To the Editor:
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial pneumonia of unknown origin that leads to severe fibrosis (1). Age is a primary risk factor in IPF because prevalence and incidence increase with age, and most of the hallmarks of aging have been described in IPF. Among those hallmarks, the accumulation of senescent cells reduces the lung’s regenerative potential and response to stress (2). Senescent cells show lower levels of proliferation, resist apoptosis, express increased levels of β-galactosidase, and secrete the senescence-associated secretory phenotype (SASP). In young individuals, the SASP directs the immune-mediated clearance of damaged cells and initiates tissue regeneration. However, the persistence of senescent cells can have unwanted side effects, namely chronic inflammation and organ fibrosis (3).
Natural killer (NK) cells are innate cytotoxic lymphocytes that clear senescent cells. Two distinct phenotypes of NK cells have been described: the cytotoxic CD56dimCD16+ or circulating NK (ci-NK) cells and the proinflammatory CD56brightCD16− or tissue-resident NK (tr-NK) cells (4). Possibly, aging of the immune system leads to inefficient clearance of senescent cells (5), and the SASP produces a dysfunctional immune microenvironment that permits these cells to evade the immune response. Our group aimed to investigate the proportion of NK cells in the lungs of patients with IPF. Because IPF disease pathology generally progresses from the lower lobes (IPF of the lower lobe [IPF-LL]) to the upper lobes (IPF of the upper lobe [IPF-UL]), we compared the NK-cell numbers in both lobes to investigate their roles in disease evolution. Some of the results of these studies have been previously reported in the form of an abstract (6, 7).
We collected tissue samples from IPF explants and rejected, healthy donor lungs. All IPF donors were nonsmokers, and control subjects were divided by age (subjects with IPF, 63.88 ± 6.88 yr; older subjects, 62.73 ± 6.56 yr; and younger subjects, 30.82 ± 8.98 yr). Blood samples were obtained from donors or from patients with IPF during their clinical follow-up. Fresh lung tissue samples were washed with phosphate-buffered saline and enzymatically digested as previously described (8), and peripheral blood mononuclear cells were isolated from heparin-collected blood. Parallel blood and lung tissue samples were stained with CD3–PE-Cy5.5, CD45–Alexa700, CD16–Brilliant Violet 421, and CD56–fluorescein isothiocyanate (BD Biosciences) and analyzed by using FlowJo software (FlowJo, LLC).
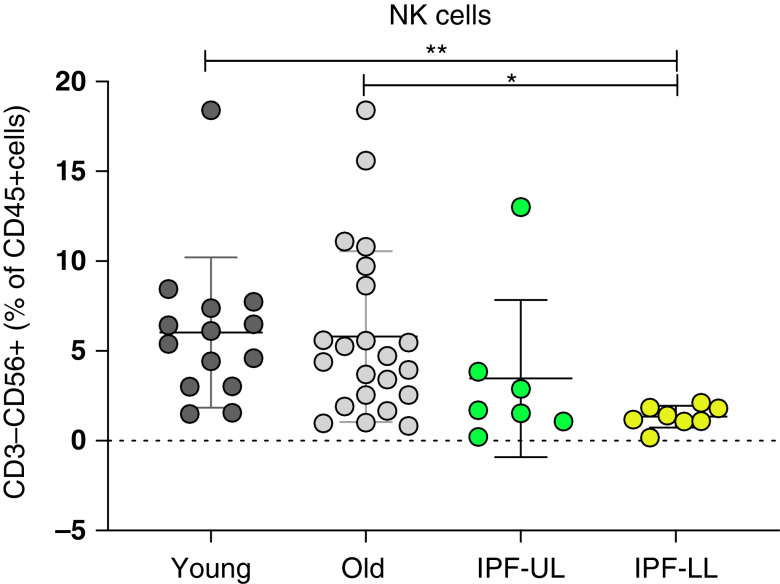
We reanalyzed the different immune-cell populations in single cell RNA sequence data from our previous publication (Gene Expression Omnibus series accession number 128033) (9), noting a profound decrease in the proportion of the NK-cell cluster in the lower lobe of the lungs in patients with IPF. We used flow cytometry to validate the decreased proportion of NK cells in IPF lungs. Total NK cells were determined as CD45+, CD3−, and CD56+ cells, and the different subpopulations of NK cells were characterized on the basis of the expression of CD56 and CD16. There was a significant reduction in the proportion of total NK cells in patients with IPF-LL compared with healthy control subjects, whereas patients with IPF-UL presented an intermediate phenotype (Figure 1). Characterization of the different NK-cell subpopulations showed an increase of the tr-NK cells and a reduction of the ci-NK cells in patients with IPF-LL compared with healthy control subjects. When we analyzed the NK cells in the blood of patients with IPF, we found the opposite phenotype—the proportion of total NK cells in the blood of patients with IPF was greater than in control subjects, with an increase in the proportion of ci-NK cells and a reduction in the proportion of tr-NK cells in patients with IPF being shown.
