Abstract
Objective. The purpose of this study was to examine psychosocial influences on exercise-induced hypoalgesia (EIH).
Design. Randomized controlled trial.
Setting. Clinical research unit in a hospital.
Subjects. Fifty-eight healthy men and women (mean age = 21 ± 3 years) participated in this study.
Methods. Participants were first asked to complete a series of baseline demographic and psychological questionnaires including the Pain Catastrophizing Scale, the Fear of Pain Questionnaire, and the Family Environment Scale. Following this, they were familiarized with both temporal summation of heat pain and pressure pain testing protocols. During their next session, participants completed the Profile of Mood States, rated the intensity of heat pulses, and indicated their pressure pain thresholds and ratings before and after three minutes of submaximal, isometric exercise. Situational catastrophizing was assessed at the end of the experimental session.
Results. Results indicated that experimental pain sensitivity was significantly reduced after exercise (P < 0.05). Men and women did not differ on any of the measured psychosocial variables (P > 0.05). Positive family environments predicted attenuated pain sensitivity and greater EIH, whereas negative and chronic pain-present family environments predicted worse pain and EIH outcomes. Situational catastrophizing and negative mood state also predicted worse pain and EIH outcomes and were additionally associated with increased ratings of perceived exertion and muscle pain during exercise.
Conclusions. This study provides preliminary evidence that psychosocial variables, such as the family environment and mood states, can affect both pain sensitivity and the ability to modulate pain through exercise-induced hypoalgesia.
Keywords: Sex, Family, Psychological, Exercise, Modulation, Catastrophizing
Introduction
It is widely acknowledged that psychosocial factors contribute to the experience of pain in both healthy [1] and patient populations [2]. Negative psychological tendencies such as pain catastrophizing [3] and depression [4] are associated with exacerbated pain states, whereas positive psychological states such as perceiving a supportive social environment are associated with lower pain severity and pain-related disability [5]. Recently, the relationship between pain and psychosocial factors, such as mood states and pain catastrophizing, has been explored with regard to these factors interfering with endogenous pain modulation [6]. Conditioned pain modulation (CPM), a commonly utilized test of endogenous pain modulation where one noxious stimulus (i.e., the conditioning stimulus) modulates the pain sensation from another noxious stimulus (i.e., the testing stimulus), is often impaired in individuals with chronic pain disorders [7,8]. Likewise, deficient CPM is associated with greater postsurgical pain and analgesic use, which can increase the risk of developing chronic pain in otherwise healthy surgical patients [9,10]. A recent meta-analysis provided preliminary evidence that select psychosocial variables could influence pain modulation depending on the type of noxious test stimulus used. For instance, anxiety was associated with an impaired ability to modulate pressure pain, depression was associated with lower heat pain modulation, and pain catastrophizing was associated with reduced electrical pain modulation [6]. Outside of CPM paradigms, there is a dearth of research examining psychosocial factors in relation to other distinct forms of endogenous pain modulation, such as exercise-induced hypoalgesia (EIH).
EIH occurs when a noxious stimulus is perceived as less painful after a bout of moderate to high intensity aerobic, resistance, and isometric exercise [11–13]. Exercise requires muscular contractions that stimulate group III (A-delta) and group IV (C) nociceptive fibers, and this stimulation has been shown to result in activation of endogenous analgesic mechanisms [14]. EIH appears to operate through mechanisms that are distinct from other modulatory paradigms (e.g., offset analgesia, CPM). Compared with these other paradigms, the analgesic effects of EIH are typically more universal across different populations [15], last longer in duration [16], and occur even after nonpainful exercise (while a painful stimulus is required for both offset analgesia and CPM) [17]. Furthermore, unlike other types of endogenous modulation, exercise can be utilized regularly, and for some clinical conditions, it can be effective for long-term pain management [18,19].
A handful of studies have provided initial evidence that EIH is influenced by psychological factors. Two studies found that greater pain catastrophizing was associated with smaller EIH responses in healthy populations [20,21], though another study found no effects of catastrophizing on pain changes after exercise [15]. In addition, two studies have reported that state anxiety was not related to EIH outcomes in both healthy participants [15] and women with fibromyalgia [22]. Other psychosocial variables that have received attention in the general pain area but have not been examined with regards to EIH include fear of pain, a family history of pain, and the family environment. Therefore, the purpose of this study was to explore the effects of select psychosocial variables on pain sensitivity and EIH in healthy men and women.
Methods
This study was part of a larger investigation examining biological mechanisms (endogenous opioids and endocannabinoids) of EIH. The larger study involved giving participants either a naltrexone (to block the actions of endogenous opioids and their potential contributions to EIH) or a placebo capsule in a randomized, counter-balanced design. Pain sensitivity was then assessed before and after isometric exercise. In order to reduce demand characteristics related to the capsule administration, the participants were told that they would be given an active (naltrexone) and an inactive agent (placebo) and that “the purpose of the research was to improve our understanding of the causes of changes in pain following exercise” (i.e., they were not primed to expect a certain pain-related outcome with either the naltrexone or placebo administration). Because research has found that placebo effects are null when the demand characteristics surrounding the participant guessing the true experimental hypothesis are attenuated [23,24] and because the results from the larger study did not indicate differences in pain sensitivity or EIH effects between the naltrexone and placebo conditions (P > 0.05; effect size estimates for EIH were negligible to small between conditions for temporal summation [d = 0.21], pressure pain ratings [d = 0.06], and pain thresholds [d = 0.004])[25], the methods and results reported presently refer only to the placebo condition of this larger study. This arm was selected in order to more clearly and concisely examine the influence of psychosocial factors on naturally occurring EIH (i.e., without the potential influence of naltrexone administration on EIH). Full details regarding the results of the primary investigation are reported in Koltyn et al. [25]. All participants completed an informed consent document, which had been approved by the University Health Sciences Institutional Review Board. All procedures were administered by a female research assistant.
Participants
As per the larger study, a power analysis was conducted to estimate the sample size required for detecting a potential difference between men and women in the effects of naltrexone on EIH using a repeated measures design, with an alpha of 0.05, a power of 0.80, and a medium effect of 0.50 [26,27]. The analysis indicated that 44 participants (22 women and 22 men) would be needed; however, the sample size was increased in anticipation of potential subject attrition. Sixty healthy participants (30 men and 30 women) between the ages of 18 and 40 years were recruited for this study. Exclusion criteria included: 1) use of tobacco products; 2) use of recreational drugs such as opioids or cannabis (to prevent precipitated withdrawal in response to naltrexone administration and/or exogenous influences on endocannabinoids [28]); 3) presence of chronic illnesses such as diabetes, cancer, chronic pain, or hypertension; and 4) previous incidence of fainting during a blood draw or receiving a shot. Participants were recruited via flyers posted around campus and were paid $100 for completing all aspects of the study.
