Abstract
The interactions of microplastics (MPs) with other chemicals and the range of outcomes are of great importance to enhance understanding of their environmental impacts and health risks. Cadmium (Cd) and cadmium compounds are widely used as pigments and stabilizers in plastics, but they readily leach out. Here we addressed the impacts of MPs, Cd, and their joint exposure in a tractable Drosophila melanogaster model. We show that exposure to MPs lead to extensive particle size depended gut damage early in life and an enhancement of Cd-induced inhibition of locomotor-behavioral function in adult flies. In addition, we show that Cd exposure induces epigenetic gene silencing via position-effect variegation (PEV) in somatic tissues that was dramatically enhanced by co-exposure with MPs. The results indicate that MPs can aggravate the toxicity of other environmental contaminants and induce adverse effects across a range of diverse outcomes in a tractable and widely used model organism. These observations raise the prospects of using Drosophila as a tool for the rapid assessment of MP-mediated toxicity.
Keywords: Microplastics, Cadmium, Toxicity, Drosophila, PEV
1. Introduction
Exposure to microplastics (MPs) have raised worldwide concern because of their continuous environmental accumulation, global occurrence, and potential health risks. In one route of exposure, MP contamination primarily occur in aquatic environments and are transmitted through the food chain. Growing evidences have demonstrated that MPs can be ingested by various aquatic organisms such as fish (Lu et al., 2016), mussels (von Moos et al., 2012), and zooplankton (Chua et al., 2014). MP can also be ingested by mosquito larvae and transfer into the adults (Al-Jaibachi R et al., 2018). Other recent studies found that half of the mayfly and caddisfly larvae in rivers contained MPs and the concentration was 0.01-0.04 MPs mg−1 dry weight (Windsor Fredric M et al., 2018). Therefore, MPs can spread via flying insects, contaminating new environments, and threatening other organisms.
Health risks of MP exposure have also been evaluated in model organism such as zebrafish and mice. Drosophila melanogaster has made major contributions to biomedical research but its use in the context of MP exposure has been limited, despite the organism’s advantages as a tractable model system (Meyer et al., 2014; Mirzoyan et al., 2019; Ong et al., 2015; Prussing et al., 2013; Zamberlan et al., 2020). In addition, it has been suggested that MPs can interact with other contaminants potentially acting as chemical carriers and influencing their bioaccumulation and toxic effects (Browne et al., 2013; Lu et al., 2018b). The interactions of MPs with other chemicals and the outcomes are of great importance to enhance the understanding of the environmental impacts and health risks of MP exposure.
Metals are used as pigments, heat stabilizers, and catalysts in plastic materials that come in contact with food products (Bakircioglu et al., 2011; Lahimer et al., 2017; Whitt et al., 2013). MPs with high surface areas and polarity can also adsorb and concentrate metals to a much greater extent than natural sediments (Holmes et al., 2012) and may serve as a point source of acute metal exposure (Munier and Bendell, 2018). In addition, MPs can act as vectors that transport adsorbed metals into organisms thereby changing their exposure route and effective dose. For instance, the adsorption of MPs increased the intake of metals through dietary pathways, resulting in higher metal accumulation in the digestive tract (Khan et al., 2015). However, the outcome of this ability of MPs to act as vectors is still debatable. Studies have shown that MPs could change the localization of metals in tissues (Khan et al., 2015). Release of metals from ingested MPs within tissues could result in elevated exposure and downstream impacts through additive or synergistic interactions (Lu et al., 2018a; Qiao et al., 2019a). On the other hand, studies have suggested that low-density MPs such as polyethylene (PE) MPs could reduce the bioavailability of metals in waters because PE-MPs tend to float on the water surface thereby limiting interactions between adsorbed metals and aquatic organisms (Chua et al., 2014; Khan et al., 2015). These contradictory studies illustrate the uncertainties that still govern our understanding of MPs-metal interactions.
Cadmium (Cd) and cadmium compounds are widely used as pigments and stabilizers in plastics such as PE, polyvinyl chloride (PVC), and acrylonitrile butadiene styrene (ABS), but they readily leach out (Abhay and Prashant, 2007; Liu et al., 2020; Turner, 2019). Here we examined the combined toxicity of MPs and Cd on Drosophila, a tractable model insect to evaluate the potential risk of toxicant exposure. To address the issues, we monitored an array of outcomes including mortality, locomotor function, and gut damage. We also monitored the ability of MPs and Cd to induce epigenetic gene silencing in Drosophila models of gene silencing via position effect variegation (PEV). Our results deepen the significance of toxicological studies of MPs exposure and highlight their interactions with heavy metal toxicants.
2. Materials and Methods
2.1. Chemicals
The 0.1-μm and 1.0-μm polystyrene plastic beads stock as dispersions (2.5% w/v, 10 mL) were purchased from Polysciences Inc. (Warrington, USA). Before the experiment, MPs were rinsed with Milli-Q water three times to remove possible contaminants. Cadmium chloride (Cd) was purchased from Alfa Aesar (Ward Hill, USA). The stock solution (100 mM) of Cd was prepared. In addition, the desired amounts of MPs and Cd were mixed and pre-incubated for 48 h before the combined Cd+MPs experiments.
