Abstract
Introduction
Epicutaneous (e.c.) allergen exposure is an important route of sensitization toward allergic diseases in the atopic march. Allergen sources such as house dust mites contain proteases that involve in the pathogenesis of allergy. Prostanoids produced via pathways downstream of cyclooxygenases (COXs) regulate immune responses. Here, we demonstrate effects of COX inhibition with nonsteroidal anti-inflammatory drugs (NSAIDs) on e.c. sensitization to protease allergen and subsequent airway inflammation in mice.
Methods
Mice were treated with NSAIDs during e.c. sensitization to a model protease allergen, papain, and/or subsequent intranasal challenge with low-dose papain. Serum antibodies, cytokine production in antigen-restimulated skin or bronchial draining lymph node (DLN) cells, and airway inflammation were analyzed.
Results
In e.c. sensitization, treatment with a nonspecific COX inhibitor, indomethacin, promoted serum total and papain-specific IgE response and Th2 and Th17 cytokine production in skin DLN cells. After intranasal challenge, treatment with indomethacin promoted allergic airway inflammation and Th2 and Th17 cytokine production in bronchial DLN cells, which depended modestly or largely on COX inhibition during e.c. sensitization or intranasal challenge, respectively. Co-treatment with COX-1-selective and COX-2-selective inhibitors promoted the skin and bronchial DLN cell Th cytokine responses and airway inflammation more efficiently than treatment with either selective inhibitor.
Conclusion
The results suggest that the overall effects of COX downstream prostanoids are suppressive for development and expansion of not only Th2 but also, unexpectedly, Th17 upon exposure to protease allergens via skin or airways and allergic airway inflammation.
Keywords: Protease allergen, Nonsteroidal anti-inflammatory drugs, Cyclooxygenases, Epicutaneous sensitization, Airway challenge
Introduction
Allergen sources such as house dust mites, fungi, and pollen produce or contain proteases, which are frequently allergens themselves. The proteolytic activity of allergens involves in the pathogenesis of allergies [1, 2, 3, 4]. The papaya fruit-derived occupational protease allergen, papain, and house dust mite major allergens Der f 1 and Der p 1 belong to the same family of cysteine proteases [5, 6]. Papain has been used as a model protease allergen that mimics those contained in allergen sources. Recent studies using murine models of airway inflammation [7, 8, 9, 10] or sensitization via skin [11, 12, 13, 14] demonstrated that the protease activity of papain is important to induce the airway and skin inflammation and serum IgE/IgG1 responses.
Clinically, development of allergic diseases such as asthma, rhinitis, and food allergy after earlier epicutaneous (e.c.) presensitization to allergens is known as the atopic march, a natural history of allergic diseases [15, 16]. e.c. administration of protease allergen in mice disrupts skin barrier function and stimulates epidermal, neuronal, and immune responses [1, 3, 4, 11, 12, 17, 18]. Our recent study showed that e.c. presensitization to papain contributed to the onset of allergic airway inflammation, which was triggered by a subsequent airway challenge with low-dose papain [10, 12, 19].
Cyclooxygenase (COX)-1 and COX-2 are the enzymes involved in the conversion of arachidonic acid to prostanoids [20, 21]. COX inhibition by treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) provokes respiratory reactions in patients suffering from NSAID-exacerbated respiratory disease [22]. Inhibition or deficiency of COXs exacerbates allergic asthma in murine models where chicken egg ovalbumin (OVA) has been used as an experimental model antigen for immunization [23, 24]. In e.c. sensitization models to OVA or hapten, COX inhibition exacerbates Th2-driven skin inflammation [25, 26] but limits Th17/Th22-driven inflammation [27].
Recently, we reported that COX inhibition promoted airway inflammation in adaptive-type and innate-type asthma models with i.n. papain administration and an innate-type model with i.n. IL-33 administration [28]. However, whether COX inhibition affects e.c. sensitization to protease allergens and subsequent airway inflammation is still unknown. In the present study, we examine the effect of COX inhibition on e.c. papain sensitization and the onset of allergic airway inflammation triggered by subsequent i.n. antigen challenge in mice.
Materials and Methods
Mice
Female 7- to 11-week-old C57BL/6J mice (Sankyo Lab Japan, Ibaraki, Japan) were maintained in a specific pathogen-free animal facility at Juntendo University and used in accordance with the guidelines of the institutional committee on animal experiments after institutional approval of experiments.
Treatment with NSAIDs
Indomethacin (Sigma, St. Louis, MO, USA), SC560 (COX-1-selective inhibitor) [23, 29] (Abcam, Cambridge, UK), and rofecoxib (COX-2-selective inhibitor) [30] (Tokyo Chemical Industry, Tokyo, Japan) were administered in drinking water (15 μg/mL, which is the half of the concentration used by Peebles et al. [23] or 30 μg/mL) starting 2 days before the first e.c. or i.n. administration of papain.
e.c. Sensitization
A volume of 12.5 μL of papain solution (Calbiochem, San Diego, CA, USA) (10, 1 or 0.1 mg/mL in PBS containing 0.5% (v/v) Tween 20; 125, 12.5, or 0.125 μg/side) was applied to each sides of the surface of the both ears of lightly anesthetized mice (500, 50, or 0.5 μg/50 μL/mouse) followed by being held with a finger wearing a plastic glove for 2 s in the present study, which was performed differently from some of our previous studies [11, 12, 31]. This procedure was repeated twice per week for 2 weeks for a total of 4 times. Aliquots of papain solution were stored at −80°C and thawed just before use.
i.n. Challenge and Bronchial Alveolar Lavage
e.c. sensitized mice with light anesthetization were i.n. challenged with low-dose papain resolved in PBS (2.5 μg/50 μL/animal) after the last e.c. administration twice with a 4-day interval. Sera and bronchial alveolar lavage fluids were collected 4 days after the last i.n. administration and analyzed, as described previously [10, 28].
