Abstract
Fibrotic diseases pose significant clinical challenges due to their broadness and complexity. Thus, a better understanding of fibrogenesis and the development of more effective treatments is imperative. Recent evidence suggests a significant antifibrotic potential of an endogenous glycoprotein, endostatin. While endostatin has been widely studied for its role as an anticancer adjuvant by inhibiting tumor angiogenesis, its possible implication in fibrosis remains largely unclear. Here, we review the role of endostatin in various cellular processes and highlight its antifibrotic activity. We hypothesize that endostatin conveys a homeostatic function in the process of fibrosis by regulating (a) TGF-β1 and its downstream signaling; (b) RhoA/ROCK pathway; (c) NF-κB signaling pathway; (d) expression of EGR-1; (e) PDGF/PDGFR pathway; (f) autophagy-related pathways; (g) pathways associated with cell proliferation and apoptosis. Finally, we propose a schematic model of the antifibrotic roles and mechanisms of endostatin; also, we outline future research directions of endostatin and aim to present a potential therapeutic approach for fibrosis.
Keywords: Endostatin, fibrosis, fibrotic diseases, TGF-β, postoperative adhesions
1. Introduction
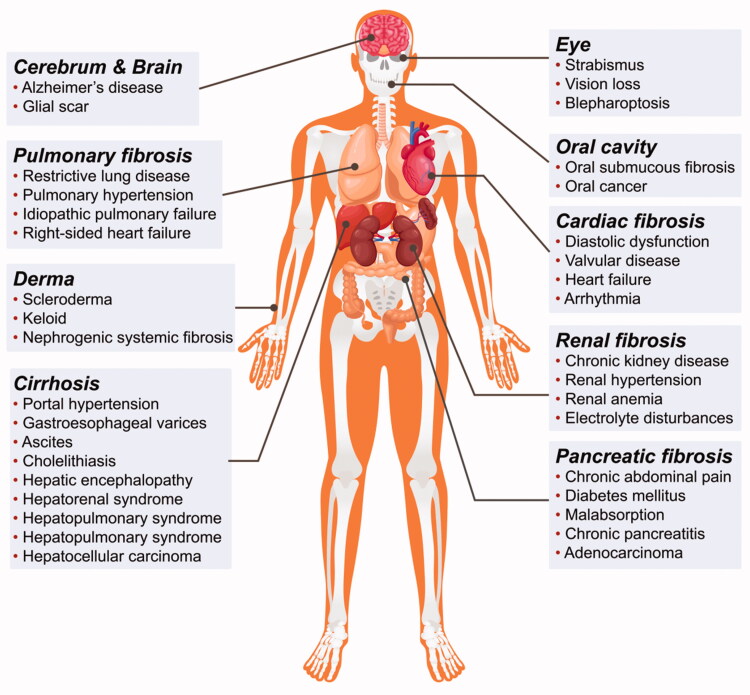
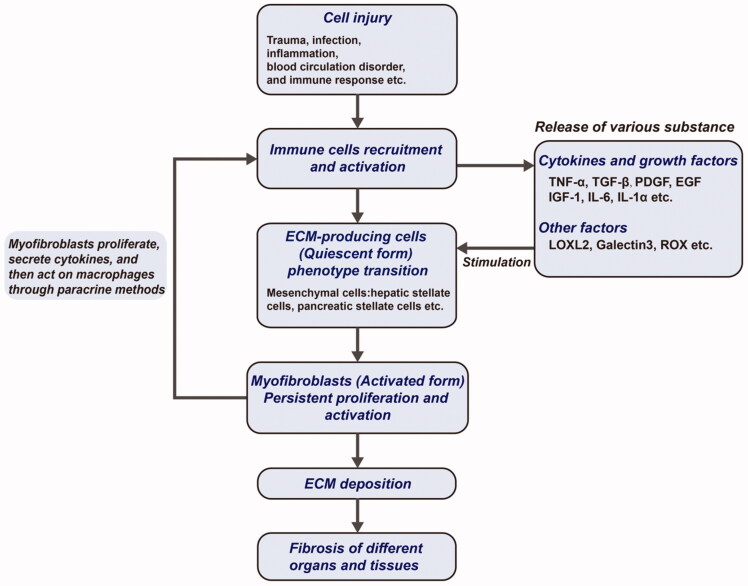
Fibrosis is a pathophysiological change characterized by excessive deposition of extracellular matrix (ECM) and is triggered by factors like trauma, infection, inflammation, blood circulation disorders, immune responses, and abnormal tissue repair (Wynn 2007; Lopez-de la Mora et al. 2015). The fibrotic process can be seen in almost all tissues and organs, examples including the liver, kidney, lung, pancreas, cardiovascular and cerebrovascular systems, bone marrow, skin, etc. (Luo et al., 2017) (Figure 1). The result of fibrosis seriously affects the function of tissues and organs, and fibrosis of important organs can endanger the lives of patients (Wynn 2007; Humphreys 2018; Jun & Lau 2018). The mechanisms underlying fibrosis development are complex and may be modulated by many factors and signaling pathways (Hu & Phan 2016; Klinkhammer et al. 2018; Tschumperlin et al. 2018; Wang et al. 2020) (Figure 2). However, the mechanisms of fibrosis are not fully understood and effective early diagnoses and treatments are still lacking in clinical practice.
Figure 1.
Distribution of fibroproliferative disorders in the human body. Nearly all organs and tissues can develop fibroproliferative disorders indicating the complexity and broadness of fibrotic diseases.
Figure 2.
Core steps of fibrogenesis. The formation mechanism of fibrosis is very complex and shares several common core steps.
Fibroproliferative disorders (FPDs) are characterized by excessive ECM accumulation and involve a variety of diseases, ranging from skin scarring and retroperitoneal fibrosis to life-threatening conditions like liver fibrosis/sclerosis, pulmonary fibrosis, glomerulosclerosis, renal interstitial fibrosis, pancreatic and gastrointestinal fibrosis, myelofibrosis, and arteriosclerosis (Huang & Ogawa 2012; Thannickal et al. 2014). The search for effective fibrosis treatments is a major research goal. Endostatin is a widely-known anticancer adjuvant that acts by inhibiting tumor angiogenesis and in recent years its anti-fibrotic effects have gained increasing attention, with cellular and animal model studies highlighting its therapeutic potential in FPDs.
2. Overview of endostatin
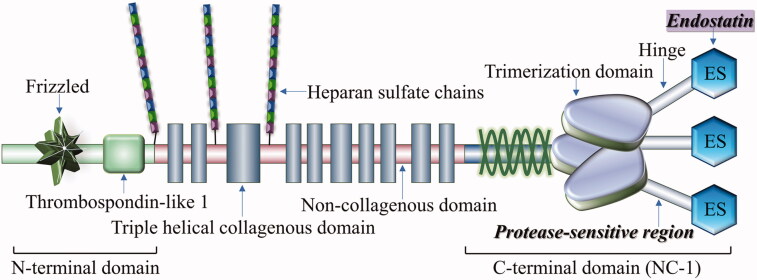
Endostatin, a bioactive product of hydrolysis of the carboxyl-terminal of extracellular matrix collagen XVII by protease (Figure 3), is an endogenous glycoprotein with remarkable anti-angiogenic effects (Pehrsson et al., 2019; Poluzzi et al., 2016). Endostatin’s amino-terminal part has anti-angiogenic activity (O'Reilly et al. 1997; Tjin Tham Sjin et al. 2005), while the carboxyl-terminal part has the anti-fibrotic function (Yamaguchi et al. 2012). Structurally modified synthetic recombinant human endostatin (Endostar) is included in various cancer treatment guidelines (Rong et al. 2012; Xiao et al. 2015) and endostatin’s anti-fibrotic effects have been validated in various FTD models.
