Abstract
Background:
Non-urothelial carcinoma (UC) malignancies are traditionally considered to yield a more aggressive clinical course and little is known about their response to neoadjuvant therapy. We examined the effect of neoadjuvant chemotherapy (NAC) on a large population of bladder cancer (BCa) patients with histological variant (HV).
Methods:
We relied on aretrospective, multicentre database including 2,858 patients with BCa, who received radical cystectomy (RC) +/− NAC, between 1990 and 2017. Pure and mixed HVs were grouped into six categories: squamous cell carcinoma (SCC) (n=283, 45%), other subtypes (n=95, 15%), micropapillary (n=85, 14%), adenocarcinoma (n=65, 10%), small-cell (n=54, 8.6%) and sarcomatous (n=47, 7.6%). Kaplan-Meier and Cox regression analyses examined the cancer-specific survival (CSS) according to HVs, using pure UC as reference. Logistic regression models examined the odds of clinic-pathological downstaging after NAC according to HV.
Results:
Overall, we identified2,229 pure UC and 629 BCa with HV at RC. Among neoadjuvant chemotherapy-treated patients (N=450), only patients with SCC (44, 9.8%) were associated with worse CSS (median CSS: 33 vs. 116 months, p<0.001) and higher mortality rates (hazard ratio: 2.1, p=0.03) compared to pure UC (328, 72.9%). Analyses were also confirmed when pure and mixed cases were considered separately. After adjusting for NAC, only SCC showed lower rate of clinical-to-pathological downstaging (odds ratio: 0.4, p=0.03) compared to UC.
Conclusions:
SCC was the variant histology exhibiting the lowest effect of NAC in terms of activity and CSS. In comparison with pure UC, SCC seems to be insensitive to traditional NAC regimens.
Keywords: Squamous-cell carcinoma, Neoadjuvant chemotherapy, Bladder cancer, Genomic profile, Immunotherapy
MICROABSTRACT
Little is known about the response to neoadjuvant therapy of non-urothelial carcinoma (UC) bladder malignancies. Using a retrospective, multicentre database, we found that squamous-cell carcinoma (SCC) was the histology exhibiting the lowest effect of neoadjuvant chemotherapy in terms of activity and cancer specific survival. Among the non-UC histologies, SCC seems to be insensitive to traditional neoadjuvant chemotherapy regimens.
Introduction
Cisplatin-based neoadjuvant chemotherapy (NAC) is the standard of care in the management of patients with bladder cancer (BCa) who are candidates for radical cystectomy (RC) [1,2]. During the past few decades, several efforts have been spent to identify more active chemotherapy regimens and to enable appropriate patient selection through the use of clinical and genomic predictors of response to chemotherapy[3]. The majority of studies in the field focused on pure or predominant urothelial carcinoma (UC), the latter being conventionally defined as cases presenting with ≥50% of UC component within the tumour specimen. Altogether, these cases comprise about 90% of BCa, whereas little is known about non-urothelial histologies, and their appropriate management is still unknown [4,5]. Interestingly, UC has a propensity for divergent differentiations, ranging from 7% to 81% [6–8]. Taken together, non-UC malignancies are traditionally considered to yield a more aggressive clinical course, resulting in a dismal prognosis for the majority of patients [9–11]. Nevertheless, caution should be applied to generalize these findings to all variant histologies[12,13]. For instance, cisplatin-based chemotherapy regimens have shown to improve survival in patients with neuroendocrine variants [14], while the beneficial role of neoadjuvant chemotherapy in the other histological variants has yet to be proven. In this context, the primary aim of the present study was to examine the efficacy of NAC on cancer-specific survival (CSS) across different HVs in BCa. We attempted to identify whether one or more BCa VHs were associated with modified/reduced NAC activity in comparison with pure UC histology, in terms of CSS.
Patients and Methods:
Study design and clinical database characteristics
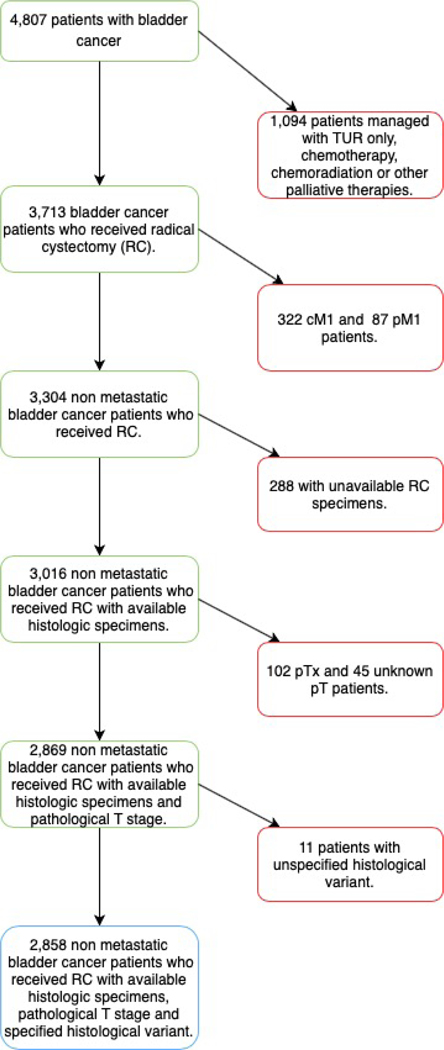
This multi-institutional database includes data gathered from hospitals in the United States, Europe, Israel, and Canada. Overall, 4,807 patients with non-metastatic BCa treated with RC between January 1990 and September 2017 were included. The criteria for initial patient selection included: any histology, clinical TanyNany M0 stage, and radical cystectomy performance with or without neoadjuvant chemotherapy. Clinical TN stages were defined according to TURB and preoperative CT scan. For those patients who received neoadjuvant chemotherapy, administration of at least 2 cycles of any regimen was required (Figure 1). The administration of any new drug, either alone or combined with neoadjuvant chemotherapy, was not allowed. The histological diagnosis was obtained from the radical cystectomy specimens, rather than from TURB specimens. Histological evaluation was not centralized but performed independently at each institution relying on expert uro-pathologists. The study was approved by the Institutional Review Board from all participating institutions that constitute this multi-institutional database.
