Abstract
In the present study, we have addressed the role of the linker for activation of T cells (LAT) in the regulation of phospholipase Cγ2 (PLCγ2) by the platelet collagen receptor glycoprotein VI (GPVI). LAT is tyrosine phosphorylated in human platelets heavily in response to collagen, collagen-related peptide (CRP), and FcγRIIA cross-linking but only weakly in response to the G-protein-receptor-coupled agonist thrombin. LAT tyrosine phosphorylation is abolished in CRP-stimulated Syk-deficient mouse platelets, whereas it is not altered in SLP-76-deficient mice or Btk-deficient X-linked agammaglobulinemia (XLA) human platelets. Using mice engineered to lack the adapter LAT, we showed that tyrosine phosphorylation of Syk and Btk in response to CRP was maintained in LAT-deficient platelets whereas phosphorylation of SLP-76 was slightly impaired. In contrast, tyrosine phosphorylation of PLCγ2 was substantially reduced in LAT-deficient platelets but was not completely inhibited. The reduction in phosphorylation of PLCγ2 was associated with marked inhibition of formation of phosphatidic acid, a metabolite of 1,2-diacylglycerol, phosphorylation of pleckstrin, a substrate of protein kinase C, and expression of P-selectin in response to CRP, whereas these parameters were not altered in response to thrombin. Activation of the fibrinogen receptor integrin αIIbβ3 in response to CRP was also reduced in LAT-deficient platelets but was not completely inhibited. These results demonstrate that LAT tyrosine phosphorylation occurs downstream of Syk and is independent of the adapter SLP-76, and they establish a major role for LAT in the phosphorylation and activation of PLCγ2, leading to downstream responses such as α-granule secretion and activation of integrin αIIbβ3. The results further demonstrate that the major pathway of tyrosine phosphorylation of SLP-76 is independent of LAT and that there is a minor, LAT-independent pathway of tyrosine phosphorylation of PLCγ2. We propose a model in which LAT and SLP-76 are required for PLCγ2 phosphorylation but are regulated through independent pathways downstream of Syk.
The platelet collagen receptor glycoprotein VI (GPVI) signals through a similar pathway to that used by immunoreceptors with pivotal roles for the Fc receptor (FcR) γ-chain and the tyrosine kinase Syk (28). Cross-linking of GPVI leads to tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif in the FcR γ-chain by a Src-like kinase, enabling the binding of Syk to the phosphorylated immunoreceptor tyrosine-based activation motif via its tandem SH2 domains (8, 13, 16). This leads to autophosphorylation and activation of Syk and regulation of downstream events that culminate in activation of phospholipase Cγ2 (PLCγ2). The activation of PLCγ2 is critical for the majority of functional responses to collagen, including aggregation and dense-granule secretion (38). Several proteins play important roles in the activation of PLCγ2 downstream of Syk in platelets. These include the adapter SLP-76, which is a substrate for Syk and is essential for tyrosine phosphorylation of PLCγ2 (12, 19); the tyrosine kinase Btk, which is implicated in the tyrosine phosphorylation of PLCγ2 (29); and the p85/110-kDa heterodimeric form of phosphatidylinositol 3-kinase (PI 3-kinase), which is required for regulation of PLCγ2 activity but not tyrosine phosphorylation (27).
Cross-linking of GPVI also leads to the tyrosine phosphorylation of a 36- to 38-kDa protein in platelets independent of PLCγ2, which is proposed to play a role in the regulation of the phospholipase (5). The 36- to 38-kDa protein has many of the characteristics of a protein with a similar molecular mass in T cells, including the ability to bind to the SH2 domains of PLCγ1 and Grb2 (31). The cloning of the 36- to 38-kDa protein from T cells, the linker for activation of T cells (LAT) (41), enabled the identification of the platelet protein as LAT (17). LAT was shown to associate with the p85α subunit of PI 3-kinase in platelets following cross-linking of GPVI by collagen or the snake venom convulxin, suggesting that it mediates recruitment to the membrane and activation of its associated p110 catalytic subunit (17). LAT has also been shown to be phosphorylated in thrombin-stimulated platelets (32).
LAT is a transmembrane protein which is found in glycolipid-enriched membrane domains (43). There are nine conserved tyrosine residues in the cytosolic domain in the murine and human sequences, five of which contain the optimal binding sequence for binding to the SH2 domain of Grb2. LAT is heavily phosphorylated on tyrosine residues upon T-cell receptor stimulation, most probably by ZAP-70 (41). Studies with a LAT-deficient Jurkat T-cell line, J.Cam2, have shown that LAT plays a critical role in the regulation of PLCγ1 and the mitogen-activated protein kinase pathway downstream of the T-cell antigen receptor (15, 42). LAT-deficient mice have no mature peripheral T cells, but they have a normal B-cell population, demonstrating that LAT is critical for T-cell but not B-cell development (42).
The present study was undertaken to investigate whether LAT is required for the activation of PLCγ2 and the onset of functional responses in platelets stimulated by cross-linking of GPVI by using platelets from mice engineered to lack the adapter (42). A collagen-related peptide (CRP) which does not bind to integrin α2β1 (25) was used rather than collagen to activate GPVI because it gives a more powerful and reproducible response between experiments and, unlike collagen, can be used in flow cytometry. Several studies have shown that activation of platelets by CRP mimics that by collagen (1, 2, 16). The results show that LAT plays a critical role in PLCγ2 tyrosine phosphorylation and activation and in subsequent functional events in response to GPVI activation. In contrast, the response to the G-protein-receptor-coupled agonist thrombin, which mediates activation through PLCβ isoforms (7) and induces minimal tyrosine phosphorylation of PLCγ2 (6), is not altered.
MATERIALS AND METHODS
Materials.