Psychosocial Instruments
Demographics/Family History of Pain
Basic demographic information including age, sex, race, ethnicity, height, weight, health status, and medication use was collected. In addition, participants were asked about the current or past occurrence of a variety of painful conditions (back, joint, jaw, neck, muscle, head, dental, etc.) among immediate family members (parents, siblings). Pain conditions in the family were totaled for each participant and categorized under the variable “family history of pain.”
Fear of Pain Questionnaire-III
The Fear of Pain Questionnaire-III (FPQ) is a 30-item questionnaire that assesses fear associated with situation-specific medical, minor, or severe pain. The FPQ has demonstrated good internal consistency for each subscale, with Cronbach’s alphas ranging from 0.87 to 0.88, and good test-retest reliability, ranging from 0.69 to 0.76 (29). The FPQ has been found to be both valid and reliable in healthy and clinical samples [29–31]. The total score on the FPQ was used in regression analyses to represent a participant’s overall fear of pain.
The Family Environment Scale
Previous work has shown that the family environment may be a contributing factor in the development and maintenance of chronic pain [32]; however, it has rarely been examined with regards to its effects on pain modulatory processes (e.g., EIH or CPM), wherein dysfunction has been shown to predict chronic pain outcomes [9]. The Family Environment Scale (FES) is a 90-item questionnaire that assesses an individual’s perception of their family environment across 10 subscales: cohesion, expressiveness, conflict, independence, achievement orientation, active-recreational orientation, intellectual-cultural, moral-religious, organization, and control [33]. Though the FES is often used in clinical settings, it has demonstrated construct validity for a variety of research applications [33]. In order to reduce the number of subscales (10 original subscales) used as potential predictors in the regression models, an exploratory factor analysis was performed on the responses from the FES. The factor analysis indicated that a two-factor solution emerged. The first factor was labeled a “positive family environment” and included the scores from the cohesion, conflict-inverted, intellectual-cultural, and active-recreational subscales. The second factor was labeled a “negative family environment” and included the scores of the expression-inverted, independence-inverted, organization, and control subscales. Both factors demonstrated acceptable internal consistency (Cronbach’s α = 0.77 for the positive FES and α = 0.66 for the negative FES). The moral-religious and achievement orientation subscales were not included as they made up a third factor with poor internal consistency (Cronbach’s α = 0.37) and an uninterpretable relationship. The summary scores of the subscales within the two emergent factors were used in the regression analyses to characterize the family environment.
The Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) is a 13-item self-report measure of pain-related catastrophic thinking that assesses three main dimensions of catastrophizing including rumination, magnification, and helplessness. The PCS requires participants to think about previous painful experiences and respond to each item on a Likert scale ranging from 0 = not at all to 4 = all the time. The PCS has good internal consistency, with an overall α index of 0.87, and has demonstrated construct validity in both clinical and nonclinical populations [34,35]. The total score on the PCS was used in the regression analyses to represent the general degree of catastrophic thinking in regards to pain.
In Vivo Pain Catastrophizing Scale
The in vivo PCS (iv-PCS) was completed after pain testing during the experimental session. Unlike the PCS, which attempts to characterize dispositional or “trait-like” catastrophizing, the iv-PCS is a shortened, six-item version of the PCS using modified wording to assess situational or “state-like” pain catastrophizing experienced during experimental laboratory procedures. Situational catastrophizing has been shown to uniquely contribute to pain experienced in the laboratory setting compared with dispositional catastrophizing [36–38]. The six items were selected to ensure equal representation of each PCS subscale: rumination, magnification, and helplessness [39]. The iv-PCS has been used previously in healthy samples and has demonstrated good internal consistency (α = 0.87) [39,40]. The total score of the iv-PCS was used in regression analyses.
Profile of Mood States
The Profile of Mood States (POMS) was administered at the beginning of the experimental session to determine the effect of mood state on pain and EIH outcomes. Six mood states are evaluated using the POMS: tension, depression, anger, vigor, fatigue, and confusion, with internal consistencies of each mood state ranging from α = 0.84–0.95. The POMS has been shown repeatedly to be a valid, reliable, and sensitive measure of general mood [41]. Total mood disturbance (TMD) was used in regression analyses and was determined by summing the negative mood subscales, subtracting the vigor subscale, and then adding 100 to adjust for potential negative total scores.
Procedures
Participants first attended a familiarization session during which they provided basic demographic information and were assessed on several psychological variables including fear of pain (FPQ), dispositional pain catastrophizing (PCS), and the family environment (FES). After completing the packet of questionnaires, participants were introduced to the pain testing protocols.
Protocol 1: Temporal Summation of Heat Pain
Temporal summation of heat pain was assessed using a standardized, previously published protocol [25,26,42]. Heat pulses (≥0.33 Hz) were administered to the thenar eminence of the dominant hand using a computer controlled Medoc sensory analyzer (CHEPS model). There were 10 pulses total, and the last six pulses were identical, reaching the same peak temperature of 51 °C. Participants were asked to provide pain ratings for the delayed sensation of each heat pulse using a 0–100 numerical scale. Pilot testing using this protocol indicated that there was a significant increase in heat pain ratings across these last six pulses, indicating that the protocol was able to elicit a temporal summation response. More details regarding the temporal summation protocol can be found in Koltyn et al. [25]. For data analysis, the difference scores in pain ratings between the fifth and the 10th pulse were used as the temporal summation outcome variable at each time point (e.g., pre- and postexercise). EIH was calculated by taking the difference score of the postexercise TS pain rating minus the pre-exercise TS pain rating (i.e., a more negative difference score indicates greater EIH).
Protocol 2: Pressure Pain Thresholds and Ratings
For the pressure pain protocol, participants were asked to place their dominant forefinger in a Forgione-Barber Pressure Stimulator [43]. A 3 kg weight was applied to the finger for a maximum of two minutes. To measure pressure pain thresholds, participants were asked to push a button attached to a timer out of their view when they first perceived pain. EIH was calculated by taking the difference in seconds of the postexercise pressure pain threshold minus the pre-exercise pressure pain threshold (i.e., a longer duration in seconds indicates greater EIH).
