalate and diflunisal usage in the same group as (A) is also associated with reduced incidence of AD in people.
(C) Subgroup analyses after excluding patients with type 2 diabetes, hypertension, or coronary artety disease (known risk factors for AD) further confirms that salsalate or diflunisal usage is associated with decreased incidence of AD.
(D) LC-MS/MS analysis shows modest penetration of diflunisal into mouse brain but the plasma:brain ratio is decreased when protein binding is taken into account and free drug levels are compared. Drug levels in plasma and brain were determined by LC-MS\MS analysis after mice were administered three different concentrations of diflunisal and euthanized 60 or 180 min later, followed by collection of blood and perfusion with saline, prior to harvesting brain tissue. Rapid equilibrium dialysis was used to determine binding of diflunisal in mouse plasma and brain homogenate. “P” and “B” denote plasma and brain, respectively.
(E) Diflunisal treatment dose-dependently reduces post-TBI elevations in ac-tau in the brain. Each group n = 4–5, ***P < 0.001, ****P < 0.0001 versus TBI+vehicIe group, one-way ANOVA; and Tukey’s post hoc analysis.
(F) Western blot and its quantification show that diflunisal dose-dependently reduced plasma ac-tau levels after TBI. Each group n = 4–5 With each lane representing a separate animal, *P< 0.05, ***P <0.001 ***p < 0.0001 versus TBI+NehicIe group, one-way ANOVA; and Tukey’s post hoc analysis.
(G) There is a significant correlation between brain and plasma ac-tau levels after brain injury (data from C and D; R = 0.857, p < 0.0001).
Because diflunisal is an even more potent inhibitor of p300/CBP than salsalate, we postulated that this medicine might also inhibit the accumulation of ac-tau after brain injury in mice. After establishing that peripherally administered diflunisal dose-dependently crossed the blood-brain barrier in mice (Figure 7D), we orally administered this medicine to animals and then euthanized at the indicated times. After peripheral blood collection, animals were perfused with I x PBS to remove blood in the brain vasculature. Total plasma and brain levels of diflunisal were evaluated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and then converted to free drug levels after measurement of binding in mouse plasma and brain. In line with previous observations of diflunisal levels in baboon cerebrospinal fluid (CSF) (Merck & Co.), there was relatively modest penetration of diflunisal into mouse brain. On average, across the two time points and three concentrations evaluated, the total diflunisal plasma to brain ratio was 000:1. However, when free drug levels were calculated, the ratio decreased to only 55:1 because the fraction unbound in brain tissue was higher than in plasma (fubrain = 0.050 ± 0.001; fuplasma = 0.014 ± 0.002). Furthermore, when absolute concentrations were taken into account, total diflunisal brain levels reached ~3.9 μM (972 ng/g), and free levels were 196 nM (49 ng/g) at the 50 mg/kg dose in mice. Total plasma levels at this dose ranged from 167–232 μg/mL in mice, a concentration similar to mean peak levels of 190 ± 33 mcg/mL observed in humans given 500 mg diflunisal twice daily for Il days (Merck & Co.), confirming the physiologic relevance of the doses employed here. Therefore, the efficacy of daily diflunisal administration, beginning 24 h after TBI, to block the expected rise in neuronal ac-tau in mice was evaluated. As shown in Figure 7E, accumulation of ac-tau in cortical brain tissue progressively decreased with escalating doses of diflunisal. Moreover, this was reflected in dose-dependent decreases in blood ac-tau as well (Figure 7F), with tight correlation between plasma and brain levels of ac-tau (Figure 7G, R = 0.857, p < 0.0001
Discussion
Taken together, our findings reveal critical cross-talk between S-nitrosylation and acetylation in neurons after brain injury, which converges on neuronal ac-tau. Although previous studies have reported phosphorylated tau in human TBI (Yang et al., 2017; Okamura et al., 2019; Gorgoraptis et al., 2019), these observations were beyond the first 24 h after injury, which was examined here. Importantly, independent analysis of the 24-h samples showed no increase in tau phosphorylation. Moreover, genetic in vitro and in vivo studies showed a direct neurotoxic effect of human ac-tau on neurons. Thus, our results support a critical role for tau acetylation in the acute period following brain injury, preceding any effects of tau phosphorylation. Future studies will focus on the interplay between tau acetylation and the myriad other pathological post-translational modifications of tau that have been reported, including phosphorylation and ubiquitination (Wesseling et al., 2020).
AD-like pathology in experimental systems had been linked previously to N-methyl-d-aspartic acid-mediated, neuronal nitric oxide synthase-dependent S-nitrosylation (Sen et al., 2018), and separately SNO-GAPDH had been implicated in modulating protein acetylation (Kornberg et al., 2010 However, understanding of the connection of protein S-nitrosylation to human-relevant in vivo models of neurodegeneration after brain injury was missing. The work here shows how this process mechanistically unfolds and how these insights form the basis of effective therapy. After injury, GAPDH S-nitrosylation leads to ac-tau accumulation and subsequent ac-tau-mediated pathology in neurons. Specifically, brain injury-induced SNO-GAPDH coordinately activates p300/CBP acetyltransferase and inhibits Sirtl deacetylase to increase amounts of neuronal ac-tau. Drugging SNO-GAPDH with CGP3466B, or p300/CBP with low-dose salsalate or diflunisal, inhibits tau acetylation and downstream consequences of brain injury. It is important to recognize that these pharmacologic agents can have pleiotropic cellular effects, and thus we are unable to define how much of their neuroprotective effect is strictly due to reducing ac-tau. Given that all the neuroprotective agents examined in this study are also shown to reduce ac-tau in vivo, however, there is compelling support for a common mechanism. This viewpoint is further bolstered by the epidemiologic association of diflunisal and salsalate usage with decreased incidence of both AD and clinically diagnosed TBI in people. Moreover, the stronger effect of diflunisal compared to salsalate correlates with the greater potency with which diflunisal inhibits p300/CBP acetyltransferase. In addition, patient usage of the NSAID aspirin, which differs from the NSAIDs diflunisal and salsalate by not being able to inhibit p300/CBP acetyltransferase, did not show a protective effect. However, it is important to note that more patients took aspirin than diflunisal or salsalate, which is an unavoidable confounding factor of the patient population. In aggregate, the animal and human data presented here offer compelling support for a neuroprotective effect of reducing ac-tau, which supports further exploration of the potential protective efficacy of diflunisal or salsalate in patients with TBI or AD.
On the other side of the balance of acetylation, SNO-GAPDH-mediated inhibition of NAD+-dependent Sirtl deacetylase is mediated by enzymatic S-nitrosylation of Siltl, thereby preventing tau deacetylation. Maintaining Sirtl activity through treatment with P7C3-A20, which preserves NAD* levels in injured neurons, also protected against the same deleterious outcomes after brain injury. Thus, multiple lines of evidence converge to show that reducing neuronal ac-tau is a successful therapeutic approach for treating TBI, whether that is through inhibiting acetylation with already existing drugs that could be repurposed for TBI, or through enhancing deacetylation through a drug to emerge from the P7C3 series of molecules. Other strategies to preserve NAD+ after injury, such as pharmacologic inhibitors of the NAD hydrolase SARMI, might be similarly effective (Pieper and McKnight, 2018; Hughes et al., 2021).
Both inhibition of tau acetylation and activation of tau deacetylation were observed following post-injury treatment with CGP34668, which selectively inhibits S-nitrosylation of GAPDH. CGP34668, also named “omigapil,” is an analog of the irreversible inhibitor of monoamine oxidase B known as deprenyl, which is employed to treat patients with Parkinson’s disease and depression. Notably, omigapil has recently shown safety in a Phase I clinical trial for patients with pediatric and adolescent congenital muscular dystrophy (CMD; NCT01805024). Abnormally frequent neurofibrillary tangles of tau in the cerebral cortex have been reported in several studies of CMD patients, and variations in tau have also been associated with CMD (Vermersch et al., 1996). Elucidation here of the downstream events in the brain following GAPDH-S-nitrosylation after brain injury may thus provide previously unanticipated strategies to preserve brain health in CMD patients. Because tau is also found in muscle fibers (Lübke et al., 1994), it is likewise possible that the signaling cascade and opportunities for therapeutic intervention that we have characterized here in the brain could apply to improving muscular health in patients with CMD.
Lastly, there is a tremendous unmet need for robust biomarkers that can establish whether a head injury has affected the brain, as well as stratify the severity and nature of the brain injury and objectively identify whether it is resolving. For example, a blood-based biomarker could overcome the limitations of current neuropsychological tests for brain injury, and also help detect brain trauma that is masked by other injuries or symptoms. Rapid and accurate field diagnosis of brain injury is also critical for assuring that athletes and military personnel are not placed at risk for a second injury before they have fully recovered. While numerous bio cerebrospinal-fluid-biomarkers-for-brain-injury-have been proposed (Zetterberg and Blennow, 2016), collection of peripheral blood samples is considerably easier.However, the low concentration of potential biomarkers in peripheral blood can be technically limiting, and the concentration of brain proteins in the blood can vary as a function of integrity of the blood-brain barrier. Thus, a robust marker that freely diffuses across the blood-brain barrier from the brain into the blood, such as tau protein, is desired. Here, we have shown that ac-tau levels rapidly rise in the blood of both rodents and humans after brain injury, and that serum levels decline proportionally with protective treatments that target the underlying signaling cascade. Other proposed brain injury markers in the field include SIOO-B and GFAP, as blood biomarkers of astroglial injury (Mondello et al., 2018). However, neither of these is derived from degenerating neurons themselves, and S 100-B is also robustly expressed in cells outside of the brain, such as adipocytes and chondrocytes (Olsson et al., 2011). Another candidate blood biomarker from brain injury is neuron-specific enolase (NSE), an enzyme involved in glycolytic energy metabolism (Mondello et al., 2018). However, the utility of NSE is limited because erythrocytes and platelets also contain high amounts of this enzyme (Tolan et al., 2013; Geisen et al., 2015), and elevated NSE is also associated with tumors, ischemic stroke, intracerebral hemorrhage, and seizures (Isgrò et al., 2015). Additional blood _biomarkers for TBI include serum NfL and UCH-LI (Shahim et al., 2020), although UCH-LI is unable to distinguish mild TBI patients from those with orthopedic trauma (Posti et al., 2017). Taken together, the quest for blood biomarkers-of-TBL is, an active and important area of investigation, and ac-tau may provide a uniquely valuable addition to the field. Importantly, ac-tau is mechanistically linked to both pathophysiology of neurodegeneration and a neuroprotective treatment strategy. Ac-tau levels in the blood correlate directly with its brain levels, and blood ac-tau appears to distinguish TBI-induced neurodegeneration from other forms of pathology, such as subarachnoid and intracerebral hemorrhage. Whether blood ac-tau levels correlate with the progression and severity of other forms of neurodegeneration, such as AD, remains to be investigated.
