Abstract
Study Objectives
Restless legs syndrome (RLS) has been hypothesized to be generated by abnormal striatal dopamine transmission. Dopaminergic drugs are effective for the treatment of RLS. However, long-term use of dopaminergic drugs causes adverse effects. We used iron-deficient (ID) and iron-replacement (IR) rats to address the neuropathology of RLS and to determine if a histamine H3 receptor (H3R) antagonist might be a useful treatment. Histamine H3R antagonists have been shown to decrease motor activity.
Methods
Control and ID rats were surgically implanted with electrodes for polysomnographic recording. After 3 days of baseline polysomnographic recordings, rats were systemically injected with the H3R agonist, α-methylhistamine, and antagonist, thioperamide. Recordings were continued after drug injection. Striatal H3R levels from control, ID, and IR rats were determined by western blots. Blood from control, ID, and IR rats was collected for the measurement of hematocrit levels.
Results
α-Methylhistamine and thioperamide increased and decreased motor activity, respectively, in control rats. In ID rats, α-methylhistamine had no effect on motor activity, whereas thioperamide decreased periodic leg movement (PLM) in sleep. Sleep–wake states were not significantly altered under any conditions. Striatal H3R levels were highest in ID rats, moderate to low in IR rats, and lowest in control rats. Striatal H3R levels were also found to positively and negatively correlate with PLM in sleep and hematocrit levels, respectively.
Conclusions
A striatal histamine mechanism may be involved in ID anemia-induced RLS. Histamine H3R antagonists may be useful for the treatment of RLS.
Keywords: animal models, periodic leg movements, movement disorders, neuropharmacology, restless legs syndrome, pharmacology
Statement of Significance.
Restless legs syndrome (RLS) affects 5%–10% of the population. Though dopamine drugs are the first line and effective in the treatment of RLS, they cause adverse effects with long-term use. We used the iron-deficient (ID) rat, an animal model of RLS, to further explore the neural mechanisms that underlies the neuropathology of RLS, and test potential drug treatments for RLS. We found that overexpression of striatal histamine H3 receptors (H3R) may participate in the generation of RLS. We also found that the H3R antagonist, thioperamide, can reverse motor hyperactivity without changing sleep time in ID rats. Thus, H3R antagonists may have the potential for the treatment of RLS.
Introduction
State-dependent changes in motor activity across the sleep–wake cycle have been well documented. Motor activity appears as high muscle tone accompanied by phasic activity during waking. During slow-wave sleep (SWS), muscle tone is relatively low with little variation. During rapid eye movement (REM) sleep, muscle atonia, interrupted by muscle twitches occurs. Periodic leg movement (PLM) in humans is defined as regular, repetitive extensions of the great toe and dorsiflexion of the ankle, knee, and hip at 10- to 90-second intervals. PLM occurs in all sleep–wake states but most prominently in SWS. PLM can occur in all age groups and affects 5%–10% of the population. PLM has been reported in patients with restless legs syndrome (RLS; also known as Willis-Ekbom disease), REM sleep behavior disorder, narcolepsy, neurodegenerative diseases, iron deficiency (ID) anemia, end-stage renal disease, peripheral neuropathy, and depression [1–8]. PLM is also frequently reported in pregnant women [9], children with attention deficit hyperactivity disorder [10], and people taking antidepressants [11].
Dopaminergic drugs are effective for the treatment of RLS and PLM disorder. However, they cause adverse effects [12–15], such as an increase in severity of RLS symptoms (augmentation), compulsive eating, nausea, insomnia, and dizziness after long-term use. Thus, it is critical that alternative drugs be investigated, using an animal model, for the treatment of RLS. However, the subjective uncomfortable and/or painful sensations made it difficult to develop an animal model of RLS. On the other hand, more than 80% of RLS patients have PLM either in wake or in sleep [16, 17]. The observable motor event, the change in sleep patterns, and the response to the therapeutic drug can be observed in animals. Based on such observations, we have developed an animal model of RLS, the ID rat [18]. Our previous studies showed that ID rats exhibit excessive sleepiness in the active (dark) phase [19], a symptom resembling daytime sleepiness in RLS patients. Furthermore, ID rats express a circadian pattern of motor hyperactivity and a skewed distribution of inter-leg movement intervals, similar to symptoms observed in RLS and PLM disorder patients [19]. ID rats also have abnormal striatal dopamine transmission and respond positively to dopaminergic drugs [18]. Changes in sleep patterns have been reported in ID mice, with an increase in wake during the last 4 hours of the active phase, indicating a delayed sleep onset [20]. ID rats show similar symptoms and similar neuropathology to human RLS, and have a positive response to therapeutic drugs useful for the treatment of RLS [18, 19]. Thus, the ID rat can serve as an ideal animal model of RLS.
Histamine H3 receptor (H3R) mechanisms have been shown to be involved in the regulation of motor activity. Animal studies show that thioperamide, a H3R antagonist, decreases stimulant-induced motor activity, such as walking, running, and climbing in mice [21, 22]. We hypothesized that H3R may be involved in the generation of the motor component of RLS and thioperamide may be capable of reversing motor hyperactivity. In this study, we used ID rats to further investigate the neuropathology of RLS/PLM disorder and to test the efficacy of thioperamide in the treatment of RLS/PLM disorder.
Methods
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC), the VA Greater Los Angeles Healthcare System.
