Abstract
SARS-COV-2 predominantly results in a respiratory illness. However, it has also been associated with a wide range of neurological disorders including a broad range of immune neuropathies. These immune neuropathies associated with SARS-COV2 infection include Guillain-Barré syndrome (GBS), recurrent GBS and exacerbation of pre-existing chronic inflammatory demyelinating polyneuropathy (CIDP). We describe a case with acute-onset CIDP presenting with three relapses of demyelinating polyradiculoneuropathy, the third relapse occurring in the 8 week of illness following a previous COVID-19 infection and a recent COVID-19 vaccination with ChAdOx1 nCoV-19 and high COVID-19 antibody level. In our knowledge, this is the ever reported case of acute-onset CIDP associated with COVID-19 vaccine and high COVID-19 antibody level.
Keywords: COVID-19, vaccination/immunisation, peripheral nerve disease
Background
Although COVID-19 predominantly has been associated with Guillain-Barré syndrome (GBS), recurrent GBS and worsening of chronic inflammatory demyelinating polyneuropathy (CIDP), we report a case of acute-onset CIDP (A-CIDP) in a patient post-COVID-19 infection and vaccination. COVID-19 is primarily a respiratory illness but may be associated with several various neurological symptoms. Mild neurological symptoms include headache, syncope, myalgia, anosmia and ageusia. Certain severe neurological illnesses associated with COVID-19 include encephalopathies, stroke, encephalitis, acute disseminated encephalomyelitis as well as other immune neuropathies like GBS,1 recurrent GBS2 and exacerbation of pre-existing CIDP.3 4 Additionally, human coronavirus 229E and OC43 have been linked to disorders like multiple sclerosis (MS), and murine coronavirus JHMV (JHM strain mouse hepatitis virus) which is likewise known to induce an MS-like disease in murine models.5
Acute inflammatory demyelinating polyneuropathy (AIDP) is characterised by a monophasic course, with a clinical peak within 4 weeks of disease onset.6 AIDP/GBS treatment-related fluctuation (TRF) is defined as an improvement in the neurological disability scale of at least one grade after completion of treatment with immunotherapy with either immunoglobulin or plasmapheresis, followed by a worsening of at least one grade on the disability scale within the first 2 months of symptom onset.7 Recurrence of AIDP/GBS is defined as two or more episodes meeting the criteria for GBS, with either a minimum interval of greater than 4 months between the episodes if the patient did not recover completely; or greater than 2 months, if there is near-complete or complete recovery after the original episode.7 CIDP is characterised by a slow and progressive course, with relapsing symptoms and gradual worsening over a period of more than 8 weeks.8 However, approximately 16% of patients with CIDP may present as acute-onset CIDP, which is characterised by a rapidly progressive onset within 8 weeks.9 The diagnosis of A-CIDP should be considered when a patient of GBS deteriorates again after 8 weeks from onset or when neurological deterioration occurs at least three times. We are presenting the first case of A-CIDP reported in association with COVID-19 disease and its vaccination.
Case presentation
A 47-year-old man with a 2-year history of well-controlled diabetes mellitus and hypertension presented to another hospital with a 2-day history of rapidly progressive pure motor-flaccid quadriparesis with bilateral facial weakness with inability to stand without support within 48 hours of disease onset. Patient had received treatment for COVID-19 Pneumonia with Computed tomography severity score (CTSS) 12/20, 7 months prior and had required remdesivir, steroids and low-molecular weight heparin. Patient had subsequently received the first dose of ChAdOx1 nCoV-19 vaccine 17 days prior to the current illness. No other recent precedent illness was reported. He was diagnosed as a case of acute inflammatory demyelinating polyradiculoneuropathy on nerve conduction velocity (NCV) study and was managed with a 5-day cycle of intravenous immunoglobulin with moderate and progressive recovery of acral motor power with ability to ambulate with single-arm support within 10 days of the immunoglobulin therapy.