In summary, ac-tau is a previously unrecognized contributor to TBI pathophysiology. Tau acetylation sites after brain injury correspond to sites implicated in human AD, reflecting a shared mechanism of aberrant signaling that may serve as a pathophysiologic mechanism for the increased risk of developing AD after TBI. This notion is supported by the observation of additionally increased ac-tau in the brains of patients with AD who also had a TBI prior to the onset of cognitive symptoms. Importantly, the presence of a small degree of baseline neuronal ac-tau in the uninjured and healthy brain prompts future investigation for a normal biological role of tau acetylation, with toxicity resulting when acetylation exceeds a threshold. At this time, our results establish that reducing ag-tau through multiple points of therapeutic intervention after brain injury offers a previously unanticipated neuroprotective strategy, and quantifying ag-tau in the blood provides a biomarker of brain injury.
Limitations of study
The utility of ac-tau as a biomarker of brain injury and neurodegeneration will need to be further established with more sophisticated measures of ac-tau and investigation of other neurodegenerative conditions, Another consideration is the presence of tau in muscle with respect to biomarker interpretation when there is comorbid muscular trauma with TBI. It is also important to note that although two sites of tau acetylation after TBI were examined here, there have been upwards of 20 tau acetylation sites identified in human tau in AD (Wesseling et al., 2020). Lastly. the retrospective human studies with diflunisal and salsalate in AD and TBI are correlative, and future prospective clinical trials are needed to confirm therapeutic efficacy.
Uncited reference
US Food and Drug Administration,.
STAR★Methods
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Andrew A. Pieper (Andrew.Pieper@HarringtonDiscovery.org)
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any new datasets or code.
Experimental model and subject details
Cell culture
SH-SY5Y cells were used to overexpress flag-tagged TauWT, TauKQ, TauKR for in vitro traumatic brain injury experiment. The cells were grown in DMEM:F12 (I:I)(GIBCO, 11320–033) containing 10% fetal bovine serum (GIBCO, 26140–079) and 1% penicillin/streptomycin (GIBCO, 15140–122).
Animals
Male and female C57BL/6J (Stock No: 000664) mice were purchased from Jackson Laboratory. WIdS (Stock No: 008820) mice were purchased from Jackson Laboratory and bred at Louis Stokes Cleveland VA Medical Center under specific pathogen-free conditions. Twelve months old male and female TauWT and TauKQhigh mice were used. Adult male Long-Evans outbred rats (Blue Spruce, HsdBlu:LE) were purchased from Envigo and approximately 3.5 months old rats were used. All animals were maintained under temperature, light, and humidity-controlled conditions with free access to food and water and randomly assigned to experimental groups. All animal work was approved by Louis Stokes Cleveland VA Medical Center Institutional Animal Care and Use Committee (animal protocol # 18–050-MS-18–015), University of California, San Francisco, Institutional Animal Care and Use Committee-approved guidelines, and Institutional Animal Care and Use Committees of the VA Western New York Healthcare System.
Human subjects
Our experiments with human blood sample were approved by Institutional Review Board (IRB) (IRB Number HSC-MS-17–0776 (Molecular and Microbiome Mechanisms after Neurological Injury), HSC ‒MH-17–0452 (Biorepository of Neurological Disorders Registry and Tissue Repository at UT Health) and #EM-15–35 (University Hospitals Case Medical Center, Center for Clinical Research and Technology, OH). Written informed consent was obtained from the patient or surrogate. The average age of blood donors are 54 ± 11 (controls) and 48 ± 20 years old (TBI) and the majority are males (control 75%, TBI 88%). Sex and age information are reported in Table S1.
Human brain samples were obtained from the holdings of Northwestern University Alzheimer’s Disease Research Center brain bank, and under approved study protocols. All donors provided written informed consent for the use of brain tissue. The average age of tissue donors is 81 ± 5 (group 1), 83 ± 7 (group 2) and 85 ± 3 years old (group 3). The history of TBI information is reported in Results.
Method details
In vitro traumatic brain injury
SH-SY5Y cells were seeded at 2 × 106 cells per well on the collagen I precoated 6-well flexible-bottomed culture plates (Flexcell International Corporation, BF-3001C). Flag-tagged TauWT, TauKQ and TauKR (provided by Dr. Li Gan) plasmids were transfected with FuGene@ HD transfection reagent (Promega, E2311) according to the manufacturer’s instructions. Six hours after transfection, cells were injured by 90-ms bust of pressurized medicinal air using the Cell Injury Controller Il system (Custom Design & Fabrication Inc., Sandston, VA). Cells and culture media were harvested two hours after injury. LDH release was measured using a commercial assay kit (Cytotoxicity Detection Kit P (LDH), Roche, 04–744-926–001) according to the manufacturer’s instructions.
In vivo multimodal traumatic brain injury
Eight-week old male, female C57BL/6J and male WldS mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal (IP) injection and placed in an enclosed chamber constructed from an air tank partitioned into two sides and separated by a port covered by a mylar membrane. The pressure in the side not containing the mouse was increased to cause membrane rupture at 20 pounds per square inch (PSI), which generates a ~1–2 ms jet airflow of 137.9 ± 13.79 kPa that passes through the animal’s head. The head remains untethered in a padded holder, while the body is fully shielded by a metal tube. The jet of air produced upon membrane rupture provides a concussive injury, which is followed by acceleration/deceleration of the head and then exposure to the ensuing blast wave within an enclosed space.
In vivo controlled cortical impact traumatic brain injury
Adult mice, 2 to 4 months of age, were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal (IP) injection and placed on a heating pad to maintain body temperature. The animal’s head was shaved and then placed on a stereotaxic frame where an incision was made through the skin, exposing the skull. For CCI injured mice, a ~5 mm diameter craniectomy was made over the right parietal cortex (bregma: −2.0 mm; lateral −2.5 mm), leaving the dura intact. Mice were then subjected to a moderate CCI injury with a piston velocity of 4.0 m/s and depth of 0.55 mm using an eCCI-6.0 device (Custom Design & Fabrication, Virginia Commonwealth, VA, United States). Sham controls underwent an identical surgical procedure with the absence of the craniectomy and injury. The incision was closed using 4–0 nylon non-absorbable sutures (Ethicon, Inc., Piscataway, NJ, United States), and mice were placed in a clean, single housed cage on a heating pad. For hydration and analgesia, animals were administered I mL of lactated ringer and 1.0 mg/mL buprenorphine. Ipsilateral CCI injured and sham cortices were harvested at 6 hr as well as 1, 3, 7, 21, and 42 days post-injury. At the desired time points, mice were anesthetized with a ketamine/xylazine cocktail and euthanized by cervical dislocation, followed by dissection and freezing of injured and sham cortices.
In vivo acoustic blast overpressure traumatic brain injury
We employed the shock tube device as described (Newman et al., 2015; Allen et al., 2018). The exit port of the shock tube is sealed by a thin brass foil diaphragm. With the diaphragm in place, the chamber behind the diaphragm is charged with compressed air, to a back pressure of 80 psi. A pressure sensor and air pressure modulator controlled by commercial hardware and custom software are used to charge the cylinder to the desired pressure. A computer-controlled (MATLAB) solenoid-driven hunting arrow mounted inside the chamber is used to pierce the diaphragm, once the criterion pressure is achieved; this triggers a blast with precise timing and sound pressure, which can be monitored by a pressure transducer probe placed at the outlet end of the shock tube and recorded by a commercial analog-to-digital converter and stored on a computer for later analysis using custom software. A wire mesh insert near the outlet end catches metal foil fragments (shrapnel), preventing penetrating injury to animals. Rats (adult male Long-Evans rats) were deeply anesthetized and placed into a custom-built metal holder, with body axis at right angles to the shock tube. Only the head was exposed (typically the left side), approximately 2.5 inches from the outlet end of the shock tube. A dual-blast paradigm, allowing a I-month interval between blast exposures, each with a sound pressure of 63 kPa (190 dB-SPL) was employed. Animals were closely monitored at regular intervals for viability and any signs of distress following blast exposure.
Metabolomics
Ten 8-week old male C57BL/6J mice were subjected to poly-traumatic brain injury as described above and ten were exposed to a sham-injury. Seven days post-injury, blood was collected retro-orbitally in K2-EDTA blood collection tubes. Plasma was separated from these blood samples and flash frozen in liquid nitrogen. Plasma samples were sent to Metabolon Inc, (Durham, North Carolina, USA) for Global Metabolomics Profiling using LC-MS. 245 biochemicals identified in these plasma samples were significantly different between TBI and Sham groups.