CRP ([GCP*(GPP*)10GCP*G]n; P* = hydroxyproline) was a kind gift of M. Barnes, R. Farndale, and G. Knight (Department of Biochemistry, Cambridge University, Cambridge, United Kingdom) (25). Tween-20, protein A-Sepharose CL-4B, phenylmethylsulfonyl fluoride, thrombin, and prostacyclin were from Sigma (Poole, United Kingdom). Rabbit anti-Syk, Btk, and anti-PLCγ2 were generous gifts from M. Tomlinson (DNAX, Palo Alto, Calif.). A second rabbit anti-Syk antibody was also used (36). Sheep anti-SLP-76 was raised as described previously (26). The polyvinylidene difluoride membrane was purchased from Bio-Rad (Hemel Hempstead, United Kingdom). Secondary antibody and enhanced chemiluminescence reagents were from Amersham International (Little Chalfont, United Kingdom). The antiphosphotyrosine monoclonal antibody (MAb) 4G10 and rabbit anti-LAT antibody were from Upstate Biotechnology Inc. (TCS Biologicals Ltd.). The rat P-selectin antibody was from Pharmingen (Becton Dickinson, Oxford, United Kingdom), and anti-rat fluorescein isothiocyanate (FITC)-conjugated secondary antibody was from Sigma. The anti-fibrinogen FITC-conjugated antibody was from DAKO (High Wycombe, United Kingdom). Lat-deficient mice (42) and Syk-deficient radiation chimeric mice (36) were obtained as previously described. All other reagents were of analytical grade or from previously described sources (20).
Preparation of platelets.
Murine blood was taken by cardiac puncture following carbon dioxide asphyxiation. Human blood was taken from drug-free volunteers on the day of the experiment, using acidic citrate dextrose (120 mM sodium citrate, 110 mM glucose, 80 mM citric acid) as anticoagulant. Platelets were isolated from platelet-rich plasma by centrifugation at 1,000 × g for 10 min in the presence of prostacyclin (0.1 μg/ml). The pellet was resuspended in a modified Tyrode’s HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2 [pH 7.3]) in the presence of prostacyclin (0.1 μg/ml). Platelets were centrifuged at 1,000 × g for 10 min and resuspended at 5 × 108 cells/ml in Tyrode’s HEPES buffer containing EGTA (1 mM) and indomethacin (10 μM).
Platelet labeling and phospholipid analysis.
Platelets suspended in Tyrode’s HEPES without phosphate were incubated with [32P]orthophosphate (0.5 mCi/ml) for 1 h at 37°C. After centrifugation, the platelets were resuspended in Tyrode’s HEPES and recentrifuged. They were resuspended at 5 × 108/ml in Tyrode’s HEPES plus 10 μM indomethacin and left for 15 min before experimentation as described above. An aliquot of each sample was suspended in Laemmli buffer for measurement of pleckstrin phosphorylation, and reactions were stopped by the addition of 1 volume of chloroform-methanol-HCl (100/200/1, vol/vol/vol). Phospholipids were extracted, and [32P]phosphatidic acid ([32P]PA) was separated by thin-layer chromatography before being counted by liquid scintillation counting (18).
Immunoprecipitation and immunoblotting.
Reactions were stopped by adding an equal volume of ice-cold Nonidet P-40 (NP-40) buffer (20 mM Tris, 300 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2% [vol/vol] NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 μg of pepstatin A per ml [pH 7.3]). Nonlysed cells and debris were removed by centrifugation. Lysates were precleared by mixing with protein A-Sepharose (20 μl of a 50% solution) for 1 h at 4°C. Platelet lysates were incubated with anti-Syk (2 μl), anti-Btk (3 μl), anti-SLP-76 (0.5 μl), and anti-PLCγ2 (2 μl) antibodies for 60 min under constant rotation. Protein A-Sepharose (20 μl of a 50% solution) was added and samples rotated for a further 60 min. The pellet of protein A-Sepharose was washed once in lysis buffer and three times in TBS-T (10 mM Tris, 160 mM NaCl, 0.1% Tween 20 [pH 7.6]), Laemmli buffer was added, and the mixture was boiled for 10 min. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide) and transferred to polyvinylidene difluoride (PVDF) membranes. Blots were developed by using the enhanced chemiluminescence detection system. Densitometry analysis was performed with NIH 1.61 software.
Flow cytometry.
Samples (5 × 106 platelets) in Tyrode’s HEPES were incubated with P-selectin antibody and anti-rat FITC-conjugated antibody (5 μl each) for 10 min at room temperature. For fibrinogen binding, samples were incubated for 30 min with FITC-conjugated anti-human fibrinogen, which cross-reacts with murine fibrinogen. After a fivefold dilution in Tyrode’s HEPES, samples were analyzed with a Becton Dickinson FACScan flow cytometer. Ten thousands particles were acquired from each sample. The light scatter and the fluorescence signals were set in logarithmic gain. The results were analyzed as a histogram of fluorescence intensity channel 1 (FL1) plotted against cell count.
Analysis of data.
Each experiment was performed at least three times. The results are shown as mean ± standard error of the mean. Differences were analyzed by an unpaired Student t test. In each case, P < 0.05 was taken as the minimum value to indicate statistical significance.
RESULTS
LAT is tyrosine phosphorylated in human and mouse platelets.
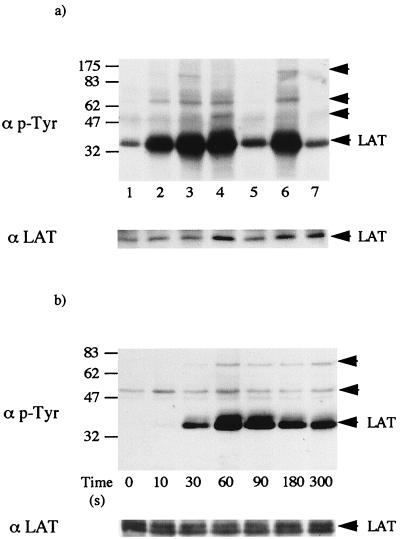
LAT was immunoprecipitated from human platelets, and the level of tyrosine phosphorylation was measured by Western blotting with MAb 4G10. LAT was weakly phosphorylated on tyrosine in nonstimulated platelets (Fig. 1a). Collagen, CRP, and FcγRIIA cross-linking stimulate marked tyrosine phosphorylation of LAT, whereas the G-protein agonist thrombin induced only a small increase in phosphorylation (Fig. 1a). Tyrosine phosphorylation of LAT in response to CRP was maintained in the presence of the protein kinase C inhibitor Ro 31-8220 and the Ca2+ chelator BAPTA (loaded as BAPTA-AM) (Fig. 1a, compare lanes 3 and 6), demonstrating that phosphorylation is independent of PLCγ2 activation and consistent with a possible role in the regulation of the phospholipase. In contrast, protein tyrosine phosphorylation in thrombin-stimulated platelets is completely inhibited in the presence of the two inhibitors, demonstrating regulation downstream of PLC (5).
FIG. 1.