2.2. Drosophila stocks
All fly strains and their different developmental stages were maintained at 24 ± 1 °C on standard Drosophila food, containing agar, corn meal, sugar, yeast, propionic acid and Tegosept. The pre-incubated solution containing MPs or metals was added to the hot food solution (> 70 °C), homogeneously mixed, poured into vials (10 mL food for each vial) and put into the refrigerator for quick solidification. Four D. melanogaster strains have been used in this study. For PEV assays, we scored the progeny of the cross between males of strain y; bw; e; ci ey; Ycs (herein labeled Ycs); and females of a strain carrying w[m4h] maintained in a background with the Su(var)3-102 (Su(var)3-102/TM3, Sb1 Ser1; w[m4h], Bloomington Drosophila Stock Center), as previously described by Lemos et al (Lemos et al., 2010). F1 males that do not inherit the Su(var) allele display PEV. For gut damage, behavioral, and mortality analyses, we used a X^X/0/X^Y strain (herein labelled 995), a X^XY/0/X^Y strain (herein labelled CS10) and the Ycs strain described above.
2.3. Survival assay
Freshly hatched CS10 and 995 virgin female flies (not more than 4 h old individuals) were respectively placed into each vial (n = 20) and 4 vials for each treatment group. For each fly strain, 6 groups were set up: Control group (only standard food), MP(L) group (treated food containing 200 μg/mL 1-μm MPs), MP(S) group (treated food containing 200 μg/mL 0.1-μm MPs), Cd group (treated food containing 1.5 mM Cd), Cd + MP(L) group (treated food containing 1.5 mM Cd and 200 μg/mL 1-μm MPs), and Cd + MP(S) group (treated food containing 1.5 mM Cd and 200 μg/mL 0.1-μm MPs). Over the course of 7 days, the number of alive files was counted every day. The concentration of MPs is based on previous toxicological studies about particulate matter on Drosophila (5-200 μg/mL) (Panacek et al., 2011; Posgai et al., 2011). The concentration of Cd is according to previous toxicity data of Cd on Drosophila (0.01-2 mM) (Courgeon et al., 1984; Jacobson et al., 1981).
2.4. Climbing assay
Virgin Ycs females were firstly crossed with Ycs males. Then the mated females were transferred to the vials containing treated food and were aged. After 9 days, the adults were removed and then the freshly hatched virginal males and females (the first filial generation, F1) were separately collected. 20 males and 20 females were respectively placed into new vials containing treated food. To evaluate the dose response of Cd on locomotor function, flies were exposed to 0, 0.01, 0.1, 0.2 mM Cd added in food. For each gender, 4 vials were used in each dose group. To evaluate the impacts of MPs and Cd on locomotor function, 6 groups were set up: Control group (only standard food), MP(L) group (treated food containing 200 μg/mL 1-μm MPs), MP(S) group (treated food containing 200 μg/mL 0.1-μm MPs), Cd group (treated food containing 0.01 mM Cd), Cd + MP(L) group (treated food containing 0.01 mM Cd and 200 μg/mL 1-μm MPs), and Cd + MP(S) group (treated food containing 0.01 mM Cd and 200 μg/mL 0.1-μm MPs). The concentration of Cd was selected based on the above dose-response climbing assay.
After exposure for 7 days, the 20 flies from each vial were transferred to a 250 mL graduated cylinder for performing the climbing assay by following a reported protocol with slight modification (Madabattula et al., 2015). The top of the cylinder was closed with parafilm to prevent the escape of any flies. The cylinder was gently tapped to drop all the flies at the bottom of the cylinder. Each trial was conducted for 2 min from the flies were last tapped and the number of flies crossed the height of 9.5 cm (90 mL line) of the cylinder was recorded at each time point (quantify every 10 sec). The percentage of flies above the threshold line was calculated. To avoid error, the height of threshold line on each cylinder was individually measured and labeled. Four replicates (20 flies for each replicate) were conducted for each group.
2.5. Trypan blue staining
Trypan blue is a dye that has been widely used for selective staining of dead tissues or cells (Strober, 2001). Third instar larvae were collected from the F1 generation of flies used in above climbing assay. Trypan blue staining was performed on the third instar larvae by following a reported protocol with slight modification (Sabat et al., 2016). Four replicates (20 larvae for each replicate) were analyzed for each group. All grouping settings were consistent with the climbing assay described above. Larvae were washed with PBS (1×) solution thoroughly and then were placed on 0.8% agarose (containing 5% sugar and 10% trypan blue stain or blue 1 dye used as controls) plates for 30 min. After that the larvae were washed thoroughly with PBS again for 15 min. The larvae were finally observed under a stereomicroscope (Olympus, Toyo, Japan). The percentage of flies with blue gut staining (i.e. presence of gut damage) was calculated in the trypan blue treatment. Blue 1 dye was used as a control because it can be visualized in the larvae gut independently of the presence of tissue damage.
