Abstract
Superagers are older adults who maintain youthful memory despite advanced age. Previous studies showed that superagers exhibit greater structural and intrinsic functional brain integrity, which contribute to their youthful memory. However, no studies, to date, have examined brain activity as superagers learn and remember novel information. Here, we analyzed functional magnetic resonance imaging data collected from 41 young and 40 older adults while they performed a paired associate visual recognition memory task. Superaging was defined as youthful performance on the long delay free recall of the California Verbal Learning Test. We assessed the fidelity of neural representations as participants encoded and later retrieved a series of word stimuli paired with a face or a scene image. Superagers, like young adults, exhibited more distinct neural representations in the fusiform gyrus and parahippocampal gyrus while viewing visual stimuli belonging to different categories (greater neural differentiation) and more similar category representations between encoding and retrieval (greater neural reinstatement), compared with typical older adults. Greater neural differentiation and reinstatement were associated with superior memory performance in all older adults. Given that the fidelity of cortical sensory processing depends on neural plasticity and is trainable, these mechanisms may be potential biomarkers for future interventions to promote successful aging.
Keywords: dedifferentiation, episodic memory, reinstatement, representational similarity analysis, successful aging
Introduction
As people age, their episodic memory typically declines (Grady and Craik 2000; Park et al. 2002; Koen and Yonelinas 2014), yet there is substantial individual variation. We and others have recently been investigating ``superagers'', who are older individuals whose episodic recall rivals that of middle-aged adults (Harrison et al. 2012; Rogalski et al. 2013; Gefen et al. 2014, 2015) and even young adults (Sun et al. 2016; Harrison et al. 2018; Zhang et al. 2019; Dang, Harrington, et al. 2019). Age-related declines in memory are typically associated with reduction in the structural and functional integrity within the default mode and salience networks (Andrews-Hanna et al. 2007; McGinnis et al. 2011; Bakkour et al. 2013; Fjell et al. 2014; Ward et al. 2015; Touroutoglou et al. 2018), which are hypothesized to subserve diverse psychological phenomena (Barrett 2017; Kleckner et al. 2017), including aspects of memory encoding, storage, and retrieval (Dickerson and Eichenbaum 2010; Kim 2010, 2011; Rugg and Vilberg 2013; Sestieri et al. 2014). Superaging, by contrast, is associated with structure (e.g., cortical thickness; Harrison et al. 2012; Rogalski et al. 2013; Sun et al. 2016) and function (e.g., intrinsic functional connectivity; Zhang et al. 2019) within these networks, which do not statistically differ from the brains of young adults. However, no studies, to date, have measured brain activity during encoding of new information and its subsequent retrieval to examine the individual differences in episodic memory related to superaging.
Inefficient encoding is one major factor contributing to age-related memory deficits (Craik and Rose 2012; Friedman and Johnson 2014). On average, older adults are less likely to encode new information with relevant contextual details when compared with young adults, resulting in less distinctive mental representations of similar episodes (Craik and Jacoby 1979; Craik and Simon 1980). Successful memory encoding draws upon the formation and binding of distinct representations of multimodal sensory experiences (Gottlieb et al. 2012; Pidgeon and Morcom 2016; Cooper and Ritchey 2020) as well as the organization of these representations based on their conceptual similarity (Kumaran et al. 2016; Rolls 2016). This is, in part, subserved by cortical regions that process certain categories of information. For example, encoding visual stimuli from different categories, such as faces and scenes, is typically associated with an increase in selective activation in the fusiform face area and parahippocampal place area of ventral visual cortex, respectively (Epstein et al. 1999; Kanwisher and Yovel 2006). On average, older adults tend to show less-selective activation in these areas when compared with young adults, a phenomenon known as age-related “neural dedifferentiation” (Baltes and Lindenberger 1997; Park et al. 2004; Voss et al. 2008; Park et al. 2010; Carp et al. 2011; Park et al. 2012; Koen and Rugg 2019).
Neural dedifferentiation is thought to be one mechanism that contributes to age-related memory decline (Li et al. 2000, 2001; Trelle et al. 2019). Memory performance depends, at least in part, on the extent to which differentiated neural activation patterns are present during encoding and are reinstated during subsequent retrieval (Kuhl et al. 2011; Staresina et al. 2012; Ritchey et al. 2013; Gordon et al. 2014; Yaffe et al. 2014; Wing et al. 2015). In both young and older adults, neural differentiation in the parahippocampal place area during memory encoding predicts subsequent recognition memory for scenes (Koen et al. 2019; Srokova et al. 2020). In the ventral visual areas that are typically sensitive to category-related information, older adults also tend to show weaker neural reinstatement during retrieval when compared with young adults (McDonough et al. 2014; St-Laurent et al. 2014; Bowman et al. 2019; Trelle et al. 2020; but see Wang et al. 2016); weaker neural reinstatement, in turn, predicts older adults’ poorer memory performance (Hill et al. 2020; Trelle et al. 2020). Some studies have reported that the degree of category-related neural reinstatement during retrieval mediates the relationship between brain activity during encoding and subsequent memory performance (Gordon et al. 2014; Trelle et al. 2020), suggesting that encoding- and retrieval-related activity related to the representation of distinct stimulus categories make partially distinct contributions to subsequent memory. Taken together, these findings suggest that age-related changes in neural differentiation during encoding and in neural reinstatement during retrieval may contribute to age-related memory differences. Accordingly, we developed the following overarching hypothesis: Individuals with youthful memory performance may show a youthful pattern of neural differentiation of perceptual signals during experiences, followed by youthful reinstatement of those signals during subsequent memory retrieval.
In the present study, we report functional magnetic resonance imaging (fMRI) data while young and older adult participants, including a sample of superagers (Sun et al. 2016; Zhang et al. 2019), performed a paired associate recognition memory task (Andreano et al. 2017) in which they encoded and later retrieved face–word or scene–word pairs. Following previous investigations of neural dedifferentiation (e.g., Park et al. 2004, 2012), we defined our regions of interest (ROIs) in the fusiform gyrus and parahippocampal gyrus using an unbiased method to select cortical vertices that were equally and maximally sensitive to both stimulus categories (Gagnon et al. 2019; Chamberlain et al. 2021). Indices of neural differentiation and reinstatement were derived using representational similarity (RS) analysis and were compared across young adults, superagers, and typical older adults. Based on the available evidence on age-related neural dedifferentiation, we hypothesized that 1) superagers would show more youthful neural differentiation during encoding of visual stimuli relative to typical older adults and that the degree of neural differentiation in superagers would be comparable to that in young adults; 2) within the entire group of older adults, greater neural differentiation during encoding would predict superior memory task performance; 3) greater neural differentiation during encoding would predict greater category-related neural reinstatement during retrieval; and that 4) the relationship between neural differentiation during encoding and subsequent memory performance would be mediated by category-related neural reinstatement during retrieval.
