Abstract
Objectives:
To describe challenges and lessons learned in conducting a remote behavioral weight loss trial.
Methods:
The Personal Diet Study is an ongoing randomized clinical trial which aims to compare two mobile health (mHealth) weight loss approaches, standardized diet vs. personalized feedback, on glycemic response. Over a six-month period, participants attended dietitian-led group meetings via remote videoconferencing and were encouraged to self-monitor dietary intake using a smartphone app. Descriptive statistics were used to report adherence to counseling sessions and self-monitoring. Challenges were tracked during weekly project meetings.
Results:
Challenges in connecting to and engaging in the videoconferencing sessions were noted. To address these issues, we provided a step-by-step user manual and video tutorials regarding use of WebEx, encouraged alternative means to join sessions, and sent reminder emails/texts about the WebEx sessions and asking participants to join sessions early. Self-monitoring app-related issue included inability to find specific foods in the app database. To overcome this, the study team incorporated commonly consumed foods as “favorites” in the app database, provided a manual and video tutorials regarding use of the app and checked the self-monitoring app dashboard weekly to identify nonadherent participants and intervened as appropriate. Among 135 participants included in the analysis, the median attendance rate for the 14 remote sessions was 85.7 % (IQR: 64.3% - 92.9%).
Conclusions:
Experience and lessons shared in this report may provide critical and timely guidance to other behavioral researchers and interventionists seeking to adapt behavioral counseling programs for remote delivery in the age of COVID-19.
Keywords: mHealth, telehealth, obesity, lifestyle intervention, lessons learned, behavioral intervention, technology
INTRODUCTION
Behavioral lifestyle interventions targeting diet and physical activity, traditionally delivered in-person, are effective at reducing body weight and improving health outcomes.1,2 Despite their well-established effectiveness, patients face numerous barriers when attending in-person behavioral counseling sessions. These barriers include the time and travel associated with appointments which may conflict with work schedules, lack of access to affordable transportation, and lack of childcare.3 Due to geographic maldistribution of professionals trained in counseling methods, those living in rural and medically underserved regions may experience absence of or delays in joining weight loss programs.4-6 The COVID-19 pandemic presents additional barriers to lifestyle counseling, as health systems struggle to limit patient and provider exposure to the virus and divert resources to where they are needed most.7
Telehealth can mitigate many of the above-mentioned barriers. Telehealth programs employ a variety of technologies including videoconference platforms, audio, email, short message services (SMS), remote monitoring devices, and smartphone applications for monitoring weight, physical activity, and diet. Telehealth programs do not require brick-and-mortar facilities, the physical presence of a counselor, or participant travel to a central location and they support recent public health guidance to minimize spread of COVID-19. Telehealth programs allow participants to discuss their health in their natural settings (e.g., home) and address some social determinants of health barriers that limit access to care (e.g., lack of transportation, childcare, and sick time).
Telehealth has been demonstrated to be effective in health promotion and disease prevention in patients with various chronic conditions, including racial and ethnic minorities.8,9 Several reports of videoconference-delivered weight loss counseling interventions appear in the literature.10-23 However, most were pilot or feasibility studies, or had small sample sizes.10,11,16-18,20 Limited details were provided regarding challenges encountered and effective solutions for implementing such interventions.
Our research team has been conducting telehealth behavioral weight loss and disease self-management interventions over the past six years, including interventions that involve SMS-based health messaging, videoconference-delivered behavioral group counseling, and dietary self-monitoring technologies.13,24-28 In this report, we describe our experience in implementing a telehealth behavioral weight loss program, challenges encountered, and lessons learned. Of note, the primary goal of the parent study was not to formally test different protocols for implementing our intervention approach (which we developed and refined with prior studies). Consequently, we did not collect evaluative data prospectively to demonstrate the effectiveness of intervention protocols. The goal of this report was to share our experience and anecdotal observations with a remote behavioral intervention over the past 4 years. This information provides timely and critical guidance to clinicians, health care systems, and research teams, who are urgently seeking new ways to adapt their programs and studies for virtual delivery in the age of COVID-19.
METHODS
The methods presented discuss the ways that we formally and informally tracked challenges and addressed them with the intent of providing support for continued participation in the remote aspects of study implementation. A brief description of the parent study is then presented, followed by challenges and strategies reported in the results section.
Methods for Informally Tracking Challenges and Evaluating Strategies
As noted above, the primary goal of the Personal Diet study was not to formally test optimal strategies to deliver WebEx counseling sessions or help participants to self-monitor their dietary intake. The challenges noted below were primarily tracked during the weekly project meetings. Each week, the study RDs and RDAs shared challenges and successes which were observed by them or reported by participants. The entire research team then discussed strategies to address these challenges. The following week, when the group met again, the RDs or RDAs would report their observations of whether the strategies helped address the challenges and if any new issues occurred. As such, the strategies presented below were based on our anecdotal observations. As challenges were being continuously troubleshot, pre- and post-implementation changes were not compared.
Methods for Formally Collecting Feedback via the End of Study Questionnaire.
At the 6-month assessment, participants completed an 11-item, investigator-developed, End of Study Questionnaire. Participants were asked to report their level of agreement to a series of statements about the relevance, feasibility, and acceptability of the WebEx counseling sessions and use of the self-monitoring app, using a 5-point Likert scale ranging from 1 (disagree) to 5 (agree). As discussed below, 2 items were added to this questionnaire after the study began and, thus, complete data are available on a subset of participants.
Brief Overview of the Parent Study
Research Design and Population
The Personal Diet Study is an ongoing, 6-month mobile-based behavioral weight loss clinical trial (NCT: NCT03336411) In general, participants were adults ages 18-80 years with overweight and obesity who had prediabetes or early-stage type 2 diabetes, and lived in the New York City metropolitan area. Measurements were obtained at baseline, 3 and 6 months.
