Abstract
Rationale: Sepsis commonly results in elevated serum troponin levels and increased risk for postsepsis cardiovascular complications; however, the association between troponin levels during sepsis and cardiovascular complications after sepsis is unclear.
Objectives: To evaluate the association between serum troponin levels during sepsis and 1 year after sepsis cardiovascular events.
Methods: We analyzed adults aged ⩾40 years without preexisting cardiovascular disease within 5 years, admitted with sepsis across 21 hospitals from 2011 to 2017. Peak serum troponin I levels during sepsis were grouped as normal (⩽0.04 ng/ml) or tertiles of abnormal (>0.04 to ⩽0.09 ng/ml, >0.09 to ⩽0.42 ng/ml, or >0.42 ng/ml). Multivariable adjusted cause-specific Cox proportional hazards models with death as a competing risk were used to assess associations between peak troponin I levels and a composite cardiovascular outcome (atherosclerotic cardiovascular disease, atrial fibrillation, and heart failure) in the year following sepsis. Models were adjusted for presepsis and intrasepsis factors considered potential confounders.
Measurements and Main Results: Among 14,046 eligible adults with troponin I measured, 2,012 (14.3%) experienced the composite cardiovascular outcome, including 832 (10.9%) patients with normal troponin levels, as compared with 370 (17.3%), 376 (17.6%), and 434 (20.3%) patients within each sequential abnormal troponin tertile, respectively (P < 0.001). Patients within the elevated troponin tertiles had increased risks of adverse cardiovascular events (adjusted hazard ratio [aHR]troponin0.04–0.09 = 1.37; 95% confidence interval [CI], 1.20–1.55; aHRtroponin0.09–0.42 = 1.44; 95% CI, 1.27–1.63; and aHRtroponin>0.42 = 1.77; 95% CI, 1.56–2.00).
Conclusions: Among patients without preexisting cardiovascular disease, troponin elevation during sepsis identified patients at increased risk for postsepsis cardiovascular complications. Strategies to mitigate cardiovascular complications among this high-risk subset of patients are warranted.
Keywords: troponin, sepsis, cardiovascular risk, mortality, infection
At a Glance Commentary
Scientific Knowledge on the Subject
Patients who survive sepsis have increased risks for adverse cardiovascular events, but the specific characteristics during sepsis associated with subsequent cardiovascular complications are unclear.
What This Study Adds to the Field
Among patients without preexisting cardiovascular disease, troponin I elevation during sepsis identified patients at an increased risk for postsepsis cardiovascular complications. Elevated troponin I levels during sepsis may be used in future prospective studies together with traditional cardiovascular risk factors to target high-risk patients for evaluation of postsepsis cardioprotective therapies.
Sepsis is life-threatening organ dysfunction owing to infection, leading to 1.7 million hospitalizations in the United States annually (1, 2). As in-hospital mortality rates of sepsis have decreased, the number of patients facing complications of postsepsis recovery have increased (3). In particular, patients who survive sepsis have increased risks for adverse cardiovascular events, including acute myocardial infarction, ischemic stroke, heart failure, arrhythmias, and sudden cardiac death (4, 5). Although multiple studies have identified higher cardiovascular risks after sepsis, the specific characteristics of sepsis that are associated with subsequent cardiovascular complications are unclear.
Troponin I is released in response to myocardial injury, and serum troponin elevations have shown prognostic value for mortality during sepsis (6–9). However, little is known about the association between troponin levels during sepsis and the risk of posthospitalization cardiac complications. At present, there are no guideline-recommended diagnostic or therapeutic management strategies for troponin elevation during sepsis or after discharge from sepsis hospitalization (10–12). Identification of risk markers for postsepsis cardiovascular events may facilitate investigations of cardiopreventive strategies to improve long-term outcomes among sepsis survivors.
Within a large, multicenter, community-based cohort of adults hospitalized for sepsis, we hypothesized that troponin I elevation during sepsis would be associated with an increased risk of postsepsis cardiovascular events.
Methods
This study was approved by the Kaiser Permanente Northern California (KPNC) Institutional Review Board. A waiver of informed consent was obtained because of the nature of the study.
Cohort Assembly
We performed a retrospective cohort study of patients aged ⩾40 years who survived to hospital discharge after an index hospitalization for sepsis across 21 KPNC hospitals between January 1, 2011, and September 30, 2017. Sepsis was defined using the Sepsis-3 international consensus definitions by establishing “suspected infection” based on a timed dyad of antibiotics and cultures with organ dysfunction as defined by a Sequential Organ Failure Assessment (SOFA) score ⩾2 (1). Patients were included with sepsis present near admission, defined by cultures performed and antibiotics provided within 48 hours of admission and SOFA criteria met within 72 hours. Patients with serum troponin I levels measured during sepsis admission were included in the primary analyses. For patients who had multiple sepsis hospitalizations during the study period, we included data only on the first hospitalization. To allow for adequate pre- and postsepsis data, we included patients who had continuous KPNC membership in the year before and after the sepsis hospitalization, unless they died during follow up after surviving the index sepsis hospitalization. Patients with a diagnosis of cardiovascular disease 5 years before hospitalization for sepsis were excluded. In the primary analysis cohort, prior atherosclerotic cardiovascular diagnoses, heart failure, or atrial fibrillation were identified using International Classification of Diseases (ICD) codes with either one inpatient visit diagnosis or at least two outpatient visit diagnoses (to avoid false-positive diagnoses based on “rule out” coding) (13, 14) or by a procedure code within 5 years of the sepsis hospitalization (see Table E1 in the online supplement). A sensitivity analysis was performed to exclude patients more broadly for prior cardiovascular-associated diagnoses by assessing ICD codes in any inpatient or outpatient claim prior to sepsis as well as employing additional ICD codes for coronary artery disease or peripheral artery disease (Table E2). An additional sensitivity analysis was performed excluding patients with any new diagnosis of acute myocardial infarction, ischemic stroke, coronary revascularization, heart failure, or atrial fibrillation during the index sepsis hospitalization.
