Abstract
Rationale: Racial residential segregation has been associated with worse health outcomes, but the link with chronic obstructive pulmonary disease (COPD) morbidity has not been established.
Objectives: To investigate whether racial residential segregation is associated with COPD morbidity among urban Black adults with or at risk of COPD.
Methods: Racial residential segregation was assessed using isolation index, based on 2010 decennial census and baseline address, for Black former and current smokers in the multicenter SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study), a study of adults with or at risk for COPD. We tested the association between isolation index and respiratory symptoms, physiologic outcomes, imaging parameters, and exacerbation risk among urban Black residents, adjusting for established COPD risk factors, including smoking. Additional mediation analyses were conducted for factors that could lie on the pathway between segregation and COPD outcomes, including individual and neighborhood socioeconomic status, comorbidity burden, depression/anxiety, and ambient pollution.
Measurements and Main Results: Among 515 Black participants, those residing in segregated neighborhoods (i.e., isolation index ⩾0.6) had worse COPD Assessment Test score (β = 2.4; 95% confidence interval [CI], 0.7 to 4.0), dyspnea (modified Medical Research Council scale; β = 0.29; 95% CI, 0.10 to 0.47), quality of life (St. George’s Respiratory Questionnaire; β = 6.1; 95% CI, 2.3 to 9.9), and cough and sputum (β = 0.8; 95% CI, 0.1 to 1.5); lower FEV1% predicted (β = −7.3; 95% CI, −10.9 to −3.6); higher rate of any and severe exacerbations; and higher percentage emphysema (β = 2.3; 95% CI, 0.7 to 3.9) and air trapping (β = 3.8; 95% CI, 0.6 to 7.1). Adverse associations attenuated with adjustment for potential mediators but remained robust for several outcomes, including dyspnea, FEV1% predicted, percentage emphysema, and air trapping.
Conclusions: Racial residential segregation was adversely associated with COPD morbidity among urban Black participants and supports the hypothesis that racial segregation plays a role in explaining health inequities affecting Black communities.
Keywords: COPD, health disparities, racial segregation, residential segregation, neighborhood
At a Glance Commentary
Scientific Knowledge on the Subject
Racial residential segregation has been associated with worse chronic disease outcomes in the United States, but the association of respiratory health and chronic obstructive pulmonary disease (COPD) is unknown.
What This Study Adds to the Field
Racial residential segregation is adversely associated with COPD morbidity among urban Black individuals. Some of the adverse associations are explained by other risk factors that are known to be linked with racial residential segregation, such as socioeconomic status, psychosocial stressors, comorbidities, and environmental exposure, but not all. There remains an unexplained association between racial residential segregation and COPD outcomes for urban Black individuals.
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease and is the third leading cause of death from chronic disease in the United States (1). Historically, the prevalence of COPD has been higher for white than for Black individuals, but recent studies suggest that Black individuals may suffer worse COPD morbidity, across a variety of measures (2–9). Several factors likely contribute to racial disparities, including biological factors (10), individual and neighborhood socioeconomic differences (11), and structural racism (12). In particular, racial residential segregation, defined as “the degree to which two or more [racial] groups live separately from one another, in different parts of the urban environment” (13), is a known risk factor for numerous health outcomes for Black individuals (14, 15). Racial residential segregation may lead to worse health status in chronic conditions via mechanisms including differential healthcare access (16, 17), environmental exposures (18), economic opportunities (11), political disenfranchisement (19), social isolation (20), and race-based discrimination and perceived stress (21). Although some studies have linked racial residential segregation to other respiratory-related outcomes (e.g., lung cancer and asthma) (22–25), to our knowledge, no studies have assessed the link between racial residential segregation and COPD morbidities, despite evidence suggesting worse COPD outcomes among Black compared with white individuals with COPD (2–9).
We hypothesize that racial residential segregation is a significant predictor of COPD morbidity for Black individuals in the United States. SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) is a multicenter U.S. cohort study focused on COPD health, with 19% recruitment of Black participants (26), and provides a valuable mix of detailed individual characterization in combination with neighborhood-level data. Geocoded data provides access to block group and tract-level census information in a subset of this study (SPIROMICS AIR [SPIROMICS Air Pollution Study]) (27), allowing insights into the association between racial segregation and COPD health.
Methods
Study Design
SPIROMICS AIR is an ancillary study of SPIROMICS (27), providing address-based geographic identification links for 2,924 participants across the United States, aged 40–80 years, current or former smokers (pack-years ⩾ 20), either with or without evidence of airflow obstruction. The current cross-sectional analysis was performed on baseline data collected between 2010 and 2015, with up to 3 years of follow-up exacerbation data, among those who self-reported as Black race, regardless of ethnicity (5.6% Hispanic). Healthy control subjects with ⩽1 pack-year smoking and participants who were missing a geographic identifier for the 2010 Census were excluded from the analysis. Participants residing in rural areas, based on census definition (28), were excluded because of low sample size (n = 3) and also because racial residential segregation has typically been operationalized within urban setting (13, 29), resulting in 515 Black individuals in the study sample (see Figure E1 in the online supplement).
Racial Residential Segregation
Racial residential segregation was measured with the isolation index, an established descriptor of segregation (13), which has been used in prior health studies (30–32) (see the online supplement for details). The isolation index for Black (“Black isolation index”) was calculated based on the 2010 decennial census (33) and measures the degree to which a Black resident is likely to be in contact with other Black residents (as opposed to members of other racial/ethnic groups) within his/her neighborhood or census tract (13). The index can range from 0 to 1, with “1” representing complete isolation or segregation of Black residents from other racial/ethnic groups within their neighborhoods.
Outcomes
Questionnaires included the COPD Assessment Test (CAT) (34), modified Medical Research Council (mMRC) scale (35), St. George’s Respiratory Questionnaire (SGRQ) (36), and ease of cough and sputum questionnaire (37). Physiologic data included FEV1% predicted (38) and 6MWD (6-minute-walk distance), performed and interpreted per American Thoracic Society standards (39). Radiographic outcomes included percentage emphysema (voxels < −950 HU on full inspiratory scan) (40) and gas trapping (voxels < −856 HU on full expiratory scan) (41) via quantitative computed tomography (CT) scan.
