Abstract
Human-induced climate change has accelerated in recent decades, causing adverse health effects. However, the impact of the changing climate on neurological disorders in the older population is not well understood. We applied time-varying Cox proportional hazards models to estimate the associations between hospital admissions for dementia and the mean and variability of summer and winter temperatures in New England. We estimated seasonal temperatures for each New England zip code using a satellite-based prediction model. By characterizing spatial differences and temporal fluctuations in seasonal temperatures, we observed a lower risk of dementia-associated hospital admissions in years when local temperatures in either summer (hazard ration [HR] = 0.98; 95% confidence interval [CI]: 0.96, 1.00) or winter (HR = 0.97; 95% CI: 0.94, 0.99) were higher than average, and a greater risk of dementia-associated admissions for older adults living in zip codes with higher temperature variations. Effect modifications by sex, race, age, and dual eligibility were considered to examine vulnerability of population subgroups. Our results suggest that cooler-than-average temperatures and higher temperature variability increase the risk of dementia-associated hospital admissions. Thus, climate change may affect progression of dementia and associated hospitalization costs.
Keywords: Climate change, Seasonal temperature, Dementia, Hospitalization
1. Introduction
Human-induced climate change has accelerated in recent decades (Haustein et al., 2017). Important elements of climate change include both short-term temperature extremes and long-term temperature variability, which are associated with adverse health outcomes. The link between short-term ambient temperature extremes (i.e., heat wave, cold wave, or both) and a variety of health outcomes is well documented (Basu, 2009; O'Neill et al., 2003; Bell et al., 2008; Guo et al., 2011; Barnett, 2007; Yin and Wang, 2017; Anderson et al., 2013; Basu et al., 2010). Increasingly, studies are investigating the health effects of long-term temperature exposure and temperature variability, especially among older populations (Zanobetti et al., 2012; Shi et al., 2015; Shi et al., 2016a).
An emerging body of evidence links temperature exposure and risk of neurological disorders. While increasing prevalence of neurological disorders is resulting in a burden on health care services (Alzheimer's Disease International, 2013; Hurd et al., 2013; Kelley et al., 2015), the long-term effects of climate change on neurological diseases are poorly understood. Body temperature decreases with advancing age and is increasingly variable in older individuals, a likely result of thermoregulatory failure, particularly relating to thermogenesis (Vogelaere and Pereira, 2005; Kenney and Hodgson, 1987; Fox et al., 1973; Frisard et al., 2007; Cheshire, 2016). Aging is also associated with brain hypometabolism, which may promote neurodegeneration and cognitive impairment (Cunnane et al., 2011). Although the underlying mechanisms are unclear, decreased body temperature is associated with aggregation of tau phosphorylation and amyloid-beta peptides, important neuropathologic markers of dementia and Alzheimer's disease (Holtzman and Simon, 2000; Planel et al., 2009; Planel et al., 2004). In mouse models of dementia, raising body temperature by increasing ambient temperature reduces the production of amyloid-beta peptides and improves memory, suggesting a potential association between temperature changes and dementia (Vandal et al., 2016). In addition, other behavior and environmental risk factors, such as physical exercise (Ahlskog et al., 2011), social isolation and the related psychosocial stress (Friedler et al., 2014), and increased fine particulate matter concentrations due to decreased temperature (Tai et al., 2010; Zanobetti et al., 2014), might also play direct and/or indirect roles in the mechanism underlying the potential relationship between temperature and neurological disorders.
Dementia is a common feature in various neurological diseases and represents a common cause of cognitive dysfunction in older individuals. Globally, there were 47 million people with dementia in 2017, and the prevalence continues to increase (Alzheimer's Disease International, 2013; United Nations et al., 2015; World Health Organization, n.d.). By 2050, a predicted 13.8 million people in the US will experience dementia, leading to $758 billion in annual medical expenditures (Hurd et al., 2013; Kelley et al., 2015). Due to the high prevalence of dementia and its burden on public health, it is important to investigate the relationship between climate change and dementia-related medical costs, especially hospitalizations.
In this study, we examined the associations between long-term exposure to summer and winter seasonal temperature average and variability and dementia-associated hospital admissions among Medicare beneficiaries aged 65 years or older. Since animal experimental study has suggested that increasing the ambient temperature coincided with memory improvement (Planel et al., 2009), we hypothesized that higher seasonal temperature average might be associated with a decreased risk of hospitalization of dementia. In addition, given impaired thermoregulatory capacity among seniors (Kelley et al., 2015; Vogelaere and Pereira, 2005; Frisard et al., 2007), we hypothesized that greater temperature variability might be associated with more dementia-related hospitalizations.