LAT is tyrosine phosphorylated in human platelets. LAT was immunoprecipitated from human platelets with a specific antibody as described in Materials and Methods. Following LAT immunoprecipitation, proteins were separated by SDS-PAGE (10% polyacrylamide) and transferred to a PVDF membrane. The membrane was probed for phosphotyrosine (p-Tyr) (top panel) and reprobed after stripping for LAT (bottom panel). (a) Platelets were incubated with buffer (lane 1), 10 μg of collagen per ml (lane 2), 3 μg of CRP per ml (lanes 3, 6, and 7), MAb IV.3 (1 μg/ml) and F(ab′)2 (30 μg/ml) for FcγRIIA cross-linking (lane 4), and 1 U of thrombin per ml (lane 5). Platelets were preincubated with 10 μM Ro31-8220 and 10 μM BAPTA-AM and then stimulated with 3 μg of CRP per ml (lanes 6 and 7). The arrows indicate the migration pattern of coimmunoprecipitated bands and LAT. The figure is representative of three to five experiments. (b) Time course of tyrosine phosphorylation of LAT. Platelets were stimulated with 3 μg of CRP per ml for the indicated time, and LAT was immunoprecipitated. Proteins were separated by SDS-PAGE (10% polyacrylamide) and transferred to a PVDF membrane. The membrane was probed for phosphotyrosine with MAb 4G10. The arrows indicate the migration pattern of coimmunoprecipitated bands and LAT. Results are from one experiment that was representative of three.
Three tyrosine-phosphorylated bands of 55, 75, and 130 kDa were present to various degrees in LAT immunoprecipitates from collagen, CRP, and FcγRIIA-stimulated platelets. The 55-kDa band was also present at a low level in basal and thrombin-stimulated cells. We have shown that Lyn and SLP-76 are components of the 55- and 75-kDa bands, respectively, by reimmunoprecipitation and blotting for phosphotyrosine (data not shown). The three bands of 55, 75, and 130 kDa were also seen in LAT immunoprecipitates from platelets stimulated by the GPVI-selective snake venom toxin convulxin. Convulxin is a more powerful stimulus than CRP and gives a greater increase in tyrosine phosphorylation of the 130-kDa band, facilitating the identification of PLCγ2 as a component protein (1a). LAT also coprecipitated with Grb2 from CRP-stimulated but not control platelets, suggesting that the interaction between the two proteins is mediated through the SH2 domain of Grb2 (data not shown). We have previously reported that LAT associates with the FcR γ-chain and the nonphosphorylated p85 subunit of PI 3-kinase following stimulation by collagen and convulxin (17). These results demonstrate that LAT associates with many of the proteins known to play major roles in GPVI receptor signalling in platelets. LAT also associates with several of these proteins downstream of the T-cell antigen receptor, including Grb2, SLP-76, PI 3-kinase, and PLCγ1 (41).
Tyrosine phosphorylation of LAT in response to CRP was detected by 30 s and was marked by 60 s (Fig. 1b). This rapid onset of phosphorylation corresponds to the general increase in tyrosine phosphorylation in whole-cell lysates and of proteins involved in GPVI signalling, including FcR γ-chain, Syk, SLP-76, Btk, and PLCγ2. The peak increase in tyrosine phosphorylation of LAT occurred after 60 to 90 s before slowly declining, whereas tyrosine phosphorylation of proteins such as Syk and SLP-76 was maintained. We have previously reported that tyrosine phosphorylation of a 36- to 38-kDa protein observed in whole-cell lysates, which comigrates with LAT, declines over time whereas phosphorylation of other proteins is maintained (2).
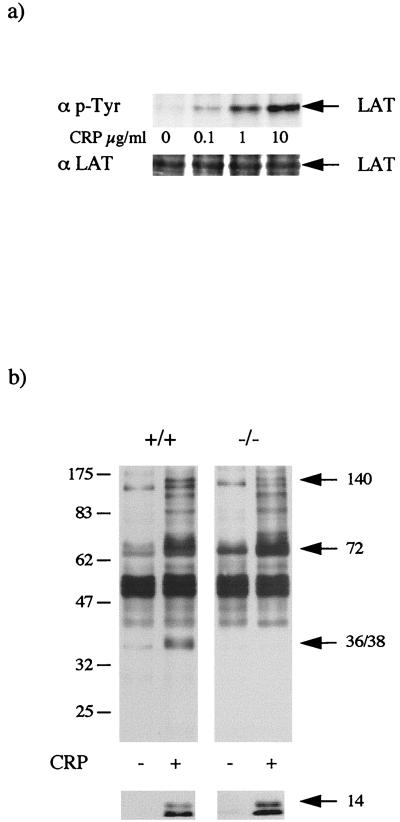
CRP also stimulated marked and rapid tyrosine phosphorylation of LAT in mouse platelets, with a similar concentration-response relationship (Fig. 2a) and time course (data not shown) to that in human platelets (Fig. 1a). The increase in LAT phosphorylation occurred in parallel with stimulation of whole-cell tyrosine phosphorylation. Coimmunoprecipitation of LAT with bands of 75 and 130 kDa, which comigrate with SLP-76 and PLCγ2, upon CRP stimulation was also seen. Tyrosine phosphorylation of LAT was completely abolished in Syk-deficient mouse platelets in response to CRP (Fig. 3a) whereas no significant difference was detected in SLP-76-deficient mouse platelets (Fig. 3b) or Btk-deficient human platelets from patients with X-linked agammaglobulinemia (XLA) (Fig. 3c). This demonstrates that tyrosine phosphorylation of LAT is downstream of Syk but independent of the tyrosine kinase Btk and the adapter SLP-76.
FIG. 2.
LAT is tyrosine phosphorylated in response to CRP in murine platelets. Washed platelets were stimulated for 2 min with CRP. (a) Concentration-response curve for tyrosine phosphorylation of LAT. LAT was immunoprecipitated with a specific antibody, and the membrane was probed for phosphotyrosine (p-Tyr) (top panel) and then stripped and reprobed for LAT (bottom panel). The experiment is representative of three. (b) Whole-cell tyrosine phosphorylation in LAT-deficient platelets. Following stimulation, proteins were separated by SDS-PAGE (10% polyacrylamide) and transferred to a PVDF membrane as described in the text. The membrane was probed for phosphotyrosine with MAb 4G10. The arrows indicate the migration pattern of PLCγ2 (140 kDa), Btk, Syk, and SLP-76 (72 kDa), and LAT (36 to 38 kDa). The bottom panel shows the tyrosine phosphorylated γ-chain (14 kDa) seen on longer exposures.
