Abstract
BACKGROUND
Inflammation and one of its mediators, NF-kappa B (NFκB), have been implicated in prostate cancer carcinogenesis. We assessed whether germline polymorphisms associated with NFκB are associated with risk of developing lethal disease (metastases or death from prostate cancer).
METHODS
Using a Bayesian approach leveraging NFκB biology with integration of publicly available datasets we used a previously defined genome-wide functional association network specific to NFκB and lethal prostate cancer. A dense-module-searching method identified modules enriched with significant genes from a genome wide association study (GWAS) study in a discovery dataset, Physicians’ Health Study and Health Professionals Follow-up Study (PHS/HPFS). The top 48 candidate single nucleotide polymorphisms (SNPs) from the dense-module-searching method were then assessed in an independent prostate cancer cohort and the one SNP reproducibly associated with lethality was tested in a third cohort. Logistic regression models evaluated the association between each SNP and lethal prostate cancer. The candidate SNP was assessed for association with lethal prostate cancer in six of 28 studies in the PRACTICAL Consortium where there was some medical record review for death ascertainment which also had SNP data from the ONCOARRAY platform. All men self-identified as Caucasian.
RESULTS
The rs1910301 SNP which was reproducibly associated with lethal disease was nominally associated with lethal disease (OR=1.40; P=0.02) in the discovery cohort and the minor allele was also associated with lethal disease in 2 independent cohorts (OR=1.35; P=0.04 and OR=1.35; p=0.07). Fixed effects meta-analysis of all three cohorts found an association: OR = 1.37 (95% CI: 1.15–1.62, P-value=0.0003). This SNP is in the promoter region of FRAS1, a gene involved in epidermal-basement membrane adhesion and is present at a higher frequency in men with African ancestry. No association was found in the subset of studies from the PRACTICAL consortium studies which had a total of 106 deaths out total of 3,263 patients and a median follow-up of 4.4 years.
CONCLUSIONS:
Through its connection with the NFκB pathway, a candidate SNP with a higher frequency in men of African ancestry without cancer was found to be associated with lethal prostate cancer across 3 well-annotated independent cohorts of Caucasian men.
Keywords: Single nucleotide polymorphisms, SNPs, African ancestry, nuclear factor kappa B
Introduction
Each year there are about 1.4 million newly diagnosed cases of prostate cancer and more than 366,000 deaths worldwide1. Some patients have indolent localized disease that does not require treatment, while others present with or develop metastatic disease that responds poorly to therapy2,3. Clinicopathologic staging can identify patients at higher risk of relapsing with metastatic disease4,5 and treatment of intermediate and high-risk localized disease decreases prostate cancer deaths6–9. Biomarkers such as PTEN loss and gene expression signatures also provide prognostic information10,11. Epidemiological and biological studies have implicated aberrant metabolism12, inflammation13 and inherited genetic exome14 and SNP variants15 with more advanced prostate cancer.
Nuclear factor kappa B (NFκB) is a transcription factor that controls inflammation and can either promote cancer progression or cancer cell death16. NFκB activation can promote proliferation, development of metastases, and evasion of apoptosis in prostate cancer17–19. Biomarkers of NFκB activation in localized disease are emerging as a possible strategy to identify patients with a higher risk of relapse with metastases after localized therapy20–24.
The identification of biomarkers related to NFκB activation that can predict aggressive prostate cancer may identify patients who are at risk of relapse after localized therapy and may benefit from adjuvant systemic therapy to prevent relapses16,25. These biomarkers may help identify men who are otherwise candidates for active surveillance but need immediate intervention. Although many SNPs predisposing to risk of prostate cancer have been identified, only a limited number of SNPs with some evidence of possible association with lethal prostate cancer have been identified to date26,27. Moreover, a germline biomarker rather than tissue-based biomarker may help identify men at risk of significant prostate cancer who should be screened for prostate cancer. To that end, we leveraged our previous work which integrated publicly available genomic datasets using a Bayesian approach and defined an NFκB-network that was enriched in patients with lethal prostate cancer after a prostatectomy28,29. This network was then used to interrogate a prostate cancer GWAS to identify SNPs associated with NFκB-activation and lethal prostate cancer.
Methods:
SNP Selection Using a Bayesian-Based Analysis Leveraging NFκB Biology
Using multiple publicly available data sets and the network approach previously described by our team28,29, we defined a genome-wide functional interaction network specific to the NFκB-pathway and metastatic disease or prostate cancer death, (“lethal cases”) compared to patients with prostate cancer who had not developed radiographic evidence of metastases on computerized tomography or technetium bone scan imaging (conventional scans) at least ten years after their diagnosis (“non-metastatic cases”). The genome-wide functional interaction network identified 8,154,133 high-confidence protein-protein functional associations. The dense-module-searching method, dmGWAS30, was then used to define a candidate subnetwork of interacting genes related to both (i) the NFκB pathway and (ii) lethal prostate cancer. We then searched the NFκB interaction network for modules enriched with genes represented by SNPs with the lowest additive model p-value for lethal disease (Supplementary Table 1) with PHS/HPFS GWAS data31 as the discovery set. After assigning each SNP on the Affymetrix 5.0 chip to a gene if the SNP is located within 20kb of the gene, a single SNP with the lowest additive model p-value for lethal disease was selected to represent each of 16,387 genes. The SNP-gene pairs were weighted by the GWAS p-values of the SNP during dense-module-searching. We arbitrarily limited our analysis to 50 genes included in the top 26 modules out of 10,171 valid modules with the highest normalized scores. Of the 52 SNPs (Supplementary Table 1) used to represent the genes in the selected subnetwork (two genes were represented by two SNPs with the same GWAS p-values each), four failed probe design using the Sequenom platform in the Gelb Center/ECOG cohort detailed below. This resulted in 48 SNPs to test in the first independent cohort (Gelb Center/ECOG cohort).
