Abstract
Lactobacilli are the predominant microorganisms of the healthy human vagina. A novel alternative for the prevention and treatment of female urogenital tract infections (UGTI) is the inclusion of these microorganisms as active pharmaceutical ingredients in probiotic formulas, and more recently in female hygienic products. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.” A list of requirements must be considered during the development of probiotic product/formula for the female urogenital tract (UGT). This review aims to resume the requirements, probiotic characteristics, and clinical trial applied to determine the effect of probiotic and potentially probiotic strains on different woman’s physiological and pathological conditions, and in preterm birth prevention. A revision of female hygienic products available in the world market is included, together with novel studies applying nanotechnology for Lactobacillus incorporation in hygienic products. Further studies and well‐designed clinical trials are urgently required to complement the current knowledge and applications of probiotics in the female UGT. The use of probiotic formulas and products will improve and restore the ecological equilibrium of the UGT microbiome to prevent and treat UGTI in women under different conditions.
Keywords: clinical trials, female hygienic products, female urogenital tract, live biotherapeutic products, probiotics, urogenital tract infections
Abbreviations
- AV
aerobic vaginitis
- BV
bacterial vaginosis
- DNA
deoxyribonucleic acid
- GBS
Group B Streptococcus
- GDM
gestational diabetes mellitus
- GRAS
generally regarded as safe
- HIV
human immunodeficiency virus
- HM
human microbiome
- HPV
human papilloma virus
- HR‐HPV
high risk human papilloma virus
- HSV‐2
Herpes simplex virus type 2
- IM
intestinal microbiota
- LBP
live biotherapeutic products
- LN
Lacto Naturel
- NIH
national institutes of health
- PPROM
preterm premature rupture of membranes
- QPS
qualified presumption of safety
- R‐VVC
recurrent‐VVC
- SR
slow‐release
- TV
trichomoniasis
- UD
undetermined
- UGT
urogenital tract
- UGTI
urogenital tract infections
- UTI
urinary tract infection
- VM
vaginal microbiota
- VVC
vulvovaginal candidiasis
1. HEALTHY VAGINAL MICROBIOTA—LACTOBACILLUS PREDOMINANCE: FUNCTIONS
The human body and their mucosa are lately considered as highly active ecosystems, where microorganisms play a very diverse list of functions and contribute to host nutrition, overall development, defenses, and immune system, response to pathogens, and mucosal cell differentiation and proliferation. The related research and the knowledge of these active communities and their gene contents have been referred collectively as the human microbiome (HM), supported mainly by an NIH‐funded project consortium.1 The Human Microbiome Project and the European MetaHIT consortium initiated almost two decades ago, aimed at detailed characterization of the structure and the composition of the microbiota from various body areas.1 It is interesting to remark that microbial numbers within an individual are estimated higher than the human cell number by an order of magnitude. In such a way that the HM is a complex system of many microbial communities, deeply described in terms of composition, diversity, and dynamics, known thanks to the use and update of massively parallel sequencing and other high throughput approaches available, indicating the long list of eukaryotes, archaea, bacteria, and viruses detected. These molecular techniques include Sanger sequencing of 16S rRNA of bacterial colonies, terminal restriction fragment length polymorphism of 16S rRNA, qPCR, and next‐generation sequencing that have modified the concept of the microbiome versatilities and more specifically Lactobacillus identification.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Then, the majority of the indigenous microbiota exists in a mutually beneficial relationship with the host, while few are opportunistic pathogens.14 An important body site providing a habitat for the development of structured microbial communities is the vaginal tract, which is broadly colonized by microorganisms known as the vaginal microbiota (VM). Unique conditions of the vagina are characterized by a few microbial species, being the vaginal microbiome a specific compartment of the HM.15 The predominance of lactobacilli was described deeply in the vaginal tract in the NIH Project, and this concept agrees with the first proclaimed by Doderlein in the early 1900.16 The most frequently isolated species are Lactobacillus crispatus, L. gasseri, L. jensenii, and L. iners. Their relative dominance was studied by Ravel et al.8 in different races and ethnic groups. They evidenced the prevalence of L. crispatus (group I), L. gasseri (group II), L. iners (III), and L. jensenii (V) in 26.2%, 6.3%, 34.1%, and 5.3% of the women sampled, respectively. A large heterogeneous group (IV) was presented in 27% of the women with a higher proportion of strictly anaerobic bacteria (Prevotella, Dialister, Atopobium, Gardnerella, Megasphaera, Peptoniphilus, Sneathia, Eggerthella, Aerococcus, Finegoldia, and Mobiluncus). These results indicate that a potential key ecological function, the production of lactic acid, seems to be conserved in all communities, and support at the same time the application of lactic acid producer and immunomodulatory‐probiotic bacteria, and are concordant with α diversity studies published later.17, 18, 19 Ma and Li20 have published recently a review on the association between vaginal community state types and species specificity index reclassifying in five groups.
The second phase of the NIH‐Human Microbiome Project was the Integrative Human Microbiome Project21 designed to explore host–microbiome interplay, including immunity, metabolism, and dynamic molecular activity to gain a more holistic view of host–microbe interactions over time, in a way to address the relationships between host and microbiome mechanistically. The enormous importance of vaginal microbiome supported its inclusion as one of the systems proposed to be studied in this second phase: pregnancy and preterm birth, to go further in the dynamics of human health and disease‐related with known microbiome interactions. In such a way that the concept of reproductive microbiome has been conceived, joining the microbiome of the vaginal tract, placenta and milk/mammary gland, and the direct relationship with fetal development, birth, and newborn features.22 In addition, the VM of the mother plays an essential role in the initial colonization of newborn babies and therefore the development of a healthy gastrointestinal and skin microbiota. The maternal microbiota exerts an indirect effect on the fetus via maternal factors, such as maternal immune responses or microbial metabolites that cross the placenta,23, 24 or other indirect factors mediating epigenetic programming in the fetus (diet, stress, or neuroendocrine exposure). Kaminska and Gajecka25 discuss the influence of human VM, not only bacteria but also viruses and fungi that constitute important components of the reproductive tract microbiome. The impact of the maternal microbiome on fetal development, and the establishment of neonatal microbiomes, including the placenta microbiome, and the hematogenous source of intrauterine infection on the health status of women were analyzed. On the other side, some evidence indicates that infertile patients harbor a different reproductive tract microbiome compared with healthy and fertile women.26
Fertilization occurs in the uterus, an immune‐protected organ, considered a sterile site maintained by the cervical plug for centuries. But the microbial communities in the endometrial cavity and its implications in reproductive health and disease, particularly chronic endometritis was published.27 Even though bacterial DNA in blood in placenta was demonstrated, mechanisms and functions of transplacental trafficking of free nucleic acids are still in discussion. Then, microbes interact with the host cells along the female reproductive tract, generating the physical, chemical, and biological environment that embryo will encounter during the peri‐implementation period and throughout pregnancy. Later, birth represents the first major exposure to a complex microbiota, because the birth canal is always adjacent to the rectum, providing an efficient mechanism for intergenerational transmission of both vaginal and gut microbes.28 The baby swallows these microorganisms, supported by DNA and live bacteria in the meconium.29 The maternal gut microbes immediately start to colonize the newborn's own gut, engaging in a kind of conversation with developing immune cells. In this way, the very early microbiome prepares the immune system for healthy functioning later in life. When a baby is born by cesarean section, the gut is seeded with different microbes not those from the mother's gut and vagina, but from her skin and breast milk, the nurse's hands, and other sources. These early differences might have implications that last a lifetime and provides differential colonization and diversity of microorganisms in the intestinal microbiome in C‐sections or vaginal born babies later in their lives.30, 31
On the other hand, the urinary tract, previously considered a sterile body niche, has emerged as the host of an array of bacteria in healthy individuals, revolutionizing the urology research field.32 Specific bacterial communities have found in the healthy urinary tract, which can change in a wide variety of urologic disorders. Then, it is also of main importance to resolve the modulation of the microbiome to improve urinary tract health.
2. FACTORS AFFECTING THE VM EQUILIBRIUM
The equilibrated/healthy ecological systems of the reproductive microbiota can be affected by a long list of intrinsic or extrinsic factors. Intrinsic factors or host factors include race, physiologic (hormonal changes, menstruation, pregnancy), immune system imbalances, maturation, and the relationship with genetic susceptibility, cancer, and the phages isolated in the tract.
The VM of reproductive‐age women was separated according to the ethnic groups (White, Black, Hispanic, and Asian) in five clusters, showing that the proportions of each community group varied among them.8
Women’s life is characterized by continuous physiological changes, from their birth through the reproductive age to menopause, and during all these phases the vaginal epithelium radically changes, and then the VM.33 Monthly ovulation, with high estrogenic levels, lead to vaginal tract, particularly acid, optimum for the lactobacilli growth, producing lactic acid.33, 34, 35 During the menstrual period there is an increment of pH in the area, and high availability of nutrients derived from menstrual bleeding for microbial growth. This period usually causes disturbance and discomfort, with lower number of lactobacilli, consequent undesirable microorganisms, increased infection rates, and recurrences.8, 36, 37 In menopause, the estrogens are no longer present and glycogen level decreases, leading to a decrease in lactobacilli.38
The human immune system restricts microbiota to their natural niches.28 There is growing evidence that the innate immune system‐antimicrobial peptides and repertoire of pattern recognition receptors, evolved in response to the need for controlling the epithelium‐colonizing microbiota.39 The human vagina consists of multiple levels of protection in innate and adaptive immunity compartmentalize into various components.40, 41 On this subject, genetic variations, such as single nucleotide polymorphisms, in different genes coding components of immune system have been shown to modulate individual´s susceptibility to acquire urogenital tract infections (UGTI).42
Bacteriophages are abundant members of the urogenital tract (UGT), most often persisting through the lysogenic life cycle as prophages integrated within the genomes of their bacterial hosts. Numerous prophages in vaginal lactobacilli were related as one of the factors affecting their depletion in vagina.43, 44
2.1. Extrinsic factors
A long list and variety of factors affect the microbiota equilibrium, as the addition of vitamins and folic acid to diet,45, 46, 47 oral anticontraceptives,48 sexual behaviors,49 hygiene habits,50 stress situations,51 sexually transmitted diseases,52 antibiotics,53 or immunosuppressor therapies,54 between others.