Figure 3.
Structure of endostatin and collagen type XVIII. Endostatin is a bioactive fragment produced by hydrolysis of carboxyl-terminal of collagen type XVIII in the extracellular matrix by proteases.
Endostatin, of which the molecular weight is about 20 kDa, has many characteristics in a structure. Firstly, its structural stability depends partly on the integrity of the amino terminus sequence. Secondly, endostatin contains a zinc-binding motif, the capacity of zinc-binding also plays a key role in modulating the molecular stability and impacts the integral nature. Research has confirmed that zinc-binding ability takes the responsibility of condensing the structure of endostatin. Thirdly, the folding pattern is determined by the correct location of disulfide bonds in the endostatin peptides, and the correct folding of endostatin peptides is crucial for obtaining the most stable structure and biological activity to the maximum extent (Folkman 2006; Han et al. 2007; Fu et al. 2009).
The molecular mechanism of endostatin is complicated and involves many targets and pathways. Endostatin acts on numerous factors, including α5 integrins, glypicans, nucleolin, flk1, and tropomyosin (MacDonald et al. 2001; Karumanchi et al. 2001; Sudhakar et al. 2003; Shi et al. 2007). Although endostatin is widely studied, most studies have focused on its anti-angiogenic and cancer adjuvant effects and its anti-fibrosis effects remain unclear. This review focuses on the mechanisms underlying endostatin as a potential anti-fibrosis agent.
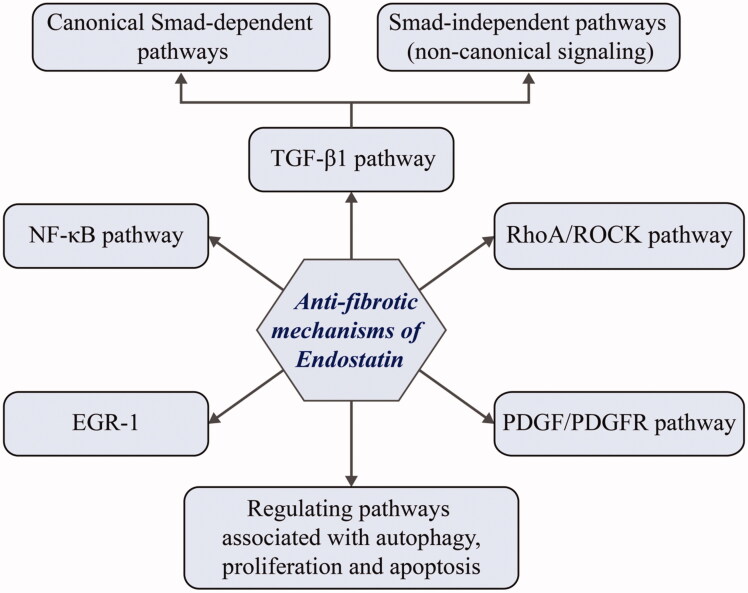
3. Anti-fibrotic mechanisms of endostatin
3.1. TGF-β1 signaling
Transforming growth factor β1 (TGF-β1) is a multifunctional cytokine, it has the functions of regulating cell proliferation, differentiation, and the production of ECM, and plays an important role in the process of organism development, wound healing, organ fibrosis, tumor generation, and metastasis. Therefore, it has always been a research hotspot in the above fields (Massague et al. 2000; Meng et al. 2016; Stewart et al. 2018). To date, three mammalian isoforms have been identified, TGF-β1, TGF-β2, and TGF-β3 (Yu et al. 2003). On the cellular membrane lies two kinds of serine/threonine kinase-type receptors, which are TGF-β targets. TGF-β signals through a heterologous receptor complex of type I and type II receptors and in most cases transmits the signal via Smads (Xu et al. 2012; Miyazawa & Miyazono 2017). The Smad family includes three categories, receptor-regulated Smads (R-Smad, including Smad1/2/3/5/8), inhibitory Smads (I-Smad, including Smad6/7), and common-mediator Smad (Co-Smad, including Smad4) (Hill 2016). R-Smads are activated by TGF-β receptor I. Smad2 and Smad3 are two main R-Smads phosphorylated by TGF-β receptor I. Phosphorylated R-Smad form heterodimeric complexes with Co-Smad. The complexes then translocate to the nucleus and regulate target gene transcription by binding to specific DNA sequences (Massague & Wotton 2000; Xu et al. 2012).
Besides canonical Smad-dependent pathways, TGF-β1 can also modulate fibrosis through Smad-independent signaling pathways, the key events of which reside in the activation of myofibroblasts, suppression of ECM degradation, and excessive deposition of ECM (Katz et al. 2016; Meng et al. 2016; Chen et al. 2018). Different Smad proteins may have distinct effects on fibrosis. There is a complex interaction between TGF-β/Smads and other signaling pathways (Dennler et al. 1998; Piek et al. 2001; Yuan & Varga 2001). Recent studies have identified many other mechanisms that modulate TGF-β1/Smads signaling in fibrosis, including epigenetic histone modifications, non-coding RNAs (Sun et al. 2010; Bian et al. 2014; McClelland et al. 2015; Yu et al. 2015). Additionally, AP1, a key pro-fibrotic transcription factor, is modulated by the non-Smad pathway via JNK activation (Jin et al. 2016). The facts above suggest that there may be a complex and huge regulation network that needs further research.
Endostatin is reported to downregulate TGF-β1 expression, thereby exerting an anti-fibrotic effect on TGF-β1-induced fibrosis. Endostatin was shown to alleviate peritoneal sclerosis by reducing TGF-β1 and α-SMA expression (Tanabe et al. 2007) in a study in which researchers induced peritoneal sclerosis via intraperitoneal injection of chlorhexidine gluconate (Tanabe et al. 2007). Immunohistochemical analysis showed that the number of TGF-β (+) and α-SMA (+) cells in the endostatin-treated peritoneum were significantly lower than in the control group. Besides, the western blotting analysis showed that endostatin suppresses TGF-β1 expression. However, whether endostatin influences processes downstream of TGF-β like the canonical Smad pathway or MAPK signaling is unknown (Yamaguchi et al. 2012).
3.2. Rhoa/ROCK pathway
Mammalian Rho GTPases fall into 8 families and the most studied subfamilies are Rho, Rac, and Cdc42. Rho GTPases are involved in cell migration, phagocytosis, contraction, and adhesion. RhoA, the best described Rho GTPase is associated with FPDs. RhoA promotes stress fiber formation and elongation, actin bundle contraction, and directional adhesion (Heasman & Ridley 2008; Hall 2012). ROCK (Rho-associated kinase) is a serine/threonine-protein kinase and direct RhoA effector. There are two subtypes of ROCK, namely ROCKI and ROCKII which differ in functions and distribution in the human body (Loirand 2015). ROCK targets include cytoskeleton-related proteins like myosin light chain and LIM kinase (LIMK) (Maekawa et al. 1999).
The RhoA/ROCK pathway is closely associated with pathological fibrosis. Hepatic stellate cells (HSCs), which play a pivotal role in liver fibrosis/sclerosis, are regulated by ROCK via multiple pathways, including p38 MAPKs, JNK, and ERK1/2 (Fukushima et al. 2005). Fasudil and Y27632 are selective ROCK inhibitors that suppress HSC proliferation and collagen synthesis in vitro (Tada et al. 2001; Fukushima et al. 2005). Fasudil ameliorates renal fibrosis in rats (Satoh et al. 2002; Han et al. 2020), while Y-27632 attenuates tubulointerstitial renal fibrosis in unilateral ureteral obstructed mice (Nagatoya et al. 2002). Rho influences α-SMA production and TGF-β1-induced cytoskeleton remodeling during epithelial-mesenchymal transition (EMT). Combined inhibition of ROCK and TGF-βRI reverses EMT (Masszi et al. 2003).
