Abstract
Mild hypoxia induced by 20% hemorrhage results in increases in few cytokine concentrations and sclerostin levels in blood, but shows no changes in bone formation, bone marrow cellularity, and gastrointestinal (GI) integrity and no systemic bacterial infection as well as no subsequent mortality. On the other hand, severe hypoxia induced by 40% hemorrhage causes significant increases in most cytokine concentrations, GI injury, lung injury, systemic bacterial infection, cellular ATP reduction and subsequent mortality. The severe hypoxia drastically damages GI and lung morphology, elevates cytokine concentrations in blood and increases inducible nitric oxide synthase (iNOS) expression in cells that is mediated by transcription factors NF-κB/NF-IL6, subsequently producing free radicals that disrupt mitochondria. ATP depletion, p53 phosphorylation, and caspase-3 activation are found, suggesting cell apoptosis. As a result, mortality occurs. However, when mild hypoxia follows ionizing radiation, the mild hypoxia significantly enhances radiation-induced mortality and acute radiation syndrome, including injury of bone marrow, GI, kidney, and lung. The synergism also occurs at the molecular level, resulting in alteration of microRNAs, amplification of iNOS expression, cytokine increases, sepsis, and ATP depletion. This is the first demonstration of synergistic effects between mild hypoxia and ionizing radiation.
Keywords: hypoxia, hemorrhage, ischemia, radiation, cytokines, NF-κB, NF-IL6, microRNA, intestine, bone marrow, survival, hematopoiesis, acute radiation syndrome
Hypoxia
Tissue hypoxia results from internal hemorrhage, external hemorrhage, chemical insults, or ischemia due to blood vessel occlusion. Depending on the severity of tissue hypoxia, the damage can vary ranging from no lethality to lethality. Severe hypoxia can cause systemic inflammation response syndrome (SIRS), multiple organ dysfunction (MOD), and multiple organ failure (MOF, ref. 4), thereby leading to fatality. This cascade reaction involves many steps at molecular levels, cellular levels, organ levels, and systemic levels.
Organ Damage after Hypoxia
Organ damage depends on the severity of hypoxia. Hemorrhage leads to hypoxia. Manning (35) classifies hemorrhage to 4 classes. Class 1 is less than 15% blood loss with no clinical sign and no treatment needed; Class 2 is 15–30% blood loss with mild clinical sign and only fluid resuscitation optionally needed; Class 3 is 30–40% blood loss with severe clinical sign and fluid resuscitation and blood transfusion needed; Class 4 is greater than 40% blood loss with fatal clinical sign and aggressive blood transfusion needed to save life. Examination of histopathology slides, hypoxia caused by mild hemorrhage (intermittent, 20% blood loss) does not alter bone (51), bone marrow (25) and small intestine morphology (Kiang, unpublished data), but hypoxia resulted from severe ischemia or severe hemorrhage induces changes in morphology of small intestine. Our laboratory and others have reported that severe hypoxia induced by ischemia with mesenteric artery occlusion for 30 min (50) or intermittent 40% hemorrhage (15) resulted in a shortening and widening of villi, indicating occurrence of villous edema. Histopathology of lungs after hemorrhage exhibits pulmonary edema (29). Histopathology of other organs are not performed and studied, yet. Since we have also found decreases in ATP levels in heart, kidney, and brain (16) and increases in caspase-3 activation in these organs (24), it was highly likely that these organs were damaged while small intestine was injured.
We have studied the bone marrow after 20% hemorrhage. No apparent alteration was observed. But circulatory blood cells acutely decreased and returned to baseline 2 days after hemorrhage (25). It is not clear whether 40% hemorrhage would change cellularity of bone marrow.
Cellular Changes after Hypoxia
Hypoxia has been shown to lead to acute increases in intracellular free calcium concentration ([Ca2+]i), 5-lipoxygenase, lipid peroxidation, cyclooxygenase (COX), constitutive nitric oxide synthase (cNOS), leukotriene B4 (LTB4), prostaglandin E2 (PGE2), interleukins, tumor necrosis factor-α (TNF-α), caspases, kruppel-like factor 6 (KLF6), inducible nitric oxide synthase (iNOS), and delayed increases in heat shock protein 70 kDa (HSP-70) and hypoxia-inducible factor-1α (HIF-1α; 9, 13, 19, 23, 39, 40, 44, 47, 50). We reported that hypoxia resulted from 40% hemorrhage decreased Bcl-2 protein and increased TNF-α, IL-6, and IL-10 concentrations, p53 protein, caspase-3 activation, and cellular ATP depletion (15).
