Abstract
Cancer is the second leading cause of death globally and its incidence and mortality are rapidly increasing worldwide. The dynamic interaction of immune cells and tumor cells determines the clinical outcome of cancer. Immunotherapy comes to the forefront of cancer treatments, resulting in impressive and durable responses but only in a fraction of patients. Thus, understanding the characteristics and profiles of immune cells in the tumor microenvironment (TME) is a necessary step to move forward in the design of new immunomodulatory strategies that can boost the immune system to fight cancer. Histamine produces a complex and fine‐tuned regulation of the phenotype and functions of the different immune cells, participating in multiple regulatory responses of the innate and adaptive immunity. Considering the important actions of histamine‐producing immune cells in the TME, in this review we first address the most important immunomodulatory roles of histamine and histamine receptors in the context of cancer development and progression. In addition, this review highlights the current progress and foundational developments in the field of cancer immunotherapy in combination with histamine and pharmacological compounds targeting histamine receptors.
Keywords: adaptive immunity, anti‐tumor immunity, breast cancer, histamine receptors, immunotherapy, innate immunity, leukemia
Targeting the histaminergic system represents a promising strategy for the potential therapeutic exploitation of new immunomodulatory drugs that can boost the immune system to fight cancer.

Abbreviations
- AC
adenylate cyclase
- AML
acute myeloid leukemia
- AOM
azoxymethane
- APCs
professional antigen‐presenting cells
- Bregs
regulatory B cells
- cAMP
cyclic adenosine monophosphate
- cDCs
conventional DCs
- CML
chronic myeloid leukemia
- CNS
central nervous system
- CR
complete remission
- CRC
colorectal cancer
- CREB
cAMP response element‐binding protein
- CTLA‐4
cytotoxic T lymphocyte antigen 4
- CXCL1
C‐X‐C motif chemokine ligand 1
- CXCL10
C‐X‐C motif chemokine ligand 10
- CXCL2
C‐X‐C motif chemokine ligand 2
- DAO
diamine oxidase
- DCs
dendritic cells
- DSS
dextran sulfate sodium
- EPO
eosinophil peroxidase
- ErbB‐2
human epidermal growth factor receptor 2
- FcεRI
receptor for immunoglobulin E
- FoxP3
transcription factor forkhead box P3
- GTP
guanosine triphosphate
- H4 receptor KO
H4 receptor knockout mice
- HDC
histamine dihydrochloride
- IFN
interferon
- IFNα
interferon alfa
- IFNγ
interferon γ
- IgE
immunoglobulin E
- LFS
leukemia‐free survival
- MAPK
mitogen‐activated protein kinase
- MBP
major basic protein
- MDSCs
myeloid‐derived suppressor cells
- MHC I
class 1 major histocompatibility complex
- MHC II
class 2 major histocompatibility complex
- Mo
monocytes
- moDCs
monocyte‐derived DCs
- MRP
resistance‐associated protein
- MSI
microsatellite instability
- NCR
natural cytotoxicity receptors
- NETs
neutrophil extracellular traps
- NHL
non‐Hodgkin lymphomas
- NK
natural killer
- NKT
natural killer T cell
- OS
overall survival
- PC
plasma cell
- PD‐1
programmed dead 1
- pDCs
plasmacytoid DCs
- PD‐L1
programmed‐death 1 ligand
- PGD2
prostaglandin D2
- PKA
protein kinase A
- PLC
phospholipase C
- RCC
renal cell carcinoma
- ROS
reactive oxygen species
- TABE
peripheral blood eosinophilia
- TAMs
tumor‐associated macrophages
- TANs
tumor‐associated neutrophils
- TATE
tumor‐associated tissue eosinophils
- T‐bet
transcription factor T‐box
- TDLN
tumor draining lymph nodes
- TGFβ
transforming growth factor β
- TILs
tumor‐infiltrating lymphocytes
- TME
tumor microenvironment
- TNBC
triple‐negative breast cancer
- TNFα
tumor necrosis factor α
- Tregs
regulatory T cells
- uPA
urokinase plasminogen activator
- WT
wild type
1. INTRODUCTION
Cancer is the second leading cause of death globally and its incidence and mortality are rapidly increasing worldwide.1 Although advances in cancer research result in improved anti‐tumor targeted therapies, they continue to have variable outcomes, associated with limited response and severe toxicity thus, several patients will suffer from overwhelming morbimortality. Extraordinary advances in the understanding of the interactions between the immune system and cancer cells have been made in the last decade, which led to the development of effective and promising immunotherapies targeting different tumor molecules and their interaction with the tumor microenvironment (TME). Consequently, immune checkpoint inhibitors were developed to successfully enhance anti‐tumor T‐cell features but resulted in durable responses only in a fraction of patients. The dynamic interaction of immune cells and tumor cells determines the clinical outcome of cancer and it can be reshaped by cancer immunotherapies. One of the most important topics in cancer immunology research today is to understand the characteristics and profiles of immune cells in the TME to design new immunomodulatory strategies that can boost the immune system to fight cancer.
Even though histamine has been the first inflammatory biogenic amine to be characterized, novel functions of histamine are still being described. In this sense, the discovery of the histamine H4 receptor by several groups in 2000/2001 significantly expanded the research field. Histamine is one of the most widely investigated molecules in biomedicine and all histamine receptor subtypes constitute well‐established or promising drug targets.2, 3
Importantly, histamine is a major mediator responsible for multiple regulatory responses of innate and adaptive immunity4, 5, 6 (Figure 1). Immune cells that are key participants in the TME can synthesize, release and respond to histamine.
FIGURE 1.
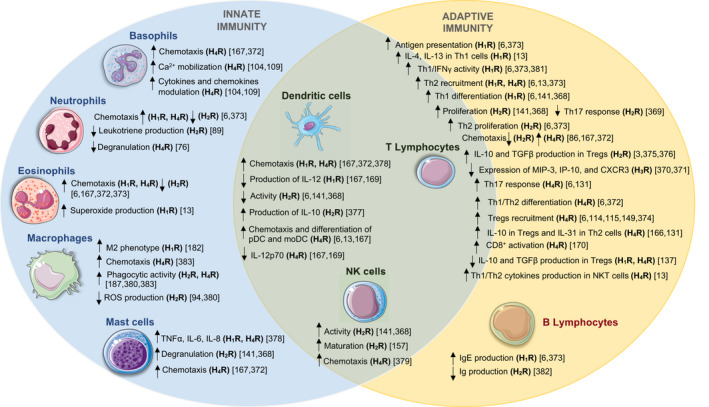
Immunomodulatory effects mediated by histamine receptor signaling in innate and adaptive immunity. The binding of histamine to its receptors can modulate the function of the immune cells, including neutrophils, eosinophils, basophils, mast cells, dendritic cells (DCs), natural killer (NK) cells, NKT cells; Th1‐, Th2‐, Th17‐, regulatory CD4+ T‐, CD8+ cytotoxic T cells, and B cells. The participation of the different histamine receptor subtypes in each cell subsets was determined through functional assays and the use of pharmacological compounds. CxCR3, C‐X‐C Motif Chemokine Receptor 3; IL, interleukin; IFNγ, interferon gamma; IP‐10, IFN‐inducible protein 10; M1, pro‐inflammatory macrophages; M2, anti‐inflammatory macrophages; MIP‐3, macrophage inflammatory protein 3; moDC, monocyte‐derived dendritic cells; NKT, invariant natural killers T cells; pDCs, plasmacytoid dendritic cells; ROS, reactive oxygen species; TGFβ, transforming growth factor‐beta; TNFα, tumor necrosis factor‐alpha; Tregs, T regulatory cells
Furthermore, there is increasing evidence indicating that histamine can modulate cell proliferation and differentiation of normal and malignant cells. High histamine biosynthesis and content have been found in different human tumors including melanoma, colon, and breast cancer, as well as in experimental cancer models. Histamine can be released to the extracellular medium and through a paracrine or autocrine regulation, it may regulate diverse biological responses related to tumor growth (reviewed in Refs. [4, 7]). From cell lines to animal models and human clinical studies, an overwhelming amount of data supports the relevance of histamine receptors in cancer development and progression. Both pro‐tumor and anti‐tumor effects of histamine receptors have been described depending on the cancer type and other important factors. Differences in histamine metabolism, TME, the concentration of histamine in the tissue, and the activation of histamine receptors may determine the biological responses in diverse neoplasias.4, 7, 8, 9, 10, 11, 12, 13 These events include angiogenesis, cell proliferation, invasion, migration, differentiation, apoptosis, and also the modulation of the immune response, indicating that histamine may be a crucial mediator in cancer formation and dissemination.
Additionally, histamine receptors are differentially expressed in benign lesions or healthy tissues compared to malignant lesions in diverse cancers, including melanoma, cholangiocarcinoma, oral, and colorectal cancers.7, 14, 15 The expression of different histamine receptor subtypes, such as H1 and H4, was associated with clinicopathological characteristics and tumor grade in different neoplasias, reinforcing the role of the histaminergic system in carcinogenesis. Therefore, in addition to a direct effect of histamine through tumor cell‐intrinsic mechanisms involving activation of histamine receptors in cancer cells (reviewed in Refs. [4, 7, 8]), histamine could contribute to the modulation of TME by regulating immune‐mediated effects.
The purpose of this review was to address the most recent findings on the immunomodulatory role of histamine and its receptors in the complex anti‐tumor immunity. In addition, this review compiles the most up‐to‐date data supporting the potential use of histamine as an adjuvant to cancer immunotherapy.
2. HISTAMINE RECEPTORS
Histamine [2‐(4‐imidazolyl)‐ethylamine; β‐imidazolylethylamine] is an endogenous biogenic amine that is synthesized by histidine decarboxylase‐mediated decarboxylation of the amino acid L‐histidine. It is catabolized intracellularly by the histamine N‐methyltransferase and extracellularly by the diamine oxidase.2, 16 Histamine is ubiquitously distributed in mammalian cells, and it exerts pleiotropic effects as a result of the existence of four G‐protein‐coupled histamine receptor subtypes that trigger distinct signaling cascades and are differentially expressed throughout the tissues.
Histamine receptors are named in the order in which they were discovered: H1, H2, H3, and H4 receptors, and have different histamine‐binding affinities.17, 18, 19, 20, 21, 22, 23, 24, 25 All four receptors show a balance between their inactive and active conformation and present constitutive activity, leading to a re‐classification of some antagonists into inverse agonists.25 To add more complexity to the matter, it has been shown that histamine receptors can appear as homo and hetero‐oligomers, which influences the repertoire of physiological and pharmacological effects.26, 27, 28, 29, 30, 31
The H1 receptor is a Gαq/11‐coupled protein receptor, which stimulates the phospholipase C (PLC) to generate inositol 1,4,5‐triphosphate and 1,2‐DAG leading to an increase in cytosolic Ca2+. Besides, it can produce cyclic adenosine monophosphate (cAMP) accumulation via Gβγ subunits of Gq.25, 26, 27, 28, 29, 30, 31, 32 It is ubiquitously distributed and plays a key role in smooth muscle contraction, stimulates nitric oxide formation, and increases vascular permeability, showing numerous roles in inflammatory processes in allergic disorders.33 As expected, H1 receptor antagonists/inverse agonists, including mepyramine, fexofenadine, loratadine, diphenhydramine, and astemizol are widely used for the treatment of allergic diseases.34, 35
Similar to H1, H2 receptor is expressed in almost all peripheral tissues as well as in the central nervous system (CNS). The H2 receptor is coupled to adenylate cyclase (AC) and its stimulation enhances the amounts of cAMP and downstream effects mediated by protein kinase A (PKA) and the transcription factor cAMP‐response element‐binding protein (CREB). However, using a different GTP‐dependent mechanism, H2 receptor also modulates phosphoinositide second messenger system.25, 36 Many of the H1 receptor‐mediated effects can be balanced by the H2 receptor, including the relaxation of smooth muscle cells, causing vasodilation. The H2 receptor activation causes marked chronotropic and inotropic effects in the heart and induces gastric acid production from parietal cells in the gastric mucosa. Most H2 receptor antagonists/inverse agonists including cimetidine, famotidine, and nizatidine are clinically used to inhibit histamine‐induced gastric acid secretion.34, 35
It is important to point out that in recombinant and native systems in which H1 and H2 receptors are coexpressed, cross‐regulation of both pathways including cross‐desensitization of the receptors and their responses occurs when cells are exposed to a sustained stimulus with H1 receptor or H2 receptor agonists.37, 38, 39
The H3 receptor is a Gαi/0‐coupled protein receptor, and its activation leads to inhibition of cAMP formation, accumulation of Ca2+, and stimulation of the MAPK pathway.40, 41 Although primarily described in the CNS, it is additionally found in other tissues including some immune cells.5, 42 The H3 receptor acts as an autoreceptor and heteroreceptor, regulating the release of histamine from histaminergic neurons and of various other neurotransmitters. Thus, the H3 receptor blocking ligands are promising agents for the treatment of CNS disorders, obesity, sleep disorders, Alzheimer's disease, and schizophrenia.43, 44, 45, 46, 47 Pitolisant is a first‐in‐class FDA‐approved agent for the treatment of daytime sleepiness in adults with narcolepsy by acting as an antagonist/inverse agonist at the H3 receptor.34, 35, 48
The H4 receptor is a Gαi/0‐coupled protein receptor that is predominantly expressed in cells of the immune system and is involved in immunomodulatory pathways. The expression of H4 receptor has been detected in various tissues including the spleen, thymus, lung, small and large intestines, and also cancer cells.8, 49, 50, 51, 52 Activation of H4 receptor leads to the inhibition of AC and downstream cAMP‐responsive elements as well as the activation of MAPK and PLC with Ca2+ mobilization.25, 34, 35, 41 Numerous in vivo studies have demonstrated that the H4 receptor plays an important role in inflammation and pruritus. Clinical trials are already in the way to assess the effectiveness of various H4 receptor antagonists.8, 53, 54, 55, 56, 57 In a phase IIa study in Japanese adult patients with moderate atopic dermatitis, JNJ39758979 (100 or 300 mg daily orally administered for 6 weeks) was effective in ameliorating pruritus and eczema but it showed agranulocytosis, a life‐threatening side effect, which seemed to be an off‐target effect.53, 58 Although toreforant (JNJ38518168), another H4 receptor antagonist with a different chemical structure to avoid the agranulocytosis‐associated side effect, failed to improve uncontrolled, eosinophilic asthma (30 mg per day for 24 weeks),54, 56 it produced a greater response than placebo in patients with moderate‐to‐severe psoriasis (30 and 60 mg per day).59 In addition, toreforant (100 mg once daily orally administered for 12 weeks) reduced the signs and the symptoms of rheumatoid arthritis in a phase IIa study, but these could not be confirmed in a phase IIb trial.60 Recently, the selective H4 receptor antagonist adriforant (ZPL‐3893787, 30 mg administered orally for 8 weeks) was well tolerated and improved eczema and severity in patients with moderate to severe atopic dermatitis.57, 61
Histamine and its four receptors represent a complex axis with multiple regulatory functions in the innate and adaptive immunity. These functions depend on the receptor subtypes involved and their differential expression and associated signaling. Therefore, in addition to histamine's classical roles in the inflammatory process, it is also recognized as a vital player in immunoregulation, balancing extensive and opposed effects in the immune system.
A summary of the distinct immunoregulatory impacts that histamine produces through its binding to each of the four subtypes of histamine receptors is depicted in Figure 1.
3. HISTAMINE MODULATION OF THE ANTI‐TUMOR IMMUNITY
Cancer is a heterogeneous and multi‐faceted disease, characterized by uncontrolled cell proliferation, evasion of growth suppressors and the immune response, avoidance of apoptosis, sustained replicative potential and angiogenesis, reprogramming of energy metabolism, genetic and epigenetic instability, tissue invasion and metastasis, and enhanced inflammation, which collectively dictate tumor progression.62, 63 Besides being a hallmark of cancer, inflammation might also contribute to the establishment of other alterations described by Hanahan and Weinberg. Infiltration of both innate and adaptive immune cells and a molecular network of soluble mediators are two key constituents of cancer‐associated inflammation.62, 63 In this regard, the complexity of cancer goes beyond the neoplastic cells and includes the TME, which is defined as the collection of cells, molecules, and vasculature that surrounds the tumor, and it is specifically adapted in response to disease. The composition of TME changes during the tumor evolution affecting the early stages of cancer progression as well as the formation of distant metastasis.
The immune system comprises a dynamic network of cells, tissues, and organs that participate in the two lines of defense called innate and adaptive immunity. Immune cells are important components of the TME because, on the one hand, they can eliminate tumor cells and, on the other hand, they can provide the necessary conditions to facilitate tumor growth and progression, which highlights the dichotomous nature of the immune system.62, 64, 65 This process is called immunoediting and refers to the ability of immune cells to intervene in the elimination of tumor cells (immunosurveillance) and, at the same time, shape the immunogenicity of tumors favoring their growth and progression (immunotolerance).64, 66 Cancer immunoediting is a dynamic process that consists of three phases: elimination, equilibrium, and escape.
In the elimination phase, the immune system detects and eliminates tumor cells that develop due to failures in their intrinsic mechanisms of tumor suppression. The elimination can be complete, meaning no tumor cells remain, or incomplete, when only a portion of them is eliminated. In the latter case, tumor cells enter an equilibrium phase, where they evolve and accumulate changes that modulate the expression of tumor antigens. In this phase, the immune system continues to act and eliminate susceptible tumor clones. However, resistant cell variants that could avoid or suppress immunity may develop, leading to the escape phase, thus allowing tumor progression.62, 64, 66
The balance between immunological surveillance and tolerance is determined by a complex interplay between different types of immune cells in the TME that include macrophages, neutrophils, mast cells, natural killer (NK) cells, dendritic cells (DCs), myeloid‐derived suppressor cells (MDSCs), B cells, and different subtypes of T cells (Table 1).
TABLE 1.