Endostatin is reported to inhibit the RhoA/ROCK pathway. Recent findings confirm that endostatin suppresses HSCs activation by inhibiting RhoA/ROCK1 signaling pathway (Ren et al. 2019). HSCs can be triggered by factors like inflammation or injury. Once activated, HSCs may turn into myofibroblasts and synthesize excessive ECM. In the study above, researchers established liver fibrosis models in vitro by treating HSCs with TGF-β1 or PDGF-BB and found that endostatin significantly reverses upregulation of collagen I, α-SMA, and F-actin by TGF-β1 or PDGF-BB. Similar results that endostatin suppresses the expression of RhoA and ROCK1 were obtained from PCR and western blot analyses.
3.3. NF-κB pathway
Nuclear factor-κB (NF-κB), a transcription factor present in the cytoplasm in inactive heterodimeric or homodimeric form, regulates multiple cellular processes. Particularly, NF-κB has crucial roles in immune cell activation, inflammation, stress response, and apoptosis. Many stimuli can activate NF-κB, which translocates from the cytoplasm to the nucleus, driving the expression of genes that contain κB binding sites (Oeckinghaus & Ghosh 2009; Hoesel & Schmid 2013). The human NF-κB family is comprised of five cellular DNA-binding subunits: p50, p52, crel, relA, and relB. In general, NF-κB binds to its inhibitory IκB partner (IκBα, IκBβ, and IκBε) and does not show transcriptional activity. The IKK protein kinase complex (IKKα, IKKβ, and IKKγ) is the core link that regulates NF-κB signaling. NF-κB must be released from IκB for activation. The activation of NF-κB mainly comprises two pathways: the canonical pathway, which is initiated due to cytokines like IL-1(interleukin-1) and TNF-α; and the alternative pathway, which plays a crucial role in B lymphocytes (Hoesel & Schmid 2013).
NF-κB signaling is implicated in fibrosis in various tissues and organs. NF-κB activation in HSCs/hepatic myofibroblasts (HMFs) promotes fibrosis in various ways, including direct pro-fibrotic function, antiapoptotic effects, and secretion of inflammatory mediators (Luedde & Schwabe 2011). A recent study showed that NF-κB signaling activation promotes pulmonary fibrosis by mediating the transactivation of fibrogenic genes in fibroblasts and myofibroblasts, suggesting a framework for further NF-κB signaling studies. Two NF-κB-related genes encoding pro-fibrotic proteins, osteopontin (OPN), and tissue inhibitor of metalloproteinase 1 (TIMP1) have been identified (Dong & Ma 2019).
Endostatin has been shown to inhibit NF-κB signaling, exerting antifibrotic activity. This is attributable to several mechanisms. Recent studies show that endostatin blocks NF-κB pathway due to its anti-inflammatory properties. Endostatin is also reported to bind to α5β1 integrin, a chondrocyte mechanoreceptor or transmembrane integrin receptor that transmits high-intensity mechanical signals. These signals are stimuli that activate NF-κB. Therefore, the interaction between α5β1 integrin and endostatin suppresses mechanoreceptor function, thereby preventing NF-κB activation (Tabruyn et al. 2009; Marcu et al. 2010). Recombinant human endostatin has been shown to antagonize the formation of mouse osteoclasts by inhibiting NF-κB and MAPKs signaling pathways (Chen et al. 2016). In summary, although there is no direct evidence, it is likely that endostatin has anti-fibrotic effects via NF-κB signaling.
3.4. EGR-1
Human EGR-1 (early growth response 1) is located on chromosome 5q31 and encodes an 80 kDa DNA binding transcription factor. EGR-1 expression levels are low or undetectable in resting cells but are elevated by many extracellular stimuli. The EGR-1 consists of an activation domain, inhibition domain, and a DNA binding domain. DNA binding domain is comprised of three zinc fingers targeting GC-rich DNA sequences. EGR-1 modulates the expression of various growth factors, cytokines, and adhesion molecules, including TGF-β, bFGF (basic fibroblast growth factor), PDGF-A, PDGF-B, IL-2, TNF-α, M-CSF (macrophage colony-stimulating factor), ICAM-1 (intercellular adhesion molecule 1), and CD44, thereby modulating signal transduction (Bhattacharyya et al. 2011; Li et al. 2020).
Numerous studies have shown that EGR-1 drives fibrosis (Bhattacharyya et al. 2011) and that EGR-1 acts downstream of Smad-independent TGF-β signaling. For example, TGF-β1 induces the phosphorylation of MEK1, upregulating ERK1/2, and its substrate Elk-1, triggering EGR-1 expression in normal human fibroblasts (Bhattacharyya et al. 2008). Ectopic EGR-1 expression drives collagen gene expression, mediating fibrosis. Similar observations have been made in a mouse model of hepatic fibrosis using TGF-β1. The results showed that TGF-β1 stimulates EGR-1 expression by activating p38 MAPK/Elk-3 signaling cascade, converging on Elk-3, and promoting liver fibrosis by modulating EMT in liver cells (Li et al. 2017). EGR-1 is a ROS-sensitive transcription factor and is activated by reactive oxygen species (ROS) produced by phagocytes at sites of tissue injury. EGR-1 activation by the Ras/Raf/ERK signaling cascade is oxidants/ROS-dependent (Roy et al. 2004).
EGR-1 downregulation is proposed as one of the endostatin’s mechanisms of action. Yamaguchi and colleagues described the potent anti-fibrotic effects of E4, a peptide derived from endostatin. Intriguingly, E4 peptide suppresses fibrosis in cell culture, human skin organ culture, and animal models. Specifically, it suppressed EGR-1 expression in fibroblasts, thereby lowering fibronectin (FN), collagen I (ColI), and lysyl oxidase (LOX) production. Lower LOX levels reduce collagen cross-linking, making the ECM more fragile (Yamaguchi et al. 2012; Nishimoto et al. 2015). It is reported that oral E4 administration has anti-fibrotic properties in a bleomycin-induced mouse model of pulmonary fibrosis, indicating that E4 has potential as an oral therapy for fibrotic disorders (Nishimoto et al. 2015).
3.5. PDGF/PDGFR pathway
Platelet-derived growth factor (PDGF) is a major mitogenic agent. There are five PDGF subtypes: PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC, and PDGF-DD. PDGF receptor (PDGFR) is a transmembrane glycoprotein with tyrosine-protein kinase activity, including three kinds of dimers, αα, αβ, and ββ, which are composed of PDGF-α and PDGFR-β subunits in a certain way. PDGF-A and PDGF-C predominantly bind to PDGFR-α chain, PDGF-B binds to both -α and -β chains, and PDGF-D binds to the β chain only. PDGF functions depend not just on the concentration of the ligand but also on ligand binding to specific receptors. Mesenchymal cells are the principal site of PDGFRs expression. Once bound by ligands, PDGFRs are phosphorylated to activate transcription factors and diverse cytoplasmic signaling cascades, like Janus kinase, mitogen-activated protein kinase, p38, phospholipase C, and Ras GTPase activating protein, driving downstream gene expression and exerting biological effects (De Donatis et al. 2008; Klinkhammer et al. 2018; Papadopoulos & Lennartsson 2018; Sil et al. 2018).
PDGF modulates fibrosis and is a promising target for anti-fibrotic therapy. PDGF promotes fibrosis in wound healing. Recombinant PDGF-BB promotes healing of chronic foot and leg ulcers (Barrientos et al. 2014). Moreover, PDGFs exert mitogenic and anti-apoptotic effects on fibroblasts and myofibroblasts that synthesize ECM, thereby facilitating the progression of fibroproliferative disorders (Klinkhammer et al. 2018).