2. Methods
2.1. Study population
We constructed an open cohort that comprised Medicare beneficiaries residing in New England (ME, VT, NH, MA, RI, CT) between 2001 and 2011. We extracted multiple variables from the Medicare denominator file for each beneficiary, including sex, race, age, state of residence, enrollment status and date of death. Individuals aged < 65 or residing outside of New England were excluded from the study.
We linked person-year Medicare denominator file with Medicare Provider Analysis and Review file to identify beneficiaries who were hospitalized due to target medical condition. As a progressive neurologic disease, dementia is very likely to be labeled as one of the coexisting secondary diagnoses rather than the principal diagnosis. Therefore, the target medical condition was defined as hospital admission for the first time, with either principal diagnosis of dementia, or any of coexisting secondary diagnoses of dementia. The diagnosis was classified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and ICD-9-CM code 290 for dementia.
Each beneficiary was followed up each calendar year until the event (first hospitalization with principal or secondary diagnosis of dementia), or death, or reached the end of the study 2011, whichever occurred earliest. The Medicare cohort consisted of beneficiaries who were alive on January 1st, 2001 and were enrolled in Medicare within the 11-year study period, i.e., 2001–2011.
2.2. Exposure
We recently presented a 3-stage statistical modeling approach to obtain spatio-temporal resolved near-surface air temperature (Ta), fusing multiple sources of data including surface temperature (Ts) derived from satellite measurements, land use, and meteorological data (Shi et al., 2016b). In the present study, we used this approach to retrieve 1 km × 1 km daily Ta in New England from 2001 to 2011 (Kloog et al., 2014). Briefly, nighttime and daytime Ts with a resolution of 1 km × 1 km were provided by Moderate Resolution Imaging Spectro-radiometer (MODIS) sensors located on NASA's Terra satellite. The nighttime Ts was used to predict Ta because it generally demonstrated higher correlation with Ta (r = 0.94) than the daytime (r = 0.87) (Kloog et al., 2012; Wan, 2008). With available Ts measurements, the relationship between Ta and Ts was calibrated from a mixed model by regressing Ta against a random intercept and calculating fixed and random slopes of Ts (model 1). To fill in grid cells without Ts measurements, we built a second model (model 2) with predicted Ta from model 1, compared against the daily mean Ta across a 60-km buffer around each monitored Ta. The estimated coefficients were used to predict Ta in areas without satellite and monitoring data. Ten-fold cross validation was performed to assess the performance of our analysis, which had an out-of-sample R2 = 0.946 with available Ts and R2 = 0.941 without Ts.
Exposures of interest were seasonal mean temperature and seasonal temperature variability during summer and winter. We used predicted daily mean Ta to calculate four temperature indices for each zip code and year: mean daily temperature in summer (June–August), daily temperature standard deviation (SD) in summer, mean daily temperature in winter (December–February), and daily temperature SD in winter. We then assigned individual temperature exposures by linking each individual's zip code of residence with the nearest center of a 1 km × 1 km temperature grid for the 2001–2011 study period.
2.3. Covariates
We included risk factors for dementia that had been identified in previous studies as potential confounders. Individual-level covariates included in the Medicare dataset were sex, race, age, dual eligibility for both Medicare and Medicaid as an indicator of individual socioeconomic status, and state of residence (Garfield et al., 2015; Lang et al., 2008). Covariates at the zip code level were median household income, median value of owner-occupied housing units, percent of population living in poverty, percent of population below high school education, population density, and homeownership rate, obtained from the 2000 U.S. Census (Karp, 2004; Fischer et al., 2009; Bennett et al., 2006). We additionally considered county-level proportion of ever smokers, obtained from the Behavioral Risk Factor Surveillance System (BRFSS) (Lang et al., 2008). These risk factors were also likely to be associated with the exposure of interest because they were tied to residential neighborhood which is used to assign the temperature indices as individualized exposures. These data were matched to person-year Medicare data based on zip code of residence.
2.4. Statistical analysis
We applied the Anderson-Gill formulation of Cox proportional hazards models to estimate the effects of the mean and variability of seasonal temperature on dementia-associated hospital admissions.