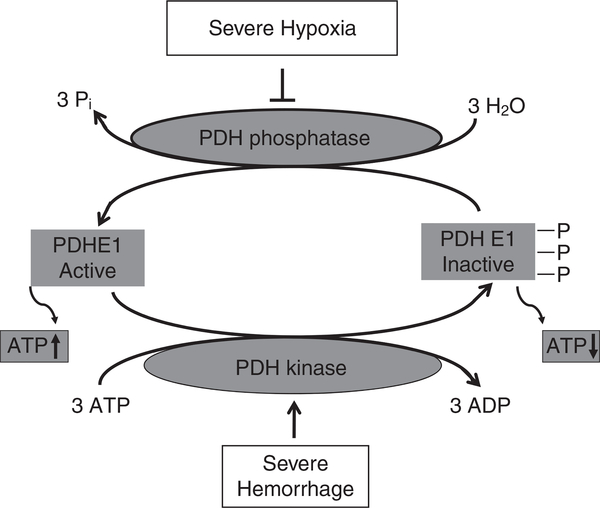
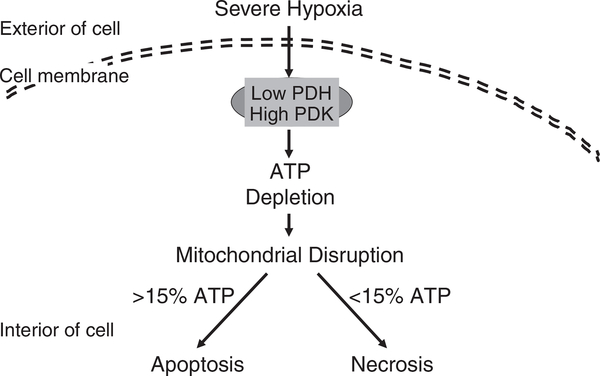
Figure 1 depicts that severe hypoxia-induced ATP depletion is mediated by inhibition of pyruvate dehydrogenase (PDH) and activation of pyruvate dehydrogenase kinase (PDK; refs 18, 51). Subsequently as shown in Fig. 2, ATP depletion results in mitochondrial disruption. When the cellular ATP level remains above 15% of the basal level, cells undergo apoptosis, whereas when the cellular ATP level is below 15% of the basal level, cells undergo necrosis (8, 30, 31, 33, 53).
Fig. 1.
Severe hypoxia inhibits PDH phosphatase to reduce active PDH E1 and activates PDH kinase (PDK) to phosphorylate PDH E1 which becomes inactive. Therefore, ATP production is attenuated. PDH: pyruvate dehydrogenase; ATP: adenosine triphosphate; ↑: increase; ↓: decrease; ⊥: inhibition.
Fig. 2.
Severe hypoxia reduces PDH and increases PDK, which leads to ATP reduction. The reduction impedes maintenance of mitochondrial membrane potential that makes cells falling into apoptosis if the cellular ATP level remains above 15% of the basal level. Otherwise, cells undergo necrosis if the cellular ATP level remains below 15% of the basal level. PDH: pyruvate dehydrogenase; PDK: pyruvate dehydrogenase kinase.
Investigation of the sequence of their appearance provides useful insights into the mechanisms that underlie hypoxia-induced tissue injury and identify therapeutic targets to prevent or reverse the injury. Experiments in iNOS-deficient mice (13, 23), treatment of animals with iNOS inhibitors such as geldanamycin, 17-DMAG (16, 19), and 5’-androstenediol (17, 48), taken together with information obtained from the treatment of cultured cells with the iNOS inhibitor LNNA (27), suggest that iNOS-derived NO participates in the injury and the inflammatory cascade produced by hypoxia.