Randomization Assignment and Intervention Approach
Participants were primarily recruited via MyChart messages sent to potentially eligible participants at New York University Langone Health (NYULH). They were randomized with equal allocation to one of two groups: Standardized or Personalized. Those in the Standardized group were instructed to follow a calorie-restricted, one-size-fits-all low-fat diet. Those in the Personalized group were provided with the same calorie restriction, but were given personalized dietary recommendations to minimize postprandial glycemic response. Glycemic variability, measured with continuous glucose monitors, is a free-living measure of postprandial glycemic responses. Reducing the postprandial glycemic response to meals may mitigate glycemic variability and slow progression to type 2 diabetes.29
Both groups received behavioral counseling based on Social Cognitive Theory30,31 and self-monitored their diet, physical activity and weight using a mobile app, and received real-time feedback relevant to the randomization assignment. Additional information regarding the Personal Diet Study methods have been previously published.28
WebEx-based Behavioral Counseling.
Behavioral counseling sessions were delivered to both groups via WebEx™. A total of 14 group counseling sessions were delivered by a Registered Dietitian (RD) weekly for the first month and then bi-weekly for the next five months. Those without Internet access or devices were provided with a study phone and 4G data plan. To foster personal connections and engagement, group size was limited to 10 participants during WebEx sessions.
Self-Monitoring via a Mobile App.
Participants in both groups were directed to use the Personalized Nutrition Project (PNP) app to log foods, beverages and exercise in real-time, as well as weekly body weights. The Standardized group received real-time feedback from the PNP app on calorie intake and macronutrient distribution. The Personalized group received the same real-time feedback from the PNP app as the Standardized group plus a predicted postprandial glycemic response score. Personalized group participants received training to use these scores for identifying potentially problematic foods and, as needed, make different food choices to reduce glycemic exposure.
Feedback Reports to Participants:
Additional feedback reports were generated from daily self-monitoring data obtained from the PNP app dashboard and sent to both groups via email, weekly for the first month, and then every other week for the remaining five months. Reports included visuals regarding the adherence with adherent defined as days in which the participant logged >50% of their target calorie intake. To minimize bias by the study RDs (PI, MLP), Research Data Associates (RDAs; KP, SC) generated the feedback reports. However, participants had opportunities to discuss feedback reports during the group intervention sessions with the study RDs. The RDs encouraged self-monitoring as a tool for maintaining dietary vigilance and emphasized empirical evidence of its importance in weight loss success.
Technology Training
Prior to initiation of the study intervention, WebEx and PNP accounts were established for each participant. A study RDA met one-on-one to guide participants in downloading WebEx and PNP apps to their own smartphone, and train them on logging-in and navigating the programs. After training, RDAs provided participants with hard-copy manuals containing step-by-step directions and associated screen shots for maneuvering through the apps. RDAs encouraged participants to reach out immediately with technical issues they encountered, or any time they had difficulty locating a food in the PNP app database.
Statistical Analyses
Descriptive analyses were used to summarize sample characteristics and overall adherence and by randomization group. Means and standard deviations (SDs) or medians and interquartile ranges (IQRs) were used for continuous variables, depending on the distribution of the variable. Frequencies and percentages were reported for categorical variables. Between-group differences in mean session attendance and self-monitoring adherence were examined using Wilcoxon rank-sum test. Changes in these variables over time were assessed using mixed logistic regressions. Between group differences in response to the End of Study Questionnaire were assed using ordered logistic regressions. All analyses were performed with Stata Version 16.1 (College Station, Texas, USA).
RESULTS
The Personalized Diet study is ongoing, and data collected through October 29 of 2020 were used for this analysis. Of 161 participants randomized and completing 6-month assessments, the majority were women (66.5%), most had a Bachelor’s degree or higher (69.6%) and an annual income of at least $50,000 (72.1%). Most participants self-identified as non-Hispanic (82.0%), nearly half as Caucasian or White (54.7%), and a quarter as African American or Black (24.8%). The mean age was 58.6 years old (SD = 11.1) and mean BMI was 34.1 kg/m2 (SD = 4.8). See Table 1 for details. Over the course of the investigation, 23 participants withdrew from the study and three were withdrawn due to no longer meeting eligibility criteria. These individuals are not included in the final analysis. With this intent-to-treat analysis, participants who were lost to follow-up were included in analysis.
Table 1.