A recent paper demonstrated that endostatin downregulates PDGFR and p-ERK protein levels, reducing scar formation in a fibroblast model of fibrosis. ERK, which is highly phosphorylated in scar fibroblasts, is located downstream of the PDGFR signaling pathway and modulates cell proliferation and migration. It is reported that endostatin does not affect ERK expression but suppresses its phosphorylation, suggesting that endostatin may alleviate dermal fibroblast fibrosis by regulating PDGFR/ERK signaling (Li & Ren 2017). Additionally, endostatin attenuates activation of hepatic stellate cells in a liver fibrosis model upon induction with PDGF-BB and TGF-β1 by inhibiting RhoA/ROCK1 signaling.
3.6. Regulating autophagy-related pathways
Autophagy is the physiological catabolic mechanism that uses lysosomes to break down harmful cellular components, including misfolded proteins, damaged organelles, and excessive lipids, to achieve the clearance and reuse of cytoplasmic waste (Mizushima et al. 2008). Autophagy is a self-survival mechanism that occurs under special circumstances such as external stress, hunger, hypoxia, and endoplasmic reticulum stress. The disruption of the autophagy mechanism is closely related to the pathogenesis of tumors, neurodegenerative diseases, metabolic-related diseases, immune diseases, and other diseases (Levine & Kroemer 2019; Morishita & Mizushima 2019). Depending on the route of cargo transport into lysosomes, autophagy pathways are divided into three main categories: macroautophagy, microautophagy, and chaperone mediated autophagy; currently, macroautophagy has been the most extensively studied. Macroautophagy is characterized by autophagosomes that swallow excess cytoplasmic components and fuze with lysosomes; it is a multistep process that involves initiation, nucleation, expansion, fusion, and degradation (Dikic & Elazar 2018). In microautophagy, the lysosome membrane directly wraps the small cytosolic components without the assistance of autophagosomes (Oku & Sakai 2018). Chaperone-mediated autophagy refers to the process in which the proteins containing a specific motif are identified and combined by a chaperone and then translocated across the lysosomal membrane (Kaushik & Cuervo 2018).
Studies have found that fibrotic diseases have a deep connection with autophagy. EMT is recognized as an important mechanism of fibrosis, and autophagy participates in the occurrence and development of fibrosis by regulating epithelial damage and activating myofibroblasts, which are the core steps of EMT (Nieto et al. 2016; Stone et al. 2016; Takagaki et al. 2020). Dysregulation of autophagy is found in pathological processes such as PF (pulmonary fibrosis), liver fibrosis, cardiac fibrosis, and peritoneal fibrosis. Specifically, autophagy slows down the pathological process of PF by regulating the apoptosis of fibroblasts and the senescence of alveolar epithelial cells. Additionally, autophagy may be an important therapeutic target for a variety of intrapulmonary fibrosis diseases, including idiopathic PF, cystic fibrosis lung disease, silicosis, and smoking-induced PF (Zhao et al. 2020). Furthermore, inhibition of autophagy can break the homeostasis of proximal tubular epithelial cells, thereby, aggravating kidney damage and further inducing renal fibrosis (Kimura et al. 2011; Tang et al. 2020). Intriguingly, autophagy upregulation can also promote fibrosis under certain circumstances. For example, studies have shown that Gα12 overexpression resulting from miR-16 dysregulation can activate HSCs by facilitating autophagy (Kim et al. 2018). In addition, PGc-1α is upregulated in systemic sclerosis (SSC) and promotes autophagy to foster TGF-β-induced fibroblast activation (Zhang et al. 2020). In summary, autophagy acts as a double-edged sword: on one hand, it can inhibit the occurrence of fibrotic diseases in most cases; on the other hand, excessive autophagy will aggravate the fibrotic process; the specific mechanism remains to be further explored.
Recent studies have indicated a new activity for endogenously-released fragments of the ECM: they can not only exert anti-angiogenic effects but also induce autophagy (Goyal et al. 2016; Neill et al. 2021). Endostatin is an autophagy inducer in endothelial cells. Soluble endostatin evokes autophagy by directly binding to the α5β1-integrin and regulating Beclin 1 and β-catenin levels (Nguyen et al. 2009). Specifically, endostatin treatment reduces Bcl-2 and Bcl-xL by interacting with α5β1-integrin and increasing the levels of Beclin 1 by inhibiting the Wnt/β-catenin pathway, thereby, disrupting the physiological Bcl-2 (or Bcl-xL)/Beclin 1 ratio and finally leading to the upregulation of autophagy. In addition, the levels of Beclin and β-catenin are negatively correlated; thus, it is reasonably speculated that β-catenin modulates Beclin 1 either by influencing its stability or by transcriptional repression (Nguyen et al. 2009). Although there is no direct evidence, it is reasonable to speculate that endostatin can also induce autophagy through similar mechanisms on epithelial cells and mesothelial cells. A reasonable explanation of the antifibrotic effects of endostatin is that it can help cells remove cytotoxic waste by increasing autophagy levels and maintaining the homeostasis of epithelial or mesothelial cells so that they can resist external negative stimuli, thereby, inhibiting EMT and reducing fibrosis. However, this mechanism still needs further experimental verification.
3.7. Regulating pathways associated with proliferation and apoptosis
Fibrosis is characterized by excessive formation and deposition of ECM by activated and expanded tissue mesenchymal stromal cells like fibroblasts, pericytes, and myofibroblasts. Fibroblast activation by various pathophysiological factors is a central link in fibrosis.
Based on the above knowledge, the possibility that endostatin has a straightforward effect on fibroblasts is worth consideration. Numerous studies have identified a potential role for endostatin indirectly modulating fibroblasts. Recombinant human endostatin (rhEndostatin) prevents synovial thickening in adjuvant arthritis (AA) rats by inhibiting synovial fibroblast proliferation and promoting apoptosis (Huang et al. 2008; Huang et al. 2014). Using MTT analysis and flow cytometry, a recent study found that rhEndostatin inhibits the proliferation of HSFs and in parallel, reduced levels of proliferative markers like p53, p27, CDK4 (Cyclin-dependent kinase 4), cyclinD1, and PCNA (proliferating cell nuclear antigen) was observed (Gong et al. 2016). In another study, transmission electron microscopy and flow cytometry revealed that rhEndostatin induces apoptosis of HSFs and significantly downregulates apoptosis-related biomarkers like bcl-2, NF-κB, c-jun, and c-fos (Gong et al. 2017).