Restimulation of Draining Lymph Node Cells
Skin draining lymph nodes (DLNs; cervical LNs) or bronchial DLNs (mediastinal LNs) were collected. DLN cells were restimulated with medium alone or E-64 (Peptide Institute, Osaka, Japan)-treated papain (E-64-papain) in the presence or absence of recombinant IL-33 (10 ng/mL; BioLegend, San Diego, CA, USA) in 96-well culture plates for 96 h, as described previously [10, 19]. We used a covalent complex between papain and the protease inhibitor E-64 as the antigen for restimulation to avoid potential protease activity-dependent effect.
ELISA
Serum total IgE and papain-specific IgE and IgG1 were measured, as described previously [10]. Cytokine and chemokine concentrations were measured with ELISA kits (R&D Systems), except for IL-9 (BioLegend).
Statistical Analysis
A one-way ANOVA with Tukey's post hoc test, the Kruskal-Wallis test with Dunn's post hoc test, the Mann-Whitney U test (two-tailed), or Student's t test (two-tailed) was used, as indicated in the figure legends. A value of p < 0.05 was regarded as statistically significant.
Results
Treatment with Indomethacin Promoted e.c. Papain Th2 and Th17 Sensitization
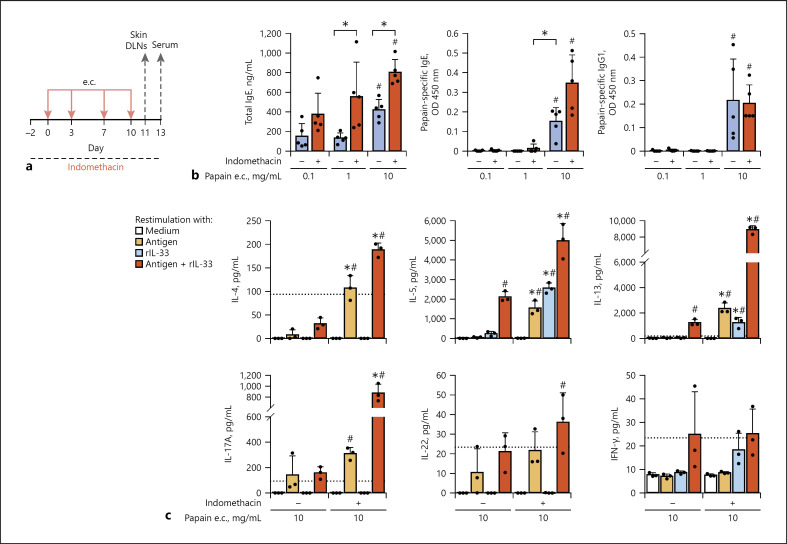
For e.c. sensitization, papain solutions with different concentrations were applied to ear skin of mice (Fig. 1a). Indomethacin was administered in drinking water. e.c. administration of 10 mg/mL of papain induced higher levels of serum total IgE and papain-specific IgE and IgG1 than that of lower concentrations of papain (Fig. 1b). COX inhibition by treatment with indomethacin enhanced serum total IgE and papain-specific IgE but not IgG1 levels in mice e.c. administered with 10 mg/mL of papain. In the model, mice were e.c. sensitized to papain via intact ear lobe skin, which caused less severe skin inflammation than other models with e.c. sensitization via skin tape-stripped or treated with detergent [12, 19], and no significant difference of ear thickness was observed between mice with and without the indomethacin treatment (unpublished observations). Skin DLN cells from mice e.c. sensitized with 10 mg/mL of papain were restimulated with the antigen for cytokine production by papain-specific Th cells in the presence or absence of IL-33 (Fig. 1c), which is indispensable to papain-induced allergic airway inflammation [7, 12, 19]. Antigen-restimulated skin DLN cells from indomethacin-treated mice with e.c. papain sensitization produced higher levels of Th2 (IL-4, IL-5, and IL-13) and Th17 (IL-17A) cytokines with a synergistic increase in the presence of IL-33 and showed a tendency of an increase in production of a Th17/Th22 cytokine, IL-22. These results indicate that COX inhibition promotes e.c. papain sensitization for papain-specific IgE, IgG1, Th2, and Th17.
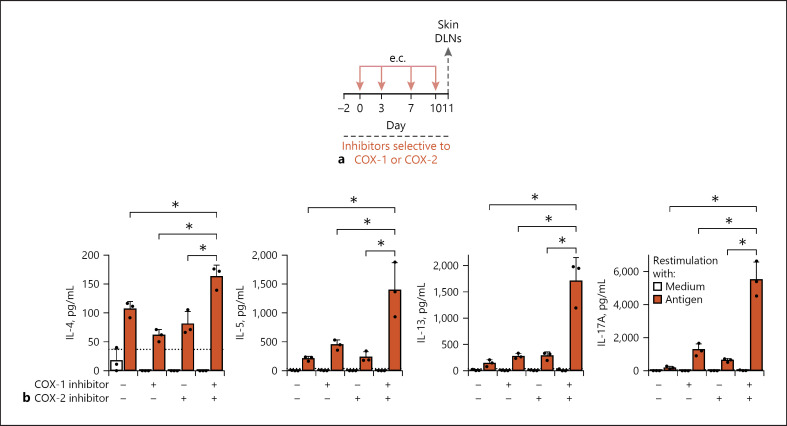
Fig. 1.
Treatment with indomethacin promoted e.c. papain Th2 and Th17 sensitization. The animal groups administered with indomethacin or vehicle were e.c. sensitized to papain (0.1, 1, or 10 mg/mL) via intact ear skin. a Time line. b Antibody responses. c Skin DLN cell cytokine responses restimulated in vitro. Data are indicated as the mean ± SD of 5 mice per group (b) and 3 wells of pooled DLN cells (c) and are representative of 3 independent experiments with similar results. c Detection limits are indicated by dotted lines. *p < 0.05 by the t test (b) or ANOVA (c: vs. no indomethacin, among the 8 groups). #p < 0.05 by ANOVA (b: vs. 0.1 mg/mL, among the 3 animal groups; c: vs. medium, among the 8 groups). DLN, draining lymph node; e.c., epicutaneous.
