Abstract
Hemianopia induced by unilateral visual cortex lesions can be resolved by repeatedly exposing the blinded hemifield to auditory–visual stimuli. This rehabilitative “training” paradigm depends on mechanisms of multisensory plasticity that restore the lost visual responsiveness of multisensory neurons in the ipsilesional superior colliculus (SC) so that they can once again support vision in the blinded hemifield. These changes are thought to operate via the convergent visual and auditory signals relayed to the SC from association cortex (the anterior ectosylvian sulcus [AES], in cat). The present study tested this assumption by cryogenically deactivating ipsilesional AES in hemianopic, anesthetized cats during weekly multisensory training sessions. No signs of visual recovery were evident in this condition, even after providing animals with up to twice the number of training sessions required for effective rehabilitation. Subsequent training under the same conditions, but with AES active, reversed the hemianopia within the normal timeframe. These results indicate that the corticotectal circuit that is normally engaged in SC multisensory plasticity has to be operational for the brain to use visual–auditory experience to resolve hemianopia.
Keywords: colliculus, ectosylvian, hemianopia, multisensory, rehabilitation
Introduction
Large unilateral lesions of visual cortex can produce an enduring and profound blindness in contralesional space (“hemianopia”; Zhang et al. 2006; Goodwin 2014). Using a cat model, we found that recovery from hemianopia could be induced by repeatedly presenting spatiotemporally congruent auditory and visual cues in the blinded hemifield (Jiang et al. 2015). Within weeks of beginning this “training,” animals began responding briskly to stimuli throughout the visual field and, in some cases, preferred visual cues in the previously blinded hemifield to those in the intact hemifield (Dakos et al. 2019, 2020). The rehabilitative paradigm proved to be effective even when the animal was anesthetized during the training sessions (Jiang et al. 2020). However, exposure to individual visual and auditory stimuli, or to cross-modal stimuli out of spatiotemporal register, did not induce visual rehabilitation (Jiang et al. 2015; Dakos et al. 2020; Stein and Rowland 2020).
Although the neurobiological bases for this striking recovery of function are not yet fully understood, there are good reasons to suspect that multisensory (i.e., visual–auditory) neurons in the deep ipsilesional superior colliculus (SC), and their inputs from association cortex (i.e., the anterior ectosylvian sulcus, AES) play a critical role (Jiang et al. 2015; see also Krauzlis et al. 2013; Hadid and Lepore 2017; Krauzlis et al. 2018; Hu et al. 2019; Kinoshita et al. 2019; Lee et al. 2020; Wang et al. 2020a). Superficial layer SC neurons retain their visual responsiveness despite the hemianopia, but there is widespread loss of visual responsiveness in neurons in the deeper and multisensory layers (Jiang et al. 2015, 2020). These deeper layer neurons mediate overt detection and localization behavior (Sprague and Meikle 1965; Casagrande et al. 1972; Stein et al. 1976; Stein and Clamann 1981; Sahibzada et al. 1986; Sparks 1986; Munoz et al. 1991; Paré et al. 1994; Lomber et al. 2001; Sommer and Wurtz 2008; Gandhi and Katnani 2011; Wolf et al. 2015), and retain their auditory (and/or somatosensory) responsiveness after the lesion (Jiang et al. 2015). It is theorized that repeated co-activation of AES visual and auditory presynaptic elements with the multisensory target neuron engage Hebbian-like mechanisms that enhance the sensitivity of the target neurons to their individual sensory (e.g., visual) channels (Yu et al. 2009; Cuppini et al. 2012). When repeatedly stimulated by congruent visual–auditory stimuli, these mechanisms can substantially alter the responsiveness of the individual channels, in some cases rendering subthreshold inputs suprathreshold (Yu et al. 2013a).
If as hypothesized, the rehabilitative training paradigm crucially involves AES to support the return of vision in hemianopic animals, deactivation of AES should render it ineffective. Alternatively, if visual recovery relies entirely on changes within the SC, or other visually-responsive or multisensory areas such as the substantia nigra or suprageniculate nucleus (Benedek et al. 1997; Nagy et al. 2006), deactivation of AES during training will not alter its effectiveness. Here, we addressed this question directly.
Materials and Methods
Data from 4 adult mongrel cats (3 males and 1 female) were obtained. The animals were obtained from a USDA-licensed commercial animal breeding facility (Liberty Labs). All procedures were performed in compliance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council of the National Academies) and approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. Animals were housed with ample living space and opportunities for behavioral enrichment, and efforts were made to minimize the number of animals used. Thus, “control” observations came from 2 of the animals whose recovery from hemianopia (without cortical deactivation) was previously reported (Jiang et al. 2020).
Behavioral task
The behavioral perimetry apparatus and test of visual function used here (Fig. 1) have been previously described (Jiang et al. 2015, 2020). Animals were motivated by food rewards and were maintained within 85% of baseline weight. After acclimation to the apparatus, they were trained with randomly interleaved trials containing an individual visual or auditory stimulus, or no stimulus (“catch trials”). At the beginning of each trial a handler gently restrained the animal so that its head and eyes were directed forward, toward an opening in the wall from which food was delivered previously so that the animal would fixate there. The trial was initiated when fixation was acquired.
Figure 1 .