Participants were followed up to 3 years in the SPIROMICS I phase, with quarterly phone calls for exacerbation frequency, based on self-report of antibiotics/steroid use, unscheduled physician visits, and emergency department visit/hospitalization for COPD. Severe exacerbations were defined as events requiring emergency department visit or hospitalization.
Statistical Analysis
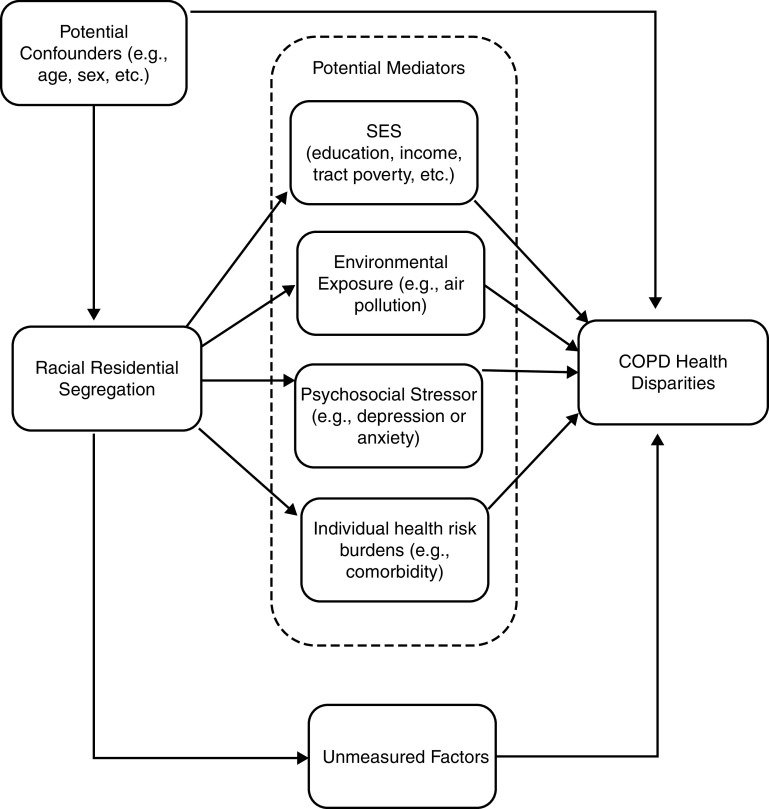
The Black isolation index at the participant’s census tract was modeled both dichotomously, as ⩾0.6 versus <0.6 (29, 30), and continuously per SD increase (0.32) to examine the association between racial residential segregation and COPD measures. Linear and negative binomial regressions of continuous and count respiratory outcomes were run respectively on the isolation index, adjusting for potential confounders: COPD status, age, sex, smoking status, pack-years, marital status, obesity, occupational exposure, and total population size of a tract. Mediation analysis was performed to estimate the direct and indirect (or mediated) association between racial segregation and COPD health according to a prespecified directed acyclic graph (Figure 1). The potential mediators consisted of participants’ individual and neighborhood socioeconomic status (SES; annual household income, educational attainment, and a composite score of neighborhood socioeconomic markers; see the online supplement for additional detail), comorbidity burden (42), and depression or anxiety (43). Analyses were additionally adjusted for ambient pollution as a potential mediator, but because <20% of the samples were missing pollutant information, multiple imputation analysis was incorporated and the analysis done separately (see the online supplement). The variables for air pollution consisted of ambient 1-year average ozone and particulate matter ⩽2.5 μm in aerodynamic diameter (PM2.5) concentrations, model-predicted pollutant concentrations outside the participant’s home over the course of 1 year (27). The recommended nonparametric bootstrap approach (44) was used to obtain the statistical inferences of the mediated effects and mediation proportion based on the 95% confidence interval (95% CI).
Figure 1.
Potential pathways between racial residential segregation and COPD health disparities. COPD = chronic obstructive pulmonary disease; SES = socioeconomic status.
As an additional sensitivity analysis, effect modification by COPD status on differences in outcomes by racial residential segregation was tested by running a multiplicative interaction model with an interaction term between COPD status and isolation index included in the adjusted model. An additional stratified model was run among participants with COPD only. Also, to explore the link between racial segregation and COPD morbidity for white individuals, the regression of COPD outcomes on isolation index (Black isolation) was run among white participants only (see the online supplement).
All analyses were performed using Stata, version 15.1 (Stata Corp). Statistical significance was defined as P < 0.05.
Results
The study population included 515 Black participants residing in 465 census block groups across 422 census tracts in 45 counties and 14 states (Figure E2). The median isolation index was 0.51 (interquartile range [IQR], 0.23–0.89) (at tract-level), ranging from 0.005 (least segregated) to 0.99 (most segregated). Nearly half of Black participants (n = 236, 46%) resided in tracts with an isolation score above 0.6—henceforth referred to as “segregated” neighborhoods. These neighborhoods had a smaller total population size (3,824 vs. 4,838; P < 0.001), higher poverty rate (25.0% vs. 17.9%; P < 0.001), lower median household income ($33,000 vs. $45,300; P < 0.001), and higher unemployment rate (9.0% vs. 6.6%; P < 0.001). There was no segregation difference in neighborhoods’ proportion of adults with less than a high school degree.
Participants had a mean age of 58.2 years, half (50%) were women, and the majority (64%) were current smokers (mean 41 pack-year smoking history). Approximately half (53%) had spirometry-confirmed airways obstruction (COPD) (Table 1). Less than a quarter (23%) were married, and less than half (44%) had education beyond high school. The mean (SD) follow-up days for prospective exacerbations was 768 (371) days. Black participants residing in segregated neighborhoods were more likely to be female, had a greater comorbidity burden, and had home addresses associated with higher concentration of ambient ozone than counterparts in nonsegregated tracts. There were no significant differences in age, educational attainment, income, marital status, occupational hazard, obesity, smoking status, pack-years, COPD status, and ambient PM2.5 level by residential segregation.