Treatment with Indomethacin Promoted Allergic Airway Inflammation in e.c. Presensitized Mice on Subsequent i.n. Challenge with Low-Dose Papain
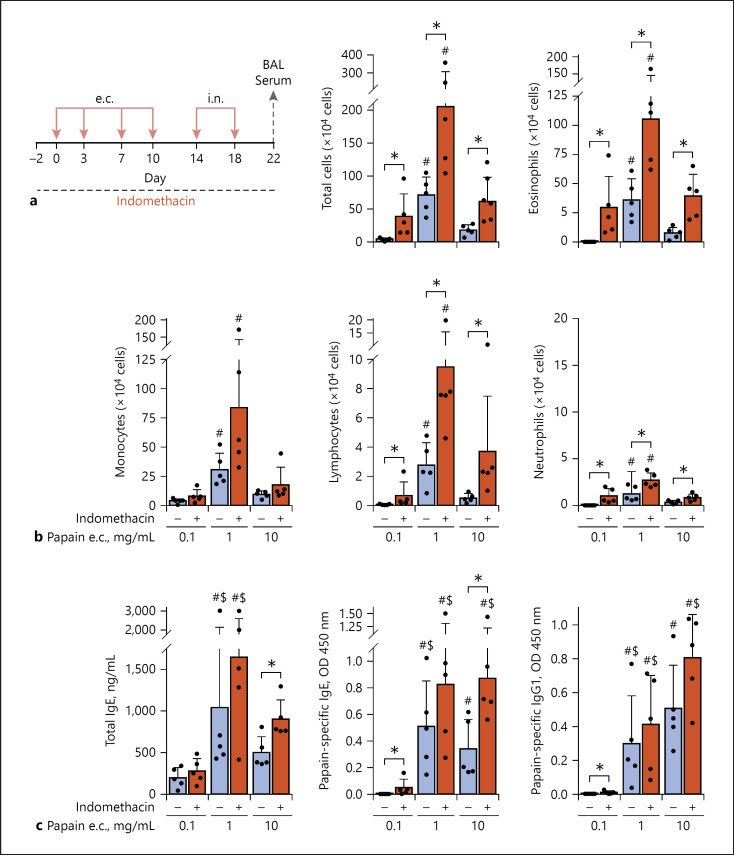
Next, we examined induction of airway inflammation by i.n. challenge with low-dose papain in mice (Fig. 2a). Mice that were e.c. presensitized with 1 mg/mL of papain showed the most severe allergic airway inflammation (Fig. 2b). COX inhibition promoted lung eosinophilia and infiltration of lymphocytes upon the i.n. challenge in mice e.c. presensitized with papain. The i.n. challenge enhanced serum levels of papain-specific IgE and IgG1 and/or total IgE in mice, which were e.c. presensitized to 10 mg/mL of papain with the indomethacin treatment or 1 mg/mL of papain with or without the indomethacin treatment (Fig. 2c), in comparison to the levels before the i.n. challenge (Fig. 1b).
Fig. 2.
Treatment with indomethacin promoted allergic airway inflammation in mice, which were e.c. papain sensitized and subsequently i.n. challenged with low-dose papain. The animal groups administered with indomethacin or vehicle were e.c. sensitized (0.1, 1 or 10 mg/mL papain) via intact ear skin and subsequently i.n. challenged (2.5 μg papain). a Time line. b Airway inflammation. c Antibody responses. Data are indicated as the mean ± SD of 5 mice per group and are representative of 3 independent experiments with similar results. *p < 0.05 by the t test or Mann-Whitney U test (b, c). #p < 0.05 (vs. 0.1 mg/mL among the 3 animal groups) by ANOVA (b) and the Kruskal-Wallis test (c). $p < 0.05 by the t test or Mann-Whitney U test (c: vs. the Fig. 1b data obtained before i.n. challenge). e.c., epicutaneous. BAL, bronchial alveolar lavage
Before the i.n. challenge, indomethacin treatment of mice e.c. sensitized to 10 mg/mL but not lower concentrations of papain promoted induction of serum papain-specific IgE (Fig. 1b). After the i.n. challenge, without the indomethacin treatment, mice e.c. presensitized to 10 mg/mL of papain showed less allergic airway inflammation than those e.c. presensitized to 1 mg/mL of papain (Fig. 2b). Focusing on these issues, next, we examined whether COX inhibition during e.c. sensitization to 10 mg/mL of papain or that during the effector phase with i.n. challenge contributed to the enhancement of airway inflammation (Fig. 3).
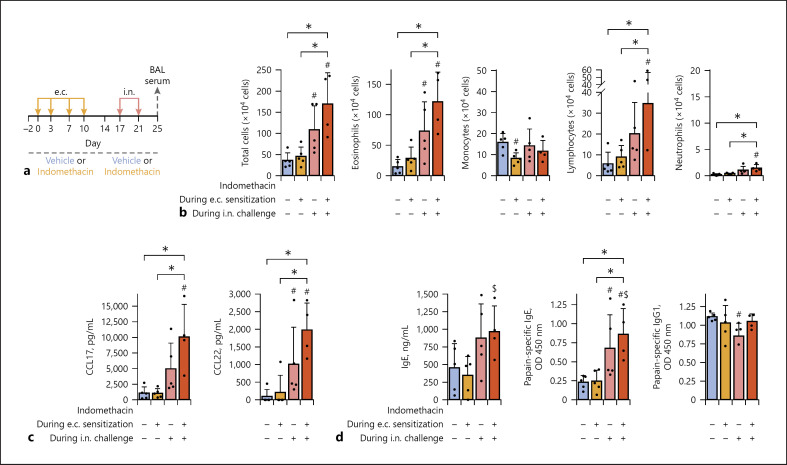
Fig. 3.