Development of ID rat
Sprague-Dawley weanling rats (21 days old) were used to develop the control and ID rat, as described in our previous study [18]. The weanling rats were purchased from Charles River Laboratory, and then, divided into two groups, control and ID. Control rats were fed a standard rodent diet containing 35 ppm iron, while ID rats were fed a rodent diet containing 4 ppm iron (TD.80396, Harlan Teklad Lab) for 2 months.
Surgery for electrode implantation
Rats were implanted with electrodes for EEG and EMG recordings. Under isoflurane (1.5%) anesthesia, three jewelers’ screws were implanted over the cortex for cortical EEG recording. Flexible multi-stranded stainless steel wires (7935, A-M Systems, Inc., Carlsborg, WA) were inserted into the nuchal and hindlimb musculature bilaterally and routed subcutaneously to the skull for EMG recording. Wires from all electrodes were soldered to a 14-pin Amphenol strip connector and encased in an acrylic head plug.
Sleep and motor activity recording in the control, ID, and iron-replacement rat
Animals were allowed to recover from surgery for at least 7 days before being used for experiments. Animals were individually housed in a sound-attenuated chamber under a 12:12 light–dark cycle. Electrophysiological signals were collected and amplified through a polygraph (Model 15LT or Model 78E, Grass, MA), and then digitized and recorded via a Micro 1401 (Cambridge Electronics Design, Cambridge, UK). Sleep and phasic leg jerks in wake and in sleep were visually scored offline with a script in Spike2 (Cambridge Electronics Design). Infrared cameras were used for video recordings. Video images were captured digitally through a four-channel surveillance video recorder card (Q-See QSPDVR04; RapidOS, New Taipei City, Taiwan). Video was recorded continuously with time stamps matched to polysomnographic recordings.
After 3 days of baseline sleep and motor activity recordings, seven each control and ID rats were injected with drugs (see section below). The rest of 16 ID rats were divided into four groups, with each group fed with standard rodent diet (iron replacement) for 1 (IR1), 2 (IR2), 3 (IR3), and 4 (IR4) weeks. Sleep and motor activity recordings in IR rats were resumed on day 6 post-standard rodent diet feeding for 2 days continuously each week, for an additional 3 weeks. The number of rats for each IRs group is 4.
Drug injection experiment
After 3-day baseline sleep and motor activity recordings, control and ID rats were intraperitoneally injected with test drugs, saline, or 30% dimethyl sulfoxide (DMSO) in saline (DMSO–saline) at Zeitgeber Time (ZT) 2. Drugs used for the experiment included α-methylhistamine (α-MH, Tocris Bioscience), a histamine H3R agonist, and thioperamide (Tocris Bioscience), a H3R antagonist. The concentration of each drug was 1, 3, and 6 mg/kg for both α-MH and thioperamide. α-MH was dissolved in Ringer’s saline, whereas thioperamide was dissolved in 30% DMSO and then diluted with Ringer’s saline, immediately before injection. Thus, the concentration of DMSO was 1%, 3%, and 6% for 1, 3, and 6 mg/kg thioperamide injection solution, respectively. Different doses of each test drug were delivered into the same animal 2 days apart, to avoid any residual effects from the previous injection. Injections of different test drugs were performed 1 week apart.
One week after completing the drug injection experiment, control and ID rats were sacrificed at 10 am. IR rats were sacrificed at 10 am on days 7 (IR1), 14 (IR2), 21 (IR3), and 28 (IR4), post-standard rodent diet feeding. All animals were deeply anesthetized with isoflurane and their brains removed. The striatum was dissected on ice and stored at −80 °C for subsequent use in western blots.
Hematocrit measurement
Blood was collected from the lateral tail vein after isoflurane anesthesia and immediately before sacrificing the animal, transferred into a heparinized hematocrit capillary, and centrifuged at 12 000 × g for 3 minutes in a hematocrit centrifuge (BD Clay Adams, 420563). Hematocrit levels were calculated by measuring the length of the red blood cell layer against the total blood layer.
Western blot experiment
Striatal tissues were taken from four animals in each control and experimental group. Striatal tissues were separately sonicated in lysis buffer (50 mM Tris, 5 mM ethylenediaminetetraacetic acid, 30% IGEPAL NP-40, 10% Na deoxycholate, and 1% sodium dodecyl sulfate, pH 7.5) and protease inhibitor tablet (Roche, 4693124), and then centrifuged at 10 000 × g for 20 minutes at 4 °C. Protein concentration of the supernatant was determined using the DC protein assay kit (Bio-Rad, 500-0112) and read at 750 nm in an Emax Precision microplate reader (Molecular Devices) using the Softmax program. Ten micrograms of striatal proteins were loaded on a 10% mini-protean TGX precast gel (Bio-Rad, 456-1034) and electrophoresed for 1.5 hours at room temperature (RT) at 120 V. Proteins were then transferred to a polyvinylidene fluoride membrane for 1.5 hours at 100 mA at RT. Membranes were washed in Tris-buffered saline with Tween (TBST: 20 mM Tris, 150 mM NaCl, 0.1% Tween), and then blocked in TBST containing 5% non-fat dry milk for 1 hour at RT. Membranes were subsequently incubated with rabbit anti-histamine H3R (1:4000, Millipore, AB15860) and mouse anti-actin (1:10 000, Millipore, MAB1501R) overnight at 4 °C, goat anti-rabbit IgG conjugated with HRP (1:10 000, Jackson Immunores Lab, 111-035-144) and goat anti-mouse IgG conjugated with HRP (1:10 000, Jackson Immunores Lab, 715-035-140) for 1 hour, and West Femto (1:80; Thermo Scientific, 34094) for 5 minutes at RT. H3R (48.6 KDa) and β-actin (42 KDa) were detected and visualized using a ChemiDoc XRS+ system (Bio-Rad). Membranes were washed three times in TBST between incubations. β-Actin was used as an internal normalizer. The optical densities of the H3R and β-actin bands were measured using a Quantity One 1-D analysis software (Bio-Rad). Striatal proteins from control, ID, and IR1-IR4 rats were analyzed simultaneously on the same gels and blots.