Patient developed recurrence of weakness of lower limbs worse than upper limbs, with a prominent girdle sensation in mid dorsal region accompanied with a horizontal binocular diplopia in right gaze with partial right sixth nerve palsy 3 weeks after the first episode. Patient was observed to have bilateral infranuclear seventh nerve palsy, right sixth nerve palsy, along with flaccid, areflexic quadriparesis with flexor plantar reflexes and with impaired joint position sensation at toes. He was extensively evaluated for other causes of flaccid areflexic quadriparesis with cranial nerve involvement but found negative, except high level of anti-IgG antibody against COVID-19. Repeat NCV revealed worsening of predominantly demyelinating polyradiculoneuropathy with abnormal facial and blink reflexes and cerebrospinal fluid (CSF) showing albuminocytological dissociation. Patient was again managed with five cycles of intravenous immunoglobulin with a possible diagnosis of TRF.
Patient developed yet again the third episode of neurological worsening after another 4 weeks (8th week from onset of illness) with rapid worsening of right more than left leg weakness, worsening of grip strength and worsening of right facial motor status with severe girdle sensation in D4–D10 distribution. Examination revealed bilateral severe seventh nerve palsy (right >left), flaccid areflexic upper and lower limb weakness with importantly absent joint position sensation over toes with preserved exteroceptive sensations. In view of third episode of relapsing demyelinating polyradiculoneuropathy with progression into 8th week, a diagnosis of acute-relapsing CIDP rather than TRFs of GBS was considered and patient was managed with a repeat 5-day course of intravenous immunoglobulin and 1 mg/kg/day of oral prednisolone and azathioprine at 100 mg/day, resulting in marked improvement of infranuclear seventh nerve palsy and quadriparesis with resolution of the exteroceptive deficit and with no further relapses.
Investigations
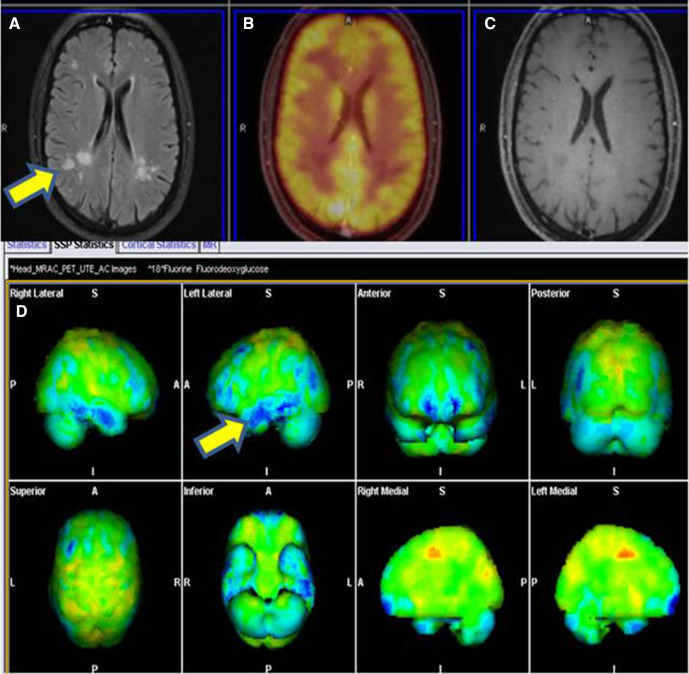
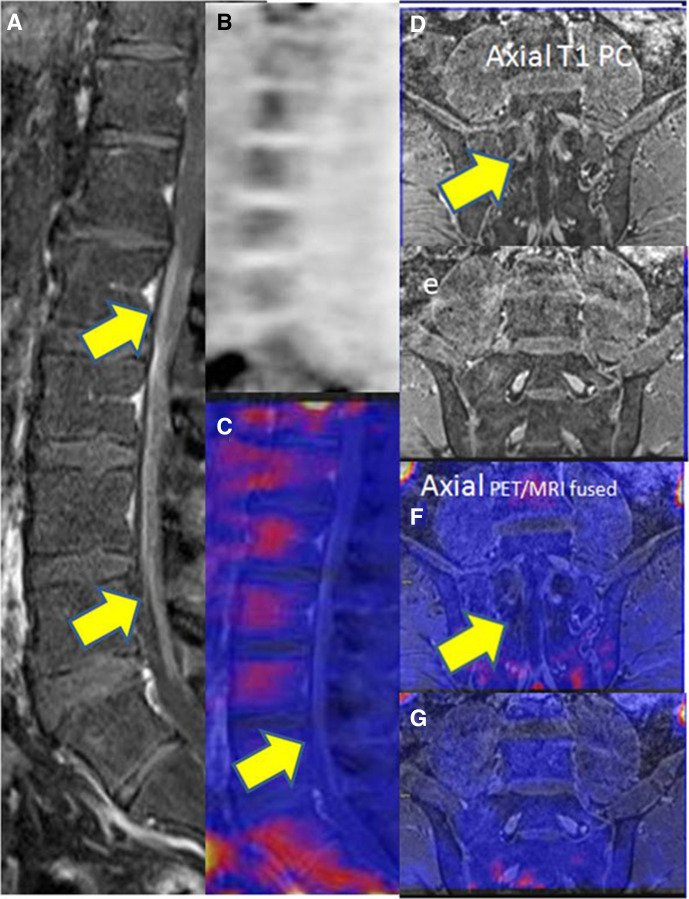
At the time of initial presentation to the hospital, patient had undergone NCV study, which was suggestive of acute inflammatory demyelinating polyradiculoneuropathy with prolonged distal latencies, dispersed Compound Motor Action Potential, reduced conduction velocity and absent F and H waves and maintained Sensory Nerve Action Potential. Urine porphobilinogen was negative. Serum protein electrophoresis revealed polyclonal gammopathy with no M spike. HIV ELISA 1 and 2 were negative. COVID-19 antibody IgG was