Abstract
Background
Alfalfa (Medicago sativa L.) production decreases under salt stress. Identification of genes associated with salt tolerance in alfalfa is essential for the development of molecular markers used for breeding and genetic improvement.
Result
An RNA-Seq technique was applied to identify the differentially expressed genes (DEGs) associated with salt stress in two alfalfa cultivars: salt tolerant ‘Halo’ and salt intolerant ‘Vernal’. Leaf and root tissues were sampled for RNA extraction at 0 h, 3 h, and 27 h under 12 dS m− 1 salt stress maintained by NaCl. The sequencing generated a total of 381 million clean sequence reads and 84.8% were mapped on to the alfalfa reference genome. A total of 237 DEGs were identified in leaves and 295 DEGs in roots of the two alfalfa cultivars. In leaf tissue, the two cultivars had a similar number of DEGs at 3 h and 27 h of salt stress, with 31 and 49 DEGs for ‘Halo’, 34 and 50 for ‘Vernal’, respectively. In root tissue, ‘Halo’ maintained 55 and 56 DEGs at 3 h and 27 h, respectively, while the number of DEGs decreased from 42 to 10 for ‘Vernal’. This differential expression pattern highlights different genetic responses of the two cultivars to salt stress at different time points. Interestingly, 28 (leaf) and 31 (root) salt responsive candidate genes were highly expressed in ‘Halo’ compared to ‘Vernal’ under salt stress, of which 13 candidate genes were common for leaf and root tissues. About 60% of DEGs were assigned to known gene ontology (GO) categories. The genes were involved in transmembrane protein function, photosynthesis, carbohydrate metabolism, defense against oxidative damage, cell wall modification and protection against lipid peroxidation. Ion binding was found to be a key molecular activity for salt tolerance in alfalfa under salt stress.
Conclusion
The identified DEGs are significant for understanding the genetic basis of salt tolerance in alfalfa. The generated genomic information is useful for molecular marker development for alfalfa genetic improvement for salt tolerance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-021-03201-4.
Keywords: Alfalfa, Differentially expressed genes, Salt stress, Transcriptome
Background
Alfalfa (Medicago sativa L.) is an important forage legume in the world. Cultivated alfalfa is an outcrossing autotetraploid (2n = 4x = 32) with a genome size of 800–1000 Mb [1]. Although alfalfa is regarded as moderately tolerant to salinity [2], alfalfa yield reduces by approximately 6–7% for each dS m− 1 increase above a salinity of 2 dS m− 1 [3]. To stabilize alfalfa production under saline regions, the development of superior salt tolerant cultivars becomes an important breeding goal. Identification of candidate genes for salt tolerance can increase the accuracy of parental selection as this trait has low heritability [4]. Salt tolerance is a complex trait controlled by multiple genes, involving different signaling pathways, osmotic tolerance, ion transport, compartmentalization of salt ions in vacuoles, the synthesis of plant hormones and photosynthesis [5].
Next-generation sequencing technologies have been used to identify candidate genes involved in salt tolerance of alfalfa. Transcriptomic studies in the 1-week old root tissue of alfalfa under salt stress found 1165 DEGs, including 86 transcription factors, which are responsible for stress tolerance, kinase, hydrolase, and oxidoreductase activities [6]. Luo et al. [7] identified 8861 DEGs in 12-day old seedlings of alfalfa under salt stress, which are responsible for ion homeostasis, antiporter, signal perception, signal transduction, transcriptional regulation, and antioxidative defense. Lei et al. [8] revealed 2237 DEGs between salt tolerant and intolerant alfalfa cultivars and found a salt tolerant alfalfa cultivar maintained relatively stable expression of genes responsible for reactive oxygen species and Ca2+ pathway, phytohormone biosynthesis and Na+/K+ transport under stress. Gruber et al. [9], using bulked genotypes as replications, studied transcriptomes in alfalfa and found genes responsible for numerous functions in a salt intolerant alfalfa cultivar. In recent years, genetic modification of certain genes controlling salt tolerance have also been conducted in alfalfa. Overexpression of salt responsive genes or transcription factors had improved salt tolerance in transgenic alfalfa. Such genes include Alfin1 [10], AVP1 [11], GmDREB1 [12], SsNHX1 [13], TaNHX2 [14], GsCBRLK [15], GsZFP1 [16], OsAPX2 [17], SeNHX1 [18], AtNDPK2 [19], AgcodA [20], and GsWRKY20 [21]. These studies have advanced our understanding of the genetic control for salt tolerance in alfalfa. However, most studies mainly focused on single time point sampling of root tissue at the seedling stage after salt stress, limiting the analysis of the temporal expression of genes affecting salt tolerance.
Tissue specific protein induction is regulated during salinity stress and is unique to roots and shoots [22]. Thus, there should be tissue specific transcriptomic responses [23–25]. Although the root is the first receptor of salt stress [6, 7], leaf tissue is the main energy source for plant growth and stress tolerance during active growth and developmental stages. To advance our knowledge about the temporal gene expression in different tissues for the genetic control under salt stress between tolerant and intolerant cultivars, we conducted a RNA-Seq analysis with the objective to simultaneously analyze gene expressions of leaf and root tissues of two alfalfa cultivars with different tolerance to salinity after exposing them to 12 dS m− 1 of electrical conductivity salt stress for 0 h, 3 h, and 27 h. The analysis was fruitful with the identification of many unique genes conditioning salt tolerance in alfalfa.
Results
High throughput sequencing and assembly
A total of 408 million raw sequence reads were generated using the Illumina HiSeq sequencing platform. The reads were reduced to 93.5% (381 million clean reads) by removing adapter contamination and reads with length lower than 36 bp (Table 1). There were 84.8% of clean reads mapped to the alfalfa reference genome using STAR (v2.6.1a). The samples showed high percentages (78.8–92.4%) of mapping with the alfalfa reference genome except for ‘Halo’ root tissue sampled at 27 h of salt stress.
Table 1.
Summary of Illumina sequencing data and mapped sequence reads for the assayed alfalfa samples
| Genotype | Tissue | Treatment | Biological replicate | Total reads | Mapped reads | Mapping rate (%) |
|---|---|---|---|---|---|---|
| Halo | Leaf | Control | 1 | 7,235,661 | 6,275,026 | 86.7 |
| Control | 2 | 7,462,991 | 6,607,722 | 88.5 | ||
| Control | 3 | 7,532,603 | 6,371,474 | 84.6 | ||
| Control | 4 | 7,163,647 | 6,286,906 | 87.8 | ||
| Stressed (3h) | 1 | 7,170,087 | 6,291,489 | 87.7 | ||
| Stressed (3h) | 2 | 8,619,083 | 7,531,679 | 87.4 | ||
| Stressed (3h) | 3 | 7,275,223 | 6,453,594 | 88.7 | ||
| Stressed (3h) | 4 | 7,346,150 | 6,417,574 | 87.4 | ||
| Stressed (27h) | 1 | 7,234,036 | 5,968,374 | 82.5 | ||
| Stressed (27h) | 2 | 7,186,154 | 6,225,839 | 86.6 | ||
| Stressed (27h) | 3 | 6,256,894 | 5,471,745 | 87.5 | ||
| Stressed (27h) | 4 | 5,696,229 | 4,741,642 | 83.2 | ||
| Root | Control | 1 | 7,930,008 | 6,833,311 | 86.2 | |
| Control | 2 | 7,568,654 | 6,104,814 | 80.7 | ||
| Control | 3 | 10,017,590 | 9,054,027 | 90.4 | ||
| Control | 4 | 6,523,142 | 5,561,719 | 85.3 | ||
| Stressed (3h) | 1 | 9,003,316 | 7,662,274 | 85.1 | ||
| Stressed (3h) | 2 | 11,023,879 | 9,201,753 | 83.5 | ||
| Stressed (3h) | 3 | 9,647,653 | 8,529,106 | 88.4 | ||
| Stressed (3h) | 4 | 6,201,499 | 4,884,633 | 78.8 | ||
| Stressed (27h) | 1 | 6,563,910 | 4,983,668 | 75.9 | ||
| Stressed (27h) | 2 | 8,321,924 | 3,727,203 | 44.8 | ||
| Stressed (27h) | 3 | 9,424,651 | 7,313,948 | 77.6 | ||
| Stressed (27h) | 4 | 7,942,407 | 2,854,933 | 35.9 | ||
| Vernal | Leaf | Control | 1 | 7,085,736 | 6,147,440 | 86.8 |
| Control | 2 | 7,149,929 | 6,449,664 | 90.2 | ||
| Control | 3 | 8,108,009 | 7,362,827 | 90.8 | ||
| Control | 4 | 10,775,421 | 9,953,409 | 92.4 | ||
| Stressed (3h) | 1 | 5,372,062 | 4,901,710 | 91.2 | ||
| Stressed (3h) | 2 | 6,180,723 | 5,477,930 | 88.6 | ||
| Stressed (3h) | 3 | 7,355,464 | 6,390,995 | 86.9 | ||
| Stressed (3h) | 4 | 6,775,443 | 6,098,490 | 90.0 | ||
| Stressed (27h) | 1 | 11,398,645 | 10,388,765 | 91.1 | ||
| Stressed (27h) | 2 | 7,517,258 | 6,754,908 | 89.9 | ||
| Stressed (27h) | 3 | 8,128,644 | 7,280,110 | 89.6 | ||
| Stressed (27h) | 4 | 7,713,476 | 7,007,553 | 90.8 | ||
| Root | Control | 1 | 11,004,685 | 9,744,748 | 88.6 | |
| Control | 2 | 10,402,513 | 9,091,041 | 87.4 | ||
| Control | 3 | 8,070,744 | 7,001,864 | 86.8 | ||
| Control | 4 | 8,936,244 | 7,616,107 | 85.2 | ||
| Stressed (3h) | 1 | 8,293,827 | 7,159,751 | 86.3 | ||
| Stressed (3h) | 2 | 8,722,568 | 7,705,076 | 88.3 | ||
| Stressed (3h) | 3 | 9,769,559 | 8,854,805 | 90.6 | ||
| Stressed (3h) | 4 | 6,402,547 | 5,317,429 | 83.1 | ||
| Stressed (27h) | 1 | 3,669,213 | 3,121,348 | 85.1 | ||
| Stressed (27h) | 2 | 9,000,614 | 7,858,878 | 87.3 | ||
| Stressed (27h) | 3 | 8,661,229 | 7,569,795 | 87.4 | ||
| Stressed (27h) | 4 | 8,640,454 | 7,481,651 | 86.6 | ||
| Total | 381,482,398 | 324,090,747 | ||||
| Average (Control) | 8,310,474 | 7,278,881 | 87.4 | |||
| Average (3 h) | 7,882,443 | 6,804,893 | 87.0 | |||
| Average (27 h) | 7,709,734 | 6,171,898 | 80.1 | |||
| Average | 7,947,550 | 6,751,891 | 84.8 | |||
Differentially expressed genes (DEGs)
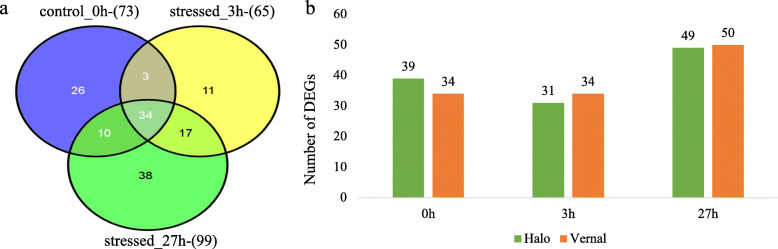
In leaf tissue, there were 237 DEGs identified between the two alfalfa cultivars. Among them, 34 DEGs were expressed at all three time points (0 h, 3 h, and 27 h) and 17 DEGs expressed after exposing to salt stress (3 h and 27 h) (Fig. 1a, b; Additional file 1: Table S1). Of these DEGs, 39, 31, and 49 DEGs were specific to ‘Halo’, and 34, 34, and 50 DEGs were specific to ‘Vernal’ at 0 h, 3 h and 27 h of salt stress, respectively (Fig. 1b). The number of DEGs in leaf tissue decreased after 3 h compared to 0 h treatment. Then, the number of DEGs increased from 3 h to 27 h of salt stress for both cultivars (Fig. 1b).