Exposure
The primary exposure was the peak serum troponin I level measured during the first 14 days of the sepsis hospitalization or until hospital discharge if length of stay was less than 14 days (Figure E1). Fourteen days was chosen as a cut off because that is the time when acute sepsis factors, rather than prior comorbidities, are most relevant for patient outcomes (15). To improve clinical interpretation of results and allow for a nonlinear association between troponin and cardiovascular outcomes, we a priori categorized troponin I as follows: normal (⩽0.04 ng/ml), tertile 1 (>0.04 to ⩽0.09 ng/ml), tertile 2 (>0.09 to ⩽0.42 ng/ml), and tertile 3 (>0.42 ng/ml).
Outcomes
We assessed the association of troponin I levels during sepsis with a composite outcome of cardiovascular events with death as competing risk. The composite outcome included: 1) atherosclerotic cardiovascular disease (ASCVD), defined as acute myocardial infarction, ischemic stroke, or coronary revascularization; 2) acute heart failure diagnosis; and 3) an atrial fibrillation diagnosis, occurring within the year after the sepsis discharge (Table E3).
Covariates
We adjusted for variables that may confound associations between troponin I levels and postsepsis cardiovascular events. These included presepsis factors potentially associated with cardiovascular risk and sepsis severity, such as age at sepsis admission; body mass index; sex; race/ethnicity; Charlson comorbidities (16); antihypertensive, antiplatelet, and statin use prior to sepsis; and smoking history. In addition, we adjusted for intrasepsis factors that may confound associations between troponin I level and postsepsis cardiovascular outcomes, including the source of sepsis, hospitalization characteristics (e.g., season at admission), acute severity of illness (based on Laboratory and Acute Physiology score, version 2 [17] and SOFA score), most extreme laboratory value measure (e.g., lowest or highest value, as appropriate) of complete blood count (e.g., white blood cell count, platelets, and bands), serum chemistry parameters (e.g., bicarbonate, creatinine), coagulation measures and lactate, use of intensive care or life-sustaining therapies (e.g., mechanical ventilation or hemodialysis), vasopressor type, fluid administration (total net intake and output and blood products transfused), and the proportion of nursing rhythm documentation indicating atrial fibrillation in cardiac rhythm flowsheets.
Missing Variables
Missing data for covariates among patients with sepsis and a troponin I measured are shown in Table 1. The most frequently missing variable was the laboratory value total bilirubin, which was missing in 29% of patients. To account for missing data, we used multiple imputation with chained equations (R version 3.6.2 MICE package) to generate imputed datasets, which relies on flexible assumptions that the data are missing at random (18). To optimize the multiple imputation procedure, we expanded the variable set beyond those included in the scientific model to include several potential transformations of a single variable. For example, for intrasepsis laboratory values, we included their first, highest, lowest, and minimum-to-maximum delta values. We varied the number of imputed datasets from 5 to 20 to confirm the stability of the imputation procedure, using 10 imputed datasets in our final approach. To evaluate the robustness of our results to assumptions about missingness, we performed two sensitivity analyses at extremes of missing data assumptions: 1) using only patients without missing covariates (i.e., a complete case analysis) and 2) using data from all cases of sepsis and imputing all missing variables, including missing troponin I.
Table 1.
Complete Case Baseline Characteristics of Adults with Sepsis Stratified by Peak Troponin I Levels
| Peak Troponin I Value during Sepsis |
||||
|---|---|---|---|---|
| Characteristics | Normal, <0.04 ng/ml | Tertile 1, >0.04–0.09 ng/ml | Tertile 2, >0.09–0.42 ng/ml | Tertile 3, >0.42 ng/ml |
| Total, n (%) | n = 4,263 | n = 1,234 | n = 1,234 | n = 1,234 |
| Peak troponin, ng/ml, median (IQR) | 0.02 (0.01, 0.03) | 0.06 (0.05, 0.08) | 0.18 (0.13, 0.27) | 1.40 (0.73, 4.08) |
| Time to peak troponin, d, median (IQR) | 0 (0, 0) | 0 (0, 1) | 0 (0, 1) | 1 (0, 1) |
| Age, median (IQR) | 73 (63, 82) | 75 (64, 84) | 74 (64, 84) | 73 (64, 83) |