Role of immune cell subsets in cancer immunoediting
| Immune cell | Tumor effect | References |
|---|---|---|
|
T cells |
Pro‐tumor effects: CD4+ Th2 cells produce IL‐4, IL‐5, IL‐13, and activate eosinophils, basophils, and B cells. Tumors characterized by a Th2 immune infiltrate are associated with a poor prognosis. IL‐17 derived from Th17 cells promotes cell migration and invasion | 65,115,125–127,149,292–295 |
|
Anti‐tumor effects: CD4+ Th1 cells produce IFNγ, TNFα, and IL‐2. They activate macrophages, NK cells, and CD8+ T cells, and eliminate tumor cells through cytolytic mechanisms or modulating the TME. They optimize DCs in antigen presentation to CD8+ T cells. In lymphoid organs, they increase the action of B cells and CTL response. They are associated with favorable prognosis in renal cell, colorectal, esophageal, and squamous carcinomas CD4+ Th17 cells have anti‐tumoral functions, inducing the recruitment of DCs into the tumor and the adjacent lymph nodes and thus, promoting tumor‐specific CTL responses CD8+ T cells display MHC I‐mediated CTL activation, which produces perforins, granzymes, serine esterases, and IFNγ or TNFα. They are associated with a better prognosis in melanoma, TNBC, ovarian, bladder, and renal cancer | ||
|
NK cells |
Anti‐tumor effects: NK cells eliminate malignant cells through perforin and granzyme B, induce target cell apoptosis via Fas/FasL and TRAIL/TRAIL pathways, and secrete cytokines including IFNγ and TNFα. They promote adaptive responses through IFNγ secretion and cDC1 regulation, eliminate immature DCs or facilitate their maturation. They discriminate between “normal and altered self” through MHC I‐specific inhibitory receptors and activate receptors that recognize ligands associated with cell stress. NK cells inhibit tumor growth, favor Th1 polarization of CD4+ T cells, and are associated with improved patient prognosis and survival | 159,296–302 |
|
Tregs |
Pro‐tumor effects: Tregs suppress effector functions of immune cells such as CD4+ and CD8+ T cells, NK cells, macrophages, and DCs. Tregs induce tumor progression by the secretion of immunosuppressive mediators IL‐10 and TGFβ, the exhaustion of T cell through the expression of LAG‐3, TIM‐3, and PD‐1, and the inhibition of DCs maturation. They inhibit the cytolytic activity on CTL and NK cells by mediators like granzyme B, the TRAIL pathway, galectin‐1, and perforin. Tregs modulate the function of DCs through the expression of Nrp‐1 and CTLA‐4 A decreased ratio of cytotoxic CD8+ T cells to Tregs correlated with poor prognosis in patients with breast, ovarian, and gastric cancers |
142,143,303–309 |
|
B cells |
Pro‐tumor effects: B cells stimulate antibody‐mediated activation of immunosuppressive myeloid cells and tumor growth by IL‐35 production. Bregs induce apoptosis in CD4+ T cells, suppress IFNγ production by NK and CD8+ cells, exacerbate inflammation, and support cancer growth by IL‐10 production. Bregs convert naïve CD4+ T cells into Foxp3+ Tregs, upregulate ROS and NO in MDSCs by TGFβ production. They are associated with a poor prognosis in ovarian cancer, glioblastoma, and clear cell renal carcinoma | 155,310–320 |
| Anti‐tumor effects: B cells induce tumor regression via a direct cytotoxic effect on tumor cells by secreting immunoglobulins (ADCC), and via Fas/FasL, TRAIL/Apo2L, and IFNγ secreted by NK cells. They act as APCs and polarize T cells toward Th1 or Th2 response. They are associated with increased overall survival in patients with melanoma, lung and pancreatic adenocarcinomas, and head and neck squamous cell carcinoma | ||
|
MDSCs |
Pro‐tumor effects: MDSCs inhibit T‐cell proliferation by depletion of essential amino acids (L‐arginine and tryptophan), production of ROS and RNS, restriction of lymphocyte trafficking (downregulation of L‐selectin), and induction of T‐cell apoptosis by decreasing Bcl‐2 expression and upregulation of FAS. They promote differentiation of CD4+ T cells to Tregs, and induce metastasis, cell migration, invasion (degradation of ECM and promotion of EMT), angiogenesis, and formation of the premetastatic niche In cancer patients, MDSCs’ expansion in the peripheral blood is correlated with poor clinical outcomes and with advanced clinical stages |
194,321–325 |
|
Dendritic cells |
Pro‐tumor effects: pDCs mediate tolerance and immunosuppression, producing IDO and inducing Tregs. pDCs in the TME are associated with poor prognosis in melanoma, head and neck, breast, and ovarian cancers | 165,326–332 |
| Anti‐tumor effects: cDCs attract primed T cells back from the lymph nodes to the tumor. cDC1 s activate CD8+ T‐cell responses through peptide cross‐presentation on MHC I. cDC2 s activate CD4+ T‐cell responses via MHC II‐dependent antigen presentation. pDCs participate in immune tolerance, produce and secrete type I interferons. Therapeutic activation of pDCs has shown efficacy in melanoma, basal cell carcinoma, and T‐cell lymphoma | ||
|
Macrophages |
Pro‐tumor effects: TAMs with a M2‐like phenotype (anti‐inflammatory role) have properties correlated with angiogenesis, immunosuppression, and promotion of cancer growth, vascular invasion, metastasis, cancer stemness, and poor prognosis. M2 macrophages produce anti‐inflammatory cytokines (e.g., IL‐10), upregulate scavenger receptors, such as mannose receptors, and suppress T‐cell recruitment and activation. M2 TAMs are associated with resistance to chemotherapy and radiotherapy | 179,180,333–337 |
| Anti‐tumor effects: TAMs with a M1‐like phenotype (pro‐inflammatory role) are associated with the early phases of tumor development or with regressing tumors. M1 macrophages mediate anti‐microbial and tumoricidal responses by secreting inflammatory cytokines, such as TNFα, IL‐12, ROS, and NO, and by upregulating the expression of MHC II and promoting a Th1‐type of response | ||
|
Mast cells |
Pro‐tumor effects: Mast cells induce the production of pro‐angiogenic and pro‐lymphangiogenic factors (chymase, tryptase, VEGF, IL‐6, PDGF, FGF‐2, MMP‐9), promote the degradation of ECM and immunosuppression, and stimulate distant metastasis. They are associated with poor prognosis in Hodgkin's lymphoma, melanoma, endometrial, cervical, esophageal, lung, gastric, colorectal, and prostate carcinomas | 108,110,112,119,338–344 |
| Anti‐tumor effects: Mast cells promote activation and recruitment of DCs, NK cells, CD8+ and CD4+ cells. They induce the inhibition of Tregs, MDSCs, and M2 phenotype, and they have cytotoxic activity. The high number of mast cells is associated with a good prognosis in breast cancer | ||
|
Eosinophils |
Pro‐tumor effects: They induce fibroblast and endothelial cell proliferation, polarization to M2 phenotype, and promote metastasis via MMP‐9, angiogenesis, and tissue healing. TABE is observed in carcinomas of the kidney, thyroid, liver, gallbladder, pancreas, breast, and Hodgkin's lymphomas and SCCs. Their presence is associated with a poor prognosis | 97,98,101,102,345–348 |
| Anti‐tumor effects: They are recruited by chemoattractants such as IL‐5, IL‐4, GM‐CSF, and CCL11 in numerous types of cancers. TATE is associated with a good prognosis in gastrointestinal and head and neck cancers. They reduce tumor growth, induce recruitment and activation of T and NK cells, and promote cytotoxic activity via degranulation. They induce inhibition and normalization of tumor vessels, polarization to M1 phenotype, and maturation of DCs | ||
|
Neutrophils |
Pro‐tumor effects: N2 TANs promote tumor growth (through the production of growth factors and NE), cell invasion and migration, angiogenesis, and lymphangiogenesis (through the release of VEGFs, MMP‐9, and Bv8). They induce inhibition of T and NK cells, ETM, metastasis, Tregs recruitment, and chemoresistance. Neutrophilia is associated with a poor prognosis. High neutrophils/lymphocytes ratio in solid tumors is correlated with poor outcomes | 349–359 |
| Anti‐tumor effects: N1 TANs induce T‐cell activation by TGFβ inhibition, recruitment of pro‐inflammatory macrophages (M1), cytotoxicity through release of ROS and RNS, apoptosis (through the release of TRAIL), and inhibition of angiogenesis (through the release of the anti‐angiogenic VEGF‐A165b) | ||
|
Basophils |
Pro‐tumor effects: They stimulate angiogenesis through the production of VEGF‐A, VEGF‐B, angiopoietin 1, CXCL8, and HGF. They promote ETM by production of CXCL8 and TNFα, the recruitment of anti‐inflammatory macrophages (M2), and they induce ECM degradation and immunosuppression | 110,173,200,360–367 |
| Anti‐tumor effects: They have cytotoxic effects via granzyme B and TNFα. Histamine secretion promotes DCs maturation and inhibition of tumor growth |
Abbreviations: ADCC, antibody‐dependent cellular cytotoxicity; APCs, antigen‐presenting cells; Apo2L, apo2 ligand or TRAIL; Bregs, B regulatory cells; Bv8, prokineticin‐2 protein; CCL11, CC‐chemokine ligand 11; cDC1 s, conventional type‐1 dendritic cells; cDC2 s, conventional type‐2 dendritic cells; CTL, cytotoxic T lymphocytes; CTLA‐4, T‐lymphocyte‐associated protein 4; CXCL8, C‐X‐C motif chemokine ligand 8; DCs, dendritic cells; ECM, extracellular matrix; Fas/FasL, Fas receptor/Fas‐ligand; FGF‐2, fibroblast growth factor 2; GM‐CSF, granulocyte‐macrophage colony stimulating factor; HGF, hepatocyte growth factor; IDO, indoleamine 2,3‐dioxygenase; LAG‐3, lymphocyte activation gene‐3; MDSCs, myeloid‐derived suppressor cells; MHC I: major histocompatibility complex class I; MHC II, major histocompatibility complex class II; MMP‐9, metalloproteinase 9; moDCs, monocyte‐derived dendritic cells; N1, tumor‐associated neutrophils type 1; N2, tumor‐associated neutrophils type 2; NE, neutrophil elastase; NK, natural killer; NO, nitric oxide; Nrp1, neuropilin; PD‐1, programmed cell death 1; pDCs, plasmacytoid dendritic cells; RNS, reactive nitrogen species; ROS, reactive oxygen species; SCC, squamous‐cell carcinoma; TABE, tumor‐associated blood eosinophilia; TANs, tumor‐associated neutrophils; TATE, tumor‐associated tissue eosinophilia; TGFβ, transforming growth factor beta; TILs, tumor‐infiltrating lymphocytes; TIM‐3, T‐cell immunoglobulin and mucin domain‐3; TME, tumor microenvironment; TNBC, triple‐negative breast cancer; TNFα, tumor necrosis factor‐alpha; TRAIL, TNF‐related apoptosis‐inducing ligand; Tregs, T regulatory cells; VEGF, vascular endothelial growth factor; VEGF‐A165b, anti‐angiogenic isoform of vascular endothelial growth factor‐A; VEGF‐B, vascular endothelial growth factor‐B.
In the last decades, advances in tumor immunology contributed to shed light on the complex mechanisms regulating cellular immune responses during cancer progression. However, the dynamic relationship between the immune system and tumor cells, which determines the clinical outcome of the disease and how it is reshaped by cancer therapy, is far from being fully understood. New research is necessary to achieve tumor control using multidisciplinary approaches.
Histamine is considered one of the most important mediators that orchestrate inflammatory responses, and it plays a central role in numerous pathological conditions, including cancer [reviewed in Refs. [4, 7]).
Considering the important role of histamine‐producing immune cells in the TME, in this section,we summarize the most important immunomodulatory roles of histamine and histamine receptors in the context of cancer development and progression.
3.1. Effect of histamine on granulocytes and mast cells
Granulocytes are immune cells that have specialized granules in the cytoplasm that contain a wide variety of substance, which may include histamine, cationic proteins, defensins, heparin, proteolytic enzymes, cathepsin G, lysozyme, and myeloperoxidase, among others. The specific types of granulocytes traditionally include neutrophils, eosinophils, and basophils. Granulocytes and mast cells are produced in the bone marrow through hematopoiesis. The process of cell maturation and proliferation occurs in the bone marrow and requires approximately 7–12 days before their release into the bloodstream (circulating leukocytes) and their homing to different tissues (resident leukocytes).67
Hematopoietic cells including mast cells, eosinophils, basophils, DCs, and T cells express histamine receptors and their histamine‐induced activation produces numerous important functions during immune responses (Figure 1).
It is important to highlight that there are uncertainties around the specificity of the commercially available antibodies used to detect histamine receptors, considering the nonspecific binding effects that have been reported. Therefore, different approaches should be used when checking the specificity of an antibody that include: the use of cells with genetic knockdown of their expression, cells recombinantly expressing closely related receptor subtypes, and/or the use of various antibodies directed against different receptors’ epitopes.34, 68, 69, 70 The verification of the expression using other identifying techniques, including qRT‐PCR, RT‐PCR, in situ hybridization, northern blot, and ligand‐binding assays, is extremely important to assess the distribution of histamine receptor subtypes.
Numerous studies showed expression of the H1, H2, and H4 receptor but not of the H3 receptor in human granulocytes and mast cells, using techniques such as RT‐PCR, northern blot, immunofluorescence, and ligand‐binding assays.71, 72, 73, 74, 75, 76, 77 However, Hofstra et al found no H4 receptor expression in murine neutrophils evaluated by RT‐PCR.78
Neutrophils are the most abundant leukocytes in the human circulatory system and are the first responders in acute inflammation. They capture invading micro‐organisms through different mechanisms such as phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs).79 In addition, neutrophils play a pivotal role in chronic inflammatory diseases such as cancer.67 Although recent evidence suggests an important role of neutrophils in the TME, the pro‐ or anti‐tumor nature of neutrophils in different cancer types is still inconclusive80 (Table 1).
Recent studies have reported that histamine plays an important role in hematopoietic stem cell proliferation and neutrophil maturation.81 During inflammatory processes, neutrophils stimulate the production and release of histamine.82 Histamine seems to have anti‐inflammatory properties via the H2 receptor and cAMP formation, inhibiting activation of neutrophils and HL‐60 leukemic cells,83 leukotriene synthesis, and chemotaxis5, 7, 84, 85, 86, 87, 88, 89 (Figure 1).
Limited information about the immunomodulatory role of histamine in tumor‐associated neutrophils (TANs) is reported. By targeting NADPH‐oxidase via the H2 receptor on monocytes90 and neutrophils,91 histamine has been proposed as an anti‐phagocyte drug‐candidate with the ability to inhibit the formation and release of reactive oxygen species (ROS).92, 93 Thus, histamine treatment potentially improves the efficacy of the immunotherapy with IL‐2 for diverse oncological conditions by protecting the anti‐tumor immune effector NK and T cells from oxidative stress‐induced inhibition and apoptosis, as described in the following section.94 In vivo treatment with histamine and H4 receptor agonists (1 mg/kg daily s.c. administration for 30 days) reduced human 1205Lu melanoma tumor growth and neovascular formation while it decreased the neutrophil‐to‐lymphocyte ratio infiltrate.10
Eosinophils are granulocytes that develop during hematopoiesis in the bone marrow and are terminally differentiated after migrating into the blood. They have multiple functions, which include cytotoxicity, inflammatory processes, modulation of innate and adaptive immunity, and anti‐tumor responses. Eosinophilic leukocytes respond to different antigenic stimuli (helminths, virus, bacteria, fungi) as well as immunostimulatory ligands (MHC II, CD40, CD80, CD86) through different receptors. They are recruited by chemokines and their function is influenced by cytokines. Together with mast cells and basophils, they control mechanisms associated with allergy and asthma. Eosinophils are characterized by basic granules composed of cationic proteins, including eosinophil cationic protein, eosinophil‐derived neurotoxin, major basic protein (MBP), eosinophil peroxidase (EPO), hydrolytic enzymes, and a diverse repertoire of preformed cytokines, chemokines, and numerous growth factors.67
Histamine has a dose‐dependent effect on chemotaxis of eosinophilic granulocytes5, 95, 96 (Figure 1).
Tissue eosinophilia (also termed tumor‐associated tissue eosinophils, TATE) and peripheral blood eosinophilia (TABE) have been associated with both favorable and unfavorable anti‐tumor response and prognosis97, 98, 99, 100, 101, 102 (Table 1). Transcriptomic and proteomic analyses of TATE revealed an activated eosinophil phenotype associated with IFNγ signaling and suggest that these cells may be targets for immunotherapy.103
Mast cells and basophils play several roles in the innate and adaptive immune responses and are mediators of type I allergy.104, 105, 106 Although both immune cell types resemble in terms of morphology and functional properties, basophils arise and mature in the bone marrow and circulate in the bloodstream, whereas mast cells develop from a different precursor in the bone marrow and usually mature in the resident tissues (e.g., skin, lung, and gastrointestinal tract). Therefore, mast cell phenotype and maturation are influenced by the local microenvironment. The activation of the receptor for immunoglobulin E (FcεRI) in mast cells and basophils, which is triggered by the crosslinking with antigen‐specific IgE, results in the release of numerous inflammatory mediators in their granule content, which are responsible for the allergic reactions. The released mediators comprise histamine, lipid mediators, proteases, cytokines, and chemokines, which may act locally on other immune cells, vessels and/or smooth muscle.67, 104, 106, 107, 108
Mast cells and basophils are the major sources of histamine in healthy tissues, which is stored in specific cytosolic granules, and it is released in large quantities during degranulation following immunological or nonimmunological activation.85 Both granulocytic immune cells express H1, H2, and H4 receptors and histamine modulates their functions, including their ability to further degranulate94, 104, 106, 109 (Figure 1).
Infiltration of mast cells has been found in numerous types of human tumors and experimental cancer models, and it was associated either with a good or a poor prognosis depending on the cancer type, tissue localization, and the ability of mast cells to interact with TME.110, 111, 112 Histamine and other secreted mediators could promote invasion and angiogenesis by shaping the TME and inducing stromal remodeling and capillary permeability112 (Table 1).
The role of histamine in the TME is complex as it can exert different immunobiological effects through the four histamine receptor subtypes.7, 113, 114, 115 The human leukemia cell line HMC‐1 expresses H1, H2, and H4 receptors evidenced by RT‐PCR and western blot, and moderate effects of H1 receptor and H2 receptor antihistamines are observed on the secretion of proinflammatory cytokines IL‐6, IL‐8, and TNFα.5 It has been recently demonstrated that the treatment with mast cell mediators exert opposite effects on the proliferation of YAC‐1 and EL4 cell lines, both derived from murine T cell lymphomas, but of different origin. The result of the co‐administration of histamine receptor antagonists and mast cell mediators on these cancer cells suggested a major involvement of H2 receptor and H4 receptor in the growth inhibition in YAC‐1 cells. On the other hand, the enhanced cell growth in EL‐4 cells was mediated by H1, H2, and H4 receptors.116 In experimental models of non‐small‐cell lung cancer, a dual effect of mast cells has been described, as they enhance tumor growth in vitro but importantly, they exert anti‐tumorigenic effects in mice as it has been shown using the mast cell‐deficient mouse Sash model.117 In some cancer types, enhancing local mast cell degranulation may induce anti‐tumor immune mechanisms, which include the recruitment of effector cells, the direct impact of released mediators on tumor cells and the secondary effects on immune regulation.118, 119 In this regard, investigating the role of mast cells in different tumors will improve the knowledge and further identify potential mechanisms involved in the paradoxical role of mast cells in the TME.
Basophils are the less abundant peripheral blood leukocytes and are key players in Th2 immune responses and allergy.120 Limited information about basophils’ role in cancer is available. Recent data show that they can be recruited into the TME by several chemotactic factors secreted by tumors or immune cells, including VEGFs, histamine, prostaglandin D2 (PGD2), urokinase plasminogen activator (uPA), and chemokines. Marked basophilia represents a relevant independent prognostic variable in chronic myeloid leukemia (CML).121 Recent evidence suggests that basophils may be a useful predictive or monitoring marker for the development of hypersensitivity against oncological treatments. In addition, the activation of basophils may be associated with improved outcomes for ovarian cancer patients.122
3.2. Effect of histamine on lymphocytes
Lymphocytes consist of three major groups: T cells, B cells, and NK cells. The major players in adaptive immunity are T and antibody‐producing B cells, which develop in the thymus and bone marrow, respectively, whereas NK cells are part of the innate immunity.123, 124 It is well‐documented that histamine through different receptor subtypes plays an important role in the modulation of lymphocytes during immune responses and inflammatory reactions5, 85, 88 (Figure 1).
T lymphocytes are one of the most powerful immune cells against cancer and they have been a major target of immunotherapy, which has emerged as a breakthrough in cancer therapeutics. CD4+ T cells, including Th1, Th2, Th17, and Tregs (CD4+CD25+ regulatory T cells) together with CD8+ cytotoxic T cells are extremely important mediating anti‐tumor immunity (Table 1). A positive correlation between the presence of tumor‐infiltrating lymphocytes (TILs) and patients’ survival has been demonstrated in numerous types of cancer.125, 126, 127
Jutel et al demonstrated through RT‐PCR and flow cytometry assays that H1 and H2 receptors are predominantly expressed in Th1 and Th2 cells, respectively.128, 129 mRNA expression studies confirmed the expression of H1, H2, and H4 receptors whereas H3 receptor mRNA was absent in CD8+, CD4+, and Th17 T cells.130, 131, 132 The expression of H2 receptor in Tregs from healthy subjects and patients with allergic rhinitis (AR) was demonstrated by flow cytometry.133 Numerous studies evaluate the important role of histamine receptors using functional assays114, 115, 133, 134, 135, 136, 137, 138 (Figure 1).