Fig. 1.
Differentially expressed genes (DEGs) between salt tolerant ‘Halo’ and intolerant ‘Vernal’ alfalfa cultivars in leaf tissue at three different time-points: control (0 h), 3 h and 27 h after salt stress. a Venn diagram for number of DEGs in leaf tissue of two alfalfa cultivars (‘Halo’ vs. ‘Vernal’) at three different time-points (0 h, 3 h, and 27 h). Number in the parenthesis represents total number of DEGs. Numbers in each intersection represent the number of DEGs detected in two or three time points. b Number of DEGs identified in leaf tissue at each time-point (0 h, 3 h, and 27 h) between tolerant and intolerant alfalfa cultivars
In root tissue, a total of 295 DEGs were identified between the two alfalfa cultivars. There were 33 DEGs expressed at all three time points and 5 DEGs expressed after exposing to salt stress (Fig. 2a, b; Additional file 1: Table S2). Of these DEGs, 68, 55, and 56 DEGs were specific to ‘Halo’ at 0 h, 3 h and 27 h, whereas 64, 42, and 10 DEGs were specific to ‘Vernal’, respectively (Fig. 2b). The number of DEGs in root tissue decreased at 3 h as compared to 0 h treatment for both cultivars, but the decrease was greater for ‘Vernal’ than for ‘Halo’. The main difference of DEGs between the two cultivars in the root was from 3 h to 27 h, with a 76% decrease in DEGs in ‘Vernal’ while there was almost no change for ‘Halo’ (Fig. 2b). After 27 h of salt stress in root tissue, the number of DEGs in ‘Halo’ were five times more than that of ‘Vernal’ (Fig. 2b).
Fig. 2.
Differentially expressed genes (DEGs) between salt tolerant ‘Halo’ and intolerant ‘Vernal’ alfalfa cultivars in root tissue at three different time-points: control (0 h), 3 h and 27 h after salt stress. a Venn diagram for number of DEGs in root tissue of two alfalfa cultivars (‘Halo’ vs. ‘Vernal’) at three different time-points (0 h, 3 h, and 27 h). Number in the parenthesis represents total number of DEGs. Numbers in each intersection represent the number of DEGs detected in two or three time points. b number of DEGs identified in root tissue at each time-point (0 h, 3 h, 27 h) between tolerant and intolerant alfalfa cultivars
Functional annotation of DEGs
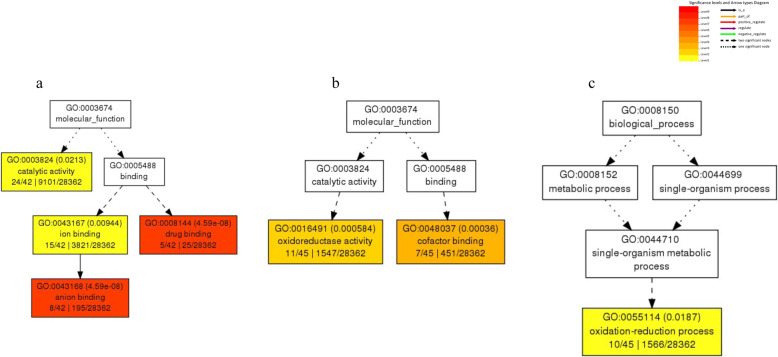
To understand what biological processes are implicated in response to salinity, we assigned the DEGs to known Gene Ontology (GO) categories. Among 237 DEGs in leaf tissue, 148 (62.4%) DEGs were assigned to three ontology classes. In ‘Halo’ leaf tissue, the most noticeable DEGs [false discovery rate (FDR) < 0.05] were “drug binding” (GO:0008144, 5), “anion binding” (GO:0043168, 8), “ion binding” (GO:0043167, 15) and “catalytic activity” (GO:0003824, 24) among molecular functions (Fig. 3a) while there was no significantly enriched functional groups from biological process and cellular component. For ‘Vernal’ leaf tissue, “cofactor binding” (GO:0048037, 7) and “oxidoreductase activity” (GO:0016491, 11) were predominant (FDR < 0.05) among molecular functions (Fig. 3b) and “oxidation-reduction process” (GO:0055114, 10) (Fig. 3c) in biological process, but there was not any significantly enriched functional groups from cellular component.
Fig. 3.
Gene ontology (GO) analysis of differentially expressed genes in leaf tissues of two alfalfa cultivars in response to salt stress (a, salt tolerant ‘Halo’ alfalfa; b and c, salt intolerant ‘Vernal’ alfalfa). Each box shows the GO term number, p-value in parenthesis, and GO term
Among the 295 DEGs in root tissue, 180 (61.0%) DEGs were annotated to three gene ontology classes. In root tissue of ‘Halo’, “anion binding” (GO:0043168, 9), “ion binding” (GO:0043167, 18) , “structural constituent of ribosome” (GO:0003735, 7), and “structural molecule activity” (GO:0005198, 7) among molecular functions (Fig. 4a) were noticeable, while “organo-nitrogen compound metabolic process” (GO:1901564, 15) was dominant among biological processes (Fig. 4b). “Ribosome” (GO:0005840, 7), “ribonucleoprotein complex” (GO:1990904, 8), “intracellular ribonucleoprotein complex” (GO:0030529, 8) were predominant in cellular components (Fig. 4c). For root tissue of ‘Vernal’, “anion binding” (GO:0043168, 9) and “drug binding” (GO:0008144, 5) (Fig. 4d) were significantly (FDR < 0.05) enriched, while no other functional group from biological processes and cellular components.
Fig. 4.
Gene ontology (GO) analysis of differentially expressed genes in leaf tissues of two alfalfa cultivars in response to salt stress (a, salt tolerant ‘Halo’ alfalfa; b and c, salt intolerant ‘Vernal’ alfalfa). Each box shows the GO term number, p-value in parenthesis, and GO term
To identify pathways involved in salt tolerance, we carried out Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis of the DEGs. In total, 64 (27%) DEGs from leaf tissue and 86 (29.15%) DEGs from root tissue were assigned to 65 KEGG pathways (Table 2). In both tissues, the most significant DEGs were represented in the pathways of metabolism and biosynthesis of secondary metabolites. Of these, five pathways were common among different time points and alfalfa tissues. The highest level of enriched DEGs were in 14 pathways in leaf tissue and 6 pathways in root tissue after 27 h of salt stress. Among these pathways, the three highest enriched DEGs were involved in plant hormone signal transduction.
Table 2.