Materials and Methods
Participants
In this work, we analyzed task-related fMRI data from the same sample of young adults, superagers, and typical older adults from which we have previously published evidence for youthful brain structure and intrinsic functional connectivity (Sun et al. 2016; Zhang et al. 2019). Specifically, 91 participants (43 females, 48 males) were initially recruited from the Greater Boston area, consisting of 47 young adults (ages: 18–35; 23 females, 24 males) and 44 older adults (ages: 60–80; 20 females, 24 males), to be part of a longitudinal study that is part of our Massachusetts General Hospital Brain Resilience in Aging: Integrated Neuroscience Studies (BRAINS) program. All participants were right-handed native English speakers with normal or corrected-to-normal vision and with no history of substance neurological or psychiatric disorder. Additionally, all participants scored within 1.5 standard deviations (SDs) of published normative values on all neuropsychological screening tests. The experimental protocols involving human subjects were approved by the Mass General Brigham Healthcare System Institutional Review Board. All experiments were undertaken with the understanding and written consent of each participant. Ten participants were excluded due to incomplete study procedure, resulting in a final sample of 41 young adults (mean age = 24.5 ± 3.6 years; 21 females, 20 males) and 40 older adults (mean age = 66.9 ± 5.5 years; 23 females, 17 males), as reported previously (Sun et al. 2016; Zhang et al. 2019). One older adult participant was excluded from the analyses of recognition memory due to being an outlier; another older adult participant was excluded from the analyses of neural reinstatement due to incomplete fMRI data acquired during retrieval. We defined superagers as those older participants who performed at or above the mean for young adults (ages: 18–32) on the long delay free recall measure of the California Verbal Learning Test (CVLT; Delis et al. 1987) (males: 13; females: 14) and no lower than 1 SD below the mean for their age group on the Trail Making Test Part B (TMT; Tombaugh 2004). Demographic information, memory task data, fMRI data derivatives, and analysis code are available at https://osf.io/yq97g/ (last accessed: June 1, 2021). Raw data are also available upon request. Demographic characteristics and neuropsychological data of our subject sample are summarized in Table 1.
Table 1.
Demographic information and neuropsychological data
| Measure | YA | SA | TOA | Group differences | ||
|---|---|---|---|---|---|---|
| n | 41 | 17 | 23 | SA versus YA | TOA versus YA | SA versus TOA |
| Sex (% female) | 51.2 | 70.6 | 34.8 | SA > YA*** | TOA < YA*** | |
| Age (years) | 24.5 (3.6) | 67.8 (6.0) | 66.2 (5.1) | |||
| Education (years) | 16.0 (2.2) | 17.2 (2.2) | 16.2 (2.0) | |||
| CVLT Long Delay Free Recall (16) | 13.2 (2.2) | 15.0 (0.9) | 11.0 (2.2) | SA > YA** | TOA < YA** | SA > TOA*** |
| TMT B (s) | 51.2 (17.0) | 59.0 (12.8) | 66.3 (30.3) | TOA < YA* | ||
| Item recognition memory (d′) | 2.2 (0.9) | 2.2 (0.7) | 1.7 (0.7) | TOA < YA* | SA > TOA* | |
| Associative recognition memory (d′) | 1.3 (0.7) | 1.1 (0.8) | 0.7 (0.6) | TOA < YA** | SA > TOA† | |
Note: The maximum score for CVLT Long Delay Free Recall is 16; s, seconds; d′, recognition memory discriminability index computed as z(Hits) − z(FAs). Table includes some data on this sample reported in Sun et al. (2016) as well as additional data on memory task performance during fMRI scanning.
*P < 0.05.
**P < 0.01.
***P < 0.001.
†P < 0.08.
Experimental Design
All participants arrived at the laboratory to first complete a comprehensive neuropsychological battery, including the CVLT and the TMT. Within 2 weeks, participants returned for a scanning session in which they performed a paired associate memory task previously described (Andreano et al. 2017; Zhang et al. 2019). The experimental stimuli consisted of 120 face–word pairs and 120 scene–word pairs, which were carefully chosen to be affectively neutral. Face stimuli were selected from the Center for Vital Longevity Face Database (Minear and Park 2004) and depicted male and female faces representing multiple age groups. Scene stimuli were obtained from the International Affective Picture System (Lang et al. 2008), which depicted a variety of outdoor scenes consisting of urban and natural environments in different weather conditions. These and similar stimuli have been widely used in previous studies of age-related neural dedifferentiation (Goh et al. 2010; Koen et al. 2019; Trelle et al. 2019, 2020; Hill et al. 2020; Srokova et al. 2020). Word stimuli were selected from the Medical Research Council Psycholinguistic Database (Coltheart 1981). All words were adjectives selected for high frequency and high concreteness. During the encoding phase, participants viewed a total of 80 image–word pairs (40 from each image category) equally distributed across four runs; each pair was presented on the screen for 6 s. To ensure depth of encoding, for each image–word pair, participants were asked to subjectively judge whether the word semantically matched the associated image. The image–word pairs included, for instance, an image of an older female face paired with a word “RESPONSIBLE,” a younger male face paired with “AVERAGE,” a cityscape paired with “INDUSTRIAL,” and an image of woods paired with “FRIENDLY,” among others. The task did not progress to the next trial until the judgment was made via a button press. Following a retention interval of approximately 10 min, participants were presented with all 80 image–word pairs that had been learned during encoding; additional 40 pairs consisting of new words and images; and 40 rearranged pairs consisting of words and images seen previously, but not previously associated with each other. During this retrieval phase, participants were asked to indicate via a button press whether they had previously seen each image–word pair during the encoding phase, or whether it was a completely new or rearranged pair (yes/no).
Behavioral Data Analysis
Participants’ responses during the retrieval phase of the memory task were coded as Hits (old image–word pairs correctly identified as old), Misses (old pairs incorrectly identified as new), Correct Rejections (CRs; new pairs correctly classified as new), or False Alarms (FA; new pairs incorrectly identified as old). Recognition accuracy was computed for each participant in terms of d′, a measure that controls for individual response bias: d′ = z(Hits) − z(FA). d′ was calculated separately to distinguish between the discriminability of previously encoded pairs versus novel pairs and that of previously encoded pairs versus rearranged pairs (Andreano et al. 2017), which were considered indices of item versus associative recognition memory, respectively (Zhang et al. 2019).
MRI Data Acquisition
Imaging data were acquired on a 3T Magnetom Tim Trio scanner (Siemens Medical Systems, Iselin, NJ). Structural images were acquired using a T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence: TR/TE/FA = 2530 ms/3.48 ms/7°, slice thickness = 1 mm, field of view = 256 × 256 mm2, 176 sagittal slices, and 0% slice gap. fMRI data were acquired using a T2*-weighted gradient-echo sequence: TR/TE/flip angle = 3000 ms/30 ms/90°, voxel resolution = 3.4 × 3.4 × 2 mm3, 56 slices, and phase encoding direction = anterior–posterior.
Structural MRI Data Preprocessing and Analysis
MRI data preprocessing and analytical steps involved in the present study are summarized in Figure 1. Each participant’s MPRAGE data underwent intensity normalization, skull stripping, and an automated segmentation of cerebral white matter to locate the gray/white boundary via the Freesurfer image analysis suite (v5.3), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/; last accessed on June 1, 2021). Defects in the surface topology were corrected (Fischl et al. 2001), and the gray/white boundary was deformed outward using an algorithm designed to obtain an explicit representation of the pial surface. Each participant’s cortical surface reconstruction was parcellated into the standard Desikan–Killiany atlas (Desikan et al. 2006), allowing delineation of the fusiform gyrus and the parahippocampal gyrus in the participants’ native surface space.
Figure 1 .