FIG. 3.
LAT tyrosine phosphorylation is lost in Syk- but not in SLP-76-deficient mouse platelets and Btk-deficient (XLA) human platelets. Platelets were stimulated with CRP (3 μg/ml) for 2 min and then lysed by addition of an equal volume of ice-cold NP-40 buffer. After preclearing, samples were immunoprecipitated for LAT as described in Materials and Methods. Membranes were probed for tyrosine phosphorylation (top panel) and reprobed for LAT (bottom panel). The arrows indicate the migration of the immunoprecipitated protein. LAT was immunoprecipitated from Syk-deficient mouse platelets (a), SLP-76-deficient mouse platelets (b), and Btk-deficient human platelets (c). Results are from one experiment representative of three. p-Tyr, phosphotyrosine.
Tyrosine phosphorylation and activation of PLCγ2 are inhibited in LAT-deficient mouse platelets in response to CRP.
CRP stimulated a marked increase in whole-cell tyrosine phosphorylation in LAT-deficient platelets which was similar to that in control cells, although there was a notable absence of the 36- to 38-kDa band, which comigrates with LAT, and a reduction in the 130-kDa band, which comigrates with PLCγ2 (Fig. 2b). A slightly reduced level of tyrosine phosphorylation of a broad 72-kDa (which comigrates with Syk, Btk, and the adapter SLP-76) was also seen, whereas tyrosine phosphorylation of a 12- to 14-kDa band, which corresponds to the FcR γ-chain (16), was similar to that in control platelets (Fig. 2b).
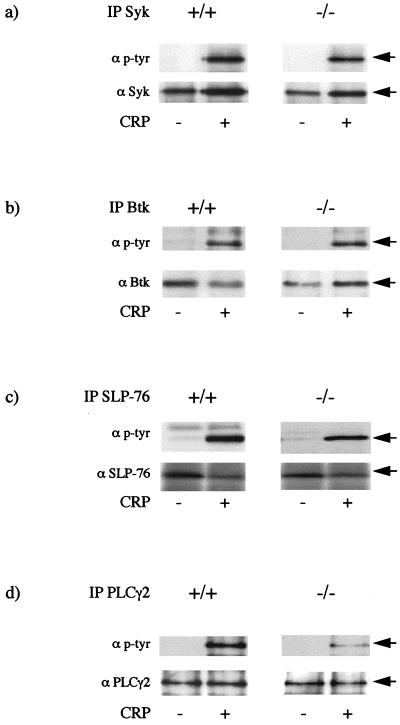
The relationship between LAT and tyrosine phosphorylation of other proteins involved in GPVI receptor signalling was investigated through immunoprecipitation. A similar level of tyrosine phosphorylation of Syk was observed in platelets from wild-type and LAT-deficient mice following stimulation by CRP, suggesting that phosphorylation of the kinase is independent or upstream of LAT (Fig. 4a). A similar level of tyrosine phosphorylation of Btk was also seen in LAT-deficient platelets, whereas tyrosine phosphorylation of SLP-76 was reduced by approximately 30% (Fig. 4b and c). In contrast, tyrosine phosphorylation of PLCγ2, the major PLCγ isoform expressed in platelets (24) and the only one reported to undergo tyrosine phosphorylation (6), was reduced by more than 75% in the LAT-deficient platelets (Fig. 4d).
FIG. 4.
Protein tyrosine phosphorylation in LAT-deficient mouse platelets. Platelets were stimulated with CRP (3 μg/ml) for 2 min and then lysed by addition of an equal volume of ice-cold NP-40 buffer. After preclearing, samples were immunoprecipitated (IP) for Syk, Btk, SLP-76, and PLCγ2 as described in Materials and Methods. Membranes were probed for tyrosine phosphorylation (top panel) and reprobed for the immunoprecipitated proteins (bottom panel). The arrows indicate the migration of the immunoprecipitated proteins. (a) Syk; (b) Btk; (c) SLP-76; (d) PLCγ2. Results are from one experiment representative of between three and five. p-tyr, phosphotyrosine.
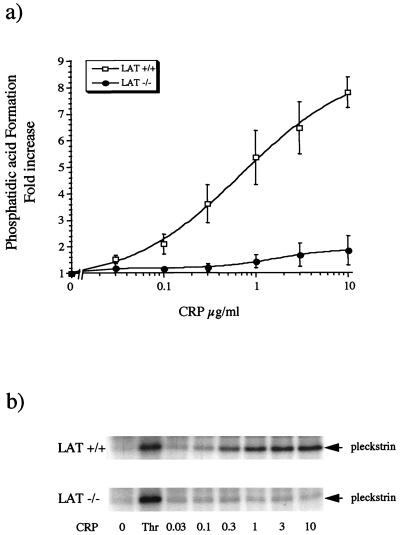
Activation of PLC can be monitored indirectly by measurement of [32P]PA, a metabolite of 1,2-diacylglycerol, and 32P-phosphorylation of pleckstrin, a protein kinase C substrate which is phosphorylated on serine and threonine residues. CRP stimulated concentration-dependent increases in [32P]PA levels in control platelets by a maximum of (7.8 ± 0.6)-fold relative to the basal level (Fig. 5a). The 50% effective concentration (EC50) for the CRP-stimulated formation of [32P]PA was approximately 0.4 μg/ml. The response to CRP (10 μg/ml) was significantly (P < 0.01) reduced, to (1.8 ± 0.6)-fold relative to the basal level in LAT-deficient cells. The residual increase in [32P]PA levels was not significantly different from the basal level at the highest concentration of CRP investigated, although there was a clear trend toward a concentration-dependent increase (Fig. 5a). In contrast, [32P]PA formation induced by thrombin was not altered in LAT-deficient platelets (the response to thrombin was [11.4 ± 0.8]- and [12.2 ± 1.4]-fold relative to the basal level in control and LAT-deficient platelets [n = 3], respectively). Phosphorylation of pleckstrin was completely inhibited in LAT-deficient platelets throughout the concentration-response curve to CRP, whereas the response to thrombin was not significantly different from that in control platelets (Fig. 5b). These results demonstrate that PLC activity is largely and possibly completely suppressed in response to CRP in LAT-deficient platelets.
FIG. 5.