3. UGTI AND FREQUENTLY APPLIED THERAPY
Female vaginal ecosystem thought to have been shaped over the years by co‐evolutionary processes occurring between the particular microbial partners and the human host. Residing at the port of entry of pathogens, the vaginal Lactobacillus species can create a barrier against pathogen invasion since the main products of their metabolism secreted in the cervicovaginal fluid can play an important role in inhibiting invaders. Therefore, a Lactobacillus‐dominated microbiota appears to be a good biomarker for a healthy vaginal ecosystem.55 Disruptions in vaginal association with the microbiomes lead to the change in the vaginal environment, which enhanced the risk of acquiring diseases. The disturbed population promotes a loss of beneficial bacteria due to the list of exogenous/endogenous factors listed above. Thus, vaginal tract can be infected by diverse pathogens resulting in diseases such as bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), trichomoniasis (TV), urinary tract infections (UTI), aerobic vaginitis (AV), and sexually transmitted diseases as those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum.14 The main viral sexually transmitted diseases are human papilloma virus (HPV), human immunodeficiency virus, and herpes simplex virus type 2 (HSV‐2). Each pathogen has its unique infection kinetics, pathology, and host evasion mechanisms. In a similar way, the genital mucosal immunity reacts differently toward each of them.
The urinary tract has emerged as the host of an array of bacteria in healthy individuals. There is a microbiome associated with the healthy urinary tract that can change in certain urological disorders.32, 56 The vagina is a key anatomical site in the pathogenesis of UTI in women, serving as a potential reservoir for infecting bacteria, and also the organ where the ascendant colonization from rectum begins.57 UTIs in females usually start as vaginal infections and ascend to the urethra and bladder.58 Women are affected with an estimated UTI lifetime risk of 60%, and 50%‐fold predominance compared with adult men59 increasing with age.60 Single episodes are very common, being Escherichia coli the most common cause of UTI57, 61 or different Gram‐positive bacteria.62 Recurrent UTI occurring in up to one‐third of women after the first episode,63 showed to be related to a decreased vaginal lactobacilli.64 Rates of UTI begin to rise at the climateric, and recurrences are one of the features of menopause.65 Vaginal tract is then a site at which probiotic interventions may decrease the risk of UTI.57, 58, 66
A wide diversity of antimicrobial compounds is applied to treat UGTI by vaginal or oral administration, including antibacterial, antifungal, antiparasitic, and antiviral agents. The antibiotic use decreases the vaginal Lactobacillus number, promotes resistance, and sometimes infection recurrences. In such a way that the resistance to antimicrobials can be transmitted either transversally or horizontally to other members of the microbiome, generating multi‐resistant microorganisms. The “resistome” concept has emerged, representing the collection and variety of genes that confer antimicrobial resistance (biocides, heavy metals, plasmids) in different microbiomes, spread over multiple areas,67 analyzed lately by targeted metagenomics techniques.68
For this reason, and supported by different concerns related to public health and the prevention of infections, the urgent requirement of adequate alternatives emerged in the last two decades.
4. PROBIOTIC AS PROPOSAL THERAPIES. STRAIN, AND HOST SPECIFICITY
One of the therapies applied for UGTI are probiotics. The definition was submitted to many discussions by government and scientific organizations during the last 30 years, and at the end is live microorganisms that, when administered in adequate amounts, confer a physiological health benefit on the host.69, 70 However, more recently, new definitions have complemented the probiotic area. The term “paraprobiotics” means dead or inactive cells of probiotics that have shown a significant effect on human health71, 72, 73, 74; while the term “postbiotics” is used to describe healthful metabolites of probiotics also showing effect on the host.75, 76, 77, 78 Lately, Zendeboodi et al.79 proposed three main classes of probiotic products, including “true probiotic” as viable and active probiotic cell, “pseudo‐probiotic” as viable and inactive cells in vegetative or spore forms, and “ghost probiotic” as dead/nonviable cell, either intact or ruptured.
Probiotics have been widely studied and applied in the field of food and food supplements, with no particular negative effect.80, 81, 82 In the last few years, the pharmaceutical industry showed a growing interest in new formulations containing beneficial microorganisms, in many cases specific to the host.83, 84 However, the increased interest in the clinical application of probiotics requires specific attention by their administration to non‐healthy population. More recently, the Food and Drug Administration has defined a new “live biotherapeutic products” (LBP) category. Then, the documents and demonstration of quality, safety, and efficacy of new products, including LBP, are framed by the characteristics and risks of the aimed population, as well as those of the strains and product components, in such a way that the global benefit–risk ratio can be assessed regarding their intended use.85
Not all the Lactobacillus strains can be considered as probiotics because the beneficial effect must be evidenced in the specific host as stated. The strains included in different products/formulas must be submitted to a long list of protocols and trials to be named as probiotics. Also, a body of different criteria applied, reviewed by different authors, to be considered as probiotic strains.69, 86 The range of functional genomics to identify genes and gene products that govern the distinctive phenotypes and health associations of probiotic strains was reviewed, recognizing the strain specificity of probiotic effects.87 This strain specificity and the disease specificity of probiotic efficacy, and the need to take these two factors: both specific strains and type of disease, when recommending the appropriate probiotic effect for each patient is also recommended.88, 89 One of the first considerations is the host specificity, which means that the strains should be isolated from the same host where will be applied. Even though some strains are used historically as probiotic or included in foods, the host specificity indicates a higher possibility to be adapted to the host conditions. Referred to the vaginal tract or mucosal specificity, Yildirim et al.90 have shown that all primates exhibited host‐specific VM and that humans were distinct from other primates in both microbiome composition and diversity. Mendes‐Soares et al.91 demonstrated that genomes of vaginal species were significantly smaller and had significantly lower GC content than those of non‐vaginal species.
More specifically, Van Der Veer et al.92 have found that L. crispatus from dysbiotic women have a more prevalent novel glycosyltransferase gene, while Pan et al.89 evidenced strain‐specific variations and phenotypic differences associated with the isolation source, either urogenital or gastrointestinal tracts.
5. REQUIREMENTS FOR PROBIOTICS FORMULAS/PRODUCTS
In the design of probiotic formulas/products, different sets of criteria and protocols must be applied according to the requirements and exigencies of regulatory organisms, described in different revisions, added to the host and tract specificity described above.86, 93, 94 They include: (a) place of isolation, (b) correct phenotypic and genetic identification of the strains, (c) functional characterization and mechanisms of action, (d) in vitro and in vivo safety evaluation, (e) technological characterization: production, formulation, and shelf life, (f) efficacy and effectiveness in clinical trials, and (g) label and health claims; and they are synthesized in Figure 1.
FIGURE 1.
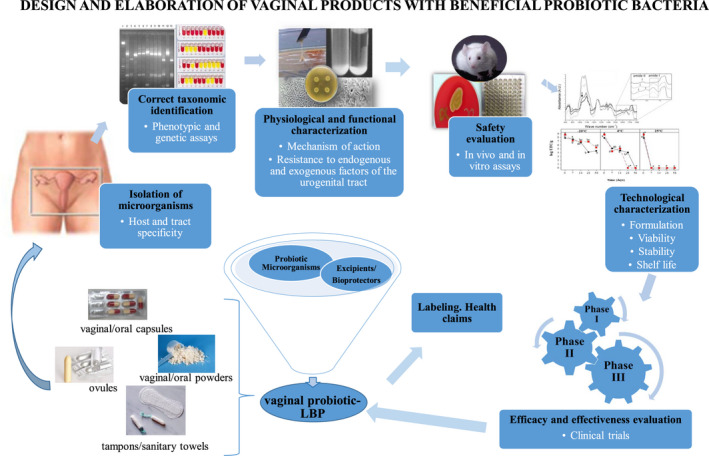
Design and elaboration of vaginal products with beneficial probiotic bacteria (modified from reference [86]). LBP, live biotherapeutic products
Most of the probiotic lactobacilli are considered as Generally Regarded as Safe and some specific species included in the Qualified Presumption of Safety (QPS) classification for inclusion in food,95 but their effectiveness and safety characteristics are strain‐specific and cannot be generalized. Then, no new strain should be assumed of sharing the same documented safety history with preexisting ones.96 Different efficacy and safety assessment protocols were recommended by the experts for a putative probiotic candidate, before confirmation and acceptance for public consumption. In order to guarantee the probiotic safety, each strain must be assayed in some aspects: antibiotic‐resistance patterns; assessment of certain metabolic activities; toxin production; determination of hemolytic activity; side‐effects in experimental animal models; phase I trials; epidemiological surveillance of adverse incidents in consumers, between other.69 Refered to the potential dissemination of the resistance mediated by genetic mechanisms, such as horizontal gene transfer where plasmids, transposons and integrons may be involved,97, 98, 99, 100, 101 is essential to evaluate the susceptibility patterns of potentially probiotic bacteria.102 Microbial resistance to clinically relevant antibiotics must be absent as part of the QPS assessment of bacteria deliberately introduced into the food chain.103 Also it is important to screen the enzymes acting as potential virulence factors, as hydrolytic enzymes (hemolysin, lecithinase, gelatinase, etc.) able to produce damage to the host.104, 105, 106, 107 The temporal persistence and colonization or permanence capability of beneficial microorganisms, and their cellular and molecular effects on the integrity of host mucosa and immune system must also be assayed.108, 109, 110
The formulas for the UGT are designed for oral or local administration. The oral delivery is supported by the microbial ability to survive through gastrointestinal tract, and to ascend to vagina after their excretion from the rectum, in ascendant colonization.111, 112, 113 Whereas vaginal application allows direct and targeted colonization of bacteria for the restoration of unbalanced urogenital microbiota.114, 115, 116, 117, 118 The vaginal drug delivery systems include a wide variety of pharmaceutical forms such as liquid, semi‐solid (gels, creams, ointments), and solid systems (tablets, vaginal suppositories, rings, films, tampons). The viable bacteria are incorporated frequently as powder, being lyophilization the gold standard technique for microbial preservation and stability during the storage for different time periods.119, 120, 121, 122 The selection of the appropriate dosage form depends on the physicochemical features of the delivered drug or formula, the target for them and women´s acceptance.123
6. EFFECTS OF PROBIOTIC OR POTENTIAL PROBIOTIC MICROORGANISMS ON THE FEMALE UGT
Pharmacological aspects of probiotics are more complex than those of conventional drugs, since their effect in the host cells‐mucosa‐organs and whole organism is very complex and difficult to evaluate, because they act by different mechanisms, either individually or in some cases synergistically. Several publications describe how vaginal lactobacilli can exert their function through different mechanisms of actions, which include: (a) production of antimicrobial substances (organic acids, hydrogen peroxide, bacteriocins, biosurfactants) or enzymes (e.g. arginine deaminase); (b) adhesion to epithelial cells, components of mucus or extracellular matrix‐colonization‐permanence; (c) autoaggregation and coaggregation; biofilm formation on vaginal mucosa; (d) competitive exclusion; (e) competition for nutrients; and (f) modulation of the immune system.86, 94, 124, 125, 126, 127, 128, 129, 130, 131, 132 The proposed mechanisms of action of probiotics in the UGT are shown in Figure 2.