Number of differentially expressed genes (DEGs) and corresponding pathways in leaf and root of salt tolerant ‘Halo’ and salt intolerant ‘Vernal’ alfalfa cultivars at 0, 3 and 27 h of salt stress
| Pathway ID | The number of differentially expressed genes | Pathway terms | |||||
|---|---|---|---|---|---|---|---|
| HL0vsVL0 | HL3vsVL3 | HL27vsVL27 | HR0vsVR0 | HR3vsVR3 | HR27vsVR27 | ||
| K00130 | 0 | 0 | 0 | 1 | 1 | 1 | betB, gbsA; betaine-aldehyde dehydrogenase |
| K00276 | 0 | 0 | 1 | 0 | 0 | 0 | AOC3, AOC2, tynA; primary-amine oxidase |
| K00430 | 0 | 0 | 1 | 0 | 0 | 0 | E1.11.1.7; peroxidase |
| K00454 | 0 | 0 | 1 | 0 | 0 | 0 | LOX2S; lipoxygenase |
| K00522 | 0 | 0 | 1 | 0 | 0 | 0 | FTH1; ferritin heavy chain |
| K00549 | 1 | 1 | 1 | 1 | 1 | 1 | metE; 5-methyltetrahydropteroyltriglutamate--homocysteine methyltransferase |
| K00660 | 0 | 0 | 0 | 0 | 0 | 1 | CHS; chalcone synthase |
| K00915 | 0 | 0 | 0 | 1 | 0 | 0 | IPMK, IPK2; inositol-polyphosphate multikinase |
| K01507 | 0 | 0 | 0 | 1 | 1 | 1 | ppa; inorganic pyrophosphatase |
| K01535 | 0 | 0 | 1 | 1 | 0 | 0 | PMA1, PMA2; H+-transporting ATPase |
| K01623 | 0 | 0 | 0 | 1 | 1 | 0 | ALDO; fructose-bisphosphate aldolase, class I |
| K01823 | 1 | 1 | 1 | 1 | 1 | 0 | idi, IDI; isopentenyl-diphosphate Delta-isomerase |
| K01859 | 0 | 0 | 0 | 0 | 0 | 1 | E5.5.1.6; chalcone isomerase |
| K02639 | 1 | 1 | 2 | 1 | 1 | 0 | petF; ferredoxin |
| K02721 | 1 | 0 | 1 | 0 | 0 | 0 | psbW; photosystem II PsbW protein |
| K02893 | 1 | 0 | 0 | 1 | 2 | 1 | RP-L23Ae, RPL23A; large subunit ribosomal protein L23Ae |
| K02906 | 0 | 0 | 1 | 0 | 0 | 0 | RP-L3, MRPL3, rplC; large subunit ribosomal protein L3 |
| K02925 | 0 | 0 | 1 | 0 | 0 | 0 | RP-L3e, RPL3; large subunit ribosomal protein L3e |
| K02971 | 0 | 0 | 1 | 1 | 1 | 0 | RP-S21e, RPS21; small subunit ribosomal protein S21e |
| K02981 | 0 | 0 | 0 | 1 | 0 | 0 | RP-S2e, RPS2; small subunit ribosomal protein S2e |
| K02985 | 1 | 0 | 0 | 0 | 0 | 0 | RP-S3e, RPS3; small subunit ribosomal protein S3e |
| K02991 | 0 | 0 | 0 | 1 | 1 | 0 | RP-S6e, RPS6; small subunit ribosomal protein S6e |
| K03231 | 2 | 2 | 2 | 2 | 2 | 2 | EEF1A; elongation factor 1-alpha |
| K03283 | 0 | 0 | 0 | 0 | 0 | 1 | HSPA1s; heat shock 70kDa protein 1/2/6/8 |
| K03364 | 0 | 0 | 1 | 0 | 0 | 0 | CDH1; cell division cycle 20-like protein 1, cofactor of APC complex |
| K05546 | 0 | 0 | 0 | 1 | 1 | 0 | GANAB; mannosyl-oligosaccharide alpha-1,3-glucosidase |
| K06617 | 0 | 0 | 0 | 0 | 1 | 0 | E2.4.1.82; raffinose synthase |
| K07374 | 0 | 0 | 0 | 1 | 0 | 0 | TUBA; tubulin alpha |
| K07466 | 1 | 1 | 1 | 1 | 1 | 0 | RFA1, RPA1, rpa; replication factor A1 |
| K08678 | 0 | 0 | 0 | 1 | 0 | 0 | UXS1, uxs; UDP-glucuronate decarboxylase |
| K09495 | 1 | 1 | 1 | 1 | 1 | 1 | CCT3, TRIC5; T-complex protein 1 subunit gamma |
| K09588 | 0 | 1 | 0 | 0 | 0 | 0 | CYP90A1, CPD; cytochrome P450 family 90 subfamily A1 |
| K09645 | 0 | 0 | 0 | 1 | 0 | 0 | CPVL; vitellogenic carboxypeptidase-like protein |
| K10534 | 0 | 0 | 0 | 0 | 1 | 0 | NR; nitrate reductase (NAD(P)H) |
| K10573 | 0 | 0 | 0 | 0 | 0 | 1 | UBE2A, UBC2, RAD6A; ubiquitin-conjugating enzyme E2 A |
| K10767 | 0 | 0 | 0 | 1 | 0 | 0 | ALKBH5; mRNA N6-methyladenine demethylase |
| K11717 | 0 | 0 | 0 | 0 | 0 | 1 | sufS; cysteine desulfurase / selenocysteine lyase |
| K12130 | 0 | 1 | 1 | 0 | 0 | 0 | PRR5; pseudo-response regulator 5 |
| K12236 | 1 | 1 | 1 | 1 | 1 | 1 | NFX1; transcriptional repressor NF-X1 |
| K12741 | 0 | 0 | 0 | 0 | 1 | 0 | HNRNPA1_3; heterogeneous nuclear ribonucleoprotein A1/A3 |
| K12891 | 0 | 1 | 0 | 0 | 0 | 0 | SFRS2; splicing factor, arginine/serine-rich 2 |
| K13946 | 0 | 0 | 1 | 1 | 1 | 0 | AUX1, LAX; auxin influx carrier (AUX1 LAX family) |
| K13963 | 0 | 0 | 0 | 1 | 1 | 0 | SERPINB; serpin B |
| K14315 | 0 | 1 | 1 | 1 | 1 | 0 | NDC1, TMEM48; nucleoporin NDC1 |
| K14404 | 0 | 0 | 0 | 0 | 1 | 0 | CPSF4, YTH1; cleavage and polyadenylation specificity factor subunit 4 |
| K14488 | 0 | 0 | 0 | 0 | 1 | 0 | SAUR; SAUR family protein |
| K14504 | 0 | 0 | 0 | 0 | 0 | 1 | TCH4; xyloglucan:xyloglucosyl transferase TCH4 |
| K14568 | 0 | 0 | 1 | 1 | 1 | 1 | EMG1, NEP1; rRNA small subunit pseudouridine methyltransferase Nep1 |
| K14842 | 0 | 0 | 0 | 1 | 0 | 0 | NSA2; ribosome biogenesis protein NSA2 |
| K15281 | 0 | 0 | 0 | 1 | 0 | 0 | SLC35D; solute carrier family 35 |
| K15378 | 0 | 1 | 0 | 0 | 1 | 0 | SLC45A1_2_4; solute carrier family 45, member 1/2/4 |
| K15397 | 1 | 0 | 0 | 0 | 0 | 0 | KCS; 3-ketoacyl-CoA synthase |
| K15747 | 1 | 0 | 1 | 0 | 0 | 0 | LUT5, CYP97A3; beta-ring hydroxylase |
| K16298 | 0 | 0 | 1 | 1 | 1 | 0 | SCPL-IV; serine carboxypeptidase-like clade IV |
| K17525 | 1 | 0 | 1 | 0 | 0 | 0 | CHID1; chitinase domain-containing protein 1 |
| K17592 | 0 | 0 | 0 | 0 | 1 | 0 | SACS; sacsin |
| K17679 | 0 | 0 | 0 | 1 | 0 | 0 | MSS116; ATP-dependent RNA helicase MSS116, mitochondrial |
| K18270 | 0 | 0 | 0 | 1 | 0 | 0 | RAB3GAP1; Rab3 GTPase-activating protein catalytic subunit |
| K18857 | 0 | 0 | 0 | 1 | 1 | 0 | ADH1; alcohol dehydrogenase class-P |
| K20471 | 0 | 0 | 1 | 0 | 0 | 0 | COPD, ARCN1, RET2; coatomer subunit delta |
| K20628 | 1 | 0 | 0 | 0 | 1 | 0 | exlX; expansin |
| K20726 | 0 | 1 | 1 | 1 | 1 | 1 | TMEM222; transmembrane protein 222 |
| K21797 | 0 | 0 | 1 | 1 | 0 | 0 | SAC1, SACM1L; phosphatidylinositol 4-phosphatase |
| K23050 | 1 | 1 | 1 | 1 | 1 | 0 | PCBER1; phenylcoumaran benzylic ether reductase |
| K23570 | 1 | 1 | 1 | 1 | 1 | 1 | EMC10; ER membrane protein complex subunit 10 |
HL0 Halo leaf control, VL0 Vernal leaf control, HL3 Halo leaf after 3 h of salt stress, VL3 Vernal leaf after 3 h of salt stress, HL27 Halo leaf after 27 h of salt stress, VL27 Vernal leaf after 27 h of salt stress, HR0 Halo root control, VR0 Vernal root control, HR3 Halo root after 3 h of salt stress, VR3 Vernal root after 3 h of salt stress, HR27 Halo root after 27 h of salt stress, VR27 Vernal root after 27 h of salt stress
Candidate genes to enhance salt tolerance in alfalfa
The detected DEGs can be classified into two major groups for the candidate genes responsible for salt tolerance in alfalfa: 1) genes consistently expressed under short-term and long-term salt stress (3 h and 27 h) in ‘Halo’, and 2) the genes consistently expressed at all three time points in ‘Halo’. In the first group, there were 13 genes (11 in leaf; 2 in root) consistently expressed at both 3 h and 27 h of salt stress. While in the second group, there were 46 genes (17 in leaf, 29 in root) consistently expressed at all three time points. Thirteen candidate genes were highly expressed in both leaf and root tissues of ‘Halo’ as compared to ‘Vernal’, while 15 and 18 candidate genes revealed tissue specific expression in the leaf and root tissues of ‘Halo’, respectively (Tables 3, 4, and 5). Among the genes expressed in both tissues, MS.gene029203 (F-box/LRR-repeat protein 4) showed increasing expression with time in both leaf and root tissues of ‘Halo’, while MS.gene049294 (caffeic acid 3-O-methyltransferase) showed increasing expression with time in leaf tissue and MS.gene01091 (T-complex protein 1 subunit gamma) and MS.gene32989 (hypothetical protein TSUD_06780) showed increasing expression with time only in root tissue. Among the genes with leaf tissue specific expression, MS.gene029201 (replication protein A 70 kDa DNA-binding subunit C), MS.gene029206 (FAD synthetase 1, chloroplastic), and MS.gene24098 (thioredoxin-like protein CDSP32 chloroplastic-like) showed increasing expression with time. Among the genes with root tissue specific expression, MS.gene011517 (14 kDa proline-rich protein DC2.15) and MS.gene013923 (histone lysine N-methyltransferase, H3 lysine-9 specific SUVH1), had higher and consistent expression with time under salt stress.