Participants’ Characteristics at Baseline
| All Participants (n= 161) |
Standard Diet Group (n = 77) |
Personalized Diet Group (n = 84) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Gender (%, n) | 0.039 A | ||||||
| Male | 33.5% | 54 | 41.6% | 32 | 26.2% | 22 | |
| Female | 66.5% | 107 | 58.4% | 45 | 73.8% | 62 | |
| Age (mean. SD) | 58.6 (11.1) | 59.7 (11.2) | 57.6 (11.0) | 0.242 B | |||
| Age Group (%, n) | 0.328 C | ||||||
| 18 - 29 | 1.2% | 2 | 1.3% | 1 | 1.2% | 1 | |
| 30 - 39 | 5.0% | 8 | 6.5% | 5 | 3.6% | 3 | |
| 40 - 49 | 13.0% | 21 | 10.4% | 8 | 15.5% | 13 | |
| 50 - 59 | 33.5% | 54 | 29.9% | 23 | 36.9% | 31 | |
| 60 - 69 | 29.2% | 47 | 31.2% | 24 | 27.4% | 23 | |
| 70 or older | 18.0% | 29 | 20.8% | 16 | 15.5% | 13 | |
| Race (%, n) | 0.723 A | ||||||
| White / Caucasian | 54.7% | 88 | 57.1% | 44 | 52.4% | 44 | |
| Black / African American | 24.8% | 40 | 26.0% | 20 | 23.8% | 20 | |
| Other | 19.3% | 31 | 16.9% | 13 | 21.4% | 18 | |
| Missing | 1.2% | 2 | 2.4% | 2 | |||
| Ethnicity (%, n) | 0.721 A | ||||||
| Non-Hispanic | 82.0% | 132 | 83.1% | 64 | 81.0% | 68 | |
| Hispanic | 18.0% | 29 | 16.9% | 13 | 19.0% | 16 | |
| Education (%, n) | 0.815 C | ||||||
| Less than high school | |||||||
| High school | 16.8% | 27 | 15.6% | 12 | 17.9% | 15 | |
| Associate degree | 7.5% | 12 | 7.8% | 6 | 7.1% | 6 | |
| Technical degree / certificate | 5.6% | 9 | 5.2% | 4 | 6.0% | 5 | |
| Bachelor's degree | 25.5% | 41 | 28.6% | 22 | 22.6% | 19 | |
| Master's degree | 32.3% | 52 | 28.6% | 22 | 35.7% | 30 | |
| Doctoral or Professional | 11.8% | 19 | 14.3% | 11 | 9.5% | 8 | |
| Missing | 0.6% | 1 | 1.2% | 1 | |||
| Employed (%, n) | 0.603 A | ||||||
| No | 28.0% | 45 | 29.9% | 23 | 26.2% | 22 | |
| Yes | 72.0% | 116 | 70.1% | 54 | 73.8% | 62 | |
| Missing | |||||||
| Income (%, n) | 0.540 C | ||||||
| < $10,000 | 0.6% | 1 | 1.3% | 1 | 0% | 0 | |
| $10,000 - $19,999 | 2.5% | 4 | 2.6% | 2 | 2.4% | 2 | |
| $20,000 - $29,999 | 1.9% | 3 | 2.6% | 2 | 1.2% | 1 | |
| $30,000 - $39,999 | 5.0% | 8 | 5.2% | 4 | 4.8% | 4 | |
| $40,000 - $49,999 | 5.0% | 8 | 5.2% | 4 | 4.8% | 4 | |
| $50,000 - $74,999 | 20.5% | 33 | 22.1% | 17 | 19.0% | 16 | |
| $75,000 - $99,999 | 14.9% | 24 | 13.0% | 10 | 16.7% | 14 | |
| > $100,000 | 36.6% | 59 | 36.4% | 28 | 36.9% | 31 | |
| Missing | 13.0% | 21 | 11.7% | 9 | 14.3% | 12 | |
| Baseline BMI (mean, SD) | 34.1 (4.8) | 33.3 (4.6) | 34.8 (4.9) | 0.046 B | |||
| Baseline BMI Category | 0.033 C | ||||||
| Overweight ( 30>BMI≥25) | 23.0% | 37 | 31.2% | 24 | 15.5% | 13 | |
| Class I Obesity (35>BMI≥30) | 37.3% | 60 | 33.8% | 26 | 40.5% | 34 | |
| Class II Obesity (40>BMI≥35) | 26.1% | 42 | 26.0% | 20 | 26.2% | 22 | |
| Class III Obesity (BMI≥40) | 13.7% | 22 | 9.1% | 7 | 17.9% | 15 | |
| Baseline hbA1c (mean, SD) | 5.79 (0.58) | 5.85 (0.65) | 5.74 (0.52) | 0.234 B | |||
| Baseline hbA1c category | 0.090 A | ||||||
| < 6.5 | 88.80% | 143 | 84.4% | 65 | 92.9% | 78 | |
| ≥ 6.5 | 11.20% | 18 | 15.6% | 12 | 7.1% | 6 | |
Attendance and Adherence.
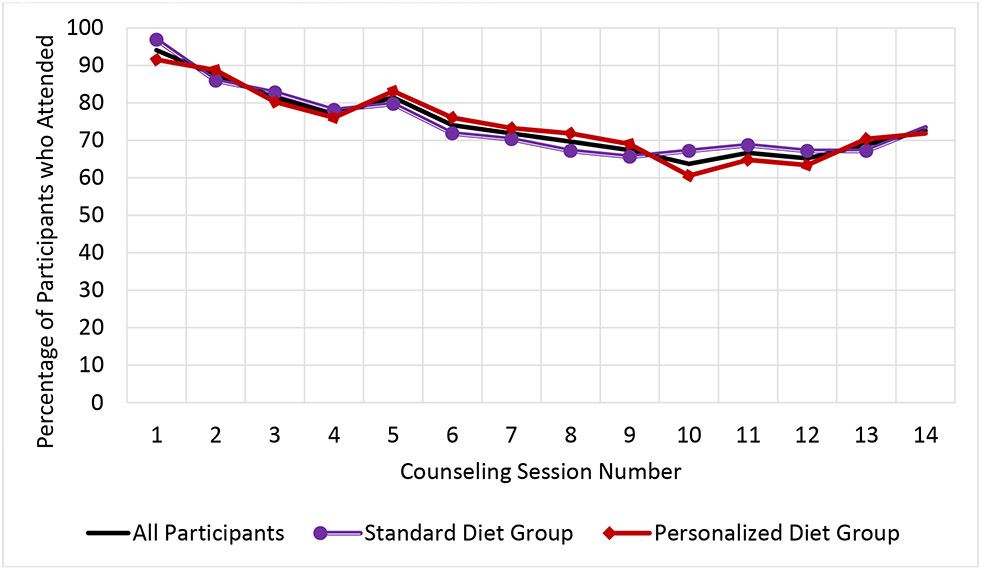
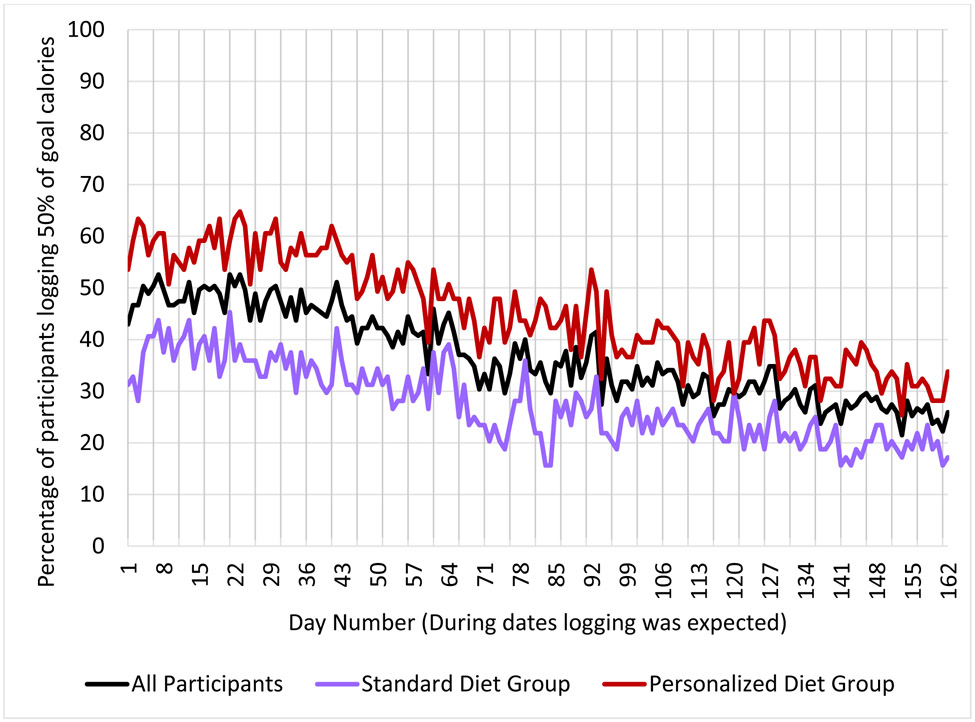
The median attendance rate for the 14 WebEx group sessions was 85.7% (IQR = 64.3% - 92.9%; mean = 74.4%, SD = 26.2%). There were no statistically significant differences between the Personalized versus Standardized groups (mean of 74.4% vs 74.3%, p = 0.98). The log odds of session attendance decreased over time (OR = 0.87, 95% CI: 0.84-0.90, p <0.001). See Figure 1 and Supplemental Table 1 for additional information. Percent adherence to meal logging, with adherence defined as a day during which the participant logged >50% of their kcal goal, was low on average (median = 23.5%, IQR = 4.3% – 68.5%; mean = 36.8%, SD = 34.4%). Overall adherence was higher for Personalized group compared to the Standardized (mean of 45.1% vs 27.5% respectively, p < 0.01). The log odds of dietary self-monitoring decreased over time (OR = 0.98, 95% CI: 0.98 – 0.99, p <0.001). See Figure 2 for additional information.
Figure 1.
Group Counseling Session Attendance
Figure 2.
Percentage of Participants Logging at Least 50% of their Daily Goal Calories in the Personalized Nutrition Program (PNP) Mobile Application
End of Study Questionnaire.
