Abstract
Focal limbic seizures can cause loss of consciousness. Previous work suggests that hippocampal seizures can increase activity in the lateral septum (LS) and decrease cholinergic output from the basal forebrain (BF), leading to deficits in conscious arousal. The mechanism by which LS and BF interact is unclear. In this study, we used anterograde and retrograde tracing to investigate anatomical pathways connecting LS and BF. We found that LS projects directly to BF and indirectly to BF via the thalamic paratenial nucleus (PT). Acute electrophysiology experiments during electrically induced focal limbic seizures showed that multiunit activity decreased in PT during the ictal period and was associated with increased cortical slow wave activity. These results suggest that LS could functionally inhibit BF during a seizure directly, or could indirectly decrease excitatory output to BF through PT. Further work investigating such parallel inhibitory and excitatory pathways to subcortical arousal may ultimately lead to new treatment targets for consciousness-impairing limbic seizures.
Keywords: basal forebrain, consciousness, epilepsy, paratenial nucleus, sleep, thalamus
1 |. INTRODUCTION
Limbic seizures with loss of consciousness result in reduced quality of life.1 The network inhibition hypothesis posits that focal limbic seizures impair consciousness by activating subcortical circuits that inhibit arousal systems including cholinergic neurons in the basal forebrain (BF). The resultant functional disassociation between cortex and subcortical arousal is evidenced by low-frequency cortical oscillations and impairment of consciousness.2–4 The lateral septum (LS), a subcortical midline structure strongly connected with the hippocampus,5 appears to play an important inhibitory role in this circuit.6 Hippocampal seizures rapidly propagate to LS, resulting in increased LS activity,7 and stimulating LS induces low-frequency oscillations and decreased choline signal in the cortex.8 Although the anatomical connections of LS and BF have been described,5,9 their functional relationship is less clear. Here, we aimed to define the importance of the projections between LS and BF by using neuronal tracers and electrophysiology in an established rat model of focal limbic seizures. Elucidating this network would help us understand mechanisms whereby limbic seizures may impair consciousness.
2 |. MATERIALS AND METHODS
2.1 |. Animals
All experiments were approved by Yale University’s Animal Care and Use Committee. Adult Long Evans or Sprague Dawley rats weighing 200–280 g were used as in our previous studies.2–4 For anterograde tracing, we used rats expressing Cre recombinase under the choline-acetyltransferase promoter (ChAT-Cre; Deisseroth laboratory)10 in anticipation of performing optogenetic experiments in the future.
2.2 |. Tracer injection
Stereotaxic coordinates are in millimeters relative to bregma.11 Animals were deeply anesthetized with ketamine/xylazine (90/15 mg/kg, intramuscular). For anterograde tracing (Figure 1A), we used a recombinant adeno-associated virus (rAAV5) vector with strong affinity for neurons.12 The vector carried archaerhodopsin (ArchT) conjugated with tdTomato under the CAG promoter (AAV-CAG-ArchT-tdTomato; #29778, Addgene)3; 0.8 μl was injected into the left LS (anteroposterior [AP], +0.6; mediolateral [ML], +0.7; superior-inferior [SI], −5.0) at 0.04 μl/min using a Quintessential Stereotaxic Injector (53 311, Stoelting) via a pulled glass micropipette (21–175B, Fisher Scientific), held in place for 10 minutes wait time after injection. After 2 weeks,3 animals were perfused with 4% paraformaldehyde and the brain was removed.
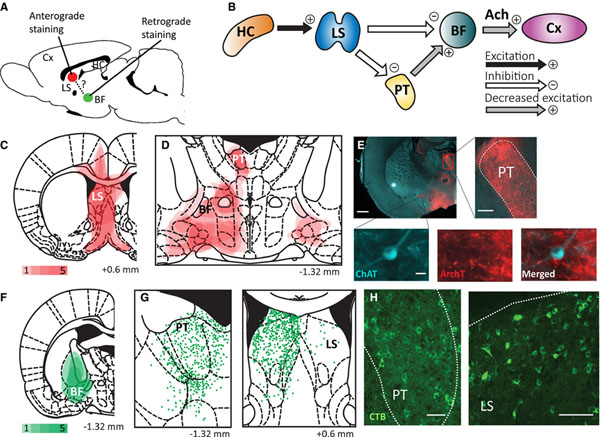
FIGURE 1.