Hypoxia Modifies MicroRNAs
MicroRNAs (miRNAs) are small non-coding RNA molecules containing about 20–22 nucleotides that function in silencing RNAs and regulating gene expression (1, 3). It is evident that miRNAs involve in several diseases such as kidney disease (43), schizophrenia, bipolar disorder, depression, and anxiety disorders (6, 10, 14), alcoholism (32), obesity (12, 46), and diabetes (12). Hypoxia has been shown to alter the global microRNA expression in kidney, GI, and blood. Hypoxia induced by 20% hemorrhage altered 56 miRNAs (Kiang et al., unpublished data). Among them, miR-145 was upregulated and miR-15b and let-7g was downregulated (Kiang et al., unpublished data). MiR-145 and niR-15b were involved in kidney diseases (43). Let-7g was thought to be involved in obesity and diabetes (12).
Radiation
Today, more than 50% of cancer patients receive radio-therapy at some time during their disease (5), resulting in radiation injury to the normal tissues as well. The acute radiation syndrome (ARS) is manifested. ARS is the signs and symptoms that occur within several months after receiving ionizing radiation (38). The most radiation sensitive organs include the hematopoietic (11, 25), GI (20, 22, 34), skin (22, 37, 41), and vascular systems (42, 45). The moderate radiation dose range (1–7 Gy in human) of ionizing radiation poses a risk of damage to the hematopoietic system, leading to decreases in blood cells and platelet counts and increases susceptibility to infection and hemorrhage (7, 52). The high-dose whole-body irradiation (8 Gy and more in human) causes acute GI syndrome including loss of intestinal crypts and breakdown of the GI mucosal barrier (20–22). The high-dose of ionizing radiation induces GI hemorrhage, endotoxemia, bacteremia, anorexia, nausea, vomiting, diarrhea, and loss of electrolytes and fluid (49); skin injury from radiation burns is characterized by loss of epidermis and dermis (2, 22), reduction of the skin stem cells, and impair of cell communication and cutaneous integrity, which may play an important role as a factor triggering the failure of other organ systems (36). In addition, vascular endothelium is damaged (42). The concomitant and interdependent injuries to various organ systems lead to multi-organ dysfunction (MOD) and failure (MOF). As a result, mortality occurs.
Hypoxia Exacerbates Radiation-Induced Mortality, Less Water Intake, and Body Weight Loss
When whole animals were exposed to 60Co γ-photon radiation at 8.75 Gy (i.e., LD50/30, CD2F1 male mice), animals began to die 10 days after irradiation (25). But when these animals were followed by hypoxia induced by intermittent 20% hemorrhage, animals started to die 9 days after irradiation. On day 30 after irradiation, hypoxia aggravated radiation-induced mortality by 20%, whereas hypoxia alone did not result in any mortality (25). It should be kept in mind that 20% hemorrhage may induce other physiological outcomes in addition to hypoxia to cells, tissues, and organs.
Radiation also transiently made animals drink less water. The average daily water intake per mouse was approximately 4 mL/day/mouse; radiation reduced it down to 1.5 mL/day/mouse; radiation followed by hypoxia further reduced it down to 0.5 mL/day/mouse. However, all mice consumed water back to normal levels on day 7 after irradiation or radiation followed by hypoxia. However, hypoxia reduced the water intake at the first day but returned to normal levels the 2nd day (25). The underlying mechanism(s) is/are not clear.
Radiation transiently made animals lose body weight on day 3 and bounced back on day 7, but then lost the body weight again on day 15. Animals exposed to radiation and hypoxia lost body weight even more than animals exposed to radiation only. However, radiation reduced organ weights of GI, lungs and spleen, but hypoxia failed to further reduce their weights. Hypoxia alone did not alter their body and organ weights (25).
Hypoxia Enhances Radiation-Induced Organ Damage
Bone marrow is sensitive to ionizing radiation and acutely injured within a day after ionizing irradiation at 8.75 Gy. Histopathology examination of bone marrow after irradiation displayed erythroid (for RBC), myloid (for WBC), and megakaryocyte (for platelets) reduction and was full of vacuoles (i.e., fat cells). Radiation followed by hypoxia further reduced bone marrow cellularity, whereas hypoxia alone did not. The reduction of bone marrow cellularity is negatively correlated with erythropoietin (EPO) concentrations in blood and its production in kidneys. It is further confirmed with concurrently increased HIF-1α concentrations in kidneys (25). Hypoxia alone due to 20% hemorrhage did not affect bone volume, trabecular thickness, trabecular number, and trabecular spacing but increased sclerostin (a protein to decrease bone formation). However, this hypoxia further aggravated radiation-induced decreases in bone volume, trabecular thickness, trabecular number, and trabecular spacing, osteocalcin (a protein secreted by osteoblasts, as a bone formation marker) and PINP (as a bone formation marker), but enhanced radiation-induced increases in sclerostin (51).