Figure 1.
Inclusion and exclusion criteria flow-chart.
Statistical analyses
Descriptive statistics included frequencies and proportions for categorical variables. Medians and interquartile ranges (IQR) were reported for continuous variables. The statistical significance of differences in medians and proportions was respectively tested with the Kruskal-Wallis and Chi-square tests. Analyses were performed by grouping histological variants into 6 categories, where pure and mixed cases of each histological variant were respectively combined. Furthermore, we analysed data by separating pure from mixed cases of each variant histology. Here, 11 histological subgroups were tested as independent predictors. For example, squamous cell carcinoma (SCC) was analysed combining pure and mixed cases, and also as pure SCC vs. UC with SCC component, separately. The primary study endpoint was CSS, which was analysed across all different histological variants and according to treatment delivered. Here, UC was used as reference category while all others histological variants were compared with. CSS was defined as the time from RC to cancer-specific death. Data were censored if the patients were alive at last follow-up, or if they died for other causes. The secondary endpoint was the clinical-to-pathological downstaging, which was defined as a pathologic nodal stage (N stage) that was at least one stage lower than the pre-NAC clinical N stage[15]. Whether no variation of the N stage occurred, we used the above-mentioned criteria using the tumour stage (pre-NAC cT and pT). Kaplan-Meier method was used for estimation of CSS. Logistic regression and Cox regression models were used to analyse the effect of NAC. Subgroup analyses specifically focused on cN0 and cisplatin-only treated patients. The reverse Kaplan-Meier method was used for follow-up quantification. All statistical tests were two-sided with a level of significance set at p<0.05. Analyses were performed using the R software environment for statistical computing and graphics.
Results:
Study population
We identified 2,858 patients with cTanyNanyM0 bladder cancer treated with RC ± NAC who were suitable for the study purposes. (Table 1). Overall, 2,229 (78%) had pure UC, while 629 (22%) exhibited pure or predominant histological variant. The most frequently represented histological variant was SCC (n=283, 45%) followed by other subtypes (n=95, 15%), micropapillary tumours (n=85, 14%), adenocarcinoma (n=65, 10%), small-cell/neuroendocrine (n=54, 8.6%) and sarcomatous components (n=47, 7.6%). NAC was administered in 450 (16%) patients. However, NAC was variably administered according to different histological variants. Specifically, small-cell carcinomas (35.4%) and micropapillary tumours (35.1%) were the histologies most frequently treated with NAC, followed by SCC (19.4%), UC (18.5%), adenocarcinoma (17%), other subtypes (14.3%), and sarcomatous variants (12.1%). Cisplatin-based chemotherapy was the preferred NAC regimen in UC, SCC, sarcoma, micropapillary and other subtypes, ranging from 73 to 57% (Supplementary Table 1).
Table 1.
Overall patient characteristics of 2,858 non-metastatic (cM0pM0) bladder cancer (BCa) patients (cTanyNany) treated with radical cystectomy at 27 tertiary care centers.