Of the 144 participants who had the opportunity to complete the End of Study Questionnaire, 3 were determined not to be eligible for the study, and 22 formally withdrew. Among 119 participants, a total of 100 participants completed the Questionnaire (response rate: 84%). Most participants agreed that the WebEx sessions were applicable (84%), motivated them to make lifestyle changes (75%), and that the WebEx mobile application was easy to use (84%), and they felt connected to other group members (61%). Forty percent of participants felt that the videoconferencing sessions contained “a lot of information” that they did not already know. Most participants reported changing meals (55%) and the type of food they ate (58%) based on the feedback received from the app. Fewer participants agreed that the app kept them engaged and motivated in the program (50%), and only 29% planned to use the app after completion of the intervention. Of the 86 participants asked whether they used an alternative self-monitoring app during the study, 29% agreed. See Table 2 for details.
Table 2.
Results from the End of Study Questionnaire
| Percentage of participants answering |
||||||||
|---|---|---|---|---|---|---|---|---|
| Question | Group | disagree (1,2) |
neutral (3) |
agree (4,5) |
mean (SD) |
n | p-value | |
| 1 | I changed the meals I ate based on the feedback that the app gave me. | all | 18.0 | 27.0 | 55.0 | 3.5 (1.3) | 100 | 0.053 |
| stnd | 25.0 | 29.2 | 45.8 | 3.3 (1.4) | 48 | |||
| persn | 11.5 | 25.0 | 63.5 | 3.8 (1.2) | 52 | |||
| 2 | I changed the types of foods I ate based on the feedback that the app gave me. | all | 20.0 | 22.0 | 58.0 | 3.5 (1.3) | 100 | 0.063 |
| stnd | 27.1 | 22.9 | 50.0 | 3.2 (1.4) | 48 | |||
| persn | 13.5 | 21.2 | 65.4 | 3.7 (1.1) | 52 | |||
| 3 | The use of the app kept me engaged and motivated to participate in the program. | all | 34.0 | 16.0 | 50.0 | 3.3 (1.6) | 100 | 0.061 |
| stnd | 41.7 | 16.7 | 41.7 | 3.0 (1.5) | 48 | |||
| persn | 26.9 | 15.4 | 57.7 | 3.5 (1.6) | 52 | |||
| 4 | I will continue using the app after the program is over. | all | 60.6 | 10.1 | 29.3 | 2.4 (1.6) | 99 | 0.194 |
| stnd | 66.7 | 8.3 | 25.0 | 2.2 (1.6) | 48 | |||
| persn | 54.9 | 11.8 | 33.3 | 2.6 (1.7) | 51 | |||
| 5 | I used a different app to track my food during the program. | all | 57.0 | 14.0 | 29.1 | 2.4 (1.7) | 86 | 0.973 |
| stnd | 60.5 | 7.0 | 32.6 | 2.4 (1.8) | 43 | |||
| persn | 53.5 | 20.9 | 25.6 | 2.4 (1.6) | 43 | |||
| 6 | The topic of the WebEx sessions were applicable to me personally | all | 5.1 | 11.1 | 83.8 | 4.3 (0.9) | 99 | 0.235 |
| stnd | 4.2 | 12.5 | 83.3 | 4.3 (0.8) | 48 | |||
| persn | 5.9 | 9.8 | 84.3 | 4.4 (1.0) | 51 | |||
| 7 | I did not know a lot of the information that the WebEx sessions contained. | all | 28.0 | 32.0 | 40.0 | 3.1 (1.2) | 100 | 0.767 |
| stnd | 27.1 | 31.3 | 41.7 | 3.1 (1.3) | 48 | |||
| persn | 28.8 | 32.7 | 38.5 | 3.1 (1.3) | 52 | |||
| 8 | I felt connected to my group members in the WebEx sessions. | all | 17.2 | 22.2 | 60.6 | 3.7 (1.4) | 99 | 0.044 |
| stnd | 20.8 | 27.1 | 52.1 | 3.5 (1.4) | 48 | |||
| persn | 13.7 | 17.6 | 68.6 | 4.0 (1.3) | 51 | |||
| 9 | The WebEx sessions motivated me to make lifestyle changes. | all | 6.0 | 19.0 | 75.0 | 4.0 (1.0) | 100 | 0.222 |
| stnd | 2.1 | 18.8 | 79.2 | 4.2 (0.8) | 48 | |||
| persn | 9.6 | 19.2 | 71.2 | 3.9 (1.2) | 52 | |||
| 10 | I found WebEx easy to use. | all | 6.0 | 10.0 | 84.0 | 4.4 (1.0) | 100 | 0.923 |
| stnd | 6.3 | 12.5 | 81.3 | 4.4 (1.0) | 48 | |||
| persn | 5.8 | 7.7 | 86.5 | 4.4 (0.9) | 52 | |||
| 11 | The group to which I was randomly assigned (low-fat or personalized) negatively affected my motivation and involvement in the study | all | 76.0 | 19.0 | 5.0 | 1.7 (1.0) | 100 | 0.217 |
| stnd | 72.9 | 20.8 | 6.3 | 1.8 (1.1) | 48 | |||
| persn | 78.8 | 17.3 | 3.8 | 1.6 (1.0) | 52 | |||
Note. Participants answered the End of Study Questionnaire using a 5-point Likert scale, 1 = disagree, 2, 3 = neutral, 4, 5 = agree. For this table responses were collapsed into 3 categories: disagree (1, 2), neutral (3), and agree (4, 5).
Differences between groups were assessed using ordered logistic regressions. The End of Study Questionnaire was added after the study was underway, and question five was added even later, which is why the number of participants who answered was smaller than for other questions.