Formation of PA and phosphorylation of pleckstrin are inhibited in LAT-deficient mouse platelets. 32P-labelled platelets (200 μl) were stimulated with CRP for 2 min, and an aliquot (10 μl) was taken for analysis of pleckstrin phosphorylation. Phospholipids were extracted from the remaining suspension as described in Materials and Methods and separated by thin-layer chromatography. [32P]PA was localized with a PhosphorImager. Spots were scraped, and radioactivity was measured by liquid scintillation counting. (a) The PA level is expressed as fold increase relative to the basal level (4,363 ± 657 cpm) and is shown as mean and standard error of the mean from three independent experiments. (b) Pleckstrin phosphorylation was detected by autoradiography. The results are from one experiment representative of three.
Functional responses in LAT-deficient platelets.
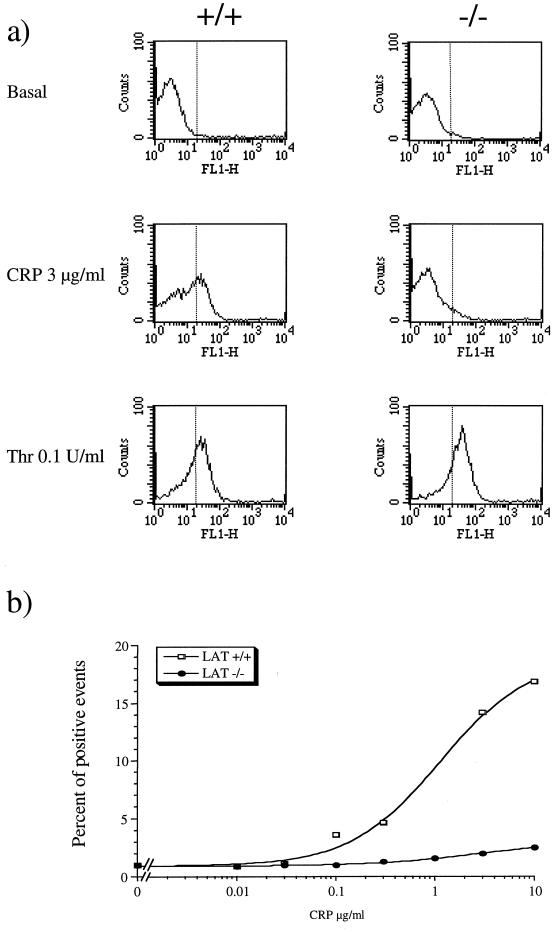
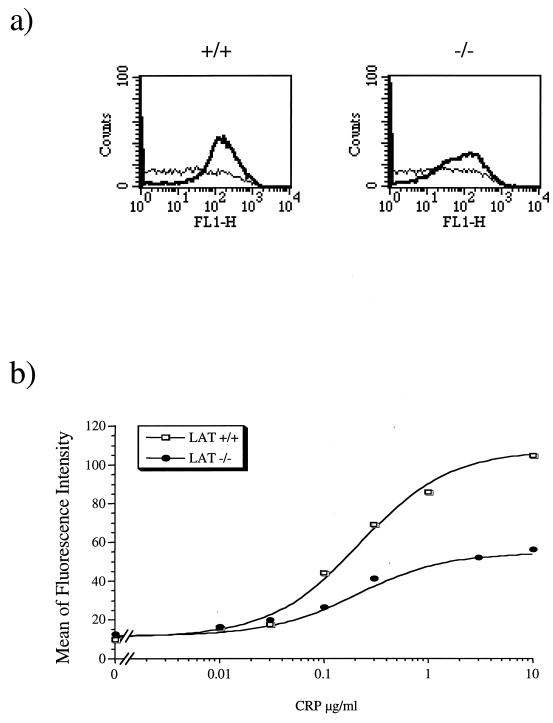
To investigate the functional significance of LAT in GPVI signalling, platelets from LAT-deficient mice were challenged with CRP and analyzed for α-granule secretion and activation of the integrin αIIbβ3 by fibrinogen binding. α-Granule secretion was measured by flow cytometry through immunodetection of P-selectin, which becomes exposed on the surface of the activated platelets. CRP stimulated a concentration-dependent increase in P-selectin exposure in platelets from control mice with EC50 and maximal responses occurring at approximately 1 and 10 μg/ml, respectively. In contrast, the response to CRP (0.1 to 10 μg/ml) was substantially inhibited in LAT-deficient platelets, with the small increase observed failing to reach statistical significance for the highest concentration of CRP that was used (Fig. 6). Nevertheless, there was a clear trend toward a concentration-dependent increase in P-selectin exposure in LAT-deficient platelets. The response to thrombin was similar in control and LAT-deficient platelets (Fig. 6a). CRP stimulated fibrinogen binding in platelets from control mice, with EC50 and maximal responses at approximately 0.2 and 3 μg/ml (Fig. 7). These values are lower than those required for PA formation and P-selectin expression. CRP stimulated fibrinogen binding in LAT-deficient platelets over a similar concentration range to that in control cells but with a reduced (∼60%) maximal response (Fig. 7b).
FIG. 6.
P-selectin secretion is impaired in LAT-deficient mouse platelets in response to CRP. Washed platelets were stimulated by incubating with CRP for 2 min and then treated with anti-P-selectin and FITC-conjugated secondary antibody for 10 min. Ten thousand events were acquired for each samples. Responsive cells are counted as positive events by delimiting a threshold between basal and stimulated platelets; the threshold was set at a position corresponding to the 98th percentile of the population (dotted line). (a) Histograms of fluorescence corresponding to basal and CRP- and thrombin (Thr)-stimulated platelets for control (+/+) and LAT-deficient (−/−) mouse platelets. The results are from one experiment representative of three. (b) Concentration-response curve of P-selectin secretion in response to CRP. The results are from one experiment representative of three.
FIG. 7.
Fibrinogen binding is decreased in LAT-deficient mouse platelets in response to CRP. Platelets were stimulated with CRP for 2 min in the presence of their own diluted platelet-poor plasma (to replace fibrinogen). Aliquots (50 μl) were incubated with FITC-conjugated anti-fibrinogen antibody for 30 min. Samples were fixed with 1% formaldehyde and analyzed by using a one-fluorescence-channel setting. (a) Histograms of fluorescence for control (+/+) and Lat-deficient (−/−) platelets in the basal state (thin line) or stimulated with 3 μg of CRP per ml (thick line). (b) Concentration-response curve of fibrinogen binding in response to CRP. The y axis denotes the geometric mean of the fluorescence. Results are from one experiment representative of three.