FIGURE 2.
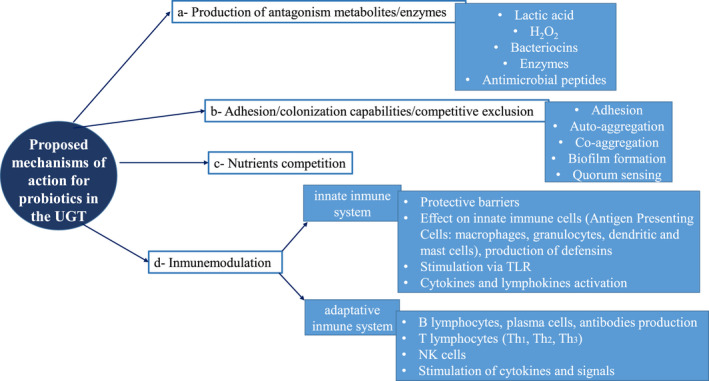
Proposed mechanisms of action for probiotics in the UGT (adapted from different bibliographic references). TLR, toll‐like receptor; UGT, urogenital tract
A very important subject to be considered in the development of probiotic formulas for the UGT is the effect of the bacteria as bioactive principle, whether for preventive or therapeutic purposes, which is supported by the reestablishment of the ecological microbial equilibrium of the tract. One of the main requirements of probiotic products is the viable cell number, between 107 and 109 colony forming units per formulation dose.70, 94, 133, 134 Since the effectiveness of probiotic formulas depends on these number of viable cells, most of the pharmacokinetic studies have described the survival capability of probiotics in the target organ and its capability to maintain their numbers and colonize to generate the probiotic effects. The survival of microorganisms depends on their resistance to the conditions of the host tract and to the technological processes and their maintenance as marketed products. The fate of probiotics (i.e., their survival, or movement) in the gastrointestinal tract (or in experimental animal models), their effect in specific target organs, or stimulation of specific cell‐populations, mainly in the gut were demonstrated. Scientific publications evidenced that oral probiotics increase intestinal antimicrobial activity and Paneth cells in order to strength the epithelial barrier against pathogens in mice, proposing a different mechanism by which probiotics protect the host mainly against infectious diseases. The whole bacteria can not enter the intestinal cells, while only the degradation products of bacteria are able to take contact with the immune cells.135, 136 A recent study have shown the probiotic properties of two vaginal lactobacilli (L. fermentum MG901 and L. plantarum MG989), the adhesion to HT‐29 cell and the inhibition of E. coli and Candida albicans adherence to these cells. The probiotic bacteria persisted up to 6 days in the feces of mice after the oral administration. The authors suggested the vaginal strains could be used as oral and vaginal probiotic helping to in vivo clear VVC.137
Referred to oral probiotics for women, supported by the ascending colonization hypothesis that promotes the permanence and colonization of beneficial bacteria in the vagina after excretion through the rectum,111, 112, 113 the capability of microorganisms to colonize the vagina was studied. De Vrese et al.138 showed that oral intake of four Lactobacillus strains improves the microbial pattern in vaginal dysbiosis. In a similar way, Vladareanu et al.139 demonstrated that oral probiotic increased the vaginal colonization of lactobacilli via cross‐contamination from the gastrointestinal tract to vagina, indicating an improvement of vaginal conditions, suggesting then the successfully prevention of VVC episodes.
On the other side, the effect, safety, and mechanisms of action of lactobacilli as probiotic products for local administration, such as vaginal capsules, suppositories, and tablets, were demonstrated in different publications. Verdenelli et al.140 registered the normalization and maintenance of pH and Nugent score and the increase of lactobacilli total number after 7 days of probiotic suppositories treatment in women. The authors highlighted that local administration promotes a quick local action, driven by the activity of probiotic bacteria that adhere and colonize the vaginal epithelium. The colonization of vaginal epithelium was shown by molecular typing test, suggesting the restoration and maintenance of VM. Cohen et al.141 published a randomized, double‐blind, placebo‐controlled, phase 2b trial to evaluate Lactin‐V (L. CTV‐05) after the treatment with vaginal metronidazole, resulting in a significantly lower incidence of BV recurrence.
The use of probiotics in the obstetrical and gynecological field has increased in the last years. Up to date, several clinical studies have evaluated the effects of probiotic or potential probiotic microorganisms, administered both orally and vaginally, on the prevention and treatment of UGTI in women under different physiological conditions: fertile and non‐pregnant, pregnant, and post‐menopausal, as well as also in the prevention of preterm birth (Table 1). In the present review, most of the available clinical trials published between 2015 and 2020 were included, because previous trials were analyzed before.86, 142
TABLE 1.
Effect of probiotic or potential probiotic microorganisms on the female urogenital tract
| Microorganisms | Isolation site | Pharmaceutical form or product | Clinical targeta | Type of study and participant type and number | Results | References |
|---|---|---|---|---|---|---|
| Lactobacillus crispatus CTV‐05 | Healthy human vagina | Vaginal powder (Lactin‐V, Osel) | Subsequent to BV therapy | Multicenter randomized double‐blind placebo‐controlled phase 2b trial. 228 BV women | Probiotic (11 weeks) after metronidazole produced lower incidence BV recurrence | 141 |
| L. plantarum P17630 | Healthy human vagina | Oral capsule (Proge Farm S.r.l.) | VM‐improvement | Randomized double‐blind placebo‐controlled study. 93 R‐VVC history women | Probiotic [3 treatment cycles (15 days/cycle) separated by 15‐day wash‐out intervals] improved Lactobacillus colonization and clinical sign (redness/swelling/discharge) | 139 |
| L. rhamnosus Lcr35® | Human feces | Vaginal capsule or slow‐release (SR) vaginal tablet (Gynophilus®) | VM‐improvement | Comparative phase I randomized open‐label pilot clinical trial. 33 healthy women | Capsules (daily) or SR‐tablets (every 3, 4, or 5 days for 21 days) did not produce adverse effects, favored Lactobacillus spp. growth, reduced non‐Lactobacillus spp. colonization | 143 |
| Vaginal capsule | Adjunct to therapy of TV in BV‐presence | Randomized placebo‐controlled double‐blind study. 90 women with TV in BV presence | Probiotic (1 capsule twice/day/7 days) increased TV and BV cure, decreased vaginal inflammation and pH, increased redox potential | 144 | ||
| L. rhamnosus BMX 54 | UD | Vaginal tablet (NORMOGIN®) | VM‐restoration | Randomized trial. 117 HPV+BV or VVC‐women receiving standard antimicrobial treatment | Probiotic (6 months) resolved HPV‐related cytological anomalies twice higher than probiotics during 3 months, induced high total HPV clearance | 145 |
| L. rhamnosus HN001 | Yoghurt | Oral capsule (Fonterra‐Cooperative) | Improvement‐maternal health‐pregnancy (prevention maternal GDM) and postpartum (depression‐anxiety), prevention infant eczema‐allergy | Two‐center randomized double‐blind placebo‐controlled trial. 423 pregnant women at 14–16 weeks gestation with personal or partner history of atopic disease and expecting infants at high risk of allergic disease | Probiotic (until delivery and then until 6 months post‐partum, if breastfeeding), reduced GDM prevalence, decreased depression and anxiety scores in the postpartum period, did not reduce infant eczema | 146, 147, 148, 149 |
| L. salivarius CECT 9145 | Healthy human vagina | Freeze‐dried | GBS‐vaginal and rectal colonization‐reduction | Prospective pilot clinical trial. 57 pregnant women | Lactobacilli (one/day from week 26/38 pregnancy) reduced rectal and vaginal GBS colonization | 150 |
| L. acidophilus La‐14, L. rhamnosus HN001 | Human feces and yoghurt, respectively | Oral capsule+bovine lactoferrin RCX™ (Respecta®) | VM‐improvement | Double‐blind randomized placebo‐controlled study. 40 healthy women | Probiotic (twice daily/2 weeks) increased probiotic species in vagina and without adverse effect | 151 |
| Adjunct to BV‐therapy | Double‐blind placebo‐controlled‐randomized clinical trial. 48 BV women | Probiotic+lactoferrin (2 capsules/day/5 days followed by 1 capsule/day/10 days/month during 6 months) reduced vaginal discharge, itching, nugent score and recurrence rate | 37 | |||
| Adjunct to R‐VVC‐therapy | Double‐blinded placebo‐prospective randomized clinical trial. 48 Candida albicans‐positive women | Probiotic+lactoferrin (2 capsules/day/5 days followed by 1 capsule/day/10 days/month during 6 months) reduced itching and discharge at 3 and 6 months, and R‐VVC | 152 | |||
| L. rhamnosus IMC 501, L. paracasei IMC 502 |
Elderly human faeces Human feces |
Vaginal suppository (SYNBIO®) | VM‐restoration | Single‐arm open‐label controlled towards the baseline (pre–post) study. 35 apparently healthy women | Probiotic for 7 days did not produce adverse effects, reduced Nugent score, increased Lactobacillus level, did not modify pH vaginal | 140 |
| L. rhamnosus GR‐1, L. reuteri RC‐14 | Healthy human urethra and vagina, respectively | Oral capsule (U‐relax®) | HR‐HPV‐clearance | Randomized double‐blinded placebo‐controlled trial. 121 HR‐HPV women | Probiotic (1 capsule/day until negative HR‐HPV testing) did not influence HR‐HPV clearance, decreased rates of mildly abnormal and unsatisfactory cervical smears | 153 |
| Oral capsule (Chr. Hansen) | VM‐modulation | Pilot randomized blinded placebo‐controlled trial. 38 pregnant women of gestational age less than 36 weeks | Probiotic (1 capsule/day/1 month) without side effects did not modify VM | 154 | ||
| VM‐maintenance/ restoration | Randomized placebo‐controlled triple‐blind parallel group trial. 320 women with <12 completed pregnancy weeks | Probiotic (1 capsule/day/8 weeks) did not modified VM | 155 | |||
| VM‐maintenance/restoration | Randomized double‐blind placebo‐controlled trial. 304 women with 9–14 pregnancy weeks | Probiotic (1 capsule/day from recruitment until pregnancy end) did not modified VM | 156 | |||
| GBS‐vaginal colonization‐reduction | Randomized controlled trial. 99 pregnant women at 35–37 weeks of gestation with vaginal and rectal‐GBS positive | Probiotic (2 capsules before bedtime until delivery) reduced the GBS colonization | 157 | |||
| GBS‐vaginal colonization‐reduction | Pilot randomized control study. 34 GBS‐positive women at 36 weeks pregnant received standard antenatal care | Probiotic (1 capsule/day/3 weeks or until birth) did not reduce vaginal GBS‐rates | 158 | |||