extremely elevated (>400 AU/mL). Vasculitic profiles including Antinuclear Antibody (Immunofluorescence), Extractable Nuclear Antigen Antibodies (immunoblot), p-ANCA (perinuclear- anti-neutrophile cytoplasmic antibodies), cANCA (Cytoplasmic -Antineutrophile Cytoplasmic Autoantibody) were negative. At the time of first relapse, repeat NCV study revealed worsening of predominantly demyelinating polyradiculoneuropathy with abnormal facial and blink reflexes. CSF examination revealed albumin cytological dissociation with protein of 250 mg/dL and sugar of 106 mg/dL and 0 cells. CSF electrophoresis was negative for an oligoclonal band. MRI brain and spine were unremarkable except for non-specific ischaemic demyelination. Antiganglioside antibodies were negative. During the second relapse, repeat CSF revealed cytoalbuminic dissociation with protein of 120 mg/dL, sugar of 65 mg/dL with 0 cells. CSF lyme serology was negative and large volume CSF was negative for malignant cells. Contrast-enhanced MRI brain (figure 1) revealed discrete and confluent hyperintensities in white matter in T2-FLAIR (T2 weighted- Fluid -attenuated inversion recovery) image (A) with non-enhancing lesion in postcontrast images (C) and non-18-FDG (Flurodeoxyglucose) avid lesion in fused PETMRI (Positron Emission Tomography/Magnetic resonance Imaging) and relative frontal and temporal hypometabolism in FDG metabolic statistical images (B and D). Post contrast (figure 2) sagittal T1W (A) and axial (D and E) images revealed enhancing thickening of cauda equina nerve roots extending from L1 vertebral level and mild diffuse leptomeningeal enhancement up to the D11/12 level with no appreciable FDG uptake in PET image and in fused PET/MRI sagittal (C) and axial (F and G) images. Serum COVID-19 IgG antibody continued to be significantly elevated (>400 AU/mL). Sural nerve biopsy revealed no significant inflammatory infiltrate nor any suggestion of vasculitis.
Figure 1.
Contrast-enhanced MRI revealed discrete and confluent hyperintensities in white matter in T2-FLAIR image (A) with no enhancing lesion in post-contrast images (C) and no 18-FDG avid lesion in fused PET/MRI (B). FDG metabolic statistical images (D) showing relative hypometabolic areas in blue colour in frontal and temporal lobes.
Figure 2.
Post-contrast sagittal T1W (A) and axial (D and E) images showing enhancing thickening of cauda equina nerve roots extending from L1 vertebral level and mild diffuse leptomeningeal enhancement up to the D11/12 level with no appreciable FDG uptake in PET image (B) and infused PET/MRI sagittal (C) and axial (F and G) images.