| Variables | Rate (%) | ||
|---|---|---|---|
| Overall | N | 2858 (100) | |
|
| |||
| Age at cystectomy (years) | Median | 68yrs | |
|
| |||
| Range | 61–7 5yrs | ||
|
| |||
| Gender | Female | 527 (18.4) | |
|
| |||
| Male | 2331 (81.6) | ||
|
| |||
| Smoke habits | Never smoked | 673 (23.5) | |
|
| |||
| Current smoker | 690 (24.1) | ||
|
| |||
| Former smoker | 1144 (40) | ||
|
| |||
| Unknown | 351 (12.3) | ||
|
| |||
| Surgical Margins status | Negative | 2454 (85.9) | |
|
| |||
| Positive | 319 (11.2) | ||
|
| |||
| Unknown | 85 (3) | ||
|
| |||
| Charlson Comorbidity Index (CCI) | >=2 | 1077 (37.7) | |
|
| |||
| 0 | 731 (25.6) | ||
|
| |||
| 1 | 347 (12.1) | ||
|
| |||
| Unknown | 703 (24.6) | ||
|
| |||
| Ethnicity | Not Hispanic/Latino, White | 2520 (88.2) | |
|
| |||
| Asian | 23 (0.8) | ||
|
| |||
| Hispanic or Latino | 106 (3.7) | ||
|
| |||
| Not Hispanic/Latino, Black | 47 (1.6) | ||
|
| |||
| Other or mixed | 162 (5.7) | ||
|
| |||
| Histology at RC specimens | UC | 2229 (78) | |
|
| |||
| Adenocarcinoma | 65 (2.3) |
||
| Pure | 38 (58.5) | ||
| UC with Glandular component | 27 (41.5) | ||
|
| |||
| Micropapillary | 85 (3) | ||
|
| |||
| Other subtypes | 95 (3.3) | ||
|
| |||
| Pure other/mixed non UC | 63 (66.3) | ||
|
| |||
| UC with others component | 32 (33.7) | ||
|
| |||
| Sarcoma | 47 (1.6) |
||
| Pure | 17 (36.2) | ||
| UC with sarcomatoid component | 30 (63.8) | ||
|
| |||
| SCC | 283 (9.9) |
||
| Pure | 127 (44.9) | ||
| UC with squamous component | 156 (55.1) | ||
|
| |||
| Small-Cells | 54 (1.9) |
||
| Pure | 41 (75.9) | ||
| UC with small-cell component | 13 (24.1) | ||
|
| |||
| Clinical T stage | <cT2 | 506 (17.7) | |
|
| |||
| cT2 | 1632 (57.1) | ||
|
| |||
| cT3–4 | 357 (12.5) | ||
|
| |||
| Unknown | 363 (12.7) | ||
|
| |||
| Clinical N stage | cN0 | 1247 (43.6) | |
|
| |||
| cN1 | 153 (5.4) | ||
|
| |||
| cN2 | 103 (3.6) | ||
|
| |||
| cN3 | 11 (0.4) | ||
|
| |||
| cNX | 462 (16.2) | ||
|
| |||
| Unknown | 882 (30.9) | ||
|
| |||
| NAC | No | 2207 (77.2) | |
|
| |||
| Unknown | 201 (7) | ||
|
| |||
| Yes | 450 (15.7) | ||
|
| |||
| NAC Regimen | Carboplatin based | 45 (1.6) | |
|
| |||
| CMV/MVEC | 19 (0.7) | ||
|
| |||
| Gemcitabine + cisplatin | 197 (6.9) | ||
|
| |||
| MVAC | 96 (3.4) | ||
|
| |||
| No NAC | 2205 (77.2) | ||
|
| |||
| Others | 95 (3.3) | ||
|
| |||
| Unknown | 201 (7) | ||
|
| |||
| RT to the primary | Yes | 143 (5) | |
|
| |||
| AC | No | 1638 (57.3) | |
|
| |||
| Unknown | 720 (25.2) | ||
|
| |||
| Yes | 500 (17.5) | ||
|
| |||
| AC Regimen | Carboplatin based | 69 (2.4) | |
|
| |||
| CMV/MVEC | 7 (0.2) | ||
|
| |||
| Gemcitabine + cisplatin | 198 (6.9) | ||
|
| |||
| MVAC | 57 (2) | ||
|
| |||
| No AC | 1637 (57.3) | ||
|
| |||
| Others | 170 (5.9) | ||
|
| |||
| Unknown | 720 (25.2) | ||
|
| |||
| LND | LND | 1655 (57.9) | |
|
| |||
| no LND | 49 (1.7) | ||
|
| |||
| Unknown | 1154 (40.4) | ||
|
| |||
| pT stage | pT0 | 123 (4.3) | |
|
| |||
| pT1 | 241 (8.4) | ||
|
| |||
| pT2 | 540 (18.9) | ||
|
| |||
| pT3 | 1214 (42.5) | ||
|
| |||
| pT4 | 527 (18.4) | ||
|
| |||
| pTa-is | 213 (7.5) | ||
|
| |||
| pTN stage | <pT2N0M0 | 399 (14) | |
|
| |||
| pT0N0M0 | 110 (3.8) | ||
|
| |||
| pT2N0M0 | 355 (12.4) | ||
|
| |||
| pT3–4N0M0 | 787 (27.5) | ||
|
| |||
| pTanyN+M0 | 1066 (37.3) | ||
|
| |||
| pT anyNX | 141 (4.9) | ||
|
| |||
| Treatment received | NAC + RC + no AC | 366 (12.8) | |
|
| |||
| NAC + RC + AC | 35 (1.2) | ||
|
| |||
| no NAC + RC + AC | 434 (15.2) | ||
|
| |||
| RC alone | 1244 (43.5) | ||
|
| |||
| Unknown | 779 (27.3) | ||
Abbreviations:
UC: Urothelial carcinoma
SCC: Squamous-cell carcinoma
RC: Radical cystectomy
NAC: Neoadjuvant chemotherapy
AC: Adjuvant chemotherapy
CSS outcomes
The median follow-up was 29 months (IQR: 12–57 months). Overall, we recorded 751 (33%) cancer-related death events. Median CSS rates for each histological variant and stratified according to received treatment are reported in Table 2. Noteworthy, we found that SCC (n=44, 9.8%) was the only histological variant associated with worse CSS compared to UC (with a median of 33 vs. 116 months, 95% confidence interval [CI]: 17-NA months, p<0.001), when only NAC-treated patients (n=450, being the 15.7% of all study population) were considered (Figure 2). Within the 450 NAC-treated patients, SCC variant exhibited higher cancer specific mortality (CSM) rates after multivariable adjustment (HR: 2.1, 95%CI: 1.1–4.2, p=0.03) compared to UC (Table 3). Similar results were obtained in the subgroup of patients who showed a bladder-confined tumour (pN0) on final (RC specimens) pathological examination (Supplementary Table 2 and Supplementary Table 3). Subgroup analyses including cN0 and cisplatin-only treated patients showed that SCC was the only histological variant associated with higher CSM after uni- and multi- variable adjustments, when only patients treated with NAC were considered (results not shown).