The PHS/HPFS GWAS was conducted on self-identified Caucasian men including 196 lethal and 368 indolent cases. Endpoints were confirmed by medical record and death certificate review by a physician.
Patients and Genotyping Sequencing Methods in Independent Cohorts
The candidate SNPs were then tested in an independent cohort Gelb Center/ECOG patients of self-identified Caucasian men who provided consent. Using the Gelb Center prostate cancer hospital registry database at Dana-Farber Cancer Institute32 we identified an independent cohort of 254 self-identified men with a blood sample available for analysis and a history of localized prostate cancer treated with curative intent with surgery or radiation and had not developed radiographic evidence of metastatic disease with a median follow-up of 8.4 years. The patients from the ECOG cohort of 256 self-identified Caucasian men with a blood sample available all had documented metastatic disease (relapsed post-local therapy or de novo metastatic)3 as determined by eligibility at time of enrolment on the therapeutic clinical trial. Patients included in this analysis provided consent and institutional review board approval were obtained for all studies conducted3. All DNA samples were extracted from peripheral whole blood using QIAamp DNABlood mini kit (QIAGEN Inc, Valencia, CA). Genotyping was completed at the same time using Sequenom iPLEX matrix-assisted laser desorption/ionization (MALDI)-time of flight mass spectrometry technology (Carlsbad, CA). SNP assays were combined into four multiplex pools in 384-well format. Approximately 5% of samples were randomly selected and genotyping duplicated for quality control. Concordance rate for duplicate genotyping was 100%. Call rate overall was greater than 99%.
The SNP which was found to be associated with lethal disease in the Gelb Center/ECOG cohort was then also tested in The Fred Hutchinson Cancer Research Center (FHCRC) study. These patients were previously enrolled in population-based prostate cancer case–control studies33,34. The subset of self-identified Caucasian patients included (n = 1,548) for these analyses were diagnosed with histologically confirmed adenocarcinoma of the prostate using the Seattle-Puget Sound Surveillance, Epidemiology, and End Results (SEER) Program cancer registry. Prostate cancer recurrence status was determined from prospectively collected information from follow-up surveys that were completed by patients in 2004–2005 and in 2010–2011, review of medical records, and/or physician follow-up as needed. Metastatic progression was confirmed by positive bone scan, MRI, CT, or biopsy. Cause-specific deaths were ascertained from the SEER registry, which links quarterly with the Washington State Vital Statistics Database and annually with the National Death Index. Endpoints were also ascertained by medical record and death certificate review by a study physician. There were 570 cases who had no evidence of recurrence or progression during a follow-up period of at least 10 years after diagnosis indolent and 104 cases of metastatic disease or death from prostate cancer. A custom designed TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA) was used for genotyping on the ABIPrism 7900HT sequence detection system according to the manufacturer’s instructions. The study was approved by the institutional review board of the Fred Hutchinson Cancer Research Center, and written informed consent was obtained from all participants. Genotyping was approved by the institutional review board of the Intramural Program for the National Human Genome Research Institute and was done using Affymetrix Human Mapping 500K array (Affymetrix, Inc).
The frequency of the SNP which was reproducibly associated with lethal prostate cancer from the three first cohorts was then assessed for frequency by ethnicity in men with and without prostate cancer in the PRACTICAL consortium35 who had SNP data from the ONCOARRAY platform. The individual PRACTICAL study principal investigators (N=63) were queried to identify studies which had some physician review of medical records in addition to use of use national registries for ascertaining cause of death. Six studies were identified out of 28 studies where investigators provided process for death ascertainment with a total of 3,263 patients and a total of 106 deaths and a median follow-up of 4.4 years.
Statistical Methods
Odds ratios (OR) and 95% confidence intervals (CI) were estimated using univariable logistic regression, assuming an additive model for the association between each SNP and lethal disease and modeled as a continuous variable (0, 1 or 2 copies), assuming constant effect going from 0 to 1 and from 1 to 2. Having identified the candidate SNP in the first three well annotated cohorts, a fixed effects meta-analysis was performed to obtain an overall OR and 95% CI for the first three cohorts. The impact of the SNP of interest on overall survival for the ECOG clinical trial data-set, the only data-set where outcome to androgen deprivation therapy data was known, was estimated using the Kaplan-Meier method and differences between survival distributions was tested using the log-rank test.
