Abstract
Background:
The incidence of oral squamous cell carcinoma (OSCC) has been increasing day by day in the Southeast Asian countries. There is variation in the incidence rates in various parts of the world, with the highest recorded in Southeast Asian countries such as Sri Lanka, India, Bangladesh, and Pakistan. The survival rate for OSCC has remained generally unchanged in the past three decades, underlining the need for more biomarkers to be developed to aid prognostication and effective treatment planning and management. The prognostic potential of epithelia-cadherin (E-cadherin) expression in OSCCs has been variable in previous studies has been correlated with improved prognosis in other cancers. The aim of the present study was to investigate and analyze the expression of E-cadherin in different histopathological grades OSCC, to understand its potential as prognostic biomarker of most common oral cancer.
Materials and Methods:
E-cadherin expression was examined by immunohistochemistry in 35 cases of OSCC of the buccal mucosa and 5 of normal buccal mucosa.
Results:
In our study, E-cadherin expression appeared in widely differentiated squamous cell carcinoma (130.75 ± 30.64) moderately differentiated squamous cell carcinoma (123.66 ± 13.17), and poorly differentiated squamous cell carcinoma (88.52 ± 30.11).
Conclusions:
E-cadherin expression is reduced with higher grades of OSCC. However, the present results suggest that E-cadherin expression may be useful as prognostic markers for OSCC.
Keywords: Epithelia-cadherin, immunohistochemistry, oral squamous cell carcinoma
INTRODUCTION
Cancer represents a global public health problem, which is currently the third cause of death resulting from diseases. Squamous cell carcinoma is the most frequently reported malignant neoplasia that affects the oral cavity. Globally, oral cancer is the sixth most common cancer with an incidence of more than 500,000 cases annually, of which 62% occurs in developing countries. In India, oral cancer is one of the common cancer affecting the individuals.[1]
This disease process has multifaceted etiology. A plethora of lifestyle and environmental factors are associated with its development, the synergistic effect of tobacco and alcohol compounds the risk.[2,3,4]
Although histological analysis of hematoxylin and eosin (H & E) stained tissue section remains at the core of the practice of head-and-neck pathology, immunohistochemistry has become a powerful tool in the armamentarium of the pathologists of newer generation, which may use to facilitate the diagnosis of various neoplastic processes. It identifies cellular or tissue constituents (antigen) by means of antigen–antibody interaction.
Epithelia-cadherin (E-cadherin) is a transmembrane glycoprotein dependent on calcium. E-cadherin is present in most epithelial cells that play an important role in intercellular adhesion, maintenance of cellular polarity and tissue architecture.[5,6,7] Alteration in E-cadherin expression is associated with the loss of differentiation, acquisition of an invasive phenotype and poor clinical course of many types of cancer.[4]
Therefore study has been designed with this aim in mind to evaluate the E-cadherin expression in different grades of oral squamous cell carcinoma (OSCC) and to compare, corroborate and correlate E-cadherin expression in different grades of OSCC for better understanding of the biological behavior of the lesion and in turn act as a prognostic marker.
MATERIALS AND METHODS
The patients attended at the out-patient department were screened clinically with a view to detect the presence of OSCC as per the clinical criterion. Initially, thirty five patients of OSCC were selected for the study. The inclusion criteria are followed like individuals within age limit 40 years–70 years with no other medical history and without any deleterious oral habits. Five samples having oral normal epithelium obtained from patients, who did not have cancer were used as a control for the immune-histochemical strainers. Special care was taken to record the clinic pathological data of the patients suffering from OSCC with a view to facilitate the accurate results.
Incisional biopsy was performed from representative sites of the lesion after achieving local anaesthesia. Initially, after 24 h of fixation in 10% buffered formalin, tissue blocks were prepared. Sections of 5 μm thickness were cut using rotary microtome. These sections were then placed on albumin coated slides for routine H & E staining and on poly lysine-coated slides for immunohistochemical staining with E-cadherin.
H & E staining done by Harris's haematoxylin and immunohistochemistry staining done by E-Cadherin-ab40772, abcam, USA, Rabbit monoclonal antibody in 1:500 dilution. Visualization was performed using freshly prepared diaminobenzidine chromogen for 10 min. The slides were finally counterstained with Harris hematoxylin.