2.6. PEV assay
PEV is a classic and widely used marker of gene silencing via heterochromatin spreading in Drosophila. We evaluated the potential for MPs and Cd to alter epigenetic states in D. melanogaster using the classic whitemottled4 (wm4) model of PEV. In the wm4 PEV, the white gene for red eye color is moved from its native X-chromosome euchromatic position to a location near the heterochromatic X-chromosome centromere. Stochastic expansion of the heterochromatin (“variegation”) into the white locus causes gene silencing and produces varying numbers of unpigmented ommatidia (eye segments) depending on whether white is silenced or expressed. Virgin 6175 females (carrying wm4) were crossed to Ycs males at 1:1 ratio on normal food for 2-3 days. Mated females (15 individuals) were transferred to vials containing treatment or control food. Same treatment groups like the climbing assay were set up. Male offspring manifesting PEV, were collected daily for 4-5 days, aged for 24 hours, and flash-frozen in liquid nitrogen. For each treatment, 4 replicates were conducted, and 16-20 eyes were scored for each replicate using a five-category scale. Eye pigment was assessed by removing heads from frozen flies and placing one head per well in an 8-well tube strip containing 10 μL 30% acidified ethanol, pH 2. Heads were incubated 24 h and 1.5 μL of each solution was used to determine the absorbance at 480 nm using Thermo Scientific NanoDrop 2000 with a background correction of 750 nm. Each treatment per genotype had 8 replicates.
2.7. Statistical analysis
In this study, the experiment was designed with four replicates (n = 20 in each replicate) for each group. The results are shown as means ± standard deviation. Data analysis was performed using Prism 8.0 software. One-way analysis of variance (ANOVA) followed by Tukey’s test was used to address differences across groups. The level of significance was set at p < 0.05.
3. Results
3.1. Survivorship upon exposure to MPs and Cd
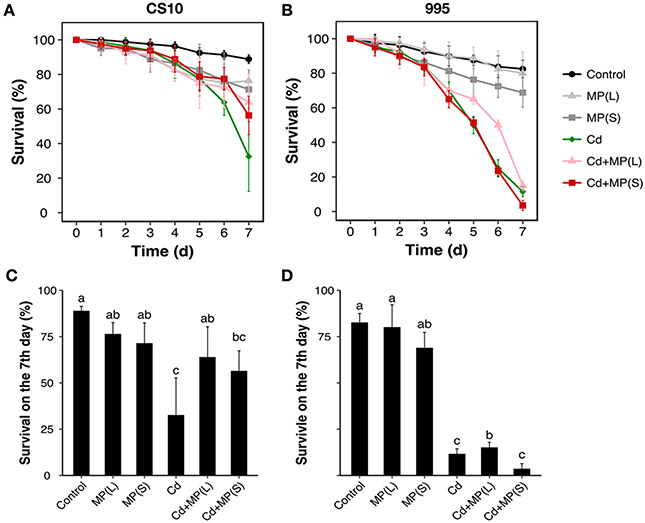
Exposure to MPs caused no significant changes in survival of either CS10 or 995 compared to controls (Figure 1). No changes in wing phenotypes or leg defects were observed. Exposure to Cd caused a time-dependent increase in mortality during the experimental period compared to the control group. Simultaneously exposure to Cd and MPs did not alter the survival of the flies compared to Cd alone. The 995 genotype (XX females) was more sensitive to metal toxicity than the CS10 genotype (XXY) females.
Figure 1.
Survivorship of Drosophila exposed to MPs and Cd. (A, B) Survival curves of two genotypes [X^XY females (CS10) and X^X females (995)] exposed to MP and/or Cd. Data were collected every 24h for 7 days. (C, D) Survival on the 7th day for Cd and/or MP treatments. Bars with different letters are significantly different (ANOVA and Tukey's tests, p < 0.05). Four vials with 20 flies in each vial were used for each treatment and genotype.