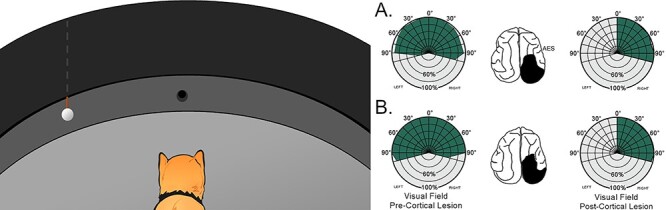
The visual localization task. Left: Animals were first trained to fixate a food reward at 0°, and then to approach a ping pong ball lowered from behind an opaque curtain in 15° (randomly presented) steps of eccentricity from 0° to ±105°. Little training was required to master this task, and each animal responded with high reliability to the stimulus at all locations. Right: Visual detection and orientation performance before (pre-) and after (post-) the hemianopia-inducing lesion in 2 animals (A, B) implanted with cryogenic coils in AES. Each circle in the polar plot represents 20% correct performance (shown in green), and each radial line = 15° of eccentricity. Schematics of the brain illustrate cortical lesion in black. Note the absence of contralesional visual responses after the lesion.
The visual stimulus was a white ping pong ball on the end of a stick introduced at a randomized location from −105° (left) to 105° (right; 15° intervals) by being lowered below an obscuring black curtain (Fig. 1, left). Auditory stimuli were produced by rapping the ball against the side of the arena behind the curtain. Animals were rewarded when they approached the location of the stimulus, or when they remained still (“No-Go”) on catch trials. Training was complete when responses were correct on 85% of trials for each trial type. This task requires very little training, and animals typically learn to perform at criterion in a few days (Jiang et al. 2015, 2020).
Surgical Procedures and Cortical Lesion
Each animal was sedated with acepromazine (0.1–0.4 mg/kg, i.m.) and buprenorphine (0.005–0.01 mg/kg, i.m.), given dexamethasone (1 mg/kg, intramuscular [i.m.]) to minimize cerebral edema, and the saphenous vein was catheterized. They were then anesthetized with pentobarbital sodium (22–30 mg/kg, i.v., followed by 1–3 mg/kg/h CRI). After loss of corneal blink reflexes, it was placed in a stereotaxic head-holder on a heating pad. An endotracheal ventilation tube was inserted. Physiological saline (50–200 mL), subcutaneous and/or intravenous was administered to compensate for fluid loss. Core body temperature, expiratory CO2, blood pressure, and heart rate were monitored (SurgiVet Advisor, Smith Medical) and maintained within normal physiological limits. The surgical site was shaved and scrubbed with Betadine. A scalp incision was made, and a craniotomy exposed the cortical areas to be removed. After reflecting the dura, the gray matter was extirpated by subpial aspiration. All contiguous areas of visual cortex were removed in either the left (n = 2) or right (n = 2) hemisphere (Brodmann areas: 17, 18, 19, 20a, 20b, 21a, 21b, DLS, VLS, PS, PMLS, PLLS, AMLS, ALLS, 5, 7, and SVA; Rosenquist and Palmer 1971). The results of previous experiments reveal that similar contralesional blindness is obtained regardless of which hemisphere is damaged (Jiang et al. 2015; Dakos et al. 2020).
In 1 animal an array of 4 Epoxylite-coated, tungsten monopolar electrodes (100–500-kΩ, electrode separation ∼1 mm) was lowered to the SC while recording auditory- or somatosensory-evoked activity. The electrodes were oriented rostral-to-caudal in an attempt to find and record from an area of the SC responsive to the training location in visual–auditory space (i.e., 45° eccentric). This was achieved best by the second electrode in the array. When the electrode array reached a depth at which multisensory recordings were obtained, it was cemented in place and its leads were fed through the head holder.
Following the ablation and implantation, the region was then packed with Gelfoam, the cranial bone plate was replaced and the scalp incision was closed with sutures. Analgesia (buprenorphine 0.01 mg/kg, i.m.) was provided BID for 48 h and an antibiotic (cefazolin, 20–30 mg/kg, i.m. bid) was administered for 7–10 days. Following surgery, animals exhibited a characteristic, albeit transient, tendency for ipsiversive circling (for 1–3 days). They were unresponsive to manually presented visual stimuli (e.g., laser pointers and threatening gestures) within the contralesional hemifield, but responded to these stimuli when presented in the ipsilesional hemifield. Contralesional auditory and somatosensory responsiveness (assessed by clicks, snaps, and tactile stimuli) was initially compromised, but recovered within the first week (see also, Sprague 1966; Wallace et al. 1990; Jiang et al. 2015). However, contralesional blindness persisted. After a 2.5–3-month observational period, multisensory training began with or without cryogenic deactivation of AES.
Cryogenic Deactivation of AES
Two animals were implanted with cryogenic coils in ipsilesional AES (right hemisphere) as described previously (Lomber et al. 1999; Jiang et al. 2001, 2002). The coils were fabricated from loops of 20–21 gauge stainless steel hypodermic tubing that were custom-fitted to the cortical curvature of each animal. Before implantation, each animal was anesthetized with a dose of ketamine hydrochloride (20–30 mg/kg, i.m.) and acepromazine maleate (0.1 mg/kg, i.m.). It was then intubated, and anesthesia was maintained with isoflurane (2–4% to effect, followed by 1–3% to maintain). The animal was placed in a stereotaxic head-holder and maintained as described above. A craniotomy exposed AES, the dura was opened, and the sulcal walls of the AES were gently separated to allow insertion of the cooling coils. Coil stems were fixed to the skull with surgical screws and dental cement. Previous studies showed that this technique induces no apparent damage to underlying neurons and produces a limited area of cortical deactivation that is restricted to AES (Jiang et al. 2001). The area overlying the implant was packed with Gelfoam. A stainless-steel well was fitted to the craniotomy and anchored to the skull with stainless-steel screws and dental acrylic, and a head-holder was similarly secured on the top of the head (McHaffie and Stein 1983). After a 10–14-day recovery period, multisensory training began. During an experiment, cryogenic blockade of cortical activity was induced by circulated cold water (~0°C) through the coils. This decreases cortical temperature to ~ 10°C near the cooling coil, but baseline temperature (36–38°C) is regained within minutes of ceasing the blockade (Jiang et al. 2001; Jiang and Stein 2003). Deactivation is conducted while animals are anesthetized within time-limited “blocks” of < 45 min, a technique that has been used successfully in the past. It avoids permanent damage to the underlying tissue and has no lasting effects on orientation behavior (Jiang et al. 2001, 2002; Jiang and Stein 2003; Alvarado et al. 2007).