Drug preparation and administration
CGP3466B (Sigma-Aldrich, SML 1941) was dissolved in DMSO and then diluted in sterile saline. The final working stock was 0.0014 mg/ml for administering the 0.014 mg/kg dose. Intraperitoneal administration of CGP3466B was initiated 15 min or 24 h after injury, and tissues were harvested 6h (for SNO-GAPDH, SNO-SIRTI measurement) or 2 weeks (for ac-tau measurement) later, respectively. Salsalate (AdipoGen, AG-CRl-3574) was dissolved in DMSO and then diluted in sterile PBS. The final working stocks were as follows: 2.5 mg/ml (25 mg/kg dose), I mg/ml (10 mg/kg dose), 0.5 mg/ml (5 mg/kg dose). Daily intraperitoneal administration of salsalate was initiated 24 hr after injury and continued throughout behavioral testing. P7C3-A20 and FK866 (Sigma-Aldrich, F8557) were first dissolved in 1 vol of DMSO, followed by addition of 4 vol of Kolliphor and vigorously vortexing. The solution was then diluted with 30 vol of filtered 5% dextrose (pH 7.0). EX527 (Selleckchem, Sl 541) was dissolved in 1% DMSO + 30% polyethylene glycol + 1% Tween 80. Daily intraperitoneal administration of P7C3-A20 was initiated 24 hr after poly-traumatic brain injury. EX527 (10 mg/kg) was administer once a day and FK866 was treated twice per day, with the first injection given at the same time as P7C3-A20 (20 mg/kg) and the second injection given 6 h later (LoCoco et al., 2017). Diflunisal was first dissolved in 1 vol of DMSO, followed by addition of 2 vol of Kolliphor and vigorously vortexing. The solution was then diluted with 7 vol of saline to the appropriate concentration for administration at 25, 50, or 100 mg/kg.
Behavioral analysis
The Barnes maze apparatus consisted of a gray circular platform (91 cm in diameter and 90 cm in height), with 20 equally spaced holes 5 cm in diameter along the perimeter (Stoelting Co.). One of these holes contained a recessed escape chamber located under the platform. Four different and equally-spaced visual cues with different shapes and colors were hung on a black circular curtain surrounding the maze. The training session consisted of four trials per day for four consecutive days. In each trial, the mouse was gently released in the middle of the maze under the cylindrical chamber, and after 5 s elapsed the covering chamber was lifted to allow the mouse to explore the maze. If a mouse failed to find the escape chamber within 60 s, it was manually guided to the escape chamber and then allowed to stay in the chamber for 30 s. Both the platform and the escape chamber were cleaned thoroughly between individual trials. On day 5, 24 h after the last training day, the escape chamber was removed and mice were allowed to explore the maze for 60 s. Total and primary latency were measured during training days, and latency to first nose pokes in escape hole and time spent in target quadrant were measured for memory test on the probe trial day. Any-maze video tracking software (Stoelting Co.) was used to acquire measurements. Analysis was conducted blind to treatment group.
For foot slip test of motor function, mice were trained to cross an 80 cm-long beam over two days and then tested on day 18. Video of the mice was recorded and analyzed by observers blind to treatment group.
Western blotting
Western blotting was performed as described previously (Min et al., 2010), with the 9AB antibody against acetylated-tau generated in the Gan laboratory. Briefly, cortical and hippocampal tissues were sonicated in RIPA buffer (Sigma-Aldrich, R0278) containing protease and phosphatase inhibitor cocktail (Thermo Scientific, #1861284), 1 mM phenylmethyl sulfonyl fluoride (Sigma Aldrich, P7626), and histone deacetylase inhibitors such as 5 mM nicotinamide (Sigma-Aldrich, 72340) and 1 11M trichostatin A (Sigma-Aldrich, T8552). Lysates were centrifuged at 170,000 g at 4°C for 15 min and at 18,000 g at 4°C for 10 min, after which protein concentrations of supernatants were measured by bicinchoninic acid assay (Thermo Scientific, A53225). Proteins were heated in a Laemmli Sample Buffer (Bio-Rad Laboratories, Inc., #1610737) with beta-mercaptoethanol (Bio-Rad Laboratories, Inc., #1610710) for 5 min, and then resolved in 4%–20% Criterion TGX Stain-Free gels (Bio-Rad Laboratories, Inc., #5678095). Stain-free gels were exposed to ultraviolet light by ChemiDocTM MP Imaging system (Bio-Rad Laboratories, Inc.) to visualize total plasma proteins. Proteins were transferred onto 0.2 μm polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc., #1704157) with the Trans-Blot Turbo system (Bio-Rad Laboratories, Inc.). Membranes were blocked with 5% nonfat dry milk in tris-buffered saline-tween 20 (TBST) for 1 h at room temperature, and then incubated with primary antibodies at 4°C overnight. The following antibodies were used to probe target proteins: rabbit anti-ac-tau (Li Gan laboratory, 1 :500), mouse anti-tau (abeam, ab80579, 1:5000), mouse anti-tau (Thermo Fisher Scientific, #13–6400, 1:5000), mouse anti-β-actin (Santa Cruz Biotechnology, sc-47778, 1:1000), mouse anti-GAPDH (ENID Millipore Corp, MAB 374, 1:5000), mouse anti-NeuN (ENID Millipore Corp, MAB 377, 1:1000), mouse anti-GFAP (Thermo Scientific, MAS-12023, 1:1000), mouse anti-SIRTI (Abeam, abi10304, 1:500), rabbit anti-p300 (Santa Cruz Biotechnology, sc-584, 1:500), rabbit anti-CBP (Cell Signaling Technology, #7389, I:IOOO), rabbit anti-acetyl K5 histone 2A (Abcam, abl 764, 1:1000), rabbit anti-histone 2A (Cell Signaling Technology, #12349, 1:1000), rabbit anti-acetyl K18 histone 3 (Abcam, ab1191, rabbit anti-histone 3 (Cell Signaling Technology, #4499, 1:1000), rabbit anti-phospho-tau (Ser 202) (Cell Signaling Technology, #11834, 1:1000), rabbit anti-phospho-tau (Ser 262) (Thermo Fisher Scientific, 44–750G, 1:1000), mouse anti-phospho-tau (Ser 396, 404) (gift from Dr. Peter Davies, 1:1000), rabbit anti-KIBRA (Santa Cruz Biotechnology, sc-133374, 1:500), rabbit anti-PSD95 (Cell Signaling Technology, #2507, 1:1000), mouse anti-AnkG (Santa Cruz Biotechnology, sc-12719, 1:1000), rabbit anti-βIV spectrin (gift from Dr. Matthew N. Rasband,l :500), rabbit anti-TNF-alpha (Cell Signaling Technology, #11948, I :2000), rabbit anti-ac-p300/CBP (Cell Signaling Technology, #4771, 1:1000), rabbit anti-Flag (Cell Signaling Technology, #2368, 1:1000), rabbit anti-cleaved caspase 3 (Cell Signaling Technology, #9661, 1:500). After primary antibody incubation, membranes were rinsed with TBST (3 × 5 min) and subsequently incubated with horseradish peroxidase conjugated-secondary antibodies. SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Scientific, #34096) was used to detect band by BioSpectrum 810 Imaging System (UVP, Upland, CA). Densitometry quantification of western blot signal was conducted by ImageJ version I .42 software (National Institutes of Health, Bethesda, MD).
Neuron and non-neuron isolation
Three cortices were pooled and collected in DPBS with Calcium, Magnesium, Glucose and Pyruvate (Thermo Fisher Scientific, 14287080) and processed using the MACS Adult Brain dissociation kit (Miltenyi Biotec, 130–107-677) to generate a single cell suspension. Briefly, each sample was digested using a combination of enzymatic and mechanical dissociation. For enzymatic dissociation, Enzyme mixes 1 and 2 (provided in the kit) were used for mechanical dissociation. Samples immersed in the enzymes mixes were placed in MACS C-tubes, which were placed in the MACS Octo dissociator with heaters for 30 min at 37°C. The dissociated samples were further processed for debris removal by first passing them through a MACS 70 μm smart strainer and then using MACS Debris Removal reagent. Lastly, RBC lysis was performed to achieve the final single cell suspension of brain cells. This single cell suspension was further processed using the MACS Neuronal isolation kit (Miltenyi Biotec, 130–115-389) to separate the single cell suspension into neuronal and non-neuronal populations. Briefly, cells were mixed with MACS Non-neuronal cell Biotin antibody cocktail for 5 min. After washing with DPBS (with 0.5% FBS), cells were incubated with Anti-Biotin Microbeads. After a 10-min incubation, cells were passed through a column attached to a magnetic stand, which results in binding of non-neuronal cells (labeled by microbead-biotin antibodies) to the column, and the flow-though was collected as neuronal population. The non-neuronal population was collected by removing the column from the magnetic stand and placing the plunger in the column to flush out the non-neuronal cells.