DISCUSSION
We show in the present study that LAT is strongly tyrosine phosphorylated in human platelets in response to collagen, CRP, and FcγRIIA cross-linking whereas only very weak phosphorylation is detected in response to the G-protein-coupled receptor agonist thrombin. Tyrosine phosphorylation of LAT in response to CRP was completely inhibited in mouse platelets deficient in Syk, placing it downstream of the tyrosine kinase. In contrast, tyrosine phosphorylation of LAT was not altered in mouse and human platelets deficient in SLP-76 and Btk, respectively. LAT associates with many of the proteins that participate in signalling by the GPVI receptor in platelets, including FcR γ-chain, Grb2, PI 3-kinase, SLP-76, and PLCγ2, suggesting that it is an important adapter molecule linking the activation of Syk to a number of downstream pathways. In support of this, we have shown that tyrosine phosphorylation of PLCγ2 is markedly reduced in LAT-deficient mouse platelets in response to CRP. LAT also associates with several of the proteins involved in T-cell receptor signalling, including Grb2, PI 3-kinase, SLP-76, and PLCγ1 (41), and tyrosine phosphorylation of PLCγ1 is largely inhibited in the LAT-deficient J.Cam2 Jurkat cell line in response to T-cell receptor activation (15). These observations suggest that LAT plays a similar role in the regulation of PLCγ in platelets and T lymphocytes following stimulation of GPVI and the T-cell receptor, respectively.
There is considerable evidence to support a critical role of PLCγ2 in the activation of platelets by GPVI. The second messengers 1,2-diacylglycerol and inositol-1,4,5-trisphosphate bring about most of the responses that constitute platelet activation in response to collagen (35, 37). We have also shown that CRP stimulate shape changes in human platelets through the mobilization of intracellular Ca2+ (4). There is direct evidence for a critical role of tyrosine phosphorylation in the regulation of PLCγ (30). The reduction in tyrosine phosphorylation of PLCγ2 in the LAT-deficient platelets is therefore likely to result in the loss of PLC activity (Fig. 5). Additionally, the inhibition of PLC activity could be due to loss of translocation to the plasma membrane through a LAT-dependent pathway. The response to thrombin was not altered in the LAT-deficient platelets, consistent with a minimal increase in tyrosine phosphorylation of PLCγ2. The mechanism of platelet activation by thrombin is believed to be via PLCβ. The significance of the small increase in tyrosine phosphorylation of LAT by thrombin in platelets is therefore unclear.
The observation that tyrosine phosphorylation of PLCγ2 in response to GPVI activation is dramatically reduced in either LAT-deficient (see above) or SLP-76-deficient mouse platelets (12, 19) demonstrates a critical role for the two adapters in the regulation of the phospholipase. These results correspond to those for tyrosine phosphorylation of PLCγ1 induced by cross-linking of the T-cell receptor in mutant Jurkat cells deficient in LAT (J.Cam2 cells) and SLP-76 (J14-v-29 cells) (15, 39). An important difference between platelets and T cells, however, is that tyrosine phosphorylation of SLP-76 is markedly reduced in the LAT-deficient J.Cam2 cell line whereas its phosphorylation is only slightly reduced in LAT-deficient mouse platelets. This suggests that SLP-76 is regulated downstream of LAT in T cells but that this is not the major pathway of regulation in platelets. In contrast, tyrosine phosphorylation of LAT is independent of SLP-76 in both systems. It is also noteworthy that we have not observed coimmunoprecipitation of SLP-76 and PLCγ2 in platelets but have observed coprecipitation of LAT and PLCγ2 and also of Lyn and PLCγ2 (19). In light of these results, we propose a model of PLCγ2 regulation in platelets in which the two adapters are regulated independently of each other but in which both are required for normal tyrosine phosphorylation of the phospholipase (Fig. 8). In this model, PLCγ2 is recruited to the membrane through association of its C-terminal SH2 domain with LAT (20) and its pleckstrin homology domain with PI-3,4,5-trisphosphate (14, 27). SLP-76 is proposed to recruit a member of the Src family of tyrosine kinases, probably Fyn and/or Lyn (19), to the vicinity of PLCγ2 via its SH2 domain, enabling tyrosine phosphorylation.
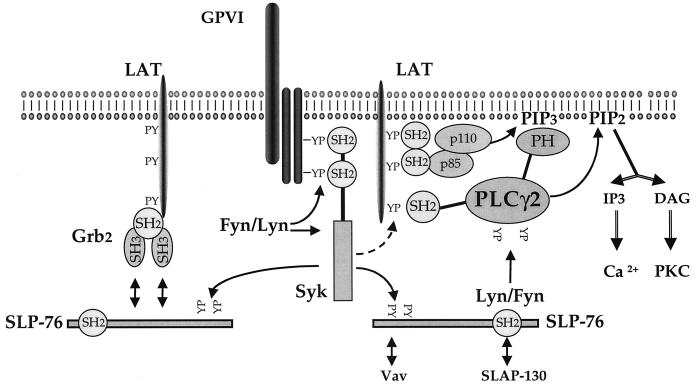
FIG. 8.
Model for the mechanism of regulation of PLCγ2 tyrosine phosphorylation and activation by LAT. CRP binding to GPVI triggers tyrosine phosphorylation of the FcR γ-chain, allowing recruitment of Syk and its activation. Syk phosphorylates LAT and SLP-76 via independent pathways. LAT is proposed to recruit PLCγ2 to the membrane through its C-terminal SH2 domain (19). LAT also associates with the p85 subunit of PI 3-kinase, leading to the formation of PI-3,4,5-trisphosphate, which is also required for recruitment of PLCγ2 (27). SLP-76 is proposed to recruit the Src kinase Fyn or Lyn to PLCγ2, leading to tyrosine phosphorylation. A separate pool of LAT is proposed to associate with SLP-76 via Grb2 and may be involved in the regulation of the Rho/Rac signalling pathways (10). This pathway gives rise to a minor route of phosphorylation of SLP-76 but not PLCγ2. PH, pleckstrin homology domain; PIP3, PI-3,4,5-trisphosphate; PIP2, PI-4,5-bisphosphate; IP3, inositol-1,4,5-triphosphate; DAG, diacylglycerol, PKC, protein kinase C.