Immunofluorescent histology from anterograde and retrograde tracing suggests a direct anatomical pathway from lateral septum to basal forebrain and an indirect pathway via the paratenial nucleus of the thalamus. A, Schematic showing anterograde tracer targeting the lateral septum (LS) and retrograde tracer targeting the basal forebrain (BF). Cx, cortex. B, Schematic circuit diagram suggested by our tracing and electrophysiology experimental results. A limbic seizure originating in the hippocampus (HC) activates LS, which can either directly inhibit BF or indirectly do so by reducing excitatory output from the thalamic paratenial nucleus (PT). Both pathways result in decreased acetylcholine (Ach) arousal to the Cx. C, Summary showing the sites of anterograde tracer injections in LS. D, Summary showing areas (red) where axon fibers were found. Projections were seen in PT and various areas of the BF (see text for details). Red density color bar key for C and D indicates the number of animals (1–5) for which staining was found in each region. E, Example of anterograde projections in a choline acetyltransferase (ChAT)-Cre rat coronal section at approximately anteroposterior (AP) −1.32 mm. Insets show dense fibers in PT, as well as fibers interfacing with a ChAT-positive neuron in BF. White scale bar indicates 1 mm in large section, 0.2 mm in the PT inset, and 20 μm in the ChAT inset. ArchT, archaerhodopsin. F, Sites of retrograde cholera toxin B (CTB) tracer injections targeting BF. Green density color bar key indicates the number of animals (1–5) for which staining was found in each region. G, Schematic showing cell bodies centered on PT and LS superimposed for all five animals. Each green point represents one neuron body. H, Histology examples showing retrograde CTB-stained neuronal cell bodies in PT (approximately AP, −1.32 mm) and LS (approximately AP, +0.6 mm). All projections and cell bodies were detected manually. White scale bars indicate 0.1 mm. Coronal section schematics are presented in AP coordinates relative to bregma from a rat atlas.11 Brain atlas sections in C, D, F, and G are reproduced with permission from G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, 6th Edition, Academic Press, 2007
For retrograde tracing (Figure 1A), identical procedures were utilized, with minor modifications. We used cholera toxin B (CTB) conjugated with Alexa Fluor 488 (C34775, Thermo Fisher Scientific)13; 1 μl was injected into BF (AP, −1; ML, +2.7; SI, −6.7) at 0.04 μl/min followed by a 5-minute wait time, and the brain was harvested after 1 week.13
2.3 |. Immunohistochemistry and microscopy
Brains were sliced into 60-μm sections incubated overnight in 5% donkey serum and 0.3% Triton X-100 in phosphate-buffered saline with primary antibody. Slices labeled with anterograde tracer were costained with anti-ChAT (1:250; AB144P, Millipore) and anti–red fluorescent protein (RFP; 1:500). Retrograde tracers were enhanced by anti–green fluorescent protein conjugated with Alexa Fluor 488 (1:1000). Sections were incubated for 2 hours in secondary antibody, Alexa Fluor 488 for anti-ChAT (1:250, Life Technologies) and 594 for anti-RFP (1:1000), and viewed with a Leica DM6B microscope at ×20 magnification, using the 594-nm channel for ArchT and 488-nm channel for CTB and ChAT. Brain section images were overlaid on a standard rat brain atlas to manually determine the location of projections and cell bodies, as performed by others.11,14
2.4 |. Electrophysiology
Multiunit activity (MUA) and local field potential (LFP) recordings were obtained as described previously.4,15 Briefly, deep anesthesia was induced as above, and the skull was exposed for electrode placement (Figure 2A). A stainless-steel bipolar LFP electrode (50- to 100-kΩ resistance; E363/2–2TW, Plastics One), with tips separated by 1 mm and insulation shaved from the distal 0.3 mm, was placed unilaterally in the dorsal hippocampus (AP, −3.8; ML, +2.5; SI, −3.2), with tips in the coronal plane. An MUA/LFP microelectrode (3- to 4-MΩ resistance; part #UEWMGGSEDNNM, FHC) was placed unilaterally in the lateral orbitofrontal cortex (LOFC) at a 20° angle from vertical (target coordinates: AP, +4.2; ML, ±2.2; SI, −4.2). Another MUA/LFP electrode was vertically placed in the thalamic paratenial nucleus (PT; AP, −1.0; ML, ±0.5; SI, −6.0). A stainless-steel skull screw (0–80 × 3/32, Plastics One) just caudal to the hippocampal electrode served as ground and reference.