Table 1.
Characteristics of Study Participants (Black Participants with or at Risk of COPD)
| All (N = 515) | Isolation < 0.6: Nonsegregated (N = 279) | Isolation ⩾ 0.6: Segregated (N = 236) | P Value | |
|---|---|---|---|---|
| Demographic | ||||
| Age, yr | 58.2 ± 9.0 | 58.8 ± 8.9 | 57.5 ± 9.0 | 0.114 |
| Sex, F | 259 (50.3) | 120 (43.0) | 139 (58.9) | <0.001 |
| More than high school education | 228 (44.4) | 131 (47.1) | 97 (41.1) | 0.171 |
| Household Income | 0.186 | |||
| <$15,000 | 203 (39.4) | 106 (38.0) | 97 (41.1) | |
| $15,000–$34,999 | 101 (19.6) | 55 (19.7) | 46 (19.5) | |
| $35,000–$49,999 | 42 (8.2) | 22 (7.9) | 20 (8.5) | |
| $50,000–$74,999 | 35 (6.8) | 23 (8.2) | 12 (5.1) | |
| >$75,000 | 16 (3.1) | 13 (4.7) | 3 (1.3) | |
| Missing/declined to answer | 118 (22.9) | 60 (21.5) | 58 (24.6) | |
| Married | 117 (22.8) | 70 (25.2) | 47 (20.0) | 0.164 |
| Occupational exposure* | 270 (53.4) | 143 (52.4) | 127 (54.5) | 0.633 |
| Obese | 204 (39.6) | 117 (41.9) | 87 (36.9) | 0.241 |
| Comorbidity count† | 2.4 ± 1.5 | 1.9 ± 1.5 | 2.4 ± 1.5 | <0.001 |
| Depression or anxiety‡ | 188 (37.4) | 91 (33.6) | 97 (41.8) | 0.057 |
| Currently smoking | 325 (63.7) | 169 (61.5) | 156 (66.4) | 0.249 |
| Pack-years | 40.8 ± 17.2 | 40.1 ± 16.4 | 41.7 ± 18.1 | 0.313 |
| COPD | 273 (53.0) | 137 (49.1) | 136 (57.6) | 0.053 |
| Ambient pollution | ||||
| Ozone, 1-yr average concentration | 22.0 ± 4.6 | 21.4 ± 4.7 | 23.0 ± 4.2 | 0.001 |
| PM2.5, 1-yr average concentration | 10.4 ± 1.5 | 10.3 ± 1.8 | 10.4 ± 1.1 | 0.411 |
| Respiratory morbidity | ||||
| FEV1% predicted | 76.9 ± 27.5 | 81.3 ± 26.9 | 71.7 ± 27.4 | <0.001 |
| CAT | 16.1 ± 8.9 | 14.6 ± 8.7 | 17.8 ± 8.8 | <0.001 |
| mMRC | 1.2 ± 1.1 | 1.0 ± 1.0 | 1.4 ± 1.1 | <0.001 |
| SGRQ | 37.6 ± 21.7 | 33.4 ± 21.4 | 42.6 ± 21.1 | <0.001 |
| Cough and sputum | 9.9 ± 3.8 | 9.4 ± 3.7 | 10.4 ± 3.9 | 0.003 |
| 6MWD | 412.1 ± 132.3 | 409.3 ± 106.6 | 415.4 ± 157.7 | 0.614 |
| CT scan findings | ||||
| % Emphysema | 7.2 ± 10.6 | 6.1 ± 9.6 | 8.4 ± 11.6 | 0.013 |
| % Air trapping | 22.1 ± 22.8 | 19.7 ± 21.1 | 24.9 ± 24.5 | 0.011 |
| Exacerbations | ||||
| Any (number per year, prospective)§ | 0.50 ± 1.06 | 0.34 ± 0.75 | 0.69 ± 1.32 | <0.001 |
| Severe (number per year, prospective)ǁ | 0.26 ± 0.77 | 0.14 ± 0.32 | 0.40 ± 1.07 | <0.001 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; CT = computed tomography; mMRC = modified Medical Research Council; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter; SGRQ = St. George’s Respiratory Questionnaire.
Data are shown as n (%) or mean ± SD.
Bold values are statistically significant.
Occupational exposure to hazardous vapor, gas, dust, or fumes in the longest job held.
Comorbidity count based on yes/no answer to the following symptoms: asthma, cardiovascular heart disease, diabetes, cardiovascular heart failure, stroke, hypertension, gastroesophageal reflux disease, anemia, and sleep apnea.
Depression or anxiety symptom (dichotomous) based on the Hospitalization Anxiety Depression Scale with the cutoff of ⩾8 for either the Depression subscale or the Anxiety subscale.
“Any” exacerbation defined as the total number of oral antibiotics or steroid use due to adverse respiratory symptoms during the entire duration of the study. The rate of any exacerbation represented the yearly average (365 × total number of any exacerbations/total days in the study).
“Severe” exacerbation defined as the total number of hospitalizations or emergency room visits due to respiratory symptoms during the duration of the study. The rate of severe exacerbation represented the yearly average (365 × total number of severe exacerbations/total days in the study).
For respiratory outcomes, Black participants in segregated neighborhoods had worse lung function, worse clinical disease severity (CAT, mMRC, SGRQ, and cough and sputum), worse CT scan findings (emphysema and air trapping), and a higher rate of prospective exacerbations as compared with counterparts in nonsegregated neighborhoods. 6MWD showed no significant differences by residential segregation (Table 1).