Multisensory Training
Following the methods of Jiang et al. (2020), each animal was anesthetized and paralyzed during each weekly multisensory training session using ketamine hydrochloride (20–30 mg/kg, i.m.) and acepromazine maleate (0.05–0.1 mg/kg, i.m.). An endotracheal tube was inserted, and the animal was artificially respired. Its head was secured by attaching the head-holder to the stereotaxic frame, and paralysis was induced with pancuronium bromide (0.1 mg/kg, i.v.) to fix the eyes and pinnae. Anesthesia, paralysis, and hydration were maintained via continuous intravenous infusion of ketamine hydrochloride (5–10 mg/kg/h) and pancuronium bromide (0.04–0.1 mg/kg/h) in 5% dextrose Ringer’s solution (3–6 mL/h). Vital signs were monitored and maintained continuously (Digital Vital Signs Monitor, SurgiVet V9200). The pupils were dilated with ophthalmic atropine sulfate (1%), and the eyes were fitted with contact lenses to focus them and prevent corneal drying.
The animal was repeatedly exposed to contralesional, spatiotemporally concordant visual–auditory stimuli (600 exposures at 6-s intervals/session divided into 3 blocks of 200). The stimulus delivery apparatus consisted of a board of LEDs and speakers to provide visual and auditory stimulation. Visual stimuli were created by activating 2 white LEDs displaced 10-mm vertically (the top ~ 2° above the animal’s eyes) and each covered with a white plastic hemisphere (half a ping pong ball) to create a diffuse circle of light (5° diameter, ~ 13.8 cd/m2, and background ~ 0.75 cd/m2). Auditory stimuli were broadband bursts of noise (75-dB SPL and background 48.4–52.7-dB SPL) delivered from speakers between the 2 LEDs. Each trial began with the illumination of a visual stimulus at 0° for 1000 ms, followed by a 1500-ms visual stimulus and 100-ms auditory stimulus co-localized at 45° of eccentricity in the contralesional field. When implemented, AES deactivation (water temperature was continuously monitored) began 5 min before the presentation of training stimuli in a block and was rewarmed for 15 min after each block.
Testing for Visual Recovery
Each animal was tested in the behavioral perimetry apparatus for visual recovery on non-training weekdays. These tests consisted of ~5 presentations of visual stimuli (the ping pong ball) at each location between −105° and 105° of eccentricity (15° increments). Other features of testing (e.g., trial initiation) were the same as in training. AES was always active during testing.
In the control animals, training sessions terminated as soon as responses were elicited by visual cues at any location in the blinded hemifield. This is because the effective field has been shown to expand thereafter without further training (see Jiang et al. 2015; Dakos et al. 2020; Jiang et al. 2020). The same paradigm was followed in the animals in which AES was cryogenically deactivated during the training sessions. However, in these cases the sessions continued without rehabilitative success for 6 and 8 weeks. Subsequent sessions of multisensory training without AES deactivation, and with the accompanying tests for the return of contralesional visual responsiveness, began immediately thereafter.
Physiological Recordings
As noted above, 1 animal was implanted with chronic indwelling electrodes in order to confirm that the training paradigm was initiating the plastic changes in SC sensory responsiveness previously documented after repeated visual–auditory stimuli (Yu et al. 2013b). After the training paradigm reached a point at which recovery of the visual responses of SC neurons was expected, probe tests were initiated to determine whether such stimulation could enhance the visual sensitivity of SC neurons. The probe consisted of 3 stages: preliminary tests to measure baseline visual and auditory responsiveness, followed by 50 repetitions of a combined visual–auditory stimulus, followed by final visual tests to measure induced changes in visual responsiveness. The visual probe was a 5° wide X 2° tall 32 cd/m2 light bar swept downward (80°/s) across the responsive area and then, 3 s later, swept upward, producing 2 effective visual stimuli separated in time. The auditory stimulus was a 70-dB SPL broadband noise burst presented against a 55-dB background. In the visual–auditory repetitions, the auditory stimulus was synchronized with the first visual stimulus. All responses were bandpass amplified, displayed on an oscilloscope, and subsequently routed to computer disc using a 1401+ hardware acquisition system (CED Systems) running CED Spike2 software. Impulses were identified with a criterion of 3X waveform amplitude over background activity. Stimulation onset and duration were recorded concurrently with the digitized neural data. Stimulus-driven response rates (reported as mean ± SEM impulses/s) were estimated from the rate observed in the 500-ms poststimulus window minus the spontaneous rate observed in the 500-ms pre-stimulus window. Significant responses (stimulus-driven rate > 0) and significant differences between response rates were determined with t-tests.