GI is another organ sensitive to ionizing radiation and acutely injured within days after ionizing irradiation. Histopathology examination of GI after irradiation showed presence of short and wide villi, opened villous tips, decreased crypt depth and reduced numbers of crypt counts. Radiation followed by hypoxia further reduced these parameters, whereas hypoxia alone did not. The enhancement was mediated by activation of the NF-κB/iNOS signaling pathway (Kiang et al., unpublished).
Hypoxia Enhances Radiation-Induced Reductions of WBC, RBC, and Platelets in Peripheral Circulation
When whole animals were exposed to 60Co γ-photon radiation at 8.75 Gy, animals began to significantly lose bone marrow cells on day 1, WBC counts on day 2, RBC counts, hemoglobin levels, hematocrit readings, and platelet counts on day 7 after irradiation. But when these irradiated animals were followed by hypoxia induced by 20% hemorrhage, their cell losses were even greater (25). This enhancement is thought due to lack of replenishment of hematopoietic progenitor cells from bone marrow, because bone marrow hematopoietic progenitor cells are acutely killed massively after irradiation combined with hypoxia.
Hypoxia Enhances Radiation-Induced Increases in Cytokine/Chemokine Concentrations in Blood and Organs
Using cytokine multiplex kits and luminex, we have shown that ionizing radiation increases cytokine/chemokine concentrations in blood (22). Whole body irradiation combined with hypoxia significantly enhanced increases in IL-1β, IL-6, IL-17A, and TNF-α in blood, IL-6 and TNF-α in bone marrow, IL-1β, IL-6, and TNF-α in ileum, and IL-6 in kidneys. Hypoxia alone slightly increased IL-17A in bone marrow, IL-1β in ileum, TNF-α in spleen and kidneys (25). The results suggest contributions of cytokines/chemokines from various organs to the blood stream that would carry these proinflammatory cytokines ubiquitously so as to systematically induce undesirable outcomes.
Hypoxia Enhances Radiation-Induced Modification on MicroRNA Expression
When animals were exposed to ionizing radiation alone, hypoxia alone, or radiation combined with hypoxia, hypoxia caused by 20% hemorrhage alone resulted in changes in 56 microRNA expressions in blood. Ionizing radiation alone resulted in changes in 34 microRNA expressions. Combination of ionizing radiation with hypoxia modified 66 microRNA expressions in blood. Among them, 19 microRNA were commonly present in blood of animals exposed to hypoxia alone, radiation alone, and combination of the two. Six out of 19 microRNAs were upregulated and other 13 microRNAs were downregulated. Hypoxia enhanced radiation-induced changes in these 16 microRNAs (Kiang et al., unpublished data). Using Ingenuine Program Analysis (IPA), mir-27a, miR-29b, miR-30e, and let-7e regulated NF-κB gene expression that was responsible for transcribing iNOS and cytokines (25).
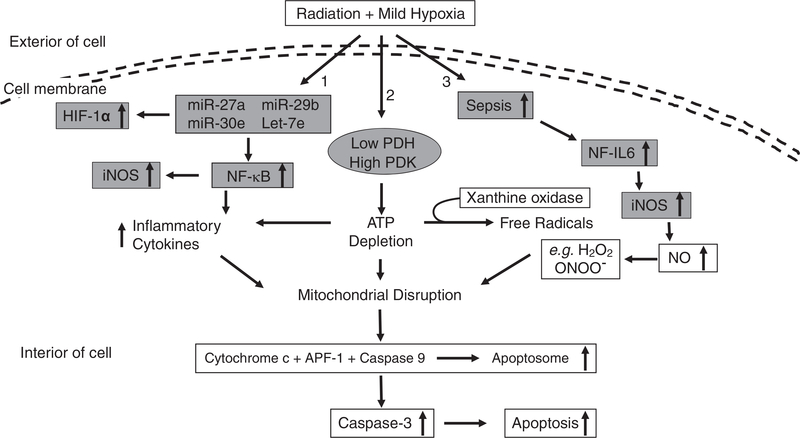
As shown in Fig. 3, mild hypoxia following ionizing radiation results in changes in 3 aspects: 1) to modify miR-27a, miR-29b, miR-30e, and let-7e expressions that lead to increases in HIF-1α and NF-κB. The latter elevates iNOS expression and inflammatory cytokine products; 2) to reduce PDH and elevate PDK that decrease ATP production; and 3) to cause sepsis that activates NF-IL6 and subsequently increases iNOS expression. Then NO production is increased and produces free radical ONOO− that can nitrate proteins. Increases in inflammatory cytokines, ATP depletion, and free radical ONOO− disrupt mitochondrial membrane potential. Then, cytochrome c leak out from mitochondria to cytoplasm, conjugate with caspase-9 and apoptotic protease activating factor-1 (APF-1) to form apoptosomes that activate various caspases. Caspase-3 activation results in cell apoptosis (26, 28).