| L. fermentum 57A, L. plantarum 57B, L. gasseri 57C | Healthy human vagina | Vaginal capsule (inVag®) | VM‐restoration | Multicenter randomized double‐blind placebo‐controlled trial. 160 abnormal VM women | Probiotic (1 capsule/day/7 days) decreased vaginal pH and Nugent score, increased Lactobacillus abundance, without adverse effect | 159 |
| Oral capsule (prOVag®) | Adjunct to BV/AV‐therapy | Randomized double‐blind placebo controlled trial. 154 of recurrent BV/AV histories and current symptoms women. | Probiotic (2 capsule/day/10 days, during follow‐up, and one capsule/day/10 days perimenstrually), lengthened time to clinical BV/AV symptoms relapse, reduced and maintained low vaginal pH and Nugent score, increased vaginal Lactobacillus counts | 160 | ||
| L. rhamnosus DSM 14870, L. gasseri DSM 14869 | Healthy human vagina | Vaginal capsule (EcoVag®) | Adjunct to BV‐therapy | Prospective partially randomized exploratory pilot study. 39 BV women | Probiotic (once/day/30 days then once/week until day 190) colonized vagina, did not improve BV cure rates or alleviate recurrence | 161 |
| Subsequent to BV/R‐VVC‐therapy | Two pilot open‐label clinical trials. 40 Scandinavian BV or VVC‐diagnosed women | Probiotic (5 days) induced a 6‐month BV cure rate of 50%. Probiotic (10 days after each antibiotic treatment followed by weekly administration of capsules for 4 months) induced 6‐ and 12‐month BV‐cure rates of 67%, while the 6‐ and 12‐month VVC‐cure rates of 100% and 89% | 162 | |||
| Adjunct to antibiotic treatment on perinatal outcome with PPROM | Prospective randomized trial. 115 PPROM between 24 and 34 weeks of gestation women | Probiotic (10 days) increased gestational age at birth, duration of latency period and birth weight. Neonates of probiotic‐group presented lower chance of entering intensive care unit, shorter total hospitalization time, lower need for oxygen administration and mechanical ventilation, and lower length of oxygen administration | 163 | |||
| L. gasseri LN40, L. rhamnosus LN113, L. fermentum LN99 | Healthy human vagina | Intimate care ointment (Ellen AB) | VM‐improvement | Double‐blind randomized pilot study. 18 healthy postmenopausal women | Probiotic (10 days) induced lactobacilli persistence in vagina for at least 10 days | 164 |
|
Formula A: L. gasseri CRL1307, CRL1263, L. reuteri CRL1324 Formula B:L. gasseri CRL1256, CRL1320, L. rhamnosus CRL1332 |
Healthy human vagina | Vaginal capsule | VM‐restoration | Double‐blind randomized clinical trial of safety. 39 normal or intermediate microbiota women | Lactobacillus formulations (1 capsule/day/7 days) tended to decrease in both Nugent score and vaginal leukocyte number, increased cultivable lactobacilli, without adverse effect | 129 |
|
Pro‐I:L. crispatus EST‐1, EST‐4, EST‐6 Pro‐II:L. crispatus EST‐2, EST‐3, EST‐5 |
Healthy human vagina | Oral capsule | VM‐restoration | Randomized double‐blind placebo‐controlled crossover trial. 40 reproductive‐age considered healthy women | Pro‐I or Pro‐II (once capsule/day/1‐week followed by 2‐week washout period, continued with second treatment and washout period) were well tolerable, and Pro‐II reduced Nugent score and Gardnerella vaginalis counts | 165 |
|
F1‐Probiotic: L. acidophilus PBS066, L. reuteri PBS072 F2‐Probiotic: L. plantarum PBS067, L. rhamnosus PBS070, Bifidobacterium animalis subsp. lactis PBS075 |
Gjjg Human feces |
Oral capsule+inulin | Recurrent UGTI‐prevention | Randomized placebo‐controlled pilot study. 60 healthy women | F1 and F2 (14 days) colonized vagina and showed in vitro anti‐microbial activity against urogenital pathogens | 166 |
| L. crispatus LbV 88, L. gasseri LbV 150 N, L. jensenii LbV 116, L. rhamnosus LbV96 | Healthy human vagina | Yoghurt | Adjunct to BV therapy | Double‐blind randomized controlled‐clinical pilot trial. 36 BV women | Yoghurt (twice/day/4 weeks) improved BV‐recovery rate and symptoms, tended to improve VM | 167 |
| Oral capsule (Florium, European‐Patent‐PCT/EP2011/065877) | IM/VM‐reconstitution of herpesvirus‐pregnant | Randomized trial. 60 women with herpes virus infection on the 14‐16th week of pregnancy | Probiotic (2 capsules/day/30 min before meals/‐week) reduced opportunistic pathogens, increased Lactobacillus in intestine and vagina, decreased 2–3 times complaints incidence (bloating/discomfort/constipation/mucus‐in‐stool/excessive‐vaginal‐discharge/itching/swelling/ redness‐of‐mucosa), reduced twofold the incidence of placental insufficiency, preeclampsia and fetal distress | 168 | ||
| L. acidophilus, L. rhamnosus, Streptococcus thermophilus, L. delbrueckii subsp. bulgaricus | UD | Vaginal capsule (Lactagyn®) | Subsequent to R‐VVC‐therapy | Randomized trial. 436 VVC women | Probiotic (10 applications/beginning 5th day after azole treatment) reduced clinical complaints, improved microbiological efficacy | 169 |
| L. acidophilus, L. casei, L. lactis, B. bifidum, B. lactis | UD | Oral powder (SimFort; Vitafor Nutrientes) | Adjunct to isoflavone to improve menopause genitourinary symptoms | Randomized trial. 60 postmenopausal‐women | Probiotic (one pack) improved isoflavones metabolism after 16 weeks, but failed to yield estrogenic effect on the urogenital tract and relieve vulvovaginal symptoms | 170 |
Abbreviations: AV, aerobic vaginitis; BV, bacterial vaginosis; GBS, Group B Streptococcus; GDM, gestational diabetes mellitus; HPV, human papilloma virus; HSV, Herpes simplex virus; IM, intestinal microbiota; PPROM, preterm premature rupture of membranes; TV, trichomoniasis; UD: undetermined; VM, vaginal microbiota; VVC, vulvovaginal candidiasis, R‐VVC, recurrent‐vulvovaginal candidiasis.
Different colors are used to include different women status in the same table. Each color represents a different state: non pregnant (white), pregnant (light gray), and post‐menopausal (dark gray).
aProducts in the market.
In non‐pregnant, fertile women (Table 1, white panel), probiotic strains were assayed to: evaluate their safety, and to improve the VM of healthy women129, 139, 143, 151; restore the abnormal VM129, 140, 159, 165; prevent recurrent UGTI141, 166; as adjunct treatment to BV,37, 160, 161, 167 AV160 antimicrobial therapy, recurrent‐VVC (R‐VVC),152, 162 and TV in BV presence144; as BV and R‐VVC subsequent treatment141, 162, 169; and HPV clearance.145, 153 Most of the publications have evaluated commercial, or potential probiotic formulas, including oral and vaginal capsules, vaginal tablets, suppositories or powders, and even yogurt, containing individual or combined Lactobacillus strains of urogenital, intestinal, or food origins, as shown in Table 1. Lactobacillus strains combined with bovine‐lactoferrin were also assayed.37, 151, 152 Independent of the administration route, the strain/s and doses evaluated, most of the trials reported positive effect on the UGT, such as the absence of adverse effects, VM improvement and restoration, permanence and colonization of probiotic microorganism, and higher cure rates and lower recurrences, mainly in BV, AV, R‐VVC, and TV patients. However, Marcotte et al.161 indicated that the probiotic adjunct therapy of BV did not improve cure rates or alleviate recurrence, probably due to treatment failures or to the limited power of the study. Referred to the use of probiotic for HPV clearance, Palma et al.145 reported the higher HPV clearance in BV women with metronidazole therapy plus 6 months vaginal Lactobacillus implementation, than the one with only 3 months’ use. Ou et al.153 published that the application of probiotic strains did not influence genital high risk‐HPV clearance, but decreased the rates of mildly abnormal and unsatisfactory cervical smears.
Probiotic application in pregnant women (Table 1, light gray panel) indicates that most of the clinical trials evaluated oral administration of commercial formulas containing urogenital Lactobacillus strains. A positive effect on the modulation of VM, increased beneficial microorganisms, and lower pathogen levels, as Group B Streptococcus (GBS) was reported.150, 157, 168 However, oral probiotics taken from early pregnancy showed no effect on vaginal health during mid and end gestation,155, 156 or 3 weeks doses or until birth in 36‐week‐pregnancy were ineffective in impacting GBS vaginal colonization.158 The addition of vaginal probiotics to standard antibiotic treatment on perinatal outcome in preterm premature rupture of membranes pregnancy prolonged the bith gestational age, the latency period length, improving newborn weight and health.163 On the other hand, the oral application of probiotic in maternal health during pregnancy, postpartum, and in infant eczema and allergy prevention was evaluated.146, 147, 148, 149 L. rhamnosus HN001 during pregnancy reduced gestational diabetes mellitus (GDM) prevalence, particularly among older women and those with previous GDM,147 and the depression and anxiety scores in women in the postpartum period.148 However, L. rhamnosus HN001 did not reduce the infant eczema.149 In other way, Anoshina168 showed that combined Lactobacillus strains to HSV‐women decreased the complaints incidence of bloating, discomfort, constipation, mucus in stool, excessive vaginal discharge, itching, swelling and mucosa redness, reducing the placental insufficiency and preeclampsia as well as fetal distress incidence.
In postmenopausal women, the application of probiotic strains (Table 1, dark gray panel) in intimate care ointment demonstrated the successful colonization of probiotic strains to improve the healthy VM.164 But, oral administration of probiotic+isoflavone was not as effective as the hormonal therapy to improve genitourinary menopause symptoms.170
In conclusion, several trials have demonstrated that single or combined probiotic or potential probiotic strains, either alone or supplemented to antimicrobial therapy, were effective for BV, AV, R‐VVC, and TV prevention and treatment. However, there is insufficient clinical evidence on the efficacy of probiotics for vaginal health in pregnant, post‐menopausal women, and for the prevention of preterm birth and its complications. Then, more studies with well‐designed randomized controlled trials evaluating larger patient size, and different: length of interventions, dosage and strains of probiotics, and administration route are urgently required.