Results:
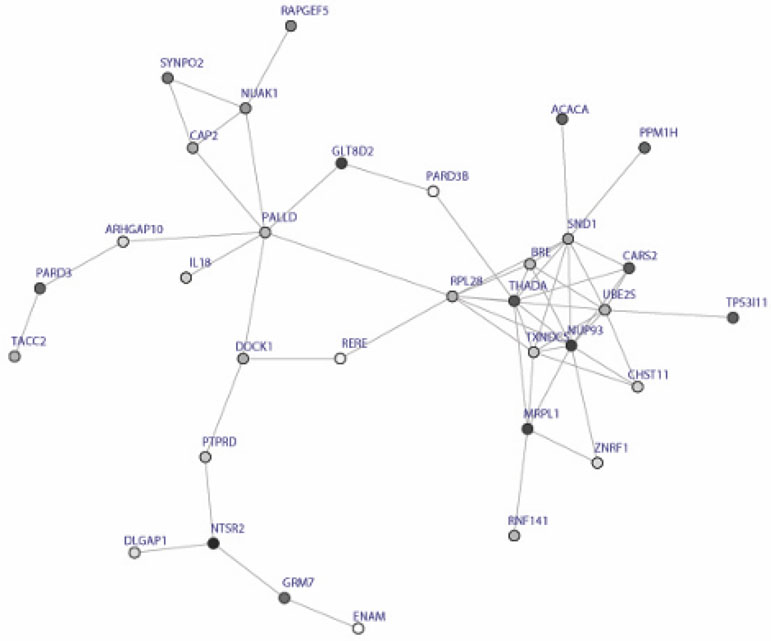
The GWAS results from the PHS/HPFS discovery cohort has been previously described31. Table 1 details patient characteristics of the two test cohorts used for assessment of reproducibility. Figure 1 shows the subnetwork consisting of the 26 modules and the top 48 SNPs from the discovery cohort are listed in Supplementary Table 1.
Table 1:
Patient Characteristics of the Test Cohorts.
| Variable | Category | ECOG Metastatic | DFCI GC No recurrence1 | FHCRC Metastasis or death | FHCRC No adverse outcomes2 |
|---|---|---|---|---|---|
|
| |||||
| Total | 256 | 254 | 104 | 570 | |
|
| |||||
| Age | Mean (SD) | 61.0 (8.5) | 60.4 (7.8) | 58.8 (8.2) | 59.6 (7.2) |
| Median (Q1,Q3) | 61 (55,67) | 61 (55,66) | 59.0 (53.0, 64.0) | 59.0 (54.0, 64.0) | |
| [Min, Max] | [38,90] | [40,81] | [42.0, 74.0] | [35.0, 74.0] | |
| Freq. of Missing | 4 | 1 | 0 | 0 | |
|
| |||||
| Local therapy | None | 181 (71) | 31 (12) | 32 (30.8) | 48 (8.4) |
| Prostatectomy | 50 (20) | 177 (70) | 41 (39.4) | 367 (64.4) | |
| Definitive RT | 25 (10) | 46 (18) | 31 (29.8) | 151 (26.5) | |
| Other | 0 | 0 | 0 | 4 (0.7) | |
| Unknown/Missing | 0 | 0 | 0 | 0 | |
|
| |||||
| Adjuvant hormonal therapy | No | 242 (94.5) | 247 (97) | 89 (85.6) | 544 (95.4) |
| Yes | 14 (5.5) | 7 (3) | 15 (14.4) | 26 (4.6) | |
| Unknown/Missing | 0 | 0 | 0 | 0 | |
|
| |||||
| Clinical stage at diagnosis | Local (T1,T2,T3) | 62 (25.3) | 225 (100) | 73 (70.2) | 565 (99.3) |
| Regional (T4,N1) | 14 (5.7) | 0 (0) | 9 (8.7) | 5 (0.7) | |
| Metastatic (M1) | 169 (69.0) | 0 (0) | 22 (21.2) | 0 | |
| Unknown/Missing | 11 | 29 | 0 | 0 | |
|
| |||||
| Gleason score | <=6 | 18 (7.6) | 148 (59) | 29 (28.4) | 356 (62.5) |
| 7 | 61 (25.7) | 81 (32) | 37 (36.3) | 185 (32.5) | |
| >=8 | 158 (66.7) | 21 (8) | 36 (35.3) | 29 (5.1) | |
| Unknown/Missing | 19 | 4 | 2 | 0 | |
|
| |||||
| Length of follow-up (years from diagnosis) | Mean (SD) | 4.3 (2.9) | 9.0 (3.5) | 9.7 (5.7) | 14.7 (4.0) |
| Median (Q1,Q3) | 3.8 (2.3,5.3) | 8.4 (6.3,11.3) | 8.9 (5.1, 13.2) | 13.1 (11.6, 19.3) | |
| [Min, Max] | [0.3,15.2] | [0.4,22.2] | [1.1, 22.4] | [10.1, 22.9] | |
| Freq. of Missing | 3 | 1 | 0 | 0 | |
No evidence of recurrence on conventional scans after curative therapy for localized disease.