3.2. Motor activity (climbing) upon exposure to MPs and Cd
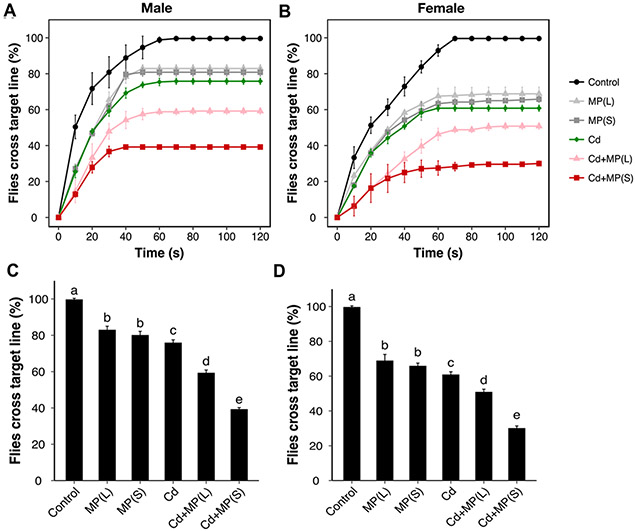
Monitoring of motor activity via the climbing assay has been widely used in Drosophila models of neurodegeneration and Parkinson disease. Here, the climbing activity of flies was first tested for a range of Cd concentrations (0, 0.01, 0.1, 0.2 mM) (Figure 2). Dose-response inhibition of climbing activity was observed. Based on these results, the minimal concentration of Cd (0.01 mM) was used for the evaluation of the impacts of MPs and Cd on locomotor function and other toxic tests. In terms of the determination of locomotor function, 100% males and females in the control group successfully crossed the target line within 120 s (Figure 3). However, 83% males and 68% females in the MP(L) group and 80% males and 66% females in the MP(S) group crossed the same distance within the same time (Figure 3). There was no significant difference between MP(L) and MP(S) treatment groups. For Cd alone group, only 76% males and 61% females could cross the target line after exposed to Cd (Figure 3). Compared with Cd alone, Cd+MP(L) significantly decreased 17% (p < 0.01) and 10% (p < 0.001) climbing activity for males and females, respectively (Figure 3). While, Cd+MP(S) decreased 37% (p < 0.001) and 31% (p < 0.001) climbing activity for males and females compared with Cd alone. Cd+MP(S) caused further decreased of climbing activity than Cd+MP(L) for both males and females (p < 0.001).
Figure 2.
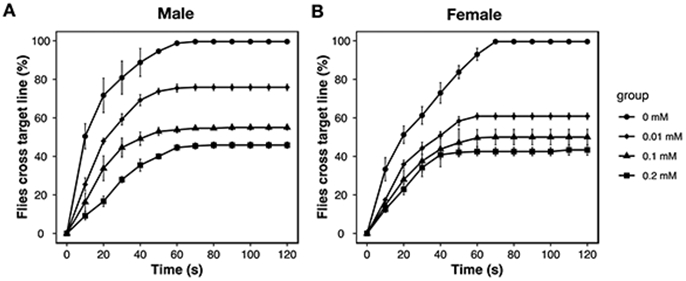
Dose response curves of Cd exposure on climbing ability in Drosophila. Four vials with 20 flies (Ycs genotype) for each vial were used for males (A) and females (B).
Figure 3.
Climbing ability of Drosophila exposed to MP and Cd. Four vials with 20 flies in each vial were used for each group. Climbing ability of (A) male and (B) female flies (Ycs genotype) exposed to MP and/or Cd. (C) Climbing ability of (A) male and (B) female flies exposed to MP and/or Cd at the end of the experiment. Bars with different letters are significantly different (ANOVA and Tukey's tests, p < 0.05).
3.3. Gut damage upon exposure to MPs and Cd
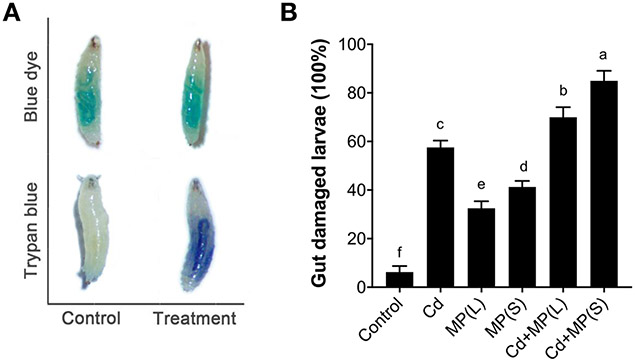
Trypan blue staining was used to identify intestinal cell damage in flies exposed to MPs and Cd. Positive trypan blue staining was only observed in treatment groups, including MPs alone groups, Cd alone group and combined exposure groups of MPs and Cd (Figure 4A), indicating that these treatments induced gut damage in flies. On the other hand, blue 1 dye staining can be visualized independently of the presence of dead tissue (i.e., gut damage); as expected, blue 1 dye staining observed in both control and treatment groups and no significant difference was observed between the groups (Figure 4A). Compared with the control, Cd alone exposure caused increased gut damage by 52% (p < 0.001) (Figure 4B). MPs also caused a certain degree of gut damage. MP(L) and MP(S) increased the gut damage rate by 32% (p < 0.001) and 41% (p < 0.001) compared with the control (Figure 4B). For the combined exposure, Cd+MP(L) and Cd+MP(S) respectively increased the gut damage rate by 64% (p < 0.001) and 79% (p < 0.001) compared with Cd alone group (Figure 4B).
Figure 4.
Gut damage in 3rd instar larvae exposed to MP and Cd. (A) Blue 1 dye is present in the gut of larvae (Ycs genotype) of both control and treatment groups (MP and/or Cd). Trypan blue staining of damaged tissue was only observed in treated larvae (presence of gut damage). No sign of tissue damage was observed in the control larvae. (B) Percentage of gut damage in larvae after exposure to MP and/or Cd. Bars with different letters are significantly different (ANOVA and Tukey's tests, p < 0.05).