Results
Hemianopia after the Cortical Lesion
In prescreening evaluations, animals easily detected and reacted to visual stimuli at every tested location on both sides of space. They approached food and objects of interest and withdrew from rapidly approaching objects and threatening gestures from all regions. However, immediately following the unilateral lesion of visual cortex, they became unresponsive to all visual stimuli in contralesional space. This included highly salient moving visual stimuli (e.g., moving spots generated by a laser pointer) and threatening gestures that had previously elicited rapid withdrawal responses. Characteristic ipsiversive “circling” behavior and diminished auditory and somatosensory responsiveness were also observed immediately following their recovery from surgery. Auditory and somatosensory deficits resolved within days, whereas unresponsiveness to contralesional visual stimuli remained stable over the next several months. During most contralesional stimulus presentations, the animals remained standing at the start position (No-Go response), as found in previous studies (Jiang et al. 2015; Dakos et al. 2019, 2020; Jiang et al. 2020).
AES Must Be Active for Effective Multisensory Rehabilitation
Control animals receiving multisensory training in the absence of AES deactivation showed an onset of visual recovery to centrally located stimuli after 4 weeks. An expansion of the effective visual field then ensued without further training, finally encompassing the entire contralesional field within 6 weeks (Jiang et al. 2020). In contrast, animals in which AES was deactivated during this training procedure failed to show visual recovery. They remained hemianopic and their contralesional blindness persisted. Training with AES deactivation was continued beyond the initial 4-week period generally required in this context (Jiang et al. 2020) for an additional 2 weeks in 1 animal, and an additional 4 weeks in the other (Fig. 2). In neither case were there any signs of visual responsiveness in the blinded hemifield.
Figure 2 .
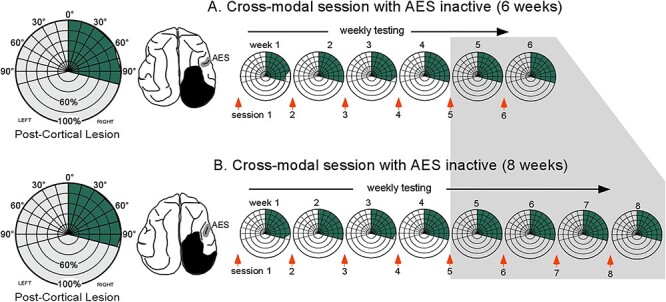
Multisensory training with AES deactivated was ineffective in resolving hemianopia. Hemianopic animals given 6 (A) or 8 (B) weeks of training with spatiotemporally congruent visual–auditory stimuli in the blinded hemifield (left) while the ipsilesional (right) AES was deactivated. On the left are radial plots showing visual localization performance prior to rehabilitative training. On the right are radial plots of visual performance in which data were averaged over each week of rehabilitative training. Note the absence of visual recovery. Shaded region indicates testing points at which the control animals had already recovered visual responsiveness. Other conventions are the same as in Fig 1.
The animals then underwent a second round of multisensory training, but now without deactivation of AES. Visual responsiveness began to return after 4 weeks, a timeline consistent with that of animals without cryogenic coils or AES deactivation (Jiang et al. 2020). Visual recovery followed a characteristic central-to-peripheral expansion, finally encompassing the entire contralesional hemifield before the end of the sixth week (Fig. 3). The re-established contralesional visual responsiveness extended to all tested visual stimuli, yielding approach and withdrawal patterns that matched those in the intact field. Once begun, the timeline for recovery was the same as that observed previously, underscoring the absence of any apparent impact of the previous training during AES deactivation.
Figure 3 .
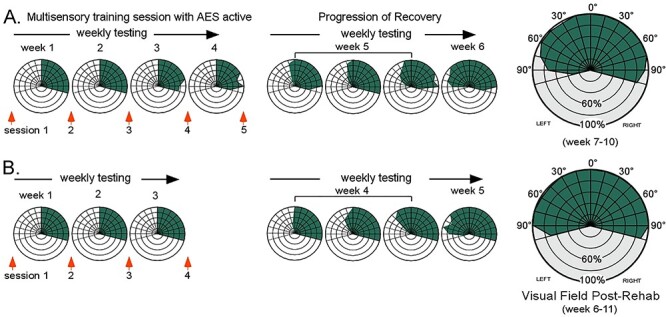
Subsequent multisensory training without AES deactivation resolved hemianopia. The same training that was previously ineffective when AES was deactivated now rehabilitated hemianopia within the normal time period in both animals (A, B). Visual responses first appeared at central locations within 4 weeks of training onset, and then progressed peripherally without further training. By week 6 vision had been restored throughout the contralesional hemifield and remained stable thereafter. Conventions are the same as previous figures.
To examine whether the plastic changes in SC neuronal responsiveness reported after multisensory stimulation in normal animals (see Yu et al. 2013a) could be found in these cortically damaged animals, single and “probe” recordings from an implanted electrode were taken in an anesthetized animal after sufficient training to expect the onset of visual recovery. This animal was previously exposed to cross-modal stimuli while AES was deactivated, but as noted earlier, this was unsuccessful in restoring vision in the contralesional hemifield (Fig 2). The training paradigm was continued with AES active, and probe recordings were conducted in week 5 (Fig 3). An isolated neuron was initially significantly (P = 0.0055) responsive to auditory stimuli (11.6 ± 3.2 impulses/s), but not to visual stimuli. Thereafter, 50 stimulus repetitions of the cross-modal stimulus (auditory+downward-moving visual stimulus) were presented with AES active. Each cross-modal stimulus was followed by an upward moving visual stimulus. During these presentations, the response to the visual–auditory combination (21.0 ± 1.7 impulses/s) was significantly (P = 0.029) greater than to the auditory stimulus alone, and the neuron began responding to the upward-moving visual stimulus as well. Tests following the repetitions of the cross-modal stimuli revealed significant responses to both the downward (8.2 ± 1.4 impulses/s, P < 2.6E−4) and upward (17.4 ± 2.8 impulses/s, P < 1.8E−4) moving visual stimuli. The on-line development of this enhanced visual sensitivity during repetitions of the cross-modal stimulus was consistent with the plastic changes in SC responsiveness previously reported in normal animals (see Yu et al. 2013a). Similar changes in other SC neurons occurring while cross-modal training was taking place (i.e., both before and after this recording) represent a possible substrate for supporting visual responsiveness in the rehabilitated animal.