SNO-resin assisted capture (SNO-RAC)
SNO-RAC was performed as described previously (Forrester et al., 2009). Mouse cerebral cortex was mechanically homogenized in lysis buffer containing 100 mM HEPES/I mM EDTA’O.I mM neocuproine (HEN), 150 mM NaCl, 0.1% (vol/vol) Nonidet P-40 (NP-40), 0.2% S-methylmethanethiosulfonate (MMTS) and protease and phosphatase inhibitor cocktail (Thermo Scientific, #1861284). After two times centrifugation (20,000 g at 4°C for 20 min), protein concentration of supernatants was determined using Coomassie protein assay (Thermo Scientific, #1856210). Total lysates were treated with 0.2% MMTS and 2.5% SDS, and then incubated at 50C for 20 min. Proteins were precipitated with pre-chilled (−20°C) acetone and centrifuged at 4,255 g at 4°C for 12 min. After washing pellets with 70% acetone three times, proteins were sonicated in HEN buffer containing 1% SDS. Precipitation of proteins was repeated with −20°C acetone, and the final pellets were resuspended in HEN/I% SDS. Proteins were incubated with freshly prepared 30 mM ascorbate and 50 μl of thiopropyl Sepharose (GE Lifesciences, 17–0420-01, Pittsburgh, PA) and rotated in the dark for 3 h. After centrifugation at 1,200 g for 30 s, the bound SNO proteins were sequentially washed three times with HEN/I %SDS and two times with 1/10 diluted HEN/I%SDS. SNO-proteins were then eluted with 2 × Laemmli Sample Buffer (Bio-Rad Laboratories, Inc., #1610737) with 10% beta-mercaptoethanol (Bio-Rad Laboratories, Inc., #1610710) and analyzed by SDS-PAGE and immunoblotting.
Postsynaptic density fractionation
To enrich the postsynaptic density (PSD), synaptosomal membranes were isolated from adult mice following Kristian et al., 2010 with minor modifications (Kristian, 2010; Cao et al., 2007). Brains were extracted, and the cortex and hippocampus were quickly dissected. Tissue samples were immediately homogenized with 8 passes of a Teflon on glass Potter-Elvehjem homogenizer in subcellular isolation buffer (SIB: 225 mM mannitol, 75 mM sucrose, 2 mM K2HP04, 5 mM HEPES, I mM EGTA, 0.1% fatty acid free bovine serum albumin). Differential centrifugation was performed at 1,500 g followed by 21,000 g on the supernatant to sequentially de-enrich unlysed cells and cytosolic and endoplasmic reticulum proteins, respectively. The resulting membrane-enriched pellet was separated at 21,000 g for 8 minutes over a discontinuous Percoll gradient comprised of 15%, 24%, and 40% Percoll steps. Synaptosomes were collected between the 15% and 24% Percoll interfaces. Synaptosomes diluted in SIB were pelleted at 10,000 g. All steps were performed on ice with centrifuging at 4°C.
Immunohistochemistry
Mice were transcardially perfused with cold I × phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS at pH 7.4 under anesthesia. Brains were carefully removed and post-fixed in 4% paraformaldehyde for 24–48h at 4°C. Brains were immersed in 30% sucrose in PBS for 48–72h at 4°C and then rapidly frozen in 2-methylbutane pre-cooled to −20°C with dry ice. Brains were cut coronally (30 or 40 μm) and sections were stored in cryoprotective solution (150 mM Ethylene glycol, 100 mM glycerol, 250 mM PBS) at - 20°C. For tau and NeuN staining, sections were washed three times with PBS for 5 min and then treated with 0.2% Triton X-100 in PBS for 15 min. Sections were washed with PBS and then incubated with 100 mM glycine for 15 min. After blocking sections using blocking buffer (1% bovine serum albumin, 10% normal goat serum, 0.3 M glycine) for 30 min, primary antibodies (Tau, Thermo Fisher Scieentific, #13–6400, 1:100, NeuN, EMD Millipore Cor., #MABN-140, 1:500) were incubated overnight at 4°C. Sections were washed three times with PBS (5 min each) and then incubated with Alexa Fluor 488 goat anti-mouse (Thermo Fisher Scientific, Al 1001, 1:200) or Alexa Fluor 568 goat anti-rabbit (Thermo Fischer Scientific, 1:200) at room temperature for 2 h. Sections were mounted on slides and then coverslipped with Prolong diamond antifade mountant (Invitrogen, P36961). AnkG and βIV spectrin staining were performed as described previously (Sohn_et al., 2016). Sections were permeabilized with 0.3% Triton X-100 and blocked with 10% normal goat serum at room temperature for lh. Sections were incubated with primary antibodies (AnkG, UC Davis’NIH NeuroMab Facility, N106/36, 1:500, βIV spectrin, gift from Dr. Matthew N. Rasband, I :500) overnight at 4°C and then with Alexa Fluor 488 goat anti-mouse (Thermo Fisher Scientific, A11001, 1:300) or Alexa Fluor 568 goat anti-rabbit (Thermo Fisher Scientific, Al 1011, 1:300) at room temperature for 1 h. For synaptic vesicle 2 staining, brain sections were first permeabilized in blocking solution containing PBS with 0.5% Triton X-100 and 10% normal donkey serum for 1 h at room temperature. Then they were incubated overnight with an SV2 antibody (Developmental Studies Hybridoma Bank) in blocking solution followed the next day by a 1 h incubation with an Alexa-conjugated secondary antibody (Life Technologies) at room temperature. For silver staining, sections were collected in 0.1 M phosphate buffer (pH 7.4) containing 4% paraformaldehyde and fixed for 7 days at 4°C. Sections were then processed for the detection of axon degeneration with FD NeuroSilver Kit Il (FD Neurotechnologies, PK301) according to the manufacturer’s instructions. Sections were subsequently mounted on slides, cleared in xylene, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ).
Quantification of immunohistochemistry
Images were acquired with Zeiss LSM710 confocal microscope, Zeiss Axiolmager.M2 with monochromatic digital camera (Zeiss AxioCam MRm Rev. 3) and Zeiss Axio Scan.Zl. To visualize the axon initial segment, twenty serial optical sections (0.5 μm steps) were projected into a single image. Confocal images of synaptic vesicle 2 were acquired on a CSU22 spinning disk confocal system (Yokogawa) with a Ti-E microscope (Nikon) using a 60x oil immersion objective lens. ImageJ version 1.42 software (National Institutes of Health, Bethesda, MD) was used to analyze the intensity of the AIS, tau, SV2 and integrated density of silver staining with the plugin of the color deconvolution method as previously described (Ruifrok and Johnston, 2001). The operator performing quantification was blinded to condition and treatment.
Quantitative real-time PCR
Total RNA was extracted from frozen cortex using High Pure RNA Isolation Kit (Roche Life Science, USA) according to the manufacturer’s protocol. RNA concentrations were determined by UV visible absorption spectra, using Nanodrop 2000 (Thermo Scientific, USA). First-strand cDNA was synthesized from total RNA (500ng) using iScript cDNA Synthesis Kit (Bio-Rad Laboratories Inc., 1708891, USA) according to the manufacturer’s instruction. Quantitative PCR was performed in triplicate using Fast SYBR Green Master Mix on a Step One Plus Real-time PCR System (Applied Biosystems, USA). Following primers (5’ to 3’) were used to examine the gene expression of CCL5, IL lβ, CCL2, and GAPDH; CCL5 GGG TAC CAT GAA GAT CTC TGC, (R) GCG AGG GAG AGG TAG GCA AAG, IL-lβ (F): GAG CAC CTT CTT TTC CTT CAT CTT, (R) CAC ACA CCA GCA GGT TAT CAT CA, CCL2 (F) GGC TCA GCC AGA TGC AGT TAA, (R) CCT ACT CAT TGG GAT CAT CTT GCT, GAPDH (F) TGT GTC CGT CGT GGA TCT GA, (R) CCT GCT TCA CCA CCT TCT TGA. Fold change of gene expression was calculated by comparative CT quantification method (Schmittgen and Livak, 2008) and normalized to the expression of GAPDH.
NAD+ measurement
Cerebral cortex was dissected as quickly as possible on a cold metal block and flash frozen in liquid nitrogen. Samples were stored at – 80°C until assay. Tissue NAD+ determination was performed according to the manufacturer’s instructions (BioVision, K337–100). Brain tissues were washed with cold PBS and homogenized in NADH/NAD extraction buffer and then centrifuged at 14,000 rpm at 4°C for 15 min. Supernatants were filtered using 3 kDa molecular weight cutoff centrifugal filters (Merck Millipore Ltd., UFC500324) to remove enzymes that consume NADH and NAD. Fifty microliters of samples were transferred into a 96 well plate for total NADH and NAD, and 50 μl of sample was heated at 60°C for 30 min to decompose NAD and then also added into a 96 well plate for measuring total NADH. Ten microliters of I nmol/gl NADH standard was diluted with 990 μl of NADWNAD extraction buffer and then transferred into a 96 well plate to make 0, 20, 40, 60, 80, and 100 pmol/well. One hundred microliters of NAD cycling enzyme mix was added into standard, and samples were then incubated in the plate at room temperature for 5 min. After adding 10 μl of NADH developer, the plate was read at OD 450 nm.
Preparation of plasma and albumin/immunoglobulin depletion
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal (IP) injection and blood samples were collected in EDTA tubes (Becton, Dickinson and Company, 365974) by retro-orbital bleeding and then plasma was separated at 2,000 g at 4°C for 15 min. Albumin and immunoglobulin depletion were performed according to the manufacturer’s instructions (Bio-Rad Laboratories, Inc., 732–6701). Briefly, Aurum serum protein columns were washed two times with 1 mL of Aurum serum protein binding buffer and centrifuged at 10,000 g for 20 s. Sixty microliter of human and mouse plasma sample was mixed with 180 μl of Aurum serum protein binding buffer and 200 μl of the diluted plasma sample were added to the top of the resin bed. Column was gently vortexed every 5 min for a total incubation time of 15 min and then centrifuged at 10,000 g for 20 s. Eluate was collected in collection tube and resin was washed with 200 μl of the binding buffer. After centrifugation (10,000 g, 20 s), the eluate was collected in a previous collection tube.