In addition, pain ratings were assessed every 30 seconds during the two-minute exposure. Participants were asked to provide pain ratings for the pressure pain stimuli using a 0–100 numerical scale. Pressure pain ratings were averaged across the four 30-second time points, and this average was used as the pressure pain outcome variable at each time point (e.g., pre- and postexercise). EIH was calculated by taking the difference score of the postexercise average pressure pain rating minus the pre-exercise average pressure pain rating (i.e., a more negative difference score indicates greater EIH).
Exercise Stimulus
To compute the exercise intensity level for the experimental session, participants were asked to perform two maximal voluntary hand grip contractions for five seconds each, separated by three minutes of recovery time. An average of the two maximal contractions was used to calculate the participant’s 25% maximal voluntary contraction (MVC in kg). The 25% MVC was used for the three-minute exercise period during the experimental session.
Experimental Session
Participants returned to the laboratory between 7:00 a.m. and 1:00 p.m. within 10 to 14 days of their familiarization session. For women, the visit was scheduled so that it occurred during the follicular phase of their menstrual cycle to control for potential hormonal effects on pain sensitivity [44]. Participants were instructed not to exercise or consume caffeine or alcohol in the four hours prior to testing and not to take any analgesic medications in the 24 hours prior to testing. Following this, the participant completed pre-exercise temporal summation and pressure pain testing on the dominant hand.
After experimental pain testing, participants performed isometric exercise (25% of their dominant hand MVC) for three minutes. Participants were asked to rate their perceived exertion and muscle pain every 30 seconds during exercise using Borg’s Ratings of Perceived Exertion (RPE) 6–20 scale [45] and a pain scale previously validated to assess muscle pain experienced specifically during exercise [46]. Immediately following exercise, they completed another round of temporal summation and pressure testing. At the end of the experimental procedures, participants completed the iv-PCS. While completing this questionnaire, participants were told to reflect on their experimental heat and pressure pain experiences (as opposed to muscle pain experienced during exercise).
Statistical Analysis
Multiple analytic approaches were conducted in order to determine the relationship between select psychosocial variables and outcome variables (muscle pain, perceived exertion, experimental pain ratings, and EIH responses). Descriptive statistics were calculated for men and women for demographic, psychosocial, RPE, and muscle pain variables, as well as for pre- and postexercise temporal summation of heat pain ratings, pressure pain thresholds, and average pressure pain ratings. Independent samples t tests were computed to test for differences in baseline characteristics between men and women. A 2 (sex) by 2 (pre-, postexercise) repeated measures analysis of variance was used to determine whether there were significant reductions in pain sensitivity after exercise in men and women. Next, total scores from the questionnaires administered during the familiarization session (FPQ, positive and negative FES, PCS), the POMS total mood disturbance, and the in vivo PCS scores from the experimental session, sex, and family history of pain variables were entered as possible predictors of experimental pain and EIH outcomes in a series of best-subsets regression analyses (e.g., the best subset of predictors for models with one predictor, two predictors, …eight predictors were computed). For each outcome of interest, models with significant adjusted R-squared values (P < 0.05) were compared using Akaike’s information criterion to select the most parsimonious model. All models were subjected to diagnostic tests inspecting for influential cases (Cook’s Distance, hat values), predictor multicollinearity (variance inflation factor), homoscedasticity (Breusch-Pagan test), and normally distributed independent errors (Durbin-Watson Test and visual inspection of residuals). This process was repeated for all of the outcome variables of interest including pre- and postexercise temporal summation and pressure pain ratings/thresholds, EIH difference scores, average ratings of perceived exertion, and average muscle pain ratings. All statistical analyses were conducted using IBM SPSS Statistics version 22.0 and R statistical computing software (version 3.1.2).
Results
Sample Characteristics and Pain Sensitivity Testing
Fifty-eight healthy and pain-free young adults (29 men and 29 women) with an average age of 21 ± 3 years completed all aspects of this study. The racial and ethnic makeup of the sample included 33 (57%) Caucasian participants (16 women, 17 men), 10 (17%) African American participants (six women, four men), eight (14%) Asian American participants (two women, six men), six (10%) Latino participants (four women, two men), and one (2%) American Indian participant (one woman). There were significant sex differences in average MVC, body mass index, and the reported number of painful conditions among immediate family members (P < 0.05, range = 0–6 painful conditions). Because there was a significant sex difference in family history of pain and because previous work has indicated that the influence of familial history of pain on experimental pain responses may vary by sex [47,48], a sex*family history of pain interaction term was also included in the regression analyses. The descriptive characteristics of the sample and psychosocial variables are listed in Table 1.
Table 1.
Means and standard deviations for sample demographics and psychological characteristics
| Men, mean (SD) | Women, mean (SD) | Overall, mean (SD) | |
|---|---|---|---|
| (N = 29) | (N = 29) | (N = 58) | |
| Age, y | 21.1 (2.9) | 20.7 (2.7) | 20.9 (2.8) |
| BMI, kg/m2 | 26.1 (4.9) | 22.2 (3.2)* | 24.2 (4.7) |
| Avg MVC, kg | 38.3 (9.9) | 22.4 (6.5)* | 30.3 (11.6) |
| Family history of pain† | 1.4 (1.7) | 2.6 (1.5)* | 2.0 (1.7) |
| FPQ | 74.8 (19.5) | 79.9 (14.0) | 77.4 (17.0) |
| FES-positive | 24.3 (6.7) | 23.7 (7.3) | 24.0 (7.0) |
| FES-negative | 17.0 (5.9) | 17.7 (6.0) | 17.4 (5.9) |
| PCS | 14.0 (6.6) | 16.5 (9) | 15.3 (8.0) |
| In vivo PCS | 5.8 (5.5) | 6.3 (4.6) | 6.0 (5.0) |
| TMD | 112.8 (28.9) | 113.8 (20.9) | 113.3 (25.0) |
BMI = body mass index; MVC = maximal voluntary contraction; FES = Family Environment Scale; TMD = total mood disturbance; PCS = Pain Catastrophizing Scale; FPQ = Fear of Pain Questionnaire.
Indicates a significant sex difference (P < 0.05).
Number of painful conditions experienced by the participant’s immediate family members.