Multivariable Regression
After adjusting for confounders, a one-SD increase in residential segregation (isolation index) was associated with more respiratory symptoms as measured by CAT (β = 1.38; 95% CI, 0.58 to 2.19), dyspnea score (mMRC; β = 0.16; 95% CI, 0.07 to 0.25), cough and sputum production (β = 0.82; 95% CI, 0.11 to 1.53), worse respiratory quality of life (SGRQ; β = 3.4; 95% CI, 1.5 to 5.3), and lower FEV1% predicted (β = −4.4; 95% CI, −6.2 to −2.5) (Table 2 and Figure 1). A one-SD increase in residential segregation was associated with a 36% higher rate of any exacerbation (odds ratio [OR], 1.36; 95% CI, 1.14 to 1.63) as well as a 51% higher rate of severe exacerbations requiring acute healthcare utilization (OR, 1.51; 95% CI, 1.21 to 1.89) over study follow-up. Furthermore, a one-SD increase in the isolation index was associated with worse CT measures, including higher percentage emphysema (β = 1.4; 95% CI, 0.7 to 2.1) and air trapping (β = 2.1; 95% CI, 0.5 to 3.7). There were no significant associations between residential segregation and 6MWD. The linearity assumption was satisfied for all outcomes; no statistically significant improvement in model fit was found with nonlinear polynomial model specifications for isolation index.
Table 2.
Adjusted Associations between Isolation Index and COPD Outcomes among Black Participants with or at Risk of COPD (N = 515)
| Segregation as Continuous Variable |
Segregated vs. Nonsegregated Neighborhoods |
|||
|---|---|---|---|---|
| Estimated Difference (95% CI) for a One-SD Increase* | P Value | Estimated Difference (95% CI) between Isolation ⩾0.6 and <0.6† | P Value | |
| Respiratory morbidity | ||||
| CAT | 1.38 (0.58 to 2.19) | 0.001 | 2.37 (0.76 to 3.97) | 0.004 |
| mMRC | 0.16 (0.07 to 0.25) | 0.001 | 0.29 (0.10 to 0.47) | 0.003 |
| SGRQ | 3.39 (1.49 to 5.28) | <0.001 | 6.09 (2.32 to 9.87) | 0.002 |
| Cough and sputum | 0.36 (0.01 to 0.71) | 0.041 | 0.82 (0.11 to 1.53) | 0.024 |
| FEV1% predicted | −4.37 (−6.21 to −2.53) | <0.001 | −7.28 (−10.93 to −3.63) | <0.001 |
| 6MWD, meters | 5.94 (−5.87 to 17.75) | 0.324 | 11.05 (−12.52 to 34.62) | 0.357 |
| Exacerbations‡ | ||||
| Any, IRR | 1.36 (1.14 to 1.63) | 0.001 | 1.70 (1.19 to 2.43) | 0.003 |
| Severe, IRR | 1.51 (1.21 to 1.89) | <0.001 | 2.04 (1.35 to 3.09) | 0.001 |
| CT scan findings | ||||
| % Emphysema | 1.41 (0.69 to 2.13) | <0.001 | 2.35 (0.84 to 3.85) | 0.002 |
| % Air trapping | 2.11 (0.54 to 3.67) | 0.008 | 4.53 (1.43 to 7.63) | 0.004 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CAT = COPD Assessment Test; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; IRR = incidence rate ratio; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
All models were adjusted for COPD status, age, sex, smoking status, pack-years, obesity, marital status, occupational exposure, and total population size. In the exacerbation models, participant total follow-up days in the study was specified as offset.
Bold values are statistically significant.
Predicted change in the level of respiratory morbidity/CT scan finding or the IRR of exacerbations for a one-SD (0.32) increase in the continuous isolation index score, adjusting for covariates.
Estimated mean difference in the level of respiratory morbidity/CT scan finding or the IRR of exacerbation between those residing in the segregated neighborhoods (isolation index ⩾0.6) and in the nonsegregated neighborhoods (isolation index <0.6), adjusting for covariates.
“Any” exacerbation defined as the total number of oral antibiotics or steroid use due to adverse respiratory symptoms during the duration of the study. “Severe” exacerbation defined as the total number of hospitalizations or emergency room visits due to adverse respiratory symptoms during the duration of the study. The effect estimates for exacerbation outcomes represent the IRR.
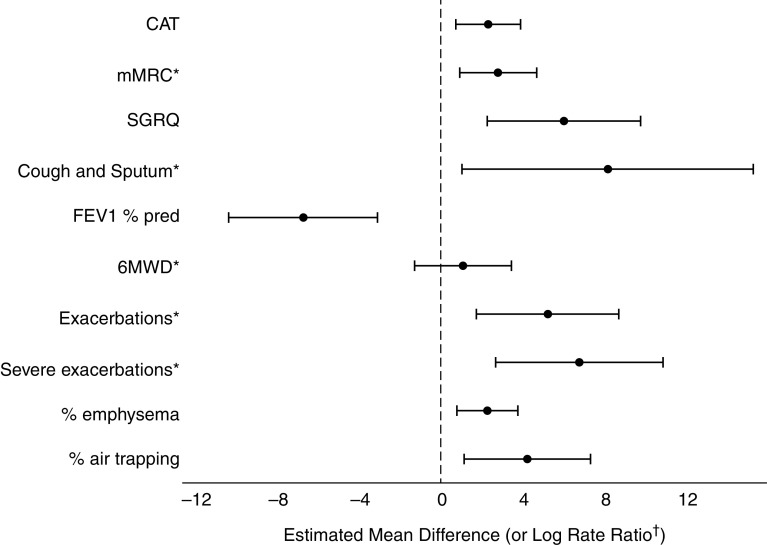
When using a dichotomized isolation index (⩾0.6 vs. <0.6), results were similar (Table 2 and Figure 2). Black participants residing in segregated tracts (vs. nonsegregated) had worse CAT and SGRQ scores, with effect sizes larger than the minimum clinically important difference for both measures (45, 46). Furthermore, those residing in segregated tracts had worse dyspnea, worse cough and sputum, and lower FEV1% predicted as well as worse percentage emphysema and air trapping. Those residing in segregated tracts had almost double the risk of any or severe exacerbations. Similarly, no significant segregation differences were found for 6MWD.
Figure 2.