Because local seasonal temperature patterns were likely to interact with each other (Portmann et al., 2009; Irons and Oswood, 1992), we entered the four exposures of interest described in Exposure section into the model simultaneously for mutual adjustment. To deal with potential non-proportionality of hazards, sex, race and age were stratified to allow for a different baseline hazard function for each stratum. We further adjusted individual risk factors including dual eligibility, state of residence, and socioeconomic status at the zip code level including median household income, median value of owner-occupied housing units, percent of population living in poverty, percent of population below high school education, population density, and homeownership rate, and county-level proportion of ever smokers. Effect modifications of sex, race, age, and dual eligibility were examined by including multiplicative terms between these variables and each temperature index in the model.
Further, to evaluate the effects of spatial and temporal pattern of seasonal temperature change and variability on dementia hospitalizations, we decomposed each temperature index into two components: spatial contrast and temporal deviation. Spatial contrast was defined as the average of each temperature index in each zip code during the entire follow-up period, and temporal deviation was defined as the difference between the temperature index and its spatial component in each zip code each year. We then applied a similar Cox proportional hazards model with eight terms (spatial contrast and temporal deviation components for each of the four indices) entering the model simultaneously, where the place-to-place difference in each temperature index was captured by the spatial contrast component and year-to-year variation within each zip code was captured by the temporal deviation component. By mutually adjusting the spatial and temporal components, we were able to simultaneously test the hypotheses whether climate patterns in different geographical regions and local climate fluctuations had significant impacts on the risk of dementia hospitalizations.
Sensitivity analyses and univariate analyses were performed to assess the robustness of results. The two types of sensitivity analyses were 1) restricting the analysis to most recent years from 2006 to 2011, and 2) adjusting for the numbers of heat waves and cold waves in each summer and winter, respectively. For each zip code, heat and cold waves were defined as periods when daily temperature of at least three consecutive days exceeds the 97th percentile daily threshold (for heat waves) or below 3rd percentile daily threshold (for cold waves) during the study period 2001–2011 (Barnett et al., 2012). The two types of univariate analyses conducted were 1) simultaneously entering general temperature indices into the model, and 2) simultaneously entering spatial and temporal components of general temperature indices into the model.
Pearson correlation coefficients were calculated to evaluate linear correlation among temperature exposures of interest. Cox regression results are expressed as hazard ratio (HR) for hospital admission diagnosis of dementia per interquartile range (IQR) increase in degrees Celsius for each temperature index. The use of IQR standardized the effect magnitude across temperature indices that distributed differently.
3. Results
Our cohort comprised 3,069,816 Medicare enrollees in New England from 2001 to 2011 (Table 1). The cohort was mostly white (93.5%) and included more females (57%) than males. Of the 80,999 subjects admitted to the hospital with either principal or secondary discharge diagnosis of dementia, whites (94.3%) and females (64.3%) were more predominant compared to the full cohort. Massachusetts had the largest proportion (44.2%) of Medicare beneficiaries among the six New England states (ME, VT, NH, MA, RI, CT), and the largest proportion (53.9%) of subjects with a dementia-associated hospital admission (Fig. 1).
Table 1.
Characteristics of all individuals and individuals with hospital admission of dementia in New England, 2001–2011.
| Variables | All individuals | Individuals with dementia admissions | |
|---|---|---|---|
| N (%) | N (%) | ||
| Beneficiaries | 3,069,816 (100) | 80,999 (100) | |
| Age at study entry | 65–74 | 2,075,948 (67.6) | 20,958 (25.9) |
| 75–84 | 721,242 (23.5) | 39,238 (48.4) | |
| > 85 | 272,626 (8.9) | 20,803 (25.7) | |
| Race | White | 2,870,849 (93.5) | 76,392 (94.3) |
| Black | 97,607 (3.2) | 2917 (3.6) | |
| Othera | 101,360 (3.3) | 1690 (2.1) | |
| Female | 1,750,400 (57.0) | 52,044 (64.3) | |
| Dual eligibility | 319,590 (10.4) | 13,103 (16.2) | |
| State of residence | ME | 314,668 (10.3) | 5298 (6.5) |
| VT | 137,909 (4.5) | 2990 (3.7) | |
| NH | 264,862 (8.6) | 6028 (7.4) | |
| MA | 1,355,628 (44.2) | 43,674 (53.9) | |
| RI | 234,848 (7.7) | 4079 (5.0) | |
| CT | 761,903 (24.8) | 18,930 (23.4) | |
| Mean | Mean | ||
| Socioeconomic status | Median household income (1999 USD) | 61,900 | 60,350 |
| Median value of owner-occupied housing units (1999 USD) | 258,300 | 249,900 | |
| Percent of population living in poverty (%) | 8.7 | 9.1 | |
| Percent of population below high school education (%) | 25.6 | 27.4 | |
| Population density (per sq. mile) | 0.002 | 0.002 | |
| Homeownership rate (%) | 67.8 | 65.3 | |
| Percent of ever smokers' prevalence (%) | 50.3 | 50.1 |
Other racial groups include Asian, Hispanic, North American Native, and unknown.