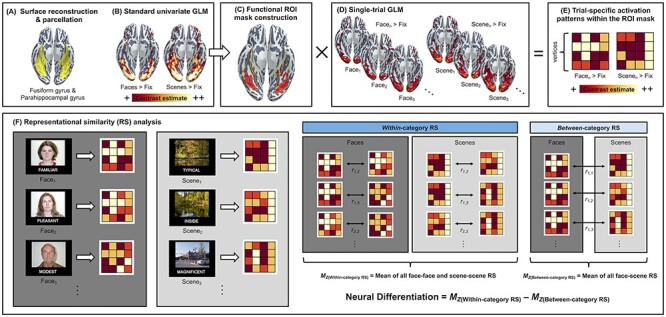
Schematic diagram of the analytical pipeline. (A) Surface reconstruction was performed using FreeSurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/; last accessed on June 1, 2021) on each participant’s T1-weighted MPRAGE structural image to derive the parcellation of bilateral fusiform and parahippocampal gyri in their native cortical surface space (highlighted in yellow). (B) For each participant, a standard univariate analysis of brain activity during encoding was performed in a GLM framework using FreeSurfer’s FS-FAST v6.0. This yielded two whole-brain contrast estimate maps identifying mean activation differences between faces versus fixation and scenes versus fixation, which were used for the creation of participant-specific ROIs. (C) For each participant, the top 5000 vertices showing maximal contrast estimates for the faces versus fixation and scenes versus fixation contrasts were identified within the anatomical boundaries of the fusiform and parahippocampal gyri, generating a participant-specific functional ROI masks (for details, see Materials and Methods). (D) For each participant, a single-trial GLM was performed to derive whole-brain contrast estimates unique to each trial. (E) Using the functional ROI masks, trial-specific activation estimates were derived for all trials. (F) Finally, these trial-specific activation estimates were used to compute within-category and between-category RS. Within-category RS was defined as the mean of Pearson’s r values calculated for all unique pairs of face–face or scene–scene trials. Between-category RS was defined as the mean of Pearson’s r values calculated for all unique pairs of face–scene trials. Neural differentiation was computed for each participant by subtracting the mean of between-category RS from the mean of within-category RS, where larger values indicate greater neural differentiation. This measure represents how distinct regional brain activation patterns are when viewing items that are from the same category relative to when viewing items that are from different categories.
fMRI Data Preprocessing and Analysis
Functional data were preprocessed via FreeSurfer’s functional analysis stream (FS-FAST, v6.0) involving template volume creation, brain masking, intensity normalization, functional-to-anatomical coregistration, motion correction, surface resampling, and surface-constrained smoothing using a 2D Gaussian kernel with full-width-half-maximum of 5 mm.
Univariate GLM Analysis
Preprocessed functional data were then used as input for participant-level vertex-wise analysis of blood oxygen level–dependent (BOLD) activation in a general linear model (GLM) framework. Specifically, task-evoked hemodynamic responses associated with the two experimental conditions (i.e., faces and scenes) were modeled by convolution with a canonical hemodynamic response function. The GLM included one regressor per condition as the events of interest; the estimated motion parameters for each run as well as a quadratic trend were also modeled as the events of no interest. This model yielded for each hemisphere and participant two contrast images identifying differential BOLD activation associated with the events of interest relative to the baseline (i.e., faces vs. fixation, scenes vs. fixation). To estimate the trial-specific BOLD activation, we constructed a GLM in which hemodynamic responses associated with each trial were modeled as a separate regressor (Rissman et al. 2004). This model yielded for each hemisphere and participant a contrast image identifying differential BOLD activation associated with each trial relative to baseline (e.g., face1 vs. fixation, face2 vs. fixation) for a total of 80 encoding and 160 retrieval trials.
Functional ROI Construction
Next, we constructed participant-specific functional ROIs based on their fusiform and parahippocampal gyrus parcellations reconstructed via FreeSurfer as well as the estimated univariate GLM contrast maps, following the procedures described in previous studies of neural differentiation (Gagnon et al. 2019; Lalwani et al. 2019; Chamberlain et al. 2021). Specifically, we first sorted (in descending order) the vertices within each participant’s fusiform/parahippocampal mask based on the magnitude of BOLD activation separately for the two experimental contrasts (faces vs. fixation, scenes vs. fixation). We then defined the functional ROIs by alternating between the two sorted lists of vertices, adding the most active vertex that has not already been included for one contrast, then adding the most active vertex that has not been included for the other contrast. This vertex identification procedure was repeated until the target ROI size was reached. Following a recent study (Chamberlain et al. 2021), we used a size of 5000 vertices in all analyses. This unbiased approach to defining the functional ROIs allowed inclusion of vertices that are guaranteed to be sensitive to both faces and scenes in each and every participant, without making any a priori assumption about the loci of group differences in activation, if any, within the anatomical boundaries of the fusiform gyrus and the parahippocampal gyrus (see Supplementary Fig. S1 for the extent of spatial overlap in the functional ROIs per group).
RS Analysis
Using the functional ROIs and a series of trial-specific contrast images, we calculated RS to define neural differentiation during encoding and category-related neural reinstatement during retrieval. Similar methods have been used by previous investigations of neural differentiation and/or reinstatement (Gagnon et al. 2019; Koen et al. 2019; Trelle et al. 2019, 2020; Hill et al. 2020; Srokova et al. 2020; Chamberlain et al. 2021). Specifically, for each participant and for each trial, we extracted vertex-wise contrast estimates from within their functional ROI masks, resulting in a vector of 5000 vertices per trial. For the analysis of neural differentiation, we computed Pearson’s r values for all unique pairs of trials during encoding, which were subsequently z-transformed. We defined neural differentiation as the difference between the mean of all within-category RS (i.e., face–face and scene–scene) and the mean of all between-category RS (i.e., face–scene). For the analysis of category-related neural reinstatement, we computed Pearson’s r values for all unique pairs of encoding and retrieval trials belonging to the same category (i.e., faceencoding − faceretrieval, sceneencoding − sceneretrieval) which were z-transformed and averaged across all trial pairs.
Brain-Behavior Regression Analysis
We conducted brain-behavior regression analyses to assess the degree to which neural differentiation or reinstatement (predictor variables) predicts recognition memory performance (outcome variables) in the entire group of older adults. In addition, we also conducted regression analyses to examine the role of neural differentiation and reinstatement as predictors for verbal recall memory as measured by the CVLT. All brain-behavior regression analyses were conducted using IBM SPSS Statistics (version 26.0; IBM Corp., 2017). Results were considered statistically significant at P < 0.05.
Mediation Analysis of the Relationship between Neural Differentiation and Memory Performance
To assess the contribution of neural differentiation and reinstatement to recognition memory performance, we performed a mediation analysis using PROCESS macro version 3.5 for SPSS (Hayes 2017; Touroutoglou et al. 2018). Specifically, we modeled neural differentiation during encoding as the predictor variable (X), item or associative recognition memory performance as the outcome variable (Y), and category-related neural reinstatement during retrieval as the mediator variable (M). In Step 1 of our mediation analysis, associative recognition memory performance was regressed on neural differentiation during encoding to examine the total effect of neural differentiation on memory (path c). In Step 2, category-related neural reinstatement during retrieval was regressed on neural differentiation during encoding (path a). In Step 3, associative recognition memory performance was regressed on category-related neural reinstatement during retrieval, while controlling for the effect of neural differentiation during encoding (path b). In Step 4, we calculated the indirect effect ab as an index of the degree to which category-related neural reinstatement during retrieval mediated the effect of neural differentiation during encoding on associative memory performance. We repeated the same analysis to examine whether category-related neural reinstatement during retrieval mediated the effect of neural differentiation during encoding on item memory performance. For each model, a bias-corrected bootstrap 95% confidence interval (CI) was generated for the indirect effect based on 5000 bootstrap samples. An empirical 95% CI not including zero indicated a significant indirect effect.