3.4. PEV upon exposure to MPs and Cd
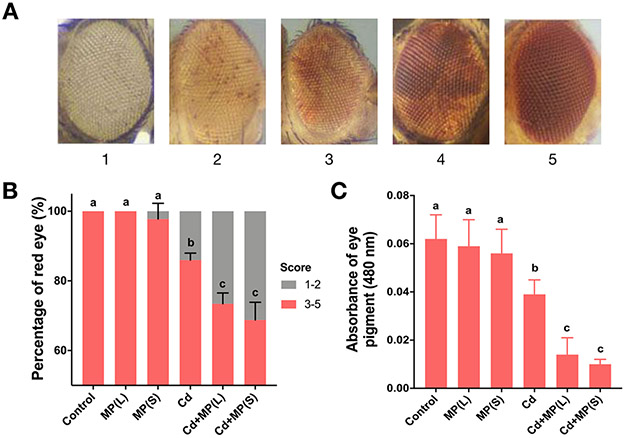
PEV is a marker of gene silencing via heterochromatin spreading in Drosophila. In this assay using the wm4h marker the DNA sequence of a gene remains unchanged but its expression at an euchromatin-heterochromatin boundary is modulated by the expansion or contraction of neighboring heterochromatin. Here we crossed wm4h virgin females to males carrying the Ycs Y-chromosome. The F1 males carrying the wm4h inversion and the Ycs chromosome display variegating red eyes due to the ability of Ycs to induce heterochromatin contraction and wm4h expression (Lemos et al., 2010). We scored variegation on a scale of 1-5, from white to red (Figure 5A). To facilitate statistical analysis, the eye color score of 1 to 2 is identified as white, and the eye color score of 3 to 5 is identified as red. The percentage of red eye in each group was calculated and the results are shown in Figure 5B. Compared with the control, MPs alone did not alter PEV, whereas Cd alone was sufficient to induce gene silencing and significantly decreased the red eye rate by 14% (p < 0.001). For the combined exposure, Cd+MP(L) and Cd+MP(S) significantly decreased the red eye rate by 15% (p < 0.001) and 20% (p < 0.001) compared to Cd alone group (Figure 5B).
Figure 5.
Gene silencing via position effect variegation (PEV; wm4h) in Drosophila exposed to MP and Cd. (A) Representative images showing eye pigment scores. (B) Distribution of eye pigment scores after exposure to MP and/or Cd. (C) Eye pigment measurement for flies after exposure to MP and Cd. Bars with different letters are significantly different (ANOVA and Tukey's tests, p < 0.05).
Similar trends were also confirmed by measurement red eye pigment (Figure 5C). Compared with the control, pigment levels were not significantly changed in the MPs alone treatment groups. Cd exposure significantly decreased the pigment level by 37% (p < 0.05) compared with the control. For the combined exposure, Cd+MP(L) and Cd+MP(S) significantly decreased the pigment level by 64% (p < 0.001) and 74% (p < 0.001) compared to Cd alone group.
4. Discussion
It has been documented that both aquatic and flying insects can accumulate MPs in their bodies (Al-Jaibachi R et al., 2018; Windsor Fredric M et al., 2018). However, the outcomes of MPs accumulation are unclear. The fruit-fly Drosophila melanogaster has been widely used a model organism in biomedical research (Mirzoyan et al., 2019; Prussing et al., 2013), partially due to its short life cycle, fully sequenced genome, and extensive gene conservation with humans and mouse. Indeed, with over 70% of the disease causing human genes also present in fruit flies, this model organism has also been used to evaluate outcomes of exposure to heavy metals (Guan et al., 2019) and particulate matter (Vecchio, 2015). To our knowledge, this is the first study to evaluate the toxicity of MPs in a Drosophila model.
Here we showed that gut health and motor activity were significantly disrupted by MPs exposure. Exposure to MP (L) for 7 days reduced climbing activity of male and female flies by 17% and 32%, respectively. Exposure to MP (S) for 7 days reduced climbing activity of male and female flies by 20% and 34%, respectively. These results indicated that locomotor functions were affected by MPs exposure. On the one hand, maintaining the locomotor functions of insects need sufficient energy supply, with MPs exposure possibly disrupting feeding behavior and energy metabolism. For instance, it has been reported that MPs can alter feeding and reduce energy allocation of oysters (Sussarellu et al., 2016). Similarly, MPs exposure affects feeding behavior of fish (Mattsson et al., 2015) and disrupts energy metabolism of fish (Lu et al., 2016) and mice (Deng et al., 2017). On the other hand, locomotor functions are also regulated by the nervous system. It is documented that MPs exposure induces neurotoxicity and motor behavior of seabass and nematode (Barboza et al., 2018b; Le et al., 2018). According to these studies, AChE inhibition, lipid peroxidation and damage affects neurons involved in motor modulation (such as cholinergic and GABAergic neurons) thereby contributing to neurotoxicity. Thus, MPs induced locomotor defects in Drosophila might have partially emerged from impaired energy metabolism or malfunctioning of neural connections.