Tau seed amplification assay (AD RT-QuIC)
Twenty percentage w/v brain homogenates were prepared from hippocampus and cortex in ice-cold IX PBS with protease inhibitors (Roche, complete, EDTA-free) using I mm zirconia/silica beads (Biospec Products) and a BeadMill 24 (Fisher Scientific). Brain homogenates were used to seed the AD RT-QuIC. Assay conditions used were as previously published (Kraus et al., 2019) with the addition that both heparin (USP, 1235820) and poly-L-glutamate (Sigma, P1818) were independently tested in evaluating seeding activity. Synthetic fibrils generated from recombinant tau encoding aa 306–378 were used as a positive control. For analysis of human TBI and control plasma samples (albumin/immunoglobulin depleted), 5 μl was used to seed the reaction, with triplicate wells analyzed for each biological replicate. 18 ng of synthetic fibrils / 5 μl of control plasma was used as a positive control to verify that plasma matrices were not inhibitory to the RT-QuIC reactions.
Quanterix
Plasma Nfl, UCH-LI, GFAP, pTau181 and Tau from control and TBI patient’s samples were measured by using Simoa@ Neurology 4-Plex B kit and Simoa@ pTau-181 advantage V2 kit by Quanterix The Science of Precision Health (Billerica, MA).
Diflunisal pharmacokinetics
Diflunisal levels in mouse plasma and brain were monitored by LC-MS/MS using an AB Sciex (Framingham, MA) 4000 QTRAP@ mass spectrometer coupled to a Shimadzu (Columbia, MD) Prominence LC. Diflunisal was detected with the mass spectrometer in negative MRM (multiple reaction monitoring) mode by following the precursor to fragment ion transitions 248.9 to 204.9 (quantifier ion) and 248.9 to 184.9 (qualifier ion). An Agilent C 18 XDB column (5 micron, 50 X 4.6 mm) was used for chromatography for PK studies with the following conditions: Buffer A: dH20 + 0.1% formic acid, Buffer B: acetonitrile + 0.1% formic acid, 0 – 1.0 min 5% B, 1.0– 1.5 min gradient to 100% B, 1.5 – 3.0 min 100% B, 3.0 – 3.2 min gradient to B, 3.2 – 4.5 5% B. Tolbutamide (transition 269.1 to 169.9) from Sigma (St. Louis, MO) was used as an internal standard (IS). Pharmacokinetic studies were performed by injecting 8 week old C57BL/6J male mice with diflunisal formulated in 10% DMSO, 20% Kolliphor EL, 70% saline. 60 or 180 minutes post-dose, animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal (IP) injection. Blood was collected using the anticoagulant ACD, and then brains were harvested after transcardial perfusion with IxPBS. Tissues were weighed before snap freezing and blood spun at 2,000x g at 40C for 15 min to collect plasma which was stored frozen along with brain tissue at −80°C until analysis. Brain tissue was homogenized in a 3-fold volume (weight by volume) of PBS to generate a homogenate. 100 μl of plasma or tissue homogenate was mixed with 100 μl of acetonitrile plus 0.2% formic acid and 100 ng/ml N-benzylbenzamide IS. The samples were vortexed 15 s, incubated at room temp for 10’ and spun twice at 16,100 × g 4°C in a refrigerated microcentrifuge. Standard curves were generated using blank plasma (Bioreclamation, Westbury, NY) or blank tissue homogenate spiked with known concentrations of diflunisal and processed as described above. The concentration of drug in each time-point sample was quantified using Analyst software (Sciex). A value of 3-fold above the signal obtained from blank plasma or tissue homogenate was designated the limit of detection (LOD). The limit of quantitation (LOQ) was defined as the lowest concentration at which back calculation yielded a concentration within 20% of theoretical.
Diflunisal protein binding
Protein binding of diflunisal in mouse plasma or brain homogenate was determined by rapid equilibrium dialysis using RED chambers (Thermo Scientific, Waltham, MA). On the day of the RED experiment, frozen mouse plasma and 4× homogenized brain was thawed in a water bath at 37°C. Then it was equilibrated for 45 minutes at 37°C in an atmosphere of 5% CO2. The pH of PBS was confirmed within 7.4 ± 0. l. The pH of plasma and brain was measured and adjusted to 7.4 ± 0.1 using concentrated acid or base. Plasma and brain were diluted at 1:20 (final) with PBS and used for all subsequent steps. An aliquot of plasma or brain was spiked with compound to a compound concentration of 5 μM and vortex mixed. Enough non-spiked matrix remained to enable matrix matching of dialysate at the end of the binding assessment. This matrix was stored at 37°C in an atmosphere of 5% CO2. For each matrix, dialysis was performed using n = 4 individual RED units with 200 μL of compound spiked plasma or brain homogenate at 5 μM in the donor chamber and 400 μl- of PBS in the dialysate chamber. The plate containing the RED units was sealed with a gas-permeable seal and incubated at 37°C for 6 hours under a 5% CO2 atmosphere in an orbital shaker set to 100 rpm. At the end of the dialysis period, aliquots were taken from the donor and dialysate chambers of each RED unit to obtain post-dialysis measures of bound and unbound compound concentration. Donor, dialysate, and plasma or brain stability samples were analyzed by using a matrix matching approach whereby each sample was mixed in a ratio with the opposite medium (blank matrix or PBS). The matrix matched samples were then crashed with 200 μl- acetonitrile + formic acid (0.1% final) + tolbutamide IS (50 ng/mL final). Tubes were vortex mixed for 10 s and incubated at room temperature for 10 minutes. Then they were centrifuged at 16,100 × g for 5 minutes. Diflunisal levels in both chambers were measured by LC-MS/MS in comparison to a standard curve prepared in matrix:PBS as described above. For each matrix, stability was assessed by maintaining individual aliquots of compound-spiked matrix at 37°C and 5% CO2 for 0 and 6 hours. At each time point, n = 2 50 μl- aliquots were matrix matched and crashed. Stability of diflunisal in plasma and brain was 99% over 6 hours as assessed by LC-MS/MS. Fraction unbound (fu) was determined based on the following equations:
Human plasma study
This is a retrospective study of plasma samples from subjects with TBI admitted to the neuroscience intensive care unit at the Memorial Herman Hospital-Texas Medical Center from December 2017 to April 2019. Inclusion criteria were age > 18, presented after TBI (ACRM criteria: loss of consciousness, posttraumatic amnesia, alteration of consciousness), underwent a brain CT, fluency in English or Spanish, ability to provide consent (or consent obtainable from surrogate), visual acuity/hearing adequate for testing and neurologically intact prior to injury. Exclusion criteria were patients with past medical history (including bipolar disorder, seizures, dementia, depression, schizophrenia, HIV, cancer (current treatment that would interfere with follow-up), end-stage renal disease (on dialysis), severe polytrauma that would interfere with follow-up, modified Rankin scale (mRS) > 1 (i.e., uses walker or need assistance with daily actives), claustrophobia, lives greater than 2 hour from hospital, low interest/low probability for follow-up, prisoner, pregnant women, penetrating TBI, current participation in interventional trial and risk of imminent death. Blood samples were collected at 5 pre-determined time-points: < 24 hours of injury (Tl), during 24–48 hours of injury (T3), during 3–5 days of injury (T4), during 6–8 days of injury (T5) and > 10 days after injury (T6). We randomly selected 45 subjects, age- and gender-matched with 25 non-neurologically-impaired healthy subjects. Eighty-nine TBI plasma samples were analyzed: 44 at Tl, 22 at T3, 6 at T4, 7 at T5, and 10 at T6. Since fewer samples were collected after Tl, results obtained from T3 and T4 (24–120 hours post-TBI), and from T5 and T6 (> 120 hours post-TBI), were grouped for analysis. Mean participant age (±SD) was 50 ± 18 years, and average age between TBI patients and healthy subjects was similar (48 ± 20 versus 54 ± 11, p > 0.05; Table Sex-ratio was equivalent across TBI and controls (89% versus 75% male, p > 0.05). Due to myriad reasons, samples from all patients at all timepoints are not available. Blood was drawn from existing lines or by venipuncture and collected into sterile vacutainers per time point. The samples were placed on ice immediately after collection and transported to the laboratory for centrifugation within an hour of draw (at 1460 × g for 10 minutes at 4°C), generating plasma. The plasma was centrifuged a second time (at 1460 × g for 10 minutes at 4°C) in order to generate platelet-poor plasma. Plasma was divided into aliquots and frozen at −80°C until analysis. Based on admission GCS-ftppend-ix+, subjects were grouped by injury grade as mild (GCS between 13 and 1 5), moderate (GCS between 9 and 12) and severe (GCS less than 9). During the study period, 85 TBI subjects were consented (27 mild, 23 moderate, 34 severe and I unknown). From these subjects, we randomly selected 45 subjects: 15 mild TBI subjects, 15 moderate TBI subjects and 15 from severe TBI so that subjects across injury severity grade (mild, moderate or severe) were matched for age. Additionally, all TBI subjects (n = 45) were also matched for age and sex with respect to the control subjects. Plasma samples from 25 nonneurological subjects were used as controls (patients were approached and enrolled at the UT Physician Cardiology clinic). Blood was drawn by venipuncture and collected into sterile vacutainers and immediately placed on ice. For processing of plasma, the tubes were centrifuged at 1460 × g for 10 minutes at 4°C followed by a second centrifugation at 1460 × g for 10 minutes at 4°C to generate platelet-poor plasma. Plasma was then aliquoted and stored at −80°C until analysis. Demographic and clinical information including past medical history, age, sex, Glasgow coma scale (GCS) at admission. Descriptive statistics were calculated for demographic variables in TBI and control cohorts (Table S1). To describe differences in age and sex, we used the Student’s t test, 12-test, and Fisher’s exact test. Statistical analyses were performed using open-source software packages in R (v3.1.3).