Discussion
The loss of contralesional vision following unilateral damage to visual cortex occurs despite the sparing of the visuomotor circuitry of ipsilesional SC, which is far from the site of physical damage. However, deep layer SC neurons outside of the rostral fixation zone (Munoz and Wurtz 1995), unlike their counterparts in the overlying superficial layers, become unresponsive to visual stimuli after the cortical lesion (Jiang et al. 2015). This renders them unable to support visual responsiveness that might otherwise be possible in the absence of visual cortex (Kinoshita et al. 2019). Presumably, their inactivation here is due to the dominance of interhemispheric inhibition created by the loss of the counterbalancing excitatory inputs from visual cortex (Sprague 1966; Wallace et al. 1989, 1990; Durmer and Rosenquist 2001).
Seminal work by Sprague and colleagues demonstrated that disrupting these interhemispheric inhibitory inputs by strategic lesions in the contralateral hemisphere (e.g., lesioning the opposite SC or intercollicular commissure) overcomes the imbalance and resolves the hemianopia (Sprague 1966; Wallace et al. 1989; Wallace et al. 1990). However, the multisensory training paradigm appears to overcome such inhibition and ameliorates hemianopia without the need for surgical intervention in cats as well as humans (Làdavas 2008; Leo et al. 2008; Dundon et al. 2015a, 2015b). That the brain’s orientation mechanics to nonvisual stimuli remain intact in hemianopia minimizes the possibility that the training paradigm corrects a significant motor impairment (see also Frassinetti et al. 2005). It is also unlikely that this rehabilitative training paradigm involves the retraining of an association between visual stimuli and an orientation response: using a spatially incongruent but predictive nonvisual stimulus to reliably elicit orientation to an ineffective visual stimulus in the blinded field does not restore visual capabilities (Dakos et al. 2020). Nor does the paradigm correct a simple attentional defect: repeatedly “priming” the animal to the location of an unperceived visual stimulus with a spatially congruent auditory stimulus preceding that visual stimulus fails to induce visual recovery (Dakos et al. 2020).
Rather, the training paradigm appears to ameliorate a sensory deficit linked to the loss of visual responsiveness in deeper layer SC neurons, most of which are multisensory (Meredith and Stein 1985; Burnett et al. 2004, 2007). To do so, the paradigm requires the repeated presentation of spatiotemporally congruent pairs of visual–nonvisual stimuli in the blinded field. These stimuli are transduced into convergent, temporally-overlapping visual and nonvisual input signals to the multisensory layers of the SC and, in the normal brain, typically elicit enhanced sensory responses (spatially or temporally disparate stimuli do not; Stein and Meredith 1993; Rowland et al. 2007a, 2007b; Stein et al. 2014; Miller et al. 2017).
The major route for relaying these convergent tectopetal sensory signals is the AES, and its deactivation eliminates multisensory enhancement in normal animals (Wilkinson et al. 1996; Jiang et al. 2001, 2002, 2006, 2007; Alvarado et al. 2009). However, AES has a significant impact on other multisensory SC processes. Most important in the current context is its mediation of multisensory (e.g., visual–nonvisual) plasticity. AES influences are essential for the brain to use visual–auditory experiences to craft the multisensory integration capabilities of these neurons during early life, and for modifications in their capabilities during adulthood (Yu et al. 2013a; Rowland et al. 2014; Wang et al. 2020b). The changes that have been observed are consistent with the operation of a Hebbian-like learning rule applied to cross-modal tectopetal inputs (Yu et al. 2009; Cuppini et al. 2012; Yu et al. 2013b), and may also operate on AES inputs during the multisensory training program to restore the visual responsiveness of multisensory SC neurons.
Electrophysiological evidence was consistent with the operation of this mechanism when AES was active in the current experiment. Responses to the visual modality were significantly enhanced (P < 0.01) by repeated exposure to visual–auditory pairings: a marginally effective visual stimulus was rendered significantly more robust, and an ineffective visual stimulus was rendered effective. This provides a plausible mechanism for the restoration of visual responsiveness, would also explain why rehabilitation is effective even under anesthesia, and would be consistent with other reorganizational multisensory dynamics observed in compromised populations (Collignon et al. 2011). It may also explain why hemianopia does not resolve readily under normal, non-laboratory conditions. The latter seems surprising given that the normal environment is rife with congruent visual–auditory stimuli everywhere in space and seems a far richer multisensory environment than a training paradigm using seemingly meaningless flashes of light and broadband noise bursts. The crucial feature in the rehabilitative training, a feature also shared by long-term potentiation, is the high-frequency repetition of the same event with a low-frequency of intervening stimuli (Dan and Poo 2004; Caporale and Dan 2008; Feldman 2012). Normal environments, in contrast, provide a far greater variety of experiences, each of which can produce different patterns of correlated and uncorrelated neural activity, and consequently reduce the effectiveness of a simple unsupervised learning algorithm (see also Xu et al. 2017).