Black participants with or at risk of chronic obstructive pulmonary disease living in segregated neighborhoods have worse chronic obstructive pulmonary disease outcomes than their counterparts living in nonsegregated neighborhoods. Above demonstrates the estimated mean difference (or the log incidence rate) and 95% confidence intervals of respiratory morbidity/computed tomography scan findings (or exacerbations) for Black participants residing in tracts with isolation ⩾0.6 (vs. isolation <0.6), adjusting for age, sex, smoking status, pack-years, obesity, marital status, occupational exposure, and total population size. In exacerbation models, participant total follow-up days in the study was specified as offset. *Estimated mean differences were rescaled to fit the chart: multiplied by 10 for mMRC, Cough and Sputum, any exacerbation (log rate), and severe exacerbation (log rate); multiplied by 1/10 for 6MWD. †For any exacerbation and severe exacerbation, the point estimate and 95% confidence interval represents the log rate ratio (multiplied by 10 to fit the chart’s scale). 6MWD = 6-minute-walk distance; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
Mediation Analysis
When additionally controlling for potential mediators—individual and neighborhood SES, comorbidity count, and depression or anxiety—the associations between segregation and COPD outcomes attenuated but generally remained significant (Table 3). The largest mediation was shown in cough and sputum score, representing 49.3% mediation of the segregation difference in the score between those residing in segregated (vs. nonsegregated) neighborhoods (Table 3). For all outcomes except CAT and cough and sputum, the effect estimates of segregation remained robust to the adjustment by the mediators (Table 3). When additionally adjusting for ambient pollution (1-year average ozone and PM2.5), there was further attenuation in the segregation effect estimates, where racial segregation was no longer statistically significantly associated with SGRQ and both exacerbation measures (Table E1). Ambient pollution and other mediators explained 54.6% of the segregation differences in SGRQ (βmediated = 3.3; 95% CI, 0.7–5.8) and 34.5% and 39.7% of any and severe exacerbation risk, respectively, although the effect estimates of mediation were themselves not statistically significant (Table E1). For dyspnea, lung function, percentage emphysema, and percentage gas trapping, the segregation differences persisted even with the additional adjustment by all measured mediators including ambient pollution (Table E1).
Table 3.
Mediation Analysis: Adjusted Associations between Dichotomous Isolation Index and COPD Outcomes among Black Participants, Additionally Adjusted for Potential Mediators
| Total* Associations: Estimated Difference (95% CI) between Isolation ⩾0.6 and <0.6† | Direct‡ Associations: Estimated Difference (95% CI) between Isolation ⩾0.6 and <0.6† | Mediated (Indirect)§ Associations: Estimated Difference (95% CI) between Isolation ⩾0.6 and <0.6† | Mediation Percentage: Mediated Association/ Total Associationǁ | |
|---|---|---|---|---|
| Respiratory morbidity | ||||
| CAT | 2.37 (0.76 to 3.97) | 1.43 (−0.10 to 2.96) | 0.94 (0.10 to 1.77) | 39.7% |
| mMRC | 0.29 (0.10 to 0.47) | 0.21 (0.03 to 0.39) | 0.07 (−0.02 to 0.16) | 25.4% |
| SGRQ | 6.09 (2.32 to 9.87) | 3.68 (0.30 to 7.06) | 2.41 (0.09 to 4.85) | 39.6% |
| Cough and sputum | 0.82 (0.11 to 1.53) | 0.42 (−0.29 to 1.12) | 0.40 (0.11 to 0.73) | 49.3% |
| FEV1% predicted | −7.28 (−10.93 to −3.63) | −5.86 (−9.60 to −2.11) | −1.42 (−3.02 to −0.06) | 19.5% |
| 6MWD, meters | 11.05 (−12.52 to 34.62) | 17.82 (−5.34 to 40.98) | −6.77 (−15.33 to 1.46) | −61.2% |
| Exacerbations¶ | ||||
| Any, IRR | 1.70 (1.19 to 2.43) | 1.60 (1.10 to 2.34) | 1.06 (0.91 to 1.31) | 11.6% |
| Severe, IRR | 2.04 (1.35 to 3.09) | 1.84 (1.16 to 2.93) | 1.11 (0.92 to 1.42) | 14.4% |
| CT scan findings | ||||
| % Emphysema | 2.35 (0.84 to 3.85) | 2.48 (0.84 to 4.12) | −0.13 (−0.65 to 0.40) | −5.7% |
| % Air trapping | 4.53 (1.43 to 7.63) | 4.54 (1.30 to 7.77) | −0.01 (−1.12 to 1.16) | −0.2% |
Definition of abbreviations: 6MWD = 6-minute-walk distance; CAT = COPD Assessment Test; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; IRR = incidence rate ratio; mMRC = modified Medical Research Council; SES = socioeconomic status; SGRQ = St. George’s Respiratory Questionnaire.
Bold values are statistically significant.
For total associations, all models were adjusted for COPD status, age, sex, smoking status, pack-years, obesity, marital status, occupational exposure, and total population size. In the exacerbation models, participants’ total follow-up days in the study was specified as offset.
Estimated mean difference in the level of respiratory morbidity/CT scan finding or the IRR of exacerbation between those residing in the segregated neighborhoods (isolation index ⩾0.6) and in the nonsegregated neighborhoods (isolation index <0.6), adjusting for covariates.
For direct association, all models were additionally adjusted for individual SES (education and income) and neighborhood SES (a composite score of neighborhood poverty rate, median household income, unemployment rate, and educational attainment), comorbidity count, and depression or anxiety. Comorbidity count was based on a yes/no answer to the following symptoms: asthma, cardiovascular heart disease, diabetes, cardiovascular heart failure, stroke, hypertension, gastroesophageal reflux disease, anemia, and sleep apnea. Depression or anxiety (dichotomous) was based on the Hospitalization Anxiety Depression Scale with the cutoff of ⩾8 for either the Depression subscale or the Anxiety subscale.
Mediated (or indirect) association is the difference between the total and direct associations and represents the mean difference in the level of respiratory morbidity/CT scan finding or the IRR of exacerbation between those residing in the segregated neighborhoods (isolation index ⩾0.6) and in the nonsegregated neighborhoods (isolation index <0.6) that is attributable to the mean differences in the mediating factors (SES, comorbidity, depression/anxiety) across the dichotomous isolation.
Mediation percentage is the ratio of mediated association to total association (or, equivalently, 1 − direct association/total association) in percentage terms and represents the proportional reduction in racial segregation’s association with COPD outcome from before and after the adjustment with the mediators.