No evidence of recurrence/progression and survival time of ≥10 years.
Figure 1:
Modules identified by dmGWAS, a dense module searching method. The subnetwork includes 50 genes and 109 interactions.
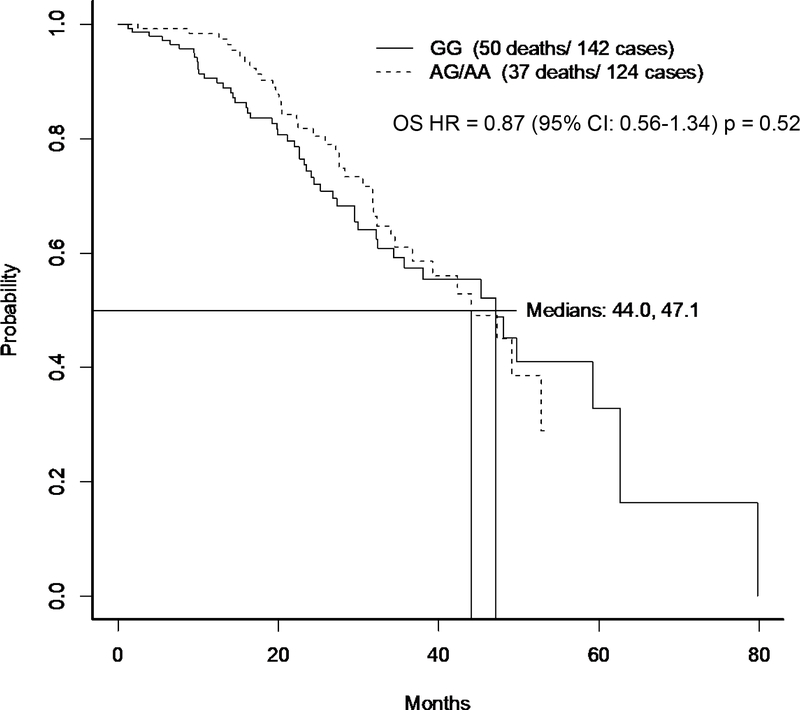
One of these 48 SNPs, rs1910301, was replicated to be associated with lethal disease in the independent cohort of GC/ECOG patients, OR: 1.35, (95%CI:1.02–1.80, P-value 0.04). In the original PHS/HPFS GWAS analysis, the rs1910301 SNP had an OR of 1.40 (95%CI: 1.05–1.84, P-value = 0.02) for lethal disease. This SNP was the only SNP taken forward to be tested in the FHCRC cohort and had an OR for lethal disease of 1.35 (95%CI: 0.97–1.85, p=0.07). A fixed effects model of all three cohorts resulted in a meta-analysis OR for lethal prostate cancer of 1.37 (95% CI:1.15–1.62; p=0.0003). We also explored whether this SNP was associated with a poorer response to androgen deprivation therapy using the using the overall survival data from the parent ECOG trial3. The presence of at least one copy of allele A was not significantly associated with shorter overall survival OS [HR=0.87 (95% CI 0.56–1.34) p=0.52], (Figure 2).
Figure 2:
Kaplan-Meier curves for overall survival by genotype for E3805 patients on androgen deprivation therapy alone with genotype data.
Using the PRACTICAL consortium data the genotype frequency was then assessed in men with prostate cancer (cases) and no cancer (controls) and reported by ethnicity (N=102,026 total, 91,309 European descent, 2,437 Asian descent and 8,280 African descent). The allele frequency was 23% for Europeans and Asians and 60% for men of African descent (Table 2B). Of the 6 studies with 3,263 patients with a median follow-up of 4.4 years we identified 258 patients alive for more than 10 years and compared them to the 106 total prostate cancer deaths resulting in an OR of 0.95 (95% CI:0.65–0.38, p=0.80). Given there were only 106 (3.2%) prostate cancer deaths recorded in 3,263 patients consistent with a short median follow-up of 4.4 years, this cohort was deemed too immature for assessment of prostate cancer death and inclusion in the meta-analysis.