Ingestion and transit of MPs in the gut has been demonstrated in animals as diverse as fish (Tanaka and Takada, 2016), mussels (von Moos et al., 2012), mice (Deng et al., 2017), and insects (Al-Jaibachi R et al., 2018). Here we documented a positive trypan blue staining in the gut of D. melanogaster larvae exposed to MPs. Trypan blue is a negatively charged dye that only stains cells with a compromised cell membrane, hence indicating cell death (Strober, 2001). Our results suggested that exposure to MPs cause significant gut damage in Drosophila larvae. On the one hand, the gut damage may be attributed to scratch or friction of the digestive tract by MPs (Choi et al., 2018). On the other hand, MPs could induce the generation of reactive oxygen species (ROS) and trigger oxidative stress (Avio et al., 2015), which may subsequently result in inflammation in the tissues. This is possibly why MP(S) with a larger specific surface area than MP(L) caused more severe gut damage in this study. Our previous studies also have demonstrated that ingestion of MPs generates reactive oxygen species (ROS), results in oxidative stress, and causes intestinal inflammation and increased intestinal permeability (Lu et al., 2016; Qiao et al., 2019b). In addition, the damage of digestive tract could disrupt nutrient absorption and energy harvesting (Fandriks, 2017), which may explain the disruption of energy metabolism mentioned above.
Although there are few studies to which our results can be directly compared, Drosophila has been established as a successful non-mammal model organism with minimal ethical issues for the risk assessment of nanomaterials (Ong et al., 2015), which can provide us with meaningful lessons. For instance, dietary uptake of carbon nanomaterials (e.g. C60, carbon black, and single-walled and multiwalled nanotubes) in Drosophila was linked to locomotor impairment and higher mortality (Liu et al., 2009). Exposure to silver nanoparticles could cause developmental and reproductive toxicity (Panacek et al., 2011) and behavioral abnormalities such as poor crawling and climbing ability (Raj et al., 2017b). Furthermore, particle size significantly influenced the toxic effects of nanomaterials on Drosophila (Raj et al., 2017a). Here, we confirm that smaller MPs lead to significantly more severe gut damage than larger MPs based on the higher percentage of larvae stained with trypan blue. Smaller MPs also lead to a significantly greater reduction in climbing ability of adult flies. As mentioned above, the smaller MPs with larger specific surface area could induce more oxidative stress. Furthermore, the smaller MPs especially nanoplastics (NPs) have higher potential to enter internal circulation through digestive tract and cause more severe damage (Browne et al., 2008). The smaller MPs also have higher interaction with cell membranes, which may change the membrane structure and pose a threat to the function of the cell (von Moos et al., 2012; Wu et al., 2019). Hence, more studies are advocated to characterize the interaction of MPs with cell membranes to better understand the chemical basis of MPs toxicity in cells and organisms.
Furthermore, compared with Cd alone, combined exposure of MPs and Cd caused aggravated toxicity manifested by reduced climbing activity and increased gut damage. Given that MPs alone or Cd alone could both individually cause these toxicities in Drosophila, the coexposure of the two contaminants may induce additive or synergistic effects. The possible mechanism is that MPs could transport Cd into tissues and locally release the metal, causing enhanced toxicity (Lu et al., 2018b). Similarly, our recent study found that co-exposure of MPs and copper could induce synergistic effects on zebrafish, as suggested by alterations in the biomarkers of malonaldehyde (MDA) and superoxide dismutase (SOD) in the liver and gut (Qiao et al., 2019a).
It is also interesting that MP co-exposure with Cd caused significantly enhanced gene silencing in models of PEV that manifested the phenotype in the adult eye. The enhanced gene silencing caused by the combined MP-Cd exposure may be due to an increase in uptake or tissue accumulation of Cd upon co-exposure with MPs. This observation is in agreement with suggestions that MPs can adsorb and transport metals into tissues which might increase the bioaccumulation of metals and induce higher toxicity (Barboza et al., 2018a; Barboza et al., 2018b; Lu et al., 2018b). This has been confirmed by the epigenetic alterations we observed here. While MPs did not by itself alter PEV, it substantially enhanced gene silencing due to Cd exposure. PEV emerges from leakage of epigenetic modifications, such as DNA methylation and histone acetylation (Zhou et al., 2012), from heterochromatin to nearby euchromatin. Cd has been linked to epigenetic alterations, such as chromatin condensation in rat lung epithelial cells (Hart et al., 1999), DNA hypermethylation in human cell lines (Benbrahim-Tallaa et al., 2007), and altered histone modifications in human urothelial cells (Sanders et al., 2014). Our results identify Cd as a strong enhancer of variegation, which is further potentiated by co-exposure with MPs
5. Conclusion
Plastics are a global problem with exposure to microplastics an emerging health concern. However, the impact of microplastic exposure is not self-evident and exploring the landscape of MP exposures and their possible outcomes remains a challenge. Drosophila melanogaster is an exceptional model for both mechanistic interrogation and screening approaches, but it has been underutilized in environmental health research despite the availability of a plethora of assays and screening endpoints. Here, we conducted a series of experiments to address the consequences of microplastic and cadmium exposure across a number of outcomes. The results reveal interactions between these contaminants, causing microplastic size-depended gut damage early in life, inhibition of locomotor-behavioral function in adult flies, and epigenetic gene silencing in somatic tissues. Moreover, the presence of MPs induces aggravated toxic effects of Cd manifested by significantly reduced climbing activity and increased gut damage, as well as epigenetic gene silencing in somatic tissues as revealed by PEV assays. We envision that Drosophila is likely to serve as a key organism to facilitate assessments of MP-mediated toxicity and interactions with other ubiquitous contaminants.