“Any” exacerbation defined as the total number of oral antibiotics or steroid use due to worsening respiratory symptoms during the duration of the study. “Severe” exacerbation defined as the total number of hospitalizations or emergency room visits due to adverse respiratory symptoms during the duration of the study. Effect estimates for exacerbation outcomes represent the IRR.
Effect Modification by COPD Status
The segregation difference in FEV1% predicted was greater among those with COPD (βinteraction = −7.5; 95% CI, −14.5 to −0.7) compared with those without, whereas the segregation difference in the odds of any exacerbation was greater among those without (compared with those with) COPD (ORinteraction = 0.32; P = 0.003). For other outcomes, segregation differences were generally larger among individuals with COPD compared with those without, although the differences by COPD status were not statistically significant. The results among only those with COPD were largely consistent with those among the combined population including at-risk individuals (Table E2).
Racial Segregation and COPD Morbidity among White Participants
There were 1,783 white participants, among whom the median Black isolation index was 0.04 (IQR, 0.02–0.11), with 2% living in Black racially segregated neighborhoods. White residents living in Black racially segregated neighborhoods similarly had worse COPD morbidity for several outcomes, but the association of segregation and dyspnea, odds of any exacerbations, and percentage emphysema were smaller in magnitude and not statistically significant for white participants in comparison with Black participants (Table E3). The Black–white difference in segregation effect estimates were not statistically significant for any outcomes (Pinteraction > 0.05 for all).
Discussion
Although racial disparities in COPD outcomes have been reported previously (6, 8, 47), this is the first study that links racial residential segregation to worse COPD outcomes among Black adults in the United States. Racial residential segregation was associated with worse outcomes across a range of outcomes (subjective symptom, quality-of-life scores, spirometry, imaging findings, and COPD exacerbation rate) among Black participants with at least a 20 pack-year smoking history residing in urban areas in the United States. As in prior work demonstrating the impact of segregation on racial health disparities (12, 14), the adverse effect of residing in a segregated area was persistent even when adjusting for potentially confounding risk factors, including participants’ smoking status/pack-year history and occupational exposures. In addition, associations of racial segregation were partially mediated by factors such as SES, comorbidity burden, depression/anxiety, and ambient pollution; however, there continue to be unmeasured mechanisms by which racial residential segregation may associate with more severe respiratory outcomes.
The detrimental health effect of residential segregation has been documented in other health outcomes (14, 48) and, specifically, has been suggested to be associated with poor outcomes in other respiratory ailments, such as lung cancer (22, 23) and asthma risk (24, 49). For example, lung cancer mortality rates among Black adults in the United States are more than 10% higher in the most segregated areas (22). In our study, the SPIROMICS cohort of Black participants resided in tracts with a substantially higher isolation index (median, 0.51; IQR, 0.23–0.89) than the national median (0.05; IQR, 0.01–0.17)—based on 2010 decennial census data (33)—and higher levels of racial residential segregation associated with worse COPD outcomes consistently across morbidity measures: higher participant-reported respiratory symptoms and exacerbations, worse quality of life, lower lung function (FEV1% predicted), and higher severity of COPD-related CT measures. Furthermore, the differences in COPD metrics between those residing in high- versus low-segregation areas exceeded standards for clinical significance, specifically for symptoms and quality of life (45, 46), and reflected a greater than doubling in the risk of acute healthcare visit for severe COPD exacerbation. These results highlight the potential for the substantial adverse clinical impact of neighborhood racial segregation on respiratory outcomes related to COPD. Importantly, the results suggest that living in a predominantly Black racially segregated neighborhood has adverse associations even for white residents, although the literature is mixed regarding the impact of residential segregation on the white population (14, 50).
There are likely a number of contributing mechanisms by which racial residential segregation may associate with more severe respiratory outcomes in this population. Several of these potential factors such as lower SES, high environmental risk exposures (e.g., air pollution) (18, 51), and higher comorbidity and psychological burden may be along the causal pathway to worsened COPD morbidity. Notably, segregated neighborhoods had lower SES; however, the association of racial segregation with COPD outcomes persisted despite adjustment for both individual and a composite neighborhood SES score. Furthermore, participants in segregated neighborhoods had higher comorbidity burden compared with those residing in less segregated neighborhoods; however, differences in outcomes by racial residential segregation generally remained after adjustment for comorbidities and specifically for psychosocial burden as measured by depression and anxiety, suggesting that the presence of chronic disease burden is not sufficient to explain the effect of racial residential segregation on respiratory outcomes. Lastly, ozone and PM2.5 exposures are known to be linked with worse COPD outcomes (52), and we found that although PM2.5 concentrations were similar, ozone exposures were higher for participants in racially segregated areas compared with those in nonsegregated areas, with suggestion of nonlinearity between isolation index and ozone levels (Figure E3). However, worse respiratory outcomes (i.e., dyspnea, lung function, and emphysema and gas trapping risk) persisted in segregated neighborhoods despite adjustment for pollutants (ozone and PM2.5) in addition to SES factors and comorbidity burden.
Our research cannot determine all the pathways between racial residential segregation and COPD morbidity, as there are many unmeasured factors that are correlated with housing patterns in segregated communities, which may include access to medical care (16, 17), healthy food access (53), access to opportunities for a healthy lifestyle (11, 12), community health norms (11), and toxic stress and trauma (21). It is likely that the most segregated neighborhoods bear the greatest historical weight of systemic racism (29), and our results suggest that additional attention is needed to fully understand the impact of racial segregation on respiratory disease.
Our study has several limitations to consider. This study was focused on COPD disparities impacting Black residents across Black residential segregation. Further work is needed to characterize other minority residential segregations and their impact on the respective minority groups. Furthermore, the reference group for the isolation index is non-Black residents, and the impacts and mechanism of racial segregation estimates could vary depending on the racial/ethnic composition of the non-Black population in the census tract. There are several measures of residential segregation that may capture distinct dimensions of segregation and that may differentially impact disease outcomes (13). However, we used a validated metric (isolation index) widely used and particularly recommended in the health literature because the index is considered to best capture the potential pathway by which segregation can impact health via concentration of negative characteristics that correlate with geographic isolation for Black populations (20, 54). In addition, our data are limited to urban areas owing to the low number of Black participants residing in rural areas; however, it is possible that differences in the effects of racial segregation in rural or by geographic regions exist. Similarly, the sample size of the study limited our ability to assess regional differences. Future studies are needed to characterize segregation and its impact on rural communities, to assess potential regional differences, and to fully understand the multiple dimensions of segregation and mechanisms by which racial residential segregation may impact respiratory health.