| BMI, median (IQR) | 27 (23, 32) | 27 (23, 32) | 27 (23, 32) | 27 (23, 33) |
| Male, n (%) | 2133 (50) | 592 (48) | 601 (49) | 529 (43) |
| Race and ethnicity, categories defined by database, n (%) | ||||
| White | 2,585 (61) | 737 (60) | 745 (60) | 771 (63) |
| Asian/Pacific Islander | 606 (14) | 193 (16) | 204 (17) | 215 (17) |
| Hispanic | 616 (14) | 150 (12) | 151 (12) | 128 (10) |
| Black | 400 (9) | 140 (11) | 117 (10) | 102 (8) |
| Native American | 44 (1) | 12 (1) | 15 (1) | 12 (1) |
| Other | 12 (<1) | 2 (<1) | 2 (<1) | 6 (<1) |
| Presepsis variables | ||||
| Medical comorbidities, n (%) | ||||
| Diabetes mellitus | 1,583 (37) | 426 (35) | 423 (34) | 465 (38) |
| Diabetes mellitus with chronic complications | 1,134 (27) | 325 (26) | 320 (26) | 375 (30) |
| Renal disease | 1,469 (35) | 446 (36) | 428 (35) | 462 (37) |
| Chronic kidney disease | 1,448 (34) | 459 (37) | 426 (35) | 413 (33) |
| Proteinuria within the prior year | 471 (11) | 149 (12) | 145 (12) | 126 (10) |
| Chronic dialysis | 45 (1) | 19 (2) | 17 (1) | 19 (2) |
| Chronic pulmonary disease | 1,485 (35) | 397 (32) | 368 (30) | 413 (33) |
| Mild liver disease | 219 (5) | 49 (4) | 41 (3) | 42 (3) |
| Moderate or severe liver disease | 145 (3) | 40 (3) | 27 (2) | 22 (2) |
| Malignancy, including leukemia or lymphoma | 942 (22) | 241 (20) | 222 (18) | 187 (15) |
| Metastatic solid tumor | 412 (10) | 93 (7) | 91 (7) | 60 (5) |
| Dementia | 191 (5) | 69 (6) | 63 (5) | 48 (4) |
| Hemiplegia or paraplegia | 82 (2) | 23 (2) | 24 (2) | 20 (2) |
| Peptic ulcer disease | 168 (4) | 46 (4) | 57 (5) | 34 (3) |
| Rheumatologic disease | 209 (5) | 54 (4) | 73 (6) | 51 (4) |
| AIDS | 22 (<1) | 10 (<1) | 4 (<1) | 2 (<1) |
| Organ transplant | 25 (<1) | 9 (<1) | 13 (1) | 6 (<1) |
| Charlson comorbidity within prior 5 yr, median (IQR) | 3 (1, 5) | 3 (1, 5) | 2 (1, 4) | 3 (1, 4) |
| Smoking history | 1,974 (46) | 564 (46) | 587 (48) | 583 (47) |
| ASCVD 10-yr risk, mean (SD) | 0.24 (0.21) | 0.26 (0.22) | 0.26 (0.22) | 0.27 (0.23) |
| Preadmission medications, n (%) | ||||
| Antihypertensive | 2,987 (70) | 862 (70) | 835 (68) | 858 (70) |
| Statin | 1,705 (40) | 490 (40) | 484 (39) | 533 (43) |
| Aspirin | 87 (2) | 15 (1) | 19 (2) | 35 (3) |
| Antiplatelet (nonaspirin) | 72 (2) | 14 (1) | 12 (1) | 24 (2) |
| Intrasepsis variables | ||||
| Maximum LAPS2 score, median (IQR) | 104 (82, 128) | 114 (92, 139) | 120 (93, 147) | 130 (102, 157) |
| Maximum SOFA score, median (IQR) | 3 (2, 5) | 4 (3, 6) | 4 (3, 6) | 5 (3, 8) |
| Lowest oxygen saturation, median (IQR) | 91 (87, 94) | 90 (86, 93) | 90 (85, 93) | 88 (82, 92) |
| Noninvasive ventilation, n (%) | 867 (20) | 299 (24) | 356 (29) | 457 (37) |
| Total days of noninvasive ventilation, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Mechanical ventilation, n (%) | 463 (11) | 169 (14) | 286 (23) | 501 (41) |
| Total days of invasive mechanical ventilation, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) |
| Intrasepsis atrial fibrillation, n (%) | 836 (20) | 424 (34) | 444 (36) | 460 (37) |
| Blood products received, n (%) | 969 (23) | 311 (25) | 294 (24) | 376 (30) |
| Total net intake and output (ml), median (IQR) | 1,605 (−677, 4,126) | 1,473 (−1,089, 4,350) | 1,170 (−1,739, 3,776) | 490 (−2,534, 3,422) |
| Infection site, n (%) | ||||
| Postoperative, central nervous system, device or endovascular | 236 (6) | 75 (6) | 80 (6) | 91 (7) |
| Gastrointestinal tract | 1,146 (27) | 283 (23) | 309 (25) | 272 (22) |
| Genitourinary tract | 1,343 (32) | 423 (34) | 443 (36) | 412 (33) |
| Respiratory tract | 1,584 (37) | 518 (42) | 492 (40) | 510 (41) |
| Skin infection | 350 (8) | 99 (8) | 104 (8) | 85 (7) |
| Infection type, n (%) | ||||
| Gram-negative bacteria | 454 (11) | 135 (11) | 153 (12) | 153 (12) |
| Gram-positive bacteria | 128 (3) | 47 (4) | 51 (4) | 55 (4) |
| Viral infection | 416 (10) | 103 (8) | 103 (8) | 105 (9) |
| Tuberculous or fungal infection | 196 (5) | 50 (4) | 45 (4) | 38 (3) |
| Culture negative | 2,945 (69) | 911 (74) | 929 (75) | 889 (72) |
| Laboratory values during sepsis, median (IQR) | ||||
| Highest WBC count, K/μl | 14.0 (9.9, 19.1) | 15.2 (10.9, 20.3) | 15.5 (11.4, 20.8) | 16.2 (12.3, 21.9) |
| Highest band count, K/μl | 0 (0, 10) | 0 (0, 11) | 1 (0, 15) | 1 (0, 14) |
| Lowest platelet count, K/μl | 154 (108, 212) | 151 (107, 201) | 146 (102, 196) | 141 (101, 192) |