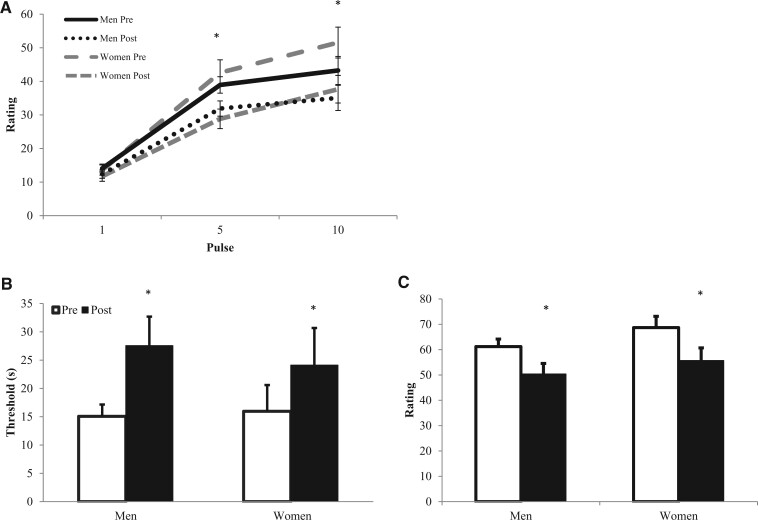
The results for experimental pain sensitivity outcomes (temporal summation, pressure pain thresholds and ratings) are depicted in Figure 1. There was a significant increase in heat pain ratings from the fifth to the 10th pulse (P < 0.05), indicating that temporal summation occurred. There was a significant decrease in heat pain ratings at pulses 5 and 10 after exercise (P < 0.05). There was an increase in pressure pain thresholds after exercise, and there was a decrease in average pressure pain ratings after exercise (P < 0.05). Men and women did not differ in heat pain ratings, pressure pain thresholds, or pressure pain ratings at any time point (P > 0.05; effect size estimates for EIH between men and women were negligible to small for temporal summation [d = 0.07], pressure pain thresholds [d = 0.21], and pressure pain ratings [d = 0.14]). Men and women also did not differ in their average ratings of perceived exertion or muscle pain during exercise (P > 0.05). RPE and muscle pain data are located in Table 2. For more information on the results of pain sensitivity outcomes, see Koltyn et al. [25].
Figure 1.
Means and standard errors for experimental pain outcome data for men and women. (A) Heat pain ratings at pulses 1, 5, and 10 pre- and postexercise. (B) Pressure pain thresholds (sec) pre- and postexercise. (C) Average pressure pain ratings pre- and postexercise. *Significant reduction in pain sensitivity after exercise (P < 0.05).
Table 2.
Means and standard deviations for ratings of perceived exercise and muscle pain ratings
| Outcome | Men, mean (SD) | Women, mean (SD) | Overall, mean (SD) |
|---|---|---|---|
| (N = 29) | (N = 29) | (N = 58) | |
| Ratings of perceived exertion | 13.4 (2.5) | 13.9 (1.9) | 13.7 (2.2) |
| Muscle pain ratings | 3.1 (2.6) | 2.4 (1.5) | 2.7 (2.2) |
Regression Analyses
Temporal Summation of Heat Pain
The regression models related to the temporal summation (TS) of heat pain outcomes are summarized in Table 3. For pre-exercise TS, situational catastrophizing (iv-PCS), total mood disturbance (TMD, from POMS), and family history of pain were included in the final model, which accounted for 12% of the variance. Higher situational catastrophizing was associated with greater TS at baseline. For postexercise TS, there were no significant models. For TS EIH scores, total mood disturbance, a positive family environment (FES), and situational catastrophizing were included in the final model, which accounted for 12% of the variance. After controlling for mood disturbance and a positive family environment, situational catastrophizing was a significant predictor, such that greater catastrophizing was associated with less TS after exercise (i.e., more EIH).
Table 3.
Regression models for temporal summation outcomes
| Outcome variable | Predictor(s) | Adj. R2 | P | B | SE B | β | P |
|---|---|---|---|---|---|---|---|
| TS pre | 0.12 | 0.02 | |||||
| iv-PCS | 0.78 | 0.35 | 0.29 | 0.03* | |||
| TMD | −0.12 | 0.08 | −0.20 | 0.13 | |||
| Fam. history of pain | 1.57 | 1.00 | 0.20 | 0.12 | |||
| TS post | No sig. models | ||||||
| TS EIH (post-pre) | 0.12 | 0.02 | |||||
| iv-PCS | −0.67 | 0.29 | −0.30 | 0.03* | |||
| TMD | 0.12 | 0.07 | 0.23 | 0.09 | |||
| Positive FES | −0.40 | 0.21 | −0.25 | 0.06 |
TS EIH refers to the difference score of the postexercise temporal summation values minus the pre-exercise temporal summation values. Therefore, negative relationships for predictors to TS EIH indicate that after controlling for other variables in the model, higher scores on that predictor variable are associated with more EIH.
FES = Family Environment Scale; iv-PCS = in vivo “situational” catastrophizing; TMD = total mood disturbance; TS = temporal summation (i.e., the increase in pain ratings from pulse 5–10).
Predictor is significant at the 0.05 level.
Pressure Pain Thresholds
The regression models related to pressure pain thresholds (PPTs) are summarized in Table 4. For pre-exercise PPTs, sex, family history of pain, the sex*family history of pain interaction, fear of pain (FPQ), and a negative family environment were included in the final model, which accounted for 14% of the variance in thresholds prior to exercise. Controlling for the other predictors in the model, females without a family history of pain had greater PPTs compared with males. When sex was interacted with a family history of pain, thresholds were approximately nine seconds shorter for women than men for each additional familial pain condition. For postexercise PPTs, four predictors were included in the final model, negative family environment, fear of pain, dispositional catastrophizing (PCS), and total mood disturbance, which accounted for 24% of the variance in postexercise thresholds. Of these predictors, both the negative family environment and fear of pain predictors were significant, such that higher scores on these indices were associated with lower thresholds. For EIH PPTs, negative family environment, fear of pain, total mood disturbance, and dispositional catastrophizing were included in the final model, which accounted for 22% of the variance in EIH-related threshold changes. After controlling for fear of pain and catastrophizing, a negative family environment and total mood disturbance were significant predictors, such that higher scores on these indices were associated with less EIH.
Table 4.