COX inhibition during the e.c. sensitization or i.n. challenge phase contributed to the enhancement of airway inflammation modestly or largely, respectively. Mice were administered with indomethacin or vehicle during e.c. sensitization, i.n. challenge, or both phases. All the animal groups were e.c. sensitized to 10 mg/mL papain via intact ear skin and subsequently i.n. challenged (2.5 μg papain). a Time line. b Airway inflammation. c Th2-attracting chemokines in BAL fluid. d Antibody responses. Data are indicated as the mean ± SD of 5 or 4 mice per group and are representative of 3 independent experiments with similar results. *p < 0.05 by ANOVA among the 4 animal groups. #p < 0.05 by the Mann-Whitney U test (vs. vehicle). $p < 0.05 by the Mann-Whitney U test (d: vs. indomethacin during e.c. sensitization). COX, cyclooxygenase; e.c., epicutaneous; BAL, bronchial alveolar lavage.
COX Inhibition during the e.c. Sensitization or i.n. Challenge Phase Contributed to the Enhancement of Airway Inflammation Modestly or Largely, Respectively
Water containing indomethacin or vehicle was fed to mice during e.c. sensitization, i.n. challenge, or both (Fig. 3a). The group with COX inhibition throughout the experiment showed the most severe allergic airway inflammation (Fig. 3b) and the highest levels of Th2-attracting chemokine release in the lung (Fig. 3c) and serum total and papain-specific IgE (Fig. 3d), being followed by the group with COX inhibition during the i.n. challenge phase only.
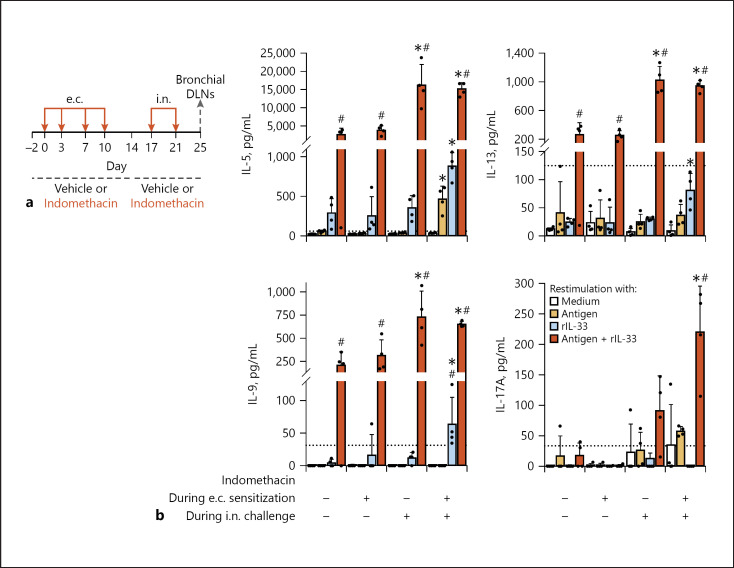
COX Inhibition during the e.c. Sensitization or i.n. Challenge Phase Contributed to the Promotion of Th2/Th9/Th17 Responses in Bronchial DLNs after the i.n. Challenge Modestly or Largely, Respectively
Using mice with the same in vivo treatment (Fig. 3a), we analyzed cytokine responses upon in vitro restimulation of bronchial DLN cells with the antigen in the presence or absence of IL-33 (Fig. 4a). On the DLN cell restimulation with the antigen plus IL-33, 2 groups with COX inhibition during the i.n. challenge showed higher levels of production of Th2 (IL-5 and IL-13) and Th9 (IL-9) cytokines than the other 2 groups without COX inhibition during the i.n. challenge (Fig. 4b). The results shown in Figures 3 and 4 indicated that the enhancement of allergic airway inflammation and Th2 and Th9 differentiation by COX inhibition was largely dependent on COX inhibition during the effector phase.
Fig. 4.
COX inhibition during the e.c. sensitization or i.n. challenge phases contributed to the promotion of Th2/Th9/Th17 expansion in bronchial DLNs after the i.n. challenge modestly or largely, respectively. Mice were administered with indomethacin or vehicle during e.c. sensitization, i.n. challenge, or both phases. All the animal groups were e.c. sensitized to 10 mg/mL papain via intact ear skin and subsequently i.n. challenged (2.5 μg papain). a Time line. b Bronchial DLN cell cytokine responses restimulated in vitro. Data are indicated as the mean ± SD of 4 wells of pooled DLN cells from 5 or 4 mice per group and are representative of 3 independent experiments with similar results. Detection limits are indicated by dotted lines. *p < 0.05 by ANOVA among the 4 animal groups (vs. no indomethacin). #p < 0.05 by ANOVA among the 4 animal groups (vs. medium). COX, cyclooxygenase; DLN, draining lymph node; e.c., epicutaneous.
We considered that the COX inhibition during e.c. sensitization had a modest contribution to the effector phase responses because the group with COX inhibition throughout the experiment showed the most significant in vivo responses (Fig. 3) and solely showed increased IL-5, IL-5/IL-13/IL-9, and IL-17A production on restimulation of bronchial DLN cells with the antigen alone, IL-33 alone, and the antigen plus IL-33, respectively (Fig. 4). However, as COX inhibition during the e.c. sensitization phase alone did not enhance the effector phase responses, the contribution seemed to be limited.
Co-Treatment with COX-1-Selective and COX-2-Selective Inhibitors Promoted Th2 and Th17 Differentiation in the e.c. Sensitization Phase
Indomethacin is a nonspecific COX inhibitor, which inhibits both COX-1 and COX-2. To clarify whether inhibition of COX-1 or COX-2 contributes to the promotion of e.c. sensitization and subsequent airway inflammation, we examined effects of treatment with COX-1-selective and/or COX-2-selective inhibitors (Fig. 5a, 6a). Antigen-restimulated skin DLN cells from e.c. sensitized mice treated with both the 2 inhibitors produced higher levels of Th2 and Th17 cytokines (Fig. 5b). The enhancement was reproducible in the group co-treated with the 2 inhibitors. Two groups treated with either one showed no enhancement, as shown in Figure 5b, but occasionally showed enhanced responses in other independent experiments (unpublished data). The results indicate that inhibition of both of the COX-1 and COX-2 activities is necessary for effective promotion of Th2 and Th17 differentiation in e.c. papain sensitization.