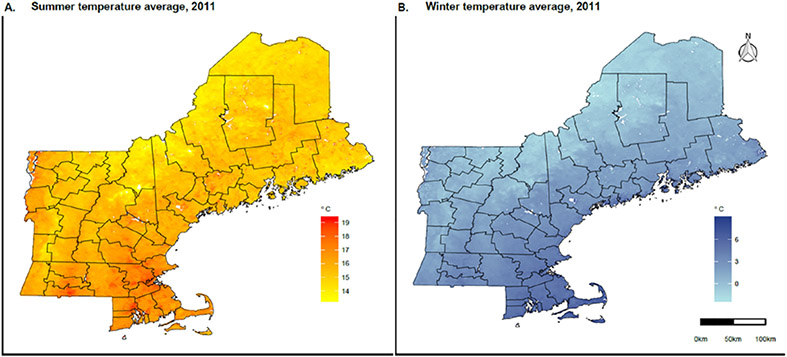
Fig. 1. Summer and winter temperature averages in New England, 2011.
Panel A shows the summer (June–August) temperature average in Celsius degree in New England, 2011. Panel B shows the winter (December–February) temperature average in Celsius degree in New England, 2011.
The average summer mean temperature, summer temperature SD, winter mean temperature, and winter temperature SD across New England between 2001 and 2011 were 17.1 °C, 2.6 °C, −1.3 °C, and 1.6 °C, respectively (Table 2).
Table 2.
Descriptive statistics of temperature indices across New England, 2001–2011.
| Temperature indices (°C) | Average | Min | Q1 | Q2 | Q3 | Max | IQR | |
|---|---|---|---|---|---|---|---|---|
| Summer mean | Generala | 17.1 | −2.5 | 16.4 | 17.1 | 17.9 | 29.2 | 1.5 |
| Spatialb | 17.1 | 11.2 | 16.6 | 17.2 | 17.8 | 19.2 | 1.2 | |
| Temporalc | 0 | −13.8 | −0.4 | 0.0 | 0.4 | 10.2 | 0.8 | |
| Summer SD | General | 2.6 | 0.5 | 2.3 | 2.6 | 2.8 | 3.6 | 0.5 |
| Spatial | 2.6 | 1.2 | 2.5 | 2.6 | 2.7 | 2.9 | 0.2 | |
| Temporal | 0 | −1.1 | −0.2 | 0.0 | 0.2 | 1.0 | 0.4 | |
| Winter mean | General | −1.3 | −9.9 | −1.8 | −1.2 | −0.6 | 0.7 | 1.2 |
| Spatial | −1.2 | −5.5 | −1.3 | −1.1 | −0.9 | 0.4 | 0.4 | |
| Temporal | 0 | −4.7 | −0.5 | 0.0 | 0.6 | 4.7 | 1.1 | |
| Winter SD | General | 1.6 | 0.0 | 1.0 | 1.5 | 2.2 | 6.2 | 1.2 |
| Spatial | 1.6 | 0.2 | 1.3 | 1.5 | 1.8 | 4.2 | 0.5 | |
| Temporal | 0 | −2.6 | −0.5 | −0.1 | 0.4 | 4.3 | 0.9 | |
General temperature indices included daily temperature average in summer (summer mean), daily temperature standard deviation in summer (summer SD), daily temperature average in winter (winter mean), and daily temperature standard deviation in winter (winter SD).
Spatial contrast was defined as the average of each temperature index in each zip code during the entire follow-up period 2001–2011.
Temporal deviation was defined as the difference between the temperature index and its spatial component in each zip code each year.