4. Brief summary of the possible mechanisms of endostatin in fibrotic diseases
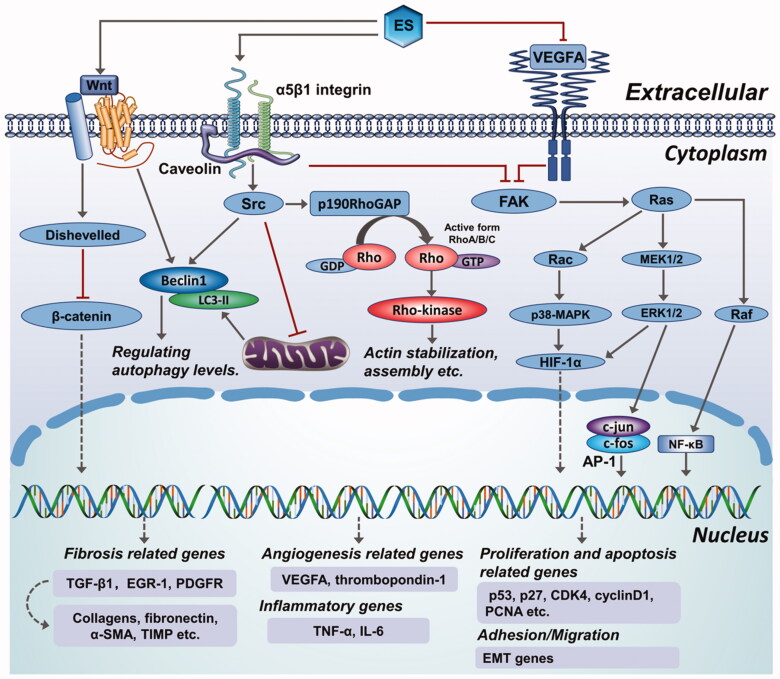
Potential endostatin targets are shown in Figure 4 but it is not clear how they are modulated by endostatin. Endostatin is reported to exert its antiangiogenic effect on endothelial cells via multiple targets and pathways (Wickstrom et al. 2005). We speculate that it may have similar effects on fibroblast-type cells and mesothelial cells, which may lead to antifibrotic effects. The mechanism of endostatin action can be summarized by three major downstream effects: inhibition of HIF-1α/VEGFA and Wnt signaling-dependent down-regulation of β-catenin (Hanai et al. 2002), suppression of FAK/Ras/p38-MAPK/ERK signaling cascade through α5β1-integrin binding (Rehn et al. 2001; Shichiri & Hirata 2001; Wickstrom et al. 2001), and actin disassembly via Src-dependent-p190RhoGAP activation (Wickstrom et al. 2002; 2003). Additionally, endostatin competitively inhibits VEGFR2 binding to its ligand, thereby suppressing VEGF-mediated downstream effector pathways involving p38-MAPK, p125FAK, and ERK (Kim et al. 2000; Hajitou et al. 2002; Eriksson et al. 2003). Based on the information above, we propose a schematic model of the antifibrotic roles of endostatin (Figure 5), however, there are still a number of details awaiting further investigation and validation.
Figure 4.
Overview of different roles of endostatin in fibrotic diseases.
Figure 5.
An elaborate scheme showing the anti-fibrotic mechanisms of endostatin and a comprehensive illustration of potential antifibrotic effects of endostatin.
5. Prospects of endostatin
5.1. Future research perspective: postoperative adhesion
Endostatin has been shown to be an exact anti-fibrotic molecule and exerts anti-fibrotic effects in multiple manners, which has been verified in many fibrosis models (Table 1). Considering the fact that many physiological and pathological conditions are characterized by fibrosis, endostatin may potentially be used on more fibrosis-related diseases. For example, postoperative adhesions, which is recognized as one of the most common clinical complications following diversified operations, such as abdominal and pelvic operation, plastic surgery, repair procedure of tendon, and the like.
Table 1.
Fibrotic diseases in which endostatin may have an effect.
| Fibrotic diseases | Target cell | Possible role in diseases | References |
|---|---|---|---|
| Liver fibrosis | Hepatic stellate cells | RhoA/ROCK1 signal pathways; inhibiting the expression of α-SMA, collagen-1, and TGF-β1 | (Chen et al., 2014; Ren et al. 2019) |
| Pulmonary fibrosis | Alveolar type II cells | Reducing the levels of TNF-α and TGF-β1; decreasing alveolar type II cell apoptosis | (Nishimoto et al. 2015; Richter et al., 2009) |
| Systemic sclerosis | Fibroblasts | Reducing levels of Egr-1, LOX | (Yamaguchi et al. 2012) |
| Hypertrophic scar | Skin fibroblasts/hypertrophic scar fibroblasts (HSFs) | PDGFRβ/ERK pathway; inhibiting HSFs proliferation and inducing its apoptosis | (Gong et al. 2016; Gong et al. 2017; Li & Ren 2017) |
| Arthritis | Synovial fibroblasts | Downregulating the level of cyclin-D1 and PCNA; inhibiting the NF-κB and MAPKs signaling pathways | (Chen et al. 2016; Huang et al. 2008; Huang et al. 2014) |
| Myocardial remodeling | Myofibroblasts | Stimulating proliferation and migration of myofibroblasts | (Isobe et al., 2010; Sugiyama et al., 2018) |
ROCK1: Rho-associated kinase 1; α-SMA: α-smooth muscle actin; TNF-α: tumor necrosis factor α; TGF-β1: transforming growth factor β1; Egr-1: early growth response-1; LOX: lysyl oxidase; PDGFRβ: platelet-derived growth factor receptor β; ERK: extracellular signal-regulated kinase; PCNA: proliferating cell nuclear antigen; NF-κB: nuclear factor-κB; MAPK: mitogen-activated protein kinase.
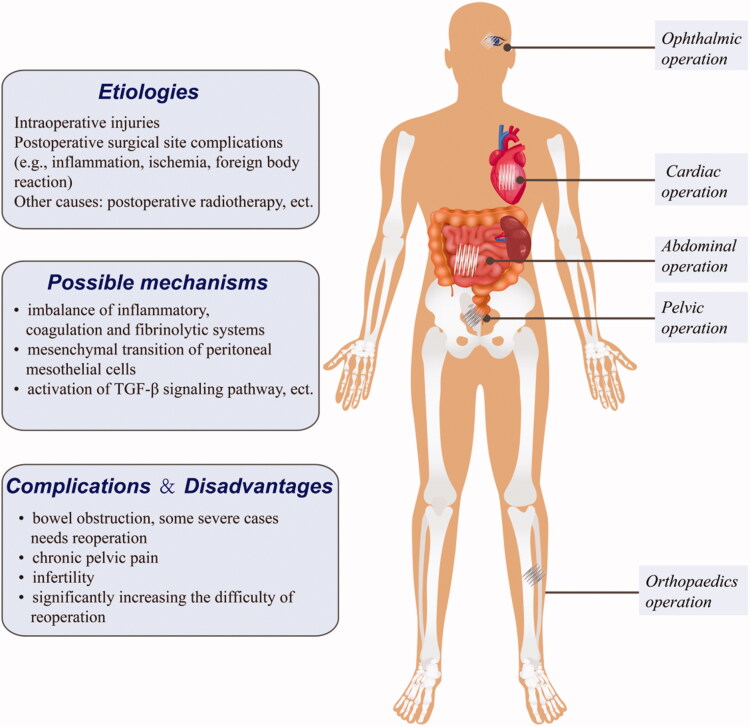
Postoperative adhesion (POA) is a manifestation of assorted aberrant histologic proliferation, characterized by hyperplastic fibrous tissue adherence to adjacent tissues and organs (Figure 6). Fibrotic adhesion bands may have various appearances, ranging from thin-layer slices to thick bands with neovascularization. More than 90% of patients undergoing abdominal surgery develop varying degrees of abdominal adhesions, which lead to complications like chronic abdominal pain, intestinal obstruction, and female infertility (Arung et al. 2011; Hellebrekers & Kooistra 2011). Besides, adhesion formation secondary to ligament and tendon healing may restrict joint movement. Moreover, POA secondary to open-heart surgery may make the pericardium fibrotic and thickened, resulting in constrictive pericarditis, which critically influences cardiac function. Furthermore, adhesions that occur after plastic surgery may seriously diminish therapeutic efficacy and fail to achieve desired outcomes. These complications frequently require multiple hospital admissions, increasing the burden on the patient and the healthcare system (ten Broek et al. 2013). These facts suggest that POA is an important clinical problem with potentially serious outcomes. Existing anti-adhesion strategies include reducing intraoperative damage, physical barriers, and anti-adhesion drugs (Moris et al. 2017; Kou et al. 2020; Li et al. 2020). However, currently, there are few effective anti-adhesion drugs.