Data analysis
The CED 1401 Spike 2 program was used to analyze EEG power spectra, as well as to detect and score phasic muscle activity during quiet wake and sleep. Periodic leg movements in quiet wake (PLMW) and in SWS (PLMS) were analyzed by adapting the criteria outlined by the World Association of Sleep Medicine [23, 24] and described in our previous study [18]. In brief, phasic motor events in the leg satisfying the following criteria were counted as PLM: (1) the amplitude was twice that of the tonic background activity, (2) the duration ranged between 0.2 and 5 seconds, (3) the interval between jerks was between 10 and 90 seconds, and (4) at least four consecutive jerks fulfilled the three criteria described above. The PLMS event ended either when the animal awakened from sleep or the inter-leg movement-interval was longer than 90 seconds. Phasic motor events in sleep, which did not meet the criteria of PLMS, were counted as isolated leg movements (ILMS). The index of PLM in quiet wake (PLMWI) and in SWS (PLMSI) was calculated as the total number of periodic motor movements divided by total time in quiet wake or in SWS, over 2 or 8 hours. Similarly, the index of ILMS (ILMSI) was calculated as the total number of ILM in sleep divided by total time in sleep, over 2 or 8 hours.
To determine long-term and short-term effects of test drugs on sleep and motor activity, the total time in wake, SWS, and REM sleep, as well as PLMWI, PLMSI, and ILMSI were determined in 2-hour epochs (short term) or total 8 hours (long term) after saline/DMSO–saline and H3R agonist and antagonist injections. One-way analysis of variance (ANOVA) with replicated measures followed by Bonferroni’s post hoc and t-tests were used for statistical analysis.
Results
Effect of α-MH and thioperamide on sleep and motor activity in control rats
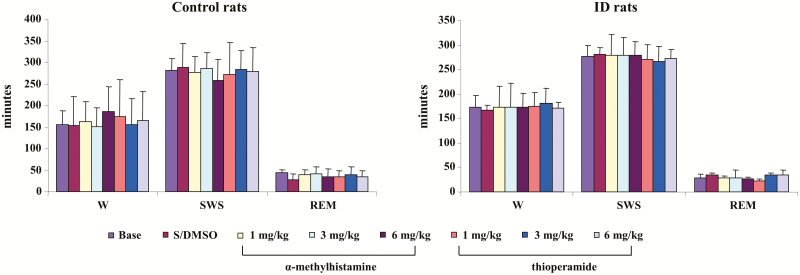
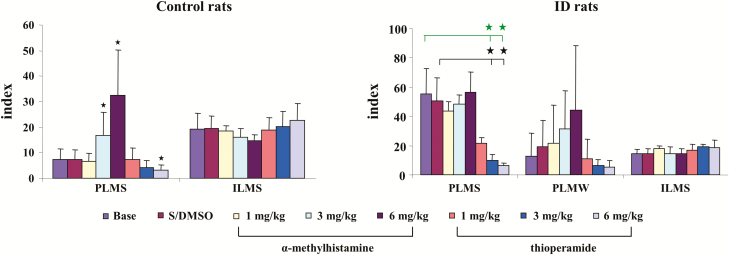
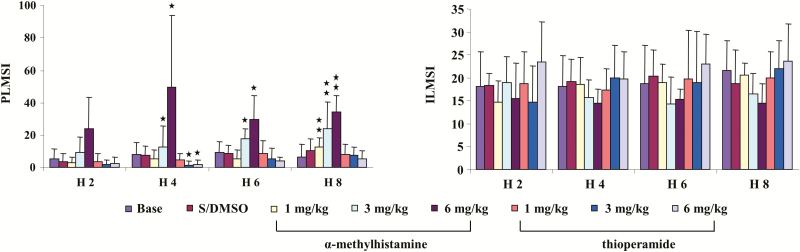
α-MH, at any dose (1, 3, and 6 mg/kg), injected intraperitoneally into control rats produced no change in wake, SWS, or REM sleep time when averaged over the 8 hours of recording post-injection (Figure 1, left panel) or scored over 2-hour epochs post-injection in the control rat. In contrast, α-MH injection produced dose-dependent increase in PLM in sleep (Figure 2, left panel, p < 0.001, df = 5, ANOVA). PLM in sleep significantly increased after α-MH at 3 mg/kg (Figure 2, left panel) and 6 mg/kg (Figure 2, left panel) injection when averaged over the 8 hours of recording. α-MH at 1 mg/kg injected into control rats had no effect on motor activity (Figure 2, left panel) over the 8 hours of recording. Increased PLM in sleep were observed starting hour 3 post-α-MH injection (Figure 3, left panel). Isolated leg movements in sleep were not changed by α-MH injection at any dose and at any time period (Figure 2, left panel; Figure 3, right panel). Similar to α-MH injection, systemic injection of thioperamide did not change time in wake, SWS, and REM sleep (Figure 1, left panel), nor the number of ILM in sleep (Figure 2, left panel; Figure 3, right panel) at any time after injection. However, thioperamide injections produced dose-dependent decreases in the number of PLM in sleep at hours 3 and 4 post-injection (Figure 3, left panel). Saline or DMSO–saline injection produced no changes in wake and sleep time (Figure 1, left panel), as well as PLM (Figure 2, left panel) and ILM (Figure 2, left panel; Figure 3, right panel) in sleep. Neither DMSO–saline nor DMSO–saline–thioperamide injection changed motor behaviors, such as scratching, grooming, climbing, and running.