The present results are consistent with the hypothesis that rehabilitation reflects the strengthening of visual influence from AES, thereby resulting in a gain recalibration that allows its inputs to activate their postsynaptic SC target neurons and, in turn, support overt visual behavior. Also, consistent with this hypothesis is prior evidence showing that damaging AES in rehabilitated animals reinstates their hemianopia (Jiang et al. 2015).
It is important to note that the SC is part of an extensive subcortical visual circuit involving a variety of nuclei (e.g., suprageniculate nucleus, pulvinar, and basal ganglia) that also have multisensory properties (Chalupa and Fish 1978; McHaffie et al. 1993; Benedek et al. 1997; McHaffie et al. 2005; Nagy et al. 2006; Rokszin et al. 2010; Nagy et al. 2011; Benedek et al. 2019). It is likely that the training paradigm also induces changes in nuclei elsewhere in the circuit that facilitate the effective use of AES–SC visual information. It is also possible that changes in the internal reorganization within AES, prompted by the training paradigm, help reinstate its ability to relay visual information. Although AES–SC inputs are primarily unisensory in the intact animal (Wallace et al. 1993), reorganizational changes in the principal visual and auditory regions of AES (e.g., see Rauschecker and Korte 1993; Rauschecker 1996) may alter the modality specificity of its descending efferents. Further physiological studies are necessary to target better the sites (s) at which the multisensory training paradigm operates.
If, as hypothesized, the impact of the multisensory rehabilitative training paradigm (at any site) effectively restores responsiveness throughout the visual field via an unsupervised Hebbian mechanism, manipulations that enhance the operation of such an algorithm should also enhance the effectiveness of the rehabilitation. For example, pharmacological agents or neuromodulators that enhance LTP, or degrade or block it (Frémaux and Gerstner 2015; Brzosko et al. 2019), should have parallel effects on the efficacy of hemianopic rehabilitation by this method, and/or an alert brain might better process the nature and impact of these inputs. In short, any mechanisms that can enhance the synchronization of AES–SC inputs, amplify presynaptic and postsynaptic responsiveness, or increase their reliability, should also enhance the effectiveness of the procedure. The therapeutic value of these and other manipulations will become important to explore as converging evidence continues to implicate the individual multisensory neuron as a key element in the restoration of sight in hemianopia.
Contributor Information
Huai Jiang, Department of Neurobiology and Anatomy, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Terrence R Stanford, Department of Neurobiology and Anatomy, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Benjamin A Rowland, Department of Neurobiology and Anatomy, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Barry E Stein, Department of Neurobiology and Anatomy, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Funding
This research was supported by National Institutes of Health (National Eye Institute) (grant EY026916).
Notes
We thank Nancy London for technical assistance. Conflict of Interest: The authors declare no conflict of interest.
References
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. 2009. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci. 29(20):6580–6592. doi: 10.1523/JNEUROSCI.0525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. 2007. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 27(47):12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Keri S, Nagy A, Braunitzer G, Norita M. 2019. A multimodal pathway including the basal ganglia in the feline brain. Physiol Int. 106(2):95–113. doi: 10.1556/2060.106.2019.09. [DOI] [PubMed] [Google Scholar]
- Benedek G, Perény J, Kovács G, Fischer-Szátmári L, Katoh YY. 1997. Visual, somatosensory, auditory and nociceptive modality properties in the feline suprageniculate nucleus. Neuroscience. 78(1):179–189. doi: 10.1016/s0306-4522(96)00562-3. [DOI] [PubMed] [Google Scholar]
- Brzosko Z, Mierau SB, Paulsen O. 2019. Neuromodulation of spike-timing-dependent plasticity: past, present, and future. Neuron. 103(4):563–581. doi: 10.1016/j.neuron.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Chaponis D, Wallace MT. 2004. Superior colliculus lesions preferentially disrupt multisensory orientation. Neuroscience. 124(3):535–547. doi: 10.1016/j.neuroscience.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Perrault TJ Jr, Wallace MT. 2007. Excitotoxic lesions of the superior colliculus preferentially impact multisensory neurons and multisensory integration. Exp Brain Res. 179(2):325–338. doi: 10.1007/s00221-006-0789-8. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. 2008. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. 1972. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science. 177(4047):444–447. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Fish SE. 1978. Response characteristics of visual and extravisual neurons in the pulvinar and lateral posterior nuclei of the cat. Exp Neurol. 61(1):96–120. doi: 10.1016/0014-4886(78)90184-x. [DOI] [PubMed] [Google Scholar]
- Collignon O, Champoux F, Voss P, Lepore F. 2011. Sensory rehabilitation in the plastic brain. Prog Brain Res. 191:211–231. doi: 10.1016/B978-0-444-53752-2.00003-5. [DOI] [PubMed] [Google Scholar]