Fig. 3.
Mild hypoxia following ionizing radiation exacerbates survival by amplifying molecular signals, cytokines, and sepsis after ionizing radiation. Mild hypoxia following ionizing radiation results in changes in 3 aspects: 1) to modify miR-27a, miR-29b, miR-30e, and let-7e expressions that lead to increases in HIF-1α and NF-κB. The latter elevates iNOS expression and inflammatory cytokine products; 2) to reduce PDH that decreases ATP production; and 3) to cause sepsis that activates NF-IL6 and subsequently increases iNOS expression. Then NO production is increased and produces free radical ONOO− that can nitrate proteins. Increases in inflammatory cytokines, ATP depletion, and free radical ONOO− disrupt mitochondrial membrane potential. Then, cytochrome c leaks out from mitochondria to cytoplasm, conjugates with caspase-9 and APF-1 to form apoptosomes that activate caspases. Caspase-3 activation results in cell apoptosis (26, 28). miR: microRNA; HIF-1α: hypoxia-inducible factor 1α; NF-κB: nuclear factor-keppaB; iNOS: inducible nitric oxide synthase; PDH: pyruvate dehydrogenase; APF-1: apoptotis protease activating factor-1; NF-IL6: nuclear factor-interleukin 6; NO: nitric oxide; ATP: adenosine triphosphate; ↑: increase; ↓: decrease.
Conclusions
There are 50% of population experiencing cancers and some of them receive radiation therapy (5). The scenario with radiation therapy followed by hypoxia ranging from mild degrees to severe degrees due to anemia, ischemia, hemorrhage, or exposure to hypoxic chemicals can happen. Although mild hypoxia is not lethal and causes transient increases in only some cytokines, mild hypoxia following ionizing radiation enhances hematopoietic and GI acute radiation syndromes that lead to increased mortality. This is the first demonstration of synergistic effects between mild hypoxia and ionizing radiation.
Acknowledgements
The author thanks the effort of her colleagues at Armed Forces Radiobiology Research Institute (AFRRI) and collaborators at Uniformed Services University of the Health Sciences (USUHS), and support of her technical staff at AFRRI and USUHS. This report has been cleared and approved by AFRRI and USUHS leadership management. The views, opinions and findings contained in this report are those of the author and do not represent official policy or positions of the AFRRI, USUHS, the United States Army, Navy, or Air Force, the United States Department of Defense, or the United States Government. The work was supported by AFRRI intramural RAB33336 and RAB33529.