| Highest creatinine, mg/dl | 1.26 (0.91, 1.90) | 1.45 (1.00, 2.33) | 1.47 (1.08, 2.19) | 1.49 (1.05, 2.31) |
| Highest glucose, mg/dl | 154 (124, 210) | 157 (128, 216) | 162 (130, 220) | 179 (140, 254) |
| Highest ALT, U/L | 35 (24, 62) | 37 (24, 65) | 39 (26, 70) | 41 (26, 80) |
| Highest total bilirubin, mg/dl | 0.9 (0.5, 1.6) | 0.9 (0.6, 1.5) | 0.9 (0.6, 1.5) | 0.8 (0.5, 1.2) |
| Highest lactic acid, mmol/L | 1.9 (1.3, 3.0) | 2.1 (1.4, 3.4) | 2.3 (1.5, 3.7) | 2.7 (1.7, 4.7) |
| Vasopressor or inotrope received during sepsis, n (%) | ||||
| Norepinephrine | 454 (11) | 183 (15) | 263 (21) | 419 (34) |
| Epinephrine | 5 (<1) | 3 (<1) | 9 (<1) | 29 (2) |
| Phenylephrine | 50 (1) | 20 (2) | 38 (3) | 97 (8) |
| Dopamine | 61 (1) | 32 (3) | 41 (3) | 127 (10) |
| Dobutamine | 61 (1) | 32 (3) | 41 (3) | 127 (10) |
| Milrinone | 2 (<1) | 0 (0) | 12 (<1) | 29 (2) |
| Season, n (%) | ||||
| Fall | 829 (19) | 256 (22) | 296 (24) | 254 (21) |
| Summer | 1,052 (25) | 280 (23) | 277 (22) | 307 (25) |
| Spring | 1,174 (28) | 330 (27) | 317 (26) | 325 (26) |
| Winter | 1,208 (28) | 368 (30) | 344 (28) | 348 (28) |
Definition of abbreviations: ALT = alanine aminotransferase; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; IQR = interquartile range; LAPS2 = Laboratory and Acute Physiology Score version 2; SOFA = Sequential Organ Failure Assessment; WBC = white blood cell count.
Covariate missingness (% missing): BMI (1.8% missing), total net intake and output (0.3% missing), ALT (26% missing), total bilirubin (29% missing), lactic acid (19% missing).
Statistical Analysis
All variables were summarized using medians with interquartile range (IQR) for continuous variables and counts with percentages for dichotomous and categorical variables. We performed univariable- and multivariable-adjusted cause-specific Cox proportional hazard models with death as a competing risk to calculate unadjusted and adjusted cause-specific hazard ratios (HRs) of cardiovascular outcomes of interest associated with troponin I levels during sepsis. The Cox proportional hazards assumption was not violated. We conducted quantitative sensitivity analyses (i.e., “eValues”) (19) to identify the strength of association between a theoretical unmeasured confounder, troponin I, and postdischarge cardiovascular events that would be necessary to move effect estimates to the null. To determine how presepsis risk for cardiovascular disease might modify the association between troponin I during sepsis and postsepsis cardiovascular events, we performed a subgroup analysis stratified by American Heart Association/American College of Cardiology Pooled Cohort Equation ASCVD 10-year predicted risk categories (low, <7.4%; moderate, 7.5–19.9%; and high, >20%) (20, 21) and tested for interaction between continuous predicted ASCVD risk and troponin I. We also performed a sensitivity analysis using natural log-transformed troponin I as a continuous variable to test the robustness of our abnormal troponin tertile categorization. All tests were two sided and conducted using a significance level of 0.05. Statistical analyses were conducted using R version 3.6.2 (2019-12-12).
Results
Patient Characteristics
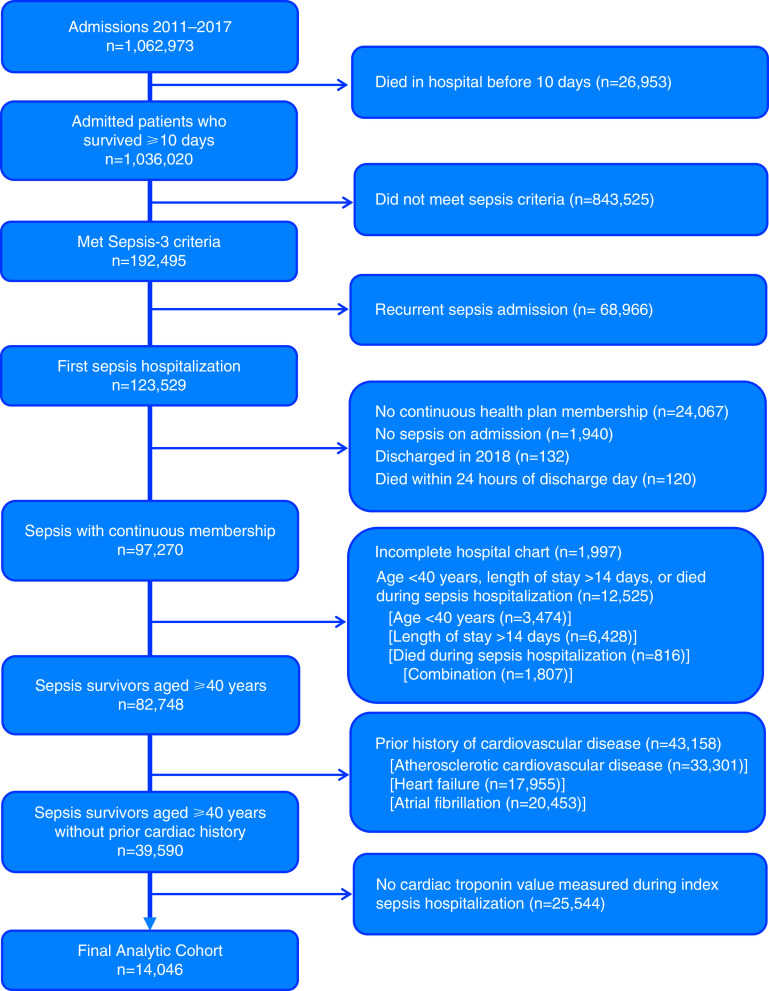
Among the 82,748 patients who met sepsis criteria, 14,046 did not have a prior diagnosis of ASCVD, heart failure, or atrial fibrillation within 5 years of the sepsis hospitalization and had a troponin I level measured; therefore, they were included in the primary analysis (Figure 1). The maximum 14-day troponin value was normal (⩽0.04 ng/ml) in 7,643 (54.4%) cases. The time from admission to peak troponin I level was a median of 0 days (IQR, 0–1 d). Patients were a median age of 75 years (IQR, 65–84 yr); 47% were men, and 62% were white. Most infections originated from the respiratory tract (40%) or genitourinary tract (33%). Although none of the patients had previous diagnosis codes for cardiovascular disease, 71% were prescribed antihypertensive agents, 41% statin therapy, 2% aspirin, and 1.6% other antiplatelet therapy prior to their index sepsis hospitalization. Table 1 shows patient characteristics stratified by troponin I level. Although baseline characteristics and comorbidities were, in general, similar across troponin I levels, indices of acute sepsis severity (e.g., Laboratory and Acute Physiology score, version 2, mechanical ventilation, and SOFA score) were increased at higher troponin I levels.
