Abstract
Objective
Diabetic peripheral neuropathy (DPN) is one of the most important risk factors of diabetic foot ulcers, and early screening and treatment of DPN are crucial. The Ipswich Touch Test (IPTT) is a new method for screening for DPN and, compared with traditional methods, is more simple to operate and requires no equipment. However, the screening accuracy of IPTT in patients with DPN has not been well characterised. We aim to conduct a systematic review and meta-analysis to characterise the sensitivity and specificity of IPTT compared with traditional methods and to understand the potential screening value of IPTT.
Design
Systematic review and meta-analysis.
Data sources
PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang, Chinese Biomedical Literature Database up to 16 April 2020.
Methods
Stata V.15.1 software was used for analysis, and the screening value of IPTT in DPN was described using 10 g monofilament (10g-MF), neuropathy disability scores (NDS), Pin prick, 128 Hz tuning fork, and ankle reflex as reference standards. Sensitivity, specificity and other measures of accuracy of IPTT for screening DPN were pooled based on a quality effects model. The protocol was registered with PROSPERO (42020168420).
Results
Of the 441 records retrieved, 7 studies were evaluated for the screening value of IPTT. Five studies with 10g-MF as the reference standard were included in the meta-analysis, and the pooled sensitivity and specificity were 0.77 (95%CI 0.69–0.84) and 0.96(95%CI 0.93–0.98), respectively, and the area under curve was 0.897. Compared with vibration perception threshold, IPTT showed a sensitivity between 0.76 and 1, and a specificity between 0.90 and 0.97. Compared with NDS, IPTT showed a sensitivity between 0.53 and 1, and a specificity between 0.90 and 0.97. Compared with Pin prick, IPTT showed a sensitivity and specificity of 0.8 and 0.88, respectively. Compared with 128 Hz tuning fork, IPTT showed a sensitivity and specificity of 0.4 and 0.27, respectively. Compared with ankle reflex, IPTT had a sensitivity of 0.2 and a specificity of 0.97.
Conclusions
IPTT shows a high degree of agreement with other commonly used screening tools for DPN screening. It can be used clinically, especially in remote areas and in primary medical institutions, and by self-monitoring patients. More high-quality studies are needed to assess and promote more effective screening practices.
PROSPERO registration number
Registration Number is CRD (42020168420).
Keywords: diabetic neuropathy, public health, diabetic foot
Strengths and limitations of this study.
This is the first meta-analysis to explore the potential screening value of Ipswich Touch Test (IPTT) in diabetic peripheral neuropathy (DPN).
A quality effects model was used to achieve optimal error estimation in the data analysis.
A Doi plots and Luis Furuya-Kanamori index were used to assess publication bias.
Although we systematically and comprehensively studied the current evidence of IPTT screening in DPN screening, the number of original studies was very limited, and the existing conclusions were based on these seven original studies. Therefore, readers should therefore proceed with caution.
Introduction
Diabetic peripheral neuropathy (DPN) is a common long-term complication and the most important risk factor for the occurrence of diabetic foot ulcers (DFU).1–4 DPN affects up to 50% of people with diabetes,5 6 with chronic painful neuropathy affecting up to 26%.7 In the early stage of DPN, the symptoms lack specificity, and about half of patients with diabetes cannot recognise the injury to the lower extremities.8 9 Once the patient has symptoms such as limb numbness and pain, it signals that pathological changes have occurred in the peripheral nerves and have advanced into the irreversible stage. If not treated promptly, serious tissue damage, such as foot ulcers, amputation, and even death, may occur.10 11 Studies have shown that early screening and detection of peripheral neuropathy can not only slow down the DPN process, but also effectively prevent DFU.12 Therefore, early screening and treatment of DPN is very important.
At present, the screening value of 10 g monofilament (10g-MF), vibration perception threshold (VPT), and 128 Hz tuning fork in DPN has been widely recognised.13 Compared with VPT and 128 Hz tuning forks, 10g-MF is the most widely used screening tool because it is more simple, objective and easy to carry, although it requires a calibration facility to confirm that the vertical pressure of the monofilament used when bending is 10 g.14–16 Commercially available 10g-MF devices exhibit significant variability within and between devices of different manufacturers and their actual bending force varies widely from their designated 10 g value. When used they have a short service life where the instrument is within 10% of their initial bending force which is not usually the stated 10 g of force.17 18 Meanwhile, medical personnel are required to be trained before using the device, and screening is limited to hospitals or clinics. For clinics and communities in remote areas, medical personnel may lack the device or the training to screen patients for DPN, resulting in a missed opportunity for patients to receive the best treatment. In recent years, Dr. Rayman proposed the Ipswich Touch Test (IPTT), which only requires the physician’s index finger. During this test, the patient is required to close their eyes while the physician lightly rests their finger on each of the patient’s first, third, and fifth toes for 1 to 2 s. Patients are instructed to respond with a ‘yes’ when they feel the physician’s touch. Compared with the current methods, IPTT requires no equipment, is more convenient and effective, and can be performed by doctors, nurses and even family caregivers after training.19 IPTT can be applied to inpatients, outpatients, community patients, self-monitoring patients at home, and to areas lacking more advanced equipment.20 21 Currently, IPTT has been applied in the UK, Spain, Brazil and Saudi Arabia,19 22–26 and was approved by the American Diabetes Association in 2015.20 The 2019 guidelines of the International Working Group on the Diabetic Foot also suggest that IPTT should be used for DPN screening in patients with diabetes in the absence of 10g-MF.27 Although these studies have achieved satisfactory results, they have not been widely promoted and applied globally. Previous studies have reported differences in the results of the screening value of DPN. However, neither a meta-analysis nor a systematic review has been conducted on the screening value of IPTT.