Systemic treatment with histamine (10 mg/kg, twice a day for 21 days beginning the day of tumor implantation) increased Colon 38 tumor growth implants in syngeneic mice by an indirect effect associated with a reduction in the anti‐tumor cytokines expression in the TME, dysregulating the balance between Th1 and Th2 cells.139 Reynolds et al reported the levels of histamine content in 31 colorectal cancer specimens and indicated that they were sufficient to inhibit lymphocyte activity.140 Lactobacillus rhamnosus‐derived histamine promotes a regulatory Foxp3‐T cell response profile in intestinal Peyer patches while altering Th1 polarization through the H2 receptor.141
The infiltrating cytotoxic cells, mainly CD8+ T lymphocytes and NK cells, are responsible for killing cancer cells. Therefore, immunosuppressive cells’ infiltrate such as Tregs and MDSCs, is usually associated with a worse prognosis in cancer patients.
Tregs are a subset of CD4+ T cells characterized by their expression of a master transcription factor forkhead box P3 (FoxP3), which is essential for Tregs’ differentiation and function. They play a central role in the maintenance of self‐tolerance, homeostasis, and resolution of inflammation through the suppression of the T‐cell population, including both CD4+ and CD8+ T cells, DCs, B cells, natural killer T (NKT) cells, Th17 cells, NK cells, monocytes, and macrophages by the secretion of suppressive cytokines like IL‐10 and TGFβ, and the expression of the inhibitory surface molecules LAG‐3, TIM‐3, PD‐1, and CTLA‐4.142, 143
In the TME, Tregs are one of the major immune cell types involved in the suppression of anti‐tumor immunity, promoting tumor immune evasion (Table 1). Histamine and its receptor ligands are capable of modulating the activity of Tregs in many pathological processes like allergies, autoimmune and inflammatory diseases, and even in various types of cancer (Figure 1). It was shown that histamine released by mast cells reduced the expression of CD25 and the Tregs‐specific transcription factor Foxp3 and inhibited Tregs’ suppressor function, enhancing the development of protective immunity. These effects were mimicked by the H1‐receptor‐specific agonist 2‐pyridylethylamine and were reversed by loratadine.136 On the other hand, several studies indicate that the immunosuppressive activity of Tregs in allergy and asthma is increased through the activation of the H2 receptor.144, 145 In line with those results, cimetidine, a H2 receptor antagonist, reduces the regulatory T‐cell‐mediated immunosuppression.84, 146, 147
In the tumor context, a reduction in the percentage of splenic Tregs was found in histidine decarboxylase‐deficient mice compared to wild‐type (WT) mice bearing syngeneic mammary‐adenocarcinoma LM2 tumors. The lack of histamine upregulated splenic T‐bet+ lymphocytes and the IL‐12/IFNγ production.148
Recently the role of the H4 receptor in the anti‐tumor immunity was described for the first time, using H4 receptor deficiency or pharmacological blockade in the experimental murine model of triple‐negative breast cancer (TNBC) developed by orthotopic inoculation of 4T1 cells. The effect of systemic treatment with histamine (1 or 5 mg/kg daily s.c. administration for 15 days, starting when tumors became palpable) or specific H4 receptor pharmacological ligands JNJ7777120 (H4 receptor antagonist, 10 mg/kg daily s.c. administration for 15 days, starting when tumors became palpable) and JNJ28610244 (H4 receptor agonist, 1 or 5 mg/kg daily s.c. administration for 15 days, starting when tumors became palpable), on tumor progression and immune response was evaluated. Histamine (5 mg/kg) reduced tumor weight, an effect that was inversely correlated with the presence of TILs. Histamine, used even in a lower concentration (1 mg/kg), was able to enhance the therapeutic effect of ionizing radiation, suggesting that it could be a potential agent to be used in combined therapies. The higher anti‐tumor and antimetastatic effects of histamine treatment compared with H4 receptor agonist's administration could be associated with the multifaceted action of histamine on different receptors and cell types, which on the one hand balanced anti‐tumor immunity and on the other hand, by acting directly through the H4 receptor on 4T1 tumor cells, reduced proliferation (Figure 2). The administered doses of the H4 receptor's agonist conditioned the outcome of its therapeutic and immunomodulatory effects in vivo. The lowest concentration (1 mg/kg) slightly, but significantly, reduced the tumor size and increased the percentage of CD4+ T cells in the tumor‐draining lymph nodes (TDLN), whereas a concentration of 5 mg/kg did not change the tumor weight probably due to an immunosuppressive effect on the TME (Figure 2). The treatment with the H4 receptor antagonist led to a reduced proportion of tumor‐infiltrating CD4+ T cells and Tregs in the TDLN, as it was observed in H4 receptor‐deficient mice (H4 receptor‐KO)114, 149 (Figure 2). H4 receptor‐KO mice showed reduced tumor growth and lung metastases, and CD4+ T cell tumor infiltration, while they exhibited a greater infiltrate of NK cells and CD19 + B lymphocytes compared to tumors developed in WT mice. The TDLN of the H4 receptor‐KO mice showed decreased percentages of the CD4+ T cells and Tregs subpopulations together with a higher percentage of NK cells.
FIGURE 2.
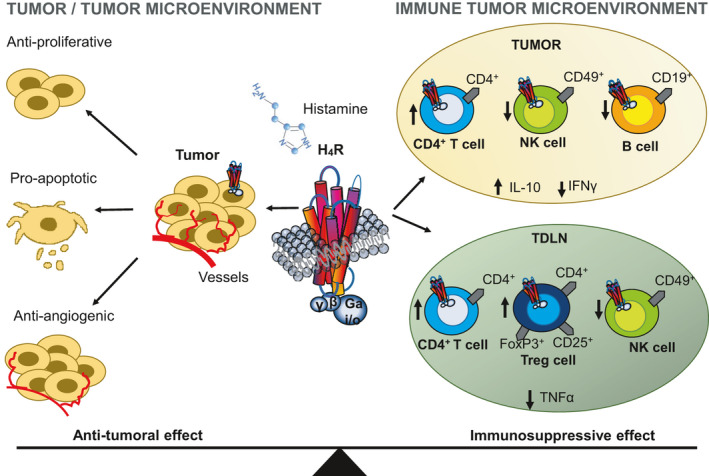
Effect of H4 receptor activation in tumor cells and the tumor microenvironment (TME). Histamine or selective H4 receptor agonists play important roles at a variety of stages during tumor development and in multiple cell types including cancer and immune cells. On the one hand, H4 receptor activation exerts a direct in vitro cytotoxic effect on TNBC cells, whereas on the other the H4 receptor selectively affects the distribution of different immune cell populations in the TME, modulating the local and systemic immune responses. In a TNBC murine model, H4 receptor stimulation increases the percentage of CD4+ tumor‐infiltrating T cells, whereas it decreases the infiltration of NK cells and CD19+ B lymphocytes. In addition, it increases IL‐10 secretion levels, whereas decreases IFNγ levels in tumor‐conditioned medium from wild‐type (WT) mice. Likewise, tumor draining lymph nodes (TDLN) of WT mice show higher proportions of CD4+ T cells and T regulatory cells (CD4+ CD25+ FoxP3+), a reduced percentage of NK cells, and decreased TNFα levels in TDLN compared with H4 receptor‐KO mice, thus suggesting an immunosuppressive effect of H4 receptor114, 149
In another model of breast cancer developed in BALB/c mice with LM3 cells (ErbB‐2 positive), the percentage of Tregs decreased significantly in TDLN from H4 receptor‐deficient animals, demonstrating that in both breast cancer models the H4 receptor exhibits an immunosuppressive effect, particularly modulating the compartment of CD4+ T lymphocytes.114, 115, 149 In line with these results, intratracheal instillation of the H4 receptor agonist 4‐methylhistamine (10 µg/100 µl) mitigated airway hyperreactivity and inflammation of allergic asthma in a murine model through increasing IL‐10 secretion levels and the recruitment of Tregs.134 Additionally, in an experimental allergic encephalomyelitis model, H4 receptor‐KO mice showed a lower proportion of Tregs in secondary lymphoid organs compared to WT mice, which increased the severity of the disease.150 In a phase IV trial, patients with acute myeloid leukemia (AML) who received immunotherapy with histamine dihydrochloride and IL‐2 during the initial cycles showed an increase in the peripheral blood Tregs’ count151 (Table 2). Furthermore, it was recently demonstrated that the number and size of tumors and the degree of colonic inflammation, associated with the expression of cyclooxygenase 2 and the production of C‐X‐C motif chemokine ligand 1 (CXCL1) and CXCL2, are reduced in H4 receptor‐deficient mice compared to WT mice in a chemically induced colorectal cancer model.152
TABLE 2.
Clinical trials with histamine or histamine receptor's ligands and immunotherapy
| Trial [references] | Phase | Disease | Patients (N) | Treatment | Drug indication | Recruitment status |
|---|---|---|---|---|---|---|
| NCT00005038 (*) | II | Kidney cancer | 60 | IL‐2 (Aldesleukin) + histamine dihydrochloride (HDC) | Aldesleukin s.c. once daily and HDC s.c. twice daily (b.i.d.) on days 1–5 of weeks 1–3 followed by 2 weeks of rest | Unknown |
| NCT00003991 [231, 232, 239] | III | Leukemia | 360 | Aldesleukin + HDC | Following consolidation chemotherapy or autologous stem cell transplantation, patients received Aldesleukin (16,400 IU/kg s.c. b.i.d.) followed by HDC (0.5 mg s.c.) over 5–7 min b.i.d. on days 1–21. Treatment was repeated every 6 weeks for 3 courses and then every 9 weeks for 7 courses in the absence of disease relapse or unacceptable toxicity | Completed |
| NCT01347996 [151, 201, 214, 230, 234, 235, 237, 238] | IV | Acute myeloid leukemia | 84 | HDC (Ceplene®) + IL‐2 | Ceplene® (0.5 mg s.c. b.i.d.) and IL‐2 (1 µg/kg or 16,400 IU/kg b.i.d. for 21 day‐cycle followed by 21 days of rest | Completed |
| NCT03040401 (*) | I/II | Chronic myelomonocytic leukemia | 15 | Ceplene® + IL‐2 (Proleukin®) | Ceplene® and/or Proleukin® s.c. b.i.d. in 3‐week periods followed by 3‐ or 6‐week rest periods | Unknown |
| NCT00039234 (*) | III | Melanoma (skin), metastatic cancer | 224 | Aldesleukin + HDC | Aldesleukin s.c. b.i.d. on days 1 and 2 of weeks 1 and 3 and days 1–5 of weeks 2 and 4. Patients also received HDC s.c. over 10–30 min on days 1–5 of weeks 1–4 | Active, not recruiting |
| NCT00002733 (*) | II | Kidney cancer, melanoma (skin) |
20–30 with melanoma 20–30 with renal cell carcinoma |
TILs + cimetidine | TILs infusion once followed by oral cimetidine every 6 h for 4 weeks | Completed |
| NCT04165096 | II | Non‐small‐cell lung carcinoma | Estimated Enrollment: 90 participants | MK‐5890 + pembrolizumab + Diphenhydramine + acetaminophen | On day 1 of each 3‐week cycle, participants receive pembrolizumab 200 mg intravenously (i.v.) plus MK‐5890 i.v. for a maximum of 35 cycles (approximately 2 years). All participants are premedicated 1.5 h (±30 min) before infusion of MK‐5890 with 50 mg oral diphenhydramine (or equivalent dose of anti‐histamine), and 500–1000 mg of oral acetaminophen (or equivalent dose of analgesic) | Recruiting |
Twice daily (b.i.d.), tumor‐infiltrating lymphocytes (TILs), aldesleukin (IL‐2), histamine dihydrochloride (HDC), Ceplene® (histamine dihydrochloride), Proleukin® (IL‐2), MK‐5890 (anti‐CD27), pembrolizumab (anti‐PD‐1 immune checkpoint blocking antibodies), diphenhydramine (H1 receptor antagonist), intravenously (i.v.). (*) Dosage is not available.
B cells are recognized as the main effector cells of humoral immunity because of their ability to produce antibodies (immunoglobulins, Ig). The naïve mature B cells differentiate into activated B cells after the first encounter with the antigen, thus proliferating and becoming plasma cells, which produce and release antibodies. They can be classified according to their location and how they are activated.153 Regulatory B cells (Bregs) can inhibit T‐cell‐mediated immunity and are characterized by producing inhibitory cytokines such as IL‐10, IL‐35, or TGFβ.154, 155
The tumor‐infiltrating B cells exert both pro‐tumor and anti‐tumor effects depending on their phenotype, the antibodies and cytokines that they produce, and the composition of the TME (Table 1).
Histamine can affect B‐cell Ig production (Figure 1). Colorectal cancer patients treated with cimetidine (8.8 or 1.2 g per day oral administration from the day of admission to the 10th postoperative day) showed elevated levels of CD19+ B cells in blood samples, which was associated with an improved local immune response.156 In line with these results, a recent study demonstrated that treatment with ranitidine, a H2 receptor antagonist, (8 mg/kg added to drinking water 1 day prior to tumor cell injection and during 21 days) enhanced anti‐tumor antibody responses and reduced tumor growth in murine models of breast cancer developed with E0771‐GFP and 4T1 cell lines, effects that were mediated by B cells and may have included the participation of NK cells.157
Natural killer cells are effector lymphocytes that play a crucial role in the defense against viruses and the surveillance of tumor insurgence. Activation of NK cells in the TME can contribute to anti‐tumor immunity through various mechanisms (Table 1).158, 159 Damaj et al evaluated the expression of histamine receptors by immunoblot analysis and staining with anti‐histamine receptors’ antibodies and flow cytometry, and showed that NK cells, monocytes, and dendritic cells express the H1 and H4 receptors but not H2 and H3 receptors.160
Treatment with histamine enhanced IL‐2 and IFNα induced NK cell‐mediated killing of human tumor cells in vitro and in tumor‐bearing mice by inhibiting phagocyte‐derived ROS.161, 162 However, the benefit of histamine does not apply to all tumors and depends on its type and origin.163 Degranulating mast cells at tumor sites can also augment NK cell function via histamine release.113 These findings are the fundamental rock for the use of histamine as an adjuvant to cancer immunotherapy, which is described in the next section.
3.3. Effect of histamine on dendritic cells
Dendritic cells (DCs) are a heterogeneous population of migratory leukocytes that play a fundamental role in the induction and regulation of innate and adaptive immunity. They are crucial as professional antigen‐presenting cells (APCs), activating CD8+ and CD4+ T cells through MHC I and MHC II molecules, respectively, and providing a wide variety of fundamental signals (costimulatory molecules and cytokines) to shape the immune response.164, 165 Three subsets of DCs have been described with specific functions, morphology, and location: conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte‐derived DCs (moDCs). cDCs phagocytose debris from apoptotic tumor cells, and they migrate to TDLN where they present these antigens to naïve CD4+ or CD8+ T cells (Table 1).
In both mature and immature DCs, expression of all histamine receptors has been demonstrated by RT‐PCR.166, 167, 168 However, the authors were not able to evaluate the expression of the H3 and H4 receptors by western blot and flow cytometry using commercially available polyclonal rabbit antibodies.167, 169 The studies investigating the H3 receptor mRNA expression in MoDCs are controversial. Some of them detected mRNA presence167, 169 whereas others found only a faint170 or no signal.171 Thus, both endogenous and exogenous histamine may influence not only the expression of surface markers but also the function, differentiation, and maturation of DCs.5, 172, 173
Histamine increases the capacity of DCs to induce the polarization of naïve CD4+ T lymphocytes into predominantly Th2 lymphocytes through H2 receptor‐mediated chemotaxis.174, 175 On the other hand, Vanbervliet et al showed in a murine model of atopic dermatitis, a significantly reduced antigen‐specific skin inflammation and diminished IL‐12 and increased IL‐23 and IL‐6 production by DCs in H1 receptor‐deficient mice compared to WT mice.176 Martner et al, demonstrated that the treatment with histamine (75 mg/kg i.p. three times a week for 2 weeks) reduced the growth of murine EL4 lymphomas while increased tumor‐infiltrating DCs in WT mice but not in NADPH oxidase type 2 (NOX2)‐deficient mice. A positive correlation between accumulation of intra‐tumoral DCs and CD8+ T cells paralleled with a reduced tumor size.173
3.4. Effect of histamine on monocytes and macrophages
Monocytes play an important role in the immune defense, inflammation, and homeostasis by sensing their local environment. They circulate in the blood and migrate to inflammatory tissues and differentiate in response to different stimuli into macrophages and monocyte‐derived dendritic cells (moDCs). Macrophages can be divided into two main groups designated M1 and M2, which can be identified by cell surface markers and their functional phenotype. M1 macrophages play a critical role in the innate defense of the host and tumor destruction. M2 macrophages have been found to participate in biological processes of angiogenesis, tissue remodeling, wound healing, and anti‐inflammatory responses.177, 178 During tumor development and progression through the metastatic cascade, macrophages are involved in shaping the primary, micro‐invasive, and premetastatic TMEs.179 Tumor‐associated macrophages (TAMs) include both M1 macrophages that harbor anti‐tumor effector functions and M2 macrophages that express tumor‐promoting and immunosuppressive factors (Table 1).179, 180
Several authors have reported that both monocytes and fully differentiated macrophages express histamine receptors, particularly H1, H2, and H4 receptors.88, 181, 182, 183, 184 However, others found no evidence of H1 and H4 receptor expression in human monocytes.184, 185 Histamine stimulates the exocytosis and the cytokine production in human lung macrophages via the H1 receptor while increasing phagocytosis by its signaling through the H2 receptor.186, 187 In both bone marrow‐derived macrophages and RAW 264.7 cells, histamine is capable of promoting macrophage differentiation and induces chemotaxis and phagocytic activity by the activation of the H4 receptor188, 189 (Figure 1). Furthermore, during in vitro differentiation from monocytes to macrophages, the H4 receptor agonist ST‐1006 modified the M1 phenotype by upregulating the macrophage differentiation marker CD68 and downregulating the production of CXCL10.182
Cimetidine treatment (400 mg twice daily, given as infusion or tablets depending on the postoperative condition) of patients with gastrointestinal cancer resulted in a better prognosis by increasing the release of the anti‐tumor cytokine IL‐18 from monocytes.190, 191 Although oral ranitidine, another H2 receptor antagonist (8 mg/kg added to drinking water 1 day prior to tumor cell injection, refreshed every other day), did not affect tumor growth in the B16‐F10 melanoma, LLC1 lung cancer, and EL4 thymoma experimental models, it consistently reduced primary tumor growth and metastasis in the breast cancer models E0771 and 4T1, respectively. Ranitidine affects monocyte populations in breast cancer, providing a reduction of tumor‐associated immune suppression.192 In addition, the simultaneous inhibition of the H1 receptor (mepyramine, 50 µM oral administration during treatment with dextran sulfate sodium, DSS) and the stimulation of the H2 receptor (cimetidine, 100 µM oral administration during DSS treatment) signaling pathways were described to effectively suppress the pro‐inflammatory signaling in macrophages, reducing the inflammation‐associated colonic tumorigenesis.11 In this response, the described mechanisms of H1 and H2 receptors’ cross‐regulation should be considered, including the cross‐desentization and cross‐internalization, which could have potential therapeutic implications in combined treatments.38
3.5. Effect of histamine on myeloid‐derived suppressor cells
Myeloid‐derived suppressor cells (MDSCs) are one of the major components of the TME and are characterized by their potent immunosuppressive activity. MDSCs are immature myeloid cells that are precursors of DCs, macrophages, and granulocytes. They are generated in the bone marrow and migrate to tumors and peripheral lymphoid organs to contribute to the formation of the TME, being the main contributors to immune dysfunction observed in cancer patients (Table 1).193, 194, 195 The accumulation of immature myeloid cells and the deficit of mature DCs is associated with increased tumor growth and poor prognosis in human and murine cancers.173, 196, 197, 198
Histamine can regulate myeloid cell differentiation199 (Figure 1). Increased inflammation‐associated carcinogenesis was observed in histamine‐deficient mice, which were associated with decreased myeloid cell differentiation and accumulation of CD11b+Gr1+ immature myeloid cells. The treatment with exogenous histamine (0.8 mg/kg i.p. per day for 20 days) induced their terminal differentiation into monocytes and neutrophils, acting through H1 receptor and H2 receptor, and suppressed their ability to support the growth of tumor allografts.200 Adoptive transplant of histidine decarboxylase‐deficient bone marrow to WT mice reproduced the cancer phenotype of histidine decarboxylase‐KO mice, associated with an increase in CD11b+Ly6G+ cell mobilization.200
Accordingly, Grauers Wiktorin et al showed in vivo that the treatment with histamine (75 µg/mouse i.p. three times a week starting 1 day before tumor inoculation) diminished tumor growth and the accumulation and immunosuppressive features of MDSCs in EL4 lymphoma. Histamine also improved the anti‐tumor efficacy of immune checkpoint blockade with anti‐PD‐1/anti‐PD‐L1 (100–240 µg/mouse of each antibody, i.p. starting 3, 6, and 10 days after tumor inoculation) in the murine EL4 lymphoma and MC‐38 colon carcinoma.201 The counts of MDSCs in blood samples from patients with AML significantly predicted leukemia‐free survival (LFS). Interestingly, their frequency and absolute counts were significantly reduced during treatment with histamine and IL‐2.201
In this line, Gao et al reported that the administration of L. reuteri, a histamine‐producing member of the gut microbiota, protects histidine decarboxylase‐deficient mice from colon carcinogenesis induced with azoxymethane/DSS, by reducing the recruitment of MDSCs and the production of inflammatory cytokines.202 Another lactobacilli, L. rhamnosus, is a source of histamine that promotes a Tregs response profile in intestinal Peyer patches.141, 203 The microbial community in the intestine is indeed an important determinant of the gut pathophysiology and its unbalance may produce other consequences outside the gastrointestinal tract. Histamine‐secreting microbes are present within the human gut microbiota and they may modulate host immunological responses.203, 204 The microbiome, which not only includes gut bacteria but also skin bacteria, and other resident microorganisms is an emerging area of research. Studies suggest that microbiome impacts both the development and progression of cancer as well as patient responses to cancer treatments, including immunotherapy.205, 206 Recent data show that each tumor type has a distinct microbiome composition and intratumor bacteria are present mostly intracellularly in both cancer and immune cells.206 Further studies are needed to unravel whether the tumor microbiome could be another source of histamine involved in tumor and TME interaction.