Figure 1.
Cohort assembly of adults without preexisting cardiovascular disease who survived sepsis hospitalization. A flow diagram identifying the cohort used in our primary analysis is shown. We identified a cohort of adults aged ⩾40 years without a prior diagnosis of cardiovascular disease who survived sepsis admission and had troponin I measured during their hospitalization.
Outcomes
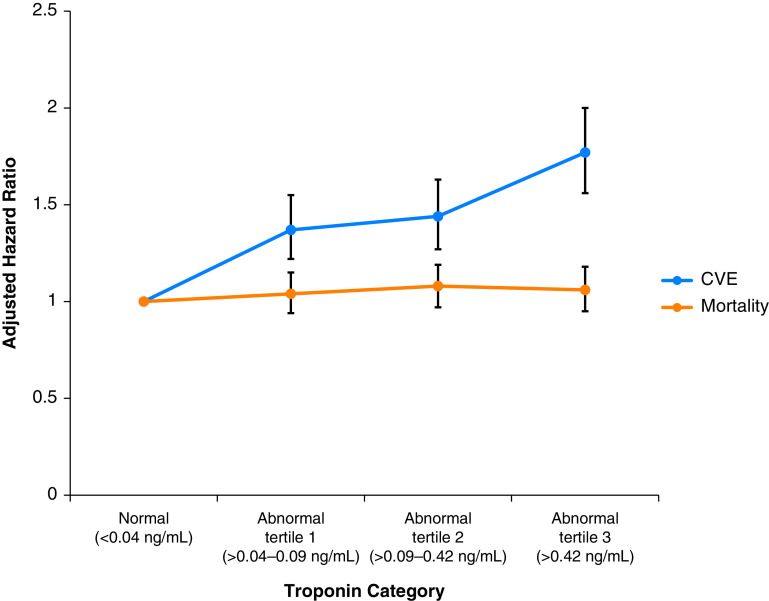
Patients were observed for 1 year following the index sepsis hospitalization, during which time 2,012 (14.3%) patients experienced the composite cardiovascular outcome: 479 (3.4%) with ASCVD events, 1,425 (10.1%) with atrial fibrillation, and 345 (2.5%) with heart failure. The composite outcome occurred among 832 (10.9%) patients with normal troponin as compared with 370 (17.3%) patients within abnormal troponin tertile 1 (>0.04 to 0.09 ng/ml), 376 (17.6%) patients within abnormal troponin tertile 2 (>0.09 to 0.42 ng/ml), and 434 (20.3%) patients within abnormal troponin tertile 3 (>0.42 ng/ml) (P < 0.001; rates for individual outcomes shown in Table E4). The median time to a cardiovascular event following sepsis hospitalization was 15 days for an ASCVD event, 12 days for a heart failure event, and 35 days for an atrial fibrillation event. Figure 2 shows the association between maximum troponin I values during sepsis and risk for cardiovascular events 1 year after discharge from a sepsis hospitalization. Both unadjusted and multivariable-adjusted models demonstrated that troponin I levels above the normal range were associated with increased risk of cardiovascular events 1 year after sepsis (Table 2), with adjusted HR (aHR)troponin 0.04–0.09 = 1.37 (95% confidence interval [CI], 1.20–1.55; P < 0.001; eValue, 2.08), aHRtroponin 0.09–0.42 = 1.44 (95% CI, 1.27–1.63; P < 0.001; eValue, 2.24), and aHRtroponin>0.42 = 1.77 (95% CI, 1.56–2.00; P < 0.001; eValue, 2.94).
Figure 2.
Multivariable association between peak troponin I level during sepsis and postdischarge risks of cardiovascular events (CVE) and all-cause death. Each circle represents a category of peak troponin I elevation during a hospitalization for sepsis and shows the adjusted hazard ratio (and associated 95% confidence interval) of that troponin I level and the outcome of interest. The blue line represents the adjusted hazards of a composite cardiovascular complication (atherosclerotic cardiovascular disease, atrial fibrillation, or heart failure), and the orange line represents the adjusted hazards of mortality at 1 year after discharge from a sepsis hospitalization. Modest troponin elevation was associated with a 37–77% increased risk of the composite outcome of CVE without an increased risk of mortality.