Figure 1.
Effect of systemic injection of α-methylhistamine and thioperamide on sleep–wake time in control (left panel) and iron deficiency (ID; right panel) rats over 8-hour recording. Both α-methylhistamine and thioperamide had no effect on time in wake (W), slow-wave sleep (SWS), and rapid eye movement (REM) sleep. Data expressed as mean ± SD. S/DMSO, saline/dimethyl sulfoxide. N = 7 each.
Figure 2.
Effect of systemic injection of α-methylhistamine and thioperamide on motor activity in control (left panel) and iron deficiency (ID; right panel) rats over 8-hour recording. Both α-methylhistamine and thioperamide had no effect on isolated leg movement in sleep (ILMS) in control and ID rats. α-Methylhistamine injection increased periodic leg movements in sleep (PLMS) in control rats (left panel), but not in ID rats (right panel). Thioperamide at 6 mg/kg decreased PLMS in control rats (left panel). On the other hand, both 3 and 6 mg/kg thioperamide injection decreased PLMS in ID rats (right panel). Both α-methylhistamine and thioperamide had no effect on PLM in quiet wake (PLMW) in ID rats. Data expressed as mean ± SD. The y-axis represents index of PLMS, PLMW, and ILMS. Green bars and stars: baseline vs. drug injection; black bars and stars: S/DMSO vs. drug injection. *p < 0.05, post hoc. N = 7 each.
Figure 3.
Effect of systemic injection of histamine H3 receptor agonist, α-methylhistamine, and antagonist, thioperamide, on motor activity in control rats over 2-hour epochs recording. α-Methylhistamine produced an increase in periodic leg movements (PLM) in sleep (PLMS; left panel), but not isolated leg movements in sleep (ILMS; right panel) in control rats. The increase in PLMS was observed starting hour 3 post-αMH injection, and lasted more than 6 hours after αMH injection. A significant decrease in PLMS was found hours 3 and 4 post-thioperamide injection. ILMSI and PLMSI: index of ILMS and PLMS, respectively. Data expressed as mean ± SD. The X-axis represents the time after test drug injection. H 2, H 4, H 6, and H 8: hours 1–2, hours 3–4, hours 5–6, and hours 7–8 post-drug injection. *p < 0.05, **p <0 .01, post hoc. N= 7 each.
Effect of histamine H3R agonist and antagonist on sleep and motor activity in ID rats
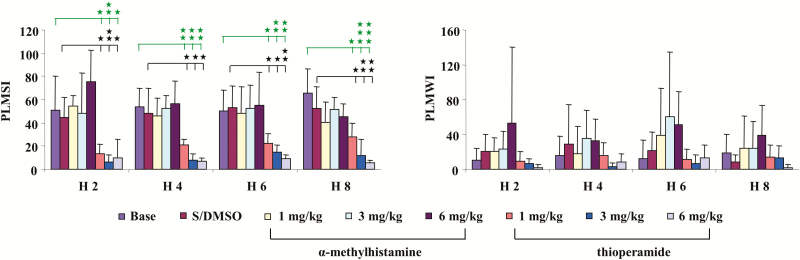
Similar to control rats, α-MH injection in ID rats produced no significant change in sleep–wake states (Figure 1, right panel) when averaged over the 8 hours post-injection period, or even when scored in 2-hour epochs. In contrast to control rats, α-MH injection in ID rats produced no change in the number of PLM (Figure 2, right panel) in sleep, even when scored in 2-hour epochs (Figure 4, left panel). Though an increase in PLM in quiet wake was found after α-MH injection in ID rats, the change was not significant (Figures 2 and 4, right panel). Similar to α-MH injection, systemic injection of thioperamide into ID rats produced no changes in total time spent in wake or sleep over 8 hours (Figure 1, right panel), or 2-hour epochs after injection. Isolated leg movements in sleep were not changed by thioperamide injection (Figure 2, right panel). However, PLM in sleep dose dependently decreased with thioperamide injection in rats (p < 0.05, df = 5, ANOVA). This effect was not only seen during the 8-hour post-injection period (Figure 2, right panel), but also in the 2-hour epoch post-injection (Figure 4, left panel). Though PLM in wake was also decreased by thioperamide injection in ID rats, the change was not significant (Figures 2 and 4, right panel).
Figure 4.
Effect of systemic injection of α-methylhistamine and thioperamide on motor activity in iron-deficient (ID) rats over 2-hour epochs recording. α-Methylhistamine failed to change periodic leg movement (PLM) in sleep (PLMS, left panel) at any time after injection. In contrast, thioperamide injection decreased PLMS in ID rats (left panel). Neither α-methylhistamine nor thioperamide changed PLM in quiet wake (right panel). Thirty percent dimethyl sulfoxide–saline (DMSO–saline) vehicle injection had no any effect on PLM in sleep (left panel) and in quiet wake (right panel). Data expressed as mean ± SD. Green bars and stars: baseline vs. thioperamide injection; black bars and stars: S/DMSO vs. thioperamide injection. H 2, H 4, H 6, and H8: hours 1–2, hours 3–4, hours 5–6, and hours 7–8 post-drug injection. *p < 0.05, **p < 0.01, ***p < 0.001, post hoc test. N = 7 each.