Table 2.
Median Cancer-specific Survival Stratified by Histologic Type and Perioperative Treatment (Compared With UC as Reference)
| Median CSS (IQR), mo; P Value |
|||||
|---|---|---|---|---|---|
| Histologic Variant | Overall (n = 2858) | NAC + RC (n = 450) | RC Alone (n = 1244) | RC + AC (n = 500) | RC + Perioperative CHT (n = 915) |
| Pure and mixed combined | |||||
| UC | 116 (103–133) | 116 (64-NR) | 127 (115–179) | 103 (88–145) | 112 (98–145) |
|
| |||||
| Adenocarcinoma | 76 (30-NR); .8 | 107 (30-NR); .64 | NR (NR); .36 | 74 (23-NR); .23 | 76 (30-NR); .57 |
|
| |||||
| Micropapillary | 65 (31-NR); .056 | 28 (22-NR); .23 | 65 (33-NR); .2 | 31 (23-NR); .091 | 31 (23-NR); .056 |
|
| |||||
| Small cell | 41 (30-NR); .15 | 46 (34-NR); .4 | 33 (30-NR); .073 | NR (NR); .35 | 46 (34-NR); .61 |
|
| |||||
| Other subtype | 74 (33-NR); .11 | NR (22-NR); .48 | 33 (10-NR); .012a | 132 (42-NR); .77 | 132 (42-NR); .64 |
|
| |||||
| Sarcoma | 14 (9-NR); < .0001a | 12 (12-NR); .98 | 9 (8-NR); < .0001a | 9 (4-NR); < .0001a | 12 (9-NR); .035a |
|
| |||||
| SCC | 47 (33-NR); .00034a | 33 (17-NR); .00059a, b | 268 (47-NR); .16 | 38 (23-NR); .093 | 34 (25-NR); .0016a |
| Pure and mixed separated | |||||
| UC | 116 (103–133) | 116 (64-NR) | 127 (115–179) | 103 (88–145) | 112 (98–145) |
|
| |||||
| Pure adenocarcinoma | 104 (76-NR); .77 | 107 (NR-NR); .5 | NR (28-NR); .9 | 76 (11-NR); .06 | 107 (76-NR); .55 |
|
| |||||
| Micropapillary | 65 (31-NR); .056 | 28 (22-NR); .23 | 65 (33-NR); .2 | 31 (23-NR); .091 | 31 (23-NR); .056 |
|
| |||||
| Pure small cell | 41 (33-NR); .2 | 46 (34-NR); .38 | 33 (30-NR); .1 | NR (NR); .35 | 46 (34-NR); .65 |
|
| |||||
| Pure other/mixed non-UC | 100 (48-NR); .7 | NR (22-NR); .87 | NR (10-NR); .38 | 132 (100-NR); .39 | 132 (100-NR); .3 |
|
| |||||
| Pure sarcoma | 9 (8-NR); < .0001a, b | 12 (NR); .0098a | 9 (8-NR); .00024a, b | 9 (NR-NR); .00052a,b | 10.5 (9-NR); < .0001a, b |
|
| |||||
| Pure SCC | 70 (40-NR); .13 | 20.5 (8-NR); .0016a, b | 268 (70-NR); .78 | 107 (15-NR); .52 | 29 (15-NR); .082 |
|
| |||||
| UC with glandular component | 74 (24-NR); .94 | 30 (NR); .87 | NR (NR); .13 | 74 (23-NR); .92 | 74 (30-NR); .83 |
|
| |||||
| UC with other component | 33 (24-NR); .016a | 24 (6-NR); .42 | 25 (6-NR); .002a | 42 (21-NR); .24 | 42 (24-NR); .4 |
|
| |||||
| UC with sarcomatoid component | 25 (14-NR); .011a, b | NR (NR); .34 | 14 (4-NR); < .0001a, b | 14.5 (4-NR); .0011a, b | 25 (25-NR); .51 |
|
| |||||
| UC with small cell component | NR (16-NR); .48 | NR (16-NR); .89 | 30 (14-NR); .36 | NA | NR (16-NR); .82 |
|
| |||||
| UC and SCC component | 34 (25-NR); 0.00017a, b | 33 (17-NR); .014a | NR (22-NR); .014a, b | 38 (23-NR); .084 | 34 (24-NR); .0053a |
Abbreviations: AC = adjuvant chemotherapy; CHT = chemotherapy; CSS = cancer-specific survival; IQR = interquartile range; NA = not applicable; NAC = neoadjuvant chemotherapy; NR = not reached; RC = radical cystectomy; SCC = squamous cell carcinoma; UC = urothelial carcinoma.