Table 2.
Univariable- and Multivariable-Adjusted Association of Peak Troponin I Levels during Sepsis with Postsepsis Cardiovascular Events and Death
| Primary Analysis |
Sensitivity Analyses |
|||||||
|---|---|---|---|---|---|---|---|---|
| Exclusion of All Patients With a Cardiovascular Diagnosis 5 yr before Sepsis Hospitalization (n = 14,046) |
Exclusion of All Patients With Presepsis* or Intrasepsis Cardiovascular Diagnoses† (n = 6,874) |
|||||||
| Model | Outcome | Troponin Category | n | HR (95% CI) | P Value | n | HR (95% CI) | P Value |
| Univariable models | Death from any cause | Normal, ⩽0.04 ng/ml | 7,643 | 1.00 (reference) | — | 4,483 | 1.00 (reference) | — |
| Abnormal tertile 1, >0.04–0.09 ng/ml | 2,135 | 1.18 (1.07, 1.30) | 0.001 | 960 | 1.12 (0.96, 1.30) | 0.14 | ||
| Abnormal tertile 2, >0.09–0.42 ng/ml | 2,134 | 1.23 (1.12, 1.36) | <0.001 | 863 | 1.14 (0.97, 1.33) | 0.11 | ||
| Abnormal tertile 3, >0.42 ng/ml | 2,134 | 1.13 (1.02, 1.25) | 0.02 | 568 | 0.96 (0.78, 1.17) | 0.68 | ||
| Cardiovascular event | Normal, ⩽0.04 ng/ml | 7,643 | 1.00 (reference) | — | 4,483 | 1.00 (reference) | — | |
| Abnormal tertile 1, >0.04–0.09 ng/ml | 2,135 | 1.71 (1.52, 1.94) | <0.001 | 960 | 1.44 (1.08, 1.91) | 0.01 | ||
| Abnormal tertile 2, >0.09–0.42 ng/ml | 2,134 | 1.77 (1.57, 2.00) | <0.001 | 863 | 1.35 (1.00, 1.83) | 0.05 | ||
| Abnormal tertile 3, >0.42 ng/ml | 2,134 | 2.02 (1.80, 2.27) | <0.001 | 568 | 1.69 (1.22, 2.35) | 0.002 | ||
| Multivariable models | Death from any cause | Normal, ⩽0.04 ng/ml | 7,643 | 1.00 (reference) | — | 4,483 | 1.00 (reference) | — |
| Abnormal tertile 1, >0.04–0.09 ng/ml | 2,135 | 1.04 (0.94, 1.15) | 0.44 | 960 | 1.01 (0.87, 1.18) | 0.90 | ||
| Abnormal tertile 2, >0.09–0.42 ng/ml | 2,134 | 1.08 (0.97, 1.19) | 0.14 | 863 | 1.09 (0.93, 1.27) | 0.31 | ||
| Abnormal tertile 3, >0.42 ng/ml | 2,134 | 1.06 (0.95, 1.18) | 0.33 | 568 | 1.02 (0.83, 1.26) | 0.86 | ||
| Cardiovascular event | Normal, ⩽0.04 ng/ml | 7,643 | 1.00 (reference) | — | 4,483 | 1.00 (reference) | — | |
| Abnormal tertile 1, >0.04–0.09 ng/ml | 2,135 | 1.37 (1.20, 1.55) | <0.001 | 960 | 1.31 (0.98, 1.74) | 0.07 | ||
| Abnormal tertile 2, >0.09–0.42 ng/ml | 2,134 | 1.44 (1.27, 1.63) | <0.001 | 863 | 1.24 (0.91, 1.69) | 0.18 | ||
| Abnormal tertile 3, >0.42 ng/ml | 2,134 | 1.77 (1.56, 2.00) | <0.001 | 568 | 1.58 (1.11, 2.24) | 0.01 | ||
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
Covariates: Presepsis factors include age, body mass index, sex, race, Charlson comorbidity score and history of diabetes mellitus, chronic kidney disease, dialysis, proteinuria, renal disease, chronic pulmonary disease, malignancy, metastatic solid tumor, organ transplant, acquired immunodeficiency disorder, mild liver disease, moderate/severe liver disease, peptic ulcer disease, rheumatologic disease, dementia, hemiplegia/paraplegia, smoking, antihypertensive use, antiplatelet use, statin use, and aspirin use. Intrasepsis factors include atrial fibrillation, maximum dose and proportion requiring vasopressor or inotropes, infection type, infection site, Laboratory Acute Physiology Score version 2, maximum Sequential Organ Failure Assessment score, lowest oxygen saturation, duration of and proportion requiring invasive and noninvasive ventilation, total volume and proportion requiring blood product transfusion, net fluid balance, admission season, and highest laboratory values for alanine aminotransferase, total bilirubin, creatinine, glucose, white blood cell count, band count, platelets, and lactate.
Strict exclusion criteria for presepsis cardiovascular diagnosis employed additional International Classification of Diseases 9/10 codes for coronary artery disease and peripheral artery disease detailed in Table E2.
Intrasepsis cardiovascular diagnosis was defined as any atherosclerotic cardiovascular disease, heart failure, or atrial fibrillation diagnosis by International Classification of Diseases 9/10 coding during sepsis hospitalization.