- Cuppini C, Magosso E, Rowland B, Stein B, Ursino M. 2012. Hebbian mechanisms help explain development of multisensory integration in the superior colliculus: a neural network model. Biol Cybern. 106(11–12):691–713. doi: 10.1007/s00422-012-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakos AS, Jiang H, Stein BE, Rowland BA. 2020. Using the principles of multisensory integration to reverse hemianopia. Cereb Cortex. 30(4):2030–2041. doi: 10.1093/cercor/bhz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakos AS, Walker EM, Jiang H, Stein BE, Rowland BA. 2019. Interhemispheric visual competition after multisensory reversal of hemianopia. Eur J Neurosci. 50(11):3702–3712. doi: 10.1111/ejn.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo M-M. 2004. Spike timing-dependent plasticity of neural circuits. Neuron. 44(1):23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dundon NM, Bertini C, Làdavas E, Sabel BA, Gall C. 2015a. Visual rehabilitation: visual scanning, multisensory stimulation and vision restoration trainings. Front Behav Neurosci. 9:192. doi: 10.3389/fnbeh.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundon NM, Làdavas E, Maier ME, Bertini C. 2015b. Multisensory stimulation in hemianopic patients boosts orienting responses to the hemianopic field and reduces attentional resources to the intact field. Restor Neurol Neurosci. 33(4):405–419. doi: 10.3233/RNN-140457. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Rosenquist AC. 2001. Ibotenic acid lesions in the pedunculopontine region result in recovery of visual orienting in the hemianopic cat. Neuroscience. 106(4):765–781. [DOI] [PubMed] [Google Scholar]
- Feldman DE. 2012. The spike-timing dependence of plasticity. Neuron. 75(4):556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti F, Bolognini N, Bottari D, Bonora A, Làdavas E. 2005. Audiovisual integration in patients with visual deficit. J Cogn Neurosci. 17(9):1442–1452. doi: 10.1162/0898929054985446. [DOI] [PubMed] [Google Scholar]
- Frémaux N, Gerstner W. 2015. Neuromodulated spike-timing-dependent plasticity, and theory of three-factor learning rules. Front Neural Circuits. 9:85. doi: 10.3389/fncir.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. 2011. Motor functions of the superior colliculus. Annu Rev Neurosci. 34:205–231. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. 2014. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol. 8:1919–1927. doi: 10.2147/OPTH.S59452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadid V, Lepore F. 2017. From cortical blindness to conscious visual perception: theories on neuronal networks and visual training strategies. Front Syst Neurosci. 11:64. doi: 10.3389/fnsys.2017.00064. [accessed 2017 Sep 7]. http://journal.frontiersin.org/article/10.3389/fnsys.2017.00064/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Kamigaki T, Zhang Z, Zhang S, Dan U, Dan Y. 2019. Prefrontal corticotectal neurons enhance visual processing through the superior colliculus and pulvinar thalamus. Neuron. 104(6):1141–1152.e4. doi: 10.1016/j.neuron.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rowland BA, Stein BE. 2020. Reversing hemianopia by multisensory training under anesthesia. Front Syst Neurosci. 14:4. doi: 10.3389/fnsys.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG. 2015. Multisensory training reverses midbrain lesion-induced changes and ameliorates haemianopia. Nat Commun. 6:7263. doi: 10.1038/ncomms8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Rowland BA, Stein BE. 2007. Multisensory orientation behavior is disrupted by neonatal cortical ablation. J Neurophysiol. 97(1):557–562. doi: 10.1152/jn.00591.2006. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. 2002. Two corticotectal areas facilitate multisensory orientation behavior. J Cogn Neurosci. 14(8):1240–1255. doi: 10.1162/089892902760807230. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. 2006. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol. 95(3):1380–1396. doi: 10.1152/jn.00880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Stein BE. 2003. Cortex controls multisensory depression in superior colliculus. J Neurophysiol. 90(4):2123–2135. doi: 10.1152/jn.00369.2003. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. 2001. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 85(2):506–522. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Kato R, Isa K, Kenta K, Kazuto K, Onoe H, Isa T. 2019. Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nat Commun. 10(1):135. doi: 10.1038/s41467-018-08058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Bogadhi AR, Herman JP, Bollimunta A. 2018. Selective attention without a neocortex. Cortex. 102:161–175. doi: 10.1016/j.cortex.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zénon A. 2013. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Làdavas E. 2008. Multisensory-based approach to the recovery of unisensory deficit. Ann N Y Acad Sci. 1124:98–110. doi: 10.1196/annals.1440.008. [DOI] [PubMed] [Google Scholar]
- Lee KH, Tran A, Turan Z, Meister M. 2020. The sifting of visual information in the superior colliculus. Elife. 9:e50678. doi: 10.7554/eLife.50678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo F, Bolognini N, Passamonti C, Stein BE, Làdavas E. 2008. Cross-modal localization in hemianopia: new insights on multisensory integration. Brain 131(Pt3):855–865. doi: 10.1093/brain/awn003. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Horel JA. 1999. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 86(2):179–194. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P. 2001. Role of the superior colliculus in analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J Comp Neurol. 441(1):44–57. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. 1983. A chronic headholder minimizing facial obstructions. Brain Res Bull. 10(6):859–860. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Norita M, Dunning DD, Stein BE. 1993. Corticotectal relationships: direct and “indirect” corticotectal pathways. Prog Brain Res. 95:139–150. [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. 2005. Subcortical loops through the basal ganglia. Trends Neurosci. 28(8):401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. 1985. Descending efferents from the superior colliculus relay integrated multisensory information. Science (New York, NY). 227(4687):657–659. doi: 10.1126/science.3969558 [DOI] [PubMed] [Google Scholar]
- Miller RL, Stein BE, Rowland BA. 2017. Multisensory integration uses a real-time unisensory-multisensory transform. J Neurosci. 37(20):5183–5194. doi: 10.1523/JNEUROSCI.2767-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Pélisson D, Guitton D. 1991. Movement of neural activity on the superior colliculus motor map during gaze shifts. Science. 251(4999):1358–1360. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. 1995. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J Neurophysiol. 73(6):2334–2348. [DOI] [PubMed] [Google Scholar]