4. HISTAMINE AS AN ADJUVANT TO CANCER IMMUNOTHERAPY
Immunotherapy comprises a series of agents designed to stimulate the immune system in order to develop a tumor‐specific immune response to eradicate cancer. Cutting‐edge immunotherapies include immune checkpoints blockade, adoptive T cellular therapies, chimeric antigen receptor T‐cell immunotherapy, oncolytic viruses, and cancer vaccines. In particular, immunotherapy with immune checkpoint inhibitors using CTLA‐4, PD‐1, and PD‐L1 neutralizing or blocking antibodies is a promising and rapidly growing field of interest with impressive success in many solid tumors.207, 208, 209, 210 It seeks to unleash anti‐tumor T‐cell responses by avoiding host immunotolerance, and results in durable clinical responses but only in a fraction of patients.211, 212
Immunotherapeutics such as IL‐2, and interferons (IFN), among others, have been used as options for the treatment of certain cancers such as metastatic malignant melanoma, AML, and renal cell carcinoma.213, 214 The basis for the anti‐tumor effects of these cytokines is correlated with their ability to activate elements of the immune system that recognize and destroy tumor cells. NK cells and a subset of T lymphocytes are among the principally activated cells. However, these agents show not sufficiently optimal results in terms of effectiveness and the development of adverse effects.215, 216 When administered in addition to IL‐2, histamine dihydrochloride improves the activation of T cells and NK cells, controlling tumor growth of various cancers. This combination therapy appears to be a useful maintenance therapy alternative for patients with AML in remission. Table 2 summarizes the most important clinical trials.
The pharmacokinetic properties of subcutaneous histamine administration (1 mg) as well as the drug–drug interactions with subcutaneously administrated IL‐2 (1.1 mg) were evaluated in a clinical study with healthy volunteers and cancer patients. Pharmacokinetic parameters showed a high inter‐individual variability. In healthy subjects, the administration of histamine for more than 10 min revealed a maximum plasma concentration peak at 18 min (C max, 38 nmol/L), a distribution volume of 59 L and an elimination rate of 6%/min. Similar results were observed in a 20‐min infusion in melanoma patients. There was no effect on histamine kinetics when IL‐2 was injected either 10 min prior to or 10 min following histamine administration.217, 218, 219 A phase I study showed no severe adverse events upon a single dose of histamine (0.5 or 1.0 mg) subcutaneous injection in healthy volunteers. The administration of a histamine dose of 0.5 and 1 mg showed a time to C max (T max) of 0.15 and 0.14 h, a mean C max of 26.59 and 71.01 nmol/L, area under the plasma concentration–time curve from time zero to the last sampling (AUC 0–∞) of 9.61 and 22.69 nmol/h/L, maximum urine excretion rates of 21.85 and 38.94 nmol/h, respectively.220
In this section,we highlight the current progress and foundational developments in the field of cancer immunotherapy in combination with histamine and pharmacological compounds targeting histamine receptors.
4.1. Leukemia and lymphoma
The initial treatment for leukemia comprises the induction and consolidation chemotherapy aimed at inducing and sustaining the disappearance of leukemic cells (complete remission, CR). Several immunotherapies have been developed to prevent relapse, including the administration of a low‐dose of IL‐2 in combination with histamine dihydrochloride (HDC/IL‐2) for the treatment of AML.220, 221, 222, 223, 224, 225
As compared to IL‐2 as a single agent, the use of histamine, acting specifically through the H2 receptor, restored the IL‐2‐induced destruction of AML blasts by preventing the inhibition of the cytotoxic lymphocytes induced by monocyte‐derived ROS, and enhancing the accumulation of CD25+ T cells in peripheral blood. In five patients with early relapse, the remission duration after the treatment with HDC/IL‐2 (0.9 MIU IL‐2 s.c. twice daily, and 0.4–0.7 mg HDC s.c. twice daily, in cycles of 21 days and separated by 6‐week intervals) has in each case exceeded that of previous remissions.225 The effect of famotidine was also investigated on the cytotoxic activity of peripheral blood mononuclear cells and TILs. Both the cytotoxic activity and DNA synthesis of activated TILs were increased by the combination of IL‐2 and famotidine, effects that were independent of a decrease in the suppressor T‐cell population.226
Patients with AML receiving post consolidation immunotherapy with HDC/IL‐2 displayed enhanced efficacy in terms of relapse prevention and overall survival (OS) in patients with CR.227, 228, 229, 230, 231, 232, 233 Nevertheless, the treatment did not affect LFS or OS in patients who required more than one cycle of induction to attain CR and was not significantly beneficial in older patients (>60 years old). Statistical analyses confirmed the consistency of the HDC/IL‐2 effects compared with untreated patients (Table 2).232
Treatment with HDC/IL‐2 aims at targeting the formation of immunosuppressive ROS produced by the NOX2 enzyme of myeloid cells (HDC component), while concomitantly activating and expanding populations of NK cells and T cells (IL‐2 component) (Table 2).214 These components act in synergy to promote the NK‐ and T‐cell function and viability demonstrated in vitro, and also synergize to inhibit tumor growth in animal models. Some studies suggest that the combined treatment activates a pool of otherwise hyporesponsive, unlicensed NK cells to exert anti‐leukemic activity and reduces MDSCs in blood of AML patients in CR (Table 2).151, 201 Furthermore, Tregs, eosinophil, and NK cell counts were markedly increased in the blood of patients, whereas the absolute counts of CD8+ T cells were not altered (Table 2).214, 225, 234, 235 In particular, a threefold increase in CD56 bright NK cells was observed upon combined treatment in AML patients after chemotherapy.183 In another clinical trial, treatment with HDC/IL‐2 resulted in a blood expansion of CD56bright and CD16+ NK cells, together with an increase in the expression of the natural cytotoxicity receptors (NCR) NKp30 and NKp46 in NK cells, mainly in older patients, being a predictor of LFS and OS (Table 2).214, 236, 237 In contrast, the counts of DCs, neutrophils, and monocytes, principally the two major monocyte populations in blood CD14++CD16− (CD14+) and CD14+CD16+ (CD16+), were reduced during the first treatment's cycle.238 This combined treatment also induced a significant increase in the frequency of T effector cells, only in older patients (Table 2).235 Additionally, it significantly improved LFS and OS of younger AML patients (<60 years) with normal karyotype versus control. These results imply that the clinical benefit of HDC/IL‐2 in AML is pronounced in patients harboring leukemic cells of normal karyotype, especially in NPM1‐mutated AML patients (Table 2).230
Post hoc analyses of efficacy in morphological subtypes of AML among patients participating in the HDC/IL‐2 phase III trial, showed a nonsignificant trend toward improvement of LFS in those patients with M0/M1 (undifferentiated/minimal maturation) AML versus controls. No benefit for the treatment was observed in M2 (myeloblastic) AML, whereas HDC/IL‐2 significantly improved LFS among patients with M4/M5 (myelomonocytic/monocytic) AML (Table 2). Interestingly, M4/M5 cells, but not M2 cells, expressed H2 receptors and produced ROS that induced apoptosis in adjacent NK cells, effects that were inhibited by HDC. Therefore, the expression of the H2 receptor could determine the effectiveness of histamine‐based immunotherapy.231, 239 The expression of H2 receptor was significantly enhanced in CD14++ monocytes during and between treatment cycles, as well as in CD16+ monocytes during the first HDC/IL‐2 treatment cycle. A high H2 receptor expression in both monocyte types could better predict LFS and OS (Table 2).238, 239
On the basis of the results of three completed clinical trials, the treatment of immunotherapy with low‐dose IL‐2 and histamine dihydrochloride was approved for relapse prevention in AML patients within the European Union.201, 231, 234, 239
The development of immunotherapies for lymphoma has undergone a revolutionary evolution over the past decades. Since the first successful immunotherapy with rituximab (monoclonal antibody) for the treatment of B‐cell non‐Hodgkin lymphoma, a plethora of new immunotherapeutic approaches has ensued.240, 241
Preclinical studies show that histamine administration (1500 µg/mouse i.p. injection three times a week starting 1 day before tumor inoculation) enhanced the efficacy of anti‐PD‐1/anti‐PD‐L1 (100–240 µg/mouse of each antibody, 3, 6 and 10 days after tumor inoculation) in reducing EL4 tumor growth developed in C57BL/6J mice. Although the treatment did not affect the intra‐tumoral proportion of MDSCs, or T and NK cells, it slightly increased the fraction of CD8+ T cells displaying an effector phenotype. Treatment of EL4 tumor‐bearing mice with histamine did not alter the expression of PD‐L1 on MDSCs or PD‐1 on CD8+ T cells.201 A clinical trial was carried out in patients with high‐grade non‐Hodgkin lymphoma who received repeated cycles of IFNα, IL‐2, and histamine [3 million international units (MIU) IFNα, 1.5 mg/kg IL‐2, and 0.5 mg histamine, s.c. 1–2 times daily administration, 5 days a week] following relapse and high‐dose chemotherapy with stem cells demonstrate that combined immunotherapy induced significant increases in the frequency of cytokine‐producing T cells and in NK‐cell‐mediated cytotoxicity, as well as a reduction in the count of CD8+ T cells that remained low during the posttreatment observation period.242
A switch of histamine receptor expression from H2 to H1 during the differentiation of monocytes into macrophages is observed in the promonocytic U‐937 cell line (derived from a histiocytic lymphoma).181 The role of cAMP pathways has been well established in hematological malignancies. Elevation of intracellular cAMP using cAMP analogs induces cell cycle arrest, cell differentiation, or apoptosis in leukemia and lymphoma cell lines.243, 244 Although histamine or H2 receptor agonists increased cAMP levels, they failed to promote U‐937 cells’ differentiation due to rapid homologous and GRK2 dependent desensitization of H2 receptors.245 To further complicate the scene, the H2 receptor agonist, amthamine, increased intracellular cAMP levels while concomitantly augmented cAMP efflux regulated by multidrug resistance‐associated proteins (MRPs), particularly MRP4 in U‐937 and other AML cell lines.246, 247
Therefore, the beneficial anti‐tumor effects of histamine in hematological malignancies could not only involve the H2‐receptor‐mediated counteraction of the ROS‐induced immunosuppressive signals from monocytes/macrophages but also a direct anti‐proliferative action via the H2 receptor expressed in tumor cells, which might further contribute to reach tumor control.
4.2. Kidney cancer
The most common subtype of kidney cancer arises from the renal epithelium and is called renal cell carcinoma (RCC). Histamine and its receptor ligands have been tested in several clinical trials, although many of the results have been inconclusive and controversial. Donskov et al, have studied the effectiveness and safety of histamine dihydrochloride in combination with low‐dose IL‐2 and IFNα (1 mg HDC s.c., b.i.d. days 1–5, weeks 1–4, and 3 MIU IFNα s.c., once daily for 1 week, followed by up to nine 4‐week cycles of 3 MIU IFNα s.c., days 1–7, weeks 1–4, and IL‐2, 2.4 MIU/m2 s.c., b.i.d., days 1–5, weeks 1 and 2) in patients with metastatic RCC. Although histamine was well tolerated, it does not seem to add efficacy in the scheduled regimen.248 Using a similar treatment scheme, the same authors found positive correlations between the absolute number of peripheral blood lymphocytes and objective response.249 However, histamine did not influence TILs, blood leukocyte count, f‐chain expression, or cytotoxicity.250 Regardless of the histamine treatment (1.0 mg HDC, slow 20 min s.c. injection twice daily, concomitantly with 18 MIU IL‐2 s.c. once daily, 5 days per week for 3 weeks followed by 2 weeks’ rest), patients with high counts of monocytes and neutrophils in peripheral blood had a poor survival.94, 251 The combined treatment of IFNα and cimetidine (5 MIU IFNα per day, five times a week or 5 MIU IFNα intramuscular plus 2400 mg cimetidine oral daily administration), did not result in a significant improvement in the response rates compared with the IFNα monotherapy in a prospective randomized phase III trial conducted in patients with advanced RCC and pulmonary metastases.252
The combination of immunotherapies with H2 receptor antagonists, such as famotidine and cimetidine, have been further investigated. A phase II study showed that combined treatment of IFNα with cimetidine, cyclooxygenase 2 inhibitor meloxicam, and renin–angiotensin system inhibitor candesartan or perindopril (3–6 MIU s.c. thrice/week IFNα, 800 mg cimetidine, 10 mg meloxicam, and 4 mg candesartan or perindopril oral administration), provides favorable responses and low toxicological profiles in patients with advanced RCC.253 Combined treatment with IL‐2 and famotidine (9–21.6 MIU/m2 IL‐2 i.v. and 20 mg famotidine i.v. twice a day) in patients with metastatic RCC suggests some benefit of the combination but the results are not conclusive or significant, probably due to the small number of patients recruited.254, 255, 256, 257, 258
4.3. Melanoma
Advanced melanoma is a disease with a very poor prognosis. Dacarbazine and IL‐2 have been approved by the FDA for a long time to treat patients with metastatic melanoma. However, overall response rates are very low (16%).216 Recent studies have shown a significantly higher success rate with the combination of immunotherapy with chemotherapy or targeted molecular therapies. Treatment with nivolumab (anti‐PD‐1) in combination with ipilimumab (anti‐CTL‐4) was approved by FDA for melanoma patients with lymph node involvement.209, 259
Several clinical trials have been performed adding histamine or H2 receptor antagonists as an adjuvant to IL‐2 therapy for patients with metastatic melanoma.219 Quan et al reported clinical trials conducted from 2004 to 2012, in patients with metastatic melanoma who were treated with famotidine combined with IL‐2 in different treatment regimens (9–21.6 MIU/m2 IL‐2 i.v. and 20 mg famotidine i.v. twice a day). Even though the results of one study show that 25% of the patients (4) treated with the combination survived at least 20 months,260, 261 the mean survival of this and other regimens was 7–13 months.262, 263, 264, 265 Another study performed with 241 patients shows that the treatment with HDC/IL‐2/IFNα was safely administered on an outpatient basis (3 MIU IFNα s.c., once daily for 7 days, 2.4 MIU/m2 IL‐2 s.c., twice a day for 5 days, and 1 mg HDC s.c., twice a day for 5 days or dacarbazine 850 mg/m i.v. every 3 weeks), but this immunotherapeutic regimen did not improve the response rate or OS compared with dacarbazine.266
A significant increase in the production of IFNγ‐producing T lymphocytes was observed in patients with melanoma and liver metastases treated with HDC/IL‐2 (HDC 1 mg s.c. daily, b.i.d., IL‐2 9 MIU/m s.c., daily week 1, 3, and 7, IL‐2 2 MIU/m2 s.c., daily week 2 and 4) compared with those who received IL‐2 alone.267 Other clinical trials investigated the tolerance and response of the combination of IL‐2 and IFNα with different concentrations of HDC or cimetidine in patients with melanoma. Treatment regimens were safe and well tolerated. Most of them did not improve the results obtained with IL‐2 or IFNα as a single agent.268, 269, 270, 271, 272, 273, 274 However, in three of them, a longer survival was observed in patients with melanoma with liver metastases when using IL‐2/HDC and IL‐2/IFNα/HDC (2–18 MIU/m2 IL‐2 s.c., 1 mg HDC by slow s.c. injection, and eventually plus 3 MU IFNα s.c. daily administration).92, 275, 276
Other studies in patients with metastatic melanoma treated with protocols comprising histamine, IFNα, and low‐dose IL‐2 (3 MIU IFNα s.c. daily, 1 mg HDC s.c., b.i.d., and 2.4 MIU/m2 IL‐2 s.c., b.i.d. for 1–2 weeks) demonstrated a trend toward a gradual increase in the absolute number of circulating CD56+ CD3+ NK cells in patients maintaining stable disease during therapy, and additional tumor infiltration of NK cells (CD56+) and monocytes during treatment was only seen in responding patients.277
In addition, preclinical studies showed that the combined treatment with IL‐2 and histamine receptor ligands (25 mg/kg histamine, 50 mg/kg ranitidine, 6000 U/kg IL‐2; all compounds were administered i.v. as a single dose 24 h before i.v. melanoma cells’ inoculation) completely blocked the development of metastasis in Swiss albino, C57BL/6 and BALB/c mice inoculated with B16 murine melanoma cells (F1 and F10 strains). On the other hand, concomitant treatment with ranitidine nullified the anti‐metastatic effects of IL‐2.278
4.4. Colorectal cancer
In colorectal cancer (CRC), immunotherapy has become an attractive option compared with conventional chemotherapy. Treatment efficiency of three FDA‐approved immune checkpoint inhibitors targeting PD‐1 and CTLA‐4 is influenced by the microsatellite instability status in each CRC patient. Multiple studies are using combination modalities to enhance immune response.279, 280
MC‐38 tumor growth was strongly reduced by the treatment with histamine (1500 µg/mouse i.p. injection 3 times a week starting 1 day before tumor inoculation) and anti‐PD‐1/anti‐PD‐L1 (100–240 µg/mouse of each antibody, 3, 6, and 10 days after tumor inoculation), tending to increase the fraction of intra‐tumoral CD8+ T cells and raised significantly the fraction of CD8+ T cells with an effector phenotype. In addition, the percentage of intra‐tumoral CD4+ T cells was not altered, and NK cells were decreased.201
lL‐2 was used alone (200 units/ml) or in combination with ranitidine (0.02 mg/ml) to improve in vitro NK cell activity in peripheral blood of CRC patients with liver metastases. Ranitidine synergizes the IL‐2‐induced NK cell activity.281
In addition to the histamine‐induced modulation of the anti‐tumor immunity, it produces numerous effects on both gastrointestinal epithelium and CRC, considering that H1, H2, and H4 receptors are expressed in both healthy tissues and CRC samples. H2 receptor signaling suppressed tumor growth in inflammation‐associated CRC. On the other hand, H1 and H4 receptors, both suppressed in CRC, may have a protective effect against CRC growth. Until now, the use of antihistamines has been used exclusively in CRC to prevent chemotherapy‐induced adverse events.7, 282, 283, 284
4.5. Prostate cancer and other cancers
In prostate cancer, immunotherapy has not yet reached a therapeutic breakthrough as compared to several other solid tumors. Sipuleucel‐T and pembrolizumab are the only registered immune‐oncology drugs to treat this malignancy.285
A study was conducted to determine whether IL‐2 and histamine alone, or in combination could modulate the effects of irradiation on Dunning (R3327) rat prostatic adenocarcinoma at the cellular level. It was demonstrated that IL‐2, especially in combination with histamine, alters the response to radiation, increasing the number of apoptotic cells, and significantly reducing tumor cells compared to irradiation alone.286, 287
Immunotherapy for sarcoma (Coley's toxins, IL‐2, adoptive T‐cell transfer, and immune checkpoint blockade) showed limited success. Ongoing research is studying the combined use of immune checkpoint blockade with other immune modulators, surgery, or radiation.288 In a rat experimental model bearing BN‐175 tumors the association of histamine and IL‐2 in the melphalan‐based isolated limb perfusion setting showed no improved response (40 µg melphalan, 1 mg histamine, and 50 µg IL‐2 in 5 ml total volume perfusate).289
On the other hand, glioblastoma is the deadliest form of brain cancer. Some interesting, though controversial, results have been obtained with immunotherapy including IL‐2 in various experimental models, as well as in the clinical setting. Combination immunotherapies or treatment regimens involving both standard therapies and immunotherapies show promising results as powerful anti‐cancer therapies in glioblastoma.290 The combination of HDC/IL‐2 (HDC 4 mg/kg s.c. as daily injections from day 6 after intracranial tumor implantation, and 1.8 MIU/ml s.c. on day 6 after tumor implantation) significantly reduced tumor growth and the microvessel density in the syngeneic BT4C rat malignant glioma model.291
5. CONCLUSIONS AND FUTURE PERSPECTIVE
Histamine produces a complex and fine‐tuned regulation of the phenotype and functions of the different immune cells, producing distinct effects depending on the activated receptor subtype and its signaling. This biogenic amine is able to promote inflammatory and immunoregulatory responses that contribute to pathological conditions, as well as homeostatic function, balancing the inflammatory reactions.