WebEx sessions refer to group counseling sessions delivered using a secure WebEx software.
Lessons learned regarding the delivery of group-based WebEx counseling sessions
Table 3 outlines several major challenges encountered and strategies our study team used during the course of the study. As anticipated, a few technical challenges were encountered when conducting WebEx sessions. First, although thorough one-on-one technology training was provided, participants sometimes encountered difficulty joining the WebEx sessions. To address this, test-run WebEx sessions with return demonstrations were introduced, and step-by-step hard copy instructions were distributed for future reference. Participants were encouraged to join meetings 15 minutes early to allow staff time to address connection difficulties without disrupting the session. With the first session of each cohort, an RDA was available to assist participants with logging-in and troubleshooting technical issues encountered.
Table 3.
Lessons Learned and Strategies Implemented in Our Remote Behavioral Weight Loss Study
| Challenges | Methods used to identity challenges |
Strategies we have implemented | Evaluation approaches |
|---|---|---|---|
| WebEx behavioral counseling related | |||
| Difficulty joining WebEx sessions |
|
|
|
| Bandwidth issues |
|
|
|
| Engagement levels vary |
|
|
|
| Self-monitoring app related | |||
| Difficulty locate foods in the app |
|
|
|
| Dissatisfaction with the app |
|
|
|
| App glitches (e.g., the meal score did not show up after participants entered planned meal) |
|
|
|
Participants were encouraged to use their own devices (smartphone, tablet, or home computer) and their own cellular connection or home WiFi to attend counseling sessions. The majority used their own devices, with only 18 (11.2%) requiring a loaner iPhone or iPad (provided at no charge for the duration of the study). While we did not prospectively collect information regarding the nature of their connections, bandwidth adequacy varied between the participant’s devices and connections, occasionally affecting the quality of the videoconference. For those joining meetings via their cellular phones, bandwidth issues may have been due to their use of cellular plans. For those joining via WiFi, bandwidth can be affected by the presence of malware, background software (e.g., automated software updates), other users on the connection, the age and efficiency of the equipment in use, and proximity to the router. When WebEx connection quality was poor, participants were advised to discontinue the video (attend via audio-only) or exit the session altogether and rejoin using a land line or mobile phone. Participants were encouraged to view the pre-recorded video after the session that was accessible from the study website.
While the median attendance rate for the 14 remote sessions was high (>85%), participants were not equally engaged. Participants were given the option of joining sessions audio-only (i.e., without showing their video image). While anecdotal, our sense was that those who shared their video image were more engaged than participants who did not. In audio-only mode, participants may be less attentive or engage in other activities and less inclined to respond or interact with the dietitian or other participants.
Lessons learned regarding use of technology-based self-monitoring and feedback
As with the WebEx sessions, one-on-one training was performed but technical challenges were occasionally encountered with the self-monitoring app. The food database within the app was derived from the USDA National Nutrition Database for Standard Reference. Foods and beverages within the USDA database are listed and labeled in a unique manner, complicating the food search. To address this, study staff worked with the app developer to save 200 commonly consumed foods as “favorites” on every participant’s account, with all participants receiving the same 200 favorites. Participants were also able to designate their own frequently consumed foods as “favorites” on their individual accounts. When a food was saved as favorite, it appeared on the top of the search results list, expediting the search.