Hematocrit levels and motor activity in IR rats, as well as striatal levels of H3R in control, ID, and IR rats
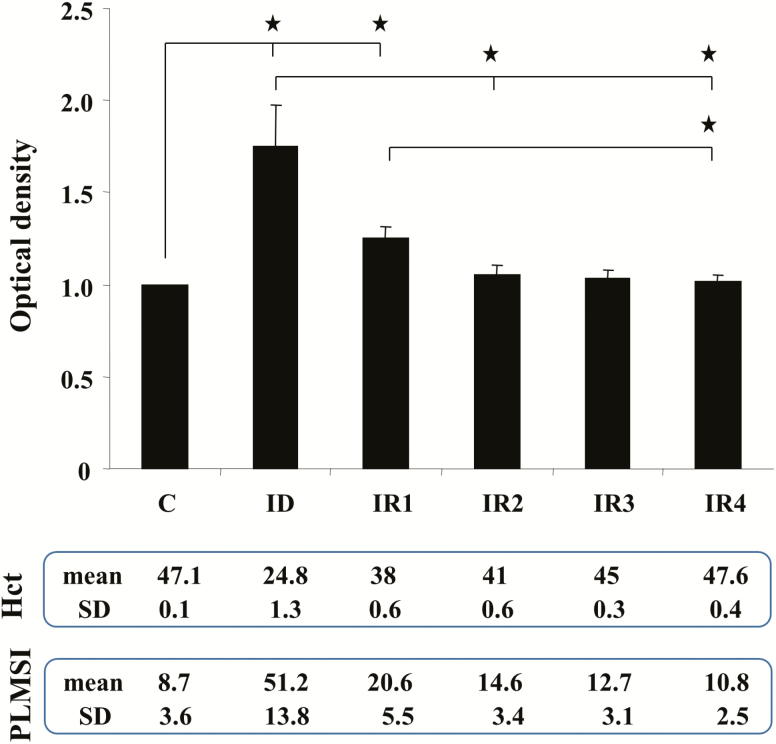
Consistent with our previously reported data [18], hematocrit levels and PLM in sleep were normalized after ID rats were fed standard rodent diet (Figure 5). The present study showed that striatal H3R levels are highest in ID rats, moderate to low in IRs rats, and lowest in control rats (Figure 5). Statistical analysis found that striatal levels of H3R are positively correlated with PLMs in sleep (R2 = 0.6, p < 0.01, n = 24, Pearson correlation test) and negatively correlated with hematocrit levels (R2 = −0.65, p <0.01, n = 24, Pearson correlation test).
Figure 5.
Striatal histamine H3 receptor (H3R) levels in control (C), iron-deficient (ID) and iron-replacement (IR) week 1 (IR1), week 2 (IR2), week 3 (IR3), and week 4 (IR4) rats. Top panel: Relative density of bands expressed as the ratio of H3R and actin in each animal group (mean ± SEM). Middle panel: average (mean) and SD of hematocrit levels (Hct) taken from the rat performing western blot experiment. Lower panel: mean and SD of index of PLMS (PLMSI) taken from the rat performing western blot experiment. *p < 0.05, post hoc test. N = 4 each.
Discussion
Dopaminergic mechanisms have been hypothesized to be involved in the pathophysiology of RLS. An increase in tyrosine hydroxylase (TH) and phosphorylated TH in the substantia nigra (SN) and an increase in phosphorylated TH and a decrease in dopamine D2 receptors in the putamen have been reported in RLS patients [25]. A decrease [26] or an increase [27] in striatal levels of dopamine transporter have also been demonstrated in RLS patients. ID rats, an animal model of RLS [18], also showed an increase in TH and phosphorylated TH in the SN [25], as well as an increase in dopamine transporter in the striatum [18]. Although symptoms of RLS and PLM disorder patients can be relieved with iron therapy [28] and disorders of RLS-like behaviors in ID rats can be corrected with iron replacement [18], the neural abnormality of the SN cannot be reversed in ID rats fed with high iron diet [25]. Thus, other mechanisms in the striatum, which affect dopamine transmission, may be involved in the generation of motor hyperactivity.
Clinical studies showed that 24% of ID anemia patients have RLS [1], indicating that systemic anemia does not always produce RLS. Allen et al. [1] reported that plasma levels of ferritin and hemoglobin are not different in idiopathic RLS patients and controls. On the other hand, brain levels of iron and ferritin were low and transferrin levels were high in idiopathic RLS patients [29]. A decrease in the brain level of iron and ferritin and an increase in the brain level of transferrin has also been found in ID weanling male Sprague-Dawley rats [30] fed with a low-iron diet for 4–6 weeks [31]. In addition, a decrease in striatal levels of dopamine D1 (D1) and dopamine D2 (D2) receptors was also found in ID rats [32]. Though brain levels of iron, ferritin, and transferrin in ID rats were not measured in the present study, on the basis of Erikson et al.’s study [31], we assume that brain levels of transferrin are high and iron and ferritin are low in our similarly treated ID rats. Our ID rats were produced by feeding the 21 weanling rats with a low-iron rodent diet for 8 weeks.