The fate of tumors depends on the levels of pro‐ versus anti‐tumorigenic signals that are provided by the tumor cells and the TME, as well as their specific interactions. Although there are numerous well‐known described effects of histamine on the immune system, the number of studies that identify its effects on anti‐tumor immunity is still poor. Experimental and clinical findings show that histamine is a crucial mediator of immune cell responses, participating in the anti‐tumor immunity in different types of cancer. On the one side, some studies support the pro‐tumorigenic effects of histamine through enhancing tumor immune escape via the generation of an immunosuppressive TME. On the other side, a vast majority of the reports demonstrated potent anti‐tumorigenic properties, shaping innate and adaptive immune responses to control tumor growth. Not only immune cells but also cancer cells can produce and respond to histamine in a paracrine or autocrine way, which denotes the complexity of the histamine/histamine receptor axis modulation of the anti‐tumor immunity. Differences in the levels of histamine, the composition of TME, or histamine receptor subtypes present in tumor cells and immune cells could ultimately determine the biological effects of histamine and pharmacological agents targeting histamine receptors. Therefore, these facts help to understand the controversial studies in cancer research.
In the modern era of cancer immunotherapy, the immuno‐oncology field is continuously expanding, with more immunotherapeutic drugs and trials that are transforming the care of cancer patients. In this scenario, the histaminergic system provides a promising strategy for the potential therapeutic exploitation of new immunomodulatory drug targets.
The potential role of histamine in cancer immunotherapy has been investigated for more than a decade. Histamine dihydrochloride is being used in numerous clinical trials as an adjuvant to IL‐2 immunotherapy based on its ability to preserve the function of T lymphocytes and NK cells by reducing the monocyte‐ and macrophage‐induced formation and release of ROS. Several studies proved the clinical benefit of the combination, especially in AML. It is important to highlight that histamine was generally well tolerated and no unexpected or irreversible adverse effects were observed, demonstrating that it can be safely administered.
Immunotherapy is now a mainstay of cancer treatment. The success in targeting immunologic checkpoints, including the PD‐1/PD‐L1 blockade in different solid tumors, has revived the interest in immunotherapies and in combinatorial strategies to achieve additive or synergistic clinical benefits.
One obstacle in the effectiveness of immunotherapy is the complexity and the dynamic nature of immune‐related responses. In this line, novel immunotherapy combinations seek immunomodulatory agents capable of manipulating the signals in the TME to boost the immune system against cancer, targeting T cells and other components including myeloid cells. Considering the promising preclinical and clinical data using the combination of histamine with immunotherapies, future clinical trials should be developed to evaluate the efficacy and safety of the combined therapy of immune checkpoint inhibitors and histamine receptor ligands. Taking into consideration the pleiotropic nature of histamine, we hypothesize that histamine could produce nonredundant and complementary anti‐tumor effects through modulation of the anti‐tumor immunity and induction of direct anti‐proliferative actions via histamine receptors expressed in tumor cells. This could further contribute to reach tumor control and gain clinical response, especially for hard‐to‐treat cancers (e.g., triple‐negative breast cancer).
One of the challenges in research on cancer immunotherapy is the lack of appropriate laboratory models to study the immune response and the TME. Several preclinical data that study the tumor response and help to drive clinical actions, are originated in xenograft models developed in immunodeficient hosts, in which the role of the immune system in the response to therapeutics could not be evaluated. One of the major limitations in clinical translation is the use of trustful mouse models that recapitulate the complexity of human cancer and immune populations within the TME. Considering the key role of the histaminergic system in immunomodulation, it is necessary to evaluate the potential therapeutic efficacy of histamine receptor ligands globally, in immunocompetent experimental models. Another challenge in cancer immunotherapy is the discovery and validation of new biomarkers to predict which patients will respond to a determined combination strategy. Further research is needed to evaluate whether any member of the family of histamine receptors could be a molecular marker to guide treatment.
Finally, a completely unexplored topic is the role of histamine‐producing bacteria in the response to cancer immunotherapy. The dynamic relationship between the microbiome, the immune system, and cancer is a topic of recent exploration. Microbiota has a key role in how the immune response develops and has a potential impact on the response to immunotherapy. Future studies should have this topic into consideration.
As immunotherapy comes to the forefront of cancer treatments, a better understanding of how histamine regulates immune cells within the TME and how this can influence anti‐tumor immunity and patient prognosis is needed and is an interesting avenue for future research.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,384 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.385
DISCLOSURE
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
We thank the National Agency for Scientific and Technological Promotion (Grant: PICT2018‐03778) for financial support.
Sarasola Md, Táquez Delgado MA, Nicoud MB, Medina VA. Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol Res Perspect. 2021;9:e00778. 10.1002/prp2.778
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2.Tiligada E, Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol. 2020;177(3):469‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128(6):1153‐1162. [DOI] [PubMed] [Google Scholar]
- 4.Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161(4):755‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäumer W, Roßbach K. Histamine as Immunmodulator. JDDG—J Ger Soc Dermatol. 2010;8(7):495‐504. [DOI] [PubMed] [Google Scholar]
- 6.Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2020;177(3):516‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoud MB, Formoso K, Medina VA. Pathophysiological role of histamine H4 receptor in cancer: Therapeutic implications. Front Pharmacol. 2019;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinel Lamas DJ, Croci M, Carabajal E, et al. Therapeutic potential of histamine H₄ receptor agonists in triple‐negative human breast cancer experimental model. Br J Pharmacol. 2013;170(1):188‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massari NA, Nicoud MB, Sambuco L, et al. Histamine therapeutic efficacy in metastatic melanoma: role of histamine H4 receptor agonists and opportunity for combination with radiation. Oncotarget. 2017;8(16):26471‐26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Z, Fultz RS, Engevik MA, et al. Distinct roles of histamine H1 and H2 receptor signaling pathways in inflammation‐associated colonic tumorigenesis. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G205‐G216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai W‐K, Zhang J‐B, Chen J‐H, et al. The HRH4 rs11662595 mutation is associated with histamine H4 receptor dysfunction and with increased epithelial‐to‐mesenchymal transition progress in non‐small cell lung cancer. Biochim Biophys Acta—Mol Basis Dis. 2017;1863(11):2954‐2963. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Hou Y, Yin C, et al. Upregulation of histamine receptor H1 promotes tumor progression and contributes to poor prognosis in hepatocellular carcinoma. Oncogene. 2020;39(8):1724‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F, Han Y, Staloch D, Francis T, Stokes A, Francis H. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology. 2011;54:1718‐1728. [DOI] [PubMed] [Google Scholar]
- 15.Salem A, Salo T. Nothing to sneeze at: histamine and histamine receptors in oral carcinogenesis. Oral Dis. 2020. 10.1111/odi.13411. [DOI] [PubMed] [Google Scholar]
- 16.Schayer RW. The metabolism of histamine in various species. Br J Pharmacol Chemother. 1956;11(4):472‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black JW, Duncan WAM, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H2—receptors. Nature. 1972;238:37‐38. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279(2):615‐620. [DOI] [PubMed] [Google Scholar]
- 19.Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto SI. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275(47):36781‐36786. [DOI] [PubMed] [Google Scholar]
- 20.Ash A, Schild HO, Black JW. Receptors mediating some actions of histamine. Br J Pharmacol. 1966;120(4 Suppl):300‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrang JM, Garbarg M, Schwartz JC. Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302(5911):832‐837. [DOI] [PubMed] [Google Scholar]
- 22.Cogé F, Guénin SP, Rique H, Boutin JA, Galizzi JP. Structure and expression of the human histamine H4‐receptor gene. Biochem Biophys Res Commun. 2001;284(2):301‐309. [DOI] [PubMed] [Google Scholar]
- 23.Morse KL, Behan J, Laz TM, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296(3):1058‐1066. [PubMed] [Google Scholar]
- 24.Nguyen T, Shapiro DA, George SR, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol. 2001;59(3):427‐433. [DOI] [PubMed] [Google Scholar]
- 25.Panula P, Chazot PL, Cowart M, et al. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev. 2015;67(3):601‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushima Y, Asano T, Saitoh T, et al. Oligomer formation of histamine H2 receptors expressed in Sf9 and COS7 cells. FEBS Lett. 1997;409(2):283‐286. [DOI] [PubMed] [Google Scholar]
- 27.Hancock AA, Esbenshade TA, Krueger KM, Yao BB. Genetic and pharmacological aspects of histamine H3 receptor heterogeneity. Life Sci. 2003;73(24):3043‐3072. [DOI] [PubMed] [Google Scholar]
- 28.Breitwieser GE. G Protein‐coupled receptor oligomerization: implications for G protein activation and cell signaling. Circ Res. 2004;94(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 29.Bongers G, Bakker RA, Leurs R. Molecular aspects of the histamine H3 receptor. Biochem Pharmacol. 2007;73(8):1195‐1204. [DOI] [PubMed] [Google Scholar]
- 30.Maggio R, Novi F, Scarselli M, Corsini GU. The impact of G‐protein‐coupled receptor hetero‐oligomerization on function and pharmacology. FEBS J. 2005;272(12):2939‐2946. [DOI] [PubMed] [Google Scholar]
- 31.Leurs R, Chazot PL, Shenton FC, Lim HD, De Esch IJP. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157(1):14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill SJ, Ganellin CR, Timmerman H, et al. International union of pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49(3):415‐472. [PubMed] [Google Scholar]
- 33.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 34.Seifert R, Strasser A, Schneider E, Neumann D, Dove S, Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci. 2013;34(1):33‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strasser A, Wittmann H‐J, Buschauer A, et al. Species‐dependent activities of G‐protein‐coupled receptor ligands:lessons from histamine receptor orthologs. Trends Pharmacol Sci. 2013;34(1):13‐32. [DOI] [PubMed] [Google Scholar]
- 36.Johnson CL. Chapter Four ‐ Histamine receptors and cyclic nucleotides. In: Ganellin CR, Parsons ME, Pharmacology of Histamine Receptors. Bristol: John Wright & Sons; 1982: 146‐216. [Google Scholar]
- 37.Alonso N, Fernandez N, Notcovich C, et al. Cross‐desensitization and cointernalization of H1 and H2 histamine receptors reveal new insights into histamine signal integration. Mol Pharmacol. 2013;83(5):1087‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monczor F, Copsel S, Fernandez N, Davio C, Shayo C. Histamine H2 receptor in blood cells: a suitable target for the treatment of acute myeloid leukemia. In: Hattori Y, Seifert R, eds. Histamine and Histamine Receptors in Health and Disease. Handbook of Experimental Pharmacology. Cham: Springer; 2016: 251‐263. [DOI] [PubMed] [Google Scholar]
- 39.Díaz Nebreda A, Zappia CD, Rodríguez González A, et al. Involvement of histamine H1 and H2 receptor inverse agonists in receptor's crossregulation. Eur J Pharmacol. 2019;847:42‐52. [DOI] [PubMed] [Google Scholar]
- 40.Dimitriadou V, Rouleau A, Tuong MDT, et al. Functional relationship between mast cells and C‐sensitive nerve fibres evidenced by histamine H3‐receptor modulation in rat lung and spleen. Clin Sci. 1994;87(2):151‐163. [DOI] [PubMed] [Google Scholar]
- 41.Shahid M, Tripathi T, Sobia F, Moin S, Siddiqui M, Khan RA. Histamine, histamine receptors, and their role in immunomodulation: an updated systematic review. Open Immunol J. 2009;2(1):9‐41. [Google Scholar]
- 42.Rouleau A, Stark H, Schunack W, Schwartz JC. Anti‐inflammatory and antinociceptive properties of BP 2–94, a histamine H (3)‐receptor agonist prodrug. J Pharmacol Exp Ther. 2000;295(1):219‐225. [PubMed] [Google Scholar]
- 43.Kantor I, Jurkiewicz D. Assessment of betahistine dihydrochloride effectiveness in the treatment of vertigo of a different aetiology based on videonystagmography test results. Pol Merkur Lekarski. 2006;20(117):318‐321. [PubMed] [Google Scholar]
- 44.Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double‐blind, randomised trial. Lancet Neurol. 2013;12(11):1068‐1075. [DOI] [PubMed] [Google Scholar]
- 45.Kasteleijn‐ Nolst Trenité D, Parain D, Genton P, et al. Efficacy of the histamine 3 receptor (H3R) antagonist pitolisant (formerly known as tiprolisant; BF2.649) in epilepsy: dose‐dependent effects in the human photosensitivity model. Epilepsy Behav. 2013;28(1):66‐70. [DOI] [PubMed] [Google Scholar]
- 46.Amini A, Heidari K, Kariman H, et al. Histamine antagonists for treatment of peripheral vertigo: a meta‐analysis. J Int Adv Otol. 2015;11(2):138‐142. [DOI] [PubMed] [Google Scholar]
- 47.Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2017;16(3):200‐207. [DOI] [PubMed] [Google Scholar]
- 48.Guevarra JT, Hiensch R, Varga AW, Rapoport DM. Pitolisant to treat excessive daytime sleepiness and cataplexy in adults with narcolepsy: Rationale and clinical utility. Nat Sci Sleep. 2020;12:709‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He G‐H, Ding J‐Q, Zhang X, et al. Activation of histamine H4 receptor suppresses the proliferation and invasion of esophageal squamous cell carcinoma via both metabolism and non‐metabolism signaling pathways. J Mol Med. 2018;96(9):951‐964. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Xiong YI, Li J, et al. Deletion and down‐regulation of HRH4 gene in gastric carcinomas: a potential correlation with tumor progression. PLoS One. 2012;7(2):e31207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka T, Kochi T, Shirakami Y, et al. Cimetidine and clobenpropit attenuate inflammation‐associated colorectal carcinogenesis in male ICR mice. Cancers. 2016;8(2):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai W‐K, Hu J, Li T, et al. Activation of histamine H4 receptors decreases epithelial‐to‐mesenchymal transition progress by inhibiting transforming growth factor‐β1 signalling pathway in non‐small cell lung cancer. Eur J Cancer. 2014;50(6):1195‐1206. [DOI] [PubMed] [Google Scholar]
- 53.Thurmond RL, Chen B, Dunford PJ, et al. Clinical and preclinical characterization of the histamine H4 receptor antagonist JNJ‐39758979. J Pharmacol Exp Ther. 2014;349(2):176‐184. [DOI] [PubMed] [Google Scholar]
- 54.Thurmond RL, Venable J, Savall B, et al. Clinical development of histamine H4 receptor antagonists. Handb Exp Pharmacol. 2017;241:301‐320. [DOI] [PubMed] [Google Scholar]
- 55.Kollmeier A, Francke K, Chen B, et al. The histamine H4 receptor antagonist, JNJ 39758979, is effective in reducing histamine‐induced pruritus in a randomized clinical study in healthy subjects. J Pharmacol Exp Ther. 2014;350(1):181‐187. [DOI] [PubMed] [Google Scholar]
- 56.Kollmeier AP, Barnathan ES, O'Brien C, et al. A phase 2a study of toreforant, a histamine H4 receptor antagonist, in eosinophilic asthma. Ann Allergy Asthma Immunol. 2018;121(5):568‐574. [DOI] [PubMed] [Google Scholar]
- 57.Werfel T, Layton G, Yeadon M, et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL‐3893787 in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;143(5):1830‐1837.e4. [DOI] [PubMed] [Google Scholar]
- 58.Murata Y, Song M, Kikuchi H, et al. Phase 2a, randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study of a H4 R‐antagonist (JNJ‐39758979) in Japanese adults with moderate atopic dermatitis. J Dermatol. 2015;42(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 59.Frankel E, Song M, Li S, Jiang J, Thurmond R, Randazzo B. Efficacy and safety of toreforant, a selective histamine H4 receptor antagonist, for the treatment of moderate‐to‐severe plaque psoriasis: results from a phase 2 multicenter, randomized, double‐blind. Placebo‐controlled trial. J Drugs Dermatol. 2018;17(8):873‐879. [PubMed] [Google Scholar]
- 60.Thurmond RL, Greenspan A, Radziszewski W, et al. Toreforant, a histamine H4 receptor antagonist, in patients with active rheumatoid arthritis despite methotrexate therapy: results of 2 phase II studies. J Rheumatol. 2016;43(9):1637‐1642. [DOI] [PubMed] [Google Scholar]
- 61.Schaper‐Gerhardt K, Rossbach K, Nikolouli E, Werfel T, Gutzmer R, Mommert S. The role of the histamine H4 receptor in atopic dermatitis and psoriasis. Br J Pharmacol. 2020;177(3):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 63.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57‐70. [DOI] [PubMed] [Google Scholar]
- 64.Jacobo Velazquez PM, Huerta JG, Cravioto P. Interacciones entre el cáncer y el sistema inmunológico. Alergia Asma e Inmunol Pediátricas. 2017;26:56‐63. [Google Scholar]
- 65.Tower H, Ruppert M, Britt K. The immune microenvironment of breast cancer progression. Cancers. 2019;11(9):1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavares LP, Peh HY, Tan WSD, et al. Granulocyte‐targeted therapies for airway diseases. Pharmacol Res. 2020;157:104881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beermann S, Seifert R, Neumann D. Commercially available antibodies against human and murine histamine H4‐receptor lack specificity. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385:125‐135. [DOI] [PubMed] [Google Scholar]
- 69.Gutzmer R, Werfel T, Bäumer W, Kietzmann M, Chazot P, Leurs R. Well characterized antihistamine 4 receptor antibodies contribute to current knowledge of the expression and biology of the human and murine histamine 4 receptor. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:853‐854. [DOI] [PubMed] [Google Scholar]
- 70.Miyauchi S, Hirasawa A, Iga T, et al. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:427‐434. [DOI] [PubMed] [Google Scholar]
- 71.Wescott S, Kaliner M. Histamine H1 binding site on human polymorphonuclear leukocytes. Inflammation. 1983;7(3):291‐300. [DOI] [PubMed] [Google Scholar]
- 72.Liu C, Ma XJ, Jiang X, et al. Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol. 2001;59:420‐426. [DOI] [PubMed] [Google Scholar]
- 73.O'Reilly M, Alpert R, Jenkinson S, et al. Identification of a histamine H4 receptor on human eosinophils–role in eosinophil chemotaxis. J Recept Signal Transduct Res. 2002;22(1–4):431‐448. [DOI] [PubMed] [Google Scholar]