Many participants had prior experience in using other commercially available dietary self-monitoring apps (e.g., MyFitnessPal and MyNetDiary). While anecdotal, our impression was that participants accustomed to using other apps were more dissatisfied with the study app. While we acknowledged that the study app may not be as user friendly as other commercially available apps, we stressed to participants that the personalized feedback on postprandial glycemic responses from the PNP app was novel and could therefore be useful for weight loss. Participants were offered additional booster training and guided in using alternative search strategies. A series of videos on how to log foods and save meals was posted on the study website, and a hard-copy instructional manual was created. Finally, occasional app issues interfered with participants’ ability to receive timely feedback about meals they were planning to consume. Participants experiencing this problem on a frequent basis were loaned a 4G phone to address possible bandwidth issues. The app developer was also contacted to troubleshoot any programming issues.
Regardless of the issue encountered, addressing nonadherence early is imperative as self-monitoring is considered to be an essential component of behavioral weight loss programs and self-monitoring declines over time.32 In this study, RDAs monitored the self-monitoring dashboard on a weekly basis. Participants logging < 3 days per week, were contacted by the study RD to understand self-monitoring barriers, troubleshoot app issues, and to encourage logging. If needed, booster sessions were scheduled to re-train participants in using the app.
DISCUSSION
Obesity and related chronic diseases affect more than half of the US population. Behavioral lifestyle interventions are recommended for weight loss and preventing or delaying the development of diabetes and its complications. Yet, numerous barriers limit the reach and scalability of traditional in-person behavioral counseling. This study demonstrates the feasibility of telehealth for delivering such programs remotely. Our WebEx-based session attendance rates are comparable to or higher than some in-person counseling interventions33-37 and show that remote counseling interventions can be as engaging as in-person programs. As our session schedule was consistent with Medicare provisions for intensive behavioral weight loss counseling, and can thus further facilitate downstream implementation.38
In a systematic review of barriers to remote health interventions, Alvarado et al39 described patient-level barriers to mHealth interventions citing digital illiteracy in roughly a quarter of studies where participants were uncomfortable with technology. While we did not assess digital literacy in this study, it may have played a role in the observed technological issues and the low level of self-monitoring adherence. To counter potential digital divide challenges, the following strategies may be useful for those who plan to implement similar interventions in patients having limited access to and familiarity with technology: (1) conduct one-on-one assessment to identify technology knowledge gaps, (2) include a training approach that includes a return demonstration of joining videoconferences and entering meals into the mobile app, (3) limit the time between technology training and the start of the intervention, (4) practice joining videoconferences immediately prior to the first scheduled session, and (5) provide video tutorials and a user manual with screenshots to illustrate how to maneuver through videoconference and self-monitoring apps.
Despite our study taking place in a large, metropolitan area, participants still experienced bandwidth issues reported in studies conducted in rural settings.40,41 To assure smooth intervention delivery, it is important to anticipate connectivity issues and provide advance guidance about alternative strategies that could be used to join videoconference sessions and track dietary intake when problems are encountered.
The use of smartphone apps to self-monitor dietary intake, body weight and physical activity has been shown to contribute to weight loss success and is becoming common-place in mHealth interventions. In this study, we chose the PNP app because, at the time the study was funded, it was the only available app that provided personalized feedback on postprandial glycemic response to meals, the cornerstone of our study intervention. Adherence to self-monitoring was lower than expected which may impact study findings. Self-monitoring is burdensome and challenging32, underscoring the importance of a user interface that enhances participant engagement.
The COVID-19 pandemic serves as a barrier to traditional face-to-face weight loss counseling. Obese patients and those with diabetes are at greater risk of poor COVID-19 outcomes and may forego in-person counseling to minimize exposure.42,43 Stay-at-home orders also may impact lifestyle behaviors in a manner that contributes to obesity (e.g., increased sedentary behavior and stress-eating).44-46 Recently, Medicare policy changes to reimburse telehealth were enacted to facilitate COVID-19 mitigation, reduce the strain on healthcare systems, maintain continuity of care while minimizing the risk of direct exposure, and enhance access to care for those who are medically or socially vulnerable. The Centers for Disease Control and Prevention noted that telehealth services could be used to “provide coaching and support for patients managing chronic health conditions, including weight management and nutrition counseling”.47 While these reimbursement policy changes were implemented as temporary measures, the Centers for Medicare and Medicaid Services is considering making them permanent.48 Of note, as our study was launched in 2017, well before the COVID-19 pandemic, the challenges and lessons shared in this report were not in response to COVID-19 per se. Yet, we believe this report could have critical and timely implications for research and health care communities as many are seeking ways to deliver behavioral interventions in a remote or hybrid mode. Our study can potentially serve as a program model for future behavioral counseling practices.
As both COVID-19 and the obesity pandemic disproportionately affect racial and ethnic minorities, there is an urgent need to find scalable solutions to increase access of evidence-based behavioral interventions to these high-risk populations. Participants in our study were primarily recruited from an urban medical center, where most had high education and income levels. The preliminary data presented on the WebEx sessions attendance rate and self-monitoring data might not generalize to underserved racial and ethnic minority groups. Additional research is needed to determine whether strategies for remote delivery of behavioral counseling intervention will be as successful in other study populations, and are equally effective in participants of diverse racial and ethnic backgrounds. In addition, the challenges and strategies we shared in this report were primarily based on our anecdotal observations. We did not have empirical data to show before and after results or demonstrate whether a particular strategy worked. However, the adherence data to our remote delivery can provide support to the feasibility of our videoconferenced group counseling approach.
CONCLUSIONS
As obesity and COVID-19 continue to affect a large population in the US, there is an urgent need to develop novel solutions to increase access to evidence-based lifestyle counseling. Our study employed existing technologies to bring behavioral interventions to patients’ homes or other natural settings. The high engagement rate with the remote group counseling sessions supports the feasibility of delivering telehealth-based behavioral support. As many behavioral researchers are adapting studies for remote delivery, the challenges, lessons learned, and strategies described in this report could serve as a timely and practical guide.