Survival analyses showed that each IQR (1.5 °C) increase in summer mean temperature was significantly associated with a 12% (95% CI: 1.09, 1.15) increased hazard of dementia-associated hospital admission (Table 3). However, winter mean temperature was not significantly associated with risk of dementia admission (HR = 1.00; 95% CI: 0.98, 1.03). Temperature variability in summer and winter increased risk of dementia-associated hospital admissions. There was a significant increased risk of dementia admission per IQR (0.5 °C) increase in standard deviation of summer temperature (HR = 1.07; 95% CI: 1.05, 1.09). Although it did not quite reach statistical significance, higher winter temperature variability might be associated with increased risk of dementia admissions (HR = 1.03; 95% CI: 1.00, 1.06).
Table 3.
Hazard ratio (95% CI) of dementia hospitalization per IQR increase in degree Celsius in temperature indices across New England, 2001–2011.
| General | Spatial | Temporal | |
|---|---|---|---|
| Summer mean | 1.12 (1.09, 1.15) | 1.03 (1.00, 1.06) | 0.98 (0.96, 1.01) |
| Summer SD | 1.07 (1.05, 1.09) | 1.15 (1.12, 1.18) | 1.03 (1.01, 1.05) |
| Winter mean | 1.00 (0.98, 1.03) | 1.07 (1.05, 1.09) | 0.97 (0.94, 0.99) |
| Winter SD | 1.02 (1.00, 1.05) | 1.11 (1.07, 1.14) | 0.99 (0.97, 1.01) |
We then analyzed the associations between dementia admissions and spatial contrasts and temporal deviations (Table 3). Each IQR increase (1.2 °C) in spatial contrast of summer mean temperature was significantly associated with a 3% (95% CI: 1.00, 1.06) increase in risk of hospital admission for dementia, suggesting that individuals living in warmer areas had an increased risk of dementia-associated hospitalization. We also found a trend suggesting a negative association between temporal deviation of summer mean temperature and dementia-associated admissions (HR = 0.98; 95% CI: 0.96, 1.01). Spatial contrast (HR = 1.07; 95% CI: 1.05, 1.09) and temporal deviation (HR = 0.97; 95% CI: 0.94, 0.99) of winter mean temperature had statistically significant but contrary effects on dementia-associated admissions, suggesting that the risk of dementia-associated admission was higher in areas with a higher overall winter temperature but was decreased in years when winter temperature was higher than average. Increased variability of the temporal and spatial components in summer and the spatial component in winter were also significantly associated with a higher risk of dementia-associated hospital admissions.
We evaluated the effects of spatial and temporal modifications by age, sex, race and dual eligibility (Fig. 2). We found consistent and significant modifications of spatial components in winter mean temperature, summer SD and winter SD by age and dual eligibility: subjects in younger age groups and dual eligible beneficiaries were more vulnerable to experiencing an increased risk for a dementia-associated hospitalization relating to a higher mean winter temperature or increased temperature variations. The spatial components of winter mean temperature and summer SD had a greater effect on the risk of dementia-associated admission in women than in men. Effects of the temporal components of general temperature indices were not consistently detected.
Fig. 2. Modifications of spatial contrasts and temporal deviations of general temperature indices.
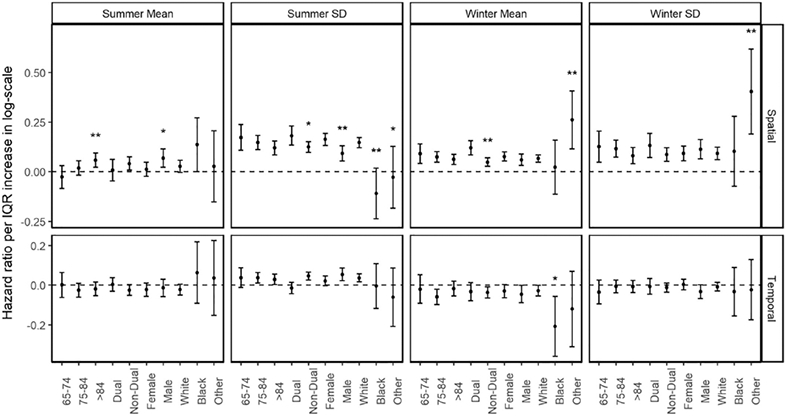
Results are expressed as hazard ratios (95% CIs) in a logarithmic scale per IQR increase in degree Celsius. Level of statistical significance when compared with reference category in each subgroup: p < 0.01 (**). Level of statistical significance when compared with reference category in each subgroup: p < 0.05 (*).