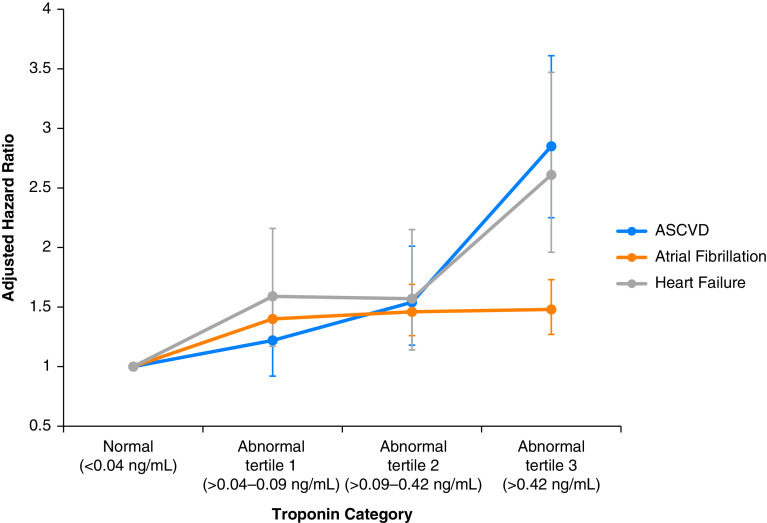
Compared with troponin I levels in the normal range, increasing troponin was associated with an increased risk for each individual cardiovascular event within 1 year after sepsis hospitalization, most pronounced for ASCVD events and heart failure (Figure 3). Although higher troponin I levels were associated with increased mortality in the unadjusted model, there were no significant differences across troponin I categories for all-cause death after adjustment for potential confounders.
Figure 3.
Multivariable association between peak troponin I level during sepsis and postdischarge risks of individual types of cardiovascular events. Each circle represents a category of peak troponin I elevation during a hospitalization for sepsis and shows the adjusted hazard ratio (and associated 95% confidence interval) of that troponin I level and an individual cardiovascular complication at 1 year after discharge. The blue line represents the adjusted hazards of atherosclerotic cardiovascular disease (ASCVD), the orange line the adjusted hazards of atrial fibrillation, and the gray line the adjusted hazards of heart failure. Modest troponin elevation was associated with increased risks of each individual cardiovascular complication, most pronounced in ASCVD and heart failure events.
Increasing troponin I levels were similarly associated with increased cardiovascular events, without increased mortality, across ASCVD risk categories (Table E5). For example, patients with low ASCVD risk (⩽7.4% 10-year risk) had aHRtroponin 0.04–0.09 = 1.52 (95% CI, 1.12–2.07; P = 0.01) and aHRtroponin>0.42 = 1.60 (95% CI, 1.11–2.29; P = 0.01). Patients with high ASCVD risk (⩾20% 10-year risk) had aHRtroponin 0.04–0.09 = 1.41 (95% CI, 1.18–1.68; P < 0.001) and aHRtroponin>0.42 = 1.88 (95% CI, 1.58–2.23; P < 0.001), with P = 0.28 for interaction between predicted ASCVD risk and troponin I level for risk of postsepsis cardiovascular events.
Sensitivity analyses using broader exclusion of patients with any prior history of cardiovascular disease (cohort n = 9,683) yielded similar results, showing increased risk of cardiovascular events with increasing troponin I levels (Table E6). The exclusion of patients with intrasepsis cardiovascular diagnosis (cohort n = 6,847) showed increased risk for postsepsis cardiovascular disease in the highest tertile of troponin I (Table 2, Table E6). Sensitivity analysis using log-transformed troponin I as a continuous variable (cohort n = 14,046), complete case analysis (cohort n = 7,965), and analysis of all sepsis hospitalizations (cohort n = 39,590) using imputed troponin I (Table E7) showed similar results as the primary analysis.
Discussion
In a multicenter cohort of more than 14,000 patients without known cardiovascular disease who were hospitalized with sepsis, patients with troponin I elevations during sepsis had higher risks for subsequent cardiovascular complications—including myocardial infarction, stroke, atrial fibrillation, and heart failure—in the year following sepsis. The association between elevated troponin I levels and postsepsis cardiovascular events was similar across multiple sensitivity analyses, though the effect was modestly attenuated when excluding patients with intrasepsis cardiovascular events. In addition, the association persisted regardless of the patients’ presepsis predicted risk of ASCVD. Our findings identify modest elevations of troponin I—sometimes colloquially called “troponin leaks”—as a marker of risk for postsepsis hospitalization cardiovascular complications that impact sepsis recovery and may identify a group of patients to target for standardized postsepsis follow-up care with additional cardiac diagnostics and cardioprotective medication strategies.
Although prior studies have identified associations between sepsis and subsequent cardiovascular events (5, 22, 23), specific aspects of sepsis that may predict long-term cardiovascular complications are understudied. Frencken and colleagues evaluated 200 patients with sepsis in two Dutch ICUs and found troponin levels during sepsis to be associated with new prescriptions of cardiovascular medications, though whether these new prescriptions were associated with cardiovascular disease events was unclear (24). Our findings clarify the association of peak troponin I level during sepsis with risks of individual cardiovascular events in the year following sepsis.
Multiple mechanisms may explain the link between troponin elevations during sepsis and postsepsis cardiovascular complications. Troponin may be released during sepsis because of direct myocardial injury from inflammation or infection (25), oxygen supply and demand mismatch from preexisting coronary artery disease, microvascular dysfunction, decreased clearance from renal injury (23), or, more rarely, acute coronary thrombus. Any of these potential mechanisms of troponin elevation during sepsis may result in increased long-term risk of ASCVD events, arrhythmias, and heart failure. For example, chronic inflammation is implicated in ASCVD risk (22), and infection may precipitate an arrhythmogenic substrate or result in myocardial injury that could contribute to development of heart failure (23). Further studies should focus on elucidating the major mechanistic pathways to optimally target candidates for further cardiovascular screening, cardiology referrals, and preventative interventions.