Table 3.
List of 13 salt responsive candidate genes simultaneously highly expressed in both leaf and root tissues of salt tolerant alfalfa cultivar ‘Halo’
| Gene ID | Nr IDa | log2FCb(Leaf) | log2FC (Root) | Putative function | ||||
|---|---|---|---|---|---|---|---|---|
| 0h | 3h | 27h | 0h | 3h | 27h | |||
| MS.gene01091 | XP_003593572.2 | 6.7 | 7.3 | 6.9 | 8.4 | 9.4 | 10.3 | T-complex protein 1 subunit gamma [Medicago truncatula (barrel medic)] |
| MS.gene013211 | XP_003602730.1 | 7.3 | 5.9 | 6.7 | 9.9 | 8.9 | 7.5 | ribonuclease TUDOR 1 [Medicago truncatula (barrel medic)] |
| MS.gene013222 | XP_003602710.1 | 5.5 | 5.9 | 5.5 | 7.6 | 6.7 | 7.3 | cleft lip and palate transmembrane protein 1 homolog [Medicago truncatula (barrel medic)] |
| MS.gene017955 | XP_003625216.1 | 5.5 | 6.9 | 5.6 | NA | 9.5 | 7.7 | 40S ribosomal protein S20-2 [Medicago truncatula (barrel medic)] |
| MS.gene029200 | PNY01153.1 | 8.3 | 7.5 | 7.5 | 7.4 | 6.3 | 7.7 | replication factor A protein [Trifolium pratense] |
| MS.gene029202 | XP_013470381.1 | 7.7 | 8.1 | 7.2 | 8.2 | 8.1 | 8.5 | E3 ubiquitin-protein ligase CIP8 [Medicago truncatula (barrel medic)] |
| MS.gene029203 | XP_013470380.1 | NA | 6.8 | 7.3 | 6.8 | 8.0 | 8.5 | F-box/LRR-repeat protein 4 [Medicago truncatula (barrel medic)] |
| MS.gene049294 | XP_003602595.1 | 4.0 | 4.1 | 5.3 | 6.3 | 5.4 | 4.3 | caffeic acid 3-O-methyltransferase [Medicago truncatula (barrel medic)] |
| MS.gene32989 | GAU34467.1 | NA | 6.8 | 6.4 | 7.0 | 7.1 | 7.6 | hypothetical protein TSUD_06780 [Trifolium subterraneum] |
| MS.gene36780 | KEH43749.1 | 9.4 | 8.4 | 8.8 | 10.4 | 11.3 | 8.2 | elongation factor 1-alpha [Medicago truncatula (barrel medic)] |
| MS.gene36960 | AET01475.1 | 8.6 | 8.5 | 8.8 | 9.8 | 9.8 | 8.8 | elongation factor 1-alpha [Medicago truncatula] |
| MS.gene52595 | XP_003624202.1 | 7.3 | 8.0 | 7.7 | 9.5 | 8.1 | 7.9 | ER membrane protein complex subunit 10 [Medicago truncatula (barrel medic)] |
| MS.gene93979 | XP_003619874.1 | 7.7 | 6.9 | 7.9 | 7.4 | 7.4 | 7.0 | NF-X1-type zinc finger protein NFXL1 [Medicago truncatula (barrel medic)] |
aNr ID is the protein accession number in NCBI non redundant protein database
blog2FC stands for log Fold Change, where it is log base 2
Table 4.
List of 15 salt responsive candidate genes highly expressed in leaf tissue of salt tolerant alfalfa cultivar ‘Halo’
| Gene ID | Nr IDa | log2FCb (Leaf) | Putative function | ||
|---|---|---|---|---|---|
| 0h | 3h | 27h | |||
| MS.gene024018 | KHN29288.1 | NA | 8.9 | 4.9 | Monothiol glutaredoxin-S14, chloroplastic [Glycine soja] |
| MS.gene029055 | AFK45194.1 | 5.2 | 7.3 | 5.8 | CDP-diacylglycerol--glycerol-3-phosphate 3-phosphatidyltransferase 2 [Medicago truncatula (barrel medic)] |
| MS.gene029201 | AET03044.2 | NA | 7.5 | 9.0 | replication protein A 70 kDa DNA-binding subunit C [Medicago truncatula (barrel medic)] |
| MS.gene029206 | XP_024628388.1 | NA | 4.7 | 5.0 | FAD synthetase 1, chloroplastic [Medicago truncatula (barrel medic)] |
| MS.gene037960 | XP_003589866.2 | NA | 2.7 | 2.7 | nuclear pore complex protein NUP1 [Medicago truncatula (barrel medic)] |
| MS.gene038586 | RHN67456.1 | NA | 6.5 | 6.2 | putative minus-end-directed kinesin ATPase [Medicago truncatula] |
| MS.gene065734 | XP_013467963.1 | 6.4 | 9.8 | 6.9 | uncharacterized LOC25483798 [Medicago truncatula (barrel medic)] |
| MS.gene07287 | XP_003591401.1 | 8.8 | 11.2 | 10.5 | calvin cycle protein CP12-2, chloroplastic [Medicago truncatula (barrel medic)] |
| MS.gene24098 | PNY14915.1 | 5.1 | 5.7 | 6.2 | thioredoxin-like protein CDSP32 chloroplastic-like [Trifolium pratense] |
| MS.gene24746 | RHN68722.1 | 6.5 | 4.9 | 5.3 | hypothetical protein MtrunA17_Chr3g0116951[Medicago truncatula] |
| MS.gene36621 | XP_003627058.1 | NA | 4.7 | 4.5 | stem 28 kDa glycoprotein [Medicago truncatula (barrel medic)] |
| MS.gene39381 | RHN38725.1 | NA | 6.6 | 6.5 | putative nucleoporin protein Ndc1-Nup [Medicago truncatula] |
| MS.gene63155 | RHN41150.1 | NA | 7.3 | 4.2 | putative protein kinase RLK-Pelle-LRR-XII-1 family [Medicago truncatula] |
| MS.gene81767 | XP_013467963.1 | NA | 4.8 | 3.6 | uncharacterized LOC25483798 [Medicago truncatula (barrel medic)] |
| MS.gene99197 | AIP98334.1 | 4.6 | 4.8 | 4.7 | ZEP [Medicago sativa] |
aNr ID is the protein accession number in NCBI non redundant protein database
blog2FC stands for log Fold Change, where it is log base 2
Table 5.