Results
Generalization of Superior Verbal Memory Performance to Visual Recognition Memory
We first examined the generalizability of youthful memory in superagers using the challenging visual–verbal paired associate recognition memory task administered during fMRI scanning. We previously reported performance on this task in Zhang et al. (2019), where we examined the relationship between this measure and intrinsic functional connectivity. Here, we report these behavioral data with additional details, given that we analyzed in this study the task-related fMRI data acquired while participants performed this memory encoding and retrieval paradigm. A one-way analysis of variance (ANOVA) with Group (young adults, superagers, and typical older adults) as a factor conducted separately for each memory type revealed that these groups differed on both item (F(2, 77) = 3.26, P < 0.044, η2 = 0.078) and associative (F(2, 77) = 4.41, P < 0.015, η2 = 0.103) recognition memory performance. Follow-up t-tests showed that, as expected, superagers performed better on item recognition (t(37) = 2.42, P < 0.011, one-tailed, Cohen’s d = 0.80, 95% CI = [0.49, 1.08]) and marginally better on associative recognition (t(37) = 1.44, P < 0.080, one-tailed, Cohen’s d = 0.48, 95% CI = [0.08, 0.72]) than typical older adults. Remarkably, superagers did not differ from young adults on this challenging memory test, whether measured by associative recognition memory (t(56) = 1.04, P < 0.30) or item recognition memory (t(56) = −0.10, P < 0.92). By contrast, typical older adults performed worse than young adults on both item recognition (t(61) = 2.29, P < 0.025, Cohen’s d = 0.61, 95% CI = [0.35, 0.89]) and associative recognition (t(61) = 3.15, P < 0.003, Cohen’s d = 0.85, 95% CI = [0.63, 1.09]) (for descriptive statistics, see Table 1).
Superagers Exhibit Youthful Neural Differentiation during Encoding of Distinct Categories of Visual Information
Next, we examined the degree of neural differentiation during encoding of face–word and scene–word pairs. To this end, we calculated RS within the participant-specific functional ROI masks for all unique pairs of trials during encoding. We defined neural differentiation as the difference between the mean of all within-category RS (i.e., face–face and scene–scene) and that of all between-category RS (i.e., face–scene), where larger values indicate greater selectivity in neural activation when encoding items from the same category versus items from different categories. A two-way mixed ANOVA with Group (young adults, superagers, and typical older adults) and Condition type (within- and between-category) as factors revealed a significant Group × Condition-type interaction: F(2, 78) = 3.63, P < 0.031, 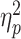 = 0.085. Follow-up t-tests showed that, as predicted, superagers exhibited greater neural differentiation when compared with typical older adults (t(38) = 2.22, P < 0.033). Superagers and young adults showed a similar pattern of neural differentiation (t(56) = −0.094, P < 0.93). By contrast, typical older adults had reduced neural differentiation when compared with young adults (t(62) = 2.56, P < 0.013) (Fig. 2A). Interestingly, these effects were driven largely by group differences in within- but not between-category RS (Fig. 2B,C), suggesting that they reflect the variability in the fidelity of neural representations within each of the two stimulus categories. As a complementary analysis, we performed a two-way ANOVA using the entire fusiform and parahippocampal gyrus masks for each participant rather than restricting the analysis space to their subregions that we identified functionally as being maximally responsive to face and scene stimuli. This analysis similarly revealed a significant Group × Condition-type interaction: F(2, 78) = 4.39, P < 0.016, ηp2 = 0.101.
= 0.085. Follow-up t-tests showed that, as predicted, superagers exhibited greater neural differentiation when compared with typical older adults (t(38) = 2.22, P < 0.033). Superagers and young adults showed a similar pattern of neural differentiation (t(56) = −0.094, P < 0.93). By contrast, typical older adults had reduced neural differentiation when compared with young adults (t(62) = 2.56, P < 0.013) (Fig. 2A). Interestingly, these effects were driven largely by group differences in within- but not between-category RS (Fig. 2B,C), suggesting that they reflect the variability in the fidelity of neural representations within each of the two stimulus categories. As a complementary analysis, we performed a two-way ANOVA using the entire fusiform and parahippocampal gyrus masks for each participant rather than restricting the analysis space to their subregions that we identified functionally as being maximally responsive to face and scene stimuli. This analysis similarly revealed a significant Group × Condition-type interaction: F(2, 78) = 4.39, P < 0.016, ηp2 = 0.101.
Figure 2 .
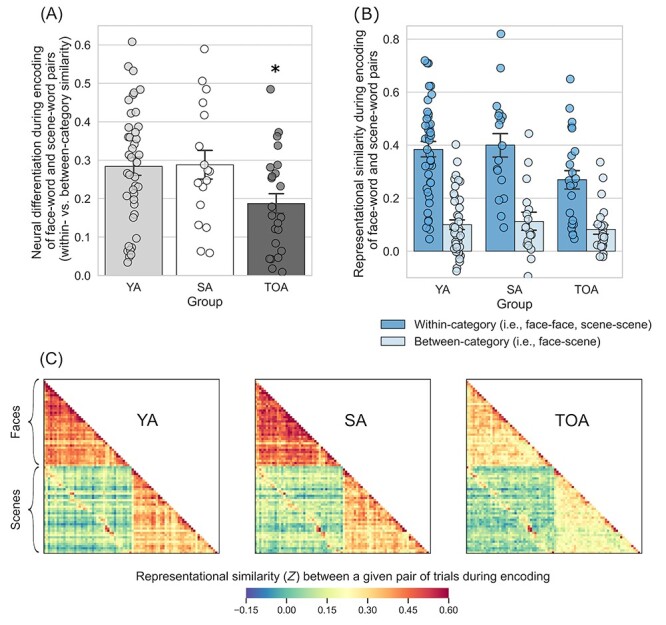
Superagers exhibit more youthful neural differentiation during encoding of distinct categories of visual information. (A) During the encoding of face–word pairs and scene–word pairs, superagers exhibited neural differentiation similar to that of young adults, while typical older adults showed reduced neural differentiation (*TOA is different from YA and SA at P < 0.05). Neural differentiation is a measure that represents how distinct regional brain activation patterns are when viewing items that are from the same category relative to when viewing items that are from different categories (see Fig. 1 for more details on this measure). (B) The effect shown in (A) was driven primarily by group differences in within-category RS (i.e., comparing face–face or scene–scene trial pairs; shown in dark blue bars). (C) Group average RS matrices illustrate the effects quantified in (A) and (B). YA, young adults; SA, superagers; TOA, typical older adults. Error bars denote one standard error of the mean.
To ensure that the observed effects were not driven by the group differences in the overall responsiveness of the ventral visual cortex, we additionally performed a two-way analysis of covariance (ANCOVA) controlling for the mean univariate activation calculated either within the entire anatomical (i.e., fusiform and parahippocampal gyrus) masks or specifically within the functional ROIs where univariate activation was maximal for each participant. Regardless of how the univariate activation was defined, the ANCOVA still yielded a significant Group × Condition-type interaction (anatomical ROIs: F(2, 77) = 4.03, P < 0.022, ηp2 = 0.095; functional ROIs: F(2, 77) = 4.49, P < 0.014, ηp2 = 0.104), suggesting that the results of our RS analysis were not driven by the differences in the responsivity of the ventral visual cortex across groups. In addition, we also performed comparisons of neural differentiation between subgroups of young adults based on relatively higher versus lower performance on the CVLT. This analysis did not reveal significant group differences, suggesting that the observed differences between superagers and typical older adults do not merely reflect differences in cognitive function irrespective of aging (see Supplementary Text S1).