Statistically significant.
Statistically significant also on multivariable analysis.
Figure 2.
Cancer-specific survival rates according to BCa histologies in 2,858 cTanyNanyM0 patients treated with radical cystectomy + neoadjuvant chemotherapy.
Table 3.
Multivariable logistic regression testing for the effect of histological variant on cancer specific mortality (CSM) according to treatment protocol in the overall population.
| Histological variant | Overall | NAC+RC | RC+AC | RC only | RC+perioperative CHT |
|---|---|---|---|---|---|
| Adenocarcinoma | 0.91 (0.49–1.67) p=0.75 | 0.36 (0.08–1.65) p=0.19 | 5.20 (1.74–15.52) p=0.003 | 0.39 (0.10–1.58) p=0.19 | 1.03 (0.42–2.51) p=0.95 |
| Pure Adenocarcinoma | 0.66 (0.27–1.63) p=0.37 | 0.19 (0.02–1.56) p=0.12 | 9.28 (1.99–43.28) p=0.005 | 0.61 (0.15–2.48) p=0.49 | 0.95 (0.29–3.15) p=0.93 |
| UC with glandular component | 1.30 (0.57–2.94) p=0.53 | 1.95 (0.22–17.03) p=0.54 | 3.75 (0.85–16.56) p=0.08 | NA | 1.87 (0.57–6.15) p=0.31 |
| Micropapillary | 1.31 (0.84–2.04) p=0.24 | 1.24 (0.49–3.15) p=0.65 | 2.48 (1.11–5.56) p=0.03 | 1.02 (0.51–2.02) p=0.96 | 1.69 (0.90–3.15) p=0.10 |
| SCC | 1.36 (1.03–1.79) p=0.03 | 2.10 (1.07–4.15) p=0.03 | 2.36 (1.32–4.23) p=0.004 | 0.94 (0.62–1.42) p=0.77 | 1.95 (1.25–3.04) p=0.003 |
| Pure SCC | 0.98 (0.63–1.52) p=0.92 | 3.38 (1.19–9.59) p=0.02 | 3.27 (1.15–9.31) p=0.03 | 0.48 (0.25–0.95) p=0.03 | 3.36 (1.60–7.03) p=0.001 |
| UC and SCC component | 1.74 (1.22–2.46) p=0.002 | 1.61 (0.68–3.80) p=0.28 | 2.11 (1.06–4.17) p=0.03 | 1.73 (1.05–2.85) p=0.03 | 1.67 (0.98–2.82) p=0.06 |
| Small cell | 1.20 (0.65–2.21) p=0.55 | 2.50 (0.85–7.32) p=0.09 | NA | 1.13 (0.46–2.78) p=0.79 | 1.47 (0.57–3.81) p=0.43 |
| Pure Small-cells | 1.24 (0.58–2.66) p=0.57 | 3.00 (0.90–10.04) p=0.07 | 1.30 (0.32–5.31) p=0.71 | 1.40 (0.51–3.84) p=0.52 | |
| UC with small-cell component | 1.17 (0.43–3.16) p=0.76 | 2.03 (0.27–15.46) p=0.49 | 1.09 (0.34–3.47) p=0.89 | 2.02 (0.27–14.82) p= 0.49 |
|
| Sarcoma | 4.67 (2.79–7.82) p<0.0001 | 1.86 (0.23–14.89) p=0.56 | 21.72 (4.79–98.56) p=0.004 | 3.97 (2.11–7.48) p<0.0001 | 6.96 (2.41–20.13) p=0.0003 |
| Pure Sarcoma | 9.02 (3.98–20.91) p=<0.0001 | 4.84 (0.58–40.62) p=0.14 | 36.86 (3.01–452.64) p=0.005 | 7.88 (2.33–26.67) p=0.001 | 9.86 (2.16–45.01) p=0.003 |
| UC with sarcomatoid component | 3.66 (1.92–6.97) p=0.0001 | NA | 16.46 (2.45–110.56) p=0.004 | 3.48 (1.67–7.28) p=0.001 | 5.11 (1.16–22.39) p=0.03 |
| Other subtypes | 1.35 (0.84–2.17) p=0.21 | 1.23 (0.28–5.39) p=0.78 | 0.99 (0.39–2.56) p=0.99 | 1.79 (0.85–3.79) p=0.13 | 0.95 (0.41–2.19) p=0.90 |
adjusted for pTN stages + LND+ RT to primary tumor + surgical margins status + age at cystectomy + gender + smoke habits + CCI.