Table 2B:
Frequency of SNP rs1910301 across the study populations
| GG | AG | AA | A Allele Frequency | ||
|---|---|---|---|---|---|
| PHS/PFS 1 | Mets or PrCa2 death (event) | 106 (32.4) | 69 (34.3) | 21 (58.3) | 28.3% |
| No Mets or prostate cancer death | 221 (67.6) | 132 (65.7) | 15 (41.7) | 22.0% | |
| ECOG 1 | Mets or PrCa death (event) | 140 (46.4) | 96 (55.2) | 20 (58.8) | 26.5% |
| Gelb Center 1 | No Mets or PrCa death | 162 (53.6) | 78 (44.8) | 14 (41.2) | 20.8% |
| Fred Hutch 1 | Mets or PrCa death | 57 (13.7) | 37 (17.1) | 10 (23.3) | 27.4% |
| No Mets or PrCa death | 358 (86.3) | 179 (82.9) | 33 (76.7) | 21.4% | |
| PRACTICAL | GG | AG | AA | ||
| European descent | Cases | 32451 (58.8) | 19585 (35.5) | 3126 (5.7) | 23.4% |
| Controls | 21085 (58.3) | 12994 (35.9) | 2068 (5.7) | 23.7% | |
| Asian Descent | Cases | 707 (57.6) | 447 (36.4) | 74 (6.0) | 24.2% |
| Control | 702 (58.0) | 437 (36.1) | 70 (5.8) | 23.9% | |
| African Descent | Cases | 721 (16.9) | 1943 (45.5) | 1603 (37.6) | 60.3% |
| Controls | 685 (17.1) | 1879 (46.8) | 1449 (36.1) | 59.5% |
All self-identified Caucasian
PrCa: prostate cancer
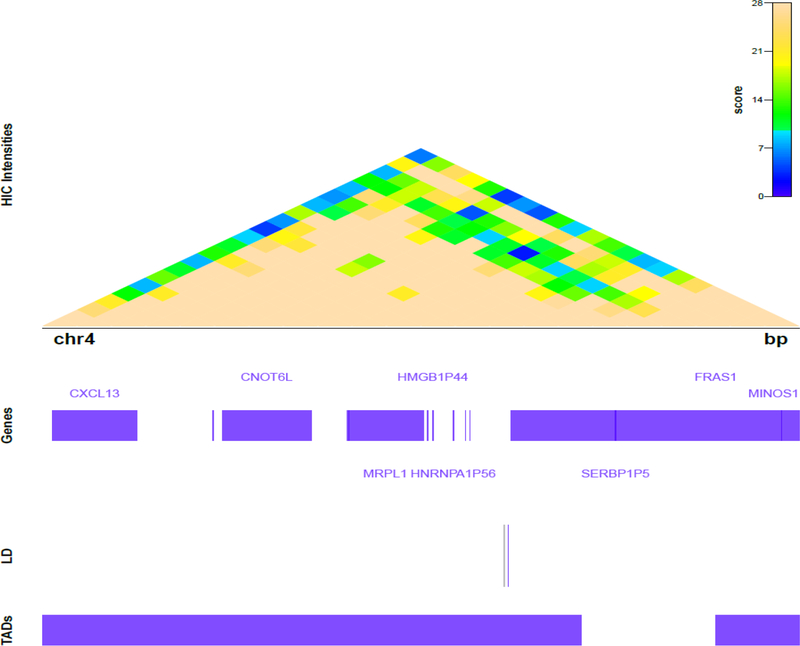
Biological plausibility of the rs1910301 finding was assessed by querying genomic data-bases and revealed it is in the promoter of FRAS1 (Supplemental Figure 1A). We further explored the regional 3D genomic structure surrounding rs1910301 using publicly available Hi-C data from human fibroblast IMR90 cell profiles36,37 This SNP was also found to reside in a topologically associating domain (TAD) of 8 genes including MRPL1 (Figure 3), a gene highly represented in the top gene modules as well as CNOT6L. The SNP also has some conservation across species and is in a region with DNAse hypersensitivity (Supplemental Figure 1B). The Human Protein Atlas reported FRAS1 immunohistochemistry protein staining from 10 prostate cancer samples: 0-high 3-medium; 4-low, 3-none. The TCGA data noted patients in the lower quartile had a better overall survival than patients with higher expression (Supplemental Figure 2). Expression quantitative trait loci (eQTL) analysis using the GTEx Portal revealed rs1910301 impacts CNOT6L RNA expression including prostate tissue (Supplemental Figure 3).
Figure 3:
Topographical map. The SNP rs1910301 was found to reside in a topologically associating domain (TAD) with 8 genes.
Discussion:
This unique approach to identifying biomarkers of NFκB activation identified SNP rs1910301 in promoter region of FRAS1 on chromosome 4q21, as a candidate SNP possibly associated with a 37% increase in risk of development of metastatic disease or death from prostate cancer in three well annotated and mature cohorts. Having identified this candidate SNP in the meta-analysis of the first three cohorts, we were unable to confirm the association in the PRACTICAL cohort. This was despite attempts to define a subset with some degree of medical review of causes of death. After the efforts to define a cohort for analysis, we were only able to define a cohort with a median follow-up 4.4 years and 3.2% prostate cancer deaths. The PRACTICAL consortium is a very robust dataset for SNPs for risk of prostate cancer15 but longer follow-up is needed for prostate outcome data.