The initial ANOVA also yielded a significant main effect of Condition type (F(1, 78) = 208.71, P < 0.001, ηp2 = 0.73), revealing, as expected, greater RS for within-category trial pairs than for between-category pairs on average. On average, RS for face–face trial pairs was greater than that for scene–scene trial pairs (see Supplementary Text S2). A main effect of Group was identified as a trend (F(1, 78) = 2.40, P < 0.097, ηp2 = 0.058) because it did not reach a conventional level of statistical significance of α < 0.05.
Youthful Patterns of Neural Differentiation during Encoding Supports Better Memory in Older Adults
To assess the behavioral significance of neural differentiation during encoding, we performed a linear regression analysis within the entire group of older adults. The predictor variable in our regression models was the degree of neural differentiation during encoding of image–word pairs, whereas the outcome variable was memory performance calculated as recognition discriminability, d′, separately for item and associative recognition memory. This analysis showed that, as predicted, greater (more youthful) neural differentiation predicted better recognition memory performance in older adults (item: r = 0.50, P < 0.001; associative: r = 0.58, P < 0.001) (Fig. 3). In addition, we analyzed neural differentiation based on RS during retrieval and its relation to recognition memory performance. These analyses yielded results similar to those observed during encoding (see Supplementary Text S3 and Supplementary Fig. S2).
Figure 3 .
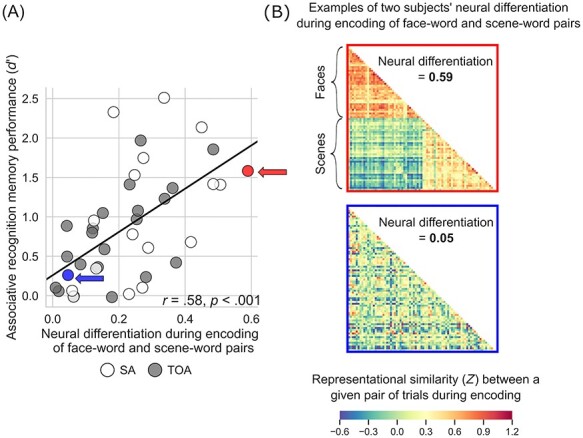
Youthful neural differentiation supports better memory in older adults. (A) Older adults with greater neural differentiation during encoding of distinct categories of visual information show better associative memory for that information after a delay. That is, older adults whose brains function more like those of young adults to differentiate all face–word pairs from all scene–word pairs when learning the information remember more individual image–word pairs when later tested on that information. Memory performance is expressed as d′ (recognition discriminability; see Materials and Methods). (B) The neural differentiation effect shown in (A) is illustrated using RS matrices from two individuals labeled in (A): a 70-year-old superager (indicated by a red circle/arrow in (A); top matrix here) whose RS for within-category trial pairs was 0.49, whose RS for between-category trial pairs was −0.10, neural differentiation was thus 0.59, and whose d′ was 1.58; and a 64-year-old typical older adult (indicated by a blue circle/arrow in (A); bottom matrix here) whose RS for within-category trial pairs was 0.10, whose RS for between-category trial pairs was 0.05, neural differentiation was thus 0.05, and whose d′ was 0.26. These matrices show, at the individual level, the effect shown at the group level in Figure 2C: When superagers learn information, their regional brain activity shows greater RS within distinct categories of information than when typical older adults learn information, and they remember that information better later.
Within-Category Neural Reinstatement during Retrieval Mediates the Relationship between Neural Differentiation during Encoding and Memory Performance
We defined “within-category neural reinstatement” during retrieval following similar procedures to derive neural differentiation during encoding. Specifically, we calculated RS within the participant-specific functional ROI masks between all unique pairs of encoding and retrieval trials belonging to the same category (i.e., faceencoding − faceretrieval, sceneencoding − sceneretrieval), which were averaged across all trial pairs. Larger values indicated a greater degree of similarity in the neural activation between the encoding and retrieval phases when viewing items of the same category. A one-way ANOVA revealed a significant effect of Group on neural reinstatement: F(2, 77) = 3.96, P < 0.023, η2 = 0.093. Follow-up t-tests revealed a pattern of group differences similar to that observed with the analysis of neural differentiation during encoding. Specifically, superagers exhibited greater category-related neural reinstatement when compared with typical older adults (t(37) = 2.84, P < 0.007) but not when compared with young adults (t(56) = 0.70, P < 0.49). Young adults also showed greater category-related neural reinstatement when compared with typical older adults (t(61) = 2.37, P < 0.021). We then performed simple linear regression analysis within the entire group of older adults to examine the relationship between neural differentiation during encoding and category-related neural reinstatement during retrieval. This analysis showed that greater neural differentiation during encoding predicted greater category-related neural reinstatement during retrieval in older adults (r = 0.79, P < 0.001) (Fig. 4A). In the entire group of older adults, both neural differentiation and reinstatement were positively associated with CVLT performance (see Supplementary Fig. S3).
Figure 4 .
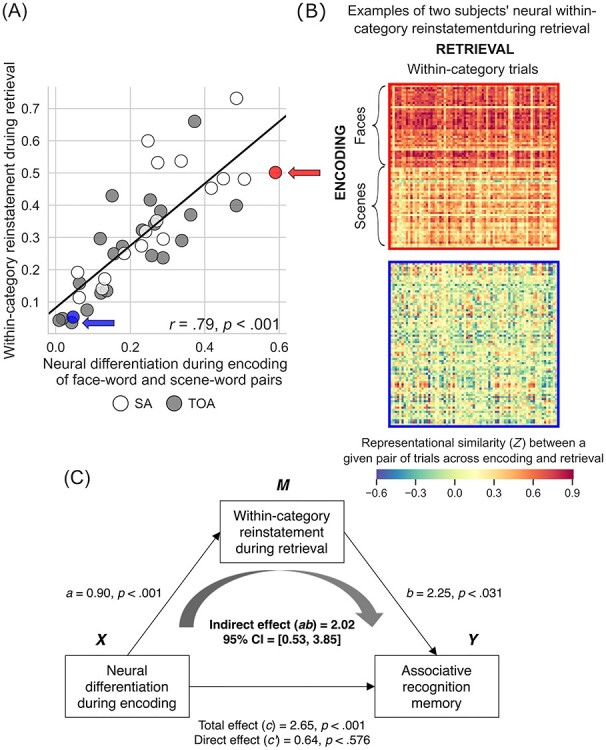
Within-category neural reinstatement during retrieval mediates the relationship between neural differentiation during encoding and memory performance. (A) Older adults with greater neural differentiation during encoding also show greater within-category neural reinstatement during retrieval. Within-category neural reinstatement was defined as the mean of Pearson’s r values calculated for all unique pairs of encoding and retrieval trials within the same categories (i.e., face–face or scene–scene) (for details, see Materials and Methods). (B) Within-category neural reinstatement depicted in (A) is illustrated using RS matrices from the same two individuals shown in (A) and in Figure 3: a 70-year-old superager (indicated by a red circle/arrow in (A); top matrix here) whose within-category neural reinstatement was 0.61/0.38 for face–word/scene–word pairs; and a 64 year-old typical older adult (indicated by a blue circle/arrow in (A); bottom matrix here) whose within-category neural reinstatement was 0.07/0.05 for face–word/scene–word pairs. These matrices show that the brains of superagers exhibit greater RS than that of typical older adults when the regional brain activity during the initial learning of categories of visual information is compared with regional brain activity when that information is subsequently retrieved from memory. (C) The degree of within-category neural reinstatement during retrieval fully mediated the relationship between neural differentiation during encoding and associative recognition memory performance. That is, older adults who show greater neural differentiation during encoding of visual information in distinct categories also show greater reinstatement (i.e., a higher degree of similarity in brain activity between encoding and retrieval); greater reinstatement, in turn, leads them to remember more individual image–word pairs when tested.