References
- 1.Ambros V The functions of animal microRNAs. Nature 431: 350–355, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Barabanova AV Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit. Pregl. 63: 477–480, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baue AE, Durham R and Faist E Systemic inflammatory response syndrome (MODS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 10: 79–89, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen SM Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer 6: 702–713, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Beveridge NJ, Gardiner E, Carroll AP, Tooney PA and Cairns MJ Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry 15: 1176–1189, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman CN, Stone HB, Moulder JE and Pellmar TC Medicine. Modulation of radiation injury. Science 304: 693–694, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Comelli M, Di Pancrazio F and Mavelli I Apoptosis is induced by decline of mitochondrial ATP synthesis in erythroleukemia cells. Free Radic. Biol. Med. 34: 1190–1199, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Conde AG, Lau SS, Dillmann WH and Mestril R Induction of heat shock proteins by tyrosine kinase inhibitors in rat cardiomyocytes and myogenic cells confers protection against simulated ischemia. J. Mol. Cell Cardiol. 29: 1927–1938, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Sun G, Yan J, Noltner K, Li W, Buzin CH, Longmate J, Heston LL, Rossi J and Sommer SS Evidence for X-chromosomal schizophrenia associated with microRNA alterations. PLoS ONE 4: e6121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fliedner TM, Graessel D, Meineke V and Dorr H Pathological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: an essential basis for an evidence-based clinical triage. Exp. Hematol. 35: 8–16, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Fros t R.J. and Olson EN Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. (U. S. A.) 108: 21075–21080, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J and Nathan CF Essential role of induced nitric oxide in the initiation of the inflammatory responses after hemorrhagic shock. J. Exp. Med. 187: 917–928, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hommers LG, Domschke K and Deckert J Heterogeneity and Individuality: microRNAs in Mental Disorders. J. Neural. Transm. 122: 79–97, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Kiang JG, Agravante NG, Smith JT and Bowman PD 17-DMAG diminishes hemorrhage-induced small intestine injury by elevating Bcl-2 protein and inhibiting iNOS pathway, TNF-α increase, and caspase-3 activation. Cell Biosci. 1: 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiang JG, Bowman DP, Lu X, Li Y, Ding XZ, Zhao B, Juang Y-T, Atkins JL and Tsokos GC Geldanmaycin treatment prevents hemorrhage-induced ATP loss by overexpressing HSP-70 and activating pyruvate dehydrogenase. Am. J. Physiol. Gastrointest. Liver Physiol. 291: G117–G127, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kiang JG, Bowman DP, Lu X, Li Y, Wu BW, Loh HH, Tsen KT and Tsokos GC Geldanmaycin treatment inhibits hemorrhage-induced increases in caspase-3 activity: Role of inducible nitric oxide synthase. J. App. Physiol. 103: 1045–1055, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kiang JG, Bowman DP, Lu X, Li Yansong, Ding XZ, Zhao B, Juang YT, Atkins JL and Tsokos GC Geldanmaycin treatment prevents hemorrhage-induced ATP loss in mouse organs by overexpressing HSP-70 and activating pyruvate dehydrogenase. Am. J. Physiol. Gastrointest. Liver Physiol. 291: G117–G127, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kiang JG, Bowman PD, Wu BW, Hampton N, Kiang AG, Zhao B, Juang Y-T, Atkins JL and Tsokos GC Geldanamycin treatment inhibits hemorrhage-induced increases in KLF6 and iNOS expression in unresuscitated mouse organs: role of inducible HSP-70. J. Appl. Physiol. 97: 564–569, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kiang JG, Garrison BR and Gorbunov NV Radiation combined injury: DNA damage, apoptosis, and autophagy. Adapt. Med. 2: 1–10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiang JG, Garrison BR, Burns TM, Zhai M, Dews IC, Ney PH, Fukumoto R, Cary LH, Elliott TB and Ledney GD Wound trauma alters ionizing radiation dose assessment. Cell Bioscience 2: 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC and Ledney GD Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat. Res. 173: 319–332, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiang JG, Krishnan S, Lu X and Li Y Inhibition of inducible nitric oxide synthase protects human T cells from hypoxia-induced injury. Mol. Pharmacol. 73: 738–747, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kiang JG, Peckham RM, Duke LE, Chaudry IH and Tsokos GC Androstenediol inhibits trauma-hemorrhage-induced increase in caspase-3 by downregulating inducible nitric oxide synthase pathway. J. App. Physiol. 102: 933–941, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kiang JG, Smith JT, Anderson MN, Swift JM, Gupta P, Balakathiresan N and Maheshwari RK Hemorrhage exacerbates radiation effects on survival, leukocytopenia, thrombopenia, erythropenia, bone marrow cell depletion and hematopoiesis, and inflammation-associated microRNAs expression in kidney. PLoS One 10: e0139271, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiang JG and Tsen KT Biology of hypoxia. Chin. J. Physiol. 49: 223–233, 2006. [PubMed] [Google Scholar]