Fig. 5.
Co-treatment with COX-1-selective and COX-2-selective inhibitors promoted Th2/Th17 differentiation in e.c. papain sensitization. Mice were administered with COX-inhibitor(s) or vehicle during e.c. sensitization. All the animal groups were e.c. sensitized to 10 mg/mL papain via intact ear skin. a Time line. b Skin DLN cell cytokine responses restimulated in vitro. Data are indicated as the mean ± SD of 3 wells of pooled DLN cells from 3 or 4 mice and are representative of 3 independent experiments with similar results. Detection limits are indicated by dotted lines. *p < 0.05 by t test (vs. no inhibitors). COX-1 inhibitor, SC560. COX-2 inhibitor, rofecoxib. COX, cyclooxygenase; DLN, draining lymph node; e.c., epicutaneous.
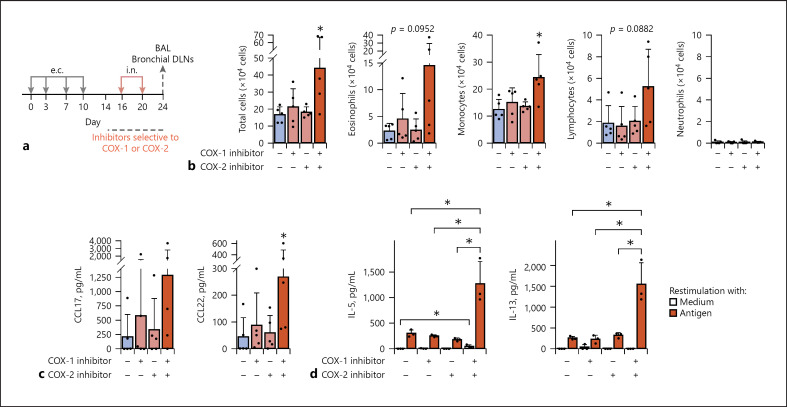
Fig. 6.
Co-treatment with COX-1-selective and COX-2-selective inhibitors promoted airway inflammation and Th2 responses in the effector phase of i.n. challenge. Mice were treated with COX inhibitor(s) or vehicle during the i.n. challenge phase. All the animal groups were e.c. sensitized to 10 mg/mL papain via intact ear skin and subsequently i.n. challenged (2.5 μg papain). a Time line. b Airway inflammation. c Th2-attracting chemokines in BAL fluid. d Bronchial DLN cell cytokine responses restimulated in vitro. Data are indicated as the mean ± SD of 5 mice per group (b, c) or that of 3 wells of pooled DLN cells from 3 mice (d), and are representative of 3 independent experiments with similar results. d Detection limits are indicated by dotted lines. *p < 0.05 by the Mann-Whitney U test (b, c: vs. no inhibitors) or ANOVA (d: vs. combination of the 2 inhibitors). COX-1 inhibitor, SC560. COX-2 inhibitor, rofecoxib.; COX, cyclooxygenase; DLN, draining lymph node; e.c., epicutaneous; BAL, bronchial alveolar lavage.
Co-Treatment with COX-1-Selective and COX-2-Selective Inhibitors Promoted Airway Inflammation and Th2 Responses in the Effector Phase of i.n. Challenge after the e.c. Presensitization
The group treated with both the COX-1-selective and COX-2-selective inhibitors during the i.n. challenge showed the most severe airway inflammation (Fig. 6a, b) and the highest levels of release of Th2 attracting chemokines in the lung (Fig. 6c, significantly for CCL22 and tendency for CCL17). Antigen-restimulated bronchial DLN cells from i.n. challenged mice treated with both the 2 inhibitors produced the highest levels of Th2 cytokines, IL-5, and IL-13 (Fig. 6d). The enhancement of the effector phase responses was reproducible in the group co-treated with the 2 inhibitors. Two groups treated with either one showed no enhancement, as shown in Figure 6b–d, but the group treated with the COX-1-selective inhibitor alone occasionally showed enhancement of some of the responses in other independent experiments (unpublished data). The results indicate that inhibition of both of the COX-1 and COX-2 activities during i.n. challenge with low-dose papain is necessary for effective promotion of airway inflammation and airway Th2 responses in e.c. presensitized mice.
Discussion
In the present study, we demonstrated that NSAID treatment promoted responses in the protease allergen-induced e.c. sensitization and subsequent airway inflammation. Treatment with indomethacin that inhibits both COX-1 and COX-2 activities promoted e.c. papain sensitization (Fig. 1) and effector phase responses on subsequent i.n. challenge with low-dose papain (Fig. 2), which does not induce responses in mice without e.c. presensitization [10]. COX inhibition during the i.n. challenge largely contributed to the enhancement of the effector phase responses and that during the e.c. sensitization also, but modestly, contributed (Fig. 3, 4). Co-treatment with COX-1-selective and COX-2-selective inhibitors was effective for the promotion of e.c. sensitization (Fig. 5) and effector phase responses (Fig. 6). Thus, the inhibition of both COX-1 and COX-2 promoted Th responses for not only Th2 but also, unexpectedly, Th17 upon antigen exposure via skin or airways and allergic airway inflammation in the e.c.-i.n. papain model.