Finally, we assessed the contribution of these neural indices to behavior by modeling both in a simple mediation analysis to predict the memory performance in the entire group of older adults. We modeled neural differentiation during encoding as the predictor variable, recognition memory performance as the outcome variable, and category-related neural reinstatement during retrieval as the mediator variable. As predicted, this analysis revealed a significant indirect effect of neural differentiation during encoding on associative recognition memory through category-related neural reinstatement during retrieval (indirect effect [ab] = 2.02, 95% CI [0.53, 3.85]; Fig. 4C); older adults who initially showed greater neural differentiation during encoding also showed greater category-related reinstatement at retrieval, which, in turn, led them to remember more individual image–word pairs when tested. We identified similarly significant results when we modeled item recognition memory as the outcome variable (ab = 1.99, 95% CI [0.54, 3.69]).
Discussion
The results reported here extend our previous findings (Sun et al. 2016; Zhang et al. 2019) by demonstrating that superagers, similar to young adults, exhibited more distinct patterns of activation in the ventral visual areas while viewing visual stimuli belonging to different categories (i.e., greater neural differentiation during encoding) than typical older adults. Within the entire group of older adults, youthful neural differentiation in these brain regions supported better subsequent memory. That is, older adults whose brains enabled more distinct representation of the different categories of visual information subsequently remembered more of that information after a delay. Additionally, older adults who showed more category-selective neural activation patterns during encoding also showed more similar activation patterns while subsequently retrieving information from memory compared with when it was first learned (i.e., greater category-related neural reinstatement during retrieval). Older adults’ ability to reinstate the initial category-related neural representations at retrieval, in turn, led them to accurately remember more image–word pairs.
Superagers are typically defined as older individuals with superior free recall of verbal material using a 15- or 16-word list (Harrison et al. 2012, 2018; Rogalski et al. 2013; Gefen et al. 2015; Sun et al. 2016; Zhang et al. 2019; Dang, Harrington, et al. 2019; Dang, Yassi, et al. 2019). Here, we employed a much more challenging 80-pair visual paired associate recognition memory task and found that superagers’ memory performance was not only better than that of typical older adults but—remarkably—not different from that of young adults. Our results demonstrate one potential neural mechanism associated with this superior memory performance: When older adults with superior memory saw distinct categories of visual images arbitrarily paired with words, the category-selectivity of ventral visual cortical activation was stronger than that of typical older adults and similar to that of young adults. Older adults with stronger category-selectivity in ventral visual activation during encoding of image–word pairs subsequently remembered them better. When participants saw image–word pairs later and were asked if they remembered the specific pairing of items, those with better memory exhibited category-selective activation patterns which were more similar to when they had initially learned these items. That is, older adults who are categorized as superagers using a test dependent on a completely different sensory modality (i.e., auditory) show greater fidelity of visual cortical processing during the learning and retrieval of image–word pairs, compared with typical older adults.
Our results revealed that greater neural differentiation during encoding in superagers when compared with typical older adults was driven by decreased similarity in the activation patterns for within-category (vs. between-category) trial pairs in typical older adults. That is, superagers exhibited more similar activation patterns across different exemplars of the same category when compared with typical older adults. This result is overall consistent with the evidence based on multivariate analyses showing that age differences in neural differentiation were driven by differences in within-category similarity (Carp et al. 2011; Chamberlain et al. 2021). Several factors could contribute to the age-related reduction in the fidelity of activation patterns within these regions. Aging is typically associated with a reduction in dopaminergic neurons and receptor density in the brain (Bäckman et al. 2006, 2010; Lindenberger 2014). Deficient dopamine function, in turn, has been associated with decreased signal-to-noise ratio in neuronal activity, possibly contributing to inefficient stimulus detection (Li et al. 2001; Lövdén et al. 2007; Li and Rieckmann 2014; Abdulrahman et al. 2017). Alternatively, the reduced activation fidelity in the visual cortex might reflect age-dependent deficits in the attentional modulation of neural representations. The prefrontal cortex—as part of the larger frontoparietal network—is thought to subserve attentional modulation of stimulus feature representations in the visual cortical areas by selectively enhancing goal-relevant (e.g., category-specific) and inhibiting goal-irrelevant information (Jehee et al. 2011; Baldauf and Desimone 2014; Lee et al. 2018; Zheng et al. 2018). Aging is commonly associated with functional alterations in the prefrontal cortex (Cabeza and Dennis 2013), which may lead to less-successful modulation of sensory representations. In this context, norepinephrine may be another relevant biomarker. Mediated by the locus coeruleus, norepinephrine facilitates selective representation of salient stimuli while suppressing that of nonsalient ones (Mather et al. 2016; Mather and Harley 2016). Reduced noradrenergic system activity is linked to impairment in selective attention in older adults (Lee et al. 2018; Dahl et al. 2020). Following prior work, future studies on superaging could incorporate magnetic resonance spectroscopy data (Cassady et al. 2019; Gagnon et al. 2019; Lalwani et al. 2019; Chamberlain et al. 2021) and analyses of task-related functional connectivity (Burianová et al. 2013) to test these hypotheses.
Additionally, our findings support the idea that superagers are able, during retrieval, to reactivate the neural representations of stimulus categories initially formed during encoding to a greater degree than typical older adults. Successful memory retrieval involves the reimplementation of representations initially active during encoding, as indexed by the extent of cortical reinstatement observed at retrieval (Rugg et al. 2015; Xue 2018). The memory of visual information is dependent on the neural reinstatement of perceptual signals in the ventral visual cortex (Kuhl et al. 2011; Gordon et al. 2014). On average, older adults tend to show category-related neural reinstatement in the ventral visual areas to a lesser degree than young adults (McDonough et al. 2014; St-Laurent et al. 2014; Bowman et al. 2019; Trelle et al. 2020; but see Wang et al. 2016). One possibility is that superagers fall at the upper end of the distribution of all older adults, capturing those who are capable of reinstating category-related brain activation at retrieval with greater fidelity; this may, in turn, facilitate their youthful memory abilities. Furthermore, we also found that superior verbal free recall as measured by the CVLT was positively associated with greater fidelity of visual cortical activation during encoding and retrieval when examined continuously beyond the categorical distinction of superagers versus typical older adults. This finding suggests that, regardless of whether the relationship is analyzed categorically or continuously, activation fidelity in the ventral visual cortex is a robust predictor of superior memory recall in older adults.
Finally, our mediation analysis demonstrated that neural differentiation during encoding and category-related neural reinstatement during retrieval make distinct contributions to the recognition memory performance in older adults. This is consistent with prior work showing similarly additive effects of encoding- and retrieval-related brain activity on subsequent memory in the young (Gordon et al. 2014) and older (Trelle et al. 2020) adults. Studies reporting age-related differences in neural differentiation have only recently begun investigating whether neural differentiation at encoding and category-related reinstatement at retrieval predict memory performance. Our findings are consistent with a recent study showing that greater neural differentiation during encoding predicts greater category-related reinstatement during retrieval across age groups (Hill et al. 2020). We extend these findings by identifying a relationship between neural differentiation and reinstatement along with their synergistic contributions predicting associative recognition memory performance in older adults. These results support the hypothesis that both the formation of distinct neural representations during initial encoding and the intact accessibility of such representations during retrieval are critical for memory performance (Trelle et al. 2019).