Human brain study
Nine human brain specimens were obtained from the holdings of Northwestern University Alzheimer’s Disease Research Center brain bank, as described in the Results section, with results displayed in Figure 6J.
Pharmacoepidemiologic validation
The IBM@ MarketScan@ Medicare Supplemental Database is one of the first in the U.S. to profile the healthcare experience of retirees with Medicare supplemental insurance paid by employers. The MarketScan@ Medicare Supplemental Database provides detailed cost, use and outcomes data for healthcare services performed in both inpatient and outpatient settings. For most of the population, the medical claims are linked to outpatient prescription drug claims and person-level enrollment data through the use of unique patient or enrollee identifiers. Beneficiaries in the MarketScan@ Medicare Supplemental Database have drug coverage; therefore, drug data are available and provide additional, often valuable, information. This feature makes the database a robust tool for pharmacoeconomic and outcomes research and helps provide insight into the drug use and spending patterns of older Americans. In this study, the pharmacoepidemiology study utilized the MarketScan@ Medicare Claims database from 2012 to 2017. The dataset included individual-level diagnosis codes, procedure codes and pharmacy claim data for 7.23 million patients. Pharmacy prescriptions of salsalate, diflunisal, and aspirin were identified by using RxNorm and National Drug Code (NDC). For a subject, a drug episode is defined as from drug initiation to drug discontinuation. Specifically, drug initiation is defined as the first day of drug supply (i.e., 1st prescription date). Drug discontinuation is defined as the last day of drug supply (i.e., last prescription date + days of supply) and without drug supply for the next 60 days. In another word, gaps of less than 60-day of drug supply were allowed within a drug episode. The drug cohort included the first drug episode for each subject. For the final cohorts, demographic variables including age, race, sex and geographical location were collected. Additionally, diagnoses of hypertension (HT), type 2 diabetes (T2D), and coronary artery disease (CAD) before drug initiation were collected (Table S3). These variables were specifically selected to address potential confounding biases. Last, a control cohort was selected from patients who were not exposed to salsalate and diflunisal. Specifically, control exposures were matched to the exposures (n strata = 10) by initiation time, enrollment history, gender, HT diagnose, T2D diagnose and CAD diagnose. The outcome was time from drug initiation to diagnose of AD and TBI defined by ICD9/10 codes (Table S4). For drug cohorts, observations without diagnosis of AD were censored at the end of drug episodes. For the control cohort, the corresponding drug episode starting date was used as the starting time. Observations without diagnosis of AD were censored at the corresponding drug episode’s end date. The detailed description are provided in a previous study (Cheng et al., 2018). For propensity score estimation, we define NE = north east, NC = north central, S = south, W = west, T2D = type 2 diabetes, HT = hypertension and CAD = coronary artery disease. The propensity score of taking a candidate drug versus a comparator drug (i.e., aspirin) was estimated by the following logistic regression model:
Stratified Cox models were used to compare the TBI or AD risks. For a candidate drug (i.e., salsalate or diflunisal) versus a comparator drug (i.e., aspirin), the analyses were stratified (n strata = 10) by the estimated propensity score. All analyses were stratified based on the subgroups defined by sex, T2D, HT and CAD diagnoses (n strata = 10). Finally, propensity score stratified Cox-proportional hazards models were used to conduct statistical inference for the hazard ratios (HR) of developing AD or TBI between cohorts.
Quantification and statistical analysis
All statistical analyses were performed using GraphPad Prism, version 9.0.0 (GraphPad Software, Inc.). Student’s t test was used to demonstrate statistical differences between two groups, and One-way ANOVA or repeated-measured two-way ANOVA with Dunnett’s or Tukey’s post hoc test was used when appropriate. Values are presented as mean ± SEM, and individual data points represent individual samples or animals. Details of statistical analysis can be found in the figure legends (statistical tests used, what n represents, etc.). Significance was determined at p value below of 0.05, while p values below or equal to 0.05, 0.01, 0.001 and 0.0001 Were represented by *, **, ***, and ****, respectively.
Supplementary Material
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-ac-tau | Li Gan | N/A |
| Mouse monoclonal anti-tau | Abeam | Cat#ab80579; RR1D:AB 1603723 |
| Mouse monoclonal anti-tau | Thermo Fisher Scientific | Cat# 13-6400. RR1D: AB 86533 |
| Mouse monoclonal anti-β-actin | Santa Cruz Biotechnology | Cat# sc-47778, RRID: AB 626632 |
| Mouse monoclonal anti-GAPDH | Millipore | Cat# MAB374, RRID: AB 2107445 |
| Mouse monoclonal anti-NeuN | Millipore | Cat# MAB377, RRID: AB 2298772 |
| Mouse monoclonal anti-GFAP | Thermo Fisher Scientific | Cat# MA5-I2023, RRID: AB 10984338 |
| Mouse monoclonal anti-SIRTl | Abeam | Cat# abl 10304. RRID: AB 10864359 |
| Rabbit polyclonal anti-p300 | Santa Cruz Biotechnology | Cat# sc-584, RRID:AB 2293429 |
| Rabbit monoclonal anti-CBP | Cell Signaling Technology | Cat# 7389. RRID:AB 2616020 |
| Rabbit polyclonal anti-Histone H2AK5 | Abeam | Cat# ab 1764. RRID: AB 10563138 |
| Rabbit monoclonal anti-Histone H2A | Cell Signaling Technology | Cat# 12349. RR1D:AB 2687875 |
| Rabbit polyclonal anti-Histone H3K18 | Abeam | Cat# ab 1191. RR1D:AB 298692 |
| Rabbit monoclonal anti-Histone H3 | Cell Signaling Technology | Cat# 4499. RRID:AB 10544537 |
| Rabbit polyclonal anti-phospho-tau (S202) | Cell Signaling Technology | Cat# 11834 |
| Rabbit polyclonal anti-phospho-tau (S262) | Thermo Fisher Scientific | Cat# 44-750G, RRID:AB 2533743 |
| Mouse monoclonal anti-phospho-tau (S396/404) | Peter Davies | N/A |
| Rabbit polyclonal anti-KIBRA | Santa Cruz Biotechnology | Cat# sc-133374. RRID:A B 2216359 |
| Rabbit polyclonal anti-PSD95 | Cell Signaling Technology | Cat# 2507. RRIDAB 56 1221 |
| Mouse monoclonal anti-AnkG | Santa Cruz Biotechnology | Cat# sc-12719. RRID: AB 626674 |
| Rabbit polyclonal anti-|3IV-spectrin | Matthew N. Rasband | N/A |
| Rabbit monoclonal anti-TNFα | Cell Signaling Technology | Cat# 1 1948. RR1D:AB 2687962 |
| Rabbit polyclonal anti-ac-p300/CBP | Cell Signaling Technology | Cat# 4771. RRID: AB 2262406 |
| Rabbit polyclonal anti-DYKDDDDK tag | Cell Signaling Technology | Cat# 2368. RR1D:AB 2217020 |
| Rabbit polyclonal anti-cleaved caspase 3 | Cell Signaling Technology | Cat# 9661. RRID: AB 2341188 |
| Rabbit monoclonal anti-NeuN | Millipore | Cat# MABN140. RRID:A B 2571567 |
| Mouse monoclonal anti-AnkG | UC Davis/NIH NeuroMab Facility | Cat# N106/36. RRID: AB 2877524 |
| Alexa Flour 488 goat anti-mouse IgG (H+L) | Thermo Fisher Scientific | Cat# A-l 1001. RRID: AB 2534069 |
| Alexa Flour 568 goat anti-rabbit IgG (H+L) | Thermo Fisher Scientific | Cat# A-l 1011. RRID:AB 143157 |
| Biological samples | ||
| Human plasma | The University of Texas Health Science Center at Houston | https://www.uth/edu |
| Human cortical brain tissue | Mesulam Center for Cognitive Neurology and Alzheimer’s Disease, Northwestern University | https://www.brain.northwestem.edu |
| Chemicals, peptides, and recombinant proteins | ||
| CGP3466B maleate salt | Sigma-Aldrich | SML1941; CAS: 200189-97-5 |
| Salsalate | AdipoGen | AG-CR1-3574; CAS: 552-94-3 |
| P7C3-A20 | Andrew A. Pieper | N/A |
| FK866 hydrochloride hydrate | Sigma-Aldrich | F8557; CAS: 658084-64-1 (free base) |
| EX527 | Selleckchem | SI541; CAS: 49843-98-3 |
| Diflunisal | Sigma-Aldrich | D3281; CAS: 22494-42-4 |
| Critical commercial assays | ||
| Cytotoxicity Detection Kit (LDH) | Roche | Cat#04744926001 |
| MACS Adult Brain Dissociation Kit | Miltenyi Biotec | Cat# 130-107-677 |
| MACS Neuronal isolation Kit | Miltenyi Biotec | Cat# 130-115-389 |
| Aurum Serum Protein Mini Kit | Bio-Rad | Cat#732-6701 |
| NAD/NADH Quantitation Colorimetric Kit | Biovision | Cat#K337 |
| FD NeuroSilver Kit | FD NeuroTechnologies, Inc | Cat#PK301 |
| Experimental models: cell lines | ||
| SH-SY5Y | ATCC | CRL-2266 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6J (Stock No: 000664) | The Jackson Laboratory | RRID:IMSR_JAX:000664 |
| Mouse: FVB.B-WldS/UmonJ (Stock No: 008820) | The Jackson Laboratory | RRID:IMSR_JAX:008820 |
| Mouse: Tg(Pmp-MAPT)#Lgn | Li Gan | RRID: MGI_5708386 |
| Mouse: Tg(Pmp-MAPT’*K274Q*K281Q)286Lgn | Li Gan | RRID: MGI_5708388 |
| Rat: Long-Evans Rat | Envigo | Cat# 5508398, RRID:RGD_5508398 |
| Oligonucleotides | ||
| Primer CCL5 Forward: GGGTACCATGAAGATCTCTGC |
This paper | N/A |
| Primer CCL5 Reverse: GCGAGGGAGAGGTAGGCAAAG |
This paper | N/A |
| Primer IL-1 β Forward: GAGCACCTTCTTTTCCTTCATCTT |
This paper | N/A |
| Primer IL-1 β Reverse: CACACACCAGCAGGTTATCATCA |
This paper | N/A |
| Primer CCL2 Forward: GGCTCAGCCAGATGCAGTTAA |
This paper | N/A |
| Primer CCL2 Reverse: CCTACTCATTGGGATCATCTTGCT |
This paper | N/A |
| Primer GAPDH Forward: TGTGTCCGTCGTGGATCTGA |
This paper | N/A |
| Primer GAPDH Reverse: CCTGCTTCACCACCTTCTTGA |
This paper | N/A |
| Recombinant DNA | ||
| Plasmid: Flag-TauWT | Li Gan | N/A |
| Plasmid: Flag-TauKQ | Li Gan | N/A |
| Plasmid: Flag-TauKR | Li Gan | N/A |
| Software and algorithms | ||
| Any-maze | Stoelting | RRID: SCR 014289 |
| Image J/Fiji | https://imaaei.net/Fiii | RRID: SCR 002285 |
| GraphPad Prism Version 9.0.0 | https://www.craDhDad.com/ | N/A |
| Other | ||
| Zeiss LSM710 confocal microscope | Carl Zeiss | N/A |
| Zeiss Axiolmager.M2 | Carl Zeiss | N/A |
| Zeiss Axio Scan.Zl | Carl Zeiss | N/A |
| CSU22 spinning disk confocal | Yokogawa Electric Corporation | N/A |
Previous Version.