In the sensitivity analysis, most estimates of general temperature indices and their spatial and temporal components remained robust after restricting study period or adjusting for heat and cold waves (Supplementary Table 1). In the univariate analyses, the directions of all associations between the hazard ratio of dementia-associated admission and temperature exposures from the univariate models remained stable, and the statistical significance of most coefficients remained consistent with main analyses (Supplementary Table 2). Pearson correlation coefficients between the temperature indices are presented in Supplementary Tables 3 and 4.
4. Discussion
This is the first reported large community-based study examining the relationship between prolonged temperature exposure and dementia-associated hospital admissions. We assessed the associations between seasonal temperature and risk of principal or secondary hospital admissions for dementia in New England Medicare enrollees over 11 years. We examined the long-term effects of spatial differences and temporal fluctuations in summer and winter temperature averages and variability, and observed a lower risk of dementia-associated admissions in years when temperatures in summer or winter were higher than average, and a greater risk of dementia-associated admissions in older persons living in areas with increased temperature variation.
Our results indicate that warmer-than-average temperatures may decrease the risk of dementia-associated hospital admissions. This finding is consistent with evidence from a recent laboratory animal study showing that increasing ambient temperature may help mitigate dementia symptoms by reducing the production of amyloid-beta peptides (Vandal et al., 2016). We also observed increased temperature variability may increase risk for dementia-associated hospital admissions. Age-related decline of thermoregulatory function may contribute to this phenomenon: older adults are at an increased risk of thermoregulatory disorders as they are less able to adapt to temperature changes and maintain body temperature (Vogelaere and Pereira, 2005; Kenney and Hodgson, 1987; Cheshire, 2016). Our findings also suggest that people living in warmer regions in New England experienced a higher risk of dementia-associated hospital admissions. A possible explanation is that, due to great disparity of population density between New England's northern and southern portions, subjects living in the south (CT, MA, and RI) may have easier access to hospital facilities, resulting in increased dementia-associated admissions (Gesler and Meade, 1988; Mayhew and Leonardi, 1982; Love and Lindquist, 1995). However, the effect of these geographical accessibility differences could not be determined from the present study.
We also found that low-income seniors receiving both Medicare and Medicaid benefits were more vulnerable to warmer climate or greater temperature variations. In addition, we observed stronger associations between dementia admissions and spatial components in winter mean temperature, summer temperature SD and winter temperature SD in younger age groups, which may be caused by increased time spent outdoors.
A major strength of this study is that it was based on a large, well-characterized Medicare cohort, allowing us to control for confounders at the individual level. We also employed high-resolution (1 km × 1 km) estimates of daily mean ambient temperatures, enabling us to avoid potential bias due to sparse air temperature data and to more precisely obtain summary statistics of seasonal temperature. Detailed information on the source population allowed us to examine the annual risk for dementia-associated admissions at the zip-code level, rather than relying on wider regions with more within-unit variation. Furthermore, by decomposing every seasonal temperature index into spatial and temporal components, we were able to capture individual acclimation and adaption to local climate and vulnerability to changes in climate in each area.
Some limitations of our study must be noted. First, the Medicare data does not provide information on potential confounding factors for hospitalization such as geographical accessibility to hospitals, which is especially important for people living in rural communities, availability of air-conditioning, weather conditions, time spent outdoors, etc. Though we believe these factors are unlikely to have affected the exposures, this remains a possible limitation. Second, multicollinearity within temperature exposures was not eliminated. However, the issue of multicollinearity is primarily a matter of statistical power with fairly large samples (i.e., 20 million person-years in this study), it should not unduly influence the coefficients. Third, the exposure assessment is subject to measurement error because zip codes (rather than home addresses) were the finest geographic unit we could use to match temperature measurements with each beneficiary.
Given the study design, our findings are important for exploring the mechanisms between dementia and temperature and the disease burden. The study outcome, first-time hospital admission for dementia, cannot be regarded as the onset of the condition. Instead, it is likely related to the progression of dementia, which could be triggered or worsened by many conditions, such as neurodegeneration (Alzheimer's Disease International, 2013), metabolic syndrome and cognitive decline (Vogelaere and Pereira, 2005; Frisard et al., 2007; Planel et al., 2004), cerebrovascular insufficiency (Kenney and Hodgson, 1987), and infectious diseases (Holtzman and Simon, 2000), among others. Other behavior and environmental pathways, such as physical activity (Ahlskog et al., 2011), social isolation and psychosocial stressors (Friedler et al., 2014), and elevated particulate matter levels due to decreased temperature (Tai et al., 2010; Zanobetti et al., 2014), may also impact dementia hospitalizations or mediate the associations between dementia and climate change.