- 74.Lippert U, Artuc M, Grützkau A, et al. Human skin mast cells express H2 and H4, but not H3 receptors. J Invest Dermatol. 2004;123(1):116‐123. [DOI] [PubMed] [Google Scholar]
- 75.Reher T, Brunskole I, Neumann D, Seifert R. Evidence for ligand‐specific conformations of the histamine H2‐receptor in human eosinophils and neutrophils. Biochem Pharmacol. 2012;84(9):1174‐1185. [DOI] [PubMed] [Google Scholar]
- 76.Dib K, Perecko T, Jenei V, et al. The histamine H4 receptor is a potent inhibitor of adhesion‐dependent degranulation in human neutrophils. J Leukoc Biol. 2014;96(3):411‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merves J, Chandramouleeswaran PM, Benitez AJ, et al. Altered esophageal histamine receptor expression in Eosinophilic Esophagitis (EoE): implications on disease pathogenesis. PLoS One. 2015;10(2):e0114831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hofstra C, Desai P, Thurmond R, et al. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305(3):1212‐1221. [DOI] [PubMed] [Google Scholar]
- 79.Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. 2020;1224:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grecian R, Whyte MKB, Walmsley SR. The role of neutrophils in cancer. Br Med Bull. 2018;128(1):5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu X, Ding S, Li H, et al. Disruption of histamine/H1R signaling pathway represses cardiac differentiation and maturation of human induced pluripotent stem cells. Stem Cell Res Ther. 2020;11(1):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corral Lugo A, Matilla MA, Martín‐Mora D, et al. High‐affinity chemotaxis to histamine mediated by the TlpQ chemoreceptor of the human pathogen Pseudomonas aeruginosa . MBio. 2018;9(6):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burde R, Seifert R, Buschauerz A, Schultz G. Histamine inhibits activation of human neutrophils and HL‐60 leukemic cells via H2‐receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989;340(6):671‐678. [DOI] [PubMed] [Google Scholar]
- 84.Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Enhancement of neutrophil infiltration in histidine decarboxylase‐deficient mice. Immunology. 2002;107(2):217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medina V, Rivera SE. Histamine as a potential adjuvant to immuno and radiotherapy for cancer treatment: discovering new functions for the oldest biogenic amine. Curr Immunol Rev. 2010;6(4):357‐370. [Google Scholar]
- 86.Mitsuhashi M, Mitsuhashi T, Payan D. Multiple signaling pathways of histamine H2 receptors. Identification of an H2 receptor‐dependent Ca2+ mobilization pathway in human HL‐60 promyelocytic leukemia cells. J Biol Chem. 1989;264(31):18356‐18362. [PubMed] [Google Scholar]
- 87.Bury TB, Corhay JL, Radermecker MF. Histamine‐induced inhibition of neutrophil chemotaxis and T‐lymphocyte proliferation in man. Allergy. 1992;47(6):624‐629. [DOI] [PubMed] [Google Scholar]
- 88.Mirossay L, Chastre E, Callebert J, et al. Histamine H2 receptors and histidine decarboxylase in normal and leukemic human monocytes and macrophages. Am J Physiol. 1994;267(2 Pt 2):R602‐R611. [DOI] [PubMed] [Google Scholar]
- 89.Flamand N, Plante H, Picard S, Laviolette M, Borgeat P. Histamine‐induced inhibition of leukotriene biosynthesis in human neutrophils: involvement of the H2 receptor and cAMP. Br J Pharmacol. 2004;141(4):552‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hellstrand K, Hermodsson S. Histamine H2‐receptor‐mediated regulation of human natural killer cell activity. J Immunol. 1986;137(2):656‐660. [PubMed] [Google Scholar]
- 91.Betten Å, Dahlgren C, Hermodsson S, Hellstrand K. Histamine inhibits neutrophil NADPH oxidase activity triggered by the lipoxin A4 receptor‐specific peptide agonist Trp‐Lys‐Tyr‐Met‐Val‐Met. Scand J Immunol. 2003;58(3):321‐326. [DOI] [PubMed] [Google Scholar]
- 92.Hellstrand K, Naredi P, Lindner P, et al. Histamine in immunotherapy of advanced melanoma: a pilot study. Cancer Immunol Immunother. 1994;39(6):416‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hellstrand K. Histamine in cancer immunotherapy: a preclinical background. Semin Oncol. 2002;29(3 Suppl 7):35‐40. [DOI] [PubMed] [Google Scholar]
- 94.Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H. Monocytes and neutrophils as “bad guys” for the outcome of interleukin‐2 with and without histamine in metastatic renal cell carcinoma–results from a randomised phase II trial. Br J Cancer. 2006;94(2):218‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehta P, Miszta P, Rzodkiewicz P, Michalak O, Krzeczyński P, Filipek S. Enigmatic histamine receptor H4 for potential treatment of multiple inflammatory, autoimmune, and related diseases. Life. 2020;10(4):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marson CM. Targeting the histamine H4 receptor. Chem Rev. 2011;111(11):7121‐7156. [DOI] [PubMed] [Google Scholar]
- 97.Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in cancer: favourable or unfavourable? Curr Med Chem. 2016;23(7):650‐666. [DOI] [PubMed] [Google Scholar]
- 98.Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol Immunother. 2019;68(5):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Munitz A, Levi‐Schaffer F. Eosinophils: “New” roles for “old” cells. Allergy Eur J Allergy Clin Immunol. 2004;59(3):268‐275. [DOI] [PubMed] [Google Scholar]
- 100.Lowe D, Jorizzo J, Hutt MSR. Tumour‐associated eosinophilia: A review. J Clin Pathol. 1981;34(12):1343‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 102.Reichman H, Karo‐Atar D, Munitz A. Emerging roles for eosinophils in the tumor microenvironment. Trends Cancer. 2016;2(11):664‐675. [DOI] [PubMed] [Google Scholar]
- 103.Reichman H, Itan M, Rozenberg P, et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res. 2019;7(3):388‐400. [DOI] [PubMed] [Google Scholar]
- 104.Ennis M, Ciz M, Dib K, et al. Histamine receptors and inflammatory cells. Histamine H4 Recept A Nov Drug Target Immunoregul Inflamm. 2013;9788376560:103‐143. [Google Scholar]
- 105.Stark H. Histamine H4 Receptor: A Novel Drug Target in Immunoregulation and Inflammation. London, UK: Versita Ltd.; 2013. [Google Scholar]
- 106.Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human mast cells and basophils‐How are they similar how are they different? Immunol Rev. 2018;282(1):8‐34. [DOI] [PubMed] [Google Scholar]
- 107.Rigoni A, Colombo MP, Pucillo C. Mast cells, basophils and eosinophils: from allergy to cancer. Semin Immunol. 2018;35:29‐34. [DOI] [PubMed] [Google Scholar]
- 108.Komi DEA, Mortaz E, Amani S, et al. The Role of mast cells in IgE‐independent lung diseases. Clin Rev Allergy Immunol. 2020;58(3):377‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mommert S, Kleiner S, Gehring M, et al. Human basophil chemotaxis and activation are regulated via the histamine H4 receptor. Allergy. 2016;71(9):1264‐1273. [DOI] [PubMed] [Google Scholar]
- 110.Faustino‐Rocha AI, Ferreira R, Gama A, Oliveira PA, Ginja M. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017;172:27‐41. [DOI] [PubMed] [Google Scholar]
- 111.Kennedy L, Hodges K, Meng F, Alpini G, Francis H. Histamine and histamine receptor regulation of gastrointestinal cancers. Transl Gastrointest Cancer. 2012;1(3):215‐227. [PMC free article] [PubMed] [Google Scholar]
- 112.Rigoni A, Colombo MP, Pucillo C. The role of mast cells in molding the tumor microenvironment. Cancer Microenviron. 2015;8(3):167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63(1):113‐124. [DOI] [PubMed] [Google Scholar]
- 114.Sterle HA, Nicoud MB, Massari NA, et al. Immunomodulatory role of histamine H4 receptor in breast cancer. Br J Cancer. 2019;120(1):128‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicoud MB, Táquez Delgado MA, Sarasola MdlP, et al. Impact of histamine H4 receptor deficiency on the modulation of T cells in a murine breast cancer model. Cancer Immunol Immunother. 2021;70(1):233‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paudel S, Mehtani D, Puri N. Mast cells may differentially regulate growth of lymphoid neoplasms by opposite modulation of histamine receptors. front. Oncol. 2019;9. 10.3389/fonc.2019.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stoyanov E, Uddin M, Mankuta D, Dubinett SM, Levi‐Schaffer F. Mast cells and histamine enhance the proliferation of non‐small cell lung cancer cells. Lung Cancer. 2012;75(1):38‐44. [DOI] [PubMed] [Google Scholar]
- 118.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1(5):269‐279. [DOI] [PubMed] [Google Scholar]
- 119.Derakhshani A, Vahidian F, Alihasanzadeh M, et al. Mast cells: a double‐edged sword in cancer. Immunol Lett. 2019;209:28‐35. [DOI] [PubMed] [Google Scholar]
- 120.Karasuyama H, Miyake K, Yoshikawa S, Kawano Y, Yamanishi Y. How do basophils contribute to Th2 cell differentiation and allergic responses? Int Immunol. 2018;30(9):391‐396. [DOI] [PubMed] [Google Scholar]
- 121.Valent P, Horny H‐P, Arock M. The underestimated role of basophils in Ph (+) chronic myeloid leukaemia. Eur J Clin Invest. 2018;48(10):e13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bax HJ, Chauhan J, Stavraka C, et al. Basophils from cancer patients respond to immune stimuli and predict clinical outcome. Cells. 2020;9(7):1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al‐Shura AN. Advanced Hematology in Integrated Cardiovascular Chinese Medicine: 7 ‐Lymphocytes. 3. Cambridge, MA: Academic Press; 2020:41–46. [Google Scholar]
- 124.Omman RA, Kini AR. Leukocyte development, kinetics, and functions. In: Keohane EM, Otto CM, Walenga JM, Rodak’s Hematology: Clinical Principles and Applications. 6th edn. Amsterdam, Netherlands: Elsevier; 2019:117‐135. [Google Scholar]
- 125.Denkert C, von Minckwitz G, Darb‐Esfahani S, et al. Tumour‐infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40‐50. [DOI] [PubMed] [Google Scholar]
- 126.Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor‐infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2019;343, 103753. [DOI] [PubMed] [Google Scholar]
- 127.Sikandar B, Qureshi MA, Naseem S, Khan S, Mirza T. Increased tumour infiltration of CD4+ and CD8+ T‐lymphocytes in patients with triple negative breast cancer suggests susceptibility to immune therapy. Asian Pacific J Cancer Prev. 2017;18(7):1827‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jutel M, Watanabe T, Klunker S, et al. Histamine regulates T‐cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413(6854):420‐425. [DOI] [PubMed] [Google Scholar]
- 129.Jutel M, Blaser K, Akdis C. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005;137(1):82‐92. [DOI] [PubMed] [Google Scholar]
- 130.Gantner F, Sakai K, Tusche M, Cruikshank W, Center D, Bacon K. Histamine h(4) and h(2) receptors control histamine‐induced interleukin‐16 release from human CD8(+) T cells. J Pharmacol Exp Ther. 2002;303(1):300‐307. [DOI] [PubMed] [Google Scholar]
- 131.Gutzmer S, Mommert M, Gschwandtner K, et al. Histamine H4 receptor is functionally expressed on TH2 cells. J Allergy Clin Immunol. 2009;123:619‐625. [DOI] [PubMed] [Google Scholar]
- 132.Mommert S, Gschwandtner M, Koether B, et al. Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol. 2012;180:177‐185. [DOI] [PubMed] [Google Scholar]
- 133.Ciebiada M, Kasztalska K, Gorska‐Ciebiada M, Górski P. Histamine type 2 receptor expression on peripheral blood regulatory lymphocytes in patients with allergic rhinitis treated with specific immunotherapy. Am J Rhinol Allergy. 2014;28(3):e130‐e135. [DOI] [PubMed] [Google Scholar]
- 134.Morgan R, McAllister B, Cross L, et al. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol. 2007;178(12):8081‐8089. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Su B, Ding Z, Du X, Wang B. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T cells. Biochem Biophys Res Commun. 2008;372(3):491‐496. [DOI] [PubMed] [Google Scholar]
- 136.Forward NA, Furlong SJ, Yang Y, Lin T‐J, Hoskin DW. Mast cells down‐regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183(5):3014‐3022. [DOI] [PubMed] [Google Scholar]
- 137.Tamaka K, Seike M, Hagiwara T, Sato A, Ohtsu H. Histamine suppresses regulatory T cells mediated by TGF‐β in murine chronic allergic contact dermatitis. Exp Dermatol. 2015;24(4):280‐284. [DOI] [PubMed] [Google Scholar]
- 138.Jafarzadeh A, Nemati M, Khorramdelazad H, Hassan ZM. Immunomodulatory properties of cimetidine: its therapeutic potentials for treatment of immune‐related diseases. Int Immunopharmacol. 2019;70:156‐166. [DOI] [PubMed] [Google Scholar]
- 139.Tomita K, Okabe S. Exogenous histamine stimulates colorectal cancer implant growth via immunosuppression in mice. J Pharmacol Sci. 2005;97(1):116‐123. [DOI] [PubMed] [Google Scholar]
- 140.Reynolds JL, Akhter J, Adams WJ, Morris DL. Histamine content in colorectal cancer. Are there sufficient levels of histamine to affect lymphocyte function? Eur J Surg Oncol. 1997;23(3):224‐227. [DOI] [PubMed] [Google Scholar]
- 141.Frei R, Ferstl R, Konieczna P, et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013;132(1):194‐204. [DOI] [PubMed] [Google Scholar]
- 142.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057‐1061. [DOI] [PubMed] [Google Scholar]
- 143.Sakaguchi S, Wing K, Onishi Y, Prieto‐Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(10):1105‐1111. [DOI] [PubMed] [Google Scholar]
- 144.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116(5):961‐968; quiz 969. [DOI] [PubMed] [Google Scholar]
- 145.Robinson DS. Regulatory T cells and asthma. Clin Exp allergy J Br Soc Allergy Clin Immunol. 2009;39(9):1314‐1323. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y, Pan T, Wang Q, Guo Z. Additional bedtime H2‐receptor antagonist for the control of nocturnal gastric acid breakthrough. Cochrane database Syst Rev. 2009;2(4):CD004275. [DOI] [PubMed] [Google Scholar]
- 147.Niu X, Yang Y, Wang J. Synergistic and additive effects of cimetidine and levamisole on cellular immune responses to hepatitis B virus DNA vaccine in mice. Scand J Immunol. 2013;77(2):84‐91. [DOI] [PubMed] [Google Scholar]
- 148.Hegyesi H, Colombo L, Pállinger É, Tóth S, Boer K, Molnár V. Full paper impact of systemic histamine deficiency on the crosstalk between mammary adenocarcinoma and T cells. J Pharmacol Sci. 2007;73:66‐73. [DOI] [PubMed] [Google Scholar]
- 149.Nicoud MB, Sterle HA, Massari NA, et al. Study of the antitumour effects and the modulation of immune response by histamine in breast cancer. Br J Cancer. 2020;122(3):348‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.del Rio R, Noubade R, Saligrama N, et al. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti‐inflammatory responses within the central nervous system. J Immunol. 2012;188(2):541‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bernson E, Hallner A, Sander FE, et al. Impact of killer‐immunoglobulin‐like receptor and human leukocyte antigen genotypes on the efficacy of immunotherapy in acute myeloid leukemia. Leukemia. 2017;31(12):2552‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schirmer B, Rother T, Bruesch I, et al. Genetic deficiency of the histamine H4‐receptor reduces experimental colorectal carcinogenesis in mice. Cancers. 2020;12(4):912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20(2):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwartz AM, Putlyaeva LV, Covich M, et al. Early B‐cell factor 1 (EBF1) is critical for transcriptional control of SLAMF1 gene in human B cells. Biochim Biophys Acta—Gene Regul Mech. 2016;1859(10):1259‐1268. [DOI] [PubMed] [Google Scholar]
- 155.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti‐tumor immunity. Cell Mol Immunol. 2017;14(8):662‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li B, Cao F, Zhu Q, et al. Perioperative cimetidine administration improves systematic immune response and tumor infiltrating lymphocytes in patients with colorectal cancer. Hepatogastroenterology. 2013;60(122):244‐247. [DOI] [PubMed] [Google Scholar]
- 157.Rogers D, Vila‐Leahey A, Pessôa AC, Oldford S, Marignani PA, Marshall JS. Ranitidine inhibition of breast tumor growth is B cell dependent and associated with an enhanced antitumor antibody response. Front Immunol. 2018;9:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cantoni C, Huergo‐Zapico L, Parodi M, et al. NK cells, tumor cell transition, and tumor progression in solid malignancies: new hints for NK‐based immunotherapy? J Immunol Res. 2016;2016:4684268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45‐56. [DOI] [PubMed] [Google Scholar]
- 160.Damaj B, Becerra C, Esber H, Wen Y, Maghazachi A.. Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol. 2007;179(11):7907‐7915. [DOI] [PubMed] [Google Scholar]
- 161.Hellstrand K, Hermodsson S, Brune M, Naredi P, Carneskog J, Mellqvist UH. Histamine in cancer immunotherapy. Scand J Clin Lab Invest. 1997;57(3):193‐202. [DOI] [PubMed] [Google Scholar]
- 162.Brune M, Hansson M, Mellqvist U‐H, Hermodsson S, Hellstrand K. NK cell‐mediated killing of AML blasts: role of histamine, monocytes and reactive oxygen metabolites. Eur J Haematol. 1996;57(4):312‐319. [DOI] [PubMed] [Google Scholar]
- 163.Nagai Y, Tanaka Y, Kuroishi T, Sato R, Endo Y, Sugawara S. Histamine reduces susceptibility to natural killer cells via down‐regulation of NKG2D ligands on human monocytic leukaemia THP‐1 cells. Immunology. 2012;136(1):103‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor‐infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015;194(7):2985‐2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol. 2019;348:123‐178. [DOI] [PubMed] [Google Scholar]
- 166.Gutzmer R, Langer K, Lisewski M, et al. Expression and function of histamine receptors 1 and 2 on human monocyte‐derived dendritic cells. J Allergy Clin Immunol. 2002;109(3):524‐531. [DOI] [PubMed] [Google Scholar]
- 167.Idzko M, la Sala A, Ferrari D, et al. Expression and function of histamine receptors in human monocyte‐derived dendritic cells. J Allergy Clin Immunol. 2002;109:839‐846. [DOI] [PubMed] [Google Scholar]
- 168.Gutzmer R, Diestel C, Mommert S, et al. Histamine H4 receptor stimulation suppresses IL‐12p70 production and mediates chemotaxis in human monocyte‐derived dendritic cells. J Immunol. 2005;174(9):5224‐5232. [DOI] [PubMed] [Google Scholar]
- 169.Mazzoni A, Young H, Spitzer J, Visintin A, Segal D. Histamine regulates cytokine production in maturing dendritic cells, resulting inaltered T cell polarization. J Clin Invest. 2001;108:1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gantner F, Sakai K, Tusche MW, et al. Histamine H4 and H2 receptors control histamine‐induced interleukin‐16 release from human CD8+ T cells”. J Pharmacol Exp Ther. 2002;303(1):300‐307. [DOI] [PubMed] [Google Scholar]
- 171.Caron G, Delneste Y, Roelandts E, et al. Histamine polarizes human dendritic cells into Th2 cell‐pro‐moting effector dendritic cells. J. Immunol. 2001;167:3682. [DOI] [PubMed] [Google Scholar]
- 172.Carroll‐Portillo A, Cannon JL, te Riet J, et al. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J Cell Biol. 2015;210(5):851‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martner A, Wiktorin HG, Lenox B, et al. Histamine promotes the development of monocyte‐derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J Immunol. 2015;194(10):5014‐5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108(12):1865‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sabatté J, Maggini J, Nahmod K, et al. Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev. 2007;18(1–2):5‐17. [DOI] [PubMed] [Google Scholar]
- 176.Vanbervliet B, Akdis M, Vocanson M, et al. Histamine receptor H1 signaling on dendritic cells plays a key role in the IFN‐γ/IL‐17 balance in T cell‐mediated skin inflammation. J Allergy Clin Immunol. 2011;127(4):943‐953. [DOI] [PubMed] [Google Scholar]