Figure 1.
The proportions of NK cells in fresh human lung tissue from healthy control subjects were determined and divided by age (younger subjects in dark gray [n = 14] and older subjects in light gray [n = 22]), the presence of IPF-UL (green, n = 8), and the presence of IPF-LL (yellow, n = 8). *P < 0.05 and **P < 0.01 by Kruskal-Wallis test with a post hoc comparison with correction for multiple tests (Dunn’s). IPF-LL = idiopathic pulmonary fibrosis of the lower lobe; IPF-UL = idiopathic pulmonary fibrosis of the upper lobe; NK = natural killer.
An analysis of single cell RNA sequence data of the NK-cell cluster showed markedly altered gene expression in NK cells from control versus IPF lungs as well as significant differences in NK cells from IPF-UL versus IPF-LL tissues (Table 1). IFN-γ was expressed more highly, in line with the increased proportion of proinflammatory tr-NK cells. Interestingly, the expression of IFNGR1 (IFN-γ receptor) was reduced, pointing toward an impaired capacity to respond to stimuli. We also detected altered expression of several IFN signaling pathway genes, which showed a predominantly reduced expression of the activator elements (IRF1) and an increased expression of the inhibitory genes (IRF4 and IRF8).
Table 1.
Differentially Expressed Genes in the Natural Killer–Cell Cluster
| Gene | IPF of Upper Lobe vs. Control |
IPF of Lower Lobe vs. Control |
|||
|---|---|---|---|---|---|
| logFC | Adjusted P Value | logFC | Adjusted P Value | ||
| IFNγ | IFNG | 0.961604 | 1.02 × 10−26 | 0.755063 | 0.428627 |
| IFNGR1 | −0.64487 | 1.13 × 10−141 | −0.37189 | 3.25 × 10−9 | |
| IRF1 | — | — | −0.28732 | 5.24 × 10−7 | |
| IRF4 | 0.268594 | 1.51 × 10−42 | — | — | |
| IRF8 | 0.784697 | 2.13 × 10−138 | 0.301498 | 5.57 × 10−5 | |
| Cytotoxicity | GNLY | 0.550427 | 9.85 × 10−58 | — | — |
| GZMA | −0.32322 | 2.89 × 10−22 | — | — | |
| GZMB | 1.426721 | 2.18 × 10−90 | −0.64957 | 0.011556 | |
| GZMH | 0.280219 | 1.29 × 10−10 | — | — | |
| GZMK | −0.35484 | 3.16 × 10−45 | 0.51977 | 9.09 × 10−38 | |
| GZMM | — | — | −0.28041 | 5.24 × 10−12 | |
| PRF1 | — | — | −0.27861 | 0.006536 | |
| ER stress | DNAJA1 | — | — | 0.288644 | 2.02 × 10−12 |
| DNAJB1 | −0.30486 | 1 | 0.93242 | 1.31 × 10−43 | |
| DUSP5 | 0.552546 | 1.87 × 10−100 | — | — | |
| HSP90AA1 | −0.27286 | 1 | 0.683977 | 2.80 × 10−32 | |
| HSPA1A | −0.62515 | 7.68 × 10−47 | 1.253565 | 2.56 × 10−44 | |
| HSPA1B | −0.35989 | 8.46 × 10−66 | 0.726816 | 1.02 × 10−21 | |
| XBP1 | — | — | −0.35802 | 5.26 × 10−38 | |
| HSPB1 | — | — | 0.372697 | 4.72 × 10−39 | |
| HSPH1 | — | — | 0.261661 | 2.59 × 10−18 | |
| HSP90AB1 | — | — | 0.317723 | 3.64 × 10−23 | |
| MT zinc metabolism | MT1A | −0.26348 | 3.40 × 10−8 | −0.29711 | 4.34 × 10−14 |
| MT1E | −0.3746 | 1.66 × 10−18 | −0.39028 | 7.47 × 10−13 | |
| MT1M | −0.30203 | 2.02 × 10−15 | −0.32251 | 3.82 × 10−10 | |
| MT1X | −0.67098 | 2.36 × 10−23 | −0.55898 | 0.04864 | |
| MT2A | — | — | −0.646 | 3.66 × 10−5 | |
Definition of abbreviations: ER = endoplasmic reticulum; FC = fold change; IPF = idiopathic pulmonary fibrosis; MT = metallothionein.