Histamine H3R mechanisms have been shown to be involved in the regulation of motor activity. Animal studies showed that both α-MH and thioperamide have no effect on spontaneous motor activity during wake in mice [21] or in rats [33]. Systemic injection of α-MH produces no effect on stimulant-induced motor activity in mice [21] or in rats [33]. However, thioperamide injection decreases stimulant-induced motor activities, as well as walking, running, and climbing activities in mice [21, 22]. Consistent with these findings, our present study showed that neither α-MH nor thioperamide changes motor activity in wake. However, we found that α-MH and thioperamide increases and decreases motor activity in sleep, respectively, which was not reported by Clapham and Kilpartrick [21] or Nosál et al. [22]. Based on our findings, we then examined the effect of these drugs on motor activity in ID rats. In contrast to control rats, systemic application of α-MH into ID rats failed to change PLM in sleep. This may be due to a change in neurotransmission in the striatum of ID rats. Iron deficiency not only changes the dopaminergic system in the SN and striatum [18, 25], but also alters the GABAergic system. Anderson et al. [34] reported that GABA uptake decreases in the striatum in ID rats. The decrease in GABA uptake results in an increase in GABA levels in the synapse and consequently decreases striatal neuronal activity. The decreased striatal activity and/or neurodegeneration of the striatopallidal neurons have been shown to increase motor activity in mice [35]. However, PLM in sleep was dramatically suppressed by all doses of thioperamide injection in ID rats. The efficacy of the thioperamide suppressive effect on motor activity in sleep and in wake in ID rats is comparable to that of pramipexole, shown in our previous study [18], a drug commonly used to treat RLS.
Mechanisms underlying motor hypoactivity caused by H3R antagonists have not been documented. Our present study showed that striatal H3R levels are positively correlated with PLM in sleep. This led us to speculate that over expression of H3R in the striatum may cause motor hyperactivity. The histamine H3R is a presynaptic and a postsynaptic receptor. At presynaptic terminals, H3R not only acts as a heteroreceptor, which suppresses transmitter release [36] and modulates striatal medium spiny neuron (MSN) activity [37], but also forms with adenosine A2a receptor (A2aR) as the heteromers [38]. Activation of H3R increases the A2aR activity-induced cAMP formation [38], and causes neurotransmitter release [39]. Adenosine A2aR has been shown to increase glutamatergic release from corticostriatal terminals [40, 41]. Thus, the increased H3R, as seen in our present study, as well as the increased A2aR in ID rats [42] may corporate into the heteromers and contribute to the increase in glutamate release from corticostriatal terminals, result in PLM generation [43]. Yepes et al. [43] reported that pramipexole, drug commonly used for the treatment of RLS, suppresses cortical stimulation-induced glutamate release. H3R has also been shown to interact with other receptors at the postsynaptic level. Ferrada et al. [44] showed that a strong interaction between H3R and dopamine D2 receptors exists at the postsynaptic striatal cell membrane level. Using radioligand binding technique, they found that H3R agonist decreases the affinity of postsynaptic dopamine D2 receptor for its agonists in the striatum. Inactivation or neurodegeneration of striatonigral D1 receptor containing MSN (D1-MSN) and striatopallidal D2 receptor containing MSN (D2-MSN) decreases and increases motor activity in mice, respectively [35]. Neuroimaging studies showed that the density of striatal postsynaptic D2 receptors in RLS patients is either not changed [45] or decreased [46]. We hypothesize that overexpression of H3R in the striatum, as seen in ID rats, causes an increase in glutamate release from the corticostriatal terminals by enhancing A2aR activity and a decrease in the ability of dopamine D2 receptors to bind dopamine agonists in the striatum. Application of H3R antagonists may thus decrease A2aR activity and restore the function of dopamine D2 receptors in the striatum and improve motor hyperactivity in sleep, as seen in the present study. In addition, the effect of thioperamide on motor activity may also be mediated through the SN pars reticulata (SNR). Activation of H3R by application of imetit, a H3R agonist, in the SNR decreases GABAergic neuronal activity [47]. Inactivation of the SNR by muscimol infusion has been shown to cause hyposomnia and PLM-like activity in wake and increase PLM-like activity in sleep in rats [48]. Therefore, application of thioperamide increases SNR GABAergic neuronal activity and reduces RLS-like activity in ID rats. However, H3R control of motor component of RLS-like activity in ID rats mediated through peripheral structures, such as skin, muscle, and spinal cord cannot be ruled out. Histamine H3Rs have been found in peripheral sensory fibers, dorsal root ganglia, and spinal dorsal horn [49]. These H3Rs act as heteroreceptors and suppress GABA and/or glycine release, resulting in sensorimotor hyperactivity. Further study is needed to address whether an upregulation of H3R occurred in the spinal cord and peripheral tissues in ID animals.
Using high-performance liquid chromatography analysis technique, Sakurai et al. [50] showed that the half-life of thioperamide in the plasma is 26.9 minutes in male Wistar rats. However, the ratio of brain and plasma concentration of thioperamide was gradually increased over the 2-hour measurement period [50]. This result may explain our findings showing that the suppressive effect of thioperamide on motor activity is lasted for 4 and 10 hours in control and ID rats, respectively. Upregulation of striatal H3R in ID rats, shown in our present study, may also explain the different time course of thioperamide effect on motor activity in the control and ID rat. Motor activity returned to the baseline level 12 hours after high-dose thioperamide (6 mg/kg) administration into the ID rat (data not shown). We also performed the experiment that 6 mg/kg thioperamide is injected into an animal for 3 consecutive days. Results from this experiment showed that the efficacy of thioperamide on motor activity is not changed (data not shown). Thus, a prolonged drug holiday (longer than 1 day) may not be necessary even after high-dose thioperamide administration. In contrast to pramipexole, which has long half-life at 8–10 hours [51], thioperamide may have the potential for the long-term treatment of RLS and PLM disorder.