In this study, we aimed to conduct a comprehensive and systematic literature review to systematically evaluate the potential screening value of IPTT in DPN, and provide evidence and guidance for the clinical application value of IPTT.
Methods
The Joanna Briggs Institute protocol28 has been registered with PROSPERO, the International Prospective Register of Systematic Reviews hosted by the Centre for Reviews and Dissemination (Registration Number is CRD (42020168420)). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.29
Data sources and searches
We systematically searched PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang Data, Chinese Biomedical Literature Database for reports published before 16 April 2020. For included studies with insufficient data, we emailed the authors to ask if they would provide data for our study. With this strategy, we combined search terms for applied technique (Ipswich Touch Test, touch test, IPTT) and disease (DPN, diabetic foot, DFU, diabetes mellitus, diabetic complications). The study design and published language were not limited. In addition, we conducted a manual search, including searching through conference papers and grey literature, and the references of all included studies were examined. All search strategies were determined by multiple pre-searches, and the search formulas were adjusted according to the characteristics of each database. A detailed search strategy is provided in online supplemental file 1. All analyses were based on previously published studies; thus, no ethical approval and patient consent were required.
bmjopen-2020-046966supp001.pdf (128.9KB, pdf)
Inclusion and exclusion criteria
Previously published studies were included in this meta-analysis if: (1) the study examined the screening accuracy of the IPTT test for detecting DPN; (2) all the research subjects were patients with diabetes, and; (3) IPTT was included as an index test. Studies were excluded from the meta-analysis if the studies had incomplete data sets or were other than original reports (commentaries/reviews). The age, sex, region, and race of the subjects were not restricted. The published language was not limited.
Data extraction and quality assessment
We imported initial search records from databases into NoteExpress V.3.2.0.7535 literature management software. Two reviewers independently screened titles and abstracts of all the included literature, following the inclusion and exclusion criteria. After screening the abstract, the full text was read in detail. Any discrepancies were resolved by discussion. The following information was extracted from the eligible studies: study characteristics (author, publication year, study period, country, reference standard, setting, operators), participant characteristics (sample number, range), and outcome indicators (sensitivity, specificity, true positive number, false positive number, false negative number, true negative number). Missing data were supplemented by contacting authors wherever possible.
The quality of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS); it is a methodological quality assessment scale, and includes 14 items.30 Quality items were weighted equally with 1 point awarded for each of the 14 items. The quality score was then calculated by summing the points awarded for each question (maximum sum 14). This score was then normalised by dividing the sum by the highest score of the listed studies, thereby ranking the studies from 1 down to a minimum of 0.31 Data extraction and quality assessment were performed independently by two reviewers. Differences were reconciled through discussion until a consensus was reached on the item in question.
Data synthesis
We calculated sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR), a value of pooled PLR greater than 10 and of pooled NLR less than 0.1 were noted as providing convincing diagnostic evidence. For each summary statistic, a 95% CI was computed, and the sensitivity, specificity, PLR, NLR, DOR and corresponding 95% CI were obtained using the quality effects model under the split component synthesis method framework.32 33 Relevant studies have proven that the quality effects model is superior to the traditional random effects model.34–36 The quality scores were used to redistribute inverse variance weights based on study deficiencies via the quality effects model,37 38 and analyses were conducted using Stata, V.15.1 (Stata Corp).
Since the number of studies included affects the Q test, we used the I2 statistic to evaluate the magnitude of heterogeneity since the value of the I2 statistic will not change with the number of studies included and the results of heterogeneity test are more reliable. An I2 ≥50% indicates the existence of significant heterogeneity.39 40 Publication bias was assessed with Doi plots and Luis Furuya-Kanamori (LFK) index, the Doi plot uses a rank-based measure (Z score) of precision (instead of the SE) and plots it against the effect size, it can visualise asymmetry, and the LFK index quantifies the extent of Doi plot asymmetry by averaging half of the sum of the Z score plus the normalised effect size across the meta-analysis, which can detect and quantify the asymmetry in the Doi plots. The closer the value of the LFK index is to zero, the more symmetrical the Doi plot.41 LFK index values outside the interval of −1 and +1 are deemed consistent with asymmetry.41 Related studies have shown that these methods can markedly improve the ability of researchers to detect bias in a meta-analysis.41
Patient and public involvement
Since the data in this study were all from previously published studies, patients and the public were not involved in this research.