List of 18 salt responsive candidate genes highly expressed in root tissue of salt tolerant alfalfa cultivar ‘Halo’
| Gene ID | Nr IDa | log2FCb (Root) | Putative function | ||
|---|---|---|---|---|---|
| 0h | 3h | 27h | |||
| MS.gene002389 | XP_003593475.1 | 7.4 | 8.0 | 7.9 | secretory carrier-associated membrane protein [Medicago truncatula (barrel medic)] |
| MS.gene011517 | XP_003608741.1 | 8.1 | 8.6 | 8.6 | 14 kDa proline-rich protein DC2.15 [Medicago truncatula (barrel medic)] |
| MS.gene013923 | XP_003624859.1 | 6.5 | 6.6 | 6.6 | histone-lysine N-methyltransferase, H3 lysine-9 specific SUVH1 [Medicago truncatula (barrel medic)] |
| MS.gene023013 | XP_013448530.1 | 8.0 | 6.0 | 6.2 | peptidyl-prolyl cis-trans isomerase FKBP62 [Medicago truncatula (barrel medic)] |
| MS.gene02427 | AFK40071.1 | 6.9 | 5.7 | 5.5 | soluble inorganic pyrophosphatase PPA1 [Medicago truncatula (barrel medic)] |
| MS.gene029223 | XP_003592714.1 | 5.9 | 6.7 | 6.1 | E3 ubiquitin ligase BIG BROTHER-related [Medicago truncatula (barrel medic)] |
| MS.gene044457 | XP_013458006.1 | 7.3 | 6.4 | 7.0 | uncharacterized LOC25493896 [Medicago truncatula (barrel medic)] |
| MS.gene049130 | RDX70942.1 | 9.2 | 10.0 | 8.5 | Aldehyde dehydrogenase family 2 member C4, partial [Mucuna pruriens] |
| MS.gene049840 | XP_024638826.1 | 7.4 | 7.2 | 8.1 | uncharacterized LOC11406476 [Medicago truncatula (barrel medic)] |
| MS.gene056386 | XP_013456308.1 | 8.2 | 8.3 | 6.7 | fructokinase-2 [Medicago truncatula (barrel medic)] |
| MS.gene058673 | PNX87529.1 | 7.9 | 5.5 | 9.5 | heavy-metal-associated domain-containing protein [Trifolium pratense] |
| MS.gene070486 | XP_003616935.1 | 5.7 | 5.5 | 7.9 | phosphatidylglycerol/phosphatidylinositol transfer protein [Medicago truncatula (barrel medic)] |
| MS.gene073760 | XP_013468212.1 | 7.1 | 7.8 | 6.9 | probable E3 ubiquitin-protein ligase LOG2 [Medicago truncatula (barrel medic)] |
| MS.gene43277 | XP_003608928.1 | 6.9 | 7.7 | 7.4 | betaine aldehyde dehydrogenase 1, chloroplastic [Medicago truncatula (barrel medic)] |
| MS.gene46459 | XP_013467706.1 | 8.6 | 9.5 | 7.7 | uncharacterized LOC25483559 [Medicago truncatula (barrel medic)] |
| MS.gene61130 | XP_004487038.1 | NA | 4.4 | 4.1 | 60S ribosomal protein L23a-2-like [ Cicer arietinum (chickpea)] |
| MS.gene67829 | XP_013454067.1 | 9.5 | 8.7 | 9.1 | ribosomal RNA small subunit methyltransferase nep-1 [Medicago truncatula (barrel medic)] |
| MS.gene95536 | XP_013469288.1 | 6.7 | 7.4 | 7.0 | acyl-CoA-binding domain-containing protein 6 [Medicago truncatula (barrel medic)] |
aNr ID is the protein accession number in NCBI non redundant protein database
blog2FC stands for log Fold Change, where it is log base 2
In addition, there were also genes consistently expressed under salt stress in leaf (Additional file 1: Table S1) and root (Additional file 1: Table S2) tissues of ‘Vernal’. In ‘Vernal’, there were 21 (17 in leaf; 4 in root) genes consistently expressed at all three time points and 9 (6 in leaf; 3 in root) genes consistently expressed at both 3 h and 27 h of salt stress.
Identification of single nucleotide polymorphisms (SNPs)
The relative distribution of identified SNPs over alfalfa chromosome are presented in Fig. 5. A total of 74,705 SNPs were identified in this study, among which 37,527 were from 'Halo' and 37,178 were from 'Vernal'. Minimum number of SNPs were found in Chr6.4 while maximum number of SNPs were detected in Chr4.4 (Fig. 5).
Fig. 5.
Distribution of SNPs identified over 32 allelic chromosomes of alfalfa (Medicago sativa L.) is represented with the Circos diagram. Histogram (1–70) showing distribution of SNPs per Mb bins across genome of alfalfa. The 8 alfalfa chromosomes (Chr1-8) are shown on outermost circle, middle (blue) and innermost (orange) circles represent SNPs distribution of salt intolerant 'Vernal' and salt tolerant 'Halo' alfalfa
Discussion
This study generated a unique set of differentially expressed genes associated with salt tolerance in alfalfa. This finding is not only significant for understanding the temporal expression of genes conditioning salt tolerance in alfalfa, but also can be used to characterize alfalfa breeding material and develop molecular markers for salt tolerance selection. First, 84.8% of sequence reads were mapped onto the alfalfa reference genome. Secondly, 237 DEGs in leaf and 295 DEGs in root tissues were identified between the two alfalfa cultivars. Third, this study was able to determine candidate genes consistently expressed under short-term and long-term salt stress in the salt tolerant cultivar. Fourth, this study found 74,705 SNPs which are valuable marker for future alfalfa breeding for salt tolerance. Fifth, we found ‘Halo’ under salt stress maintained 5 five times more DEGs in the root than ‘Vernal’. Finally, this study found seven candidate genes for salt tolerance (MS.gene32989, MS.gene065734, MS.gene24746, MS.gene81767, MS.gene044457, MS.gene049840, and MS.gene46459) with unknown functions, suggesting a need for further research to understand their role in salt tolerance.
Due to polyploidy and its out-crossing nature, alfalfa has encountered many challenges in genomic studies [26] as compared to self-pollinated crops such as wheat [27] and soybean (Glycine max) [28]. Thus, this transcriptomic study had considered several factors to overcome certain technical difficulties. First, identical clones were sampled at different time points from different alfalfa tissues. Unlike previous alfalfa transcriptomic studies, two technical replicates for each treatment were included to minimize technical errors. Furthermore, this study focused on both leaf and root tissues of alfalfa cultivars to capture tissue specific gene expression. These considerations seemed to be effective in capturing about 381 million high quality reads, which likely represents most of the genome of M. sativa. The raw reads showed a high percentage of mapping with the reference genome. These outputs should have enhanced our detection of DEGs. For example, both alfalfa cultivars showed a similar trend in the number of DEGs in leaf tissue with the increase of salt exposure time. In this study, we also selected three different time points (0 h, 3 h, and 27 h) to capture gene activation under short- and long- term salt stress. It has been established that salt responsive defense response is activated within 24 h of stress [29].
One of the main differences between the two cultivars was the number of DEGs in roots. In the root of salt tolerant alfalfa, the number of DEGs was similar between 3 h and 27 h of salt stress, but a sharp decrease was observed between 3 h and 27 h in the intolerant cultivar ‘Vernal’. We speculate that such earlier activation of salt responsive genes and maintenance of a large number of DEGs might be a key characteristic for salt tolerance in alfalfa, suggesting alfalfa tolerance is associated with upregulation of key genes from short term salt stress. About 60% of DEGs were assigned to GO categories, while KEGG pathways for less than 30% DEGs were identified in this study. The DEGs were mainly involved in metabolic pathways as revealed by KEGG pathway analysis. Although certain pathways involved in salt tolerance may be conserved in plant species such as in halophytes, there was still variation among plant species, cultivars, and tissues [5]. This study demonstrated that transcriptional variation in adaptation to salt stress exists not only among the alfalfa cultivars but also between the different tissues. ‘Ion binding’ (GO:0043167) was significantly enriched in both leaf and root tissues of ‘Halo’, but not in ‘Vernal’ under salt stress. This suggested that the genes responsible for ‘ion binding’ should be unique for salt tolerance of ‘Halo’ alfalfa. Therefore, the tissue- and genotype-specific salt responsive genes might be useful in identification of salt tolerant genotypes in the future.
Among 13 candidate genes expressed in leaf and root tissues of ‘Halo’ under salt stress (Table 3) in this study, two genes (MS.gene013222 and MS.gene52595) are responsible for transmembrane protein function. These transmembrane proteins control gateways and selective transport of salt ions to facilitate salt tolerance in plants. Likewise, MS.gene013211, a homologous gene to ribonuclease TUDOR1, is involved in stress adaptation and highly expressed in leaf and root tissues of ‘Halo’ in our study [30]. MS.gene93979, a homologous gene to NF-X1-type zinc finger protein, is part of mechanisms that regulate growth under salt stress and was highly expressed in leaf and root tissues of ‘Halo’ in our study [31]. In addition, MS.gene029202 (E3 ubiquitin-protein ligase CIP8), MS.gene029203 (F-box/LRR-repeat protein 4), MS.gene36780 and MS.gene36960 (elongation factor 1-alpha) were highly expressed in leaf and root tissues of salt tolerant alfalfa in our study. These genes are involved in regulation of a number of biological processes including biotic and abiotic stress tolerances [32–34]. For example, MS.gene049294, which is a homologous gene of O-methyltransferase, was found to improve salt tolerance in transgenic Arabidopsis [35]. MS.gene01091, a homologous gene to the T-complex protein 1 subunit gamma, showed high expression in both root and leaf tissue and is involved in intracellular assembly and folding of various proteins [36]. MS.gene029200, a homologous gene to replication factor A protein, was highly expressed in both leaf and root tissues of ‘Halo’ in our study, which might play a role in binding, replication, repair, and recombination of DNA under stress conditions [37].
In this study, we found 15 and 18 candidate genes specific to leaf and root tissues of salt tolerance ‘Halo’ alfalfa (Tables 4, 5). In leaf tissue, nine genes showed consistent expression under salt stress, while six of them were expressed at all three time points. In our study, salt tolerant alfalfa showed an increased expression of MS.gene024018 and MS.gene24098 with putative functions of chloroplastic glutaredoxin and thioredoxin-like protein CDSP32, respectively. The two genes (MS.gene024018, MS.gene24098) were found to be important for defense against protein oxidative damage in other studies [38, 39]. This is important because salt stress results in the formation of reactive oxygen species, which damage protein, membrane lipids, and nucleic acids [40]. MS.gene63155, a homologous gene to receptor-like kinases (RLKs), are a family of transmembrane proteins, showed lowered expression with time under salt stress. This gene is involved in plant growth as well as stress response [41]. Two nucleoporin proteins (MS.gene037960 and MS.gene39381) were expressed consistently under salt stress in leaf tissue of ‘Halo’. These proteins connect cytoplasm and nucleoplasm, and are involved in abiotic stress tolerance [42]. MS.gene038586 is a homologous gene to kinesin super family proteins which plays a significant role in intracellular transport and are critical for cellular functioning and survival [43]. MS.gene029206, a homologous gene to FAD synthetase 1, is a co-factor for various enzymes that participate in numerous metabolic processes like photosynthesis, electron transport, fatty acid oxidation and biosynthesis of secondary metabolites [44]. MS.gene36621, a homologous gene to stem 28 kDa glycoprotein, which is known as a vegetative storage protein, was highly expressed under salt stress in our study. This protein plays a certain role as a somatic storage protein during early seedling development [45]. Salt tolerant alfalfa showed a high expression of MS.gene07287 in leaf, a homologous gene to calvin cycle protein CP12-2. This gene is involved in photosynthesis and improved plant growth [46]. The Calvin–Benson cycle is the primary pathway of carbon fixation, producing carbon compounds. CP12 facilitates the formation of a complex between glyceraldehyde-3-phosphate dehydrogenase and phosphoribulokinase, thereby increasing the photosynthetic capacity of the plant [46]. MS.gene99197, a homologous gene to zeaxanthin epoxidase (ZEP), was highly expressed at all three time points in leaf tissue of ‘Halo’. It is an important enzyme in ABA biosynthesis and plays an important role in osmotic tolerance [47].