Clinical studies have shown that histamine-related substances affect symptoms of RLS. Allen et al. [52] showed that daytime symptom of RLS, PLM in wake, is worsened in patients taking diphenhydramine, a histamine H1 receptor antagonist. Animal studies demonstrated that activation of the histamine H1 receptor has no effect on motor activity [21, 22, 33]. However, diphenhydramine not only acts as a histamine H1 receptor antagonist, but it also bind with serotonin transporter [53] and inhibits serotonin re-uptake [54]. Serotonin has been reported to have excitatory effects on the motor system [55]. Systemic injection of diphenhydramine increased walking in rats [53]. Thus, the aggravating effect on daytime RLS in patients taking diphenhydramine may be mediated through a serotonergic mechanism. Histamine H2 receptor-related substances may also generate RLS. O’Sullivan and Greenberg [56] reported that a chronic esophageal reflux patient treated with cimetidine, a histamine H2 receptor antagonist, developed RLS. However, the mechanism underlying histamine H2 receptor antagonist inducing RLS is not clear. Zhou et al. [47] reported that application of histamine H2 receptor antagonist, ranitidine, suppresses SNR GABAergic neuronal activity. A decrease in SNR GABAergic neuronal activity produced motor hyperactivity [48], as described above. This may explain it that patients taking cimetidine develop RLS.
Histamine mechanisms have been documented to modulate sleep–wake states. Monti et al. [57] reported that α-MH (1–10 mg/kg) failed to change wake and sleep times. On the other hand, a high dose (4 mg/kg), but not low doses (1 and 2 mg/kg) of thioperamide, increased wake and decreased sleep in rats [57]. In contrast, our present study showed that sleep patterns are not altered after any dose (1, 3, and 6 mg/kg) of thioperamide application in control or ID rats. This discrepancy may be attributed to the different strain of rats. Monti et al. [57] used Wistar rats, while we used Sprague-Dawley rats. Kalivas [58] reported that Sprague-Dawley rats purchased from different sources showed a different histamine antagonism to pentobarbital induced necrosis and hyperthermia, with highly positive response in rats purchased from Tylers, intermediate response in rats purchased from Charles River, and no response in rats purchased from Zivic-Miller.
In conclusion, our present study indicates that histamine H3R mechanisms in the striatum may play a role in the neuropathology of RLS and PLM disorder. Iron therapy not only reverses RLS-like symptoms but also decreases striatal H3R levels. H3R antagonist, thioperamide, produces no change in sleep–wake states, but suppresses motor hyperactivity in sleep in ID rats. Thus, H3R antagonists may have potential for the treatment of RLS and PLM disorder.
Funding
This work supported by the Restless Legs Syndrome Foundation (Y.-Y.L.), the National Institute of Health grants, NS082242 (Y.-Y.L.) and DA034748 (J.M.S.), and the Department of Veterans Affairs.
Conflict of interest statement. None declared.
This work was conducted at VAGLAHS Sepulveda, 16111 Plummer Street, North Hills, CA 91343.
References
- 1.Allen RP, et al. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88(4):261–264. [DOI] [PubMed] [Google Scholar]
- 2.Bhalsing K, et al. Prevalence and profile of restless legs syndrome in Parkinson’s disease and other neurodegenerative disorders: a case-control study. Parkinsonism Relat Disord. 2013;19(4):426–430. [DOI] [PubMed] [Google Scholar]
- 3.Iannaccone S, et al. Evidence of peripheral axonal neuropathy in primary restless legs syndrome. Mov Disord. 1995;10(1):2–9. [DOI] [PubMed] [Google Scholar]
- 4.Lee HB, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20(1):101–105. [DOI] [PubMed] [Google Scholar]
- 5.Plazzi G, et al. Periodic leg movements during sleep in narcoleptic patients with or without restless legs syndrome. J Sleep Res. 2012;21(2):155–162. [DOI] [PubMed] [Google Scholar]
- 6.Polydefkis M, et al. Subclinical sensory neuropathy in late-onset restless legs syndrome. Neurology. 2000;55(8): 1115–1121. [DOI] [PubMed] [Google Scholar]
- 7.Walters AS, et al. A preliminary look at the percentage of patients with restless legs syndrome who also have Parkinson disease, essential tremor or Tourette syndrome in a single practice. J Sleep Res. 2003;12(4):343–345. [DOI] [PubMed] [Google Scholar]
- 8.Winkelman JW, et al. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis. 1996;28(3):372–378. [DOI] [PubMed] [Google Scholar]
- 9.Lee KA, et al. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335–341. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree VM, et al. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12(1):73–81. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, et al. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510–514. [DOI] [PubMed] [Google Scholar]
- 12.García-Borreguero D, et al. Augmentation as a treatment complication of restless legs syndrome: concept and management. Mov Disord. 2007;22(Suppl. 18):S476–S484. [DOI] [PubMed] [Google Scholar]
- 13.Godau J, et al. Rotigotine in the long-term treatment of severe RLS with augmentation: a series of 28 cases. Sleep Disord. 2011;2011:468952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silber MH, et al. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26(7): 819–821. [DOI] [PubMed] [Google Scholar]
- 15.von Scheele C, et al. Long-term effect of dopaminergic drugs in restless legs. A 2-year follow-up. Arch Neurol. 1990;47(11):1223–1224. [DOI] [PubMed] [Google Scholar]
- 16.Montplaisir J, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–65. [DOI] [PubMed] [Google Scholar]
- 17.Montplaisir J, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord. 1998;13(2):324–329. [DOI] [PubMed] [Google Scholar]