7. HYGIENE PRODUCTS FOR THE PREVENTION OF UGTI
The probiotic administration in hygiene products, as tampons and pads, has emerged as a new possibility to carry beneficial strains and exert the claimed effect. The pharmacological aspects of the probiotic products for feminine hygiene including sanitary towels and tampons, classified in several countries as medical devices, are scarcely described. They can act in the VM restoration, and in some cases, could reduce the risk associated with toxic shock syndrome produced by Staphylococcus aureus strains, with higher incidence in women who use tampons.171 The availability of everyday feminine hygiene products (e.g., tampons, sanitary napkins, panty liners) containing probiotic microorganisms from vaginal origin is limited in the market and available only in some regions of the world. Table 2 shows the feminine hygiene products containing vaginal probiotic lactobacilli assayed for pH balancing and protection during menstrual bleeding. Most of them were designed with Lacto Naturel, LN® formula, as tampons, sanitary pads, foams, and creams, with patented probiotic strains: L. gasseri LN40, L. fermentum LN99, L. casei ssp. rhamnosus LN113, Pediococcus acidilactici LN23. These strains were isolated from vagina and incorporated as freeze‐dried cells. In tampons and towels, they are included in the inner part, and once hydrated, they diffuse to the surface in the vaginal cavity. Different trials of Ellen® probiotic tampons are included in the scientific summary, but the scientific‐reviewed publications were not found.172 This summary describes the clinical studies performed, indicating that the use of probiotic tampon can lead to vaginal colonization of LN® probiotic bacteria in asymptomatic women. These devices are aimed to prevent a disturbed VM condition, while the LN‐bacteria exert their effect exclusively in the vaginal cavity and on/in the mucus covering the squamous vaginal epithelial cells. The release of probiotic bacteria and the production of lactic acid are the main proposed effects.
TABLE 2.
Feminine hygiene products containing vaginal probiotic lactobacilli
| Microorganisms | Brand name and type | Claimed effect | References |
|---|---|---|---|
| Lacto Naturel, LN®: L. gasseri LN40, L. fermentum LN99, L. casei ssp rhamnosus LN 113, Pediococcus acidilactici LN23 | ELLEN® probiotic tampon | VM‐improvement/restoration | 172 |
| Muvagyn® probiotic tampon | |||
| Florgynal® probiotic tampon‐Saforelle | |||
| Natura Femina‐Ellen Tampon | |||
| Ellen® LN intimate cleansing foam and intimate grooming/shaving cream | Vaginal pH‐maintenance | ||
| Ellen® probiotic intimate topical cream | pH‐maintenance, hydration dry mucous | ||
| Natura Femina probiotic paste in cotton sanitary towels | VM and pH‐maintenance, reduce discomfort (itching/irritation/discharge/odors), infection or inflammation | 173 | |
| L. acidophilus pure culture | Carin/Oasis/Micci ProBiotic ultra wings‐sanitary napkins | VM‐improvement/restoration | 174 |
| Intimea LACTOPROBIOTIC (probiotics+lactic acid), ultra‐thin‐dairy use, incontinences | VM‐improvement/restoration+lactic acid to prevent infection and vaginal inflammation | 175, 176 |
Abbreviation: VM, vaginal microbiota.
A group of experts from the Polish Gynecological Society evaluated the available bibliography and issued a statement concluding that this product is an innovative solution allowing the vaginal application of strains with beneficial probiotic properties during menstrual bleeding.177 Sauperl et al.173 have later proposed the incorporation of a probiotic paste with the same LN strains to the surface of sanitary napkins in the design of functionalized sanitary products.
Other sets of products are Carin/Oasis/Micci ProBiotic, containing L. acidophilus in the inner area of feminine sanitary napkins, while Intimea LACTOPROBIOTIC feminine pads include L. acidophilus on the surface, registered in United States and Europe patents.175, 176 Dried lactobacilli are added through contact sorption drying carriers in a lipid phase. The isolation source of these strains neither their effect is unknown.
On the other hand, nanotechnology applying the electrospinning technique has recently been proposed to cover different devices for vaginal uses with viable Lactobacillus in a single step.178, 179 The coating system was successfully applied in several products, as stent, soybeans, cellulose paper, with a wide variety of bioactive immobilized in nanofibers through electrospinning.180, 181, 182 Then, this Lactobacillus immobilization system offers advantages when compared with lyophilized powders, since modifications of the final product are not evidenced, and subsequent processing for their incorporation in the product design are not required. There are few publications on the application of probiotics immobilized in nanofibers for vaginal application available. Škrlec et al.183 immobilized probiotic L. plantarum ATCC 8014 (unknown origin) in nanofibers prolonging the strain viability. In the case of vaginal strains, Nagy et al.184 immobilized L. acidophilus in polyvinylalcohol and polyvinylpyrrolidone nanofibers, suggesting them for BV therapy, but did not prove their efficacy. The immobilization of a L. rhamnosus CRL 1332 vaginal strain in polyvinylalcohol nanofibers by electrospinning was successfully, maintaining the required viable cell numbers during 360 days at 4°C and the inhibition to urogenital pathogens.178 Up to date, clinical trials to determine the safety, efficacy and effectiveness of immobilized probiotics in these products for UGTI treatment were not published.
8. CONCLUSIONS
Lactobacillus species are the predominant microorganisms in the healthy human vaginal microbiome, and their inclusion as probiotic for the UGT in medical clinical practice is widely recommended. Evidence of their multiple and potential effects must be demonstrated, mainly referred to the VM reestablishment and to the preventive/therapeutic effect against UGTI. Urogenital probiotics designed with different bacterial strains, doses, treatment schemes, routes, and vehicles of administration were clinically evaluated. The application in the preterm birth area, in the improvement of maternal health pregnancy and postpartum, and prevention of infant eczema‐allergy must be encouraged. A promising area is the probiotic inclusion in daily use‐hygiene products with nanofiber‐immobilized lactobacilli, with interesting application possibilities. The potential benefits of probiotics use on the health of women around the world strongly support the requirement of further studies to complement the current knowledge and to encourage clinical applications of probiotics in the UGT, either as preventive or therapeutic agents.
DISCLOSURE
The authors declare there is no conflict of interest. Some of the results of the research group were included in a patent presentation (INPI, 2018, N° 20180103893).
ACKNOWLEDGMENTS
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET) [PIP 545] and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) [PICT 2017‐4324 and PICT 2018‐00670].
REFERENCES
- 1.Human Microbiome Project Consortium . A framework for human microbiome research. Nature. 2012;486(7402):215‐221. 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhelst R, Verstraelen H, Claeys G, et al. Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora. BMC Microbiol. 2005;5:61. 10.1186/1471-2180-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, Hansmann MA, Davis CC, et al. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol Med Microbiol. 2010;58(2):169‐181. 10.1111/j.1574-695X.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sroka‐Oleksiak A, Gosiewski T, Pabian W, et al. Next‐generation sequencing as a tool to detect vaginal microbiota disturbances during pregnancy. Microorganisms. 2020;8(11):1813. 10.3390/microorganisms8111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fettweis JM, Serrano MG, Girerd PH, Jefferson KK, Buck GA. A new era of the vaginal microbiome: advances using next‐generation sequencing. Chem Biodivers. 2012;9(5):965‐976. 10.1002/cbdv.201100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jespers V, Menten J, Smet H, et al. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol. 2012;12(1):83. 10.1186/1471-2180-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datcu R, Gesink D, Mulvad G, et al. Vaginal microbiome in women from Greenland assessed by microscopy and quantitative PCR. BMC Infect Dis. 2013;13(1):480. 10.1186/1471-2334-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci U S A. 2011;1:4680‐4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012;343(1):2‐9. 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummelen R, Fernandes AD, Macklaim JM, et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One. 2010;5(8):e12078. 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forney LJ, Gajer P, Williams MEF, et al. Comparison of self‐collected and physician‐collected vaginal swabs for microbiome analysis. J Clin Microbiol. 2010;48(5):1741‐1748. 10.1128/JCM.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drell T, Lillsaar T, Tummeleht L, et al. Characterization of the vaginal micro‐ and mycobiome in asymptomatic reproductive‐age estonian women. PLoS One. 2013;8(1):e54379– 10.1371/journal.pone.0054379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Lee S, Park YS. Molecular typing tools for identifying and characterizing lactic acid bacteria: a review. Food Sci Biotechnol. 2020;29(10):1301‐1318. 10.1007/s10068-020-00802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Kakkar V, Bhushan I. Crosstalk between vaginal microbiome and female health: a review. Microb Pathog. 2019;136:103696. 10.1016/j.micpath.2019.103696. [DOI] [PubMed] [Google Scholar]
- 15.Buchta V. Vaginal microbiome. Vaginální mikrobiom. Ceska Gynekol. 2018;83(5):371‐379. [PubMed] [Google Scholar]
- 16.Hunter CA, Long KR, Schumacher RR. A study of döderlein’s vaginal bacillus*. Ann N Y Acad Sci. 1959;83(2):217‐226. 10.1111/j.1749-6632.1960.tb40894.x. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Huang Y, Zhang Z, et al. Exploring profile and potential influencers of vaginal microbiome among asymptomatic pregnant Chinese women. PeerJ. 2019;7:e8172. 10.7717/peerj.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado‐Diaz DJ, Tyssen D, Hayward JA, et al. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non‐optimal vaginal microbiota. Front Cell Infect Microbiol. 2020;9:446. 10.3389/fcimb.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hočevar K, Maver A, Vidmar Šimic M, et al. Vaginal microbiome signature is associated with spontaneous preterm delivery. Front Med. 2019;6:201. 10.3389/fmed.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma ZS, Li L. Quantifying the human vaginal community state types (CSTs) with the species specificity index. PeerJ. 2017;2017(5):e3366. 10.7717/peerj.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor LM, Creasy HH, Fettweis JM, et al. The integrative human microbiome project. Nature. 2019;569(7758):641‐648. 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Power ML, Quaglieri C, Schulkin J. Reproductive microbiomes: a new thread in the microbial network. Reprod Sci. 2017;24(11):1482‐1492. 10.1177/1933719117698577. [DOI] [PubMed] [Google Scholar]