Abbreviations:
UC: Urothelial carcinoma
SCC: Squamous-cell carcinoma
RC: Radical cystectomy
NAC: Neoadjuvant chemotherapy
AC: Adjuvant chemotherapy
CHT: Chemotherapy
Clinical-to-pathological downstaging
After either univariable (odds ratio [OR]: 0.4, 95% CI: 0.2–0.7, p=0.01) or multivariable (OR: 0.4, 95% CI: 0.1–0.8, p=0.03) adjustment for NAC administration and Charlson comorbidity index [CCI], SCC was the only histological variant associated with significant lower odds of clinical-to-pathological downstaging compared to UC (Supplementary Table 4).
Effect of neoadjuvant chemotherapy regimen on CSS
After NAC-regimen stratification, patients with SCC still exhibited worse CSS rates compared to UC both in cisplatin-based (33 vs. NA months, p=0.009) and other regimens-based (5 vs. 49 months, p<0.0001) groups. In contrast, no CSS rate difference (29 vs. 76 months, p=0.7) was found between SCC and UC patients who received a carboplatin-based chemotherapy. On multivariable models, SCC exhibited higher CSM rates in cisplatin-treated patients (HR: 2.5, 95% CI: 1.2–5.2, p=0.02) after adjustment for age, CCI, gender and lymph node dissection, but not after pTN stage adjustment (HR: 1.6, 95% CI: 0.8–3.2, p=0.2). Furthermore, SCC exhibited higher CSM rates in the other-based regimen group after adjustment for age, CCI, gender and lymph node dissection (HR: 33, 95% CI: 2.1-NA, p=0.001) or pTN stages (HR: 46, 95% CI: 4-NA, p=0.002).
Discussion:
SCC of the bladder has been considered an aggressive form of BCa, frequently diagnosed in advanced stages and characterized by rapid progression[16]. Moreover, it has been postulated that this specific histology might exhibit a remarkable resistance to traditional platinum-based regimens in either neoadjuvant[17] or adjuvant[18] settings[19]. In our report, we integrated different sources to comprehensively characterize bladder SCC, and we reported several key findings. Firstly, we found that SCC was the only histological variant associated with worse CSS after NAC and RC, confirming previous results on the overall poor effectiveness of chemotherapy in SCC [21–23]. Indeed, our large, multi-institutional dataset allowed us to achieve a level of data granularity which is not possible in population-based dataset, as previously reported by other authors[9,18,20]. For instance, we were able to correctly capture the chemotherapeutic regimen in both the neoadjuvant and adjuvant settings, and to stratify our analyses for such variables. Thus, we found that CSS differences may occur in patients with SCC when different NAC regimens are considered. Taken together, our study can be considered a strong, although retrospective, evidence of overall poor efficacy of traditional NAC in patients with SCC.
Data from the PURE-01 study, which is investigating neoadjuvant immunotherapy in patients with MIBC, showed a potential benefit of immunotherapy in terms of pathologic response in the sub-group of patients with SCC variant treated with neoadjuvant pembrolizumab[21]. The identification of predictive biomarkers underlying response to immunotherapy[22], as well as chemotherapy resistance, is required to better stratify patients at the diagnosis of a VH. For example, the role of PI3K-AKT-mTOR signalling pathway, to which PIK3CA gene belong, has been robustly characterized in cancer and it is known to regulate most hallmarks of cancer, including proliferation, survival, and genomic instability[23], as well as chemotherapy resistance[24]. Interestingly, there is increasing evidence that this network also regulates the immunosuppressive microenvironment. For instance, PI3K pathway inhibition enhanced CD8+ T cell infiltration within tumour tissue in various preclinical models, resulting in significant survival benefit[25,26]. Therefore, it is clear that therapeutic inhibition of the PI3K-AKT-mTOR axis may have the dual benefit of putting the brakes on tumour progression and also increasing local tumour immunotherapy surveillance. Such therapeutic strategy may also be implemented in the clinics, either as single- or multiple-agent regimens, as already proved for SCC in the metastatic settings[27]. Taken together, data elaborated from this multi-institutional database suggest that SCC histology is associated with inferior neoadjuvant chemotherapy sensibility, as compared to UC. Such findings may justify the lower clinical-to-pathological downstaging and shorter cancer-specific survival, which have been captured by our analyses. Our study is not devoid of limitations. First and foremost, collapsing pure and mixed SCC cases might have introduced a bias related to the heterogeneity of the SCC cohort. On the other hand, sub-analyses were provided keeping SCC and UC with SCC component separated. Here, pure SCC remained significantly associated with worse CSS after NAC in either overall and localized (pN0) cohorts. Additionally, in support of our methodology, previous results of a retrospective study, investigating thr potential CSS differences between pure and mixed SCC histologies, did not find differences between the two groups[28]. Another similar limitation is the lack of quantification of the percentage of VH in mixed cases. Thirdly, the inclusion of muscle invasive and non-muscle