Dysplastic fields with cellular and nuclear alterations from H & E were selected. Similar fields as that of H- & E-stained sections were also chosen from E-cadherin stained sections. Photomicrographs of selected sites from the tissue sections (both H & E and Immunohistochemical [IHC]) of different grades of OSCC and normal oral mucosa (NOM) were taken with an inverted microscope in bright field mode fitted with a Computer aided camera [Figures 1a, b and 2a, b and 3a, b]. Gray scale value obtained with computer software with appropriate standardization.
Figure 1.
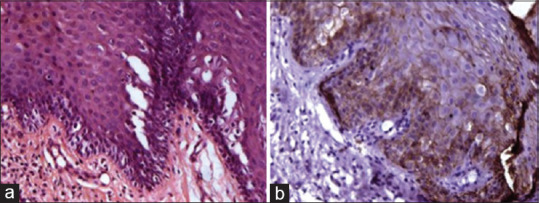
(a) Photomicrograph showing well-differentiated oral squamous cell carcinoma tissue section stained with H & E stain. (b) Photomicrograph showing well-differentiated oral squamous cell carcinoma tissue section stained with epithelia-cadherin
Figure 2.
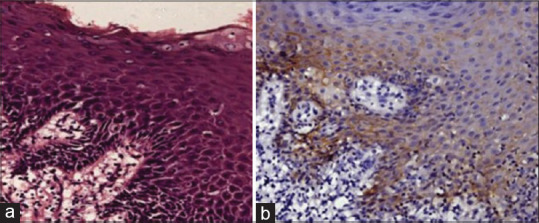
(a) Photomicrograph showing moderately differentiated squamous cell carcinoma tissue section stained with H & E stain. (b) Photomicrograph showing moderately differentiated squamous cell carcinoma tissue section stained with epithelia-cadherin
Figure 3.
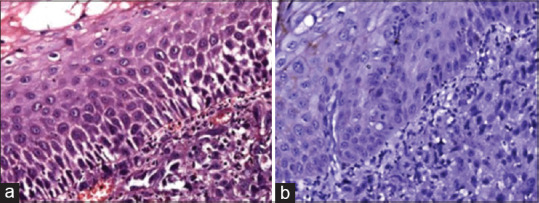
(a) Photomicrograph showing poorly differentiated squamous cell carcinoma tissue section stained with H & E stain. (b) Photomicrograph showing poorly differentiated squamous cell carcinoma tissue section stained with epithelia-cadherin
Statistical analysis
To have an idea regarding statistical importance and significance, both descriptive statistics and probabilistic statistics have been employed. In descriptive statistics, primarily, the data were plotted as bar diagram. Thereafter, Whisker's wide notched box plot was drawn based on the distribution of the data in terms of minimum-maximum and percentile distribution. Thereafter, to understand the statistical significance, Unpaired T-test was performed.
RESULTS
The data obtained from the present study of E-cadherin expression in NOM and different histopathological grades of OSCC were evaluated semi-quantitatively and compiled, tabulated, and subjected to statistical analysis. The results that were obtained are presented in the following manner. A total number of 35 cases of OSCC of different histopathological grades and 5 normal individuals including both genders, with age ranging from 40 to 70 years, were included in this study.
The staining patterns and localization of E-cadherin in NOM were brightly decorated within the epithelium in a circumferentially membranous basolateral fashion. No cytoplasmic or nuclear staining was noted in all five cases, the basal and parabasal cells displayed the greatest intensity of staining, whereas the most external and differentiated layers were not stained. Furthermore, there was no stromal staining.
The semi-quantitative evaluation of E-cadherin expression in cell membrane of NOM is found to be 130.8, while in widely differentiated squamous cell carcinoma (WDOSCC) it was 123.7, moderately differentiated squamous cell carcinoma (MDOSCC) it was 103.6 and in poorly differentiated squamous cell carcinoma (PDOSCC) 88.52. There was a no significant relation between the cytoplasmic expression of E-cadherin and the histological degree of OSCC. No nuclear staining was detected.
Bar diagram representation of grey scale value [Figure 4] for membranous expression of E-cadherin in NOM (basal and supra basal layers) and different histopathological grades of OSCC (in the islands) depicted that the mean grey scale value for membranous expression of E-Cadherin was found to be gradually decreasing in NOM to WDOSCC to MDOSCC and PDOSCC. Whisker's Notched box plot was derived for better understanding of the concentration of the available data, the maximum and minimum of the range of the data and its median. It demonstrates that there is a decrease in the median value of the gray scale intensity from NOM to WDOSCC, MDOSCC and PDOSCC [Figure 5]. When P value of the control (i.e., NOM) was compared with the diseased ones (WDOSCC, MDOSCC, PDOSCC) and in-between various grades of OSCC, [Figure 6] by using Unpaired T-test, it was found that all of them (p2, p3, p4, p5 and p6) were significant; except p1 as depicted in Figure 6.