- 23.De Agüero MG, Ganal‐Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296‐1302. 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 24.Romano‐Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1–2):189‐195. 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamińska D, Gajecka M. Is the role of human female reproductive tract microbiota underestimated? Benef Microbes. 2017;8(3):327‐343. 10.3920/BM2015.0174. [DOI] [PubMed] [Google Scholar]
- 26.Wee BA, Thomas M, Sweeney EL, et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust New Zeal J Obstet Gynaecol. 2018;58(3):341‐348. 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 27.Moreno I, Simon C. Relevance of assessing the uterine microbiota in infertility. Fertil Steril. 2018;110(3):337‐343. 10.1016/j.fertnstert.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez‐Bello MG, Godoy‐Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019;68(6):1108‐1114. 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagpal R, Tsuji H, Takahashi T, et al. Sensitive quantitative analysis of the meconium bacterial microbiota in healthy term infants born vaginally or by cesarean section. Front Microbiol. 2016;7:1997. 10.3389/fmicb.2016.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stearns JC, Simioni J, Gunn E, et al. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci Rep. 2017;7(1):1‐9. 10.1038/s41598-017-16606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korpela K, Costea P, Coelho LP, et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28(4):561‐568. 10.1101/gr.233940.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aragón IM, Herrera‐Imbroda B, Queipo‐Ortuño MI, et al. The urinary tract microbiome in health and disease. Eur Urol Focus. 2018;4(1):128‐138. 10.1016/j.euf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Fuochi V, Volti GL, Furneri PM. Commentary: Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol. 2017;8:1815. 10.3389/fmicb.2017.01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168(9–10):782‐792. 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Moreno I, Simon C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod Med Biol. 2019;18(1):40‐50. 10.1002/rmb2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eschenbach DA, Thwin SS, Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30(6):901‐907. 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 37.Russo R, Karadja E, De Seta F. Evidence‐based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Benef Microbes. 2019;10(1):19‐26. 10.3920/BM2018.0075. [DOI] [PubMed] [Google Scholar]
- 38.Nunn KL, Forney LJ. Unraveling the dynamics of the human vaginal microbiome. Yale J Biol Med. 2016;89(3):331‐337. https://pubmed.ncbi.nlm.nih.gov/27698617/. Accessed October 29, 2020. [PMC free article] [PubMed] [Google Scholar]
- 39.Bosch TCG. Rethinking the role of immunity: lessons from hydra. Trends Immunol. 2014;35(10):495‐502. 10.1016/j.it.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Yarbrough VL, Winkle S, Herbst‐Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353‐377. 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 41.Nasu K, Narahara H. Pattern recognition via the toll‐like receptor system in the human female genital tract. Mediators Inflamm. 2010;2010:976024. 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalia N, Singh J, Kaur M. Immunopathology of recurrent vulvovaginal infections: new aspects and research directions. Front Immunol. 2019;10:2034. 10.3389/fimmu.2019.02034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller‐Ensminger T, Mormando R, Maskeri L, Shapiro JW, Wolfeid AJ, Putontiid C. Introducing Lu‐1, a novel Lactobacillus jensenii phage abundant in the urogenital tract. PLoS One. 2020;15(6):e0234159. 10.1371/journal.pone.0234159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiliç AO, Pavlova SI, Alpay S, Kiliç SS, Tao L. Comparative study of vaginal Lactobacillus phages isolated from women in the United States and Turkey: prevalence, morphology, host range, and DNA homology. Clin Diagn Lab Immunol. 2001;8(1):31‐39. 10.1128/CDLI.8.1.31-39.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao W, Hao M, Wang Y, et al. Association between folate status and cervical intraepithelial neoplasia. Eur J Clin Nutr. 2016;70(7):837‐842. 10.1038/ejcn.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel EM, Salemi JL, Villa LL, Ferenczy A, Franco EL, Giuliano AR. Dietary consumption of antioxidant nutrients and risk of incident cervical intraepithelial neoplasia. Gynecol Oncol. 2010;118(3):289‐294. 10.1016/j.ygyno.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo SS, Oh HY, Lee JK, Kong JS, Lee DO, Kim MK. Combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin Nutr. 2016;35(6):1434‐1441. 10.1016/j.clnu.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Brooks JP, Edwards DJ, Blithe DL, et al. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel‐releasing intrauterine system on the vaginal microbiome. Contraception. 2017;95(4):405‐413. 10.1016/j.contraception.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noyes N, Cho K‐C, Ravel J, Forney LJ, Abdo Z. Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLoS One. 2018;13(1):e0191625. 10.1371/journal.pone.0191625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Heal Dis. 2013;24: 10.3402/mehd.v24i0.19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amabebe E, Anumba DOC. Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front Endocrinol. 2018;9:568. 10.3389/fendo.2018.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. 2017;129(4):643‐654. 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogacka A, Salazar N, Suárez M, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full‐term neonates. Microbiome. 2017;5(1):93. 10.1186/s40168-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mei C, Yang W, Wei X, Wu K, Huang D. The unique microbiome and innate immunity during pregnancy. Front Immunol. 2019;10:2886. 10.3389/fimmu.2019.02886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajic P, Wolfe AJ, Gupta GN. The urinary microbiome: implications in bladder cancer pathogenesis and therapeutics. Urology. 2019;126:10‐15. 10.1016/j.urology.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Stapleton AE. The vaginal microbiota and urinary tract infection. Microbiol Spectr. 2016;4(6). 10.1128/microbiolspec.uti-0025-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta V, Nag D, Garg P. Recurrent urinary tract infections in women: How promising is the use of probiotics? Indian J Med Microbiol. 2017;35(3):347‐354. 10.4103/ijmm.IJMM_16_292. [DOI] [PubMed] [Google Scholar]
- 59.Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web‐based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):107‐117. 10.1080/14737167.2017.1359543. [DOI] [PubMed] [Google Scholar]
- 60.Foxman B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1‐13. 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against uropathogenic Escherichia coli . Mol Immunol. 2019;108:56‐67. 10.1016/j.molimm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Kline KA, Lewis AL. Gram‐positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2): 10.1128/microbiolspec.uti-0012-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renard J, Ballarini S, Mascarenhas T, et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infect Dis Ther. 2015;4(1):125‐135. 10.1007/s40121-014-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirjavainen PV, Pautler S, Baroja ML, et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clin Vaccine Immunol. 2009;16(1):29‐36. 10.1128/CVI.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muhleisen AL, Herbst‐Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42‐50. 10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 66.Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15(12):750‐776. 10.1038/s41585-018-0106-x. [DOI] [PubMed] [Google Scholar]
- 67.Singh S, Verma N, Taneja N. The human gut resistome: current concepts & future prospects. Indian J Med Res. 2019;150(4):345‐358. 10.4103/ijmr.IJMR_1979_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanza VF, Baquero F, Martínez JL, et al. In‐depth resistome analysis by targeted metagenomics. Microbiome. 2018;6(1):11. 10.1186/s40168-017-0387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ISAPP . Probiotics: A Consumer Guide for Making Smart Choices Developed by the International Scientific Association for Probiotics and Prebiotics. International Scientific Association for Probiotics and Prebiotics; 2013. http://www.isapp.net. Accessed January 25, 2021. [Google Scholar]
- 70.Hill C, Guarner F, Reid G, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506‐514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 71.Lopez M, Li N, Kataria J, Russell M, Neu J. Live and ultraviolet‐inactivated Lactobacillus rhamnosus GG decrease flagellin‐induced interleukin‐8 production in Caco‐2 cells. J Nutr. 2008;138(11):2264‐2268. 10.3945/jn.108.093658. [DOI] [PubMed] [Google Scholar]
- 72.Ostad SN, Salarian AA, Ghahramani MH, Fazeli MR, Samadi N, Jamalifar H. Live and heat‐inactivated lactobacilli from feces inhibit Salmonella typhi and Escherichia coli adherence to Caco‐2 cells. Folia Microbiol. 2009;54(2):157‐160. 10.1007/s12223-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 73.Rampengan NH, Manoppo J, Warouw SM. Comparison of efficacies between live and killed probiotics in children with lactose malabsorption. Southeast Asian J Trop Med Public Health. 2010;41(2):474‐481. [PubMed] [Google Scholar]
- 74.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 2011;6(3):261‐274. 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsilingiri K, Rescigno M. Postbiotics: what else? Benef Microbes. 2013;4(1):101‐107. 10.3920/BM2012.0046. [DOI] [PubMed] [Google Scholar]
- 76.Aguilar‐Toalá JE, Garcia‐Varela R, Garcia HS, et al. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105‐114. 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- 77.Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics‐A step beyond pre‐ and probiotics. Nutrients. 2020;12(8):2189. 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cuevas‐González PF, Liceaga AM, Aguilar‐Toalá JE. Postbiotics and paraprobiotics: from concepts to applications. Food Res Int. 2020;136:109502. 10.1016/j.foodres.2020.109502. [DOI] [PubMed] [Google Scholar]
- 79.Zendeboodi F, Khorshidian N, Mortazavian AM, da Cruz AG. Probiotic: conceptualization from a new approach. Curr Opin Food Sci. 2020;32:103‐123. 10.1016/j.cofs.2020.03.009. [DOI] [Google Scholar]
- 80.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics—a review. J Food Sci Technol. 2015;52(12):7577‐7587. 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu N, Huang S, Xiao J, Chen XD. Producing powders containing active dry probiotics with the aid of spray drying. Adv Food Nutr Res. 2018;85:211‐262. 10.1016/bs.afnr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Champagne CP, da Cruz AG, Daga M. Strategies to improve the functionality of probiotics in supplements and foods. Curr Opin Food Sci. 2018;22:160‐166. [Google Scholar]