Supplementary Material
Acknowledgments:
This research is supported by grants from the American Heart Association (grant #17SFRM33590133) and the National Institute of Health (NIH UL1TR001445). Dr. Hu received support from the National Institute of Health grants K99MD012811, R00MD012811, U54MD000538-15 pilot award, and P30DK111022 pilot award. Dr. Kharmats received support from an NIH grant 5T32HL129953-04.
Footnotes
Conflict of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Curry SJ, Krist AH, Owens DK, et al. Behavioral weight loss interventions to prevent obesity-relatedmorbidity and mortality in adults US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2018;320(11):1163–1171. doi: 10.1001/jama.2018.13022 [DOI] [PubMed] [Google Scholar]
- 2.Krist AH, Davidson KW, Mangione CM, et al. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(20):2069–2075. doi: 10.1001/jama.2020.21749 [DOI] [PubMed] [Google Scholar]
- 3.Fukunaga LL, Uehara DL, Tom T. Perceptions of diabetes, barriers to disease management, and service needs: A focus group study of working adults with diabetes in Hawaii. Prev Chronic Dis. 2011;8(2). www.cdc.gov/pcd/issues/2011/mar/09_0233.htm.Accessed December 16, 2020. [PMC free article] [PubMed] [Google Scholar]
- 4.Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129(6):611–620. doi: 10.1016/j.puhe.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes self-management education programs in nonmetropolitan counties - United States, 2016. MMWR Surveill Summ. 2017;66(10):1–6. doi: 10.15585/mmwr.ss6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibounheuang P, Olson PS, Kittiboonyakun P. Patients’ and healthcare providers’ perspectives on diabetes management: A systematic review of qualitative studies. Res Soc AdmPharm. 2020;16(7):854–874. doi: 10.1016/j.sapharm.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Mehta P, Stahl MG, Germone MM, et al. Telehealth and Nutrition Support During the COVID-19 Pandemic. J Acad Nutr Diet. 2020;120(12):1953–1957. doi: 10.1016/j.jand.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly JT, Reidlinger DP, Hoffmann TC, Campbell KL. Telehealth methods to deliver dietary interventions in adults with chronic disease: A systematic review and meta-analysis1,2. Am J Clin Nutr. 2016;104(6):1693–1702. doi: 10.3945/ajcn.116.136333 [DOI] [PubMed] [Google Scholar]
- 9.Mayberry LS, Lyles CR, Oldenburg B, Osborn CY, Parks M, Peek ME. mHealth Interventions for Disadvantaged and Vulnerable People with Type 2 Diabetes. Curr Diab Rep. 2019;19(12). doi: 10.1007/s11892-019-1280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batsis JA, McClure AC, Weintraub AB, et al. Feasibility and acceptability of a rural, pragmatic, telemedicine-delivered healthy lifestyle programme. Obes Sci Pract. 2019;5(6):521–530. doi: 10.1002/osp4.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KE, Alencar MK, Coakley KE, et al. Telemedicine-Based Health Coaching Is Effective for Inducing Weight Loss and Improving Metabolic Markers. Telemed e-Health. 2019;25(2):85–92. doi: 10.1089/tmj.2018.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shell AL, Hsueh L, Vrany EA, et al. Depressive symptom severity as a predictor of attendance in the HOME behavioral weight loss trial. J Psychosom Res. 2020;131. doi: 10.1016/j.jpsychores.2020.109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevick MA, Woolf K, Mattoo A, et al. The Healthy Hearts and Kidneys (HHK) study: Design of a 2 × 2 RCT of technology-supported self-monitoring and social cognitive theory-based counseling to engage overweight people with diabetes and chronic kidney disease in multiple lifestyle changes. Contemp Clin Trials. 2018;64:265–273. doi: 10.1016/j.cct.2017.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JD, Hales S, Evans TE, et al. Description, utilisation and results from a telehealth primary care weight management intervention for adults with obesity in South Carolina. J Telemed Telecare. 2020;26(1-2):28–35. doi: 10.1177/1357633X18789562 [DOI] [PubMed] [Google Scholar]
- 15.Clark DO, Keith N, Weiner M, Xu H. Outcomes of an RCT of videoconference vs. in-person or in-clinic nutrition and exercise in midlife adults with obesity. Obes Sci Pract. 2019;5(2):111–119. doi: 10.1002/osp4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taetzsch A, Gilhooly CH, Bukhari A, et al. Development of a videoconference-adapted version of the community diabetes prevention program, and comparison of weight loss with in-person program delivery. Mil Med. 2019;184(11-12):647–652. doi: 10.1093/milmed/usz069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azar KMJ, Aurora M, Wang EJ, Muzaffar A, Pressman A, Palaniappan LP. Virtual small groups for weight management: an innovative delivery mechanism for evidence-based lifestyle interventions among obese men. Transl Behav Med. 2015;5(1):37–44. doi: 10.1007/s13142-014-0296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West DS, Stansbury M, Krukowski RA, Harvey J. Enhancing group-based internet obesity treatment: A pilot RCT comparing video and text-based chat. Obes Sci Pract. 2019;5(6):513–520. doi: 10.1002/osp4.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson KE. A Comparison of Video Conferencing and In-Person Health Coaching Approaches in Combination with MHealth Devices on Weight Loss, Physical Activity, and Glycemic Control Recommended Citation. https://digitalrepository.unm.edu/educ_hess_etds. Accessed December 16, 2020.