To date, while numerous SNPs from GWAS have been associated with risk of developing prostate cancer, there has been limited success in reproducibly finding SNPs associated with lethal prostate cancer. This may be partly due to relatively few events of metastatic disease in these studies resulting in limited statistical power. Previously rs5993891, in ARVCF on chromosome 22q11 has been found to be associated with a 48% reduction in risk of prostate cancer specific mortality in a meta-analysis of four cohorts38. In another meta-analysis of seven cohorts with 12,082 patients with 1,544 prostate cancer deaths after adjustment for clinicopathological factors, rs2308327 in the MGMT, rs2070874 in IL4 and in rs2494750 in AKT1 were associated with risk of prostate cancer specific mortality; and in a cohort of men with an inherited susceptibility to the disease, prostate cancer specific mortality was associated with rs635261 at RNASEL; rs915927 in XRCC1; rs2494750 in AKT127,39. In a case-only GWAS of 12,518 prostate cancer cases, two loci were associated with higher Gleason score (rs35148638 in RASA1; rs78943174 in NAALADL2)40.
To address the potential for false negative findings from GWAS level statistics, we used a Bayesian-based analysis leveraging the biology connecting NFκB to lethal prostate cancer and the dmGWAS analytical approach were used. This was a parsimonious biology based-approach to select candidate SNPs. To further minimize false positive findings, we assessed for reproducibility across cohorts and saw the rs1910301 SNP is consistently associated with approximately a 37% greater risk of having lethal prostate cancer disease across three cohorts of Caucasian men with prostate cancer. In short, by using a Bayesian approach and showing reproducibility across three cohorts we have been able to identify one of the few candidate SNPs that are possibly associated with lethal disease (metastatic disease or prostate cancer death).
Notably, the frequency of rs1910301 differs across ethnic groups in the 1000 Genomes Project and was more frequent in patients with African ancestry (allele A frequency, 64%) in comparison to a population of European ancestry without prostate cancer (23%). The new data presented in this paper from the three cohorts for assessment of association with lethal prostate cancer is restricted to men of European ancestry (N=1,748) and the allele frequency of the risk carrying variant, the A-variant, was identical (23%) to that reported by 1,000 Genomes Project. Furthermore, this was the same frequency in the PRACTICAL consortium as it was 23% in patients with prostate case and 23% in the controls with no prostate cancer of European descent (N=91,309). Patients of Asian descent had the same A allele frequency in men with prostate cancer (24%) and no prostate cancer (23%). The A allele frequency in the 8,280 men of African descent confirmed the frequency as 60% in men with prostate cancer and 59% in men without prostate cancer. The clinical implications of racial/ethnic allele frequency heterogeneity are not well defined. Emerging evidence points to an association between racial/ethnic differences in SNP frequency with differential treatment response/relapse risk in localized prostate cancer41,42, though data remains limited. It is interesting to note that rs1910301 is found at a higher frequency in African-Americans – a group of men who after even accounting for socio-economic factors, still have a higher rate of metastatic disease43. Moreover, African-Americans treated with androgen deprivation therapy alone have the same benefit with androgen deprivation therapy as Caucasians43 and it is notable we did not find an association of this SNP with poorer overall survival in the E3805 analysis in Caucasian men treated with androgen deprivation alone.
The biological plausibility of this SNP impacting the biology of prostate cancer development can be seen by the genes it is associated with topographically. Reassuringly, rs1910301 is located in the promoter region of a gene that may be relevant to metastatic disease biology, FRAS1. Together with FREM2, FRAS1 forms a gene unit that regulates epidermal-basement membrane adhesion and cell migration44. FREM2 is an NFκB regulated gene and mutations in FREM2 and FRAS1 are associated with the Fraser syndrome – a congenital syndrome with craniofacial, urogenital and respiratory system abnormalities44. In cancer cells in vitro, silencing of FRAS1 leads to decreased ability of non-small lung cancer, A549 cells to migrate and invade45. In addition, FRAS1 was found to be more frequently mutated in metastatic breast cancer than primary breast cancer46. It is therefore possible that a SNP that alters FRAS1 activity causes dysfunction of FRAS1-FREM2 gene unit and increases metastatic potential. We also found rs1910301 to reside in a topologically associating domain (TAD) with MRPL1 (Figure 3), a gene highly represented in the top gene modules, potentially helping to explain the identification of rs1910301 through the dmGWAS approach. MRPL1 encodes the 39S subunit protein that belongs to the L1 ribosomal protein family and altered function may play a role in prostate cancer progression through dysregulation of translation of proteins – for example – of cellular adhesion or cell cycle47. The finding that the rs1910301 SNP has some conservation across species and is in a region with some DNAse hypersensitivity also adds biological plausibility. The latter suggests the SNP is in an area where the chromatin is accessible and functionally related to transcriptional activity as it is amenable for the protein binding including transcription factors. The observation using the expression quantitative trait locus showed an association between rs1910301 and CNOT6L is also notable as this is one of the 8 genes found to reside in the topologically associated domain of rs1910301. CNOT6L is cytoplasmic deadenylase with 3-prime-to-5-prime exoribonuclease activity48.
However, it is recognized that this work is hypothesis generating and fine-mapping and mechanistic studies to define the exact gene and the biological basis for the possible association of rs1910301 with lethal prostate cancer are needed. It is unknown if rs1910301 is pathogenic or in linkage disequilibrium with the actual gene driving the metastatic biology. Other limitations of this study include not inferring ancestry from GWAS data. We also only chose a gene if the SNP was located within 20kb of the gene and thus excluding long regulatory-range elements.