Figure 6.
Representative postoperative adhesion, and a brief overview of its etiology, mechanisms and complications. Postoperative adhesion (POA) is a comprehensive manifestation of assorted aberrant histologic proliferation, characterized by hyperplastic fibrous tissues adhering to adjacent tissues and organs.
The process of POA is very similar to fibrosis (Chegini 2008; Capobianco et al. 2017), and in both the core steps are the activation of fibroblasts, production of large amounts of fibrous collagen, other matrix proteins, and α-smooth muscle actin (α-SMA). Broadly speaking, the formation of POA is also a fibrotic process. The transforming growth factor-β system has been proved to be the main pro-fibrotic mediator of POA formation, and TGF-β1 can induce the formation of postoperative peritoneal adhesion model (Chegini 2008; Jin et al. 2016). Besides, studies have confirmed that the formation of postoperative peritoneal adhesions involves activation of RhoA/ROCK signaling pathway and the EGR-1 pathway (Roy et al. 2004; Liu et al. 2020). These mechanisms are consistent with the anti-fibrotic mechanism of endostatin described above. Thus, we have sufficient reasons to speculate that endostatin can also inhibit the formation of postoperative adhesions, but this hypothesis still needs further investigation. The application of anti-fibrotic drugs to POA is a promising research direction. On top of the existing anti-adhesion formation strategy, combining anti-fibrotic strategy may more effectively inhibit POA formation, reducing fibrotic disease burden and benefiting patients.
5.2. Future challenges
Despite its good anti-fibrotic effects, the use of endostatin has many challenges. First, it is an endogenous molecule and its structural stability needs improvement. Future studies should improve the structure of endostatin to give it stronger anti-fibrotic activity while reducing its antiangiogenic properties and other side effects. Many studies have yielded good results in this regard, including the development of recombinant human endostatin (Endostar) by Chinese researchers. Compared with natural endostatin, Endostar has an added 9 amino acid sequence at the N-terminus, which prolongs its half-life, significantly improving biological activity and stability (Han et al. 2007; Xiao et al. 2015). Moreover, Endostar nano-carriers are reported to maintain it at good concentrations in plasma and tissues (Hu & Zhang 2010; Tong et al. 2010; Chen & Hu 2011). Endostar is now widely used in anticancer therapy. A Japanese study also found that E4, an endostatin-derived peptide, may suppress organ fibrosis. Relative to natural endostatin or recombinant endostatin, E4 has only the C-terminal structure and no N-terminal anti-angiogenic properties, which minimizes side effects. The molecular structure becomes more stable due to C-terminus chemical modification (Yamaguchi et al. 2012; Nishimoto et al. 2015).
There are many obstacles in anti-fibrotic treatment and it is nearly impossible to completely reverse pathological fibrosis. Many reasons contribute to this condition, including the fact that there are too many etiologies of fibrosis; the mechanisms of fibrosis are complex and involve many pathophysiological processes; and last but not least, single-target therapy may not be effective. Future research should consider combining treatment with endostatin and other anti-fibrotic drugs targeting distinct fibrosis pathogenic factors, which may have better therapeutic effects. Currently, endostatin treatment of fibrotic diseases has only been tested in cells and animal models, which do not recapitulate in vivo human conditions. Moreover, fibrosis mechanisms may differ across organs and tissues. Thus, further research is needed to determine if endostatin can effectively inhibit fibrosis in humans.
6. Conclusion
Pathological fibrosis causes many diseases and a heavy medical burden worldwide. However, there are few effective anti-fibrosis treatments. In this review, we have discussed the anti-fibrotic mechanisms of endostatin and its therapeutic value based on fibrosis disease models and highlight potential future research directions. Further comprehensive studies on the anti-fibrotic mechanisms of endostatin and its derivatives or analogs will uncover new strategies for the treatment of fibrosis-related diseases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arung W, Meurisse M, Detry O. (2011). Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol 17:4545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Brem H, Stojadinovic O, et al. (2014). Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 22:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Chen SJ, Wu M, et al. (2008). Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol 173:1085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Wu M, Fang F, et al. (2011). Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol 30:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian EB, Huang C, Wang H, et al. (2014). Repression of smad7 mediated by dnmt1 determines hepatic stellate cell activation and liver fibrosis in rats. Toxicol Lett 224:175–85. [DOI] [PubMed] [Google Scholar]
- Capobianco A, Cottone L, Monno A, et al. (2017). The peritoneum: healing, immunity, and diseases. J Pathol 243:137–47. [DOI] [PubMed] [Google Scholar]
- Chegini N. (2008). Tgf-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin Reprod Med 26:298–312. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu D-G, Yang G, et al. (2014). Endostar, a novel human recombinant endostatin, attenuates liver fibrosis in CCl 4-induced mice. Exp Biol Med (Maywood) 239:998–1006. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang T, Lu DW, et al. (2018). Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother 101:670–81. [DOI] [PubMed] [Google Scholar]
- Chen N, Gao RF, Yuan FL, et al. (2016). Recombinant human endostatin suppresses mouse osteoclast formation by inhibiting the NF-κB and MAPKs Signaling Pathways . Front Pharmacol 7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hu S. (2011). Suitable carriers for encapsulation and distribution of endostar: Comparison of endostar-loaded particulate carriers. Int J Nanomedicine 6:1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Donatis A, Comito G, Buricchi F, et al. (2008). Proliferation versus migration in platelet-derived growth factor signaling: The key role of endocytosis. J Biol Chem 283:19948–56. [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, et al. (1998). Direct binding of smad3 and smad4 to critical tgf beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. Embo J 17:3091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Elazar Z. (2018). Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19:349–64. [DOI] [PubMed] [Google Scholar]
- Dong J, Ma Q. (2019). In vivo activation and pro-fibrotic function of NF-κB in Fibroblastic Cells During Pulmonary Inflammation and Fibrosis Induced by Carbon Nanotubes . Front Pharmacol 10:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K, Magnusson P, Dixelius J, et al. (2003). Angiostatin and endostatin inhibit endothelial cell migration in response to fgf and vegf without interfering with specific intracellular signal transduction pathways. FEBS Lett 536:19–24. [DOI] [PubMed] [Google Scholar]
- Folkman J. (2006). Antiangiogenesis in cancer therapy-endostatin and its mechanisms of action. Exp Cell Res 312:594–607. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tang H, Huang Y, et al. (2009). Unraveling the mysteries of endostatin. IUBMB Life 61:613–26. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Nakamuta M, Kohjima M, et al. (2005). Fasudil hydrochloride hydrate, a rho-kinase (rock) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int 25:829–38. [DOI] [PubMed] [Google Scholar]
- Gong YF, Zhang XM, Liu F, et al. (2016). Inhibitory effect of recombinant human endostatin on the proliferation of hypertrophic scar fibroblasts in a rabbit ear model. Eur J Pharmacol 791:647–54. [DOI] [PubMed] [Google Scholar]
- Gong YF, Zhang XM, Yu J, et al. (2017). Effect of recombinant human endostatin on hypertrophic scar fibroblast apoptosis in a rabbit ear model. Biomed Pharmacother 91:680–6. [DOI] [PubMed] [Google Scholar]