From the perspective of disease burden, the increasing prevalence of dementia in hospital admissions prompts a need for more health care services. With a growing number of people living with dementia, the rising demand for health care is placing a staggering burden on society in areas of public health and economic consideration (Kelley et al., 2015). In this study, in addition to analyzing seasonal temperature variability, we also considered year-to-year variations within each zip code, adding considerably to our understanding of the impact of climate change on hospitalization costs of dementia patients.
In conclusion, long-term exposure to warmer climate and greater temperature variation significantly increased the risk of dementia-associated hospitalization. For individual years with warmer summers or winters, we observed a decreased risk for dementia-associated admissions. Our results provide useful insights into how climate change may affect disease progression and healthcare costs, and lay a foundation for further studies to identify the predominant mechanism explaining how dementia is related to climate change and fluctuations.
Supplementary Material
Acknowledgments
This research was funded by the National Institute of Environmental Health Sciences (NIEHS) grant ES000002, Environmental Protection Agency (EPA) grant RD-83479801.
Footnotes
Declarations of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2018.12.054.
References
- Ahlskog J, Geda Y, Graff-Radford N, Petersen R, 2011. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc 86 (9), 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Disease International, 2013. Policy Brief for Heads of Government. The Global Impact of Dementia 2013–2050. Alzheimer's Disease International, London. [Google Scholar]
- Anderson GB, Dominici F, Wang Y, et al. , 2013. Heat-related emergency hospitalizations for respiratory diseases in the Medicare population. Am. J. Respir. Crit. Care Med 187 (10), 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, 2007. Temperature and cardiovascular deaths in the US elderly: changes over time. Epidemiology 18 (3), 369–372. [DOI] [PubMed] [Google Scholar]
- Barnett A, Hajat S, Gasparrini A, Rocklöv J, 2012. Cold and heat waves in the United States. Environ. Res 112, 218–224. [DOI] [PubMed] [Google Scholar]
- Basu R, 2009. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ. Health 8 (1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Malig B, Ostro B, 2010. High ambient temperature and the risk of preterm delivery. Am. J. Epidemiol 172 (10), 1108–1117. [DOI] [PubMed] [Google Scholar]
- Bell ML, O'Neill MS, Ranjit N, et al. , 2008. Vulnerability to heat-related mortality in Latin America: a case-crossover study in Sao Paulo, Brazil, Santiago, Chile and Mexico City, Mexico. Int. J. Epidemiol 37 (4), 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Schneider J, Tang Y, Arnold S, Wilson R, 2006. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 5 (5), 406–412. [DOI] [PubMed] [Google Scholar]
- Cheshire WP, 2016. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci 196, 91–104. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Nugent S, Roy M, et al. , 2011. Brain fuel metabolism, aging and Alzheimer's disease. Nutrition 27 (1), 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Yeung E, Hansen T, Gibbons S, Fornazzari L, Ringer L, et al. , 2009. Impact of socioeconomic status on the prevalence of dementia in an inner city memory disorders clinic. Int. Psychogeriatr 21 (06), 1096. [DOI] [PubMed] [Google Scholar]
- Fox RH, Woodward PM, Exton-Smith AN, et al. , 1973. Body temperatures in the elderly: a national study of physiological, social, and environmental conditions. Br. Med. J 1 (5847). 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedler B, Crapser J, McCullough L, 2014. One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol. 129 (4), 493–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisard MI, Broussard A, Davies SS, et al. , 2007. Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. J. Gerontol. A Biol. Sci. Med. Sci 62 (7), 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield R, Musumeci MB, Reaves LE, Damico A, 2015. Medicaid's Role for People With Dementia. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/medicaids-role-for-people-with-dementia/, Accessed date: 9 December 2018. [Google Scholar]
- Gesler WM, Meade MS, 1988. Locational and population factors in health care-seeking behavior in Savannah, Georgia. Health Serv. Res 23 (3), 443–462. [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Pan X, et al. , 2011. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag nonlinear model. Environ. Health Perspect 119 (12), 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustein K, Allen MR, Forster PM, et al. , 2017. A real-time global warming index. Sci. Rep 7 (1), 15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman A, Simon EW, 2000. Body temperature as a risk factor for Alzheimer's disease. Med. Hypotheses 55 (5), 440–444. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Langa KM, 2013. Monetary costs of dementia in the United States. N. Engl. J. Med 369 (5), 489–490. [DOI] [PubMed] [Google Scholar]