Unlike prior studies (6, 8, 9), we did not identify associations between elevated troponin during sepsis and 1-year mortality rates after sepsis in adjusted analyses. Potential reasons for this difference with prior studies include highly granular covariate data in our study that allowed for greater adjustment for confounding variables. Additional differences included a lower acuity of illness in our study (i.e., patients in our study had a wide range of sepsis severity rather than only severe sepsis and shock in prior studies) and our focus on postdischarge—rather than in-hospital—deaths.
Strengths of the study include a large, diverse, and representative cohort of patients with a wide array of medical comorbidities, sepsis etiologies, and severity of illness. Long-term longitudinal multicenter evaluation of patient risk factors and diagnoses ranging from 5 years before sepsis, through sepsis, and up to 1 year after sepsis is often not possible without granular electronic health record data from an integrated healthcare system. Furthermore, we used methods to account for measured and unmeasured confounding and the competing risk of death and included sensitivity analyses that accounted for various missing data assumptions.
This study has several limitations. First, this is a retrospective analysis with missing data that was handled by multiple imputation techniques; although missing data may influence results, we found similar results across a range of complete case to imputed data analyses. Second, confounding by unmeasured clinical factors, such as occult coronary artery disease or undiagnosed structural heart disease, may also affect risk estimates; however, unmeasured confounders would need to have associations of HRs of 2–3 (associations stronger than the measured covariates) with the outcome and troponin to move effect estimates to the null. In addition, a sensitivity analysis with increasingly broad exclusion criteria for any prior cardiovascular disease showed similar associations between troponin I level and postsepsis cardiovascular events; the additional exclusion of patients with intrasepsis cardiovascular events resulted in a statistically significant association between troponin I level and postsepsis cardiovascular events only within the highest abnormal troponin tertile compared with normal troponin levels. Finally, we did not assess cardiovascular events beyond 1 year after sepsis hospitalizations; conclusions regarding long-term cardiovascular risks in this population remain unclear.
Conclusions
Among a cohort of more than 14,000 patients without known prior cardiovascular disease, modestly elevated troponin I levels during sepsis were associated with an increased risk of cardiovascular events in the year after sepsis hospitalization. Elevated troponin I during sepsis may be used in future prospective studies together with traditional cardiovascular risk factors to target high-risk patients for evaluation of postsepsis cardioprotective therapies.
Footnotes
Supported by funding from the National Institutes of Health National Heart, Lung and Blood Institute grants R01HL151607, R01HL139751, and R01HL136660; Agency of Healthcare Research and Quality grant R01HS026485; Boston Biomedical Innovation Center/NIH/NHLBI grant 5 U54HL119145–07; and royalties from UptoDate (all to A.J.W.).
Author Contributions: M.A.G., J.M.R., N.A.B., V.X.L., and A.J.W. were involved in conceptual design, data acquisition, analysis, interpretation, and manuscript preparation. K.K.T., Y.L., P.K., A.S.G., M.D., A.M., H.C., Y.D., and L.C.M. were involved in data acquisition, analysis, and interpretation. All authors listed have been involved in revising the manuscript for important intellectual content, approved the final version submitted for publication, and are in agreement with the accuracy and integrity of the submitted work.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202103-0613OC on May 26, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189:1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ou SM, Chu H, Chao PW, Lee YJ, Kuo SC, Chen TJ, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors. A nationwide population-based study. Am J Respir Crit Care Med. 2016;194:209–217. doi: 10.1164/rccm.201510-2023OC. [DOI] [PubMed] [Google Scholar]
- 6. John J, Woodward DB, Wang Y, Yan SB, Fisher D, Kinasewitz GT, et al. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270–275. doi: 10.1016/j.jcrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7. Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart Lung. 2015;44:75–81. doi: 10.1016/j.hrtlng.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8. Cheng H, Fan WZ, Wang SC, Liu ZH, Zang HL, Wang LZ, et al. N-terminal pro-brain natriuretic peptide and cardiac troponin I for the prognostic utility in elderly patients with severe sepsis or septic shock in intensive care unit: a retrospective study. J Crit Care. 2015;30:654, .e9–.e14. doi: 10.1016/j.jcrc.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 9. Vasile VC, Chai HS, Abdeldayem D, Afessa B, Jaffe AS. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am J Med. 2013;126:1114–1121. doi: 10.1016/j.amjmed.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 10. Eggers KM, Jernberg T, Lindahl B. Cardiac troponin elevation in patients without a specific diagnosis. J Am Coll Cardiol. 2019;73:1–9. doi: 10.1016/j.jacc.2018.09.082. [DOI] [PubMed] [Google Scholar]
- 11. Januzzi JL, Jr, McCarthy CP. Trivializing an elevated troponin: adding insult to injury? J Am Coll Cardiol. 2019;73:10–12. doi: 10.1016/j.jacc.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 12. Pierpont GL, McFalls EO. Interpreting troponin elevations: do we need multiple diagnoses? Eur Heart J. 2009;30:135–138. doi: 10.1093/eurheartj/ehn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 14. Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305:1113–1118. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4:566–573. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 20. Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 23. Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CCH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frencken JF, Donker DW, Spitoni C, Koster-Brouwer ME, Soliman IW, Ong DSY, et al. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11:e004040. doi: 10.1161/CIRCOUTCOMES.117.004040. [DOI] [PubMed] [Google Scholar]
- 25. Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Anzueto A, Babu BL, et al. Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med. 2017;196:609–620. doi: 10.1164/rccm.201701-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]