No study is without limitations. First, with a sample size of 17 superagers, our study was limited in its power to detect small effect sizes. Indeed, some of the observed effects, particularly the behavioral differences between superagers and typical older adults (e.g., associative recognition), were rather modest and would not reach conventional levels of statistical significance if a two-tailed test was used, making it important for future studies to replicate these effects in a larger sample of participants. Second, the present study focused on the fusiform gyrus and the parahippocampal gyrus as a priori ROIs in order to increase the robustness of results. It therefore remains unknown whether superagers might differ from typical older adults in the engagement of other regions that play a role in episodic memory. This seems plausible given that superagers’ youthful functional connectivity in the default mode and salience networks is associated with superior memory task performance (Zhang et al. 2019). The frontoparietal network may also be relevant, given its role in the attentional modulation of sensory representations via noradrenergic modulation (Mather and Harley 2016; Lee et al. 2018). Third, our older adult sample as a whole was relatively young, with the mean age of 66.9 years old corresponding to the typical retirement age. Future studies should therefore investigate samples consisting of even older participants (e.g., 80+). Furthermore, in the current study, participants were aware that their memory for image–word pairs would be subsequently tested. Future studies might examine the degree to which similar results are obtained when participants incidentally encode such stimuli. Finally, to ensure the robustness of the observed effects, future studies should also employ experimental stimuli depicting a variety of content beyond faces and scenes drawn from common databases.
Conclusion
In sum, the findings reported here advance our understanding of the neural substrates of superaging by pointing to the role of neural differentiation and reinstatement in the ventral visual cortex as one of the potential mechanisms subserving the superagers’ superior memory performance. These findings have implications not only for our understanding of the possible mechanisms of successful cognitive aging but also for possible interventions to promote brain health. Given that the fidelity of cortical sensory processing is dependent on neural plasticity and is possible to improve with training (Seger and Miller 2010; van der Linden et al. 2013; Lindenberger and Lövdén 2019), neural differentiation may be one potential biomarker to be targeted by future interventions to promote superaging.
Supplementary Material
Contributor Information
Yuta Katsumi, Department of Psychology, Northeastern University, Boston, MA 02115, USA; Japan Society for the Promotion of Science, Tokyo 1020083, Japan; Frontotemporal Disorders Unit, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Joseph M Andreano, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Lisa Feldman Barrett, Department of Psychology, Northeastern University, Boston, MA 02115, USA; Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Bradford C Dickerson, Frontotemporal Disorders Unit, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Alexandra Touroutoglou, Frontotemporal Disorders Unit, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Notes
This research was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at MGH, using the resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant that is supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the Neuroimaging Analysis Center, P41EB015902, a P41 supported by NIBIB. This work also involved the use of instrumentation supported by the National Institutes of Health (NIH) Shared Instrumentation Grant Program; specifically, S10RR017208-01A1, S10RR026666, S10RR022976, S10RR019933, S10RR023043, and S10RR023401. Conflict of Interest: None declared.
Funding
Research reported in this publication was supported by the National Institute of Aging under Award Numbers R01AG030311 and R21AG061743 and the National Institute On Deafness and Other Communication Disorders of the National Institutes of Health under Award Number K23DC016912. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional personnel support was provided by the Japan Society for the Promotion of Science.
References
- Abdulrahman H, Fletcher PC, Bullmore E, Morcom AM. 2017. Dopamine and memory dedifferentiation in aging. Neuroimage. 153:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Touroutoglou A, Dickerson BC, Barrett LF. 2017. Resting connectivity between salience nodes predicts recognition memory. Soc Cogn Affect Neurosci. 12:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S-C, Nyberg L. 2010. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. 34:670–677. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. 2006. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 30:791–807. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC. 2013. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 76:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf D, Desimone R. 2014. Neural mechanisms of object-based attention. Science. 344:424–427. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. 1997. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 12:12–21. [DOI] [PubMed] [Google Scholar]
- Barrett LF. 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CR, Chamberlain JD, Dennis NA. 2019. Sensory representations supporting memory specificity: age effects on Behavioral and neural discriminability. J Neurosci. 39:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianová H, Lee Y, Grady CL, Moscovitch M. 2013. Age-related dedifferentiation and compensatory changes in the functional network underlying face processing. Neurobiol Aging. 34:2759–2767. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. 2013. Frontal lobes and aging: deterioration and compensation. In: Principles of frontal lobe function. 2nd ed. New York: Oxford University Press, [Google Scholar]. [Google Scholar]
- Carp J, Park J, Polk TA, Park DC. 2011. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage. 56:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, et al. 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage. 186:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JD, Gagnon H, Lalwani P, Cassady KE, Simmonite M, Seidler RD, Taylor SF, Weissman DH, Park DC, Polk TA. 2021. GABA levels in ventral visual cortex decline with age and are associated with neural distinctiveness. Neurobiol Aging. 102:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M. 1981. The MRC psycholinguistic database. Q J Exp Psychol Sect A. 33:497–505. [Google Scholar]
- Cooper RA, Ritchey M. 2020. Progression from feature-specific brain activity to hippocampal binding during episodic encoding. J Neurosci. 40:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Jacoby LL. 1979. Elaboration and Distinctiveness in Episodic Memory. In: Nilsson L, editor. Perspectives on memory research. Hillsdale, NJ: Halsted Press Division of Wiley. [Google Scholar]
- Craik FIM, Rose NS. 2012. Memory encoding and aging: a neurocognitive perspective. Neurosci Biobehav Rev. 36:1729–1739. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Simon E. 1980. Age differences in memory: the roles of attention and depth of processing. In: New directions in memory and aging. Hillsdale (NJ): Erlbaum, p. 20. [Google Scholar]
- Dahl MJ, Mather M, Sander MC, Werkle-Bergner M. 2020. Noradrenergic responsiveness supports selective attention across the adult lifespan. J Neurosci. 40:4372–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C, Harrington KD, Lim YY, Ames D, Hassenstab J, Laws SM, Yassi N, Hickey M, Rainey-Smith SR, Robertson J, et al. 2019. Superior memory reduces 8-year risk of mild cognitive impairment and dementia but not amyloid β-associated cognitive decline in older adults. Arch Clin Neuropsychol. 34:585–598. [DOI] [PubMed] [Google Scholar]
- Dang C, Yassi N, Harrington KD, Xia Y, Lim YY, Ames D, Laws SM, Hickey M, Rainey-Smith S, Sohrabi HR, et al. 2019. Rates of age- and amyloid β-associated cortical atrophy in older adults with superior memory performance. Alzheimers Dement Diagn Assess Dis Monit. 11:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. 1987. California verbal learning test: adult version. Manual. San Antonio (TX): The Psychological Corporation. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. 2010. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 35:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. 1999. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 23:115–125. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. 2001. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB. 2014. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 24:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R. 2014. Inefficient encoding as an explanation for age-related deficits in recollection-based processing. J Psychophysiol. 28:148–161. [Google Scholar]
- Gagnon H, Simmonite M, Cassady K, Chamberlain J, Freiburger E, Lalwani P, Kelley S, Foerster B, Park DC, Petrou M, et al. 2019. Michigan neural distinctiveness (MiND) study protocol: investigating the scope, causes, and consequences of age-related neural dedifferentiation. BMC Neurol. 19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, Bigio EH, Weintraub S, Rogalski E, Mesulam M-M, et al. 2015. Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci. 35:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Shaw E, Whitney K, Martersteck A, Stratton J, Rademaker A, Weintraub S, Mesulam M-M, Rogalski E. 2014. Longitudinal neuropsychological performance of cognitive super agers. J Am Geriatr Soc. 62:1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. 2010. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 51:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. 2014. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex. 24:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb LJ, Wong J, de CM, Rugg MD. 2012. Neural correlates of the encoding of multimodal contextual features. Learn Mem. 19:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI. 2000. Changes in memory processing with age. Curr Opin Neurobiol. 10:224–231. [DOI] [PubMed] [Google Scholar]