In summary, the rs1910301 SNP was identified by a Bayesian interrogation of GWAS data focused on the cancer-promoting NFκB activation network and reproducibly had an OR for metastatic prostate cancer of about 1.37 across three independent cohorts. The association was not confirmed in the PRACTICAL consortium with limited follow-up for cancer outcomes. The higher frequency of the SNP in the African American population may be a clue to the biology underlying the greater propensity for metastatic disease in this patient population. Based on this data, biological mechanistic work to define the exact biological underpinnings for the association and assessment in prospective trials of localized disease with detailed endpoint ascertainment and accounting for clinico-pathological and treatment variables is needed to determine whether it is a biomarker that can be used in the clinical setting such as identifying men who should be (i) targeted for screening or (ii) with low risk disease who should not be managed with active surveillance or (iii) who need adjuvant systemic therapy.
Supplementary Material
Table 2A:
Odds ratio (OR)1 and p-value for association between SNP rs1910301 and risk of metastatic disease or prostate cancer death in three independent cohorts
| Cohort | OR for metastatic disease | P value |
|---|---|---|
| PHS/HPFS | 1.40 (1.05 – 1.84) | 0.02 |
| DFCI GC-EA | 1.35 (1.02 – 1.80) | 0.04 |
| FHCRC | 1.35 (0.97 – 1.85) | 0.07 |
| Meta-analysis of 3 cohorts [fixed effects model] | 1.37 (95% CI: 1.15–1.62) | 0.0003 |
PHS/HPFS: Physicians’ Health Study and Health Professionals Follow-up Study; DFCI GC: Dana-Farber Cancer Institute Gelb Center; EA: The ECOG-ACRIN Cancer Research Group; FHCRC: Fred Hutchinson Cancer Research Center.
OR modeled as ordinal for the minor allele
Acknowledgments
Funding: DOD W81XWH-11-1-0379 (CS). The Physicians’ Health Study was supported by grants CA34944, CA40360, CA097193, HL26490 and HL34595. The Health Professionals Follow-Up Study was supported by grants CA133891 and UM1CA167552. This study was additionally supported by CA136578, CA141298, CA131945, and P50CA090381. This work was supported by grants RO1 CA056678 (J.L. Stanford), R01 CA092579 (J.L. Stanford), R03 CA121871 (J.L. Stanford), RO3 CA137799 (J.L. Stanford). Partial financial support and drug supply by Sanofi. For the E3805: CHAARTED: Sanofi provided docetaxel for early use and financial grant support; NCI-CTEP and ECOG-ACRIN; Public Health Service Grants CA180794, CA180820, CA23318, CA66636, CA21115, CA49883, CA16116, CA21076, CA27525, CA13650, CA14548, CA35421, CA32102, CA31946, CA04919, CA107868, CA184734 and support from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services; Frontier Science Award. Prostate Cancer Foundation Mazzone Awards.
We are grateful to the participants and staff of the Physicians’ Health Study and Health Professionals Follow-Up Study for their valuable contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. We are grateful to E3805: CHAARTED Investigators and the patients who were accrued patients to the trial; We are grateful to the PRACTICAL Consortium investigators and patients.
Conflict of Interest Statement:
Drs Sweeney, Mucci, Lee, Börnigen, and Huttenhower hold a patent for rs1910301 as a biomarker in prostate cancer. No other potential conflicts were disclosed by the other authors.
Footnotes
Data Use and Consent Statement
Data was made available by data use agreements and are stored locally by the relevant researchers. All patients were consented for germline SNP analyses and secondary use of the data was conducted with the approval by DF/HCC IRB.