We have also shown that LAT associates with Grb2 and SLP-76 in stimulated platelets, suggesting that the three proteins may form a complex. If this is the case, the interaction of SLP-76 and LAT is likely to be indirect, being mediated via the SH3 and SH2 domains of Grb2, respectively (see Fig. 8). This interaction may explain the weak inhibition of tyrosine phosphorylation of SLP-76 in LAT-deficient mouse platelets. The functional significance of the complex may be related to the regulation of Rho/Rac signalling pathways rather than PLCγ2 as proposed by Cantrell (10).
The small but significant increase in tyrosine phosphorylation of PLCγ2 in the LAT-deficient mice in response to CRP demonstrates that residual phosphorylation can occur through a pathway that is independent of the adapter. A similar residual increase in tyrosine phosphorylation of PLCγ2 in response to collagen and CRP occurs in FcR γ-chain- and SLP-76-deficient platelets, whereas tyrosine phosphorylation of the phospholipase is completely inhibited in Syk-deficient platelets (references 19 and 28) and results not shown). A low level of tyrosine phosphorylation of PLCγ1 is also observed in the LAT-deficient J.Cam2 cell line (15). In this case, however, it is unclear whether the residual phosphorylation was due to expression of a very small amount of LAT (15).
The residual increase in tyrosine phosphorylation of PLCγ2 observed in LAT-deficient platelets demonstrates a second (LAT-independent) pathway of regulation of the phospholipase. The existence of this pathway may reflect the expression in platelets of a second protein that is closely related to LAT, similar to the recent identification of two new members of the membrane-associated adapter protein family in T cells, namely, TRIM and SIT (9, 11). A role for Btk in the regulation of PLCγ2 in platelets (29) and in B cells (34) has also been proposed, with a similar role proposed for inducible T-cell kinase in T cells (3, 33). The present study has shown that tyrosine phosphorylation of Btk by CRP is not altered in LAT-deficient platelets, suggesting that it may mediate the LAT-independent pathway of phosphorylation. Syk has also been proposed to play a role in tyrosine phosphorylation of PLCγ2 in B cells (23) and is therefore also a candidate kinase for this pathway.
Although the residual tyrosine phosphorylation of PLCγ2 in the LAT-deficient mice was not associated with significant formation of PA, there was a trend toward an increase in phosphorylation with higher concentrations of CRP, suggesting that there may have been weak activation of the phospholipase. A similar set of results was observed for the expression of P-selectin. In contrast, CRP stimulated significant binding of fibrinogen in LAT-deficient mice, albeit at a lower level than in controls. Since the concentration-response curve for activation of αIIbβ3 lies to the left of that for expression of P-selectin and formation of PA, residual PLCγ2 activity could account for the activation of αIIbβ3 in the LAT-deficient mice. Alternatively, the increase in fibrinogen binding to αIIbβ3 may be independent of PLCγ2. For example, the PI 3-kinase pathway has been shown to stimulate αIIbβ3 activation in platelets (22, 40), although it is noteworthy that Gibbins et al. (17) have proposed that LAT is also required for activation of this pathway downstream of GPVI.
Mice deficient in Syk and SLP-76 undergo subcutaneous and intraperitoneal hemorrhaging and show poor viability, and it has been suggested that this may be caused by an impairment of collagen-induced platelet activation (12, 21, 28). The same phenotype, however, is not seen in mice with platelets deficient in the FcR γ-chain or LAT, despite the dramatic reduction in response to activation of GPVI. It is therefore important to establish whether the reduced level of αIIbβ3 activation in the LAT-deficient platelets in response to activation of GPVI is sufficient to prevent the abdominal hemorrhaging that is characteristic of Syk- and SLP-76-deficient mice. Alternatively, the phenotype of the Syk- and SLP-76-deficient mice may be the consequence of impairment of the function of macrophages, as proposed by Kiefer et al. (21).
In summary, the present study has shown a critical role for LAT in the regulation of PLCγ2 downstream of GPVI, adding to the growing evidence that the collagen receptor signals through a pathway that shares many features with signalling by the T-cell receptor. This also demonstrates that LAT plays a more widespread role than is suggested by its name (the linker for activation of T cells) and that LAT or an equivalent protein may play a general role in linking activation of immunoreceptors to PLCγ2.
ACKNOWLEDGMENTS
This work was supported by the British Heart Foundation and Wellcome Trust. L.Q. holds a BHF studentship. B.S.G. holds a Wellcome Prize Studentship. J.-M.P. is supported by the Fondation pour la Recherche Médicale and the Institut National de la Santé et de la Recherche Médicale.
We are grateful to M. Barnes, R. Farndale, and G. Knight for the gift of CRP.
REFERENCES
- 1.Achison M, Joel C, Hargreaves P G, Sage S O, Barnes M J, Farndale R W. Signals elicited from human platelets by synthetic, triple helical, collagen-like peptides. Blood Coagul Fibrinolysis. 1996;7:149–152. doi: 10.1097/00001721-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 1a.Asazuma, N., and S. P. Watson. Unpublished data.