This is the first study to demonstrate and quantify an adverse association between racial residential segregation and increased COPD morbidity and severity in the U.S. Black population. This study includes outcomes encompassing multiple domains of respiratory morbidity including quality of life, respiratory symptoms, functional status, and objective lung function and CT metrics. Our results suggest that other neighborhood SES measures, comorbidity burden and ambient pollution burden, do not explain a large component of the adverse association. With such insight, it is important that healthcare providers recognize that individuals residing in segregated or disadvantaged neighborhoods may have often unmeasured contextual factors contributing to disease risk. Furthermore, safeguarding the public's health may include identifying regions and neighborhoods that warrant an allocation of resources to assure a proactive attempt at mitigating health disparities in a manner that clinical interventions alone often cannot. Future interventional studies to reduce the racial disparity in COPD morbidity and severity need to consider the implications and strong associations between racial residential segregation and health for Black communities.
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. The authors also acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, M.D.; Wayne H. Anderson, Ph.D.; Mehrdad Arjomandi, M.D.; Igor Barjaktarevic, M.D., Ph.D.; R. Graham Barr, M.D., Dr.P.H.; Lori A. Bateman, M.Sc.; Surya P. Bhatt, M.D.; Eugene R. Bleecker, M.D.; Richard C. Boucher, M.D.; Russell P. Bowler, M.D., Ph.D.; Stephanie A. Christenson, M.D.; Alejandro P. Comellas, M.D.; Christopher B. Cooper, M.D., Ph.D.; David J. Couper, Ph.D.; Gerard J. Criner, M.D.; Ronald G. Crystal, M.D.; Jeffrey L. Curtis, M.D.; Claire M. Doerschuk, M.D.; Mark T. Dransfield, M.D.; Brad Drummond, M.D.; Christine M. Freeman, Ph.D.; Craig Galban, Ph.D.; MeiLan K. Han, M.D., M.S.; Nadia N. Hansel, M.D., M.P.H.; Annette T. Hastie, Ph.D.; Eric A. Hoffman, Ph.D.; Yvonne Huang, M.D.; Robert J. Kaner, M.D.; Richard E. Kanner, M.D.; Eric C. Kleerup, M.D.; Jerry A. Krishnan, M.D., Ph.D.; Lisa M. LaVange, Ph.D.; Stephen C. Lazarus, M.D.; Fernando J. Martinez, M.D., M.S.; Deborah A. Meyers, Ph.D.; Wendy C. Moore, M.D.; John D. Newell Jr., M.D.; Robert Paine, III, M.D.; Laura Paulin, M.D., M.H.S.; Stephen P. Peters, M.D., Ph.D.; Cheryl Pirozzi, M.D.; Nirupama Putcha, M.D., M.H.S.; Elizabeth C. Oelsner, M.D., M.P.H.; Wanda K. O’Neal, Ph.D.; Victor E. Ortega, M.D., Ph.D.; Sanjeev Raman, M.B.B.S., M.D.; Stephen I. Rennard, M.D.; Donald P. Tashkin, M.D.; J. Michael Wells, M.D.; Robert A. Wise, M.D.; and Prescott G. Woodruff, M.D., M.P.H.. The project officers from the Lung Division of the NHLBI were Lisa Postow, Ph.D., and Lisa Viviano, B.S.N.
Footnotes
SPIROMICS AIR was supported by the NIH/National Institute of Environmental Health Sciences (R01ES023500). SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici SpA, Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Nycomed GmbH, Takeda Pharmaceutical Company, Novartis Pharmaceuticals Corporation, Regeneron Pharmaceuticals, Inc., and Sanofi. Also supported by National Institute of Minority Health and Health Disparities grant DP50MD010431/Environmental Protection Agency grant 83615001 (N.N.H.), National Institute of Environmental Health Sciences grant K23ES029105 (E.P.B.), and NHLBI grant T32 HL007534 (C.E. and D.B.). The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. The U.S. Environmental Protection Agency does not endorse any products or commercial services mentioned in this publication.