In root tissue, one (MS.gene61130) of the 18 genes detected was consistently expressed under salt stress, while the rest were expressed at all three time points in our study (Table 5). MS.gene002389, a homologous gene to secretory carrier-associated membrane proteins, is involved in membrane trafficking and found to influence accumulation of secondary cell wall components in Poplus [48]. MS.gene011517, a homologous gene to 14-kDa proline-rich protein DC2.15, is involved in cell wall modification and organization [49]. The plant cell wall is not only a physical barrier between the plant and the environment but also is a responsive part of the plant to biotic and abiotic stresses. The finding of tissue specific salt tolerant candidate genes responsible for the plant cell wall is promising and underlines the need for further research on its role in response to salt stress. In addition, salt stress causes lipid peroxidation, resulting in damage of membrane lipids and eventual cell leakage. This study showed salt tolerant alfalfa had an increased expression of MS.gene049130, a homologous gene to aldehyde dehydrogenase, responsible for oxidation of aldehydes produced during lipid peroxidation thereby detoxifying cells [50]. MS.gene95536 is a homologous gene to acyl-CoA-binding domain-containing protein 6, which is associated with phospholipid metabolism. This gene also was shown to play a role in the freezing tolerance of Arabidopsis [51]. MS.gene070486, a homologous gene to phosphatidylinositol transfer proteins, plays an important role in signal transduction and facilitates lipid transfer between membranes [52]. MS.gene056386, a homologous gene to fructokinases, are important enzymes catalyzing fructose phosphorylation and are involved in plant growth and development [53]. MS.gene058673, a homologous gene to heavy-metal-associated domain-containing protein conferring tolerance to abiotic stress [54]. MS.gene073760, a homologous gene to probable E3 ubiquitin-protein ligase LOG2, which induces amino acid secretion. This is the main form of organic nitrogen in the plant [55]. MS.gene02427, a homologous gene to soluble inorganic pyrophosphatase, is tightly linked with carbohydrate metabolism. It plays an important role in stress adaptive responses [56]. Carbohydrate metabolism produces soluble carbohydrates that are important for salt tolerance because of its osmotic adjustment function in the root.
Conclusion
Our study generated a unique set of DEGs for alfalfa salt tolerance studies and breeding efforts. The information is useful for better understanding of temporal expression of genes in response to salt stress. Furthermore, GO annotation and KEGG pathway analysis of the DEGs provided insights to the different molecular and biological processes between salt tolerant and intolerant alfalfa cultivars. In particular, ‘ion binding activity’ was found as a key molecular activity specific to salt tolerant alfalfa cultivar ‘Halo’. Based on this finding, salt tolerance in alfalfa appears to be associated with consistent expression of genes for selective transport of salt ions and compounds, increasing photosynthetic capacity as well as carbohydrate metabolism, enhancing defense against oxidative damage, modification of root cell wall and protection against lipid peroxidation. The SNPs discovered in this study will be valuable for molecular marker-assisted breeding for the development of salt tolerant alfalfa.
Methods
Plant material and salt treatment
Two alfalfa cultivars, ‘Halo’ (obtained from Agriculture and Agri-Food Canada, Swift Current Research and Development Centre) and ‘Vernal’ (sourced from Dr. Biligetu’s lab, Crop Development Centre, University of Saskatchewan) were chosen for the study. Cultivar ‘Halo’ was selected for improved salinity tolerance for germination, seedling growth, and mature plant regrowth at 100 mM NaCl in the greenhouse conditions [57], and cultivar ‘Vernal’ was considered as a salinity intolerant cultivar [58, 59]. Four genotypes (biological replicates) of each cultivar were grown from seeds in the College of Agriculture and Bioresources greenhouse at the University of Saskatchewan (45 Innovation Blvd., Saskatoon, SK) for 12 weeks. Six identical clones of each biological replicate were produced by stem cuttings. Salt stress of 120 mM NaCl approximately corresponding to 12 dS m− 1 electrical conductivity was applied on 4 week old seedlings. Salt stress of 12 dS m− 1 was selected from our earlier greenhouse study where alfalfa was grown at various gradients of salt stress and alfalfa cultivars showed variation in response to salt stress at 12 dS m− 1, with increase in salt stress from 12 dS m− 1 all alfalfa cultivars showed very high mortality (Bhattarai et al., unpublished). Leaf and root samples were collected immediately before salt treatment (control, 0 h), and at 3 h and 27 h of salt treatments. The samples were immediately frozen in liquid nitrogen and then stored at − 80 °C for 2 weeks until total RNA extraction carried out.
Tissue sample and RNA isolation
About 100 mg of tissue samples were disrupted using TissueLyser II and total RNA was extracted with RLT buffer using the Qiagen RNeasy Plant Mini Kit (Qiagen Inc., Mississauga, ON, Canada) according to the manufacturer’s protocol. DNase treatment was performed using the Ambion DNA-free DNase treatment and removal reagents (Life Technologies, Carlsbad, CA, USA) to remove contaminant genomic DNA from the isolated total RNA. Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) was used to measure the total RNA concentration. RNA integrity number was evaluated for 12 samples using RNA 6000 Nano labchip on 2100 Agilent Bioanalyzer (Agilent Technologies, Waldbronn, Germany) (Additional file 1: Table S3; Additional file 2: Fig. S1).
Library preparation and sequencing
Poly (A) RNA was purified from total RNA using Magnosphere MS150 OligodT beads according to the manufacturer’s protocol. The RNA samples were subsequently used in cDNA library preparation. Two cDNA libraries were prepared using Lexogen’s SENSE mRNA-Seq Library Prep Kit V2 (Lexogen, Vienna, Austria). To minimize technical errors, two technical replicates of each treatment were divided into two cDNA libraries. The technical replicates represented two clones of the same genotype (biological replicate) by separately extracting RNA. Thus, 96 samples (2 cultivars × 2 tissue types × 3 time points × 4 biological replicates × 2 technical replicates) were collected for the study. The cDNA libraries were sequenced using the Illumina HiSeq v4 system at the National Research Council of Canada, Saskatoon, Canada. Raw reads were deposited in the National Center for Biotechnology Information (NCBI) and received BioProject ID PRJNA657410.
Reference-based mapping, differential gene expression analysis and annotation
The quality of the raw sequence was assessed using the FastQC software [60]. The raw reads were cleaned by removing adapters and low-quality sequences using Trimmomatic v.0.36 based on the default setting of paired-end mode, phred 33 and threads 6 [61]. The trimmed high-quality reads of samples from the two technical replicates were merged and mapped with the alfalfa reference genome (10.6084/m9.figshare.12327602.v3) [62, 63] using STAR (v2.6.1a) [64] with “quantMode” as “GeneCounts”. The obtained “ReadsPerGene” of each sample were extracted as count matrix and the differentially expressed genes were analyzed using DeSeq2 package [65] where data were normalized by the median of the ratios. The threshold of padj < 0.001 and the Log fold change (Log2FC) > 2 were used to determine the significance of gene expression differences. The functional annotation of the DEGs were also extracted via searches of NR databases as available in “query.blastp.db.out” and gene ontologies were obtained via searches of the GO databases as available in “Msa.GO.list.up” likewise Kyoto Encyclopedia of Genes and Genomes (KEGG) Ortholog (KO) were obtained via search of KO databases as available in “query.ko” from Zeng [63]. Gene ontology analysis of the DEGs was done for biological process, cellular components, and molecular function by AgriGO v2.0 software [66]. Venn diagrams were produced using the Venny tool [67].
Identification of single nucleotide polymorphisms (SNPs)
SNPs calling was done using freebayes software using the bam file generated in the mapping process where at least 5 supporting observations were required to be consider a variant [68]. To visualize the relative distribution of SNPs over chromosomes, Circos tool was used [69].
Supplementary Information
Additional file 1 : Table S1. List of 237 differentially expressed genes in leaf tissue at the control (0 h), 3 h, and 27 h of salt stress between salt tolerant ‘Halo’ and salt intolerant ‘Vernal’ cultivars of alfalfa. Table S2. List of 295 differentially expressed genes in root tissue at the control (0 h), 3 h, and 27 h of salt stress between salt tolerant ‘Halo’ and salt intolerant ‘Vernal’ cultivars of alfalfa. Table S3. RNA quality of 12 RNA samples determined with 2100 Agilent Bioanalyzer.
Additional file 2 : Fig. S1. Electropherogram of 12 RNA samples.