Regression models for pressure pain threshold outcomes
| Outcome variable | Predictor(s) | Adj. R2 | P | B | SE B | β | P |
|---|---|---|---|---|---|---|---|
| PPT pre | 0.14 | 0.03 | |||||
| Sex(f) | 20.89 | 8.17 | 0.54 | 0.01* | |||
| Fam. history of pain | 3.50 | 2.22 | 0.31 | 0.12 | |||
| Sex(f)*fam. history of pain | −8.86 | 3.12 | −0.82 | 0.007** | |||
| FPQ | −0.26 | 0.16 | −0.23 | 0.10 | |||
| Negative FES | −0.72 | 0.42 | −0.22 | 0.09 | |||
| PPT post | 0.24 | <0.01 | |||||
| Negative FES | −2.02 | 0.63 | −0.37 | 0.002** | |||
| FPQ | −0.57 | 0.24 | −0.30 | 0.02* | |||
| TMD | −0.34 | 0.18 | −0.23 | 0.06 | |||
| PCS | 0.97 | 0.54 | 0.24 | 0.08 | |||
| PPT EIH (post-pre) | 0.22 | <0.01 | |||||
| TMD | −0.27 | 0.13 | −0.27 | 0.03* | |||
| FPQ | −0.33 | 0.17 | −0.26 | 0.06 | |||
| Negative FES | −1.28 | 0.45 | −0.34 | 0.006** | |||
| PCS | 0.65 | 0.38 | 0.23 | 0.09 |
FES = Family Environment Scale; FPQ = Fear of Pain Questionnaire; PCS = Pain Catastrophizing Scale; PPT = pressure pain thresholds in seconds; TMD = total mood disturbance.
Predictor is significant at the 0.05 level.
Predictor is significant at the 0.01 level.
Pressure Pain Ratings
The regression models related to pressure pain ratings (PPRs) are summarized in Table 5. For pre-exercise PPRs, negative family environment, sex, family history of pain, and sex*family history of pain interaction were included in the final model, which accounted for 11% of the variance in ratings at baseline. Females with a family history of pain and higher scores for the negative family environment were associated with higher PPRs. For postexercise PPRs, negative family environment and situational catastrophizing were included in the final model, which accounted for 13% of the variance. Higher scores for the negative family environment were associated with higher pain ratings after exercise. For EIH PPRs, total mood disturbance and fear of pain were included in the final model, which accounted for 14% of the variance in EIH-related PPR changes. Both fear of pain and mood disturbance were significant predictors, such that higher scores on these scales were associated with less EIH.
Table 5.
Regression models for pressure pain ratings outcomes
| Outcome variable | Predictor(s) | Adj. R2 | P | B | SE B | β | P |
|---|---|---|---|---|---|---|---|
| PPR pre | 0.11 | 0.04 | |||||
| Negative FES | 0.94 | 0.44 | 0.27 | 0.04* | |||
| Sex(f) | −6.55 | 8.49 | −0.16 | 0.44 | |||
| Fam. history of pain | −3.63 | 2.20 | −0.30 | 0.11 | |||
| Sex(f)*fam. history of pain | 6.42 | 3.20 | 0.61 | 0.05* | |||
| PPR post | 0.13 | <0.01 | |||||
| Negative FES | 1.34 | 0.52 | 0.32 | 0.01** | |||
| iv-PCS | 1.11 | 0.61 | 0.22 | 0.08 | |||
| PPR EIH (post-pre) | 0.14 | <0.01 | |||||
| TMD | 0.17 | 0.08 | 0.28 | 0.03* | |||
| FPQ | 0.22 | 0.10 | 0.29 | 0.03* |
Positive relationships for predictors to PPR EIH indicate that after controlling for other variables in the model, higher scores on that predictor variable are associated with less EIH.
FES = Family Environment Scale; FPQ = Fear of Pain Questionnaire; iv-PCS = in vivo “situational” catastrophizing; PPR = average pressure pain ratings across the two minutes; TMD = total mood disturbance.
Predictor is significant at the 0.05 level.
Predictor is significant at the 0.01 level.
Ratings of Perceived Exertion and Muscle Pain
The regression models related to ratings of perceived exertion and muscle pain are summarized in Table 6. For average RPE during exercise, situational catastrophizing, dispositional catastrophizing, and total mood disturbance were included in the final model, which accounted for 20% of the variance in average RPE ratings. Both catastrophizing scales were significant predictors, such that greater dispositional and situational catastrophizing were associated with higher RPE. For average muscle pain, situational catastrophizing and total mood disturbance were included in the final model, which accounted for 20% of the variance in muscle pain. When controlling for mood disturbance, situational catastrophizing was a significant predictor and was associated with greater muscle pain during exercise.
Table 6.
Regression models for RPE and muscle pain during exercise
| Outcome variable | Predictor(s) | Adj. R2 | P | B | SE B | β | P |
|---|---|---|---|---|---|---|---|
| Average RPE | 0.20 | <0.01 | |||||
| iv-PCS | 0.15 | 0.05 | 0.34 | 0.008** | |||
| PCS | 0.09 | 0.03 | 0.32 | 0.02* | |||
| TMD | −0.02 | 0.01 | −0.23 | 0.07 | |||
| Average muscle pain | 0.20 | <0.001 | |||||
| iv-PCS | 0.21 | 0.05 | 0.48 | 0.0002*** | |||
| TMD | −0.02 | 0.01 | −0.22 | 0.08 |
iv-PCS = in vivo “situational” catastrophizing; PCS = Pain Catastrophizing Scale; RPE = ratings of perceived exertion during exercise; TMD = total mood disturbance.
Predictor is significant at the 0.05 level.
Predictor is significant at the 0.01 level.
Predictor is significant at the 0.001 level.
Discussion
The purpose of this study was to explore the effects of various psychosocial variables on experimental pain ratings and EIH responses. Overall, there were several different predictors that were included in the final regression models; however, there were few consistent and significant predictors of pain ratings or EIH. Despite these varied findings, there were apparent psychosocial themes that emerged from the data, which should be taken into consideration when designing studies intended to examine mechanisms and contributors to endogenous pain modulation.