The FC was calculated with the expression by the patients with IPF versus that by control subjects, and a Wilcoxon rank sum test was used to determine the P value. Cells without data mean that the statistics were not significant.
Regarding genes involved in NK-cell cytotoxicity, we noted differential expression of the granzyme family genes when comparing NK cells from the upper and lower lobes. The IPF-UL NK cells expressed higher levels of granzyme B, the most potent NK-cell cytotoxic enzyme, whereas the IPF-LL NK cells expressed the less cytotoxic granzyme K and showed reduced expression of the main cytotoxic genes: granzyme B and perforin. This elevated expression of granzyme K accords with the recent publication of granzyme K–producing CD8 cells as an inflammaging hallmark (10). Thus, gene expression by IPF-LL NK cells indicated an aging phenotype with impaired cytotoxic capacity, whereas the IPF-UL NK cells showed gene expression similar to that of controls. The spliced form of Xbp1 has been described as an essential factor downstream of IL-15 in NK-cell proliferation and granzyme B production (11). IPF-LL NK cells expressed slightly lower levels of total Xbp1 and of the splice variant of Xbp1. This reduced level of Xbp1 could be one of the mechanisms leading to reduced expression of granzyme B by IPF-LL NK cells.
Endoplasmic reticulum stress–responding genes showed increased expression in IPF-LL and also, to a lesser extent, in IPF-UL. Furthermore, we identified a reduced expression of MT (metallothionein) enzymes, zinc metabolism enzymes whose deficiency has been linked to immunosenescence and premature aging (12).
Ingenuity pathways analysis (IPA) identified ULBP1 as a primary upstream regulator of IPF-UL (z-score, 2.00), indicating NK-cell activation through this specific pathway for the clearance of senescent cells. The IPA also indicated upregulation of pathways related to NK-cell activation (type I diabetes and reactive oxygen species production; Lg P values of 3.72 and 3.09). On the other hand, an IPA of IPF-LL NK cells indicated pathways related to liver inflammation or fibrosis (Lg P values of 2.46 and 2.96). These analyses point toward a shift in the IPF NK-cell phenotype from clearance of senescent cells in the upper lobe toward immune exhaustion in the lower lobe or with disease progression.
In this study, we have demonstrated that the number of lung NK cells and their activity is profoundly compromised in IPF lungs. This altered NK-cell response is more severe in the lower lobe, correlating with a more severe pathology. We observed the opposite tendency in IPF blood, which had an increased proportion of NK cells, specifically of ci-NK cells. These results may reflect an accumulation of NK cells in the blood due to impaired recruitment. In addition, the recruited NK cells have an altered phenotype toward immunosenescence as well as impaired activity, which may lead to an ineffective removal of senescent cells in IPF lungs.
Footnotes
Supported by grants 1 U01 HL14555-01, P50AR060780, and 5R01HL123766-04. D.V. was supported by P50CA097190. This project used the University of Pittsburgh Medical Center Cancer Biomarkers Facility: Luminex Core Laboratory, which is supported in part by award P30CA047904.
Originally Published in Press as DOI: 10.1164/rccm.202012-4418LE on June 2, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis. Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 4. Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34:573–582. doi: 10.1016/j.it.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013;12:1069–1078. doi: 10.1016/j.arr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz T, Jia M, Tabib T, Sembrat J, Cardenes N, Bruno T, et al. Impaired NK activity in the IPF lungs [abstract] Eur Respir J. 2020;56:3708. [Google Scholar]
- 7.Cruz Ta, Jia M, Tabib T, Sembrat J, Bondonese A, Cardenes N, et al. Impaired NK activity in the IPF lungs [abstract] Am J Respir Crit Care Med 2020201A3077. [Google Scholar]
- 8. Cruz T, López-Giraldo A, Noell G, Casas-Recasens S, Garcia T, Molins L, et al. Multi-level immune response network in mild-moderate chronic obstructive pulmonary disease (COPD) Respir Res. 2019;20:152. doi: 10.1186/s12931-019-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J. 2019;54:1802441. doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity. 2021;54:99–115, e12. doi: 10.1016/j.immuni.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Zhang Y, Yi P, Dong W, Nalin AP, Zhang J, et al. The IL-15-AKT-XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat Immunol. 2019;20:10–17. doi: 10.1038/s41590-018-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swindell WR. Metallothionein and the biology of aging. Ageing Res Rev. 2011;10:132–145. doi: 10.1016/j.arr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]