fu - fraction unbound
Cdialysate – concentration of compound in dialysate chamber after dialysis
Cdonar – concentration of compound in donor chamber after dialys
DF – dilution factor for plasma dilution
– fraction unbound after correcting for plasma dilution
– fraction unbound of diluted plasma, calculated using the fu equation above if using diluted plasma
Updated Version.
fu – fraction unbound
Cdialysate – concentration of compound in dialysate chambcr after dialysis
Cdonor – concentration of compound in donor chamber after dialysis
DF – dilution factor for plasma dilution
– fraction unbound after correcting for plasma dilution
– fraction unbound of diluted plasma, calculatcd using the fu equation above if using diluted plasma
Highlights.
Brain injury induces Alzheimer’s disease-like neuronal ac-tau
Neurodegenerative brain injury is reflected by ac-tau blood levels in mice and people
Decreasing ac-tau after brain injury at multiple signaling nodes is neuroprotective
Ac-tau-inhibiting medicines are associated with reduced neurodegenerative disease
Acknowledgments
We thank those who provided blood and brain samples, Matthew N. Rasband for β1V-spectrin antibodies, Peter Davies for PHFI (p-tau S396/S404) antibodies, Mikayla Huntley for tau seeding assay help, and Gloria Lee for providing brain tissue from tau knockout mice. Postmortem brain tissues were provided by the Neuropathology Core of Northwestern University (NIH P30 AG013854 24). We thank the Translational Therapeutics Core of the Cleveland Alzheimefs Disease Research Center (NIH/NIA I P30 AG062428-01) for assisting in the study with human postmortem brain tissues. The SV2 monoclonal antibody developed by K.M. Buckley was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH, and maintained at The University of Iowa, Department of Biology (Iowa City, IA). This work was supported by a grant to A.A.P. from the Brockman Foundation. A.A.P. was also supported by Elizabeth Ring Mather and William Gwinn Mather Fund, S. Livingston Samuel Mather Trust, G.R. Lincoln Family Foundation, Wick Foundation, the Leonard Krieger Fund of the Cleveland Foundation, Gordon and Evie Safran, and Louis Stokes VA Medical Center resources and facilities. Acknowledgment is made to the donors of Alzheimer’s Disease Research, a program of BrightFocus Foundation, for support of M. -K.S. in this (A2019551F). A.A.P., M.K.J., J.D.R., and J.S.S. are suppo,ted by Project 19PABH134580006-AHA/Allen Initiative in Brain Health and Cognitive Impairment. E.V.-R. was supported by Free Radical and Radiation Biology, University of lowa (T32 CA078586). F.C. and A.A.P. are supported together by NIA/NIH (ROIAG066707) and also the Translational Therapeutic Core of the Cleveland Alzheimer’s Disease Research Center (NIH/NIA 1 P30 AG062428-01), which assisted in the study of human postmortem brain tissue and longitudinal analysis of patient data. D.J.L. was supported by The Miami Project to Cure Paralysis and NIH/NINDS (NS098740). S.J.F. and M.T.P. were supported by VA BLR&D MERIT Award (2 101 BX002439-04Al) and Research Career Scientist Awards from Department of Veterans Affairs, with resources of VA Western NY Healthcare System (Buffalo, NY), and Atlanta VA Medical Center (Decatur, GA). S.J.F. was supported by Clinical and Translational Science Award (ULITR001412) to University of Buffalo from National Center for Advancing Translational Sciences (NCATS/NIH). R.S.A. was supported by Career Development Award (CDA-2 RX002928) from Department of Veterans Affairs. T.G. was supported by NIA/NIH (ROIAG062566). M.E.F. was supported by NIA/NIH (K08AG065463).
Footnotes
Declaration of interests
A.A.P. is an inventor on patents related to P7C3. L.G. is a founder of Aeton Therapeutics, Inc. No other authors declare competing interests.
Supplemental information
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2021.03.032.
References
- Allen RS, Motz CT, Feola A, Chesler KC, Haider R, Ramachandra Rao S, Skelton LA, Fliesler SJ, Pardue MT, 2018. Long-term functional and structural consequences of primary blast overpressure to the eye. J. Neurotrauma 35, 2104–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P, Abu-Taleb R, Oguntayo S, Tanaka M, Wang Y, Valiyaveettil M, Long JB, Zhang Y, Nambiar MP, 2013. Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: role of compromised cell membrane integrity. Neurosci. Lett. 552, 87–91. [DOI] [PubMed] [Google Scholar]
- Bolós M, Llorens-Martín M, Perea JR, Jurado-Arjona J, Rábano A, Hernández F, Avila J, 2017. Absence ofCX3CR1 impairs the intemalization of Tau by microglia. Mol. Neurodegener. 12, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AKF, Gao Y, Yin X-M, Clark RSB, Graham SH, Chen J, 2007. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J. Neurosci. 27, 9278–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control: Division of Unintentional Injury Prevention. [Google Scholar]
- Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabási A-L, Loscalzo J, 2018. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 9, 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Guo JL, Hunado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee v.M., 2011. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Carlomagno Y, Gendron TF, Dunmore J, Scheffel K, Stetler C, Davis M, Dickson D, Jarpe M, DeTure M, Petrucelli L, 2014. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 23, 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutca LM, Stasheff SF, Hedberg-Buenz A, Rudd DS, Batra N, Biodi FR, Yorek MS, Yin T, Shankar M, Herlein JA, et al. , 2014. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest. Ophthalmol. Vis. Sci. 55, 8330–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DB, Rank KB, Bhattacharya K, Thomsen DR, Gurney ME, Sharma SK, 2000. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J. Biol. Chem. 275, 24977–24983. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS, 2009. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat. Biotechnol. 27, 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen U, Benk C, Beyersdorf E, Klemm R, Tnmmer G, Özbek B, Kern E, Heilmann C, 2015. Neuron-specific enolase correlates to laboratory markers of haemolysis in patients on long-term circulatory support. Eur. J. Cardiothorac. Surg. 48, 416–420 discussion 420. [DOI] [PubMed] [Google Scholar]
- Gorgoraptis N, Li LM, Whittington A, Zimmerman KA, Maclean LM, McLeod C, Ross E, Heslegrave A, Zetterberg H, Passchier J, et al. , 2019. In vivo detection of cerebral tau pathology in long-term survivors of traumatic brain injury. Sci. Transl. Med. 11, eaaw1993. [DOI] [PubMed] [Google Scholar]
- Hara MR, Snyder SH, 2006. Nitric oxide-GAPDH-Siah: a novel cell death cascade. Cell. Mol. Neurobiol. 26, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RO, Bosanac T, Mao X, Engber TM, DiAntonio A, Milbrandt J, Devraj R, Krauss R, 2021. Small molecule SARMI inhibitors recapitulate the SARMI−/− phenotype and allow recovery of a metastable pool of axons fated to degenerate. Cell Rep. 34, 108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Phelps c.H, Beach TG, Bigio, Caims NJ, Carrillo, Dickson D.w., Duyckaetts C, Frosch MP, Masliah E, et al. , 2012. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Cohen TJ, Grossman M, Amold SE, Xie SX, Lee v.M., Trojanowski JQ, 2012. Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain 135, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgrò MA, Bottoni P, Scatena R, 2015. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv. Exp. Med. Biol. 867, 125–143. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2010. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci. Il, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JVK, Snowman AM, Law L, Hester LD, Snyder SH, 2010. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 12, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Saijo E, Metrick MA 2nd, Newell K, Sigurdson CJ, Zanusso G, Ghetti B, Caughey B, 2019. Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease. Acta Neuropathol. 137, 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian T, 2010. Isolation of Mitochondria from the CNS. Curr. Protoc. Neurosci. Chapter 7, Unit 7.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, Tian G, 2017. Head injury as a risk factor for dementia and Alzheimer’s disease: a systematic review and meta-analysis of 32 observational studies. PLoS ONE 12, e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP, 2017. Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy. eLife 6, e29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke U, Six J, Villanova M, Boons J, Vandermeeren M, Ceuterick C, Cras P, Martin JJ, 1994. Microtubule-associated protein tau epitopes are present in fiber lesions in dive1N muscle disorders. Am. J. Pathol. 145, 175–188. [PMC free article] [PubMed] [Google Scholar]