Differential diagnosis
At first instance in view of sudden-onset areflexic quadriparisis, a diagnosis of AIDP or GBS was considered on the basis of the clinical profile and NCV study. Subsequently, after the first relapse, a possibility of TRF of GBS was considered and patient was managed accordingly. But after second relapse, diagnosis of acute-relapsing CIDP was evident.
Treatment
Each episode of polyradiculoneuropathy responded to intravenous immunoglobulin therapy at a dose of 0.4 gm/kg body weight per day for 5 days but only to relapse in the next 3–4 weeks. No further relapses occurred after considering the possibility of A-CIDP and introduction of 1 mg/kg/day of prednisolone and 100 mg/day of azathioprine.
Outcome and follow-up
Patient had progressive and near-complete recovery of the neurological deficit with no further relapses.
Discussion
Prior to COVID-19, GBS had been associated to an inflammatory response in response to multiple infections, including campylobacter jejuni and lyme disease. GBS is a commonly identified neurological complication of COVID-19. Zhao et al described the first care of GBS in a patient with SARS-CoV-2 infection.10 Almost expectedly, this was followed by multiple reports of GBS occurring either during the acute illness from SARS-CoV-2 infection or within a few weeks of the illness. Most of the cases were reported in the form of case reports or small case series. A systematic review of all published cases until 20 July 2020 included 73 patients reported in 52 publications. Mean age among the patients was 55 years with a range from 11 to 94 years with male predominance (68.5%). Most patients had respiratory and/or systemic symptoms and developed GBS manifestations following COVID-19. However, some patients were asymptomatic but had tested positive for SARS-CoV2. Although the clinical variants and electrophysiological subtypes resembled those of classic GBS less often seen variants like Miller Fisher syndrome were also reported. CSF albumin cytological dissociation was reported in around 71% cases, and SARS-CoV-2 RNA was absent in the CSF of all tested cases. Approximately, 70% of the patients showed a favourable response to immunoglobulin therapy. Older age was associated with a less favourable outcome. COVID-19-associated GBS seems to share the clinical profile as that of classic postinfectious GBS and possibly shares the same immune-mediated pathogenesis.1
A case of recurrent GBS, occurring secondary to COVID-19 infection, has also been described, wherein the patient had two prior flares of GBS, each episode being precipitated by a viral illness followed by complete recovery and a recent event following COVID-19 infection.2
CIDP is a relapsing-remitting or progressive inflammatory demyelinating polyneuropathy, which has a varied clinical presentation. CIDP can be a challenging diagnosis for physicians due to the heterogeneity of presentations, ranging from distal versus proximal onset, symmetric versus asymmetric onset and sensory versus motor variants.
Approximately, 16% of CIDP patients may present acutely simulating GBS and developing in less than 8 weeks. This entity is classified as acute-onset CIDP (A-CIDP) with initial presentation overlapping clinical and electrophysiological findings with GBS, but subsequently followed by a chronic course beyond 8 weeks. Patients with three or more TRFs are also included in this definition.11
Differentiating between A-CIDP from GBS is easy when GBS presents as a monophasic illness but is difficult when GBS is associated with relapses due to recurrences and TRFs following immunotherapy (immunoglobulins or plasma exchange). TRF needs to be differentiated from A-CIDP in order to guide treatment strategies without delay. In one series of 91 patients (AIDP n=77; A-CIDP n=14), the median ages were 55.5 years in patients with A-CIDP versus 43 years in AIDP (p=0.07). Diabetes mellitus was more common in patients with A-CIDP (29% vs 8%, p=0.04). No difference among the two groups was observed in respect of presence of underlying malignancy, autoimmune disorder or HIV. Cranial nerve, motor and autonomic nervous system involvement rates were equal in either groups. Patients in the A-CIDP group showed a greater tendency of disturbances of propioception (83% vs 28%; p<0.001), sensory ataxia (46% vs 16%; p=0.01) and the use of combined immunotherapy with corticosteroids (29% vs 3%; p=0.005). There were no significant differences among two groups in term of CSF abnormalities, intensive care unit admission, nor in the mortality rates. During the first 8 weeks, both the illness were practically indistinguishable. It was suggested that alterations in proprioception could be a red flag to suggest A-CIDP.