Acknowledgements
We would like to thank Carolee Horbach, and Gregory Peterson at the Saskatoon Research and Development Centre, Agriculture and Agri-Food Canada, Saskatoon, SK, for their technical assistance in RNA extraction and library preparation and Fangqin Zeng and Raju Chaudhary for their help and suggestions during data analysis. This research was enabled in part by support provided by WestGrid and Compute Canada. We would also like to thank greenhouse staff at the College of Agriculture and Bioresources, University of Saskatchewan, for their support and assistance.
Abbreviations
- ABA
Abscisic acid
- AVP1
Arabidopsis type I proton-pumping pyrophosphatase
- DEGs
Differentially expressed genes
- dS m− 1
DeciSiemens per metre
- DREB
Dehydration-responsive element binding protein
- NHX
Na+/H+ antiporter
- CBRLK
Calcium/calmodulin-binding receptor-like kinase
- ZFP
Zinc finger protein
- APX
Ascorbate peroxidase
- NDPK2
Nucleoside diphosphate kinase 2
- codA
Choline oxidase
- RLK
Receptor-like kinase
- FAD
Flavin adenine dinucleotide
- ZEP
Zeaxanthin epoxidase
Authors’ contributions
B.B. conceived the project; S.B., B.B. designed experiments; K.T. provided guidance on the salt stress system; B.B. prepared the study materials; S.B. performed experiments; S.B., Y.-B.F., B.B. analyzed data; S.B. wrote a first version of the manuscript and Y.-B.F., B.C., B.B., C.K., K.T. substantially contributed to the last version of the manuscript. All authors read and approved the final manuscript.
Funding
The research was funded by an NSERC Discovery grant.
Availability of data and materials
Raw reads have been deposited in the National Center for Biotechnology Information (NCBI) and received BioProject ID PRJNA657410. The data will be accessible with the following link: “https://www.ncbi.nlm.nih.gov/bioproject/PRJNA657410”.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blondon F, Marie D, Brown S, Kondorosi A. Genome size and base composition in Medicago siativa and M. truncatula species. Genome. 1994;37(2):264–270. doi: 10.1139/g94-037. [DOI] [PubMed] [Google Scholar]
- 2.Maas EV, Hoffman GJ. Crop salt tolerance-current assessment. J Irrig Drain Div. 1977;103(2):115–134. doi: 10.1061/JRCEA4.0001137. [DOI] [Google Scholar]
- 3.Johnson DW, Smith SE, Dobrenz AK. Selection for increased forage yield in alfalfa at different NaCl levels. Euphytica. 1992;60(1):27–35. doi: 10.1007/BF00022255. [DOI] [Google Scholar]
- 4.Gregorio GB, Senadhira D. Genetic analysis of salinity tolerance in rice (Oryza sativa L.) Theoret Appl Genet. 1993;86:333–338. doi: 10.1007/BF00222098. [DOI] [PubMed] [Google Scholar]
- 5.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–81. 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed]
- 6.Postnikova OA, Shao J, Nemchinov LG. Analysis of the alfalfa root transcriptome in response to salinity stress. Plant Cell Physiol. 2013;54(7):1041–1055. doi: 10.1093/pcp/pct056. [DOI] [PubMed] [Google Scholar]
- 7.Luo D, Zhou Q, Wu YG, Chai XT, Liu WX, Wang YR, Yang QC, Wang ZY, Liu ZP. Full length transcript sequencing and comparative transcriptomic analysis to evaluate the contribution of osmotic and ionic stress components towards salinity tolerance in the roots of cultivated alfalfa (Medicago sativa L.) BMC Plant Biol. 2019;19:32. doi: 10.1186/s12870-019-1630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei Y, Xu Y, Hettenhausen C, Lu C, Shen G, Zhang C, Li J, Song J, Lin H, Wu J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018;18(1):35. doi: 10.1186/s12870-018-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber M, Xia J, Yu M, Steppuhn H, Wall K, Messer D, Sharpe A, Acharya S, Wishart D, Johnson D, Miller D, Taheri A. Transcript analysis in two alfalfa salt tolerance selected breeding populations relative to a non-tolerant population. Genome. 2017;60(2):104–127. doi: 10.1139/gen-2016-0111. [DOI] [PubMed] [Google Scholar]
- 10.Winicov I. Alfin1 transcription factor overexpression enhances plant root growth under normal and saline conditions and improves salt tolerance in alfalfa. Planta. 2000;210(3):416–422. doi: 10.1007/PL00008150. [DOI] [PubMed] [Google Scholar]
- 11.Bao AK, Wang SM, Wu GQ, Xi JJ, Zhang JL, Wang CM. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.) Plant Sci. 2009;176:232–240. doi: 10.1016/j.plantsci.2008.10.009. [DOI] [Google Scholar]
- 12.Jin T, Chang Q, Li W, Yin D, Li Z, Wang D, Liu B, Liu L. Stress-inducible expression of GmDREB1 conferred salt tolerance in transgenic alfalfa. Plant Cell Tissue Organ Cult. 2010;100(2):219–227. doi: 10.1007/s11240-009-9628-5. [DOI] [Google Scholar]
- 13.Li W, Wang D, Jin T, Chang Q, Yin D, Xu S, Liu B, Liu L. The vacuolar Na+/H+ antiporter gene SsNHX1from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.) Plant Mol Biol Rep. 2011;29(2):278–290. doi: 10.1007/s11105-010-0224-y. [DOI] [Google Scholar]
- 14.Zhang YM, Liu ZH, Wen ZY, Zhang HM, Yang F, Guo XL. The vacuolar Na+/H+ antiport gene TaNHX2 confers salt tolerance on transgenic alfalfa (Medicago sativa L.) Funct Plant Biol. 2012;39(8):708–716. doi: 10.1071/FP12095. [DOI] [PubMed] [Google Scholar]
- 15.Bai X, Liu J, Tang L, Cai H, Chen M, Ji W, Liu Y, Zhu Y. Overexpression of GsCBRLK from Glycine soja enhances tolerance to salt stress in transgenic alfalfa (Medicago sativa) Funct Plant Biol. 2013;40(10):1048–1056. doi: 10.1071/FP12377. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Cai H, Ji W, Luo X, Wang Z, Wu J, Wang X, Cui L, Wang Y, Zhu Y, Bai X. Overexpression of GsZFP1enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.) Plant Physiol Biochem. 2013;71:22–30. doi: 10.1016/j.plaphy.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Ma C, Xue X, Xu M, Li J, Wu JX. Overexpression of a cytosolic ascorbate peroxidase gene, OsAPX2, increases salt tolerance in transgenic alfalfa. J Integr Agric. 2014;13(11):2500–2507. doi: 10.1016/S2095-3119(13)60691-7. [DOI] [Google Scholar]
- 18.Zhang LQ, Niu YD, Huridu H, Hao JF, Qi Z, Hasi A. Salicornia europaea L. Na+/H+ antiporter gene improves salt tolerance in transgenic alfalfa (Medicago sativa L.) Genet Mol Res. 2014;13(3):5350–5360. doi: 10.4238/2014.July.24.14. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Li H, Ke Q, Jeong JC, Lee HS, Xu B, Deng XP, Lim YP, Kwak SS. Transgenic alfalfa plants expressing AtNDPK2 exhibit increased growth and tolerance to abiotic stresses. Plant Physiol Biochem. 2014;84:67–77. doi: 10.1016/j.plaphy.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Wang Z, Ke Q, Ji CY, Jeong JC, Lee H, Lim YP, Xu B, Deng XP, Kwak SS. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol Biochem. 2014;85:31–40. doi: 10.1016/j.plaphy.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Tang L, Cai H, Zhai H, Luo X, Wang Z, Cui L, Bai X. Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.) Plant Cell Tissue Organ Cult. 2014;118(1):77–86. doi: 10.1007/s11240-014-0463-y. [DOI] [Google Scholar]
- 22.Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedling. Plant Physiol. 1987;84(2):324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Beena AS, Awana M, Singh A. Salt-induced tissue-specific cytosine methylation downregulates expression of HKT genes in contrasting wheat (Triticum aestivum L.) genotypes. DNA Cell Biol. 2017;36:283–294. doi: 10.1089/dna.2016.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villarino GH, Hu Q, Scanlon MJ, Mueller L, Bombarely A, Mattson NS. Dissecting tissue-specific transcriptomic responses from leaf and roots under salt stress in Petunia hybrida Mitchell. Genes. 2017;8(8):195. doi: 10.3390/genes8080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Chen T, Ma L, Zhao Z, Zhao PX, Nan Z, Wang Y. Global transcriptome sequencing using the Illumina platform and the development of EST-SSR markers in autotetraploid alfalfa. PLoS One. 2013;8(12):e83549. doi: 10.1371/journal.pone.0083549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman M, Levy AA. Allopolyploidy – a shaping force in the evolution of wheat genomes. Cytogenet Genome Res. 2005;109(1-3):250–258. doi: 10.1159/000082407. [DOI] [PubMed] [Google Scholar]