Acknowledgments
This research work was financially supported by the National Natural Science Foundation of China (No. 21777068). Work in the Lemos lab has been partially supported by NIEHS grants R01ES027981. Thanks to Andrew Driscoll for providing technical assistance.
Reference
- Abhay K, Prashant P. Lead and cadmium in soft plastic toys. Current Science 2007; 93: 818–822. [Google Scholar]
- Al-Jaibachi R, Cuthbert RN, A. C. Up and away: ontogenic transference as a pathway for aerial dispersal of microplastics. Biology Letters 2018; 14: 20180479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d'Errico G, et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution 2015; 198: 211–222. [DOI] [PubMed] [Google Scholar]
- Bakircioglu D, Kurtulus YB, Ucar G. Determination of some traces metal levels in cheese samples packaged in plastic and tin containers by ICP-OES after dry, wet and microwave digestion. Food and Chemical Toxicology 2011; 49: 202–207. [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Vieira LR, Branco V, Carvalho C, Guilherminol L. Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Scientific Reports 2018a; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, et al. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquatic Toxicology 2018b; 195: 49–57. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterlandz RA, Dill AL, Webber MM, Waalkes MP. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexprression of de Novo DNA methyltransferase. Environmental Health Perspectives 2007; 115: 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental Science & Technology 2008; 42: 5026–5031. [DOI] [PubMed] [Google Scholar]
- Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Current Biology 2013; 23: 2388–2392. [DOI] [PubMed] [Google Scholar]
- Choi JS, Jung YJ, Hong NH, Hong SH, Park JW. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Marine Pollution Bulletin 2018; 129: 231–240. [DOI] [PubMed] [Google Scholar]
- Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes Compressa. Environmental Science & Technology 2014; 48: 8127–8134. [DOI] [PubMed] [Google Scholar]
- Courgeon AM, Maisonhaute C, Bestbelpomme M. Heat-shock proteins are induced by cadmium in Drosophila cells. Experimental Cell Research 1984; 153: 515–521. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports 2017; 7:46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandriks L Roles of the gut in the metabolic syndrome: an overview. Journal of Internal Medicine 2017; 281: 319–336. [DOI] [PubMed] [Google Scholar]
- Guan DL, Ding RR, Hu XY, Yang XR, Xu SQ, Gu W, et al. Cadmium-induced genome-wide DNA methylation changes in growth and oxidative metabolism in Drosophila melanogaster. Bmc Genomics 2019; 20: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BA, Lee CH, Shukla GS, Shukla A, Osier M, Eneman JD, et al. Characterization of cadmium-induced apoptosis in rat lung epithelial cells: Evidence for the participation of oxidant stress. Toxicology 1999; 133: 43–58. [DOI] [PubMed] [Google Scholar]
- Holmes LA, Turner A, Thompson RC. Adsorption of trace metals to plastic resin pellets in the marine environment. Environmental Pollution 2012; 160: 42–48. [DOI] [PubMed] [Google Scholar]
- Jacobson KB, Opresko L, Owenby RK, Christie NT. Effects of cadmium on Drosophila - Toxicity, proteins, and transfer-RNAs. Toxicology and Applied Pharmacology 1981; 60: 368–378. [DOI] [PubMed] [Google Scholar]
- Khan FR, Syberg K, Shashoua Y, Bury NR. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environmental Pollution 2015; 206: 73–79. [DOI] [PubMed] [Google Scholar]
- Lahimer MC, Ayed N, Horriche J, Belgaied S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arabian Journal of Chemistry 2017; 10: S1938–S1954. [Google Scholar]
- Le LL, Liu MT, Song Y, Lu SB, Hu JN, Cao CJ, et al. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environmental Science-Nano 2018; 5: 2009–2020. [Google Scholar]
- Lemos B, Branco AT, Hartl DL. Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proceedings of the National Academy of Sciences of the United States of America 2010; 107: 15826–15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu K, Fu H, Ji R, Qu X. Sunlight mediated cadmium release from colored microplastics containing cadmium pigment in aqueous phase. Environmental Pollution 2020; 263: 114484. [DOI] [PubMed] [Google Scholar]
- Liu XY, Vinson D, Abt D, Hurt RH, Rand DM. Differential toxicity of carbon nanomaterials in Drosophila: Larval dietary uptake is benign, but adult exposure causes locomotor impairment and mortality. Environmental Science & Technology 2009; 43: 6357–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Qiao R, An H, Zhang Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018a; 202: 514–520. [DOI] [PubMed] [Google Scholar]
- Lu K, Qiao RX, An H, Zhang Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018b; 202: 514–520. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environmental Science & Technology 2016; 50: 4054–4060. [DOI] [PubMed] [Google Scholar]