Our patient had three relapses of demyelinating polyradiculoneuropathy with the third episode occurring in the eighth week of illness, thus suggestive of a diagnosis of A-CIDP. Notably, patient had a history of diabetes and prominent posterior column symptoms as girdle sensations in mid-dorsal region and impaired proprioception in legs, which has been seen more commonly in A-CIDP. No other precedent illness except for recent COVID-19 vaccination within 3 weeks of the onset of illness was reported. Markedly elevated IgG COVID-19 antibody was identified possibly linking the association between the disease to the immunological process created by the combined effect of COVID-19 infection and the vaccination. neither aetiological cause for the A-CIDP including an underlying vasculitis nor a malignancy was identified. Neither previous cases of A-CIDP have been reported in association with COVID-19 infection nor with its vaccination. Two cases of exacerbation of pre-existing CIDP associated with COVID-19 infection, however, have been described.3 4
Patient’s perspective.
This disease came to me rapidly after COVID-19 vaccination and it paralysed me. I was fortunate to have an early diagnosis and treatment of my illness. Now, I have recovered fully and am thankful to the treating doctor.
Learning points.
Acute-onset chronic inflammatory demyelinating polyneuropathy (CIDP) associated with COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination is extremely rare and has not been reported before.
The potential association between exacerbations of neuroimmunological illnesses, especially immune-mediated neuropathies and SARS-CoV-2 should be speculated.
Clinician should be aware of the fact that SARS-CoV-2 infection or its vaccination may be a possible trigger factor for clinical worsening in immune-mediated polyneuropathies.
In order to avoid delay in treatment, an awareness about A-CIDP is mandatory to reach an early diagnosis.
Acknowledgments
Dr Raju Vaishya (senior consultant orthopaedics, Indraprastha Apollo Hospital, New Delhi) had been a great help in writing the case report.
Footnotes
Contributors: VS drafted the article, JS and SP helped in research work and conceptualisation. AJ reported the MRI and PET images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 2021;268:1133–70. 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonnell EP, Altomare NJ, Parekh YH, et al. COVID-19 as a trigger of recurrent Guillain–Barré syndrome. Pathogens 2020;9:965. 10.3390/pathogens9110965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Looy E, Veenker L, Steyaert A, et al. COVID-19-induced exacerbation of chronic inflammatory demyelinating polyneuropathy. J Neurol 2021;268:3129–31. 10.1007/s00415-021-10417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Rumeileh S, Garibashvili T, Ruf W, et al. Exacerbation of chronic inflammatory demyelinating polyneuropathy in concomitance with COVID-19. J Neurol Sci 2020;418:117106. 10.1016/j.jns.2020.117106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan A, Johnson RT. Infections and multiple sclerosis. Handb Clin Neurol 2014;122:151–71. 10.1016/B978-0-444-52001-2.00007-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Meché FG, Van Doorn PA, Meulstee J, et al. Diagnostic and classification criteria for the Guillain-Barré syndrome. Eur Neurol 2001;45:133–9. 10.1159/000052111 [DOI] [PubMed] [Google Scholar]
- 7.Kuitwaard K, van Koningsveld R, Ruts L, et al. Recurrent Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 2009;80:56–9. 10.1136/jnnp.2008.156463 [DOI] [PubMed] [Google Scholar]
- 8.Van den Bergh PY, Hadden RD, Bouche P. Eur J Neurol. 2010, 17:356-63. Eur Neurol 2001;45:133–9. [DOI] [PubMed] [Google Scholar]
- 9.Ruts L, van Koningsveld R, van Doorn PA. Distinguishing acute-onset CIDP from Guillain-Barré syndrome with treatment related fluctuations. Neurology 2005;65:138–40. 10.1212/01.wnl.0000167549.09664.b8 [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020;19:383–4. 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alessandro L, Pastor Rueda JM, Wilken M, et al. Differences between acute-onset chronic inflammatory demyelinating polyneuropathy and acute inflammatory demyelinating polyneuropathy in adult patients. J Peripher Nerv Syst 2018;23:154–8. 10.1111/jns.12266 [DOI] [PubMed] [Google Scholar]