Results
Study selection
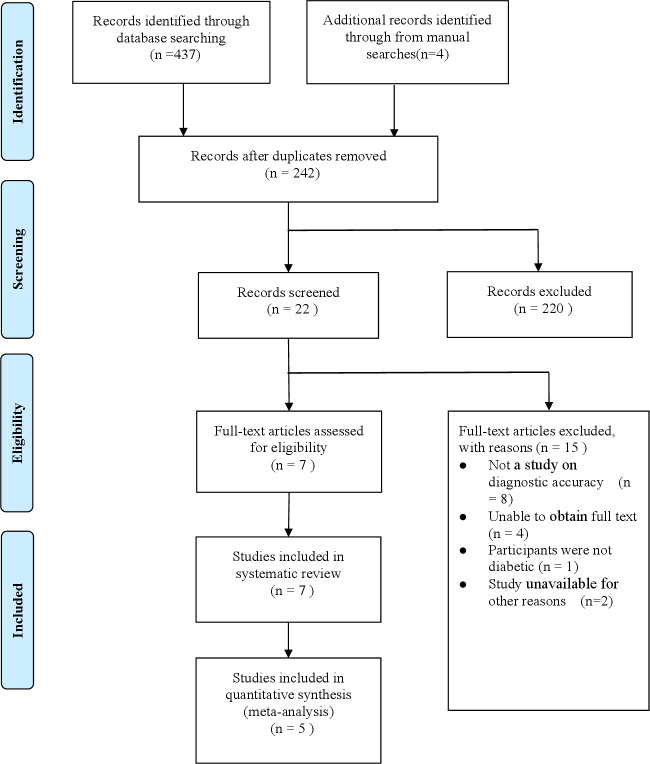
Our initial search resulted in a total of 441 records: 437 from database searching and four records from manual searches of references. After duplicates were removed, 242 records were identified, and 220 records were excluded as irrelevant. After reading the full-text articles, seven studies met the inclusion criteria (figure 1). Two studies were excluded for lacking necessary data for meta-analysis. Finally, 5 studies with 6 datasets were included in the final meta-analysis, involving a total of 1162 patients.22–26
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Characteristics and quality of the included studies
The characteristics of the included studies are presented in table 1. The seven studies included a total of 1510 participants with diabetes and were published between 2011 and 2020.19 22–27 To explore the accuracy of IPTT in DPN screening, 10g-MF, VPT, neuropathy disability scores (NDS), pin prick, 128 Hz tuning fork, and ankle reflex were used as the reference standard. The research setting included homes of patients, clinics, care centres and outpatient centres, and the assessors included doctors, nurses and family caregivers.
Table 1.
Basic characteristics of the included studies
| Study | Year | Country | N | Setting | Operators | Quality score (Qi) | Reference standard | TP | FP | FN | TN | Se (%) | Sp (%) |
| Sharma et al22 | 2012 | UK | 130 | Home | Families | 0.074 | 10g-MF | 24 | 4 | 6 | 96 | 80.0 | 96.0 |
| Sharma et al23 | 2014 | UK | 331 | Home | Families | 0.714 | 10g-MF | 65 | 15 | 18 | 233 | 78.3 | 93.9 |
| Clinic | Doctors/nurses | 0.786 | 10g-MF | 67 | 9 | 16 | 239 | 81.2 | 96.4 | ||||
| Amal Madanat et al24 | 2015 | Saudi Arabia | 351 | Care centres | Doctors/nurses | 0.357 | 10g-MF | 29 | 6 | 28 | 288 | 51.0 | 98.0 |
| VPT | 48 | 24 | 9 | 270 | 85 | 92 | |||||||
| NDS | 30 | 9 | 27 | 285 | 53 | 97 | |||||||
| Basir et al25 | 2020 | Spain | 100 | Care centres | Doctors/nurses | 0.429 | 10g-MF | 4 | 30 | 1 | 65 | 80.0 | 68.0 |
| Pin prick | 4 | 1 | 11 | 84 | 80.0 | 88.0 | |||||||
| Tuning fork 128 Hz | 2 | 3 | 69 | 26 | 40.0 | 27.0 | |||||||
| Ankle reflex | 1 | 4 | 2 | 93 | 20.0 | 97.0 | |||||||
| Dutra et al26 | 2020 | Brasília | 250 | Outpatient centre | Doctors/nurses | 0.643 | 10g-MF | 30 | 5 | 6 | 209 | 83.3 | 97.7 |
| Rayman et al19 | 2011 | UK | 265 | Clinic | – | – | VPT | – | – | – | – | 76.0 | 90.0 |
| Bowling et al27 | 2012 | UK | 83 | Clinic | Doctors/nurses | – | VPT | – | – | – | – | 100 | 96.6 |
| NDS | – | – | – | – | 100 | 90.3 |
FN, false negative number; FP, false positive number; 10g-MF, 10 g monofilament; NDS, neuropathy disability scores; SE, sensitivity; Sp, specificity; TN, true negative number; TP, true positive number; VPT, vibration perception threshold.