Figure 4.
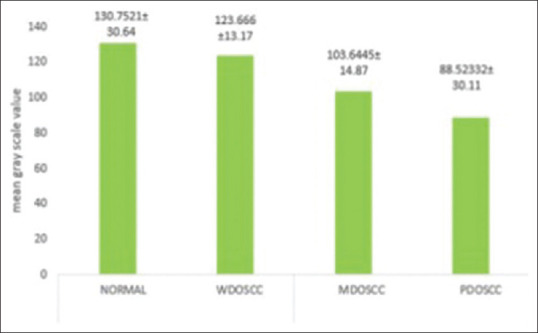
Bar diagram representation of gray scale intensity for membranous expression of epithelia-cadherin in normal oral mucosa (basal and supra basal layers) and different grades of oral squamous cell carcinoma (in the islands)
Figure 5.
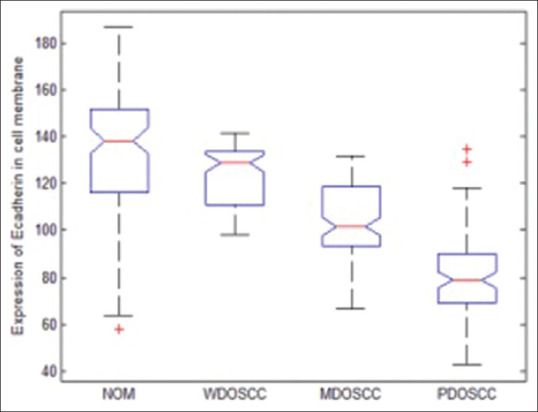
The Whisker's notched box plot representation of the distribution of membranous gray scale intensity of epithelia-cadherin in normal oral mucosa and different grades of oral squamous cell carcinoma
Figure 6.
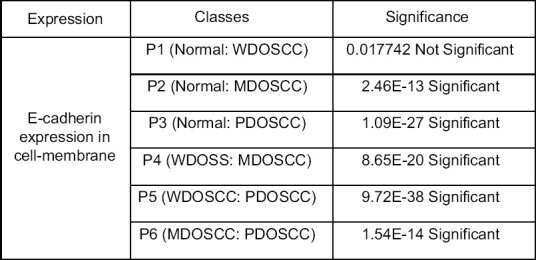
Comparison of the P value of cell cytoplasmic expression of epithelia-cadherin between normal oral mucosa and various grades of oral squamous cell carcinoma; and comparison in between various grades of oral squamous cell carcinoma
DISCUSSION
The most common type of cancer involving the oral cavity is squamous cell carcinoma (OSCC), which accounts for approximately 90% of all oral malignancies. Although the postoperative life quality in these patients is improved in recent years, the 5-year survival rate has not been raised significantly.
At present, the diagnosis of OSCC is based on comprehensive clinical examination and histopathological analysis of suspicious areas. But, heterogeneous complex, biological nature of OSCC and the qualitative microscopic analysis of cellular and nuclear pleomorphism are always not focused in nature and unable to determine the prognosis of the disease processes. Currently, biomarkers related to cell–cell adhesion are of particular interest as they may be able to predict the various early sequences in the development of OSCC. Thus, in future, specific and personalized diagnostics can guide treatment against the disease and thereby consequently improving the chances of cure.[3,4,5,6,7]
IHC studies using anti E-cadherin antibody that participate in the cell-to-cell adhesion process in epithelial tissues, have been performed to understand the significance of the expression of these proteins in human cancers so as to use as the prognostic marker. The comparative evaluation between the NOM and different histopathological grades of OSCC to that of their expression of E-cadherin was corroborated through IHC staining. Structural integrity is a key property of epithelial tissues which is maintained by cell-cell interactions. The zonula adherence provides lateral adhesion between epithelial cells which is composed of the transmembrane cell adhesion molecule E-cadherin. On the cytoplasmic side, the tail of E-cadherin is bound to catenin (e.g., α-, β-catenin). The resulting E-cadherin–catenin complex binds to vinculin and α-actin and is required for the interaction of cadherin with the actin filaments of the cytoskeleton. Studies by various researchers have established that tumor invasion and metastasis involve a series of steps including proteolytic degradation of basement membrane and extracellular matrix, changes in cell adhesion and tumor cells movement.[2,7,8,9]
In this study E-cadherin expression appeared in WDOSCC (130.75 ± 30.64), MDOSCC (123.66 ± 13.17) and PDOSCC (88.52 ± 30.11) which significantly shows gradual decrease or loss of E-cadherin expression in cell membrane as the disease processes from normal to PDOSCC. This expression was closely correlated with the histological differentiation of the OSCC.