The most transparent theme that emerged from the results was the presence of family-related predictors in several of the resulting models. It is well documented that children reared in families where one or more parents has a chronic pain condition are more likely than children with healthy parents to report numerous pain complaints and develop chronic pain conditions as adults [49]. In the present study, a family history of pain contributed to greater TS prior to exercise. In addition, a family history of pain was particularly influential for women in determining pressure pain thresholds and pressure pain ratings. Controlling for other predictors, women without a family history of pain had longer pressure pain thresholds and lower pressure pain sensitivity on average than men; however, female thresholds were significantly shorter and their pain sensitivity significantly greater than men for each additional familial pain condition. This is in agreement with previous findings indicating that women are more likely than men to report a higher number of pain complaints and demonstrate greater experimental pain sensitivity when a family history of pain is also present [47,48,50]. While familial genetics and biological sex differences between men and women are certainly contributing to this interaction, it is also possible that the family environment could be influencing this relationship. For instance, it has been hypothesized that women are more sensitive to and are better able to interpret nonverbal cues related to pain (e.g., grimacing) than men [51,52]. It has also been hypothesized that daughters are more likely than sons to assume caregiving roles for chronically ill family members and thus have more exposure to pain and pain-related behaviors, which could impact their own future pain-related responses and behaviors via social learning theory [53,54].
Though not a frequently examined topic today, there is a fair amount of early research suggesting that the family environment influences pain. For instance, studies have found that “pain-prone” patients come from families with high amounts of aggression and hostility and, conversely, that patterns of rigidity and conflict are often present in “psychosomatic families” (for a review, see Turk et al. 1987 [32]). In addition, one study reported that adolescent surgical patients reared in supportive, expressive environments report less pain and discomfort and need less analgesic medication postoperatively compared with children who come from families higher in conflict and control. These results suggest that the family environment may interact with the pain modulatory capabilities of analgesics [55]. In the present study, a positive family environment, which included the subscales of cohesion and conflict-inverted, was indicative of a greater EIH response for temporal summation (i.e., a greater reduction in temporal summation postexercise). A negative family environment, which included the subscales of control, expressiveness-inverted, and independence-inverted, was a consistent predictor of worse outcomes for pressure pain thresholds and ratings.
Fear of pain is another psychological variable that has been regularly associated with increased pain sensitivity [56] and pain-related disability [57]. In this study, fear of pain was predictive of lower PPTs before and after exercise. It was also associated with less EIH during the pressure pain protocol. To date, one other study has assessed the relationship between fear of pain and EIH, and they found that responses on an abbreviated, nine-item version of the FPQ were not correlated with pressure pain ratings after a painful isometric elbow flexion exercise or during a CPM task (while controlling for baseline pain ratings) [15]. Similarly, the present study did not find a significant correlation between total scores on the FPQ and pressure pain ratings after exercise (P = 0.067); however, in the regression analysis, fear of pain was a significant predictor for pressure pain ratings when controlling for total mood disturbance scores (P = 0.03). Fear of pain also more consistently predicted pressure pain thresholds rather than pain ratings, suggesting it may better relate to anticipatory or arousal aspects of painful experiences. Beyond EIH paradigms, it has been reported that individuals who regularly engage in high-intensity exercise exhibit greater CPM than less active controls [58,59]. Highly active individuals also report less fear of pain overall, and when present, fear of pain negatively correlates with their ability to modulate pain [59].
Finally, total mood disturbance contributed to several of the models, which predicted lower pain thresholds and lower EIH; however, it was a significant predictor only for the EIH effect observed for PPTs and PPRs. In both cases, greater mood disturbance was associated with less EIH. These findings are in agreement with the general pain literature, which has found increased rates of anxiety and depressive symptoms among chronic pain patients [4]. In healthy individuals, it has also been found that depressed mood is a predisposing factor to developing chronic pain [60]. Conversely, reductions in depressive symptoms over the course of treatment have been found to be related to improved pain and disability outcomes at long-term follow-up [61]. Acutely, experimentally inducing a positive mood state has been found to reduce pain unpleasantness ratings and also increase activity in regions of the brain thought to be involved in pain modulation, although mood state was assessed with nonstandardized measures in this particular study and it is not clear whether the investigators were truly able to manipulate mood state [62]. Regarding EIH, the effects of mood state have rarely been reported. To date, only two studies have touched on this relationship and have found that state anxiety was not associated with EIH in healthy adults or women with fibromyalgia [15,22]. In sum, there is preliminary evidence that mood states are able to affect endogenous pain modulation; however, it is too early to say whether this applies to EIH as well.
Also notable in the present study was the surprisingly small predictive contributions of sex and dispositional catastrophizing in many of the resulting models. When sex was included in a final model, it was generally overshadowed by its interaction term with a family history of pain. In general, sex and catastrophizing are frequently and strongly associated with clinical pain outcomes. It has been reported that women have a greater incidence of chronic pain conditions, tend to be more sensitive to experimental pain procedures than men, and also engage in more catastrophic thinking in regards to pain [63]. However, the literature for sex differences in pain in healthy, young adult samples has been more inconsistent, especially across various types of pain modalities assessed in the laboratory setting [64]. One potential explanation for these inconsistencies could be the influence of gonadal hormones on pain sensitivity, and much of the research conducted examining sex differences in pain has not controlled for menstrual cycle phase [63]. In the present study, experimental pain testing was conducted during the follicular phase of the women’s menstrual cycles, which potentially reduced the variability that sex hormones may have on pain. Currently, the relationship between female reproductive hormones and experimental pain is unclear; however, a meta-analysis conducted by Riley et al. (1998) [44] concluded that pain thresholds for mechanical, thermal, and ischemic muscle pain were higher during the follicular phase when progesterone and estrogen levels are lower than during the luteal phase. The research regarding sex differences in EIH at this point remain equivocal, with some previous studies documenting no sex differences [25,27,65] and other studies reporting mixed findings [16,20,66–68]. Also potentially contributing to the lack of sex differences in this study was that men and women did not differ on any psychological variable including pain catastrophizing, which has often been reported to be higher in women and in some instances has been found to mediate the relationship between sex and experimental pain modulation [21,69].