- 83.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688‐693. 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LeBegue CE, Love BL, Wyatt MD. Microbes as drugs: the potential of pharmabiotics. Pharmacotherapy. 2020;40(2):102‐106. 10.1002/phar.2357. [DOI] [PubMed] [Google Scholar]
- 85.Cordaillat‐Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52(9):1397‐1406. 10.1038/s12276-020-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nader‐Macías MEF, Juárez Tomás MS. Profiles and technological requirements of urogenital probiotics. Adv Drug Deliv Rev. 2015;92:84‐104. 10.1016/j.addr.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 87.Salvetti E, O’Toole PW. The genomic basis of lactobacilli as health‐promoting organisms. In: Bugs as Drugs. Vol 5. American Society of Microbiology; 2017:49‐71. 10.1128/microbiolspec.bad-0011-2016. [DOI] [PubMed] [Google Scholar]
- 88.McFarland LV, Evans CT, Goldstein EJC. Strain‐specificity and disease‐specificity of probiotic efficacy: a systematic review and meta‐analysis. Front Med. 2018;5:124. 10.3389/fmed.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan M, Hidalgo‐Cantabrana C, Goh YJ, Sanozky‐Dawes R, Barrangou R. Comparative analysis of Lactobacillus gasseri and Lactobacillus crispatus isolated from human urogenital and gastrointestinal tracts. Front Microbiol. 2020;10:3146. 10.3389/fmicb.2019.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yildirim S, Yeoman C, Janga S, et al. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J. 2014;8:2431‐2444. 10.1038/ismej.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mendes‐Soares H, Suzuki H, Hickey RJ, Forneya LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 2014;196(7):1458‐1470. 10.1128/JB.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Der Veer C, Hertzberger RY, Bruisten SM, et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome. 2019;7(1):49. 10.1186/s40168-019-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.FAO/WHO . Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, ON: FAO/WHO; 2002. [Google Scholar]
- 94.Reid G. The development of probiotics for women’s health. Can J Microbiol. 2017;63(4):269‐277. 10.1139/cjm-2016-0733. [DOI] [PubMed] [Google Scholar]
- 95.European Food Safety Authority . Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA J. 2007;587(12):1‐16. 10.2903/j.efsa.2007.587. [DOI] [Google Scholar]
- 96.Alayande KA, Aiyegoro OA, Nengwekhulu TM, Katata‐Seru L, Ateba CN. Integrated genome‐based probiotic relevance and safety evaluation of Lactobacillus reuteri PNW1. PLoS One. 2020;15(7):e0235873. 10.1371/journal.pone.0235873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Reenen CA, Dicks LM. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Arch microbiol. 2011;193(3):157‐168. [DOI] [PubMed] [Google Scholar]
- 98.Gueimonde M, Sánchez B, de Los Reyes‐Gavilán GC, Margolles A. Antibiotic resistance in probiotic bacteria. Front Microbiol. 2013;4:202. 10.3389/fmicb.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imperial IC, Ibana JA. Addressing the antibiotic resistance problem with probiotics: reducing the risk of its double‐edged sword effect. Front Microbiol. 2016;7:1983. 10.3389/fmicb.2016.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Wintersdorff CJ, Penders J, van Niekerk JM, et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7:173. 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daniali M, Nikfar S, Abdollahi M. Antibiotic resistance propagation through probiotics. Expert Opin Drug Metab Toxicol. 2020;16(12):1207‐1215. 10.1080/17425255.2020.1825682. [DOI] [PubMed] [Google Scholar]
- 102.Sharma K, Attri S, Goel G. Selection and evaluation of probiotic and functional characteristics of autochthonous lactic acid bacteria isolated from fermented wheat flour dough babroo. Probiotics Antimicrob Proteins. 2019;11(3):774‐784. 10.1007/s12602-018-9466-z. [DOI] [PubMed] [Google Scholar]
- 103.EFSA . Update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 7: suitability of taxonomic units notified to EFSA until September 2017. EFSA J. 2017;16(1):5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wojnicz D, Tichaczek‐Goska D, Korzekwa K, Kicia M, Hendrich AB. Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections. Int J Food Sci Nutr. 2016;67(8):1005‐1016. 10.1080/09637486.2016.1211996. [DOI] [PubMed] [Google Scholar]
- 105.Granato PA. The microbiota of humans and microbial virulence factors. In: Biological Safety: Principles and Practices. 2017:1‐17. 10.1128/9781555819637.ch1. [DOI] [Google Scholar]
- 106.Touret T, Oliveira M, Semedo‐Lemsaddek T. Putative probiotic lactic acid bacteria isolated from sauerkraut fermentations. PLoS One. 2018;13(9):e0203501. 10.1371/journal.pone.0203501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hernández‐García JE, Sebastián‐Frizzo L, Rodríguez‐Fernández JC, et al. Evaluación in vitro del potencial probiótico de Lactobacillus acidophilus SS80 y Streptococcus thermophilus SS77. Rev Salud Anim. 2019;41(1):e09. [Google Scholar]
- 108.Yao XY, Yuan MM, Li DJ. Molecular adjuvant C3d3 improved the anti‐hCGbeta humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCGbeta‐C3d3 fusion protein. Vaccine. 2007;25(32):6129‐6139. 10.1016/j.vaccine.2007.04.090. [DOI] [PubMed] [Google Scholar]
- 109.Saksena S, Goyal S, Raheja G, et al. Upregulation of P‐glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1115‐G1123. 10.1152/ajpgi.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ballini A, Santacroce L, Cantore S, et al. Probiotics improve urogenital health in women. Open Access Maced J Med Sci. 2018;6(10):1845‐1850. 10.3889/oamjms.2018.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patrignani F, Siroli L, Parolin C, Serrazanetti DI, Vitali B, Lanciotti R. Use of Lactobacillus crispatus to produce a probiotic cheese as potential gender food for preventing gynaecological infections. PLoS One. 2019;14(1):e0208906. 10.1371/journal.pone.0208906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lagenaur LA, Lee PP, Hamer DH, Sanders‐Beer BE. Demonstration of vaginal colonization with GusA‐expressing Lactobacillus jensenii following oral delivery in rhesus macaques. Res Microbiol. 2011;162(10):1006‐1010. 10.1016/j.resmic.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reid G. Has knowledge of the vaginal microbiome altered approaches to health and disease? F1000Res. 2018;7:460. 10.12688/f1000research.13706.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buggio L, Somigliana E, Borghi A, Vercellini P. Probiotics and vaginal microecology: fact or fancy? BMC Womens Health. 2019;19(1): 10.1186/s12905-019-0723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Araújo Pereira RR, Bruschi ML. Vaginal mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 2012;38(6):643‐652. 10.3109/03639045.2011.623355. [DOI] [PubMed] [Google Scholar]
- 116.Baloglu E, Senyigit ZA, Karavana SY, Bernkop‐Schnürch A. Strategies to prolong the intravaginal residence time of drug delivery systems. J Pharm Pharm Sci. 2009;12(3):312‐336. 10.18433/j3hp41. [DOI] [PubMed] [Google Scholar]
- 117.Dobaria N, Mashru R, Vadia NH. Vaginal drug delivery systems: a review of current status. East Cent African J Pharm Sci. 2008;10(1):3‐13. 10.4314/ecajps.v10i1.9754. [DOI] [Google Scholar]
- 118.Sahoo CK, Kumar Nayak P, Sarangi DK, Sahoo TK. Intra vaginal drug delivery system: an overview. Am J Adv Drug Deliv. 2013;2013:43‐55. www.ajadd.co.uk. Accessed October 29, 2020. [Google Scholar]
- 119.Juárez Tomás MS, Bru E, Martos G, Nader‐Macías ME. Stability of freeze‐dried vaginal Lactobacillus strains in the presence of different lyoprotectors. Can J Microbiol. 2009;55(5):544‐552. 10.1139/w08-159. [DOI] [PubMed] [Google Scholar]
- 120.Vitali B, Abruzzo A, Parolin C, et al. Association of Lactobacillus crispatus with fructo‐oligosaccharides and ascorbic acid in hydroxypropyl methylcellulose vaginal insert. Carbohydr Polym. 2016;136:1161‐1169. 10.1016/j.carbpol.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 121.Borges S, Costa P, Silva J, Teixeira P. Effects of processing and storage on Pediococcus pentosaceus SB83 in vaginal formulations: lyophilized powder and tablets. Biomed Res Int. 2013;2013:680767. 10.1155/2013/680767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Juárez Tomás MS, De Gregorio PR, Leccese Terraf MC, Nader‐Macías MEF. Encapsulation and subsequent freeze‐drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur J Pharm Sci. 2015;79:87‐95. 10.1016/j.ejps.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 123.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11(1):78‐87. 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nader‐Macias ME. Probiotics in the prevention of urogenital tract infections. Mechanisms involved. Curr Womens Health Rev. 2006;2(2):103‐117. 10.2174/157340406776931025. [DOI] [Google Scholar]
- 125.Leccese Terraf MC, Juárez Tomás MS, Nader‐Macías ME, Silva C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J Appl Microbiol. 2012;113(6):1517‐1529. 10.1111/j.1365-2672.2012.05429.x. [DOI] [PubMed] [Google Scholar]
- 126.Leccese Terraf MC, Mendoza LM, Juárez Tomás MS, et al. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J Appl Microbiol. 2014;117(6):1761‐1772. 10.1111/jam.12642. [DOI] [PubMed] [Google Scholar]
- 127.Leccese Terraf MC, Juarez Tomás MS, Rault L, et al. In vitro effect of vaginal lactobacilli on the growth and adhesion abilities of uropathogenic Escherichia coli . Arch Microbiol. 2017;199(5):767‐774. 10.1007/s00203-016-1336-z. [DOI] [PubMed] [Google Scholar]
- 128.Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30(1):17‐25. 10.1016/j.bpg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 129.De Gregorio PR, Maldonado NC, Pingitore EV, et al. Intravaginal administration of gelatine capsules containing freeze‐dried autochthonous lactobacilli: a double‐blind, randomised clinical trial of safety. Benef Microbes. 2020;11(1):5‐17. 10.3920/BM2019.0081. [DOI] [PubMed] [Google Scholar]
- 130.De Gregorio PR, Tomás MSJ, Terraf MCL, Nader‐Macías MEF. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J Med Microbiol. 2014;63(Pt 5):685‐696. 10.1099/jmm.0.069401-0. [DOI] [PubMed] [Google Scholar]
- 131.De Gregorio PR, Juárez Tomás MS, Leccese Terraf MC, Nader‐Macías MEF. Preventive effect of Lactobacillus reuteri CRL 1324 on group B Streptococcus vaginal colonization in an experimental mouse model. J. Appl. Microbiol. 2015;118(4):1034‐1047. 10.1111/jam.12739. [DOI] [PubMed] [Google Scholar]
- 132.De Gregorio PR, Juárez Tomás MS, Nader‐Macías ME. Immunomodulation of Lactobacillus reuteri CRL1324 on group B Streptococcus vaginal colonization in a murine experimental model. Am J Reprod Immunol. 2016;75(1):23‐35. 10.1111/aji.12445. [DOI] [PubMed] [Google Scholar]
- 133.Mastromarino P, Vitali B, Mosca L. Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol. 2013;36(3):229‐238. [PubMed] [Google Scholar]
- 134.Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34(12):2735‐2737. 10.1007/s11606-019-05077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galdeano CM, Perdigón G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J Appl Microbiol. 2004;97(4):673‐681. 10.1111/j.1365-2672.2004.02353.x. [DOI] [PubMed] [Google Scholar]