- 177.Yona S, Kim K‐W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12(4):e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nasrollahzadeh E, Razi S, Keshavarz‐Fathi M, Mazzone M, Rezaei N. Pro‐tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol Immunother. 2020;69:1673‐1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor‐associated macrophages in the tumor microenvironment. Cancer Med. 2019;8(10):4709‐4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang K‐Y, Arima N, Higuchi S, et al. Switch of histamine receptor expression from H2 to H1 during differentiation of monocytes into macrophages. FEBS Lett. 2000;473(3):345‐348. [DOI] [PubMed] [Google Scholar]
- 182.Mommert S, Ratz L, Stark H, Gutzmer R, Werfel T. The histamine H4 receptor modulates the differentiation process of human monocyte‐derived M1 macrophages and the release of CCL4/MIP‐1β from fully differentiated M1 macrophages. Inflamm Res. 2018;67(6):503‐513. [DOI] [PubMed] [Google Scholar]
- 183.Mommert S, Aslan D, Ratz L, et al. The anaphylatoxin C3a receptor expression on human M2 macrophages is down‐regulated by stimulating the histamine H4 receptor and the IL‐4 receptor. J Innate Immun. 2018;10(4):349‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Capelo R, Lehmann C, Ahmad K, et al. Cellular analysis of the histamine H4 receptor in human myeloid cells. Biochem Pharmacol. 2016;103:74‐84. [DOI] [PubMed] [Google Scholar]
- 185.Werner K, Neumann D, Buschauer A, Seifert R. No evidence for histamine H4 receptor in human monocytes. J Pharmacol Exp Ther. 2014;351(3):519‐526. [DOI] [PubMed] [Google Scholar]
- 186.Marone G, Gentile M, Petraroli A, De Rosa N, Triggiani M. Histamine‐induced activation of human lung macrophages. Int Arch Allergy Immunol. 2001;124(1–3):249‐252. [DOI] [PubMed] [Google Scholar]
- 187.Fultz R, Engevik MA, Shi Z, et al. Phagocytosis by macrophages depends on histamine H2 receptor signaling and scavenger receptor 1. Microbiologyopen. 2019;8(10):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu L, Cheng D, Huang Z, et al. Histamine promotes the differentiation of macrophages from CD11b+ myeloid cells and formation of foam cells through a Stat6‐dependent pathway. Atherosclerosis. 2017;263:42‐52. [DOI] [PubMed] [Google Scholar]
- 189.Czerner CP, Klos A, Seifert R, Neumann D. Histamine induces chemotaxis and phagocytosis in murine bone marrow‐derived macrophages and RAW 264.7 macrophage‐like cells via histamine H4‐receptor. Inflamm Res. 2014;63(3):239‐247. [DOI] [PubMed] [Google Scholar]
- 190.Tonnesen T, Knigge U, Bulow S, et al. Effect of cimetidine on survival after gastric cancer. Lancet. 1988;2:990‐991. [DOI] [PubMed] [Google Scholar]
- 191.Takahashi HK, Watanabe T, Yokoyama A, et al. Cimetidine induces interleukin‐18 production through H2‐agonist activity in monocytes. Mol Pharmacol. 2006;70(2):450‐453. [DOI] [PubMed] [Google Scholar]
- 192.Vila‐Leahey A, Rogers D, Marshall JS. The impact of ranitidine on monocyte responses in the context of solid tumors. Oncotarget. 2016;7(10):10891‐10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid‐derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro‐angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anani W, Shurin MR. Targeting myeloid‐derived suppressor cells in cancer. Adv Exp Med Biol. 2017;1036:105‐128. [DOI] [PubMed] [Google Scholar]
- 196.Gabrilovich D. Mechanisms and functional significance of tumour‐induced dendritic‐cell defects. Nat Rev Immunol. 2004;4(12):941‐952. [DOI] [PubMed] [Google Scholar]
- 197.Pinzon‐Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83(5):451‐461. [DOI] [PubMed] [Google Scholar]
- 198.Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martin RK, Saleem SJ, Folgosa L, et al. Mast cell histamine promotes the immunoregulatory activity of myeloid‐derived suppressor cells. J Leukoc Biol. 2014;96(1):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang XD, Ai W, Asfaha S, et al. Histamine deficiency promotes inflammation‐associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2010;17(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grauers Wiktorin H, Nilsson MS, Kiffin R, et al. Histamine targets myeloid‐derived suppressor cells and improves the anti‐tumor efficacy of PD‐1/PD‐L1 checkpoint blockade. Cancer Immunol Immunother. 2019;68(2):163‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gao C, Ganesh BP, Shi Z, et al. Gut microbe‐mediated suppression of inflammation‐associated colon carcinogenesis by luminal histamine production. Am J Pathol. 2017;187(10):2323‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Castelo Branco A, Yoshikawa F, Pietrobon A, Sato M. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:9524075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Barcik W, Wawrzyniak M, Akdis C, O'Mahony L. Immune regulation by histamine and histamine‐secreting bacteria. Curr Opin Immunol. 2017;48:108‐113. [DOI] [PubMed] [Google Scholar]
- 205.Helmink B, Wadud Khan M, Hermann A, Gopalakrishnan V, Wargo J. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377‐388. [DOI] [PubMed] [Google Scholar]
- 206.Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type‐specific intracellular bacteria. Science. 2020;368(6494):973‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ding Q‐Q, Chauvin J‐M, Zarour HM. Targeting novel inhibitory receptors in cancer immunotherapy. Semin Immunol. 2020;49: 101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu M, Guo F. Recent updates on cancer immunotherapy. Precis Clin Med. 2018;1(2):65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seidel JA, Otsuka A, Kabashima K. Anti‐PD‐1 and anti‐CTLA‐4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA‐4 and PD‐1 blockade. Semin Oncol. 2010;37(5):430‐439. [DOI] [PubMed] [Google Scholar]
- 211.Gonzalez‐Cao M, Martinez‐Picado J, Karachaliou N, Rosell R, Meyerhans A. Cancer immunotherapy of patients with HIV infection. Clin Transl Oncol. 2019;21(6):713‐720. [DOI] [PubMed] [Google Scholar]
- 212.Król A, Gawlik T, Jarząb B. Endocrine complications of cancer immunotherapy. Endokrynol Pol. 2018;69(6):722‐733. [DOI] [PubMed] [Google Scholar]
- 213.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Martner A, Rydström A, Riise RE, et al. Role of natural killer cell subsets and natural cytotoxicity receptors for the outcome of immunotherapy in acute myeloid leukemia. Oncoimmunology. 2016;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Walker PR, Khuder SA, Quan WDYJ. Continuous infusion interleukin‐2 and antihistamines in metastatic kidney cancer. Cancer Biother Radiopharm. 2005;20(5):487‐490. [DOI] [PubMed] [Google Scholar]
- 216.Atkins MB, Lotze MT, Dutcher JP, et al. High‐dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105‐2116. [DOI] [PubMed] [Google Scholar]
- 217.Middleton M, Sarno M, Agarwala SS, et al. Pharmacokinetics of histamine dihydrochloride in healthy volunteers and cancer patients: implications for combined immunotherapy with interleukin‐2. J Clin Pharmacol. 2002;42(7):774‐781. [DOI] [PubMed] [Google Scholar]
- 218.Yang L, Perry C. Histamine dihydrochloride: in the management of acute myeloid leukaemia. Drugs. 2011;71(1):109‐122. [DOI] [PubMed] [Google Scholar]
- 219.Perz J, Ho A. Histamine dihydrochloride for the treatment of acute myeloid leukemia, malignant melanoma and renal cell carcinoma. Future Oncol. 2008;4(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 220.Li J, Huang X, Wang Q, et al. Pharmacokinetic properties and safety profile of histamine dihydrochloride injection in Chinese healthy volunteers: a phase I, single‐center, open‐label, randomized study. Clin Ther. 2015;37(10):2352‐2364. [DOI] [PubMed] [Google Scholar]
- 221.Weinstock M, Rosenblatt J, Avigan D. Dendritic cell therapies for hematologic malignancies. Mol Ther—Methods Clin Dev. 2017;5:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Martner A, Thorén FB, Aurelius J, Hellstrand K. Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood Rev. 2013;27(5):209‐216. [DOI] [PubMed] [Google Scholar]
- 223.Romero AI, Thorén FB, Aurelius J, Askarieh G, Brune M, Hellstrand K. Post‐consolidation immunotherapy with histamine dihydrochloride and interleukin‐2 in AML. Scand J Immunol. 2009;70(3):194‐205. [DOI] [PubMed] [Google Scholar]
- 224.Thorén FB, Romero AI, Brune M, et al. Histamine dihydrochloride and low‐ dose interleukin‐2 as post‐consolidation immunotherapy in acute myeloid leukemia. Expert Opin Biol Ther. 2009;9(9):1217‐1223. [DOI] [PubMed] [Google Scholar]
- 225.Brune M, Hellstrand K. Remission maintenance therapy with histamine and interleukin‐2 in acute myelogenous leukaemia. Br J Haematol. 1996;92(3):620‐626. [DOI] [PubMed] [Google Scholar]
- 226.Tsunoda T, Tanimura H, Yamaue H, et al. In vitro augmentation of cytotoxic activity of peripheral blood mononuclear cells and tumor‐infiltrating lymphocytes by famotidine in cancer patients. Int J Immunopharmacol. 1992;14(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 227.Kiffin R, Grauers Wiktorin H, Nilsson MS, Aurelius J. Anti‐Leukemic Properties of Histamine in Monocytic Leukemia: The Role of NOX2. Front Oncol. 2018;8:1‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Berry SM, Broglio KR. antagonist for the control of nocturnalAddressing the incremental benefit of histamine dihydrochloride when added to interleukin‐2 in treating acute myeloid leukemia: a Bayesian meta‐analysis. Cancer Invest. 2011;29(4):293‐299. [DOI] [PubMed] [Google Scholar]
- 229.Rashidi A, Walter RB, Tallman MS. Maintenance therapy in acute myeloid leukemia: an evidence‐based review of randomized trials. Boold. 2016;128(6):763‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nilsson MS, Hallner A, Brune M. Immunotherapy with HDC/IL‐2 may be clinically efficacious in acute myeloid leukemia of normal karyotype. Hum Vaccin Immunother. 2020;16(1):109‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nilsson MS, Hallner A, Brune M, et al. Complete remission after the first cycle of induction chemotherapy determines the clinical efficacy of relapse‐preventive immunotherapy in acute myeloid leukaemia. Br J Haematol. 2020;188(4):e49‐e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Buyse M, Michiels S, Squifflet P, et al. Leukemia‐free survival as a surrogate end point for overall survival in the evaluation of maintenance therapy for patients with acute myeloid leukemia in complete remission. Haematologica. 2011;96(8):1106‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hellstrand K, Mellqvist UH, Wallhult E, et al. Histamine and interleukin‐2 in acute myelogenous leukemia. Leuk Lymphoma. 1997;27(5–6):429‐438. [DOI] [PubMed] [Google Scholar]
- 234.Sander FE, Nilsson M, Rydström A, et al. Role of regulatory T cells in acute myeloid leukemia patients undergoing relapse‐preventive immunotherapy. Cancer Immunol Immunother. 2017;66(11):1473‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sander FE, Rydström A, Bernson E, et al. Dynamics of cytotoxic T cell subsets during immunotherapy predicts outcome in acute myeloid leukemia. Oncotarget. 2016;7(7):7586‐7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cuapio A, Post M, Cerny‐Reiterer S, et al. Maintenance therapy with histamine plus IL‐2 induces a striking expansion of two CD56bright NK cell subpopulations in patients with acute myeloid leukemia and supports their activation. Oncotarget. 2016;7(29):46466‐46481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Martner A, Rydström A, Riise RE, et al. NK cell expression of natural cytotoxicity receptors may determine relapse risk in older AML patients undergoing immunotherapy for remission maintenance. Oncotarget. 2015;6(40):42569‐42574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rydström A, Hallner A, Aurelius J, et al. Dynamics of myeloid cell populations during relapse‐preventive immunotherapy in acute myeloid leukemia. J Leukoc Biol. 2017;102(2):467‐474. [DOI] [PubMed] [Google Scholar]
- 239.Aurelius J, Martner A, Brune M, et al. Remission maintenance in acute myeloid leukemia: impact of functional histamine H2 receptors expressed by leukemic cells. Haematologica. 2012;97(12):1904‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Heyman B, Yang Y. New developments in immunotherapy for lymphoma. Cancer Biol Med. 2018;15(3):189‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pishko A, Nasta SD. The role of novel immunotherapies in non‐Hodgkin lymphoma. Transl Cancer Res. 2017;6(1):93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Åhlberg R, MacNamara B, Andersson M, et al. Stimulation of T‐cell cytokine production and NK‐cell function by IL‐2, IFN‐α and histamine treatment during remission of non‐Hodgkin’s lymphoma. Hematol J. 2003;4(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 243.Perez D, Sklar L, Chigaev A, Matlawska‐Wasowska K. Drug repurposing for targeting cyclic nucleotide transporters in acute leukemias—a missed opportunity. Semin Cancer Biol. 2021;68:199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yin Y, Allen PD, Jia L, MacEy MG, Kelsey SM, Newland AC. Constitutive levels of cAMP‐dependent protein kinase activity determine sensitivity of human multidrug‐resistant leukaemic cell lines to growth inhibition and apoptosis by forskolin and tumour necrosis factor alpha. Br J Haematol. 2000;108(3):565‐573. [DOI] [PubMed] [Google Scholar]
- 245.Fernández N, Monczor F, Lemos B, et al. Reduction of G protein‐coupled receptor kinase 2 expression in U‐937 cells attenuates H2 histamine receptor desensitization and induces cell maturation. Mol Pharmacol. 2002;62(6):1506‐1514. [DOI] [PubMed] [Google Scholar]
- 246.Copsel S, Garcia C, Diez F, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. 2011;286(9):6979‐6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rodríguez González A, Sahores A, Díaz‐Nebreda A, et al. MRP4/ABCC4 expression is regulated by histamine in acute myeloid leukemia cells, determining cAMP efflux. FEBS J. 2021;288(1):229‐243. [DOI] [PubMed] [Google Scholar]
- 248.Donskov F, Von Der Maase H, Henriksson R, et al. Outpatient treatment with subcutaneous histamine dihydrochloride in combination with interleukin‐2 and interferon‐α in patients with metastatics renal cell carcinoma: results of an open single‐armed multicentre phase II study. Ann Oncol. 2002;13(3):441‐449. [DOI] [PubMed] [Google Scholar]
- 249.Donskov F, Bennedsgaard KM, Von Der MH, et al. Intratumoural and peripheral blood lymphocyte subsets in patients with metastatic renal cell carcinoma undergoing interleukin‐2 based immunotherapy: association to objective response and survival. Br J Cancer. 2002;87:194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Donskov F, Bennedsgaard KM, Hokland M, et al. Leukocyte orchestration in blood and tumour tissue following interleukin‐2 based immunotherapy in metastatic renal cell carcinoma. Cancer Immunol Immunother. 2004;53(8):729‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Donskov F, Middleton M, Fode K, et al. Two randomised phase II trials of subcutaneous interleukin‐2 and histamine dihydrochloride in patients with metastatic renal cell carcinoma. Br J Cancer. 2005;93(7):757‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kinouchi T, Sakamoto J, Tsukamoto T, et al. Prospective randomized trial of natural interferon‐alpha versus natural interferon‐alpha plus cimetidine in advanced renal cell carcinoma with pulmonary metastasis. J Cancer Res Clin Oncol. 2006;132(8):499‐504. [DOI] [PubMed] [Google Scholar]
- 253.Tatokoro M, Fujii Y, Kawakami S, Saito K, Koga F, Matsuoka Y. Phase‐II trial of combination treatment of interferon‐a, cimetidine, cyclooxygenase‐2 inhibitor and renin‐angiotensin‐system inhibitor (I‐CCA therapy) for advanced renal cell carcinoma. Cancer Sci. 2010;102(1):137‐143. [DOI] [PubMed] [Google Scholar]
- 254.Quan WDY, Vinogradov M, Quan FM, Khan N, Liles DK, Walker PR. Continuous infusion interleukin‐2 and famotidine in metastatic kidney cancer. Cancer Biother Radiopharm. 2006;21(5):515‐519. [DOI] [PubMed] [Google Scholar]
- 255.Quan WDY, Quan FM, King LA, Walker PR. Low‐dose cyclophosphamide and continuous‐infusion interleukin‐2 with famotidine in previously treated metastatic melanoma or kidney cancer. Cancer Biother Radiopharm. 2008;23(1):108‐113. [DOI] [PubMed] [Google Scholar]
- 256.Quan WDY, Quan FM. High‐dose intensity pulse interleukin‐2 with famotidine in metastatic kidney cancer. Cancer Biother Radiopharm. 2009;24(2):181‐183. [DOI] [PubMed] [Google Scholar]
- 257.Quan WDY, Gagnon GA, Walker PR, Quan FM. Pulse interleukin‐2 with famotidine induces CD56+ lymphocytes in the peripheral blood of patients with metastatic melanoma or kidney cancer. Cancer Biother Radiopharm. 2011;26(1):65‐67. [DOI] [PubMed] [Google Scholar]
- 258.Walter DY, Quan JFMQ. Activity of outpatient intravenous interleukin‐2 and famotidine in metastatic clear cell kidney. Cancer. 2014;29(2):58‐61. [DOI] [PubMed] [Google Scholar]
- 259.Gellrich F, Schmitz M, Beissert S, Meier F. Anti‐PD‐1 and novel combinations in the treatment of melanoma—an update. J Clin Med. 2020;9(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Quan WDY, Milligan KS, Quan FM, et al. Continuous infusion interleukin‐2 and intravenous famotidine in metastatic melanoma. Cancer Biother Radiopharm. 2006;21(6):607‐612. [DOI] [PubMed] [Google Scholar]
- 261.Quan WDY, Quan FM, Perez M, Johnson E. Outpatient intravenous interleukin‐2 with famotidine has activity in metastatic melanoma. Cancer Biother Radiopharm. 2012;27(7):442‐445. [DOI] [PubMed] [Google Scholar]
- 262.Quan W, Ramirez M, Taylor C, Vinogradov M, Quan F, Khan N. High‐dose continuous infusion plus pulse interleukin‐2 and famotidine in metastatic kidney cancer. Cancer Biother Radiopharm. 2004;20(1):36‐40. [DOI] [PubMed] [Google Scholar]
- 263.Quan WDY, Walker PR, Picton M, et al. High‐dose intensity pulse interleukin‐2 with famotidine has activity in metastatic melanoma. Cancer Biother Radiopharm. 2008;23(5):641‐646. [DOI] [PubMed] [Google Scholar]
- 264.Quan WDY, Quan FM. Activity of continuous infusion + pulse interleukin‐2 with famotidine in metastatic melanoma. Cancer Biother Radiopharm. 2009;24(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 265.Quan W, Knupp C, Quan F, Walker P. Pulse infusion interleukin‐2 with famotidine and cyclophosphamide has activity in previously treated metastatic melanoma. Cancer Biother Radiopharm. 2010;25(2):179‐183. [DOI] [PubMed] [Google Scholar]
- 266.Middleton M, Hauschild A, Thomson D, et al. Results of a multicenter randomized study to evaluate the safety and efficacy of combined immunotherapy with interleukin‐2, interferon‐α2b and histamine dihydrochloride versus dacarbazine in patients with stage IV melanoma. Ann Oncol. 2007;18(10):1691‐1697. [DOI] [PubMed] [Google Scholar]
- 267.Asemissen AM, Scheibenbogen C, Letsch A, et al. Addition of histamine to interleukin 2 treatment augments type 1 T‐cell responses in patients with melanoma in vivo: Immunologic results from a randomized clinical trial of interleukin 2 with or without histamine (MP 104). Clin Cancer Res. 2005;11(1):290‐297. [PubMed] [Google Scholar]
- 268.Lindnér PER, Rizell M, Mattsson JAN, et al. Combined treatment with histamine in patients with metastatic melanoma. Anticancer Res. 2004;24:1837‐1842. [PubMed] [Google Scholar]
- 269.Beusterien KM, Ackerman SJ, Plante K, et al. The health‐related quality‐of‐life impact of histamine dihydrochloride plus interleukin‐2 compared with interleukin‐2 alone in patients with metastatic melanoma. Support Care Cancer. 2003;11(5):304‐312. [DOI] [PubMed] [Google Scholar]
- 270.Schmidt H, Larsen S, Bastholt L, Fode K, Rytter C, von der Maase H. A phase II study of outpatient subcutaneous histamine dihydrochloride, interleukin‐2 and interferon‐α in patients with metastatic melanoma. Ann Oncol. 2002;13(12):1919‐1924. [DOI] [PubMed] [Google Scholar]