- Madabattula ST, Strautman JC, Bysice AM, O'Sullivan JA, Androschuk A, Rosenfelt C, et al. Quantitative analysis of climbing defects in a Drosophila model of neurodegenerative disorders. Jove-Journal of Visualized Experiments 2015: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson K, Ekvall MT, Hansson LA, Linse S, Malmendal A, Cedervall T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environmental Science & Technology 2015; 49: 553–561. [DOI] [PubMed] [Google Scholar]
- Meyer S, Schulz J, Jeibmann A, Taleshi MS, Ebert F, Francesconi KA, et al. Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster. Metallomics 2014; 6: 2010–2014. [DOI] [PubMed] [Google Scholar]
- Mirzoyan Z, Sollazzo M, Allocca M, Valenza AM, Grifoni D, Bellosta P. Drosophila melanogaster: A model organism to study cancer. Frontiers in Genetics 2019; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munier B, Bendell LI. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. Plos One 2018; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C, Yung LYL, Cai Y, Bay BH, Baeg GH. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015; 9: 396–403. [DOI] [PubMed] [Google Scholar]
- Panacek A, Prucek R, Safarova D, Dittrich M, Richtrova J, Benickova K, et al. Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster. Environmental Science & Technology 2011; 45: 4974–4979. [DOI] [PubMed] [Google Scholar]
- Posgai R, Cipolla-McCulloch CB, Murphy KR, Hussain SM, Rowe JJ, Nielsen MG. Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: Size, coatings and antioxidants matter. Chemosphere 2011; 85: 34–42. [DOI] [PubMed] [Google Scholar]
- Prussing K, Voigt A, Schulz JB. Drosophila melanogaster as a model organism for Alzheimer's disease. Molecular Neurodegeneration 2013; 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao RX, Lu K, Deng YF, Ren HQ, Zhang Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Science of the Total Environment 2019a; 682: 128–137. [DOI] [PubMed] [Google Scholar]
- Qiao RX, Sheng C, Lu YF, Zhang Y, Ren HQ, Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Science of the Total Environment 2019b; 662: 246–253. [DOI] [PubMed] [Google Scholar]
- Raj A, Shah P, Agrawal N. Dose-dependent effect of silver nanoparticles (AgNPs) on fertility and survival of Drosophila: An in-vivo study. Plos One 2017a; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Shah P, Agrawal N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Scientific Reports 2017b; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat D, Patnaik A, Ekka B, Dash P, Mishra M. Investigation of titania nanoparticles on behaviour and mechanosensory organ of Drosophila melanogaster. Physiology & Behavior 2016; 167: 76–85. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Smeester L, Rojas D, DeBussycher T, Wu MC, Wright FA, et al. Cadmium exposure and the epigenome. Epigenetics 2014; 9: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W Trypan blue exclusion test of cell viability. Current protocols in immunology 2001; Appendix 3: Appendix 3B. [DOI] [PubMed] [Google Scholar]
- Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proceedings of the National Academy of Sciences of the United States of America 2016; 113: 2430–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Takada H. ; Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Scientific Reports 2016; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A Cadmium pigments in consumer products and their health risks. Science of The Total Environment 2019; 657: 1409–1418. [DOI] [PubMed] [Google Scholar]
- Vecchio G A fruit fly in the nanoworld: Once again Drosophila contributes to environment and human health. Nanotoxicology 2015; 9: 135–137. [DOI] [PubMed] [Google Scholar]
- von Moos N, Burkhardt-Holm P, Kohler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environmental Science & Technology 2012; 46: 11327–11335. [DOI] [PubMed] [Google Scholar]
- Whitt M, Vorst K, Brown W, Baker S, Gorman L. Survey of heavy metal contamination in recycled polyethylene terephthalate used for food packaging. Journal of Plastic Film & Sheeting 2013; 29: 163–173. [Google Scholar]
- Windsor Fredric M, Tilley Rosie M, Tyler Charles R, Ormerod Steve J. Microplastic ingestion by riverine macroinvertebrates. Science of The Total Environment 2018; 646: 68–74. [DOI] [PubMed] [Google Scholar]
- Wu B, Wu XM, Liu S, Wang ZZ, Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019; 221: 333–341. [DOI] [PubMed] [Google Scholar]
- Zamberlan DC, Halmenschelager PT, Silva LFO, da Rocha JBT. Copper decreases associative learning and memory in Drosophila melanogaster. Science of the Total Environment 2020; 710: 6. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sackton TB, Martinsen L, Lemos B, Eickbush TH, Hartl DL. Y chromosome mediates ribosomal DNA silencing and modulates the chromatin state in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 2012; 109: 9941–9946. [DOI] [PMC free article] [PubMed] [Google Scholar]