Individuals who engage in greater pain catastrophizing typically have higher experimental pain sensitivity and demonstrate deficient endogenous pain modulation, as assessed via both CPM and EIH paradigms [1,20,21,39,70]. In this study, dispositional catastrophizing (the PCS) appeared only as a nonsignificant predictor for experimental pain outcomes. It did, however, significantly predict greater perceived exertion during exercise. Conversely, in vivo catastrophizing (i.e., situational catastrophizing; iv-PCS) appeared much more frequently in the resulting models. In particular, situational catastrophizing predicted increased TS before exercise; however, it also predicted greater EIH during the TS protocol. While it is not clear why situational catastrophizing would be associated with larger EIH during the TS protocol, it could be related to the fact that iv-PCS scores were strongly associated with higher RPEs and muscle pain during exercise. If those who engaged in high situational catastrophizing were also more likely to have experienced greater muscle pain during exercise, there could have been carryover conditioned pain modulatory effects from residual muscle pain during TS testing, which immediately followed exercise (prior to pressure pain testing). These results also suggest that experimental pain catastrophizing should be assessed using both dispositional and situational measures of catastrophizing. It may be especially important to differentiate between dispositional and situational catastrophizing in healthy, young adult samples wherein individuals are less likely to have experienced many painful events, as completing dispositional pain catastrophizing questionnaires (e.g., the PCS) typically depends on the recall of previous painful experiences.
There are limitations associated with the present study. First, these results pertain to the placebo arm of the larger study, which could suggest that placebo effects influenced some of the observed relationships between psychosocial variables and EIH responses. However, the neutral language of the consent form (i.e., not priming the participants to expect a certain pain-related outcome with either capsule administration) lessens the likelihood of the introduction of placebo effects [23,24], and when participants were informally asked whether they thought they had received the placebo or the naltrexone capsule after each experimental day, they did not guess which capsule they received significantly better than chance (P = 0.20). Regardless, if participants were indeed able to discern the true purpose of the study, nocebo effects rather than placebo effects would have been expected because participants would expect their pain sensitivity to increase as a result of naltrexone administration and expect no change in their pain sensitivity following placebo administration. However, there was a significant decrease in pain sensitivity following exercise in both the placebo and naltrexone conditions, and this magnitude of pain reduction was not different between the two conditions [25]. Second, due to the exploratory nature of this study, which used the psychosocial data collected from a larger study designed to examine biological mechanisms related to EIH, the resulting regression models cannot be generalized to other EIH studies with healthy young adults, and these results are certainly not generalizable to clinical populations (e.g., chronic pain or psychiatric). However, the results from this study support the notion that endogenous pain modulation via exercise can be influenced by psychosocial factors. In particular, family-related factors such as the family environment and a family history of pain appear to be especially important in predicting pain outcomes and should continue to be assessed in pain sensitivity and EIH protocols. Likewise, transient psychological variables such as situational catastrophizing and mood state frequently contributed to the resulting models and may be more revealing than dispositional variables when exploring determinants of pain modulation in nonclinical samples. Rather than simply adding psychological questionnaires to traditional EIH protocols, future studies hoping to expand upon these relationships should consider recruiting specific sample populations and designing experiments that are intended to directly assess the effects of these psychosocial variables on EIH.
References
- 1.Goodin BR, McGuire L, Allshouse M, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain 2009;10(2):180–90. [DOI] [PubMed] [Google Scholar]
- 2.George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain 2007;23(1):76–84. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan M, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17(1):52–64. [DOI] [PubMed] [Google Scholar]
- 4.Burke ALJ, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: A meta-analytic review. Br J Clin Psychol 2015;54(3):345–60. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MP, Moore MR, Bockow TB, Ehde DM, Engel JM. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: A systematic review. Arch Phys Med Rehabil 2011;92(1):146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahman-Averbuch H, Nir R-R, Sprecher E, Yarnitsky D. Psychological factors and conditioned pain modulation: A meta-analysis. Clin J Pain 2016; 32(6):541–54. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J Pain 2012;13(10):936–44. [DOI] [PubMed] [Google Scholar]
- 8.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23(5):611–5. [DOI] [PubMed] [Google Scholar]
- 9.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;138(1):22–8. [DOI] [PubMed] [Google Scholar]
- 10.Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: A pilot study. J Pain Palliat Care Pharmacother 2010;24(2):119–28. [DOI] [PubMed] [Google Scholar]
- 11.Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012;13(12):1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltyn KF. Analgesia following exercise: A review. Sport Med 2000;29(2):85–98. [DOI] [PubMed] [Google Scholar]
- 13.Koltyn KF. Exercise-induced hypoalgesia and intensity of exercise. Sport Med 2002;32(8):477–87. [DOI] [PubMed] [Google Scholar]
- 14.Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: Physiological mechanisms and clinical implications. Med Sci Sports Exerc 1990;22(4):417–28. [PubMed] [Google Scholar]
- 15.Lemley KJ, Hunter SK, Bement MKH. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015;47(1):176–84. [DOI] [PubMed] [Google Scholar]
- 16.Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014;155(1):158–67. [DOI] [PubMed] [Google Scholar]
- 17.Ellingson LD, Koltyn KF, Kim J-S, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 2014;51(3):267–76. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147(7):492–504. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (US); 2011. 3, Care of People with Pain. [PubMed]
- 20.Naugle KM, Naugle KE, Fillingim RB, Riley JL. Isometric exercise as a test of pain modulation: Effects of experimental pain test, psychological variables, and sex. Pain Med 2014;15(4):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res 2008;186(1):79–85. [DOI] [PubMed] [Google Scholar]
- 22.Hoeger Bement MK, Weyer A, Hartley S, et al. Pain perception after isometric exercise in women with fibromyalgia. Arch Phys Med Rehabil 2011;92(1):89–95. [DOI] [PubMed] [Google Scholar]
- 23.Lindheimer JB, O’Connor PJ, McCully KK, Dishman RK. The effect of low intensity cycling on mood and working memory in response to a randomized, placebo-controlled design. Psychosom Med 2016; in press. [DOI] [PubMed] [Google Scholar]