- Goyal A, Gubbiotti MA, Chery DR, et al. (2016). Endorepellin-evoked autophagy contributes to angiostasis. J Biol Chem 291:19245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajitou A, Grignet C, Devy L, et al. (2002). The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. Faseb J 16:1802–4. [DOI] [PubMed] [Google Scholar]
- Hall A. (2012). Rho family gtpases. Biochem Soc Trans 40:1378–82. [DOI] [PubMed] [Google Scholar]
- Han Q, Fu Y, Zhou H, et al. (2007). Contributions of zn(ii)-binding to the structural stability of endostatin. FEBS Lett 581:3027–32. [DOI] [PubMed] [Google Scholar]
- Han Z, Wang Z, Song C, et al. (2020). Fasudil suppresses renal fibrosis in diabetic rats through pi3k/akt signaling pathway. Panminerva Medica. doi: 10.23736/S0031-0808.19.03793-5. [DOI] [PubMed] [Google Scholar]
- Hanai J, Gloy J, Karumanchi SA, et al. (2002). Endostatin is a potential inhibitor of wnt signaling. J Cell Biol 158:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. (2008). Mammalian rho gtpases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9:690–701. [DOI] [PubMed] [Google Scholar]
- Hellebrekers BW, Kooistra T. (2011). Pathogenesis of postoperative adhesion formation. Br J Surg 98:1503–16. [DOI] [PubMed] [Google Scholar]
- Hill CS. (2016). Transcriptional control by the smads. Cold Spring Harb Perspect Biol 8. doi: 10.1101/cshperspect.a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA. (2013). The complexity of NF-κB signaling in inflammation and cancer . Mol Cancer 12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Phan SH. (2016). Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res 108:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Zhang Y. (2010). Endostar-loaded peg-plga nanoparticles: In vitro and in vivo evaluation. Int J Nanomedicine 5:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ogawa R. (2012). Fibroproliferative disorders and their mechanobiology. Connect Tissue Res 53:187–96. [DOI] [PubMed] [Google Scholar]
- Huang XY, Chen FH, Li J, et al. (2008). Mechanism of fibroblast-like synoviocyte apoptosis induced by recombinant human endostatin in rats with adjuvant arthritis. Anat Rec (Hoboken) 291:1029–37. [DOI] [PubMed] [Google Scholar]
- Huang XY, Zhang XM, Chen FH, et al. (2014). Anti-proliferative effect of recombinant human endostatin on synovial fibroblasts in rats with adjuvant arthritis. Eur J Pharmacol 723:7–14. [DOI] [PubMed] [Google Scholar]
- Humphreys BD. (2018). Mechanisms of renal fibrosis. Annu Rev Physiol 80:309–26. [DOI] [PubMed] [Google Scholar]
- Isobe K, Kuba K, Maejima Y, et al. (2010). Inhibition of endostatin/collagen XVIII deteriorates left ventricular remodeling and heart failure in rat myocardial infarction model. Circ J 74:109–19. 10.1253/circj.cj-09-0486 19966499 [DOI] [PubMed] [Google Scholar]
- Jin X, Ren S, Macarak E, et al. (2016). Pathobiological mechanisms of peritoneal adhesions: The mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-β1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation . Matrix Biol 51:55–64. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. (2018). Resolution of organ fibrosis. J Clin Invest 128:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumanchi SA, Jha V, Ramchandran R, et al. (2001). Cell surface glypicans are low-affinity endostatin receptors. Mol Cell 7:811–22. [DOI] [PubMed] [Google Scholar]
- Katz LH, Likhter M, Jogunoori W, et al. (2016). TGF-β signaling in liver and gastrointestinal cancers . Cancer Lett 379:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. (2018). The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19:365–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Han CY, Kim JY, et al. (2018). Gα12 overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J Hepatol 68:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Jang JW, Lee OH, et al. (2000). Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res 60:5410–3. [PubMed] [Google Scholar]
- Kimura T, Takabatake Y, Takahashi A, et al. (2011). Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22:902–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhammer BM, Floege J, Boor P. (2018). Pdgf in organ fibrosis. Mol Aspects Med 62:44–62. [DOI] [PubMed] [Google Scholar]
- Kou L, Jiang X, Xiao S, et al. (2020). Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J Control Release 318:25–37. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. (2019). Biological functions of autophagy genes: A disease perspective. Cell 176:11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TT, Liu MR, Pei DS. (2020). Friend or foe, the role of egr-1 in cancer. Med Oncol 37:7. [DOI] [PubMed] [Google Scholar]
- Li TZ, Kim SM, Hur W, et al. (2017). Elk-3 contributes to the progression of liver fibrosis by regulating the epithelial-mesenchymal transition. Gut and Liver 11:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ren HT. (2017). Endostatin inhibits fibrosis by modulating the pdgfr/erk signal pathway: An in vitro study. J Zhejiang Univ Sci B 18:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu L, Chen Y. (2020). Dual dynamically crosslinked thermosensitive hydrogel with self-fixing as a postoperative anti-adhesion barrier. Acta Biomater 110:119–28. [DOI] [PubMed] [Google Scholar]
- Liu W, Qin F, Wu F, et al. (2020). Sodium aescinate significantly suppress postoperative peritoneal adhesion by inhibiting the rhoa/rock signaling pathway. Phytomedicine 69:153193. [DOI] [PubMed] [Google Scholar]
- Loirand G. (2015). Rho kinases in health and disease: From basic science to translational research. Pharmacol Rev 67:1074–95. [DOI] [PubMed] [Google Scholar]
- Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, et al. (2015). Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci 12:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Schwabe RF. (2011). NF-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma . Nat Rev Gastroenterol Hepatol 8:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Xie X, Luo D, et al. (2017). The role of halofuginone in fibrosis: more to be explored?. J Leukoc Biol 102:1333–45. 10.1189/jlb.3RU0417-148RR 28986385 [DOI] [PubMed] [Google Scholar]
- MacDonald NJ, Shivers WY, Narum DL, et al. (2001). Endostatin binds tropomyosin. A potential modulator of the antitumor activity of endostatin. J Biol Chem 276:25190–6. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, et al. (1999). Signaling from rho to the actin cytoskeleton through protein kinases rock and lim-kinase. Science (New York, N.Y.) 285:895–8. [DOI] [PubMed] [Google Scholar]
- Marcu KB, Otero M, Olivotto E, et al. (2010). Nf-kappab signaling: Multiple angles to target oa. Curr Drug Targets 11:599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Wotton D. (2000). Transcriptional control by the tgf-beta/smad signaling system. Embo J 19:1745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Blain SW, Lo RS. (2000). Tgfbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309. [DOI] [PubMed] [Google Scholar]
- Masszi A, Ciano CD, Sirokmany G, et al. (2003). Central role for rho in tgf-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 284:F911–924. [DOI] [PubMed] [Google Scholar]
- McClelland AD, Herman-Edelstein M, Komers R, et al. (2015). Mir-21 promotes renal fibrosis in diabetic nephropathy by targeting pten and smad7. Clinical Science (London, England: 1979) 129:1237–49. [DOI] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY. (2016). TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–38. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Miyazono K. (2017). Regulation of tgf-beta family signaling by inhibitory smads. Cold Spring Harbor Perspect Biol 9. doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, et al. (2008). Autophagy fights disease through cellular self-digestion. Nature 451:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris D, Chakedis J, Rahnemai-Azar AA, et al. (2017). Postoperative abdominal adhesions: Clinical significance and advances in prevention and management. J Gastrointest Surg 21:1713–22. [DOI] [PubMed] [Google Scholar]
- Morishita H, Mizushima N. (2019). Diverse cellular roles of autophagy. Annu Rev Cell Dev Biol 35:453–75. [DOI] [PubMed] [Google Scholar]
- Nagatoya K, Moriyama T, Kawada N, et al. (2002). Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int 61:1684–95. [DOI] [PubMed] [Google Scholar]