- 136.Cazorla SI, Maldonado‐Galdeano C, Weill R, De Paula J, Perdigón GDV. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front Microbiol. 2018;9:736. 10.3389/fmicb.2018.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang CH, Kim Y, Han SH, Kim JS, Paek NS, So JS. In vitro probiotic properties of vaginal Lactobacillus fermentum MG901 and Lactobacillus plantarum MG989 against Candida albicans . Eur J Obstet Gynecol Reprod Biol. 2018;228:232‐237. 10.1016/j.ejogrb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 138.de Vrese M, Laue C, Papazova E, Petricevic L, Schrezenmeir J. Impact of oral administration of four Lactobacillus strains on Nugent score—systematic review and meta‐analysis. Benef Microbes. 2019;10(5):483‐496. 10.3920/BM2018.0129. [DOI] [PubMed] [Google Scholar]
- 139.Vladareanu R, Mihu D, Mitran M, et al. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): a randomized double‐blind placebo‐controlled study. Eur Rev Med Pharmacol Sci. 2018;22:262‐267. 10.26355/eurrev_201801_14128. [DOI] [PubMed] [Google Scholar]
- 140.Verdenelli MC, Cecchini C, Coman MM, et al. Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr Microbiol. 2016;73(4):483‐490. 10.1007/s00284-016-1085-x. [DOI] [PubMed] [Google Scholar]
- 141.Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin‐V to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382(20):1906‐1915. 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nader‐Macias M, de Ruiz C, Ocana V, Juarez TM. Advances in the knowledge and clinical applications of lactic acid bacteria as probiotics in the urogenital tract. Curr Womens Health Rev. 2008;4(4):240‐257. 10.2174/157340408786848214. [DOI] [Google Scholar]
- 143.Dausset C, Patrier S, Gajer P, et al. Comparative phase I randomized open‐label pilot clinical trial of Gynophilus® (Lcr regenerans®) immediate release capsules versus slow release muco‐adhesive tablets. Eur J Clin Microbiol Infect Dis. 2018;37(10):1869‐1880. 10.1007/s10096-018-3321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sgibnev AKE. Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: a randomized, placebo‐controlled, double‐blind study. Eur J Clin Microbiol Infect Dis. 2020;39:345‐351. [DOI] [PubMed] [Google Scholar]
- 145.Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A. Long‐term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV‐infection. BMC Infect Dis. 2018;18(1):13. 10.1186/s12879-017-2938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barthow C, Wickens K, Stanley T, et al. The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double‐blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth. 2016;16(1):133. 10.1186/s12884-016-0923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117(6):804‐813. 10.1017/S0007114517000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double‐blind placebo‐controlled trial. EBioMedicine. 2017;24:159‐165. 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wickens K, Barthow C, Mitchell EA, et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr Allergy Immunol. 2018;29(3):296‐302. 10.1111/pai.12874. [DOI] [PubMed] [Google Scholar]
- 150.Martín V, Cárdenas N, Ocaña S, et al. Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius cect 9145, a target‐specific probiotic strain. Nutrients. 2019;11(4):810. 10.3390/nu11040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Alberti D, Russo R, Terruzzi F, et al. Lactobacilli vaginal colonisation after oral consumption of Respecta® complex: a randomised controlled pilot study. Arch Gynecol Obstet. 2015;292(4):861‐867. 10.1007/s00404-015-3711-4. [DOI] [PubMed] [Google Scholar]
- 152.Russo R, Superti F, Karadja E, De Seta F. Randomised clinical trial in women with recurrent vulvovaginal candidiasis: efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses. 2019;62(4):328‐335. 10.1111/myc.12883. [DOI] [PubMed] [Google Scholar]
- 153.Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H. The influence of probiotics on genital high‐risk human papilloma virus clearance and quality of cervical smear: a randomized placebo‐controlled trial. BMC Womens Health. 2019;19(1):103. 10.1186/s12905-019-0798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McMillan A, Rulisa S, Gloor GB, Macklaim JM, Sumarah M, Reid G. Pilot assessment of probiotics for pregnant women in Rwanda. PLoS One. 2018;13(6): 10.1371/journal.pone.0195081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;215(5):608.e1‐608.e7. 10.1016/j.ajog.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 156.Husain S, Allotey J, Drymoussi Z, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double‐blind, placebo‐controlled trial with microbiome analysis. BJOG: An Int J Obstet Gynaecol. 2020;127(2):275‐284. 10.1111/1471-0528.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ho M, Chang YY, Chang WC, et al. Oral Lactobacillus rhamnosus GR‐1 and Lactobacillus reuteri RC‐14 to reduce group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol. 2016;55(4):515‐518. 10.1016/j.tjog.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 158.Olsen P, Williamson M, Traynor V, Georgiou C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: a pilot randomised control study. Women Birth. 2018;31(1):31‐37. 10.1016/j.wombi.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 159.Tomusiak A, Strus M, Heczko PB, et al. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: a multicenter, randomized, double‐blind, and placebo‐controlled trial. Drug Des Devel Ther. 2015;9:5345‐5354. 10.2147/DDDT.S89214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heczko PB, Tomusiak A, Adamski P, et al. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double‐blind, placebocontrolled trial. BMC Womens Health. 2015;15(1). 10.1186/s12905-015-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marcotte H, Larsson PG, Andersen KK, et al. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in South African women with bacterial vaginosis. BMC Infect Dis. 2019;19(1): 10.1186/s12879-019-4425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pendharkar S, Brandsborg E, Hammarström L, Marcotte H, Larsson PG. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect Dis. 2015;15(1):255. 10.1186/s12879-015-0971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Daskalakis GJ, Karambelas AK. Vaginal probiotic administration in the management of preterm premature rupture of membranes. Fetal Diagn Ther. 2017;42(2):92‐98. 10.1159/000450995. [DOI] [PubMed] [Google Scholar]
- 164.Kwak YK, Daroczy K, Colque P, Kühn I, Möllby R, Kopp KH. Persistence of lactobacilli in postmenopausal women—a double‐blind, randomized, Pilot study. Gynecol Obstet Invest. 2017;82(2):144‐150. 10.1159/000446946. [DOI] [PubMed] [Google Scholar]
- 165.Rostok M, Hütt P, Rööp T, et al. Potential vaginal probiotics: safety, tolerability and preliminary effectiveness. Benef Microbes. 2019;10(4):385‐393. 10.3920/BM2016.0123. [DOI] [PubMed] [Google Scholar]
- 166.Mezzasalma V, Manfrini E, Ferri E, et al. Orally administered multispecies probiotic formulations to prevent uro‐genital infections: a randomized placebo‐controlled pilot study. Arch Gynecol Obstet. 2017;295(1):163‐172. 10.1007/s00404-016-4235-2. [DOI] [PubMed] [Google Scholar]
- 167.Laue C, Papazova E, Liesegang A, et al. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—a double‐blind, randomised, controlled clinical pilot trial. Benef Microbes. 2018;9(1):35‐50. 10.3920/BM2017.0018. [DOI] [PubMed] [Google Scholar]
- 168.Anoshina TM. Role of microbiota correction in complex treatment of pregnant women with herpesvirus infection. Perinatol I Pediatr. 2016;4:22‐25. 10.15574/PP.2016.68.22. [DOI] [Google Scholar]
- 169.Kovachev SM, Vatcheva‐Dobrevska RS. Local probiotic therapy for vaginal Candida albicans infections. Probiotics Antimicrob Proteins. 2015;7(1):38‐44. 10.1007/s12602-014-9176-0. [DOI] [PubMed] [Google Scholar]
- 170.Ribeiro AE, Monteiro NES, De MAVG, Costa‐Paiva LH, Pedro AO. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2019;26(6):643‐652. 10.1097/GME.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 171.MacPhee RA, Miller WL, Gloor GB, et al. Influence of the vaginal microbiota on toxic shock syndrome toxin 1 production by Staphylococcus aureus . Appl Environ Microbiol. 2013;79(6):1835‐1842. 10.1128/AEM.02908-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.ELLEN® Probiotic Tampon . SCIENTIFIC SUMMARY product description. https://www.ellentampon.ch/sites/all/themes/ellen_tampon_ch/media/downloads/probiotic‐tampon‐scientific‐information‐public.pdf. Accessed November 2, 2020.
- 173.Sauperl O, Zabret A, Fras ZL. Development of advanced sanitary materials with the use of probiotic paste. J Eng Fiber Fabr. 2020;15:155892502092221. 10.1177/1558925020922215. [DOI] [Google Scholar]
- 174.PRODUCTS . www.fide.cz. http://www.fide.cz/PRODUCTS.#unique‐PRODUCTS. Accessed November 2, 2020.
- 175.Husmark UM, Gustafsson IA; Essity Hygiene and Health AB . Hygiene product with a probiotic composition. Pub.No.: US 2004/0243076 A1. December 2, 2004.
- 176.Husmark UM, Gustafsson IA; Essity Hygiene and Health AB . Hygiene product with a probiotic composition. Pub.No.: DE 602004007788 T2. July 5, 2008.
- 177.Zespoł Ekspertów Polskiego Towarzystwa Ginekologicznego . Statement of the Polish Gynecological Society Expert Group on the use of ellen probiotic tampon. Ginekol Pol. 2012;83(8):633‐638. [PubMed] [Google Scholar]
- 178.Silva JA, De Gregorio PR, Rivero G, Abraham GA, Nader‐Macías MEF. Immobilization of vaginal Lactobacillus in polymeric nanofibers for its incorporation in vaginal probiotic products. Eur J Pharm Sci. 2021;156:105563. 10.1016/j.ejps.2020.105563. [DOI] [PubMed] [Google Scholar]
- 179.De Gregorio PR, Silva JA, Rivero G, Abraham GA, Nader‐Macías MEF; CERELA‐CONICET‐Universidad Nacional de Mar del Plata‐Argentina . Method of immobilization of probiotic lactic acid bacteria textile substrate and product obtained. INPI N ° 20180103893. December 27, 2018
- 180.Cherpinski A, Szewczyk PK, Gruszczyński A, Stachewicz U, Lagaron JM. Oxygen‐scavenging multilayered biopapers containing palladium nanoparticles obtained by the electrospinning coating technique. Nanomaterials. 2019;9(2): 10.3390/nano9020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Gregorio PR, Michavila G, Muller LR, et al. Beneficial rhizobacteria immobilized in nanofibers for potential application as soybean seed bioinoculants. PLoS One. 2017;12(5):e0176930. 10.1371/journal.pone.0176930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kersani D, Mougin J, Lopez M, et al. Stent coating by electrospinning with chitosan/poly‐cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention. Eur J Pharm Biopharm. 2020;150:156‐167. 10.1016/j.ejpb.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 183.Škrlec K, Zupančič Š, Prpar Mihevc S, Kocbek P, Kristl J, Berlec A. Development of electrospun nanofibers that enable high loading and long‐term viability of probiotics. Eur J Pharm Biopharm. 2019;136:108‐119. 10.1016/j.ejpb.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 184.Nagy ZK, Wagner I, Suhajda Á, et al. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. Expreess Polym Lett. 2014;8(5):352‐361. 10.3144/expresspolymlett.2014.39. [DOI] [Google Scholar]