- Harrison TM, Maass A, Baker SL, Jagust WJ. 2018. Brain morphology, cognition, and β-amyloid in older adults with superior memory performance. Neurobiol Aging. 67:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam M-M, Rogalski E. 2012. Superior memory and higher cortical volumes in unusually successful cognitive aging. J Int Neuropsychol Soc. 18:1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. 2017. Introduction to mediation, moderation, and conditional process analysis: second edition: a regression-based approach. New York: Guilford Press. [Google Scholar]
- Hill PF, King DR, Rugg MD. 2020. Age differences in retrieval-related reinstatement reflect age-related dedifferentiation at encoding. Cereb Cortex. 31:106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehee JFM, Brady DK, Tong F. 2011. Attention improves encoding of task-relevant features in the human visual cortex. J Neurosci. 31:8210–8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. 2006. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc B Biol Sci. 361:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. 2010. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 50:1648–1657. [DOI] [PubMed] [Google Scholar]
- Kim H. 2011. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 54:2446–2461. [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF. 2017. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. 1:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Hauck N, Rugg MD. 2019. The relationship between age, neural differentiation, and memory performance. J Neurosci. 39:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Rugg MD. 2019. Neural dedifferentiation in the aging brain. Trends Cogn Sci. 23:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. 2014. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: a meta-analytic review. Neuropsychol Rev. 24:332–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. 2011. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci U S A. 108:5903–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, McClelland JL. 2016. What learning systems do intelligent agents need? Complementary learning systems theory updated. Trends Cogn Sci. 20:512–534. [DOI] [PubMed] [Google Scholar]
- Lalwani P, Gagnon H, Cassady K, Simmonite M, Peltier S, Seidler RD, Taylor SF, Weissman DH, Polk TA. 2019. Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. Neuroimage. 201:116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Gainesville (FL): University of Florida. [Google Scholar]
- Lee T-H, Greening SG, Ueno T, Clewett D, Ponzio A, Sakaki M, Mather M. 2018. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat Hum Behav. 2:356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Frensch PA. 2000. Unifying cognitive aging: from neuromodulation to representation to cognition. Neurocomputing. 32–33:879–890. [Google Scholar]
- Li S-C, Lindenberger U, Sikström S. 2001. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 5:479–486. [DOI] [PubMed] [Google Scholar]
- Li S-C, Rieckmann A. 2014. Neuromodulation and aging: implications of aging neuronal gain control on cognition. Curr Opin Neurobiol. 29:148–158. [DOI] [PubMed] [Google Scholar]
- Lindenberger U. 2014. Human cognitive aging: Corriger la fortune? Science. 346:572–578. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Lövdén M. 2019. Brain plasticity in human lifespan development: the exploration-selection-refinement model. Annu Rev Dev Psychol. 1:197–222. [Google Scholar]
- Lövdén M, Li S-C, Shing YL, Lindenberger U. 2007. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: longitudinal data from the Berlin Aging Study. Neuropsychologia. 45:2827–2838. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. 2016. Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci. 39:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Harley CW. 2016. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 20:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Cervantes SN, Gray SJ, Gallo DA. 2014. Memory’s aging echo: age-related decline in neural reactivation of perceptual details during recollection. Neuroimage. 98:346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. 2011. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 24:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M, Park DC. 2004. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 36:630–633. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. 2002. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. 2004. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 101:13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Hebrank A, Park DC, Polk TA. 2010. Neural specificity predicts fluid processing ability in older adults. J Neurosci. 30:9253–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang C-M, Rieck JR, Polk TA, Park DC. 2012. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 32:2154–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon LM, Morcom AM. 2016. Cortical pattern separation and item-specific memory encoding. Neuropsychologia. 85:256–271. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. 2004. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 23:752–763. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R. 2013. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex. 23:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Mesulam M-M. 2013. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci. 25:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2016. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol Learn Mem. 129:4–28. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Uncapher MR. 2015. Encoding and retrieval in episodic memory: insights from fMRI. In: Addis DR, Barense M, Duarte A, editors. The Wiley handbook on the cognitive neuroscience of memory. Chichester, UK: John Wiley & Sons, Ltd., pp. 84–107. [Google Scholar]
- Rugg MD, Vilberg KL. 2013. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Miller EK. 2010. Category learning in the brain. Annu Rev Neurosci. 33:203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. 2014. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 26:551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srokova S, Hill PF, Koen JD, King DR, Rugg MD. 2020. Neural differentiation is moderated by age in scene- but not face-selective cortical regions. eNeuro. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RNA, Kriegeskorte N, Alink A. 2012. Episodic reinstatement in the medial temporal lobe. J Neurosci. 32:18150–18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Bondad A, Buchsbaum BR. 2014. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. J Neurosci. 34:4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC. 2016. Youthful brains in older adults: preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. J Neurosci. 36:9659–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN. 2004. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 19:203–214. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Zhang J, Andreano JM, Dickerson BC, Barrett LF. 2018. Dissociable effects of aging on salience subnetwork connectivity mediate age-related changes in executive function and affect. Front Aging Neurosci. 10:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelle AN, Carr VA, Guerin SA, Thieu MK, Jayakumar M, Guo W, Nadiadwala A, Corso NK, Hunt MP, Litovsky CP, et al. 2020. Hippocampal and cortical mechanisms at retrieval explain variability in episodic remembering in older adults. Elife. 9:e55335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelle AN, Henson RN, Simons JS. 2019. Neural evidence for age-related differences in representational quality and strategic retrieval processes. Neurobiol Aging. 84:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M, Wegman J, Fernández G. 2013. Task- and experience-dependent cortical selectivity to features informative for categorization. J Cogn Neurosci. 26:319–333. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF. 2008. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Res. 1244:121–131. [DOI] [PubMed] [Google Scholar]
- Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. 2016. The effects of age on the neural correlates of recollection success, recollection-related cortical reinstatement, and post-retrieval monitoring. Cereb Cortex. 26:1698–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, Sperling RA. 2015. Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiol Aging. 36:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. 2015. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci. 27:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G. 2018. The neural representations underlying human episodic memory. Trends Cogn Sci. 22:544–561. [DOI] [PubMed] [Google Scholar]
- Yaffe RB, Kerr MSD, Damera S, Sarma SV, Inati SK, Zaghloul KA. 2014. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc Natl Acad Sci U S A. 111:18727–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Andreano JM, Dickerson BC, Touroutoglou A, Barrett LF. 2019. Stronger functional connectivity in the default mode and salience networks is associated with youthful memory in superaging. Cereb Cortex. 30:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Gao Z, Xiao X, Ye Z, Chen C, Xue G. 2018. Reduced fidelity of neural representation underlies episodic memory decline in normal aging. Cereb Cortex. 28:2283–2296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