We used papain as a model of protease allergen, which could be more relevant to natural exposure to protease-containing airborne allergens than OVA because the proteolytic activity of allergens involves in the pathogenesis of allergies [1, 2, 3, 4]. In the effector phase responses on the i.n. challenge after the e.c. presensitization, COX inhibition promoted airway inflammation, serum papain-specific and total IgE production, and bronchial DLN cell Th2, Th9, and Th17 cytokine responses (Fig. 2, 3, 4). Costimulation with the antigen and IL-33 of bronchial DLN cells synergistically promoted the cytokine responses (Fig. 4), supporting the IL-33-dependency of the papain-induced allergic airway inflammation [7, 12, 19] and suggesting contribution of IL-33-responsive Th2 and ILC2 [10, 28]. COX inhibition during i.n. challenge largely contributed to the enhancement of the effector phase responses, although that during both the e.c. sensitization and i.n. challenge periods showed the most severe responses, indicating a modest contribution of COX inhibition during e.c. sensitization (Fig. 3, 4). Both Th2 and ILC2 contribute to the adaptive i.n. papain model without e.c. presensitization [7, 9, 28], and Th2 seems to contribute to the e.c.-i.n. papain model, in which antigen-specific adaptive immunity should be established during the e.c. sensitization [10]. Therefore, the prostaglandin (PG) E2-EP2/EP4, PGI2-IP, and/or PGD2-DP1 pathways [20] could be considered as candidate pathways responsible to the prostanoid-mediated suppression of the effector phase of the e.c.-i.n. papain model.
COX inhibition promoted serum total and papain-specific IgE response and Th2 and Th17 cytokine production in skin DLN cells upon e.c. papain sensitization via intact ear skin (Fig. 1). Costimulation with the antigen and IL-33 of skin DLN cells synergistically promoted the cytokine responses (Fig. 1c). Although we cannot exclude the possibility that IL-33 enhanced the in vitro Th recall responses via indirect pathways such as stimulation of antigen-presenting cells, the results may suggest that IL-33-responsive Th cells were differentiated during the e.c. sensitization.
The Th2 subset appears to be a key factor for inflammation in allergic diseases; however, contribution of other subsets such as Th9, Th17, and Th22 has been suggested. In OVA asthma models, treatment with each of COX-1-selective and COX-2-selective inhibitors or each of COX-1−/− and COX-2−/− mice showed exacerbated allergic airway responses [23, 24, 32]. COX-1−/− mice showed increased Th2 cytokine responses [32], while COX-2−/− mice showed increased Th9 and attenuated Th17 cytokine responses [33, 34]. In an e.c. OVA sensitization model via shaved and tape-stripped back skin, treatment with a COX-2-selective inhibitor showed exacerbated skin inflammation, increased serum OVA-specific IgE and IgG1, and increased IL-4 and attenuated IFN-γ responses in spleen cells [25]. Thus, the indomethacin promotion of Th2/Th9 response and/or IgE production is common between the papain model (Fig. 1, 2, 3, 4) and other models [23, 24, 25, 26, 32, 33]. In contrast, the indomethacin promotion of Th17 response in the papain model (Fig. 1, 4) is apparently different from results obtained in the OVA asthma model [34] and the hapten-induced contact dermatitis model [27]. Mechanisms for the unexpected differential results for Th17 responses could be due to unknown mechanisms dependent on the allergen protease activity, which has potential to disrupt skin/airway barrier function and stimulate epidermal/epithelial, neuronal, and innate/adaptive immune responses [1, 2, 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 17, 18, 35, 36, 37, 38, 39, 40], the route of exposure, and/or the strategy how to evaluate the contribution of COXs (chemical inhibitors or deficient mice). Generally, PGE2 has been considered to upregulate Th17 responses [41]; however, some studies showed PGE2-mediated suppression and NSAID-induced promotion of Th17 responses in different models [42, 43].
Molecular species of prostanoids and their receptors are multiple, and they play a variety of roles in different contexts [21]. In e.c. sensitization models to OVA or hapten, COX inhibition exacerbates allergic skin inflammation [25, 26], while deficiency of CRTH2, a receptor for PGD2, attenuated it [44]. PG signaling has regulatory roles in not only T-cell-mediated allergic inflammation but also innate-type responses in ILC2 and epithelial cells including keratinocytes [20, 26, 28, 45, 46], and NSAIDs decrease epithelial barrier integrity in gastrointestinal epithelial cells [47]. Further mechanisms downstream of COXs in the papain model should be investigated in future studies.
In the present papain model, co-treatment with COX-1-selective and COX-2-selective inhibitors promoted the responses in both the e.c. sensitization (Fig. 5) and airway effector phase responses (Fig. 6). As mentioned in the Results section, treatment with either one of the 2 selective inhibitors occasionally showed enhancement of the responses (unpublished data). Therefore, we speculate that in our present model, prostanoid production mediated by each of COX-1 and COX-2 is barely sufficient to negatively regulate the responses and/or that downstream prostanoid actions are not the same between the 2 COXs due to possible differences in prostanoid producer cell types, production sites, timing, amounts, target cell types, and so on. We cannot exclude possibilities that relatively long half-lives in blood of the 3 NSAIDs used in the present study and/or their potential nonspecific effects independent of COX inhibition partially contributed to the results.
Very recently, we demonstrated that prophylactic e.c. administration of papain with or without protease activity prevented papain-induced Th2-mediated airway inflammation [31]. Without COX inhibition, mice e.c. presensitized to 10 mg/mL of papain showed markedly less airway inflammation on i.n. challenge than those e.c. presensitized to 1 mg/mL of papain, also suggesting induction of tolerance by e.c. presensitization with 10 mg/mL of papain against the onset of airway inflammation (Fig. 2b) [12]. Interestingly, the COX inhibition canceled the tolerance (Fig. 3, 4), the mechanism of which is yet to be investigated. In the OVA asthma model, treatment with indomethacin during the intraperitoneal immunization abrogates PGI2-mediated airway tolerance, which was induced by OVA inhalation prior to the immunization [48].
In conclusion, we demonstrated that COX inhibition promoted the Th differentiation or expansion upon antigen exposure via skin (Th2 and Th17) or airways (Th2, Th9, and/or Th17), respectively, and allergic airway inflammation in the protease allergen model. The protease allergen model may assist in elucidating the roles and mechanisms of prostanoid-mediated regulation of e.c. sensitization and the onset of effector phase responses induced by protease-containing natural allergen sources.
Statement of Ethics
The animal experiments were approved by the committee on animal experiments of Juntendo University School of Medicine (approval numbers: 2020244, 310039, 3000066, 290001, 280143, and 270113) and conducted according to the guidelines of the committee.