REFERENCES:
- 1.Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066–73. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM Jr., Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 2006;296:2329–35. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 8.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin.[see comment]. New England Journal of Medicine 1997;337:295–300. [DOI] [PubMed] [Google Scholar]
- 9.Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011;12:451–9. [DOI] [PubMed] [Google Scholar]
- 10.Ahearn TU, Pettersson A, Ebot EM, et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J Natl Cancer Inst 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein EA, Yousefi K, Haddad Z, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur Urol 2015;67:778–86. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008;9:1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med 2003;349:366–81. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh-Le MP, Fan CC, Karunamuni R, et al. A Genetic Risk Score to Personalize Prostate Cancer Screening, Applied to Population Data. Cancer Epidemiol Biomarkers Prev 2020;29:1731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer 2012;12:121–32. [DOI] [PubMed] [Google Scholar]
- 17.Min J, Zaslavsky A, Fedele G, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med 2010;16:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney C, Li L, Shanmugam R, et al. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res 2004;10:5501–7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Altuwaijri S, Deng F, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol 2009;175:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lessard L, Karakiewicz PI, Bellon-Gagnon P, et al. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res 2006;12:5741–5. [DOI] [PubMed] [Google Scholar]
- 21.Okera M, Bae K, Bernstein E, Cheng L, Lawton C, Wolkov H, Pollack A, Dicker A, Sandler H, Sweeney CJ. Evaluation of nuclear factor κB and chemokine receptor CXCR4 co-expression in patients with prostate cancer in the Radiation Therapy Oncology Group (RTOG) 8610. BJU Int 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gannon PO, Lessard L, Stevens LM, et al. Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. Eur J Cancer 2013;49:2441–8. [DOI] [PubMed] [Google Scholar]
- 23.Gerke T, Beltran H, Wang X, et al. Low Tristetraprolin Expression Is Associated with Lethal Prostate Cancer. Cancer Epidemiol Biomarkers Prev 2019;28:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rounbehler RJ, Berglund AE, Gerke T, et al. Tristetraprolin Is a Prognostic Biomarker for Poor Outcomes among Patients with Low-Grade Prostate Cancer. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanmugam R, Kusumanchi P, Cheng L, et al. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkappaB and generating reactive oxygen species. Prostate 2010;70:1074–86. [DOI] [PubMed] [Google Scholar]
- 26.Shui IM, Lindstrom S, Kibel AS, et al. Prostate cancer (PCa) risk variants and risk of fatal PCa in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Eur Urol 2014;65:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karyadi DM, Zhao S, He Q, et al. Confirmation of genetic variants associated with lethal prostate cancer in a cohort of men from hereditary prostate cancer families. Int J Cancer 2015;136:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bornigen D, Moon YS, Rahnavard G, et al. A reproducible approach to high-throughput biological data acquisition and integration. PeerJ 2015;3:e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bornigen D, Tyekucheva S, Wang X, et al. Computational Reconstruction of NFkappaB Pathway Interaction Mechanisms during Prostate Cancer. PLoS Comput Biol 2016;12:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia P, Zheng S, Long J, Zheng W, Zhao Z. dmGWAS: dense module searching for genome-wide association studies in protein-protein interaction networks. Bioinformatics 2011;27:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penney KL, Pyne S, Schumacher FR, et al. Genome-wide association study of prostate cancer mortality. Cancer Epidemiol Biomarkers Prev 2010;19:2869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh WK, Hayes J, Evan C, et al. Development of an integrated prostate cancer research information system. Clin Genitourin Cancer 2006;5:61–6. [DOI] [PubMed] [Google Scholar]
- 33.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J Epidemiol 2008;168:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanford JL, Wicklund KG, McKnight B, Daling JR, Brawer MK. Vasectomy and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 1999;8:881–6. [PubMed] [Google Scholar]
- 35.Dadaev T, Saunders EJ, Newcombe PJ, et al. Fine-mapping of prostate cancer susceptibility loci in a large meta-analysis identifies candidate causal variants. Nat Commun 2018;9:2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon JR, Selvaraj S, Yue F, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012;485:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creed JH MA, Gerke TA. epiTAD: a web application for visualizing high throughput chromosome conformation capture data in the context of genetic epidemiology. bioRxiv 243840.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penney KL, Shui IM, Feng Z, Sesso HD, Stampfer MJ, Stanford JL. Replication of a genetic variant for prostate cancer-specific mortality. Prostate Cancer Prostatic Dis 2015;18:260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FitzGerald LM, Zhao S, Leonardson A, et al. Germline variants in IL4, MGMT and AKT1 are associated with prostate cancer-specific mortality: An analysis of 12,082 prostate cancer cases. Prostate Cancer Prostatic Dis 2018;21:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berndt SI, Wang Z, Yeager M, et al. Two susceptibility loci identified for prostate cancer aggressiveness. Nat Commun 2015;6:6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto N, Shiota M, Tomisaki I, Minato A. Gene Polymorphism-related Individual and Interracial Differences in the Outcomes of Androgen Deprivation Therapy for Prostate Cancer. Clin Genitourin Cancer 2017. [DOI] [PubMed] [Google Scholar]
- 42.Whitman EJ, Pomerantz M, Chen Y, et al. Prostate cancer risk allele specific for African descent associates with pathologic stage at prostatectomy. Cancer Epidemiol Biomarkers Prev 2010;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard B, Muralidhar V, Chen YH, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiyozumi D, Sugimoto N, Sekiguchi K. Breakdown of the reciprocal stabilization of QBRICK/Frem1, Fras1, and Frem2 at the basement membrane provokes Fraser syndrome-like defects. Proc Natl Acad Sci U S A 2006;103:11981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan Q, Huang RF, Liang XH, et al. FRAS1 knockdown reduces A549 cells migration and invasion through downregulation of FAK signaling. Int J Clin Exp Med 2014;7:1692–7. [PMC free article] [PubMed] [Google Scholar]
- 46.Lefebvre C, Bachelot T, Filleron T, et al. Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med 2016;13:e1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelletier J, Thomas G, Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer 2018;18:51–63. [DOI] [PubMed] [Google Scholar]
- 48.Morita M, Suzuki T, Nakamura T, Yokoyama K, Miyasaka T, Yamamoto T. Depletion of mammalian CCR4b deadenylase triggers elevation of the p27Kip1 mRNA level and impairs cell growth. Mol Cell Biol 2007;27:4980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.