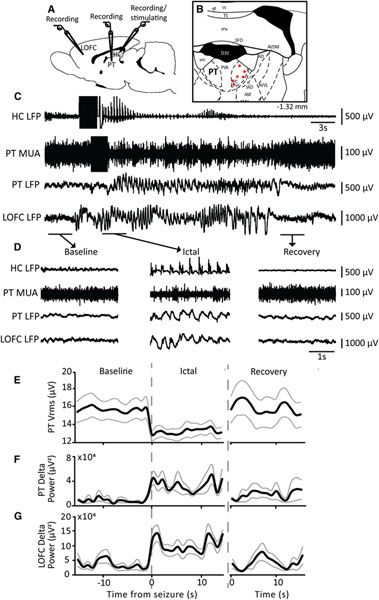
FIGURE 2.
Decreased multiunit activity in the thalamic paratenial nucleus (PT) and increased delta power in the cortex during focal limbic seizures. A, Schematic showing placement of monopolar recording electrodes in the lateral orbitofrontal cortex (LOFC) and PT, and a bipolar recording/stimulating electrode in the hippocampus (HC). B, Schematic showing electrode tip locations in PT (red; n = 6) to record multiunit activity (MUA) on a coronal section of a rat brain atlas.11 Number on bottom right is anteroposterior coordinates in mm relative to bregma. C, Seizure induced by 2-second stimulation of HC (black rectangular segments) results in polyspike activity in HC local field potential (LFP). PT exhibits decreased MUA, and LOFC shows low-frequency oscillations. D, Magnified segments of data from the baseline, ictal, and recovery periods in C. E, Time course of average MUA root-mean-square voltage (Vrms) in PT shows decreased activity in the ictal period followed by a return back to baseline during the recovery period. F, Time course of average delta band power (0–4 Hz) in PT LFP shows a nonsignificant trend toward increased power during the ictal period. G, Time course of average delta band power (0–4 Hz) in LOFC LFP shows increase in delta power during the ictal period followed by a return to baseline during the recovery period. Time courses in E, F and G are plotted in 1s overlapping bins for the 15s immediately before the seizure (Baseline), the first 15 s after seizure onset (Ictal), and the 15s after seizure offset and disappearance of cortical slow waves (Recovery). Traces in E-G are mean ± standard error of the mean of n = 6 rats, 10 seizures. LS, lateral septum. The brain atlas section in B is reproduced with permission from G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, 6th Edition, Academic Press, 2007
After animals recovered to light anesthesia, defined by <3 slow waves per 10 seconds in the LOFC tracing and unresponsive to toe pinch,2–4 a focal seizure was electrically induced in the hippocampus with a 2-second, 60-Hz train of square biphasic pulses (Model 2100, A-M Systems), with a pulse width of 1 millisecond per phase.7 Current was titrated to the minimum required to induce a seizure of >15-second duration, with amplitudes ranging from 30 to 300 μA. Secondarily generalized seizures characterized by propagation of polyspike activity to LOFC were excluded.
Hippocampal signals were amplified (×1000) and filtered (1–500 Hz; Model 1800, A-M Systems). LOFC and PT signals were amplified (×1000) and filtered (0.1 Hz-10 kHz), followed by further analog filtering (unity gain; Model 3364, Krohn-Hite) to obtain LFP (0.1–100 Hz) and MUA (0.4–10 kHz). Signals were digitized at 1 kHz for LFP and 20 kHz for MUA (Power 1401 and Spike2 v8 software, CED).