Conflict of Interest Statement
The authors have no conflict of interest in relation to this work.
Funding Sources
This study was supported by JSPS KAKENHI (grant Nos. JP18K08417, JP17K10007, and JP25461711) and Juntendo University.
Author Contributions
P.S. and T.T. wrote the manuscript. T.T. organized the study. P.S., T.T., S.K., and N.M. performed the experiments, analyzed the data, and/or interpreted the data. P.S., T.T., T.Y., Y.S., K.O., S.I., and H.O. contributed to the study design. All the authors read and approved the final manuscript.
Acknowledgements
The authors thank Mayu Suzuki, Saya Shimizu, Mutsuko Hara, Toru Kimitsu, Tomoko Yoshimura, Yurie Masutani, and Shinya Kunimine for their technical assistance; Mai Ohba and Toshiaki Okuno for lipidomics; Toyoko Hidano, Fumio Kanai, Takatoshi Kuhara, and Norihiro Tada for animal care; and Michiyo Matsumoto and Emi Yamaguchi for their secretarial assistance.
References
- 1.Takai T, Ikeda S. Barrier dysfunction caused by environmental proteases in the pathogenesis of allergic diseases. Allergol Int. 2011;60((1)):25–35. doi: 10.2332/allergolint.10-RAI-0273. [DOI] [PubMed] [Google Scholar]
- 2.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19((4)):375–85. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- 3.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol. 2019;20((11)):1435–43. doi: 10.1038/s41590-019-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity. 2020;53((5)):1063–e7.. doi: 10.1016/j.immuni.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015;64((4)):304–11. doi: 10.1016/j.alit.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM, et al. Sequence analysis of cDNA coding for a major house dust mite allergen Der p1. Homology with cysteine proteases. J Exp Med. 1988;167((1)):175–82. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013;190((9)):4489–99. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, et al. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192((9)):4032–42. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40((3)):425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishioka I, Takai T, Maruyama N, Kamijo S, Suchiva P, Suzuki M, et al. Airway inflammation after epicutaneous sensitization of mice requires protease activity of low-dose allergen inhalation. J Allergy Clin Immunol. 2018;141((6)):2271–e7.. doi: 10.1016/j.jaci.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Iida H, Takai T, Hirasawa Y, Kamijo S, Shimura S, Ochi H, et al. Epicutaneous administration of papain induces IgE and IgG responses in a cysteine protease activity-dependent manner. Allergol Int. 2014;63((2)):219–26. doi: 10.2332/allergolint.13-OA-0621. [DOI] [PubMed] [Google Scholar]
- 12.Shimura S, Takai T, Iida H, Maruyama N, Ochi H, Kamijo S, et al. Epicutaneous allergic sensitization by cooperation between allergen protease activity and mechanical skin barrier damage in mice. J Invest Dermatol. 2016;136((7)):1408–17. doi: 10.1016/j.jid.2016.02.810. [DOI] [PubMed] [Google Scholar]
- 13.Kamijo S, Suzuki M, Hara M, Shimura S, Ochi H, Maruyama N, et al. Subcutaneous allergic sensitization to protease allergen is dependent on mast cells but not IL-33 distinct mechanisms between subcutaneous and intranasal routes. J Immunol. 2016;196((9)):3559–69. doi: 10.4049/jimmunol.1500717. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo S, Hara M, Suzuki M, Nakae S, Ogawa H, Okumura K, et al. Innate IL-17A enhances IL-33-independent skin eosinophilia and IgE response on subcutaneous papain sensitization. J Invest Dermatol. 2021;141((1)):105–e14.. doi: 10.1016/j.jid.2020.05.088. [DOI] [PubMed] [Google Scholar]
- 15.Paller AS, Spergel JM, Mina-Osorio P, Irvine AD. The atopic march and atopic multimorbidity many trajectories, many pathways. J Allergy Clin Immunol. 2019;143((1)):46–55. doi: 10.1016/j.jaci.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121((6)):1331–6. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Hirasawa Y, Takai T, Mitsuishi K, Okuda M, Kato T, et al. Reduction of skin barrier function by proteolytic activity of a recombinant house dust mite major allergen Der f 1. J Invest Dermatol. 2006;126((12)):2719–23. doi: 10.1038/sj.jid.5700584. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa Y, Takai T, Nakamura T, Mitsuishi K, Gunawan H, Suto H, et al. Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J Invest Dermatol. 2010;130((2)):614–7. doi: 10.1038/jid.2009.257. [DOI] [PubMed] [Google Scholar]
- 19.Ochi H, Takai T, Shimura S, Maruyama N, Nishioka I, Kamijo S, et al. Skin treatment with detergent promotes protease allergen-dependent epicutaneous sensitization in a manner different from tape stripping in mice. J Invest Dermatol. 2017;137((7)):1578–82. doi: 10.1016/j.jid.2017.02.970. [DOI] [PubMed] [Google Scholar]
- 20.Samuchiwal SK, Boyce JA. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J Allergy Clin Immunol. 2018;141((4)):1182–90. doi: 10.1016/j.jaci.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Honda T, Kabashima K. Prostanoids in allergy. Allergol Int. 2015;64((1)):11–6. doi: 10.1016/j.alit.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease new prime suspects. N Engl J Med. 2016;374((5)):484–8. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 23.Peebles RS, Jr, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, et al. Selective cyclooxygenase-1 and −2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2002;165((8)):1154–60. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 24.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, et al. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest. 1999;104((6)):721–32. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laouini D, Elkhal A, Yalcindag A, Kawamoto S, Oettgen H, Geha RS. COX-2 inhibition enhances the TH2 immune response to epicutaneous sensitization. J Allergy Clin Immunol. 2005;116((2)):390–6. doi: 10.1016/j.jaci.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Sawada Y, Honda T, Nakamizo S, Nakajima S, Nonomura Y, Otsuka A, et al. Prostaglandin E2 (PGE2)-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2019;144((5)):1265–e9.. doi: 10.1016/j.jaci.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Robb CT, McSorley HJ, Lee J, Aoki T, Yu C, Crittenden S, et al. Prostaglandin E2 stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol. 2018;141((1)):152–62. doi: 10.1016/j.jaci.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama N, Takai T, Kamijo S, Suchiva P, Ohba M, Takeshige T, et al. Cyclooxygenase inhibition in mice heightens adaptive- and innate-type responses against inhaled protease allergen and IL-33. Allergy. 2019;74((11)):2237–40. doi: 10.1111/all.13831. [DOI] [PubMed] [Google Scholar]