- 28.Gill N, Findley S, Walling JG, Hans C, Ma J, Doyle J, Stacey G, Jackson SA. Molecular and chromosomal evidence for allopolyploidy in soybean. Plant Physiol. 2009;151(3):1167–1174. doi: 10.1104/pp.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez JA, Almansa MS. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant. 2002;115(2):251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- 30.Yan C, Yan Z, Wang Y, Yan X, Han Y. Tudor-SN, a component of stress granules, regulates growth under salt stress by modulating GA20ox3 mRNA levels in Arabidopsis. J Exp Bot. 2014;65(20):5933–5944. doi: 10.1093/jxb/eru334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisso J, Altmann T, Müssig C. The AtNFXL1 gene encodes a NF-X1 type zinc finger protein required for growth under salt stress. FEBS Lett. 2006;580(20):4851–4856. doi: 10.1016/j.febslet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 32.Mazzucotelli E, Belloni S, Marone D, De Leonardis A, Guerra D, Di Fonzo N, Cattivelli L, Mastrangelo A. The e3 ubiquitin ligase gene family in plants: regulation by degradation. Curr Genomics. 2006;7(8):509–522. doi: 10.2174/138920206779315728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song JB, Wang YX, Li HB, Li BW, Zhou ZS, Gao S, Yang ZM. The F-box family genes as key elements in response to salt, heavy metal and drought stresses in Medicago truncatula. Funct Integr Genomics. 2015;15(4):495–507. doi: 10.1007/s10142-015-0438-z. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Ma J, Zheng JC, Chen J, Chen M, Zhou YB, Fu JD, Xu ZS, Ma YZ. The elongation factor GmEF4 is involved in the response to drought and salt tolerance in soybean. Int J Mol Sci. 2019;20(12):3001. doi: 10.3390/ijms20123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niron H, Türet M. A putative common bean chalcone o-methyltransferase improves salt tolerance in transgenic Arabidopsis thaliana. J Plant Growth Regul. 2020;39(3):957–969. doi: 10.1007/s00344-019-10040-z. [DOI] [Google Scholar]
- 36.Bhaskar KN, Goyal N. Cloning, characterization and sub-cellular localization of gamma subunit of T-complex protein-1 (chaperonin) from Leishmania donovani. Biochem Biophys Res Commun. 2012;429:70–74. doi: 10.1016/j.bbrc.2012.10.090. [DOI] [PubMed] [Google Scholar]
- 37.Longhese MP, Plevani P, Lucchini G. Replication factor A is required in vivo for DNA replication, repair, and recombination. Mol Cell Biol. 1994;14(12):7884–7890. doi: 10.1128/mcb.14.12.7884-7890.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broin M, Rey P. Potato plants lacking the CDSP32 plastidic thioredoxin exhibit overoxidation of the BAS1 2-cysteine peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress. Plant Physiol. 2003;132(3):1335–1343. doi: 10.1104/pp.103.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD. AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem. 2006;281(36):26280–26288. doi: 10.1074/jbc.M601354200. [DOI] [PubMed] [Google Scholar]
- 40.Foyer CH, Noctor G. Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;28(8):1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x. [DOI] [Google Scholar]
- 41.Shiu SH, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132(2):530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong CH, Hu X, Tang W, Zheng X, Kim YS, Lee BH, Zhu JK. A putative Arabidopsis nucleoporin, AtNUP160, is critical for RNA export and required for plant tolerance to cold stress. Mol Cell Biol. 2006;26:9533–9543. doi: 10.1128/MCB.01063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88(3):1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 44.Sandoval FJ, Zhang Y, Roje S. Flavin nucleotide metabolism in plants: monofunctional enzymes synthesize fad in plastids. J Biol Chem. 2008;283(45):30890–30900. doi: 10.1074/jbc.M803416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason HS, Guerrero FD, Boyer JS, Mullet JE. Proteins homologous to leaf glycoproteins are abundant in stems of dark-grown soybean seedlings. Analysis of proteins and cDNAs. Plant Mol Biol. 1988;11(6):845–856. doi: 10.1007/BF00019524. [DOI] [PubMed] [Google Scholar]
- 46.López-Calcagno PE, Abuzaid AO, Lawson T, Raines CA. Arabidopsis CP12 mutants have reduced levels of phosphoribulokinase and impaired function of the Calvin-Benson cycle. J Exp Bot. 2017;68(9):2285–2298. doi: 10.1093/jxb/erx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HY, Seok HY, Park BK, Kim SH, Goh CH, Lee BH, Lee CH, Moon YH. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem Biophys Res Commun. 2008;375(1):80–85. doi: 10.1016/j.bbrc.2008.07.128. [DOI] [PubMed] [Google Scholar]
- 48.Obudulu O, Mähler N, Skotare T, Bygdell J, Abreu IN, Ahnlund M, Gandla ML, Petterle A, Moritz T, Hvidsten TR, Jönsson LJ, Wingsle G, Trygg J, Tuominen H. A multi-omics approach reveals function of secretory carrier-associated membrane proteins in wood formation of Populus trees. BMC Genomics. 2018;19(1):11. doi: 10.1186/s12864-017-4411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan W, Lou HQ, Gong YL, Liu MY, Wang ZQ, Yang JL, Zheng SJ. Identification of early Al-responsive genes in rice bean (Vigna umbellata) roots provides new clues to molecular mechanisms of Al toxicity and tolerance. Plant Cell Environ. 2014;37(7):1586–1597. doi: 10.1111/pce.12258. [DOI] [PubMed] [Google Scholar]
- 50.Koncitikova R, Vigouroux A, Kopecna M, Andree T, Bartos J, Sebela M, Morera S, Kopecny D. Role and structural characterization of plant aldehyde dehydrogenases from family 2 and family 7. Biochem J. 2015;468(1):109–123. doi: 10.1042/BJ20150009. [DOI] [PubMed] [Google Scholar]
- 51.Chen QF, Xiao S, Chye ML. Arabidopsis ACBP6 is an acyl-CoA-binding protein associated with phospholipid metabolism. Plant Signal Behav. 2008;3(11):1019–1020. doi: 10.4161/psb.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garner K, Hunt AN, Koster G, Somerharju P, Groves E, Li M, Raghu P, Holic R, Cockcroft S. Phosphatidylinositol transfer protein, cytoplasmic 1 (PITPNC1) binds and transfers phosphatidic acid. J Biol Chem. 2012;287(38):32263–32276. doi: 10.1074/jbc.M112.375840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein O, Granot D. Plant Fructokinases: evolutionary, developmental, and metabolic aspects in sink tissues. Front Plant Sci. 2018;9:339. doi: 10.3389/fpls.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun XH, Yu G, Li JT, Jia P, Zhang JC, Jia CG, Zhang YH, Pan HY. A heavy metal-associated protein (AcHMA1) from the halophyte, Atriplex canescens (Pursh) Nutt., confers tolerance to iron and other abiotic stresses when expressed in Saccharomyces cerevisiae. Int J Mol Sci. 2014;15(8):14891–14906. doi: 10.3390/ijms150814891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pratelli R, Guerra DD, Yu S, Wogulis M, Kraft E, Frommer WB, Callis J, Pilot G. The ubiquitin E3 ligase LOSS OF GDU2 is required for GLUTAMINE DUMPER1-induced amino acid secretion in Arabidopsis. Plant Physiol. 2012;158(4):1628–1642. doi: 10.1104/pp.111.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SK, Jeon JS. Review: Crucial role of inorganic pyrophosphate in integrating carbon metabolism from sucrose breakdown to starch synthesis in rice endosperm. Plant Sci. 2020;298:110572. doi: 10.1016/j.plantsci.2020.110572. [DOI] [PubMed] [Google Scholar]
- 57.Steppuhn H, Acharya SN, Iwaasa AD, Gruber M, Miller DR. Inherent responses to root-zone salinity in nine alfalfa populations. Can J Plant Sci. 2012;92(2):235–248. doi: 10.4141/cjps2011-174. [DOI] [Google Scholar]
- 58.Peel MD, Waldron BL, Jensen KB, Chatterton NJ, Horton H, Dudley LM. Screening for salinity tolerance in alfalfa. Crop Sci. 2004;44(6):2049–2053. doi: 10.2135/cropsci2004.2049. [DOI] [Google Scholar]
- 59.Rahman MA, Alam I, Kim YG, Ahn NY, Heo SH, Lee DG, Liu G, Lee BH. Screening for salt responsive proteins in two contrasting alfalfa cultivars using a comparative proteome approach. Plant Physiol Biochem. 2015;89:112–122. doi: 10.1016/j.plaphy.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27(6):863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Zeng Y, Yang Y, Huang L, Tang B, Zhang H, Hao F, Liu W, Li Y, Liu Y, Zhang X, Zhang R, Zhang Y, Li Y, Wang K, He H, Wang Z, Fan G, Yang H, Bao A, Shang Z, Chen J, Wang W, Qiu Q. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat Commun. 2020;11(1):2494. doi: 10.1038/s41467-020-16338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Y. Genome fasta sequence and annotation files. figshare. Dataset. 2020. [Google Scholar]
- 64.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. AgriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45(W1):W122–W129. doi: 10.1093/nar/gkx382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliveros JC. VENNY. An interactive tool for comparing lists with Venn Diagrams. 2007. [Google Scholar]
- 68.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. 2012. [Google Scholar]
- 69.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Table S1. List of 237 differentially expressed genes in leaf tissue at the control (0 h), 3 h, and 27 h of salt stress between salt tolerant ‘Halo’ and salt intolerant ‘Vernal’ cultivars of alfalfa. Table S2. List of 295 differentially expressed genes in root tissue at the control (0 h), 3 h, and 27 h of salt stress between salt tolerant ‘Halo’ and salt intolerant ‘Vernal’ cultivars of alfalfa. Table S3. RNA quality of 12 RNA samples determined with 2100 Agilent Bioanalyzer.
Additional file 2 : Fig. S1. Electropherogram of 12 RNA samples.
Data Availability Statement
Raw reads have been deposited in the National Center for Biotechnology Information (NCBI) and received BioProject ID PRJNA657410. The data will be accessible with the following link: “https://www.ncbi.nlm.nih.gov/bioproject/PRJNA657410”.