invasive BCa may increase the heterogeneity of our cohorts. However, no defined guidelines are available in SCC BCa and no uniform treatment protocol exists that specify the correct and appropriate use of chemotherapy and/or RC according to clinical stage in histological variants. Consequently, wider inclusion criteria should be chosen, where guidelines are lacking. For this reason, we focused on patients with BCa who were treated with RC rather than on muscle invasive BCa only, excluding those patients with recurrent, BCG resistant or multifocal non-muscle invasive BCa that may be associated with adverse prognosis when associate with histological variants[29]. Fourth, it is also important to highlight that some HVs were probably poorly represented in the current analyses. Indeed, we probably lacked of adequate power to identify statistically significant differences in the NAC treated groups between UC and non-SCC patients. This seems the case for the sarcomatoid variant, which showed absolute inferior CSS compared to UC, although not statistically significant. Taken together, it should be kept in mind that HVs are rare entities and that adequate power is difficult to reach even in large multi-institutional studies, such as our one. Five, the definition of clinical TN stage was also subjected to a certain degree of bias. Under-staging derived from incomplete TURB or variable slide thickness of the CT scans should be taken into account when examining our data. Six, the time frame of the current study crossed the past three decades, when surgical techniques, NAC regimens, lymph node dissection templates, as well as management of BCa patients have evolved and changed. Unfortunately, we could not adjust for all these confounding factors, which have undoubtedly affected the results of the present study. Last but not least, being a retrospective multi-institutional study, there was no centralized pathology revision, standardized chemotherapy regimens, and homogeneous imaging surveillance protocol after treatment. These limitations should be acknowledged, since they might have markedly influenced our results and their reproducibility.
Conclusions:
When treated with traditional neoadjuvant chemotherapy, squamous-cell bladder cancer variant is characterized by lower clinical-to-pathological downstaging and shorter cancer-specific survival compared to pure UC histology, representing the variant histology with the lowest activity of NAC in our multicenter database. For this group of patients, new therapeutic strategies should be explored, including neoadjuvant immunotherapy. Coming trials, including detailed biomarker analyses, are largely awaited, to better address the specific role of neoadjuvant modalities in bladder cancer variant histologies.
Supplementary Material
CLINICAL PRACTICE POINTS.
The efficacy of chemotherapy on non-urothelial bladder cancer has never been examined accurately.
Using a multicentre, international dataset, it was shown that squamous-cell bladder cancer variant is characterized by low clinical-to-pathological downstaging and short cancer-specific survival when treated with traditional neoadjuvant chemotherapy (NAC), representing the variant histology with the lowest activity of NAC.
For this group of patients, new therapeutic strategies should be explored, including neoadjuvant immunotherapy.
Acknowledgments
Funding:
None. This study has not received funding
Footnotes
Ethical approval and consent to participate:
The protocol for the research project has been approved by the institutional review boards and it conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Consent for publication:
Not requested for this study
Data availability:
Data are not available for free access. No hyperlink can be provided
Competing interests:
The authors declare no competing interests
Authors disclose no conflict of interest on the topic of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- [2].Metcalfe MJ, Ferguson JE, Li R, Xiao L, Guo CC, Czerniak BA, et al. Impact of High-risk Features and Effect of Neoadjuvant Chemotherapy in Urothelial Cancer Patients with Invasion into the Lamina Propria on Transurethral Resection in the Absence of Deep Muscle Invasion. Eur Urol Focus 2017;3:577–83. 10.1016/j.euf.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol 2017;14:221–34. 10.1038/nrclinonc.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016;70:106–19. 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- [5].Burger M, Kamat AM, McConkey D. Does Variant Histology Change Management of Non-muscle-invasive Bladder Cancer? Eur Urol Oncol 2019. 10.1016/j.euo.2019.06.012. [DOI] [PubMed] [Google Scholar]
- [6].Domanowska E, Jozwicki W, Domaniewski J, Golda R, Skok Z, Wiśniewska H, et al. Muscle-invasive urothelial cell carcinoma of the human bladder: multidirectional differentiation and ability to metastasize. Hum Pathol 2007;38:741–6. 10.1016/j.humpath.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [7].Jozwicki W, Domaniewski J, Skok Z, Wolski Z, Domanowska E, Jozwicka G. Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis: a mapping study. Urology 2005;66:1122–6. 10.1016/j.urology.2005.06.134. [DOI] [PubMed] [Google Scholar]