After each experiment, histology was performed with Cresyl violet to confirm electrode placement using a standard atlas as done previously.4,11
2.5 |. Data analysis
Analysis epochs were as follows: baseline = 15 seconds preceding seizure; ictal = first 15 seconds of seizure (polyspike discharges in hippocampus); recovery = 15 seconds following disappearance of postictal cortical slow waves (Figure 2C,D). LFP delta power (0–4 Hz) and MUA root-mean-square voltage (Vrms) were analyzed in Spike-2 (v8, CED). Vrms was calculated in overlapping 1-second windows. LFP delta power was calculated using fast Fourier transform with 1-second overlapping windows. Group averages yielded similar results when taken across seizures (n = number of seizures) or by first pooling using within-animal means (n = number of animals), so we presented the latter more conservative results. Signals are reported as mean ± standard error of the mean and were analyzed by two-tailed paired t test with significance threshold P < .05.
3 |. RESULTS
3.1 |. LS has direct connections to cholinergic BF nuclei, and indirect connections through the PT
ArchT was injected into the LS unilaterally in five ChAT-Cre rats to delineate output projections (Figure 1A,C). In all five animals, dense axonal projections were found in the PT, BF, and peduncular part of the hypothalamus ipsilateral to the injection site (Figure 1D,E). Anterogradely stained axons were seen interfacing with ChAT-positive neurons in BF (Figure 1E inset). Projections were also seen in the anterior hypothalamus, horizontal limb of the diagonal band, bed nucleus of the stria terminalis, and amygdala. Three of five animals had projections to the contralateral peduncular hypothalamus (Figure 1D); however, in all three cases there was off-target tracer uptake in the contralateral dorsal LS.
For retrograde tracing, we injected CTB into BF of five wild-type rats (Figure 1F). Cell bodies were seen centered on PT and LS in all five rats (Figure 1G,H), confirming our anterograde staining findings. Cell bodies were also seen in the nearby paracentral, interanterodorsal, anterior paraventricular, and central medial thalamic nuclei. In combination, these anterograde and retrograde staining patterns suggest the anatomical connectivity between LS and BF includes a direct pathway from LS to BF, and a parallel indirect pathway from LS to BF via the thalamic PT (Figure 1B), consistent with other rodent tracing experiments.16
3.2 |. MUA in PT decreases during focal hippocampal seizures in association with increase in cortical LFP delta power
To assess the functional importance of the indirect pathway between LS and BF through PT in focal seizures, we measured PT activity during electrically induced limbic seizures (Figure 2A,C). Ten seizures were recorded in six rats (Figure 2B). MUA Vrms in PT decreased by 15.16 ± 9.13% in the ictal period compared to baseline (P = .02) and returned to baseline during the recovery period (P = .21; Figure 2D,E). Meanwhile, LFP delta (0–4 Hz) power in LOFC increased by 213.42 ± 86.90% during the ictal period (P = .02) and returned to baseline during the recovery period (P = .43; Figure 2D,G), consistent with previous findings of ictal slow waves and decreased MUA representing depressed cortical function.2,4,7 PT LFP delta power showed a nonsignificant increasing trend during the ictal period (P = .058; Figure 2D,F), suggesting reduced activity. Taken together with the above histological findings (Figure 1), these results suggest that decreased activity in PT during focal limbic seizures could contribute to decreased arousal from BF to cortex.
4 |. DISCUSSION
Previous work suggested that limbic seizures can cause cortical low-frequency oscillations by inhibiting cholinergic arousal systems in the forebrain,2–4,7 although the pathway was unclear. Here, we used anterograde tracing in LS and retrograde tracing in BF to show parallel direct and indirect connectivity between these two nuclei. We further demonstrated that PT may contribute functionally via this pathway, as hippocampal seizures that resulted in cortical slow waves were associated with decreased PT firing, possibly due to inhibitory input from LS. Although the hippocampus has direct connections to PT in the rodent brain,16 hippocampal seizures resulted in decreased PT activity and not seizure propagation. These results suggest that LS could inhibit BF directly as well as indirectly via PT (Figure 1B).