- 29.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95((22)):13313–8. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, et al. Rofecoxib [Vioxx MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone] a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999;290((2)):551–560. PMID: 10411562. [PubMed] [Google Scholar]
- 31.Kunimine S, Takai T, Kamijo S, Maruyama N, Kimitsu T, Masutani Y, et al. Epicutaneous vaccination with protease inhibitor-treated papain prevents papain-induced Th2-mediated airway inflammation without inducing Th17 in mice. Biochem Biophys Res Commun. 2021;546:192–9. doi: 10.1016/j.bbrc.2020.12.090. [DOI] [PubMed] [Google Scholar]
- 32.Carey MA, Germolec DR, Bradbury JA, Gooch RA, Moorman MP, Flake GP, et al. Accentuated T helper type 2 airway response after allergen challenge in cyclooxygenase-1−/− but not cyclooxygenase-2−/− mice. Am J Respir Crit Care Med. 2003;167((11)):1509–15. doi: 10.1164/rccm.200211-1383OC. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Edin ML, Bradbury JA, Graves JP, DeGraff LM, Gruzdev A, et al. Cyclooxygenase-2 inhibits T helper cell type 9 differentiation during allergic lung inflammation via down-regulation of IL-17RB. Am J Respir Crit Care Med. 2013;187((8)):812–22. doi: 10.1164/rccm.201211-2073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med. 2011;184((1)):37–49. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato T, Takai T, Mitsuishi K, Okumura K, Ogawa H. Cystatin A inhibits IL-8 production by keratinocytes stimulated with Der p 1 and Der f 1 biochemical skin barrier against mite cysteine proteases. J Allergy Clin Immunol. 2005;116((1)):169–76. doi: 10.1016/j.jaci.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa T, Takai T, Kato T, Kikuchi Y, Niyonsaba F, Ikeda S, et al. Upregulation of the release of granulocyte-macrophage colony-stimulating factor from keratinocytes stimulated with cysteine protease activity of recombinant major mite allergens Der f 1 and Der p 1. Int Arch Allergy Immunol. 2008;146((1)):27–35. doi: 10.1159/000112500. [DOI] [PubMed] [Google Scholar]
- 37.Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, et al. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy. 2009;64((9)):1366–74. doi: 10.1111/j.1398-9995.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 38.Kamijo S, Nunomura S, Ra C, Kanaguchi Y, Suzuki Y, Ogawa H, et al. Innate basophil IL-4 responses against allergens and cytokines require the Fc receptor γ-chain. J Allergy Clin Immunol. 2016;137((5)):1613–e2.. doi: 10.1016/j.jaci.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi Y, Takai T, Kuhara T, Ota M, Kato T, Hatanaka H, et al. Crucial commitment of proteolytic activity of a purified recombinant major house dust mite allergen Der p1 to sensitization toward IgE and IgG responses. J Immunol. 2006;177((3)):1609–17. doi: 10.4049/jimmunol.177.3.1609. [DOI] [PubMed] [Google Scholar]
- 40.Takai T, Kato T, Hatanaka H, Inui K, Nakazawa T, Ichikawa S, et al. Modulation of allergenicity of major house dust mite allergens Der f 1 and Der p 1 by interaction with an endogenous ligand. J Immunol. 2009;183((12)):7958–65. doi: 10.4049/jimmunol.0713276. [DOI] [PubMed] [Google Scholar]
- 41.Tsuge K, Inazumi T, Shimamoto A, Sugimoto Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int Immunol. 2019;31((9)):597–606. doi: 10.1093/intimm/dxz021. [DOI] [PubMed] [Google Scholar]
- 42.Dejani NN, Orlando AB, Niño VE, Penteado LA, Verdan FF, Bazzano JMR, et al. Intestinal host defense outcome is dictated by PGE2 production during efferocytosis of infected cells. Proc Natl Acad Sci U S A. 2018;115:E8469–78. doi: 10.1073/pnas.1722016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41((10)):2840–51. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 44.He R, Oyoshi MK, Wang JY, Hodge MR, Jin H, Geha RS. The prostaglandin D2 receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. J Allergy Clin Immunol. 2010;126:784–90. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou W, Zhang J, Toki S, Goleniewska K, Norlander AE, Newcomb DC, et al. COX inhibition increases alternaria-induced pulmonary group 2 innate lymphoid cell responses and IL-33 release in mice. J Immunol. 2020;205((4)):1157–66. doi: 10.4049/jimmunol.1901544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueta M, Matsuoka T, Yokoi N, Kinoshita S. Prostaglandin E receptor subtype EP3 downregulates TSLP expression in human conjunctival epithelium. Br J Ophthalmol. 2011;95((5)):742–3. doi: 10.1136/bjo.2010.188748. [DOI] [PubMed] [Google Scholar]
- 47.Thakre-Nighot M, Blikslager AT. Indomethacin induces increase in gastric epithelial tight junction permeability via redistribution of occludin and activation of p38 MAPK in MKN-28 cells. Tissue Barriers. 2016;4((3)):e1187325.. doi: 10.1080/21688370.2016.1187325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou W, Goleniewska K, Zhang J, Dulek DE, Toki S, Lotz MT, et al. Cyclooxygenase inhibition abrogates aeroallergen-induced immune tolerance by suppressing prostaglandin I2 receptor signaling. J Allergy Clin Immunol. 2014;134((3)):698–e5.. doi: 10.1016/j.jaci.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]