Various researchers also demonstrated a reduction of E-cadherin expression in OSCC compared to NOM.[7,8,9] This study also revealed a gradual reduction of E-cadherin expression demonstrated in gray scale value.
A significant correlation of P value of membranous expression of E-cadherin between NOM and various grades of OSCC; and also between various grades was noted. Therefore, it can be stated that the strong expressions of E-cadherin are usually found in membranes of basal cell layers which are decreasing gradually when it gradually progresses toward the disease process. The expression of E-cadherin significantly correlated with the histological degree of OSCC. A significant correlation was seen in normal and aberrant expressions between E-cadherin.
E-cadherin is the most important mediator in maintaining the epithelial structure forming the cadherin/catenin complex within cells by binding to catenin. This function of E-cadherin was proven experimentally, in which intercellular binding increases so that tumor invasiveness could be prevented when E-cadherin cDNA is transplanted in cell lines with poor prognosis having no expression of E-cadherin and tumor invasiveness could be recovered with the introduction of E-cadherin antibody.[8] However, these data are not always reliable so that researches have been focused on finding more objective and reliable diagnostic and tents according to the ideal marker. The treatment outcomes could be increased drastically in high risk-patients through more aggressive treatment such as surgery combined with adjunctive radiotherapy, chemotherapy or hormone therapy and various economic and medical damages could be reduced by preventing unnecessary treatment in low-risk patients.
CONCLUSIONS
The results of the present study proved that the aberrant expression of E-cadherin is the significant factor in predicting the histological grade in patients with OSCC. Thus, it could be stated that analyzing the expression patterns of E-cadherin complex is very useful with other previously determined clinic-pathologic indices in differential grades of OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to acknowledge Prof. (Dr) R. R. Paul, Prof. (Dr.) Mousumi Pal, H.O.D., Prof. (Dr.) S. Kundu, Professor, Department of Oral and Maxillofacial Pathology, and Prof. (Dr.) Jyotirmoy Chatterjee, Professor, SMST IIT Kharagpur for giving immense support and help throughout the study.
REFERENCES
- 1.Wünsch-Filho V. The epidemiology of oral and pharynx cancer in Brazil. Oral Oncol. 2002;38:737–46. doi: 10.1016/s1368-8375(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Global Cancer Facts & Figures. 4th Edition. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 3.Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–30. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vartanian JG, Carvalho AL, de Araújo Filho MJ, Junior MH, Magrin J, Kowalski LP. Predictive factors and distribution of lymph node metastasis in lip cancer patients and their implications on the treatment of the neck. Oral Oncol. 2004;40:223–7. doi: 10.1016/j.oraloncology.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998;4:70–7. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 6.Krisanaprakornkit S, Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma? ISRN Oncol. 2012;2012:681469. doi: 10.5402/2012/681469. doi: 10.5402/2012/681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow V, Yuen AP, Lam KY, Tsao GS, Ho WK, Wei WI. A comparative study of the clinicopathological significance of E-cadherin and catenins (alpha, beta, gamma) expression in the surgical management of oral tongue carcinoma. J Cancer Res Clin Oncol. 2001;127:59–63. doi: 10.1007/s004320000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda G, Sunakawa H, Nakamori K, Shinya T, Tsuhako W, Tamura Y, et al. Aberrant expression of beta- and gamma-catenin is an independent prognostic marker in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2006;35:356–61. doi: 10.1016/j.ijom.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Lopes FF, da Costa Miguel MC, Pereira AL, da Cruz MC, de Almeida Freitas R, Pinto LP, et al. Changes in immunoexpression of E-cadherin and beta-catenin in oral squamous cell carcinoma with and without nodal metastasis. Ann Diagn Pathol. 2009;13:22–9. doi: 10.1016/j.anndiagpath.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Chen XY, Huang KJ, Wu WD, Jiang T, Cao J, et al. Expression of FoxM1 and the EMT-associated protein E-cadherin in gastric cancer and its clinical significance. Oncol Lett. 2016;12:2445–50. doi: 10.3892/ol.2016.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]