- 20.Alencar MK, Johnson K, Mullur R, Gray V, Gutierrez E, Korosteleva O. The efficacy of a telemedicine-based weight loss program with video conference health coaching support. J Telemed Telecare. 2019;25(3):151–157. doi: 10.1177/1357633X17745471 [DOI] [PubMed] [Google Scholar]
- 21.Vadheim LM, Patch K, Brokaw SM, et al. Telehealth delivery of the diabetes prevention program to rural communities. Transl Behav Med. 2017;7(2):286–291. doi: 10.1007/s13142-017-0496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ventura Marra M, Shotwell M, Nelson K, Malone J. IMPROVING WEIGHT STATUS IN OBESE MIDDLE-AGED AND OLDER MEN THROUGH TELENUTRITION. Innov Aging. 2017;1(suppl_1):635–636. doi: 10.1093/geroni/igx004.2242 [DOI] [Google Scholar]
- 23.Ahrendt AD, Kattelmann KK, Rector TS, Maddox DA. The effectiveness of telemedicine for weight management in the move! program. J Rural Heal. 2014;30(1):113–119. doi: 10.1111/jrh.12049 [DOI] [PubMed] [Google Scholar]
- 24.Rogers E, Aidasani SR, Friedes R, et al. Barriers and facilitators to the implementation of a mobile insulin titration intervention for patients with uncontrolled diabetes: A qualitative analysis. JMIR mHealth uHealth. 2019;7(7). doi: 10.2196/13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy NK, Orzeck-Byrnes NA, Aidasani SR, et al. Transition of a text-based insulin titration program from a randomized controlled trial into real-world settings: Implementation study. J Med Internet Res. 2018;20(3). doi: 10.2196/jmir.9515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford AT, Wang B, Orzeck-Byrnes NA, et al. Sociodemographic and clinical correlates of key outcomes from a Mobile Insulin Titration Intervention (MITI) for medically underserved patients. Patient Educ Couns. 2019;102(3):520–527. doi: 10.1016/j.pec.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 27.Hu L, St-Jules DE, Popp CJ, Sevick MA. Determinants and the Role of Self-Efficacy in a Sodium-Reduction Trial in Hemodialysis Patients. J Ren Nutr. 2019;29(4). doi: 10.1053/j.jrn.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popp CJ, St-Jules DE, Hu L, et al. The rationale and design of the personal diet study, a randomized clinical trial evaluating a personalized approach to weight loss in individuals with pre-diabetes and early-stage type 2 diabetes. Contemp Clin Trials. 2019;79. doi: 10.1016/j.cct.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 29.Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31 Suppl 2(Supplement 2):S150–S154. doi: 10.2337/dc08-s241 [DOI] [PubMed] [Google Scholar]
- 30.Bandura A Social Cognitive Theory: An Agentic Perspective. Annu Rev Psychol. 2001;52(1):1–26. doi: 10.1146/annurev.psych.52.1.1 [DOI] [PubMed] [Google Scholar]
- 31.Bandura A Health promotion from the perspective of social cognitive theory. Psychol Heal. 1998;13(4):623–649. doi: 10.1080/08870449808407422 [DOI] [Google Scholar]
- 32.Burke LE, Wang J, Sevick MA. Self-Monitoring in Weight Loss: A Systematic Review of the Literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med (Baltim). 2010;51(2):123–128. doi: 10.1016/j.ypmed.2010.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newton RL, Carter LA, Johnson W, et al. A church-based weight loss intervention in African American adults using text messages (LEAN Study): Cluster randomized controlled trial. J Med Internet Res. 2018;20(8). doi: 10.2196/jmir.9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perri MG, Shankar MN, Daniels MJ, et al. Effect of Telehealth Extended Care for Maintenance of Weight Loss in Rural US Communities: A Randomized Clinical Trial. JAMA Netw open. 2020;3(6):e206764. doi: 10.1001/jamanetworkopen.2020.6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. 2016;10:1547–1559. doi: 10.2147/PPA.S103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke LE, Ewing LJ, Ye L, et al. The SELF trial: A self-efficacy-based behavioral intervention trial for weight loss maintenance. Obesity. 2015;23(11):2175–2182. doi: 10.1002/oby.21238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=IntensiveBehavioralTherapyforObesity&bc=ACAAAAAAIAAA&NCAId=253.Accessed December 16, 2020.
- 39.Alvarado MM, Kum HC, Coronado KG, Foster MJ, Ortega P, Lawley MA. Barriers to remote health interventions for type 2 diabetes: A systematic review and proposed classification scheme. J Med Internet Res. 2017;19(2). doi: 10.2196/jmir.6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson JA, Chubak J, O’Connell J, Ramos MC, Jensen J, Jobe JB. Design of a randomized controlled trial of a web-based intervention to reduce cardiovascular disease risk factors among remote reservation-dwelling American Indian adults with type 2 diabetes. J Prim Prev. 2012;33(4):209–222. doi: 10.1007/s10935-012-0276-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young H, Miyamoto S, Ward D, Dharmar M, Tang-Feldman Y, Berglund L. Sustained effects of a nurse coaching intervention via telehealth to improve health behavior change in diabetes. Telemed e-Health. 2014;20(9):828–834. doi: 10.1089/tmj.2013.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020;132(8):1–7. doi: 10.1080/00325481.2020.1786964 [DOI] [PubMed] [Google Scholar]
- 43.Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Z, Lin X, Kaminga AC, Xu H. Impact of the COVID-19 epidemic on lifestyle behaviors and their association with subjective well-being among the general population in Mainland China: Cross-sectional study. J Med Internet Res. 2020;22(8). doi: 10.2196/21176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almandoz JP, Xie L, Schellinger JN, et al. Impact of COVID -19 stay-at-home orders on weight - related behaviours among patients with obesity . Clin Obes. 2020; 10(5). doi: 10.1111/cob.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6). doi: 10.3390/nu12061583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Using Telehealth to Expand Access to Essential Health Services during the COVID-19 Pandemic ∣ CDC. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html. Accessed December 16, 2020. [Google Scholar]
- 48.CMS Unveils Major Organizational Change to Reduce Provider and Clinician Burden and Improve Patient Outcomes ∣ CMS. https://www.cms.gov/newsroom/press-releases/cms-unveils-major-organizational-change-reduce-provider-and-clinician-burden-and-improve-patient. Accessed December 16, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.