- [8].Shah RB, Montgomery JS, Montie JE, Kunju LP. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: impact of mandatory central pathology review at a large referral hospital. Urol Oncol 2013;31:1650–5. 10.1016/j.urolonc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- [9].Vetterlein MW, Wankowicz SAM, Seisen T, Lander R, Löppenberg B, Chun FK-H, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017;123:4346–55. 10.1002/cncr.30907. [DOI] [PubMed] [Google Scholar]
- [10].Krasnow RE, Drumm M, Roberts HJ, Niemierko A, Wu C-L, Wu S, et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur Urol 2017;72:54–60. 10.1016/j.eururo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [11].Li Q, Assel M, Benfante NE, Pietzak EJ, Herr HW, Donat M, et al. The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy. Eur Urol Focus 2019;5:104–8. 10.1016/j.euf.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zahoor H, Elson P, Stephenson A, Haber G-P, Kaouk J, Fergany A, et al. Patient Characteristics, Treatment Patterns and Prognostic Factors in Squamous Cell Bladder Cancer. Clin Genitourin Cancer 2018;16:e437–42. 10.1016/j.clgc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- [13].Diamantopoulos LN, Khaki AR, Vakar-Lopez F, Tretiakova MS, Gore JL, Schade GR, et al. Patient (pt) characteristics, treatment patterns, outcomes and prognostic factors in plasmacytoid urothelial carcinoma (PUC). J Clin Oncol 2019;37:e16007– e16007. 10.1200/JCO.2019.37.15_suppl.e16007. [DOI] [Google Scholar]
- [14].Geynisman DM, Handorf E, Wong Y-N, Doyle J, Plimack ER, Horwitz EM, et al. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med 2016;5:192–9. 10.1002/cam4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martini A, Daza J, Poltiyelova E, Gul Z, Heard JR, Ferket BS, et al. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int n.d;0. 10.1111/bju.14719. [DOI] [PubMed] [Google Scholar]
- [16].Matulay JT, Woldu SL, Lim A, Narayan VM, Li G, Kamat AM, et al. The impact of squamous histology on survival in patients with muscle-invasive bladder cancer. Urol Oncol 2019. 10.1016/j.urolonc.2019.01.020. [DOI] [PubMed] [Google Scholar]
- [17].Kassouf W, Spiess PE, Siefker-Radtke A, Swanson D, Grossman HB, Kamat AM, et al. Outcome and patterns of recurrence of nonbilharzial pure squamous cell carcinoma of the bladder: a contemporary review of The University of Texas M D Anderson Cancer Center experience. Cancer 2007;110:764–9. 10.1002/cncr.22853. [DOI] [PubMed] [Google Scholar]
- [18].Berg S, D’Andrea D, Vetterlein MW, Cole AP, Fletcher SA, Krimphove MJ, et al. Impact of adjuvant chemotherapy in patients with adverse features and variant histology at radical cystectomy for muscle-invasive carcinoma of the bladder: Does histologic subtype matter? Cancer 2019. 10.1002/cncr.31952. [DOI] [PubMed] [Google Scholar]
- [19].Comparative Effectiveness of Treatment Strategies for Squamous Cell Carcinoma of the Bladder - European Urology Oncology n.d. https://euoncology.europeanurology.com/article/S2588-9311(18)301950/fulltext (accessed April 25, 2019). [DOI] [PubMed]
- [20].Fischer-Valuck BW, Michalski JM, Contreras JA, Brenneman R, Christodouleas JP, Abraham CD, et al. A propensity analysis comparing definitive chemo-radiotherapy for muscle-invasive squamous cell carcinoma of the bladder vs. urothelial carcinoma of the bladder using the National Cancer Database. Clin Transl Radiat Oncol 2019;15:38–41. 10.1016/j.ctro.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol 2019. 10.1016/j.eururo.2019.10.026. [DOI] [PubMed] [Google Scholar]
- [22].Necchi A, Madison R, Raggi D, Jacob J, Bratslavsky G, Shapiro O, et al. Comprehensive assessment of immuno-oncology biomarkers in adenocarcinoma, urothelial carcinoma and squamous-cell carcinoma of the bladder. Eur Urol 2020. (In Press). [DOI] [PubMed] [Google Scholar]
- [23].O’Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol 2018;48:91–103. 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- [24].Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang LY, et al. Suppression of esophageal tumor growth and chemoresistance by directly targeting the PI3K/AKT pathway. Oncotarget 2014;5:11576–87. 10.18632/oncotarget.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov 2016;6:202–16. 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dong Y, Richards J-A, Gupta R, Aung PP, Emley A, Kluger Y, et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 2014;33:4632–42. 10.1038/onc.2013.409. [DOI] [PubMed] [Google Scholar]
- [27].Kao C, McNamara M, Alley C, Spector N, Jauhari S, Gupta RT, et al. A Complete Response After Pseudo-progression: Pembrolizumab for Metastatic Squamous Cell Carcinoma (SCC) of the Bladder. Clin Genitourin Cancer 2019;17:e672–7. 10.1016/j.clgc.2019.03.019. [DOI] [PubMed] [Google Scholar]
- [28].Ehdaie B, Maschino A, Shariat SF, Rioja J, Hamilton RJ, Lowrance WT, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol 2012;187:74–9. 10.1016/j.juro.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abufaraj M, Shariat SF, Foerster B, Pozo C, Moschini M, D’Andrea D, et al. Accuracy and prognostic value of variant histology and lymphovascular invasion at transurethral resection of bladder. World J Urol 2018;36:231–40. 10.1007/s00345-017-2116-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.