- 2.Asselin J, Gibbins J M, Achison M, Lee Y-H, Morton L F, Farndale R W, Barnes M J, Watson S P. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1. Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- 3.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, M., M. Retzer, J. I. Wilde, P. Maschberger, M. Essler, M. Aepfelbacher, S. Watson, and W. Siess. Dichotomous regulation of myosin phosphorylation and shape change by Rho-kinase and calcium in intact platelets. Blood, in press. [PubMed]
- 5.Blake R A, Asselin J, Walker T, Watson S. Fcγ receptor II stimulated formation of inositol phosphates in human platelets is blocked by tyrosine kinase inhibitors and associated with tyrosine phosphorylation of the receptor. FEBS Lett. 1994;342:15–18. doi: 10.1016/0014-5793(94)80575-x. [DOI] [PubMed] [Google Scholar]
- 6.Blake R A, Schieven G L, Watson S P. Collagen stimulates tyrosine phosphorylation of phospholipase C-γ2 but not phospholipase C-γ1 in human platelets. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- 7.Brass L F, Hoxie J A, Kieber-Emmons T, Manning D, Poncz M, Woolkalis M. Agonist receptors and G proteins as mediators of platelet activation. Adv Exp Med Biol. 1993;344:17–37. doi: 10.1007/978-1-4615-2994-1_2. [DOI] [PubMed] [Google Scholar]
- 8.Briddon S J, Watson S P. Evidence for the involvement of p59fyn and p53/56lyn in signalling via the collagen receptor in human platelets. Biochem J. 1999;337:203–209. [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyns E, Marie-Cardine A, Kirchgessner H, Sagolla K, Shevchenko A, Mann M, Autschbach F, Bensussan A, Meuer S, Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-ζ complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantrell D. The real LAT steps forward. Trends Cell Biol. 1998;8:180–182. doi: 10.1016/s0962-8924(98)01264-1. [DOI] [PubMed] [Google Scholar]
- 11.Cardine A-M, Kirchgessner H, Bruyns E, Shevchenko A, Mann M, Autschbach F, Ratnofsky S, Meuer S, Schraven B. SHP2-interacting transmembrane adapor protein (SIT), a novel disulfide-linked dimer regulating human T cell activation. J Exp Med. 1999;189:1181–1194. doi: 10.1084/jem.189.8.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements J L, Lee J R, Gross B, Yang B, Olson J D, Sandra A, Watson S P, Lentz S R, Koretzky G A. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J Clin Investig. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor γ chain complex on human platelets. J Exp Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finco T S, Kadlecek T, Zhang W, Samelson L E, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 16.Gibbins J, Asselin J, Farndale R, Barnes M, Law C-L, Watson S P. Tyrosine phosphorylation of the Fc receptor γ-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 17.Gibbins J M, Briddon S, Shutes A, van Vugt M J, van de Winkel J G J, Saito T, Watson S P. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor γ-chain and LAT in platelets stimulated by collagen and convulxin. J Biol Chem. 1998;273:34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- 18.Gratacap, M.-P., B. Payrastre, C. Viala, G. Mauco, M. Plantavid, and H. phospholipase C-γ2 is an early key event in FcγRIIA-mediated activation of human platelets. J. Biol. Chem. 273:24314–24321. [DOI] [PubMed]
- 19.Gross B, Lee J R, Clements J L, Turner M, Tybulewicz V L J, Findell P R, Koretzky G A, Watson S P. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor GPVI in platelets. J Biol Chem. 1999;274:5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 20.Gross B S, Melford S K, Watson S P. Evidence that PLC-γ2 interacts with SLP-76, Syk, Lat, Lyn and the FcR γ-chain following stimulation of the collagen receptor in human platelets. Eur J Biochem. 1999;263:1–13. doi: 10.1046/j.1432-1327.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 21.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcγ receptor signaling macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacsovics T J, Bachelot C, Toker A, Vlahos C J, Duckworth B, Cantley L C, Hartwig J H. Phosphoinositide 3-kinase inhibition spares actin assembly in activating platelets but reverses platelet aggregation. J Biol Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]
- 23.Law C-L, Chandran K A, Sidorenko S P, Clark E A. Phospholipase C-γ1 interacts with conserved phosphotyrosyl residues in the linker region of syk and is a substrate for syk. Mol Cell Biol. 1996;16:1305–1315. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S B, Rao A K, Lee K H, Yang X, Bae Y S, Rhee S G. Decreased expression of phospholipase C-β2 isozyme in human platelets with impaired function. Blood. 1996;88:1684–1691. [PubMed] [Google Scholar]
- 25.Morton L F, Hargreaves P G, Farndale R W, Young R D, Barnes M J. Integrin alpha 2 beta 1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for alpha 2 beta 1-independent platelet reactivity. Biochem J. 1995;306:337–344. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motto D G, Ross S E, Wu J, Hendricks-Taylor L R, Koretsky G A. Implication of the GRB2-associated phosphoprotein SLP-76 in cell receptor-mediated interleukin 2 production. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquet J-M, Bobe R, Gross B, Gratacap M-P, Tomlinson M, Payrastre B, Watson S P. A collagen related peptide regulates phospholipase Cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem J. 1999;342:171–177. [PMC free article] [PubMed] [Google Scholar]
- 28.Poole A, Gibbins J M, Turner M, van Vugt M, van de Winkel J, Saito T, Tybulewicz V L J, Watson S P. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quek L S, Bolen J, Watson S P. Regulation of Bruton’s tyrosine kinase and phospholipase Cγ2 in collagen-stimulated platelets. Curr Biol. 1998;8:1137–1140. doi: 10.1016/s0960-9822(98)70471-3. [DOI] [PubMed] [Google Scholar]
- 30.Rhee S G, Choi K D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992;267:12393–12396. [PubMed] [Google Scholar]
- 31.Robinson A, Gibbins J, Rodriguez-Linares B, Finan P, Wilson L, Kellie S, Findall P, Watson S. Characterisation of Grb2-binding proteins in human platelets activated by FcγRIIA cross-linking. Blood. 1996;88:522–530. [PubMed] [Google Scholar]
- 32.Sarkar S. Tyrosine phosphorylation and translocation of LAT in platelets. FEBS Lett. 1998;441:357–360. doi: 10.1016/s0014-5793(98)01584-1. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer E M, Debnath J, Yap G, McVicar D, Liao X C, Littman D R, Sher A, Varmus H E, Lenardo M J, Schwartzberg P L. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 34.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J-P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siess W. Molecular mechanism of platelet activation. Physiol Rev. 1989;69:158–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- 36.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L J. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 37.Walker T, Watson S P. Synergy between Ca2+ and protein kinase C is the major factor in determining the level of secretion from human platelets. Biochem J. 1993;289:277–282. doi: 10.1042/bj2890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson S P, Gibbins J. Collagen receptor signalling in platelets: extending the role of the ITAM. Immunol Today. 1998;19:260–265. doi: 10.1016/s0167-5699(98)01267-5. [DOI] [PubMed] [Google Scholar]
- 39.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases form PLC-γ1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Zhang J, Shattil S J, Cunningham M C, Rittenhouse S E. Phosphonositide 3-kinase gamma and p85/phosphoinositide 3-kinase in platelets. Relative activation by thrombin receptor or beta-phorbol myristate acetate and roles in promoting the ligand-binding function of alphaII-beta3 integrin. J Biol Chem. 1996;271:6265–6272. doi: 10.1074/jbc.271.11.6265. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the zap-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Sommers C L, Burshtyn D N, Stebbins C C, DeJarnette J B, Trible R P, Grinberg A, Tsay H C, Jacobs H M, Kessler C M, Long E O, Love P E, Samelson L E. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Trible R P, Samelson L E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]