- 24.Desharnais R, Jobin J, Côté C, Lévesque L, Godin G. Aerobic exercise and the placebo effect: A controlled study. Psychosom Med 1993;55(2):149–54. [DOI] [PubMed] [Google Scholar]
- 25.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain 2014;15(12):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koltyn KF, Knauf MT, Brellenthin AG. Temporal summation of heat pain modulated by isometric exercise. Eur J Pain 2013;17(7):1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeda M, Newcomb LW, Ellingson LD, Koltyn KF. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biol Psychol 2010;85(1):90–6. [DOI] [PubMed] [Google Scholar]
- 28.McPartland JM, Guy GW, Di Marzo V. Care and feeding of the endocannabinoid system: A systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One 2014;9(3):e89566.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeil DW, Rainwater AJ. 3rd Development of the Fear of Pain Questionnaire–III. J Behav Med 1998;21(4):389–410. [DOI] [PubMed] [Google Scholar]
- 30.Albaret M-C, Muñoz Sastre MT, Cottencin A, Mullet E. The Fear of Pain Questionnaire: Factor structure in samples of young, middle-aged and elderly European people. Eur J Pain 2004;8(3):273–81. [DOI] [PubMed] [Google Scholar]
- 31.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The Fear of Pain Questionnaire-III: Further reliability and validity with nonclinical samples. J Behav Med 2002;25(2):155–73. [DOI] [PubMed] [Google Scholar]
- 32.Turk DC, Flor H, Rudy TE. Pain and families. I. Etiology, maintenance, and psychosocial impact. Pain 1987;30(1):3–27. [DOI] [PubMed] [Google Scholar]
- 33.Moos R. Family Environment Scale. Menlo Park, CA: Mind Garden, Inc.; 1974. [Google Scholar]
- 34.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 35.Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20(6):589–605. [DOI] [PubMed] [Google Scholar]
- 36.Grosen K, Drewes AM, Pilegaard HK, et al. Situational but not dispositional pain catastrophizing correlates with early postoperative pain in pain-free patients before surgery. J Pain 2016;17(5):549–60. [DOI] [PubMed] [Google Scholar]
- 37.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain 2004;112(1–2):188–96. [DOI] [PubMed] [Google Scholar]
- 38.Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: A cross-lagged panel analysis among healthy, pain-free participants. J Pain 2010;11(9):876–84. [DOI] [PubMed] [Google Scholar]
- 39.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain 2006;22(8):730–7. [DOI] [PubMed] [Google Scholar]
- 40.Campbell CM, Kronfli T, Buenaver LF, et al. Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. J Pain 2010;11(5):443–53.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNair D, Lorr M, Droppleman L. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 42.Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (windup). J Pain 2006;7(8):575–82. [DOI] [PubMed] [Google Scholar]
- 43.Forgione AG, Barber TX. A strain gauge pain stimulator. Psychophysiology 1971;8(1):102–6. [DOI] [PubMed] [Google Scholar]
- 44.Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain 1998;74(2–3):181–7. [DOI] [PubMed] [Google Scholar]
- 45.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 46.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: Assessment and experimental evidence. Med Sci Sports Exerc 1997;29(8):999–1012. [DOI] [PubMed] [Google Scholar]
- 47.Fillingim RB, Edwards RR, Powell T. Sex-dependent effects of reported familial pain history on recent pain complaints and experimental pain responses. Pain 2000;86(1–2):87–94. [DOI] [PubMed] [Google Scholar]
- 48.Edwards PW, Zeichner A, Kuczmierczyk AR, Boczkowski J. Familial pain models: The relationship between family history of pain and current pain experience. Pain 1985;21(4):379–84. [DOI] [PubMed] [Google Scholar]
- 49.Higgins KS, Birnie KA, Chambers CT, et al. Offspring of parents with chronic pain: A systematic review and meta-analysis of pain, health, psychological, and family outcomes. Pain 2015;156(11):2256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buskila D, Neumann L. Fibromyalgia syndrome (FM) and nonarticular tenderness in relatives of patients with FM. J Rheumatol 1997;24(5):941–4. [PubMed] [Google Scholar]
- 51.Koutantji M, Pearce SA, Oakley DA. The relationship between gender and family history of pain with current pain experience and awareness of pain in others. Pain 1998;77(1):25–31. [DOI] [PubMed] [Google Scholar]
- 52.Violon A. Family etiology of chronic pain. Int J Fam Ther 1985;7(4):235–46. [Google Scholar]
- 53.Chambers CT, Craig KD, Bennett SM. The impact of maternal behavior on children’s pain experiences: An experimental analysis. J Pediatr Psychol 2002;27(3):293–301. [DOI] [PubMed] [Google Scholar]
- 54.Stoller EP. Parental caregiving by adult children. J Marriage Fam 1983;45(4):851–8. [Google Scholar]
- 55.Gil KM, Ginsberg B, Muir M, Sullivan F, Williams DA. Patient controlled analgesia: The relation of psychological factors to pain and analgesic use in adolescents with postoperative pain. Clin J Pain 1992;8(3):215–21. [PubMed] [Google Scholar]
- 56.Horn ME, Alappattu MJ, Gay CW, Bishop M. Fear of severe pain mediates sex differences in pain sensitivity responses to thermal stimuli. Pain Res Treat 2014;2014:897953.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zale EL, Lange KL, Fields SA, Ditre JW. The relation between pain-related fear and disability: A meta-analysis. J Pain 2013;14(10):1019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naugle KM, Riley JL. Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc 2014;46(3):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geva N, Defrin R. Enhanced pain modulation among triathletes: A possible explanation for their exceptional capabilities. Pain 2013;154(11):2317–23. [DOI] [PubMed] [Google Scholar]
- 60.Shahidi B, Curran-Everett D, Maluf KS. Psychosocial, physical, and neurophysiological risk factors for chronic neck pain: A prospective inception cohort study. J Pain 2015;16(12):1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wideman TH, Scott W, Martel MO, Sullivan MJL. Recovery from depressive symptoms over the course of physical therapy: A prospective cohort study of individuals with work-related orthopaedic injuries and symptoms of depression. J Orthop Sports Phys Ther 2012;42(11):957–67. [DOI] [PubMed] [Google Scholar]
- 62.Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from ssattention. J Neurosci 2009;29(3):705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain 2009;10(5):447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and pain perception—Part 2: Do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012;153(3):619–35. [DOI] [PubMed] [Google Scholar]
- 65.Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc 2008;40(11):1880–9. [DOI] [PubMed] [Google Scholar]
- 66.Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sport Exerc 2001;33(2):282–90. [DOI] [PubMed] [Google Scholar]
- 67.Sternberg WF, Bokat C, Kass L, Alboyadjian A, Gracely RH. Sex-dependent components of the analgesia produced by athletic competition. J Pain 2001;2(1):65–74. [DOI] [PubMed] [Google Scholar]
- 68.Lemley KJ, Drewek B, Hunter SK, Hoeger Bement MK. Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc 2014;46(1):185–91. [DOI] [PubMed] [Google Scholar]
- 69.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: Differential effects for daily pain versus laboratory-induced pain. Pain 2004;111(3):335–41. [DOI] [PubMed] [Google Scholar]
- 70.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006;120(3):297–306. [DOI] [PubMed] [Google Scholar]