- 271.Creagan ET, Schaid DJ, Ahmann DL, Frytak S. Disseminated malignant melanoma and recombinant interferon: analysis of seven consecutive phase II investigations. J Invest Dermatol. 1990;95(6 Suppl):S188‐S192. [DOI] [PubMed] [Google Scholar]
- 272.Creagan WT, Ahmann DL, Frytak S, et al. Three consecutive phase I/ studies of recombinant interferon aifa‐2a in advanced malignant melanoma. Cancer. 1987;59:638‐646. [DOI] [PubMed] [Google Scholar]
- 273.Creagan BET, Ahmann DL, Green SJ, Long HJ, Frytak S, Itri LM. Phase II study of recombinant leukocyte A interferon (IFN‐rA) plus cimetidine in disseminated malignant melanoma. J Clin Oncol. 1985;3:977‐981. [DOI] [PubMed] [Google Scholar]
- 274.Schmidt H, Geertsen PF, Fode K, Rytter C, Bastholt L, von der Maase H. Subcutaneous interleukin‐2 and interferon‐alpha plus cisplatin with and without prophylactic cimetidine in patients with metastatic malignant melanoma: a phase II study. Melanoma Res. 2000;10(1):66‐77. [PubMed] [Google Scholar]
- 275.O’Day SJ, Agarwala SS, Naredi P, Kass CL, Gehlsen KR, Glaspy J. Treatment with histamine dihydrochloride and interleukin‐2 in patients with advanced metastatic malignant melanoma: a detailed safety analysis. Melanoma Res. 2003;13(3):307‐311. [DOI] [PubMed] [Google Scholar]
- 276.Agarwala SS, Glaspy J, O’Day SJ, et al. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin‐2 versus interleukin‐2 alone in patients with metastatic melanoma. J Clin Oncol. 2002;20(1):125‐133. [DOI] [PubMed] [Google Scholar]
- 277.Jørkov AS, Donskov F, Steiniche T, et al. Immune response in blood and tumour tissue in patients with metastatic malignant melanoma treated with IL‐2, IFNα and histamine dihydrochloride. Anticancer Res. 2003;23(1 B):537‐542. [PubMed] [Google Scholar]
- 278.Hellstrand K, Asea A, Hermodsson S. Role of histamine in natural killer cell‐mediated resistance against tumor cells. J Immunol. 1990;145(12):4365‐4370. [PubMed] [Google Scholar]
- 279.Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol. 2020;13:1756284820917527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nielsen J, Henning J. Effect of ranitidine and low‐dose interleukin‐2 in vitro on NK‐cell activity in peripheral blood from patients with liver metastases from colorectal cancer. Eur J Surg Oncol. 1995;21:526‐530. [DOI] [PubMed] [Google Scholar]
- 282.Losurdo G, Principi M, Girardi B, et al. Histamine and histaminergic receptors in colorectal cancer: from basic science to evidence‐based medicine. Anticancer Agents Med Chem. 2018;18(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 283.Shi Z, Fultz RS, Engevik MA, et al. Distinct roles of histamine H1‐ and H2‐receptor signaling pathways in inflammation‐associated colonic tumorigenesis. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G205‐G216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pietrangeli P, Seguella L, Annunziata G, et al. Lathyrus sativus diamine oxidase counteracts histamine‐induced cell proliferation, migration and pro‐angiogenic mediators release in human colon adenocarcinoma cell line Caco‐2. Phytother Res. 2019;33(7):1878‐1887. [DOI] [PubMed] [Google Scholar]
- 285.Tsaur I, Brandt MP, Juengel E, Manceau C, Ploussard G. Immunotherapy in prostate cancer: new horizon of hurdles and hopes. World J Urol. 2020. 10.1007/s00345-020-03497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Johansson S, Landström M, Hellstrand K, Henriksson R. The response of Dunning R3327 prostatic adenocarcinoma to IL‐2, histamine and radiation. Br J Cancer. 1998;77(8):1213‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Johansson S, Landström M, Henriksson R. Alterations of tumour cells, stroma and apoptosis in rat prostatic adenocarcinoma following treatment with histamine, interleukin‐2 and irradiation. Anticancer Res. 1999;19(3A):1961‐1969. [PubMed] [Google Scholar]
- 288.Klemen ND, Kelly CM, Bartlett EK. The emerging role of immunotherapy for the treatment of sarcoma. J Surg Oncol. 2020. 10.1002/jso.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brunstein F, Hoving S, Aan De Wiel‐Ambagtsheer G, et al. Decreased response rates by the combination of histamine and IL‐2 in melphalan‐based isolated limb perfusion. Cancer Immunol Immunother. 2007;56(4):573‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Patel MA, Pardoll DM. Concepts of immunotherapy for glioma. J Neurooncol. 2015;123(3):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Johansson M, Henriksson R, Bergenheim AT, Koskinen LOD. Interleukin‐2 and histamine in combination inhibit tumour growth and angiogenesis in malignant glioma. Br J Cancer. 2000;83(6):826‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mahmoud SMA, Paish EC, Powe DG, et al. Tumor‐infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949‐1955. [DOI] [PubMed] [Google Scholar]
- 293.Hadrup S, Donia M, Thor SP. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mitra R, Singh S, Khar A. Antitumour immune responses. Expert Rev Mol Med. 2003;5(3):1‐19. [DOI] [PubMed] [Google Scholar]
- 295.Birbrair A. Tumor Microenvironment Hematopoietic Cells—Part A. Vol 1224. Basingstoke: Springer Nature; 2020. [Google Scholar]
- 296.Konjević GM, Vuletić AM, Mirjačić Martinović KM, Larsen AK, Jurišić VB. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine. 2019;117:30‐40. [DOI] [PubMed] [Google Scholar]
- 297.Nicholson SE, Keating N, Belz GT. Natural killer cells and anti‐tumor immunity. Mol Immunol. 2019;110:40‐47. [DOI] [PubMed] [Google Scholar]
- 298.Dadi S, Chhangawala S, Whitlock BM, et al. Cancer immunosurveillance by tissue‐resident innate lymphoid cells and innate‐like T cells. Cell. 2016;164(3):365‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Guillerey C, Smyth MJ. NK cells and cancer immunoediting. Assess Eval High Educ. 2015;37:435. [Google Scholar]
- 300.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121(6):1058‐1063. [DOI] [PubMed] [Google Scholar]
- 301.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577‐583. [PubMed] [Google Scholar]
- 302.Coca S, Perez‐Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colerectal carcinoma. Cancer. 1997;79(12):2320‐2328. [DOI] [PubMed] [Google Scholar]
- 303.Hashemi V, Maleki LA, Esmaily M, et al. Regulatory T cells in breast cancer as a potent anti‐cancer therapeutic target. Int Immunopharmacol. 2020;78:106087. [DOI] [PubMed] [Google Scholar]
- 304.Ghalamfarsa G, Kazemi MH, Raoofi Mohseni S, et al. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Targets. 2018;23(2):127‐142. [DOI] [PubMed] [Google Scholar]
- 305.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells. Nature. 2011;475(7355):226‐230. [DOI] [PubMed] [Google Scholar]
- 306.Shevach EM. Mechanisms of Foxp3+ T regulatory cell‐mediated suppression. Immunity. 2009;30(5):636‐645. [DOI] [PubMed] [Google Scholar]
- 307.Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene‐3 engagement of MHC class II. J Immunol. 2008;180(9):5916‐5926. [DOI] [PubMed] [Google Scholar]
- 308.Loser K, Apelt J, Voskort M, et al. IL‐10 controls ultraviolet‐induced carcinogenesis in mice. J Immunol. 2007;179(1):365‐371. [DOI] [PubMed] [Google Scholar]
- 309.Garín MI, Chu NC, Golshayan D, Cernuda‐Morollón E, Wait R, Lechler RI. Galectin‐1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109(5):2058‐2065. [DOI] [PubMed] [Google Scholar]
- 310.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294‐307. [DOI] [PubMed] [Google Scholar]
- 311.Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J. The B‐side of cancer immunity: the underrated tune. Cells. 2019;8(5):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti‐tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jahrsdörfer B, Blackwell SE, Wooldridge JE, et al. B‐chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin‐21‐based activation. Blood. 2006;108(8):2712‐2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mohammed ZMA, Going JJ, Edwards J, McMillan DC. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev. 2012;38(8):943‐955. [DOI] [PubMed] [Google Scholar]
- 315.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2796‐2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Li Q, Lao X, Pan Q, et al. Adoptive transfer of tumor reactive B cells confers host T‐cell immunity and tumor regression. Clin Cancer Res. 2011;17(15):4987‐4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Olkhanud PB, Damdinsuren B, Bodogai M, et al. Tumor‐evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T‐regulatory cells. Cancer Res. 2011;71(10):3505‐3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B‐cell‐derived lymphotoxin promotes castration‐resistant prostate cancer. Nature. 2010;464(7286):302‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Andreu P, Johansson M, Affara NI, et al. FcRγ activation regulates inflammation‐associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.De Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411‐423. [DOI] [PubMed] [Google Scholar]
- 321.Fleming V, Hu X, Weber R, et al. Targeting myeloid‐derived suppressor cells to bypass tumor‐induced immunosuppression. Front Immunol. 2018;9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid‐derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol. 2017;233(4):3024‐3036. [DOI] [PubMed] [Google Scholar]
- 323.Cao Y, Slaney CY, Bidwell BN, et al. BMP4 inhibits breast cancer metastasis by blocking myeloid‐derived suppressor cell activity. Cancer Res. 2014;74(18):5091‐5102. [DOI] [PubMed] [Google Scholar]
- 324.Brimnes MK, Vangsted AJ, Knudsen LM, et al. Increased level of both CD4+FOXP3+ Regulatory t Cells and CD14+HLA‐DR‐/low myeloid‐derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540‐547. [DOI] [PubMed] [Google Scholar]
- 325.Ostrand‐Rosenberg S, Sinha P, Beury DW, Clements VK. Cross‐talk between myeloid‐derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor‐induced immune suppression. Semin Cancer Biol. 2012;22(4):275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, et al. Dendritic cell therapy in cancer treatment; the state‐of‐the‐art. Life Sci. 2020;254:117580. [DOI] [PubMed] [Google Scholar]
- 327.Giovanelli P, Sandoval TA, Cubillos‐Ruiz JR. Dendritic cell metabolism and function in tumors. Trends Immunol. 2019;40(8):699‐718. [DOI] [PubMed] [Google Scholar]
- 328.Binnewies M, Mujal AM, Pollack JL, et al. Unleashing type‐2 dendritic cells to drive protective antitumor CD4+ T Cell Immunity. Cell. 2019;177(3):556‐571.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fu C, Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol. 2018;9:3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Böttcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4(11):784‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Enamorado M, Iborra S, Priego E, et al. Enhanced anti‐tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun. 2017;8:1‐11. 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Quaranta V, Schmid MC. Macrophage‐mediated subversion of anti‐tumour immunity. Cell. 2019;8(7):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor‐associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Prenen H, Mazzone M. Tumor‐associated macrophages: a short compendium. Cell Mol Life Sci. 2019;76(8):1447‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Najafi M, Hashemi Goradel N, Farhood B, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120(3):2756‐2765. [DOI] [PubMed] [Google Scholar]
- 338.Sammarco G, Varricchi G, Ferraro V, et al. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int J Mol Sci. 2019;20(9):2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Carpenco E, Ceauşu RA, Cimpean AM, et al. Mast cells as an indicator and prognostic marker in molecular subtypes of breast cancer. In Vivo. 2019;33(3):743‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Glajcar A, Szpor J, Pacek A, et al. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch. 2017;470(5):505‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rao Q, Chen Y, Yeh CR, et al. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget. 2016;7(7):7842‐7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Li L, Dang Q, Xie H, et al. Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA‐HOTAIR/PRC2‐androgen receptor (AR)‐MMP9 signals and increased stem/progenitor cell population. Oncotarget. 2015;6(16):14179‐14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19(1):149‐159. [DOI] [PubMed] [Google Scholar]
- 344.Elpek GO, Gelen T, Aksoy NH, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54(12):940‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lucarini V, Ziccheddu G, Macchia I, et al. IL‐33 restricts tumor growth and inhibits pulmonary metastasis in melanoma‐bearing mice through eosinophils. Oncoimmunology. 2017;6(6):e1317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.O’Flaherty SM, Kripitch Sutummaporn WLH, Worrall AP, et al. Comparison between different D‐Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int J Lab Hematol. 2017;38(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 347.Xing Y, Tian Y, Kurosawa T, et al. CCL11‐induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells. 2016;21(6):624‐638. [DOI] [PubMed] [Google Scholar]
- 348.Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti‐tumor response. Cancer Immunol Immunother. 2012;61(9):1527‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159‐2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Shaul ME, Fridlender ZG. Cancer‐related circulating and tumor‐associated neutrophils—subtypes, sources and function. FEBS J. 2018;285(23):4316‐4342. [DOI] [PubMed] [Google Scholar]
- 351.Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G. Roles of neutrophils in cancer growth and progression. J Leukoc Biol. 2018;103(3):457‐464. [DOI] [PubMed] [Google Scholar]
- 352.Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83‐91. [DOI] [PubMed] [Google Scholar]
- 354.Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen‐presenting cell. J Leukoc Biol. 2015;98(4):489‐496. [DOI] [PubMed] [Google Scholar]
- 355.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor‐associated neutrophils stimulate T cell responses in early‐stage human lung cancer. J Clin Invest. 2014;124(12):5466‐5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Raccosta L, Fontana R, Maggioni D, et al. The oxysterol‐cxcr2 axis plays a key role in the recruitment of tumor‐promoting neutrophils. J Exp Med. 2013;210(9):1711‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor‐associated neutrophil phenotype by TGFb “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wislez M, Rabbe N, Marchal J, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63(6):1405‐1412. [PubMed] [Google Scholar]
- 360.Montfort A, Colacios C, Levade T, Andrieu‐Abadie N, Meyer N, Ségui B. The TNF paradox in cancer progression and immunotherapy. Front Immunol. 2019;10:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Piliponsky AM, Shubin NJ, Lahiri AK, et al. Basophil‐derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol. 2019;20(2):129‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Visciano C, Liotti F, Prevete N, et al. Mast cells induce epithelial‐to‐mesenchymal transition and stem cell features in human thyroid cancer cells through an IL‐8‐Akt‐Slug pathway. Oncogene. 2015;34(40):5175‐5186. [DOI] [PubMed] [Google Scholar]
- 364.Prevete N, Staiano RI, Granata F, et al. Expression and function of Angiopoietins and their tie receptors in human basophils and mast cells. J Biol Regul Homeost Agents. 2013;27(3):827‐839. [PubMed] [Google Scholar]
- 365.Cerny‐Reiterer S, Ghanim V, Hoermann G, et al. Identification of basophils as a major source of hepatocyte growth factor in chronic myeloid leukemia: a novel mechanism of BCR‐ABL1‐independent disease progression. Neoplasia. 2012;14(7):572‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev. 2011;242(1):144‐160. [DOI] [PubMed] [Google Scholar]
- 367.Tschopp CM, Spiegl N, Didichenko S, et al. Granzyme B, a novel mediator of allergic inflammation: Its induction and release in blood basophils and human asthma. Blood. 2006;108(7):2290‐2299. [DOI] [PubMed] [Google Scholar]
- 368.Meiler F, Zumkehr J, Klunker S, et al. In vivo switch to IL‐10‐secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887‐2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Smolinska S, Groeger D, Perez NR, et al. Histamine receptor 2 is required to suppress innate immune responses to bacterial ligands in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1575‐1586. [DOI] [PubMed] [Google Scholar]
- 370.Toyota H, Dugovic C, Koehl M, et al. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002;62:389‐397. [DOI] [PubMed] [Google Scholar]
- 371.Teuscher C, Subramanian M, Noubade R. Central histamine H3 receptor signaling negatively regulates susceptibility to autoimmune inflammatory disease of the CNS. Proc Natl Acad Sci U S A. 2007;104:10146‐10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Dunford PJ, O’Donnell N, Riley JP, et al. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062‐7070. [DOI] [PubMed] [Google Scholar]
- 373.Jutel JM, Blaser K, Akdis CA. The role of histamine in regulation of immune responses. Chem Immunol and Allergy. 2006;2006(91):174‐187. [DOI] [PubMed] [Google Scholar]
- 374.Kim CH, Lee JM, Yoo JK, et al. Inhibitory effect of imiquimod‐induced psoriasis‐like skin inflammation in mice by histamine H4 receptor agonist 4‐methylhistamine. Scand J of Immunol. 2016;83:409‐417. [DOI] [PubMed] [Google Scholar]
- 375.Takagaki K, Osawa S, Horio Y, et al. Cytokine responses of intraepithelial lymphocytes are regulated by histamine H2 receptor. J Gastroenterol. 2009;44(4):285‐296. [DOI] [PubMed] [Google Scholar]
- 376.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors, and their role in immune pathology. Clin Exp Allergy. 2009;39(12):1786‐1800. [DOI] [PubMed] [Google Scholar]
- 377.Renkl A, Berod L, Mockenhaupt M, et al. Distinct effects of sphingosine‐1‐phosphate, lysophosphatidic acid and histamine in human and mouse dendritic cells. Int J Mol Med. 2004;13:203‐209. [PubMed] [Google Scholar]
- 378.Lippert U, Moller A, Welker P, et al. Inhibition of cytokine secretion from human leukemic mast cells and basophils by H1‐ and H2‐receptor antagonists. Exp Dermatol. 2000;9:118‐124. [DOI] [PubMed] [Google Scholar]
- 379.Ehling S, Roßbach K, Dunston SM, et al. Allergic inflammation is augmented via histamine H4 receptor activation: The role of natural killer cells in vitro and in vivo. J Dermatol Sci. 2016;83(2):106‐115. [DOI] [PubMed] [Google Scholar]
- 380.Hellstrand K, Brune M, Naredi P, et al. Histamine: a novel approach to cancer immunotherapy. Cancer Invest. 2000;18(4):347‐355. [DOI] [PubMed] [Google Scholar]
- 381.Noubade R, Milligan G, Zachary JF, et al. Histamine receptor H1 is required for TCR‐mediated p38 MAPK activation and optimal IFN‐gamma production in mice. J Clin Invest. 2007;117(11):3507‐3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Fujimoto M, Kimata H. Histamine inhibits immunoglobulin production via histamine H2 receptors without affecting cell growth in human B cells. Clin Immunol Immunopathol. 1994;73(1):96‐102. [DOI] [PubMed] [Google Scholar]
- 383.Czerner CP, Klos A, Seifert R, et al. Histamine induces chemotaxis and phagocytosis in murine bone marrow‐derived macrophages and RAW 264.7 macrophage‐like cells via histamine H4‐receptor. Inflamm Res. 2014;63(3):239‐247. [DOI] [PubMed] [Google Scholar]
- 384.Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2019: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Alexander SPH, Christopoulos A, Davenport AP, et al. The concise guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(Suppl 1):S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.