Author Contributions: H.W., N.N.H., and J.D.K. were responsible for the concept, design, analysis, and interpretation of data. H.W., N.N.H., E.P.B., and K.A. were responsible for drafting of the manuscript. All authors contributed to data analysis, drafting, revision, and final approval of the version submitted for publication and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202009-3721OC on May 10, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1–77. [PubMed] [Google Scholar]
- 2. Gilkes A, Ashworth M, Schofield P, Harries TH, Durbaba S, Weston C, et al. Does COPD risk vary by ethnicity? A retrospective cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2016;11:739–746. doi: 10.2147/COPD.S96391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ejike CO, Woo H, Galiatsatos P, Paulin LM, Krishnan JA, Cooper CB, et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021;203:987–997. doi: 10.1164/rccm.202002-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarrabian B, Mirsaeidi M. A trend analysis of COPD mortality in the United States by race and sex. Ann Am Thorac Soc. 2021;18:1138–1146. doi: 10.1513/AnnalsATS.202007-822OC. [DOI] [PubMed] [Google Scholar]
- 5. Foreman MG, Zhang L, Murphy J, Hansel NN, Make B, Hokanson JE, et al. COPDGene Investigators. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184:414–420. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ejike CO, Dransfield MT, Hansel NN, Putcha N, Raju S, Martinez CH, et al. Chronic obstructive pulmonary disease in America’s black population. Am J Respir Crit Care Med. 2019;200:423–430. doi: 10.1164/rccm.201810-1909PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansel NN, Washko GR, Foreman MG, Han MK, Hoffman EA, DeMeo DL, et al. COPDGene Investigators. Racial differences in CT phenotypes in COPD. COPD. 2013;10:20–27. doi: 10.3109/15412555.2012.727921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisner MD, Blanc PD, Omachi TA, Yelin EH, Sidney S, Katz PP, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65:26–34. doi: 10.1136/jech.2009.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarrazin MV, Cannon KT, Rosenthal GE, Kaldjian LC. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc. 2009;101:656–662. doi: 10.1016/s0027-9684(15)30974-3. [DOI] [PubMed] [Google Scholar]
- 10. Celedón JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, et al. The transforming growth factor-β1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD) Hum Mol Genet. 2004;13:1649–1656. doi: 10.1093/hmg/ddh171. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WJ. 2012. [Google Scholar]
- 12. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 13. Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67:281–315. [Google Scholar]
- 14. Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiol Rev. 2009;31:178–194. doi: 10.1093/epirev/mxp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shavers VL, Fagan P, Jones D, Klein WM, Boyington J, Moten C, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102:953–966. doi: 10.2105/AJPH.2012.300773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. ‘Pharmacy deserts’ are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood) 2014;33:1958–1965. doi: 10.1377/hlthaff.2013.1397. [DOI] [PubMed] [Google Scholar]
- 18. Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, et al. Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Public Health. 2014;104:2130–2137. doi: 10.2105/AJPH.2014.302135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Purtle J. Felon disenfranchisement in the United States: a health equity perspective. Am J Public Health. 2013;103:632–637. doi: 10.2105/AJPH.2012.300933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shihadeh ES, Flynn N. Segregation and crime: the effect of black social isolation on the rates of black urban violence. Soc Forces. 1996;74:1325–1352. [Google Scholar]
- 21. Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18:1–10. doi: 10.3109/10253890.2014.989204. [DOI] [PubMed] [Google Scholar]
- 22. Hayanga AJ, Zeliadt SB, Backhus LM. Residential segregation and lung cancer mortality in the United States. JAMA Surg. 2013;148:37–42. doi: 10.1001/jamasurgery.2013.408. [DOI] [PubMed] [Google Scholar]
- 23. Fairley T, Tai E, Townsend J, Stewart S, Steele C, Davis S, et al. Racial/ethnic disparities and geographic differences in lung cancer incidence-38 States and the District of Columbia, 1998-2006. MMWR Morb Mortal Wkly Rep. 2010;59:1433–1438. [PubMed] [Google Scholar]
- 24. Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. Geographic variability in childhood asthma prevalence in Chicago. J Allergy Clin Immunol. 2008;121:639–645. doi: 10.1016/j.jaci.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 25. Bellin M, Osteen P, Collins K, Butz A, Land C, Kub J. The influence of community violence and protective factors on asthma morbidity and healthcare utilization in high-risk children. J Urban Health. 2014;91:677–689. doi: 10.1007/s11524-014-9883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansel NN, Paulin LM, Gassett AJ, Peng RD, Alexis N, Fan VS, et al. Design of the Subpopulations and Intermediate Outcome Measures in COPD (SPIROMICS) AIR Study. BMJ Open Respir Res. 2017;4:e000186. doi: 10.1136/bmjresp-2017-000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau 2018www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural.html.
- 29.Massey DS, Denton NA. 1993. [Google Scholar]
- 30. Hearst MO, Oakes JM, Johnson PJ. The effect of racial residential segregation on black infant mortality. Am J Epidemiol. 2008;168:1247–1254. doi: 10.1093/aje/kwn291. [DOI] [PubMed] [Google Scholar]
- 31. Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA, et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113:2166–2172. doi: 10.1002/cncr.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acevedo-Garcia D. Zip code-level risk factors for tuberculosis: neighborhood environment and residential segregation in New Jersey, 1985-1992. Am J Public Health. 2001;91:734–741. doi: 10.2105/ajph.91.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Census Bureau. 2010. https://www.census.gov/data/developers/data-sets/decennial-census.html
- 34. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 35. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 37. Rubin BK, Ramirez O, Ohar JA. Iodinated glycerol has no effect on pulmonary function, symptom score, or sputum properties in patients with stable chronic bronchitis. Chest. 1996;109:348–352. doi: 10.1378/chest.109.2.348. [DOI] [PubMed] [Google Scholar]
- 38. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 39. Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respiratory Soc. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 40. Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 44. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 45. Kon SS, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. doi: 10.1016/S2213-2600(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 46. Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 47. Dransfield MT, Bailey WC. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med. 2006;27:463–471, vii. doi: 10.1016/j.ccm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 48. Landrine H, Corral I. Separate and unequal: residential segregation and black health disparities. Ethn Dis. 2009;19:179–184. [PubMed] [Google Scholar]
- 49. Barnthouse M, Jones BL. The impact of environmental chronic and toxic stress on asthma. Clin Rev Allergy Immunol. 2019;57:427–438. doi: 10.1007/s12016-019-08736-x. [DOI] [PubMed] [Google Scholar]
- 50. Do DP, Frank R, Iceland J. Black-white metropolitan segregation and self-rated health: Investigating the role of neighborhood poverty. Soc Sci Med. 2017;187:85–92. doi: 10.1016/j.socscimed.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 51. Levy JI, Quirós-Alcalá L, Fabian MP, Basra K, Hansel NN. Established and emerging environmental contributors to disparities in asthma and chronic obstructive pulmonary disease. Curr Epidemiol Rep. 2018;5:114–124. doi: 10.1007/s40471-018-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paulin LM, Gassett AJ, Alexis NE, Kirwa K, Kanner RE, Peters S, et al. for SPIROMICS investigators. Association of long-term ambient ozone exposure with respiratory morbidity in smokers. JAMA Intern Med. 2020;180:106–115. doi: 10.1001/jamainternmed.2019.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bower KM, Thorpe RJ, Jr, Rohde C, Gaskin DJ. The intersection of neighborhood racial segregation, poverty, and urbanicity and its impact on food store availability in the United States. Prev Med. 2014;58:33–39. doi: 10.1016/j.ypmed.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health. 2003;93:215–221. doi: 10.2105/ajph.93.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]