- Neill T, Kapoor A, Xie C, et al. (2021). A functional outside-in signaling network of proteoglycans and matrix molecules regulating autophagy. Matrix Biol 100-101:118–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TM, Subramanian IV, Xiao X, et al. (2009). Endostatin induces autophagy in endothelial cells by modulating beclin 1 and beta-catenin levels. J Cell Mol Med 13:3687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, et al. (2016). Emt: 2016. Cell 166:21–45. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Mlakar L, Takihara T, et al. (2015). An endostatin-derived peptide orally exerts anti-fibrotic activity in a murine pulmonary fibrosis model. Int Immunopharmacol 28:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S. (2009). The nf-kappab family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku M, Sakai Y. (2018). Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays 40:e1800008. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, et al. (1997). Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–85. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Lennartsson J. (2018). The pdgf/pdgfr pathway as a drug target. Mol Aspects Med 62:75–88. [DOI] [PubMed] [Google Scholar]
- Pehrsson M, , Bager CL, , Karsdal MA.Biochemistry of Collagens, Laminins and Elastin, 2019, Second Edition, Chapter 18-Type XVIII collagen:149-162. doi.org/ 10.1016/B978-0-12-817068-7.00018-5. [DOI] [Google Scholar]
- Piek E, Ju WJ, Heyer J, et al. (2001). Functional characterization of transforming growth factor beta signaling in smad2- and smad3-deficient fibroblasts. J Biol Chem 276:19945–53. [DOI] [PubMed] [Google Scholar]
- Poluzzi C, , Iozzo RV, , Schaefer L. (2016). Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev 97:156–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn M, Veikkola T, Kukk-Valdre E, et al. (2001). Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A 98:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Li Y, Chen Y, et al. (2019). Endostatin attenuates pdgf-bb- or tgf-beta1-induced hscs activation via suppressing rhoa/rock1 signal pathways. Drug design. DDDT Volume 13:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AG, McKeown S, Rathinam S, et al. (2009). Soluble endostatin is a novel inhibitor of epithelial repair in idiopathic pulmonary fibrosis. Thorax 64:156–61. [DOI] [PubMed] [Google Scholar]
- Rong B, Yang S, Li W, et al. (2012). Systematic review and meta-analysis of endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World Journal of Surgical Oncology 10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Clark CJ, Mohebali K, et al. (2004). Reactive oxygen species and egr-1 gene expression in surgical postoperative peritoneal adhesions. World J Surg 28:316–20. [DOI] [PubMed] [Google Scholar]
- Satoh S, Yamaguchi T, Hitomi A, et al. (2002). Fasudil attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Eur J Pharmacol 455:169–74. [DOI] [PubMed] [Google Scholar]
- Shi H, Huang Y, Zhou H, et al. (2007). Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood 110:2899–906. [DOI] [PubMed] [Google Scholar]
- Shichiri M, Hirata Y. (2001). Antiangiogenesis signals by endostatin. Faseb J 15:1044–53. [DOI] [PubMed] [Google Scholar]
- Sil S, Periyasamy P, Thangaraj A, et al. (2018). Pdgf/pdgfr axis in the neural systems. Mol Aspects Med 62:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AG, Thomas B, Koff J. (2018). Tgf-beta: Master regulator of inflammation and fibrosis. Respirology (Carlton, Vic.) 23:1096–7. [DOI] [PubMed] [Google Scholar]
- Stone RC, Pastar I, Ojeh N, et al. (2016). Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, et al. (2003). Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A 100:4766–71. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Sugiyama A, , Hirano Y, , Okada M, , Yamawaki H. (2018). Endostatin Stimulates Proliferation and Migration of Myofibroblasts Isolated from Myocardial Infarction Model Rats. Int J Mol Sci 19: 10.3390/ijms19030741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Reddy MA, Yuan H, et al. (2010). Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol 21:2069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabruyn SP, Memet S, Ave P, et al. (2009). Nf-kappab activation in endothelial cells is critical for the activity of angiostatic agents. Mol Cancer Ther 8:2645–54. [DOI] [PubMed] [Google Scholar]
- Tada S, Iwamoto H, Nakamuta M, et al. (2001). A selective rock inhibitor, y27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. J Hepatol 34:529–36. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Lee SM, Dongqing Z, et al. (2020). Endothelial autophagy deficiency induces il6 - dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 16:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Maeshima Y, Ichinose K, et al. (2007). Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int 71:227–38. [DOI] [PubMed] [Google Scholar]
- Tang C, Livingston MJ, Liu Z, et al. (2020). Autophagy in kidney homeostasis and disease. Nat Rev Nephrol 16:489–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Broek RP, Issa Y, van Santbrink EJ, et al. (2013). Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 347:f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Zhou Y, Gaggar A, et al. (2014). Fibrosis: Ultimate and proximate causes. J Clin Invest 124:4673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjin Tham Sjin RM, Satchi-Fainaro R, Birsner AE, et al. (2005). A 27-amino-acid synthetic peptide corresponding to the nh2-terminal zinc-binding domain of endostatin is responsible for its antitumor activity. Cancer Res 65:3656–63. [DOI] [PubMed] [Google Scholar]
- Tong Y, Zhong K, Tian H, et al. (2010). Characterization of a monopeg20000-endostar. Int J Biol Macromol 46:331–6. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Ligresti G, Hilscher MB, et al. (2018). Mechanosensing and fibrosis. J Clin Invest 128:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun L, Nie Y, et al. (2020). Protein kinase c δ (PKCδ) Attenuates Bleomycin Induced Pulmonary Fibrosis via Inhibiting NF-κB Signaling Pathway. Front Physiol 11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom SA, Alitalo K, Keski-Oja J. (2002). Endostatin associates with integrin alpha5beta1 and caveolin-1, and activates src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Research 62:5580–9. [PubMed] [Google Scholar]
- Wickstrom SA, Alitalo K, Keski-Oja J. (2003). Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of rhoa activity. J Biol Chem 278:37895–901. [DOI] [PubMed] [Google Scholar]
- Wickstrom SA, Alitalo K, Keski-Oja J. (2005). Endostatin signaling and regulation of endothelial cell-matrix interactions. Adv Cancer Res 94:197–229. [DOI] [PubMed] [Google Scholar]
- Wickstrom SA, Veikkola T, Rehn M, et al. (2001). Endostatin-induced modulation of plasminogen activation with concomitant loss of focal adhesions and actin stress fibers in cultured human endothelial cells. Cancer Res 61:6511–6. [PubMed] [Google Scholar]
- Wynn TA. (2007). Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Yang S, Hao J, et al. (2015). Endostar attenuates melanoma tumor growth via its interruption of b-fgf mediated angiogenesis. Cancer Lett 359:148–54. [DOI] [PubMed] [Google Scholar]
- Xu P, Liu J, Derynck R. (2012). Post-translational regulation of tgf-beta receptor and smad signaling. FEBS Lett 586:1871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takihara T, Chambers RA, et al. (2012). A peptide derived from endostatin ameliorates organ fibrosis. Sci Transl Med 4:136ra71–ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Guo Y, Chen B, et al. (2015). Microrna-17-5p activates hepatic stellate cells through targeting of smad7. Lab Invest 95:781–9. [DOI] [PubMed] [Google Scholar]
- Yu L, Border WA, Huang Y, et al. (2003). Tgf-beta isoforms in renal fibrogenesis. Kidney Int 64:844–56. [DOI] [PubMed] [Google Scholar]
- Yuan W, Varga J. (2001). Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves smad3. J Biol Chem 276:38502–10. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen L, Zhu H, et al. (2020). PGC-1α regulates autophagy to promote fibroblast activation and tissue fibrosis. Ann Rheum Dis 79:1227–33. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wang Y, Qiu T, et al. (2020). Autophagy, an important therapeutic target for pulmonary fibrosis diseases. Clin Chim Acta 502:139–47. [DOI] [PubMed] [Google Scholar]