- Nagy A, Eördegh G, Paróczy Z, Márkus Z, Benedek G. 2006. Multisensory integration in the basal ganglia. Eur J Neurosci. 24(3):917–924. doi: 10.1111/j.1460-9568.2006.04942.x. [DOI] [PubMed] [Google Scholar]
- Nagy AJ, Berényi A, Gulya K, Norita M, Benedek G, Nagy A. 2011. Direct projection from the visual associative cortex to the caudate nucleus in the feline brain. Neurosci Lett. 503(1):52–57. doi: 10.1016/j.neulet.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. 1994. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 101(1):123–139. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. 1996. Substitution of visual by auditory inputs in the cat’s anterior ectosylvian cortex. Prog Brain Res. 112:313–323. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Korte M. 1993. Auditory compensation for early blindness in cat cerebral cortex. J Neurosci. 13(10):4538–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokszin A, Márkus Z, Braunitzer G, Berényi A, Benedek G, Nagy A. 2010. Visual pathways serving motion detection in the mammalian brain. Sensors (Basel). 10(4):3218–3242. doi: 10.3390/s100403218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist AC, Palmer LA. 1971. Visual receptive field properties of cells of the superior colliculus after cortical lesions in the cat. Exp Neurol. 33(3):629–652. [DOI] [PubMed] [Google Scholar]
- Rowland B, Jiang W, Stein B. 2014. Brief cortical deactivation early in life has long-lasting effects on multisensory behavior. J Neurosci. 34:7198–7202. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland B, Stanford T, Stein B. 2007a. A Bayesian model unifies multisensory spatial localization with the physiological properties of the superior colliculus. Exp Brain Res. 180(1):153–161. doi: 10.1007/s00221-006-0847-2. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Quessy S, Stanford TR, Stein BE. 2007b. Multisensory integration shortens physiological response latencies. J Neurosci. 27(22):5879–5884. doi: 10.1523/JNEUROSCI.4986-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. 1986. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 6(3):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. 2008. Visual perception and corollary discharge. Perception. 37(3):408–418. doi: 10.1068/p5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL. 1986. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 66(1):118–171. [DOI] [PubMed] [Google Scholar]
- Sprague JM. 1966. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 153(3743):1544–1547. [DOI] [PubMed] [Google Scholar]
- Sprague JM, Meikle TH. 1965. The role of the superior colliculus in visually guided behavior. Exp Neurol. 11:115–146. [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. 1981. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol. 19(3–4):180–192. [DOI] [PubMed] [Google Scholar]
- Stein BE, Goldberg SJ, Clamann HP. 1976. The control of eye movements by the superior colliculus in the alert cat. Brain Res. 118(3):469–474. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. 1993. The merging of the senses. Cambridge, Mass: MIT Press (Cognitive neuroscience series). [Google Scholar]
- Stein BE, Rowland BA. 2020. Using superior colliculus principles of multisensory integration to reverse hemianopia. Neuropsychologia. 141:107413. doi: 10.1016/j.neuropsychologia.2020.107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. 2014. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 15(8):520–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. 1993. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 69(6):1797–1809. [DOI] [PubMed] [Google Scholar]
- Wallace SF, Rosenquist AC, Sprague JM. 1989. Recovery from cortical blindness mediated by destruction of nontectotectal fibers in the commissure of the superior colliculus in the cat. J Comp Neurol. 284(3):429–450. doi: 10.1002/cne.902840309. [DOI] [PubMed] [Google Scholar]
- Wallace SF, Rosenquist AC, Sprague JM. 1990. Ibotenic acid lesions of the lateral substantia nigra restore visual orientation behavior in the hemianopic cat. J Comp Neurol. 296(2):222–252. doi: 10.1002/cne.902960204. [DOI] [PubMed] [Google Scholar]
- Wang L, McAlonan K, Goldstein S, Gerfen CR, Krauzlis RJ. 2020a. A causal role for mouse superior colliculus in visual perceptual decision-making. J Neurosci. 40(19):3768–3782. doi: 10.1523/JNEUROSCI.2642-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu L, Xu J, Stein BE, Rowland BA. 2020b. Experience creates the multisensory transform in the superior colliculus. Front Integr Neurosci. 14:18. doi: 10.3389/fnint.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA, Stein BE. 1996. The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp Brain Res. 112(1):1–10. [DOI] [PubMed] [Google Scholar]
- Wolf AB, Lintz MJ, Costabile JD, Thompson JA, Stubblefield EA, Felsen G. 2015. An integrative role for the superior colliculus in selecting targets for movements. J Neurophysiol. 114(4):2118–2131. doi: 10.1152/jn.00262.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yu L, Rowland BA, Stein BE. 2017. The normal environment delays the development of multisensory integration. Sci Rep. 7(1):4772. doi: 10.1038/s41598-017-05118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Rowland BA, Xu J, Stein BE. 2013a. Multisensory plasticity in adulthood: cross-modal experience enhances neuronal excitability and exposes silent inputs. J Neurophysiol. 109(2):464–474. doi: 10.1152/jn.00739.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Stein BE, Rowland BA. 2009. Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci. 29(50):15910–15922. doi: 10.1523/JNEUROSCI.4041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Xu J, Rowland BA, Stein BE. 2013b. Development of cortical influences on superior colliculus multisensory neurons: effects of dark-rearing. Eur J Neurosci. 37:1594–1601. doi: 10.1111/ejn.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. 2006. Natural history of homonymous hemianopia. Neurology. 66(6):901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]