The inhibitory role of LS in hippocampal seizures is supported by prior LS recording, disconnection, stimulation, and neurotransmitter experiments.2,4,7,8,15 LS innervates PT, as well as the interanteromedial, paraventricular, and reuniens thalamic nuclei,5 and prior work also shows depressed activity in the thalamic intralaminar central lateral nucleus.15 These previous studies suggest that depressed cortical activity may occur through multiple thalamic pathways. We chose to investigate PT further, as it was the most consistently labeled area in our experiments, and because prior work had shown projections from PT to the ventral prefrontal cortex and BF cholinergic regions.14 The present retrograde tracing studies further support the connections from PT to BF. Depressed subcortical cholinergic activity in focal limbic seizures has been confirmed by direct recordings from both BF and brainstem cholinergic neurons.4,17 Reduced cholinergic neuronal firing could arise from either increased inhibitory or reduced excitatory inputs. Reduced excitatory synaptic input was demonstrated in brainstem cholinergic neurons in focal limbic seizures, supporting the possible role of decreased excitation as well as increased inhibition in depressed subcortical arousal.17
Our results support parallel pathways from LS to BF with direct inhibitory and indirect reduced excitatory function. These findings add to a growing body of work investigating mechanisms of decreased subcortical arousal in focal limbic seizures.2–4,7,8,15,17,18 Importantly, the proposed parallel pathways with converging function investigated here likely represent only one such example. The possible modulatory role of PT and BF in arousal adds to potential targets for therapeutic intervention, such as thalamic stimulation (https://braininitiative.nih.gov/funded-awards/thalamic-stimulation-prevent-impaired-consciousness-epilepsy), in an effort to restore conscious arousal in refractory limbic seizures.3,18 The impact of modulating these pathways is clinically relevant and may offer new treatments for people with epilepsy.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS066974, R01 NS096088), the Betsy and Jonathan Blattmachr family, and the Mark Loughridge and Michele Williams Foundation.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01 NS066974 and R01 NS096088; Mark Loughridge and Michele Williams Foundation; Betsy and Jonathan Blattmachr family
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position of issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Vickrey BG, Berg AT, Sperling MR, Shinnar S, Langfitt JT, Bazil CW, et al. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia. 2000;41:760–4. [DOI] [PubMed] [Google Scholar]
- 2.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;3(28):9066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman M, Zhan Q, McCafferty C, Lerner BA, Motelow JE, Meng J, et al. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: effects on cortical physiology. Epilepsia. 2015;56:e198–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;4(85):561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;19(24):115–95. [DOI] [PubMed] [Google Scholar]
- 6.Garrido Sanabria ER, Castaneda MT, Banuelos C, Perez-Cordova MG, Hernandez S, Colom LV. Septal GABAergic neurons are selectively vulnerable to pilocarpine-induced status epilepticus and chronic spontaneous seizures. Neuroscience. 2006;27(142):871–83. [DOI] [PubMed] [Google Scholar]
- 7.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;14(29):13006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Motelow JE, Zhan Q, Hu YC, Kim R, Chen WC, et al. Cortical network switching: possible role of the lateral septum and cholinergic arousal. Brain Stimul. 2015;8:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostinelli LJ, Geerling JC, Scammell TE. Basal forebrain subcortical projections. Brain Struct Funct. 2019;224:1097–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;8(72):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Amsterdam, the Netherlands; Boston, MA: Elsevier; 2007. [Google Scholar]
- 12.Haery L, Deverman BE, Matho KS, Cetin A, Woodard K, Cepko C, et al. Adeno-associated virus technologies and methods for targeted neuronal manipulation. Front Neuroanat. 2019;13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte WL, Kamishina H, Reep RL. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat Protoc. 2009;4:1157–66. [DOI] [PubMed] [Google Scholar]
- 14.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;10(508):212–37. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Motelow JE, Ma C, Biche W, McCafferty C, Smith N, et al. Seizures and sleep in the thalamus: focal limbic seizures show divergent activity patterns in different thalamic nuclei. J Neurosci. 2017;22(37):11441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;10(508):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews JP, Yue Z, Ryu JH, Neske G, McCormick DA, Blumenfeld H. Mechanisms of decreased cholinergic arousal in focal seizures: in vivo whole-cell recordings from the pedunculopontine tegmental nucleus. Exp Neurol. 2019;314:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundishora AJ, Gummadavelli A, Ma C, Liu M, McCafferty C, Schiff ND, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 2017;1(27):1964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]