We assessed the methodological quality of the studies using QUADAS. The assessment results of the research methodological quality of each study are presented in figure 2.42
Figure 2.
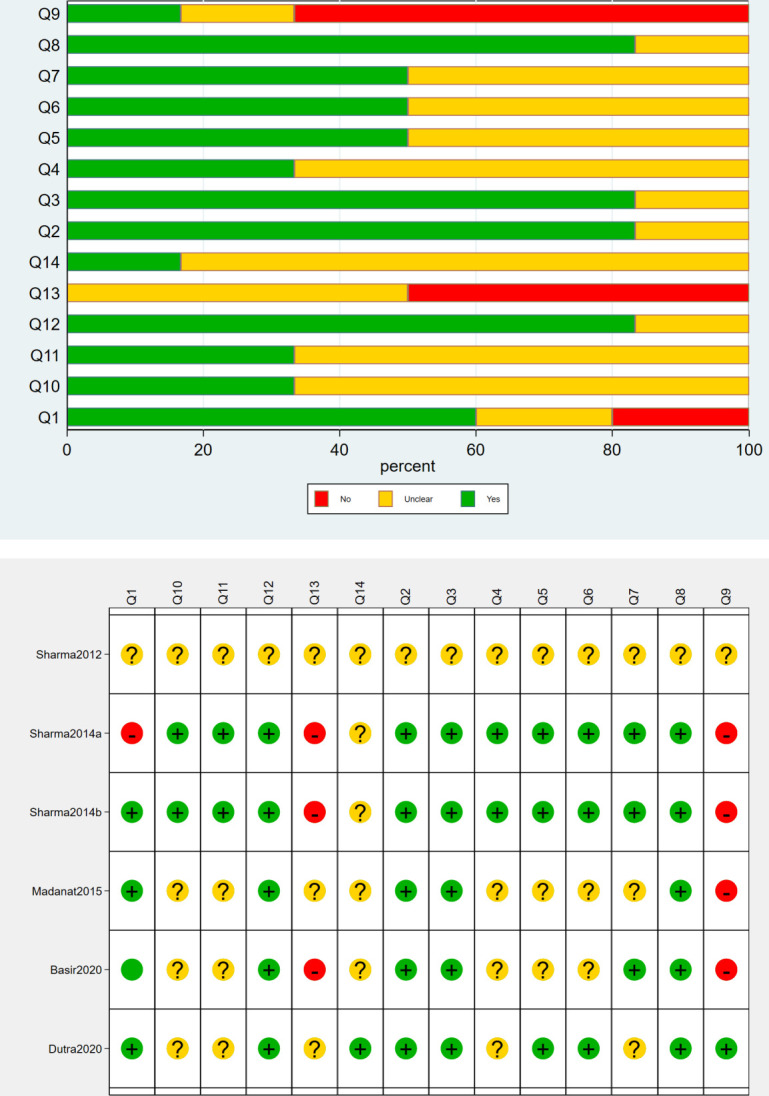
Quality assessment of the included studies.
Screening accuracy
In the included studies, the researchers used a variety of different test methods as the standard to observe the sensitivity and specificity of IPTT for screening for DPN, such as 10g-MF, VPT, NDS, tuning fork 128 Hz and ankle reflex. The differences in the sensitivity and specificity of IPTT obtained by using different test methods as reference standards are presented. In general, when 10g-MF and VPT were used as reference standards, the sensitivity and specificity of IPTT were relatively high. For the five studies comprising six data pools that used 10g-MF as the reference standard, the sensitivity ranged from 51.0% to 83.3%, and the specificity ranged from 68.0% to 98.0%.22–26 For the three studies that used VPT as a reference standard, the sensitivity ranged from 76.0% to 100.0%, and the specificity ranged from 90.0%–96.6%. Using NDS as the reference standard, the sensitivity of IPTT to be 0.53, and the specificity to be 0.97. Compared with the pin prick, the sensitivity and specificity of IPTT were 0.8 and 0.88, respectively.24 Compared with 128 Hz tuning fork, the sensitivity and specificity of IPTT were only 0.4 and 0.27, respectively.25 Compared with ankle reflex, IPTT had a sensitivity of 0.2 and a specificity of 0.97 (table 1).25
Meta-analysis results using 10g-MF as the reference standard
Screening accuracy
In the literature we retrieved, there were a total of five studies with IPTT as the target test and 10g-MF as the reference standard.22–26 Among these five studies, one study contained two datasets because it was conducted at the patient’s homes and in the clinic.22 Therefore, six datasets were included to evaluate the overall effect of IPTT in the screening of DPN.22–26 The combined sensitivity and specificity were 0.77 (95% CI 0.69 to 0.84) and 0.96 (95% CI 0.93 to 0.98), respectively. The results show I - squared is 40.5%. In addition, the DOR was 75.24 (39.90 to 141.89). The Summary Receiver Operating Characteristic (SROC) analysis for the studies yielded an overall weighted area under the curve of 0.897 (0.86 to 0.92) (figure 3, table 2 and online supplemental file 2).