- Lucke-Wold B, Seidel K, Udo R, Omalu B, Ornstein M, Nolan R, Rosen C, Ross J, 2017. Role of tau acetylation in Alzheimer’s disease and chronic traumatic encephalopathy: the way forward for successful treatment. J. Neurol. Neurosurg. 4, 140. [PMC free article] [PubMed] [Google Scholar]
- Ma VY, Chan L, Canuthers KJ, 2014. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 95, 986–995.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S-W, Cho s.-H., Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, et al. , 2010. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Sorinola A, Czeiter E, Vamos Z, Amrein K, Synnot A, Donoghue E, Sandor J, Wang KKW, Diaz-Arrastia R, et al. , 2018. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. J. Neurotrauma Published online July 2, 2018 doi: 10.1089/neu.2017.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Phelps c.H, Beach, Bigio EH, N.J., Dickson D.w., Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. , National Institute on Aging; Alzheimer’s Association, 2012. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA, 2020. Nitric oxide-dependent protein post-translational modifications impair mitochondrial function and metabolism to contribute to neurodegenerative diseases. Antioxid. Redox Signal. 32, 817–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeftler T, Niederkofler V, Daum G, Attems J, Hutter-Paier B, 2018. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Hayes SH, Rao AS, Allman BL, Manohar S, Ding D, Stolzberg D, Lobarinas E, Mollendorf JC, Salvi R, 2015. Low-cost blast wave generator for studies of hearing loss and brain injury: blast wave effects in closed spaces. J. Neurosci. Methods 242, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Kawakami, Watanabe K, Oshima K, Niizato K, Ikeda K, Akiyama H, Hasegawa M, 2019. Tau progression in single severe frontal traumatic brain injury in human brains. J. Neurol. Sci. 407, 116495. [DOI] [PubMed] [Google Scholar]
- Olsson B, Zetterberg H, Hampel H, Blennow K, 2011. Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 95, 520–534. [DOI] [PubMed] [Google Scholar]
- Orešič M, Posti JP, Kamstrup-Nielsen MH, Takala RSK, Lingsma HF, Mattila I, Jäntti S, Katila AJ, Carpenter KLH, Ala-Seppälä H, et al. , 2016. Human serum metabolites associate with severity and patient outcomes in traumatic brain injury. EBioMedicine 12, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea JR, López E, Díez-Ballesteros JC, Ávila J, Hernndez F, Bolos M, 2019. Extracellular monomeric tau is internalized by astrocytes. Front. Neurosci. 13, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, McKnight SL, 2018. Benefits of enhancing nicotinamide adenine dinucleotide levels in damaged or diseased nerve cells. Cold Spring Harb. Symp. Quant. Biol. 83, 207–217. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen C-H, et al. , 2010. Discovery of a proneurogenic, neuroprotective chemical. Cell 142, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posti JP, Hossain I, Takala RSK, Liedes H, Newcombe V, Outtrim J, Katila AJ, Frantzén J, Ala-Seppälä H, Coles JP, et al. , 2017. Glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 are not specific biomarkers for mild CT-negative traumatic brain injury. J. Neurotrauma 34, 1427–1438. [DOI] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA, 2001. Quantification of histochemical staining by color deconvolution. Anal. Quant. cytol. Histol. 23, 291–299. [PubMed] [Google Scholar]
- Ryan J, storey E, Murray AM, Woods RL, Wolfe R, Reid CM, Nelson MR, Chong TTJ, Williamson JD, Ward SA, et al. , ASPREE Investigator Group, 2020. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 95, e320–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Jha S, Liu E, Akella T, McCullough LD, Rasband MN, 2009. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 29, 13242–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ, 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Sen T, Saha P, Sen N, 2018. Nitrosylation of GAPDH augments pathological tau acetylation upon exposure to amyloid-β. Sci. Signal. Il, eaa06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Politis A, van der Merwe A, Moore B, Ekanayake V, Lippa SM, Chou Y-Y, Pham DL, Butman JA, Diaz-Arrastia R, et al. , 2020. Time course and diagnostic utility of NtL, tau, GFAP, and UCH-Ll in subacute and chronic TBI. Neurology 95, e623–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S, Kumar R, Rai N, Kumar V, Singh K, Upadhyay AD, Tripathi M, Dwivedi S, Dey AB, Dey S, 2016. Estimation of tau and phosphorylated tau181 in serum of Alzheimer’s disease and mild cognitive impairment patients. PLoS ONE Il, eol 59099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M-K, Vázquez-Rosa E, Cintr6n-Pérez C, Riegel A, Harper MM, Ritzel D, Pieper AA, 2021. Characterization of the jet-flow overpressure model of traumatic brain injury in mice. Neurotrauma Rep. 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Wang L, Man N, Maksimoska J, Sorum AW, Lim HW, Lee IS, Shimazu T, Newman JC, Schröder S, et al. , 2016. Salicylate, diflunisal and their metabolites inhibit CBP/p300 and exhibit anticancer activity. eLife 5, ell 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- sohn RD., Tracy TE, son, Zhou, Leite REP, Miller BL, Seeley WW, Grinberg LT, Gan L, 2016. Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol. Neurodegener. 11, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Kryscio RJ, Sabbagh MN, Ziolkowski C, Lin Y, Sparks LM, Liebsack C, Johnson-Traver S, 2012. Tau is reduced in AD plasma and validation of employed ELISA methods. Am. J. Neurodegener Dis. 1, 99–106. [PMC free article] [PubMed] [Google Scholar]
- Tolan NV, Vidal-Folch N, Algeciras-Schimnich A, Singh RJ, Grebe SK, 2013. Individualized correction of neuron-specific enolase (NSE) measurement in hemolyzed serum samples. Clin. Chim. Acta 424, 216–221. [DOI] [PubMed] [Google Scholar]
- Tracy TE, Sohn PD., Minami SS, Wang, Min S-W, Li Y, Zhou Y, Le D, Lo L, Ponnusamy R, et al. , 2016. Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron 90, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi z.-Q., Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA, 2006. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Manufacturer data sheet 9676203, Dolobid (Diflunisal). Merck & Co, Inc. https://www.accessdata.gov/drugsatfada_docsflabeV2007/018445s0581bl.pdf. [Google Scholar]
- Vázquez-Rosa E, Watson MR, Sahn JJ, Hodges TR, Schroeder RE, Cintrón-Pérez CJ, Shin MK, Yin TC, Emery JL, Martin SF, et al. , 2019. Neuroprotective efficacy of a sigma 2 receptor/TMEM97 modulator (DKR-1677) after traumatic brain injury. ACS Chem. Neurosci. 10, 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Rosa E, Shin M-K, Dhar M, Chaubey K, Cintrón-Pérez CJ, Tang X, Liao X, Miller E, Koh Y, Barker S, et al. , 2020. P7C3-A20 treatment one year after TBI in mice repairs the blood-brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc. Natl. Acad. Sci. USA Il 7, 27667–27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermersch P, Sergeant N, Ruchoux MM, Hofmann-Radvanyi H, Wattez A, Petit H, Dwailly P, Delacourte A, 1996. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology 47, 711–717. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Spooren WP, Hengerer B, 2000. CGP 3466 protects dopaminergic neurons in lesion models of Parkinson’s disease. Naunyn Schmiedebergs Arch. Pharmacol. 362, 526–537. [DOI] [PubMed] [Google Scholar]
- Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL, 2014. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiez A-S, Castonguay WC, Gaul OJ, Waite JS, Schmidt CM, Reis A, Rea BJ, Sowers LP, Cintr6n-Pérez C, Vázquez-Rosa E, et al. , 2021. Different forms of traumatic brain injury cause different tactile hypersensitivity profiles. Pain 162, 1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, Fatou B, Guise AJ, Cheng L, Takeda S, et al. , 2020. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. cell 183, 1699–1713.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W-J, Chen W, Chen L, Guo Y-J, zeng J-S, Li G-Y, Tong W-S, 2017. Involvement of tau phosphorylation in traumatic brain injury patients. Acta Neurol. Scand. 135, 622–627. [DOI] [PubMed] [Google Scholar]
- Yin TC, Britt JK, De Jesüs-C0flés H, Lu Y, Genova RM, Khan M.z., Voorhees JR, Shao J, Katzman AC, Huntington PJ, et al. , 2014. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 8, 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Voorhees JR, Genova RM, Davis KC, Madison AM, Britt JK, Cintrón-Pérez CJ, McDaniel L, Harper MM, Pieper AA, 2016. Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. eNeuro 3 ENEURO.0220‒16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K, 2016. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 12, 563–574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any new datasets or code.