- Irons J, Oswood M, 1992. Seasonal temperature patterns in an arctic and two subarctic Alaskan (USA) headwater streams. Hydrobiologia 237 (3), 147–157. [Google Scholar]
- Karp A, 2004. Relation of education and occupation-based socioeconomic status to incident Alzheimer's disease. Am. J. Epidemiol 159 (2), 175–183. [DOI] [PubMed] [Google Scholar]
- Kelley AS, McGarry K, Gorges R, et al. , 2015. The burden of health care costs for patients with dementia in the last 5 years of life. Ann. Intern. Med 163 (10), 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL, Hodgson JL, 1987. Heat tolerance, thermoregulation and ageing. Sports Med. 4 (6), 446–456. [DOI] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky A, Koutrakis P, Schwartz J, 2012. Temporal and spatial assessments of minimum air temperature using satellite surface temperature measurements in Massachusetts, USA. Sci. Total Environ 432, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Nordio F, Coull BA, et al. , 2014. Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the Northeastern USA. Remote Sens. Environ 150, 132–139. [Google Scholar]
- Lang I, Llewellyn D, Langa K, Wallace R, Huppert F, Melzer D, 2008. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J. Am. Geriatr. Soc 56 (2), 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D, Lindquist P, 1995. The geographical accessibility of hospitals to the aged: a geographic information systems analysis within Illinois. Health Serv. Res 29, 629–651. [PMC free article] [PubMed] [Google Scholar]
- Mayhew LD, Leonardi G, 1982. Equity, efficiency, and accessibility in urban and regional health-care systems. Environ. Plan. A 14 (11), 1479–1507. [Google Scholar]
- O'Neill MS, Zanobetti A, Schwartz J, 2003. Modifiers of the temperature and mortality association in seven US cities. Am. J. Epidemiol 157 (12), 1074–1082. [DOI] [PubMed] [Google Scholar]
- Planel E, Miyasaka T, Launey T, et al. , 2004. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease. J. Neurosci 24 (10), 2401–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Bretteville A, Liu L, et al. , 2009. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 23 (8), 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann R, Solomon S, Hegerl G, 2009. Spatial and seasonal patterns in climate change, temperatures, and precipitation across the United States. PNAS 106 (18), 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Kloog I, Zanobetti A, et al. , 2015. Impacts of temperature and its variability on mortality in New England. Nat. Clim. Chang 5, 988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu P, Wang Y, et al. , 2016a. Chronic effects of temperature on mortality in the Southeastern USA using satellite-based exposure metrics. Sci. Rep 6, 30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu P, Kloog I, et al. , 2016b. Estimating daily air temperature across the Southeastern United States using high-resolution satellite data: a statistical modeling study. Environ. Res 146, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai A, Mickley L, Jacob D, 2010. Correlations between fine particulate matter (PM2.5) and meteorological variables in the United States: implications for the sensitivity of PM2.5 to climate change. Atmos. Environ 44 (32), 3976–3984. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division, 2015. World Population Ageing 2015. [Google Scholar]
- Vandal M, White PJ, Tournissac M, et al. , 2016. Impaired thermoregulation and beneficial effects of thermoneutrality in the 3×Tg-AD model of Alzheimer's disease. Neurobiol. Aging 43, 47–57. [DOI] [PubMed] [Google Scholar]
- Vogelaere P, Pereira C, 2005. Thermoregulation and aging. Rev. Port. Cardiol 24 (5), 747–761. [PubMed] [Google Scholar]
- Wan Z, 2008. New refinements and validation of the MODIS land-surface temperature/emissivity products. Remote Sens. Environ 112 (1), 59–74. [Google Scholar]
- World Health Organization, 2004. 10 facts on dementia. http://www.who.int/features/factfiles/dementia/en/, Accessed date: 9 December 2018. [Google Scholar]
- Yin Q, Wang J, 2017. The association between consecutive days' heat wave and cardiovascular disease mortality in Beijing, China. BMC Public Health 17 (1), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O'Neill MS, Gronlund CJ, et al. , 2012. Summer temperature variability and long-term survival among elderly people with chronic disease. PNAS 109 (17), 6608–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Dominici F, Wang Y, Schwartz J, 2014. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health 13 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.