Figure 3.
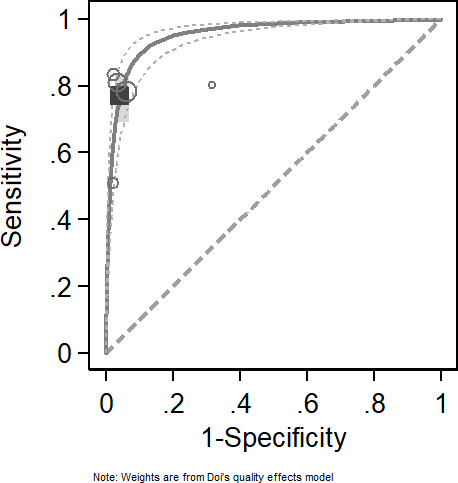
Sensitivity and specificity of Ipswich Touch Test (IPTT) in the diagnosis of diabetic peripheral neuropathy (DPN).
Table 2.
Meta-analysis of screening accuracy under the quality effect model
| Variables | Se (95% CI) | Sp (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
| Pooled value | 0.77 (0.69 to 0.84) | 0.96 (0.93 to 0.98) | 18.06 (10.75 to 30.36) | 0.24 (0.17 to 0.35) | 75.24 (39.90 to 141.89) | 0.897 (0.86 to 0.93) |
SE, Sensitivity; Sp, specificity.
Publication bias
Minor asymmetry was present in the Doi plot and the results of the LFK index also suggested minor negative asymmetry of the Doi plot (LFK index=−1.68). The findings might provide unequivocal evidence for publication bias, implying that studies with negative or equal outcomes are lacking. However, these findings might also be attributable to chance, given the few number of studies included in the analyses (figure 4).
Figure 4.
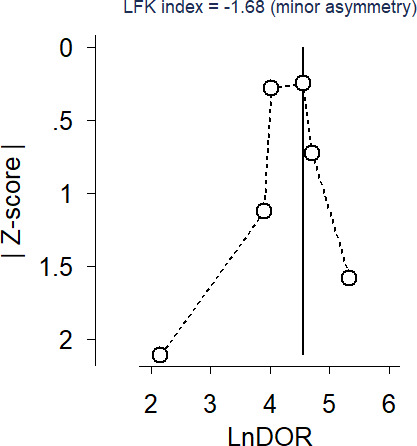
Doi plot and Luis Furuya-Kanamori (LFK) index.
Discussion
DPN is the most important risk factor for the occurrence of DFU and one of the more common chronic complications associated with diabetes. However, it is often ignored. Once the patient develops DPN, it is likely to cause DFUs, gangrene and even amputation, and many patients experience numbness and tingling in their limbs. Early identification of DPN can greatly reduce the burden of chronic diseases on society. In this study, we systematically reviewed the relevant literature on the identification of DPN by IPTT. A total of 7 studies were included, involving 1510 participants with diabetes to explore the value of IPTT screening. Previous studies have disputed the diagnostic value of IPTT, mainly due to the use of different test methods, such as VPT, NDS, pinprick, tuning fork 128 Hz, and ankle reflex, as the reference standard, compared with NDS, acupuncture, 128 Hz tuning fork, and ankle reflex, IPTT has higher screening accuracy when 10g-MF and VPT were used as the reference standard.10 19 20 Basir et al25 observed that when the 128 Hz tuning fork was used as a reference standard, the sensitivity and specificity of IPTT were only 40% and 27%, respectively. This may be due to the lower predictive level of the tuning fork compared with the monofilament. However, Miller et al21 observed that combining a tuning fork with a monofilament would result in a more effective evaluation. Regarding the quality of the current studies, some studies lacked rigour in study design, such as the interval between target tests and unclear reference standard tests, and most studies failed to describe the reference methods in detail. The overall quality of the included studies was rated as low to medium quality.
Compared with 10g-MF, the results of the meta-analysis found the combined sensitivity and specificity of IPTT to be 0.77 (95% CI 0.69 to 0.84) and 0.96 (95% CI 0.93 to 0.98), respectively, and the AUC to be 0.897 (95% CI 0.86 to 0.93). The results indicated that IPTT cannot well rule out DPN, but can confirm DPN effectively. In our study, the PLR and the NLR were 18.06 (95% CI 10.75 to 30.36) and 0.24 (95% CI 0.17 to 0.35), respectively, it means that the ratio of the true positive rate to the false-positive rate of IPTT screening for DPN is 18.06, and the ratio of false negative rate to true negative rate is 0.24. A DOR equal to 1 indicated that a test was unable to distinguish between patients with or without the disease. Our study yielded a DOR value of 75.24 (95% CI 39.90 to 141.89), indicating that IPTT had good discrimination in patients with DPN. We also found that when VPT is used as a reference standard, IPTT shows a higher sensitivity and specificity. At present, 10g-MF and VPT are the most widely used clinical screening methods for DPN. Basir et al explored the accuracy of using IPTT in detecting neuropathy in patients with small fibre and large fibre neuropathy, and found that there was no difference between IPTT and the gold standard, indicating that IPTT can be used as an alternative assessment method.25 Therefore, the current evidence shows that IPTT has a high screening value for DPN and can be used for preliminary screening of DPN in areas lacking more advanced equipment.