- 18.Lai YY, et al. Motor hyperactivity of the iron-deficient rat—an animal model of restless legs syndrome. Mov Disord. 2017;32(12):1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai YY, et al. Reply: the iron-deficient rat as a model of restless legs syndrome: was anything lost in translation? Mov Disord. 2018;33(1):182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean T Jr, et al. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med. 2006;7(8):634–640. [DOI] [PubMed] [Google Scholar]
- 21.Clapham J, et al. Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol. 1994;259(2):107–114. [DOI] [PubMed] [Google Scholar]
- 22.Nosál R, et al. Central histaminergic H3-receptors and locomotor activity. Inflamm Res. 2001;50 (Suppl. 2):S76–S77. [DOI] [PubMed] [Google Scholar]
- 23.Allen RP, et al. ; International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. [DOI] [PubMed] [Google Scholar]
- 24.Zucconi M, et al. ; International Restless Legs Syndrome Study Group (IRLSSG). The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7(2):175–183. [DOI] [PubMed] [Google Scholar]
- 25.Connor JR, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earley CJ, et al. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. Sleep. 2011;34(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KW, et al. Increased striatal dopamine transporter density in moderately severe old restless legs syndrome patients. Eur J Neurol. 2012;19(9):1213–1218. [DOI] [PubMed] [Google Scholar]
- 28.Aurora RN, et al. ; American Academy of Sleep Medicine. The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno S, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. [DOI] [PubMed] [Google Scholar]
- 30.Erikson KM, et al. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127(10):2030–2038. [DOI] [PubMed] [Google Scholar]
- 31.Han J, et al. Gene expression of transferrin and transferrin receptor in brains of control vs. iron-deficient rats. Nutr Neurosci. 2003;6(1):1–10. [PubMed] [Google Scholar]
- 32.Erikson KM, et al. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69(3-4):409–418. [DOI] [PubMed] [Google Scholar]
- 33.Pillot C, et al. Ciproxifan, a histamine H3-receptor antagonist/inverse agonist, potentiates neurochemical and behavioral effects of haloperidol in the rat. J Neurosci. 2002;22(16):7272–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson JG, et al. Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci. 2007;95(1):188–195. [DOI] [PubMed] [Google Scholar]
- 35.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107(33):14845–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina-Hernández A, et al. Histamine H3 receptor activation inhibits glutamate release from rat striatal synaptosomes. Neuropharmacology. 2001;41(8):928–934. [DOI] [PubMed] [Google Scholar]
- 37.Ellender TJ, et al. Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci. 2011;31(43):15340–15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Márquez-Gómez R, et al. Functional histamine H3 and adenosine A2A receptor heteromers in recombinant cells and rat striatum. Pharmacol Res. 2018;129:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa WS, Yu SC, Liewald JF, et al. Fast cAMP modulation neurotransmission via neuropeptide signals and vesicle loading. Curr Biol. 2017;27:495–507. [DOI] [PubMed] [Google Scholar]
- 40.Ciruela F, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26(7):2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popoli P, et al. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol. 1995;287(2):215–217. [DOI] [PubMed] [Google Scholar]
- 42.Quiroz C, et al. Up-regulation of striatal adenosine A(2A) receptors with iron deficiency in rats: effects on locomotion and cortico-striatal neurotransmission. Exp Neurol. 2010;224(1):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yepes G, et al. Targeting hypersensitive corticostriatal terminals in restless legs syndrome. Ann Neurol. 2017;82(6):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrada C, et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisensehr I, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57(7):1307–1309. [DOI] [PubMed] [Google Scholar]
- 46.Michaud M, et al. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249(2):164–170. [DOI] [PubMed] [Google Scholar]
- 47.Zhou FW, et al. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol. 2006;96(3):1581–1591. [DOI] [PubMed] [Google Scholar]
- 48.Lai YY, et al. Role of the substantia nigra in the control of sleep and motor activity in sleep. Sleep. 2009;32(Suppl.):A302. [Google Scholar]
- 49.Cannon KE, et al. Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain. 2007;129(1-2):76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakurai E, et al. The disposition of thioperamide, a histamine H3-receptor antagonist, in rats. J Pharm Pharmacol. 1994;46(3):209–212. [DOI] [PubMed] [Google Scholar]
- 51.Eisenreich W, et al. Pramipexole extended release: a novel treatment option in Parkinson’s disease. Parkinsons Dis. 2010;2010:612619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen RP, et al. Anti-histamine and benzodiazepines exacerbate daytime restless legs syndrome (RLS) symptoms. Sleep. 2005;28(Suppl.):A279. [Google Scholar]
- 53.Tanda G, et al. Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem. 2008;106(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T, et al. Diphenhydramine overdose mimicking serotonin syndrome. Psychiatry Clin Neurosci. 2011;65(5):534. [DOI] [PubMed] [Google Scholar]
- 55.Jacob BL, et al. 5-HT and motor control: a hypothesis. TINS. 1995;16:342–356. [Google Scholar]
- 56.O’Sullivan RL, et al. H2 antagonists, restless leg syndrome, and movement disorders. Psychosomatics. 1993;34(6):530–532. [DOI] [PubMed] [Google Scholar]
- 57.Monti JM, et al. Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol. 1991;205(3):283–287. [DOI] [PubMed] [Google Scholar]
- 58.Kalivas PW. Histamine-induced arousal in the conscious and pentobarbital-pretreated rat. J Pharmacol Exp Ther. 1982;222(1):37–42. [PubMed] [Google Scholar]