- 27.Kiang JG, Warke VG and Tsokos GC NaCN-induced chemical hypoxia is associated with altered gene expression. Mol. Cell. Biochem. 254: 211–216, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kiang JG Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell. Res. 14: 450–459, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kiang JG, Lu X, Tabaku LS, Bentley TB, Atkins JL and Tsokos GC Resuscitation with lactated Ringers solution limits the expression of molecular events associated with lung injury after hemorrhage. J. Appl. Physiol. 98: 550–556, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Leist M, Single B and Castoldi AF Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185: 1481–1486, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemasters JJ Mechanisms of Hepatic Toxicity V. Necrapoptosis and the mitochondrial permeability transition: shared pathways for necrosis and apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 276: G1–G6, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA and Mayfield RD Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin. Exp. Res. 35: 1928–1937, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberthal W, Menza SA and Levine JS Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. Renal Physiol. 274: F314–F327, 1998. [DOI] [PubMed] [Google Scholar]
- 34.MacNaughton WK Review article: new insights into the pathologenesis of radiation-induced intestinal dysfunction. Aliment. Pharmacol. Ther. 14: 523–528, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Manning JE Fluid and Blood Resuscitation. In: Emergency Medicine: A Comprehensive Study Guide. Tintinalli JE Ed. McGraw-Hill: New York: 2004. p227. [Google Scholar]
- 36.Meineke V The role of damage to the cutaneous system in radiation-induced multi organ failure. Brit. J. Radiol. Suppl. 27: 85–99, 2005. [Google Scholar]
- 37.Meistrich ML and Kangasniemi M Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J. Androl. 18: 80–87, 1997. [PubMed] [Google Scholar]
- 38.Mettler FA Jr, Gus’kova AK, and Gusev I Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 93: 462–469, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI and Chaudry IH Preinduction of heat shock proteins protects cardiac and hepatic functions following trauma and hemorrhage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278: R352–R359, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Moore WM, Webber RK, Jerome GM, Tjoeng FS, Misko TP and Currie MG L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J. Med. Chem. 37: 3886–3888, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Muller K and Meineke V Radiation-induced alterations in cytokine production by skin cells. Exp. Hematol. 35: 96–104, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Pena LA, Fuks Z and Kolesnick RN Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 60: 321–327, 2000. [PubMed] [Google Scholar]
- 43.Phua YL, Chu JY, Marrone AK, Bodnar AJ, Sims-Lucas S and Ho J Renal stromal miRNAs are required for normal nephrogenesis and glomerular mesangial survival. Physiol. Rep. 3(10). pii: e12537, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittet JF, Lu LN, Geiser T, Lee H, Matthay A and Welch WJ Stress preconditioning attenuates oxidative injury to the alveolar epithelium of the lung following haemorrhage in rats. J. Physiol. 538: 583–597, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodemann HP and Blaese MA Responses of normal cells to ionizing radiation. Semin. Radiat. Oncol. 17: 81–88, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Romao JM, Jin W, Dodson MV, Hausman GJ, Moore SS and Guan LL MicroRNA regulation in mammalian adipogenesis. Exp. Biol. Med. (Maywood) 236: 997–1004, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Shea-Donohue T, Anderson J and Swiecki C Ischemia/reperfusion injury. In: Combat Medicine – Basic and clinical research in military, trauma, and emergency medicine (eds Tsokos GC and Atkins JL), Humana Press, New Jersey, 2003. pp. 219–248. [Google Scholar]
- 48.Shimizu T, Szalay L, Choudhry MA, Schwacha MG, Rue LW III, Bland KI and Chaudry IH Mechanism of salutary effects of androstenediol on hepatic function following trauma-hemorrhage: the role of endothelin 1 and inducible nitric oxide synthase. Am. J. Physiol. Gastrointest. Liver Physiol. 288: G244–G250, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Somosy Z, Horvath G, Telbisz A, Rez G and Palfia Z Morphological aspects of ionizing radiation response of small intestine. Micron 33: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Stojadinovic A, Kiang JG, Smallridge RC, Galloway RL and Shea-Donahue T Heat shock protein 72 kD induction protects rat intestinal mucosa from ischemia/reperfusion injury. Gastroenterology 109: 505–515, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Swift JM, Smith JT and Kiang JG Ciprofloxacin therapy mitigates ATP loss after irradiation combined with wound trauma: Preservation of pyruvate dehydrogenase and inhibition of pyruvate dehydrogenase kinase 1. Radiat. Res. 183: 684–692, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Waselenko JK, MacVitte TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO and Dainiak N Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 140: 1037–1051, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Wiegele G, Brandis M and Zimmerhackl LB Apoptosis and necrosis during ischemia in renal tubular cells (LLC-PK1 and MDCK). Nephrol. Dial. Transplant 13: 1158–1167, 1998. [DOI] [PubMed] [Google Scholar]