Heterogeneity is an important factor of this meta-analysis.43 In this study, we chose the quality effects model because it has been proven to be significantly better than the traditional random effects model and fixed effects model and attempts bias adjustment. When 10g-MF was used as the reference standard, the I2 was only 40.5%, indicating there was good consistency among the five studies included in the meta-analysis. In addition, the existence of heterogeneity may be related to other factors, such as differences in research methodology, operators, or other factors. Due to the limited number of included studies, we did not analyse the heterogeneity through subgroup analysis in this study. In terms of methodology, although we systematically and comprehensively studied the current evidence of using IPTT for DPN screening, the number of original studies is very limited, and the current conclusions are based only on these seven studies. Therefore, caution should be taken when generalising these results. About using other test methods as the reference standard (except 10g-MF), we only described the relevant indicators as there were too few related studies to merge data. In addition, despite our efforts to conduct a thorough search for eligible studies, symmetry was detected. Thus, the pooled effect may have been overstated; this was also one of the limitations of our study.
Studies have shown that routine foot examinations and rapid risk stratification are often difficult to implement in busy primary care institutions. Additionally, the lack of awareness of standardised testing for DPN among healthcare professionals is a concern, which may be due to a shortage of material and personnel resources in primary care institutions. This is concerning because identifying foot neuropathy and the patients at risk for ulceration has been shown to prevent the incidence of foot ulcers.44–46 IPTT is a new method for screening DPN that does not require any tools and can be carried out after minimal training. It is not affected by time, venue, or its operators.20 The advancement of IPTT is of great significance for the early screening of DPN to impede the progression of DFUs, as it can be used to quickly and reliably screen and manage patients at high risk for ulceration, especially in remote areas or places lacking screening tools.47 48 Kerry et al49 reported that in the first year IPTT was introduced as a screening tool, the relative risk reduction (RRR) of DFU was 64%, and in the second year, the RRR was 70%, thereby reducing hospital-acquired foot ulcers in patients with diabetes by two-thirds and negating the excess risk associated with diabetes.20 50 51 Meanwhile, it can effectively improve patients’ disease-related knowledge, which plays a positive role in promoting the self-management of patients and their families. At the same time, IPTT has a predictive effect on DFUs and reduces delays in patient visits.21 However, more thorough studies are needed for verification.
Most of the literature on IPTT is focused on screening tests and some commentary-type studies, and the number of studies is small. These studies were carried out in the UK, Spain, Brazil and Saudi Arabia, and although they achieved satisfactory results, have not been carried out globally. However, it has not been applied in developing countries such as China. China is a country with a large population and a relatively small number of medical personnel, especially in some remote areas where the medical allocation is in short supply. In these areas, the application and promotion of IPTT can effectively alleviate the challenges associated with the allocation of medical resources and play an important role in the management of patients with diabetes. IPTT has also recently been approved for use in a number of countries.21 24–26 However, Kempegowda et al45 reported that 88.4% of physicians are not familiar with IPTT. Therefore, we suggest that IPTT be further promoted among physicians and medical staff, especially in remote areas and areas lacking screening tools.36 Future large-scale, high-quality and multicentre studies on populations of different ethnicities will verify the potential applicability of IPTT alone or in combination with other DPN screening methods.
Conclusions
In summary, IPTT shows a high degree of agreement with commonly used screening tools for DPN; it can be used clinically, especially in remote areas and primary medical institutions, and self-monitoring patients. This is also the first meta-analysis of the accuracy of IPTT identification of DPN, and a systematic quantitative evaluation of its screening value, which can provide evidence for the clinical application of IPTT in the future. However, due to a limited number of studies of low or medium quality from limited geographical areas, more high-quality studies are needed to promote more effective screening practices.
bmjopen-2020-046966supp002.pdf (50.6KB, pdf)
Supplementary Material
Acknowledgments
Thanks all authors and patients of the previous research for providing data for our article.
Footnotes
Contributors: NZ conducted the database search, screened and extracted data for the meta-analysis, prepared extracted data for the procedures, and had primary responsibility in writing this article. X-Y L, JZ and FZ performed statistical analysis and contributed to article screening, data collection and extraction. JX, Q-HZ, and J-HL contributed to the discussion and editing. JX and J-RC critically revised the draft manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Funding: This research was supported by the Natural Science Foundation of Hunan Province (Grant No. 2019JJ80087).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All free text entered below will be published.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–75. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 3.Banik PC, Barua L, Moniruzzaman M, et al. Risk of diabetic foot ulcer and its associated factors among Bangladeshi subjects: a multicentric cross-sectional study. BMJ Open 2020;10:e034058. 10.1136/bmjopen-2019-034058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep 2014;14:473. 10.1007/s11910-014-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan MWJ, Reynolds RM, Marioni RE, et al. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 2011;7:108–14. 10.1038/nrendo.2010.228 [DOI] [PubMed] [Google Scholar]
- 6.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 2019;19:86. 10.1007/s11892-019-1212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks CW, Selvarajah S, Mathioudakis N, et al. Trends and determinants of costs associated with the inpatient care of diabetic foot ulcers. J Vasc Surg 2014;60:1247–54. 10.1016/j.jvs.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care 2017;40:136–54. 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javed S, Hayat T, Menon L, et al. Diabetic peripheral neuropathy in people with type 2 diabetes: too little too late. Diabet Med 2020;37:573–9. 10.1111/dme.14194 [DOI] [PubMed] [Google Scholar]
- 10.Feldman EL, Nave K-A, Jensen TS, et al. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–313. 10.1016/j.neuron.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Li N, Zhao Y, et al. Painful diabetic peripheral neuropathy study of Chinese outpatients (PDN-SCOPE): protocol for a multicentre cross-sectional registry study of clinical characteristics and treatment in China. BMJ Open 2019;9:e025722. 10.1136/bmjopen-2018-025722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carls GS, Gibson TB, Driver VR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc 2011;101:93–115. 10.7547/1010093 [DOI] [PubMed] [Google Scholar]
- 13.International Diabetes Federation . Diabetic foot screening pocket chart. Brussels: IDF, 2017. https://www.idf.org/e-library/guidelines/124-diabetic-foot-screening-pocket-chart.html [Google Scholar]
- 14.Standards of medical care in Diabetes-2017: summary of revisions. Diabetes Care 2017;40:S4–5. 10.2337/dc17-S003 [DOI] [PubMed] [Google Scholar]
- 15.Craig AB, Strauss MB, Daniller A, et al. Foot sensation testing in the patient with diabetes: introduction of the quick & easy assessment tool. Wounds 2014;26:221–31. [PubMed] [Google Scholar]
- 16.Slater RA, Koren S, Ramot Y, et al. Interpreting the results of the Semmes-Weinstein monofilament test: accounting for false-positive answers in the International consensus on the diabetic foot protocol by a new model. Diabetes Metab Res Rev 2014;30:77–80. 10.1002/dmrr.2465 [DOI] [PubMed] [Google Scholar]
- 17.Lavery LA, Lavery DE, Lavery DC, et al. Accuracy and durability of Semmes-Weinstein monofilaments: what is the useful service life? Diabetes Res Clin Pract 2012;97:399–404. 10.1016/j.diabres.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Spruce MC, Bowling FL. Diabetic foot screening: new technology versus 10g monofilament. Int J Low Extrem Wounds 2012;11:43–8. 10.1177/1534734612438055 [DOI] [PubMed] [Google Scholar]
- 19.Rayman G, Vas PR, Baker N, et al. The Ipswich touch test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care 2011;34:1517–8. 10.2337/dc11-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vas PRJ, Sharma S, Rayman G. Distal sensorimotor neuropathy: improvements in diagnosis. Rev Diabet Stud 2015;12:29–47. 10.1900/RDS.2015.12.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JD, Carter E, Shih J, et al. How to do a 3-minute diabetic foot exam. J Fam Pract 2014;63:646–56. [PubMed] [Google Scholar]
- 22.Sharma S, Kerry C, Rosier J. The Ipswich touch test (IpTT): screening for diabetic neuropathy at home. Diabet Med 2012;29:20. [Google Scholar]
- 23.Sharma S, Kerry C, Atkins H, et al. The Ipswich touch test: a simple and novel method to screen patients with diabetes at home for increased risk of foot ulceration. Diabet Med 2014;31:1100–3. 10.1111/dme.12450 [DOI] [PubMed] [Google Scholar]
- 24.Madanat A, Sheshah E, Badawy E-B, et al. Utilizing the Ipswich touch test to simplify screening methods for identifying the risk of foot ulceration among diabetics: the Saudi experience. Prim Care Diabetes 2015;9:304–6. 10.1016/j.pcd.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Basir IS, Syam Y, Yusuf S, et al. Accuracy of Ipswich touch test (IpTT) to detect small fiber neuropathy and large fiber neuropathy as a risk factor of diabetic foot ulcers in public health centers. Enferm Clin 2020;30 Suppl 2:308–12. 10.1016/j.enfcli.2019.07.108 [DOI] [Google Scholar]
- 26.Dutra LMA, Moura MC, do Prado FA, et al. Is it possible to substitute the monofilament test for the Ipswich touch test in screening for peripheral diabetic neuropathy? Diabetol Metab Syndr 2020;12:27. 10.1186/s13098-020-00534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaper NC, van Netten JJ, Apelqvist J, et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3266. 10.1002/dmrr.3266 [DOI] [PubMed] [Google Scholar]
- 28.Atlantis E, Cochrane B. The association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and functional capacity in chronic obstructive pulmonary disease: a systematic review. Int J Evid Based Healthc 2016;14:53–63. 10.1097/XEB.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 30.Whiting P, Rutjes AWS, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foxlee N, Stone JC, Doi SAR. A comparison of univariate and bivariate models in meta-analysis of diagnostic accuracy studies. Int J Evid Based Healthc 2015;13:28–34. 10.1097/XEB.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 32.Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology 2008;19:94–100. 10.1097/EDE.0b013e31815c24e7 [DOI] [PubMed] [Google Scholar]
- 33.Doi SAR, Barendregt JJ, Khan S, et al. Advances in the meta-analysis of heterogeneous clinical trials II: the quality effects model. Contemp Clin Trials 2015;45:123–9. 10.1016/j.cct.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 34.Doi SAR, Furuya-Kanamori L. Selecting the best meta-analytic estimator for evidence-based practice: a simulation study. Int J Evid Based Healthc 2020;18:86–94. 10.1097/XEB.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 35.Furuya-Kanamori L, Kostoulas P, Doi SAR. A new method for synthesizing test accuracy data outperformed the bivariate method. J Clin Epidemiol 2021;132:51–8. 10.1016/j.jclinepi.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 36.Doi SAR, Furuya-Kanamori L, Thalib L, et al. Meta-analysis in evidence-based healthcare: a paradigm shift away from random effects is overdue. Int J Evid Based Healthc 2017;15:152–60. 10.1097/XEB.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 37.Doi SAR. Evidence synthesis for medical decision making and the appropriate use of quality scores. Clin Med Res 2014;12:40–6. 10.3121/cmr.2013.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone JC, Glass K, Munn Z, et al. Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches. J Clin Epidemiol 2020;117:36–45. 10.1016/j.jclinepi.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Mou Z-yun, Zhai J-xia, et al. [Application of Stata software to test heterogeneity in meta-analysis method]. Zhonghua Liu Xing Bing Xue Za Zhi 2008;29:726–9. [PubMed] [Google Scholar]
- 40.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 41.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 2018;16:195–203. 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 42.Furuya-Kanamori L, Xu C, Hasan SS, et al. Quality versus risk-of-bias assessment in clinical research. J Clin Epidemiol 2021;129:172–5. 10.1016/j.jclinepi.2020.09.044 [DOI] [PubMed] [Google Scholar]
- 43.Lo K, Stephenson M, Lockwood C. Analysis of heterogeneity in a systematic review using meta-regression technique. Int J Evid Based Healthc 2019;17:131–42. 10.1097/XEB.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 44.Bowling FL, Abbott CA, Harris WE, et al. A pocket-sized disposable device for testing the integrity of sensation in the outpatient setting. Diabet Med 2012;29:1550–2. 10.1111/j.1464-5491.2012.03730.x [DOI] [PubMed] [Google Scholar]
- 45.Kempegowda P, Saeed MA, Foot S. Short-burst teaching aimed at medical professionals): impact of ’shortburst' teaching on the knowledge regarding the ipswich Touch Test. Diabet Med 2016;33:134.26053687 [Google Scholar]
- 46.Bailey TS, HM Y, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am Med J 1985;78:371–4. [DOI] [PubMed] [Google Scholar]
- 47.Baker N. An alternative to a 10-g monofilament or tuning fork? two new, simple, easy-to-use screening tests for determining foot ulcer risk in people with diabetes. Diabet Med 2012;29:1477–9. 10.1111/j.1464-5491.2012.03731.x [DOI] [PubMed] [Google Scholar]
- 48.O'Loughlin A, Dinneen SF. ACP Journal Club: the Ipswich Touch Test at home had 78% sensitivity and 94% specificity for detecting loss of foot sensation. Ann Intern Med 2015;162:C10. 10.7326/ACPJC-2015-162-4-010 [DOI] [PubMed] [Google Scholar]
- 49.Kerry CD, Sharma S, Rayman G. Reduction in hospital-acquired diabetes foot lesions using the Ipswich touch test (IpTT). Diabetic Med 2013;30:141–2. [Google Scholar]
- 50.VPRR KC. Reduction in the incidence of hospital acquired foot pressure ulcers in people with diabetes. Diabetic Med 2015;1:26. [Google Scholar]
- 51.Madanat A, Sheshah E, Badawy E, et al. Utilizing the Ipswich touch test to simplify screening methods for identifying the risk of foot ulceration among diabetics: comment on the Saudi experience. Prim Care Diabetes 2015;9:401–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-046966supp001.pdf (128.9KB, pdf)
bmjopen-2020-046966supp002.pdf (50.6KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All free text entered below will be published.